Abstract
In this study, the antioxidative activity and polyphenolic content of Syzygium aromaticum's flower bud were compared under different extraction solvents including chloroform, ethyl acetate, methanol, and aqueous. The antioxidant activity was assessed via established in vitro assay models such as 2, 2-diphenyl-1-Picrylhydrazyl (DPPH) radical scavenging assay, NO− radical scavenging assay, H2O2 scavenging assay and Fe3+ reducing capacity. Total phenolic content was measured according to Folin–Ciocalteu's method, and total flavonoid content was estimated by using the aluminum chloride colorimetric method. The results showed that aqueous extract possessed the highest TPC (19.11 ± 2.76 mg GAE/g DW) and TFC (15.32 ± 1.53 mg CtE/g DW). Among the extracts, methanol extract exerted the strongest radical DPPH quenching activity with an IC50 value of 303.56 ± 13.14 μg/mL. The highest NO− radical scavenging activity was shown by methanol extract (IC50192.94 ± 1.9 μg/mL) which is stronger than BHT (IC50247.64 ± 12.89 μg/mL). Methanol extract showed a strong H2O2 scavenging activity (IC50233.71 ± 3.72 μg/mL). The highest Fe3+ reducing capacity was shown by methanol extract (Absorbance = 0.36 ± 0.05). Strong and positive correlations were observed between total phenolic and flavonoid contents and the antioxidant assays. The results of the present work revealed that the tested spice demonstrated high antioxidant activity, total phenolics, and flavonoids. Thus, this spice is worth considering as important source of natural antioxidant agents.
1. Introduction
Key threats to biomolecules of the cells are free radicals in the form of reactive oxygen species (ROS) and reactive nitrogen species (RNS). They are the root cause for several degenerative ailments in humans [1]. Antioxidants are known to hamper cells' damage mediated by the oxidizing free radical chain reactions [2]. One of the sources of antioxidants are spices that are used to flavor foods since antiquity. Epidemiological studies validated the role of spice consumption to prevent the degenerative diseases like cardiovascular diseases, carcinogenesis, and atherosclerosis [3]. Spices have been considered as one among topmost five foods with the highest polyphenol compounds such as flavonoids, phenolic acids, and tannins [4–7]. Many previous studies [8–13] have shown antioxidant properties of spices.
The secondary metabolites, especially phenolics, have antioxidant property [14, 15]. Phenolic compounds (including flavonoids) of edible plants are the most abundant secondary metabolites in the plant kingdom, they play a protective role as nonenzymatic antioxidants in cells, protecting cells from oxidative stress damage, and are rich sources of human health-promoting metabolites [16, 17]. The type of solvent used to extract secondary metabolites has impact on their quality and quantity [18, 19]. The influence of extracting solvents on total phenolics and antioxidant activities have also been reported by different authors [20–22]. The chemical components of the extracts and essential oils are dependent upon the species, genotype, environment, organ type, developmental phase, extraction methods, and other variables [17].
Ethiopia is an abode of a variety of spices and condiments like Cinnamomum zeylanicum, Lippia adoensis, Myristica fragrans, Nigella sativa, Piper capense, Rhamnus prinoides, Ruta chalepensis, Syzygium aromaticum, Thymus shimperi, and Trachyspermum ammi. These spices have been used to improve taste and aroma of several local dishes [23, 24]. The tested spice in the present study, Syzygium aromaticum (Myrtaceae) locally known as kerunfud, is one of the spices used in the preparation of Ethiopian local dishes like “Mekelesha” and “Nitirkibie” [24] and “wot” [23]. In addition to its aroma and taste enhancing property, it is also used to treat tooth ache, wounds, warts, and erectile dysfunction [25, 26] in Ethiopia. The buds of S. Aromaticum are rich in eugenol, eugenyl acetate and β-caryophyllene [27]. Phenolic acids such as hydroxibenzoic acids, hydroxicinamic acids, and hydroxiphenyl propens, caffeic, ferulic, elagic, and salicylic acids were also isolated from extracts of S. aromaticum [28]. Buds of S. aromaticum exhibited antihyperglycemic, hypolipidemic, and hepatoprotective activities [29]. Previously, S. aromaticum hydrosols and essential oil were investigated for antimicrobial activity [30, 31] in Ethiopia. Across the world, studies have been conducted on antioxidant activity of S. aromaticum [32–38]. Though most of the above mentioned Ethiopian spices were studied for their antioxidant activity [13], to the best of our knowledge, Ethiopian cultivars of S. aromaticum have not yet been evaluated to relate extraction solvent to the quantity of phenolic compounds and antioxidant capacity. Thus, we hypothesized that different solvents yield in different amounts of phenolics to exhibit varying antioxidant activities. Our objective of this study was to quantify total phenolic and flavonoid contents of the flower bud of S. aromaticum extracted by using different solvents and evaluate their antioxidant activities.
2. Materials and Methods
2.1. Chemicals
Chloroform, ethyl acetate, methanol, ferric chloride (FeCl3), aluminum chloride (AlCl3), sodium hydroxide (NaOH), sodium carbonate (Na2CO3), sodium nitroprusside, hydrogen peroxide (H2O2), butylated hydroxytoluene (BHT), trichloroacetic acid (TCA), potassium ferricyanide [K3Fe3(CN)6], and N-1-naphthyl ethylene diamine dihydrochloride, catechin, and gallic acid, and 2,2-diphenyl-2-picrylhydrazyl (DPPH) were procured from Merck Co. India. All chemicals are of analytical grade.
2.2. Spice Extract Preparation
Flower buds were collected from Bate village around Haramaya University (9°23' N of latitude and 42° 01' E of longitude), Eastern Hararghe, Ethiopia. The collected material was identified by experts in the School of Biological Sciences and Biotechnology and its voucher specimen was kept in the herbarium of Haramaya University. The collected flower buds were air-dried in a hot air oven (Bluefic, India) at 40°C for 96 hours and then pulverized using mortar and pestle. The powder of the spice (50°g) was extracted (in triplicate) by separately dissolving in 250 ml of chloroform, ethyl acetate, methanol, and distilled water for 24 hours. The extract was filtered using Whatman no. 1 filter paper and the filtered solvent was evaporated under reduced pressure using a rotary evaporator (Heidolph rotary evaporator, Laborata 4001) at 55°C to remove the solvent. The obtained crude extracts were stored at -4°C until further analysis. The obtained extracts were labeled as chloroform extract (CE), ethyl acetate extract (EAE), methanol extract (ME), and aqueous extract (AQE).
2.3. Quantitative Analysis of Total Polyphenolic Compounds
2.3.1. Total Phenolic Quantification
The total phenolic content in the extracts was determined according to the Folin–Ciocalteu procedure [39]. About 4 mg of the dried crude extracts were mixed with 5 ml 80% acetone, shaken well in a vortex-shaker, and centrifuged at 2,200 × g for 2 min at room temperature. The supernatant was transferred to a 10 mL volumetric flask with a Pasteur pipette. The remaining residue was extracted twice with 2.5 mL 80% acetone, shaken well in a vortex-shaker, and centrifuged as before after standing for 5 min, and the supernatants were transferred to the same 10 mL volumetric flask with a Pasteur pipette. Aliquots (100 μL) of supernatant from each sample were put into a 10 mL volumetric flask and mixed with 1.9 mL deionized water. The Folin–Ciocalteu–phenol reagent (1 mL) was added and the solution was shaken vigorously and mixed with 5 mL sodium carbonate (20%). After 20 min, the solution was centrifuged at 2,200 × g for 2 min at room temperature. Absorbance at 735 nm was measured in a spectrophotometer (Shimadzu UV-2401PC) and the results were expressed as gallic acid equivalents from a gallic acid standard curve (mg GAE/g Extract DW; r2 = 0.9867). The analyses were performed in triplicate.
2.3.2. Total Flavonoid Quantification
The total flavonoid content in the extracts was determined by aluminum chloride colorimetric method based on the method indicated by Ordonez et al. [40]. Briefly, a volume of 0.5 mL of AlCl3 ethanol solution (2%) was added to 0.5 mL of extract. After one hour incubation at room temperature, the absorbance was measured at 420 nm using UV-Vis spectrophotometer. The analysis was performed in triplicate. The total flavonoid content was estimated from a catechin standard curve and the results are expressed as mg catechin equivalents (mg CtE/g Extract DW; r2 = 0.9675).
2.4. Assessment of Antioxidant Capacity
2.4.1. Scavenging Capacity on DPPH Stable Radical
The determination of DPPH stable radical scavenging activities of the extracts and standard were evaluated based on the method described by Singh et al. [41]. Extracts (1 mL) of the different concentrations (i.e., 25, 50, 100, 200, 400, 800, and 1000 μg/mL−1) made by reconstituting in respective solvents were added to DPPH solution (5 mL, 0.1 mM) in methanol and vortexed. After 20 minutes of reaction at 25°C, the absorbance was measured at 517 nm against a blank (methanol) in a UV-Vis spectrophotometer (Shimadzu UV-2401PC). Methanolic DPPH solution (5 ml) without antioxidant was used as control. The DPPH scavenging activity of the extract was expressed as IC50 (inhibitory concentration), that is, the concentration of the extract at which DPPH radicals were scavenged by 50%. Butyl hydroxy toluene (BHT) was used as standard antioxidant.
The percentage quenching of DPPH was calculated as follows:
| (1) |
where Abs sample is the absorbance of the sample (extract and standard antioxidant) and Abs control is DPPH solution without the added extract.
2.4.2. Scavenging Capacity on Nitric Oxide Radical
Nitric oxide (NO) generated from sodium nitroprusside (SNP) in aqueous solution at physiological pH was estimated by the use of the Griess reaction [42]. The reaction mixture (3 mL) containing SNP (10 mM, 2 mL), phosphate buffer saline (0.5 mL, pH 7.4), and the extracts (0.5 mL) at different concentrations (25, 50, 100, 200, 400, 800, and 1000 μg mL−1) were incubated at 25°C for 150 min. After incubation, 0.5 mL of the incubated solution containing nitrite was pipetted and mixed with 1 mL of sulfanilic acid reagent (0.33% in 20% glacial acetic acid) and allowed to stand for 5 min for completing diazotization. Then, 1 mL of N-(1-naphthyl) ethylenediamine dihydrochloride was added, mixed, and allowed to stand at 25°C for 30 min. The absorbance of pink colored chromophore formed during diazotization was measured at 540 nm. The NO scavenging activity of the extract was expressed as IC50 (inhibitory concentration), that is, the concentration of the extract at which NO radicals were quenched by 50%. Butyl hydroxy toluene (BHT) was used for comparison.
| (2) |
where Abs sample is absorbance of the sample and Abs control is absorbance of control.
2.4.3. Scavenging Capacity on H2O2
Scavenging capacity of the extracts towards Hydrogen peroxide (H2O2) was carried out by adopting the method of Ruch et al. [43]. Solution of H2O2 (50 mM) was prepared in phosphate buffer (50 mM, pH 7.4). Extracts' concentrations (25, 50, 100, 200, 400, 800, and 1000 μg mL−1, 0.3 mL) were mixed separately with H2O2 solution (0.6 ml). The resulting solution was kept for 10 minutes and its absorbance was measured at 230 nm thereafter. The H2O2 scavenging activity of the extract was expressed as IC50 (inhibitory concentration), that is, the concentration of the extract at which H2O2 radicals were quenched by 50%. Butyl hydroxy toluene (BHT) was used for comparison.
| (3) |
where Abssample is absorbance of the sample and Abscontrol is absorbance of control.
2.4.4. Reducing Capacity of Fe3+
Reducing capacity of the spice's extracts towards Fe3+ was performed according to Oyaizu [44]. From extracts' solution (25, 50, 100, 200, 400, 800, and 1000 μg mL−1), 0.5 mL portion was mixed with 2.5 mL of sodium phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferricyanide [K3Fe3(CN)6] (1%) in test tubes. The resulting solution was vortexed and incubated at 50°C for 20 min. Then, 2.5 mL of trichloroacetic acid (TCA) (10%, w/v) was added to all tubes and the solutions were centrifuged at 3000 × g for 10 min. The aqueous solution of FeCl3 (1%, 1 ml) was diluted by adding deionized water (5 mL). To this solution, the upper layer of the centrifuged solution (5 mL) was mixed and incubated at 35°C for 10 min. Absorbance of the developed color was measured at 700 nm. BHT was used as standard. Reducing capacity of the spice extracts towards Fe3+ was interpreted from relation of absorbance and reducing capacity: when absorbance increases, reducing capacity also increases.
2.5. Statistical Analysis
Triplicates were made for the experiments. Statistical Package for Social Sciences version 20 and Microsoft excel were used for data analysis. One-way analysis of variance (ANOVA) with Duncan's multiple range tests was used for data analysis. A statistically significant difference was considered at p < 0.05. The Pearson correlation coefficient (squared > 0.700) was used to analyze correlation between the amounts of TPC, TFC, and antioxidant capacities.
3. Results
3.1. Total Phenolic and Total Flavonoid Contents
Results showed that TPC and TFC from S. aromaticum extracts significantly (p < 0.05) varied between extraction solvents used. AQE had the highest TPC and TFC values followed by ME, EAE, and CE (Table 1).
Table 1.
Total phenolic and flavonoid contents of different solvents' extracts of S. aromaticum flower buds.
| Extract solvent | TPC (mg GAE/g) | TFC (mg CtE/g) |
|---|---|---|
| Chloroform extract (CE) | 6.23 ± 1.09c | 5.63 ± 1.08c |
| Ethyl acetate extract (EAE) | 7.98 ± 1.25bc | 5.83 ± 1.17c |
| Methanol extract (ME) | 10.74 ± 2.00b | 13.25 ± 1.95b |
| Aqueous extract (AQE) | 19.11 ± 2.76a | 15.32 ± 1.53a |
Note: the values are mean ± standard deviation (n = 3). Small letter superscripts compare means in a column. Means with similar letters are not varied significantly, whereas means with different letters are significantly varied at p < 0.05. GAE: gallic acid equivalents; CtE: catechin equivalents.
3.2. Antioxidant Capacity
3.2.1. Scavenging Capacity on DPPH Stable Radical
The DPPH radical scavenging capacity of each extract type (i.e., CE, EAE, ME, and AQE) from S. aromaticum significantly (p < 0.05) varied between the different concentrations. DPPH scavenging capacity also varied significantly (p < 0.05) between the extraction solvents used to get the extracts (Figure 1). The DPPH scavenging activity of CE (r2 = 0.885, p = 0.008), EAE (r2 = 0.931, p = 0.002), ME (r2 = 0.988, p ≤ 0.001), and AQE (r2 = 0.970, p ≤ 0.001) showed strong positive correlations with total phenolic contents. Likewise, the DPPH scavenging activity of CE (r2 = 0.992, p = 0.078), EAE (r2 = 0.927, p = 0.073), ME (r2 = 0.746, p = 0.254), and AQE (r2 = 0.896, p = 0.104) had also strong positive correlations with total flavonoid contents, though values were not statistically significant. Assessment by IC50 values showed significant (p < 0.05) variation between extracts in their DPPH scavenging activity with values in a decreasing order of scavenging activity being ME (IC50 = 303.56 ± 13.14 μg/mL) > EAE (IC50 = 413.14 ± 10.52 μg/mL) > AQE (IC50 = 502.40 ± 10.17 μg/mL) > CE (IC50 = 509.48 ± 9.88 μg/mL). The scavenging activity of all extracts, however, was found to be lower than the standard antioxidant, BHT, with an IC50 value of 256.38 ± 25.41 μg/mL.
Figure 1.
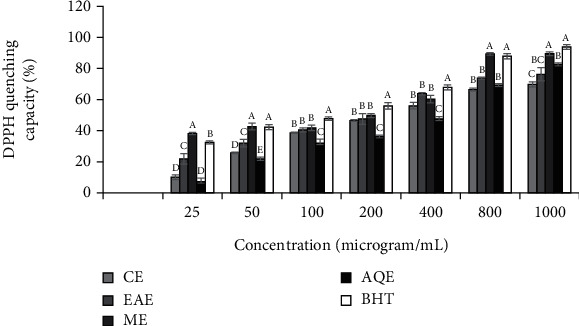
DPPH radical scavenging capacity of extracts of S. aromaticum flower buds. Values are Mean ± SE, n = 3.
3.2.2. Scavenging Capacity on NO− Radical
The NO− radical inhibition potentials of each extract type (i.e., CE, EAE, ME, and AQE) varied significantly (p < 0.05) between the different concentrations, and inhibitory effects of extracts increased with concentration (Figure 2). Extraction solvent used to get the extracts had also significantly impacted (p < 0.05) NO− radical inhibition potential. Strong positive correlations were observed between the NO− radical scavenging activity of the extracts: CE: r2 = 0.884, p = 0.012; EAE: r2 = 0.940, p = 0.002; ME: r2 = 0.916, p = 0.004; AQE: r2 = 0.895, p = 0.006; total phenolic contents. The total flavonoid contents also showed strong correlations with NO− radical scavenging activity of CE (r2 = 0.942, p = 0.058), EAE (r2 = 0.992, p = 0.008), ME (r2 = 0.941, p = 0.059), and AQE (r2 = 0.914, p = 0.086). Assessment by IC50 values showed significant (p < 0.05) variation between extracts in their NO− radical inhibition activity with values in a decreasing order of NO−inhibition activity being ME (IC50 = 192.94 ± 1.9 μg/mL) > EAE (IC50 = 290.37 ± 3.20 μg/mL) > AQE (IC50 = 296.16 ± 6.72 μg/mL) > CE (IC50 = 605.84 ± 5.36 μg/mL). The NO− scavenging activity of extracts, except ME, was found to be lower than the standard antioxidant, BHT, with an IC50 value of 247.64 ± 12.89 μg/mL.
Figure 2.
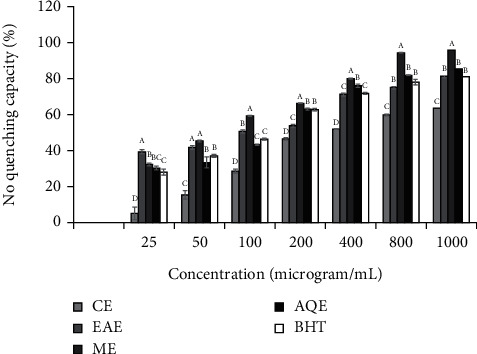
NO− scavenging capacity of extracts of S. aromaticum flower buds. Values are Mean ± SE, n = 3.
3.2.3. H2O2 Scavenging Capacity
With regard to H2O2 scavenging activity of the extracts of S. aromaticum flower bud, significant difference was observed between the concentrations of each extract type and scavenging activity increased with extract concentrations (Figure 3). Extraction solvent used to get the extracts had also significantly influenced (p < 0.05) H2O2 scavenging capacity. The H2O2 scavenging capacity of CE (r2 = 0.988, p ≤ 0.001), EAE (r2 = 0.992, p ≤ 0.001), ME (r2 = 0.969, p ≤ 0.001), and AQE (r2 = 0.977, p ≤ 0.001) exhibited strong positive correlation with total phenolic contents. Strong positive correlations were also found between the H2O2 scavenging capacity of CE (r2 = 0.823, p = 0.177), EAE (r2 = 0.960, p = 0.040), ME (r2 = 0.934, p = 0.066), and AQE (r2 = 0.728, p = 0.272) and total flavonoid contents. Assessment by IC50 values exhibited significant (p < 0.05) variation between extracts in their H2O2 scavenging capacity with values in a decreasing order of scavenging activity being ME (IC50 = 233.71 ± 3.72 μg/mL) > EAE (IC50 = 312.98 ± 23.15 μg/mL) > AQE (IC50 = 532.61 ± 25.16 μg/mL) > CE (IC50 = 621.84 ± 9.65 μg/mL). The H2O2 scavenging capacity of BHT (IC50 = 683.42 ± 11.27 μg/mL) was found to be lower than the extracts.
Figure 3.
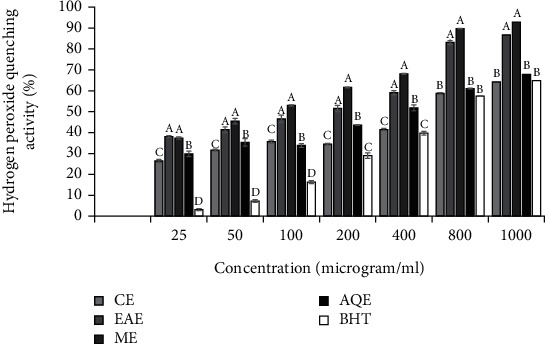
H2O2 scavenging capacity of extracts of S. Aromaticum flower buds. Values are Mean ± SE, n = 3.
3.2.4. Reducing Capacity of Fe3+
There was significant (p < 0.05) difference between the concentration of each extract type and between the different solvents used to get the extracts in reducing ferric ion (Fe3+) to ferrous ion (Fe2+) (Figure 4). Among the extracts, ME of S. aromaticum exhibited the highest reducing power, whereas CE was the least. Strong positive correlations were observed between total phenolic contents of CE (r2 = 0.0.953, p ≤ 0.001), EAE (r2 = 0.0.994, p ≤ 0.001), ME (r2 = 0.0.978, p ≤ 0.001), and AQE (r2 = 0.0.970, p ≤ 0.001) and reducing capacity. The Fe3+ reducing capacity by the extracts in a decreasing order was ME >AQE > EAE > CE based on the absorbance value. High absorbance value indicates high reducing capacity. The reducing capacity of the standard antioxidant BHT (absorbance 1.20 ± 0.06) was found to be higher than the extracts.
Figure 4.
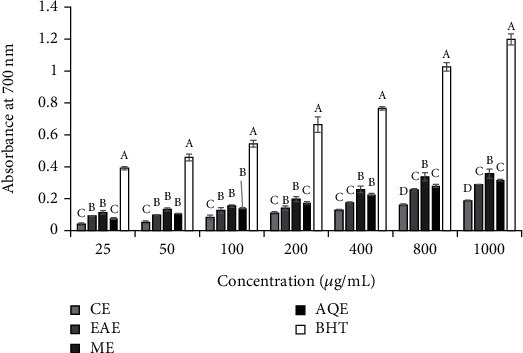
Reducing capacity of extracts of S. aromaticum flower buds. Values are Mean ± SE, n = 3.
4. Discussion
4.1. Total Phenolic and Flavonoid Contents
Despite an ample number of studies on this spice across the world, S. aromaticum from Ethiopia was examined for the first time for its antioxidant capacity and polyphenolic content. The secondary metabolite profile, which in turn influences antioxidant activity, could differ between regions due to growth environmental factors and plants' genotypes [17]. In this study, the total phenolic and flavonoid values significantly varied between extraction solvents. The highest values were recorded in AQE followed by ME, EAE, and CE. The extraction solvents used were of varying polarity, with water being the highest polar solvent and chloroform the least polar solvent. Therefore, the variation observed between the different solvents extracts is attributed to their polarity difference. Previously, Egigu et al. [45] reported that the extraction potential of solvents depend on their polarity strength. Several studies have proved the significance of high polar organic solvents for the improvement of extraction yield of phenolic compounds [46–48]. Highly hydroxylated aglycone forms of phenolic compounds are soluble in high polar solvents such as water and alcohols (ethanol and methanol) and hydroxy groups of phenolic compounds contribute to antioxidant activity [49]. Concomitant with variations in values of total phenolics and total flavonoids, the antioxidant capacity of the tested plant also varied with the solvents used to extract. Correlation analysis also showed strong positive correlation between total phenolic and flavonoid contents of the extracts and the different antioxidant assays, suggesting that differences of antioxidant capacities between the extracts can be ascribed to polyphenolic contents. Previously, several researchers [50, 51] showed the presence of strong correlation between antioxidant capacities and the plant's total phenolic contents that confirms phenolic compounds as important contributors to antioxidant activities. These plant-derived versatile products are in high demand for cosmetics, food, and pharmaceutical industries due to their safety, being less prone to risk, relatively low extraction cost, easy storage of products, and ease of scaling-up [17].
4.2. Antioxidant Capacity
In this study, four different antioxidant assays, namely, DPPH (2, 2-diphenyl-2-picrylhydrazine) radical scavenging, NO− radical inhibition, H2O2 scavenging capacity, and Fe3+ reducing capacity were conducted to evaluate the antioxidant potential of the extracts of the S. aromaticum flower bud. This is so the antioxidant property of the compounds in a crude extract may vary depending on the nature of oxidants [52]. Our results showed that all solvents' extracts of S. aromaticum flower bud significantly scavenged DPPH radical in a concentration dependent manner. The control solution without the extracts, however, had no inhibitory effect at all. Previously, Molyneux [53] and Adefegha and Oboh [54] explained that the DPPH radical scavenging assay is a commonly used cost-effect approach of evaluating the antioxidant property of secondary metabolites in crude plant extracts. The antioxidant property of natural products, for example, phenolic compounds is ascribed to electron donation in the form of hydrogen to DPPH radical [55]. Pietta [56] also reported that the antioxidant property of phenolic compounds due to their redox properties that enable them act as reducing agents, hydrogen donors, and/or singlet oxygen quenchers.
Comparison between the different solvents' extracts showed that ME had the highest DPPH radical scavenging capacity, which is on a par with that of the standard BHT followed by EAE. The DPPH radical quenching capacities of AQE and CE, however, were less than that of ME and CE. The same was also verified by their IC50 values. Here, it is noteworthy that extracts obtained by highly polar solvent (water) and highly nonpolar solvent (chloroform) had lesser power of responding to DPPH radical. This may be related to the variation in secondary compounds profile of the different solvents' extracts that should be verified through GC-MS and HPLC analyses. According to Egigu et al. [45], solvents with different polarity show difference in their extraction potential, which in turn determine the quantity and quality of compounds extracted. Previous studies [9, 35] on S. aromaticum also confirmed that extracts of highly polar solvents such as ethanol and water possess strong DPPH scavenging activity. Generally, the DPPH radical scavenging capacities of extracts showed significant positive correlation with the amounts of total phenolics and flavonoids measured. Earlier, Adebiyiet al. [57], Sasikumar et al. [13], and Soulef et al. [58] reported a strong positive correlation between in vitro antioxidant activity and phenolic contents of plant extracts.
Compared to the control (solution without extracts) all solvents' extracts of S. aromaticum significantly scavenged nitric oxide (NO−) in a concentration dependent manner. Nitric oxide (NO−) is physiologically important molecule that reacts with different molecules such as proteins and DNA [59]. Coupled with superoxide anion radical (O2∗−), nitric oxide forms peroxynitrite anion (ONOO−), which is more potent Reactive Nitrogen Species (RNS) resulting in nitrosative stress [60]. Plant natural products, for example, phenolics compete for superoxide with nitric oxide and prevent nitrosative stress caused by peroxynitrite anion [61]. In the present study, the in vitro nitric oxide scavenging capacity varied with the extract type where ME had the highest value followed by EAE, AQE, and CE. Our results of ME, which was on a par with the standard, BHT, accords with that of Sasikumar et al. [13]. We also noticed strong positive correlation between nitric oxide scavenging capacity and total phenolics of the extracts. Our finding is also supported by that of Sasikumar et al. [13] who evaluated the nitric oxide scavenging capacities of different spices.
Though a weak oxidant, H2O2 can oxidize and inactivate some enzymes as it is. However, it may react with some cations such as Fe2+ and Cu2+ within a cell and produce a toxic hydroxyl radical (OH•) that oxidizes many cellular molecules [62]. Results of this study showed that all solvents' extracts of S. aromaticum scavenged H2O2 and the scavenging capacity increased with extracts' concentration. Highest scavenging capacity was by ME followed by EAE, AQE, and CE. Hydrogen peroxide scavenging capacities of ME, EAE, and AQE were superior to the standard used at all concentration levels while that of CE was higher than the standard at concentrations below 100 μg/mL and on a par at concentrations ≥200 μg/mL. Formerly, a study by [54] vindicated the effect of high polar solvent extracts of S. aromaticum for Fe3+ reduction. Moreover, a study by [63] showed the effective scavenging activity of clove (S. aromaticum) oil against H2O2. Scavenging capacities of extracts correlated positively with total phenolic content, suggesting phenolic compounds are reductants responsible to scavenge H2O2 [56]. In vitro reducing power of a plant extract can be considered as an indicator for the presence of potential antioxidant compounds. In this study, the reducing power of extracts was determined by the intensity of the resultant Prussian blue color complex which absorbs at 700 nm. Our result suggests that all solvents' extracts have antioxidant compounds that convert Fe3+ to Fe2+ and reducing power of extracts appeared to increase with extracts' concentration. However, the extracts had significantly lower reducing power when compared to the standard.
5. Conclusions
In conclusion, different extraction solvents resulted in different concentrations of TPC and TFC with the highest values measured in strongly polar solvents. The antioxidant properties of the extracts showed strong positive correlation with TPC and TFC, suggesting that the difference in antioxidant properties of extracts of S. aromaticum flower bud is attributed to polyphenolic content.
Acknowledgments
The authors express their gratitude to the Ministry of Education of Ethiopia for funding the research and the School of Biological Sciences and Biotechnology for providing lab facilities.
Data Availability
All relevant data are included in the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
The first author, Shibiru Temesgen, conducted laboratory work and contributed to manuscript writing. J. M. Sasikumar contributed to research design, laboratory work, data analysis, and writing. Meseret C. Egigu contributed to data analysis and writing.
References
- 1.Halliwell B., Gutteridge J. M. C. Free radicals, ageing, and disease, free radicals in biology and medicine . 2nd. New York, NY, USA: Oxford: Clarendron Press; 1985. [Google Scholar]
- 2.Velioglu Y. S., Mazza G., Gao L., Oomah B. D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. Journal of Agricultural and Food Chemistry . 1998;46(10):4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- 3.Jungbauer A., Medjakovic S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas . 2012;71(3):227–239. doi: 10.1016/j.maturitas.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Cai Y. Z., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sciences . 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hossain M., Brunton N., Barry-Ryan C., Martin-Diana A., Wilkinson M. Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Rasayan Journal of Chemistry . 2008;1:751–756. [Google Scholar]
- 6.Loizzo M. R., Tundis R., Conforti F., et al. _Salvia leriifolia_ Benth (Lamiaceae) extract demonstrates in vitro antioxidant properties and cholinesterase inhibitory activity. Nutrition Research . 2010;30(12):823–830. doi: 10.1016/j.nutres.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Viuda-Martos M., Ruiz-Navajas Y., Fernandez-Lopez J., Pérez-Alvarez J. A. Spices as functional foods. Critical Reviews in Food Science and Nutrition . 2010;51(1):13–28. doi: 10.1080/10408390903044271. [DOI] [PubMed] [Google Scholar]
- 8.Kahkonen M. P., Hopia A. I., Vuorela H. J., et al. Antioxidant activity of plant extracts containing phenolic compounds. Journal of Agricultural and Food Chemistry . 1999;47(10):3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 9.Gulcin I., Sat I. G., Beydemir S., Elmastas M., Kufrevioglu O. I. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.) Food Chemistry . 2004;87(3):393–400. doi: 10.1016/j.foodchem.2003.12.008. [DOI] [Google Scholar]
- 10.Nagy M., Socaci S. A., Tofană M., et al. Determination of total phenolics, antioxidant capacity and antimicrobial activity of selected aromatic spices. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Food Science and Technology . 2015;72(1):82–85. doi: 10.15835/buasvmcn-fst:11094. [DOI] [Google Scholar]
- 11.Słowianek M., Leszczyńska J. Antioxidant properties of selected culinary spices. Herba Polonica . 2016;62(1):29–41. doi: 10.1515/hepo-2016-0003. [DOI] [Google Scholar]
- 12.Hashem M. A., Sen N., Hussain M. S., et al. A comparative study of antioxidant potential of three commonly used spices in Bangladesh. Free Radicals and Antioxidants . 2018;8:6–10. [Google Scholar]
- 13.Sasikumar J. M., Erba O., Egigu M. C. In vitro antioxidant activity and polyphenolic content of commonly used spices from Ethiopia. Heliyon . 2020;6(9, article e05027) doi: 10.1016/j.heliyon.2020.e05027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charles D. J. Antioxidant Properties of Spices, Herbs and Other Sources . New York, NY, USA: Springer; 2013. [DOI] [Google Scholar]
- 15.Srinivasan K. Antioxidant potential of spices and their active constituents. Critical Reviews in Food Science and Nutrition . 2014;54(3):352–372. doi: 10.1080/10408398.2011.585525. [DOI] [PubMed] [Google Scholar]
- 16.Kiani R., Arzani A., Maibody S. A. M. M. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Frontiers in Plant Science . 2021;12, article 646221 doi: 10.3389/fpls.2021.646221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soleimani M., Arzani A., Arzani V., Roberts T. H. Phenolic compounds and antimicrobial properties of mint and thyme. Journal of Herbal Medicine . 2022;36, article 100604 doi: 10.1016/j.hermed.2022.100604. [DOI] [Google Scholar]
- 18.Dailey A., Vuong Q. V. Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food & Agriculture . 2015;1, article 1115646 doi: 10.1080/23311932.2015.1115646. [DOI] [Google Scholar]
- 19.Venkatesan T., Choi Y., Kim Y. Impact of Different Extraction Solvents on Phenolic Content and Antioxidant Potential of _Pinus densiflora_ Bark Extract. BioMed Research International . 2019;2019:14. doi: 10.1155/2019/3520675.3520675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J., Chen C., Zhao S., Ge F., Liu D. Effect of solvents on the antioxidant activity of walnut(Juglans regia L.) shell extracts. Journal of Food and Nutrition Research . 2014;2(9):621–626. doi: 10.12691/jfnr-2-9-15. [DOI] [Google Scholar]
- 21.Iloki-Assanga S. B., Lewis-Luján L. M., Lara-Espinoza C. L., et al. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Research Notes . 2015;8(1):1–14. doi: 10.1186/s13104-015-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobuj M. K. A., Islam M. A., Islam M. S., Islam M. M., Mahmud Y., Rafiquzzaman S. M. Effect of solvents on bioactive compounds and antioxidant activity of _Padina tetrastromatica_ and _Gracilaria tenuistipitata_ seaweeds collected from Bangladesh. Scientific Reports . 2021;11(1):1–14. doi: 10.1038/s41598-021-98461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen P. C. M. Spices, Condiments and Medicinal Plants in Ethiopia, their Taxonomy and Agricultural Significance . Centre for Agricultural Publishing and Documentation Wageningen, The Netherlands; 1981. [Google Scholar]
- 24.Demeke D. Assessing ethnobiological use of spices and condiment in prepared dishes in Bahir Dar city market, Bahir Dar, Ethiopia: Ethnobiological study. Journal of Biology, Agriculture and Healthcare . 2020;10(13):10–16. [Google Scholar]
- 25.Asmerom D., Kalay T. H., Araya T. Y., Desta D. M., Wondafrash D. Z., Tafere G. G. Medicinal plants used for the treatment of erectile dysfunction in Ethiopia: a systematic review. BioMed Research International . 2021;2021:12. doi: 10.1155/2021/6656406.6656406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tegen D., Dessie K., Damtie D. Candidate anti-COVID-19 medicinal plants from Ethiopia: a review of plants traditionally used to treat viral diseases. Evidence-based Complementary and Alternative Medicine . 2021;2021:20. doi: 10.1155/2021/6622410.6622410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zachariah T. J., Krishnamoorthy B., Rema J., Mathew P. A. Oil constituents in bud and pedicel of clove (Syzygium aromaticum) Indian Perfumer . 2005;49:313–316. [Google Scholar]
- 28.Shan B., Cai Y. Z., Sun M., Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. Journal of Agriculture and Food Chemistry . 2005;53(20):7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- 29.Adefegha S. A., Oboh G., Adefegha O. M., Boligond A. A., Athayded M. L. Antihyperglycemic, hypolipidemic, hepatoprotective and antioxidative effects of dietary clove (Szyzgium aromaticum) bud powder in a high-fat diet/streptozotocin-induced diabetes rat model. Journal of Science in Food and Agriculture . 2014;94(13):2726–2737. doi: 10.1002/jsfa.6617. [DOI] [PubMed] [Google Scholar]
- 30.Gemeda N., Urga K., Tadele A., Lemma H., Melaku D., Mudie K. Antimicrobial activity of topical formulation containing Eugenia caryophyllata L. (Krunfud) and Myritus communis L. (Ades) essential oils on selected skin disease causing microorganisms. Ethiopian Journal of Health Sciences . 2008;18:101–107. [Google Scholar]
- 31.Hussien J., Teshale C., Mohammed J. Assessment of the antimicrobial effects of some Ethiopian aromatic spice and herb hydrosols. International Journal of Pharmacology . 2011;7(5):635–640. doi: 10.3923/ijp.2011.635.640. [DOI] [Google Scholar]
- 32.Zhang K. Chemical composition and antioxidant activities of the essential oil from the clove buds (Syzygium aromaticum) toward various oxidative stresses in vitro. Agriculture and Food Science Research . 2015;2:19–24. [Google Scholar]
- 33.Al Mashkor M. A. Evaluation of antioxidant activity of clove (Syzygium aromaticum) International Journal of Chemical Sciences . 2015;13:23–30. [Google Scholar]
- 34.Turgay O., Esen Y. Antioxidant, total phenolic and antimicrobial characteristics of some species. Bulgarian Journal of Agricultural Sciences . 2015;21:498–503. [Google Scholar]
- 35.El-Maati M. F. A., Mahgoub S. A., Labib S. M., Al-Gaby A. M. A., Ramadan M. F. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. European Journal of Integrative Medicine . 2016;8(4):494–504. doi: 10.1016/j.eujim.2016.02.006. [DOI] [Google Scholar]
- 36.Gaspar E. M., Duarte R., Santan J. C. Volatile composition and antioxidant properties of clove products. Biomedical Journal of Scientific and Technical Research . 2018;9:7270–7276. [Google Scholar]
- 37.Kim D. E., Hwang Y. S., Chang B. Y., Kim D. S., Cho H. K., Kim S. Y. Effects of the Syzygium aromaticum L. extract on antioxidation and inhibition of matrix metalloproteinase in human dermal fibroblast. Asian Pacific Journal of Tropical Biomedicine . 2019;9(2):53–59. doi: 10.4103/2221-1691.250850. [DOI] [Google Scholar]
- 38.Selles S. M. A., Kouidri M., Belhamiti B. T., Amrane A. A. Chemical composition, in-vitro antibacterial and antioxidant activities of Syzygium aromaticum essential oil. Journal of Food Measurement and Characterization . 2020;13:1–7. [Google Scholar]
- 39.Singleton V. L., Orthofer R., Lamuela-Raventos R. M. Oxidants and Antioxidants Part A . Vol. 299. Elsevier; 1999. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent; pp. 152–178. [DOI] [Google Scholar]
- 40.Ordonez A. A. L., Gomez J. D., Vattuone M. A., Isla M. I. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chemistry . 2006;97(3):452–458. doi: 10.1016/j.foodchem.2005.05.024. [DOI] [Google Scholar]
- 41.Singh R. P., Chidambara Murthy K. N., Jayaprakash G. K. Studies on the antioxidant activity of pomegranate (Punicagranatum) peel and seed extracts using in vitro models. Journal of Agriculture and Food Chemistry . 2002;50(1):81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- 42.Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry . 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 43.Ruch R. J., Cheng S. J., Klaunig J. E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis . 1989;10(6):1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 44.Oyaizu M. Studies on products of browning reaction prepared from glucosamine. Japanese Journal of Nutrition . 1986;44:307–315. [Google Scholar]
- 45.Egigu M. C., Ibrahim M. A., Yahya A., Holopainen J. K. Yeheb (Cordeauxia edulis) extract deters feeding and oviposition of Plutella xylostella and attracts its natural enemy. BioControl . 2010;55(5):613–624. doi: 10.1007/s10526-010-9287-9. [DOI] [Google Scholar]
- 46.Zhang Q. W., Lin L. G., Ye W. C. Techniques for extraction and isolation of natural products: a comprehensive review. Chinese Medicine . 2018;13(1):p. 20. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alara O. R., Abdurahman N. H., Ukaegbu C. I. Extraction of phenolic compounds: A review. Current Research in Food Science . 2021;4:200–214. doi: 10.1016/j.crfs.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Autor E., Cornejo A., Bimbela F., Maisterra M., Gandía L. M., Martínez-Merino V. Extraction of phenolic compounds from Populus Salicaceae bark. Biomolecules . 2022;12(4):p. 539. doi: 10.3390/biom12040539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaczorová D., Karalija E., Dahija S., Bešta-Gajevi’c R., Pari’c A., Zeljkovi’c S. C. Influence of extraction solvent on the phenolic profile and bioactivity of two Achillea species. Molecules . 2021;26(160) doi: 10.3390/molecules26061601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar S., Sandhir R., Ojha S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Research Notes . 2014;7(1):1–9. doi: 10.1186/1756-0500-7-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takao L. K., Imatomiand M., Gualtieri S. C. J. Antioxidant activity and phenolic content of leaf infusions of Myrtaceae species from Cerrado (Brazilian savanna) Brazilian Journal of Biology . 2015;75(4):948–952. doi: 10.1590/1519-6984.03314. [DOI] [PubMed] [Google Scholar]
- 52.Prior R. L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agriculture and Food Chemistry . 2005;53(10):4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 53.Molyneux P. The use of the stable free radical diphenyl picrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin Journal of Science and Technology . 2004;26:211–219. [Google Scholar]
- 54.Adefegha S. A., Oboh G. Effect of diets supplemented with Ethiopian pepper [ _Xylopia aethiopica_ (Dun.) A. Rich (Annonaceae)] and Ashanti pepper [ _Piper guineense_ Schumach. et Thonn (Piperaceae)] on some biochemical parameters in normal rats. Asian Pacific Journal of Tropical Biomedicine . 2012;2(2):S558–S566. doi: 10.1016/S2221-1691(12)60274-3. [DOI] [Google Scholar]
- 55.Mao L.-C., Pan X., Que F., Fang X.-H. Antioxidant properties of water and ethanol extracts from hot air-dried and freeze-dried daylily flowers. European Journal of Food Research Technology . 2006;222:236–241. [Google Scholar]
- 56.Pietta P. G. Flavonoids as antioxidants. Journal of Natural Products . 2000;63(7):1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 57.Adebiyi O. E., Olayemi F. O., Ning-Hua T., Guang-Zhi Z. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef University Journal of Basic Applied Sciences . 2017;6(1):10–14. doi: 10.1016/j.bjbas.2016.12.003. [DOI] [Google Scholar]
- 58.Soulef S., Seddik K., Nozha M., Smain A., Saliha D., Hosni K. Phytochemical screening and in vivo and in vitro evaluation antioxidant capacity of Fargaria ananassa, Prunus armeniaca and Prunus persica fruits growing in Algeria. Progress in Nutrition . 2020;22:236–252. [Google Scholar]
- 59.Knott A. B., Bossy-Wetzel E. Nitric oxide in health and disease of the nervous system. Antioxidants Redox Signalling . 2009;11(3):541–553. doi: 10.1089/ars.2008.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang F., Yuan Q., Chen F., et al. Fundamental mechanisms of the cell death caused by nitrosative stress. Frontiers in Cell Development and Biology . 2021;9:1–10. doi: 10.3389/fcell.2021.742483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nimse S. B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Advances . 2015;5:27986–28006. [Google Scholar]
- 62.Hazra B., Biswas S., Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complementary and Alternative Medicine . 2008;8:1–10. doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gulcin I., Elmastas M., Aboul-Enein H. Y. Antioxidant activity of clove oil - a powerful antioxidant source. Arabian Journal of Chemistry . 2012;5(4):489–499. doi: 10.1016/j.arabjc.2010.09.016. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included in the paper.


