Abstract
The approved worldwide use of two messenger RNA (mRNA) vaccines (BNT162b2 and mRNA-1273) in late 2020 has proven the remarkable success of mRNA therapeutics together with lipid nanoformulation technology in protecting people against coronaviruses during COVID-19 pandemic. This unprecedented and exciting dual strategy with nanoformulations and mRNA therapeutics in play is believed to be a promising paradigm in targeted cancer immunotherapy in future. Recent advances in nanoformulation technologies play a prominent role in adapting mRNA platform in cancer treatment. In this review, we introduce the biologic principles and advancements of mRNA technology, and chemistry fundamentals of intriguing mRNA delivery nanoformulations. We discuss the latest promising nano-mRNA therapeutics for enhanced cancer immunotherapy by modulation of targeted specific subtypes of immune cells, such as dendritic cells (DCs) at peripheral lymphoid organs for initiating mRNA cancer vaccine-mediated antigen specific immunotherapy, and DCs, natural killer (NK) cells, cytotoxic T cells, or multiple immunosuppressive immune cells at tumor microenvironment (TME) for reversing immune evasion. We highlight the clinical progress of advanced nano-mRNA therapeutics in targeted cancer therapy and provide our perspectives on future directions of this transformative integrated technology toward clinical implementation.
Keywords: mRNA delivery, Nanoformulations, Cancer immunotherapy, mRNA therapeutics, Targeted cancer therapy
Graphical abstract
Highlights
-
•
Introduction and discussion of the fundamentals and advancement of mRNA biology and nanoformulation chemistry.
-
•
Clarification of the latest paradigms of nano-mRNA therapeutics for targeted cancer immunotherapy by regulation of specific subtype of immune cells.
-
•
Clinical progress of nano-mRNA integrated formulations in cancer immunotherapy.
-
•
Perspectives on the future directions of nano-mRNA-based targeted cancer immunotherapy toward clinical practices.
1. Introduction
The authorized use and increasing global vaccination rate of two effective and safe mRNA-based vaccines (BNT162b2 [1] and mRNA-1273 [2]) against COVID-19 have successfully reduced the transmission of coronaviruses and deadly illness, making extraordinary contributions to helping people get through the difficult pandemic. The success of these lipid nanoformulation-mRNA-based COVID-19 vaccines have provided practical insights into the treatment of cancer patients by introducing tumor antigen-encoding nano-mRNA technology [3].
Malone and colleagues first reported the successful protein expression of mRNA delivered by a synthetic cationic lipid nanoparticle (LNP) in vitro in 1989 [4]. Soon after, Wolff and co-workers demonstrated the success of in vivo transfection of reporter luciferase protein by direct injection of naked mRNA into mice in 1990 [5]. The study of administration of mRNA expressing vasopressin into rats first demonstrated the biological response of mRNA platforms in 1992, though the biological function did not last long [6]. In 1993, liposome-mRNA expressing influenza virus protein showed the induction of antigen specific cytotoxic T lymphocytes (CTLs) against virus infected cells in mice [7], indicating immunogenic features of mRNAs. In 1995, the first naked cancer mRNA vaccine encoding carcinoembryonic antigen presented antigen specific antibody immune response, protecting mice from tumor challenge [8]. Despite these early promising results, the development of mRNA therapeutics was not well invested at that time, largely owning to inadequate knowledge in mRNA biology and poor control on mRNA stability, inherent high immunogenicity, and in vivo delivery strategies.
Upon pathogen invasion, the innate immune system can recognise the foreign by sensing pathogen associated molecules (such as RNA), thereby orchestrating adaptive immune response [9]. The path from RNA sensing to the licensed mRNA products for human use took decades. Toll-like receptors (TLRs, such as TLR3 [10], TLR7 [11], TLR8 [12]) of antigen presenting cells (APCs) were found sensing RNA molecules in the early 21st century, but it was unknown how APCs can distinguish pathogen RNA from self-RNA released from apoptotic self-cells until 2005. The historic collaboration between two pivotal researchers, Karikó and Weissman from different disciplines, led to a landmark in RNA biology research [13]. It was discovered that RNA modifications, such as base alternations and pseudouridine incorporation, could ablate RNA mediated activation of TLRs 3, 7 and 8, subsequently reducing immunomodulatory function [13]. This significant finding uncovered the mechanism by which the innate immune cells can sense non-self RNA molecules, and fuelled the studies on RNA modifications and associated receptor-based immune recognition [14]. However, the precise mechanistical understanding of “how” and “to what degree” the receptors can tolerate the modified RNA molecules remains incompletely clear. Despite these knowledge gaps, this finding paved the way for current authorized use of synthetic COVID-19 mRNA vaccines in human, where pseudouridine incorporation enables acceptable immunostimulatory function (reducing adverse effects) and increased translation capability of target proteins (enhancing antigen production). Given the advances on mRNA optimization and purification, synthetic mRNA has been explored as a versatile technology to produce peptides and proteins as therapeutics in the host.
To enhance the efficacy of synthetic mRNA therapeutics, various nanoparticles have been engineered to protect mRNA molecules from degradation by ubiquitous RNases in vivo, deliver them to specific organs, and achieve endosomal escape after entering cells [15]. The prevalent mRNA delivery system at advanced clinical development is ionizable LNPs [16]. Accumulated knowledge in nano-mRNA technology is expected to advance the development of a wide range of therapeutics, such as preventative viral vaccines [17], therapeutic cancer vaccines [18], and replacement of protein-based immunotherapeutics [18].
To fight against infectious diseases, the prophylactic mRNA vaccines require robust antigen specific antibody immune response (humoral immunity) produced by B cells, which can neutralize pathogens with the help of CD4+ T cells [19]. In this context, CD8+ CTLs at a low level might be involved in the early control on viral infections prior to the production of sufficient antibodies. To combat existing cancer, it is vital for therapeutic cancer vaccines to evoke T-cell based immune response (cellular immunity), particularly potent and multifunctional CTLs, thereby eliminating tumor cells. The help signals from CD4+ T-helper 1 (Th1) cells is essential to promote the proliferation, functionality, and longevity of CTLs [20]. Guided by advanced nanoformulations, mRNA cancer vaccines enable simultaneous activation of CD8+ and CD4+ T cell immunity by targeting regulation of APCs in lymphoid organs, inducing potent and durable cytotoxic activity of CTLs against cancer [[21], [22], [23], [24], [25]]. Recently, Sahin and colleagues tuned the surface charge of mRNA-lipoplexes that encoded tumor neoantigens to be slight negative or near-neutral, which surprisingly allowed exclusive target to splenic plasmacytoid DCs (pDCs) and macrophages post intravenous administration, and elicited exceptionally potent and broad effector T cell immunity against tumor in both preclinical and clinical settings [21]. Instead of activation of TLR receptors like in the case of lipoplexes, the screened heterocyclic amine of LNPs reported recently enabled the activation of intracellular stimulator of interferon genes (STING) pathway, thus enhancing the immunogenicity, in vivo translation of tumor antigen and anti-tumor efficacy of nano-mRNA cancer vaccines [22].
Beyond the progress in targeted therapeutic vaccination, recent emerging nanoformulation technologies have advanced the development of mRNA therapeutics encoding immunostimulatory cytokines [[25], [26], [27]] (ClinicalTrials.gov number: NCT04455620, NCT03946800), therapeutic antibodies [[28], [29], [30]] (NCT05262530, NCT04683939), or transcription factors [31], creating new modalities in targeted cancer immunotherapy. Success has been reported in nano-mRNA programming tumor associated macrophages (TAMs) [31], cytotoxic (adoptive) T cells [[32], [33], [34]] and NK cells [30], or indirect modulation of multiple immune cells [35]. The rapid advancement in engineered nanoformulations and mRNA technology enables nano-mRNA therapeutics functioning better in vivo, with increased safety, stability, translation, non-TLR associated immunogenicity, modulation of tumor infiltrating immune cells, and anti-tumor immunity.
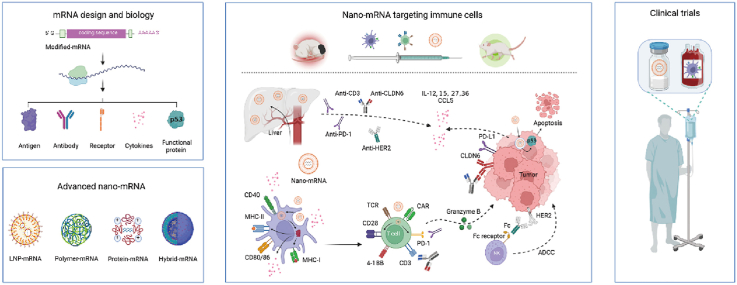
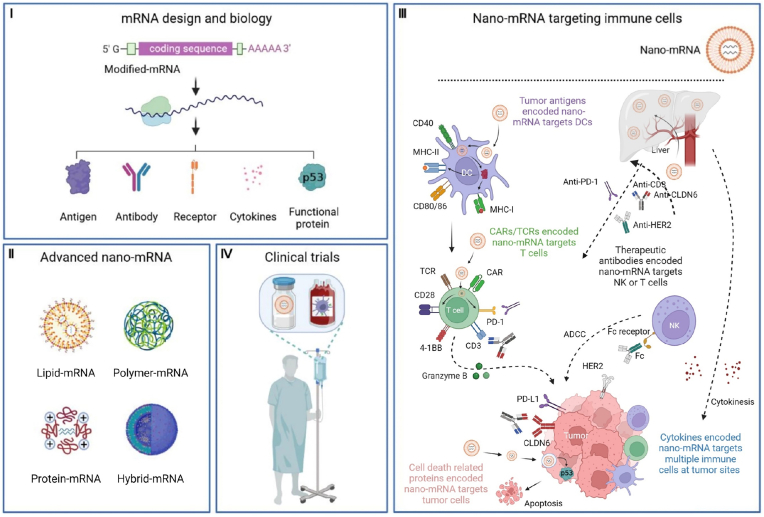
The technology of nano-mRNA has been revolutionizing the development of new drugs for prevention of infectious diseases and cancer treatment. There is a large pipeline of advanced and innovative mRNA therapeutics under clinical studies, expecting to address many unmet clinical needs in future. Recent reviews on mRNA therapeutics offer timely summary data, focusing on mRNA vaccines for infectious diseases and/or cancers [17,18], LNPs [16] or diverse nano-platforms [15] for delivery of mRNA therapeutics. In this review, we discuss the basic principles and new developments of mRNA technology (Fig. 1-I), and fundamental chemistry and advanced engineering technology of fascinating nanoformulations (Fig. 1-II). We highlight the latest preclinical (Fig. 1-III) and clinical (Fig. 1-IV) paradigms of nano-mRNA therapeutics in regulation of specific subtypes of immune cells of interest for enhanced cancer immunotherapy, such as therapeutic cancer vaccines and protein-based cancer immunotherapy (e.g., nano-mRNA encoding cytokines or specific antibodies). We conclude this review by providing our perspectives on future directions of the integrated nano-mRNA technology from basic science to clinical practices in cancer immunotherapy. This review offers an introduction of nano-mRNA technology and up-to-date reference point in targeted cancer immunotherapy for researchers in this interdisciplinary field of nanotechnology, immunology, and RNA biology.
Fig. 1.
The fundamental science and emerging concepts in mRNA biology and mRNA delivery nanoformulations underline new modalities of targeted cancer immunotherapy under clinical investigation. (I) The advancements in mRNA biology and modification strategies accelerate the progress of mRNA therapeutics encoding immunological molecules, such as tumor antigens and therapeutic antibodies. (II) Scientific advances in nanotechnology led to the development of cutting-edge platforms for mRNA delivery, including the representative lipid, polymer, protein, or lipid/polymer hybrid nanoparticles. (III) Nanoformulations can encapsulate and protect mRNA molecules from degradation and promote in vivo expression of encoded therapeutic immuno-molecules for targeted modulation of immune cells against cancerous cells in preclinical models. (IV) The encouraging outcomes from preclinical studies together with funding efforts have moved several fascinating nano-mRNA technologies from lab bench to clinical trials. Created with permission by BioRender.
2. Basic principles of mRNA design and manufacture
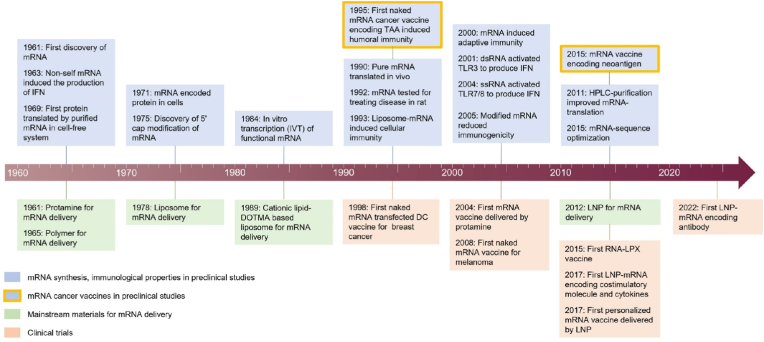
mRNA is a single stranded of nucleotides, which was first discovered in 1961 by Brenner and colleagues [36]. It was found that endogenous mRNA was in an unstable intermediate step between the translation of genetic information of DNA and production of DNA encoded proteins by cytoplasmic ribosomes. Since then, numerous scientific breakthroughs in mRNA biology have brought the use of mRNA from basic science to clinical reality (top light blue rectangles, Fig. 2). For instance, Lockard and Lingrel in 1969 provided the first evidence of in vitro production of mouse proteins encoded by purified mRNA in a cell-free system (rabbit-reticulocyte lysate), demonstrating the success of translation of exogenous mRNA in different systems [37]. In 1971, intracellular production of mRNA encoded proteins was achieved in cells [38]. The success of first in vitro transcription (IVT) of functional mRNA using SP6 RNA polymerase in 1984 [39] was an important milestone in the development of mRNA-based therapy. This pioneer work enables commercial scale production of synthetic mRNAs.
Fig. 2.
A timeline of key milestones in the development of mRNA technology and nano-mRNA formulations in preclinical and clinical studies, of which targeted cancer immunotherapy related are highlighted with orange outlines.
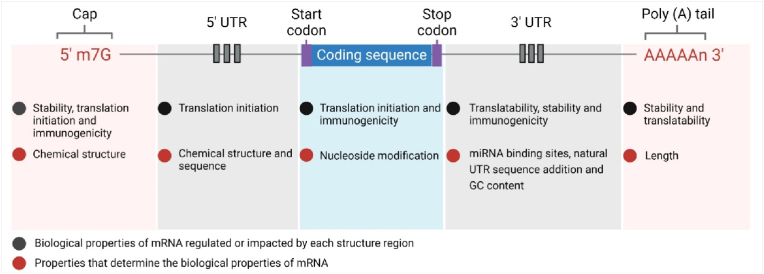
In the synthesis of IVT mRNA, the construction of plasmid DNA (pDNA) is the first step, which contains the corresponding sequence of targeted proteins. Then, linearized pDNA is used as a template for DNA-dependent RNA polymerases (such as SP6, T3 or T7 RNA polymerases derived from bacteriophages) to synthesize mRNA in a cell-free approach [40,41]. Finally, pDNA template is degraded in the presence of DNases for mRNA purification. The synthesized mRNA contains five structural regions as shown in Fig. 3. From 5′ to 3′ end, mRNA includes a 5′ cap, a 5′ UTR (untranslated region), an ORF (open reading frame) encoding protein of target, a 3′ UTR and a 3′ poly (A) tail that consists of repeated sequences of adenine nucleotides.
Fig. 3.
A scheme of IVT mRNA construct and key functions of each structural region. Created with permission by BioRender.
The discovery of mRNA 5′ cap modification by Muthukrishnan and colleagues in 1975 [42] was essential for the control on mRNA recognition, immunogenicity and translation. The evidence discovered in 1963 revealed that non-self mRNA from pathogens triggered the production of interferon (IFN), suggesting that host cells can recognise foreigners by sensing non-self nucleic acids [43]. Mechanistically, unmodified exogenous RNA acting as PAMP (pathogen-associated molecular pattern), can be recognised by some key PRRs (pattern recognition receptors) in cells, such as TLR 7/8 [12], TLR 3 [10] and retinoic acid-inducible gene (RIG)-like receptors (RIG-I and MDA-5(melanoma differentiation-associated protein 5)) [44]. The activation of these innate immune sensors leads to the secretion of type I IFN, degradation of mRNA and inhibition of mRNA translation. In contrast, 7-methylguanosine (m7G) modification of 5′ terminal can protect mRNA from degradation by 5′–3′ exonucleases, thereby increasing the stability of mRNA. Beyond that, 5′ cap plays a crucial role in recruiting the translation initiation factor, such as eukaryotic initiation factor 4F (eIF4F) [45], and assisting ribosomes to bind with mRNA sequence, thus improving mRNA translation efficiency. Thus, correct and complete cap structure is critically important in reducing immunogenicity and promoting translation of synthesized mRNA. Capped mRNA can be produced by incorporating a cap analogue in the DNA template during the process of IVT, or by enzymatic reaction after transcription [46,47].
Along with 5′ cap, 3′ Poly (A) tail plays an active role as in increasing the stability and translation efficiency of mRNA. The average length of Poly (A) tail is 100–250 nucleotides. Studies have shown that the length of poly (A) influences the translation efficiency of mRNA, but the optimal length is still controversial [[48], [49], [50]]. The poly (A) tail can be synthesized by adding poly (T) sequences to a DNA template during the process of IVT, or by using poly (A) polymerases post transcription [51]. The former method can more precisely control the length of poly (A) tail. In the enzymatic polyadenylation, poly (A) polymerases are added to RNA 3′-OH terminal to ensure the formation of a sufficient length of poly (A) tail, however; the length can be influenced by the concentration of 3′ end OH, reaction time, enzyme amount and ATP (adenosine triphosphate) concentration. The final product obtained from this approach is often a mixture of mRNAs with heterogeneous tail lengths, which leads to inconsistent quality control in different batches and hardly meets the regulatory requirements [52]. In contrast, the co-transcription of added poly-T nucleotides together with poly (A) enables production of mRNA molecules with a homologous tail length [50].
UTRs regulate the translation and half-life of mRNA. 5′ UTR, together with 5′ cap, affects ribosome recruitment and the initiation process of mRNA translation. For example, highly stable secondary and tertiary structures of 5′ UTR prevent the recruitment of ribosome, but internal ribosome entry sites (IRES) that are in the regions of mRNA can bypass the 5′ cap structure and recruit ribosomes directly [[53], [54], [55]]. Therefore, optimizing 5′ UTR, such as reducing advanced structures and adding IRES sequences, is vital for enhanced mRNA translation. 3′ UTR containing regulatory elements governs mRNA translatability, stability, and immunogenicity. For example, 3′ UTR contains binding sites with its partner microRNA (miRNA, a translation repressor), which can regulate the interaction of miRNA and subsequent translation silence mediated by miRNA [56]. Thus, removing miRNA-binding sites from 3′ UTR is an approach to promote encoded protein expression. Besides, 3′ UTR is a key determinant of intracellular kinetics of mRNAs [57]. Long 3′ UTR tends to decrease half-life of mRNAs though short 3′ UTR facilitates mRNA translation. To optimise the length of UTRs, natural UTR sequences screened from endogenous long-lived mRNAs, such as globin mRNA [58], are broadly used in synthetic IVT mRNAs. Furthermore, optimizing the proportion of different bases in the 3’ UTR sequences, such as increasing GC (guanine, cytosine) contents and reducing U (uracil) contents [59], can improve the stability and decrease the immunogenicity of synthetic mRNA.
ORF is the most important part of mRNA, which directs the synthesis of proteins of interest. The amino acid composition and spatial structure of each functional protein are specific, so the overall room for adjustment is limited. Codon optimization is one efficacious strategy for increasing translatability and minimizing immunogenicity of mRNAs [60]. Except methionine and tryptophan, each amino acid can be encoded by more than one codon. Therefore, replacing the low frequency codon with the high frequency codon speeds up the translation [61]. However, codon replacement strategy needs to consider the effect of translation speed on the formation of correct spatial structure of proteins. Some rare codons may decrease translational speed but benefit optimization of protein folding [62]. Nucleoside modification appears the most attractive and widely used strategy. Studies confirmed that modified nucleosides profoundly avoid the recognition of mRNAs by PRRs [13,63], and reduce the negative regulation of type I-IFN response on adaptive immunity. Currently, modified nucleosides used in synthetic mRNAs include pseuduridine (ψ) [64,65], N1-methylpseuduridine [66,67], 5-methylcytidine (m5C), 5-methyluridine (m5U), N6-methyladenosine, and 2-thiouridine.
In addition to the optimization and post-transcriptional modification of mRNA, purification process used for the synthetic mRNA substantially impacts mRNA intrinsic immunogenicity. Upon IVT process, a wide variety of potentially immunogenic contaminants would induce inflammatory response associated side effects, such as residual templates, nucleotides, and double-stranded RNA (dsRNA). Most of these contaminants can evoke type I-IFN response by activating PRRs, thus inhibiting mRNA translation. The most common method used to purify IVT mRNA is high performance liquid chromatography (HPLC). In 2011, Kariko and colleagues proved that HPLC purification remarkably increased the translational efficacy of unmodified or m5C/Ψ-nucleoside modified mRNA, and almost completely eliminated the immunogenicity [68]. Moreover, another study in 2015 demonstrated that the sequence optimized mRNA purified by HPLC can also obviously avoid innate immune activation in large animals [69]. Nevertheless, the disadvantages of HPLC hamper its wide applications in large-scale production of mRNAs: 1) a large amount of mRNA consumed (with a low recovery rate of about 50%); 2) high economic costs; 3) hazardous waste generated during treatment; 4) long processing time (1–2 days). Currently, some new purification methods with a high recovery and low cost have been developed and applied in mRNA production, such as oligo(dT)-cellulose chromatography that can remove at least 90% dsRNA contaminants and achieve more than 65% recovery rate in less than 2 h [70], and ribonuclease III (RNase III) specific digestion with process time of approximately 30 min [71].
IVT is a rapid, versatile, and controllable method for manufacturing mRNAs, which addresses the major challenges associated with the perception of using mRNA molecules as therapeutic medicines. The first evidence of in vivo translation of naked mRNAs was reported by Wolff and colleagues in 1990 (Fig. 2) [5], laying the foundation of the concept that mRNAs can be utilised as therapeutics. Soon after, Jirikowski and colleagues demonstrated that intrahypothalamic injection of mRNA encoding angiotensin can temporarily reverse diabetes insipidus in rats in 1992 [6], which was the first preclinical studies of mRNA used for treating disease. The concept of mRNA vaccines can be dated back to 1993, when induction of cellular immunity was observed in mice immunised with liposome-mRNA encoding influenza virus proteins [7]. The first naked mRNA cancer vaccine designed in 1995 proved the successful induction of humoral immune response against encoded tumor associated antigens (TAAs) in mice [8]. In 2000, mRNA vaccine constructed in a liposome system was able to induce comparable balanced antigen specific adaptive immunity, but showed improved stability compared to the unprotected naked mRNA [72]. Continued research efforts on mechanisms by which host immune system senses endogenous or modified RNAs shared a determination to move mRNA platform from the lab to medical inventions. For example, dsRNA was found recognised by TLR3 in 2001 [10], while ssRNA was found sensed by TLR7/8 in 2004 [12]. The first evidence in 2005 that modified mRNAs were able to completely shut down the TLR-medicated massive inflammation [13]. These key hallmarks have inspired numerous studies on mRNA modifications to balance the immunologic effects and translation efficacy of mRNAs, enabling acceptable safety profile and potency. Naked mRNAs are easily degraded by ubiquitous RNases in vivo. As a result, a great variety of delivery platforms (Please refer to next section) have been established to encapsulate and protect mRNAs from degradation.
Compared with DNA-based therapy, mRNA therapeutics have several unique advantages: 1) The translational process of mRNA occurs immediately in the cytoplasm, without entering the cell nucleus. 2) mRNA will not integrate into the host genomic DNA, with reduced risk of mutagenesis and carcinogenesis [73]. 3) Conventional IVT mRNA has no self-replicating ability and degrade rapidly after translation, so mRNA regulation of host cells is transient, exhibiting a favourable safety profile.
Over the past decades, mRNA technology along with advanced delivery nanoplatforms served as the backbone for the success of mRNA therapeutics in human use. In regard of cancer treatment, mRNA therapeutics are expanding well beyond cancer vaccines, including the replacement of therapeutic antibodies, proteins, cytokines, receptors for immunotherapy. This paradigm is a potential modality with great therapeutic prospects for clinical translation in cancer therapy.
3. Delivery strategies for mRNA therapeutics
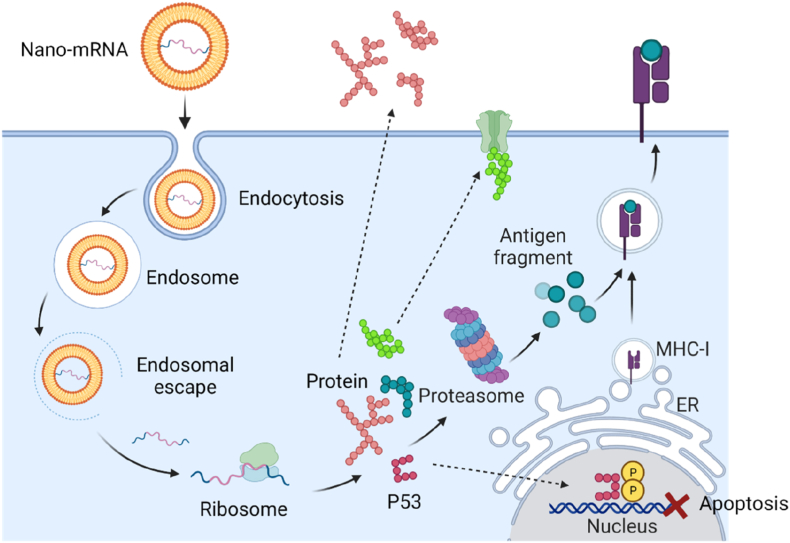
Inside the body, mRNA is easily engulfed by innate immune cells and degraded by nucleases. Given the negative charge and a relatively large molecular size of 104–106 Da (dalton), mRNA hardly cross the anionic cell membrane [17]. Diverse delivery strategies have been developed for intracellular delivery of mRNA molecules, including physical electroporation methods and nanotechnology-based solutions. The nanoformulation platforms usually contain cationic compositions that can capture mRNA inside the nano-space (Fig. 4), thereby protecting mRNA from in vivo degradation. The flexibility in the modulation of chemophysical features of nanoformulations enables targeting delivery to specific organs without causing unwanted toxicities. In terms of intracellular delivery, mRNA encapsulated nanoformulations enter the cells via endocytosis pathway and are trapped in the acidic environment, where the nanoformulations usually facilitate the cytosolic release of mRNA by disrupting the endo/lysosome membrane (Fig. 4). The delivered mRNA can then exert its function to guide ribosome to produce the encoded immunogenetic proteins, which will be secreted out to extracellular space, tethered to the cell membrane, processed by proteasome and presented on cell surface, or function inside the transfected cells. During the past decades, numerous advanced nanoformulations have proven their capability in protecting, delivering and potentizing mRNA therapeutics in animal and human studies (bottom light green and orange rectangles, Fig. 2). In this part, we will introduce the basic physicochemical properties of nanoparticle delivery platforms, advanced development in the formulation of nano-mRNA therapeutics, and the structure-function relationships in targeted cancer immunotherapy.
Fig. 4.
A scheme showing the intracellular delivery and translation process of mRNA encapsulated in the nanoformulations, and the function modes of produced proteins. Created with permission by BioRender.
3.1. Physical delivery for mRNA therapeutics
The commonly used physical methods for mRNA delivery includes electroporation [74,75], gene guns [76], photoporation [77] and direct local injection [78]. Electroporation, also termed electrotransfection, is defined as the use of an external electric field between two electrodes to form small pores in the cell membrane, so that negatively charged mRNA can pass through the membrane barrier. Electroporation approach is often applied locally and relatively superficial area to transfect targeted cells rapidly and efficiently. Currently, electroporation is an efficient and rapid method utilised to generate genetically engineered DCs or T cells for cancer treatment in animal models and humans [[79], [80], [81]]. However, in vitro electroporation will inevitably damage the function of some target cells and even cause cell death. While at the same time, in vivo electroporation is also affected by the electrode contact area, so it can only be used in superficial area. Gene gun shots mRNA loaded on gold nanoparticles at a high speed into target cells by using a gas shock wave [82]. However, potential cell death caused by the high-pressure shock wave and high costs limit the practical application of gene gun in mRNA delivery. Photoporation is a relatively new technique, in which transient pores are created in the cell membrane using a femtosecond laser to increase cell membrane permeability of delivered molecules [83]. A recent study demonstrated that vapor nanobubble assisted photoporation increased mRNA transfection of T cells in vitro under a reduced laser intensity [77]. But until now, it has been mainly used at the single-cell or subcellular levels.
Direct local injection of naked mRNA molecules has shown some efficacy in cancer immunotherapy, while the protein expression level is relatively low. Enhanced protein expression was observed post intranodal injection of mRNA dissolved in buffers containing calcium ions [84]. Modification of mRNA construct is an alternative approach to increase the potency of direct injected mRNA in vivo. For example, Christian and colleagues constructed N1-methylpseudouridine modified and cellulose-purified mRNA that encoded multiple cytokines of GM-CSF (granulocyte-macrophage colony-stimulating factor), IL-12 (interleukin-12), IL-15, and IFN-α. Intratumoral injection of the modified mRNA enhanced the translation of mRNA and induced potent anti-tumor immunity in preclinical tumor models [78]. However, the promising results of naked mRNA therapeutics in preclinical animal models cannot be reflected in clinical studies, which could be contributed by species-specific difference in immune system. For instance, intradermal injection of naked mRNA encoding TAAs in patients with melanoma under a clinical study, did not show clinical effectiveness [85], though mice intradermally immunised with naked mRNA demonstrated successful protein expression and development of immune response against encoded proteins. Altogether, direct administration of naked mRNA locally could be a promising therapeutic utility in certain circumstances, such as certain administration routes, mRNA modification and research settings.
3.2. Nanoformulations for delivery of mRNA therapeutics
Physical methods are simple but have some intrinsic disadvantages, such as potential side effects in cell damage, limited access to the sites or cells of interest, inconsistent efficiency in small and large animals. Therefore, chemical nanomaterial-based delivery strategies have been developed as alternative strategies for delivery of mRNA therapeutics, greatly assisting the translation of promising mRNA therapeutics into clinical use. As immunotherapy landscape evolved, delivery nanotechnologies have rapidly adapted to meet specific delivery need for mRNA immuno-therapeutics [86]. The advantages of nanoplatforms in mRNA delivery are profound compared to other viral delivery methods, such as low immunogenicity, excellent biocompatibility, high payload, and easy scale-up fabrication. The widely used nanoplatforms for mRNA delivery mainly include lipid/liposome [16], protein/peptide [87], lipid/polymer hybrid [88], and inorganic nanoparticles [89]. Each type of nanoparticles possesses unique chemical compositions and physical properties of solubility, size, surface charge and softness. Upon in vivo administration, these physicochemical features are determinants for their biodistribution, immunogenicity, and transfection efficacy of mRNA. Improved fundamental understanding on the mechanisms on mRNA-nanoformulation mediated immunological response will provide guidelines on further optimization toward the development of next generation of nanoparticle delivery systems.
3.2.1. Peptide or protein-based nanoparticles
Peptides and proteins are biological polymers comprising of amino acids as building blocks. Proteins but not peptides appear in a complex spatial structure. Protamine is a natural cationic polypeptide, which was the first mRNA delivery nanoparticles proposed by Amos in 1961 (Fig. 2) for enhanced transfection in cells [90]. The tight nanocomplexes of protamine-mRNA increased the stability of delivered mRNA molecules against degradation by serum RNases [72]. The first clinical trial of mRNA cancer vaccine delivered by protamine was initiated by Benjamin and co-workers in 2004 [91], revealing that protamine was a safe mRNA delivery system and was able to induce T cell immunity. The encouraging preclinical data supported the development of protamine-based mRNA cancer vaccine products under early clinical investigation (see the details of clinical trials in Section 5).
Cationic cell-penetrating peptide (CPP) is a class of small molecule polypeptide, generally composed of 8–30 amino acids that are capable of mediating cell membrane penetration and endosomal membrane disruption [92]. Thus, CPP-based nanoparticles were explored as antigen mRNA delivery systems, enabling induction of antigen specific CTLs [93]. Different from protamine that delivers mRNA into cells through classical endocytic pathway, CPP assists intracellular delivery of mRNA molecules via receptor independent direct transmembrane, which is more efficient and less limited by the type of cells to be delivered [94]. In addition, CPP, as a short peptide, shows advantages in large-scale production compared with natural extracted protamine with a spatial structure [95]. CPP started late in the field of mRNA delivery and the development of CPP-mRNA in cancer immunotherapy has significantly lagged behind protamine systems. However, the application prospects of CPP in mRNA delivery for cancer immunotherapy are very broad.
Virus-like particles (VLPs) are formed by the self-assembly of one or several viral structural proteins in the absence of viral DNA or RNA genomes. Natural VLPs (20–300 nm) often act as subunit antigen sources in prophylactic vaccine products [96], such as human papillomavirus (HPV) vaccine Gardasil. The potential of VLPs as delivery platforms was recently investigated for exogenous mRNA encoding reporter proteins [97]. VLPs encapsulate mRNA by binding internal amino acid residues of VLPs with specific motif sequence in mRNA molecules [98]. In some cases, the encapsulation of mRNA in VLPs is driven by the electrostatic interaction between the positively charged arginine sequence at the N-terminal of the capsid protein and the negatively charged RNA skeleton [99]. Recently, VLP-mRNA paradigm was used for cancer immunotherapy in preclinical animal models. For instance, recombinant bacteriophage MS2 VLPs was reported to deliver mRNA encoding prostate cancer associated antigen PAP (prostatic acid phosphatase) and GM-CSF to DCs, inducing balanced Th1/Th2 responses against murine prostate cancer [100]. Recently, Zhang and colleagues reported that PEG10 (paternally expressed 10), a mammalian retrovirus like protein, enabled successful encapsulation of exogenous mRNA by flanking delivered mRNA genes with 5′ and 3′ UTRs of PEG10 [87]. VLPs resemble the capsid structure of viruses that can be recognised by PRRs of innate immune cells, possessing immunogenic effects [101]. Beyond that, the ease of large-scale production and purification endows VLPs as potential mRNA delivery platforms in targeted cancer immunotherapy, though this paradigm is still under early investigations.
3.2.2. Lipid nanoparticles
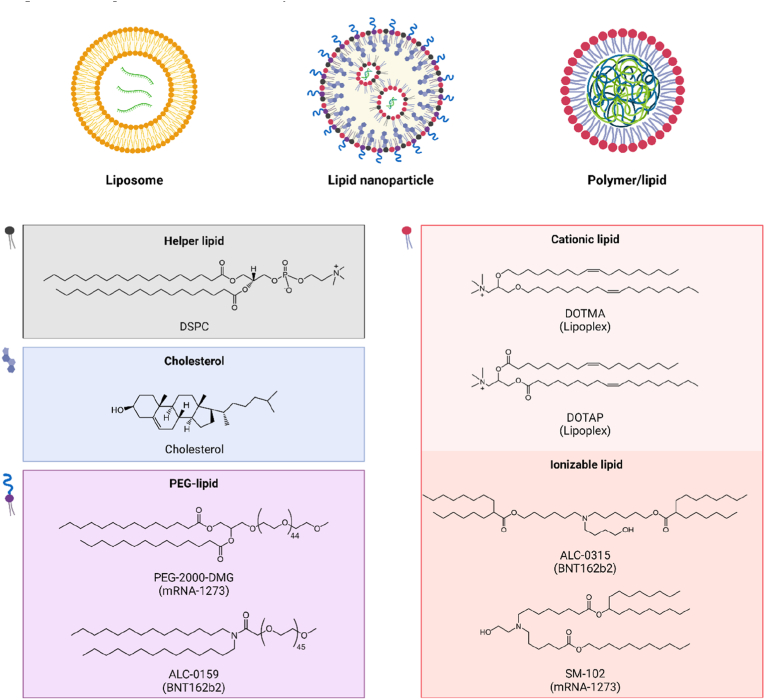
Lipids are a class of amphiphilic molecules comprising a hydrophilic head, a hydrophobic tail, and a linker conjugated two domains. Different types of lipids, such as natural phospholipid, ionizable lipids and cationic lipids, have been extensively investigated for mRNA delivery in preclinical animal models and humans. Liposomes are spherical nano-vesicles with single or double layers formed by phospholipids and cholesterols (Fig. 5). In 1978 (Fig. 2), scientists used membrane-structured liposomes to transport mRNA into mouse [102] and human [103] cells, inducing intracellular expression of encoded proteins. Now, solid LNPs that were first explored in delivering self-amplifying mRNA vaccines in 2012 [104], are favourably used in clinic. Solid LNPs are made of four-lipid cocktail (Fig. 5): 1) a helper phospholipid that resembles the cell membrane lipids forming a bilayer structure; 2) a cholesterol that modulates the integrity, rigidity and stability of the bilayer structure, 3) a polyethylene glycol (PEG)–lipid that increases the colloidal stability and reduces opsonization, and 4) an ionizable lipid that is protonated with a positive charge at a low pH and cling to mRNA molecules in the core of LNPs. Of note, the ionizable lipids lose the positive charge under bloodstream alkaline conditions, decreasing systemic toxicity. In addition, the protonation of ionizable lipids in acidic endo/lysosomes promote endosomal escape of delivered mRNA by destabilizing the membrane. The incorporation of ionizable lipids is the key for the success of LNP-mRNA vaccines, such as ionizable lipid SM102 in mRNA-1273 and ALC-0315 in BNT162b2 (Fig. 5). In addition to delivery function, zwitterionic ionizable lipids containing a heterocyclic amine group in the head domain were found capable of activating STING pathway and potentiating anti-tumor immunity of LNP-mRNA vaccines [22].
Fig. 5.
Schematic illustration of representative cationic or ionizable lipid-based nanoformulations complexed with mRNA and chemical structures of the components in LNPs. Created with permission by BioRender.
By contrast, cationic lipids have a permanent positively charged head [105]. The cationic lipids of DOTMA (1,2-di-O-octadecenyl-3-trimethylammonium propane (chloride salt)) and DOTAP (1,2-dioleoyl-3-trimethylammonium-propane, chloride) (As demonstrated in Fig. 5) were the first lipids explored for mRNA delivery in 1989 [4] (Fig. 2). In 2015, cationic lipids demonstrated successful delivery of mRNA expressing neo-antigens that can be recognised by CD4+ T cells, with complete rejection of established tumors in mice [106]. Cationic lipids together with cholesterol and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) (Fig. 5) are commonly used in the LNP-nanoformulations for mRNA delivery, such as the therapeutic mRNA cancer vaccine product of lipoplexes that are actively investigated under clinical studies [21,23]. DOPE is the most widely used auxiliary lipid, which can stabilize the bilayer membrane structure of LNP and reduce the cytotoxicity of positive components of LNPs. In addition, the inverted hexagonal H(II) phase of DOPE leads to the instability of endosomes, which plays a crucial role in assisting endosome escape of LNPs [107]. By modulating the surface properties of lipoplexes to slightly negative charge, lipoplexes can direct mRNA specifically to spleens for potent vaccine-induced immunity (see details in Sections 4, 5). Diverse cationic lipids have been developed to adapt into the need of specific nano-mRNA therapeutics, which are clarified in detail in an excellent review focusing on the LNPs for mRNA delivery [16]. The delivery efficacy and inherent adjuvancity of cationic-based LNPs can be widely adjusted by alternating the chemical compound structures of each component as well as physical properties of nanoformulations.
3.2.3. Polymer nanoparticles
A polymer is a class of macromolecules composing numerous repeating natural or synthetic subunits, which can be formed in a variety of nano-constructs [108]. Cationic diethylaminoethyl-dextran was the first type of polymer, being found capable of enhancing poliovirus RNA infection in 1965 [109]. This chemical now is a product in the market for gene transfection. Compared to LNPs, polymer-based nanoparticles are less clinically advanced, but offer similar functions for mRNA delivery. For instance, the cationic polymers condense mRNA molecules into the nanoformulations, promoting cellular up by cells and cytosolic delivery by proton sponge mediated osmotic swell and endosome membrane rupture [110].
Polyethylenimine (PEI) is the most often used polymers for gene delivery, while its applications are limited by high charge density mediated toxicity [111]. To mitigate the toxicity, biocompatible or degradable components are incorporated into PEI-based formulations, such as PEG [112] and redox sensitive disulfide linkers [113]. Alternatively, biodegradable polymers that contain with disulfides have been explored for mRNA delivery, such as poly (β-amino ester)s (PBAE) that combined with PEG-lipid specifically targeting lungs [88,114,115]. In addition, pH-responsive poly(aspartamide)s linked with ionizable aminoethylene facilitated cytosolic delivery of mRNA, of which the hydrophobic properties and length of aminoethylene impacted delivery efficacy [116]. However, most of these polymers alone were not often studied in nano-mRNA-based cancer immunotherapy.
3.2.4. Hybrid nanoparticles and other candidates
Hybrid nanoparticles integrate the physicochemical features of dual or multiple materials, evolving as a promising platform for mRNA delivery, such as liposome-protamine nanoparticles [117] and lipid/polymer nanoparticles (Fig. 5) [28,[118], [119], [120], [121]]. One lipid/polymer product, called TransIT-mRNA Transfection kit that is manufactured by Mirus Bio, remarkably enhanced the efficacy of mRNA encoding bispecific antibodies against established large tumors [28]. This strategy is currently under clinical evaluation (see Section 5). Recently, cationic lipids termed G0-C14 formed complexes with mRNA, which was then coated with PLGA (poly(lactic-co-glycolic acid)/PEG. The hybrid nanoformulation adhered to local mucosal sites, increasing mRNA-mediated inhibition of bladder cancer [118].
Organic/inorganic hybrid nanoparticles were also examined for mRNA delivery in cancer cells, such as natural polymer chitosan/selenium nanoparticles that were decorated with tumor targeting folic acid molecules [122], and dendrimer polymer/gold/folic acid nanoparticles [123]. However, in vitro validation of nano-mRNA efficacy is limited. In contrast, inorganic calcium phosphate nanoparticles that were coated with LNPs demonstrated as a powerful delivery system in vivo for targeted cancer immunotherapy [124].
Other candidates that are at the early stage in mRNA delivery potentially offer new opportunities in cancer immunotherapy, mainly including mesoporous silica nanoparticles with a high porosity [89], Zn2+ ions [125], outer membrane vesicles (OMVs) derived from bacteria [24]. The advantages and disadvantages of representative types of mRNA delivery nanoparticles are summarized in Table 1.
Table 1.
Summary of the advantages of disadvantages of representative types of nanoparticles for mRNA delivery.
| Type of nanomaterials | Advantages | Disadvantages |
|---|---|---|
| Peptides | Low toxicity | Short half-life |
| Easy membrane penetration | Lack of targeting | |
| Low immunogenicity | ||
| Proteins | Low toxicity | Undesired immunogenicity |
| Good stability | Lack of targeting | |
| Natural sources | ||
| Lipid | High biocompatibility | Potential immunogenicity |
| Adjustable compositions | Low encapsulation efficiency | |
| Easy surface modification and functionalization | ||
| Easy mass production | ||
| Polymer | Adjustable compositions | Potential immunogenicity and toxicity |
| Easy surface modification and functionalization | Low degradation | |
| Hybrid | Customizability of functions | Complex design and composition |
| Potential immunogenicity and toxicity |
4. Recent advanced nanoformulation-mediated mRNA immunotherapies in preclinical studies
4.1. Nano-mRNA targeting DCs
Cancer vaccines are an appealing immunotherapeutic approach to stimulate the immune system to produce antigen specific immune response against cancerous cells [126,127]. Such vaccines typically consist of exogenous well-defined tumor specific antigens (TSAs) or TAAs and immunogenetic adjuvants that can efficiently activate DCs and boost immune response against tumor antigens [126]. APCs comprise a heterogenous group of immune cells that can process and present endogenous and exogenous antigens via the major histocompatibility complex (MHC) molecules, which is essential for initiating the interaction between innate and adaptive immunity. DCs are the main subtype of APCs with a key function of priming T cell immunity. Therefore, the advancements in cancer vaccines focus on targeted delivery of antigens and adjuvants to DCs [21,22,24]. T cells rely on DCs to capture and transport the antigens to the draining lymph nodes (dLNs) from the immunization sites. Generally, the captured exogenous protein or peptide antigens are processed in endo/lysosomes and then presented on MHC class II molecules, which can be recognised by TCRs (T-cell receptors) of CD4+ Th cells [128]. By contrast, CTLs are restricted to MHC class I presented antigens [129]. The mRNA cancer vaccine platform promotes MHC class I antigen presenting pathway by endogenously translating encoded tumor antigens, thereby effectively priming CTLs [130], which are the key mediators for tumor eradication. Beyond that, mRNA itself can be sensed by TLRs of DCs, showing inherent immunogenicity to boost immune response of mRNA vaccines against antigens [131]. Apart from these characteristics, mRNA strategy is versatile and can be used to encode multiple peptides or proteins, not only providing tumor antigens but also offering additional molecule adjuvants to peripheral or lymphatic DCs [132]. The mRNA platform also shows potential in in situ vaccination, by reprogramming DCs to restore their functions in antigen presentation and T cell priming [133]. The success of this deliberate mRNA strategy heavily relies on the powerful delivery systems, promoting the translation of mRNA in vivo [16]. Transformative advances in nanoformulations have demonstrated their unique capability in harnessing the potential of mRNA cancer vaccines in preclinical and clinical studies [15].
4.1.1. Ex vivo nano-mRNA DC vaccines
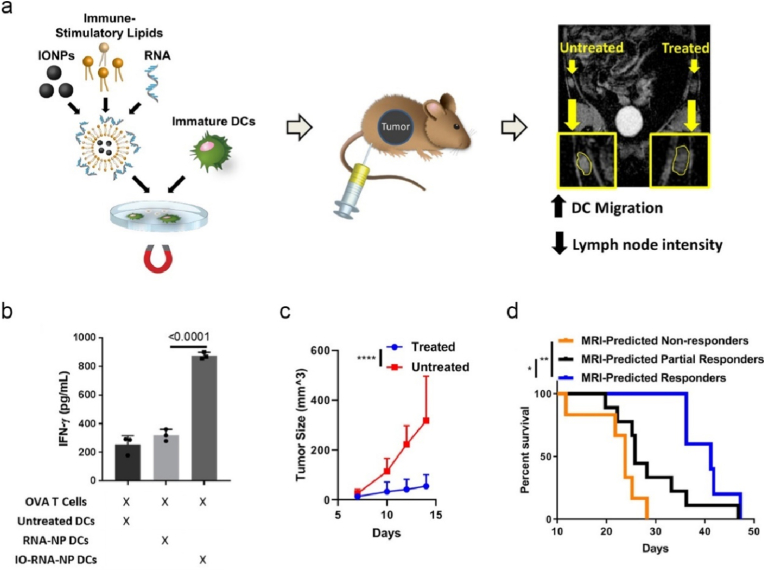
DC vaccines are generated ex vivo prior to infusion back patients, where the isolated DCs are appropriately activated by adjuvants and loaded with tumor antigens [134]. DCs are often stimulated with whole tumor lysate derived from debulking surgery to prime the immune system of patients [135], while this strategy might potentially induce a tolerogenic transformation caused by immunoregulatory cytokines. DC vaccines loaded with nano-mRNA encoding TAAs or TSAs offer a complementary approach [136]. In term of tumor antigens, ovalbumin (OVA) derived from egg white is commonly used as model antigens in preclinical studies of cancer immunotherapy, which can sensitize immune reactions and elicit antigen specific antibodies (humoral) and T cell (cellular) immune response. To enhance the potency of nano-mRNA DC vaccines, Mitchell and colleagues screened cationic liposomes for optimized DC activation and antigen translation ex vivo, and then engineered hybrid nanoformulations by incorporation of commercially available iron oxide nanoparticles (IONPs) and OVA-mRNA in the optimized liposomes (denoted as IO-RNA-NPs, Fig. 6a) [137]. Under magnetic field, IO-RNA-NPs enhanced DC activation and T cell priming ex vivo (Fig. 6b). Mice received IO-RNA-NPs-loaded DC vaccine and adoptive OT-I T cells with transgenic TCRs that specifically recognise MHC I restricted OVA peptide, significantly inhibited the growth of B16F10-OVA melanoma. The incorporated IONPs enabled tracking DC migration from the injection site to dLNs under magnetic resonance imaging (MRI) (Fig. 6a), which acted as predictors in anti-tumor performance of DC vaccines (Fig. 6c and d). The potential of vitamin E-scaffolds/lipoplex/cationic peptide-delivered OVA-mRNA was explored in the development of ex vivo DC vaccine against E.G7-OVA lymphoma in mice [138]. Instead of pulsing DCs with mRNA encoding tumor antigens, Moghaddam and co-workers engineered DCs stimulated with chitosan nanoparticles-mRNA encoding costimulatory molecules of CD40 and ICOSL (inducible costimulator ligand) and isolated tumor cell mRNA, which enabled T cell proliferation and significant inhibition of 4T1 mouse breast tumor post intratumor injection [139].
Fig. 6.
Cationic liposome/IONPs promote OVA-mRNA translation, ex vivo DC maturation, tracking DC vaccines in vivo with MRI, thereby effectively predicting anti-tumor effects. (a) Schematic illustration of the synthesis of IO-RNA-NPs, activating DCs ex vivo under magnetic field, and monitoring trained DC migration from the injection site to dLNs with an MRI-instrument. (b) Ex vivo DCs pre-treated with IO-RNA-NPs or RNA-NPs were co-cultured with OVA experienced T-cells for 48 h. IFN-gamma levels were measured by enzyme-linked immunosorbent assay (ELISA). C) Average B16F10-OVA tumor growth curves of mice received IO-RNA-NPs loaded-DC vaccine and adoptive OT-I T cells, and untreated mice. (d) Survival curves of mice grouped based on the response prediction by MRI data Reprinted with permission from Ref. [137]. Copyright 2019, American Chemical Society.
4.1.2. Nano-mRNA therapeutic cancer vaccines activating TLRs
The clinical efficacy of ex vivo generated DC vaccines are often comprised by the poor control on their migration in vivo to the therapeutic sites. In contrast, the advancement in nanoformulations allowed targeting delivery of nano-mRNA cancer vaccines to endogenous DCs in lymphoid organs. Sahin who is known for BioNTech company and COVID-19 vaccine (BNT162b2) and his team discovered that the surface charge of lipoplex-mRNA determined its biodistribution post intravenous administration [21]. Tuning lipoplex-mRNA to be slight negative or neutral led to exclusive target to splenic DCs as well as macrophages, thereby substantially promoting translation of encoded tumor antigens in DCs and inducing profound expansion of antigen specific T cells. Lipoplex-mRNA demonstrated universal and potent anti-tumor capability in multiple aggressive murine tumor models, including B16-OVA and B16F10-Luc (luciferase) melanoma, CT26 and CT26-Luc colon carcinoma, and TC-1-Luc cervical cancer [21]. Mechanically, lipoplex-mRNA activated splenic DCs via TLR-7 signalling pathway, leading to type 1 IFNα secretion and cooperation of innate and adaptive immune response against tumor. The preliminary data of this intriguing nano-mRNA platform in 3 patients with advanced malignant melanoma displayed supportive results of systemic IFNα secretion and amplification of T cells against encoded tumor antigens. The full report on the results of lipoplex-mRNA clinical trial (NCT02410733) will be introduced in Section 5.
Instead of priming endogenous T cells, Sahin team demonstrated a great success of lipoplex-mRNA vaccine in promoting the proliferation of adoptively transferred chimeric antigen receptor (CAR)-T cells to treat solid tumors [34]. CAR-T cell therapy has shown clinical success in curing patients with B-cell malignancies [140,141], while remains challenging in the treatment of patients with solid tumors [142]. To increase cancer specific targets and expansion of CAR-T cells, Sahin team engineered a lipoplex-mRNA vaccine encoding TAAs of claudin (CLDN) 6 (denoted as CLDN6-LPX), which showed effective expression of CLDN6 in ex vivo human DCs under an optimal dose of 100 μg/ml. Following intravenous administration, CLDN6-LPX enabled targeted transfection of splenic CD8+DCs, CD8-DCs, and F4/80+ macrophages, providing proliferation signals to engrafted CLDN6-CAR-T cells against established solid OV-90 human ovarian cancer in immunodeficient NSG (NOD scid gamma) mice. Following engraftment, the adoptive CLDN6-CAR-T cells alone even at a high dose tended to decline rapidly in vivo, while repeated vaccination with CLDN6-LPX maintained CLDN6-CAR-T cells at therapeutic levels, even at an initial low dose. The kinetic expansion results explained the superior capability of vaccine-stimulated CLDN6-CAR-T cells in the treatment of solid tumors. This fascinating nano-mRNA platform in combined with CLDN6-CAR-T cell therapy currently is under clinical studies, which will be discussed in next section.
LNPs not only enable protection and targeting delivery of mRNA to endogenous DCs, but also offer self-adjuvancity via activation of TLRs that are not associated with mRNA sensing [25]. Xia and colleagues screened a library of cationic LNPs via the interaction of poly(-amidoamine) (PAMAM) and epoxide consisting of different R structures [25]. The optimal C1 LNP containing C12 tail enabled effective targeting of mRNA to dLNs, promoted encoded antigen expression and presentation via stimulating TLR4 pathway and secreting proinflammatory cytokines (such as IL-12 and IL-1β). Mice immunised with C1 LNP-mRNA encoding OVA exhibited potent anti-tumor immunity in B16-OVA tumor model.
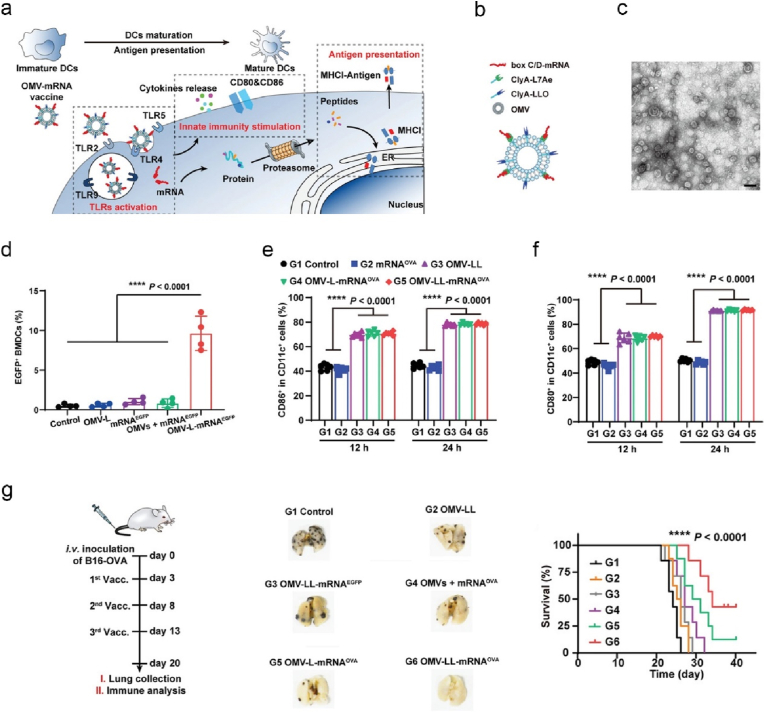
To complement LNPs, OMVs have been exploited as delivery vehicles in the development of potent cancer vaccines [143]. OMVs possess heterogeneity in size and composition, and immunomodulatory features endowed by abundant PAMPs [143,144]. Very recently, Nie and colleagues first explored the potential of OMV-platform derived from E. coli in targeted delivery of mRNA cancer vaccines to endogenous DCs and stimulating DCs via multiple TLRs (Fig. 7a) [24]. The authors genetically engineered OMVs with surface expressed L7Ae (RNA binding protein) and listeriolysin O (lysosomal escape protein) (denoted as OMV-LL, ∼30 nm in diameter) (Fig. 7b and c). OMV-LL enabled antigen expression (Fig. 7d) and cross presentation, and DC maturation (Fig. 7e and f). Mice vaccinated with OMV-LL-mRNA encoding OVA or ADPGK (ADP-dependent glucokinase) exhibited remarkable regression of B16-OVA metastatic melanoma (Fig. 7g) or MC38 colon cancer, respectively.
Fig. 7.
An OMV-mRNA vaccine activates DCs via multiple TLR receptor pathways, and promotes cross-presentation of expressed antigens, thereby exerting profound anti-tumor effect. (a) A scheme for DC activation mechanism mediated by OMV-mRNA vaccine. (b) Schematic diagram of the composition of OMV-mRNA. (c) A TEM (transmission electron microscope) image of L7Ae-modified OMVs. Scale bar: 50 nm (d) The percentages of enhanced green fluorescent protein (EGFP)+ BMDCs (bone marrow-derived dendritic cells) transfected with different mRNA nanoformulations as indicated. (e–f) The maturation markers of CD86 (e) and CD80 (f) expressed on CD11c + DCs were analysed by flow cytometry after incubated with various nanoformulations. (g) The experimental timeline of mice inoculated intravenously with B16-OVA cells and vaccinated with different mRNA nanoformulations. Shown are the representative digital photos of resected lungs with tumor nodules and survival curves of mice [24].
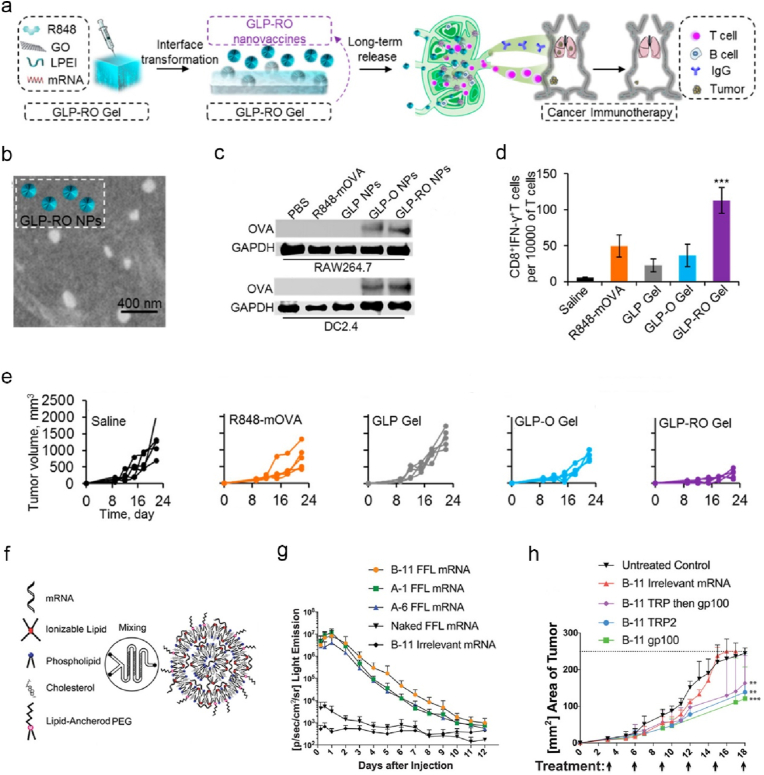
Direct incorporation of TLR agonists provides an alternative approach to promote immunogenicity of nano-mRNA cancer vaccines [119,[145], [146], [147]]. Wang lab engineered a transformable hydrogel that was formed by small graphene oxide nanoparticles and low molecular weight PEI (denoted as GLP-RO Gel, Fig. 8a), to deliver a nano-mRNA vaccine consisting of R848 (TLR7/8 agonist), and OVA encoding mRNA (denoted as GLP-RO, Fig. 8b) [145]. The hydrogel enabled sustained release of GLP-RO nanoparticles, effective OVA expression in DCs in vitro (Fig. 8c), and targeted delivery to skin dLN-DCs post subcutaneous injection. Mice immunised with GLP-RO Gel induced strong systemic functional CD8+ T cells against OVA (Fig. 8d), thus significantly regressed B16-OVA tumor (Fig. 8e). Blankschtein and co-workers developed PEG-LNPs to deliver mRNA encoding melanoma TAAs (TPR2 (terminal protein region 2) and gp100 (glycoprotein 100)) and lipopolysaccharide (LPS, TLR4 agonist) (Fig. 8f) [146]. The optimized B11 LNPs among three promising candidates (B-11, A-1, and A-6) that were screened from a lipid library with various components, induced the strongest antigen specific CD8+ T cells, and effectively increased translation of mRNA encoding firefly luciferase (FFL) at the injection sites in mice (Fig. 8g). Along with lipid component optimization, mRNA modification was also optimized. Unmodified mRNA potentially activates TLRs 3, 7 and 8 [148], which could enhance vaccine immunogenicity but also hinder the translation and promote degradation of mRNA [149]. To address these issues, the authors designed modified mRNAs replaced with ψ and 5-mC. The optimized B11-modified mRNA displayed potent therapeutic effects against B16F10 tumor in mice (Fig. 8h). Beyond these representative platforms, the potential of other types of LNPs were also explored by co-delivering of mRNA encoding tumor antigens and TLR agonists to endogenous DCs [119,147].
Fig. 8.
Nanoformulations incorporated with TLR7/8 or TLR4 agonists target dLN-DCs, promoting translation of mRNA and anti-tumor T cell immunity. (a) Illustrated is the preparation of GLP-RO nano-mRNA vaccines in the transformable hydrogel and associated mechanism for enhanced cancer immunotherapy. (b) A SEM (scanning electron microscope) image of released GLP-RO nanoparticles. (c) Western blot analysis of OVA expression levels of RAW264.7 and DC2.4 cells treated with different formulations as indicated. (d, e) Splenic CD8+IFN-γ+ T cells (d) and individual B16-OVA tumor growth curves (e) of mice received different treatments as indicated. (f) A schematic illustration of the construct of optimized LNP-mRNA. (g) Kinetic expression profiles of FFL at the injection sites of mice received different nano-mRNA formulations as indicated. (h) Average B16F10 tumor areas of mice treated with different nano-mRNA formulations as indicated Reprinted with permission from Refs. [145,146]. Copyright 2017 and 2021, American Chemical Society.
4.1.3. Nano-mRNA therapeutic cancer vaccines activating STING pathway
Type I IFNs consisting of IFNα and IFN-β, can potentiate the induction of CD8+ T cell immunity of mRNA vaccines [150]. Thus, it is desired in the development of nano-mRNA cancer vaccines that mRNA platforms or nanoformulations are capable to induce strong type I IFNs for enhanced function and durability of CTLs against cancer. TLRs signal activates the transcription factor myeloid differentiation primary response 88 (MyD88), inducing production of inflammatory cytokine IFNα [151]. Different from RNA sensing TLRs, STING is activated by cytosolic dsDNA (double stranded DNA) in response to viral infection [152]. STING signalling plays a central role in production of type I IFNs in innate immune system [152]. STING now is an appealing target for therapeutic cancer vaccines and cancer immunotherapy [153]. Anderson and co-workers engineered LNPs containing heterocyclic amine groups (A18), which enabled targeted activation of dLN-DCs via STING pathway, mRNA translation, subsequent secretion of type 1 IFNs and initiation of T cell priming. Mice vaccinated with A18 LNP-mRNA cancer vaccines showed profound therapeutic effect in multiple murine tumor models. In addition to LNP-mediated STING activation, Huang and co-workers designed a mRNA encoding active mutations in STING (V155M) [154] that caused hyperactivity in STING activation, which was encapsulated into LNPs together with HPV-E6/E7 antigen mRNA [155]. This nanoformulation enabled potent secretion of type I IFN cytokines via STING pathway, which in turn increased the expression and cross presentation of HPV-E6/E7 and subsequent proliferation of CD8+ T cells against E7. Vaccination with LNP-antigen mRNA-STINGV155M significantly regressed HPV + TC-1 tumor and prolonged the survival of mice.
4.1.4. Nano-mRNA therapeutic cancer vaccines activating the receptors of polysaccharides
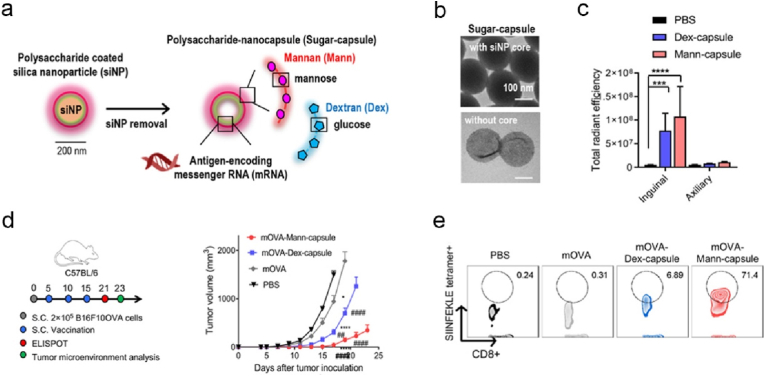
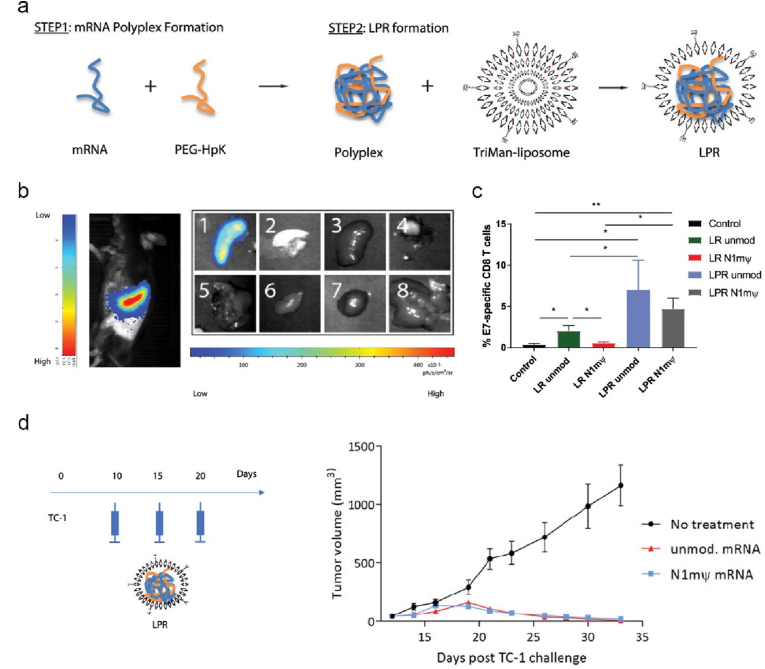
PRR activation is critical to establish a pro-inflammatory milieu and initiate the cross-talk between non-specific innate and specific adaptive immune response, thus the strategies targeting PRRs have been harnessed in the development of therapeutic cancer vaccines [126]. Among PRRs, TLRs and STING have been extensively explored, while the immunology of CLRs (C-type lectin receptors) and their immunostimulatory potentials in vaccine development, particularly in nano-mRNA cancer vaccines, have been much less investigated. In response of fungal infection, CLRs will be activated by sensing cell wall polysaccharides, leading to potent cytokine production and subsequent development of humoral and cellular immune response. Polysaccharides β-glucans and mannans can activate CLRs Dectin-1 and Dectin-2, respectively, while these CLRs are not the sole receptors for the defined polysaccharides, depending on their source and physical properties (such as soluble or particulate) [156,157]. Thus, the studies on polysaccharides-based formulations in nanoscale for mRNA delivery would provide new understandings on their receptor signalling and associated vaccine immunological activity, which potentially offers guidelines in engineering advanced nano-mRNA systems. For this reason, Moon and colleagues engineered sugar nanocapsules (Fig. 9a and b) by coating silica nanoparticles (around 200 nm, Fig. 9b) with mannan (Mann) or dextran (Dex) and core removal, to deliver antigen mRNA with the aid of PEI [158]. Mann-capsule and Dex-capsule were found activating DCs via Dectin-2/TLR4 and CD206/CD209 signalling pathways, receptively. Follow subcutaneous injection, Mann-capsule allowed more efficient drainage to dLNs (Fig. 9c) compared to Dex-capsule, potentially owning to the abundant mannan receptors on DCs. Mice vaccinated with Mann-capsule-mRNA encoding OVA antigen displayed substantially enhanced regression of B16-OVA than those with Dex-capsule-mRNA (Fig. 9d), which can be explained by the higher frequency of systemic and tumor infiltrating OVA-specific CD8+ T cells (Fig. 9e). In the formulation of nano-mRNA cancer vaccine, mannan showed superior capability over Dextran in targeting DCs and priming T cells. In another study, Pichon and colleagues decorated lipopolyplex with trimannans to deliver pseudouridine nucleoside modified mRNA (denoted as LPR, Fig. 10a), to reduce unmodified mRNA associated inflammatory sides effects while maintain the immunogenicity of modified mRNA. LPR enabled targeting splenic DCs (Fig. 10b) post intravenous administration [132]. Mice immunization with LPR delivered a mixture of mRNAs (encoding E7, and stimulatory molecules of CD40L, CD70, and TLR4) elicited strong antigen E7 specific CD8+ T cell immune response (Fig. 10c), and profound efficacy in the treatment of TC-1 tumor (Fig. 10d). Cytotoxic T cell response was dependent on type I IFN induced by LPR, irrespective of encoded stimulatory molecules. It would be interesting to explore the specific receptor signalling pathway activated by the unique trimannans-nanostructure.
Fig. 9.
mRNA-sugar-capsule decorated with mannan or dextran promotes DC maturation via the activation of multiple pattern recognition receptors, cross-presentation of translated antigens, thus elicits effective anti-tumor T cell response. (a) Illustrated is the preparation process of mRNA-sugar-capsule. (b) Representative TEM images of sugar-capsules. (c) Cy5.5 signal intensity of Cy5.5 (cyanine5.5) tagged Man-capsule or Dex-capsule in inguinal and axiliary LNs harvested from C57BL/6 mice post injection. (d) The experimental timeline of mouse B16F10-OVA model and the average tumor growth curves of mice received with different formulations. (e) Flow cytometry data showing the frequency of tumor-infiltrating OVA specific tetramer + CD8+ T cells. Reprinted with permission from Ref. [158]. Copyright 2020, American Chemical Society.
Fig. 10.
Trimannose decorated lipopolyplex targets splenic DCs for enhanced delivery and translation of modified mRNA, hence increases anti-tumor immunity. (a) Schematic illustration of the preparation of LPR nanoparticles. (b) Bioluminescence imaging revealed that FFL-LPR-mRNA targeted the spleens of mice at 24 h after systemic administration. (c) E7-specific CD8+ T cells of mice immunised with different nanoformulations as indicated. (d) An experimental timeline of a therapeutic TC-1 tumor model and average TC-1 tumor growth curves of mice vaccinated with different nanoformulations as indicated. Reprinted with permission from Ref. [132]. Copyright 2018, American Chemical Society.
To promote cytosolic delivery of mRNA vaccines in targeted DCs, inorganic calcium phosphate was incorporated into LNPs, followed by decoration with mannoses [124]. The designed hybrid nano-mRNA encoding antigen mucin 1 (MUC1) significantly inhibited the growth of breast cancer in combination with anti-CTLA-4 (anti-cytotoxic T lymphocyte-associated antigen 4) monoclonal antibody (mAb). The strategies targeting polysaccharide receptors expressed on APCs are a versatile approach in nano-mRNA vaccine paradigm.
4.1.5. Nano-mRNA therapeutic cancer vaccines enhanced by other therapeutic modalities
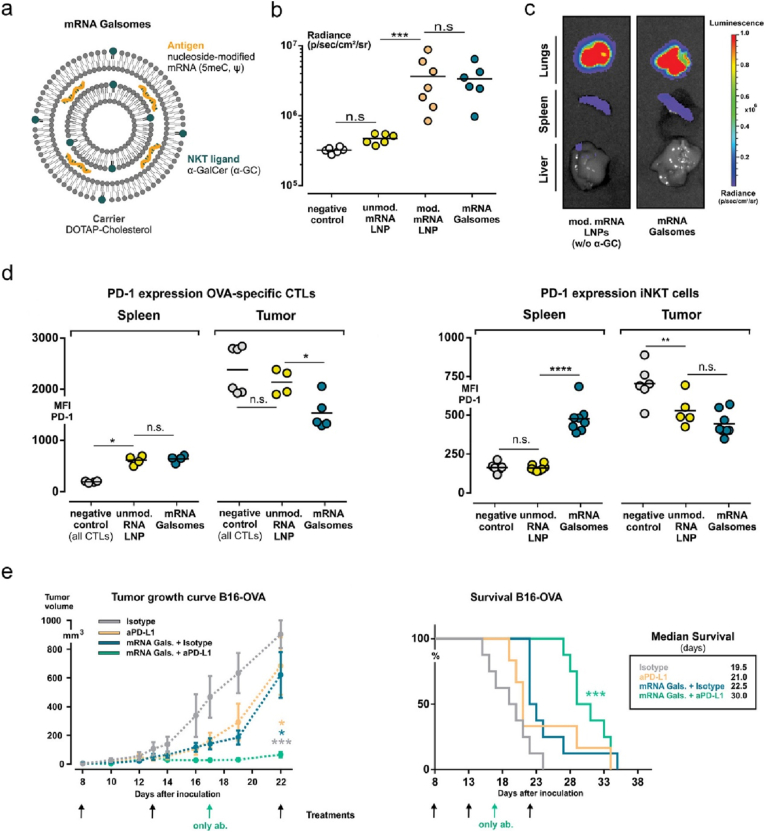
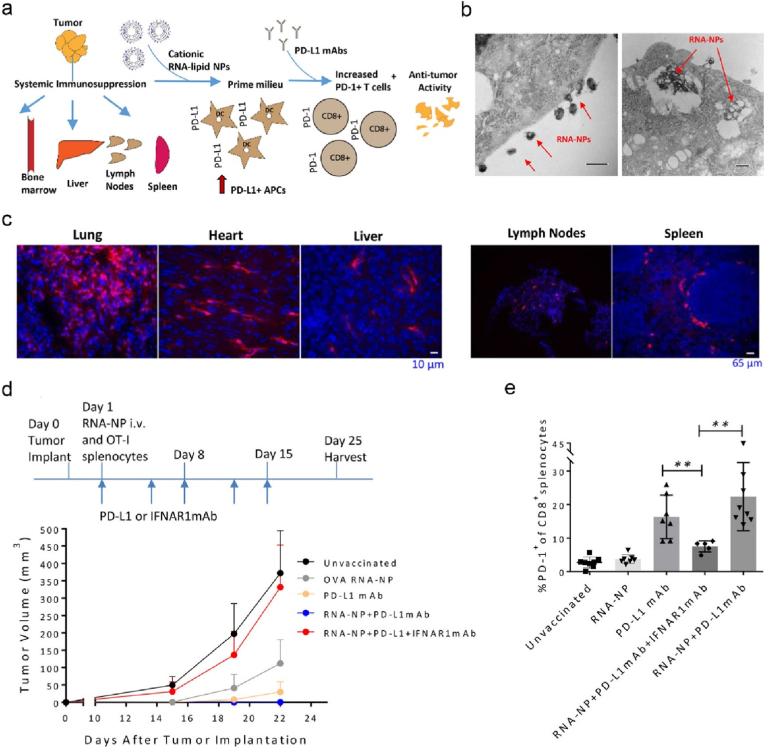
The discoveries of inhibitory markers on immune cells and associated pathway mechanisms that modulate the immune system against cancer led to the great success of non-antigen specific cancer immunotherapy, such as immune checkpoint inhibitors (ICIs). ICIs that block the inhibitory receptor of PD-1(programmed cell death protein 1) or CTLA-4 expressed on T cells have demonstrated potent clinical efficacy in the treatment of patients with advanced cancer. However, only a small fraction of cancer patients can benefit from this treatment modality. Low prevalence of tumor infiltrating T cells expressing such receptors could be one of the key factors for non-responders. Thus, vaccination with therapeutic cancer vaccines could potentially increase response rate of cancer patients to ICI treatment by expansion of antigen specific T cells. To this end, Dewitte and colleagues evaluated the potency of engineered nano-mRNA vaccine combined with anti-PD-1 mAbs in cancer treatment [159]. To increase the potency, the authors co-encapsulated α-GC (glucosidase) that activated iNKT (invariant NKT) cells and nucleoside-modified antigen mRNA in LNPs (denoted as mRNA Galsomes, Fig. 11a). The modified mRNA promoted its in vivo translation (Fig. 11b) and the nanoformulation of mRNA Galsomes enabled targeting splenic DCs post intravenous administration (Fig. 11c). Mice vaccinated with OVA mRNA Galsomes elicited strong systemic OVA specific CD8+ T cells, non-antigen specific iNKT and NKT cells, while these effector cells displayed upregulated expression of PD-1 after infiltrating into B16-OVA tumor sites (Fig. 11d). As expected, vaccination with OVA mRNA Galsomes significantly promoted the response of mice with B16-OVA tumor to anti-PD-1 mAbs therapy (Fig. 11e). It remains a challenge to identify TAAs for vaccine devolvement. For this reason, Mitchell lab developed a personalised nano-mRNA vaccine, where whole tumor derived mRNA molecules were loaded into LNPs (Fig. 12a) [160]. Follow intravenous injection, the nanoformulation systemically targeted the peripheral (such as lung, liver, and lymphoid organs, Fig. 12c) and tumor PD-L1(programmed cell death ligand 1)+ myeloid cells, most of which were PD-L1+ APCs (Fig. 12a). DC transfection by mRNA-LNP was visualised (Fig. 12b) with a high expression of PD-L1. Vaccination with this mRNA-LNP potentiated the therapeutic effect of anti-PD-1 mAbs against B16-OVA (Fig. 12d), owning to the significantly increased frequency of PD-1+CD8+ T cells infiltrated in tumor sites (Fig. 12e). In contrast, co-administration of anti-IFNα receptor 1 (IFNAR1) mAbs that block type I IFN receptor signalling led to abrogation of anti-tumor efficacy (Fig. 12d and e), suggesting that the therapeutic effect of mRNA-LNP was dependent on type I IFN.
Fig. 11.
Liposomes co-deliver nucleoside modified mRNA and immunopotentiator α-GC for enhancing antigen cross-presentation and iNKT activation. (a) Illustrated is the structure and composition of mRNA Galsomes. (b, c) The bioluminescence expression levels in the whole body (b) and major organs (c) in mice vaccinated with different fluc-mRNA nanoformulations. (d) Flow cytometry analysis of PD-1 expressed on OVA specific CTLs and iNKT cells harvested from spleen and tumor tissues of mice treated with different nanoformulations as indicated. (e) Average B16-OVA-tumor growth and survival curves of mice received different treatments. Reprinted with permission from Ref. [159]. Copyright 2019, American Chemical Society.
Fig. 12.
Personalised LNP-mRNA induces systematic DC maturation, and reverses the immunosuppression in TME, therefore enhances ICI cancer immunotherapy efficacy. (a) Schematic representation of anti-tumor mechanism of cationic lipid-tumor derived mRNA vaccine combined with anti-PD-L1 mAbs. (b) Representative TEM images of LNP-mRNA internalized by DC2.4 cells (scale bars: 500 nm). (c) In vivo distribution of LNP-mRNA encoding Cre (cAMP response element) in different organs. (d) The experimental timeline and B16F10-OVA tumor growth curves of mice received different nanoformulations as indicated. (e)The percentages of PD-1+CD8+ splenocytes derived from B16F10-OVA bearing mice Reprinted with permission from Ref. [160]. Copyright 2018, American Chemical Society.
Inorganic mesoporous silica nanoparticles were also explored their potential in delivery of mRNA cancer vaccines in combined with other therapeutic inhibitors [89]. The designed inorganic platform enabled the slow-release of loaded PKR (protein kinase R) inhibitor C16 that blockades the inhibitory factor of translation initiation, thereby improving antigen mRNA-translation in DCs and eliciting strong anti-tumor immunity.
Local radiotherapy promoted the capability of tumor associated DCs in sensing endogenous antigens from dying cancer cells, thereby potentiating the therapeutic effect of systematically administrated nano-mRNA cancer vaccines [[161], [162], [163]]. The combined strategy of nano-mRNA cancer vaccines with therapeutic immuno-molecules (e.g., ICIs [164]) or other treatment modalities represents a promising approach for potent and durable anti-tumor immunity, with some fascinating paradigms under clinical investigation [23].
4.2. Nano-mRNA targeting cytotoxic T cells and NK cells
Tumor antigen specific T cells and NK cells are two central cytotoxic effector cells in eliminating cancer. T cell-based therapy requires prior antigen exposure to target tumor cells by recognition of epitopes presented by MHC class I molecules on tumor cells. By contrast, NK cells that are a subtype of innate immune cells can recognise and target tumor cells with absent expression of MHC class I molecules or TAAs/TSAs. Instead of single receptor mediated recognition like CTLs, NK cells target the altered antigen MICA/B (MHC class I chain related proteins A and B) expressed by tumor cells via an array of stimulatory (such as NKG2D and NKp46) and inhibitory (such as NKG2A and KIRs) receptors. In fact, the inhibitory receptors were activated by MHC class I molecules, thereby avoiding undesired destruction on healthy cells.
Adoptive transfer of genetically engineered cytotoxic T cells have emerged as potentially curative options in patients with certain cancers, such as B-cell malignancies, making extraordinary contributions to new cancer treatment modalities in clinic [165]. In these personalised cancer immunotherapies, the isolated patient T cells are engineered with expression of CARs or TCRs ex vivo prior infusion back to patients, enabling the redirection of T cells against tumor cells by recognizing tumor specific antigens. Beyond T cells, CAR-based therapeutics have recently explored the potential in CAR-NK cell-based cancer therapy [166]. To reduce the complexity and high cost associated with ex vivo engineering strategy, the advanced nano-mRNA technology that allows specific target to T cells, has been investigated in engineering circulating T cells with CARs or TCRs in both preclinical and clinical studies.
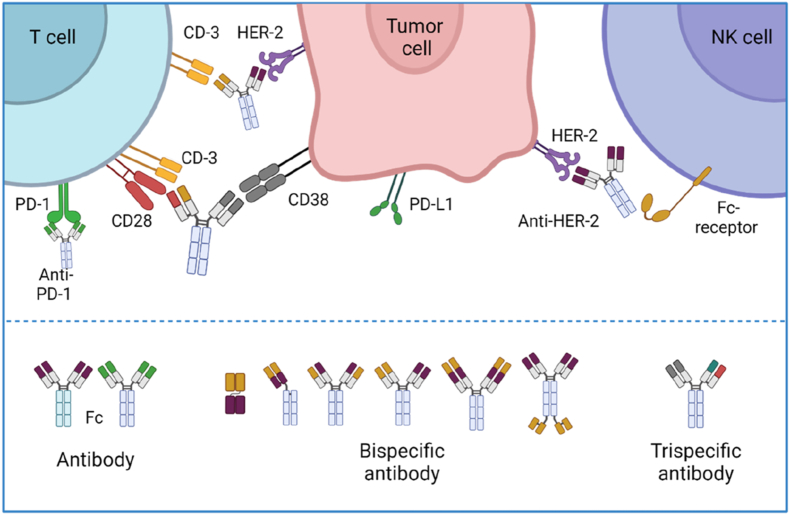
Along with CARs or TCRs therapies, engineered therapeutic antibodies (Fig. 13) with one or multiple targets offer an alternative efficacious approach to boost the therapeutic efficacy of T or NK cells against cancer, by enhancing the target of these immune cells to cancer cells in preclinical and clinical studies [167,168]. However, serum short life together with manufacturing challenges are the key factors limiting their far-reaching potential of T/NK cell-engaging specific antibodies. For this reason, Sahin and his colleagues have explored the potential of their nano-mRNA technology encoding antibodies in producing endogenous long-lasting therapeutic antibodies for the treatment of established large tumors [28]. The encouraging results from the clinical trials might pave the way to another breakthrough of nano-RNA technology in therapeutic mAbs-based cancer immunotherapy.
Fig. 13.
The action modes and representative structures of therapeutic antibodies. Monospecific, bispecific and triple-specific antibodies that target antigens expressed on cancer and/or NK or T cells enable increased engagement of cytotoxic T/NK cells with cancer cells, thereby promoting cancer cell lysis (top). Engineering strategies on the chain structures of therapeutic antibodies allow a wide range of biological functions (bottom). Created with permission by BioRender.
Far beyond the encoded therapeutics introduced above, the versatile and adaptable nano-mRNA could potentially replace a wide range of therapeutic immuno-molecules, such as IL-12 cytokines [121] or T cell inhibitory receptors [29], with improved local modulation of T/NK cell-mediated anti-tumor immunity.
4.2.1. Nano-mRNA engineering cancer-specific T cells
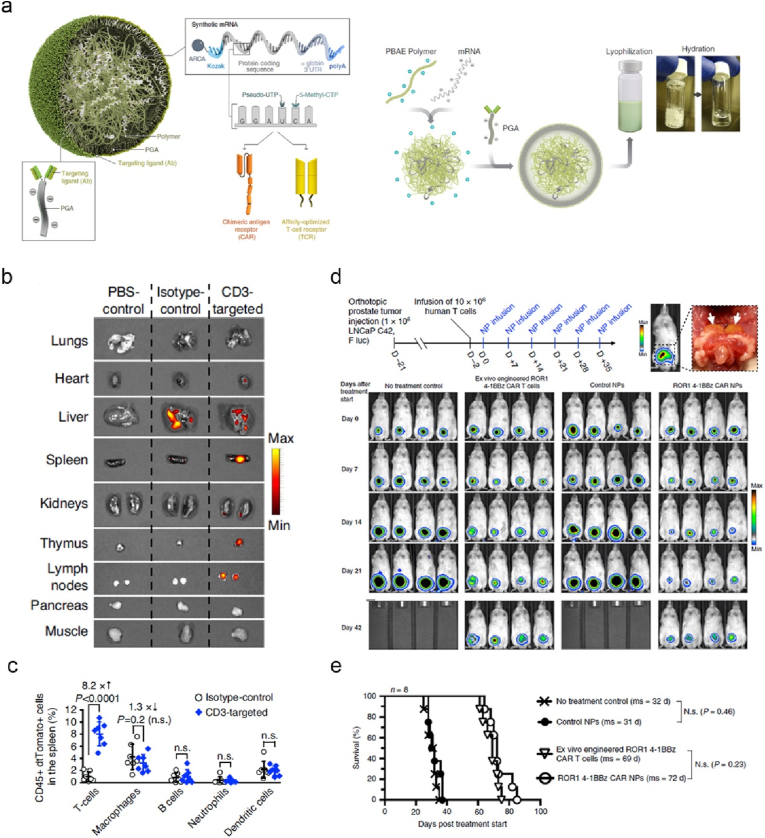
To enhance the potency of CAR-T cells, Stephan and colleagues developed cationic polymer PBAE nanoparticles modified with anti-CD3 and anti-CD28 mAbs for targeted translation of encoded transcription factor Foxo1 in human CAR-T cells in vitro, reprogramming the differentiation and phenotypic changes to memory CAR-T cells with competent functions [33]. The nano-mRNA engineered CAR-T significantly enhanced therapeutic activity against CD19+ human B-cell lymphoma established in NSG mice. To bypass T cell isolation and ex vivo culture of CAR-T cells, the authors recently utilised PBAE-mRNA technology for in vivo genetically engineering T cells with encoded CARs or TCRs (Fig. 14a) [32]. The decoration of anti-CD3 mAbs allowed active targeting to the spleen and lymph nodes (Fig. 14b), exclusively transfecting CD45+CD3+ T cells, but not APCs (Fig. 14c). The infusion of PBAE-mRNA engineered adoptive human T cells with CARs, showed profound regression of leukemia and solid LNCap C42 prostate tumor (Fig. 14d and e) in NSG mice with comparable efficacies to ex vivo engineered CAR-T cell therapy. To advance the implementation of this intriguing paradigm in clinic, the authors proposed to perform comprehensive biosafety evaluation in large animal models.
Fig. 14.
PBAE nanoparticles functionalized with anti-CD3 enable targeted delivery of CAR or TCR mRNA for reprogramming circulating T cells in vivo, thereby effectively recognizing and eliminating tumor cells. (a) Illustrated is the preparation process of PBAE-mRNA NPs. (b) Fluorescence imaging revealed dtTomato expression in the organs of mice after intravenous injection of different dtTomato-mRNA-formulations as shown. (c) Flow cytometry analysed the percentages of different types of transfected CD45+dtTomato + cells harvested from the spleen of mice. (d) The experimental timeline and representative bioluminescent images of mice with orthotopically transplanted luciferase-LNCap C42 prostate tumor and treated with different formulations as indicated. (e) Survival curves of mice received different treatments [32]. Reproduced under the terms of the Creative Commons CC BY license. Copyright 2020, The Author(s), published by Springer Nature.
4.2.2. Nano-mRNA engineering T cell stimulatory receptor
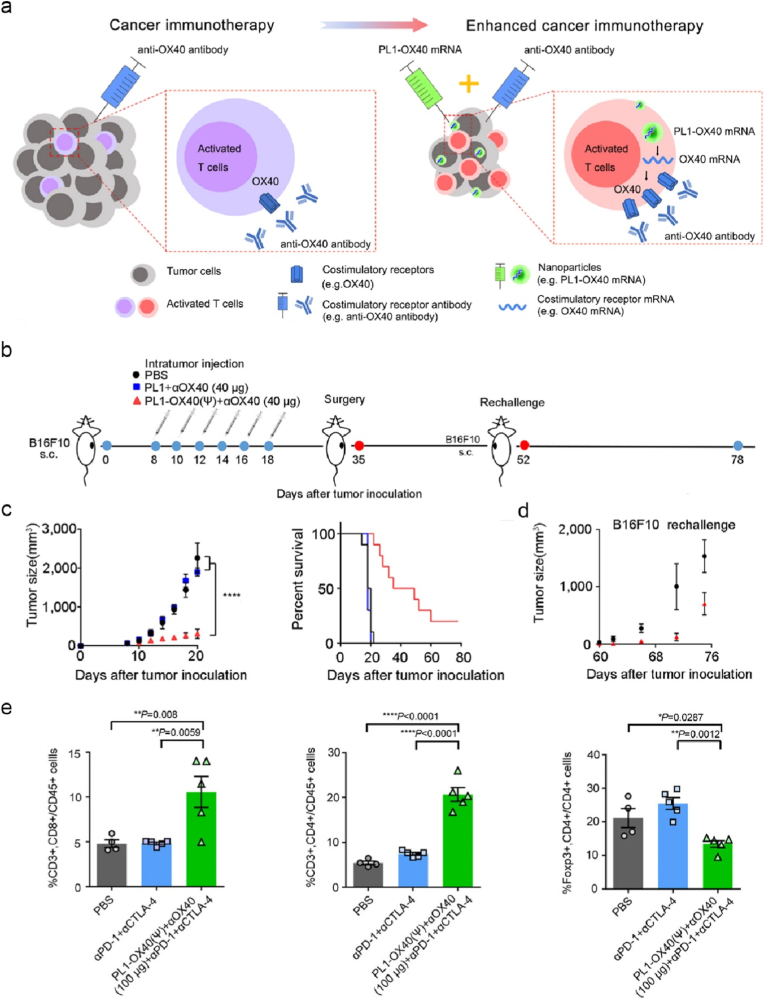
In contrast to ICI-based immunotherapies that target and obstruct tumor or T cell inhibitory receptor signalling pathway, agonistic mAbs against CD137 (4-1BB) or CD134 (OX40) promote the activation of T cell stimulatory receptors of 4-1BB or OX-40, thereby stimulating the proliferation and expansion of CD8+ T cells with profound anti-tumor activity in animal models [169]. Despite the success in preclinical studies, the low clinical efficacy (e.g., anti-4-1BB mAb agonist, Utomilumab) [170] and severe side effects (e.g., anti-4-1BB mAb, Urelumab) [171] have hampered their implementation in clinic. Several anti-OX40 mAb agonists entered clinical trials with a very low clinical activity [172]. To increase the therapeutic efficacy of anti-OX40 mAb agonists, Dong and co-workers engineered biomimetic phospholipid nanoformulations (denoted as PL1) for intratumor delivery of mRNA encoding OX40 (Fig. 15a) [98]. Intratumor injection of PL1-OX40 mRNA enabled upregulation of OX-40 expressed on tumor infiltrating T cells, which significantly promoted the therapeutic activity of anti-OX40 mAb against established B16F10 melanoma (Fig. 15b and c) or A20 tumor in mice, and enhanced the prevention immunity against the same tumor rechallenge (Fig. 15d). Mechanistically, the administration of PL1-OX40 mRNA substantially increased the infiltration of CD8+ and CD4+ T cells at tumor sites and decreased the ratio of Foxp3+ regulatory T (Treg) cells/CD4+ T cells (Fig. 15e). This nano-mRNA strategy was also applicable under systematic administration. Of note, the combination of PL1-OX40 mRNA and ICIs (anti-PD-L1 and anti-CTLA-4 mAbs) dramatically reduced tumor metastasis, indicating a potential treatment regimen in future clinical studies. Incorporating mRNAs encoding cytokines (such as IL-23 and IL-36γ) is an alternative strategy to promote the efficacy of nano-OX40 mRNA platform with long-term anti-tumor immunity [173].
Fig. 15.
Biomimetic phospholipid nanoparticles effectively deliver OX40 mRNA to tumor-infiltrating T cells with increased OX40 expression, thus potentiate the therapeutic effect of anti-OX40 mAbs against tumor. (a) Illustrated is the mechanism of PL1-OX40 mRNA nanoformulation for enhanced cancer immunotherapy of anti-OX40 mAbs. (b–d) Shown are the experimental timeline of B16F10 tumor bearing mice received different treatments as indicated (b), tumor growth profiles and survival curves of mice with primary tumor (c), and tumor sizes of rechallenged mice (d). (e) The percentages of CD8+, CD4+ T cells and Tregs infiltrated in A20-tumor derived from mice received different treatments as shown [98]. Reproduced under the terms of the Creative Commons CC BY license. Copyright 2021, The Author(s), published by Springer Nature.
4.2.3. Nano-mRNA expressing therapeutic antibodies
HER2 (human epidermal growth factor receptor type 2) overexpressed on breast cancer is associated with overall low survival rate. Trastuzumab is a clinically approved humanised antibody targeting HER2 for the treatment of invasive breast cancer [174]. Mechanistically, trastuzumab targets HER2 and abrogates the down-stream proliferative pathway of breast cancers. Additionally, trastuzumab induces NK-mediated ADCC (antibody dependent cell mediated cytotoxicity, Fig. 13), where Fc receptor expressed on NK cells interacts with Fc domain of trastuzumab, promoting NK activation and lysis of breast cancer cells. To improve the pharmacokinetic prolife of trastuzumab with increased serum lifetime, Anderson and co-workers engineered LNP-mRNA targeting liver for in vivo production of trastuzumab, showing profound therapeutic effect against HER2-positve human breast cancer [30]. This nano-mRNA paradigm was applicable in expression of other types of therapeutic antibodies in vivo, such as anti-human CD20 mAbs (rituximab) against non-Hodgkin's lymphoma [175], anti-PD-1 mAbs for the treatment of intestinal cancer [29].
Instead of monospecific antibodies, Sahin team engineered a lipid/polymer nanoformulation encapsulated with nucleoside-modified mRNA for in vivo production of CD3 × CLDN6 bispecific T-cell engager antibodies against established large tumors [28]. Post intravenous injection, the nano-mRNA enabled endogenous expression of encoded CD3 × CLDN6 bispecific antibodies in liver, with potent pharmacologically activity. NSG mice injected with three doses of lipid/polymer-CD3 × CLDN6 mRNA completely rejected the established large OV-90 human ovarian carcinoma (200–300 mm3). Abundant T cells were found infiltrated into CLDN6+ tumor, proving bispecific antibodies mediated strong T cell engagement with tumor cells for potent anti-tumor activity. Exogenous CD3 × CLDN6 bispecific antibodies was able to achieve comparable therapeutic efficacy but requiring as much as ten doses. The safety and potency of this nano-mRNA platform is currently evaluated under clinical trials (Please refer to next section for details).
4.3. Nano-mRNA targeting multiple immune cells in TME
Cytokines are stimulatory proteins that can target and modulate multiple subsets of immune cells by activating cytokine receptor downstream signalling. Clinically, administration of cytokines is one central approach to potentially increase the fraction of cancer patients responding to ICIs [176]. However, the systematic administration of stimulatory cytokines, such as IL-12, inevitably induces off-target associated toxicities [176,177]. Direct administration of cytokines into local sites is an alternative strategy with reduced toxicities to non-malignant regions. Luheshi and colleagues engineered non-toxic LNP-mRNA nanoformulation for intratumor injection in mice, inducing systemic immune response against primary and distant tumors [133]. The authors designed IL-12 mRNA incorporating the sequences of IL-12a and IL-12b together with a linker IL12p70, which was then formulated into LNPs. LNP-mRNA encoding IL-12 provoked strong anti-tumor immunity in a CD8+ T cell dependent manner. Locally administrated LNP-IL-12-mRNA in primary tumor sites induced systematic preventive immunity against distant tumor rechallenge in multiple tumor models (A20 murine B cell lymphoma, PD-L1 sensitive and resistant MC38 colon carcinoma). The secreted IL-12 showed advantages over membrane-tethered isoform of IL-12 in potentiating anti-PD-L1 mAbs. Anti-tumor immunity of LNP-IL-12-mRNA was correlated with promoted Th1 immunity in TME, though in-situ production of secreted IL-12 broadly activated DCs, NK and NKTs. To develop synergistic effect, LNPs locally delivered both IL-12 mRNA and IL-27 mRNA that targeted tumor cells and multiple immune cells (B cells, monocytes and macrophages), inducing robust tumor infiltration of cytotoxic T cells and NK cells against murine B16F10 melanoma [26].
In addition to local administration, systematic injections (such as intravenous and intraperitoneal injection) of nano-mRNA can target malignant tissues via ERP (enhanced permeability and retention) effect [35,178,179], or driven by the interactions between active targeting molecules and receptors expressed on tumor cells [180]. Following administration into bloodstream, it is crucial for the nanoformulation strategy to protect mRNA molecules from degradation during circulation and transportation period before entering tumor sites. For example, PEG are hydrophilic and neutral, which are often anchored on nanoformulations to increase the circulation time of nanoformulations, avoiding non-specific binding with proteins in serum components that can be recognised and cleared by innate immune cells (e.g., macrophages) [181]. Ionizable LNPs that are commonly used in clinical studies are neutral, which demonstrate enhanced circulation time and improved safety profiles compared with cationic LNPs [182]. Once ionizable LNPs enter acidic subcellular compartment of endosomes, they turn to be positively charged and promoted the release of mRNA molecules into the cytoplasm by fusing with endosomal membrane [183].
To modulate local immunosuppressive environment against cancer, nanoformulation strategies have been engineered for targeted delivery of mRNA encoded cytokines of interest into tumor sites, such as IL-12 [184], IL-15 [120]. Beyond cytokines-mediated immune modulation of TME, targeted nano-mRNA platform can translate other stimulatory proteins, dramatically altering the functions and crosstalk of tumor infiltrated immune cells towards anti-tumor immunity, such as transcription factors driving polarization of M1-type macrophages [31], proinflammatory chemokine CCL5 (C–C motif ligand 5) recruiting multiple leukocytes [27], combined co-stimulatory molecules of OX-40L/CD80/CD86 activating APCs and T cells [185].
4.4. Indirect targeting immune cells by nano-mRNA mediated immunogenetic cancer cell death
Along with direct modulation of immune cells in TME, nano-mRNA encoding functional proteins can indirectly trigger immune cells infiltration in tumor through reprogramming tumor cells. Clinical studies revealed that the loss of suppressor gene, such as PTEN (gene of phosphate and tension homology deleted on chromosome ten), in human cancers was significantly associated with immunosuppressive environment and resistance to immunotherapy of anti-PD-1 mAbs [186]. To understand whether PTEN restoration could promote immunogenetic cell death-mediated immune modulation at TME, Shi and colleagues developed polymeric nanoparticles encapsulated with PTEN mRNA (denoted as mPTEN@NPs, with a size of around ∼ 200 nm) to genetically reprogram cancer cells [179]. Following intravenous administration, mPTEN@NPs restored the expression of PTEN in PTEN-mutated B16F10 tumor, thereby inducing autophagy and associated immunogenic cell death. mPTEN@NPs enhanced the therapeutic effect against established B16F10 melanoma and prostate tumor. Mechanistically, cancer cell death induced the release of DAMPs (damage associated molecular patterns), which remarkedly activated DCs and T cells, thereby promoting T cell infiltration in tumor sites with increased response to anti-PD-1 mAbs.
The loss of p53 tumor suppressor gene was also found associated with therapeutic resistance to cancer immunotherapy. Recently, the same lab engineered polymer/lipid hybrid nanoparticles decorated with peptide CTCE to encapsulate p53 mRNA (referred as CTCE-p53 NPs, Fig. 16a and b), which enabled active targeting to chemokine receptor CXCR4 expressing hepatocellular carcinoma (HCC) [35]. Intravenously administrated CTCE-p53 NPs accumulated in orthotopic p53-null RIL-175 murine HCC and genetically restored the expression of p53 (Fig. 16c). CTCE-p53 NPs alone or combined with anti-PD1 mAbs exhibited a significant regression of established RIL-175 tumor (Fig. 16d). The restoration of p53 in HCC reversed the immunosuppressive environment, with increased secretion of inflammatory cytokines and infiltration of anti-tumor immune cells, such as M1 macrophages, effector T cells (Fig. 16e) and NK cells. Liposome-protamine hybrid nanoparticles were investigated as another promising delivery platform for in vivo targeting delivery of mRNA encoding survivin-T34A, inducing potent anti-tumor efficacy [187].
Fig. 16.
CTCE decorated p53 mRNA-nanoparticles restore the expression of encoded p53 protein in HCCs, secreting multiple cytokines that modulate immune cells for efficacious cancer treatment. (a) Illustrated are the composition of synthetic CTCE-p53 NPs and the mechanism of CTCE-p53 NPs evoking strong anti-tumor response. (b) A representative TEM image of CTCE-p53 NPs. (c) Immunofluorescence images of p53 knockout RIL-175 cells showed the expression of encoded p53 (scale bar: 50 μm). (d, e) p53 null RIL-175 tumor growth curves (d), and the percentages of tumor infiltrating CD3+CD8+ T cells and M2-like macrophages (e) of mice treated with different formulations as indicated [35]. Reproduced under the terms of the Creative Commons CC BY license. Copyright 2022, The Author(s), published by Springer Nature.
Immunosuppressive characteristics of specific type of tumor heavily rely on the crosstalk among malignant cells and heterogenous populations of immune cells. Genetically reprogramming tumor cells by nano-mRNA therapeutics provides a potential opportunity to remodel the distribution and function of immune cells infiltrated at TME for enhanced anti-tumor immunity.
Recent advanced nano-mRNA formulations that we introduced in preclinical studies for targeted cancer immunotherapy are summarized in Table 2. In the context of modulating tumor infiltrating immune cells or cancer cells, it is crucial to increase cancer selectivity of nano-mRNA, preventing potential chronic immune-related adverse events (irAEs) [188]. For example, nano-mRNA expressing anti-PD-1 or anti-CTLA-4 mAbs can concurrently inhibit non-cancer immune cells, augmenting autoimmune toxicities and increasing the incidences of irAEs. By precisely engineering their physiochemical properties, nano-mRNA formulations can selectively target the organs of interest, promoting their clinical implementation in targeted cancer immunotherapy (please refer to the last section for our discussion on this point).
Table 2.
Summary of advanced nanoformulations for targeted delivery of mRNA to specific subsets of immune cells.
| Targeted immune cells | Nanoformulations | mRNA encoded molecules | Size (nm) | Surface charge (mV) | Tumor models | Mechanisms | Therapeutic outcome | Administration routes | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Nano-mRNA targeting DCs | |||||||||
| Ex vivo DCs | Cationic liposomes/iron oxide NPs | OVA | 207.9 ± 67.2 | 44.1 ± 4.5 | B16F10-OVA murine melanoma | Activating DCs and promoting antigen expression | Suppressing tumor growth | i.d. | [137] |
| Ex vivo DCs | Lipoplex/vitamin E scaffold/peptide | OVA | / | / | E.G7-OVA murine lymphoma | As above | Suppressing tumor growth | s.c. | [138] |
| Ex vivo DCs | Chitosan NPs | CD40+ICOSL | 35 | / | 4T1 murine breast cancer | Activating DCs, promoting T proliferation and accelerating cytokine secretion | Suppressing tumor growth | i.t. | [139] |
| Splenic conventional DCs, pDCs and macrophage | Lipoplex | OVA; gp70; TRP-1; CT26-M90; HPV E6/E7 | 200–400 | / | B16-OVA and B16F10-Luc murine melanoma, CT26 and CT26-Luc murine colon carcinoma and TC-1-Luc murine cervical cancer | Activating DC and priming CD4+ and CD8+ T cells via TLR7-IFNα signaling pathway | Regression of tumor/suppressing tumor growth | i.v. | [21] |
| As above | Lipoplex | CLDN6 | / | / | xenograft OV-90 human ovarian cancer | Driving proliferation of adoptive CLDN6-CAR-T cells | Regression of tumor/suppressing tumor growth | i.v. | [34] |
| DCs | C1 LNP | OVA | 150 | 16.37 ± 0.404 | B16-OVA murine melanoma and MC38-OVA murine colon carcinoma | Activating DCs via TLR4 pathway | Suppressing tumor growth | s.c. | [25] |
| dLN-DCs, NK cells, macrophages and B cells | OMV/clyA-L7Ae/clyA-LLO | OVA; | 28.1 | / | B16-OVA murine melanoma and MC38 murine colon carcinoma | Activating DC via multiple TLR pathways | Regression of tumor/suppressing tumor growth | s.c. | [24] |
| ADPGK | |||||||||
| dLN-DCs | Hydrogel | OVA | 220 | −0.52 | B16-OVA murine melanoma | Sustained release of delivered R848 and mRNA for enhanced DC activation via TLR7/8 pathway | Suppressing tumor growth | s.c. | [145] |
| (PEI + GO) | |||||||||
| DCs | PLGA/lipid NPs | OVA | 400 | 20 | B16-OVA murine melanoma | Delivery of gardiquimod for DC activation via TLR7 pathway | Suppressing tumor growth | i.v. | [119] |
| dLN-DCs, neutrophils, macrophages, and B cells | LNPs | Gp100; TRP2; OVA; | 200 | −14.1–2.0 | B16F10 and B16-OVA murine melanoma | LNPs delivered modified mRNA and LPS for enhanced DC activation via TLR4 pathway | Suppressing tumor growth | s.c. | [146] |
| DCs | PEG/lipid/polymer NPs | OVA | 137 ± 2.8 | 11.2 ± 2.1 | E.G7-OVA murine lymphoma and RM1-OVA murine prostate cancer | Delivery of C16-R848 for enhanced DC activation via TLR7/8 pathway | Suppressing tumor growth | s.c. | [147] |
| dLN-macrophages, DCs, | Heterocyclic LNPs | OVA; | 100 | / | B16F10-OVA murine melanoma and TC1 murine cervical cancer | Activating DCs via heterocyclic amine group-mediated STING pathway | Suppressing tumor growth | s.c. | [22] |
| and monocytes | E7 | ||||||||
| DCs | LNPs | E6/7; STINGV155M | 80–100 | / | TC-1 murine cervical cancer | Activating DCs via hypersensitive STINGV155M pathway | Suppressing tumor growth | i.m. | [155] |
| DCs | Manna/PEI nanocapsules | OVA | 220 | 32 ± 0.5 | B16F10-OVA murine melanoma | Activating DCs via dectin-2/TLR-4 | Suppressing tumor growth | s.c. | [158] |
| Splenic DCs and macrophages | Trimannose/lipid/polymer hybrid NPs | OVA; | 230 | 45 | TC-1 murine cervical cancer and B16-OVA murine melanoma | Priming anti-tumor T cells via nanoparticle mediated-type I-IFN pathway | Regression of tumor/suppressing tumor growth | i.v. | [132] |
| E7; | |||||||||
| CD40L + CD70+ cATLR4 | |||||||||
| dLN-DCs | Mannose/lipid-calcium/phosphate NPs | MUC1 | 58 | 38 | 4T1 murine breast cancer | Activating DCs via mannose receptor pathway | Suppressing tumor growth | s.c. | [124] |
| Splenic DCs | α-GC/liposomes | OVA | 190 | 47 | B16-OVA murine melanoma and E.G7-OVA murine lymphoma models | Activating DCs, iNKT and NK cell | Suppressing tumor growth | i.v | [159] |
| Systematic PD-L1+ APCs | LNPs | Whole tumor antigens; | 70–200 | 40–50 | B16F10 and B16-OVA murine melanoma; glioma | Activating PD-L1+DC | Suppressing tumor growth | i.v. | [160] |
| OVA | |||||||||
| DCs | Mesoporous silica NPs | OVA; | 115 | / | E.G7-OVA murine lymphoma | Promoting mRNA translation by loaded PKR inhibitor C16 | Regression of tumor/suppressing tumor growth | s.c. | [89] |
| GM-CSF; | |||||||||
| Lymphoid organ resident DCs | Lipoplex | PME1 | 340 | / | CT26 murine colon carcinoma | Local radiotherapy induced CD8+ T cells combined with nano-mRNA induced CD4+ T immunity for enhanced therapeutic effect | Regression of tumor/suppressing tumor growth | i.v. | [161] |
| DCs | Lipoplex | E7 | 200–250 | −20–30 | TC-1 murine cervical cancer | Activating DCs and inducing antigen-specific effector and memory CD8+ T cells | Regression of tumor/suppressing tumor growth | i.v. | [164] |
| DCs | Lipoplex | E7 | 200–250 | −20–30 | TC-1 murine cervical cancer | Local radiotherapy promoted nano-mRNA vaccine efficacy | Regression of tumor/Suppressing tumor growth | i.v. | [162] |
| DCs | Protamine | OVA | / | / | E.G7-OVA murine lymphoma | As above | Suppressing tumor growth | s.c. | [163] |
| Nano-mRNA targeting cytotoxic T cells and NK cells | |||||||||
| T cells | Anti-CD3/CD28 mAbs/PbAE/PGA(polyglycolic acido) polymer NPs | Foxo1 | 109.6±/26.6 | 1.1 ± 5.3 | Raji murine lymphoma | Reprogramming CAR-T cells to a memory phenotype with competent functions | Suppressing tumor growth | i.v. | [33] |
| T cells | Anti-CD8 mAbs/PbAE/PGA polymer NPs | CARs; TCRs | 106.9 ± 7.2 | / | Eμ-ALL01 leukemia, | In vivo reprograming of circulating T cells with CARs or TCRs | Suppressing tumor growth | i.v. | [32] |
| Raji lymphoma, LNCaP C42 prostate cancer and HepG2 hepatitis B-induced hepatocellular carcinoma models | |||||||||
| T cells | Phospholipid NPs | OX40; | 120–230 | 8–36 | B16F10 murine melanoma, A20 B cell lymphoma | Promoting T cell activation and therapeutic response to anti-OX40mAbs | Regression of tumor/Suppressing tumor growth | i.t./i.v. | [98] |
| CD137 | |||||||||
| T cells, macrophages, monocytes, granulocytes, DCs, tumor cells | LNPs | IL-23, IL-36γ, OX40L | / | / | H22 murine hepatocellular carcinoma, MC38 murine colon carcinoma, and B16F10-AP3 murine melanoma | Promoting the infiltration of multiple immune cells into tumor sites via cytokine- and OX-40L-mediated pathways | Regression of tumor/Suppressing tumor growth | i.t./s.c./i.d. | [173] |
| NK cells | LNPs | Anti-HER2 mAbs | / | / | mouse xenograft MDA-MB-231-HER2 human breast cancer | Enhancing NK-mediated ADCC | Suppressing tumor growth | i.v. | [30] |
| T cells | LNPs | Rituximab antibody | / | / | mouse xenograft human Raji lymphoma | CDC and ADCC | Regression of tumor/Suppressing tumor growth | i.v. | [175] |
| T cells | LNPs | Anti-PD-1 mAbs | / | / | MC38 murine colon carcinoma | Activating T cells by in vivo expression of therapeutic anti-PD-1 mAbs | Suppressing tumor growth | i.v. | [29] |
| T cells | Lipid/polymer NPs | CD3× CLDN6 bispecific antibodies | / | / | mouse xenograft OV-90, ES-2, ES-2/hCLDN6 human ovarian cancer | Bispecific T cell-engager mediated cytotoxic T cells to tumor cells | Regression of tumor | i.v. | [28] |
| Nano-mRNA targeting multiple immune cells in TME | |||||||||
| T cells, NK cells, macrophages | LNPs | IL-12a + IL12b | / | / | MC38-S and MC38-R murine colon carcinoma, B16F10-AP3 melanoma and A20 B cell lymphoma. | Driving Th1 immunity and activating DCs, T cells and NK cells | Regression of tumor/Suppressing tumor growth | i.t. | [133] |
| PDX model: ME 12057, ME 12058, HN 5111, HM5116 | |||||||||
| T cells, DCs, NK cells | LNPs | IL-12, IL-27, GM-CSF | 150–200 | / | B16F10 murine melanoma | Inducing robust infiltration of NK and CD8 T cells in tumor | Suppressing tumor growth | i.t. | [26] |
| T cells, NK cells, macrophages | LNPs | IL-12 | / | / | MYC-driven murine hepatocellular carcinoma | Promoting tumor infiltrating activated Th cells and production of IFN-γ | Suppressing tumor growth | i.v. | [184] |
| NK cells, T cells, | liposome-protamine complex | IL-15 | 221.33 ± 2.52 | 48.03 ± 1.429 | C26 murine colon cancer | Enhancing proliferation of NK, B, and T cells in tumor | Suppressing tumor growth | i.p./i.t/i.v. | [120] |
| TAMs, monocytes, DCs, neutrophils | Di-mannose/PbAE/PGA polymer NPs | IRF5 and IKKβ | 99.8 ±/24.5 | 3.40 ± /2.15 | ID8 murine ovarian cancer, B16F10 melanoma, and glioblastoma | Driving the polarization from M2-like macrophages to M1-like macrophages in tumor | Suppressing tumor growth | i.p./i.v. | [31] |
| T cells, DCs, NK cells | LNPs | CCL5 | / | / | B16F10 murine melanoma | CCL5 chemokine mediated recruitment of multiple | Suppressing tumor growth | i.t. | [27] |
| Leukocytes in tumor | |||||||||
| Tumor cells, T cells, DCs, macrophages | Charge-altering releasable transporters (CART) | CD70, OX40L, CD80, CD86, IL-12, IFN-γ | / | / | A20 murine B cell lymphoma and CT26 murine colon carcinoma | Cytokine mediated robust local T-cell activation and systematic immune response | Regression of tumor/Suppressing tumor growth | i.t. | [185] |
| Indirect targeting immune cells by nano-mRNA mediated immunogenetic cancer cell death | |||||||||
| Tumor cells | Lipid/mPEG-PLGA/G0-C14 hybrid NPs | PTEN | 111.8 ± 15.3 | / | Pten-mutated B16F10 murine melanoma and Pten-null/Pten-Cap8 murine prostate cancer | Inducing autophagy of cancer cells and release of immunogenic DAMPs | Suppressing tumor growth | i.v. | [179] |
| Tumor cells | Lipid/PLGA/G0-14/DSPE-PEG/CTCE NPs | p53 | 110 | negative | p53-null RIL-175 murine hepatocellular carcinoma | Promoting anti-tumor immune cells and decreasing pro-tumor immune cells | Suppressing tumor growth | i.v. | [35] |
| Tumor cells | liposome-protamine lipoplex | survivin-T34A | 186.1 ± 3.1 | / | C26 murine colon cancer | Inducing caspase-dependent cell apoptosis, and modulation of tumor microenvironment | Suppressing tumor growth | i.p./i.t./i.v. | [187] |
Note: i.v.: intravenous injection; i.m.: intramuscular injection; s.c.: subcutaneous injection; i.d.: intradermal injection; DSPE: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine.
Despite immense potential of nano-mRNA strategies in cancer immunotherapy, their clinical translation has faced some obstacles with nanotoxicity being one of the most often-encountered challenges. Once mRNA delivery systems engineered to nanoscale sizes, these nanoformulations actively interact with biological systems, potentially resulting in damage to human body at cellular or intercellular levels. The associated nanotoxicity is accentuated by the chemical components, morphology, surface charge and softness of nanoformulations [189]. For example, inorganic nanoparticles that can release metal ions (e.g., Cd2+. Mn2+) can promote the production of reactive oxygen species (ROS) via biochemical reactions, causing ROS-mediated cell damage [189]. Increased accumulation of Mn2+ can also trigger nanotoxicity in central nervous system, such as Parkinson diseases [190]. To capsulate negatively charged mRNA, nanoformulation delivery systems often contain cationic components, which cause their extensive interactions with cellular components (such as endosome membrane) and subsequent apoptotic cell death [191]. Beyond nanotoxicity, cationic nano-mRNA delivery systems potentially induce pro-inflammatory responses [192]. As discussed above, ionizable LNPs are one of the most promising candidates in clinical studies, which could potentially reduce nanotoxicity caused by cationic LNPs. The candidates with excellent safety profile and therapeutic efficacy have entered clinical trials, which will be discussed in next section.
5. Progress of clinical trials
The first clinical trial of mRNA therapeutics was conducted in patients with breast cancer (NCT00003432) in 1998, where patients received DC vaccines pulsed with naked mRNA expressing carcinoembryonic antigen for evaluating cancer immunotherapy efficacy. In the context of nano-mRNA immunotherapeutics, most clinical trials have focused on cancer vaccines, of which protamine-delivered mRNA vaccines were at advanced clinical development (Table 3). The early studies on mRNA vaccine delivery using protamine nanoformulations revealed that mRNA-protamine acted as adjuvants via activation of TLR7/8 pathway, induing balanced and potent cellular immunity after easy intradermal administration [193], while the translation efficacy of tightly bounded mRNA was moderate. To overcome this hurdle, CureVac developed RNActive® by combination of protamine-delivered mRNA and naked nucleoside modified mRNA with a ratio of 1:1. RNActive® encoding multiple TAAs were clinically evaluated in patients with metastatic prostate cancer, stage IIIB/IV NSCLC (non-small-cell lung cancer) or metastatic melanoma [[194], [195], [196], [197], [198]] (Table 3). This vaccination strategy alone or in combination with radiation therapy was proven well tolerated and immunogenic in patients under phase I/II clinical studies. The development of RNActive® was halted by current preference of LNPs delivered mRNA therapeutics in clinic.
Table 3.
Summary of representative nano-mRNA therapeutics for targeted cancer immunotherapy under clinical trials.
| Targeted immune cells | Nanoformulations | mRNA encoded molecules | Tumor types | Administration routes | Nano-mRNA name | Phase | Status |
NCT Number |
|---|---|---|---|---|---|---|---|---|
| (Start year) | (Ref) | |||||||
| Splenic cDCs, pDCs, and macrophages | Lipoplex | Three TAAs | Triple-negative breast cancer | i.v. | BNT114 | Phase-I | Active, not recruiting | NCT02316457 |
| (2016) | ||||||||
| As above | Lipoplex | Three TAAs | Ovarian cancer | i.v. | BNT115 | Phase-I | Recruiting | NCT04163094 |
| (2019) | ||||||||
| (W_ova1) | ||||||||
| As above | Lipoplex | TAAs | Non-small-cell lung cancer | i.v. | BNT116 | Phase-I | Not yet recruiting | NCT05142189 |
| (Estimated in 2022) | ||||||||
| As above | Lipoplex | Kallikrein-2 +Kallikrein-3 + | Metastatic castration-resistant prostate cancer | i.v. | BNT112 | Phase-I/II | Recruiting | NCT04382898 |
| ACP-Prostate + HOXB13 + | (W_pro1) | (2019) | ||||||
| NK3 homeobox 1 | ||||||||
| As above | Lipoplex | NY-ESO-1 + MAGE-A3 + TPTE + Tyrosinase | melanoma | i.v. | BNT111 | Phase-I/II | Recruiting (2021)/Active, not recruiting (2015) | NCT04526899/NCT02410733 |
| ([23]) | ||||||||
| Ex vivo human DCs | Protamine | NY-ESO-1 + MAGE-C2 + MUC1 | metastatic castration-resistant prostate cancer | i.n. | / | Phase-II | Completed | NCT02692976 |
| (2015) | ||||||||
| ([194]) | ||||||||
| Skin-APCs | Protamine | NY-ESO-1 + MAGE-C1 + 5T4 + | Non-small-cell lung cancer | i.d. | CV9202 | Phase-I/II | Completed (2017)/Terminated (2013) | NCT03164772/NCT01915524 |
| (RNActive®) | MAGE-C2 + Survivin + MUC-1 | (BI-1361849) | ||||||
| ([195,196]) | ||||||||
| As above | Protamine | NY-ESO-1 + MAGE-C1 + 5T4 + | Stage IIIB/IV non-small-cell | i.d. | CV9201 | Phase-I/II | Completed | NCT00923312 |
| (RNActive®) | MAGE-C2 + Survivin | lung cancer | (2009) | ([197]) | ||||
| As above | Protamine | Melan-A + Mage-A1 + GP100 + | Stages III/IV metastatic melanoma | i.d. | / | Phase-I/II | Completed | NCT00204607 |
| Mage-A3 + Survivin + Tyrosinase | (2004) | |||||||
| As above | Protamine | PSA + PSCA + PSMA + STEAP1 | Hormone-resistant | i.d. | CV9103 | Phase-I/II | Completed | NCT00831467 |
| (RNActive®) | prostate cancer | (2009) | ([198]) | |||||
| As above | Protamine | PSA + PSCA + PSMA + STEAP1 + | High/Intermediate risk | i.d. | CV9104 | Phase-II | Terminated | NCT02140138 |
| (RNActive®) | PAP + Muc1 | prostate cancer | (2014) | |||||
| Splenic cDCs, pDCs, and macrophages | Lipoplex | E6 + E7 | HPV16+ head and neck squamous cell carcinoma | i.v./i.d. | BNT113 | Phase-I/II | Recruiting (2021)/Recruiting (2017) | NCT04534205/NCT03418480 |
| As above | DOTAP Liposome | Total tumor mRNA + | Pediatric high-grade | i.v. | RNA-LP | Phase-I | Recruiting | NCT04573140 |
| pp65 full length LAMP mRNA | glioma/glioblastoma | (2021) | ||||||
| dLN-APCs | LNP | Personalised cancer vaccine | Multiple cancers | i.m. | NCI-4650 | Phase-I/II | Terminated (2018) | NCT03480152 |
| (mRNA-4650) | ([200]) | |||||||
| As above | LNP | As above | Solid tumor/melanoma | i.m | mRNA-4157 | Phase-I/II | Recruiting (2017)/Active, not recruiting (2019) | NCT03313778/NCT03897881 |
| As above | Lipopolyplex | As above | Multiple advanced | s.c. | / | Not Applicable | Unknown | NCT03468244 |
| digestive system cancers | (2018) | |||||||
| APCs | Protamine | As above | Multiple | / | PGV002 | Not Applicable | Recruiting | NCT05192460 |
| digestive system cancers | (Estimated in 2022) | |||||||
| Splenic cDCs, pDCs, and macrophages | Lipoplex | As above | Multiple resected/advanced cancers | i.v. | BNT122 | Phase-I/II | Recruiting (2021)/Active, not recruiting (2019)/NCT03815058 (2019)/Withdrawn (2021)/Active, not recruiting (2017) | NCT04486378/NCT04161755/NCT03815058/NCT04267237/NCT03289962 |
| (RO7198457) | ||||||||
| dLN-APCs | LNP | Four KRAS mutated proteins | Multiple KRAS mutant | i.m. | mRNA-5671 | Phase-I | Active, not recruiting (2019) | NCT03948763 |
| (G12D + G12V + G13D + G12C) | advanced/metastatic cancers | |||||||
| Splenic cDCs, pDCs, and macrophages | Lipoplex | CLDN6 | Solid Tumor | i.v. | BNT211 | Phase-I/II | Recruiting | NCT04503278 |
| (2020) | ([199]) | |||||||
| Tumor-T cells, Tumor cells | Cationic polymer/lipid formulation | CD3 × CLDN6 bispecific antibodies | Solid Tumor | i.v. | BNT142 | Phase-I/II | Recruiting | NCT05262530 |
| (2022) | ||||||||
| T cells | LNP | Optimized IL-2 | Solid Tumor | i.v. | BNT151 | Phase-I/II | Recruiting | NCT04455620 |
| (2021) | ||||||||
| As above | LNP | IL-2 + IL-7 | Solid Tumor | i.v. | BNT152 + BNT153 | Phase-1 | Recruiting | NCT04710043 |
| (2021) | ||||||||
| Tumor-T cells, NK cells, macrophages | LNP | IL-12 | Advanced Solid tumor | i.t. | MEDI1191 | Phase-I | Recruiting | NCT03946800 |
| (2019) | ||||||||
| Tumor - NK cells | LNP | Anti-Claudin 18.2 antibodies | Solid Tumor | i.v. | BNT141 | Phase-I/II | Recruiting | NCT04683939 |
| (2022) | ||||||||
| Tumor and TdLN - T cells, macrophages, monocytes, granulocytes, DCs, Tumor cells | LNP | OX40L | Recurrent/refractory solid malignant tumor or lymphoma | i.t. | mRNA-2416 | Phase-I | Active, not recruiting | NCT03323398 |
| (2017) | ([201]) | |||||||
| As above | LNP | OX40L + IL-23 + IL-36γ | multiple advanced cancers | i.t. | mRNA-2752 | Early Phase-I | Recruiting (2018)/Recruiting (2017) | NCT03739931/NCT02872025 |
Note: i.n.: intranodal injection; TdLN: Tumor draining lymph node.
Lipoplex nanoformulations developed by BioNTech are the most advanced LNP-mRNA cancer vaccines, of which BNT111 encoding four melanoma TAAs is being evaluated in phase I trial in melanoma patients (NCT02410733). The clinical results of BNT111 have been reported [23], showing that patients intravenously administrated with BNT111 vaccine induced potent and broad T cell response against TAAs. Antigen specific CTLs with a high magnitude and long durability were observed in some responders who experienced the treatment of anti-PD-1 mAbs. Patients vaccinated with BNT111 appeared with flu-like symptoms in mild to moderate levels, which were resolved within 1 day. Given the encouraging clinical safety profile and immunogenic responses, BNT111 now is under an active phase II study with or without anti-PD-1 mAbs to evaluate the efficacy as well as safety in patients with unresectable melanoma (NCT04526899).
Lipoplex cancer vaccine technology is expanding its clinical evaluations in patients with other types of malignancies (Table 3), including BNT112 vaccine encoding five prostate cancer antigens in prostate cancer patients (NCT04382898), BNT113 encoding viral neoantigen HPV16 E6/E7 in patients with HPV16 positive HNSCC (head and neck squamous cell carcinoma) (NCT04534205, NCT03418480), BNT114 encoding three breast cancer antigens in breast cancer patients (NCT023164570), BNT115 encoding mixed ovarian cancer TAAs in patients with ovarian cancer (NCT04163094), and BNT116 encoding mixed NSCLC TAAs in NSCLC patients (NCT05142189). BioNTech also developed lipoplex-based neoantigen mRNA vaccine of BNT122 for multiple cancer treatment. BNT122 encoding up to 20 neoepitopes for individual patient is currently under clinical evaluation in four studies (Table 3). These vaccine product candidates are to augment the production of antigen specific endogenous T cells, while the newly investigated BNT211 vaccine product encodes CLDN6 for in vivo expansion of adoptive CLDN6 CAR-T cells. The brief report on phase I/II clinical results of BNT211 (NCT04503278) demonstrated encouraging safety profile and efficacy in patients with CLDN6+ solid tumors [199], which might be a hallmark in CAR-T mediated solid tumor treatment.
Moderna developed an alternative LNP-based nanoformulation for delivery of personalised cancer mRNA vaccines. It is a key but remains a challenge to select appropriate immunogenic neoantigens. Moderna has developed different pipelines for identification and selections of neoantigens that can be recognised by functional tumor-infiltrating T cells in patients. For example, Moderna identified 15 human leukocyte antigen (HLA) class I neoantigens from driver gene mutations by exome sequencing of patient tumor samples, which were then encoded into a mRNA vaccine together with the defined antigens (named mRNA-4650). The personized mRNA-4650 vaccine was administrated intramuscularly in patients with metastatic gastrointestinal cancer in a phase I/II study (NCT03480152) to evaluate the safety, tolerability, and immunogenic efficacy. The clinical results from 4 patients displayed no severe side effects, while this vaccine did not stimulate increased neoantigen specific T cell immunity [200]. Given that no clinical response was detected, Moderna decided to terminate this clinical trial. Instead, a closely related mRNA-4157 vaccine was developed via a different neoantigen selection protocol, where a single mRNA encoded up to 34 selected neoantigens. mRNA-4157 vaccine alone or in combination with pembrolizumab (anti-PD1 mAbs) currently is in a phase I study in patients with solid tumors (NCT03313778, starting from 2017) and phase II study in patients with complete resected cutaneous melanoma (NCT03897881). Moderna also developed TAA encoded mRNA vaccine, such as mRNA-5671 incorporating four mutated KRAS (kirsten rat sarcoma viral oncogene homolog) antigens. mRNA-5671 vaccine as monotherapy and combination therapy with pembrolizumab currently is evaluated in patients with multiple KRAS mutant advanced or metastatic cancers under a phase I study (NCT03948763). Another newly developed DOTAP liposome vaccine containing autologous total tumor mRNA and mRNA encoding lysosome membrane-associated protein (LAMP) is under a first human phase I study in patients with glioblastoma (NCT04573140).
Along with therapeutic nano-mRNA cancer vaccines, both BioNTech and Moderna recently are remarkedly increasing investments on nano-mRNA development pipelines for the replacement of immunotherapeutic antibodies or cytokines and in vivo production of T/NK cells with CARs and TCRs. One such product, BNT142 encoding CD3 × CLDN6 bispecific antibodies, is just starting the first human phase I/II studies (NCT05262530) this year to evaluate its safety and immunogenetic efficacy in patients with CLDN6+ advanced solid tumors. Another type of products, BNT151 encoding optimized IL-2, BNT151 encoding IL-2 and BNT153 encoding IL-7, are under clinical trials (NCT04455620, NCT04710043) alone or combined with other therapeutic agents in solid tumor patients via intravenous administration. An ongoing study just started in 2022 is evaluating the safety and pharmacokinetics of nano-mRNA product (BNT141) encoding therapeutic mAbs against tumor antigen CLDN18.2 in patients with CLDN18.2+ tumor (NCT04683939). An intratumor administrated product (MEDI1191) that encodes fusion proteins of IL-12α and IL-12β is in a phase I study in patients with solid tumor (NCT03946800). Another two products, mRNA-2416 encoding OX40L and mRNA-2752 (NCT02872025, starting from 2017) incorporating encoded OX-40L, IL-36γ and IL-23, are being evaluated in phase I studies (Table 3). The tumor samples from NSCLC patients in mRNA-2416 clinical trials were spatially mapped to evaluate the clinical significance of OX40/OX40L pathway [201]. The results demonstrated that local injection of mRNA-2416 increased the expression of OX40L in TME, which was associated with increased infiltrated CD4+ and CD8+ T cells and conventional type 1 DCs (cDC1s) in NSCLC. The preliminary clinical response data are encouraging.
Recent explosion and success of new immunotargets, genetically engineered adoptive T cell therapy and mRNA COVID-19 vaccines have fuelled research interest and investment from biotechnology companies in the use of nano-mRNA therapeutics for targeted cancer immunotherapy. As seen from Table 3, organic nanoparticles, such as LNPs and polymer nanoparticles, are more prevalent in clinical studies compared with inorganic counterparts, contributing by the advantages of organic nanoparticles (listed in Table 1), such as high biocompatibility and simplicity in scale-up fabrication. Most LNP-mRNA therapeutics in cancer immunotherapy are at their early phase of clinical trials and have not yet employed in clinic. It had been a long journey for researchers to address the issues of mRNA instability and low transfection efficacy in vivo prior to their recent clinical trials. The preliminary results from current clinical trials are encouraging, while there are still some unsolved critical issues (discussed in the last section) that might impede their clinical translation in future.
6. Conclusions and future directions
The global recognition of the integrated technology of nano-mRNA in the rapid development of safe and effective COVID-19 vaccines has reignited the research interest and investment in mRNA therapeutics. The increasing knowledge in mRNA biology allows appropriate modulation on mRNA construct, inherent immunogenicity and production process and functional adaptability. Nanotechnologies that can encapsulate, protect, and deliver mRNA to specific sites of the body are indispensable for the extraordinary success of mRNA therapeutics in clinic. In parallel, immunotherapy has revolutionized the modalities of cancer treatment, such as ICIs and adoptive cell therapy. Given tumor heterogenicity, only 10–30% patients can benefit from cancer immunotherapy. Short half-life and autoimmune related adverse effects of immuno-drugs are additional barriers for the broad implementation of cancer immunotherapy in clinical practices. The rapid progress of nano-mRNA promotes the promise of cancer immunotherapy by targeted delivery of encoded immuno-drugs to specific immune cells, enabling production of long-acting therapeutics and reducing off-target associated toxicities. As exemplified above, recent technological advances in delivery platforms and mRNA constructs have led to diverse promising paradigms in targeted cancer immunotherapy under preclinical and clinical studies. The flexibility of mRNA technology in encoding a great variety of immuno-molecules has created numerous clinical products, ranging from therapeutic cancer vaccines to immunostimulatory cytokines, antigen specific therapeutic antibodies, and in vivo genetically engineered T cells with CARs. Despite the stunning success of nano-mRNA technology in targeted cancer immunotherapy, there are still some challenges to be addressed for advancing the use of nano-mRNA as therapeutic immuno-medicines in clinic.
-
(1)
Improved optimization of mRNA technology and understanding of molecular mechanisms of constructed mRNA. The efforts in the past decade have driven the rapid development of transformative mRNA technologies to clinical reality. The incredible speed in mRNA vaccine deployment globally has proven the safety, efficacy, and adaptability of mRNA therapeutics in medicine. The discoveries in mRNA modifications are the key for the success of mRNA vaccines. Nevertheless, the remaining technical obstacles facing mRNA platforms are apparent, such as limited stability in vivo and low expression of encoded molecules. Continued development of mRNA technology with increased stability, immunogenicity and translatability could lead to the next generation of mRNA-based immuno-drugs, fulfilling the medical potential of mRNA technology. To this end, it is essential to deepen the understanding of the relationship between the construct of modified mRNA and its stability/translation/immune modulation. High throughput methods are an appealing strategy to fast and accurately establish the correlations between the performance of mRNA and its massive structure variants. For example, the newly developed sequencing-based evaluation platform called PERSIST-seq enables systematic delineation of mRNA stability of a library of mRNA with diverse constructs [202]. Beyond conventional nonreplicating mRNA technology widely explored in cancer immunotherapy, self-amplify mRNAs have emerged as vaccine candidates for preventing infectious diseases [203]. Compared to conventional mRNA, self-amplified mRNA can copy itself and promote expression of encoded proteins under a low dose. However, it remains a challenge to precisely control the modification, transport, and strong immunogenicity-mediated side effects of large sized self-amplifying mRNAs. Very recently, an self-amplifying mRNA was reported inducing potent protective immunity in non-human primates against SARS-CoV-2 [204]. These encouraging preclinical data along with further technological discoveries might expand the applications of self-amplifying mRNA technology in therapeutic cancer vaccines and therapeutic protein-based immunotherapy (Fig. 17).
-
(2)
Optimization of mRNA delivery platforms with a precise organ selectivity and high encapsulating capacity. The stability of naked mRNA is substantially enhanced by mRNA delivery platforms. Currently ionizable LNPs are the preferred formulations to transport mRNA therapeutics in animal models and humans, with improved safety profile and therapeutic efficacy. Selective delivery of mRNA therapeutics to the action site in an organism is crucial to achieve the therapeutic outcome and reduce off-target associated toxicity. Targeting DCs in lymphoid organs is essential to initiate T cell immunity of delivered mRNA vaccines, thus spleen-target lipoplex products have been developed and applied in severval cancer mRNA vaccine formulations that are under extensive clinical investigations. In addition to specific targeting efficacy, mRNA delivery platform is expected to have a high payload with multiple antigen candidates, which is normally comprised by the consideration of dose tolerability of LNPs. Thus, redesigning mRNA delivery platforms with increased loading capability and enhanced the translation efficacy of mRNA is a potential solution to balance the safety and efficacy. In the context of targeting immune cells at tumor sites, nano-mRNA encoding immune stimulatory proteins can be passively delivered to tumors driven by the small nanosize-mediated EPR effect. Nonetheless, the accumulation efficacy is limited by the heterogenous physical barriers inside tumor [205]. Therefore, selective LNPs recently have been rationally engineered for active targeting delivery of mRNA to specific organs of lung, liver, or spleen [206,207]. This intriguing mRNA delivery system holds immense potential in targeted cancer immunotherapy. Alternatively, VLPs that have been approved as antigens in human use could potentially serve as promising delivery vehicles for mRNA therapeutics. The reengineering strategies are required to promote the translatability and immunogenicity of VLPs in the body.
-
(3)
Improved understanding on the clinical response of nano-mRNA immunotherapeutics. As reflected in Table 3, most LNP-mRNA modalities in the treatment of cancer patients are still under early phases of clinical trials. The positive therapeutic outcomes from these clinical studies will support the progress of promising candidates toward next evaluation stage. More importantly, the clinical data will expand our understanding on how and to what degree the patient response to the newly developed LNP-mRNA immuno-drug, which is extremely valuable in determining optimization directions for both mRNA and delivery technologies. The positive feedback loop will advance the translation of nano-mRNA technology in clinic.
-
(4)
Exploration of uncovered mechanisms at the interface of nano-mRNA and tumor microenvironment. Tumors are made up of heterogenous groups of malignant, stromal, and immune cells, with substantial levels of heterogenicity in each type of cells. The crosstalk among these cells creates an amalgam of signals that promote or suppress tumor growth and evolution. The bulk transcriptomic analysis tends to obscure specific subtype and status of cells that might be crucial for a therapeutic target. The integration of single-cell and bulk analyses is particularly vital in cancer immunotherapy, which can provide the insights into tumor or patient specific mechanisms driving a therapeutic response [208]. The emerging high-dimensional technologies, such as single-cell sequencing, single cell T cell receptor analysis, spatial transcriptomics, high dimensional flow cytometry, are revolutionary tools, which will advance our understanding of the interaction mechanisms between nano-mRNA and patient TME. The detailed molecular mechanisms that drive the response or resistance to nano-mRNA immunotherapy will yield informative results for the development of next generation of nano-mRNA technologies.
-
(5)
Improved research collaborations across disciplines. Multidisciplinary research is vital to address research problems and society challenges. For example, the success of mRNA vaccines is a product of close and intensive collaboration with scientists at universities from different fields. Similarly, combined advances from nanotechnology, mRNA biology, immunology, and oncology, have furthered our knowledge of nano-mRNA technology in cancer immunotherapy. To advance the translation of nano-mRNA technology from basic science into medical products, it is indispensable to overcome the barriers caused by distinct technical vocabulary and commutation cultures, thereby fostering mutual understanding in research goals.
Fig. 17.
An illustrated scheme showing the proposed future directions for implementation of nano-mRNA integrated technology in targeted cancer immunotherapy. Created with permission by BioRender.
The advances in mRNA technology and nanoformulation strategies have demonstrated immense potential in cancer immunotherapy by targeted modulation of specific subsets of immune cells. The integrated nano-mRNA technology mediated immuno-therapeutics have been broadly studied for cancer treatment in preclinical and clinical studies. Continued research efforts and discoveries in this multidisciplinary field is expected to fulfil the far-reaching potential of nano-mRNA therapeutics in clinical practices for cancer treatment as well as other medical applications [209,210].
Ethics statement
This is a review-type manuscript. No ethics approval and consent to participate are required for this review-type manuscript.
CRediT authorship contribution statement
Wei Yang: Conceptualization, Literature research, Investigation, Writing – original draft. Jianwei Cao: Investigation, Writing – original draft. Hui Cheng: Investigation, Visualization. Liang Chen: Investigation, Visualization. Meihua Yu: Conceptualization, Supervision, Writing – review & editing, Funding acquisition. Yu Chen: Conceptualization, Supervision, Writing – review & editing, Funding acquisition. Xingang Cui: Supervision, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgment
This work was supported by the National Natural Science Foundation of China [32101146, 81974391, 82072806, 82173265]; Shanghai Science and Technology Program [21010500100, 22140901700]; Basic Research Program of Shanghai Municipal Government [21JC1406002]; Shanghai Pujiang Program [21PJ1404500]; Shanghai Excellent Overseas Young Scientists; the Clinical Research Plan of SHDC [SHDC2020CR4025]; the Natural Science Foundation of Shanghai [20ZR1470500]; Hospital Funded Clinical Research, Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine [21XHDB06].
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Meihua Yu, Email: myu@shu.edu.cn.
Yu Chen, Email: chenyuedu@shu.edu.cn.
Xingang Cui, Email: cuixingang@xinhuamed.com.cn.
Abbreviations
- DCs
dendritic cells
- CTLs
cytotoxic T lymphocytes
- TLRs
Toll-like receptors
- APCs
antigen presenting cells
- STING
intracellular stimulator of interferon genes
- NK cells
natural killer cells
- IVT
in vitro transcription
- pDNA
plasmid DNA
- UTR
untranslated region
- ORF
open reading frame
- PAMP
pathogen-associated molecular pattern
- PRRs
pattern recognition receptors
- IFN
interferon
- TAA
tumor-associated antigen
- HPLC
high performance liquid chromatography
- LNP
lipid nanoparticle
- DOTMA
1,2-di-O-octadecenyl-3-trimethylammonium propane (chloride salt)
- RIG-1
recombinant retinoic acid inducible gene 1 protein
- MDA-5
melanoma differentiation-associated protein 5
- m7G
7-methylguanosine;
- eIF4F
eukaryotic initiation factor 4F
- IRES
internal ribosome entry sites
- miRNA
microRNA
- GC
guanine cytosine;
- U
uracil
- dsRNA
double-stranded mRNA
- RNase
ribonuclease
- Da
dalton
- MHC
major histocompatibility complex
- ER
endoplasmic reticulum
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IL-12
interleukin-12
- CPP
cationic cell-penetrating peptide;
- VLPs
virus-like particles
- HPV
human papillomavirus
- PEG10
paternally expressed 10
- PEG
polyethylene glycol
- DSPC
1,2-distearoyl-sn-glycero-3-phosphocholine;
- DOTAP
1,2-Dioleoyl-3-trimethylammonium-propane, chloride;
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine;
- PEI
polyethylenimine;
- PBAE
poly (β-amino ester)s
- PLGA
poly(lactic-co-glycolic acid)
- OMVs
outer membrane vesicles
- dLNs
draining lymph nodes
- TCRs
T-cell receptors
- IONPs
iron oxide nanoparticles
- IO-RNA-NPs
OVA-mRNA in the optimized liposomes
- MRI
magnetic resonance imaging
- OVA
ovalbumin
- ICOSL,
inducible costimulator ligand;
- CD40
cluster of differentiation 40
- Luc
luciferase labeling
- CLDN6
claudin 6
- CAR-T cells
chimeric antigen receptor-T cells
- LPX
lipoplex
- NSG
NOD scid gamma
- PAMAM
poly(-amidoamine)
- DiR
1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyaine iodide;
- EGFP
enhanced green fluorescent protein
- R848
a TLR7/8 agonist
- LPS
lipopolysaccharide;
- FFL,
firefly luciferase
- SEM
scanning electron microscope
- TEM
transmission electron microscope
- IRF
interferon regulatory factor
- dsDNA
double stranded DNA
- NF-κB
nuclear factor kappa-B;
- ISRE
interferon stimulated response element
- CLRs
C-type lectin receptors; Mann, mannan
- Dex
dextran
- CTLA-4
cytotoxic T lymphocyte-associated antigen-4
- mAbs
monoclonal antibody; cy5.5, cyanine5.5
- Fluc
firefly luciferin
- ICIs
immune checkpoint inhibitors
- PD-1
programmed death 1
- α-GC
α-Glucosidase
- iNKT cells
invariant natural killer T cells
- NKT cells
natural killer T cells
- PKR inhibitor
protein kinase inhibitor
- Cre
cAMP response element
- HER2
human epidermal growth factor receptor type 2
- ADCC
antibody dependent cell mediated cytotoxicity
- CCL5
C–C motif ligand 5
- DLS
dynamic light scattering
- NP
nanoparticle
- PTEN
gene of phosphate and tension homology deleted on chromosome ten
- CRT
calreticulin
- CTCE
CXCR4 antagonist
- HCC
hepatic cell carcinoma
- pDCs
plasmacytoid dendritic cells
- GO
graphite oxide;
- PGA
polyglycolic acido
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- Di
dialkylcarbocyanines
- CART
charge-altering releasable transporter
- DSPE
1,2-distearoyl-sn-glycero-3-phosphoethanolamine;
- i.v.
intravenous injection
- i.m.
intramuscular injection
- s.c.
subcutaneous injection
- i.d.
intradermal injection
- NSCLC
non-small-cell lung cancer
- HNSCC
head and neck squamous cell carcinoma
- HLA
human leukocyte antigen
- KRAS
kirsten rat sarcoma viral oncogene homolog
- PAP
prostatic acid phosphatase
- TME
tumor microenvironment
- DAMPs
damage associated molecular patterns
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Tureci O., Nell H., Schaefer A., Unal S., Tresnan D.B., Mather S., Dormitzer P.R., Sahin U., Jansen K.U., Gruber W.C., Grp C.C.T. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W.P., Zhou H.H., Han S., Ivarsson M., Miller J., Zaks T., Grp C.S. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris V.K., Kopetz S. Nat Rev Cancer; 2022. Don't Blame the Messenger: Lessons Learned for Cancer mRNA Vaccines during the COVID-19 Pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malone R.W., Felgner P.L., Verma I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. U.S.A. 1989;86:6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science (New York, N.Y.) 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 6.Jirikowski G.F., Sanna P.P., Maciejewski-Lenoir D., Bloom F.E. Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA. Science (New York, N.Y.) 1992;255:996–998. doi: 10.1126/science.1546298. [DOI] [PubMed] [Google Scholar]
- 7.Martinon F., Krishnan S., Lenzen G., Magné R., Gomard E., Guillet J.-G., Lévy J.-P., Meulien P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993;23:1719–1722. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- 8.Conry R.M., LoBuglio A.F., Wright M., Sumerel L., Pike M.J., Johanning F., Benjamin R., Lu D., Curiel D.T. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 1995;55:1397–1400. [PubMed] [Google Scholar]
- 9.Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 10.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappa B by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 11.Diebold S.S., Kaisho T., Hemmi H., Akira S., Sousa C.R.E. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 12.Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., Lipford G., Wagner H., Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 13.Kariko K., Buckstein M., Ni H.P., Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao Y.F., Tang Z.M., Huang X.G., Chen W., Zhou J., Liu H.J., Liu C., Kong N., Tao W. Emerging mRNA technologies: delivery strategies and biomedical applications. Chem. Soc. Rev. 2022;51:3828–3845. doi: 10.1039/d1cs00617g. [DOI] [PubMed] [Google Scholar]
- 16.Hou X.C., Zaks T., Langer R., Dong Y.Z. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhary N., Weissman D., Whitehead K.A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021;20:817–838. doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huff A.L., Jaffee E.M., Zaidi N. Messenger RNA vaccines for cancer immunotherapy: progress promotes promise. J. Clin. Invest. 2022;132 doi: 10.1172/JCI156211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cagigi A., Lore K. Immune responses induced by mRNA vaccination in mice, monkeys and humans. Vaccines. 2021;9(61–14):61. doi: 10.3390/vaccines9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bevan M.J. Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 21.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., Meng M., Fritz D., Vascotto F., Hefesha H., Grunwitz C., Vormehr M., Husemann Y., Selmi A., Kuhn A.N., Buck J., Derhovanessian E., Rae R., Attig S., Diekmann J., Jabulowsky R.A., Heesch S., Hassel J., Langguth P., Grabbe S., Huber C., Tureci O., Sahin U. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 22.Miao L., Li L.X., Huang Y.X., Delcassian D., Chahal J., Han J.S., Shi Y.H., Sadtler K., Gao W.T., Lin J.Q., Doloff J.C., Langer R., Anderson D.G. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019;37:1174–1185. doi: 10.1038/s41587-019-0247-3. [DOI] [PubMed] [Google Scholar]
- 23.Sahin U., Oehm P., Derhovanessian E., Jabulowsky R.A., Vormehr M., Gold M., Maurus D., Schwarck-Kokarakis D., Kuhn A.N., Omokoko T., Kranz L.M., Diken M., Kreiter S., Haas H., Attig S., Rae R., Cuk K., Kemmer-Bruck A., Breitkreuz A., Tolliver C., Caspar J., Quinkhardt J., Hebich L., Stein M., Hohberger A., Vogler I., Liebig I., Renken S., Sikorski J., Leierer M., Muller V., Mitzel-Rink H., Miederer M., Huber C., Grabbe S., Utikal J., Pinter A., Kaufmann R., Hassel J.C., Loquai C., Tureci O. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585:107. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Ma X.T., Yue Y.L., Zhang K.Y., Cheng K.M., Feng Q.Q., Ma N.N., Liang J., Zhang T.J., Zhang L.Z., Chen Z.Q., Wang X.W., Ren L., Zhao X., Nie G.J. Rapid surface display of mRNA antigens by bacteria-derived outer membrane vesicles for a personalized tumor vaccine. Adv. Mater. 2022;34 doi: 10.1002/adma.202109984. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H.X., You X.R., Wang X.J., Cui L., Wang Z.N., Xu F.F., Li M.Y., Yang Z.G., Liu J.Y., Huang P., Kang Y., Wu J., Xia X.J. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. P Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2005191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J.Q., Zhang C.X., Zhang X.F., Yan J.Y., Zeng C.X., Talebian F., Lynch K., Zhao W.Y., Hou X.C., Du S., Kang D.D., Deng B.B., McComb D.W., Bai X.F., Dong Y.Z. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J. Contr. Release. 2022;345:306–313. doi: 10.1016/j.jconrel.2022.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu A.Y., Zhu J.M., Zeng C.X., Lin C.H., Yu J.Y., Liu J.Q., Lynch K., Talebian F., Pan X.L., Yan J.Y., Dong Y.Z., Li Z.H., Bai X.F. IL-27 induces CCL5 production by T lymphocytes, which contributes to antitumor activity. J. Immunol. 2022;208:2239–2245. doi: 10.4049/jimmunol.2100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stadler C.R., Bahr-Mahmud H., Celik L., Hebich B., Roth A.S., Roth R.P., Kariko K., Tureci O., Sahin U. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nat. Med. 2017;23:815–817. doi: 10.1038/nm.4356. [DOI] [PubMed] [Google Scholar]
- 29.Wu L.P., Wang W.W., Tian J.L., Qi C.R., Cai Z.X., Yan W.H., Xuan S.A., Shang A.Q. Intravenous delivery of RNA encoding anti-PD-1 human monoclonal antibody for treating intestinal cancer. J. Cancer. 2022;13:579–588. doi: 10.7150/jca.63991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rybakova Y., Kowalski P.S., Huang Y.X., Gonzalez J.T., Heartlein M.W., DeRosa F., Delcassian D., Anderson D.G. mRNA delivery for therapeutic anti-HER2 antibody expression in vivo. Mol. Ther. 2019;27:1415–1423. doi: 10.1016/j.ymthe.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F., Parayath N.N., Ene C.I., Stephan S.B., Koehne A.L., Coon M.E., Holland E.C., Stephan M.T. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 2019;10:3974. doi: 10.1038/s41467-019-11911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parayath N.N., Stephan S.B., Koehne A.L., Nelson P.S., Stephan M.T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 2020;11:6080. doi: 10.1038/s41467-020-19486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moffett H.F., Coon M.E., Radtke S., Stephan S.B., McKnight L., Lambert A., Stoddard B.L., Kiem H.P., Stephan M.T. Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers. Nat. Commun. 2017;8 doi: 10.1038/s41467-017-00505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinhard K., Rengstl B., Oehm P., Michel K., Billmeier A., Hayduk N., Klein O., Kuna K., Ouchan Y., Woll S., Christ E., Weber D., Suchan M., Bukur T., Birtel M., Jahndel V., Mroz K., Hobohm K., Kranz L., Diken M., Kuhlcke K., Tureci O., Sahin U. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. 2020;367:446–453. doi: 10.1126/science.aay5967. [DOI] [PubMed] [Google Scholar]
- 35.Xiao Y.L., Chen J., Zhou H., Zeng X.D., Ruan Z.P., Pu Z.Y., Jiang X.Y., Matsui A., Zhu L.L., Amoozgar Z., Chen D.S., Han X.F., Duda D.G., Shi J.J. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-28279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenner S., Jacob F., Meselson M. An Unstable Intermediate Carrying Information from Genes to Ribosomes for Protein Synthesis. 1961;190:576–581. doi: 10.1038/190576a0. [DOI] [PubMed] [Google Scholar]
- 37.Lockard R.E., Lingrel J.B. The synthesis of mouse hemoglobin beta-chains in a rabbit reticulocyte cell-free system programmed with mouse reticulocyte 9S RNA. Biochem. Biophys. Res. Commun. 1969;37:204–212. doi: 10.1016/0006-291x(69)90720-7. [DOI] [PubMed] [Google Scholar]
- 38.Gurdon J.B., Lane C.D., Woodland H.R., Marbaix G. Vol. 233. 1971. pp. 177–182. (Use of Frog Eggs and Oocytes for the Study of Messenger RNA and its Translation in Living Cells). [DOI] [PubMed] [Google Scholar]
- 39.Krieg P.A., Melton D.A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984;12:7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krupp G. RNA synthesis: strategies for the use of bacteriophage RNA polymerases. Gene. 1988;72:75–89. doi: 10.1016/0378-1119(88)90129-1. [DOI] [PubMed] [Google Scholar]
- 41.Paschal B.M., McReynolds L.A., Noren C.J., Nichols N.M. Vol. 8. 2008. RNA Polymerases, Current Protocols in Molecular Biology. (Chapter 3) [DOI] [PubMed] [Google Scholar]
- 42.Muthukrishnan S., Both G.W., Furuichi Y., Shatkin A.J. Vol. 255. 1975. 5′-Terminal 7-methylguanosine in Eukaryotic mRNA Is Required for Translation; pp. 33–37. [DOI] [PubMed] [Google Scholar]
- 43.Isaacs A., Cox R.A., Rotem Z. Foreign nucleic acids as the stimulus to make interferon. Lancet (London, England) 1963;2:113–116. doi: 10.1016/s0140-6736(63)92585-6. [DOI] [PubMed] [Google Scholar]
- 44.Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 45.Gingras A.C., Raught B., Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 46.Muttach F., Muthmann N., Rentmeister A. Synthetic mRNA capping. Beilstein J. Org. Chem. 2017;13:2819–2832. doi: 10.3762/bjoc.13.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stepinski J., Waddell C., Stolarski R., Darzynkiewicz E., Rhoads R.E. Synthesis and properties of mRNAs containing the novel "anti-reverse" cap analogs 7-methyl(3'-O-methyl)GpppG and 7-methyl (3'-deoxy)GpppG. RNA. 2001;7:1486–1495. [PMC free article] [PubMed] [Google Scholar]
- 48.Elango N., Elango S., Shivshankar P., Katz M.S. Optimized transfection of mRNA transcribed from a d(A/T)(100) tail-containing vector. Biochem Bioph Res Co. 2005;330:958–966. doi: 10.1016/j.bbrc.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 49.Peng J., Schoenberg D.R. mRNA with a <20-nt poly(A) tail imparted by the poly(A)-limiting element is translated as efficiently in vivo as long poly(A) mRNA. RNA. 2005;11:1131–1140. doi: 10.1261/rna.2470905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holtkamp S., Kreiter S., Selmi A., Simon P., Koslowski M., Huber C., Tureci O., Sahin U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108:4009–4017. doi: 10.1182/blood-2006-04-015024. [DOI] [PubMed] [Google Scholar]
- 51.Heine A., Juranek S., Brossart P. Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol. Cancer. 2021;20:52. doi: 10.1186/s12943-021-01339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weissman D. mRNA transcript therapy. Expert Rev. Vaccines. 2015;14:265–281. doi: 10.1586/14760584.2015.973859. [DOI] [PubMed] [Google Scholar]
- 53.Vagner S., Galy B., Pyronnet S. Irresistible IRES - attracting the translation machinery to internal ribosome entry sites. EMBO Rep. 2001;2:893–898. doi: 10.1093/embo-reports/kve208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leppek K., Das R., Barna M. Functional 5 ' UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 2018;19:158–174. doi: 10.1038/nrm.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaspar P., Moura G., Santos M.A.S., Oliveira J.L. mRNA secondary structure optimization using a correlated stem-loop prediction. Nucleic Acids Res. 2013;41:e73. doi: 10.1093/nar/gks1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linares-Fernandez S., Lacroix C., Exposito J.Y., Verrier B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol. Med. 2020;26:311–323. doi: 10.1016/j.molmed.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Guhaniyogi J., Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265:11–23. doi: 10.1016/s0378-1119(01)00350-x. [DOI] [PubMed] [Google Scholar]
- 58.Jiang Y., Xu X.S., Russell J.E. A nucleolin-binding 3 ' untranslated region element stabilizes beta-globin mRNA in vivo. Mol. Cell Biol. 2006;26:2419–2429. doi: 10.1128/MCB.26.6.2419-2429.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kudla G., Lipinski L., Caffin F., Helwak A., Zylicz M. High guanine and cytosine content increases mRNA levels in mammalian cells. PLoS Biol. 2006;4:933–942. doi: 10.1371/journal.pbio.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Hawash A.B., Zhang X.Y., Ma F.Y. Strategies of codon optimization for high-level heterologous protein expression in microbial expression systems. Gene Rep. 2017;9:46–53. [Google Scholar]
- 61.Yan X.W., Hoek T.A., Vale R.D., Tanenbaum M.E. Dynamics of translation of single mRNA molecules in vivo. Cell. 2016;165:976–989. doi: 10.1016/j.cell.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spencer P.S., Siller E., Anderson J.F., Barral J.M. Silent substitutions predictably alter translation elongation rates and protein folding efficiencies. J. Mol. Biol. 2012;422 doi: 10.1016/j.jmb.2012.06.010. 328-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Durbin A.F., Wang C., Marcotrigiano J., Gehrke L. RNAs containing modified nucleotides fail to trigger RIG-I conformational changes for innate immune signaling. mBio. 2016;7 doi: 10.1128/mBio.00833-16. e00833-00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kariko K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kariko K., Muramatsu H., Keller J.M., Weissman D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol. Ther. 2012;20:948–953. doi: 10.1038/mt.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andries O., Mc Cafferty S., De Smedt S.C., Weiss R., Sanders N.N., Kitada T. N-1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Contr. Release. 2015;217:337–344. doi: 10.1016/j.jconrel.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 67.Svitkin Y.V., Cheng Y.M., Chakraborty T., Presnyak V., John M., Sonenberg N. N1-methyl-pseudouridine in mRNA enhances translation through eIF2 alpha-dependent and independent mechanisms by increasing ribosome density. Nucleic Acids Res. 2017;45:6023–6036. doi: 10.1093/nar/gkx135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kariko K., Muramatsu H., Ludwig J., Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39:e142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thess A., Grund S., Mui B.L., Hope M.J., Baumhof P., Fotin-Mleczek M., Schlake T. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 2015;23:1456–1464. doi: 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baiersdorfer M., Boros G., Muramatsu H., Mahiny A., Vlatkovic I., Sahin U., Kariko K. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids. 2019;15:26–35. doi: 10.1016/j.omtn.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foster J.B., Choudhari N., Perazzelli J., Storm J., Hofmann T.J., Jain P., Storm P.B., Pardi N., Weissman D., Waanders A.J., Grupp S.A., Karik K., Resnick A.C., Barrett D.M. Purification of mRNA encoding chimeric antigen receptor is critical for generation of a robust T-cell response. Hum. Gene Ther. 2019;30:168–178. doi: 10.1089/hum.2018.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoerr I., Obst R., Rammensee H.G., Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur. J. Immunol. 2000;30:1–7. doi: 10.1002/1521-4141(200001)30:1<1::AID-IMMU1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 73.Weng Y.H., Li C.H., Yang T.R., Hu B., Zhang M.J., Guo S., Xiao H.H., Liang X.J., Huang Y.Y. The challenge and prospect of mRNA therapeutics landscape. Biotechnol. Adv. 2020;40 doi: 10.1016/j.biotechadv.2020.107534. [DOI] [PubMed] [Google Scholar]
- 74.Van Hoecke L., Van Lint S., Roose K., Van Parys A., Vandenabeele P., Grooten J., Tavernier J., De Koker S., Saelens X. Treatment with mRNA coding for the necroptosis mediator MLKL induces antitumor immunity directed against neo-epitopes. Nat. Commun. 2018;9:3417. doi: 10.1038/s41467-018-05979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bai Y., Kan S.F., Zhou S.X., Wang Y.T., Xu J., Cooke J.P., Wen J.H., Deng H.K. Enhancement of the in vivo persistence and antitumor efficacy of CD19 chimeric antigen receptor T cells through the delivery of modified TERT mRNA. Cell Discov. 2015;1 doi: 10.1038/celldisc.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qiu P., Ziegelhoffer P., Sun J., Yang N.S. Gene gun delivery of mRNA in situ results in efficient transgene expression and genetic immunization. Gene Ther. 1996;3:262–268. [PubMed] [Google Scholar]
- 77.Raes L., Stremersch S., Fraire J.C., Brans T., Goetgeluk G., De Munter S., Van Hoecke L., Verbeke R., Van Hoeck J., Xiong R.H., Saelens X., Vandekerckhove B., De Smedt S., Raemdonck K., Braeckmans K. Intracellular delivery of mRNA in adherent and suspension cells by vapor nanobubble photoporation. Nano-Micro Lett. 2020;12:185. doi: 10.1007/s40820-020-00523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hotz C., Wagenaar T.R., Gieseke F., Bangari D.S., Callahan M., Cao H., Diekmann J., Diken M., Grunwitz C., Hebert A., Hsu K., Bernardo M., Kariko K., Kreiter S., Kuhn A.N., Levit M., Malkova N., Masciari S., Pollard J., Qu H., Ryan S., Selmi A., Schlereth J., Singh K., Sun F.X., Tillmann B., Tolstykh T., Weber W., Wicke L., Witzel S., Yu Q.Y., Zhang Y.A., Zheng G., Lager J., Nabel G.J., Sahin U., Wiederschain D. Local delivery of mRNA-encoding cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abc7804. [DOI] [PubMed] [Google Scholar]
- 79.Liu X.J., Jiang S.G., Fang C.Y., Li H., Zhang X.H., Zhang F.Q., June C.H., Zhao Y.B. Novel T cells with improved in vivo anti-tumor activity generated by RNA electroporation. Protein Cell. 2017;8:514–526. doi: 10.1007/s13238-017-0422-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Nuffel A.M.T., Benteyn D., Wilgenhof S., Pierret L., Corthals J., Heirman C., van der Bruggen P., Coulie P.G., Neyns B., Thielemans K., Bonehill A. Dendritic cells loaded with mRNA encoding full-length tumor antigens prime CD4(+) and CD8(+) T cells in melanoma patients. Mol. Ther. 2012;20:1063–1074. doi: 10.1038/mt.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beatty G.L., O'Hara M.H., Lacey S.F., Torigian D.A., Nazimuddin F., Chen F., Kulikovskaya I.M., Soulen M.C., McGarvey M., Nelson A.M., Gladney W.L., Levine B.L., Melenhorst J.J., Plesa G., June C.H. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology. 2018;155:29–32. doi: 10.1053/j.gastro.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vassilev V.B., Gil L.H.V.G., Donis R.O. Microparticle-mediated RNA immunization against bovine viral diarrhea virus. Vaccine. 2001;19:2012–2019. doi: 10.1016/s0264-410x(00)00438-2. [DOI] [PubMed] [Google Scholar]
- 83.Tirlapur U.K., Konig K. Cell biology - targeted transfection by femtosecond laser. Nature. 2002;418:290–291. doi: 10.1038/418290a. [DOI] [PubMed] [Google Scholar]
- 84.Van Lint S., Goyvaerts C., Maenhout S., Goethals L., Disy A., Benteyn D., Pen J., Bonehill A., Heirman C., Breckpot K., Thielemans K. Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy. Cancer Res. 2012;72:1661–1671. doi: 10.1158/0008-5472.CAN-11-2957. [DOI] [PubMed] [Google Scholar]
- 85.Weide B., Carralot J.P., Reese A., Scheel B., Eigentler T.K., Hoerr I., Rammensee H.G., Garbe C., Pascolo S. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J. Immunother. 2008;31:180–188. doi: 10.1097/CJI.0b013e31815ce501. [DOI] [PubMed] [Google Scholar]
- 86.Kubiatowicz L.J., Mohapatra A., Krishnan N., Fang R.H., Zhang L. Exploration; 2022. mRNA Nanomedicine: Design and Recent Applications. n/a, 20210217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Segel M., Lash B., Song J.W., Ladha A., Liu C.C., Jin X., Mekhedov S.L., Macrae R.K., Koonin E.V., Zhang F. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science. 2021;373:882–889. doi: 10.1126/science.abg6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaczmarek J.C., Patel A.K., Kauffman K.J., Fenton O.S., Webber M.J., Heartlein M.W., DeRosa F., Anderson D.G. Polymer-lipid nanoparticles for systemic delivery of mRNA to the lungs. Angew. Chem., Int. Ed. 2016;55:13808–13812. doi: 10.1002/anie.201608450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang W., Liu Y., Chin J.M., Phua K.K.L. Sustained release of PKR inhibitor C16 from mesoporous silica nanoparticles significantly enhances mRNA translation and anti-tumor vaccination. Eur. J. Pharm. Biopharm. 2021;163:179–187. doi: 10.1016/j.ejpb.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 90.Amos H. Protamine enhancement of RNA uptake by cultured chick cells. 1961;5:1–4. [Google Scholar]
- 91.Weide B., Pascolo S., Scheel B., Derhovanessian E., Pflugfelder A., Eigentler T.K., Pawelec G., Hoerr I., Rammensee H.G., Garbe C. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. Journal of immunotherapy (Hagerstown, Md. 1997;32:498–507. doi: 10.1097/CJI.0b013e3181a00068. 2009. [DOI] [PubMed] [Google Scholar]
- 92.Lundin P., Johansson H., Guterstam P., Holm T., Hansen M., Langel U., Andaloussi S.E.L. Distinct uptake routes of cell-penetrating peptide conjugates. Bioconjugate Chem. 2008;19:2535–2542. doi: 10.1021/bc800212j. [DOI] [PubMed] [Google Scholar]
- 93.Udhayakumar V.K., De Beuckelaer A., McCaffrey J., McCrudden C.M., Kirschman J.L., Vanover D., Van Hoecke L., Roose K., Deswarte K., De Geest B.G., Lienenklaus S., Santangelo P.J., Grooten J., McCarthy H.O., De Koker S. Arginine-rich peptide-based mRNA nanocomplexes efficiently instigate cytotoxic T cell immunity dependent on the amphipathic organization of the peptide. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201601412. [DOI] [PubMed] [Google Scholar]
- 94.Ruseska I., Zimmer A. Internalization mechanisms of cell-penetrating peptides. Beilstein J. Nanotechnol. 2020;11:101–123. doi: 10.3762/bjnano.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodriguez V., Lascani J., Asenjo J.A., Andrews B.A. Production of cell-penetrating peptides in Escherichia coli using an intein-mediated system. Appl. Biochem. Biotechnol. 2015;175:3025–3037. doi: 10.1007/s12010-015-1484-7. [DOI] [PubMed] [Google Scholar]
- 96.Mohsen M.O., Zha L.S., Cabral-Miranda G., Bachmann M.F. Major findings and recent advances in virus like particle (VLP)-based vaccines. Semin. Immunol. 2017;34:123–132. doi: 10.1016/j.smim.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 97.Jekhmane S., de Haas R., Filho O.P.D.S., van Asbeck A.H., Favretto M.E., Garcia A.H., Brock R., de Vries R. Virus-like particles of mRNA with artificial minimal coat proteins: particle formation, stability, and transfection efficiency. Nucleic Acid Therapeut. 2017;27:159–167. doi: 10.1089/nat.2016.0660. [DOI] [PubMed] [Google Scholar]
- 98.Li W.Q., Zhang X.F., Zhang C.X., Yan J.Y., Hou X.C., Du S., Zeng C.X., Zhao W.Y., Deng B.B., McComb D.W., Zhang Y.B., Kang D.D., Li J.N., Carson W.E., Dong Y.Z. Biomimetic nanoparticles deliver mRNAs encoding costimulatory receptors and enhance T cell mediated cancer immunotherapy. Nat. Commun. 2021;12:7264. doi: 10.1038/s41467-021-27434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yildiz I., Lee K.L., Chen K., Shukla S., Steinmetz N.F. Infusion of imaging and therapeutic molecules into the plant virus-based carrier cowpea mosaic virus: cargo-loading and delivery. J. Contr. Release. 2013;172:568–578. doi: 10.1016/j.jconrel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li J.M., Sun Y.L., Jia T.T., Zhang R., Zhang K., Wang L.N. Messenger RNA vaccine based on recombinant MS2 virus-like particles against prostate cancer. Int. J. Cancer. 2014;134:1683–1694. doi: 10.1002/ijc.28482. [DOI] [PubMed] [Google Scholar]
- 101.Mohsen M.O., Gomes A.C., Vogel M., Bachmann M.F. Interaction of viral capsid-derived virus-like particles (VLPs) with the innate immune system. Vaccines-Basel. 2018;6:37. doi: 10.3390/vaccines6030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dimitriadis G.J. Vol. 274. 1978. Translation of Rabbit Globin mRNA Introduced by Liposomes into Mouse Lymphocytes; pp. 923–924. [DOI] [PubMed] [Google Scholar]
- 103.Ostro M.J., Giacomoni D., Lavelle D.O.N., Paxton W., Dray S. Vol. 274. 1978. Evidence for Translation of Rabbit Globin mRNA after Liposomemediated Insertion into a Human Cell Line; pp. 921–923. [DOI] [PubMed] [Google Scholar]
- 104.Geall A.J., Verma A., Otten G.R., Shaw C.A., Hekele A., Banerjee K., Cu Y., Beard C.W., Brito L.A., Krucker T., O'Hagan D.T., Singh M., Mason P.W., Valiante N.M., Dormitzer P.R., Barnett S.W., Rappuoli R., Ulmer J.B., Mandl C.W. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. U.S.A. 2012;109:14604–14609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019;27 doi: 10.1016/j.ymthe.2019.02.012. 710-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kreiter S., Vormehr M., van de Roemer N., Diken M., Lower M., Diekmann J., Boegel S., Schrors B., Vascotto F., Castle J.C., Tadmor A.D., Schoenberger S.P., Huber C., Tureci O., Sahin U. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520 doi: 10.1038/nature14426. 692-U269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kauffman K.J., Dorkin J.R., Yang J.H., Heartlein M.W., DeRosa F., Mir F.F., Fenton O.S., Anderson D.G. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15:7300–7306. doi: 10.1021/acs.nanolett.5b02497. [DOI] [PubMed] [Google Scholar]
- 108.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vaheri A., Pagano J.S. Infectious poliovirus RNA: a sensitive method of assay. Virology. 1965;27:434–436. doi: 10.1016/0042-6822(65)90126-1. [DOI] [PubMed] [Google Scholar]
- 110.Bus T., Traeger A., Schubert U.S. The great escape: how cationic polyplexes overcome the endosomal barrier. J. Mater. Chem. B. 2018;6:6904–6918. doi: 10.1039/c8tb00967h. [DOI] [PubMed] [Google Scholar]
- 111.Moghimi S.M., Symonds P., Murray J.C., Hunter A.C., Debska G., Szewczyk A. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol. Ther. 2005;11:990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 112.Ke X.Y., Shelton L., Hu Y.Z., Zhu Y.N., Chow E., Tang H.Y., Santos J.L., Mao H.Q. Surface-functionalized PEGylated nanoparticles deliver messenger RNA to pulmonary immune cells. Acs Appl Mater Inter. 2020;12:35835–35844. doi: 10.1021/acsami.0c08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Breunig M., Lungwitz U., Liebl R., Goepferich A. Breaking up the correlation between efficacy and toxicity for nonviral gene delivery. P Natl Acad Sci USA. 2007;104:14454–14459. doi: 10.1073/pnas.0703882104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaczmarek J.C., Kauffman K.J., Fenton O.S., Sadder K., Patel A.K., Heartlein M.W., DeRosa F., Anderson D.G. Optimization of a degradable polymer-lipid nanoparticle for potent systemic delivery of mRNA to the lung endothelium and immune cells. Nano Lett. 2018;18:6449–6454. doi: 10.1021/acs.nanolett.8b02917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Patel A.K., Kaczmarek J.C., Bose S., Kauffman K.J., Mir F., Heartlein M.W., DeRosa F., Langer R., Anderson D.G. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater. 2019;31 doi: 10.1002/adma.201805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim H.J., Ogura S., Otabe T., Kamegawa R., Sato M., Kataoka K., Miyata K. Fine-tuning of hydrophobicity in amphiphilic polyaspartamide derivatives for rapid and transient expression of messenger RNA directed toward genome engineering in brain. Acs Central Sci. 2019;5:1866–1875. doi: 10.1021/acscentsci.9b00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y.H., Su H.H., Yang Y., Hu Y.X., Zhang L., Blancafort P., Huang L. Systemic delivery of modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Mol. Ther. 2013;21:358–367. doi: 10.1038/mt.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kong N., Zhang R.N., Wu G.W., Sui X.B., Wang J.Q., Kim N.Y., Blake S., De D.B., Xie T., Cao Y.H., Tao W. Intravesical delivery of KDM6A-mRNA via mucoadhesive nanoparticles inhibits the metastasis of bladder cancer. P Natl Acad Sci USA. 2022;119 doi: 10.1073/pnas.2112696119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang J.N., Arya S., Lung P.S., Lin Q.B., Huang J.D., Li Q. Hybrid nanovaccine for the co-delivery of the mRNA antigen and adjuvant. Nanoscale. 2019;11:21782–21789. doi: 10.1039/c9nr05475h. [DOI] [PubMed] [Google Scholar]
- 120.Lei S.B., Zhang X.Y., Men K., Gao Y., Yang X.J., Wu S.S., Duan X.M., Wei Y.Q., Tong R.S. Efficient colorectal cancer gene therapy with IL-15 mRNA nanoformulation. Mol. Pharm. 2020;17:3378–3391. doi: 10.1021/acs.molpharmaceut.0c00451. [DOI] [PubMed] [Google Scholar]
- 121.Zhang R., Men K., Zhang X.Y., Huang R., Tian Y.M., Zhou B.L., Yu C.H., Wang Y.T., Ji X., Hu Q.Y., Yang L. Delivery of a modified mRNA encoding IL-22 binding protein (IL-22BP) for colon cancer gene therapy. J. Biomed. Nanotechnol. 2018;14:1239–1251. doi: 10.1166/jbn.2018.2577. [DOI] [PubMed] [Google Scholar]
- 122.Maiyo F., Singh M. Folate-targeted mRNA delivery using chitosan-functionalized selenium nanoparticles: potential in cancer immunotherapy. Pharmaceut. Biol. 2019;12:164. doi: 10.3390/ph12040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mbatha L.S., Maiyo F., Daniels A., Singh M. Dendrimer-coated gold nanoparticles for efficient folate-targeted mRNA delivery in vitro. Pharmaceutics. 2021;13:900. doi: 10.3390/pharmaceutics13060900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu L.N., Wang Y.H., Miao L., Liu Q., Musetti S., Li J., Huang L. Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol. Ther. 2018;26:45–55. doi: 10.1016/j.ymthe.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zou Z., He L.B., Deng X.X., Wang H.X., Huang Z.Y., Xue Q., Qing Z.H., Lei Y.L., Yang R.H., Liu J.W. Zn2+-Coordination-Driven RNA assembly with retained integrity and biological functions. Angew. Chem., Int. Ed. 2021;60:22970–22976. doi: 10.1002/anie.202110404. [DOI] [PubMed] [Google Scholar]
- 126.Saxena M., van der Burg S.H., Melief C.J.M., Bhardwaj N. Therapeutic cancer vaccines. 2021;21:360–378. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 127.van der Burg S.H., Arens R., Ossendorp F., van Hall T., Melief A.J.M. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Cancer. 2016;16:219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 128.Pishesha N., Harmand T.J., Ploegh H.L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 2022 doi: 10.1038/s41577-41022-00707-41572. [DOI] [PubMed] [Google Scholar]
- 129.Wculek S.K., Cueto F.J., Mujal A.M., Melero I., Krummel M.F., Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 130.Bonehill A., Heirman C., Tuyaerts S., Michiels A., Breckpot K., Brasseur F., Zhang Y., van der Bruggen P., Thielemans K. Messenger RNA-electroporated dendritic cells presenting MAGE-A3 simultaneously in HLA class I and class II molecules. J. Immunol. 2004;172:6649–6657. doi: 10.4049/jimmunol.172.11.6649. [DOI] [PubMed] [Google Scholar]
- 131.Pastor F., Berraondo P., Etxeberria I., Frederick J., Sahin U., Gilboa E., Melero I. An RNA toolbox for cancer immunotherapy. Nat. Rev. Drug Discov. 2018;17:751–767. doi: 10.1038/nrd.2018.132. [DOI] [PubMed] [Google Scholar]
- 132.Van der Jeught K., De Koker S., Bialkowski L., Heirman C., Joe P.T., Perche F., Maenhout S., Bevers S., Broos K., Deswarte K., Malard V., Hammad H., Baril P., Benvegnu T., Jaffres P.A., Kooijmans S.A.A., Schiffelers R., Lienenklaus S., Midoux P., Pichon C., Breckpot K., Thielemans K. Dendritic cell targeting mRNA lipopolyplexes combine strong antitumor T-cell immunity with improved inflammatory safety. ACS Nano. 2018;12:9815–9829. doi: 10.1021/acsnano.8b00966. [DOI] [PubMed] [Google Scholar]
- 133.Hewitt S.L., Bailey D., Zielinski J., Apte A., Musenge F., Karp R., Burke S., Garcon F., Mishra A., Gurumurthy S., Watkins A., Arnold K., Moynihan J., Clancy-Thompson E., Mulgrew K., Adjei G., Deschler K., Potz D., Moody G., Leinster D.A., Novick S., Sulikowski M., Bagnall C., Martin P., Lapointe J.M., Si H., Morehouse C., Sedic M., Wilkinson R.W., Herbst R., Frederick J.P., Luheshi N. Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin. Cancer Res. 2020;26:6284–6298. doi: 10.1158/1078-0432.CCR-20-0472. [DOI] [PubMed] [Google Scholar]
- 134.Bol K.F., Schreibelt G., Gerritsen W.R., de Vries I.J.M., Figdor C.G. Dendritic cell-based immunotherapy: state of the art and beyond. Clin. Cancer Res. 2016;22:1897–1906. doi: 10.1158/1078-0432.CCR-15-1399. [DOI] [PubMed] [Google Scholar]
- 135.Harari A., Graciotti M., Bassani-Sternberg M., Kandalaft L.E. Antitumour dendritic cell vaccination in a priming and boosting approach. Nat. Rev. Drug Discov. 2020;19:635–652. doi: 10.1038/s41573-020-0074-8. [DOI] [PubMed] [Google Scholar]
- 136.Boczkowski D., Nair S.K., Snyder D., Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grippin A.J., Wummer B., Wildes T., Dyson K., Triyedi V., Yang C.L., Sebastian M., Mendez-Gomez H.R., Padala S., Grubb M., Fillingim M., Monsalve A., Sayour E.J., Dobson J., Mitchell D.A. Dendritic cell-activating magnetic nanoparticles enable early prediction of antitumor response with magnetic resonance imaging. ACS Nano. 2019;13:13884–13898. doi: 10.1021/acsnano.9b05037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tateshita N., Miura N., Tanaka H., Masuda T., Ohtsuki S., Tange K., Nakai Y., Yoshioka H., Akita H. Development of a lipoplex-type mRNA carrier composed of an ionizable lipid with a vitamin E scaffold and the KALA peptide for use as an ex vivo dendritic cell-based cancer vaccine. J. Contr. Release. 2019;310:36–46. doi: 10.1016/j.jconrel.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 139.Daneshmandi S., Pourfathollah A.A., Forouzandeh-Moghaddam M. Enhanced CD40 and ICOSL expression on dendritic cells surface improve anti-tumor immune responses; effectiveness of mRNA/chitosan nanoparticles. Immunopharmacol. Immunotoxicol. 2018;40:375–386. doi: 10.1080/08923973.2018.1510959. [DOI] [PubMed] [Google Scholar]
- 140.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y., Timmerman J.M., Stiff P.J., Friedberg J.W., Flinn I.W., Goy A., Hill B.T., Smith M.R., Deol A., Farooq U., McSweeney P., Munoz J., Avivi I., Castro J.E., Westin J.R., Chavez J.C., Ghobadi A., Komanduri K.V., Levy R., Jacobsen E.D., Witzig T.E., Reagan P., Bot A., Rossi J., Navale L., Jiang Y., Aycock J., Elias M., Chang D., Wiezorek J., Go W.Y. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., Qayed M., De Moerloose B., Hiramatsu H., Schlis K., Davis K.L., Martin P.L., Nemecek E.R., Yanik G.A., Peters C., Baruchel A., Boissel N., Mechinaud F., Balduzzi A., Krueger J., June C.H., Levine B.L., Wood P., Taran T., Leung M., Mueller K.T., Zhang Y., Sen K., Lebwohl D., Pulsipher M.A., Grupp S.A. Tisagenlecleucel in children and Young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Scarfo I., Maus M.V. Current approaches to increase CAR T cell potency in solid tumors: targeting the tumor microenvironment. J Immunother Cancer. 2017;5:28. doi: 10.1186/s40425-017-0230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schwechheimer C., Kuehn M.J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 2015;13:605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim O.Y., Park H.T., Dinh N.T.H., Choi S.J., Lee J., Kim J.H., Lee S.W., Gho Y.S. Bacterial outer membrane vesicles suppress tumor by interferon-gamma- mediated antitumor response. Nat. Commun. 2017;8:626. doi: 10.1038/s41467-017-00729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yin Y., Li X.Y., Ma H.X., Zhang J., Yu D., Zhao R.F., Yu S.J., Nie G.J., Wang H. In situ transforming RNA nanovaccines from polyethylenimine functionalized graphene oxide hydrogel for durable cancer immunotherapy. Nano Lett. 2021;21:2224–2231. doi: 10.1021/acs.nanolett.0c05039. [DOI] [PubMed] [Google Scholar]
- 146.Oberli M.A., Reichmuth A.M., Dorkin J.R., Mitchell M.J., Fenton O.S., Jaklenec A., Anderson D.G., Langer R., Blankschtein D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Islam M.A., Rice J., Reesor E., Zope H., Tao W., Lim M., Ding J.X., Chen Y.H., Aduluso D., Zetter B.R., Farokhzad O.C., Shi J.J. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials. 2021;266 doi: 10.1016/j.biomaterials.2020.120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jensen S., Thomsen A.R. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J. Virol. 2012;86:2900–2910. doi: 10.1128/JVI.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sahin U., Kariko K., Tureci O. mRNA-based therapeutics - developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 150.De Beuckelaer A., Grooten J., De Koker S. Type I interferons modulate CD8+ T Cell Immunity to mRNA Vaccines. 2017;23:216–226. doi: 10.1016/j.molmed.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 151.Gutjahr A., Tiraby G., Perouzel E., Verrier B., Paul S. Triggering intracellular receptors for vaccine adjuvantation (vol 37, pg 573, 2016) Trends Immunol. 2016;37 doi: 10.1016/j.it.2016.07.001. 716-716. [DOI] [PubMed] [Google Scholar]
- 152.Stetson D.B., Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 153.Crunkhorn S. Strengthening the sting of immunotherapy. Nat. Rev. Drug Discov. 2020;19 doi: 10.1038/d41573-020-00148-3. 669-669. [DOI] [PubMed] [Google Scholar]
- 154.Jeremiah N., Neven B., Gentili M., Callebaut I., Maschalidi S., Stolzenberg M.C., Goudin N., Fremond M.L., Nitschke P., Molina T.J., Blanche S., Picard C., Rice G.I., Crow Y.J., Manel N., Fischer A., Bader-Meunier B., Rieux-Laucat F. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J. Clin. Invest. 2014;124:5516–5520. doi: 10.1172/JCI79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tse S.W., McKinney K., Walker W., Nguyen M., Iacovelli J., Small C., Hopson K., Zaks T., Huang E. mRNA-encoded, constitutively active STING(V155M) is a potent genetic adjuvant of antigen-specific CD8(+) T cell response. Mol. Ther. 2021;29:2227–2238. doi: 10.1016/j.ymthe.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Borriello F., Poli V., Shrock E., Spreafico R., Liu X., Pishesha N., Carpenet C., Chou J., Di Gioia M., McGrath M.E., Dillen C.A., Barrett N.A., Lacanfora L., Franco M.E., Marongiu L., Iwakura Y., Pucci F., Kruppa M.D., Ma Z.C., Lowman D.W., Ensley H.E., Nanishi E., Saito Y., O'Meara T.R., Seo H.S., Dhe-Paganon S., Dowling D.J., Frieman M., Elledge S.J., Levy O., Irvine D.J., Ploegh H.L., Williams D.L., Zanoni I. An adjuvant strategy enabled by modulation of the physical properties of microbial ligands expands antigen immunogenicity. Cell. 2022;185:614–629. doi: 10.1016/j.cell.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Qi C.J., Cai Y.H., Gunn L., Ding C.L., Li B., Kloecker G., Qian K.Q., Vasilakos J., Saijo S., Iwakura Y., Yannelli J.R., Yan J. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived beta-glucans. Blood. 2011;117:6825–6836. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Son S., Nam J., Zenkov I., Ochyl L.J., Xu Y., Scheetz L., Shi J.J., Farokhzad O.C., Moon J.J. Sugar-nanocapsules imprinted with microbial molecular patterns for mRNA vaccination. Nano Lett. 2020;20:1499–1509. doi: 10.1021/acs.nanolett.9b03483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Verbeke R., Lentacker I., Breckpot K., Janssens J., Van Calenbergh S., De Smedt S.C., Dewitte H. Broadening the message: a nanovaccine Co-loaded with messenger RNA and alpha-GalCer induces antitumor immunity through conventional and natural killer T cells. ACS Nano. 2019;13:1655–1669. doi: 10.1021/acsnano.8b07660. [DOI] [PubMed] [Google Scholar]
- 160.Sayour E.J., Grippin A., De Leon G., Stoyer B., Rahman M., Karachi A., Wummer B., Moore G., Castillo-Caro P., Fredenburg K., Sarkisian M.R., Huang J.P., Deleyrolle L.P., Sahay B., Carrera-Justiz S., Mendez-Gomez H.R., Mitchell D.A. Personalized tumor RNA loaded lipid-nanoparticles prime the systemic and intratumoral milieu for response to cancer immunotherapy. Nano Lett. 2018;18:6195–6206. doi: 10.1021/acs.nanolett.8b02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Salomon N., Vascotto F., Selmi A., Vormehr M., Quinkhardt J., Bukur T., Schrors B., Loewer M., Diken M., Tureci O., Sahin U., Kreiter S. A liposomal RNA vaccine inducing neoantigen-specific CD4(+)T cells augments the antitumor activity of local radiotherapy in mice. OncoImmunology. 2020;9 doi: 10.1080/2162402X.2020.1771925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Salomon N., Selmi A., Grunwitz C., Kong A., Stanganello E., Neumaier J., Petschenka J., Diken M., Kreiter S., Tureci O., Sahin U., Vascotto F. Local radiotherapy and E7 RNA-LPX vaccination show enhanced therapeutic efficacy in preclinical models of HPV16(+) cancer. Cancer Immunol. Immunother. 2022;71:1975–1988. doi: 10.1007/s00262-021-03134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Basler L., Kowalczyk A., Heidenreich R., Fotin-Mleczek M., Tsitsekidis S., Zips D., Eckert F., Huber S.M. Abscopal effects of radiotherapy and combined mRNA-based immunotherapy in a syngeneic, OVA-expressing thymoma mouse model. Cancer Immunol. Immunother. 2018;67:653–662. doi: 10.1007/s00262-018-2117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Grunwitz C., Salomon N., Vascotto F., Selmi A., Bukur T., Diken M., Kreiter S., Tureci O., Sahin U. HPV16 RNA-LPX vaccine mediates complete regression of aggressively growing HPV-positive mouse tumors and establishes protective T cell memory. OncoImmunology. 2019;8 doi: 10.1080/2162402X.2019.1629259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xie G.Z., Dong H., Liang Y., Ham J.D., Rizwan R., Chen J.Z. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Budde L.E., Assouline S., Sehn L.H., Schuster S.J., Yoon S.S., Yoon D.H., Matasar M.J., Bosch F., Kim W.S., Nastoupil L.J., Flinn I.W., Shadman M., Diefenbach C., O'Hear C., Huang H., Kwan A., Li C.C., Piccione E.C., Wei M.C., Yin S., Bartlett N.L. Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-cell lymphomas: phase I dose-escalation study. J. Clin. Oncol. : official journal of the American Society of Clinical Oncology. 2022;40:481–491. doi: 10.1200/JCO.21.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Seung E., Xing Z., Wu L., Rao E.R., Cortez-Retamozo V., Ospina B., Chen L.Q., Beil C., Song Z.L., Zhang B.L., Levit M., Deng G.J., Hebert A., Kirby P., Li A.Q., Poulton E.J., Vicente R., Garrigou A., Piepenhagen P., Ulinski G., Sanicola-Nadel M., Bangari D.S., Qiu H.W., Pao L., Wiederschain D., Wei R.N., Yang Z.Y., Nabel G.J. A trispecific antibody targeting HER2 and T cells inhibits breast cancer growth via CD4 cells. Nature. 2022;603:328–334. doi: 10.1038/s41586-022-04439-0. [DOI] [PubMed] [Google Scholar]
- 169.Melero I., Shuford W.W., Newby S.A., Aruffo A., Ledbetter J.A., Hellstrom K.E., Mittler R.S., Chen L.P. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 170.Tolcher A.W., Sznol M., Hu-Lieskovan S., Papadopoulos K.P., Patnaik A., Rasco D., Di Gravio D., Huang B., Gambhire D., Chen Y., Thall A.D., Pathan N., Schmidt E.V., Chow L.Q.M. Phase ib study of Utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in combination with pembrolizumab (MK-3475) in patients with advanced solid tumors. Clin. Cancer Res. 2017;23:5349–5357. doi: 10.1158/1078-0432.CCR-17-1243. [DOI] [PubMed] [Google Scholar]
- 171.Segal N.H., Logan T.F., Hodi F.S., McDermott D., Melero I., Hamid O., Schmidt H., Robert C., Chiarion-Sileni V., Ascierto P.A., Maio M., Urba W.J., Gangadhar T.C., Suryawanshi S., Neely J., Jure-Kunkel M., Krishnan S., Kohrt H., Sznol M., Levy R. Results from an integrated safety analysis of Urelumab, an agonist anti-CD137 monoclonal antibody. Clin. Cancer Res. 2017;23:1929–1936. doi: 10.1158/1078-0432.CCR-16-1272. [DOI] [PubMed] [Google Scholar]
- 172.Garber K. Immune agonist antibodies face critical test. Nat. Rev. Drug Discov. 2020;19:3–5. doi: 10.1038/d41573-019-00214-5. [DOI] [PubMed] [Google Scholar]
- 173.Hewitt S.L., Bai A., Bailey D., Ichikawa K., Zielinski J., Karp R., Apte A., Arnold K., Zacharek S.J., Iliou M.S., Bhatt K., Garnaas M., Musenge F., Davis A., Khatwani N., Su S.V., MacLean G., Farlow S.J., Burke K., Frederick J.P. Durable anticancer immunity from intratumoral administration of IL-23, IL-36 gamma, and OX40L mRNAs. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aat9143. [DOI] [PubMed] [Google Scholar]
- 174.Hudis C.A. Drug therapy: trastuzumab - Mechanism of action and use in clinical practice. N. Engl. J. Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 175.Thran M., Mukherjee J., Ponisch M., Fiedler K., Thess A., Mui B.L., Hope M.J., Tam Y.K., Horscroft N., Heidenreich R., Fotin-Mleczek M., Shoemaker C.B., Schlake T. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Mol. Med. 2017;9:1434–1447. doi: 10.15252/emmm.201707678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Berraondo P., Sanmamed M.F., Ochoa M.C., Etxeberria I., Aznar M.A., Perez-Gracia J.L., Rodriguez-Ruiz M.E., Ponz-Sarvise M., Castanon E., Melero I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Leonard J.P., Sherman M.L., Fisher G.L., Buchanan L.J., Larsen G., Atkins M.B., Sosman J.A., Dutcher J.P., Vogelzang N.J., Ryan J.L. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90:2541–2548. [PubMed] [Google Scholar]
- 178.Coman D.R., deLong R.P., McCutcheon M. Studies on the mechanisms of metastasis. The distribution of tumors in various organs in relation to the distribution of arterial emboli. Cancer Res. 1951;11:648–651. [PubMed] [Google Scholar]
- 179.Lin Y.X., Wang Y., Ding J.X., Jiang A.P., Wang J., Yu M.A., Blake S., Liu S.S., Bieberich C.J., Farokhzad O.C., Mei L., Wang H., Shi J.J. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.aba9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Meyer R.A., Neshat S.Y., Green J.J., Santos J.L., Tuesca A.D. Targeting strategies for mRNA delivery. 2022;14 [Google Scholar]
- 181.Barbero F., Russo L., Vitali M., Piella J., Salvo I., Borrajo M.L., Busquets-Fité M., Grandori R., Bastús N.G., Casals E., Puntes V. formation of the protein corona: the interface between nanoparticles and the immune system. Semin. Immunol. 2017;34:52–60. doi: 10.1016/j.smim.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 182.Cullis P.R., Hope M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017;25:1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Patel S., Kim J., Herrera M., Mukherjee A., Kabanov A.V., Sahay G. Brief update on endocytosis of nanomedicines. Adv. Drug Deliv. Rev. 2019;144:90–111. doi: 10.1016/j.addr.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lai I., Swaminathan S., Baylot V., Mosley A., Dhanasekaran R., Gabay M., Felsher D.W. Lipid nanoparticles that deliver IL-12 messenger RNA suppress tumorigenesis in MYC oncogene-driven hepatocellular carcinoma. J Immunother Cancer. 2018;6:125. doi: 10.1186/s40425-018-0431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Haabeth O.A.W., Blake T.R., McKinlay C.J., Tveita A.A., Sallets A., Waymouth R.M., Wender P.A., Levy R. Local delivery of Ox40l, Cd80, and Cd86 mRNA kindles global anticancer immunity. Cancer Res. 2019;79:1624–1634. doi: 10.1158/0008-5472.CAN-18-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.George S., Miao D., Demetri G.D., Adeegbe D., Rodig S.J., Shukla S., Lipschitz M., Amin-Mansour A., Raut C.P., Carter S.L., Hammerman P., Freeman G.J., Wu C.J., Ott P.A., Wong K.K., Van Allen E.M. Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity. 2017;46:197–204. doi: 10.1016/j.immuni.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang X.Y., Men K., Zhang Y.F., Zhang R., Yang L., Duan X.M. Local and systemic delivery of mRNA encoding survivin-T34A by lipoplex for efficient colon cancer gene therapy. Int. J. Nanomed. 2019;14:2733–2751. doi: 10.2147/IJN.S198747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Johnson D.B., Nebhan C.A., Moslehi J.J., Balko J.M. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022;19:254–267. doi: 10.1038/s41571-022-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Soenen S.J., Rivera-Gil P., Montenegro J.M., Parak W.J., De Smedt S.C., Braeckmans K. Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano Today. 2011;6:446–465. [Google Scholar]
- 190.Peres T.V., Schettinger M.R.C., Chen P., Carvalho F., Avila D.S., Bowman A.B., Aschner M. Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. Bmc Pharmacol Toxico. 2016;17:57. doi: 10.1186/s40360-016-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zabirnyk O., Yezhelyev M., Seleverstov O. Nanoparticles as a novel class of autophagy activators. Autophagy. 2007;3:278–281. doi: 10.4161/auto.3916. [DOI] [PubMed] [Google Scholar]
- 192.Cui S.H., Wang Y.Y., Gong Y., Lin X., Zhao Y.N., Zhi D.F., Zhou Q., Zhang S.B. Correlation of the cytotoxic effects of cationic lipids with their headgroups. Toxicol Res-Uk. 2018;7:473–479. doi: 10.1039/c8tx00005k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Scheel B., Teufel R., Probst J., Carralot J.P., Geginat J., Radsak M., Jarrossay D., Wagner H., Jung G., Rammensee H.G., Hoerr I., Pascolo S. Toll-like receptor-dependent activation of several human blood cell types by protamine-condensed mRNA. Eur. J. Immunol. 2005;35:1557–1566. doi: 10.1002/eji.200425656. [DOI] [PubMed] [Google Scholar]
- 194.Westdorp H., Creemers J.H.A., van Oort I.M., Schreibelt G., Gorris M.A.J., Mehra N., Simons M., de Goede A.L., van Rossum M.M., Croockewit A.J., Figdor C.G., Witjes J.A., Aarntzen E.H.J.G., Mus R.D.M., Bruning M., Petry K., Gotthardt M., Barentsz J.O., de Vries I.J.M., Gerritsen W.R. Blood-derived dendritic cell vaccinations induce immune responses that correlate with clinical outcome in patients with chemo-naive castration-resistant prostate cancer. J Immunother Cancer. 2019;7:302. doi: 10.1186/s40425-019-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Papachristofilou A., Hipp M.M., Klinkhardt U., Fruh M., Sebastian M., Weiss C., Pless M., Cathomas R., Hilbe W., Pall G., Wehler T., Alt J., Bischoff H., Geissler M., Griesinger F., Kallen K.J., Fotin-Mleczek M., Schroder A., Scheel B., Muth A., Seibel T., Stosnach C., Doener F., Hong H.S., Koch S.D., Gnad-Vogt U., Zippelius A. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J Immunother Cancer. 2019;7:38. doi: 10.1186/s40425-019-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sebastian M., Papachristofilou A., Weiss C., Fruh M., Cathomas R., Hilbe W., Wehler T., Rippin G., Koch S.D., Scheel B., Fotin-Mleczek M., Heidenreich R., Kallen K.J., Gnad-Vogt U., Zippelius A. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive (R)) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer. 2014;14 doi: 10.1186/1471-2407-14-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hong H.S., Koch S.D., Scheel B., Gnad-Vogt U., Schroder A., Kallen K.J., Wiegand V., Backert L., Kohlbacher O., Hoerr I., Fotin-Mleczek M., Billingsley J.M. Distinct transcriptional changes in non-small cell lung cancer patients associated with multi-antigenic RNActive (R) CV9201 immunotherapy. OncoImmunology. 2016;5 doi: 10.1080/2162402X.2016.1249560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kubler H., Scheel B., Gnad-Vogt U., Miller K., Schultze-Seemann W., vom Dorp F., Parmiani G., Hampel C., Wedel S., Trojan L., Jocham D., Maurer T., Rippin G., Fotin-Mleczek M., von der Mulbe F., Probst J., Hoerr I., Kallen K.J., Lander T., Stenzl A. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J Immunother Cancer. 2015;3:26. doi: 10.1186/s40425-015-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mackensen A., Koenecke C., Haanen J., Alsdorf W., Desuki A., Wagner-Drouet E., Heudobler D., Borchmann P., Wiegert E., Schulz C., Rengstl B., Preussner L., Tuereci O., Sahin U. Bnt211: a phase I/ii trial to evaluate safety and efficacy of Cldn6 car-T cells and vaccine-mediated in vivo expansion in patients with cldn6-positive advanced solid tumors. J Immunother Cancer. 2021;9 A1008-A1008. [Google Scholar]
- 200.Cafri G., Gartner J.J., Zaks T., Hopson K., Levin N., Paria B.C., Parkhurst M.R., Yossef R., Lowery F.J., Jafferji M.S., Prickett T.D., Goff S.L., McGowan C.T., Seitter S., Shindorf M.L., Parikh A., Chatani P.D., Robbins P.F., Rosenberg S.A. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Invest. 2020;130:5976–5988. doi: 10.1172/JCI134915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Porciuncula A., Morgado M., Gupta R., Syrigos K., Meehan R., Zacharek S.J., Frederick J.P., Schalper K.A. Spatial mapping and immunomodulatory role of the OX40/OX40L pathway in human non-small cell lung cancer. Clin. Cancer Res. 2021;27:6174–6183. doi: 10.1158/1078-0432.CCR-21-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Leppek K., Byeon G.W., Kladwang W., Wayment-Steele H.K., Kerr C.H., Xu A.D.F., Kim D., Topkar V.V., Choe C., Rothschild D., Tiu G.C., Wellington-Oguri R., Fujii K., Sharma E., Watkins A.M., Nicol J.J., Romano J., Tunguz B., Diaz F., Cai H., Guo P.B., Wu J.W., Meng F.Y., Shi S., Participants E., Dormitzer P.R., Solorzano A., Barna M., Das R. Combinatorial optimization of mRNA structure, stability, and translation for RNA-based therapeutics. Nat. Commun. 2022;13:1536. doi: 10.1038/s41467-022-28776-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bloom K., van den Berg F., Arbuthnot P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021;28:117–129. doi: 10.1038/s41434-020-00204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rappaport A.R., Hong S.J., Scallan C.D., Gitlin L., Akoopie A., Boucher G.R., Egorova M., Espinosa J.A., Fidanza M., Kachura M.A., Shen A.N., Sivko G., Van Abbema A., Veres R.L., Jooss K. Low-dose self-amplifying mRNA COVID-19 vaccine drives strong protective immunity in non-human primates against SARS-CoV-2 infection. Nat. Commun. 2022;13:3289. doi: 10.1038/s41467-022-31005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jain R.K. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990;50:814s–819s. [PubMed] [Google Scholar]
- 206.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020;15:313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Qiu M., Tang Y., Chen J.J., Muriph R., Ye Z.F., Huang C.F., Evans J., Henske E.P., Xu Q.B. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. P Natl Acad Sci USA. 2022;119:119. doi: 10.1073/pnas.2116271119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gohil S.H., Iorgulescu J.B., Braun D.A., Keskin D.B., Livak K.J. Applying high-dimensional single-cell technologies to the analysis of cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021;18:244–256. doi: 10.1038/s41571-020-00449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xu C., Lu Z., Luo Y., Liu Y., Cao Z., Shen S., Li H., Liu J., Chen K., Chen Z., Yang X., Gu Z., Wang J. Targeting of NLRP3 inflammasome with gene editing for the amelioration of inflammatory diseases. Nat. Commun. 2018;9:4092. doi: 10.1038/s41467-018-06522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang Y., Shen S., Zhao G., Xu C.F., Zhang H.B., Luo Y.L., Cao Z.T., Shi J., Zhao Z.B., Lian Z.X., Wang J. In situ repurposing of dendritic cells with CRISPR/Cas9-based nanomedicine to induce transplant tolerance. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119302. [DOI] [PubMed] [Google Scholar]