Abstract
Strawberry (Fragaria × ananassa), the family Rosaceae, is a small fruit that has great importance. It is triggered by a number of physiological, genetic, and biochemical processes. Phytohormones or plant growth regulators are organic substances produced naturally in many plants and responsible for controlling growth and other physiological functions. Therefore, plant growth regulators such as Gibberellin, NAA (auxin) and triacontanol, and chlormequat are essential factors that cause strawberry ripening, maturity indices, and determine the quality of fruits. Moreover, Gibberellin stimulates cell division and breaks dormancy whereas NAA (auxin) stimulates root growth. Similarly, triacontanol plays a special role in plant growth and development. Additionally, chlormequat is effective in controlling the height of the plant. The main objective of this review is to study the effect of various plant growth regulators that have a great potential effect on growth, development, and fruit yield.
Keywords: Fragaria × ananassa, Gibberellin, NAA, TRIA
Fragaria; Ananassa; Gibberellin; NAA; TRIA.
1. Introduction
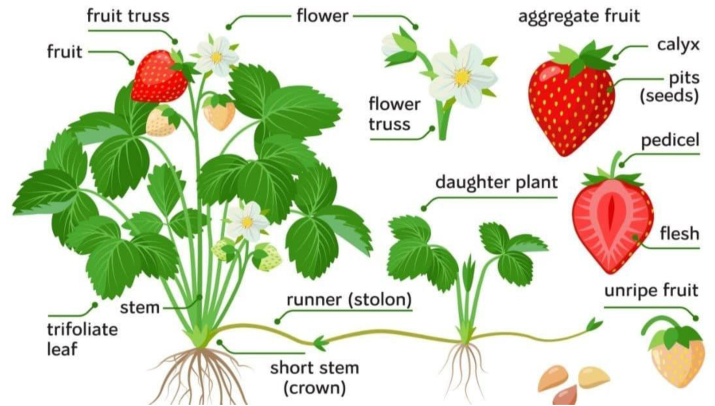
Strawberry is soft, luscious, nutritious, tasty, and perishable fruit which are grown in temperate climatic conditions where the plant behaves like a small perennial herb and also grown in a sub-tropical climate whose plant behaves as an annual belonging to the family Rosaceae (Salentijn et al., 2003; Srivastav et al., 2018; Cv et al., 2016). The cultivated strawberry (Fragaria × ananassa Duch.) is a monoecious octaploid hybrid of two largely dioecious, octaploid species, Fragaria chiloensis Duch. and Fragaria virginiana Duch (Cv et al., 2016). Strawberry is a non-climacteric fruit and characterized by a high softening rate, short post-harvest life, and fast decay (Bustamante et al., 2009). Strawberry (Fragaria ∗ ananassa) is a short day plant that has antioxidant, anti-inflammatory, anti-neurodegenerative and anti-cancer component called ellagic acid, contain phenolics and flavonoids and also rich in vitamins, minerals like potassium, phosphorus, calcium, and iron (Roussos et al., 2009). It is propagated through the runners and is red in colour due to the presence of anthocyanin, pelarogonidin, 3-monoglucoside, and traces of cyanide (Srivastav et al., 2018). Consumption of strawberries leads to health benefits against cancer, aging, inflammation, and neurological diseases (Cv et al., 2016). Camarosa, Laguna, Seascape Chandler, Sweet Charlie, Fern, Douglas, Redgauntlet, Talisman, Cambridge Favourite, Domanil, Fanil, Gorella, Goupil, Senga gigana, Senga precosana, Surprise des Hailes are different cultivars of strawberry (Sharma and Singh, 2009; Paroussi et al., 2002; (Tehranifar and Battey, 1997) Terms, 2017). Strawberry is rich in Vitamin A (60 IU/100 g of edible portion), vitamin C (30–120 mg/100 g of edible portion), fiber, pectin (0.55%) and has a low calorie carbohydrate content and is high in carotenoids, flavonoids, phenols, and glutathione ( Sharma and Negi, 2019; Nautiyal and Shukla, 2015). Strawberry is delicious, nutritious, red fruit and its taste depend upon three compounds i.e. sugar (0.5%), acid (0.90–1.85%), and aromatic compounds (Bj et al., 2020). Strawberry's edible parts are receptacle, petioles, achenes or real fruit, and seed. It has short stems known as crown which produces leaves along the stem axis and flowers (Srivastav et al., 2018) (Figure 1).
Figure 1.
Whole strawberry plant (strawberryplants.org).
Different plant growth regulators perform different function on strawberry. Various PGR like Auxin, gibberellin and cytokinin are used in strawberry in order to increase the fruit size, enhance fruit set, growth, and yields. Among them, auxin are used for enlargement of receptacle, fruit size growth and delay fruit ripening, gibberellin inhibit the fruit ripening, abscisic acid develop a color on fruit and nitric oxide extent the post-harvest life of ripe fruit (Roussos et al., 2009; Marcos et al., 2009). Auxins are also called as key phytohormones, applied in controlling the growth and ripening the fruit which is associated with increase in pectin and reduction of hemicelluloses content (Bustamante et al., 2009).
1.1. Influence of gibberellin on strawberry
Plant heights, number of runners, number of flowers, fruit set percentage, number of fruits, fruit size, fruit weight, and fruit quality are all affected by gibberellic acid (Kumra et al., 2018) Gibberellic acid (GA3) treatment promoted flowering in non-chilled strawberry plants, shortened the cropping season, and increased vegetative growth and fruit number (Paroussi et al., 2002). It acts as a fruit ripening inhibitor (Marcos et al., 2009). It Increases vegetative development, increases runner formation, lengthens the main stem internode, initiates flower development, promotes stolon formation, petiole length, and leaf area, destroys rosette habit, and slows blossom initiation ( Sharma and Singh, 2009; Guttridge and Thompson, 1964; Tafazoli & Vince-prue, 2015). The effects of a long photoperiod or chilling are also caused by GA3 (Guttridge, 1970). Gibberellins are well-known for acting as a long-day hormone in short-day plants. Gibberellin treatment increases vegetative growth but limits flower development (Kender et al., 1971). The GA 3-oxidase enzyme prevents runner and crown branch development, increasing berry output (Hytonen, Elomaa, Moritz and Junttila, 2009). By hydrolyzing protein and releasing tryptophan, GA promotes pollen germination, pollen tube expansion, and auxin biosynthesis. GA3 boosts diphenols while inhibiting IAA oxidase activity, resulting in a high auxin level. In the absence of fertilizer, the use of GA resulted in fruit set (Kumar, Saravanan, Bakshi, & Sharma, 2013).
1.2. Influence of auxin (NAA) and tricontanol on growth yield and quality
NAA is a synthetic auxin that is most commonly employed in the production of high-quality strawberries in terms of total sugars, ascorbic acid content, and titrable acidity percentage (Bhople et al., 2020). NAA is a synthetic version of auxin that aids in cell elongation, division, vascular tissue differentiation, root initiation, apical dominance, leaf senescence, leaf and fruit abscission, fruit setting ratio, fruit dropping prevention, and flower sex ratio promotion (Mehraj et al., 2015). Naphthalene acetic acid is one of auxin's most important members, and early application of Napthalene acetamide in early stages induces cell division in cambium cells, resulting in the production of xylem tissue in lower internodes, which provides mechanical support to plants while also preventing lodging (Thakur et al., 2017). It has been reported that a medium containing a low concentration of NAA 0.1 mg/l and a relatively high concentration of BA 1.0 mg/l is best for shoot generation, and that a medium containing a high concentration of cytokinin and a low concentration of auxin (a medium with a cytokinin to auxin ratio greater than 1) is best for shoot bud induction. (Lal et al., 2003).
Auxins such as IBA (Indol-3-butyric acid) and NAA (Naphthyl acetic acid) are used to promote rapid and abundant rooting of cuttings from a variety of trees, vines, shrubs, annual and perennial ornamentals (Rademacher, 2015). The effect of NAA on plant growth is greatly reliant on the time and concentration of entry, as well as promoting cellulose production and limiting fruit drop (Suman et al., 2017). GA treatment could only maintain emasculated flower receptacle growth for 6 days, according to Archbold and Dennis (1985), whereas growth of fruit treated with synthetic auxin Naphthalene acetic acid (NAA) could continue for up to 30 days, albeit at a slower rate than pollinated flowers (Roberts and Hooley, 1988). The application of NAA to strawberry fruits enhances fruit size, delays ripening, and boosts anthocyanin accumulation, as well as delaying the flowering time and enhancing fruit output and quality (Csrl, 2016). As stated, using GA3 and Napthalene acetic acid alone or in combination enhances plant height, number of crowns, runners, and leaf area. Plants treated with NAA at a concentration of 19.97 mg/l produced berries with the highest total soluble solids, total sugars, and titrable acidity (Kumar and Tripathi, 2009). Because developing leaves are one of the primary sites of auxin biosynthesis, the elongating petiole tissues could directly receive sufficient amounts of auxin from young leaves, resulting in increased petiole length due to rapid cell division and cell enlargement, NAA at 19.97 mg/l and 49.94 mg/l produced significantly longer petioles than the control (Manandhar and Shrestha, 2008). It was reported that using NAA improved the output of berries with greater width and length, as well as weight (Wang et al., 2015). The dormant bud has a lot of auxin activity, while the non-dormant bud has a lot of cytokinin activity, according to researchers. Decapitation and pharmacological studies on dormant buds also demonstrated that reducing auxin levels and administering exogenous cytokinins increases strawberry vegetative shoot regrowth (Li et al., 2021). Auxin plays a vital function in fruit growth and ripening by transcriptionally activating Aux/IAA genes (Liu et al., 2011). The skin hardness and hardiness of the underlying flesh define the firmness of strawberry fruit, and this hardiness is linked to the formation of hard achene growth, resulting in the hardiest fruit in NAA treated plants (Rathod et al., 2021). Tricontanol (TRIA) is a natural plant growth regulator found in epicuticular waxes which is used to increase fruit production. TRIA is a saturated primary alcohol found in epicuticular waxes of a variety of plant species, including Croton californicus, Copernica cerifera, and Jatropha curcas. It was first discovered in Alfalfa hay (Islam and Mohammad, 2020). Tricontanol is a widely distributed saturated primary alcoholic plant hormone discovered by Chibnallet al.(1933) and has been shown to modulate a variety of physiological and biochemical activities in plants (Zaid et al., 2020). TRIA-mediated improvements in growth yield, photosynthesis, protein synthesis, water and nutrient uptake, nitrogen fixation, enzyme activities, free amino acids, reducing sugars, and soluble protein content have been reported by numbers of researchers (Suman et al., 2017). TRIA affects blossom amount and quality in Vigna radiata L., Chrysanthemum morifolium Ramat., Bougainvillea glabra Choisy., and Fragaria ananassa Duch., which may be connected with TRIA providing an element for bud arrangement, improvement, and better-quality blossoms, as revealed by (Islam and Mohammad, 2020). Photosynthesis has been connected as a key plant reaction to TRIA, greater photosynthesis and higher photosynthate accumulation are connected to increased plant development and dry weight (Altintas, 2011; Emam et al., 2020; Naeem et al., 2012). Plants treated with tricontanol increased root number causing plant to absorb more nutrients from the soil and increased production per plant and similarly the tricontanol treated strawberry had the highest number of fruit, yield per hectare and B:C ratio (KhunteDas et al., 2019a, KhunteDas et al., 2019b). Tricontanol also enhances vital plant physiological processes such as water and mineral nutrient uptake, essential oil yield, secondary metabolites, early bolting, nitrogen assimilation, proline metabolism, and glycine betaine accumulation thereby protecting plants from variety of environment stresses (Zaid et al., 2020). TRIA controls the activation of stress resilience components in farmed plants, which helps the plants to cope with lightning-induced alterations (Islam and Mohammad, 2020; Zaid et al., 2020). TRIA has been shown in several studies to play an important role in regulating a wide range of plant morphological responses, including increasing plant height, biomass, leaf number, and leaf area per plant in most harvests. Foliar application of TRIA up to 1 ppm also resulted in twice the fresh and dry weight of shoot and root of Solanum lycopersicon (Roberts and Hooley, 1988). Triacontanol, Activol (GA3, a plant growth regulator), and NAA all increased strawberry vegetative development compared to the control. The largest crown tallness (7.2 cm) was achieved with 100 ppm Activol, although 50 ppm triacontanol treated plants had the most notable leaf number/plant (7.2) and leaf region (49.4 m2) (Zaid et al., 2020; Emam et al., 2020; Islam and Mohammad, 2020; Kumra and Reena, 2018; Zaid et al., 2020). Emam et al. (2020) was reported that spraying strawberry cv. with 400 ppm NAA resulted in the greatest natural product width, weight, volume, causticity percent (as citrus extract), and lowest sugar: corrosive ratio in Strawberry cv. Tioga. has increased fruit quality and plant stature, spread, number of leaves per plant, petiole length, leaf region records, days to first blooming and days to fruit bud advancement, fruit yield per plant, best fruit yield per hectare, and days to first blooming and fruit bud advancement in Sweet Charlie with NAA levels of 0, 10, 15, 20, 25, 30, and 35 ppm. A preharvest treatment with NAA 25 ppm has an impact on strawberry cv. Chandler's greater L-ascorbic acid concentration (49.30 mg/100 g mash) after capacity. Moreover, treating strawberries with naphthalene acidic corrosive (NAA) restrains aging and anthocyanin amassing (Ali et al., 2021; Altintas, 2011; Khunte et al., 2020). Whenever strawberries (Fragaria x ananassa Duch.) cv. Sweet Charlie plants were treated with triacontanol 5 ppm, the greatest number of natural products (23.31), yield per hectare (27.90 tons), length measurement (1.50), and money-saving advantage proportion (1:3.1) were accounted for (Kumari et al., 2018). Also, Pang and Chen (2020) asserts that TA treatment (50 M) advances fruit improvement by upregulating factors associated with natural product maturing and improvement. The best length measurement proportion of natural product (1.58) was accounted for in research led by (KhunteDas et al., 2019a, KhunteDas et al., 2019b) with the treatment Poultry Manure 2.50 tons + Triacontanol 100 ppm. Besides, a shower got from Moringa leaf separate upgraded strawberry yield, recommending that it very well to be utilized as a foliar splash to accelerate the development of youthful plants (Hind Musa Ibrahim Mohamed, 2021; Islam and Mohammad, 2020). The salicylic corrosive at 2 mM and the Tria at 10 mM medicines showed the most advancement in strawberry vegetative qualities, as well as improved botanical and fruiting characters (Islam and Mohammad, 2020; Khunte et al., 2020; Zaid et al., 2020). At 80 ppm, GA3 upgrades vegetative development, sprinter creation (Kumari et al., 2018; Suman et al., 2017b). In contrast with different medicines, research directed by Sachs and Iszak (2015) and Sood et al. (2022) found that consolidating bio-manures and development controllers (i.e., PSB at 6 kg/ha + GA3 at 100 ppm) increments normal plant tallness, spread, number of leaves, and leaf region with the briefest opportunity to foster the first sprout. Plants treated with PSB (6 kg/ha) + Triacontanol considerably affected the morphological characteristics of strawberry fruit (5 ppm).
1.3. Influence of chlormequat
Plant growth regulators are broadly utilized in fruit crops harvests to advance vegetative development, blossoming, and fruit improvement. Plant development controllers have been found to indirectly affect sprouting by lessening the vegetative turn of events (Islam and Mohammad, 2020; Kumra and Reena, 2018). CCC (Chlormequat), the first plant growth regulator was discovered by professor Tolbert at Michigan state university in the 1950s which is a synthetic PGR antagonist to GAs. The CCC has been shown in studies to efffectively reduce the growth of potato stems, leaves, and runners and thicken the stem of mung bean by controlling vein growth and lodging. Dwarfed plants, thickened stalks, increased chorophyll contents and well developed root systems are results of CCC application ( Liu et al., 2019). Likewise, as indicated by Tiwari et al. (2017) and Zaid et al., 2020a, Zaid et al., 2020b, foliar sprays of (GA3 at 200 ppm) at 30, 60, 90, and 120 days after relocating were deemed the best plant development controllers (PGR's) in terms of vegetative development limits, while (GA3 at 150 ppm) was deemed the best in terms of fruit quality for strawberry development. As per Kumra and Reena (2018), strawberry plants treated with cycocel in September and additionally October yielded before and to some degree more prominent yields in three-year preliminaries. Moreover, contrasted with untreated Fragaria ananassa, Fragaria ananassa getting two shower treatments of 10 lM TRIA showed a significant effect on plant tallness and leaf number (Ali et al., 2021; Altintas, 2011; Islam and Mohammad, 2020). As per Deyton et al. (1991), the development of smothering synthetic substances such paclobutrazol aid strawberry creation on plastic. This substance is valuable for limiting the development of sprinters. It created more fruit per plant, in any event, when contrasted with plants on which sprinters had been eliminated manually, and brought about a 22 percent increment in yield. Altintas (2011) and Khunte et al. (2020) observed that under drawn-out day conditions, the most extreme number of days it took to produce the principal blooming strawberry following relocating with 1000 ppm cycocel. Strawberry cv. crown tallness, the number of leaves, and leaf region were completely decreased. Besides, GA3 125 ppm treated strawberry plants had the most extreme plant stature (24.13 cm), while treatment T10 (GA3 75 ppm + CCC 500 ppm) treated strawberry plants had the most elevated length measurement proportion of fruits (2.10). As per the aftereffects of the test directed by Altintas (2011), favorable to Calcium (Ca) has a restraining impact on stem stretching with no adverse consequence on absolute and early yields and fruit quality for quite a long time; furthermore, with 100, and 300 mg l-1supportive of Calcium applications presenting a benefit over control plants, they would be adequate to control stem prolongation.
Crops grown with chlormequat chloride have shorter internodes but thicker, darker leaves and the chemical control of plant growth to reduce size through the use of PGR is a common practice to make plant more compact and commercially acceptable (Kumra et al., 2018). High NPK increased both leaf area and adjusted leaf area with CCC treated plants benefitting more than PP333 treated plants (McArthur and Eaton, 1988). In Europe cereal production chlormequat chloride was the first PGR to be used on large scale as antilodging agent. Similarly, chlormequat chloride applied in winter wheat at early stage of tillering increases the number of fertile tillers also reducing length of stem (Rademacher, 2015). Strawberry vegetative growth has been found to be aided by GA3 and the use of cycocel increased strawberry yield and quality. The use of cycocel at 500 ppm increased the number of flowers, fruit per plant and yield followed by NAA at 30 ppm (Kumar et al., 2012).
1.4. Effect of plant growth regulators on quality of strawberry
The physical and chemical features of the strawberry fruit are modified by the use of growth regulators. The use of plant growth regulators improves the quality of strawberry fruits, according to several studies. All doses of GA3 improves strawberry vegetative development, but cycocel at 500 ppm, followed by NAA at 30 ppm, is the best in terms of strawberry output and quality (Bakshi, 2018; Sood et al., 2022). In strawberries, skin hardness is connected to the production of hard achenes, and auxin is known to control this process, resulting in the hardiest fruit in NAA-treated plants. The use of NAA in strawberry plants raises the TSS level by increasing the concentration of volatile compounds as well as the hydrolysis of starchy compounds (Rathod et al., 2021; Saima et al., 2014; Thakur et al., 2017). Likewise, Strawberry is also quite susceptible to salt, and it has been noted that the quality of strawberry fruit has decreased. The ratio of TSS to TA has a significant impact on strawberry flavor. This ratio tended to grow when 30 mg L−1 PP333 is applied in both saline and non-saline environments (Jamalian et al., 2008). (Rathod et al., 2021) discovered that foliar spraying of plant growth regulators did not affect the firmness and acidity of strawberry fruits. The plants treated with 125 mg L−1NAA, on the other hand, generate the hardiest fruit. With a foliar spray of 125 mg L−1 NAA, strawberry fruits had the highest TSS and sugar content. Similarly, Nautiyal & Shukla (2015) claimed that the use of nitrogen-fixing bacteria and GA3 with a lower nitrogen dosage may have a regulatory effect on the absorption and translocation of several metabolites, the most significant of which is carbohydrates, which influences the quality of fruits. Furthermore, SA3 and SA4 treatments increased certain fruit-quality parameters like TSS, AA, fruit color, and strawberry production (Babalar and Asghari, 2007; Karlidag et al., 2011). SA therapy improves overall quality, and SA at 2 mmol L−1 is the most effective without causing any side effects, although SA at 4 mmol L−1 causes some fruit damage.
2. Conclusion
Plant growth regulators are the tools in flowering, fruiting, and ripening. The use of PGRs is increasing day by day mainly in many agricultural fruit crops. Therefore, numbers of synthetic chemicals are used for the regulations of growth and development of cultivated plants. Moreover, these growth regulators can be utilized for sustainable and ecologically sound fruit production. In addition, promote the less use of chemical fertilizers to a great extent. The review focuses on the influence of PGRs on growth, yield, and fruit quality of fruit crops.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Ali A., Kumar A., Rasool K., Ganai N.A., Lone A., Baba T.R., Hamid M., Haq A. Triacontanol spray mediated plant growth and productivity in fruits crops : a review. The Pharma Innov. J. 2021;10(7):789–792. [Google Scholar]
- Altintas S. Effects of chlormequat chloride and different rates of prohexadione-calcium on seedling growth , flowering , fruit development and yield of tomato. Afr. J. Biotechnol. 2011;10(75):17160–17169. [Google Scholar]
- Archbold D.D., Dennis F.G. Strawberry receptacle growth and endogenous IAA content as affected by growth regulator application and achene removal. J. Amer. Soc. Hort. Sci. 1985;110:816–820. [Google Scholar]
- Babalar M., Asghari M. Effect of pre- and postharvest salicylic acid treatment on ethylene production , fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007;105:449–453. [Google Scholar]
- Bakshi M. Influence of PGRs on growth, yield and quality of strawberry under U.P subtropics. The Asian Journal of Horticulture, Volume 7/Issue 2/434-436. Asian J. Horticult. 2018;7(2):434–437. [Google Scholar]
- Bhople A.A., Kullarkar P.P., Singh S.K., Singh S.K., Saxena D. Studies on impact of growth regulators on performance of strawberry cv. Camarosa under polyhouse condition. Ann. Agri Bio. Res. 2020;25(2):234–238. [Google Scholar]
- Bj S., Madaiah D., Bs S., Sridhara S., Pradeep S. Vol. 9. 2020. Influence of organic manures on growth , yield and quality of strawberry (Fragaria × ananassa Duch) under naturally ventilated polyhouse; pp. 3284–3287. [Google Scholar]
- Bustamante C.A., Civello P.M., Martı G.A. Vol. 177. 2009. Cloning of the promoter region of b -xylosidase (FaXyl1) gene and effect of plant growth regulators on the expression of FaXyl1 in strawberry fruit; pp. 49–56. [Google Scholar]
- Csrl E. Vol. 5. 2016. Strawberry Yield and Yield Attributes a er Application of Plant Growth Regulators and Micronutrients on Cv . Related papers. [Google Scholar]
- Cv A., Under C., Condition O. 2016. International Journal of Recent Scientific. April. [Google Scholar]
- Deyton D.E., Sams C.E., Cummins J.C. Strawberry growth and photosynthetic responses to paclobutrazol. Hortscience. 1991;26(9):1178–1180. [Google Scholar]
- Emam A.S., Samia M.A., Marwa M.M., Samira M.N., Wahed A.-E. Egyptian academic journal of biological sciences. Egypt. Acad. J. Biolog. Sci. 2020;13(4):259–266. [Google Scholar]
- Guttridge C.G. 1970. Interaction of Photoperiod, Chilling and Exogenous Gibberellic Acid on Growth of Strawberry Petioles; pp. 349–364. [Google Scholar]
- Guttridge C.G., Thompson P.A. Vol. 15. 1964. The Effect of Gibberellins on Growth and Flowering of Fragaria and Duchesnea; pp. 631–646. [Google Scholar]
- Ibrahim Mohamed Hind Musa. 2021. Investigations on the Bio-Stimulating Potential of Some Selected Local flora on Growth and Productivity of Lemongrass (Cymbopogon Citratus) [Google Scholar]
- Islam S., Mohammad F. Triacontanol as a dynamic growth regulator for plants under diverse environmental conditions. Physiol. Mol. Biol. Plants. 2020;26(5):871–883. doi: 10.1007/s12298-020-00815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamalian S., Tehranifar A., Tafazoli E., Eshghi S., Davarynejad G.H. Paclobutrazol application ameliorates the negative effect of salt stress on reproductive growth , yield , and fruit quality of strawberry plants. Hort. Environ. Biotechnol. 2008;49(4):1–6. [Google Scholar]
- Karlidag H., Yildirim E., Turan M. Salt tolerance of Physalis during germination and seedling growth Exogenous applications of salicylic acid affect quality and yield of strawberry grown under antifrost heated greenhouse conditions. J. Plant Nutr. Soil Sci. 2011;2009(270–276):172. [Google Scholar]
- Das Khunte S., Kumar A., Ansari N. Influence of PGRs and Poultry manure on physico-chemical parameters of influence of PGRs and Poultry manure on physico-chemical parameters of strawberry (Fragaria x ananassa duch) cv. Chandler. Int. J. Curr. Microbiol. Appl. Sci. 2019;8(12) [Google Scholar]
- Das Khunte S., Kumar A., Ansari N., Saravanan S. Influence of PGRs and Poultry manure on physico-chemical parameters of strawberry (Fragaria x ananassa duch.) cv. Chandler. Int. J. Curr. Microbiol. Appl. Sci. 2019;8(12):108–117. [Google Scholar]
- Das Khunte S., Kumar A., Ansari N., Saravanan S. Effect of different levels of PGRs with organic manure on growth characters and economics of strawberry (Fragaria x ananassa duch) cv. Chandler in northern region effect of different levels of PGRs with organic manure on growth characters and economic. Int. J. Curr. Microbiol. Appl. Sci. 2020;9(1) [Google Scholar]
- Kumar Raghu, Tripathi V. Influence of NAA, GA3 and boric acid on growth, yield and quality of strawberry cv. Chandler. Progress. Hortic. 2009;41(1):113–115. [Google Scholar]
- Kender W.J., Carpenter S., Braun J.W. Runner formation in everbearing strawberry as influenced by growth-promoting and inhibiting substances. Ann. Botany. 1971;35(143):1045–1052. http://www.jstor.org/stable/42751990 [Google Scholar]
- Khunte S., Das Kumar, Ansari N., Saravanan S. Effect of Different Levels of PGRs with Organic Manure on Growth Characters and Economics of Strawberry ( Fragaria x ananassa Duch.) cv. Chandler in Northern region Effect of Different Levels of PGRs with Organic Manure on Growth Characters and Economic. Int. J. Curr. Microbiol. Appl. Sci. 2020;9(1) [Google Scholar]
- Kumar Rajesh, Bakshi M., Singh D.B. Vol. 7. 2012. Influence of plant growth regulators on growth, yield and quality of strawberry (Fragaria x ananassa Duch) cv. p. 2012. [Google Scholar]
- Kumari S., Bakshi P., Sharma A., Wali V.K. Use of plant growth regulators for improving fruit production in sub tropical crops use of plant growth regulators for improving fruit production in sub tropical crops amit jasrotia and simrandeep Kour. Int. J. Curr. Microbiol. Appl. Sci. 2018;7(3):659–668. [Google Scholar]
- Kumra Rakesh, Reena S.S. Influence of plant growth regulators on strawberry : a review Related papers. Int. J. Chem. Stud. 2018;6(1):1236–1239. [Google Scholar]
- Reena Kumra Raksh, Saravana S., Bakshi P., Kumar A., Singh M., Kumar V. Influence of plant growth regulators on strawberry : a review. Int. J. Chem. Stud. 2018;6(1):1236–1239. [Google Scholar]
- Lal M., Sharma S., Hegde M. Micropropagation of strawberry (Fragaria x ananassa duch.) Indian J. Agric. Res. 2003;37(3):231–234. [Google Scholar]
- Li Y., Hu J., Xiao J., Guo G., Jeong B.R. Foliar thidiazuron promotes the growth of axillary buds in Strawberry. Agronomy. 2021;11(3):1–11. [Google Scholar]
- Liu D.J., Chen J.Y., Lu W.J. Expression and regulation of the early auxin-responsive Aux/IAA genes during strawberry fruit development. Mol. Biol. Rep. 2011;38(2):1187–1193. doi: 10.1007/s11033-010-0216-x. [DOI] [PubMed] [Google Scholar]
- Liu C., Guo Z., Park Y.G., Wei H., Jeong B.R. PGR and its application method affect number and length of runners produced in ‘Maehyang’ and ‘Sulhyang’ strawberries. Agronomy. 2019;9(2) [Google Scholar]
- Manandhar S., Shrestha G.K. Vol. 6. Nepalese Horticulture; 2008. Response of strawberry to plant growth regulators and their effect in vegetative parameters, flowering and fruiting. pp. 60–65. [Google Scholar]
- Marcos P., Marina N., Martı G.A. Vol. 176. 2009. Plant Science Influence of plant growth regulators on polygalacturonase expression in strawberry fruit; pp. 749–757. [Google Scholar]
- McArthur D.A.J., Eaton G.W. Strawberry yield response to fertilizer, paclobutrazol and chlormequat. Sci. Hortic. 1988;34(1–2):33–45. [Google Scholar]
- Mehraj H., Taufique T., Ali M.R., Mehraj H., Taufique T., Ali M.R., Sikder R.K., Uddin A.F.M.J. Impact of GA3 and NAA on horticultural traits of Abelmoschus esculentus. World Appl. Sci. J. 2015;33(11):1712–1717. [Google Scholar]
- Naeem M., Khan A., Masroor M., Moinuddin Triacontanol: a potent plant growth regulator in agriculture. J. Plant Interact. 2012;7(2):129–142. [Google Scholar]
- Nautiyal B.P., Shukla A.C. Influence of bio-fertilizers and bio-regulators on growth , yield and quality of strawberry (Fragaria × ananassa) Influence of bio-fertilizers and bio-regulators on growth , yield and quality of strawberry (Fragaria × ananassa) Indian J. Agric. Sci. 2015;85(9):1201–1205. [Google Scholar]
- Pang Q., Chen X. Triacontanol promotes the fruit development and retards fruit senescence in strawberry. Plants. 2020;9(448):1–22. doi: 10.3390/plants9040488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroussi G., Voyiatzis D.G., Paroussis E., Drogoudi P.D. Vol. 96. 2002. Growth , flowering and yield responses to GA 3 of strawberry grown under different environmental conditions; pp. 103–113. [Google Scholar]
- Rademacher W. Plant growth regulators: backgrounds and uses in plant production. J. Plant Growth Regul. 2015;34(4):845–872. [Google Scholar]
- Rathod K.D., Ahlawat T.R., Kumar S., Sarkar M., Chakraborty B. Effect of plant growth regulators on growth, yield and quality of strawberry (Fragaria × ananassa duch.) Cv. winter dawn under open field conditions of south Gujarat. Agric. Sci. Digest. 2021;41(2):329–333. [Google Scholar]
- Roberts J.A., Hooley R. Leaf, flower and fruit development. Plant Growth Regul. 1988:114–133. [Google Scholar]
- Roussos P.A., Denaxa N., Damvakaris T. Vol. 119. 2009. Scientia Horticulturae Strawberry fruit quality attributes after application of plant growth stimulating compounds; pp. 138–146. [Google Scholar]
- Sachs M., Iszak E. Effect of 2 (3-chlorophenylcarbamoyloxy) propionic acid and ethephon on runner development , flowering and fruiting behaviour of strawberries. J. Hortic. Sci. 2015;1589:37–41. [Google Scholar]
- Saima Z., Sharma A., Umar I., Wali V.K. Effect of plant bio-regulators on vegetative growth , yield and quality of strawberry cv . Chandler. Afr. J. Agric. Res. 2014;9(7):1694–1699. [Google Scholar]
- Salentijn E.M.J., Aharoni A., Schaart J.G., Boone M.J., Krens F.A. 2003. Differential Gene Expression Analysis of Strawberry Cultivars that Differ in Fruit-Firmness; pp. 571–578. [Google Scholar]
- Sharma K., Negi M. Vol. 8. 2019. Effect of organic manures and inorganic fertilizers on plant growth of strawberry (Fragaria x ananassa) cv . Shimla delicious under mid-hill conditions of Uttarakhand; pp. 1440–1444. [Google Scholar]
- Sharma R.R., Singh R. Vol. 119. 2009. Scientia Horticulturae Gibberellic acid influences the production of malformed and button berries , and fruit yield and quality in strawberry (Fragaria  ananassa Duch) pp. 430–433. [Google Scholar]
- Sood M.K., Kachawaya D.S., Singh M.C. Effect of bio-fertilizers and plant growth regulators on growth , flowering , fruit ion content , yield and fruit quality of strawberry. Int. J. Agric. Environ. Biotechnol. 2022;11(3):439–449. [Google Scholar]
- Srivastav A., Singh B.K., Pandey R., Singh K. Vol. 7. 2018. Effect of organic manures and bio-fertilizers on vegetative growth and yield of strawberry cv . chandler; pp. 2841–2844. [Google Scholar]
- Suman M., Ram Meghawal D., Prakash Sahu O., Sangma P.D. Effect of plant growth regulators on fruit crops. J. Pharmacogn. Phytochem. 2017;6(2):331–337. http://www.phytojournal.com/archives/2017/vol6issue2/PartF/6-2-70-493.pdf [Google Scholar]
- Suman M., Sangma P.D., Meghawal D.R. Effect of plant growth regulators on fruit crops Effect of plant growth regulators on fruit crops. J. Pharmacogn. Phytochem. 2017;6(2):331–337. [Google Scholar]
- Tafazoli E., Vince-prue D. Vol. 1589. 2015. A Comparison of the Effects of Long Days and Exogenous Growth Regulators on Growth and Flowering in Strawberry , Fragaria χ Ananassa Growth Regulators on Growth and Flowering in Strawberry. [Google Scholar]
- Terms F. Vol. 1589. 2017. The production of strawberry plants by in vitro; pp. 1–5. [Google Scholar]
- Thakur Y., Chandel J.S., Verma P. Effect of plant growth regulators on growth, yield and fruit quality of strawberry (Fragaria x ananassa Duch.) under protected conditions. J. Appl. Nat. Sci. 2017;9(3):1676–1681. [Google Scholar]
- Tiwari A.K., Saravanan S., Lall D. Influence of different plant growth regulators on vegetative growth and physico-chemical properties of strawberry (fragaria x ananassa duch) cv . chandler. Plant Archives. 2017;17(1):367–370. [Google Scholar]
- Wang H., Li M., Yang Y., Dong J., Jin W. Histological and endogenous plant growth regulators changes associated with adventitious shoot regeneration from in vitro leaf explants of strawberry (Fragaria × ananassa cv. ‘Honeoye’) Plant Cell Tissue Organ Cult. 2015;123(3):479–488. [Google Scholar]
- Zaid A., Asgher M., Wani I.A., Wani S.H. Role of triacontanol in overcoming environmental stresses. Protect. Chem. Agents in the Amelioration of Plant Abiotic Stress. 2020:491–509. [Google Scholar]
- Zaid A., Wani I.A., Wani S.H. Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives. 2020. Role of triacontanol in overcoming environmental stresses role of triacontanol in overcoming environmental stresses; pp. 491–509. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.