Abstract
Aims
This study aimed to explore the incidence of severe cardiac events in paediatric arrhythmogenic right ventricular cardiomyopathy (ARVC) patients and ARVC penetrance in paediatric relatives. Furthermore, the phenotype in childhood-onset ARVC was described.
Methods
Consecutive ARVC paediatric patients and genotype positive relatives ≤18 years of age were followed with electrocardiographic, structural, and arrhythmic characteristics according to the 2010 revised Task Force Criteria. Penetrance of ARVC disease was defined as fulfilling definite ARVC criteria and severe cardiac events were defined as cardiac death, heart transplantation (HTx) or severe ventricular arrhythmias. Childhood-onset disease was defined as meeting definite ARVC criteria ≤12 years of age.
Results
Among 62 individuals [age 9.8 (5.0–14.0) years, 11 probands], 20 (32%) fulfilled definite ARVC diagnosis, of which 8 (40%) had childhood-onset disease. The incidence of severe cardiac events was 23% (n = 14) by last follow-up and half of them occurred in patients ≤12 years of age. Among the eight patients with childhood-onset disease, five had biventricular involvement needing HTx and three had severe arrhythmic events. Among the 51 relatives, 6% (n = 3) met definite ARVC criteria at time of genetic diagnosis, increasing to 18% (n = 9) at end of follow-up.
Conclusions
In a paediatric ARVC cohort, there was a high incidence of severe cardiac events and half of them occurred in children ≤12 years of age. The ARVC penetrance in genotype positive paediatric relatives was 18%. These findings of a high-malignant phenotype in childhood-onset ARVC indicate a need for ARVC family screening at younger age than currently recommended.
Keywords: Arrhythmogenic right ventricular cardiomyopathy, Children, Family screening, Disease penetrance, Ventricular arrhythmia, Heart transplantation
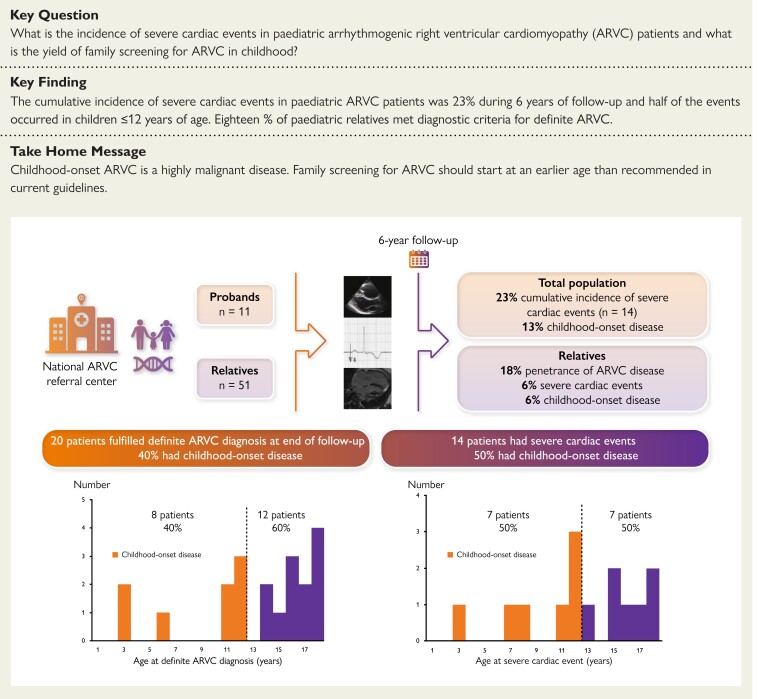
Structured Graphical Abstract
Structured Graphical Abstract.
Highly malignant disease in childhood-onset arrhythmogenic right ventricular cardiomyopathy: study design and summary results. In a paediatric ARVC cohort, childhood-onset ARVC was found in 40% of all patients with a definite diagnosis. There was a 23% cumulative incidence of severecardiac events and half of them occurred in children ≤12 years of age. The ARVC penetrance in genotype positive paediatric relatives was 18%.
See the editorial comment for this article ‘Arrhythmogenic cardiomyopathies in children: seek and you shall find’, by Juan Pablo Kaski, https://doi.org/10.1093/eurheartj/ehac585.
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inheritable and progressive heart muscle disease characterized by high risk of ventricular tachyarrhythmias and sudden cardiac death, in addition to morphological abnormalities and eventually heart failure.1 The disease has autosomal dominant inheritance with variants in genes encoding cardiac desmosomal proteins,2 and it exhibits variable penetrance and expressivity.3 ARVC diagnosis is made by combining multiple sources of information as described by the consensus-based revised 2010 Task Force Criteria (TFC).4
ARVC is considered a disease most relevant to the young adult population, with onset typically observed in the third and fourth decade of life.5 Penetrance of ARVC disease has been described in adolescents,6 but is currently considered to be extremely rare under the age of 10.5 Ten perecent to 25% of sudden deaths in children have been shown to be associated with ARVC.7,8 However, children with ARVC are underrepresented in research publications. The clinical characteristics of paediatric ARVC are largely unknown and data is lacking on both the incidence of events in children with ARVC and the penetrance of disease in ARVC genotype positive paediatric relatives.
The current expert consensus document in arrhythmogenic cardiomyopathy (AC)5 recommends that first-degree relatives undergo clinical evaluation every 1–3 years starting at 10–12 years of age. However, the clinical value of this approach has not been systematically evaluated.
We aimed to explore the incidence of severe cardiac events related to ARVC in paediatric patients (≤18 years) and to describe the phenotype of childhood-onset ARVC. Furthermore, we aimed to assess the penetrance of ARVC disease in genotype positive paediatric relatives. We hypothesized that the frequency of childhood-onset ARVC in children is underestimated and that the current screening recommendations leads to under-diagnosis of childhood-onset disease, which can impact timely interventions aimed at preventing severe outcomes.
Methods
Study population
In a single-centre cohort study, we included all consecutive ARVC probands and genotype-positive relatives ≤18 years old followed at the Department of Paediatric Cardiology, Oslo University Hospital, Rikshospitalet, Norway, between 2007 and 2021.
Inclusion was defined as the time of first echocardiographic examination. Last clinical follow-up was the last clinical visit before death, cardiac transplantation, turning 19 years of age or August 2021.
We defined phenotype positive patients as those fulfilling definite 2010 TFC. These diagnostic criteria were applied retrospectively for patients identified before 2010. We defined a proband as the first person in a family to exhibit clinical symptoms or signs that triggered an evaluation of ARVC. Genotype positive relatives were included regardless of fulfilling definite, borderline, or possible diagnosis at time of inclusion. Genotype negative probands and their relatives were only included if fulfilling a definite ARVC diagnosis by the revised 2010 TFC.4 Patients with other cardiopulmonary comorbidities were excluded. Penetrance of ARVC disease in family members was defined as fulfilling definite ARVC criteria. Written informed consent was given by the study participants or by the legal guardians of the participants if they were under the age of 16. The study complied with the Declaration of Helsinki and was approved by the Regional Committees for Medical Research Ethics (REK: 185666).
Electrocardiogram
Twelve-lead electrocardiograms (ECGs) were evaluated for repolarization (precordial T-wave inversion in leads V1 and V2 or beyond) and/or depolarization (epsilon waves or terminal activation duration ≥55 ms). In agreement with the revised 2010 TFC,4 precordial T-wave inversions were not considered for patients <14 years of age. Holter recordings were evaluated for premature ventricular complex (PVC) count and regarded abnormal if >500 PVCs per 24 h were recorded.4
Signal-averaged electrocardiographic recordings, obtained using time-domain analysis with a bandpass filter of 40 Hz, were evaluated for evidence of late potentials using age appropriate criteria.9
Genetic analyses
From 2007 to 2018, we used Sanger-based gene panels consisting of DSC2, DSG2, DSP, TMEM43 and PKP2 genes. From 2018, we used larger next-generation sequencing (NGS)-based gene panels for genetic testing. If no findings on Sanger sequencing or NGS, multiplex ligation-dependent probe amplification (MLPA, SALSA MLPA Probemix P168 ARVC-PKP2, MRC Holland) was performed. All identified variants were manually curated according to the 2015 American College of Medical Genetics and Genomics (ACMG) guidelines10 and only variants classified as pathogenic/likely pathogenic (P/LP) were included.
In case of a genotype positive proband, first-degree relatives were offered genetic cascade screening at the time ARVC was diagnosed in the family. Genotype positive relatives were called in to first clinical evaluation at time of genetic diagnosis, regardless of patient age.
Cardiac imaging
Two-dimensional echocardiography
All patients underwent an echocardiographic examination at time of study inclusion (Vivid 7, E9 or E95, GE Vingmed, Horten, Norway). Cine loops from three standard apical views (4 chamber, 2 chamber, and apical long-axis) were recorded. All echocardiographic data were analysed (EchoPac, GE, Vingmed, Horten, Norway) blinded to clinical data.
We measured right ventricular (RV) outflow tract (RVOT) diameter in parasternal short-axis view, RV basal diameter (RVD1) and RV fractional area change (RVFAC). All echocardiographic views, including the subcostal view, were used to detect RV akinesia, dyskinesia, or aneurysms.11 Left ventricular (LV) function by LV ejection fraction (LVEF) was measured by Simpson`s biplane method.12
Cardiac magnetic resonance imaging
Cardiac magnetic resonance (CMR) imaging was performed in a subset of 22 patients. CMR was performed on clinical indication including severe ventricular arrhythmias and reduced ventricular function and not routinely for diagnostic work-up. The late gadolinium enhancement (LGE) technique was performed in 21 patients. We used 1.5 Tesla units (Magnetom Vision Plus or Magnetom Sonata, Siemens, Erlangen, Germany) and a phased array body coil. Axial and sagittal T1 turbo spin echo images, multiple axial images, and one sagittal cineloop covering the RV and LV were recorded. RV and LV chamber dimensions, wall thickness, and myocardial function were assessed. A negative CMR study was defined as normal RV and LV dimensions, normal global or regional wall motion, no fatty infiltration, and no aneurysm formation.
Both echocardiography and CMR imaging were assessed to determine the severity and extent of structural abnormalities according to the revised 2010 TFC.4 LV involvement was defined by identification of at least one of the following: LVEF <55%; epicardial LGE; end-diastolic volume ≥120/ml/m²; and wall motion abnormalities. Patients included before 2010 were retrospectively re-evaluated for fulfilment of revised 2010 TFC.4
Outcomes
This study collected three outcomes:
Definitive ARVC diagnosis by the revised 2010 TFC4 established ≤18 years of age.
Severe cardiac events, defined as cardiac death, heart transplantation (HTx) or severe ventricular arrhythmias. A severe ventricular arrhythmic event was defined as aborted cardiac arrest (ACA), sustained ventricular tachycardia (VT) documented on 12-lead ECG or Holter, or VT or ventricular fibrillation (VF) terminated by appropriate implantable cardioverter defibrillator (ICD)-therapy.
Childhood-onset ARVC, defined as meeting definite ARVC criteria at 12 years of age or younger.
Statistical analysis
Continuous data were presented as mean ± standard deviation or median [interquartile range (IQR)], and categorical variables as number (percentage). Continuous variables were compared using the independent Student t test and categorical data using chi-square tests, Fisher exact test or Kruskal-Wallis rank test, as appropriate. We performed exact logistic regression analysis and provided odds ratio (95% confidence interval) for risk markers for severe cardiac events. Survival analyses were performed for age at outcomes with groups compared by log-rank test and displayed in Kaplan–Meyer plots (SPSS statistics version 26). Two-sided P-values <0.05 were considered significant.
Results
Study population
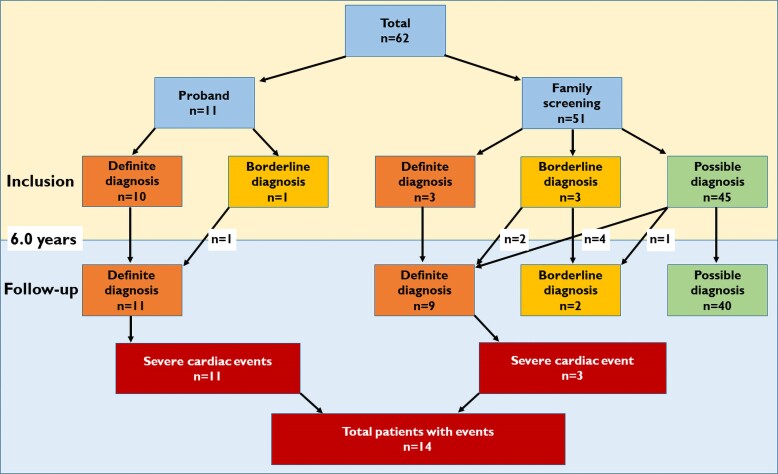
We included 62 patients, 34 (55%) females, 11 (18%) probands, age 9.8 (range 0.4–18.4, IQR 5.0–14.0) years, with follow-up time of 6.0 (2.9–9.6) years. Genetic analyses were performed in all patients, and 57 (92%) had a disease-causing variant (P/LP variant) (Table 1). Patients were included from 29 families, ranging from 1 to 6 members per family (see Supplementary material online, Table S1). No patients had homozygote or digenic variants (see Supplementary material online, Table S2).
Table 1.
Baseline characteristics of 62 paediatric ARVC patients, comparing probands and relatives
| Overall (n = 62) |
Proband (n = 11) |
Relative (n = 51) |
P-value | |
|---|---|---|---|---|
| Female sex | 34 (55) | 4 (36) | 30 (59) | 0.18 |
| Age at inclusion, years | 9.8 (5.0–14.0) | 12.3 (11.4–16.4) | 9.0 (4.3–12.9) | 0.02 |
| Mutation carrier | 57 (92) | 7 (64) | 50 (98) | <0.01 |
| Duration of follow-up, years | 6.0 (2.9–9.6) | 6.6 (1.8–9.9) | 5.8 (3.5–9.2) | 0.72 |
| Clinical phenotype | ||||
| Repolarization criteriaa | 13 (21) | 9 (82) | 4 (8) | <0.01 |
| Major | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Minor | 13 (21) | 9 (82) | 4 (8) | <0.01 |
| Depolarization criteria | 6 (10) | 4 (36) | 2 (4) | <0.01 |
| Epsilon wave | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Late potentials | 6 (10) | 4 (36) | 2 (4) | <0.01 |
| Arrhythmia criteria | 16 (26) | 9 (82) | 7 (14) | <0.01 |
| Major | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Holter monitor, >500 PVCs/24 h | 16 (26) | 9 (82) | 7 (14) | <0.01 |
| Imaging results abnormal | 12 (19) | 10 (91) | 2 (4) | <0.01 |
| Major | 12 (19) | 10 (91) | 2 (4) | <0.01 |
| Minor | 0 (0) | 0 (0) | 0 (0) | 1.00 |
ARVC, arrhythmogenic right ventricular cardiomyopathy; PVC, premature ventricular complex; h, hours.
Values are frequency (%) or median (interquartile range). P-values by Student’s t-test, chi-square test or Fisher’s exact test, as appropriate.
T-wave inversions were not counted in patients under the age of 14 years.
Outcome
Diagnosis of ARVC
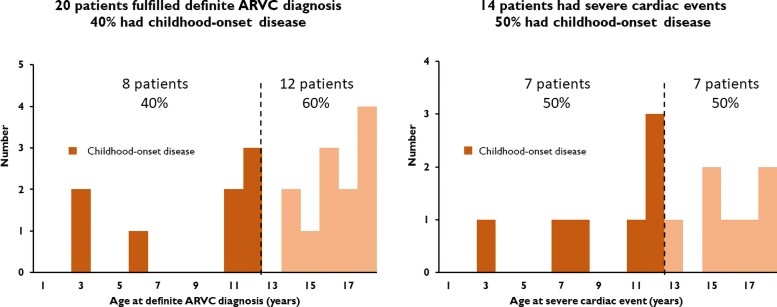
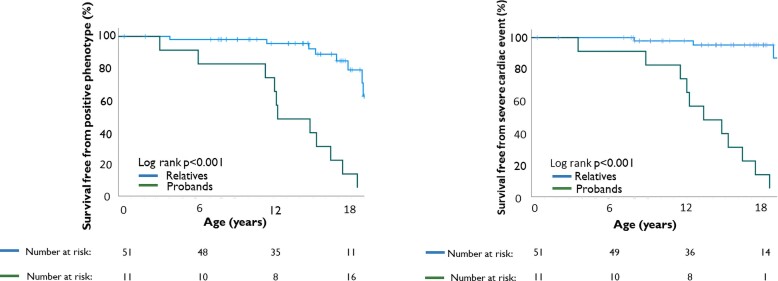
At inclusion, 13 (21%) of the 62 included patients fulfilled definite ARVC criteria, increasing to 20 patients (32%) by the end of follow-up (Figure 1). Median age at definite diagnosis was 15.0 (11.6–17.6) years and eight (40%) patients had childhood-onset disease (Figure 2, Structured Graphical Abstract). The two youngest patients with a definite diagnosis were three years old.
Figure 1.
Flowchart of included patients, diagnostic criteria, and outcome.
Figure 2.
Age distribution of definite arrhythmogenic right ventricular cardiomyopathy diagnosis and severe cardiac events. Eight (40%) of the 20 phenotype-positive children had childhood-onset disease. Seven (50%) of 14 children with a severe cardiac event experienced it ≤12 years of age.
Severe cardiac events
Among the 62 included patients, 6 had a severe cardiac event at inclusion as the first sign of ARVC disease. These six were all arrhythmic events (3 ACA, 3 sustained VT). Additional eight patients experienced a severe cardiac event during follow-up, giving a cumulative incidence of 23% (n = 14/62) at age ≤ 18. Median age at severe cardiac event was 12.9 [10.9–16.6] years (Figure 3) and 7 (50%) events occurred in patients ≤12 years of age (Table 2, Figure 2, Structured Graphical Abstract). Of the 14 events, 9 (64%) were arrhythmic events (3 ACA, 5 sustained VT, one VF terminated by appropriate ICD therapy) and 5 (36%) were HTx (Table 2). All 14 patients with severe cardiac events met diagnostic criteria for definite ARVC according to the revised 2010 TFC4 (Table 3).
Figure 3.
Survival free from definite arrhythmogenic right ventricular cardiomyopathy diagnosis and severe cardiac events. Twenty (32%) of the patients were diagnosed with definite arrhythmogenic right ventricular cardiomyopathy ≤18 years of age (end of follow-up). Median age at time of diagnosis was 15.0 (11.6–17.6) years. Fourteen (23%) of the patients had experienced a severe cardiac event by ≤18 years of age (end of follow-up). Median age at time of event was 12.9 (10.9–16.6) years.
Table 2.
Characteristics of patients with severe cardiac events during follow-up
| Patient no | Age at diagnosis | Age at first event | Genetic test results | Sex | Proband | Severe cardiac event | Circumstances of events | Phenotype positive at event | Major imaging criteria | Inverted T-waves in precordial leads | PVCs/ 24 h |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) | (years) | (P/LP variant) | (n) | ||||||||
| 1 | 3.2 | 3.6 | + | M | Yes | HTx | Yes | Yes | No | 47868 | |
| 2 | 3.9 | 7.9 | + | F | No | HTx | Yes | Yes | v1-v5a | 7000 | |
| 3 | 6.2 | 8.8 | + | F | Yes | HTx | Yes | Yes | v1-v5a | 900 | |
| 4 | 11.4 | 11.5 | - | F | Yes | HTx | Yes | Yes | v1-v4a | 105 | |
| 5 | 11.5 | 12.6 | - | F | No | HTx | Yes | Yes | v1-v3a | 103 | |
| 6 | 12.1 | 12.1 | + | M | Yes | Sustained VT | Rest | Yes | Yes | v1-v2a | 800 |
| 7 | 12.2 | 12.2 | - | M | Yes | ACA | Exercise | Yes | Yes | v1-v6a | 1587 |
| 8 | 12.3 | 13.3 | - | M | Yes | Sustained VT | Rest | Yes | Yes | v1-v4a | 1600 |
| 9 | 14.8 | 14.8 | + | F | Yes | Sustained VT | Exercise | Yes | Yes | v1-v3 | 23450 |
| 10 | 15.3 | 15.3 | - | F | Yes | ACA | Exercise | Yes | Yes | v1-v4 | 461 |
| 11 | 16.4 | 16.4 | + | M | Yes | ACA | Exercise | Yes | Yes | v1-v3 | 6428 |
| 12 | 17.3 | 17.3 | + | M | Yes | Sustained VT | Rest | Yes | Yes | v1-v3 | 8000 |
| 13 | 17.7 | 18.8 | + | M | No | Sustained VT | Exercise | Yes | Yes | v1-v3 | 10 691 |
| 14 | 18.5 | 18.5 | + | M | Yes | Sustained VT | Exercise | Yes | Yes | v1-v3 | 5300 |
P/LP, pathogenic/likely pathogenic; PVC, premature ventricular complex; M, male; F, female; HTx, heart transplantation; ACA, aborted cardiac arrest.
T-wave inversions were not counted in patients under the age of 14 years.
Table 3.
Clinical, electrical and cardiac imaging characteristics of 62 paediatric ARVC patients and mutation carriers, stratified by presence of severe cardiac events
| Severe cardiac event (n = 14) |
No severe cardiac event (n = 48) |
P-value | Exact logistic regression analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) P-value | |||||
| Female sex | 6 (43) | 21 (44) | 0.67 | ||
| Proband | 11 (79) | 0 (0) | <0.01 | a | a |
| Previous syncope | 3 (21) | 0 (0) | <0.01 | ||
| Repolarization criteriab | 6 (43) | 6 (13) | 0.01 | a | a |
| Major | 6 (43) | 6 (13) | 0.01 | ||
| Minor | 0 (0) | 0 (0) | 1.00 | ||
| Depolarization criteria | 7 (50) | 4 (8) | <0.01 | 14.0 (2.8–96.3) | <0.01 |
| Epsilon wave | 4 (29) | 0 (0) | <0.01 | ||
| Late potentials | 3 (21) | 4 (8) | 0.14 | ||
| Holter monitor >500 PVCs/24 h | 11 (79) | 4 (8) | <0.01 | 25.9 (4.8–199.0) | <0.01 |
| Number of PVCs | 1600 (800–7000) | 3.5 (1–10) | <0.01 | ||
| Imaging results abnormal | 14 (100) | 4 (8) | <0.01 | a | a |
| Major | 14 (100) | 2 (4) | <0.01 | ||
| Minor | 0 (0) | 2 (4) | 0.45 | ||
| LVEF, % | 45 ± 13 | 60 ± 4 | <0.01 | 0.8 (0.6–0.9) | <0.01 |
| RVOTi, cm/m² | 2.5 ± 0.9 | 2.0 ± 0.5 | 0.04 | 5.1 (1.5–21.6) | 0.01 |
| RVDi, cm/m² | 3.3 ± 0.9 | 2.4 ± 0.7 | 0.24 | 3.7 (1.5–11.5) | <0.01 |
| RVFAC, % | 26 ± 9 | 44 ± 4 | <0.01 | 0.6 (0.4–0.8) | <0.01 |
| LVEDDi, cm/m² | 3.6 ± 1.0 | 3.1 ± 0.8 | 0.08 | 1.7 (0.9–3.4) | 0.10 |
| PKP2 variant c.2146–1G > C | 3 (21) | 14 (29) | 0.57 | 0.7 (0.1–3.1) | 0.84 |
| PKP2 variant c.2197_2202delinsG | 2 (14) | 12 (25) | 0.40 | 0.5 (0.1–2.8) | 0.65 |
ARVC, arrhythmogenic cardiomyopathy; PVC, premature ventricular complex; h, hours, LVEF, left ventricular ejection fraction; PKP2, plakophilin-2; RVOTi, right ventricular outflow tract indexed; RVDi, right ventricular diameter indexed; RVFAC, right ventricular fractional area change; LVEDDi, left ventricular end-diastolic diameter indexed.
Values are reported as frequency (%), mean ± standard deviation, or median (interquartile range). On the left side of the table, P-values by Student’s t-test, chi-square test, Fisher’s exact test or Kruskal–Wallis rank test, as appropriate.
Model did not converge.
T-wave inversions were not counted in patients under the age of 14 years. Results from exact logistic regression analysis were reported as odd ratios (95% confidence interval) and P-values.
Eleven patients (18%) received an ICD; eight as secondary prevention and three as primary prevention therapy. No patients died during the study period (Table 2).
Cardiac phenotype in childhood-onset disease
The phenotypes identified in the 20 patients with a definite ARVC diagnosis at end of follow-up were right predominant in 11 (55%) and biventricular in 9 (45%) patients. In the nine patients with biventricular involvement, the ARVC diagnosis was confirmed by CMR in eight and histologically in one patient. The eight patients with childhood-onset disease had more frequently biventricular disease (63% vs. 33%, P < 0.01) with reduced LVEF of 37 ± 14% and RVFAC 23 ± 7% at end of follow-up. All five children who underwent HTx had childhood-onset disease and the youngest patient was transplanted at age 3.
Penetrance of AC disease in paediatric relatives
The 51 genotype positive paediatric relatives identified by screening were 9.0 (4.3–12.9) years of age at genetic diagnosis. At inclusion, three fulfilled definite ARVC criteria. Another six relatives progressed to definite diagnosis during 5.8 (3.5–9.2) years of follow-up (Figures 1 and 2), giving a cardiac penetrance of 18% (9/51). Three of the nine (33%) relatives with a definitive ARVC diagnosis had childhood-onset ARVC.
Three (6%) of the relatives experienced severe cardiac events (Figure 1) at a mean age of 12.6 (range 7.9–18.8) years (Figure 3) and at median 1.1 (range 1.0–5.2) years after genetic diagnosis. Two of these patients underwent cardiac transplantation at 7 and 12 years of age, and one experienced exercise induced sustained VT at 18 years of age (Table 2).
Sex differences
No sex differences were found, neither regarding penetrance of ARVC disease nor regarding the incidence of cardiac events.
Discussion
This study reports a 23% cumulative incidence of severe cardiac events in paediatric ARVC patients ≤ age 18. Of those with a definite ARVC diagnosis, 40% had childhood-onset disease, all with a highly malignant phenotype including ventricular arrhythmias and biventricular cardiac involvement and end-stage heart failure, reflecting the severity of ARVC disease at young age. Severe cardiac events also occurred in 6% of paediatric relatives and disease penetrance among relatives was 18%, indicating that genetic testing and clinical evaluation of relatives might be warranted at a younger age than currently recommended.
Outcome
Our study showed that 40% of patients with definite ARVC diagnosis had childhood-onset disease. Previous studies, however, have suggested that paediatric ARVC is a disease of adolescents. In the study of Roudijk et al.13 the youngest relative fulfilling ARVC diagnosis was 11 years old, while one proband was 9 years old. Te Riele et al.6 reported the youngest patient with a definite ARVC diagnose being 11 years old. According to the 2019 HRS expert consensus document ARVC is considered to be extremely rare under the age of 10 and exclusively seen in probands.5 A recent review of ARVC in children declared that identifying ARVC prior to age 10 is exceedingly unlikely.14 Our findings question these statements as we found a high frequency of childhood-onset disease defined as meeting definite ARVC criteria at ≤12 years of age.
This study is to the best of our knowledge, the first to report the incidence of severe cardiac events in ARVC patients ≤18 years of age. Our study demonstrated that ARVC is a highly malignant paediatric disease with a high incidence of ventricular arrhythmias, heart failure and need of HTx. Importantly, 50% of the severe cardiac events occurred in patients ≤12 years of age.
Risk assessment of VT and SCD in ARVC is challenging. There are published risk calculators for VT and SCD derived from the adult ARVC population.15,16 As always when working with decision support tools, these should be regarded as guidance in decision making and should not be followed blindly.17 As discussed in a recent commentary,17 risk calculators do generally not incorporate the effect of treatment. They evaluate the baseline arrhythmic risk to predict future malignant events, without accounting for changes in the risk resulting from treatment and which may overestimate risk.17 We did not use arrhythmic risk calculators in this study, nor do we use them in clinical paediatric practise due to the lack of validation in children. Implanting cardiac devices in children requires extreme care, involving unique patients’ characteristics, technical issues due to growth, and parents’ and patients’ preferences.
Severe cardiac phenotype in childhood-onset ARVC
Patients with childhood-onset ARVC showed more frequently biventricular involvement and heart failure necessitating heart transplantation. Five out of 8 children with childhood-onset ARVC showed a grave progression and needed HTx, illustrating the severity of ARVC structural disease in childhood. One of the 2 patients diagnosed with definite ARVC at 3 years of age (PKP2 positive), presented with neonatal cardiac failure, a period of recovery, and then later disease progression leading to HTx at age 3.6 years.
During the recent years, the AC clinical spectrum has grown to include both RV-, LV- and biventricular-predominant patterns of disease.5 AC include a broader spectrum of diseases with other features.18 Our patient population represented a classical ARVC cohort, with RV disease in all phenotype positive patients. Despite the ‘classical’ phenotype in our study, nine patients had biventricular disease emphasizing that paediatric-onset ARVC can involve both RV and/or LV at the time of presentation, as also recently shown by deWitt et al.19
We used the revised 2010 TFC4 to ascertain ARVC diagnosis in study subjects. It is important to recognize that the revised 2010 TFC were derived using a predominantly adult population (mean 38 ± 13 years of age). More specifically, only 8% of probands in the original TFC document20 were diagnosed between 12 and 18 years of age.4 As such, extrapolation of the TFC for use in paediatric cases might be speculative. Of note, all 14 children with severe cardiac events in our study met diagnostic criteria for definite ARVC according to the revised 2010 TFC,4 indicating that severity of disease is covered by the TFC also in paediatric patients.
Clinical yield of screening during childhood
In genotype positive paediatric relatives, ARVC penetrance at end of follow-up was as high as 18%, confirming ARVC disease also in paediatric relatives.
Cascade family screening to identify asymptomatic individuals is widely accepted as an important part of ARVC management. However, the 2019 HRS expert consensus5 recommends that first-degree relatives undergo clinical evaluation every 1–3 years starting as late as at 10–12 years of age. This recommendation is based on previous studies with underrepresentation of children that reported that most ARVC does not manifest until adulthood. However, the high penetrance of ARVC disease in our paediatric patients indicates a need of an earlier screening approach and is emphasized by the findings of childhood-onset disease and severe cardiac events among young paediatric relatives. This highlights the importance of starting clinical and genetic family screening earlier than current recommendations.
Study limitations
We performed a longitudinal cohort study with inherent limitations. The study may have entailed survival bias and we did not cover out of hospital ARVC deaths. Undiagnosed ARVC patients dying suddenly were not included. We can therefore not report the true incidence of ARVC related SCD in children which would require a nationwide autopsy-based study. Childhood-onset ARVC could be underestimated, as screening did not consequently start at birth. We did not perform CMR in all genotype positive paediatric relatives due to the need of anaesthesia, which may have underestimated the number of relatives fulfilling definite 2010 TFC. In addition, recent reports have shown that the TFC are relatively insensitive for paediatric diagnosis,21 which further may have underestimated the number of patients with definite ARVC diagnosis. Age-specific norms for T-wave amplitude are anticipated to be useful,22 although this is a more complex measurement and not included in the TFC that were applied in our study. Start of genetic testing in 2007 in Norway and changes in awareness of ARVC disease23 may have influenced the dynamics of presentation of disease.
Although this study represents one of the largest studies on paediatric ARVC to date, the sample size was limited. The design of our study was a longitudinal cohort study with a descriptive aim and it was not powered for risk analyses nor sex differences. Future multicenter studies with larger number of participants should explore age-, sex-, gene, and variant -specific features to improve management of ARVC in childhood. The issue of dependency within data should be considered when including data from families. Random effect analysis of family could not be included in our model due to absence of convergence. However, the families were relatively small with maximum six members in the biggest family (see Supplementary material online, Table S1). Furthermore, the analysis on clustering of genetic variant did not show association with severe cardiac events, suggesting that the relevance of dependency was not likely to be too significant in our model.
Conclusions
We found a 23% cumulative incidence of severe cardiac events, including VA and HTx, in a paediatric ARVC cohort of probands and genotype positive relatives ≤ 18 years of age. Childhood-onset ARVC was found in 40% of all patients with a definite diagnosis and appeared with a highly malignant phenotype, including biventricular involvement and end-stage heart failure necessitating HTx. Genotype positive paediatric relatives had a 18% disease penetrance and 6% experienced severe cardiac events. Increased awareness of childhood-onset, aggressive ARVC disease has important implications for patient management, suggesting that clinical screening should begin at a younger age than currently recommended.
Supplementary Material
Contributor Information
Marit Kristine Smedsrud, Department of Paediatric Cardiology, Oslo University Hospital, Rikshospitalet, Sognsvannsveien 20, 0372 Oslo, Norway; ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Sognsvannsveien 9, 0372 Oslo, Norway.
Monica Chivulescu, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Sognsvannsveien 9, 0372 Oslo, Norway.
Marianne Inngjerdingen Forså, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Sognsvannsveien 9, 0372 Oslo, Norway; Faculty of Medicine, Institute of Clinical Medicine, University of Oslo, Postboks 1078 Blindern, 0316 Oslo, Norway.
Isotta Castrini, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Sognsvannsveien 9, 0372 Oslo, Norway; Faculty of Medicine, Institute of Clinical Medicine, University of Oslo, Postboks 1078 Blindern, 0316 Oslo, Norway.
Eivind Westrum Aabel, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Sognsvannsveien 9, 0372 Oslo, Norway; Faculty of Medicine, Institute of Clinical Medicine, University of Oslo, Postboks 1078 Blindern, 0316 Oslo, Norway.
Christine Rootwelt-Norberg, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Sognsvannsveien 9, 0372 Oslo, Norway; Faculty of Medicine, Institute of Clinical Medicine, University of Oslo, Postboks 1078 Blindern, 0316 Oslo, Norway.
Martin Prøven Bogsrud, Unit for Cardiac and Cardiovascular Genetics, Oslo University Hospital, Ullevål, Kirkeveien 166, 0424 Oslo, Norway.
Thor Edvardsen, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Sognsvannsveien 9, 0372 Oslo, Norway; Faculty of Medicine, Institute of Clinical Medicine, University of Oslo, Postboks 1078 Blindern, 0316 Oslo, Norway.
Nina Eide Hasselberg, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Sognsvannsveien 9, 0372 Oslo, Norway.
Andreas Früh, Department of Paediatric Cardiology, Oslo University Hospital, Rikshospitalet, Sognsvannsveien 20, 0372 Oslo, Norway.
Kristina Hermann Haugaa, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Sognsvannsveien 9, 0372 Oslo, Norway; Faculty of Medicine, Karolinska Institute and Cardiovascular Division, Karolinska University Hospital, Nobels väg 6, 17177 Stockholm, Sweden.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
Funding for this project was provided by the Research Council of Norway #309762 ProCardio Center for Innovation.
Data availability
The authors do not have the authority to share the data reported in the present article, due to the sensitive nature of the data collected for this study. The Approval of the Regional Committee for Medical Research Ethics limits sharing data with researchers inside or outside Norway for purposes of reproducing the results or replicating the procedures. The data can be made available to any additional research after formal application to the Regional Committee for Medical Research Ethics and explicit consent given from every study subject.
References
- 1. Gilotra NA, Bhonsale A, James CA, Te Riele ASJ, Murray B, Tichnell C, et al. . Heart failure is common and under-recognized in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Heart Fail 2017;10:e003819. 10.1161/circheartfailure.116.003819 [DOI] [PubMed] [Google Scholar]
- 2. Gandjbakhch E, Redheuil A, Pousset F, Charron P, Frank R. Clinical diagnosis, imaging, and genetics of arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Am Coll Cardiol 2018;72:784–804. 10.1016/j.jacc.2018.05.065 [DOI] [PubMed] [Google Scholar]
- 3. Haugaa KH, Haland TF, Leren IS, Saberniak J, Edvardsen T. Arrhythmogenic right ventricular cardiomyopathy, clinical manifestations, and diagnosis. Europace 2016;18:965–972. 10.1093/europace/euv340 [DOI] [PubMed] [Google Scholar]
- 4. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. . Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur Heart J 2010;31:806–814. 10.1093/eurheartj/ehq025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, et al. . 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019;16:e301–e372. 10.1016/j.hrthm.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 6. Te Riele A, James CA, Sawant AC, Bhonsale A, Groeneweg JA, Mast TP, et al. . Arrhythmogenic right ventricular dysplasia/cardiomyopathy in the pediatric population: clinical characterization and comparison with adult-onset disease. JACC Clin Electrophysiol 2015;1:551–560. 10.1016/j.jacep.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 7. Tabib A, Loire R, Chalabreysse L, Meyronnet D, Miras A, Malicier D, et al. . Circumstances of death and gross and microscopic observations in a series of 200 cases of sudden death associated with arrhythmogenic right ventricular cardiomyopathy and/or dysplasia. Circulation 2003;108:3000–3005. 10.1161/01.Cir.0000108396.65446.21 [DOI] [PubMed] [Google Scholar]
- 8. Pilmer CM, Kirsh JA, Hildebrandt D, Krahn AD, Gow RM. Sudden cardiac death in children and adolescents between 1 and 19 years of age. Heart Rhythm 2014;11:239–245. 10.1016/j.hrthm.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 9. Fallah-Najmabadi H, Dahdah NS, Palcko M, Mehta SK. Normal values and methodologic recommendations for signal-averaged electrocardiography in children and adolescents. Am J Cardiol 1996;77:408–412. 10.1016/s0002-9149(97)89373-3 [DOI] [PubMed] [Google Scholar]
- 10. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. . Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018;19:591–600. 10.1093/ehjci/jey042 [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 13. Roudijk RW, Verheul L, Bosman LP, Bourfiss M, Breur J, Slieker MG, et al. . Clinical characteristics and follow-up of pediatric-onset arrhythmogenic right ventricular cardiomyopathy. JACC Clin Electrophysiol 2022;8:306–318. 10.1016/j.jacep.2021.09.001 [DOI] [PubMed] [Google Scholar]
- 14. Corrado D, van Tintelen PJ, McKenna WJ, Hauer RNW, Anastastakis A, Asimaki A, et al. . Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J 2020;41:1414–1429. 10.1093/eurheartj/ehz669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A, et al. . A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2022;43:e1–e9. 10.1093/eurheartj/ehac180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jordà P, Bosman LP, Gasperetti A, Mazzanti A, Gourraud JB, Davies B, et al. . Arrhythmic risk prediction in arrhythmogenic right ventricular cardiomyopathy: external validation of the arrhythmogenic right ventricular cardiomyopathy risk calculator. Eur Heart J 2022;43:3041–3052. 10.1093/eurheartj/ehac289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corrado D, Link MS, Schwartz PJ. Implantable defibrillators in primary prevention of genetic arrhythmias. A shocking choice? Eur Heart J 2022;43:3029–3040. 10.1093/eurheartj/ehac298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corrado D, Migliore F, Zorzi A. Arrhythmic risk stratification in arrhythmogenic cardiomyopathy: new predictors for left-sided variants? Eur Heart J 2021;42:2851–2853. 10.1093/eurheartj/ehab355 [DOI] [PubMed] [Google Scholar]
- 19. DeWitt ES, Chandler SF, Hylind RJ, Beausejour Ladouceur V, Blume ED, VanderPluym C, et al. . Phenotypic manifestations of arrhythmogenic cardiomyopathy in children and adolescents. J Am Coll Cardiol 2019;74:346–358. 10.1016/j.jacc.2019.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, et al. . Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task force of the working group myocardial and pericardial disease of the European Society of Cardiology and of the scientific council on cardiomyopathies of the international society and federation of cardiology. Br Heart J 1994;71:215–218. 10.1136/hrt.71.3.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roudijk RW, Evertz R, Teske AJ, Marcelis C, Bosboom D, Velthuis BK, et al. . Arrhythmogenic right ventricular cardiomyopathy in a pediatric patient. JACC Case Rep 2020;2:919–924. 10.1016/j.jaccas.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imamura T, Sumitomo N, Muraji S, Yasuda K, Nishihara E, Iwamoto M, et al. . Impact of the T-wave characteristics on distinguishing arrhythmogenic right ventricular cardiomyopathy from healthy children. Int J Cardiol 2021;323:168–174. 10.1016/j.ijcard.2020.08.088 [DOI] [PubMed] [Google Scholar]
- 23. Rootwelt-Norberg C, Lie Ø H, Dejgaard LA, Chivulescu M, Leren IS, Edvardsen T, et al. . Life-threatening arrhythmic presentation in patients with arrhythmogenic cardiomyopathy before and after entering the genomic era; a two-decade experience from a large volume center. Int J Cardiol 2019;279:79–83. 10.1016/j.ijcard.2018.12.066 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have the authority to share the data reported in the present article, due to the sensitive nature of the data collected for this study. The Approval of the Regional Committee for Medical Research Ethics limits sharing data with researchers inside or outside Norway for purposes of reproducing the results or replicating the procedures. The data can be made available to any additional research after formal application to the Regional Committee for Medical Research Ethics and explicit consent given from every study subject.