Abstract
In general, gamma interferon (IFN-γ)-producing CD4+ Th1 cells are important for the immunological control of intracellular pathogens. We previously demonstrated an association between parasite-specific induction of IFN-γ responses and resistance to the intracellular protozoan Trypanosoma cruzi. To investigate a potential causal relationship between Th1 responses and T. cruzi resistance, we studied the ability of Th1 cells to protect susceptible BALB/c mice against virulent parasite challenges. We developed immunization protocols capable of inducing polarized Th1 and Th2 responses in vivo. Induction of parasite-specific Th1 responses, but not Th2 responses, protected BALB/c mice against virulent T. cruzi challenges. We generated T. cruzi-specific CD4+ Th1 and Th2 cell lines from BALB/c mice that were activated by infected macrophages to produce their corresponding cytokine response profiles. Th1 cells, but not Th2 cells, induced nitric oxide production and inhibited intracellular parasite replication in T. cruzi-infected macrophages. Despite the ability to inhibit parasite replication in vitro, Th1 cells alone could not adoptively transfer protection against T. cruzi to SCID mice. In addition, despite the fact that the adoptive transfer of CD4+ T lymphocytes was shown to be necessary for the development of immunity protective against primary T. cruzi infection in our SCID mouse model, protective secondary effector functions could be transferred to SCID mice from memory-immune BALB/c mice in the absence of CD4+ T lymphocytes. These results indicate that, although CD4+ Th1 cells can directly inhibit intracellular parasite replication, a more important role for these cells in T. cruzi systemic immunity may be to provide helper activity for the development of other effector functions protective in vivo.
Trypanosoma cruzi is the protozoan parasite causing Chagas' disease in South and Central America. The T. cruzi life cycle is complex and includes both extracellular and intracellular forms in the mammalian host. Extracellular blood form trypomastigotes (BFT) circulate in the blood and lymph and can infect many different types of nucleated mammalian target cells. After infection of host cells, BFT differentiate into intracellular amastigotes (AMA), the life stage of T. cruzi responsible for replication within the mammalian host. During the first few weeks of T. cruzi infection in humans, BFT may be detected by microscopic examination of fresh blood. By the end of the first 2 months of infection, BFT decrease to undetectable levels as intracellular AMA proliferation is controlled by innate and adaptive immune responses, but low levels of intracellular tissue parasitism persist for the life of the host (18, 40). Mice infected with T. cruzi have been used as a model for the human disease because they also develop detectable parasitemias during acute infection, followed by chronic tissue parasitism. Different strains of mice exhibit various patterns of acute disease, which also vary depending on the different isolates of T. cruzi used. BALB/c mice are relatively susceptible to infection with the Tulahuén strain of T. cruzi in that they develop high-level parasitemias which can lead to mortality in a large proportion of animals after challenge with infective parasites (3, 31, 43, 49, 52). C57BL/6 mice are relatively resistant to similar challenges (26, 35, 36, 48, 49). Patterns of susceptibility and resistance to T. cruzi have been shown to be determined by factors other than the genetic haplotype at the H-2 locus alone (47, 53). Comparisons of immune responses activated by the Tulahuén strain of T. cruzi infection in these different mouse strains can be useful as one model system for the identification of factors associated with resistance.
CD4+ Th1 lymphocytes that produce the cytokines interleukin-2 (IL-2) and gamma interferon (IFN-γ) have been shown to be important for systemic protection against a wide spectrum of intracellular pathogens (reviewed in reference 1). This type of CD4+ T cell induces macrophage activation leading to the inhibition of intracellular replication of many pathogens. In addition, CD4+ Th1 cells can be directly cytolytic for infected cells and can help in the expansion of cytotoxic CD8+ T lymphocytes, which recognize and destroy infected cells. Therefore, it is predicted that CD4+ Th1 lymphocytes are important for protection against T. cruzi infection. IFN-γ has been identified as a resistance factor in T. cruzi infections (20, 32, 46). The administration of recombinant IFN-γ to mice increases their resistance, while the in vivo neutralization of IFN-γ with monoclonal antibodies increases susceptibility. These studies demonstrated that circulating IFN-γ is crucial for the control of an ongoing acute T. cruzi infection but did not address the potential relevance of IFN-γ responses for memory immunity induced by protective vaccines. Parasite antigens have been shown to induce increased IFN-γ mRNA and protein levels in lymphocytes from T. cruzi-infected mice, indicating that antigen-specific immune cells capable of secreting IFN-γ are present during parasite infection (27). Furthermore, we have shown that during acute T. cruzi infection, antigen-specific lymphocytes that secrete high levels of IFN-γ after stimulation with parasite lysate in vitro develop in T. cruzi-resistant C57BL/6 mice but not in susceptible BALB/c mice (9). These results suggest that the induction of T. cruzi-specific CD4+ Th1 lymphocytes by vaccines prior to T. cruzi infection could be protective against parasite challenge.
In the present work, we have extended these earlier observations by directly investigating the relationship between parasite-specific CD4+ Th1 responses and protection against T. cruzi infection. We first examined the ability of immunization protocols that induce T. cruzi-specific CD4+ Th1 cell responses to protect relatively susceptible BALB/c mice against virulent parasite challenges. We generated stable parasite-specific Th1 and Th2 cell lines and studied the ability of these cells to inhibit T. cruzi replication in vitro. We also investigated the ability of our T. cruzi-specific CD4+ Th1 cell lines to adoptively transfer protection against T. cruzi infection. Finally, we studied total and CD4-depleted, naive and memory immune lymphocytes for their ability to transfer protection to SCID mice. The combined results of these experiments indicate that although CD4+ Th1 cells can mediate both direct effector and helper functions for protective T. cruzi immunity, the helper functions may be more important in vivo.
MATERIALS AND METHODS
Cytokine, antibody, and antigen reagents.
Recombinant murine IFN-γ was supplied by Genentech, Inc. (South San Francisco, Calif.) and had a specific activity of 9.8 × 106 U/ml (7.35 × 106 U/mg). Recombinant murine IL-12 (5.6 × 106 U/mg) was obtained from Stanley F. Wolf (Genetics Institute, Cambridge, Mass.). Concentrated recombinant murine IL-4 was produced in a Cellmax chamber (Spectrum, Laguna Hills, Calif.) inoculated with P815 cells transfected with the murine IL-4 cDNA (obtained from H. Karasuyama, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan [11]). Murine IL-2 was purchased from Boehringer Mannheim Corp., Indianapolis, Ind. The recombinant murine cytokines IL-4, IL-5, and IL-10 were purchased from Genzyme Corp., Cambridge, Mass. The hybridoma cell lines producing the IFN-γ- and IL-4-neutralizing monoclonal antibodies R4-6A2 and 11B11, respectively, were obtained from the American Type Culture Collection (ATCC) and were grown in Cellmax chambers (Spectrum) to produce concentrated antibodies. The monoclonal antibodies GK1.5, Lyt2, and PK136 were prepared similarly from hybridoma cells obtained from ATCC. Antigenic lysates of T. cruzi culture-derived metacyclic trypomastigotes grown in Grace's media were prepared as described previously (9).
Parasites, mice, and T. cruzi challenge protocols.
The life cycle of the Tulahuén strain of T. cruzi was maintained by passage through mice and the reduviid vector Dipetalogaster maximus (16). To collect insect-derived metacyclic trypomastigotes (IMT), infected reduviid bugs were allowed to feed on anesthetized mice and then engorged insects were incubated in glass vials for 3 to 4 h. Excreta from multiple reduviids were pooled, and the concentration of IMT was determined by direct hemocytometer count. BFT were maintained by intraperitoneal passage in BALB/c mice. To prepare BFT for virulent challenges, C.B-17 SCID mice were infected intraperitoneally with 20,000 to 50,000 BFT and 2 to 3 weeks later these animals were sacrificed by decapitation and bled into 50-ml conical tubes containing 5 μl of preservative-free heparin (1,000 U/ml) per animal. The mononuclear cell layers of heparinized blood containing T. cruzi BFT were purified over Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) density gradients spun at 800 × g for 15 min at room temperature. After the purified cells were washed with PBS, the concentrations of BFT were determined by hemocytometer.
BALB/c female mice were obtained from Harlan Sprague-Dawley, Inc. (Indianapolis, Ind.) at 6 to 8 weeks of age and were housed in pathogen-free conditions in the Department of Comparative Medicine at Saint Louis University. C.B-17 SCID mice were obtained from Charles River Laboratories (Wilmington, Mass.) and housed in sterile pathogen-free conditions. SCID mice used in adoptive transfer experiments were 4- to 6-week-old weanlings.
An aggressive challenge with T. cruzi BFT was used in immunocompetent BALB/c mice to study systemic protective immunity. BALB/c mice were injected subcutaneously with 5,000 to 20,000 BFT, and parasitemia and mortality levels were assessed. After adoptive transfer of different immune-cell preparations to C.B-17 SCID mice, these animals were challenged with either 1,000 IMT or 5,000 to 20,000 BFT subcutaneously in the base of the tail. The IMT challenge was chosen for experiments involving transfers of Th1 cells alone or naive BALB/c mouse spleen cells. This challenge was found in preliminary experiments to be universally fatal to control SCID mice but sublethal to naive BALB/c mice and to SCID mice reconstituted with total naive BALB/c mouse spleen cells. The BFT challenge was used in SCID mice adoptively transferred with lymphocytes from memory-immune mice. This challenge was lethal for naive control BALB/c mice, but BALB/c mice previously surviving a T. cruzi challenge (memory immune) almost always survived.
Preparation of lymphocyte populations.
Lymphocytes were harvested from control and memory-immune BALB/c mice. Memory immune BALB/c mice had survived acute T. cruzi infection and had been rechallenged subcutaneously with 5,000 to 20,000 BFT 2 weeks before harvesting the draining lymph nodes and/or spleen cells. In studies of the cytokine responses produced by purified CD4+ T cells from immunized mice and in experiments involving adoptive transfers of different fractions of BALB/c splenic lymphocytes into C.B-17 SCID mice, immunomagnetic purifications and depletions of CD4+ T cells were performed. Draining lymph node cells from immunized mice or spleen cells from uninfected mice and memory immune mice were incubated with Miltenyi anti-CD4 MicroBeads, and then CD4+ and CD4− cells were separated in MiniMACS columns as recommended by the manufacturer (Miltenyi Biotec Inc., Auburn, Calif.). Fractionated populations were passed through a second set of MiniMACS columns, resulting in greater than 99% purity of CD4+ T-cell populations and greater than 99% depletion of CD4+ T cells from the CD4− populations.
Induction of T. cruzi-specific Th1 and Th2 cell responses in vivo.
Several variations of Th1 and Th2 immunization bias protocols were studied in early experiments. Vaccination parameters investigated included intraperitoneal versus subcutaneous routes, the delivery of cytokines for 1 to 3 days with each immunization, and doses of cytokine-neutralizing antibodies. Later experiments focused on the following protocols found to yield the most reproducible results. BALB/c mice were immunized subcutaneously with 10 to 25 μg of T. cruzi whole lysate in 50 μl of phosphate-buffered saline. To bias for Th1 responses, 1 μg of recombinant IL-12 was injected subcutaneously on three consecutive days beginning 1 day prior to immunization (mixed with antigen on the day of immunization). For additional Th1 bias, 0.5 mg of 11B11 monoclonal anti-IL-4 was injected intraperitoneally 1 day before and then again 2 days after immunization. To bias for Th2 responses, 500 U of recombinant IL-4 was injected subcutaneously on three consecutive days beginning 1 day prior to immunization (mixed with antigen on the day of immunization). For additional Th2 bias, 0.5 mg of R4-6A2 monoclonal anti-IFN-γ was injected intraperitoneally 1 day before and then again 2 days after immunization. Draining lymph node and spleen cells from immunized animals were harvested 3 to 14 days later and stimulated in vitro with 2 or 20 μg of T. cruzi whole lysate/ml without IL-12, IL-4, or cytokine-specific antibodies. After 3 to 4 days of incubation at 37°C with 5% CO2, culture supernatants were harvested and tested for levels of IFN-γ and IL-4 as previously described (9). In immunization and challenge experiments BALB/c mice received three vaccinations (the first two with, and the third without, cytokines and/or antibodies) 10 to 14 days apart prior to challenge with T. cruzi BFT 2 to 4 weeks after the last immunization.
Generation of T. cruzi-specific CD4+ Th1 and Th2 cell lines.
BALB/c mice were immunized three times with 25 μg of T. cruzi whole lysate including the Th1 and Th2 biasing protocols described above. Ten days after the last immunization, draining lymph node cells were harvested and stimulated in vitro (4 × 106 cells/ml) with parasite lysate (10 μg/ml) in 24-well tissue culture plates plus IL-12 (1 ng/ml) for Th1 bias or IL-4 (100 U/ml) for Th2 bias. One week later viable cells were purified over Ficoll-Paque (Pharmacia Biotech) and expanded with recombinant IL-2 in fresh media. Two weeks after the initial in vitro stimulation, this 2-week cycle of parasite lysate plus either IL-12 or IL-4 stimulation followed by IL-2 expansion was repeated. On the day of parasite lysate antigen stimulation, irradiated syngeneic spleen cells from normal BALB/c mice were added as antigen-presenting cells (APC). After these first two antigen stimulation cycles, T cells were passaged with alternating weekly schedules of parasite lysate stimulation (without IL-12 or IL-4) followed by IL-2 expansion. Similar results were obtained when spleen cells from BALB/c mice hyperimmunized by multiple T. cruzi infectious challenges were harvested and subjected to the same in vitro Th1 and Th2 bias protocols.
After at least four cycles of in vitro expansion, T-cell supernatants were evaluated 2 days after stimulation with antigen and irradiated APC for IFN-γ and IL-4 levels with the methods described above. To determine the subset of T cells present in established T-cell lines, viable cells were stained with fluorescently labeled anti-CD3, anti-CD4, and anti-CD8 antibodies (PharMingen, San Diego, Calif.) 2 weeks after the last antigen stimulation and analyzed by three-color flow cytometry (see below). SCID mice were subjected to adoptive transfer with 5 × 106 of the Th1 cells 14 days after antigen stimulation and 7 days after IL-2 expansion. SCID mice were challenged with T. cruzi 1 day after adoptive transfer with T-cell populations.
In vitro protection assays.
BALB/c mice were given 100 μg of concanavalin A intraperitoneally to activate peritoneal macrophages. Four days later, the peritoneal exudate macrophages (PEM) were harvested from these mice and cultured in eight-well tissue culture slide chambers (Nalge Nunc International, Naperville, Ill.) at 1.25 × 106 cells/well in 500-μl of Dulbecco modified Eagle medium (DMEM)–10% fetal calf serum (FCS). PEM were allowed to adhere for 2 to 5 h at 37°C, washed twice with DMEM–2% FCS, and infected with 6.25 × 106 culture-derived metacyclic trypomastigotes (multiplicity of infection of 5). These cultures were incubated 3 h longer at 37°C and then washed three times to remove extracellular parasites. Ficoll-Paque-purified T cells were added at 5 × 104 cells/well. Forty-eight hours after the addition of T cells, supernatants were collected from these cocultures. Adherent PEM were then fixed with 1% formalin and Giemsa stained (Diff Quik; Dade International Inc., Miami, Fla.). The number of infected cells per 200 total cells and the average number of intracellular AMA per infected cell were determined by microscopic examination of the stained slides. IFN-γ and IL-4 levels in the culture supernatants were measured by enzyme-linked immunosorbent assay as described previously (9), and nitric oxide (NO) levels in the supernatants were measured by a modified Greiss reaction (7, 8). Controls included uninfected wells to confirm the antigen specificity of cytokine production and wells to which T cells were not added to determine the level of infection in the absence of any immune response. Inhibitions of the percentage of cells infected and the average number of AMA/infected cell were defined as 1 − (value with T cells/value without T cells) × 100.
Detection of T. cruzi-specific Th1 cells expanded in vivo after adoptive transfer.
Spleen cell suspensions were prepared from adoptively transferred SCID mice and stimulated in vitro with parasite lysate or media. After 4 days of stimulation, culture supernatants were tested for T. cruzi-specific induction of IFN-γ and IL-4 as described above. The spleen cell cultures were fed fresh media and incubated for three additional days before harvesting viable mononuclear cells over Ficoll-Paque for fluorescence-activated cell sorter (FACS) staining.
FACS analyses.
Viable cells were stained with fluorescently labeled antibodies specific for T- and B-cell surface markers and analyzed by two- or three-color flow analysis with a Becton Dickinson FACSCalibur flow cytometer. The following antibody combinations were used: (i) anti-CD3-fluorescein isothiocyanate (FITC), anti-CD4-phosphatidylethanolamine (PE), and anti-CD8-Cy-Chrome; (ii) anti-CD3-FITC and anti-CD4-PE; (iii) anti-CD8-FITC and anti-CD4-PE; and (iv) anti-CD3-FITC and anti-B220-PE. All antibodies were obtained from PharMingen. Lymphocytes were selected for analysis based upon appropriate forward- and side-scatter gating, and the percentages of positively stained cells with each lymphocyte surface marker were determined by using isotype-matched fluorescent control antibodies to set quadrant demarcations in two-parameter dot plots.
RESULTS
Induction of T. cruzi-specific Th1 and Th2 cell responses in vivo.
To directly address a potential causal relationship between CD4+ Th1 cells and natural resistance to T. cruzi, we developed immunization protocols that could differentially induce Th1 and Th2 cells specific for T. cruzi antigens. BALB/c mice were immunized with whole T. cruzi lysate mixed with cytokines and anticytokine antibodies as described in Materials and Methods. Total draining lymph node cells and purified CD4+ T cells were harvested from these mice after immunization and were stimulated in vitro with whole T. cruzi lysate prior to testing the culture supernatants for levels of IFN-γ and IL-4. Figure 1 shows the results of one typical immunization experiment. Immunization with parasite lysate, IL-12, and neutralizing anti-IL-4 monoclonal antibody resulted in CD4+ T cells capable of producing high levels of IFN-γ but no IL-4 after in vitro stimulation. After immunization with parasite lysate, IL-4, and neutralizing anti-IFN-γ monoclonal antibody, CD4+ T cells produced IL-4 and not IFN-γ in vitro. These polarized cytokine response profiles were reproducibly seen especially after identical booster vaccinations (see Th1×2 responses in Fig. 1). Cytokine supernatants containing IFN-γ without IL-4 were also found to contain IL-2 but not IL-5 or IL-10, while cytokine supernatants containing IL-4 without IFN-γ were found to contain IL-5 and IL-10 but not IL-2 (data not shown). The latter results further document the selective Th1 and Th2 cytokine profiles induced by these differential immunization protocols.
FIG. 1.
Immunization protocols including cytokines and cytokine-neutralizing monoclonal antibodies as biological modifiers can induce highly polarized T. cruzi-specific Th1 and Th2 responses. BALB/c mice were immunized subcutaneously with T. cruzi lysate and either IL-12 plus the IL-4-neutralizing monoclonal antibody 11B11 (Th1) or IL-4 plus the IFN-γ-neutralizing monoclonal antibody R4-6A2 (Th2). Two weeks later, half of the animals were given a booster immunization identical to their first immunization. Two weeks after the booster vaccinations (4 weeks after the primary vaccinations), all mice were injected subcutaneously with T. cruzi lysate alone. Three days later, spleen cells were harvested from these different groups of immunized mice and were stimulated in vitro with T. cruzi lysate. The levels of IFN-γ and IL-4 secreted into culture supernatants were compared. Th1×1 and Th2×1, responses from mice given single vaccinations with cytokine bias; Th1×2 and Th2×2, responses from mice given booster vaccinations with cytokine bias. Shown are the mean (± standard errors) levels of cytokines in culture supernatants from one experiment representative of three separate experiments. LNC, lymph node cells.
Immunization protocols inducing T. cruzi-specific Th1 cells are protective against parasite challenge.
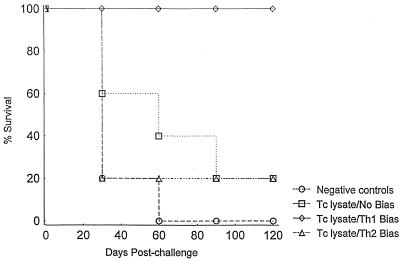
After developing immunization protocols that could induce differential Th1 and Th2 parasite-specific responses prior to infection, we next studied the effects of these immunization protocols on the levels of protective immunity against T. cruzi challenge. BALB/c mice were immunized with the Th1 and Th2 immunization protocols described above three times 10 to 14 days apart and challenged with 5,000 to 20,000 T. cruzi BFT. Presented in Table 1 are the parasitemia and overall mortality results, and presented in Fig. 2 are the detailed survival curves from one representative experiment. Unimmunized negative-control mice all died of overwhelming parasitemias within the first 2 months after BFT challenge. Mice immunized with parasite lysate alone or with the Th2-inducing immunization protocol also developed high-level parasitemias and almost all died. However, all mice immunized with the protocol known to induce Th1 cell responses were protected against the T. cruzi challenge. Mean peak parasitemia levels were 1 log unit lower in the Th1 induction group, and 100% of these animals survived for more than 4 months after T. cruzi challenge. Furthermore, the parasitemias detected in the Th2 bias group, but not in the control immunized group, were significantly increased compared with the parasitemias detected in the Th1 bias group (P < 0.05 by t test), suggesting that the induction of Th2 responses actually led to a decrease in protective immunity. Similar results were seen in multiple experiments with the following cumulative results for mortality in the experimental groups: (i) controls, 14 of 15 died; (ii) immunization with Th1 bias, 1 of 15 died; and (iii) immunization with Th2 bias, 9 of 10 died. Survival was significantly greater in the Th1 bias group than in either of the other groups, as determined by two-tailed Fisher exact tests (P < 0.01). Overall, these results demonstrated that the selective induction of T. cruzi-specific Th1 responses, but not Th2 responses, was associated with protection against a normally lethal systemic parasite challenge.
TABLE 1.
Th1 immunizations are protective against lethal T. cruzi challengea
| Immunization | Mean peak parasitemiab (BFT/ml) ± SE | Mortalityc 2 mo postchallenge (%) |
|---|---|---|
| None (negative control) | >2 × 106 | 5/5 (100) |
| T. cruzi lysate | 9.9 × 105 ± 3 × 105 | 4/5 (80) |
| T. cruzi lysate + Th1 biasd | 1.5 × 105 ± 5.4 × 104 | 0/5 (0) |
| T. cruzi lysate + Th2 biasd | 2 × 106 ± 8.7 × 105 | 4/5 (80) |
Shown are the results of one representative immunization and challenge experiment. Mice were unimmunized negative-control mice or mice immunized three times with parasite lysate. The first two immunizations were biased for Th1 or Th2 response, as described in Materials and Methods. Two weeks after the last immunization, mice were challenged with T. cruzi BFT.
Parasitemia levels were determined by microscopic examination of fresh blood every 3 to 4 days after T. cruzi infection. Shown are peak parasitemia levels which occurred between 3 and 4 weeks after challenge in all experimental groups (n = 5 for each group). All deaths occurred within 6 weeks of infection. All surviving mice were alive 3 months postchallenge.
Number of mice that died/total number of mice in the group.
Th1 (Th2) bias, immunization designed to bias for a Th1 (Th2) response.
FIG. 2.
Survival after Th1- and Th2-polarized immunizations and virulent T. cruzi challenge. Mice were immunized with T. cruzi lysate with no bias, Th1 bias, or Th2 bias, similar to the experiments described in the legend for Fig. 1. Each mouse received three vaccinations 2 weeks apart. The Th1 and Th2 groups received the cytokine-antibody treatments with the first two vaccinations. All immunized mice received only parasite lysate with the third vaccination. Two weeks after the last vaccination, mice were challenged with virulent T. cruzi BFT. Cumulative survival is shown for each group (n = 5 mice/group) from one experiment representative of three separate experiments. Tc, T. cruzi.
T. cruzi-specific CD4+ T cells induced in vivo can be expanded in vitro to generate stable Th1 and Th2 cell lines.
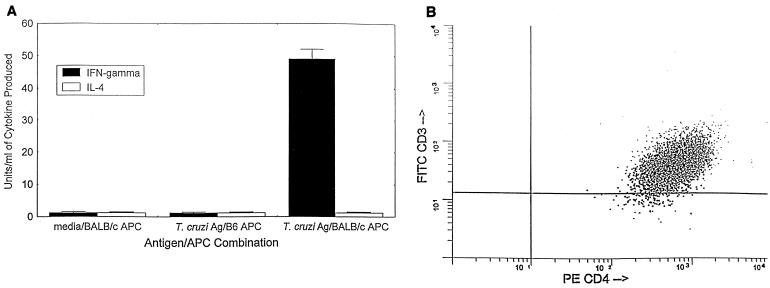
Our findings that (i) Th1 cells develop during T. cruzi infection in resistant mice and (ii) the induction of Th1 cells in relatively susceptible mice reduces parasitemias and mortality after parasite challenge strongly indicated that CD4+ Th1 cells are important for protective immunity against T. cruzi. However, previous investigators have published studies indicating that CD8+ T lymphocytes (33, 34, 44, 45) and antibody responses (13–15, 19, 21, 36, 38, 39, 42) are important for protection against this parasite, and the results described above have not addressed whether these other immune responses were important in our resistant-mouse model or in the Th1 immunization model. Therefore, our next goal was to study the possibility that purified CD4+ Th1 cells could protect SCID mice from T. cruzi challenge in the absence of other antigen-specific immune responses. We generated long-term CD4+ Th1 and Th2 cells specific for T. cruzi whole lysate as described in Materials and Methods. Figure 3 demonstrates that the Th1 cells were T. cruzi specific, major histocompatibility complex (MHC) restricted, and greater than 99% CD3+ and CD4+. In the experiments presented in Fig. 3A, we stimulated the T. cruzi-specific Th1 cell line with either medium alone or parasite lysate, in the presence of irradiated normal spleen cells harvested from either syngeneic BALB/c mice (H-2d) or allogeneic C57BL/6 mice (H-2b) as APC, and then measured the levels of IFN-γ and IL-4 secreted into the culture supernatants. The T. cruzi-specific T cells did not produce detectable levels of either IFN-γ or IL-4 after incubation with media alone in the presence of MHC-compatible APC or after incubation with parasite lysate in the presence of MHC-mismatched APC. However, after stimulation with parasite lysate in the presence of MHC-compatible APC, these same T cells produced almost 50 U of IFN-γ/ml in the absence of detectable IL-4. These results demonstrate that these T cells are parasite antigen specific and MHC restricted and that they produce a cytokine profile consistent with the Th1 type. These T cells also produced IL-2, but not IL-5, IL-6, or IL-10, in an antigen-specific and MHC-restricted fashion (data not shown), further confirming their Th1 cytokine profile. In Fig. 3B, it can be seen that virtually all of these T cells express both the CD3 and CD4 proteins, detectable by FACS, on their surfaces. Coexpression of CD8 on these T cells was not detectable at any time point (data not shown). These results confirm that these T. cruzi-specific T cells are classical CD4+ Th1 cells. Similar experiments confirmed the antigen specificity, MHC restriction, and CD4+ CD8− surface phenotype of the Th2 cells generated (data not shown).
FIG. 3.
T. cruzi-specific and MHC-restricted Th1 cells were generated from BALB/c mice immunized with a Th1 bias protocol similar to that for the experiments described in the legends for Fig. 1 and 2. Long-term T. cruzi-specific Th1 cell lines were generated as described in Materials and Methods. These T cells were antigen specific and MHC restricted and produced a Th1 cytokine profile (A). This T-cell line was incubated with either medium alone or parasite lysate in the presence of irradiated spleen cells harvested from either syngeneic BALB/c (H-2d) or allogeneic C57BL/6 mice (H-2b), and then the levels of IFN-γ and IL-4 secreted into the culture supernatants were measured. (B) Results of FACS staining confirming that essentially all of the T cells present in this T. cruzi-specific Th1 cell line were CD3+ and CD4+. Three-color analyses demonstrated that these T cells did not coexpress CD8 (data not shown). Ag, antigen.
T. cruzi-specific Th1 cells, but not Th2 cells, prevent intracellular parasite replication in vitro.
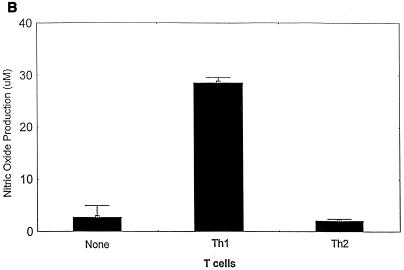
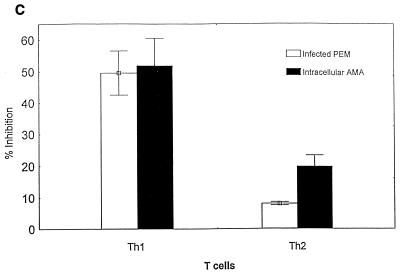
The ability of parasite-specific Th1 and Th2 cells to protect against T. cruzi infection was tested in an in vitro model (Fig. 4). T cells were incubated with T. cruzi-infected macrophages, and cytokine and NO responses were measured as described in Materials and Methods. Cocultures of Th1 or Th2 cells with uninfected macrophages did not produce IFN-γ or IL-4 levels above background (data not shown). In addition, infected macrophages cultured in the absence of T cells did not produce IFN-γ, IL-4, or NO levels above background (Fig. 4A and B), and as many as 50 to 75% of these macrophages were found to contain intracellular AMA by Giemsa staining. However, the supernatants of cocultures of parasite-specific Th1 cells and infected macrophages contained high levels of IFN-γ (Fig. 4A) and NO (Fig. 4B). Associated with these increases in IFN-γ and NO production in Th1 cell cultures were 50% reductions in the proportion of infected macrophages and 50% reductions in the average number of intracellular AMA in each infected cell, compared with control cultures incubated in the absence of T cells (Fig. 4C). Cocultures of parasite-specific Th2 cells with infected macrophages contained high levels of IL-4 (Fig. 4A), confirming that these Th2 cells could recognize parasite antigens expressed during infection. However, the Th2 cells did not induce infected macrophages to produce NO (Fig. 4B), and only minimal decreases in infection were detected microscopically compared with control cultures without T cells (Fig. 4C). In addition to these experiments analyzed microscopically after 48 h of incubation, similar cocultures of T cells and infected macrophages were set up and incubated at 37°C for more than 1 week. In the absence of T cells or in the presence of Th2 cells that were associated with only minimal inhibition of parasite infection at 48 h, trypomastigotes were easily detected in the supernatants by 72 h (>8 × 105 trypomastigotes/ml). However, trypomastigotes were not detected in the culture supernatants, during the entire period of observation (greater than 1 week), from cultures of infected macrophages incubated with Th1 cells. These data indicate that parasite-specific Th1 cells, but not Th2 cells, can mediate protection against intracellular T. cruzi infection.
FIG. 4.
T. cruzi-specific Th1 cells, but not Th2 cells, inhibit intracellular parasite replication in infected macrophages. PEM cultured in tissue culture slide chambers were infected with T. cruzi trypomastigotes. Parasite-specific Th1 cells, Th2 cells, or no T cells were added, and these cultures were incubated for 48 h prior to harvesting supernatants for measurements of IFN-γ (A) and NO (B) production. The percentages of T. cruzi-infected macrophages and the average numbers of intracellular AMA per infected cell were determined microscopically (C). The effects of added T cells on T. cruzi infection are expressed as the percent inhibition compared with results for macrophages infected in the absence of T cells. Shown are the means (± standard errors) from replicate cultures. Similar results were seen in multiple experiments.
T. cruzi-specific Th1 cells provide an essential function but are not sufficient for the development of immunity protective against primary infection in vivo.
To further investigate a causal relationship between T. cruzi-specific Th1 cells and protective immunity against T. cruzi, we performed adoptive transfer experiments with SCID mice (Table 2). We found that subcutaneous injection of 1,000 T. cruzi IMT was uniformly lethal to control SCID mice. Adoptive transfer of 5 million total spleen cells from naive BALB/c mice into SCID mice prior to challenge could reproducibly protect these animals (79% survival). However, CD4-depleted spleen cells from BALB/c mice were not protective after adoptive transfer to SCID mice (9% survival only). In addition, adoptive transfer of parasite-specific Th1 cells alone did not protect against T. cruzi challenge. These mice died despite the fact that we could demonstrate expansion of the parasite-specific Th1 cells in these animals by flow-cytometric and cytokine stimulation studies. Ten to twenty percent of total spleen cells harvested 2 to 4 weeks postinfection from SCID mice transferred with Th1 cells were CD3+ CD4+ (≥5 to 10 million CD4+ T cells), and these spleen cells produced 50 ± 2 U of IFN-γ/ml in response to in vitro stimulation with parasite lysate. CD3+ CD4+ cells were not detectable in spleen cell preparations harvested from untreated control SCID mice 2 to 4 weeks postinfection, and these control SCID spleen cells did not produce IFN-γ in response to in vitro stimulation with parasite lysate. Finally, we challenged SCID mice with T. cruzi after adoptive transfer of both our parasite-specific Th1 cells and CD4-depleted BALB/c spleen cells. We found that seven of nine SCID mice reconstructed with adoptively transferred Th1 cells and CD4-depleted BALB/c spleen cells were able to control their T. cruzi infection and survived for more than 3 months after the challenge. Overall, these results demonstrate that CD4+ T cells are necessary for the development of immunity protective against primary T. cruzi infection but clearly indicate that Th1 cells alone are not sufficient for protection against even a low-level T. cruzi challenge. Our results also have demonstrated that Th1 cells can provide the immune function(s) required from CD4+ T cells for the development of immunity protective against primary T. cruzi infection. In addition, these results indicate that the failure to develop protective immunity in SCID mice reconstructed with Th1 cells alone or CD4-depleted spleen cells alone could not be explained by an inability of these cell populations to provide their important immune effector functions in vivo after adoptive transfer.
TABLE 2.
Adoptive transfer of immunity protective against primary T. cruzi infectiona
| Cells used for adoptive transfer | Mortality postchallenge (%)c |
|---|---|
| None | 15/15 (100) |
| Naive total SC | 4/19 (21)b |
| Naive CD4-depleted SC | 10/11 (91) |
| Th1 cells | 15/15 (100) |
| Th1 cells + naive CD4-depleted SC | 2/9 (22)b |
The indicated Th1 cell lines or cells from BALB/c mice were adoptively transferred to C.B-17 SCID mice 1 day prior to subcutaneous challenge with 1,000 T. cruzi IMT. A total of 5 × 106 cells of each cell preparation were injected per mouse. All deaths occurred within 6 weeks of infection. Mice surviving infection were still alive 3 months postchallenge. SC, spleen cells.
Survival significantly greater than that of groups receiving no adoptive transfer, CD4-depleted SC, and Th1 cells alone prior to T. cruzi challenge by Fisher's two-tailed exact tests (P < 0.01).
Number of mice that died/total number of mice in group.
CD4+ T cells are not required for immune memory effector function protective against T. cruzi challenge.
The previous experiments demonstrated that CD4+ T cells were necessary as helper and/or effector immune cells for the control of primary T. cruzi infection. We next studied in the SCID mouse adoptive-transfer model whether CD4+ T lymphocytes were required for memory immune control of more aggressive secondary challenges with T. cruzi. Shown in Table 3 are the overall mortality results for three cumulative experiments comparing the protective effects of naive and memory immune spleen cell populations. CD4-depleted memory immune cells provided significant levels of protection, which were similar to those provided by total memory immune cells. These results indicate that CD4+ T cells were not necessary for memory immune effector functions protective in this adoptive-transfer SCID mouse model. Our combined results presented in this report suggest that although CD4+ Th1 cells can mediate effector inhibition of intracellular T. cruzi inside macrophages, a more important function relevant for vaccine development may be the ability of CD4+ Th1 cells to provide helper activity for the development of other effector functions.
TABLE 3.
Adoptive transfer of memory immunity against secondary T. cruzi challengesa
| Cells used for adoptive transfer | Mortality postchallenge (%)c |
|---|---|
| None | 9/9 (100) |
| Naive total SC | 8/8 (100) |
| Memory CD4+ SC | 4/4 (100) |
| Memory CD4-depleted SC | 2/6 (33)b |
| Memory CD4+ + memory CD4-depleted SC | 1/6 (17)b |
| Memory total SC | 2/8 (25)b |
No cells or the indicated BALB/c mouse spleen cells (SC) were adoptively transferred to C.B-17 SCID mice prior to subcutaneous challenge with 5,000 to 20,000 T. cruzi BFT. A total of 2.5 × 107 cells were injected per mouse. All deaths occurred within 6 weeks of infection. Mice surviving infection were still alive 3 months postchallenge.
Survival significantly greater than that of groups receiving no adoptive transfer or total naive SC prior to T. cruzi challenge by Fisher's two-tailed exact tests (P ≤ 0.01).
Number of mice that died/total number of mice in the group.
DISCUSSION
Previous investigations have documented important roles for CD4+ T lymphocytes and IFN-γ responses in T. cruzi protective immunity (4, 20, 32–34, 46). In addition, CD4+ Th1 cells have been shown to adoptively transfer protective T. cruzi immunity into resistant C57BL/6 mice (23, 28, 29). However, previous studies have not clearly defined the relative importance of CD4+ Th1 and Th2 cells for T. cruzi systemic immunity.
The ability of highly purified parasite-specific Th1 cells to protect relatively resistant C57BL/6 mice demonstrates that this immune subset is involved in natural T. cruzi systemic resistance. Of potentially greater significance, we have shown that the induction of predominant Th1 immunity can protect a highly susceptible murine strain against T. cruzi challenge. The induction of T. cruzi-specific Th1 cell responses, and not Th2 responses, in susceptible BALB/c mice was associated with lower-level parasitemias and nearly universal survival after virulent parasite challenges (Table 1 and Fig. 2). These data indicate that a T. cruzi vaccine strategy capable of biasing for Th1 immunity may be useful in populations with variable levels of natural resistance and susceptibility. The ability to induce protective immune responses in heterogeneous outbred populations, including highly susceptible individuals, will be an important requirement for any human vaccine strategy.
To more specifically address the importance, in susceptible mice, of differential CD4+ T-cell responses for T. cruzi systemic immunity, we generated highly polarized Th1 and Th2 cell lines reactive with parasite antigens from BALB/c lymphocytes. We studied the ability of these Th1 and Th2 cell lines to inhibit intracellular T. cruzi replication in vitro (Fig. 4) and to protect SCID mice against T. cruzi challenge in vivo (Table 2). Th1, but not Th2, cells induced NO production and inhibited T. cruzi intracellular replication in infected macrophages. Despite their ability to prevent intracellular parasite replication in vitro, these Th1 cells were not protective when transferred alone into SCID mice. However, when transferred with naive CD4-depleted spleen cell populations into SCID mice, these same Th1 cells were shown to provide an essential immune function required for the development of natural primary immunity protective against T. cruzi infection. Therefore, CD4+ Th1 cells appear to be necessary but not sufficient for the initial control of T. cruzi infection in mice. These results suggested to us that CD4+ Th1 cells perform a critical helper function for the development of initial adaptive immune responses protective against T. cruzi.
To explore the possibility that Th1 cells are required as effector cells for T. cruzi immunity in vivo, we conducted adoptive-transfer experiments reconstituting SCID mice with spleen cells from memory-immune BALB/c mice (Table 3). The spleen cell donors had survived challenge multiple times with virulent T. cruzi BFT and were found to develop potent Th1 cell responses early after parasite rechallenge (data not shown). We found that similar levels of protection were transferred by memory immune cells depleted of essentially all CD4+ T cells and by total memory immune cells. Therefore, CD4+ Th1 cells were not required for the secondary effector functions protective against T. cruzi in this SCID mouse adoptive-transfer model. Combined with the results shown in Table 2, these data indicate that helper functions associated with Th1 cells may be more important for T. cruzi immunity than direct effector functions mediated by these cells.
T. cruzi infects many different types of nucleated mammalian cells, including nonhematopoietic cells that do not normally express surface MHC class II molecules. Recognition and destruction of infected cells not expressing MHC class II probably require CD8+ cytotoxic T lymphocytes (CTL), which respond to parasite peptides presented by MHC class I molecules. Previous investigations have demonstrated that CTL are important for T. cruzi protective immunity in mice (30, 33, 34, 44, 45). The cytoplasmic location of T. cruzi replication may bias for antigen presentation through the endogenous processing pathway, making CTL even more important for the recognition and destruction of T. cruzi-infected cells. Our results demonstrating that CD4+ Th1 lymphocytes provide an essential immune function necessary for primary but not memory T. cruzi immunity could be explained by a role for Th1 cells in the optimal induction of protective CTL responses. Other studies of a wide variety of antigenic systems have indicated that CD4+ T lymphocytes can provide important helper functions for the generation of effective CTL (2, 5, 6, 10, 12, 22, 25, 37, 41, 50, 51).
CD4+ Th1 cells could provide an important helper function for the development of protective humoral immune responses. Generally, humoral immune mechanisms are important for the control of extracellular pathogens. Extracellular life stages of T. cruzi infect mucosal surfaces and circulate in the blood and lymph during acute systemic infection, and therefore humoral immunity may be required for optimal protection against T. cruzi infection. Passive transfer of immune serum has been shown to protect against aggressive intraperitoneal challenges with large doses of extracellular BFT (17). However, natural transmission of T. cruzi through infected reduviid excreta or even from contaminated blood transfusions does not involve large numbers of extracellular parasites introduced directly into the systemic circulation. In addition, all BFT must mature intracellularly, such that immunity capable of targeting T. cruzi-infected cells should be sufficient to protect against this parasite. Previous investigators demonstrated that vaccine-induced immunity protective against T. cruzi could be adoptively transferred with B-cell-depleted lymphocyte populations (24). We also have found that the adoptive transfer of B-cell-depleted spleen cells from naive BALB/c mice can protect SCID mice against primary T. cruzi systemic challenge (data not shown). Further studies are required to determine whether or not mucosal antibody responses (e.g., secretory immunoglobulin A) could be important for the prevention of initial T. cruzi mucosal infection.
In summary, we have shown that the induction of predominant Th1 immunity can be protective against systemic T. cruzi infection, even in highly susceptible mice. Future investigations will need to determine the effector immune mechanisms critical for the protective immunity induced by our Th1 immunization protocol. Immunocompetent mice can generate multiple subsets of CD4+ T lymphocytes, CD8+ T lymphocytes, CD4− CD8− αβTCR+ T cells, γδTCR+ T cells, and B cells, any of which may be involved in a Th1-biased immune response. Regardless of which immune subsets are the final effector cells that prevent parasite growth in vivo, our results indicate that CD4+ Th1 responses are involved in the development of protective T. cruzi systemic immunity, and immunization strategies designed to bias for Th1 responses should be included in future vaccine trials.
ACKNOWLEDGMENT
This work was supported by National Institutes of Health grant AI34912-03.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Alter B J, Schendel D J, Bach M L, Bach F H, Stimpfling J H. Cell-mediated lympholysis: importance of serologically defined H-2 regions. J Exp Med. 1973;137:1303–1309. doi: 10.1084/jem.137.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade V, Barral-Netto M, Andrade S G. Patterns of resistance of inbred mice to Trypanosoma cruzi are determined by parasite strain. Braz J Med Biol Res. 1985;18:499–506. [PubMed] [Google Scholar]
- 4.Araujo F. Development of resistance to Trypanosoma cruzi in mice depends on a viable population of L3T4+ (CD4+) T lymphocytes. Infect Immun. 1989;57:2246–2248. doi: 10.1128/iai.57.7.2246-2248.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantor H, Boyse E A. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975;141:1390–1399. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassell D, Forman J. Two roles for CD4 cells in the control of the generation of cytotoxic T lymphocytes. J Immunol. 1991;146:3–10. [PubMed] [Google Scholar]
- 7.Gazzinelli R T, Oswald I P, Hieny S, James S L, Sher A. The microbicidal activity of interferon-γ-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 8.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 9.Hoft D F, Lynch R G, Kirchhoff L V. Kinetic analysis of antigen-specific immune responses in resistant and susceptible mice during infection with Trypanosoma cruzi. J Immunol. 1993;151:7038–7047. [PubMed] [Google Scholar]
- 10.Husmann L A, Bevan M J. Cooperation between helper T cells and cytotoxic T lymphocyte precursors. Ann N Y Acad Sci. 1988;532:158–169. doi: 10.1111/j.1749-6632.1988.tb36335.x. [DOI] [PubMed] [Google Scholar]
- 11.Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4, or 5, using modified cDNA expression vectors. Eur J Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 12.Kast W M, Bronkhorst A M, de Waal L P, Melief C J. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC restricted and MHC regulated: a model for MHC-disease associations. J Exp Med. 1986;164:723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kierszenbaum F. Protection of congenitally athymic mice against Trypanosoma cruzi infection by passive antibody transfer. J Parasitol. 1980;66:673–675. [PubMed] [Google Scholar]
- 14.Kierszenbaum F, Howard J G. Mechanisms of resistance against experimental Trypanosoma cruzi infection: the importance of antibodies and antibody-forming capacity in the Biozzi high and low responder mice. J Immunol. 1976;116:1208–1211. [PubMed] [Google Scholar]
- 15.Kierszenbaum F, Lima M F, Wirth J J. Effects of antiserum to Trypanosoma cruzi on the uptake and rate of killing of vector-borne, metacyclic forms of the parasite by macrophages. Int J Parasitol. 1985;15:409–413. doi: 10.1016/0020-7519(85)90026-8. [DOI] [PubMed] [Google Scholar]
- 16.Kirchhoff L V, Hoft D F. Immunization and challenge of mice with insect-derived metacyclic trypomastigotes of Trypanosoma cruzi. Parasite Immunol (Oxford) 1990;12:65–74. doi: 10.1111/j.1365-3024.1990.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 17.Krettli A U, Brener Z. Resistance against Trypanosoma cruzi associated to anti-living trypomastigote antibodies. J Immunol. 1982;128:2009–2012. [PubMed] [Google Scholar]
- 18.Laranja F S, Dias E, Nobrega G, Miranda A. Chagas' disease—a clinical, epidemiologic and pathologic study. Circulation. 1956;14:1035–1060. doi: 10.1161/01.cir.14.6.1035. [DOI] [PubMed] [Google Scholar]
- 19.Lima-Martins M V C, Sanchez G A, Krettli A U, Brener Z. Antibody-dependent cell cytotoxicity against Trypanosoma cruzi is only mediated by protective antibodies. Parasite Immunol (Oxford) 1985;7:367–376. doi: 10.1111/j.1365-3024.1985.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 20.McCabe R, Meagher S, Mullins B. Gamma interferon suppresses acute and chronic Trypanosoma cruzi infection in cyclosporin-treated mice. Infect Immun. 1991;59:1633–1638. doi: 10.1128/iai.59.5.1633-1638.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHardy N. Passive protection of mice against infection with Trypanosoma cruzi with plasma: the use of blood- and vector bug-derived trypomastigote challenge. Parasitology. 1980;80:471–478. doi: 10.1017/s0031182000000937. [DOI] [PubMed] [Google Scholar]
- 22.Melief C J, van der Meulen M, Christiaans B J, de Greeve P. Cooperation between subclasses of T lymphocytes in the in vitro generation of cytotoxicity against a mutant H-2K difference: an analysis with anti-Lyt antisera. Eur J Immunol. 1979;9:7–12. doi: 10.1002/eji.1830090103. [DOI] [PubMed] [Google Scholar]
- 23.Millar A E, Wleklinski-Lee M, Kahn S J. The surface protein superfamily of Trypanosoma cruzi stimulates a polarized Th1 response that becomes anergic. J Immunol. 1999;162:6092–6099. [PubMed] [Google Scholar]
- 24.Miller M J, Wrightsman R A, Stryker G A, Manning J E. Protection of mice against Trypanosoma cruzi by immunization with paraflagellar rod proteins requires T cell, but not B cell, function. J Immunol. 1997;158:5330–5337. [PubMed] [Google Scholar]
- 25.Mizuochi T, Golding H, Rosenberg A S, Glimcher L H, Malek T R, Singer A. Both L3T4+ and Lyt-2+ helper T cells initiate cytotoxic T lymphocyte responses against allogenic major histocompatibility antigens but not against trinitrophenyl-modified self. J Exp Med. 1985;162:427–443. doi: 10.1084/jem.162.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murfin D J, Choromanski L, Kuhn R E. Production and characterization of clones of the Brazil strain of Trypanosoma cruzi. J Parasitol. 1985;7:525–529. [PubMed] [Google Scholar]
- 27.Nabors G S, Tarleton R L. Differential control of IFN-gamma and IL-2 production during Trypanosoma cruzi infection. J Immunol. 1991;146:3591–3598. [PubMed] [Google Scholar]
- 28.Nickell S P, Gebremichael A, Hoff R, Boyer M H. Isolation and functional characterization of murine T cell lines and clones specific for the protozoan parasite Trypanosoma cruzi. J Immunol. 1987;138:914–921. [PubMed] [Google Scholar]
- 29.Nickell S P, Keane M, So M. Further characterization of protective Trypanosoma cruzi-specific CD4+ T-cell clones: T helper type 1-like phenotype and reactivity with shed trypomastigote antigens. Infect Immun. 1993;61:3250–3258. doi: 10.1128/iai.61.8.3250-3258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nickell S P, Stryker G A, Arevalo C. Isolation from Trypanosoma cruzi-infected mice of CD8+, MHC-restricted cytotoxic T cells that lyse parasite-infected target cells. J Immunol. 1993;150:1446–1457. [PubMed] [Google Scholar]
- 31.Nogueira N, Ellis J, Chaplan S, Cohn Z. Trypanosoma cruzi: in vivo and in vitro correlation between T-cell activation and susceptibility in inbred strains of mice. Exp Parasitol. 1981;51:325–334. doi: 10.1016/0014-4894(81)90120-x. [DOI] [PubMed] [Google Scholar]
- 32.Reed S. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988;140:4342–4347. [PubMed] [Google Scholar]
- 33.Rottenberg M E, Bakhiet M, Olsson T, Kristensson K, Mak T, Wigzell H, Örn A. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect Immun. 1993;61:5129–5133. doi: 10.1128/iai.61.12.5129-5133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rottenberg M E, Riarte A, Sporrong L, Altcheh J, Petray P, Ruiz A M, Wigzell H, Orn A. Outcome of infection with different strains of Trypanosoma cruzi in mice lacking CD4 and/or CD8. Immunol Lett. 1995;45:53–60. doi: 10.1016/0165-2478(94)00221-c. [DOI] [PubMed] [Google Scholar]
- 35.Rowland E C, Kuhn R E. Suppression of cellular responses in mice during Trypanosoma cruzi infections. Infect Immun. 1978;20:393–397. doi: 10.1128/iai.20.2.393-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowland E C, Ritter D M. Corpus Christi strain-induced protection to Trypanosoma cruzi infection in C3H(He) mice: transfer of resistance to Brazil strain challenge with lymphocytes. J Parasitol. 1984;70:760–766. [PubMed] [Google Scholar]
- 37.Schendel D J, Alter B J, Bach F H. The involvement of LD- and SD-region differences in MLC and CML: a three cell experiment. Transplant Proc. 1973;5:1651–1655. [PubMed] [Google Scholar]
- 38.Scott M T. The nature of immunity against Trypanosoma cruzi in mice recovered from acute infection. Parasite Immunol (Oxford) 1981;3:209–218. doi: 10.1111/j.1365-3024.1981.tb00400.x. [DOI] [PubMed] [Google Scholar]
- 39.Scott M T, Moyes L. 35S-methionine labelled Trypanosoma cruzi blood trypomastigotes: opsonization by chronic infection serum facilitates killing in spleen and liver. Clin Exp Immunol. 1982;48:754–757. [PMC free article] [PubMed] [Google Scholar]
- 40.Shikanai-Yasuda M A, Lopes M H, Tolezano J E, Umezawa E, Amato Neto V, Pereira Barreto A C, Higaki Y, Moreira A A B, Funayama G, Barone A A, Duarte A, Odone V, Gerri G C, Sato M, Possi D, Shiroma M. Doenca de Chagas aguda: vias de transmissao, aspectos clinicos e resposta a tereapeutica especifica em casos diagnosticados em um centro urbano. Rev Inst Med Trop Sao Paulo. 1990;32:16–27. doi: 10.1590/s0036-46651990000100004. [DOI] [PubMed] [Google Scholar]
- 41.Stuhler G, Walden P. Collaboration of helper and cytotoxic T lymphocytes. Eur J Immunol. 1993;23:2279–2286. doi: 10.1002/eji.1830230934. [DOI] [PubMed] [Google Scholar]
- 42.Takehara H A, Perini A, Da Silva M H, Mota I. Trypanosoma cruzi: role of different antibody classes in protection against infection in the mouse. Exp Parasitol. 1981;52:137–146. doi: 10.1016/0014-4894(81)90069-2. [DOI] [PubMed] [Google Scholar]
- 43.Tanowitz H B, Minato N, LaLonde R, Wittner M. Trypanosoma cruzi: correlation of resistance and susceptibility in infected mice with the in vivo primary antibody response to sheep red blood cells. Exp Parasitol. 1981;52:233–242. doi: 10.1016/0014-4894(81)90078-3. [DOI] [PubMed] [Google Scholar]
- 44.Tarleton R L. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J Immunol. 1990;144:717–724. [PubMed] [Google Scholar]
- 45.Tarleton R L, Koller B H, Latour A, Postan M. Susceptibility of β2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992;356:338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- 46.Torrico F, Heremans H, Ribera M T, Van Marck E, Billiau A, Carlier Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991;146:3626–3632. [PubMed] [Google Scholar]
- 47.Trischmann T, Tanowitz H, Wittner M, Bloom B. Trypanosoma cruzi: role of the immune response in the natural resistance of inbred strains of mice. Exp Parasitol. 1978;45:160–168. doi: 10.1016/0014-4894(78)90055-3. [DOI] [PubMed] [Google Scholar]
- 48.Trischmann T M. Non-antibody-mediated control of parasitemia in acute experimental Chagas' disease. J Immunol. 1983;130:1953–1957. [PubMed] [Google Scholar]
- 49.Trischmann T M. Early parasite proliferation and host resistance in inbred strains of mice. Exp Parasitol. 1986;62:194–201. doi: 10.1016/0014-4894(86)90023-8. [DOI] [PubMed] [Google Scholar]
- 50.von Boehmer H, Haas W. Distinct Ir genes for helper and killer cells in the cytotoxic response to H-Y antigen. J Exp Med. 1979;150:1134–1142. doi: 10.1084/jem.150.5.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner H, Starzinski-Powitz A, Pfizenmaier K, Rollinghoff M. T-T cell collaboration during in vivo response to antigens coded by the peripheral and central regions of the MHC. Nature. 1976;163:235–237. doi: 10.1038/263235a0. [DOI] [PubMed] [Google Scholar]
- 52.Wrightsman R A, Krassner S, Watson J. Genetic control of responses to Trypanosoma cruzi in mice: multiple genes influencing parasitemia and survival. Infect Immun. 1982;36:637–644. doi: 10.1128/iai.36.2.637-644.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wrightsman R A, Krassner S M, Watson J D, Manning J E. Role of the H2S haplotype in survival of mice after infection with Trypanosoma cruzi. Infect Immun. 1984;44:351–354. doi: 10.1128/iai.44.2.351-354.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]