Abstract
Chlamydia trachomatis is a major cause of sexually transmitted disease (STD) for which a vaccine is needed. CD4+ T-helper type 1 (Th1) cell-mediated immunity is an important component of protective immunity against murine chlamydial genital infection. Conventional vaccine approaches have not proven effective in eliciting chlamydial-specific CD4 Th1 immunity at the genital mucosa. Thus, it is possible that the development of a highly efficacious vaccine against genital infection will depend on the generation of a live attenuated C. trachomatis vaccine. Attenuated strains of C. trachomatis do not exist, so their potential utility as vaccines cannot be tested in animal models of infection. We have developed a surrogate model to study the effect of chlamydial attenuation on infection and immunity of the female genital tract by treating mice with a subchlamydiacidal concentration of oxytetracycline following vaginal infection. Compared to untreated control mice, antibiotic-treated mice shed significantly fewer infectious organisms (3 log10) from the cervico-vagina, produced a minimal inflammatory response in urogenital tissue, and did not experience infection-related sequelae. Antibiotic-treated mice generated levels of chlamydia-specific antibody and cell-mediated immunity equivalent to those of control mice. Importantly, antibiotic-treated mice were found to be as immune as control untreated mice when rechallenged vaginally. These findings demonstrate that subclinical chlamydial infection of the murine female genital tract is sufficient to stimulate a potent protective immune response. They also present indirect evidence supporting the possible use of live attenuated chlamydial organisms in the development of vaccines against chlamydial STDs.
Chlamydia trachomatis infections are the most common bacterial sexually transmitted disease (STD) in the United States (20). An estimated 4 million cases of chlamydial genital infection occur annually (26). Infection of women constitutes a significant risk because of serious sequelae such as pelvic inflammatory disease, ectopic pregnancy, and reproductive disability (2, 3, 5, 11). The cost of treating these infections approaches 4 billion dollars annually, with 80% of these costs attributed to infection and disease of women (26). Prevention of chlamydial STD depends on the development of an efficacious chlamydial vaccine.
A murine model of C. trachomatis infection of the female genital tract (1) has been extensively studied to define the immunological parameters of infection and immunity. The combined evidence generated from this model overwhelmingly supports an important effector function for CD4+ T-helper type 1 (Th1) immunity in the clearance of chlamydiae from the murine female genital tract (6, 8–10, 13, 16, 18, 22, 27); however, the mechanisms that function in mediating clearance remain unclear. Successful immunization against chlamydial infection of the murine female genital tract by passive transfer of bone marrow-derived dendritic cells pulsed ex vivo with nonviable chlamydiae has been described (24). Immunization with pulsed dendritic cells produced a potent chlamydial-specific CD4+ Th1 immune response and levels of protective immunity against chlamydial vaginal challenge that were equivalent to those observed in postinfection immune mice. Although these findings are very encouraging because they demonstrate the feasibility of immunization against chlamydial genital infection, this approach is not applicable for use in humans due to its complexity. Using a more conventional vaccine approach, Zhang et al. (28) found that intramuscular immunization of mice with chlamydial DNA encoding the chlamydial major outer membrane protein induced both cellular and humoral immune responses suggestive of Th1-biased immunity. DNA-vaccinated mice were challenged intranasally and exhibited smaller chlamydial burdens in lung tissue than did controls. Unfortunately, similar DNA immunization strategies have not been efficacious when mice were challenged by the intravaginal route (15; H. D. Caldwell et al., unpublished observations). The reason(s) for the differences in protective efficacy between mice challenged by the lung and those challenged by the genital tract is not understood. It is possible that protective immunity at these sites is elicited by different effector mechanisms specific to distinct host target cells or that compartmentalized mucosal immune responses are operative in the genital mucosa. Regardless, conventional vaccines with a high degree of protective efficacy against chlamydial infection of the genital mucosa have yet to be produced.
The use of a live attenuated C. trachomatis organism as a vaccine to prevent genital infection has not been explored. This is in part because genetic systems have not been developed for chlamydiae, which has hampered the generation of attenuated strains by mutation of targeted virulence factors. A live attenuated C. trachomatis vaccine may have important advantages over recombinant-subunit- or DNA-based immunogens because of the pathogen's obligate intracellular life style, biologic and antigenic complexity, and propensity to infect the genital mucosa, a site that may require induction of a region-specific immune response.
Here, we have investigated whether C. trachomatis with attenuated in vivo growth characteristics might be useful as a vaccine to prevent genital infection. To investigate this possibility, we developed a surrogate model of attenuated infection that depends on treatment of mice with a subchlamydiacidal concentration of oxytetracycline following vaginal infection. The subchlamydiacidal antibiotic treatment model produces infections with a marked reduction of the chlamydial load and infection duration with a minimum inflammatory response. Interestingly, antibiotic-modified infections did not significantly affect the ability of mice to produce a chlamydial-specific immune response. Importantly, mice that spontaneously resolved antibiotic-mediated subclinical genital infections were highly resistant to chlamydial reinfection of the genital tract. These findings suggest that if live attenuated C. trachomatis strains with reduced virulence characteristics were available, they might be useful as vaccines for the prevention of C. trachomatis STDs.
MATERIALS AND METHODS
Chlamydiae.
The mouse pneumonitis strain of C. trachomatis (MoPn) was grown in HeLa 229 cells. Infectious elementary bodies (EBs) were purified by density gradient centrifugation, and infection-forming units (IFUs) were determined as previously described (4). A single preparation of MoPn seed stock was used as the challenge inoculum for all of the experiments described.
Mice.
Female C57BL/10 (H-2b) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and used between 8 and 12 weeks of age. Animals were housed in microisolator-type caging under standard environmental conditions and received food and water ad libitum. All animal care and husbandry procedures were in accordance with the Guide for the Care and Use of Laboratory Animals. The animal facilities are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Chlamydial infection.
Mice were given 2.5 mg of medroxyprogesterone acetate (Depo-Provera; Upjohn Company, Kalamazoo, Mich.) in 0.1 ml of saline subcutaneously 7 days prior to chlamydial cervicovaginal infection to synchronize estrus. Mice were infected by inoculation of 5 μl of MoPn (1,500 IFUs, 100 50% infectious doses [ID50]) in 10 mM phosphate, pH 7.2, containing 0.25 M sucrose and 5 mM l-glutamic acid into the vaginal vault. For rechallenge experiments, mice were treated with progesterone as described above and challenged intravaginally with 100 ID50 of MoPn 40 days following resolution of the primary infection.
Cervicovaginal chlamydial shedding.
Cervicovaginal chlamydial shedding was assessed by quantifying the number of IFUs recovered from cervicovaginal swabs (Calgiswab type 1; Hardwood Products Company, Guilford, Maine) taken at different times postinfection (13, 23). Monolayers of HeLa 229 cells grown in 96-well tissue culture plates were inoculated with cervicovaginal samples, and chlamydial inclusions were detected by indirect immunofluorescence staining using the chlamydial lipopolysaccharide genus-specific monoclonal antibody EVI-H1. Entire genital tracts were removed from mice at 40 to 60 days postinfection and scored for the presence of hydrosalpinx. Hydrosalpinx in this model accurately reflects infertility and is therefore thought to be a reasonable correlate and model for chlamydial salpingitis in women (7, 25).
Oxytetracycline treatment.
Mice were injected subcutaneously with 0.1 ml of oxytetracycline HCl (12 mg/ml in sterile distilled water) every 48 h for a period of 2 weeks. Antibiotic treatment of mice was started on the day of intravaginal challenge.
Antibody and cytokine ELISA.
Chlamydia-specific immunoglobulin G (IgG) and IgA in sera and vaginal washes, respectively, were assayed by enzyme-linked immunosorbent assay (ELISA) using formalin-fixed MoPn EBs as the antigen and alkaline phosphatase-conjugated goat anti-mouse IgG and IgA antibodies (Zymed Laboratories, San Francisco, Calif., and Southern Biotechnology Associates, Birmingham, Ala.) as described previously (13, 24). Chlamydial antigen-specific cytokine responses of cultured splenocytes were assayed as described previously (16, 22). Briefly, 107 splenocytes (pooled from the spleens of two mice) were cultured with 4 × 107 heat-killed (56°C, 30 min) MoPn EBs at 37°C for 1.5 h, washed once with medium, and cultured in 24-well tissue culture plates (Costar, Corning Incorporated, Corning, N.Y.). Following 72 h of incubation at 37°C, culture supernatants were collected in duplicate and assayed for gamma interferon (IFN-γ) and interleukin-10 (IL-10) by ELISA using cytokine-specific capture and detection antibodies (PharMingen, San Diego, Calif.).
Histopathology.
Naive infected, infected oxytetracycline-treated, and normal uninfected mice (three per group) were sacrificed at days 6 and 12 postinfection, and the entire genital tracts were removed. Genital tracts were fixed in 10% buffered formalin and submitted to Histopath of America (Millersville, Md.) for embedding, sectioning, and evaluation of hematoxylin-and-eosin-stained tissue by a veterinary pathologist. The severity of the inflammatory response and tissue changes was graded on a scale of 0 to 4+ according the following criteria: 0, normal; 1+, minimal response (small numbers of inflammatory cells); 2+, mild response (increased numbers of inflammatory cells with slight thickening of the stroma and extension into the surrounding adipose tissue); 3+, moderate response (pronounced presence of inflammatory cells with obliteration of the adjacent adipose tissue); 4+, marked response (extension of the moderate-severity reaction with obliteration of the affected tissue and multifocal necrosis of adjacent adipose tissue). Inflammatory scores were recorded for genital tissues of the cervix, uterus, and oviduct. The mean values of the inflammatory grades recorded for the cervix, uterus, and oviduct from three mice were calculated and used to determine an inflammatory score.
Experimental design.
Sixty mice were infected vaginally and divided into two groups of 30. One group received antibiotic treatment, and the other was not treated. Ten mice in each group were cultured, and the results were used to determine clearance rates of treated and untreated mice. These same animals were bled, and vaginal washes were obtained immediately following resolution of infection to determine serum and local chlamydial antibody responses. These same animals were rechallenged intravaginally approximately 3 weeks following resolution of the primary infection to assess levels of protective immunity. Three mice in each group were sacrificed at 6 and 12 days postinfection for histopathological analysis. Three mice from each group were sacrificed 2 to 3 weeks following resolution of infection, and their spleens were used as a source of cells for antigen-specific cytokine analysis. Five mice in each group were sacrificed at 40 days following clearance of the primary infection and scored for the presence or absence of hydrosalpinx.
RESULTS AND DISCUSSION
Infection in oxytetracycline-treated mice.
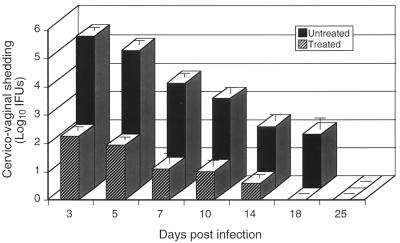
Mice were challenged intravaginally with chlamydiae and then either treated or not treated with oxytetracycline. Treated mice were administered the antibiotic immediately following infection and at 2-day intervals for a period of 14 days. Mice in both groups were cultured at various time points throughout the infection period, and the numbers of infectious chlamydiae shed from the cervicovagina were determined (Fig. 1). Oxytetracycline-treated mice shed approximately 1,000-fold fewer organisms from the genital tissue than did untreated control mice throughout the infection period. The duration of genital infection was also shorter in treated animals than in controls (18 versus 25 days). Thus, this antibiotic treatment regimen failed to completely eradicate the infection; however, it did significantly alter the normal course of infection in terms of both the chlamydial burden in genital tissue and infection duration. We reasoned that these altered in vivo growth characteristics could mimic those of an attenuated C. trachomatis strain(s) and hence might be useful for ascertaining the disease-causing characteristics and immune induction properties of such a putative strain.
FIG. 1.
Chlamydial clearance from the genital tracts of untreated or oxytetracycline-treated female mice. Groups of 10 mice were infected intravaginally with chlamydiae. One group was injected subcutaneously with oxytetracycline (1.2 mg/mouse) on the day of challenge and at 2-day intervals for the next 14 days, and the other group was not treated. Mice were cultured at different time points postinfection, and the numbers of infectious organisms shed from the cervicovagina were determined by calculating recoverable IFUs on monolayers of HeLa 229 cells. The values are means ± the standard errors of the means.
Disease in oxytetracycline-treated mice.
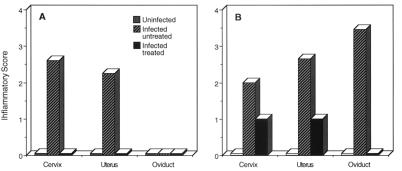
We next investigated whether the antibiotic-attenuated infections described above produced an inflammatory response in the genital tract. Inflammatory scores calculated from histopathological analyses of genital tracts taken from uninfected, infected untreated, and infected treated mice at days 6 and 12 postinfection are shown in Fig. 2. The genital tracts of uninfected control mice showed normal reproductive tissues at both time points. Infected untreated mice exhibited a marked inflammatory response in the cervical and uterine tissues at day 6 with no to minimal inflammation of the oviduct. At day 12, infected untreated mice all exhibited a moderate to marked level of inflammation in the cervix, uterus, and oviduct. In contrast, infected oxytetracycline-treated mice showed no inflammatory response in genital tissues at day 6 postinfection. At day 12, treated mice showed only minimal inflammatory responses (1+) in the cervix and uterus and no inflammation in the oviduct. Genital tissues from all three groups of mice were essentially normal following spontaneous clearance of chlamydiae from the genital tract (data not shown).
FIG. 2.
Histopathological analyses of genital tissues of chlamydia-infected mice untreated or treated with oxytetracycline following intravaginal infection. Inflammatory scores were recorded for the cervix, uterus, and oviduct following infection. Panels: A, 6 days postchallenge; B, 12 days postchallenge. The scores shown are the mean inflammatory scores of tissues from three separate mice at each time point. Inflammation scores (0 to 4+) were assigned by a veterinary pathologist as described in Materials and Methods.
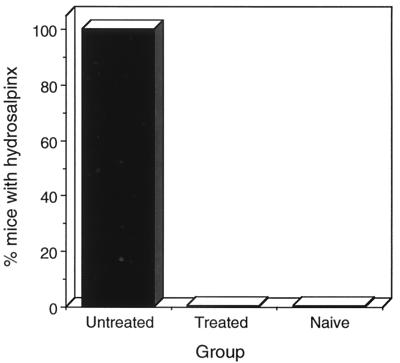
Hydrosalpinx formation is a common postinfection sequela in the mouse model that results in oviduct blockage and subsequent infertility (7, 25). Mice from each of the experimental groups were sacrificed 40 days following infection and scored for hydrosalpinx (Fig. 3). Hydrosalpinx was not found in uninfected or infected oxytetracycline-treated mice. In contrast, hydrosalpinx was present in all five of the infected untreated mice. The absence of hydrosalpinx in infected antibiotic-treated mice is consistent with the absent, or markedly reduced, inflammatory response in the genital tissues of these mice (Fig. 2). Thus, the attenuated genital infections produced in oxytetracycline-treated mice were subclinical. These infections elicited minimal to no inflammatory changes throughout the genital tissue that predictably did not produce postinfection related sequelae. It was therefore important to investigate whether these subclinical infections of the genital tract impacted the ability of mice to generate a chlamydia-specific immune response.
FIG. 3.
Incidence of hydrosalpinx in untreated or oxytetracycline-treated mice. Mice (five per group) were sacrificed 40 days following clearance of the primary infection and scored for the presence of bilateral hydrosalpinx. The results shown are the percentage of mice in each group exhibiting bilateral hydrosalpinx.
Chlamydia-specific antibody responses in the sera and vaginal washes of oxytetracycline-treated mice.
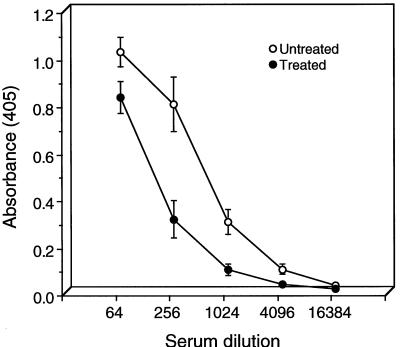
The chlamydia-specific IgG and IgA antibody responses in the sera and vaginal washes of untreated and oxytetracycline-treated mice as determined by ELISA are shown in Fig. 4. The end point titer of sera was 1:1,024 for the treated mice, compared to 1:4,096 for untreated animals. We also tested vaginal washes from both groups of mice for chlamydia-specific IgA (Fig. 5). Washes of untreated animals were found to have greater concentrations of IgA; however, the concentrations were not significantly different from those found in the treated group. Thus, there were only marginal differences in both the serum IgG and local chlamydia-specific IgA antibody responses between untreated and oxytetracycline-treated mice, despite the marked difference between the groups in the chlamydial antigen burden present in genital tissue (Fig. 1).
FIG. 4.
Serum antichlamydial IgG titers of untreated and oxytetracycline-treated mice. The sera of 10 mice were assayed individually by ELISA for IgG antibodies specific to formalin-killed MoPn EBs. End point titers were calculated as the highest dilution of serum producing an optical density of 0.2 or greater. The results are expressed as the mean antibody titer ± the standard deviation.
FIG. 5.
IgA antichlamydial antibodies in the vaginal washes of untreated and oxytetracycline-treated mice. Washes were collected by injecting two 60-μl volumes of sterile phosphate-buffered saline containing 0.5% bovine serum albumin into the vaginal vault of each mouse. The washes of individual mice (5 to 10 per treatment group) were tested for IgA antibodies specific to formalin-killed MoPn EBs. The results shown are means ± the standard deviations.
Antigen-specific cytokine responses of oxytetracycline-treated mice.
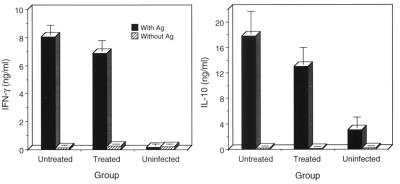
Cell-mediated immunity, particularly CD4+ Th1 immunity, plays an important role in the clearance of chlamydiae from the murine genital tract (10, 13, 16, 22, 28). CD4+ T cells are classified as Th1 or Th2, depending on the specific cytokine(s) they secrete following antigen stimulation (14). CD4+ Th1 cells produce IFN-γ, whereas Th2 cells produce IL-10. To investigate whether oxytetracycline treatment affected the development of chlamydia-specific CD4+ T-cell immunity, we assayed culture supernatants of antigen-pulsed splenocytes obtained from untreated and oxytetracycline-treated mice for IFN-γ and IL-10. The results of these experiments are shown in Fig. 6. Interestingly, and similar to what we observed for antibody responsiveness, the antigen-specific production of IFN-γ and IL-10 by splenocytes did not significantly differ between the antibiotic-treated and untreated animals.
FIG. 6.
Cytokine profiles of splenocytes from untreated and oxytetracycline-treated mice. Splenocytes (pooled from the spleens of three mice in each experimental group) were cultured in the presence of heat-inactivated MoPn EBs for 72 h. Culture supernatants were collected and assayed for IFN-γ and IL-10 by ELISA. Individual samples were tested in triplicate, and the results are expressed as means ± the standard deviations. Ag, antigen.
Genital rechallenge of oxytetracycline-treated mice.
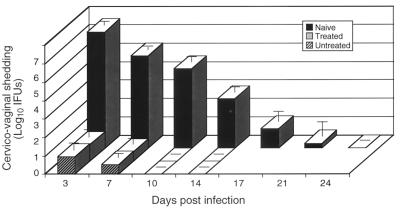
The immunological findings presented above indicate that oxytetracycline-treated mice, despite their marked reduction in infectious burden and antigenic load, responded similarly to untreated mice. It was therefore important to rechallenge treated and untreated mice to assess if these immunological findings correlated with protective immunity as measured by resistance to a secondary intravaginal challenge. Mice were rechallenged 3 weeks after they had spontaneously cleared the primary infection and then cultured at different times postchallenge to assess protective immunity. Naive mice were similarly challenged in this experiment to compare the levels of protective immunity produced in untreated or oxytetracycline-treated animals. Secondary rechallenge infections in both groups of mice yielded very low numbers of recoverable organisms from cervicovaginal swabs, and infections were significantly shortened in duration compared to those of immunologically naive mice (Fig. 7). Thus, there was virtually no difference between the abilities of treated and untreated mice to generate an adaptive protective immune response, despite the differences in infectious burden within the genital tissue and inflammatory response severity.
FIG. 7.
Chlamydial rechallenge of untreated and oxytetracycline-treated mice. Mice were challenged with 100 ID50 of MoPn 40 days after clearance of a primary infection. Naive mice were infected intravaginally, and chlamydial shedding in these mice was compared to that of secondarily rechallenged oxytetracycline-treated and untreated animals. Mice (10 per group) were cultured for chlamydiae at different time points after rechallenge, and the numbers of infectious organisms shed from the vaginas of the mice were determined by calculating the numbers of recoverable IFUs. The values are means ± the standard errors of the means.
In summary, the results of this work demonstrate that a self-limiting subclinical infection of the murine genital tract with C. trachomatis is as efficient as a clinically apparent acute infection in generating a protective antichlamydial immune response. We believe that these findings are important, since they imply that if a genetically attenuated C. trachomatis strain(s) were available, that possessed attenuated in vivo growth properties similar to those of the surrogate antibiotic-mediated attenuated infection model described here, it would be useful as a live attenuated vaccine for the prevention of chlamydial STDs. Temperature-sensitive mutants of C. psittaci have been previously shown to be effective in preventing chlamydial abortion in sheep (19), a finding that further supports the concept of using live attenuated C. trachomatis as a vaccine for the prevention of chlamydial STDs. The use of a live attenuated vaccine becomes more attractive in light of the recent findings of Ramsey et al. (17), who showed that C. trachomatis infection of the murine genital tract is capable of evoking heterotypic protective immunity, an important finding because human genital isolates of C. trachomatis exist as multiple serovars. Although genetic systems for manipulating chlamydial genes do not exist, the recent sequencing of the chlamydial genome (21) and the description of a plaque assay for the selection of C. trachomatis variants (12) make the development of genetic systems a likely possibility. Once genetic systems are developed, it should be possible to attenuate chlamydiae by targeted mutagenesis of virulence genes and then test these strains as vaccines in models of genital chlamydial infection. Live attenuated vaccines might prove to be the most effective strategy for eliciting a multifunctional, antigen-specific, and long-lasting heterotypic adaptive protective immune response at the genital mucosa.
ACKNOWLEDGMENTS
We thank Chris Fox for secretarial help and Gary Hettrick for help with graphic arts.
REFERENCES
- 1.Barron A L, White H J, Rank R G, Soloff B L, Moses E B. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981;143:63–66. doi: 10.1093/infdis/143.1.63. [DOI] [PubMed] [Google Scholar]
- 2.Brunham R C, Binns B, Guijon F, Danforth D, Kosseim M L, Rand F, McDowell J, Rayner E. Etiology and outcome of acute pelvic inflammatory disease. J Infect Dis. 1998;158:510–517. doi: 10.1093/infdis/158.3.510. [DOI] [PubMed] [Google Scholar]
- 3.Brunham R C, Peeling R, Maclean I, Kosseim M L, Paraskevas M. Chlamydia trachomatis-associated ectopic pregnancy: serologic and histologic correlates. J Infect Dis. 1992;165:1076–1081. doi: 10.1093/infdis/165.6.1076. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow J M, Yonekura M L, Richwald G A, Greenland S, Sweet R L, Schachter J. The association between Chlamydia trachomatis and ectopic pregnancy. JAMA. 1990;263:3164–3167. [PubMed] [Google Scholar]
- 6.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Maza L M, Pal S, Khamesipour A, Peterson E M. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Igietseme J U, Ramsey K H, Magee D M, Williams D M, Kincy T J, Rank R G. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, TH1 lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 9.Johansson M, Schön K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson M, Schon K, Ward M, Lycke N. Studies in knockout mice reveal that anti-chlamydial protection requires TH1 cells producing IFN-γ: is this true for humans? Scand J Immunol. 1997;46:546–552. doi: 10.1046/j.1365-3083.1997.d01-167.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones R B, Ardery B R, Hui S L, Cleary R E. Correlation between serum antichlamydial antibodies and tubal factor as a cause of infertility. Fertil Steril. 1982;38:553–558. doi: 10.1016/s0015-0282(16)46634-3. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto A, Izutsu H, Miyashita N, Ohuchi M. Plaque formation by and plaque cloning of Chlamydia trachomatis biovar trachoma. J Clin Microbiol. 1998;36:3013–3019. doi: 10.1128/jcm.36.10.3013-3019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosmann T R, Coffman R L. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 15.Pal S, Barnhart K M, Wei Q, Abai A M, Peterson E M, de la Maza L M. Vaccination of mice with DNA plasmids coding for the Chlamydia trachomatis major outer membrane protein elicits an immune response but fails to protect against a genital challenge. Vaccine. 1999;17:459–465. doi: 10.1016/s0264-410x(98)00219-9. [DOI] [PubMed] [Google Scholar]
- 16.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 17.Ramsey K H, Cotter T W, Salyner R D, Miranpuri G S, Yanez M A, Poulsen C E, DeWolfe J L, Byrne G I. Prior genital tract infection with a murine or human biovar of Chlamydia trachomatis protects mice against heterotypic challenge infection. Infect Immun. 1999;67:3019–3025. doi: 10.1128/iai.67.6.3019-3025.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rank R G, Ramsey K H, Pack E A, Williams D M. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodolakis A, Bernard F. Vaccination with temperature-sensitive mutant of Chlamydia psittaci against enzootic abortion of ewes. Vet Rec. 1984;114:193–194. doi: 10.1136/vr.114.8.193. [DOI] [PubMed] [Google Scholar]
- 20.Schachter J. Chlamydial infections (first of three parts) N Engl J Med. 1978;298:428–434. doi: 10.1056/NEJM197802232980805. [DOI] [PubMed] [Google Scholar]
- 21.Stephens R A, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov R L, Zhao Q, Koonin E V, Davis R W. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 22.Su H, Caldwell H D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su H, Feilzer K, Caldwell H D, Morrison R P. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su H, Messer R, Whitmire W, Fischer E, Portis J C, Caldwell H D. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable chlamydiae. J Exp Med. 1998;188:809–818. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swenson C E, Donegan E, Schachter J. Chlamydia trachomatis-induced salpingitis in mice. J Infect Dis. 1983;148:1101–1107. doi: 10.1093/infdis/148.6.1101. [DOI] [PubMed] [Google Scholar]
- 26.Washington A E, Johnson R E, Sanders L L., Jr Chlamydia trachomatis infections in the United States: what are they costing us? JAMA. 1987;257:2070–2072. [PubMed] [Google Scholar]
- 27.Williams D M, Grubbs B G, Pack E, Kelly K, Rank R G. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect Immun. 1997;65:2876–2882. doi: 10.1128/iai.65.7.2876-2882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D, Yang X, Berry J, Shen C, McClarty G, Brunham R C. DNA vaccination with the major outer membrane protein gene induces acquired immunity to Chlamydia trachomatis (mouse pneumonitis) infection. J Infect Dis. 1997;176:1035–1040. doi: 10.1086/516545. [DOI] [PubMed] [Google Scholar]