Abstract
There is limited knowledge regarding the cardiovascular impact of coronavirus disease 2019 (COVID-19) on emerging adults aged 18-25, a group that disproportionately contracts COVID-19. To guide future cardiovascular disease (CVD) research, policy, and practice, a scoping review was conducted to: (i) examine the impact of the COVID-19 pandemic on the cardiovascular health of emerging adults; and (ii) identify strategies to screen for and manage COVID-19–related cardiovascular complications in this age group. A comprehensive search strategy was applied to several academic databases and grey literature sources. An updated search yielded 6738 articles, 147 of which were extracted and synthesized. Reports identified COVID-19–associated cardiac abnormalities, vascular alterations, and multisystem inflammatory syndrome in emerging adults; based on data from student-athlete samples, prevalence estimates of myocarditis and cardiac abnormalities were 0.5%-3% and 0%-7%, respectively. Obesity, hypertension, CVD, congenital heart disease, and marginalization are potential risk factors for severe COVID-19, related cardiovascular complications, and mortality in this age group. As a screening modality for COVID-19–associated cardiac involvement, it is recommended that cardiac magnetic resonance imaging be indicated by a positive cardiac history and/or abnormal “triad” testing (cardiac troponin, electrocardiogram, and transthoracic echocardiogram) to improve diagnostic utility. To foster long-term cardiovascular health among emerging adults, cardiorespiratory fitness, health literacy and education, and telehealth accessibility should be priorities of health policy and clinical practice. Ultimately, surveillance data from the broader emerging adult population will be crucial to assess the long-term cardiovascular impact of both COVID-19 infection and vaccination, guide screening and management protocols, and inform CVD prevention efforts.
Résumé
Il existe peu de données portant sur les répercussions de la maladie à coronavirus 2019 (COVID-19) sur le plan cardiovasculaire chez les jeunes adultes âgés de 18 à 25 ans, un groupe contractant la COVID-19 de façon disproportionnée. Afin d’orienter la recherche, les poli-tiques et les pratiques en matière de maladies cardiovasculaires (MCV), un examen exploratoire a été réalisé dans le but i) d’examiner les conséquences de la pandémie de la COVID-19 sur la santé cardiovasculaire des jeunes adultes, et ii) de proposer des stratégies de dépistage et de prise en charge des complications cardiovasculaires associées à la COVID-19 chez les personnes de cette tranche d’âge. Une recherche initiale exhaustive a été réalisée dans plusieurs bases de données universitaires et sources de littérature grise. Les résultats actualisés de cette recherche ont permis de recenser 6 738 articles, dont 147 ont été extraits et synthétisés. Les rapports faisaient état d’anomalies cardiaques, d’altérations vasculaires et de cas du syndrome inflammatoire multisystémique, tous associés à la COVID-19 chez les jeunes adultes. À la lumière des données sur les échantillons d’étudiants-athlètes, la prévalence des myocardites et des anomalies cardiaques se situait respectivement entre 0,5 et 3 %, et entre 0 et 7 % environ. Chez ce même groupe d’âge, l’obésité, l’hypertension, les MCV, les cardiopathies congénitales et la marginalisation constituent des facteurs de risque de COVID-19 sévère, de complications cardiovasculaires associées à la COVID-19 et de mortalité. Dans le cadre du dépistage des atteintes cardiaques associées à la COVID-19, il est recommandé, pour améliorer l’utilité diagnostique, d’indiquer l’imagerie par résonance magnétique cardiaque lors de l’existence d’antécédents cardiaques ou à la suite d’une « triade » de dépistages anormaux (la troponine cardiaque, l’électrocardiogramme et l’échocardiographie transthoracique). Afin de favoriser une bonne santé cardiovasculaire à long terme chez les jeunes adultes, il est recommandé que la capacité cardiorespiratoire, la littératie dans le domaine de la santé, l’éducation et l’accès à la télésanté soient intégrés à titre de priorités dans les politiques de santé et la pratique clinique. En définitive, les données de surveillance portant sur cette large tranche d’âge seront essentielles pour évaluer les répercussions cardiovasculaires à long terme (autant celles d'infections à la COVID-19 que celles de la vaccination), pour orienter les protocoles de dépistage et de prise en charge, ainsi que pour éclairer les efforts de prévention des MCV.
Coronavirus disease 2019 (COVID-19) illness severity varies among those infected, and though the majority of individuals present with a mild cough and flu-like symptoms, others may experience pneumonia, virus-related cardiac injury, and/or death.1 Individuals with cardiovascular comorbidities are at a greater risk of experiencing more severe COVID-19, likely due to the virus exacerbating pre-existing complications through cardiac involvement.2,3 However, even in otherwise healthy individuals, infection can lead to alterations in heart structure (eg, fibrosis) and function. Karbalai Saleh et al.4 found that nearly 30% of their COVID-19 hospitalized patients (mean age = 59 years) experienced cardiac injury, which was associated with a nearly 2-fold increase in risk of short-term mortality. Moreover, there are data to suggest that individuals may be delaying or avoiding medical treatment for cardiovascular emergencies in response to the pandemic, likely resulting in worse cardiovascular outcomes and mortality. For example, cardiac centres in Canada reported a 30% reduction in emergency visits for ST-elevation myocardial infarctions earlier in 2020.5
The impact of COVID-19 on the cardiovascular health of emerging adults
The majority of research on COVID-19 has focused on cardiovascular complications in vulnerable populations, such as older adults and those with pre-existing chronic conditions.4,6 Research specific to emerging adults (ie, those aged 18-257,8) is lacking; however, there is preliminary evidence demonstrating myocarditis and cardiac abnormalities in as many as 58% of college athletes following COVID-19.9, 10, 11 As of February 2022, Canadians between the ages of 20 and 29 (accounting for 13% of the total population12) represented nearly one-fifth (n = 610,151) of COVID-19 cases and accounted for 4.8% (n = 6346), 3.1% (n = 699), and 0.3% (n = 106) of hospitalizations, intensive care unit (ICU) admissions, and deaths, respectively.13 Cardiovascular complications, including cardiogenic shock and arrhythmias, have been observed in COVID-19 patients 18 years old and younger.14 Multisystem inflammatory syndrome in children/adults (MIS-C/A) and life-threatening cardiovascular presentations (eg, myocarditis) have also been reported among young adults with or after COVID-19, the long-term consequences of which remain unknown.15 Furthermore, in light of global vaccination efforts, new evidence has emerged concerning the cardiovascular safety of COVID-19 vaccines (eg, vaccine-related myocarditis) in younger age groups.16,17 Because emerging adults disproportionately contract COVID-19,18,19 determining the short- and long-term impacts of infection on cardiovascular function and cardiovascular disease (CVD) risk will be of value to clinicians and policymakers and better inform COVID-19 vaccination policy.
Objectives
The current knowledge gaps surrounding the impact of COVID-19 on the cardiovascular health of emerging adults relate to: (i) the prevalence of COVID-19–related cardiovascular presentations/complications; (ii) the appropriate screening and management of such conditions; and (iii) cardiovascular care. Therefore, to address the needs of various knowledge users (eg, policymakers, programme planners, and health care providers), a scoping review was conducted to: (i) describe the impact of the COVID-19 pandemic on the cardiovascular health of emerging adults; and (ii) identify strategies to screen for and manage cardiovascular complications in emerging adults.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR)20 guided this scoping review. Scoping reviews are conducted to examine the extent, range, and nature of the evidence surrounding a given topic and often precede systematic reviews when evidence in an area is new or limited. These reviews map findings from a broader evidence set and identify current gaps in the literature to support future research.20 In comparison, systematic reviews additionally appraise the literature (eg, assess risk of bias) and are more appropriate when there are clearly defined research questions.
Search strategy
In consultation with a research librarian from McMaster University, the search strategy was developed and applied to the following bibliographic databases: MEDLINE (via Ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, Web of Science, PsycInfo (via ProQuest), and Sociological Abstracts. All identified keywords and index terms related to COVID-19, emerging adults, and the cardiovascular conditions of interest were included in the search and adapted for each database (Supplemental Table S1). Bibliographic databases were initially searched on January 22, 2021, and updated on January 16, 2022; no date limits were applied.
Additional searches were conducted up until January 16, 2022, to locate grey literature from national and international sources, including the Cardiac Health Foundation of Canada, Heart and Stroke Foundation of Canada, Hypertension Canada, McMaster Health Forum, and World Health Organization.
Inclusion criteria
English-language articles were required to include the following: (i) emerging adults (individuals aged approximately 18-25 ± 2) as the main participant group or subgroup, or postsecondary student samples with a mean age between 18 and 25; (ii) a COVID-19 context; and (iii) at least one of the cardiovascular conditions of interest (Box 1). The age range defining emerging adults varies across the literature, often focusing on 18- to 25-year-olds but occasionally spanning 18-29.7,8 The former definition (ie, ages 18-25) was selected by the research team; however, when applicable, 18- to 29-year-old cohorts were included in the review. All study designs were considered for inclusion.
Box 1 Cardiovascular conditions included in the screening criteria.
-
•
Hypertension or high blood pressure
-
•
Arrhythmia (includes tachycardia, bradycardia, atrial fibrillation)
-
•
Myocardial infarction
-
•
Heart failure
-
•
Cardiac arrest
-
•
Coronary heart disease or ischemic heart disease
-
•
Stroke or cerebrovascular accident
-
•
Transient ischemic attack
-
•
Valvular heart disease
-
•
Cardiomyopathy (includes myocarditis)
-
•
Cardiovascular abnormalities
Exclusion criteria
Studies were excluded if they: (i) focused on emerging adults who were pregnant or studying/working in health care; (ii) did not reference COVID-19 or the cardiovascular conditions outlined in the inclusion criteria; or (iii) were not available in full text, with the exception of case reports and case series. Articles related to COVID-19 vaccines were excluded in the updated search as they were not part of the initial scope of this project; however, these articles were collected during the screening phase to provide additional context and evidence for discussion.
Selection of studies
Citations were collated and imported into Covidence,21 and duplicates were automatically removed. After a pilot test of the screening protocol, each title and abstract were screened independently by 2 reviewers (MI, HK, KH, Y-HL, JC) and assessed against the established inclusion/exclusion criteria. Sources that appeared to satisfy the inclusion criteria were then retrieved as full-text articles and examined by 2 independent reviewers (MI, HK, KH, Y-HL, JC). Disagreements that occurred between reviewers were resolved by the primary author (ZVR).
Data extraction, quality assessment, and analysis
A standardized data extraction form was completed for each included study to gather the following information: publication details (publication year and full citation), participant characteristics (country, population, and age), study characteristics (aim, design, methods, inclusion/exclusion criteria, sample size, measures, and interventions), cardiovascular health outcomes, pertinent findings (eg, risk and protective factors and prevalence and incidence estimates), conclusions, and implications. Data extracted from included articles were organized into Supplemental Tables S2 and S3. In accordance with the PRISMA-ScR,20 articles were not appraised for risk of bias or methodological quality. Thematic content analysis, based on the scoping review objectives, was conducted by the primary author (ZVR) with assistance from researcher assistants (EK, EJ, MI, CP) and guidance from 2 senior researchers (KMD, VT).
Results
Search results
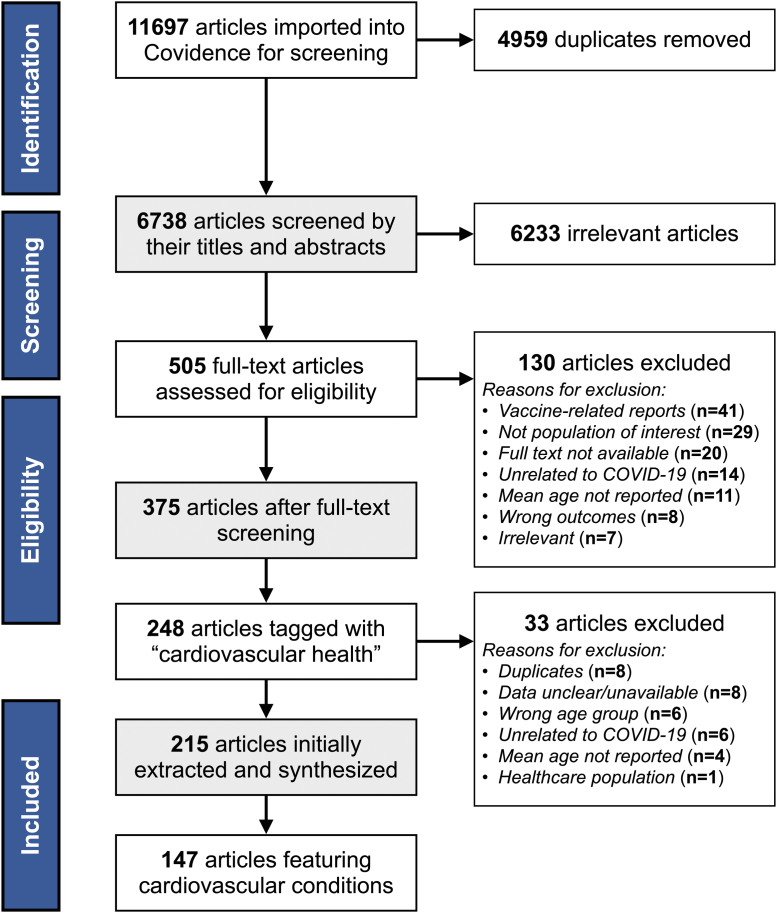
The academic database searches yielded 6738 articles after the automatic removal of duplicates, of which 6233 were deemed irrelevant during abstract and title screening. A total of 505 articles then underwent full-text screening, of which 130 were excluded for reasons such as being COVID-19 vaccine related (n = 41), including participants outside of the established age criteria (n = 24), not being available in full text (n = 19), not being directly related to COVID-19 (n = 14), or failing to report the mean age of participants (n = 11) (see Fig. 1). In total, 248 articles captured cardiovascular health outcomes, though 33 were excluded during the data extraction phase as they were either duplicates or did not meet the inclusion criteria. Of these 215 articles, 147 featured cardiovascular conditions among emerging adult populations with a COVID-19 context: 36 and 111 articles from the initial and updated search, respectively. The grey literature search located no additional sources. Altogether, the evidence in this review includes 117 case reports/series,22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138 28 observational studies,10,11,139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164 and 2 reviews/editorials.165,166 Nearly half (49%; n = 72) are from the United States (US), followed by Iran (6.1%; n = 9), the United Kingdom (5.4%; n = 8), and France (5.4%; n = 8).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of the search results.
Objective #1: Impact of the COVID-19 Pandemic on the Cardiovascular Health of Emerging Adults
Of the 147 articles included, 117 case reports/series identified 123 cases of emerging adults aged 18-25 (mean age = 21; 66% male) with active or previous COVID-19 and cardiovascular presentations/complications, 47 of which were classified by authors as hyperinflammatory syndromes, namely, MIS. Tachycardia (n = 65) was the most common cardiovascular presentation (accompanied by MIS or another cardiovascular complication in 89% of cases), followed by ventricular dysfunction (n = 44), hypotension (n = 37), thrombosis (n = 23), including pulmonary embolism and stroke, cardiomyopathy (n = 28), including myocarditis and myopericarditis, heart failure (n = 27), hypertension (n = 8), bradycardia (n = 9), myocardial infarction (n = 5), and cardiac arrest (n = 4); a detailed report of these cases can be found in Supplemental Table S2. The majority of these cases occurred in emerging adults with no reported history of CVD (91%) or other comorbidities (60%), suggesting that COVID-19 can lead to severe and sometimes fatal cardiovascular health outcomes (8.9%; n = 11) in otherwise healthy young adults. The following describes the types of cardiovascular manifestations in emerging adults who contracted COVID-19 as well as related impacts on cardiovascular care.
Reports of myocarditis and cardiac abnormalities
In the initial search, 2 observational studies10,11 focusing on COVID-19–associated myocarditis and cardiac abnormalities (ie, findings not meeting the 2018 Lake Louise Criteria167) among emerging adults were identified. Starekova et al.11 examined electronic health records of 145 university student-athletes aged 17-23 recovering from COVID-19 and found that 1.4% (n = 2) had cardiac magnetic resonance imaging (cMRI) findings consistent with myocarditis. In a cross-sectional study by Brito et al.,10 sequential cMRI was performed on 48 college student-athletes (mean age = 19) who experienced mild or asymptomatic COVID-19; cardiac abnormalities were observed in 56% (n = 27) of participants, including pericardial (n = 13), myocardial (n = 8), and myopericardial involvement (n = 6). These results initially suggested that myocarditis and general cardiac effects may be relatively common among healthy emerging adults post-infection. Since then, 10 additional articles have been identified,139, 140, 141, 142, 143, 144, 145,147,165,166 providing further insight into the prevalence and associations of myocarditis and cardiac abnormalities after COVID-19 in emerging adults. The majority of these data come from observational studies on student-athlete populations.139, 140, 141, 142, 143, 144, 145,165,166 In the broader emerging adult population, hospital-based administrative data from 2019 through 2021 from over 900 sites in the US identified 121 inpatients aged 16-24 diagnosed with COVID-19 and myocarditis, yielding an adjusted risk 7.4 times greater than that of their uninfected counterparts (0.098% vs 0.013%, respectively).147
A recent systematic review by van Hattum et al.166 provided prevalence estimates of COVID-19–associated myocarditis and other cardiac abnormalities (eg, arrhythmias) among student-athletes after infection. Among 2326 college athletes (median age = 22), the weighted prevalence of myocarditis found with cMRI was 2.1% using the established Lake Louise Criteria. The majority (59%) of these individuals were mildly symptomatic while infected; 22% were asymptomatic, and the remainder experienced moderate (19%) or severe (0.2%) illness. There were no observed arrhythmias and only 1 resuscitated cardiac arrest unlikely attributable to COVID-19. Several studies in our evidence set specific to COVID-19–related myocarditis were included in the review;166 prevalence estimates ranged from 0% to 15%,10,11,139, 140, 141, 142, 143, 144, 145, 146,165,166 which included both asymptomatic and symptomatic cases of COVID-19.
Additional evidence has accumulated regarding the prevalence of general cardiac involvement (eg, myocardial oedema and pericardial effusion) in emerging adults with COVID-19. In a large cohort of 3018 college athletes (mean age = 21 years), 0.7% (95% confidence interval [CI]: 0.4, 1.1) were determined to have definite, probable, or possible severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related myocardial or pericardial involvement with cMRI or “triad” testing: cardiac troponin levels, an electrocardiogram (ECG), and a transthoracic echocardiogram.140 An analysis by Petek et al.141 used data from the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA), representing 44 US colleges, with a sample of 3597 student-athletes (mean age = 20) diagnosed with COVID-19. Investigation of individuals with exertional cardiopulmonary symptoms (n = 137) revealed 10 cases of cardiovascular sequelae (7.3%) and 5 cases (3.6%) of definite or probable cardiac involvement via cMRI, representing 21% of athletes with chest pain after COVID-19. In another group of 170 athletes aged 18-25, 3.5% (n = 6) had abnormal cardiac rhythms and 1.2% (n = 2) were diagnosed with viral pericarditis using cMRI.139 In a sample of 137 student-athletes aged 18-27, 82% (n = 112) of whom were symptomatic, algorithm-guided screening after COVID-19 identified trace pericardial effusions with echocardiography in 2.9% (n = 4) of participants.143 Similarly, although Małek et al.144 identified no cases of myocarditis via cMRI in a cohort of 26 athletes recovering mainly from asymptomatic or mild infections (median age = 24), 19% (n = 5) demonstrated cardiac abnormalities, including signs of myocardial oedema and pericardial effusion. Compared with myocarditis, most studies suggest that general cardiac involvement among emerging adults following COVID-19 is more common (0%-58%).10,11,139, 140, 141, 142, 143, 144, 145, 146,166 However, it is of note that data from larger cohorts (ie, N > 100) provided smaller prevalence estimates in athletes imaged with cMRI (0%-7%).11,139, 140, 141,143
Risk factors
Cohort analyses using cMRI or triad testing identified several risk factors for cardiac involvement, including White Hispanic race (odds ratio [OR]: 7.6; 95% CI: 2.2, 26.1), cardiopulmonary symptoms before or during infection or return to exercise (adjusted OR [aOR]: 3.1; 95% CI: 1.2-7.8), or 1 or more abnormal triad test results potentially associated with COVID-19 (aOR: 37.4; 95% CI: 13.3-105.3).140,141 The association between biological sex and COVID-19–associated cardiac abnormalities (eg, abnormal ECG findings and diagnosis of myocarditis) was unclear.139,147 With respect to age, the risk of COVID-19–associated myocarditis in hospitalized patients was lowest among individuals aged 16-24 when compared with older age cohorts.147
Ultimately, our review of the evidence suggests the prevalence of COVID-19–associated myocarditis among otherwise healthy emerging adult student-athletes to be nearing the lower end of 0.5%-3%,11,139, 140, 141,143,165,166 though general cardiac involvement (eg, myocardial oedema and pericardial effusions) is likely more prevalent (0%-7%),11,139, 140, 141,143 particularly in those with lingering cardiopulmonary symptoms.141 Other cardiac events, such as arrhythmias and cardiac arrest, seem to be less common in this age cohort after infection.166 There was a relatively low risk of clinical cardiac events (ie, significant arrhythmias, heart failure, sudden cardiac arrest, or death) in short-term follow-up (approximately 0.03%).140
Reports of hyperinflammatory syndromes
Belay et al.148 conducted the largest US cohort study to date (n = 1733) describing the clinical characteristics and geographical and temporal distribution of patients under 21 years of age with MIS-C. Of the 55 emerging adults aged 18-20, 58% (n = 32) were admitted to the ICU, and 11% (n = 6) died. Cardiovascular manifestations in this age group included hypotension in 53%, cardiac dysfunction in 42%, myocarditis in 31%, pericardial effusion in 27%, and coronary artery dilation or aneurysm in 15%. Compared with the paediatric population, patients aged 18-20 had the highest proportion of myocarditis (31% in 18- to 20-year-olds vs 9.2%-28% in 0- to 17-year-olds; P < 0.001). The 18- to 20-year-old cohort, however, had the lowest incidence of MIS at 0.4 per 100,000 infected (P < 0.001).
In this review, 47 cases (mean age = 21; 70% male) of emerging adults with cardiovascular complications in the context of confirmed or suspected hyperinflammatory syndromes and active or previous COVID-19 were identified.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 If not concomitant COVID-19, previous infections typically occurred 3-8 weeks before the onset of symptoms.23,30,32, 33, 34,40,43, 44, 45, 46,52,53,56 Alongside MIS-C/A, cardiovascular presentations/complications included ventricular dysfunction (n = 33), tachycardia (n = 28), hypotension (n = 16), cardiogenic shock (n = 14), cardiomyopathy (n = 11), including myocarditis and pericarditis, valve insufficiency (n = 4), atrial fibrillation (n = 2), and non–ST-elevation myocardial infarction (n = 2); refer to Supplemental Table S2 for additional case details. Cardiac involvement with echocardiographic changes (eg, reduced ejection fraction and global hypokinesia) and elevated N-terminal pro-B-type natriuretic peptide and troponin were frequently reported among these cases. Of note, 79% (n = 37) of cases were previously healthy with no past medical history, and 98% (n = 46) had no reported history of CVD.
Due to prompt identification and treatment, there were few reports (4.3%; n = 2) of emerging adults with MIS who died.48,55 A case series described the postmortem examinations of 4 deaths due to maternal or paediatric antemortem COVID-19. In this report, the death of a 19-year-old woman was attributed to MIS causing coagulopathy.55 A separate retrospective cohort study by Whitworth et al.149 identified the incidence of thrombosis in children and adolescents under 21 years of age hospitalized with COVID-19 or MIS-C (n = 564). Eleven of the 20 cases of thrombosis (55%) occurred in patients aged 16-21, 36% (n = 4) of whom died; all cases were either African American or Hispanic individuals. Patients 12 and older with MIS-C displayed the highest rate of thrombotic events (19%). Despite the incidence of MIS among emerging adults being lower than younger age cohorts, those affected seem to be at an elevated risk of cardiovascular complications, including myocarditis, and death. Evidence outlining risk factors and long-term prognosis after recovery in this age group is lacking.
Reports of vascular alterations
Multiple studies reported signs of vascular dysfunction among young adults after COVID-19.122,160, 161, 162, 163, 164
Ratchford et al.160 performed a cross-sectional analysis on healthy young adults (mean age = 20) to examine the effects of COVID-19 on markers of vascular function and arterial stiffness. When compared with uninfected individuals, those with COVID-19 experienced significantly lower brachial artery flow-mediated dilation (FMD) (2.7% ± 1.2% vs 8.8% ± 3.0%; P < 0.01) and femoral artery blood flow response (−3 ± 91 mL vs 118 ± 114 mL; P < 0.01) 3-4 weeks after infection. This same group performed another cross-sectional study161 and found higher carotid artery stiffness among young adults with COVID-19 (6 ± 1 m/s) compared with healthy controls (5 ± 1 m/s; P = 0.02). Aortic augmentation index was also greater in the COVID-19 group (12.7% ± 9.1% vs 3.3% ± 12.6%; P = 0.03), suggesting aortic stiffening and potential atherosclerotic risk progression. Another study by Stute et al.163 similarly found that resting muscle sympathetic nerve activity, a measure of arterial stiffness, was higher in individuals recovering from COVID-19 (n = 16; mean age = 20) compared with uninfected controls (285 ± 101 a.u./min vs 159 ± 46 a.u./min, respectively; P = 0.001).
Growing evidence indicates that endothelial inflammation associated with COVID-19 may contribute to cerebrovascular disease. A case series by Arandela et al.122 identified 2 patients between the ages of 18 and 25 who developed reversible cerebral vasoconstriction syndrome in the context of COVID-19, suggesting a potential risk in this age cohort among those using vasoactive agents (eg, marijuana). Alterations in cerebral and peripheral vasculature were further investigated in a cohort study conducted by Nandadeva et al.162 Analysis found that only peripheral vascular function was impaired in young adults with lingering COVID-19 symptoms (n = 8; mean age = 24); this impairment was not seen in those who were no longer symptomatic (n = 8; mean age = 22). Taken together, these findings suggest that the effects of COVID-19 on central large arteries may be a transient phenomenon.
Whereas the previously mentioned studies reported vascular alterations at rest, Stute et al.164 aimed to elucidate the effect of COVID-19 on central and peripheral haemodynamics during a rhythmic handgrip exercise. Brachial artery blood flow was significantly lower in the COVID-19 group (n = 13; mean age = 21) compared with controls (n = 13; mean age = 27): 386.3 ± 132.5 mL/min vs 507.4 ± 109.9 mL/min, respectively (P = 0.002). The COVID-19 group also displayed greater increases in systolic blood pressure, systolic arterial pressure, and rate pressure product on exertion.
Reports of individuals with cardiovascular-related vulnerabilities that increase risk for severe COVID-19, related cardiovascular complications, and mortality
Cardiovascular disease, hypertension, and obesity
A few studies offered insight into cardiovascular-related risk factors that may confer an increased risk of severe COVID-19. Fathi et al.157 developed a model to predict 2-week mortality using data from 57,705 inpatients with COVID-19, which included a “young” cohort (n = 1049; aged 15-24). Among those who died in this subsample (n = 50; 4.8%), hypertension was significantly associated with 2-week mortality (OR: 54.3; 95% CI: 19.9, 168.2). A retrospective study of young adult COVID-19 patients aged 18-35 admitted to New York City public hospitals also found that cardiac comorbidities and hypertension were associated with increased mortality; however, those with these comorbidities in the 18- to 23-year-old cohort all recovered.155 To provide context, data from the National Health Interview Survey and an undergraduate student sample during the pandemic show that pre-existing heart conditions affect 0.5%-1.9% of this age group.153,156 Furthermore, evidence from after a COVID-19 lockdown found that the prevalence of hypertension among undergraduate students (n = 325; mean age = 22) remained at 1%.154
A hospital system-based retrospective chart review159 conducted in Texas identified risk factors for severe disease and readmission among young adults aged 18-29 diagnosed with COVID-19 (mean age = 24). The study identified 1853 patients with COVID-19, 8% (n = 148) of whom experienced a composite disease outcome (eg, a severe respiratory or cardiovascular event) within 30 days of their first encounter. In this cohort of young adults, older age, obesity, previous CVD (ie, myocardial infarction, congestive heart failure, and cerebrovascular disease), and diabetes were among significant risk factors for composite disease outcomes (P ≤ 0.03). A history of CVD and obesity were also predictors of severe disease and/or readmission within 30 days (P < 0.05). In addition, the authors highlighted the relationship between race and ethnicity (eg, Hispanic ethnicity) and poorer health outcomes. A preliminary analysis (abstract) by Sands-Lincoln et al.168 of patients aged 18-24 with COVID-19 (n = 6648; mean age = 22) found African American (OR: 2.4; 95% CI: 1.6, 3.5) and “other race” identity (OR: 5.0; 95% CI: 2.6, 9.1), CVD (OR: 4.0; 95% CI: 2.8, 5.7), and obesity (OR: 3.0; 95% CI: 2.1, 4.3) to be associated with increased odds of hospitalization. Another study by Richardson et al.158 analysed data from hospitalized patients aged 18-39 at acute care hospitals in New York City. Notably, among patients aged 18-24 (n = 119), those who died (n = 4) or required invasive mechanical ventilation (n = 7) were all obese, and 5 of these patients had additional comorbidities, including Down syndrome and congestive heart failure.
In summary, the majority of cardiovascular-related medical vulnerabilities in emerging adults are rare;153,154,156 however, when present, data suggest that the risk for severe COVID-19, related cardiovascular complications, and mortality in this age group is not insignificant, particularly among those with comorbidities.
Congenital heart disease and genetic syndromes
Predicting the COVID-19 response in emerging adults with existing congenital heart disease (CHD) is challenging given the heterogeneity of the population. Two separate retrospective reviews investigating factors associated with severe COVID-19 and mortality across the CHD population found that the presence of a structural congenital heart defect did not confer an increased morbidity or mortality risk.150,151 Lewis et al.150 detailed the experience of 4 emerging adults aged 21-25 with CHD who were described to have moderate-to-severe COVID-19. These individuals did not appear to be disproportionately impacted unless they were at an advanced physiological stage (ie, class C or D) (OR: 19.4) and/or had genetic syndromes (OR: 35.8), such as Down syndrome and DiGeorge syndrome (P ≤ 0.002). Similarly, Broberg et al.151 found that a worse physiological stage of CHD (eg, Eisenmenger physiology and cyanosis) was associated with mortality (P = 0.001), whereas anatomic complexity or defect group was not. These findings suggest that susceptibility to severe COVID-19 among emerging adults with CHD is based primarily on physiological factors, and, when accompanied by certain genetic conditions, such as Down syndrome and DiGeorge syndrome, CHD is associated with increased hospitalization from COVID-19.71,150, 151, 152,158
The role of genetic disorders in COVID-19 severity among emerging adults with CVD seems to vary depending on the underlying disorder. Adults with Duchenne muscular dystrophy (DMD) are at risk for cardiorespiratory compromise (ie, cardiomyopathy) and so were thought to be vulnerable to worse COVID-19 outcomes.138 However, Quinlivan et al.138 reported on 5 emerging adult males aged 18-23 with DMD who contracted COVID-19 and did not develop moderate or severe disease. Despite a history of moderate-to-severe cardiomyopathy, long-term immunosuppressive treatment, and respiratory insufficiency, all patients recovered fully with no complications. This evidence indicates that emerging adults with DMD may not be at an elevated risk of severe COVID-19 and related cardiovascular complications.
Impacts on cardiovascular care
Cardiovascular care for emerging adults was impacted by the COVID-19 pandemic, which in some cases affected cardiovascular outcomes. For example, Warraich et al.130 described a 19-year-old patient who delayed treatment by self-isolating for 2 weeks during the pandemic due to a persistent cough that was later understood to be a symptom of a posterior circulation ischemic (POCI) stroke. Other reports of emerging adults with cardiovascular presentations, including stroke,130,132 intracardiac thrombosis,135 pulmonary hypertension,131 and sinus tachycardia with atrioventricular block,133 found that many avoided seeking treatment due to lockdown measures or fear of contracting COVID-19. In addition, the pandemic resulted in misguided clinical judgement and management. In the case presented by Warraich et al.,130 the young patient with POCI stroke lacked risk factors for stroke, and so an initial diagnosis of COVID-19 was made. In another case, a 19-year-old man presenting to the emergency department with constitutional symptoms was repeatedly tested for COVID-19, treated with antibiotics for COVID-19 pneumonia, discharged, and later diagnosed with Coxsackie A myocarditis on readmission.134 Similarly, Balfe et al.137 presented the case of an 18-year-old girl suffering from rheumatic mitral stenosis whose condition was worsened by the intravenous fluids she was administered under the presumption that she had COVID-19. Reports were also identified in which life-saving cardiovascular care for emerging adults (eg, extracorporeal cardiopulmonary resuscitation) was nearly prevented or complicated due to the patient’s COVID-19 status.60,63,136 Overall, these cases highlight evidence of crucial cardiovascular care being delayed, misguided, or complicated as a result of the COVID-19 pandemic influencing public and clinical decision-making.
Objective #2: Strategies to Screen for and Manage Cardiovascular Complications in Emerging Adults
Myocarditis and cardiac abnormalities
The majority of evidence surrounding screening protocols for COVID-associated myocarditis and cardiac abnormalities in emerging adults focuses on student-athletes.10,11,139, 140, 141, 142, 143, 144, 145, 146,165,166
Initial evidence explored the utility of extensive cardiorespiratory and haematological screening in athletes recovering from COVID-19 to identify postinfection cardiovascular abnormalities. Gervasi et al.146 examined a cohort of 30 professional soccer players aged 19-27, 18 of whom tested positive for SARS-CoV-2 IgG antibodies and reported previous asymptomatic or mild COVID-19. After comprehensive screening (eg, blood tests, spirometry, and ECG), none of the participants demonstrated clinically relevant cardiovascular abnormalities (eg, myocarditis). Furthermore, both Starekova et al.11 and Brito et al.10 conducted studies in which university athletes recovering from COVID-19 underwent screening with cMRI, most of whom were recovering from mild-to-moderate or asymptomatic illness. Starekova et al.,11 with a sample of athletes aged 17-23, found that only 1.4% (n = 4) of those screened met the Lake Louise Criteria for myocarditis. Brito et al.10 identified myocardial, pericardial, or myopericardial abnormalities in 58% of athletes, but no signs of ongoing myocarditis. Because of the low prevalence of clinical myocarditis in these cohorts, cMRI as a standard screening tool for myocarditis was deemed unwarranted, especially for individuals with asymptomatic or mild COVID-19 and those with a normal ECG and cardiac troponin.
Evidence from the updated search continues to support cMRI as a sensitive and specific screening modality for myocarditis and other cardiac abnormalities in this population.140, 141, 142,145,165,166 Still, larger cohort studies recommend cMRI only for individuals with a heightened risk based on an initial, comprehensive cardiac evaluation.139, 140, 141, 142, 143, 144 Multiple studies screened for elevated cardiac troponin139, 140, 141, 142, 143, 144,165 or abnormal ECG or echocardiogram findings139, 140, 141,143,144,165 to indicate cMRI for further diagnostic workup. A sample of 1597 athletes screened for post-COVID-19 cardiac abnormalities demonstrated the utility of 4 respective screening strategies:165,169
-
1.
A positive cardiac history (eg, chest pain) to indicate triad testing; abnormal triad testing to indicate cMRI
-
2.
Abnormal triad testing to indicate cMRI
-
3.
A positive cardiac history (eg, chest pain) or abnormal triad testing to indicate cMRI
-
4.
cMRI without prior screening.
These approaches would have identified 5 (0.3%), 13 (0.8%), 17 (1.1%), and 37 (2.3%) cases of myocarditis in the cohort, respectively. Moulson et al.140 similarly found that 82% of athletes diagnosed with definite or probable cardiac involvement after COVID-19 would have been identified with a stepwise approach using moderate symptom severity, cardiopulmonary symptoms, or abnormal triad testing to indicate cMRI. Despite such evidence showcasing an increased specificity when screening algorithms included a cardiac history and triad testing,140,165,169 the previously discussed systematic review by van Hattum et al.166 found that, among 10 studies (n = 4171), there was no clear association between postrecovery cardiac troponin levels and cardiac abnormalities. In contrast, a comprehensive history appears to have clinical utility, as the Moulson et al.140 and Petek et al.141 analyses of data from more than 40 US academic institutions found cardiopulmonary symptoms, specifically chest pain and dyspnea, to increase the risk of COVID-19–related cardiac involvement (aOR: 3.1; 95% CI: 1.2-7.8). Of those who had chest pain after return to exercise, 21% had evidence of myocardial and/or pericardial involvement on cMRI.141 Ultimately, the impact of these screening algorithms on the clinical course of emerging adult athletes recovering from COVID-19 remains unknown, as few, if any, adverse cardiac events have been reported among this population following return to play.140,165,166
MIS-C/A
Regarding the identification of MIS-C/A in emerging adults with concomitant or previous COVID-19, Belay et al.148 found that only 71% of the 18- to 20-year-old MIS cases demonstrated SARS-CoV-2 polymerase chain reaction (PCR) positivity, and 58% had positive serology. In our review of MIS case reports/series involving cardiovascular complications, 47% (n = 17) reported negative PCR results, whereas 70% (n = 33) had positive serology. Given the significant proportion of patients with MIS and negative PCR results, the authors have recommended SARS-CoV-2 antibody testing to classify MIS patients without active COVID-19 or those with atypical COVID-19 presentations.24 Compared with younger age groups, 18- to 20-year-olds were more likely to report COVID-19–like illness 7 or more days before MIS onset (63% vs 18%-44%; P < 0.001).148 In the cohort study by Belay et al.,148 mainstay treatments for MIS included intravenous immunoglobulin (IVIG) and steroids. Across the 47 cases included in this review,22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 70% (n = 33) were treated with steroids (eg, methylprednisolone, prednisolone, and prednisone) and 62% (n = 29) with IVIG. Additional therapies included interleukin-1 and interleukin-6 receptor antagonists and supportive care measures, such as aspirin and anticoagulation (Supplemental Table S2).
Discussion
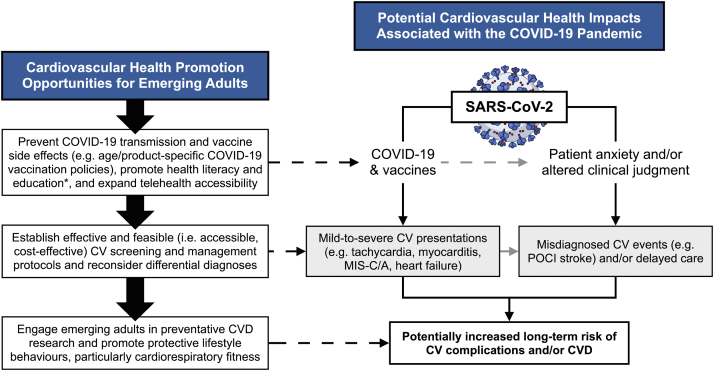
This scoping review examined current literature to describe the impact of the COVID-19 pandemic on the cardiovascular health and care of emerging adults. We identified multiple cases of cardiovascular presentations (eg, tachycardia and ventricular dysfunction), cardiac abnormalities (eg, myocardial/pericardial involvement), MIS-C/A, and vascular alterations among emerging adults who contracted COVID-19, the long-term effects of which remain unknown. The evidence is insufficient to determine the true incidence and prevalence of these complications in this age cohort; however, prevalence estimates from student-athlete samples of COVID-19–associated myocarditis and cardiac involvement on cMRI ranged from 0.5% to 3%11,139, 140, 141,143,165,166 and 0% to 7%,11,139, 140, 141,143 respectively. In some groups, medical vulnerabilities such as obesity,158,159,168 hypertension,155,157 previous CVD,159,168 and certain genetic syndromes71,150,152,158 posed an increased risk of severe illness, cardiovascular complications, and/or mortality from COVID-19. There were also reports of emerging adults with cardiovascular presentations where the provision of cardiovascular care was negatively impacted due to COVID-19.60,130, 131, 132, 133, 134, 135,137 Regarding the appropriate screening and management of these cardiovascular abnormalities, the majority of age-specific evidence pertains to COVID-19–associated myocarditis and MIS. Based on the findings of this scoping review, a framework that highlights the negative impacts of the COVID-19 pandemic on the cardiovascular health of emerging adults and corresponding health promotion opportunities to prevent and mitigate these effects was constructed (see Fig. 2).
Figure 2.
A framework to promote cardiovascular health among emerging adults in the context of the COVID-19 pandemic (created in part with BioRender.com). The dashed lines represent pathways to prevent or manage COVID-19–related cardiovascular health concerns. ∗Health literacy and education initiatives may benefit from including parents/guardians as part of the target audience as they often play a role in an emerging adult’s decision to seek medical care. CV, cardiovascular; CVD, cardiovascular disease; MIS-C/A, multisystem inflammatory syndrome in children/adults; POCI, posterior circulation ischemic; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In the following, accumulated evidence from the review is highlighted with corresponding suggestions for future research, policy, and practice.
Investigations surrounding COVID-19 and cardiovascular health
In this review, cardiovascular presentations among emerging adults with current or previous COVID-19 ranged from milder manifestations such as sinus tachycardia,170 to more serious complications, including myocarditis, stroke, cardiogenic shock, heart failure, thrombosis, and MIS. Investigators suggest that these cardiovascular complications may lead to cardiomyopathy, cardiac arrhythmias, and sudden cardiac arrest in the long term.169,171, 172, 173 Therefore, ongoing research involving emerging adults who contract COVID-19 and develop cardiovascular sequelae is recommended. Long-term surveillance of these patients can help assess and inform cardiovascular screening and treatment protocols. An emphasis should also be placed on ensuring that study samples are representative of the broader emerging adult population, given most of the cohort evidence found in this review focused on student-athletes.10,11,139, 140, 141, 142, 143, 144, 145,148,165,166
Myocarditis and cardiac abnormalities
Extrapolating prevalence estimates of COVID-19–associated myocarditis and cardiac abnormalities from student-athlete cohorts to the entire emerging adult population is cautioned. Experts believe that athletes who exercise with COVID-19 are at an increased risk of developing myocarditis.169,174 At the same time, their overall heightened cardiovascular health is not representative of the broader population. Regarding the long-term implications of these findings, updated ORCCA data175 demonstrate complete or partial resolution of cMRI abnormalities in 80% (n = 8) of participants. Follow-up after more than 1 year identified no adverse cardiac outcomes in the subsample of athletes with initial cardiac involvement. Though promising, additional surveillance is required to establish the true long-term risk of COVID-19–associated myocarditis and other cardiovascular sequelae in emerging adults.
With respect to screening, data from student-athletes may be used to aid clinical decision-making given limited evidence from the broader emerging adult population. Additional analysis of the ORCCA cohort176 continues to indicate the limited diagnostic utility of cardiac troponin as a screening modality for those with COVID-19–associated myocardial involvement; it is recommended only for those with a high clinical pretest probability of disease. Moreover, the data in this review do not support routine cMRI in lower-risk individuals.10,11,139, 140, 141, 142, 143, 144,146 This approach would be quite costly and increase the likelihood of false positives.165,177 Rather, a screening approach guided by cardiac symptoms (eg, chest pain) and/or triad testing to indicate cMRI will increase its diagnostic yield and feasibility.140,169 A 2022 expert consensus paper from the American College of Cardiology178 is in agreement, recommending against cardiac testing for asymptomatic cases, those with only mild-to-moderate noncardiopulmonary symptoms, or those with previous COVID-19 in the absence of ongoing cardiopulmonary symptoms. Individuals with COVID-19–related cardiopulmonary symptoms are recommended for triad testing and, if results are abnormal, a cardiology consultation to consider subsequent cMRI and additional cardiac testing for diagnosis. The body of evidence in this review is limited with respect to treating COVID-19–associated myocarditis in this age group. However, case reports66,75,98,120 and supporting literature179 indicate that in the absence of additional cardiovascular complications (eg, acute heart failure), rest, supportive measures (eg, intravenous/oral hydration, beta-blockers), and immunosuppressive therapy yield promising outcomes.
MIS-C/A
Emerging adults with MIS demonstrated significant mortality (Supplemental Table S2) and were more often reported to have COVID-19–like illness before presentation and subsequent findings of myocarditis.148 Ultimately, these findings emphasize the importance of recognizing and including MIS as a differential in the context of recent COVID-19 in this cohort.
As of now, it remains unclear whether MIS is a manifestation of acute COVID-19 or an entirely postacute phenomenon. Given that not all MIS patients present with positive PCR test results, SARS-CoV-2 antibody testing is recommended as a potentially crucial diagnostic measure to classify and recognize these patients.24 However, in the current state of the pandemic, the majority of adults demonstrate seroprevalence of SARS-CoV-2 antibodies,180 limiting its clinical utility. The additional use of laboratory tests for inflammation, hypercoagulability, and organ damage (eg, C-reactive protein, D-dimer, and cardiac enzymes) may assist in early identification and subsequent management. To our knowledge, no consensus guidelines are available for MIS-A; current recommendations are extrapolated from evidence specific to MIS-C, applicable to ages less than 21 years. The Centers for Disease Control and Prevention (CDC) supports serologic testing in addition to PCR testing when feasible, as well as workup for cardiac involvement in affected individuals.181 Both the CDC and National Institutes of Health discuss IVIG in combination with glucocorticoids (eg, methylprednisolone) as the first-line treatment strategy, citing its benefits for faster recovery of cardiac function.181,182 The latest clinical guideline from the American College of Rheumatology additionally recommends higher doses of steroids, anakinra, or infliximab for refractory disease.183 Future research into MIS is required to better elucidate pertinent risk factors, facilitate diagnosis and management, and identify any long-term cardiovascular implications in this age group.
Changes in systemic vasculature
Reports of young adults recovering from COVID-19 found transient alterations in arterial, cerebral, and peripheral vasculature in those with lingering symptoms and even after recovery.122,160, 161, 162, 163, 164 Participants in these studies were all assessed 3-8 weeks after their first positive COVID-19 test; therefore, there is limited long-term data to substantiate these findings. Of note, 1 study reported a 6% difference in brachial artery FMD among those with previous COVID-19 compared with those who were uninfected.160 A meta-analysis by Inaba et al.184 (mean age > 50) found that for every 1% decrease in brachial artery FMD, there is a corresponding 8% increase in risk of future cardiovascular events, including stroke, heart attack, and death. In addition, these findings represent dysautonomia after COVID-19, a phenomenon observed among those experiencing “long COVID,” the symptoms of which also include fatigue and shortness of breath.185, 186, 187 These sequelae may lead to exercise intolerance among those recovering from COVID-19 and pose future implications for CVD risk among emerging adults. Moving forward, follow-up in this group is needed to better understand the relationship between COVID-19 and long-term vascular function.
Risk stratification of emerging adults for COVID-19 severity, related cardiovascular complications, and mortality
There is increasing evidence that certain cardiovascular comorbidities (eg, obesity, hypertension, CVD)153, 154, 155, 156, 157, 158, 159,168 are relevant risk factors among emerging adults in the context of COVID-19. Among emerging adults with CHD who contract COVID-19, these patients may still be considered high-risk due to the variability in this population’s clinical presentation and response to treatments.150,151 In the future, retrospective analyses should focus on emerging adults who have experienced severe COVID-19 and related cardiovascular complications to further clarify the relationship between these outcomes and presumptive risk factors, particularly CVD. Because most sample sizes in the literature were modest, it is recommended that multicentre registries specific to emerging adults, such as the ORCCA141 and COVID-19 CVD Registry,188 be established to enable the aggregation of data and provide adequate statistical power to conduct such analyses.
The cardiovascular safety of COVID-19 vaccines for emerging adults and public education
As vaccination efforts progress around the globe, there have been signals of adverse cardiovascular events from COVID-19 vaccines, specifically cases of myocarditis and pericarditis among emerging adults, which were not observed in clinical trials.16,17,189 In a sample of approximately 23 million Nordic residents, the number of excess cases of myocarditis among males aged 16-24 associated with a second dose of the BNT162b2 (Pfizer) and mRNA-1273 (Moderna) vaccines 28 days after administration was estimated at 4-7 and 9-28 per 100,000 doses, respectively.16 A US study using the Vaccine Adverse Event Reporting System (VAERS) data17 similarly found an elevated risk among males aged 18-24, with 52 and 56 cases of myocarditis per million after second doses of the Pfizer and Moderna vaccine, respectively; comparatively, there were only 4-7 cases of myocarditis per million doses in the female cohort. No reported deaths in vaccinated individuals younger than 30 were attributed to myocarditis, apart from 1 potential case. Of note, VAERS reports can be submitted by any member of the public and are not verified for cause-and-effect relationships, making this passive surveillance system particularly susceptible to false reports and under- or over-reporting. Still, meta-analysis reveals the incidence of myopericarditis associated with COVID-19 vaccines to be either comparable to or lower than that of other vaccines.189
The cardiovascular safety concerns of COVID-19 vaccines must be contextualized with the cardiovascular risks associated with COVID-19 infection. A recent CDC analysis found that the risk of cardiovascular complications (ie, myocarditis, pericarditis, or MIS) among males aged 18-29 was 7-8 times greater after COVID-19 compared with vaccination; a similar observation was found for females.190 A population-based cohort study191 of individuals in Ontario, Canada, found that the highest rate was among males aged 18-24 receiving Moderna vs Pfizer as the second dose (300 vs 59 cases per million doses, respectively) and was significantly higher when the interdose interval was ≤30 days compared with ≥56 days (95-377 vs 11-132 per million doses, respectively). In addition, UK data192 found an association between COVID-19 and pericarditis and cardiac arrhythmias among 16- to 29-year-olds, though this same association was not established with vaccines. For additional context, data suggest that 1 million second mRNA COVID-19 vaccine doses could prevent 11,000 cases, 560 hospitalizations, 138 ICU admissions, and 6 deaths among 12- to 29-year-olds, compared with 39-47 potential cases of vaccine-related myocarditis.193 Vaccine-related myocarditis also tends to be milder when compared with COVID-19–related cases, which have been associated with a greater risk of hospitalization and death.192
Concerns regarding cardiovascular complications following COVID-19 in this cohort are partly due to the fact that emerging adults contract COVID-19 at disproportionately higher rates than older adults.156 Though in agreement with the recommendation for COVID-19 vaccination in this cohort, we encourage continued surveillance and analysis of data in this area.194 This measure is especially important in light of waning vaccine immunity, increasing natural immunity, and the emergence of new SARS-CoV-2 variants that seem to pose a lower risk of hospitalization and severe disease.195 Therefore, a risk-benefit analysis of COVID-19 vaccines should be regularly updated for emerging adults as the pandemic progresses. To mitigate public hesitancy, as well as the cardiovascular risks associated with COVID-19 vaccines and infection in this age group, we recommend researchers, policymakers, and clinicians consider the following:
-
•
The development and distribution of strain-specific boosters that improve vaccine efficacy against transmission and symptomatic illness;196,197
-
•
Longer COVID-19 vaccine interdose intervals and age-based product considerations191 to reduce the incidence of cardiovascular side effects;
-
•
Ongoing surveillance of COVID-19 and vaccine-associated cardiovascular sequelae (eg, myocarditis, pericarditis, and MIS);15,194 and
-
•
Clinician and public education (for emerging adults and parents/guardians) surrounding the signs and symptoms and expected timelines of both infection- and vaccine-associated cardiovascular sequelae; see Table 1.
Table 1.
Signs and symptoms of COVID-19–associated cardiovascular sequelae that affect emerging adults and recommendations for when to seek medical care, as per the Centers for Disease Control and Prevention15,194
| Cardiovascular sequelae | Recommendation |
|---|---|
| Myocarditis and pericarditis | Seek medical care if you or your child have any of the following symptoms after COVID-19 infection or vaccination:∗
|
| MIS-C/A | Seek medical care if you or your child have any of the following signs/symptoms after COVID-19:†
|
Seek emergency medical attention if you or your child have any of the additional signs/symptoms:†
|
MIS-C/A, multisystem inflammatory syndrome in children/adults.
More often after the second dose, usually within 1 week after vaccination (median time = 3 days).191
Usually within 6 weeks after infection or exposure.
Preventing misdiagnoses, delayed care, and future COVID-19–related CVD
This review suggests that when emerging adults present with COVID-19–like symptoms, the differential diagnosis should include potentially life-threatening cardiovascular manifestations, such as POCI stroke and other forms of viral myocarditis.130,134,137 In addition, emerging adults who have cardiovascular symptoms, along with their parents/guardians, need to be educated on the critical importance of seeking medical care despite the risk of contracting COVID-19.130, 131, 132, 133, 134, 135,137 The increasing availability of telehealth and digital health interventions198 may assist younger patients in receiving timely diagnoses and treatment for potentially life-threatening cardiovascular events.
From a developmental perspective, emerging adulthood is a critical time to establish behaviours that promote long-term cardiovascular health. In a national longitudinal study by Clark et al.,199 the average 30-year risk of developing CVD for young adults (mean age = 29) ranged from 4.4% to 18%. Health literacy and education initiatives198,200,201 that emphasize CVD prevention (eg, healthy diets, physical activity, and avoiding substance use) and the recognition of cardiovascular events need to be developed and targeted at emerging adults and parents/guardians.202, 203, 204, 205 Promoting cardiorespiratory fitness is also an integral aspect of CVD prevention. An analysis of over 1.5 million Swedish men conscripted to the military (mean age = 18.3) found that higher cardiorespiratory fitness in late adolescence and early adulthood was significantly protective against COVID-19–associated hospitalization (OR: 0.76), ICU admission (OR: 0.61), and mortality later in life (OR: 0.56).206 Encouraging cardiorespiratory fitness among emerging adults now will likely improve cardiovascular outcomes if faced with future pandemics.
Strengths and limitations
The strengths of this review include its comprehensive search strategy, an updated search, and an in-depth analysis of the acquired evidence. As per scoping review guidelines,20 included articles were not critically appraised. Therefore, any conclusions drawn from this evidence set should act as a foundation, rather than a guideline, for future research, policy, and practice. The authors also acknowledge the potential for bias and subjectivity in how data were reported given the qualitative nature of scoping reviews. In addition to the writing team including multiple members as a means of limiting individual bias, data are reported thoroughly in the supplemental files and available for review. The major limitation of this review, however, is that the majority of available cohort analyses focused on student-athlete populations; this reduces the generalizability of our findings to the broader emerging adult population. In addition, despite including articles from non–English-speaking countries when available in English, excluding non–English-language articles likely skews the evidence set and prevents extrapolation to the global population. Moreover, the COVID-19 pandemic has been dynamic with respect to the transmissibility and pathogenicity of the current variants at large, rendering older evidence less relevant. Nonetheless, this review provides a novel perspective and foundation for future research, policy, and practice.
Conclusions
To the best of our knowledge, this review is the first to focus on studies that describe the impact of COVID-19 on the cardiovascular health of emerging adults. Among otherwise healthy emerging adults who contracted COVID-19, rare and sometimes fatal cardiovascular presentations were reported. Compared with younger age cohorts, MIS in emerging adults has been associated with a greater risk of cardiovascular involvement, specifically myocarditis, thrombosis, and mortality. Limited data for emerging adults suggest that obesity, hypertension, CVD, and/or belonging to marginalized groups increase the risk of severe COVID-19, related cardiovascular complications, and mortality. Future research needs to further define the prevalence and risk factors of COVID-19–associated cardiovascular complications in this demographic. Screening and treatment protocols for these complications (eg, triad testing and cMRI) still require further development and validation using data from the broader emerging adult population. Within health care practice, several measures are encouraged to foster long-term cardiovascular health among emerging adults: (i) conducting differential diagnoses for cardiovascular issues in those with COVID-19–like symptoms; (ii) promoting health literacy and education among emerging adults and parents/guardians, including cardiorespiratory fitness; and (iii) expanding telehealth accessibility. Although COVID-19 vaccines are still recommended for emerging adults, policymakers and clinicians should be aware of the potential cardiovascular risks and use the most recent surveillance data to guide vaccination policies for this age group. These actions should prove useful while the scientific community continues to unravel the long-term cardiovascular implications of COVID-19 among emerging adults.
Acknowledgements
The authors express their gratitude to Denise Smith, a librarian at the McMaster University Health Sciences Library, for helping develop and refine the final search strategy; Dr Philip Joseph, MD, associate professor at McMaster University, for his feedback; and several research assistants, including Bismah Jameel, who assisted with article screening and data extraction.
Ethics Statement
The research reported in this review has adhered to the relevant ethical guidelines.
Funding Sources
Funding for publication was provided by the senior co-authors: (i) KMD, supported by the Canada Research Chair in Nutrition Informatics grant; and (ii) VT, supported by the Canadian Institutes of Health Research Health System Impact Fellowship grant (#177475). The agencies responsible for these grants had no involvement in this project.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
To access the supplementary material accompanying this article, visit CJC Pediatric and Congenital Heart Disease at https://www.cjcpc.ca// and at https://doi.org/10.1016/j.cjcpc.2022.11.005
Supplementary Material
References
- 1.Grant M.C., Geoghegan L., Arbyn M., et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsushita K., Ding N., Kou M., et al. The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: a systematic review and meta-analysis. Glob Heart. 2020;15:64. doi: 10.5334/gh.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karbalai Saleh S., Oraii A., Soleimani A., et al. The association between cardiac injury and outcomes in hospitalized patients with COVID-19. Intern Emerg Med. 2020;15:1415–1424. doi: 10.1007/s11739-020-02466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heart and Stroke Foundation of Canada. Don’t wait for COVID-19 to end to address health concerns. Available at: https://www.heartandstroke.ca/en/articles/don-t-wait-for-covid-19-to-end-to-address-serious-health-concerns/. Accessed March 31, 2021.
- 6.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnett J.J. Emerging adulthood: a theory of development from the late teens through the twenties. Am Psychol. 2000;55:469. [PubMed] [Google Scholar]
- 8.Lally M., Valentine-French S. In: Parenting and Family Diversity Issues. Hanson A., Elder A., editors. Iowa State University Digital Press; Ames, IA: 2020. Emerging and early adulthood. [Google Scholar]
- 9.American College of Cardiology. Intermediate and long-term impact of COVID-19 on cardiovascular disease. Available at: https://www.acc.org/latest-in-cardiology/articles/2021/04/21/13/08/intermediate-and-long-term-impact-of-covid-19-on-cardiovascular-disease. Accessed June 26, 2021.
- 10.Brito D., Meester S., Yanamala N., et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging. 2020;14:541–555. doi: 10.1016/j.jcmg.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starekova J., Bluemke D.A., Bradham W.S., et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6:945–950. doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Statistics Canada. Population estimates on July 1st, by age and sex. Available at: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501. Accessed November 11, 2022.
- 13.Government of Canada COVID-19 daily epidemiology update. https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html Available at:
- 14.Valverde I., Singh Y., Sanchez-de-Toledo J., et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143:21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Multisystem inflammatory syndrome in adults (MIS-A) https://www.cdc.gov/mis-c/mis-a.html Available at:
- 16.Karlstad Ø., Hovi P., Husby A., et al. SARS-CoV-2 vaccination and myocarditis in a Nordic cohort study of 23 million residents. JAMA Cardiol. 2022;7:600–612. doi: 10.1001/jamacardio.2022.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oster M.E., Shay D.K., Su J.R., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oster A.M., Caruso E., DeVies J., et al. Transmission dynamics by age group in COVID-19 hotspot counties—United States, April-September 2020. Morb Mortal Wkly Rep. 2020;69:1494–1496. doi: 10.15585/mmwr.mm6941e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvatore P.P., Sula E., Coyle J.P., et al. Recent increase in COVID-19 cases reported among adults aged 18-22 years—United States, May 31-September 5, 2020. Morb Mortal Wkly Rep. 2020;69:1419–1424. doi: 10.15585/mmwr.mm6939e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tricco A.C., Lillie E., Zarin W., et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 21.Covidence. Covidence systematic review software. Veritas Health Innovation, Melbourne, Australia. Available at: https://www.covidence.org. Accessed January 22, 2021.
- 22.Chau V.Q., Giustino G., Mahmood K., et al. Cardiogenic shock and hyperinflammatory syndrome in young males with COVID-19. Circ Heart Fail. 2020;13:e007485. doi: 10.1161/CIRCHEARTFAILURE.120.007485. [DOI] [PubMed] [Google Scholar]
- 23.Razavi A.C., Chang J.L., Sutherland A., Niyogi A., Menard G.E. A 23-year-old man with multisystem inflammatory syndrome after mild COVID-19. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris S.B., Schwartz N.G., Patel P., et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection—United Kingdom and United States, March–August 2020. Morb Mortal Wkly Rep. 2020;69:1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kofman A.D., Sizemore E.K., Detelich J.F., Albrecht B., Piantadosi A.L. A young adult with COVID-19 and multisystem inflammatory syndrome in children (MIS-C)-like illness: a case report. BMC Infect Dis. 2020;20:716. doi: 10.1186/s12879-020-05439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Othenin-Girard A., Regamey J., Lamoth F., et al. Multisystem inflammatory syndrome with refractory cardiogenic shock due to acute myocarditis and mononeuritis multiplex after SARS-CoV-2 infection in an adult. Swiss Med Wkly. 2020;150 doi: 10.4414/smw.2020.20387. [DOI] [PubMed] [Google Scholar]
- 27.Vieira C.B., Ferreira A.T., Cardoso F.B., Paulos J.P., Germano N. Kawasaki-like syndrome as an emerging complication of SARS-CoV-2 infection in young adults. Eur J Case Rep Intern Med. 2020;7:001886. doi: 10.12890/2020_001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cogan E., Foulon P., Cappeliez O., et al. Multisystem inflammatory syndrome with complete Kawasaki disease features associated with SARS-CoV-2 infection in a young adult. A case report. Front Med. 2020;7:428. doi: 10.3389/fmed.2020.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh V.P., Thalji M., Singh S., et al. Fulminant myocarditis and multisystem inflammatory syndrome in children, in the light of corona virus. J Am Coll Cardiol. 2021;77:1964. [Google Scholar]
- 30.Wojnowski K., Ladna J., Kumar S. Multisystem inflammatory syndrome of the adult: an important consequence of COVID-19. Chest. 2021;160 [Google Scholar]
- 31.Nwachukwu I., Fernandes M. Severe multisystem inflammatory syndrome in an adult with myocarditis. Chest. 2021;160(suppl) [Google Scholar]
- 32.Bulut H., Herbers A.H.E., Hageman I.M.G., et al. SARS-CoV-2-induced multisystem inflammatory syndrome in a young adult: case report. SN Compr Clin Med. 2021;3:1773–1779. doi: 10.1007/s42399-021-00998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald A., Hussein R., Gowtham S., et al. Multisystem inflammatory syndrome in Pennsylvania: the intersection of adult and pediatric care. Crit Care Med. 2021;49:77. [Google Scholar]
- 34.Bonnet M., Champagnac A., Lantelme P., Harbaoui B. Endomyocardial biopsy findings in Kawasaki-like disease associated with SARS-CoV-2. Eur Heart J. 2020;41:3863–3864. doi: 10.1093/eurheartj/ehaa588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carvalho V., Damasco P.H., Mello T.S., Gonçalves B. Para-aortic lymphadenopathy associated with adult COVID-19 multisystem inflammatory syndrome. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-246884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moghadam P., Blum L., Ahouach B., et al. Multisystem inflammatory syndrome with particular cutaneous lesions related to COVID-19 in a young adult. Am J Med. 2021;134:e36–e37. doi: 10.1016/j.amjmed.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faller E., Barry R., O’Flynn O., Kearney P., Sadlier C. Kawasaki-like multisystem inflammatory syndrome associated with SARS-CoV-2 infection in an adult. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2020-240845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campoy N.R., Gulati K., Morris K., Delgado F. Multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19/incomplete Kawasaki disease. Consultant. 2021;61:e8–e11. [Google Scholar]
- 39.Aggarwal A., Cohen E., Figueira M., et al. Multisystem inflammatory syndrome in an adult with COVID-19—a trial of anakinra: a case report. Infect Dis Clin Pract (Baltim Md) 2021;29:e420–e423. doi: 10.1097/IPC.0000000000001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Mashdali A.F., Al Samawi M.S. A case of post COVID-19 multisystem inflammatory syndrome and Bell’s palsy in a young adult. Clin Case Rep. 2021;9 doi: 10.1002/ccr3.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mittal N., Abohelwa M., Brogan J., Nichols J. A case report of multi-system inflammatory syndrome in adults (MIS-A) associated with heart failure. Eur Heart J Case Rep. 2021;5 doi: 10.1093/ehjcr/ytab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ejaz K., Patel N., Ramos J., Sharma A. A young adult with COVID-19 associated multisystem inflammatory syndrome. Am J Respir Crit Care Med. 2021;203 [Google Scholar]
- 43.Rajendraprasad S., Ahmad F., Nair S. Case of multisystem inflammatory syndrome in children (MIS-C) presenting with rash and shock. Chest. 2021;160 [Google Scholar]
- 44.Pombo F., Seabra C., Soares V., et al. COVID-19-related multisystem inflammatory syndrome in a young adult. Eur J Case Rep Intern Med. 2021;8 doi: 10.12890/2021_002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vujaklija Brajković A., Zlopaša O., Gubarev Vrdoljak N., et al. Acute liver and cardiac failure in multisystem inflammatory syndrome in adults after COVID-19. Clin Res Hepatol Gastroenterol. 2021;45:101678. doi: 10.1016/j.clinre.2021.101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chug L., Cabrera N.M., Mathew J., et al. Multisystem inflammatory syndrome in an adult associated with COVID-19. Crit Care Med. 2021;49:92. [Google Scholar]
- 47.Dabas R., Varadaraj G., Sandhu S., Bhatnagar A., Pal R. Kawasaki-like multisystem inflammatory syndrome associated with COVID-19 in an adult: a case report. Br J Dermatol. 2021;185:859–861. doi: 10.1111/bjd.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salzman M.B., Huang C.W., O’Brien C.M., Castillo R.D. Multisystem inflammatory syndrome after SARS-CoV-2 infection and COVID-19 vaccination. Emerg Infect Dis. 2021;27:1944–1948. doi: 10.3201/eid2707.210594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ciochetto Z., Havens P.L., Aldrete S. Two cases of multi-inflammatory syndrome in children (MIS-C) in adults in 2020. BMC Infect Dis. 2021;21:1228. doi: 10.1186/s12879-021-06911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ronit A., Jørgensen S.E., Roed C., et al. Host genetics and antiviral immune responses in adult patients with multisystem inflammatory syndrome. Front Immunol. 2021;12:718744. doi: 10.3389/fimmu.2021.718744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hékimian G., Kerneis M., Zeitouni M., et al. Coronavirus disease 2019 acute myocarditis and multisystem inflammatory syndrome in adult intensive and cardiac care units. Chest. 2021;159:657–662. doi: 10.1016/j.chest.2020.08.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bastug A., Aslaner H., Aybar Bilir Y., et al. Multiple system inflammatory syndrome associated with SARS-CoV-2 infection in an adult and an adolescent. Rheumatol Int. 2021;41:993–1008. doi: 10.1007/s00296-021-04843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bulathsinghala M., Samson R. A case of COVID-19 associated multisystem inflammatory syndrome resulting in new onset heart failure in an adult. J Invest Med. 2021;69:431. [Google Scholar]
- 54.Szawarski P., Whittaker R., Riyat M., Mandal A. Adult with a paediatric diagnosis after COVID-19 infection. Intensive Care Med Exp. 2021;9(suppl 1) [Google Scholar]
- 55.Malik F., Kasten J., VandenHeuvel K., Leino D., Bernieh A. Spectrum of autopsy findings in COVID-19 related pediatric and fetal deaths: 4 cases from a tertiary care children’s center. Pediatr Dev Pathol. 2021;24:592–617. [Google Scholar]
- 56.Nguyen V.T., Zaccarini C., Klawonn M.A., Turk M. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection: a case report. PM&R. 2021;13(suppl 1):S1–S229. [Google Scholar]
- 57.Molina M.F., Al Saud A.A., Al Mulhim A.A., Liteplo A.S., Shokoohi H. Nitrous oxide inhalant abuse and massive pulmonary embolism in COVID-19. Am J Emerg Med. 2020;38:1549.e1–1549.e2. doi: 10.1016/j.ajem.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez J.A., Rubio-Gomez H., Roa A.A., Miller N., Eckardt P.A. Co-infection with SARS-CoV-2 and parainfluenza in a young adult patient with pneumonia: case report. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mantovani Cardoso E., Hundal J., Feterman D., Magaldi J. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin Rheumatol. 2020;39:2811–2815. doi: 10.1007/s10067-020-05310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kandori K., Narumiya H., Iizuka R. Extracorporeal cardiopulmonary resuscitation should not be performed on confirmed or suspected COVID-19 patients. Resuscitation. 2020;153:6–7. doi: 10.1016/j.resuscitation.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wongkittichote P., Watson J.R., Leonard J.M., et al. Fatal COVID-19 infection in a patient with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: a case report. JIMD Rep. 2020;56:40–45. doi: 10.1002/jmd2.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeantin L., Pichereau C., Pineton de Chambrun M., et al. Myocarditis, paraparesia and ARDS associated to COVID-19 infection. Heart Lung. 2021;50:6–8. doi: 10.1016/j.hrtlng.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soquet J., Rousse N., Moussa M., et al. Heart retransplantation following COVID-19 illness in a heart transplant recipient. J Heart Lung Transplant. 2020;39:983–985. doi: 10.1016/j.healun.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garau G., Joachim S., Duliere G.-L., et al. Sudden cardiogenic shock mimicking fulminant myocarditis in a surviving teenager affected by severe acute respiratory syndrome coronavirus 2 infection. ESC Heart Fail. 2021;8:766–773. doi: 10.1002/ehf2.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crippa S., Kagi G., Graf L., Meyer Sauteur P.M., Kohler P. Stroke in a young adult with mild COVID-19 suggesting endotheliitis. New Microbes New Infect. 2020;38:100781. doi: 10.1016/j.nmni.2020.100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beşler M.S., Arslan H. Acute myocarditis associated with COVID-19 infection. Am J Emerg Med. 2020;38:2489.e1–2489.e2. doi: 10.1016/j.ajem.2020.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garot J., Amour J., Pezel T., et al. SARS-CoV-2 fulminant myocarditis. JACC Case Rep. 2020;2:1342–1346. doi: 10.1016/j.jaccas.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim I.-C., Kim J.Y., Kim H.A., Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alizadehasl A., Salehi P., Roudbari S., Peighambari M.M. Infectious endocarditis of the prosthetic mitral valve after COVID-19 infection. Eur Heart J. 2020;41:4604. doi: 10.1093/eurheartj/ehaa852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fatehi P., Hesam-Shariati N., Abouzaripour M., Fathi F., Hesam Shariati M.B. Acute ischemic and hemorrhagic stroke and COVID-19: case series. SN Compr Clin Med. 2020;2:2396–2401. doi: 10.1007/s42399-020-00559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krishnan U.S., Krishnan S.S., Jain S., et al. SARS-CoV-2 infection in patients with Down syndrome, congenital heart disease, and pulmonary hypertension: is Down syndrome a risk factor? J Pediatr. 2020;225:246–248. doi: 10.1016/j.jpeds.2020.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carneiro T., Dashkoff J., Leung L.Y., et al. Intravenous tPA for acute ischemic stroke in patients with COVID-19. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2020;29:105201. doi: 10.1016/j.jstrokecerebrovasdis.2020.105201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calhoun A., Fatade Y., Garcia M., et al. Fulminant myocarditis in COVID-19 with rapid recovery of systolic function. J Am Coll Cardiol. 2021;77(suppl 1):2012. [Google Scholar]
- 74.Choudhary K., Regante R., Fitzgibbons T., Ram S., Miskovsky J. Acute myocarditis and acute decompensated heart failure with reduced ejection fraction in COVID-19. J Am Coll Cardiol. 2021;77(suppl 1):3015. [Google Scholar]
- 75.Volis I., Livneh I., Hussein K., Raz-Pasteur A. COVID-19-associated suspected myocarditis as the etiology for recurrent and protracted fever in an otherwise healthy adult. Am J Med Sci. 2021;361:522–525. doi: 10.1016/j.amjms.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laleh Far V., Najafizadeh S.R., Eslami M., Mollazadeh R. A flare up of idiopathic hypereosinophilic syndrome due to COVID-19. Eur Heart J. 2021;42:954. doi: 10.1093/eurheartj/ehaa714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Venditti L., Rousseau A., Ancelet C., Papo T., Denier C. Susac syndrome following COVID-19 infection. Acta Neurol Belg. 2021;121:807–809. doi: 10.1007/s13760-020-01554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhattarai P., Allen H., Aggarwal A., Madden D., Dalton K. Unmasking of Addison’s disease in COVID-19. SAGE Open Med Case Rep. 2021;9 doi: 10.1177/2050313X211027758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bozan Ö., Atiş Ş.E., Çekmen B. A rare complication of COVID-19: spontaneous pneumothorax following pneumomediastinum; case report. Am J Emerg Med. 2021;47:342.e1–342.e2. doi: 10.1016/j.ajem.2021.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Connor A., Loganathan S., Aziz R. COVID-19 related myocarditis following laparoscopic appendicectomy. Br J Surg. 2021;108(suppl 7) [Google Scholar]
- 81.Recalcati S., Piconi S., Franzetti M., et al. Colchicin treatment of COVID-19 presenting with cutaneous rash and myopericarditis. Dermatol Ther. 2020;33 doi: 10.1111/dth.13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dahou S., Leach T., Bostock K., et al. Challenges in treating new onset systemic lupus erythematosus with lupus nephritis and COVID-19 infection overlap. Rheumatol Adv Pract. 2020;4(suppl 1) [Google Scholar]
- 83.Sheha D., El-Shayeb M., Eid Y., et al. Unfolding of sickle cell trait by coronavirus disease 2019 (COVID-19) infection. Br J Haematol. 2020;191:e38–e40. doi: 10.1111/bjh.17089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh S., Foster A., Khan Z., et al. COVID-19-induced diabetic ketoacidosis and acute respiratory distress syndrome in an obese 24-year-old type I diabetic. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.925586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han D., Sun Y., Xie R., Zhu X., Zhong Z. A case of severe COVID-19 in a patient with acute graft-versus-host disease after haploidentical transplantation. Case Rep Hematol. 2021;2021:8878803. doi: 10.1155/2021/8878803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strause J., Atsina K.B., Bialo D., et al. Ischemic stroke associated with aneurysmal lenticulostriate vasculopathy and symmetric reversible basal ganglia lesions in COVID-19. J Neurol Sci. 2021;426:117484. doi: 10.1016/j.jns.2021.117484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Merdad G.A., Seadawi L.E., Mustafa A.A. Peptic ulcer associated with COVID-19 in Saudi Arabia. Saudi Med J. 2021;42:1036–1040. doi: 10.15537/smj.2021.42.9.20210224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bunawan N.C., Mokoagow M.I., Djojo A.Y., et al. Delayed RT-PCR time-to-positivity in an adult with SARS-CoV-2 infection. J Infect Dev Ctries. 2021;15:913–917. doi: 10.3855/jidc.14766. [DOI] [PubMed] [Google Scholar]
- 89.Gaglani B., Westphal N., Bryant C., et al. Successful management of COVID-19-induced ARDS using VV ECMO in a patient with a BMI of 73 kg/m2. Crit Care Med. 2021;49:70. [Google Scholar]
- 90.Pasqualetto M.C., Sorbo M.D., Vitiello M., et al. Pulmonary hypertension in COVID-19 pneumoniae: it is not always as it seems. Eur J Case Rep Intern Med. 2020;7:002160. doi: 10.12890/2020_002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rashed E., Cagliostro M., Kamran M. Acute myocardial infarction in a young man in the COVID-19 era: a case report. Circulation. 2020;142(suppl 3):A17112. [Google Scholar]
- 92.Freeman H., Moulton B. Propofol-related infusion syndrome in a patient with ARDS secondary to the novel COVID-19. Chest. 2021;160:A815. [Google Scholar]
- 93.Ouzts P., McKeag I., Asif I. Elevated troponins in a cross-country athlete diagnosed with COVID-19. Clin J Sport Med. 2021;31 [Google Scholar]
- 94.Edwards K., Hussain I. Two cases of severe autoimmune thyrotoxicosis following SARS-CoV-2 infection. J Investig Med High Impact Case Rep. 2021;9 doi: 10.1177/23247096211056497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Munoz D., Malik H., Eickenhorst D., et al. Cardiac screening in a young adult male leading to discovery of post-COVID myocarditis with asymptomatic large apical left ventricular thrombus. CASE (Phila) 2021;5:309–312. doi: 10.1016/j.case.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eid M.M. Co-infection with COVID-19 and malaria in a young man. Dubai Med J. 2021;4:164–166. [Google Scholar]
- 97.Clerico M., Dogliotti I., Calcagno A., et al. COVID-19 in a post-transplant heart recipient who developed aggressive lymphoma: a biphasic course during rituximab treatment. Hemasphere. 2021;5 doi: 10.1097/HS9.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hasnie U., Andrikopoulou E., Lloyd S. False start: delayed COVID-19 myocarditis in a young athlete. J Am Coll Cardiol. 2021;77(suppl 1):1996. [Google Scholar]
- 99.Sikandar B.H., Butler S., Battula A., Shetty R. ST-elevation myocardial infarction in a 23-year-old female: the mystery of thrombus formation. Cureus. 2021;13 doi: 10.7759/cureus.15302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hekmatikar A.H.A., Shamsi M.M., Ashkazari Z.S.Z., Suzuki K. Exercise in an overweight patient with COVID-19: a case study. Int J Environ Res Public Health. 2021;18:5882. doi: 10.3390/ijerph18115882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guendouz C., Quenardelle V., Riou-Comte N., et al. Pathogeny of cerebral venous thrombosis in SARS-CoV-2 infection. Med (Baltimore) 2021;100 doi: 10.1097/MD.0000000000024708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bozorgmehr R., Tajabadi Z. Hemoptysis and hematuria as the initial symptoms of COVID-19: a case report. Tanaffos. 2021;20:75–78. [PMC free article] [PubMed] [Google Scholar]
- 103.Hirschbaum J.H., Bradley C.P., Kingsford P., Mehra A., Kwan W. Recurrent massive pulmonary embolism following catheter directed thrombolysis in a 21-year-old with COVID-19: a case report. Eur Heart J Case Rep. 2021;5 doi: 10.1093/ehjcr/ytab140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hussein M.H., Alabdaljabar M.S., Alfagyh N., Badran M., Alamiri K. Splanchnic venous thrombosis in a nephrotic patient following COVID-19 infection: a case report. BMC Nephrol. 2021;22:1–5. doi: 10.1186/s12882-021-02643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doğan A.C., Güner A., Avcı Y., Zencirkiran Agus H., Güner E.G. Pulmonary embolism in a young man infected with COVID-19 pneumonia. Turk Kardiyol Dern Ars. 2020;48:714. doi: 10.5543/tkda.2020.03688. [DOI] [PubMed] [Google Scholar]
- 106.Bhasin V., Carrillo M., Ghosh B., et al. Reversible complete heart block in a patient with coronavirus disease 2019. Pacing Clin Electrophysiol. 2021;44:1939–1943. doi: 10.1111/pace.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abdullah A., Neurath M.F., Atreya R. Mild COVID-19 symptoms in an infliximab-treated ulcerative colitis patient: can ongoing anti-TNF therapy protect against the viral hyperinflammatory response and avoid aggravated outcomes? Visc Med. 2020;36:338–342. doi: 10.1159/000508740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ghosh R., Roy D., Sengupta S., Benito-León J. Autonomic dysfunction heralding acute motor axonal neuropathy in COVID-19. J Neurovirol. 2020;26:964–966. doi: 10.1007/s13365-020-00908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Long A., Grimaldo F. Spontaneous hemopneumothorax in a patient with COVID-19. Am J Emerg Med. 2021;40:228.e1–228.e2. doi: 10.1016/j.ajem.2020.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seneviratna A. Fat embolism syndrome or COVID-19 pneumonia: a diagnostic dilemma. Sri Lankan J Anaesthesiol. 2021;29:109–113. [Google Scholar]
- 111.Flower L., Gale A., Elfar E., et al. Adult onset PIMS-TS with secondary haemophagocytic lymphistiocytosis: into the eye of the cytokine storm. Rheumatol Adv Pract. 2020;4(suppl 1) [Google Scholar]
- 112.Simpson H.D., Johnson E., Britton J., Braksick S. Alternating hemiparesis in the context of hemolytic uremic syndrome and COVID-19 positivity. Epilepsy Behav Rep. 2021;16:100468. doi: 10.1016/j.ebr.2021.100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pąchalska M., Goral-Polrola J., Chojnowska-Cwiakala I. Effect of individually-tailored TDCS and symbolic art therapy for chronic associative prosopagnosia after infection by SARS-COV-2, neuroCOVID-19 and ischemic stroke. Acta Neuropsychologica. 2021;19:329–345. [Google Scholar]
- 114.Aikawa T., Ogino J., Kudo T., Kashiwagi Y. Late-onset endocarditis after coronavirus disease 2019 infection. Eur Heart J. 2021;42:3108. doi: 10.1093/eurheartj/ehab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marcinkiewicz K., Petryka-Mazurkiewicz J., Nowicki M.M., et al. Acute heart failure in the course of fulminant myocarditis requiring mechanical circulatory support in a healthy young patient after coronavirus disease 2019. Kardiol Pol. 2021;79:583–584. doi: 10.33963/KP.15888. [DOI] [PubMed] [Google Scholar]
- 116.Al-Kaf F.A., Garni T.A., Nahes A.H., Sandokji H., Samargandy S. Cardiac tamponade, severe hypothyroidism and acute respiratory distress syndrome (ARDS) with COVID-19 infection. J Saudi Heart Assoc. 2021;33:71–76. doi: 10.37616/2212-5043.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krishna V., Morjaria J., Jalandari R., Omar F., Kaul S. Autoptic identification of disseminated mucormycosis in a young male presenting with cerebrovascular event, multi-organ dysfunction and COVID-19 infection. IDCases. 2021;25:e01172. doi: 10.1016/j.idcr.2021.e01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alam A., Kumar D. A case of COVID-19 myocarditis presenting as inferior wall MI. Chest. 2021;160 [Google Scholar]
- 119.O’Sullivan J.S., Lyne A., Vaughan C.J. COVID-19-induced postural orthostatic tachycardia syndrome treated with ivabradine. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hedayat B., Hosseini K. Chest pain and high troponin level without significant respiratory symptoms in young patients with COVID-19. Caspian J Intern Med. 2020;11(suppl 1):561–565. doi: 10.22088/cjim.11.0.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kenniff S., Mawed S.A., Al-Ghazawi S., Corcho A.R., Qureshi M. COVID-19 associated diffuse myoclonus and dystonia (CADMAD) syndrome: an immune mediated para-infectious or post-infectious condition? Neurology. 2021;96(suppl):1765. [Google Scholar]
- 122.Arandela K., Samudrala S., Abdalkader M., et al. Reversible cerebral vasoconstriction syndrome in patients with coronavirus disease: a multicenter case series. J Stroke Cerebrovasc Dis. 2021;30:106118. doi: 10.1016/j.jstrokecerebrovasdis.2021.106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Petracek L.S., Suskauer S.J., Vickers R.F., et al. Adolescent and young adult ME/CFS after confirmed or probable COVID-19. Front Med. 2021;8:668944. doi: 10.3389/fmed.2021.668944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Elikowski W., Fertała N., Zawodna-Marszałek M., et al. Marked self-limiting sinus bradycardia in COVID-19 patients not requiring therapy in the intensive care unit—case series report. Pol Merkur Lekarski. 2021;49:295–302. [PubMed] [Google Scholar]
- 125.Heidarpour M., Vakhshoori M., Haghighatpanah M.A., et al. Rhabdomyolysis plus hypocalcemia and diabetic ketoacidosis as concurrent rare COVID-19 manifestations. Case Rep Med. 2021 doi: 10.1155/2021/6625086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grisanti S., Schenone C., Biassoni E., et al. Neurological complications of COVID-19: a monocentric experience of a neurological outpatient clinic. J Neurol Sci. 2021:429. [Google Scholar]
- 127.Croci G.A., Vaira V., Trabattoni D., et al. Emergency lung transplantation after COVID-19: immunopathological insights on two affected patients. Cells. 2021;10:611. doi: 10.3390/cells10030611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mitchell W.B., Davila J., Keenan J., et al. Children and young adults hospitalized for severe COVID-19 exhibit thrombotic coagulopathy. Pediatr Blood Cancer. 2021;68 doi: 10.1002/pbc.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sawalha K., Habash F.J., Vallurupalli S., Paydak H. Theophylline in treatment of COVID-19 induced sinus bradycardia. Clin Pract. 2021;11:332–336. doi: 10.3390/clinpract11020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Warraich M., Bolaji P., Das S. Posterior circulation stroke presenting as a new continuous cough: not always COVID-19. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2020-240270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cruz-Utrilla A. Segura De la Cal T, Escribano-Subias P. Giant T wave inversion and dyspnea in the time of coronavirus pandemic. Circulation. 2020;142:906–909. doi: 10.1161/CIRCULATIONAHA.120.049194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cankay T.U., Besenek M. What do we over-look during COVID-19 pandemic? An adolescent stroke case presumed conversion disorder. Psychiatr Danub. 2020;32:300–302. doi: 10.24869/psyd.2020.300. [DOI] [PubMed] [Google Scholar]
- 133.Toth E., Dancy L., Amin-Youssef G., Papachristidis A., Dworakowski R. Collateral implications of the COVID-19 pandemic: belated presentation of infective endocarditis in a young patient. Eur Heart J. 2020;41:4365. doi: 10.1093/eurheartj/ehaa633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Atuaka C., Foziljonova K., Duarte C.D.A., et al. Not everything is COVID: persistent fever and malaise mimicking COVID-19 pneumonia. Chest. 2021;160:A422–A423. [Google Scholar]
- 135.Greenfeld S.M., Tadmor T. ‘Catastrophic’ thrombosis in a young patient with acute myeloid leukemia presenting early in the COVID-19 pandemic—a case report. Vivo. 2021;35:2951–2955. doi: 10.21873/invivo.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Daliri M., Hosseini S., Amin A., et al. Possible role of ECMO in multiorgan failure and prolonged CPR: aluminum phosphide poisoning. Asia Pac J Med Toxicol. 2020;9:154–158. [Google Scholar]
- 137.Balfe C., O’Connor C., Giblin G., et al. Presentation of severe rheumatic mitral stenosis at the peak of the COVID-19 pandemic and the presumptive treatment as severe coronavirus illness. Eur J Case Rep Intern Med. 2020;7:001957. doi: 10.12890/2020_001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Quinlivan R., Desikan M., Cruces F., Pietrusz A., Savvatis K. Clinical outcome of SARS-CoV-2 infection in 7 adults with Duchenne muscular dystrophy attending a specialist neuromuscular centre. Neuromuscul Disord. 2021;31:603–606. doi: 10.1016/j.nmd.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Erickson J.L., Poterucha J.T., Gende A., et al. Use of electrocardiographic screening to clear athletes for return to sports following COVID-19 infection. Mayo Clin Proc Innov Qual Outcomes. 2021;5:368–376. doi: 10.1016/j.mayocpiqo.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moulson N., Petek B.J., Drezner J.A., et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021;144:256–266. doi: 10.1161/CIRCULATIONAHA.121.054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Petek B.J., Moulson N., Baggish A.L., et al. Prevalence and clinical implications of persistent or exertional cardiopulmonary symptoms following SARS-CoV-2 infection in 3597 collegiate athletes: a study from the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) Br J Sports Med. 2022;56:913–918. doi: 10.1136/bjsports-2021-104644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vago H., Szabo L., Dohy Z., Merkely B. Cardiac magnetic resonance findings in patients recovered from COVID-19. JACC Cardiovasc Imaging. 2021;14:1279–1281. doi: 10.1016/j.jcmg.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hendrickson B.S., Stephens R.E., Chang J.V., et al. Cardiovascular evaluation after COVID-19 in 137 collegiate athletes: results of an algorithm-guided screening. Circulation. 2021;143:1926–1928. doi: 10.1161/CIRCULATIONAHA.121.053982. [DOI] [PubMed] [Google Scholar]
- 144.Małek Ł.A., Marczak M., Miłosz-Wieczorek B., et al. Cardiac involvement in consecutive elite athletes recovered from COVID-19: a magnetic resonance study. J Magn Reson Imaging. 2021;53:1723–1729. doi: 10.1002/jmri.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rajpal S., Tong M.S., Borchers J., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gervasi S.F., Pengue L., Damato L., et al. Is extensive cardiopulmonary screening useful in athletes with previous asymptomatic or mild SARS-CoV-2 infection? Br J Sports Med. 2021;55:54–61. doi: 10.1136/bjsports-2020-102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Boehmer T.K., Kompaniyets L., Lavery A.M., et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. Morb Mortal Wkly Rep. 2021;70:1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Belay E.D., Abrams J., Oster M.E., et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr. 2021;175:837–845. doi: 10.1001/jamapediatrics.2021.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Whitworth H., Sartain S.E., Kumar R., et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021;138:190–198. doi: 10.1182/blood.2020010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lewis M.J., Anderson B.R., Fremed M., et al. Impact of coronavirus disease 2019 (COVID-19) on patients with congenital heart disease across the lifespan: the experience of an academic congenital heart disease center in New York City. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Broberg C.S., Kovacs A.H., Sadeghi S., et al. COVID-19 in adults with congenital heart disease. J Am Coll Cardiol. 2021;77:1644–1655. doi: 10.1016/j.jacc.2021.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fisher J.M., Badran S., Li J.T., Votava-Smith J.K., Sullivan P.M. Characteristics and outcomes of acute COVID-19 infection in paediatric and young adult patients with underlying cardiac disease. Cardiol Young. 2022;32:1261–1267. doi: 10.1017/S1047951121004029. [DOI] [PubMed] [Google Scholar]
- 153.Kibbey M.M., Fedorenko E.J., Farris S.G. Anxiety, depression, and health anxiety in undergraduate students living in initial US outbreak “hotspot” during COVID-19 pandemic. Cogn Behav Ther. 2021;50:409–421. doi: 10.1080/16506073.2020.1853805. [DOI] [PubMed] [Google Scholar]
- 154.Majumdar P., Biswas A., Sahu S. COVID-19 pandemic and lockdown: cause of sleep disruption, depression, somatic pain, and increased screen exposure of office workers and students of India. Chronobiol Int. 2020;37:1191–1200. doi: 10.1080/07420528.2020.1786107. [DOI] [PubMed] [Google Scholar]
- 155.Altonen B.L., Arreglado T.M., Leroux O., Murray-Ramcharan M., Engdahl R. Characteristics, comorbidities and survival analysis of young adults hospitalized with COVID-19 in New York City. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Adams S.H., Park M.J., Schaub J.P., Brindis C.D., Irwin C.E. Medical vulnerability of young adults to severe COVID-19 illness—data from the National Health Interview Survey. J Adolesc Health. 2020;67:362–368. doi: 10.1016/j.jadohealth.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fathi M., Markazi Moghaddam N., Kheyrati L. Development and validation of models for two-week mortality of inpatients with COVID-19 infection: a large prospective cohort study. Stat Anal Data Min. 2022;15:586–597. [Google Scholar]
- 158.Richardson S., Gitlin J., Kozel Z., et al. In-hospital 30-day survival among young adults with coronavirus disease 2019: a cohort study. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sandoval M., Nguyen D.T., Vahidy F.S., Graviss E.A. Risk factors for severity of COVID-19 in hospital patients age 18-29 years. PLoS One. 2021;16 doi: 10.1371/journal.pone.0255544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ratchford S.M., Stickford J.L., Province V.M., et al. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol Heart Circ Physiol. 2021;320:H404–H410. doi: 10.1152/ajpheart.00897.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Szeghy R.E., Province V.M., Stute N.L., et al. Carotid stiffness, intima-media thickness and aortic augmentation index among adults with SARS-CoV-2. Exp Physiol. 2022;107:694–707. doi: 10.1113/EP089481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nandadeva D., Young B.E., Stephens B.Y., et al. Blunted peripheral but not cerebral vasodilator function in young otherwise healthy adults with persistent symptoms following COVID-19. Am J Physiol Heart Circ Physiol. 2021;321:H479–H484. doi: 10.1152/ajpheart.00368.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stute N.L., Stickford J.L., Province V.M., et al. COVID-19 is getting on our nerves: sympathetic neural activity and haemodynamics in young adults recovering from SARS-CoV-2. J Physiol. 2021;599:4269–4285. doi: 10.1113/JP281888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stute N.L., Stickford A.S.L., Stickford J.L., et al. Altered central and peripheral haemodynamics during rhythmic handgrip exercise in young adults with SARS-CoV-2. Exp Physiol. 2022;107:708–721. doi: 10.1113/EP089820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Udelson J.E., Rowin E.J., Maron B.J. Return to play for athletes after COVID-19 infection: the fog begins to clear. JAMA Cardiol. 2021;6:997–999. doi: 10.1001/jamacardio.2021.2079. [DOI] [PubMed] [Google Scholar]
- 166.van Hattum J.C., Spies J.L., Verwijs S.M., et al. Cardiac abnormalities in athletes after SARS-CoV-2 infection: a systematic review. BMJ Open Sport Exerc Med. 2021;7:e001164. doi: 10.1136/bmjsem-2021-001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 168.Sands-Lincoln M., Huang H., Jackson G.P., Wang S. Towards a better understanding of COVID-19 among young adults ages 18-24. Value Health. 2021;24:S121. [Google Scholar]
- 169.Daniels C.J., Rajpal S., Greenshields J.T., et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021;6:1078–1087. doi: 10.1001/jamacardio.2021.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.National Library of Medicine. Sinus tachycardia. Available at: https://www.ncbi.nlm.nih.gov/books/NBK553128/. Accessed August 29, 2022.
- 171.Mitrani R.D., Dabas N., Goldberger J.J. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17:1984–1990. doi: 10.1016/j.hrthm.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yadav R., Bansal R., Budakoty S., Barwad P. COVID-19 and sudden cardiac death: a new potential risk. Indian Heart J. 2020;72:333–336. doi: 10.1016/j.ihj.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bohm P., Scharhag J., Egger F., et al. Sports-related sudden cardiac arrest in Germany. Can J Cardiol. 2021;37:105–112. doi: 10.1016/j.cjca.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 174.Tilles J.G., Elson S.H., Shaka J.A., et al. Effects of exercise on Coxsackie A9 myocarditis in adult mice. Proc Soc Exp Biol Med. 1964;117:777–782. doi: 10.3181/00379727-117-29696. [DOI] [PubMed] [Google Scholar]
- 175.Petek B.J., Moulson N., Drezner J.A., et al. Cardiovascular outcomes in collegiate athletes after SARS-CoV-2 infection: 1-year follow-up from the outcomes registry for cardiac conditions in athletes. Circulation. 2022;145:1690–1692. doi: 10.1161/CIRCULATIONAHA.121.058272. [DOI] [PubMed] [Google Scholar]
- 176.Moulson N., Petek B.J., Churchill T.W., et al. Cardiac troponin testing as a component of return to play cardiac screening in young competitive athletes following SARS-CoV-2 infection. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.122.025369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ibrahim E.S.H., Frank L., Baruah D., et al. Value CMR: towards a comprehensive, rapid, cost-effective cardiovascular magnetic resonance imaging. Int J Biomed Imaging. 2021;2021:8851958. doi: 10.1155/2021/8851958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Writing Committee. Gluckman T.J., Bhave N.M., et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;79:1717–1756. doi: 10.1016/j.jacc.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Haussner W., DeRosa A.P., Haussner D., et al. COVID-19 associated myocarditis: a systematic review. Am J Emerg Med. 2022;51:150–155. doi: 10.1016/j.ajem.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.COVID-19 Immunity Task Force Seroprevalence in Canada. https://www.covid19immunitytaskforce.ca/seroprevalence-in-canada/ Available at:
- 181.Centers for Disease Control and Prevention Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C) https://www.cdc.gov/mis/mis-c/hcp/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fmis%2Fhcp%2Findex.html Available at:
- 182.National Institutes of Health Therapeutic management of hospitalized pediatric patients with multisystem inflammatory syndrome in children (MIS-C) (with discussion on multisystem inflammatory syndrome in adults [MIS-A]) https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-children/hospitalized-pediatric-patients-therapeutic-management-of-mis-c/ Available at:
- 183.Henderson L.A., Canna S.W., Friedman K.G., et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19. version 3Arthritis Rheumatol. 2022;74:e1–e20. doi: 10.1002/art.42062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Inaba Y., Chen J.A., Bergmann S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26:631–640. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 185.Lopez-Leon S., Wegman-Ostrosky T., Ayuzo del Valle N.C., et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. 2022;12:9950. doi: 10.1038/s41598-022-13495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Centers for Disease Control and Prevention Long COVID—household pulse survey. https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm Available at:
- 187.Han Q., Zheng B., Daines L., Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. 2022;11:269. doi: 10.3390/pathogens11020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.American Heart Association COVID-19 CVD registry. https://www.heart.org/en/professional/quality-improvement/covid-19-cvd-registry Available at:
- 189.Ling R.R., Ramanathan K., Tan F.L., et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med. 2022;10:679–688. doi: 10.1016/S2213-2600(22)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Block J.P., Boehmer T.K., Forrest C.B., et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. Morb Mortal Wkly Rep. 2022;71:517. doi: 10.15585/mmwr.mm7114e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Buchan S.A., Seo C.Y., Johnson C., et al. Epidemiology of myocarditis and pericarditis following mRNA vaccination by vaccine product, schedule, and interdose interval among adolescents and adults in Ontario, Canada. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Patone M., Mei X.W., Handunnetthi L., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moreira H.G., Oliveira Júnior M.T., Valdigem B.P., Martins C.N., Polanczyk C.A. Position statement on cardiovascular safety of vaccines against COVID-19—2022. Arq Bras Cardiol. 2022;118:789–796. doi: 10.36660/abc.20220179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Centers for Disease Control and Prevention Investigating long-term effects of myocarditis. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myo-outcomes.html Available at:
- 195.Wolter N., Jassat W., Walaza S., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.World Health Organization Interim statement on decision-making considerations for the use of variant updated COVID-19 vaccines. https://www.who.int/news/item/17-06-2022-interim-statement-on-decision-making-considerations-for-the-use-of-variant-updated-covid-19-vaccines Available at:
- 197.Chalkias S., Harper C., Vrbicky K., et al. A bivalent Omicron-containing booster vaccine against COVID-19. N Engl J Med. 2022;387:1279–1291. doi: 10.1056/NEJMoa2208343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Widmer R.J., Collins N.M., Collins C.S., et al. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta-analysis. Mayo Clin Proc. 2015;90:469–480. doi: 10.1016/j.mayocp.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Clark C.J., Alonso A., Spencer R.A., et al. Predicted long-term cardiovascular risk among young adults in the National Longitudinal Study of Adolescent Health. Am J Public Health. 2014;104:e108–e115. doi: 10.2105/AJPH.2014.302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sørensen K., Van den Broucke S., Fullam J., et al. Health literacy and public health: a systematic review and integration of definitions and models. BMC Public Health. 2012;12:80. doi: 10.1186/1471-2458-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gandrakota N., Ali M.K., Shah M.K. Trends in health information technology use among the US population with and without cardiovascular risk factors, 2012–2018: evidence from the National Health Interview Survey. JMIR Public Health Surveill. 2021;7 doi: 10.2196/29990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lynch E.B., Liu K., Kiefe C.I., Greenland P. Cardiovascular disease risk factor knowledge in young adults and 10-year change in risk factors: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J of Epidemiol. 2006;164:1171–1179. doi: 10.1093/aje/kwj334. [DOI] [PubMed] [Google Scholar]
- 203.Afroze R., Gulati C., Mora-Almanza J.G., Thakkar V., Davison K.M. Nutrition and other wellness indicators are associated with healthy weight status in emerging adults. J Nutr Educ Behav. 2020;52:S34. [Google Scholar]
- 204.Lawrence E., Mollborn S., Hummer R. Health lifestyles across the transition to adulthood: implications for health. Soc Sci Med. 2017;193:23–32. doi: 10.1016/j.socscimed.2017.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gooding H.C., Gidding S.S., Moran A.E., et al. Challenges and opportunities for the prevention and treatment of cardiovascular disease among young adults: report from a National Heart, Lung, and Blood Institute Working Group. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Af Geijerstam A., Mehlig K., Börjesson M., et al. Fitness, strength and severity of COVID-19: a prospective register study of 1 559 187 Swedish conscripts. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.