Summary
Background
HIV transmission can occur with a viral load of at least 200 copies per mL of blood and low-level viraemia can lead to virological failure; the threshold level at which risk for virological failure is conferred is uncertain. To better understand low-level viraemia prevalence and outcomes, we analysed retrospective longitudinal data from a large cohort of people living with HIV on antiretroviral therapy (ART) in Nigeria.
Methods
In this retrospective cohort study using previously collected longitudinal patient data, we estimated rates of virological suppression (≤50 copies per mL), low-level viraemia (51–999 copies per mL), virological non-suppression (≥1000 copies per mL), and virological failure (≥2 consecutive virological non-suppression results) among people living with HIV aged 18 years and older who initiated and received at least 24 weeks of ART at 1005 facilities in 18 Nigerian states. We analysed risk for low-level viraemia, virological non-suppression, and virological failure using log-binomial regression and mixed-effects logistic regression.
Findings
At first viral load for 402 668 patients during 2016–21, low-level viraemia was present in 64 480 (16·0%) individuals and virological non-suppression occurred in 46 051 (11·4%) individuals. Patients with low-level viraemia had increased risk of virological failure (adjusted relative risk 2·20, 95% CI 1·98–2·43; p<0·0001). Compared with patients with virological suppression, patients with low-level viraemia, even at 51–199 copies per mL, had increased odds of low-level viraemia and virological non-suppression at next viral load; patients on optimised ART (ie, integrase strand transfer inhibitors) had lower odds than those on non-integrase strand transfer inhibitors for the same low-level viraemia range (eg, viral load ≥1000 copies per mL following viral load 400–999 copies per mL, integrase strand transfer inhibitor: odds ratio 1·96, 95% CI 1·79–2·13; p<0·0001; non-integrase strand transfer inhibitor: 3·21, 2·90–3·55; p<0·0001).
Interpretation
Patients with low-level viraemia had increased risk of virological non-suppression and failure. Programmes should revise monitoring benchmarks and targets from less than 1000 copies per mL to less than 50 copies per mL to strengthen clinical outcomes and track progress to epidemic control.
Funding
None.
Introduction
Effective HIV antiretroviral therapy (ART) decreases patient morbidity and mortality,1 and prevents sexual transmission when viral load is below 200 copies per mL of blood.2,3 Expanding ART coverage and viral load monitoring are key components of the UNAIDS global strategy to achieve HIV epidemic control by 2030.3 In 2020, an estimated 73% of people living with HIV globally were receiving treatment, and 66% of individuals receiving ART had a viral load of less than 1000 copies per mL.4
Viral load suppression rates have increased with optimised integrase strand transfer inhibitor-based ART, such as regimens containing dolutegravir (eg, tenofovir, lamivudine, and dolutegravir).5 However, low-level viraemia, defined as a viral load between 51 copies per mL and 999 copies per mL, threatens progress to reach epidemic control.6 Low-level viraemia has been associated with non-suppression,4–8 drug resistance,9–11 chronic inflammation and serious non-AIDS events,12 and death.13 In 2021, the WHO Guideline Development Group updated their global guidance to include monitoring and management of a viral load of more than 50 copies per mL.4
Nigeria has a generalised HIV epidemic, with an estimated 1·7 million people living with HIV in 2020.14 Since 2019, Nigeria concurrently increased ART coverage, scaled up use of dolutegravir-based regimens, and expanded viral load monitoring, even during the COVID-19 pandemic.15–17 Under the National ART Guidelines,14 viral load monitoring is recommended at 6 months and 12 months after ART initiation and annually thereafter. People with virological non-suppression (≥1000 copies per mL) should repeat viral load testing 3 months after enhanced adherence counselling; patients with at least two consecutive tests indicating virological non-suppression should be reviewed for an ART regimen switch. To better understand low-level viraemia prevalence and outcomes, we aimed to analyse retrospective longitudinal patient-level data from a large cohort of people living with HIV receiving ART in Nigeria during 2016–21 through support from the US President’s Emergency Plan for AIDS Relief.
Methods
Study design, participants, and procedures
This cohort study used retrospective longitudinal patient data to assess the prevalence of virological suppression (defined as ≤50 copies per mL), three categories of low-level viraemia (51–199 copies per mL, 200–399 copies per mL, and 400–999 copies per mL), and virological non-suppression (≥1000 copies per mL) in Nigeria in 2016–21. We estimated risk of subsequent virological non-suppression and virological failure (ie, at least two consecutive virological non-suppression results) among individuals with and without low-level viraemia. We included adult patients (aged ≥18 years) who initiated and received ART between Jan 1, 2016, and Sept 30, 2021, at 1005 facilities in 18 Nigerian states, and who had at least one viral load result after at least 24 weeks on ART. We excluded patients who received third-line or non-standard ART regimens. For outcomes analyses, we excluded patients who had one or fewer viral load result, had virological non-suppression at first viral load result after at least 24 weeks of ART (first viral load result), or received second-line ART without previous documented first-line ART. For the virological failure outcomes analyses, patients with two or fewer viral load results were excluded. Key available demographic, clinical, and programme-related variables were extracted from the Nigerian National Data Repository, a centralised data warehouse of regularly reported data from facility electronic medical record systems. Quality checks removed entries due to missing, invalid, or duplicate data.
Ethical approval was granted from the Nigeria National Health Research Ethics Committee (NHREC/01/01/2007–13/11/2020). The Institutional Review Board of University of Maryland, Baltimore project (HP-00095094) and review in accordance with the US Centers for Disease Control and Prevention human research protection procedures determined this project as non-research (ie, the purpose being to prevent or control disease or injury and improve health, or to improve a public health programme or service).
Statistical analysis and outcomes
Included and excluded patients were compared using clustered Wilcoxon rank-sum test for continuous variables and Rao-Scott χ2 test for categorical variables. We estimated the proportion of patients with virological suppression, low-level viraemia, and virological non-suppression at the first viral load result, comparing by sex, age, and first-line and second-line ART (second-line ART defined as either a switch to dolutegravir-based ART following virological non-suppression, or a ritonavir-boosted protease inhibitor). Prevalence of virological suppression, low-level viraemia, and virological non-suppression was calculated by calendar year and duration on ART using the first viral load result per year. ART adherence was assessed by calculating a documented dispense ratio, defined as the cumulative number of documented ART doses dispensed divided by the number of days since ART initiation.
For analyses of virological non-suppression and virological failure outcomes, patients were censored after the relevant endpoint of interest was met; individuals not meeting endpoints were censored after their last documented viral load. Predictors of low-level viraemia, virological non-suppression, and virological failure were identified using log-binomial regression with 95% CI calculated using cluster-robust standard errors to account for facility-level clustering. Virological suppression after non-suppression was assessed using log-binomial models among patients with at least one viral load result following the non-suppressed measurement. All models were adjusted for clinically relevant (eg, sex, age at ART initiation, ART duration, and regimen) and programme-related (eg, time to first viral load and documented dispense ratio) variables. Following a purposeful selection procedure,18 variables with p<0·25 in univariable models were included in multivariable models; variables with p>0·05 in multivariable models were excluded if coefficients in the resulting smaller model changed by less than 20%. Interactions between age, sex, time on ART, integrase strand transfer inhibitor exposure, and low-level viraemia level were assessed for inclusion at p<0·05. Final model fits were assessed using the area under the receiver operating characteristic curve. Unadjusted relative risks (RRs) were calculated for univariable models and adjusted RRs (aRRs) were calculated for multivariable models. Unadjusted odds ratios (ORs) and adjusted ORs (aORs) were calculated for the association between viral load result range and subsequent viral load result of more than 50 copies per mL, at least 200 copies per mL, or virological non-suppression using mixed-effects logistic regression, with patient and facility incorporated as random intercepts. Analyses were conducted in Python (version 3.7.6, Scotts Valley, CA, USA) and R (version 4.0.2).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. HIV programme support was provided by the US President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention. Funding sources for the participating ART facilities had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
521 212 patients were abstracted between 2016 and 2021, of whom 402 668 (77·3%) of 521 212 were included in the prevalence analysis (median age 34 years [IQR 28–41]) of whom 129 620 [32·2%] were men and 273 048 (67·8%) were women (figure 1; appendix p 2). Excluded patients’ demographic characteristics were similar to those of included patients (appendix p 2) For the virological non-suppression outcomes analyses, 197 729 patients were included, of whom 197 071 (99·7%) had at least one viral load result while on first-line ART (406 689 patient-years’ follow-up) and 1616 (0·8%) had at least one viral load result while on second-line ART (2292 patient-years’ follow-up). The median time to first viral load of at least 24 weeks after ART initiation was 44 weeks (IQR 29–79) for patients in the prevalence group, and the median documented dispense ratio was 63% (IQR 41–94; table 1). 112 316 patients with at least three viral load results were included in analysis of virological failure (figure 1). Among patients included in outcomes analyses, 69 405 (35·1%) of 197 729 had at least one viral load result of 51–999 copies per mL (appendix p 3). Patients included in outcomes analyses contributed a total of 598 313 viral load results.
Figure 1: Study profile.
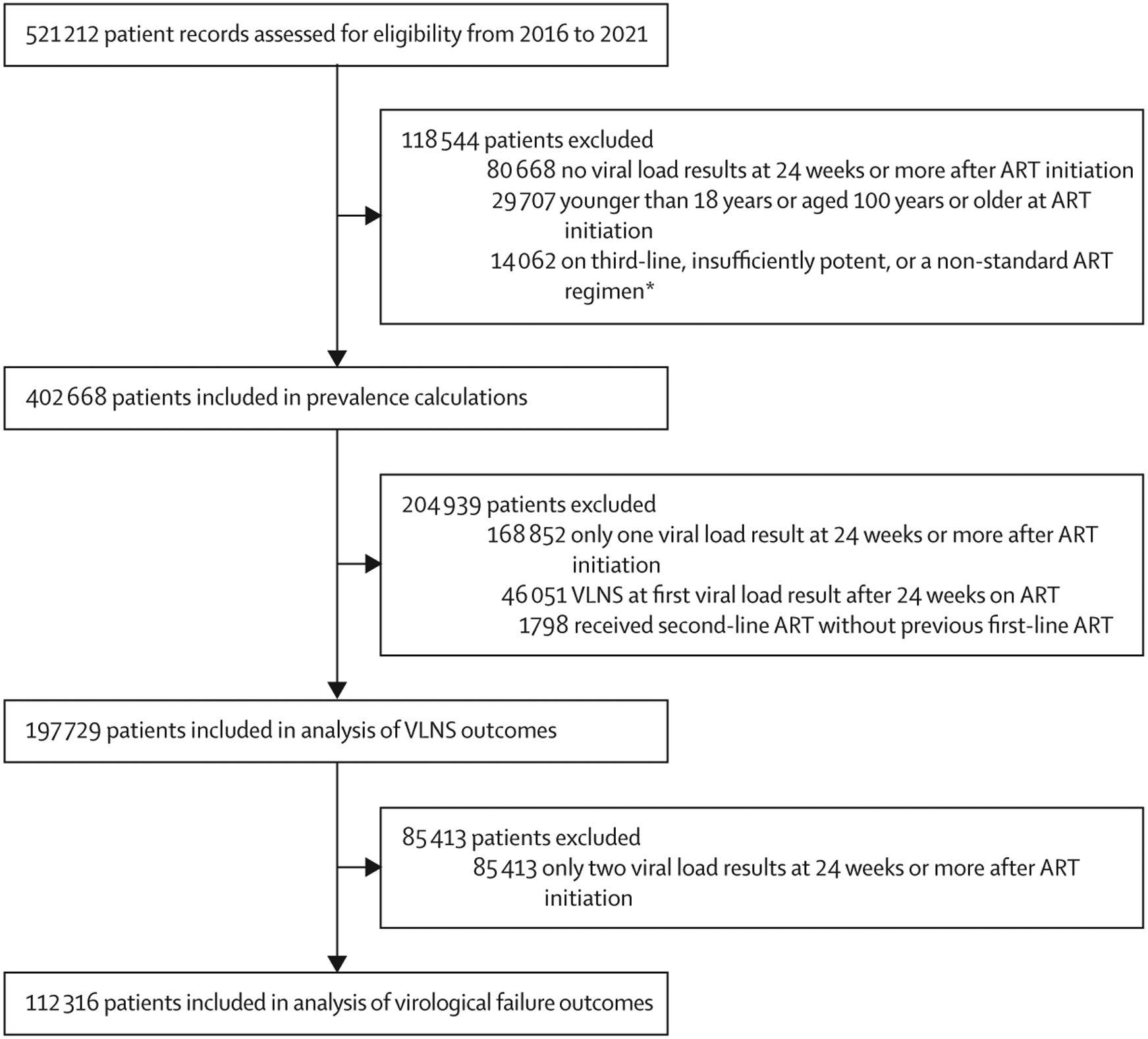
Exclusion criteria were applied independently and patients could have met several exclusion criteria; therefore disaggregated exclusion criteria numbers do not sum to the total number of patients excluded. ART=antiretroviral therapy. VLNS=virological non-suppression. *Non-standard regimens include ART combinations other than nucleos(t)ide reverse transcriptase inhibitor plus non-nucleoside reverse transcriptase inhibitor, nucleos(t)ide reverse transcriptase inhibitor plus integrase strand transfer inhibitor, or nucleos(t)ide reverse transcriptase inhibitor plus protease inhibitor. Insufficiently potent ART denotes monotherapy or dual therapy.
Table 1:
Patient characteristics
| Patients included in prevalence calculation (n=402 668) | Patients included in outcomes analyses (n=197 729) | |||
|---|---|---|---|---|
| First-line ART (n=399 684)* | Second-line ART (n=5986)* | First-line ART (n=197 071)* | Second-line ART (n=1616)* | |
| Age at ART initiation, years | 34 (28–41) | 39 (32–46) | 35 (28–42) | 39 (32–47) |
| Sex | ||||
| Male | 128 633 (32·2%) | 1891 (31·6%) | 58 566 (29·7%) | 485 (30·0%) |
| Female | 271 051 (67·8%) | 4095 (68·4%) | 138 505 (70·3%) | 1131 (70·0%) |
| Calendar year of ART initiation | ||||
| 2016 | 71 331 (17·8%) | 350 (5·8%) | 50 523 (25·6%) | 50 (3·1%) |
| 2017 | 68 934 (17·2%) | 596 (10·0%) | 48 023 (24·4%) | 149 (9·2%) |
| 2018 | 67 790 (17·0%) | 1172 (19·6%) | 46 680 (23·7%) | 308 (19·1%) |
| 2019 | 77 586 (19·4%) | 1845 (30·8%) | 41 855 (21·2%) | 454 (28·1%) |
| 2020 | 113 781 (28·5%) | 1782 (29·8%) | 9990 (5·1%) | 581 (36·0%) |
| 2021† | 262 (<0·1%) | 241 (4·0%) | ·· | 74 (4·6%) |
| Duration of follow-up on ART | 105·6 (52·7–185·3) | 85·0 (51·4–134·0) | 163·3 (107·0–218·0) | 82·1 (49·1–132·8) |
| NRTI exposure‡ | ||||
| TDF plus emtricitabine or lamivudine | 397 731 (99·5%) | 5039 (84·2%) | 196 530 (99·7%) | 1421 (87·9%) |
| Zidovudine plus lamivudine | 42 401 (10·6%) | 2706 (45·2%) | 28 694 (14·6%) | 688 (42·6%) |
| Abacavir plus lamivudine | 4226 (1·1%) | 1078 (18·0%) | 2589 (1·3%) | 307 (19·0%) |
| NNRTI exposure‡ | ||||
| Efavirenz | 195 529 (48·9%) | 1000 (16·7%) | 135 108 (68·6%) | 348 (21·5%) |
| Nevirapine | 47 889 (12·0%) | 511 (8·5%) | 32 199 (16·3%) | 155 (9·6%) |
| INSTI (eg, dolutegravir-based) exposure‡ | 375 200 (93·9%) | 3003 (50·2%) | 190 885 (96·9%) | 997 (61·7%) |
| Protease inhibitor exposure‡ | ||||
| Ritonavir-boosted lopinavir | ·· | 3903 (65·2%) | ·· | 1045 (64·7%) |
| Ritonavir-boosted atazanavir | ·· | 3671 (61·3%) | ·· | 978 (60·5%) |
| Viral load test results during follow-up§ | ||||
| 1¶ | 198 422 (49·6%) | 2823 (47·2%) | 425 (0·2%) | 511 (31·6%) |
| 2 | 93 136 (23·3%) | 1537 (25·7%) | 92 669 (47·0%) | 539 (33·4%) |
| 3 | 56 577 (14·2%) | 908 (15·2%) | 55 493 (28·2%) | 300 (18·6%) |
| ≥4 | 51 549 (12·9%) | 718 (12·0%) | 48 484 (24·6%) | 266 (16·5%) |
| Virological status at first viral load result ≥24 weeks on ART§ | ||||
| Virological suppression (≤50 copies per mL) | 290 441 (72·7%) | 3271 (54·6%) | 157 399 (79·9%) | 1137 (70·4%) |
| LLV (51–999 copies per mL)∥ | 63 832 (16·0%) | 1445 (24·1%) | 39 672 (20·1%) | 370 (22·9%) |
| 51–199 | 40 438 (63·4%) | 861 (59·6%) | 25 623 (64·6%) | 225 (60·8%) |
| 200–399 | 11 646 (18·2%) | 278 (19·2%) | 7211 (18·2%) | 57 (15·4%) |
| 400–999 | 11 748 (18·4%) | 306 (21·2%) | 6838 (17·2%) | 88 (23·8%) |
| Viral load non-suppression (≥1000 copies per mL)** | 45 411 (11·4%) | 1270 (21·2%) | ·· | 109 (6·7%) |
| Timing of first viral load, weeks§ | 44 (29–79) | 21 (5–39) | 53 (32–92) | 24 (9–41) |
| <24 weeks | ·· | 2621 (43·8) | ·· | 796 (49·3) |
| 24–52 weeks | 227 995 (57·0) | 2159 (36·1) | 95 884 (48·7) | 569 (35·2) |
| ≥52 weeks | 171 689 (43·0) | 1206 (20·1) | 101 187 (51·3) | 251 (15·5) |
| Documented dispense ratio | 63% (41–94) | 79% (55–100) | 56% (39–77) | 90% (61–100) |
Data are n (%), median (IQR), or % (IQR). Patient characteristics (age, sex, and calendar year of initiation) were measured at the start of the respective ART line, whereas all other values were measured during follow-up. ART=antiretroviral therapy. INSTI=integrase strand transfer inhibitor. LLV=low-level viraemia. NNRTI=non-nucleoside reverse transcriptase inhibitor. NRTI=nucleos(t)ide reverse transcriptase inhibitor. TDF=tenofovir disoproxil fumarate.
Patients were counted according to their ART regimen line at the time of viral load measurements and could contribute to counts of both first-line and second-line patients. Some patients who received second-line ART did not have any documented viral load results before the switch to second-line ART; therefore first-line and second-line counts do not sum to the total number of patients.
Includes patients who initiated ART in 2021 and met inclusion criteria with a sufficient number of viral load measurements after at least 24 weeks on ART.
Measured as cumulative exposure (ie, all regimens received) while on the respective regimen line.
Includes viral load results received while on the relevant line after at least 24 weeks on ART. For patients on second-line ART, the 24-week period includes previous exposure to first-line ART. All patients included in outcomes analyses who received second-line ART have at least one previous documented receipt of first-line ART. Viral load results received before the 24th week of ART were not included in analysis.
Patients with only one viral load result on the respective regimen line were included in outcomes analyses if they had at least two viral load results.
The proportion of patients within each LLV range was calculated using the total number of patients with LLV (51–999 copies per mL) as the denominator.
Includes patients without initial viral load non-suppression who switched from first-line to second-line ART and had viral load non-suppression at the first viral load result after ART regimen switch.
At first viral load result, 290 441 (72·7%) of 399 684 patients on first-line ART and 3271 (54·6%) of 5986 patients on second-line ART were suppressed (table 1), and 292 137 (72·6%) of 402 668 (appendix p 4) had virological suppression at first overall viral load result. Low-level viraemia at the first viral load result occurred among 64 480 (16·0%) of 402 668 patients. Virological non-suppression at first viral load result occurred among 46 051 (11·4%) of 402 668 patients (appendix p 4).
Between 2016 and 2021, the prevalence of virological suppression increased whereas low-level viraemia and virological non-suppression decreased (figure 2). Among patients with low-level viraemia, the relative prevalence of 51–199 copies per mL increased (appendix pp 5–6). After 2 years on ART, prevalence of virological suppression increased, whereas low-level viraemia and virological non-suppression decreased (figure 2).
Figure 2: Prevalence of low-level viraemia (LLV) and virological non-suppression* 2016–2021, by calendar year (A) and years on ART (B).
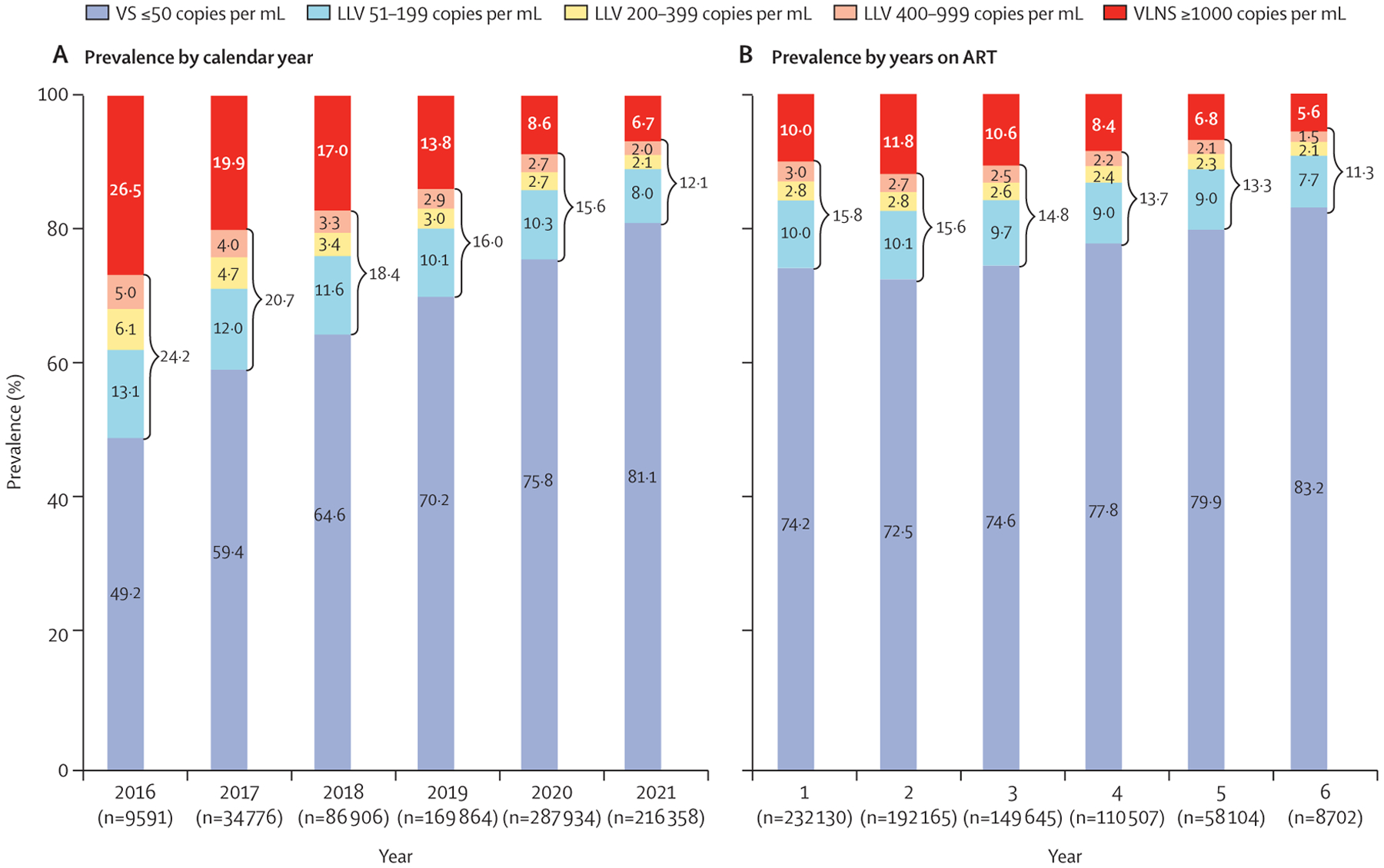
ART=antiretroviral therapy. VLNS=viral load non-suppression. VS=virological suppression. *Prevalence was calculated using the first viral load result of each year to avoid bias from repeated measurements within the same year.
An increased risk of low-level viraemia while on first-line ART was associated with male sex (aRR 1·14, 95% CI 1·10–1·18; p<0·0001) and longer duration on ART (1·07 per year, 1·06–1·09; p<0·0001), whereas ART initiation with a dolutegravir-based regimen was associated with a decreased risk of low-level viraemia (0·83 vs non-dolutegravir-based regimens, 0·80–0·87; p<0·0001; appendix p 7). Of patients included in outcomes analyses, 19 199 (9·7%) of 197 729 had virological non-suppression, and of those with at least three viral load results, 2232 (2·0%) of 112 316 experienced virological failure (appendix p 3). Relative to non-dolutegravir-based regimens, dolutegravir-based ART was associated with a decreased risk of virological non-suppression (aRR 0·73, 95% CI 0·68–0·79; p<0·0001) and virological failure (0·40, 0·35–0·47; p<0·0001; appendix p 8), with a larger decreased risk of virological non-suppression among patients who had been initiated with integrase strand transfer inhibitors compared with individuals who transitioned to integrase strand transfer inhibitors (0·67, 0·61–0·74 vs 0·85, 0·80–0·92; p<0·0001; table 2). Patients aged 18–40 years were associated with increased risk of both virological non-suppression and virological failure, relative to those who were aged 50 years or older, and men had increased risk of virological non-suppression (aRR 1·07, 95% CI 1·02–1·13; p=0·0080; table 2). Compared with patients on first-line ART with virological suppression at first viral load result, patients with low-level viraemia had an increased risk of subsequent virological non-suppression (aRR 1·61, 95% CI 1·55–1·67; p<0·0001) and virological failure (2·20, 1·98–2·43; p<0·0001; appendix p 8). All low-level viraemia ranges had increased risk of virological non-suppression and virological failure (table 2).
Table 2:
Unadjusted and adjusted relative risks of virological non-suppression and virological failure among patients on first-line antiretroviral therapy (ART)
| Virological non-suppression | Virological failure | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted analysis | Adjusted analysis | Unadjusted analysis | Adjusted analysis | |||||
| RR (95% CI) | p value | RR (95% CI) | p value | RR (95% CI) | p value | RR (95% CI) | p value | |
| Sex | ||||||||
| Male | 1·02 (0·99–1·06) | 0·20 | 1·07 (1·02–1·13) | 0·0080 | 1·07 (0·96–1·19) | 0·20 | 1·08 (0·97–1·19) | 0·17 |
| Female | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Age at ART initiation, years | ||||||||
| 18–19 | 1·22 (1·07–1·40) | 0·0032 | 1·28 (1·14–1·45) | <0·0001 | 1·71 (1·23–2·37) | 0·0015 | 1·70 (1·22–2·37) | 0·0016 |
| 20–29 | 1·12 (1·04–1·21) | 0·0031 | 1·16 (1·07–1·25) | 0·0002 | 1·26 (1·06–1·51) | 0·0093 | 1·31 (1·10–1·22) | 0·0021 |
| 30–39 | 1·11 (1·04–1·17) | 0·0008 | 1·12 (1·06–1·18) | <0·0001 | 1·20 (1·03–1·41) | 0·022 | 1·22 (1·04–1·42) | 0·012 |
| 40–49 | 1·03 (0·98–1·10) | 0·18 | 1·04 (0·99–1·10) | 0·16 | 1·06 (0·91–1·22) | 0·45 | 1·05 (0·91–1·22) | 0·49 |
| ≥50 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Time on ART, years | 1·21 (1·17–1·25) | <0·0001 | 1·16 (1·13–1·20) | <0·0001 | 1·11 (1·05–1·18) | 0·0007 | 1·23 (1·15–1·32) | <0·0001 |
| First viral load ≥12 months after ART initiation | 0·97 (0·91–1·03) | 0·32 | ·· | ·· | 0·78 (0·71–0·87) | <0·0001 | 0·66 (0·59–0·75) | <0·0001 |
| Documented dispense ratio ≥51% | 0·82 (0·78–0·87) | <0·0001 | 0·91 (0·86–0·97) | 0·0009 | 0·78 (0·69–0·88) | 0·0001 | 0·83 (0·73–0·95) | 0·0055 |
| INSTI (eg, dolutegravir) exposure (cumulative)* | 0·79 (0·74–0·85) | <0·0001 | ·· | ·· | 0·40 (0·34–0·46) | <0·0001 | ·· | ·· |
| INSTI exposure, by ART naive or experienced status* | ||||||||
| Did not receive an INSTI | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| ART naive | 0·60 (0·54–0·67) | <0·0001 | 0·67 (0·61–0·74) | <0·0001 | 0·37 (0·31–0·44) | <0·0001 | 0·45 (0·37–0·54) | <0·0001 |
| ART experienced | 0·94 (0·87–1·00) | 0·052 | 0·85 (0·80–0·92) | <0·0001 | 0·41 (0·35–0·48) | <0·0001 | 0·40 (0·34–0·46) | <0·0001 |
| Range of first on-treatment viral load | ||||||||
| Virological suppression (≤50 copies per mL) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| LLV (51–999 copies per mL)† | 1·65 (1·58–1·71) | <0·0001 | ·· | ·· | 2·27 (2·05–2·51) | <0·0001 | ·· | ·· |
| LLV range (copies per mL)† | ||||||||
| 51–199 | 1·49 (1·43–1·55) | <0·0001 | 1·48 (1·42–1·54) | <0·0001 | 1·85 (1·64–2·09) | <0·0001 | 1·80 (1·60–2·03) | <0·0001 |
| 200–399 | 1·78 (1·66–1·91) | <0·0001 | 1·73 (1·61–1·85) | <0·0001 | 2·39 (2·01–2·84) | <0·0001 | 2·31 (1·93–2·76) | <0·0001 |
| 400–999 | 2·09 (1·96–2·24) | <0·0001 | 2·03 (1·91–2·17) | <0·0001 | 3·68 (3·13–4·32) | <0·0001 | 3·54 (3·01–4·15) | <0·0001 |
Log-binomial analysis, unadjusted, and adjusted relative risks (RRs) using first on-treatment viral load after at least 24 weeks of ART. 95% CIs were calculated using cluster-robust standard errors to account for clustering at the facility level. Only patients with three or more viral load results (n=112 316) were included in analysis of virological failure, defined as two consecutive viral load results of at least 1000 copies per mL (figure 1). INSTI=integrase strand transfer inhibitor. LLV=low-level viraemia.
Patients who received INSTI as their initial ART are denoted ART naive, and patients who received non-INSTI regimens but later received INSTI are denoted ART experienced. INSTI exposure was treated as both a dichotomous variable and as a categorical variable in the univariable model, and as a categorical variable in the multivariable model. A version of the multivariable analysis was conducted using INSTI exposure as a dichotomous variable (appendix p 8).
LLV range was treated as both a dichotomous variable and as a categorical variable in the univariable model, and as a categorical variable in the multivariable model. A version of the multivariable analysis was run using LLV as a dichotomous variable (appendix p 8).
Virological non-suppression was observed at the next viral load result in 5595 (10·1%) of 55 161 patients with at least one viral load result after a low-level viraemia result. Virologic failure was observed in 819 (2·7%) of 29 958 patients with at least two viral load results after a low-level viraemia result. Among patients who had virological non-suppression, 11 399 (59·4%) of 19 199 had at least one subsequent viral load result; of these, 9167 (80·4%) of 11 399 had 999 copies per mL or fewer (7862 [69·0%] of 11 399 had 50 copies per mL or fewer, 1305 [11·4%] of 11 399 had 51–999 copies per mL), whereas 2232 (19·6%) of 11 399 had at least 1000 copies per mL and, thus, showed virological failure (appendix p 3). Of the 2232 individuals who had virological failure, at the viral load result preceding the first of the two non-suppressed measurements, 1419 (63·6%) had 50 copies per mL or fewer, whereas 813 (36·4%) had low-level viraemia (51–999 copies per mL; appendix p 9). Patients with virological non-suppression while on a dolutegravir-based ART regimen were more likely to have subsequent suppression to 50 copies per mL or fewer (aRR 1·11, 95% CI 1·07–1·14; p<0·0001) and had decreased risk of virological failure (0·76, 0·70–0·83; p<0·0001) than those with virological non-suppression while on non-dolutegravir-based ART, whereas patients with low-level viraemia at first viral load result who later experienced virological non-suppression were less likely to suppress to 50 copies per mL or fewer (0·83, 0·80–0·86; p<0·0001) and had an increased risk of virological failure (1·32, 1·21–1·44; p<0·0001) than those with initial virological suppression (appendix p 10).
Among the total viral load results included in outcomes analyses, 197 729 (33·0%) of 598 313 were the last documented viral load result for each patient, whereas 400 584 were followed by another viral load result. Of those with another viral load result, 35 975 (9·0%) had a next viral load result of 51–199 copies per mL, 16 229 (4·1%) had 200–999 copies per mL, and 22 742 (5·7%) had at least 1000 copies per mL. Using mixed-effects logistic regression to examine three outcome thresholds, viral load results in the low-level viraemia range were associated with increased odds of a next viral load result of more than 50 copies per mL, at least 200 copies per mL, and at least 1000 copies per mL. Compared with patients on non-dolutegravir-based ART, patients on dolutegravir-based ART had lower odds for the same viral load range, for each viral load outcome (eg, viral load ≥1000 copies per mL following viral load 400–999 copies per mL, integrase strand transfer inhibitor: aOR 1·96, 95% CI 1·79–2·13; p<0·0001; non-integrase strand transfer inhibitor: 3·21, 2·90–3·55; p<0·0001; tables 3, 4).
Table 3:
Prevalence of viral loads at specific thresholds by previous viral load result
| ≤50 copies per mL | 51–199 copies per mL | 200–999 copies per mL | ≥1000 copies per mL | |
|---|---|---|---|---|
| Range of most recent viral load result (non-dolutegravir-based ART) | ||||
| ≤50 copies per mL (n=82 091) | 69 144 (84·2%) | 5997 (7·3%) | 2835 (3·5%) | 4115 (5·0%) |
| 51–999 copies per mL (n=19 618) | 12 267 (62·5%) | 3588 (18·3%) | 1705 (8·7%) | 2058 (10·5%) |
| 51–199 copies per mL (n=12 687) | 8131 (64·1%) | 2628 (20·7%) | 813 (6·4%) | 1115 (8·8%) |
| 200–399 copies per mL (n=3599) | 2227 (61·9%) | 508 (14·1%) | 465 (12·9%) | 399 (11·1%) |
| 400–999 copies per mL (n=3332) | 1909 (57·3%) | 452 (13·6%) | 427 (12·8%) | 544 (16·3%) |
| ≥1000 copies per mL (n=3381) | 1675 (49·5%) | 401 (11·9%) | 302 (8·9%) | 1003 (29·7%) |
| Total (n=105 090) | 83 086 (79·1%) | 9986 (9·5%) | 4842 (4·6%) | 7176 (6·8%) |
| Range of most recent viral load result (dolutegravir-based ART) | ||||
| ≤50 copies per mL (n=237 621) | 203 295 (85·6%) | 16 875 (7·1%) | 7316 (3·1%) | 10 135 (4·3%) |
| 51–999 copies per mL (n=47 699) | 32 879 (68·9%) | 7820 (16·4%) | 3335 (7·0%) | 3665 (7·7%) |
| 51–199 copies per mL (n=32 093) | 22 266 (69·4%) | 5794 (18·1%) | 1826 (5·7%) | 2207 (6·9%) |
| 200–399 copies per mL (n=8066) | 5497 (68·2%) | 1098 (13·6%) | 766 (9·5%) | 705 (8·7%) |
| 400–999 copies per mL (n=7540) | 5116 (67·9%) | 928 (12·3%) | 743 (9·9%) | 753 (10·0%) |
| ≥1000 copies per mL (n=10 174) | 6378 (62·7%) | 1294 (12·7%) | 736 (7·2%) | 1766 (17·4%) |
| Total (n=295 494) | 242 552 (82·1%) | 25 989 (8·8%) | 11 387 (3·9%) | 15 566 (5·3%) |
| Total, all regimens (n=400 584) | 325 638 (81·3%) | 35 975 (9·0%) | 16 229 (4·1%) | 22 742 (5·7%) |
Data are n (%). ART=antiretroviral therapy.
Table 4:
Adjusted ORs of VLs at specific thresholds by previous VL result
| Next VL >50 copies per mL | Next VL ≥200 copies per mL | Next VL ≥1000 copies per mL | ||||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
| Sex | ||||||
| Male | 1·19 (1·17–1·21) | <0·0001 | 1·11 (1·09–1·14) | <0·0001 | 1·09 (1·06–1·13) | <0·0001 |
| Female | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Age at ART initiation, years* | 0·99 (0·98–1·00) | 0·22 | 0·95 (0·94–0·96) | <0·0001 | 0·91 (0·90–0·92) | <0·0001 |
| Time on ART, years† | 0·92 (0·91–0·93) | <0·0001 | 0·94 (0·93–0·95) | <0·0001 | 0·95 (0·94–0·97) | <0·0001 |
| First VL ≥12 months after ART initiation | 1·02 (1·00–1·04) | 0·14 | 1·03 (1·00–1·05) | 0·054 | 1·02 (0·98–1·05) | 0·38 |
| Documented dispense ratio since last VL ≥51%‡ | 0·92 (0·90–0·94) | <0·0001 | 0·89 (0·87–0·91) | <0·0001 | 0·85 (0·82–0·87) | <0·0001 |
| Patients on non-dolutegravir-based ART§ | ||||||
| Most recent VL ≤50 copies per mL | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Most recent VL 51–199 copies per mL | 2·63 (2·52–2·74) | <0·0001 | 1·75 (1·66–1·86) | <0·0001 | 1·72 (1·61–1·85) | <0·0001 |
| Most recent VL 200–399 copies per mL | 2·77 (2·58–2·98) | <0·0001 | 2·76 (2·54–3·01) | <0·0001 | 2·12 (1·89–2·37) | <0·0001 |
| Most recent VL 400–999 copies per mL | 3·24 (3·01–3·49) | <0·0001 | 3·47 (3·19–3·77) | <0·0001 | 3·21 (2·90–3·55) | <0·0001 |
| Most recent VL ≥1000 copies per mL | 4·27 (3·97–4·59) | <0·0001 | 4·64 (4·29–5·01) | <0·0001 | 4·51 (4·14–4·91) | <0·0001 |
| Patients on dolutegravir-based ART§ | ||||||
| Most recent VL ≤50 copies per mL | 0·99 (0·96–1·01) | 0·26 | 0·93 (0·90–0·96) | <0·0001 | 0·93 (0·90–0·97) | 0·0008 |
| Most recent VL 51–199 copies per mL | 2·07 (2·01–2·14) | <0·0001 | 1·45 (1·38–1·51) | <0·0001 | 1·40 (1·32–1·48) | <0·0001 |
| Most recent VL 200–399 copies per mL | 1·98 (1·87–2·09) | <0·0001 | 1·81 (1·70–1·94) | <0·0001 | 1·71 (1·57–1·87) | <0·0001 |
| Most recent VL 400–999 copies per mL | 2·00 (1·89–2·11) | <0·0001 | 1·98 (1·85–2·12) | <0·0001 | 1·96 (1·79–2·13) | <0·0001 |
| Most recent VL ≥1000 copies per mL | 2·60 (2·48–2·73) | <0·0001 | 2·51 (2·38–2·66) | <0·0001 | 2·43 (2·28–2·60) | <0·0001 |
Mixed-effects logistic regression analysis, adjusted ORs using each VL result with at least one available subsequent VL result. In each model, facility and patient identifications were treated as random effects (intercepts) to account for facility-level clustering and repeated measurements among patients. All other variables were set as fixed effects. OR=odds ratio. VL=viral load. ART=antiretroviral therapy.
Reference is age 18 years.
Per year.
Documented dispense ratio since last VL was calculated as the number of documented doses received since the previous VL measurement divided by the number of days since that measurement.
The effect of low-level viraemia at the previous VL is reported separately for patients on dolutegravir-based and non-dolutegravir-based ART at time of outcome VL.
Discussion
Low-level viraemia was associated with subsequent virological non-suppression and virological failure among a large cohort of patients receiving ART under non-investigational conditions in Nigeria. Patients with higher levels of low-level viraemia were at an increased risk of both outcomes. Although smaller cohort studies have found conflicting results of the range of low-level viraemia associated with increased risk of poor outcomes,5,18–22 we found all ranges of low-level viraemia conferred risk for subsequent virological non-suppression and virological failure. During 2016–21, the number of patients with virological suppression at 50 copies per mL or fewer increased by approximately 5% annually. Accelerated increases in the prevalence of virological suppression in 2019, 2020, and 2021 coincide with programmatic efforts to increase the number of patients on ART and optimise regimens, namely through scale-up of dolutegravir-based ART.15,16 Beyond expanding ART coverage, our study shows that these efforts have improved patient outcomes, with patients receiving ART with integrase strand transfer inhibitors, especially dolutegravir-based regimens, having decreased likelihood of future low-level viraemia, virological non-suppression, and virological failure.
Current guidelines that use 1000 copies per mL to initiate patient-level strategies to improve suppression, such as enhanced adherence counselling, miss an important opportunity to prevent virological non-suppression for patients with low-level viraemia. Compared with patients with 50 copies per mL or fewer, even patients with low-level viraemia at 51–199 copies per mL had an increased risk of virological non-suppression and failure. Of patients who experienced virological failure, over a third had low-level viraemia preceding the first of their two non-suppressed measurements. Early interventions at more than 50 copies per mL could prevent clinical complications stemming from virological non-suppression. Since May, 2022, the Nigerian HIV programme has proactively taken a multipronged approach to support patients with low-level viraemia by increasing the quantity and capacity of facility-level and community-based cadres who can deliver enhanced adherence counselling, including via telephone, and apply a differentiated care multidisciplinary team model and quality improvement approach. Additionally, the programme tracks weekly performance of low-level viraemia and virological non-suppression cascade management by age, sex, and pregnancy status for data-driven performance improvement efforts.
At the population level, tracking viral load of at least 1000 copies per mL misses patients with low-level viraemia (ie, 200–999 copies per mL) who can transmit HIV,2,3 which has implications for HIV epidemic control. Although we found that the prevalence of patients with viral load results of less than 200 copies per mL increased from 62·3% in 2016 to 89·1% in 2021, we also showed that patients with low-level viraemia (51–199 copies per mL) are at risk of subsequent viral load of at least 200 copies per mL, which could result in HIV transmission. Modelling the extent of HIV infections and deaths averted, including from patients with less than 200 copies per mL, could improve understanding of HIV transmission dynamics resulting from ART scale-up in Nigeria. As Nigeria approaches epidemic control, aggregate viral load indicators that track virological suppression rates at 50 copies per mL or fewer, 51–199 copies per mL, and at least 200 copies per mL can refine programmatic monitoring to maintain progress; global HIV programmes could also consider revising viral load monitoring benchmarks and programmatic targets, and expand the use of viral load diagnostic assays with lower limits of detection.
Continual monitoring and strategies to tackle poor virological outcomes resulting from ART adherence and pathophysiological conditions are needed to reach and sustain HIV epidemic control globally. Patients and clinicians need effective tools to help address the socioeconomic, structural, and psychosocial barriers affecting adherence.23–26 Higher-level analytics using routinely collected data to continuously evaluate risk for virological non-suppression could also help prevent harmful clinical outcomes.27 Tracking drug resistance prevalence and its implications for changes in antiretroviral virological potency are crucial for low-level viraemia clinical management, such as drug resistance-guided treatment modification for improved virological control.28 Although laboratory capacity in countries supported by the President’s Emergency Plan for AIDS Relief is expected to increase in the near future, HIV drug resistance testing, including at lower viral load thresholds, at the scale needed for HIV programmes in high-burden countries to make a greater clinical impact will require increased laboratory capacity and lower costs.29 Sequencing cost reductions, greater efficiencies, and the availability of simplified automated bioinformatic analyses of drug resistance data are essential, and efforts are ongoing to make integrase genotyping more widespread and affordable. Finally, additional research is needed to understand the association of pathophysiological conditions, such as immune activation, chronic inflammation, non-communicable diseases, and other serious non-AIDS events with low-level viraemia and clinical outcomes.
Our study has several limitations. Although we analysed retrospective longitudinal patient-level data from a large cohort of patients available through the Nigerian National Data Repository, data quality might have affected results. Abstracted data might have differed from patients’ clinical reality, despite routine efforts to assess and improve data quality. We used rigorous data exclusion criteria to limit analysis to only patients with quality electronic records. Analyses were further limited by data availability, including potential gaps in regimen dispense documentation; we could therefore not directly assess the effects of treatment adherence or ART regimen switch given low numbers of therapeutic switches after virological failure. The dates covered by the dataset precluded many individuals who initiated ART in 2020 or later from inclusion in the outcomes analysis due to an insufficient number of subsequent viral load results for analysis.
In conclusion, our study shows that low-level viraemia as low as 51–199 copies per mL is associated with poor virological outcomes and that integrase strand transfer inhibitors, namely dolutegravir-based regimens, decrease the likelihood of future low-level viraemia, virological non-suppression, and virological failure. Our large cohort was composed of patients from many sites across diverse geographical regions in Nigeria, contributing to the generalisability of results. To support efforts to reach HIV epidemic control, our results add evidence that revising viral load monitoring benchmarks and programmatic targets down from 1000 copies per mL to less than 50 copies per mL is warranted to strengthen efforts to prevent virological non-suppression, improve patient outcomes, and prevent HIV transmission.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for previous published studies with no language, date, or country income status restrictions using the following search terms: “low-level viremia” AND “HIV” AND “virologic failure”, OR “treatment outcomes“ OR “virologic outcomes” OR “outcomes”. We identified studies in adult patients that reported virological failure as an outcome. Multiple studies showed the association of low-level viraemia with virological non-suppression, HIV drug resistance, and mortality. Studies varied with respect to the viral load threshold and frequency of low-level viraemia episodes that conferred greater risk for poor outcomes, including virological failure. The largest analysis of patients from a WHO-guided treatment programme included 70 930 patients in South Africa during the pre-dolutegravir era and showed increased risk of virological failure and switch to second-line antiretroviral therapy (ART).
Added value of this study
To our knowledge, this is the largest analysis of HIV low-level viraemia (51–999 viral copies per mL) to date, based on more than 400 000 people living with HIV on the US President’s Emergency Plan for AIDS Relief-supported ART in Nigeria during 2016–21. We analysed the prevalence of HIV low-level viraemia and assessed treatment outcomes (eg, viral load non-suppression and virological failure), by age, sex, ART regimen, and regimen line. It is also the largest analysis of this topic inclusive of patients on dolutegravir-based ART. Our study shows an association between low-level viraemia and poor treatment outcomes (eg, viral load non-suppression and virological failure) with a significantly increased odds of virological non-suppression at the next viral load following a low-level viraemia result, with a dose response beginning in the 51–199 copies per mL range, indicating the threat to treatment programmes. We showed significant decreased odds for virological non-suppression and failure in people receiving dolutegravir-based ART.
Implications of all the available evidence
Low-level viraemia threatens the progress to reach HIV epidemic control. Findings from this very large cohort support the updated WHO global guidance for monitoring and interventions to manage patients with low-level viraemia and reinforce the benefits of treatment with a dolutegravir-based ART regimen. Our study findings strengthen support for programmatic prevention and control strategies to achieve the 2030 global targets for HIV epidemic control.
Acknowledgments
We thank the Nigerian Federal Ministry of Health, the National AIDS and STD Control Programme, viral load laboratories, implementing partners, and patients. No specific funding was received for this analysis. HIV programme support was provided by the President’s Emergency Plan for AIDS Relief. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies. Applicable federal law for ethical review includes: 45 CFR. part 46.102(l)(2), 21 CFR part 56; 42 USC. §241(d); 5 USC §552a; 44 USC §3501 et seq.
Footnotes
Declaration of interests
We declare no competing interests.
Data sharing
No new primary data were collected for this study. Data is owned by the Nigerian Federal Ministry of Health and requests for additional use can be directed to the National Coordinator, Akudo Ikpeazu (aikpeazu@yahoo.com), at the National AIDS and STD Control Programme.
References
- 1.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet 2006; 367: 817–24. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375: 830–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016; 316: 171–81. [DOI] [PubMed] [Google Scholar]
- 4.Labhardt ND, Bader J, Lejone TI, et al. Should viral load thresholds be lowered?: revisiting the WHO definition for virologic failure in patients on antiretroviral therapy in resource-limited settings. Medicine 2016; 95: e3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandenhende MA, Ingle S, May M, et al. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS 2015; 29: 373–83. [DOI] [PubMed] [Google Scholar]
- 6.Bernal E, Gómez JM, Jarrín I, et al. Low-level viremia is associated with clinical progression in HIV-infected patients receiving antiretroviral treatment. J Acquir Immune Defic Syndr 2018; 78: 329–37. [DOI] [PubMed] [Google Scholar]
- 7.Fleming J, Mathews WC, Rutstein RM, et al. Low-level viremia and virologic failure in persons with HIV infection treated with antiretroviral therapy. AIDS 2019; 33: 2005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermans LE, Moorhouse M, Carmona S, et al. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis 2018; 18: 188–97. [DOI] [PubMed] [Google Scholar]
- 9.Delaugerre C, Gallien S, Flandre P, et al. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PLoS One 2012; 7: e36673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swenson LC, Min JE, Woods CK, et al. HIV drug resistance detected during low-level viraemia is associated with subsequent virologic failure. AIDS 2014; 28: 1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li JZ, Gallien S, Do TD, et al. Prevalence and significance of HIV-1 drug resistance mutations among patients on antiretroviral therapy with detectable low-level viremia. Antimicrob Agents Chemother 2012; 56: 5998–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elvstam O, Marrone G, Medstrand P, et al. All-cause mortality and serious non-AIDS events in adults with low-level human immunodeficiency virus viremia during combination antiretroviral therapy: results from a Swedish nationwide observational study. Clin Infect Dis 2021; 72: 2079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JS, Cole SR, Richardson DB, et al. Incomplete viral suppression and mortality in HIV patients after antiretroviral therapy initiation. AIDS 2017; 31: 1989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nigeria Federal Ministry of Health. National guidelines for HIV prevention, treatment, and care. Abuja: National AIDS and STIs Control Programme, 2020. [Google Scholar]
- 15.Dirlikov E, Jahun I, Odafe SF, et al. Rapid scale-up of an antiretroviral therapy program before and during the COVID-19 pandemic—nine states, Nigeria, March 31, 2019–September 30, 2020. MMWR Morb Mortal Wkly Rep 2021; 70: 421–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nigeria Federal Ministry of Health. Rapid advice: recommendations for first line antiretroviral therapy in Nigeria. Abuja: National AIDS and STIs Control Programme, 2018. [Google Scholar]
- 17.Boyd AT, Jahun I, Dirlikov E, et al. Expanding access to HIV services during the COVID-19 pandemic—Nigeria, 2020. AIDS Res Ther 2021; 18: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boillat-Blanco N, Darling KE, Schoni-Affolter F, et al. Virological outcome and management of persistent low-level viraemia in HIV-1-infected patients: 11 years of the Swiss HIV Cohort Study. Antivir Ther 2015; 20: 165–75. [DOI] [PubMed] [Google Scholar]
- 19.Esber A, Polyak C, Kiweewa F, et al. Persistent low-level viremia predicts subsequent virologic failure: is it time to change the third 90? Clin Infect Dis 2019; 69: 805–12. [DOI] [PubMed] [Google Scholar]
- 20.Navarro J, Caballero E, Curran A, et al. Impact of low-level viraemia on virological failure in HIV-1-infected patients with stable antiretroviral treatment. Antivir Ther 2016; 21: 345–52. [DOI] [PubMed] [Google Scholar]
- 21.Leierer G, Grabmeier-Pfistershammer K, Steuer A, et al. A single quantifiable viral load is predictive of virological failure in human immunodeficiency virus (HIV)-infected patients on combination antiretroviral therapy: the Austrian HIV cohort study. Open Forum Infect Dis 2016; 3: ofw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joya C, Won SH, Schofield C, et al. Persistent low-level viremia while on antiretroviral therapy is an independent risk factor for virologic failure. Clin Infect Dis 2019; 69: 2145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med 2016; 13: e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalichman S, Kalichman MO, Cherry C. Medication beliefs and structural barriers to treatment adherence among people living with HIV infection. Psychol Health 2016; 31: 383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Q, Tso LS, Rich ZC, et al. Barriers and facilitators of interventions for improving antiretroviral therapy adherence: a systematic review of global qualitative evidence. J Int AIDS Soc 2016; 19: 21166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrd KK, Hou JG, Bush T, et al. Adherence and viral suppression among participants of the patient-centered human immunodeficiency virus (HIV) care model project: a collaboration between community-based pharmacists and HIV clinical providers. Clin Infect Dis 2020; 70: 789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebrezgi MT, Fennie KP, Sheehan DM, et al. Developing a triage tool for use in identifying people living with HIV who are at risk for non-retention in HIV care. Int J STD AIDS 2020; 31: 244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McConnell MJ, Mier-Mota J, Flor-Parra F, et al. Improved viral suppression after treatment optimization in HIV-infected patients with persistent low-level viremia. J Acquir Immune Defic Syndr 2011; 58: 446–49. [DOI] [PubMed] [Google Scholar]
- 29.Brumme CJ, Poon AFY. Promises and pitfalls of Illumina sequencing for HIV resistance genotyping. Virus Res 2017; 239: 97–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new primary data were collected for this study. Data is owned by the Nigerian Federal Ministry of Health and requests for additional use can be directed to the National Coordinator, Akudo Ikpeazu (aikpeazu@yahoo.com), at the National AIDS and STD Control Programme.


