Summary
The emergence and rapid spread outside of monkeypox virus (MPXV) to non-endemic areas has led to another global health emergency in the midst of the COVID-19 pandemic. The scientific community has sought to rapidly develop in vitro and in vivo models that could be applied in research with MPXV. In vitro models include two-dimensional (2D) cultures of immortalized cell lines or primary cells and three-dimensional (3D) cultures. In vitro models are considered cost-effective and can be done in highly controlled conditions; however, they do not always resemble physiological conditions. In this way, several in vivo models are being characterized to meet the growing demand for new studies related to MPXV. In this review, we summarize the main MPXV models that have already been developed and discuss how they can contribute to advance the understanding of its pathogenesis, replication, and transmission, as well as identifying antivirals to treat infected patients.
Subject areas: Virology
Graphical abstract
Virology
Introduction
The etiologic agent of zoonotic monkeypox disease (MPX) is the monkeypox virus (MPXV). Belonging to the family Poxviridae and the genus Orthopoxvirus, this virus was isolated for the first time in 1958 from vesico-pustular lesions of infected cynomolgus monkeys kept for research.1 The virus can be divided into two viral clades: the Congo Basin (clade I) and the West African (clade II). The Congo Basin viruses are more virulent, with human case fatality rates during outbreaks in parts of Africa estimated to be around 10%. Although monkeypox is so named because researchers first detected it in monkeys, the virus is believed to be transmitted by wild animals such as rodents or infected people.2 These animals can transmit the disease to humans and secondarily the disease is limited through person-to-person transmission.3
Patients affected by the disease may initially present with fever, headache, back pain, asthenia, myalgia, rash, and lymphadenopathy. Lymphadenopathy is a distinctive feature of the disease that can be used to differentiate it from other infections that have similar clinical presentation at onset (chickenpox, measles, smallpox).3 In more severe cases, monkeypox complications can cause pneumonitis, encephalitis, vision hazard keratitis, and secondary bacterial infections.4,5 Previous studies show that mortality rates vary substantially and are vulnerable to case finding bias.6 Historically, the case fatality rate of monkeypox ranges from 0% to 11%, with young children being the most vulnerable group. In the recent epidemics, the case fatality ratio has varied from 3% to 6%.3
Since the first human monkeypox infection was reported in 1970, most outbreaks have been confined to central Africa. The first significant outbreak outside Africa occurred in 2003 in the U.S. and was epidemiologically linked to imported exotic pets (Gambian pouched rats and dormice) from Ghana that spread the virus to pet prairie dogs and from them to humans.7 In recent years, both travel-related and non-travel-related cases have been reported outside of West and Central Africa, which are considered endemic areas. Europe, North America, Australia, and Asia had records of the disease.8 However, the number of cases registered outside endemic areas has increased considerably. As of November 7, 2022, more than 78 thousand cases of monkeypox with 43 fatalities have been reported in a total of 109 countries (102 non-endemic and 7 endemic countries).9 On July 23, 2022, World Health Organization (WHO) declared the current monkeypox epidemic represents a Public Health Emergency of International Concern (PHEIC).10
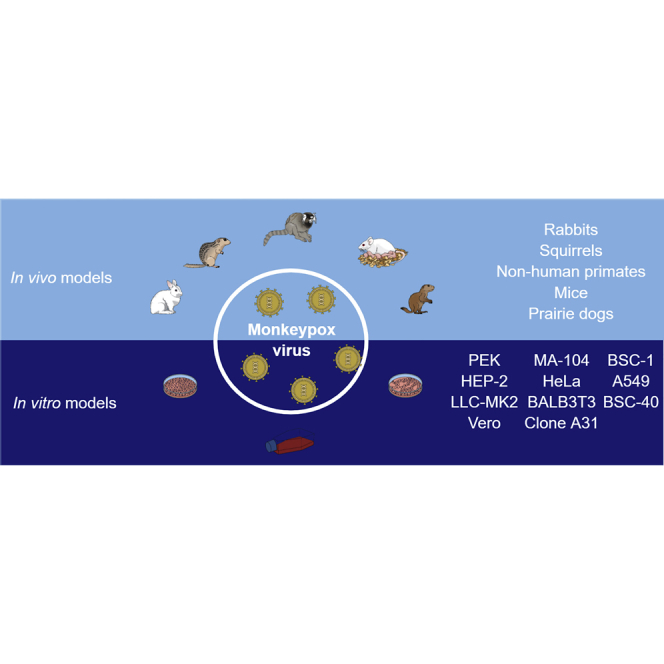
Since its discovery to date, several research models have already been developed mimicking the pathophysiology of the disease. In this review, we summarize the main models in vitro (2D and 3D cell culture) and in vivo developed to study monkeypox biology and discuss its advantages, disadvantages, and how these models can contribute to coping with the disease.
In vitro models
In order to study viral infections, tools rather than the natural host are needed due to the impact and lethality of the disease caused by viruses.11 As a solution, Renato Dulbecco and Marguerite Vogt proposed the use of cell lineages in viral research in late 1950, which revolutionized how knowledge is acquired by facilitating the study of viral infection and its replication kinetics.12,13 It also resulted in the evolution of the previous time-consumption drug discovery process to an optimized and high selective one,14 added to the alignment with the ethical desire for reducing animal use.15 In this context, it is possible to hypothesize the importance of knowing the most suitable cell culture model to be applied in the studying of the MPXV pathogenesis or to the screening and identification of active molecules that might be capitalized into the clinical treatment of the disease. The in vitro assays have shown that the cells Vero, Vero 76, Vero E6, LLC-MK2, BSC-40, BSC-1, PEK, HEP-2, HeLa, MA-104, HFF, and Balb/3T3 clone A31 are susceptible and capable of maintaining the MPXV replication, resulting in a high titer stock of virus, added of interested applicability into antiviral identification. The cell culture models, their characteristics, as well as their advantages and disadvantages are detailed below and summarized in Table 1.
Table 1.
Cells models used in the study of MPXV
| Cell/tissue type | Virus strain | Viral cultivation | Main applications | Reference |
|---|---|---|---|---|
| Vero | Congo-8 virus; VARV-UK-60/Ind-3a, MPXV-Copenhagen/Z79-I-005, VACV- Copenhagen, CPXV-Grishak, and ECTV-K-1 | Cells were cultured in RPMI-1640 or MEM supplemented with 2–10% fetal bovine serum, at 37°C in a 5% CO2 atmosphere. Some authors mentioned the use of antibiotics and antimycotics | Virus isolation, plaque-forming assay, virus amplification, infection characterization, evaluation of host responses, and evaluation of antiviral activity | (Marennikova et al.16; Rogers et al.17) |
| PEK, and HEP-2 | Congo-8 virus; VARV-UK-60, MPXV-Copenhagen/Liberia-1/Liberia-2/V-70 1 266 | a | Cell susceptibility | (Marennikova et al.16) |
| LLC-MK2 | MPXV-V79-1-005-Scab/Katako Kombe and MPXV-GFP | Cells were cultured in RPMI-1640 or OPTI-MEM-I supplemented with 2-10% fetal bovine serum, with antibiotics and antimycotics, at 37°C in a 5% CO2 atmosphere. Cells were infected with an MOI of 0.001, 0.01, 0.1, or 1 and incubated for 1–8 days | Plaque-forming assay, neutral red uptake assay, MTT assay, virus amplification, virus isolation, infection characterization, evaluation of host responses, evaluation of antiviral activity, and siRNA transfection | (Baker et al.18; Alkhalil et al.19) |
| Vero 76 | MPXV-V79-1-005-Scab, VACV-Elstree/Copenhagen, CPXV-Marina/Brighton, and CMLV-Somalia | Cells were cultured in RPMI-1640 supplemented with 2 or 10% fetal bovine serum or Eagle’s EMEM with Hanks’ salts and 5% fetal calf serum, supplemented with antibiotics and antimycotics, at 37°C in a 5% CO2 atmosphere. Cells were infected with an MOI of 0.01–1 or with 100 plaque-forming units (p.f.u) of virus/well and incubated for 3–6 days | Plaque-forming assay, neutral red uptake assay, virus amplification, virus isolation, infection characterization, evaluation of host responses, and evaluation of antiviral activity | (Baker et al.18; Yang and Schneller,20; Smee et al.,21) |
| Vero E6 | MPXV-V79-1-005-Scab, MPXV-GFP-tdTR, Katako Kombe and MPXV-GFP | Cells were cultured in RPMI-1640, EMEM, or DMEM supplemented with 2%–10% fetal bovine serum, with antibiotics and antimycotics, at 37°C in a 5% CO2 atmosphere. Cells were infected with an MOI of 0.1 or 5 and incubated for 1–5 days | Plaque-forming assay, neutral red uptake assay, virus amplification, virus isolation, infection characterization, evaluation of host responses, evaluation of antiviral activity, and transfection | (Baker et al.18; Johnston et al.22; Alkhalil et al.19) |
| MA-104 | MPXV-GFP-tdTR/Z79-I-005 | Cells were cultured in MEM supplemented with 10% fetal bovine serum, with antibiotics and antimycotics, at 37°C in a 5% CO2 atmosphere. Cells were infected with an MOI of 0.01 or 5 and incubated for 24 h | Infection characterization, evaluation of host responses, evaluation of antiviral activity | (Johnston et al.22) |
| HeLa | MPXV-GFP-tdTR/Z79-I-005 CPXV-Brighton Red, VACV-WR-GFP/NLS/WR-A4-YFP/LUC and AKMV |
Cells were cultured in DMEM supplemented with 2%–10% fetal bovine serum or calf serum, at 37°C in a 5% CO2 atmosphere. Some authors mentioned the use of antibiotics and antimycotics. Cells were infected with an MOI of 0.01 or 5 and incubated for 16–24 h | MPXV isolation, plaque-forming assay, infection characterization, evaluation of host responses, evaluation of antiviral activity, immunofluorescent cell staining, transfection, IFN I response analysis | (Magnus et al.1; Johnston et al.22; Priyamvada et al.23; Fernández de Marco et al.24) |
| Balb/3T3 clone A31 | MPXV-Z79-I005, VARV-Copenhagen, CPXV-Brighton, and CMLV-Somalia | Cells were cultured in medium supplemented with 2% serum and infected with 100 plaque-forming units (p.f.u) of virus/well and incubated for 3–6 days | Plaque-forming assay, neutral red uptake assay, infection characterization, evaluation of host responses, evaluation of antiviral activity | (Smee et al.21) |
| BSC-40 | MPXV-V79-1-005-Scab)/V70-I-266/V78-I-3945/V81-I-179/V77-I-823/V1979-I-005/2003-RCG-358/2003-USA-039/2003-USA-044/RCG2003-RCG-358, VARV-SOM77-ali/NEP73-175/BSH74-sol/SUD47-juba/SLN68-258/BRZ66-39, CPXV-Brighton Red, VACV-WR-GFP/NLS/WR-A4-YFP/LUC, and AKMV |
Cells were cultured at 37°C in a 5% CO2 atmosphere, in Opti-MEM (Invitrogen) or DMEM/RPMI-1640 supplemented with 2%–10% fetal bovine serum, antibiotics, and antimycotics. Cells were infected with an MOI of 0.1 or 10 PFU/cell and incubated for 2–5 days | Plaque-forming assay, neutral red uptake assay, virus amplification, infection characterization, evaluation of host responses, evaluation of antiviral activity, transfection | (Baker et al.18; Smith et al.25; Priyamvada et al.23; Fernández de Marco et al.24; Fogg et al.26) |
| BSC-1 | CPXV-Brighton/GER_1990_2/GER_1991_3/GER_2002_MKY/CPXV-Br (ATCC VR-302)/CPXV-GFP, MPXV-Z76/Z79-I-005/MSF6 B16R, VACV -WR (ATCC VR-1354)/IHD-J/MVA (ATCC VR-1508)/WR B18, VARV-BSH1975 B17R |
DMEM, RPMI supplemented with 2%–10% fetal bovine Serum or with 5%–10% fetal calf serum. Cells were infected with an MOI of 0.01 or 1 | Plaque-forming assay, infection characterization, analysis of the virus-cell fusion process, evaluation of host responses, evaluation of antiviral activity, and yield reduction assay | (Altmann et al.27,28; Bengali et al.29; Fernández de Marco et al.24) |
| A549 | MPXV 2003-USA-044/RCG2003-RCG-358 | DMEM with 5%–10% fetal calf serum | Transfection, IFN-III response analysis | (Fernández de Marco et al.24) |
| RK 13 | MPXV WR-7-61 | Cells were maintained in MEM supplemented with 5% fetal bovine serum, at 37°C with 5% CO2 | Plaque-forming assay | (Arndt et al.30) |
Not reported; VARV: variola virus; MPXV: monkeypox virus; VACV: vaccinia virus; CPXV: cowpox virus; ECTV: ectromelia virus; CMLV: camelpox virus; AKMV: Akhmeta virus; MOI: MOI; p.f.u: plaque formation unit.
Cell lineages directly employed in the MPXV study
MPXV was isolated in non-human primates in 1958 from pustules present on cynomolgus monkeys.1 For this, HeLa, monkey kidney, and human amnion cells were incubated with the supernatant of the emulsified pustules. The presence of cytopathic effect was observed 2–3 days after infection. The three types of infected cells were characterized by presenting alterations in their morphological structure.1
In humans the MPXV was first isolated from a human host in 1970 in the Democratic Republic of the Congo by material collected from skin lesions.16 The isolation and characterization of the cytopathic effect were performed by infecting four immortalized cell lines Vero (African green monkey kidney), PEK (pig embryonic kidney cells), and HEP-2 (Homo sapiens epithelial carcinoma cells) with a 10-fold TCID50 serial dilution of MPXV and analyzed up to 7 days post-infection (d.p.i.). In Vero cells, the MPXV formed larger plates than the variola virus with a pattern of internal structure characteristic of other MPXV isolated from monkeys. In addition, when comparing all cell lines, Vero cells were more sensitive to MPXV when compared with HEP-2, and PEK, producing higher titers (about 107 to 108 TCID50). The MPXV can be distinguished from variola and vaccinia viruses in PEK cells by its lower replication and the lack of hemadsorption phenomena.
In the attempt to identify antiviral molecules capable of inhibiting the MPXV replication cycle as well as understanding its replication mechanism, several methodologies were employed throughout the literature. Baker and coworkers used Vero 76 (African green monkey kidney), Vero E6 (African green monkey kidney deficient in type I interferon [IFN-I]), LLC-MK2 (Rhesus monkey kidney cells), and BSC-40 cells. The BSC-40 is a continuous cell line derived from BSC-1 cells (Vervet monkey kidney cell)18,31 and is suitable for amplification and isolation of large viruses such as the poxviruses due to their resemblance with the natural host. Therefore, this cell lineage was used by the authors for inoculation and amplification for 5 days and collected by centrifugation associated with freeze-thaw cycles. As for the title of the MPXV, Vero E6 cells were employed because it is deficient in an IFN-I pathway and might favor viral replication.32 The protocol used by the authors was based on incubating the virus with cells for 2 h, adding a medium with carboxymethyl cellulose (CMC), analyzed for up to 3 or 5 days p.i. through fixation with crystal violet staining solution (1.3 mg/mL crystal violet, 5% ethanol, 30% formalin), and plaques counted visually. For antiviral assays, the authors employed the neutral red uptake assay, an absorbance technique to assess cell viability where healthy, uninfected cells will take up neutral red dye through pinocytosis, whereas cells infected with a cytopathic virus will not. The Vero 76 and LLC-MK2 cell lines were infected with MOI of 0.01, 0.1, and 1 in the presence of serial dilutions of each one of the 24 compounds analyzed in 96 well plates. The incubation period was related to the presence of CPE in virus-infected and untreated cell control, and the assessment of the potential compounds by IC50 values was better visualized in 5-day incubation and MOI of 0.1. Alternatively, the plaque-forming assay was also used, and the MPXV took 4–5 days to produce plaques of similar size in Vero 76, Vero E6, or BSC-40 cells, whereas other poxviruses produced small plaques after 5–6 days with lower cytopathic effect. Following the technical protocol of plaque reduction assay, Rogers and collaborators (2008) evaluated the effect of silver nanoparticles against MPXV, however employing only the Vero cells in 12 well plates. The cells were infected with 50 p.f.u/well for 1 h and further treated with a serial dilution of the compound diluted into the medium with methylcellulose for 72 h.17
In another study, BSC-40 cells were also infected with MPXV V70-I-266 (Sierra Leone) to propagation finality, but also for the antiviral screening protocols. The cells were seeded in 96 well plates, infected with MOI of 0.1 for 1 h, removed, and replaced with ST-246 in 8 concentrations. After 3 days, the plate was stained with 2x crystal violet, and absorbance was measured at 570 nm, where the intensity of treatment was compared with the virus-infected control.25 Alternatively, the plaque reduction assay was performed by infecting BSC-40 cells in six-well plates with 25 p.f.u for 1 h, added of media with CMC with the compound in five concentrations for 3 days, fixed with 10% formalin, and submitted to immunohistochemistry polyclonal rabbit anti-variola virus antibody and goat anti-rabbit immunoglobulin G-horseradish peroxidase conjugate. The effect of the compound ST-246 was observed by the protection of reducing the CPE, which was seen even in low concentrations (0.015 μM). The BSC-40 was also employed by the authors in further studies to confirm and evaluate results encountered in vivo and in clinical trials to find viral titer, evaluate drug resistance, and perform serological analysis following the same protocols cited earlier.33,34
Differently, other authors employed the original BSC-1 cell line instead of the BSC-40 with interesting results. The BSC-1 cells seeded in 24 well plates were infected with 100 PFU for 1 h, overlaid with agarose 2% in the presence or absence of EB peptide (NH2- RRKKAAVALLPAVLLALLAP-COOH) to assess the reduction effect on plaque formation. To this, BSC-1 cells were infected at an MOI of 0.01 with the MPXV or with an inoculum containing the virus pre-treated with EB. Three days post-infection, cells were harvested by scraping, lysed by repeated cycles of freezing and thawing, and tittered on BSC-1 cells. As a result, the MXPV infectivity was significantly inhibited by the EB peptide, mainly in its attachment stage.27 To confirm, the authors based on the fact orthopoxvirus cores are only accessible to antibodies after being released into the cytoplasm, which allows the differentiation between attached and entered viruses using virion-specific or core-specific antibodies, respectively.35 To examine whether EB was disrupting virus attachment or entry into the cells, the number of attached and entered viruses per cell for 100 cells in the absence or presence of EB at 100 μM was quantified in a fluorescence microscope. Samples for attachment staining were fixed with 4% paraformaldehyde, quenched for 5 min with 100 mM glycine, blocked with 10% FBS in PBS, and stained with 1:200 anti-VACPXV antibody. Even if the compound is not capitalized in the clinic, it possesses the greatest potential as a novel in vitro tool to study the poorly characterized early steps in infection with poxviruses.
Further studies conducted by Altmann and collaborators also employed the BSC-1 cells in the assays with MPXV.28 Because the MXN is an intercalating agent and inhibits topoisomerase II function resulting in impaired cellular DNA replication and RNA synthesis,36 the authors suggested the possibility of inhibiting MPXV replication. Interestingly, to identify the activity of the MXN, a growth curve with MPXV at an MOI of 3 in the presence of the mitoxantrone (MXN) at 1 μM was generated. The supernatant was collected in time intervals of 1, 2, 6, 12, 24, and 36 h post-infection (h.p.i.). The authors also used an MPXV containing a GFP marker with MOI 1 in the presence of the serial dilutions of the MXN, which resulted in inhibition of MPXV replication with a low EC50 (0.25 μM). In addition, Bengali et al. (2012) used BSC-1 cells to analyze and compare characteristics involved in the cellular invasion of some orthopoxviruses strains in vitro, including MPXV. For this, MPXV expressing luciferase was constructed, and its expression was measured during the entry of the virus into the cell, using a luminometer. The authors observed that MPXV utilizes a low pH-dependent endocytic pathway during cell invasion.29
Johnston and coworkers took a different approach and employed several cell lineages: Vero-E6, HeLa, and MA-104. The Vero E6 cells were infected with MPXV and then transfected with a DNA plasmid containing the GFP from the early viral synthetic E/L promoter and tandem dimer Tomato Red (TR). The assay resulted in the production of MPXV-GFP-tdTR. After harvesting, the virus was amplified in MA-104 cells. These are non-human primate cells isolated from a pure cell population of African green monkey kidneys, which are also derived from the MPXV’s natural host. Further, the HeLa cells, naturally non-producing IFN-β cells37,38 were pre-treated with IFN-β in serial dilutions and then infected with the MPXV-GFP-tdTR in high (5) or low (0.01) MOIs. The effect was assessed by titration in a plaque reduction assay and resulted in the identification that MPXV is susceptible to IFN-β, even in low concentrations.22
In an attempt to identify antiviral molecules capable of inhibiting the MPXV replication cycle, Alkhalil and coworkers evaluated the RNAi pathway as a new approach in drug discovery against poxviruses. For antiviral screening, siRNAs targeted against various monkeypox viral proteins were developed and transfected into LLC-MK2 cells. The morphology of the transfected cells was analyzed with phase-contrast light microscopy after transfection, and no signs of cytotoxicity were observed in cells treated with the siRNA pools. The antiviral properties of the most potent siRNA constructs were characterized by transfecting LLC-MK2 cells with a single serial dilution of each construct at six concentrations between 40 and 1.25 nM. Later, the transfected cells were infected with 100 p.f.u/well of MPXV, and viral replication was examined at 48 h.p.i. The siA6-a siRNA was able to inhibit completely the MPXV infection at concentrations of ≥20 nM. The antiviral effects of siA6-a target the virus directly, silencing gene expression or interfering with vDNA replication without disrupting host cell biology. The study of chemical modification and the development of a siRNA delivery system are necessary before the evaluation of siA6-a in animal models.19
Yang and Schneller (2005) investigated the antiviral potential of S-adenosyl-L-hemocosteine (AdoHcy) hydrolase inhibitors, important for interrupting methylation reaction processes essential for viral replication. The experiments were performed using Vero 76 cells, MPXV, and 5′Homoaristeromycin inhibitor. The authors observed that the use of 5′homoaristeromycin 0.12 μg/mL showed satisfactory results against MPXV replication.20
To assess the antiviral activity of ribavirin and mycophenolic acid, Vero 76 and Balb/3T3 clone A31 cells were infected with MPXV (Zaire strain). The reduction effect on plaque formation was observed from cells infected with 100 plaque-forming units (p.f.u) of virus per well incubated for 1.5–2 h. Antivirals were incubated for 6 days, and plate sizes were analyzed.21 In addition, Priyamvada et al. (2021) used HeLa cells to test the antiviral effects of methylene blue derivatives against orthopoxviruses, including the MPXV WA clade. For the plaque reduction assay, HeLa cells were incubated with MPXV and treated with PAV-164 derived from PAV-866, a methylene blue analog, for 24 h. PAV-164 demonstrated efficiency in inhibiting viral replication at concentrations of 5 μM and 1.6 μM.23
It has been reported that poxvirus evades the immune response through the expression of secreted interferon (IFN) decoy receptors such as IFNα/β-binding protein (IFNα/βBP). These proteins prevent the interaction of IFN with cell receptors. Thus, de Marco et al. (2009) investigated the expression of IFNα/βBP in MPXV, using BSC-1 cells. For this analysis, BSC-1 cells were infected with RCG or USA MPXV strains, and after 40 h of incubation, their supernatant was collected and analyzed using the western blotting technique. It was observed that MPXV secretes proteins with type I IFN inhibitory activity, which leads to a failure in the antiviral response and disease progression. Furthermore, the antiviral activity induced by hIFNα-2b, hIFNα-A, or hIFNβ was inhibited.24 In addition, Arndt et al. (2015) observed that in RK13 (rabbit kidney cells) and BSC-40 cells treated with IFN-α A/D and infected with MPXV, viral replication was not affected. Similar result was found in cells treated and infected with VACV.30
BSC-40 cells were used in a study to investigate the neutralizing potential of monkey sera previously immunized with recombinant VACV protein. The results showed that MPXV incubated with monkey serum were neutralized and had their ability to spread prevented.26
From what has been presented here, it is possible to suggest that MPXV can infect several mammalian cell lineages independently of being a primate or non-primate host. This agrees with previous data described in literature because poxviruses do not need specific receptors for attaching and entering the cells,39 as they can interact with glycosaminoglycans or components of the extracellular matrix,40,41,42,43 and thus cytokine receptors may favor the infection.39 In this context, poxviruses can enter permissive and restrictive cells, but what will differentiate the establishment of infection will be the presence of machinery that can control downstream intracellular events to favor or abort viral replication. It was demonstrated by Marennikova and coworkers that HEP-2 and PEK cells produced low viral titer when compared with Vero cells, confirming that those would not be a good fit for viral isolation, amplification, or research purposes.16 What is more interesting is that a tissue-cell lineage is permissible and has the machinery needed for viral replication, which can result in a good fit for in vitro infection; however, in normal conditions, the vertebrate species might not be considered a permissive host.39 Therefore, the appropriated cell choice will be dependent on the purpose and/or hypothesis to be evaluated. Given the continuous accelerated evolution of MPXV, new studies with the currently circulating strains will contribute to understanding of viral infection mechanism.
In vivo models
What is expected of an ideal animal model is that it can develop the viral infection in a similar way to what occurs in humans, taking into account the pathogenesis of the disease and the clinical signs presented by patients. It is possible to elucidate virus biology and the mechanisms of infection using in vivo approaches; such findings are important for the definition of a suitable animal model for the development of studies aimed at the search for new preventive and therapeutic therapies. Although each animal species has some limitations as to inoculation route, dose, and age, in vivo experimental studies have demonstrated a wide variety of species capable of efficiently modeling the cycle of infection caused by MPXV.44,45 The main animal models already used in monkeypox studies will be detailed below, and their main characteristics are summarized in Tables 2, 3, 4, 5, and 6. Because some of these studies were done with past MPXV strains, these data should be interpreted with caution in the context of emerging viral lineages and strains.
Table 2.
Mice used in the study of MPXV
| Animal species | Strain | Age | Viral strain | Route of infection/dose | Major findings | Reference |
|---|---|---|---|---|---|---|
| Mouse (Mus muscullus) | a | Adults and 2-day-old | Egg passage material of the monkey agent | Intracerebral and intranasal/agent diluted 104 | Adult mice inoculated intracerebrally showed signs of encephalitis followed by 100% lethality. Two-day-old infant mice inoculated intranasally showed 100% lethality | (Magnus et al.1) |
| C57BL/6, SCID, DBA, A/Ncr, C3HeJ, IFN-γR−/−, BALB/c, IFN-α/βR−/−, 129 stat1−/−, C57BL/6 stat1−/− | 6- to 12-week-old | MPXV-ZAI-79 | Intranasal/a and footpad 102–104 p.f.u/mL | Footpad inoculation in adult mice was not efficient in infecting strains characterized as not responding to IFN-1 or type-2-dependent signaling pathways, on the other hand, infection occurred satisfactorily in C57BL/6 stat1−/− and 129 stat1−/− mice. In contrast, the immunodeficient SCID strain was susceptible to an intranasal MPXV infection, as were C57BL/− and 129 stat1−/− 129 stat1−/−. Overall, high mortality was a feature observed in STAT1-deficient mice, in addition to weight loss and viral presence in internal organs | (Stabenow et al.46) | |
| CAST/EiJ, C57BL/6, B6.129S7-IFNγ, BALB/c | a | MPXV-Z79-I-005 and MPXV-Z79-CB2 | Intranasal102–106 p.f.u/mL | MPXV replicated in the lungs to previous titers from other sites in CAST/EiJ mice. Surprisingly, lung titers in dose-infected BALB/c mice were similar to titers in CAST/EiJ mice, although all mice survived. Animals without IFN-γ, treated by gravity, or left by weight presented an upright posture and became moribund. In contrast, similar disease symptoms were delayed and markedly less pronounced in the animals that received IFN-γ, and these animals fully recovered. Inoculation of IFN in CAST/EiJ mice led to protection against MPXV. In addition, C57BL/6 mice with inactivation of the IFN gene or the IFN receptor gene are more sensitive to the disease | (Earl et al.47) | |
| a | 2-, 8-, 12-, and 15-day-old | Copenhagen | Intraperitoneal and intranasal/1,2x106 p.f.u/mL, footpad/6x102 p.f.u/mL and oral/a | When inoculated intraperitoneally, all animals showed similar symptoms and occurred as a result of infection, whereas 50% died infected via the footpad and 40% died orally. Sequential evaluations showed that the virus can be found in the blood, lungs, liver, spleen, and kidneys, with considerable amounts of virus detected in the lungs and other organs in the acute phase of the disease | (Marennikova and Seluhina,48) | |
| BALB/c and SCID | 3- to 4-week-old | MPXV-2003-USA-044 and MPXV-Congo-Luc+ | Intraperitoneal/a | In BALB/c mice, the luminescent signal had the highest peaks between 96 and 120 h. Greater replication and faster dissemination were observed mainly to organs of the peritoneal cavity, with eventual dissemination to axillary lymph. In SCID mice, a more intense luminescent light was observed after 96 h. It was spread to organs and tissues in the regions of the abdominal, thoracic, and axillary lymph nodes, in addition to showing visible signs in the tail, feet, and nasal region. Biophotonic images also revealed the tropism of MPXV for ovarian tissues | (Osorio et al.49) | |
| CAST/EiJ and BALB | 9-week-old | MPXV-z06 and MPXV-z79-CB2 | Intranasal/a | High luminescence in the nasal area with a peak between 7 and 12 days after infection was observed in the animals. With the recombinant strain MPXV-z79-CB2, CAST/EiJ mice infected intranasally showed lethargy, hunched posture, ruffled hair, and severe weight loss | (Earl et al.50) | |
| ICR | 8- to 10-day-old | MPXV-Z79-I-005 | Intranasal/104–105 p.f.u/mL | Mice by MPXV accumulations of nasal, lung, and brain pathogens. The presence and replication in primary target cells and traditional observations of MPXV (mononuclear phagocytic cells and tract epitheliocytes) are present, as well as some other cell types (endothelial cells, reticular cells, connective tissue cells) were also observed | (Sergeev et al.51) | |
| 129S1/SvlmJ, A/J, BALB/cByJ, C3H/HeJ, C57BL/6J, CAST/EiJ (WD), DBA/2J, FVB/NJ, SJL/J, SPRET/EiJ (WD), AKR/J, C57 L/J, C58/J, MOLF/EiJ (WD), NOD/ShiLtJ, NZB/BINJ, PERA/EiJ(WD), PL/J, SM/J, SWR/J, BUB/BnJ, C57BL/10J, C57BLKS/J, CBA/J, CZECHII/EiJ (WD), LP/J, RIIIS/J, WSB/EiJ (WD), BTBR T+ tf/J, C57BR/cdJ, CE/J, I/LnJ, MA/MyJ, NON/ShiLtJ, NZW/LacJ, PWK/PhJ (WD), SEA/GnJ and BALB/c | 4- to 8- week-old and 5- to 11-month- old | MPXV-Z79-I-005 and MPXV-USA-2003-044 | Intranasal and intraperitoneal/102–106 p.f.u/mL | Thirty-eight inbred mouse strains were tested for MPXV susceptibility. Three strains were developed, from which CAST/ were developed. CAST/EiJ exhibit weight loss, morbidity, and death in a dose-dependent manner, whereas there were no deaths of BALB/c mice at high doses. Both routes of inoculation resulted in replication in the spleen | (Americo et al.52) | |
| CAST/EiJ, C57BL/6, B6:129X1-Il15ratm1Ama/J and B6-129X1 | 9- to 13-week-old | MPXV-z06 | Intraperitoneal/a | Administration of IL-15 to CAST mice transiently increased NK and CD8+ T cells that could express IFN-γ, indicating that progenitor cells were able to respond to cytokines. However, the number of NK cells rapidly decreased, indicating a defect in their homeostasis. In addition, antibodies to interferon-γ abrogated the protection by activated NK cells. Thus, the inherent susceptibility of CAST mice to orthopoxviruses may be explained by a low level of natural killer (NK) cells | (Earl et al.53) | |
| C57BL/6J, CAST/EiJ, MOLF/EiJ, C58/J, NZW/Lacj, CASA/Rkj and BALB/c | a | MPXV-Z79-CB2 and MPXV-Z79-005 | Intranasal/104–106 p.f.u/mL | NZW/Lac and C58 mice exhibited more weight loss than other classical inbred strains, but all survived intranasal MPXV challenges. Mice from three naturally derived strains, in addition to CAST, exhibited severe weight loss and died or were euthanized | (Earl et al.54) | |
| C57BL/6 and BALB/c | 6- to 7-week-old | MPXV-2003-044 and MPXV-2003-358 | Footpad and intranasal/ 105 p.f.u/mL | Mice inoculated on the footpad with the Congo Basin strain showed clinical signs of the disease, with BALB/c mice showing greater edema compared with C57BL/6 mice. One mouse of the BALB/c strain showed weight loss, whereas no mouse of the C57/BL6 strain showed this clinical sign. When inoculation took place intranasally, weight loss was observed in both strains of mice. On the other hand, mice inoculated on the footpad with MPXV from West Africa showed only mild swelling at the inoculation site. None of the mice in the group inoculated with intranasal MPXV from West Africa developed any obvious signs of morbidity | (Hutson et al.44) | |
| CAST/EiJ mice | 4- to 6-week-old | 2022 MPXV isolate (SP2833) | Inhalation/104 or 106 PFU | Although the virus replicated efficiently in the respiratory tract, mice did not succumb to the infection | (Warner et al.55) |
Not reported; p.f.u: plaque formation unit; mL: milliliter; MPXV: monkeypox virus.
Table 3.
Non-human primates used in the study of MPXV
| Animal specie | Age | Viral strain | Route of infection/dose | Major findings | Reference |
|---|---|---|---|---|---|
| Cynomolgus (Macaca fascicularis) | a | Virus strain isolated from pustules of naturally infected animals | Intradermally/a | The infected animal developed a local pustule surrounded by edema 7 days after inoculation. Body temperature increased slightly between the 5th and 9th day after inoculation, but no propagation of the eruption occurred | (Magnus et al.1) |
| a | MPXV- Z79-005 | Intravenous/5x106 and 5x107 p.f.u/mL | Unimmunized monkeys became seriously ill with fever and weight loss, with between 575 and 820 skin lesions. There were fewer lesions in 65–140 immunized monkeys each, and they were generally smaller and atypical, developed less synchronously, and healed quickly | (Fogg et al.26) | |
| a | Strain recovered from naturally infected monkey (Strain 1744) | Intravenous, intradermally and subcutaneous/a | Acute disease was observed, characterized by marked facial swelling that extends to the cervical region. Severe difficulty in breathing, papular eruptions throughout the body, ulcerative lesions of the oral mucous membrane, and generalized lymphadenopathy | (Prier and Sauer,56; Prier et al.57) | |
| a | Strain recovered from the blood of an infected monkey (Strain 10,001) | Intramuscular/10−1–10−6 | After inoculation of graduated doses of MPVX, the clinical features emerging from the infection at each dilution were visibly indistinguishable. The mortality rate reached 30%, and a more intense rash was observed in these animals, followed by progressive disease, prostration, hypothermia, and collapse. Severe illness lasted from 4 to 11 days; during this time the animals became progressively dehydrated and lost weight. In some cases, there was evidence of pyoderma | (Wenner et al.58) | |
| a | a | Intravenous, intramuscular, intradermally or intradermally combined and conjunctival routes/a | The main events during the course of the infection were fever and cutaneous and oral mucosal lesions. Some animals developed a generalized smallpox rash, whereas in others only a few scattered pustules were seen | (Wenner et al.59) | |
| Immature | a | Intramuscular/a | The skin lesions progressed rapidly through the papular and vesicular stages. The pustular phase was of short duration with formation of hard crusts. Pustules were observed on the scrotum of males, lesions on the oral mucosa, on the amygdala with histological characteristics similar to those described for the skin and lymph nodes. Generalized lymphadenopathy developed during the first week and lasted until the 3rd week of the disease; there was moderate enlargement of the spleen, histological and cytological changes in a combination of diffuse and follicular hyperplasia, reticular lymphoblast proliferation | (Wenner et al.60) | |
| 6.2 ± 0.7 year-old | MPXV- Z79-005 | Intravenous/5x107 p.f.u/mL | MPXV infection caused a vesiculopustular rash, fever, lymphadenopathy, splenomegaly, pulmonary edema, increased white blood cells, and the death of some animals | (Huggins et al.61) | |
| 3- to 6-year-old | MPXV- Z79-005 | Intravenous/5x107 p.f.u/mL | Infected animals developed severe clinical syndromes consisting of anorexia, extremely low temperature, shock, disseminated intravascular coagulation, or respiratory distress leading to death. Severe pathology was characterized by generalized smallpox lesions on the skin and oral mucosa, notable lymphadenopathy and splenomegaly, and pulmonary congestion/edema with gross lesions | (Wei et al.62) | |
| a | MPXV- Z79-005 | Intravenous/5x107 p.f.u/mL | All infected and untreated animals presented skin lesions observed first in the mouth and head that spread to the rest of the body, progressive signs of the disease that led to their death | (Jordan et al.63) | |
| a | MPXV- Z79-005 | Intravenous/5x107 p.f.u/mL | Although the PCR data suggested a low viremia, all immunized animals remained clinically well, whereas the non-immunized animals became extremely ill with fever, weight loss, and decreased activity in addition to having a large number of smallpox skin lesions that exhibited a pustular progression | (Earl et al.64) | |
| a | MPXV- Z79-005 | Intravenous/5x106 and 5x107 p.f.u/mL | A large number of skin lesions appeared in control animals in the first few days, coinciding with high viremia, but disappeared in 4 out of 6 animals. Four untreated animals had severe illness requiring euthanasia. Although treated animals had a high lesion count, the viral load was much lower than that of unvaccinated animals | (Earl et al.65) | |
| a | MPXV- Z79-005 | Intravenous/1 mL 2x107 p.f.u/mL | Clinical symptoms and post-challenge survival, lesion counts, and viral loads were analyzed. Unvaccinated animals exhibited typical signs of the disease, depression, lethargy, pustule lesions, and high peaks of viral load after challenge | (Buchman et al.66) | |
| Adults | MPXV- Z79-005 | Intravenous/5x107 p.f.u/mL | Even the vaccinated animals showed signs of the disease. Untreated animals showed increased body temperature, weight loss, and a high number of skin lesions succumbing to the infection | (Denzler et al.67) | |
| 2- to 8-year-old | MPXV- Z79-005 | Intravenous/1,65x107 and 5,4x107 p.f.u/mL | After the lethal challenge with MPXV, the unvaccinated control animals succumbed to monkeypox 11 days after the challenge, numerous lesions were observed throughout the body and a weak primary immune response when compared with the treated animals | (Russo et al.68) | |
| a | a | Exposure to aerosols/a | The first sign of infection after exposure was a rise in temperature. From the 4th or 5th day after exposure, cough, runny nose, apathy, and loss of appetite were observed. Mild rash with papules or pustules limited to face and hands is observed. Histologically, ulcerative bronchiolitis, bronchitis, and peribronchitis were observed; in addition, fibrinous necrosis was found in the bronchial walls, peribronchial lymphoid tissues, and bronchopulmonary lymph nodes | (Hahon and McGavran,69) | |
| 2-4 years-old | MPXV- Z79-005 | Exposure to aerosols/2,6x105 p.f.u/mL | Untreated animals showed severe infection, weight decline, progressive depression, dyspnea, and nasal discharge. Skin lesions, first appearing on day 6 after challenge, live virus, and viral DNA were detected in the throats of all challenged animals. Most of the tissues analyzed were positive for the virus, and there were histological changes consistent with focal acute necrotizing bronchitis and bronchopneumonia; focal, fibrinous necrotizing alveolitis often accompanied by edema; and acute focal vasculitis, sometimes accompanied by thrombosis and perivascular edema | (Hatch et al.70) | |
| Youth to adults | MPXV- Z79-005 | Exposure to aerosols/a | The animals presented exanthema, enanthema, mild anorexia, fever, cough, nasal secretion, cutaneous, oral, and gastrointestinal lesions. Dyspnea, depression, severe anorexia, and signs of weakness were evident in all animals. Clinical signs progressed to the animals' natural death or euthanasia | (Zaucha et al.71) | |
| a | MPXV- Z79-005 | Intratracheal/3,42×106, 8,37×106 and 3,53×107 p.f.u/mL | Animals challenged with the lowest dose had fever, weight loss, pustular skin lesions, and lymphadenopathy. Animals exposed to the 8.37 × 106 p.f.u dose showed similar clear signs but with some animals dying and animals exposed to the higher dose had a similar disease course but with an accelerated progression to death | (Goff et al.72) | |
| 2- to 4-year-old | MPXV-MSF#6 (Isolated human) | Intratracheal/107 p.f.u i/5 mL | Severe morbidity, extensive skin lesions, dyspnea, and low saturation were observed in all control animals. Treated animals showed mild morbidity despite the typical signs of the disease | (Stittelaar et al.73) | |
| a | MPXV- Z79-005 | Exposure to aerosols/3×104, 1×105, 3×105 and 9×105 p.f.u/mL | Lesions appeared 6 days after exposure in two animals at the highest dose. One animal succumbed with no apparent injury and a single animal had severe injuries. Another animal that died presented histopathologically with pneumonia and intranuclear inclusion bodies consistent with smallpox infection. Discoloration of the lung and enlargement of the bronchial lymph nodes were also observed | (Barnewall et al.74) | |
| a | MPXV- Z79-005 | Exposure to aerosols/4×104, 1×105, 4×105 and 1×106 p.f.u/mL | Clinical signs observed were fever, decreased appetite and activity, drowsiness, depression, inguinal and axillary lymphadenopathy, and macules that progressed to pustules. Necrotizing lesions were also present on the skin, gastrointestinal tract, mucosal surfaces, and gonads. The histological findings of animals sacrificed during acute illness were similar to those previously reported for aerosolized MPXV | (Nalca et al.75) | |
| 2- to 7-year-old | MPXV- Z79-005 | Exposure to aerosols/1×105 p.f.u/mL | Challenged animals showed typical signs of the disease, fever, weight loss, papules and pustules, weight loss, and viremia detected in the first day’s post infection. When treated, they have a greater survival with rapid improvement of clinical signs | (Russo et al.76) | |
| 2-year-old | MPXV- Z79-005 | Exposure to aerosols/1×105 p.f.u/mL | Typical signs of the disease were observed, which included increased weight loss, dyspnea, anorexia and a spike in body temperature. Viral RNA was also identified in body tissues such as lungs, spleen, amygdala, tongue, kidney, heart, cerebrospinal fluid, and mediastinal lymph nodes. Histological changes were restricted to the respiratory bronchioles in the lungs | (Tree et al.77) | |
| a | MPXV- Z79-005 | Intravenous/5x104 to 5x107 p.f.u/mL and Intrabronchial/5x104 to 5x106 p.f.u/mL | Both routes of inoculation resulted in a rapid spread of the virus; typical signs of the disease from moderate to severe were observed. Disease progression was twice as rapid for animals infected via the intravenous route compared with those infected via the intrabronchial route. All challenged animals resolved the infection | (Johnson et al.78) | |
| Rhesus (Macaca mullata) | a | Virus strain isolated from pustules of naturally infected animals | Intradermally/a | After inoculation none of the challenged animals developed any signs of disease | (Magnus et al.1) |
| a | Strain recovered from naturally infected monkey (Strain 1744) | Intravenous, Intradermally and Subcutaneous/a | Intravenous infection resulted in generalized rashes, the subcutaneous one produced a similar granuloma, and the intracutaneous one caused local lesions without spreading to other parts of the body | (Prier and Sauer,56; Prier et al.57) | |
| a | a | Intramuscular/a | Signs of typical disease in cynomolgus were much less pronounced in rhesus; however, rash, dermal lesions, and short-lived exanthema were observed. None of the animals looked remarkably sick, and none specifically died of the disease, but all developed antibodies against the virus | (Wenner et al.59) | |
| 2-5 years-old | MPXV- Z79-005 | Intravenous/1,65x107 and 5,4x107 p.f.u/mL | Lethally challenged with MPXV, the animals showed the typical clinical signs of the disease, skin lesions, strong anamnestic responses to the challenge, and a weak primary immune response when compared with treated animals. Treated animals were protected from lethal challenge with MPXV | (Russo et al.68) | |
| 4- to 9-year old | MPXV- Z79-005 | Intrabronchial/2×105 p.f.u/mL | The challenge resulted in the appearance of numerous disseminated lesions, initially as pustules on the skin and oral mucosa and then progressing from pustular stages to crusts. The animals also developed coughing, symptoms of labored breathing, and fever | (Estep et al.79) | |
| Sagui (Callithrix jacchus) | Adults | MPXV- Z79-005 | Intravenous/2,4x10,7 9,5 x 105, 7,8 x 104, 5x10,3 510 or 48 p.f.u/mL | All animals developed a similar disease course and died or were sacrificed. The different doses also showed different clinical progression, mainly the temporal onset of the disease and the phenotypic presentation of rash. In general, all animals had a skin rash, significant lymphadenopathy, and pronounced lethargy, some of which needed to be euthanized | (Mucker et al.80) |
| Adults | MPXV- Z79-005 | Intranasal/100, 1000, 5000 p.f.u/mL | The disease progressed in all animals; the animals became increasingly unresponsive and lay down until they were sacrificed or succumbed to the disease. Other signs included light sensitivity, swelling, runny nose, swelling around the eyes, skin lesions, papules, petechiae, and crusts. They also showed an increase in white blood cells, a decrease in platelets, lymphocytes, and very high neutrophils | (Mucker et al.81) |
Not reported; p.f.u: plaque formation unit; mL: milliliter; MPXV: Monkeypox virus.
Table 4.
Prairie dogs used in the study of MPXV
| Animal specie | Age | Viral strain | Route of infection/dose | Major findings | Reference |
|---|---|---|---|---|---|
| Prairie dog (Cynomys ludovicianus) | 3-year-old | MPXV ROC-2003-358 | Intranasal/105 p.f.u/mL | Infected and untreated group: facial swelling, nasal discharge, nasal crust, bloody nose, inappetence, weight loss, pustular lesions, petechial rash, mouth breathing. The treated groups: prophylaxis (0) and post-infection (3) did not present symptoms of the disease, whereas the therapeutic group (after the appearance of lesions) presented facial swelling, nasal discharge, nasal crust, bloody nose, lack of appetite, weight loss, pustular lesions | (Smith et al.33) |
| Adults | MPXV-USA-2003-044 | Intraperitoneal/10x1,5 p.f.u/mL and intranasal/10x6.1 p.f.u/mL | Animals infected by the intraperitoneal route died approximately 8–11 days after infection and no mucosal or cutaneous lesions were observed. Via the intranasal route, 60% died similarly to the animals in the intraperitoneal group, and the survivors had vesicular lesions on the lips and tongue, along with nasal congestion and mucopurulent nasal secretion, but recovered | (Xiao et al.82) | |
| 3-year-old | MPXV-USA-2003-044 and MPXV-ROC-2003-358 | Intranasal/104, 105, and 106 p.f.u/mL | Clinical signs were dose dependent, ranging from inappetence, facial edema, forced breathing, nasal pus, crusty nose, nasal blood, swollen paws, crusted lesion on the face, and death | (Hutson et al.83) | |
| 2-year-old | MPXV-USA-2003-044 and MPXV-ROC-2003-358 | Intranasal and intradermal by scarification/104,5 p.f.u/mL | The animals presented skin lesions on the head, limbs, and trunk. Animals infected with the Congo strain showed a greater increase in temperature and a tendency to lose weight compared with the African strain | (Hutson et al.84) | |
| 2- to 4- year-old | MPXV-USA-2003-044 | Intranasal/8,8x105 p.f.u/mL | It was observed that animals from all groups began to show clinical signs about 8 days after infection; among these signs are inappetence, decreased activity with recumbency and reluctance to move, as well as skin lesions; in addition, some animals needed to be euthanized due to clinical condition. The survival rate of the animals varied according to the time of initiation of treatment; the earlier it was started, the greater the effectiveness | (Hutson et al.85) | |
| 2-year-old | MPXV-USA-2003-044 | Intranasal/5x104 p.f.u/mL (actual dose confirmed by 5.9x104 p.f.u/10 μL of WT and 4.3x104 p.f.u/10 μL of luc+ MPXV) | West African (WA) MPXV can be visualized using in vivo imaging in the nose, lymph nodes, intestines, heart, lung, kidneys, and liver at day 6 post-infection. On day 9, lesions became visible on the skin and, in some cases, the spleen. After day 9 post-infection, the luminescent signal representing replication either increased, indicating a progression to what would be a fatal infection, or decreased as the infection resolved | (Weiner et al.86) | |
| 20-month-old | MPXV-USA-2003-044 | Intranasal/9x103 p.f.u/mL | Sharing contaminated bedding led to all healthy animals developing the disease. In the group in which healthy animals were in contact with the challenged animal, clinical signs of the disease also developed. All four animals that were experimentally challenged recovered from the infection. The euthanized animals had severe diarrhea and countless skin lesions | (Hutson et al.87) | |
| 2-3-years-old | MPXV-USA-2003-044 and MPXV-ROC-2003-358 | Intranasal/6x103 and 5x103 p.f.u/mL | Animals challenged with the West African strain developed skin lesions, crusty noses, dehydration, and inappetence. In the group challenged with Congo Basin, all animals showed inappetence, dehydration, nasal congestion, pus/blood in the mouth, breathing difficulties, facial edema, pus in the genitals, and swollen paws, in addition to skin lesions | (Hutson et al.88) | |
| 10-month-old | MPXV-USA-2003-044 and MPXV-ROC-2003-358 | Intranasal/8x103 p.f.u/mL | Animals infected with the Congo Basin (CB) strain had virus recovered from the nasal mucosa, oropharyngeal lymph nodes, and spleen in animals challenged with on day 4 and in animals challenged with the West African (WA) strain on day 6. For In both groups, primary viremia was observed from days 6–9 to day 17. The CB strain spread faster and accumulated at higher levels, causing greater morbidity in the animals when compared with the WA strain. The viral antigen was abundant in all organs tested, except for the brain. Splenocytes were labeled positive for apoptosis more often than hepatocytes in both groups | (Hutson et al.89) | |
| Adults | MPXV-ROC-2003-358 | Intranasal/4,3x106 and 2,25x104 p.f.u/mL | The incubation period presented by prairie dogs were similar to that observed in humans with systemic orthopoxvirus infection. Regarding the clinical signs observed in the animals, the occurrence of inappetence and weight loss, as well as cutaneous and mucosal lesions, varying in degree of intensity, stands out | (Shannon Keckler et al.90) |
Not reported; p.f.u: plaque formation unit; mL: milliliter; MPXV: monkeypox virus.
Table 5.
Squirrels used in the study of MPXV
| Animal specie | Age | Viral strain | Route of infection/dose | Major findings | Reference |
|---|---|---|---|---|---|
| Squirrel (S. tridecemlineatus) | Adults | MPXV-USA-2003-044 | Intraperitoneal/105,1 and intranasal/106,1 p.f.u/mL | The infection caused severe and fatal disease in all animals in both inoculation routes. High rate of viral load and wide distribution of the virus through the organs. The squirrels were lethargic and anorexic within 4 or 5 days, with death within 9 days. No apparent skin lesions were observed; however, severe liver and spleen lesions were seen in both groups; in addition, necrosis of peribronchial lymphoid tissue and lymph nodes from other sites was also observed in the group challenged by the intranasal route | (Tesh et al.91) |
| a | MPXV-ZAI-1979-005 and MPXV-USA-2003-044 | Subcutaneous/3,7x104 and 1,8x104 p.f.u/mL | Clinical symptoms were earlier and more severe in animals that received strain Z79 and mortality in these animals occurred between days 6 and 11; on the other hand, despite presenting a milder symptomatology, death in animals that received strain US03 occurred in a very similar period. Animals infected with the Z79 virus strain showed consistently higher viral titer in blood and lung tissue compared with those infected with US03 virus alone, but it was also possible to identify virus titers in the spleen and liver of these animals | (Sbrana et al.92) | |
| a | MPXV-ZAI-1979-005 | Subcutaneous/106,3 p.f.u/mL | Infected and untreated animals show signs of disease on day 4 post-infection, and all died between 6 and 9 days post-infection. The animals had a high viral load; however, none of the challenged and treated squirrels developed detectable viremia | (Sbrana et al.93) | |
| Squirrel (Marmota bobak) | 1–2-year-old | MPXV-ZAI-1979-005 | Subcutaneous/2.6, 4.1, 5.6, 7.1 log10 p.f.u/mL and intranasal/1.8, 0.2, 2.2, 3.7, 4.2, 5.0, 6.6 and 7.8 log10 p.f.u/mL | Virus was recovered from nasal mucosa, oropharyngeal lymph nodes, and spleen in animals challenged by MPXV Congo Basin (CB) on day 4 and in animals challenged with the West African (WA) strain on day 6. For both groups, viremia primary was seen from days 6–9 through day 17. Although the histopathology and immunohistochemistry findings were similar, CB MPXV spread faster and accumulated to higher levels, causing greater morbidity in animals when compared to WA MPXV. Two animals that succumbed to the disease showed abundant viral antigen in all organs tested, except the brain. Interestingly, splenocytes were labeled positive for apoptosis more often than hepatocytes in both MPXV groups | (Sergeev et al.94) |
| Squirrel (Funisciurus anerythrus) | a | Congo Basin MPXV/Luc+ | Intranasal and intradermal/106 p.f.u/mL | MPXV infection in these animals caused moderate to severe morbidity and mortality, with clinical signs including smallpox lesions on the skin, eyes, mouth, and nose, dyspnea, and profuse nasal discharge. Both intranasal and intradermal exposures induced high levels of viremia, rapid systemic spread, and long periods of viral shedding. The sentinel animal showed clinical signs of infection including increased respiratory rate, nasal discharge and mouth lesions, respiratory problems, weight loss, and severe lethargy | (Falendysz et al.95) |
Not reported; p.f.u: plaque formation unit; mL: milliliter; MPXV: Monkeypox virus.
Table 6.
Rabbits used in the study of MPXV
| Animal species | Age | Viral strain | Route of infection/dose | Major findings | Reference |
|---|---|---|---|---|---|
| Rabbit (Oryctolagus cuniculus) | Adults and 2-day -old | Serial dilutions of virus recovered from infected monkeys | Intradermal, scarification, and intracutaneous/a | Intradermal inoculation led to severe hemorrhagic reactions, but unlike what is observed with the vaccinia virus, pustules followed by necrosis were also observed. Lesions and pustules were also observed by scarification. Infection was fatal for two-day-old rabbits after scarification or intracutaneous inoculation | (Magnus et al.1) |
| a | a | Scarification, intravenous, intradermally, and subcutaneous/a | Scarification produced confluent lesions according to the concentration used. The virus was also infectious by the intravenous, intradermal, and subcutaneous routes. The intradermal rout caused after pulse lesions followed by secondary pustules and the intravenous route resulted in generalized disease although the animals recovered | (Prier and Sauer,56; Prier et al.57) | |
| Adults | a | Intradermally/a | Intradermal infection led to hemorrhagic-necrotic lesion in rabbits | (Gispen and Brand-Saathof,96) | |
| Adults and 2-day-old | Copenhagen and MPXV-6-7255 | Intracerebral and intradermally/a | All strains caused hemorrhagic necrotic skin lesions. Intracerebral infections in adult and 2-day-old rabbits were fatal with the development of adult meningoencephalitis | (Gispen et al.97) | |
| Rabbit (Oryctolagus cuniculus domesticus) | 10-day-old | Copenhagen | Intravenous/107 p.f.u and scarification/105 and 106 p.f.u/0, 1 mL. Oral route/1,4 x 106; 107; 108; 109 p.f.u/2 mL. | Animals inoculated with MPXV generally had a generalized process of fever, conjunctivitis, rhinitis, rash on the skin and mucous membranes, and weight loss. Papules also appeared that developed into pustules and in some cases became hemorrhagic, with younger animals being more susceptible than adults | (Marennikova and Seluhina,48) |
Not reported; p.f.u: plaque formation unit; mL: milliliter; MPXV: monkeypox virus.
Murines
The mouse (Mus musculus) has been a widely used model since the discovery of MPXV. The pathogenesis of the disease has been modeled largely from animal studies, with mice initially used in the evaluation of ectromelia infection and to make kinetic observations of the spread of some viruses.98
Initial studies were devoted to investigating the susceptibility of mice to MPXV. In these tests, adult mice were inoculated intracerebrally with MPXV recovered from infected monkeys. After infection, the animals showed signs of encephalitis followed by 100% lethality. The brains of the animals were collected, processed, and the supernatant reinoculated into a new group of animals, and all these succumbed to infection. A group of two-day-old suckling mice was also evaluated in this study. The animals were inoculated intranasally. Both the virus passed into monkeys, and the virus passed into mice killed 100% of the animals.1 Mortality rates related to contemporary strains of MPXV are low compared with data with past strains.99 Lethal models certainly helped to elucidate the mechanisms that lead patients to death and contributed to the prophylactic measures that are available. It should also be considered that the current mortality rate may be underestimated considering the lack of adequate surveillance in some countries, in addition to the possibility of new, more virulent variants as the virus circulates. For all these possibilities, lethal models may contribute to further investigations on MPXV. Still, there is a need to develop new non-lethal characterized models for studies of current circulating strains. Recently, Warner and coworkers demonstrated that CAST/EiJ mice, a wild-derived inbred strain, does not succumb to infection with the 2022 circulating strain following intranasal exposure, although they are highly susceptive to clade 1 and 2 MPXV and developed fatal disease upon challenge.55
The subcutaneous paw pad inoculation route in adult mice was not efficient in infecting the A129, C57BL/6, DBA, A/Ncr, C3HeJ, IFNγR−/−, and IFN-α/βR−/− strains. In animals characterized as not responding to IFN-1-dependent or type 2 signaling pathways, on the other hand, infection occurred satisfactorily induced by strain line 1 in C57BL/6 stat1−/− and 129 stat1−/− mice. Twenty-five percent death was observed in female C57BL/6 stat1−/− mice 21 days post-infection, whereas 50% death was observed in male mice 12 days post-infection. When infected at the higher dose (4,700 p.f.u), all animals died within 9 days post-infection showing high viral titers in the lung. The immunodeficient SCID (severe combined immunodeficient) strain was susceptible to an intranasal MPXV infection, as were C57BL/6 and 129 stat1−/−, which are due to their failure to respond to STAT1 induced by IFN type 1 and 2 pathway signaling. Overall, high mortality was a feature observed in STAT1-deficient mice, in addition to weight loss and viral presence in internal organs.46 Stabenow et al. (2010) showed a gender effect on MPXV-induced mortality in STAT1-deficient C57BL/6 mouse, with males showing higher mortality rate than females. New studies are needed to assess the role of sex in monkeypox susceptibility and may elucidate mechanisms of host-pathogen interaction.
The importance of IFN in protection against MPXV infection has also been demonstrated by intranasal administration of the cytokine in CAST/EiJ mice, which led to protection against MPXV. In addition, C57BL/6 mice with inactivation of the IFN gene or the IFN receptor gene show increased sensitivity to the disease.47 Interferon knockout mice are historically known as suitable models in studies of viral infections. Many viruses have infection mechanisms that can be easily interrupted by the natural defense of mice with an intact immune system; this can be considered one of the reasons why the characterization of immunocompetent models for studies on the development of antivirals and vaccines is still a difficult task. For this reason, knockout models and/or immunocompetent mice at an age when the immune system is still immature are well accepted. However, the use of these models increases the chances that the tests were underestimated due to the absence of the antiviral defense of the model.
To address the limitation of using immunocompetent mice and to allow assessment of susceptibility and the course of infection in this model, S. S. Marennikova and E. M. Seluhina, inoculated 1-, 2-, 8-, 12-, and 15-day-old mice with the Copenhagen strain of MPXV. The 8-day-old animals showed clinical signs such as asthenia and loss of appetite when inoculated intraperitoneally or intranasally. Besides these signs, the animals inoculated via plantar cushion showed edema in the foot. In these inoculation routes, all animals died as a result of infection, whereas the intradermal route resulted in the death of 50% of the inoculated animals. Mice inoculated orally became flabby and lost their appetite, leading to a 40% mortality rate in the infected group. For 12-day-old mice, mortality occurred in only 14% of cases. On the other hand, 100% mortality was observed in 15-day-old animals after intranasal inoculation. Sequential evaluations showed that the virus could be found in the blood, lungs, liver, spleen, and kidneys, with a considerable amount of virus detected in the lungs and other organs in the acute phase of the disease.48 The fact that young mice succumb to the disease caused by MPXV suggests the possibility of using this model for viral adaptation, which would allow the development of an immunocompetent adult mouse capable of modeling the disease. Serial passage studies of MPXV in young mice with intact immune system could result in selection of an adapted strain that would cause disease in adult animals, considering the high rate of viral mutation and adaptation by natural selection. These studies will be useful for the development of challenge models for antivirals and vaccines testing using contemporary strain of MPXV.
The progression of infection caused by MPXV was also compared in mice with an intact immune system and in immunodeficient mice using viruses with MPXV engineered to express luminescent markers. Viral infection of Balb/c (immunocompetent) and SCID (immunodeficient) mice was monitored using biophoton imaging. In BALB/c mice, the luminescent signal was visualized in the first 24 h extending to 96–120 h, where higher peaks were detected. Higher replication and faster dissemination were observed mainly to organs in the peritoneal cavity, with eventual dissemination to axillary lymph. After 240 h, the animals were able to clear the infection. Balb/c mice have an immune response skewed toward a Th2 profile that not only negatively regulates the secretion of Th1 cytokines but also inhibits and counteracts the activating actions of IFNγ and tumor necrosis factor alpha (TNF-α), deactivating the transcription and translation of several genes in macrophages. Furthermore, interleukin-10 (IL-10) acts by negatively controlling the immune response in general and mainly the inflammatory response and tissue damage.100,101 Therefore, although they are immunocompetent mice, it is possible to observe the infection cycle with the appearance of some disease phenotypes. In SCID mice, luminescence indicative of MPXV infection was also visible at 24 h and limited to the peritoneal cavity. A more intense luminescent light was observed, and after 96 h it had already spread to other organs and tissues in the abdominal, thoracic, and axillary lymph node regions, in addition to showing visible signs in the tail, feet, and nasal region when the evaluation reached 264 h. At 168 h after inoculation the animals died, biophotonics images also revealed the tropism of MPXV for ovarian tissues.49
A study by Duggal and collaborators demonstrated the presence of MPXV in the interstitial cells and seminiferous tubules of the testes, as well as in the lumen of the epididymis, which are the sites of sperm production and maturation, in non-human primates.102 These findings, together with the work of Osorio et al. (2009), support the potential sexual transmission of MPXV. New studies addressing the questions are urgently needed. Studies carried out with Zika virus, a virus known to be sexually transmitted, can help as a basis for investigations for MPXV. Viral load should be quantified in vaginal washes and semen from male testes to have a better understanding of viral tropism for cells of the genitourinary system. Once this potential has been identified, crossovers between infected and healthy mice should be performed to determine the possibility and rates of sexual transmission. Vertical transmission also deserves attention. Murine models may also help to discover whether contemporary strains of MPXV can infect fetuses and what the consequences of infection to the offspring are.
Also using bioluminescence imaging, Earl et al. found high luminescence in the nasal area peaking between 7 and 12 days post-infection, moving on to the lung. Using the recombinant strain MPXV-z79-CB2, the study also demonstrated that CAST/EiJ mice infected intranasally showed lethargy, arched posture, raised hairs, and severe weight loss.50
Intact 8- to 10-day-old male and female ICR mice were also challenged intranasally with MPXV showing no lethality; however, after day 7 post-infection, clinical signs of disease such as purulent conjunctivitis, blepharitis, and raised hairs were observed, which disappeared after 11–13 days.51
The CAST/EiJ mouse also proved to be an efficient model of MPXV infection, among 37 mouse strains evaluated. In addition to CAST/Eij, MOLF/EiJ and PERA/EiJ also succumbed to the disease. Females between 5 and 11 weeks of age inoculated intranasally showed weight loss and lethality of 100%, 75%, and 40%, respectively. CAST/EiJ mice showed greater sensitivity to MPXV when infected intraperitoneally. Both routes of inoculation resulted in MPXV replication in the lung, spleen, and liver of the infected animals.52 Intranasal infection in CAST/EiJ mice also led to viral replication in lungs and dissemination to other organs (liver, spleen, kidneys, and brain).47
The inherent susceptibility of CAST/EiJ mice can be explained by a low level of NK cells.53 Crossing of CAST/EiJ mice with C57BL/6 or BALB/c was performed to investigate whether resistance or sensitivity to MPXV is a dominant factor. The F1 progeny was relatively resistant to MPXV. However, there was a sex difference; some crossbred male mice succumbed to the disease, whereas all females survived.54
Mice were also models used in evaluations of phylogenetically distinct strains of MPXV. Hutson and coworkers infected 6- to 7-week-old mice subcutaneously in the footpad or intranasally with a dose of 10.5 West African or Congo Basin MPXV strain. Mice inoculated in the footpad with the Congo Basin strain showed edema in the inoculation region, which led to impaired locomotion of some animals, and BALB/c mice had greater edema compared with C57BL/6 mice. BALB/c mice showed weight loss, whereas no mice from the C57/BL6 strain showed this clinical sign. In general, 13 days after infection, the animals had already recovered from the disease. When inoculation took place intranasally, weight loss was observed in both strains of mice. Although weight loss has been verified, no obvious signs of morbidity (ie, lesions) were observed, and all mice survived. On the other hand, mice inoculated in the footpad with MPXV from West Africa showed mild swelling in the inoculation site. None of the animals lost weight over the course of the study or developed some lesion, and all animals in this group survived the infection. None of the mice in the group inoculated with intranasal MPXV from West Africa developed any obvious signs of morbidity.103
Non-human primate models
Reports of MXPV infection in non-human primates (NHPs) have been described in several studies, most of which were carried out in cynomolgus (Macaca fascicularis) and rhesus (M. mulatta) monkeys,1,56 but there are studies with Macaca philippinensis104 and Callithrix jacchus.80,81
MPXV was first identified and isolated in 1958 in Copenhagen, Denmark, following the observation of two non-fatal outbreaks of a disease in Asian macaques (mainly M. fascicularis) that had come from Singapore for research of polio vaccines. The infected animals presented generalized petechial eruption in the skin that quickly evolved to a maculopapular eruption, with lesions observed throughout the body, particularly abundant and developed on the palms of the hands and soles of the feet.1 Magnus et al. (1959), after isolating the virus from these animals, intradermally inoculated the palm of M. fascicularis with 0.2 mL of tissue culture material from the pustules, which developed a local pustule surrounded by edema 7 days after inoculation and elevation of body temperature between the fifth and the ninth day, but without propagation of the rash.
In 1960, Prier et al. confirmed the findings of Magnus et al. by performing challenges through the intradermal, subcutaneous, and intravenous routes and demonstrated again the susceptibility of cynomolgus monkeys to MXPV infection. Intravenous inoculation resulted in generalized eruptions; the subcutaneous one developed a granuloma and the intracutaneous one generated only local lesions.56,57,105
Subsequently, a series of experiments with non-human primate models were conducted. In the first experiment, immature cynomolgus monkeys (M. fascicularis) were inoculated by the intravenous, intramuscular, intradermal, or intradermal combined and conjunctival routes, and in the second experiment the monkeys were inoculated by the intramuscular route. Assessed daily, the animals developed a typical papular rash observed throughout the body, buccal mucosa, and soft palate.58,59,60
Cynomolgus monkeys infected with MPXV intravenously developed a uniformly lethal disease and had lesions like what is seen in human infection. After challenge, the animals developed a generalized vesiculopustular eruption including fever, elevated white blood cell count, lymphadenopathy, splenomegaly, and pulmonary edema that led to death between 7 and 15 days after infection.61 Severe pathology characterized by disseminated lesions, lymphadenopathy, pulmonary edema, and splenomegaly was also observed in vaccine studies performed.62 In a drug trial in cynomolgus monkeys, similar results were found; untreated infected animals had disease progression presenting the main phenotypes and a high mortality rate.63
In human smallpox vaccine research, non-human primates are often challenged for MPXV, and cynomolgus are the most used animal model. In the studies carried out, the animals were infected by the intravenous route; those previously immunized were healthy without the appearance of lesions or with the appearance of few and small lesions of rapid healing, unlike the non-immunized monkeys (control), which became seriously ill with depression, lethargy, fever, weight loss, high number of lesions, high viremia, lymphadenopathy, even death.26,64,65,66,67,68
In studies carried out with aerosolized virus, these animals showed clinical signs of the disease and elevation of body temperature, cough, coryza, anorexia, eruptions, papules, and deaths in addition to histological changes such as focal acute necrotizing bronchitis and bronchopneumonia; focal, fibrinous necrotizing alveolitis often accompanied by edema; and acute focal vasculitis, sometimes accompanied by thrombosis and perivascular edema.69,70 Animals also showed exanthema and enanthema, depression, and anorexia in addition to weakness and progression to natural death or euthanasia.71
Because of the efficiency of MPXV infection by aerosol exposure, new studies were carried out. In one of the studies, the virus was deposited directly in the tracheal carina of the animals, whereas in others, aerosol exposure systems were used for infection. In general, cynomolgus of both sexes with different ages and weights succumb to the disease and develop similar signs such as decreased appetite and activity, elevation of body temperature, respiratory stress followed by bronchopneumonia, vesiculopustular lesions, crusted skin lesions, oral ulcers, enlarged and proliferative peripheral lymph nodes, and necrotizing or ulcerative lesions in the esophagus, stomach, and urinary bladder. In addition, some animals still presented subpleural hemorrhage and testicular hemorrhage, reaching death. In all animals, the presence of MPXV was identified in blood, tissues, and mucosal smears.72,73,74,75,76,77
A comparison of the disease course after an intravenous and intrabronchial inoculation in cynomolgus monkeys was performed using serial doses of MPXV. In both inoculation routes, a classic smallpox-like disease was observed. Animals infected by the intravenous route showed fever, appearance of lesions, peak viremia, and viral shedding in nasal and oral smears while intrabronchial exposure beyond typical signs led to the development of pneumonia and increased disease progression. The development of cutaneous lesions in relation to viremia also demonstrated that both routes of inoculation resulted in a rapid spread of the virus to surrounding tissues, resulting in moderate to severe lesional disease.78
As identified in cynomolgus, Magnus et al. (1959) also observed a generalized picture of the disease in rhesus monkeys. These animals showed the same signs, petechial eruption with evolution to maculopapular eruption and lesions throughout the body, but when performing the same challenge intradermally inoculating the palm of two rhesus monkeys with 0.2 mL of tissue culture material from the pustules, the animals did not develop any signs of the disease.1
Rhesus monkeys challenged by inoculating the virus by intradermal, subcutaneous, and intravenous routes resulted in generalized and/or local rashes and granulomas.56,57,105 In intramuscular inoculations, after daily evaluation, it was observed that the animals developed less evident cutaneous eruptions along the body and oral mucosa and short-lived exanthema. Although apparently healthy, all animals developed antibodies against the virus.59
When inoculated intravenously, rhesus monkeys challenged with MPXV were shown to be highly susceptible to the disease. The animals presented a severe condition with all the typical signs and progressive death. Viral titers were detected in the blood of all animals.68
To understand the differences in pathogenicity of Congo Basin (clade I) and the West African (clade II) viruses, Estep and collaborators deleted the MPXV inhibitor of complement enzymes (MOPICE) from the MPXV-Zaire strain, which is not expressed by viruses of the West African clade and infected rhesus monkeys (n = 4) with wild type and a virus lacing MOPICE through the intrabronchial route. Typical signs of the disease were observed in both groups and, in general, the disease manifestations associated with both viruses were similar. However, infection with the recombinant MPXV lacking MOPICE resulted in lethal disease in one animal, and all animals in the wild type group survived infection. Analysis of viral loads in infected animals showed similar patterns between bronchial alveolar lavage and whole blood samples, but viral load levels were higher in animals infected with the virus lacking, suggesting that this protein is not the sole virulence factor of clade I viruses.79
Another animal model of non-human primates is the marmoset (C. jacchus). Adult males infected intravenously through the tail vein, or through the saphenous vein, develop the normal course of the disease caused by MPXV and died 15 days post-infection. Marmosets that receive higher doses show definable clinical signs already on the second day post-infection in addition to decreased activity. Rash, significant lymphadenopathy and pronounced lethargy were also observed in marmosets experimentally infected with MPXV. In contrast, lymphadenopathy and rash in the lower-dose infected group were not observed. Lesions in this group were much more discrete and were flat, well-defined lesions and never progressed through the typical stages of the disease. In addition, later examinations showed high viremia, decreased platelets, and an abbreviated acute phase, reflecting early type hemorrhagic smallpox.80
New experiments by Mucker et al. (2018) with adult male and female marmosets infected via the intranasal route were performed. All animals showed signs of disease and were euthanized or succumbed to the disease 15 days after exposure. The only animal that was exposed to a dose of 5,000 p.f.u had dyspnea, a runny nose, and a temperature rise of about 0.5°C above the pre-exposure temperature. Animals that received doses of 1,000 p.f.u had different degrees of dyspnea. In general, all animals, regardless of the dose received, showed other signs such as sensitivity to light, swelling around the eyes, some signs of photophobia, and skin lesions. Subsequent immunological evaluations revealed an increase in white blood cells and a decrease in platelet counts, in addition to the presence of the virus in the oral cavity of all animals from the 15th day after infection, and the animal with the highest dose was identified as having the virus on the sixth day post-infection.81
The evolutionary kinship of non-human primates with humans makes them effective models for comparative studies. A great example of this is the fact that in the studies carried out so far, mice do not present one of the main characteristics of the disease caused by MPXV—the formation of pustules, which is seen only in non-human primates.
Prairie dogs
Prairie dogs (PD) (Cynomys ludovicianus) are highly susceptible to monkeypox virus (MPXV) infection, and the pathogenesis and severity of the disease vary according to the route of infection, being 100% fatal by the intraperitoneal route, whereas the intranasal route had a mortality of 60%,82 being the main route of choice for further studies. This animal species is an important model for the study of virulence factors, pathogenesis, transmission routes, and elimination of the MPXV virus, in view of its ability to mimic the way the disease occurs in humans, including the different manifestations caused by different strains of the virus.83,84 It has also been a widely used model for testing new prophylactic and therapeutic measures.33,85
A study using two groups of animals, males and females approximately 2 years old, inoculated intranasally with the West African strain, helped to understand the pathogenesis of the disease, and showed that both showed similar symptoms, despite the difference when different doses were used. The results showed that West African MPXV could be visualized using in vivo imaging in the nose, lymph nodes, intestines, heart, lung, kidneys, and liver as early as day six post-infection. By day nine, it was possible to visualize the skin lesions and, in some cases, also the spleen. After day nine, the luminescent signal representing MPXV replication increased in some animals, indicating a progression to what would be a fatal infection, and decreased in others as the infection resolved. The use of recombinant MPXV luc+ allowed for a greater understanding of how MPXV spreads throughout the body in prairie dogs during the course of infection.86
Studies on the transmission routes using West African and Congo Basin strains showed that infected animals developed the disease and started shedding the virus at 6 and 10 dpi, through oral, nasal, ocular, and fecal secretions, and that contamination of healthy animals occurred either by sharing bedding, fomites and physical contact, or by respiratory secretion.84,87 Respiratory transmissibility was questioned in a later study, according to which no healthy animals were infected by the West African MPXV carrier and only 25% of healthy animals became infected from the Congo Basin MPXV carrier, indicating that transmission respiration appears to be less efficient than contact as a transmission mechanism within this model.88 It is noteworthy that the course of the disease was similar regardless of the transmission route, including weight loss, the development of disseminated skin lesions, and antibody production, among others.87 In these studies, the age of the animals ranged from 20 to 36 months, and the viral inoculation doses used were 5x103 96, 9x103 87 and 104.5 84 p.f.u, demonstrating the ability of the virus to develop disease even at lower doses. Of note, the Congo Basin strain proved to be more pathogenic than the West African strain.84
Still on the study that compared the pathogenicity of the Congo Basin strain with that of West Africa, it is noteworthy that the clinical symptoms observed in animals challenged with West African MPXV and that developed the infection (3 out of 5) included skin lesions, crusty nose, dehydration, and inappetence. In the group challenged with Congo Basin MPXV, all animals developed the infection and presented inappetence, dehydration, nasal congestion, pus/blood in the mouth, breathing difficulties, facial edema, pus in the genitals, and swollen paws, in addition to skin lesions. Of this group, 3 of the 5 infected animals were euthanized due to the severity of the degree of morbidity presented, showing once again that Congo Basin MPXV is more pathogenic than West African MPXV.88
A study carried out to verify the effectiveness of the antiviral ST-246 in prairie dogs aged approximately 3 years showed that the infected and untreated group had a mortality rate of 75%, in contrast to all animals in the groups treated prophylactically at day 0 and at 3 days post-infection showed no symptoms of the disease and survived, whereas the therapeutic group, although treated after the appearance of lesions, also resulted in a survival rate of 100%. The clinical symptoms observed in the animals that developed the disease were inappetence, facial swelling, nasal discharge, congestion, weight loss, and, to a lesser extent, mucosal and cutaneous lesions in different degrees of severity. The results of this study suggest that this anti-orthopoxvirus compound proved effective for prophylactic treatment, although some viruses were recovered from treated animals, suggesting the possibility of resistant strains. The study was performed with adult animals, inoculated intranasally with the strain from the Congo Basin.33
In order to better understand the differences in the pathogenesis of MPXV strains, groups of 10-month-old prairie dogs were infected intranasally with the Congo Basin (CB) strain and the West African MPXV strain (WA), and tissues were collected on days 2, 4, 6, 9, 12, 17, and 24 post-infections. Samples were evaluated for the presence of viruses and macro and microscopic lesions. Virus was recovered from nasal mucosa, oropharyngeal lymph nodes, and spleen in CB-challenged animals on day 4 and in WA-challenged animals on day 6. For both groups, primary viremia was seen on days 6–9 through day 17. Although the histopathology and immunohistochemistry findings were similar, CB MPXV spread faster and accumulated to higher levels, causing greater morbidity in animals when compared with WA MPXV. Two animals that succumbed to the disease showed abundant viral antigen in all organs tested, except the brain. Interestingly, splenocytes were labeled positive for apoptosis more often than hepatocytes in both MPXV groups. These findings allow further characterization of differences between the pathogenesis of MPXV strains, including the identification of important sites during early viral replication and cellular response to viral infection. Clinical manifestations, when they occurred, were similar in both groups and included inappetence, maculopapular skin lesions, nasal discharge, and respiratory depression under anesthetic effect.89
Subsequently, a study was carried out using smallpox vaccines post-monkeypox infection. Dryvax, ACAM2000, and IMVAMUNE vaccines were tested in 2 groups of adult animals infected with the Congo Basin strain intranasally at different doses. The study found that, for high doses of infection, none of the vaccines showed efficacy, culminating in the death of all individuals in the groups tested, but for lower doses of infection, vaccine efficacy is related to the interval of post-infection application and the vaccine applied. The incubation period presented by prairie dogs was like that observed in humans with systemic orthopoxvirus infection. In general, the animals showed prodromal symptoms, such as inappetence and weight loss, in addition to an inflammatory reaction and cutaneous and mucosal lesions such as smallpox. The incubation period presented by prairie dogs was like that observed in humans with systemic orthopoxvirus infection. In general, the animals showed prodromal symptoms, such as inappetence and weight loss, in addition to an inflammatory reaction and cutaneous and mucosal lesions similar to smallpox.90
Recently, another study was carried out to test the effectiveness of Brincidofovir (BCV) against monkeypox virus. Adult animals infected with the West African strain intranasally were used. The results showed that the plasma exposure to BCV observed in prairie dogs after 20 or 5 mg/kg was 2–4 times lower than the exposure parameters observed in mice (ectromelia) and rabbits (cotopor) given the same doses. Our findings suggest potentially suboptimal exposure; however, higher doses have been shown to be toxic to prairie dogs. It is possible that the lower plasma exposure is due to the drug metabolization pathway by prairie dogs. It was observed that animals from all groups began to show clinical signs about 8 days after infection; the signs observed were inappetence, decreased activity with recumbency and reluctance to move, as well as cutaneous lesions, which varied in intensity between the animals from each group. Some animals needed to be euthanized due to the clinical condition. The survival rate of the animals varied according to the time of initiation of treatment; the earlier it was started, the greater the effectiveness.85
Although prairie dogs show a satisfactory result in modeling MPXV infection; these animals are not considered conventional experimental models. The use of unconventional animals implies specialized labor, differentiated facilities, and compliance with specific environmental legislation for this type of species. Compared with conventional laboratory species, these animals do not have well-characterized genome sequence, neither hematological and biochemical profile, and immunological reagents are very scarce.
Squirrels
The use of ground squirrels (Sciuridae) in studies related to MPXV were considered when natural infections began to be identified in the species, evidencing its importance for the epidemiology of the disease. In addition, the data found suggested that the species has the potential to become a great experimental model aimed at understanding the pathophysiology caused by MPXV and for testing therapeutic and prophylactic countermeasures,98 such as antiviral medications and vaccines.
In 2004, a study was carried out in order to study the effects of MPXV infection in adult squirrels of the species S. tridecemlineatus. Animals were infected with the MPX 2003 strain by the intraperitoneal and intranasal routes with the dose of 106 p.f.u/mL. The infection caused severe and fatal illness in all animals in both groups, suggesting that these animals are highly susceptible to the virus. The viral load and the wide distribution of the virus through the organs suggest that the infection was systemic. The squirrels were lethargic and anorexic within 4 or 5 days, with death occurring within 9 days. No apparent skin lesions were observed; however, severe liver and spleen lesions were seen in both groups. In addition, necrosis of peribronchial lymphoid tissue and lymph nodes from other sites were also observed in the group challenged by the intranasal route. It is noteworthy that the pathological features of MPXV in S. tridecemlineatus were similar to severe smallpox virus infection in humans.91
In 2007, another study using the same species of squirrel was performed comparing the pathogenic effects of two strains of MPXV, MPX Z79 and MPX US03, inoculated subcutaneously at doses of 3.7 × 104 p.f.u/mL and 1 0.8 × 104 p.f.u/mL US03, respectively. The research showed that clinical symptoms were earlier and more severe in animals that received the Z79 strain and that mortality in these animals occurred between days 6 and 11; on the other hand, despite presenting a milder symptomatology, death in animals that received the US03 strain occurred in a very similar period, between days 7 and 11 after infection. Animals infected with the Z79 MPX virus strain showed consistently higher viral titer in blood and lung tissue compared with those infected with US03 virus alone, but it was also possible to identify virus titers in the spleen and liver of these animals. Therefore, the results indicate that the US03 virus, belonging to the West African MPX virus family, was less virulent than the Central African MPX virus strains.93
In the same year, squirrels (S. tridecemlineatus) were used to investigate the efficacy of the antipoxvirus compound ST-246, after infection by the strain MPX-ZAI-1979-005, inoculated by the subcutaneous route. The results showed that all ground squirrels inoculated with the MPX virus and treated with ST-246 at 0, 24, 48, or 72 h after the challenge survived and none of these animals showed any apparent clinical symptoms. However, 33% of the animals that received treatment from 96 h after infection died; the others survived although some of them showed symptoms of the disease. In contrast, all animals infected with the MPX virus and treated with placebo showed clinical symptoms and died. All animals inoculated with MPXV and treated with placebo had a high MPXV load. However, none of the squirrels challenged with MPX virus and treated with ST-246 at 0, 24, 48, and 72 h post-infection developed detectable viremia. Squirrels in the ST-246-96h group that were humanely killed had low-titer viremia. None of the animals in the placebo or ST-246-0h, ST-246-24h, ST-246-48h, and ST-246-72h treatment groups developed detectable antibodies to the MPX virus. However, the two squirrels in the ST-246-96h group that became ill and recovered had detectable MPX virus antibody titers at the end of the experiment.92
Subsequently, squirrels of the Marmota bobak species were inoculated with the V79-1-005 strain by either subcutaneous or intranasal routes, at different doses, to verify the infective dose (ID50) and the lethal dose (LD50), as well as the effects of antiviral drugsNIOC-14 and ST-246. The study demonstrated that all doses of subcutaneous inoculation of the pathogen caused evident clinical symptoms, such as hyperthermia, skin rash on the body and mucous membranes, serous and purulent rhinitis, conjunctivitis, and incoordination, among others, culminating in the death of all infected animals. Clinical manifestations were similar in both groups. However, the skin and mucosal eruptions were milder and only 41.67% of the sick animals died. In the surviving animals, after the disappearance of clinical signs, scars were formed at the sites of eruptive elements, like smallpox. It is noteworthy that no sign of the disease was recorded in animals from experimental groups treated with NIOC-14 and ST-246 during the entire observation period. In addition, sufficiently high MPXV antibody titers were detected in the neutralization reaction in all animals in the experimental groups and surviving animals in the control group.94
In 2017, a study aimed at characterizing the infection by the MPXV/Congo/Luc+ strain in squirrels (Funisciurus anerythrus) was carried out. Animals were inoculated by intranasal and intradermal routes at a dose of 1x106 p.f.u/mL. MPXV infection caused moderate to severe morbidity and mortality of 75% in the intranasal group and 50% in the intradermal group, with clinical signs including lesions on the skin, eyes, mouth, and nose, as well as dyspnea and profuse nasal discharge. Both intranasal and intradermal exposure induced high levels of viremia, rapid systemic spread, and long periods of viral shedding. Housed in the same room, albeit in a different cage, was the sentinel animal, which showed clinical signs of MPXV infection, including increased respiratory rate, nasal discharge, and oral lesions and needed to be euthanized due to respiratory problems, weight loss, and lethargy. This study suggested that African rope squirrels develop severe pathology when infected with MPXV and that they shed large amounts of virus, evidencing their role as a source of transmission of MPXV to humans and other animals in MPXV endemic regions.95
Similar to prairie dogs, squirrels should be considered sparingly a model for MPXV as they are also unconventional species. In addition, there are countries where access to these animals is restricted.
Rabbits
Rabbits (Oryctolagus cuniculus) are generally resistant to infection caused by MPXV as adults, except in albino rabbits by inoculations performed by the intracerebral route or by scarification. In contrast, animals up to 10 days of age can be easily infected by other routes of infection considering the absence of a robust immune system at this stage of life. Intradermally, for example, hemorrhagic conditions, the appearance of pustules, and lesions have already been reported.1,48,96
Intracerebral infections in adult and 2-day-old rabbits were fatal with the development of adult meningoencephalitis.97 Infection by scarification was fatal for 2-day-old rabbits. Albino adults had fever and lesions, but recovered from the disease.1,48
Intravenous infection was more efficient in causing severe disease in adult rabbits when compared with the subcutaneous route. When infected subcutaneously, the animals presented generalized smallpox lesions, whereas in the intravenous route, an acute disease was observed with the presentation of papules that later became pustules followed by crusts on the skin and mucous membranes. Although the disease was more severe, the animals recovered.1,56,57
Oral infection in 10-day-old rabbits resulted in generalized disease and lesions on the skin, ears, and lips, whereas intranasal infection resulted in weight loss in animals. In both infections the animals died. In adult animals, no clinical signs of the disease were observed.48
The intravenous route seems promising for the development of a non-lethal adult model in rabbits. Considering the low lethality of the new circulating strains of MPXV, this animal model has the potential to become a cost-effective option, because it is an animal widely disseminated in animal experimentation units. Depending on the scientific question to be answered, other animal model options should be considered, as adult rabbits, except for the intravenous route, do not present satisfactory answers, requiring the use of animals with an incomplete immune system and/or inoculation routes with greater complexity.
Others animal models
Guinea pigs (Cavia porcellus) infected via the footpad showed swelling and edema at the inoculation site with the development of a granulomatous lesion. The animals showed antibody titers 14 days after infection. No viral titers were found in collected organs. By scarification, a mild inflammatory reaction occurred on the second day after infection and the development of discrete papules on the fourth day, followed by crusting and subsequent healing. Other routes of infection (intravenous, intracerebral, subcutaneous, intraperitoneal, intradermal, and oral) were evaluated, but the animals did not develop the disease.1,48,56,57,106
Similar to guinea pigs, hamsters (Cricetinae) were also not susceptible to attempts at infection by different routes of inoculation by MPXV, although intraperitoneal and intracardiac infection of the virus was detected in the lungs, liver, and spleen of the animals 7 days after infection. Focal lesions were observed only in the liver, kidney, and brain. The main manifestations were perivascular lymphocytic infiltrate and damage to the endothelial structure of blood vessels.48,107
Intravenous inoculation or scarification did not lead to overt in clinical disease in white rats (Rattus sp.) to. However, in newborn rats (1–3 days old) infection reached 100% mortality with virus replication in the lungs and liver after intranasal challenge.48
Cotton rats (Sigmodon sp.) can be infected intranasally, and these animals develop generalized disease that led the animals to develop difficulty breathing, cough, rhinitis, conjunctivitis, and progressive weight loss, which led to 50% mortality in the experimental group. High concentrations of virus were detected in blood and organs.108
The rodent Kellen’s dormice (Graphiurus kelleni) challenged with MPXV had 100% mortality. In general, the animals showed weight loss, dehydration, conjunctivitis, rhinitis, and lymphadenopathy. Necrosis in the submandibular lymph nodes, spleen, and thymus; hepatocellular necrosis; and hemorrhage in the lungs, stomach, and small intestine were also observed.109
Chickens (Gallus Gallus) aged 3 to 4 days or 4 to 8 weeks are permissible to infection when inoculated intradermally but develop mild disease phenotypes. Once infected with MPXV, they present vesicles heal 10 days after infection. The same does not occur in adult animals.1,106
Although the findings using guinea pigs, hamsters, rats, Kellen’s dormice, and chickens as an experimental model have been limited, further research using the contemporary strain of MPXV may change what we know about infection in these species and therefore further studies should be considered.
Recently, Seang and colleagues described a case of possible human-dog transmission of human MPXV of B.1 lineage, demonstrating the potential of the virus to perform host-jumps. The dog had no contact with other animals, but co-slept with the infected tutors.110
Concluding remarks
The number of monkeypox cases has increased outside endemic areas putting the world on alert. However, great discoveries have already occurred and through them it was possible to develop new in vivo and in vitro models capable of mimicking aspects of viral biology and the pathology caused by the disease. Using the models described herein, the characterization of different viral strains and the development of vaccines and antiviral therapies has been successfully achieved. In experimental studies, the most adequate models must provide reliable results that can be extrapolated to humans. Given the rapid evolution that MPXV has undergone over the past few years, care should be taken in translating the findings produced with historical strains to contemporary ones.
Many animal models have been shown to be susceptible to infection and develop clinical disease with varying degrees of severity, including cases of lethality. Each model has its own applicability for virus and disease studies. The choice of model is linked to the question to which one wants to answer, and each species will have an adequate morphological or physiological particularity to contribute to the experimental response. Mice and rabbits, for example, are readily available, easy to handle, have low maintenance costs, and have a widely known genome, unlike unconventional laboratory species such as prairie dogs and squirrels. However, translating knowledge from rodent studies to the clinic can be a challenge. Non-human primates are genetically closer models to humans, but handling these animals can be costly, in addition to having a longer life cycle, which makes the development of the model difficult.
The in vitro and in vivo models developed so far can contribute to study several aspects of the disease and mechanism of virus-host interactions. These models will be key to the development of new drugs and therapies capable of blocking the advance of MPXV, as the virus is spreading around the world (Figure 1).
Figure 1.
Cellular and animal models for monkeypox studies
In vitro models (PEK, HEP-2, LLC, MK2, Vero, MA-104, HeLa, BALB3T3, Clone A31, BSC-40, BSC-1, A549, and RK13 cells) and in vivo models (mice, rabbits, prairie dogs, non-human primates, and squirrels) are the basis for the elucidation the biology of new viral strains and for the discovery of new drugs, treatments, and vaccines. Created with Mindthegraph.com.
Acknowledgments
The authors thank the postgraduate program in Biosciences and Biotechnology in Health at the Instituto Aggeu Magalhães (FIOCRUZ - PE) for the institutional assistance and support. Dr. Pena's lab is supported by grants APQ-0560-2.12/19 and 441030/2020-3 from the Pernambuco State Foundation for Science and Technology (FACEPE) and National Council for Scientific and Technological Development (CNPq), respectively.
ACGJ is grateful to CAPES (Coordination for the Improvement of Higher Education Personnel)—Brazil, Prevention and Combat of Outbreaks, Endemics, Epidemics and Pandemics-Finance Code #88881.506794/2020-01, and to FAPEMIG (Minas Gerais Research Foundation APQ-03385-18 and APQ-01487-22). MSM thanks FAPEMIG for the scholarship # 12152. IAS thanks CNPq for the scholarship # 142495/2020-4 and CAPES. Print scholarship # 88887.700246/2022-00.
Author contributions
R.B.R., E.F.d.C., D.C.P.V., I.A.S., M.d.S.M., and F.B.F.F participated in the search for articles to be reviewed and carried out the writing of the review. R.B.R. designated graphical abstract. L.J.P. participated in the supervision. L.J.P., M.V.d.S., and A.C.G.J. participated in the writing and review of this paper. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
The authors declare no conflict of interest.
References
- 1.Magnus P.v., Andersen E.K., Petersen K.B., Birch-Andersen A. A Pox-like disease in Cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 1959;46:156–176. doi: 10.1111/j.1699-0463.1959.tb00328.x. [DOI] [Google Scholar]
- 2.Kozlov M. Monkeypox goes global: why scientists are on alert. Nature. 2022;606:15–16. doi: 10.1038/d41586-022-01421-8. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . 2022. Monkeypox. World Heal. Organ.https://www.who.int/news-room/fact-sheets/detail/monkeypox [Google Scholar]
- 4.Learned L.A., Reynolds M.G., Wassa D.W., Li Y., Olson V.A., Karem K., Stempora L.L., Braden Z.H., Kline R., Likos A., et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am. J. Trop. Med. Hyg. 2005;73:428–434. doi: 10.4269/ajtmh.2005.73.428. [DOI] [PubMed] [Google Scholar]
- 5.Jezek Z., Grab B., Szczeniowski M., Paluku K.M., Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull. World Health Organ. 1988;66:459–464. [PMC free article] [PubMed] [Google Scholar]
- 6.Beer E.M., Rao V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl. Trop. Dis. 2019;13:e0007791. doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., Steffen R. The changing epidemiology of human monkeypox—a potential threat? a systematic review. PLoS Negl. Trop. Dis. 2022;16:e0010141. doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z. Monkeypox: a potential global threat? J. Med. Virol. 2022;94:4034–4036. doi: 10.1002/jmv.27884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention . 2022. Monkeypox Outbreak Global Map. Centers Dis. Control Prev.https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html [Google Scholar]
- 10.World Health Organization . World Health Organization; 2022. Who Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Emergency of International Concern.https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern [Google Scholar]
- 11.Goeijenbier M., van Wissen M., van de Weg C., Jong E., Gerdes V.E.A., Meijers J.C.M., Brandjes D.P.M., van Gorp E.C.M. Modeling human viral diseases: trials and triumphs. J. Med. Virol. 2012;84:1680–1696. doi: 10.1002/jmv.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dulbecco R. Production of plaques in monolayer tissue cultures by single particles of an animal virus. Proc. Natl. Acad. Sci. USA. 1952;38:747–752. doi: 10.1073/pnas.38.8.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dulbecco R., Marguerite Vogt M.D. Plaque formation and isolation of pure lines with poliomyelitis viruses. J. Exp. Med. 1953;99:167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pena L.J., Miranda Guarines K., Duarte Silva A.J., Sales Leal L.R., Mendes Félix D., Silva A., De Oliveira S.A., Junqueira Ayres C.F., Júnior A.S., De Freitas A.C. In vitro and in vivo models for studying Zika virus biology. J. Gen. Virol. 2018;99:1529–1550. doi: 10.1099/jgv.0.001153. [DOI] [PubMed] [Google Scholar]
- 16.Marennikova S.S., Seluhina E.M., Mal’ceva N.N., Cimiskjan K.L., Macevic G.R. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull. World Health Organ. 1972;46:599–611. [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers J.V., Parkinson C.V., Choi Y.W., Speshock J.L., Hussain S.M. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 2008;3:129–133. doi: 10.1007/s11671-008-9128-2. [DOI] [Google Scholar]
- 18.Baker R.O., Bray M., Huggins J.W. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 2003;57:13–23. doi: 10.1016/S0166-3542(02)00196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alkhalil A., Strand S., Mucker E., Huggins J.W., Jahrling P.B., Ibrahim S.M. Inhibition of Monkeypox virus replication by RNA interference. Virol. J. 2009;6:188. doi: 10.1186/1743-422X-6-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang M., Schneller S.W. 5′-Homoaristeromycin. Synthesis and antiviral activity against orthopox viruses. Bioorg. Med. Chem. Lett. 2005;15:149–151. doi: 10.1016/j.bmcl.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Smee D.F., Bray M., Huggins J.W. Antiviral activity and mode of action studies of ribavirin and mycophenolic acid against orthopoxviruses in vitro. Antivir. Chem. Chemother. 2001;12:327–335. doi: 10.1177/095632020101200602. [DOI] [PubMed] [Google Scholar]
- 22.Johnston S.C., Lin K.L., Connor J.H., Ruthel G., Goff A., Hensley L.E. In vitro inhibition of monkeypox virus production and spread by Interferon-β. Virol. J. 2012;9:5. doi: 10.1186/1743-422X-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Priyamvada L., Burgado J., Baker-Wagner M., Kitaygorodskyy A., Olson V., Lingappa V.R., Satheshkumar P.S. New methylene blue derivatives suggest novel anti-orthopoxviral strategies. Antiviral Res. 2021;191:105086. doi: 10.1016/j.antiviral.2021.105086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernández de Marco M.d.M., Alejo A., Hudson P., Damon I.K., Alcami A. The highly virulent variola and monkeypox viruses express secreted inhibitors of type I interferon. FASEB J. 2010;24:1479–1488. doi: 10.1096/fj.09-144733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith S.K., Olson V.A., Karem K.L., Jordan R., Hruby D.E., Damon I.K. In vitro efficacy of ST246 against smallpox and monkeypox. Antimicrob. Agents Chemother. 2009;53:1007–1012. doi: 10.1128/AAC.01044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fogg C.N., Americo J.L., Lustig S., Huggins J.W., Smith S.K., Damon I., Resch W., Earl P.L., Klinman D.M., Moss B. Adjuvant-enhanced antibody responses to recombinant proteins correlates with protection of mice and monkeys to orthopoxvirus challenges. Vaccine. 2007;25:2787–2799. doi: 10.1016/j.vaccine.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altmann S.E., Brandt C.R., Jahrling P.B., Blaney J.E. Antiviral activity of the EB peptide against zoonotic poxviruses. Virol. J. 2012;9:6. doi: 10.1186/1743-422X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altmann S.E., Smith A.L., Dyall J., Johnson R.F., Dodd L.E., Jahrling P.B., Paragas J., Blaney J.E. Inhibition of cowpox virus and monkeypox virus infection by mitoxantrone. Antiviral Res. 2012;93:305–308. doi: 10.1016/j.antiviral.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bengali Z., Satheshkumar P.S., Moss B. Orthopoxvirus species and strain differences in cell entry. Virology. 2012;433:506–512. doi: 10.1016/j.virol.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arndt W.D., Cotsmire S., Trainor K., Harrington H., Hauns K., Kibler K.V., Huynh T.P., Jacobs B.L. Evasion of the innate immune type I interferon system by monkeypox virus. J. Virol. 2015;89:10489–10499. doi: 10.1128/jvi.00304-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brockman W.W., Nathans D. The isolation of simian virus 40 variants with specifically altered genomes. Proc. Natl. Acad. Sci. USA. 1974;71:942–946. doi: 10.1073/pnas.71.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Earley E., Johnson K. Vero Cells : Origin, Properties and Biomedical Applications. 1988. The lineage of the Vero, Vero 76 and its clone C1008 in the United States; pp. 26–29. [Google Scholar]
- 33.Smith S.K., Self J., Weiss S., Carroll D., Braden Z., Regnery R.L., Davidson W., Jordan R., Hruby D.E., Damon I.K. Effective antiviral treatment of systemic orthopoxvirus disease: ST-246 treatment of prairie dogs infected with monkeypox virus. J. Virol. 2011;85:9176–9187. doi: 10.1128/jvi.02173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berhanu A., Prigge J.T., Silvera P.M., Honeychurch K.M., Hruby D.E., Grosenbach D.W. Treatment with the smallpox antiviral tecovirimat (ST-246) alone or in combination with ACAM2000 vaccination is effective as a postsymptomatic therapy for monkeypox virus infection. Antimicrob. Agents Chemother. 2015;59:4296–4300. doi: 10.1128/AAC.00208-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Law M., Smith G.L. Studying the binding and entry of the intracellular and extracellular enveloped forms of vaccinia virus. Methods Mol. Biol. 2004;269:187–204. doi: 10.1385/1-59259-789-0:187. [DOI] [PubMed] [Google Scholar]
- 36.Fox E.J. Mechanism of action of mitoxantrone. Neurology. 2004;63:S15–S18. doi: 10.1212/WNL.63.12_suppl_6.S15. [DOI] [PubMed] [Google Scholar]
- 37.Blach-Olszewska Z., Halasa J., Matej H., Cembrzyńska-Nowak M. Why HeLa cells do not produce interferon? Arch. Immunol. Ther. Exp. 1977;25:683–691. [PubMed] [Google Scholar]
- 38.Konishi K., Yamaji T., Sakuma C., Kasai F., Endo T., Kohara A., Hanada K., Osada N. Whole-genome sequencing of Vero E6 (VERO C1008) and comparative analysis of four Vero cell sublines. Front. Genet. 2022;13:801382. doi: 10.3389/fgene.2022.801382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lalani A.S., Masters J., Zeng W., Barrett J., Pannu R., Everett H., Arendt C.W., McFadden G. Use of chemokine receptors by poxviruses. Science. 1999;286:1968–1971. doi: 10.1126/science.286.5446.1968. [DOI] [PubMed] [Google Scholar]
- 40.Blasco R., Sisler J.R., Moss B. Dissociation of progeny vaccinia virus from the cell membrane is regulated by a viral envelope glycoprotein: effect of a point mutation in the lectin homology domain of the A34R gene. J. Virol. 1993;67:3319–3325. doi: 10.1128/jvi.67.6.3319-3325.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin C.-L., Chung C.-S., Heine H.G., Chang W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 2000;74:3353–3365. doi: 10.1128/jvi.74.7.3353-3365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsiao J.-C., Chung C.-S., Chang W. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 1999;73:8750–8761. doi: 10.1128/jvi.73.10.8750-8761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsiao J.-C., Chung C.-S., Chang W. Cell surface proteoglycans are necessary for A27L protein-mediated cell fusion: identification of the N-terminal region of A27L protein as the glycosaminoglycan-binding domain. J. Virol. 1998;72:8374–8379. doi: 10.1128/jvi.72.10.8374-8379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hutson C.L., Damon I.K. Monkeypox virus infections in small animal models for evaluation of anti-poxvirus agents. Viruses. 2010;2:2763–2776. doi: 10.3390/v2122763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosa R.B., Dantas W.M., Do Nascimento J.C.F., da Silva M.V., de Oliveira R.N., Pena L.J. In vitro and in vivo models for studying sars-cov-2, the etiological agent responsible for covid-19 pandemic. Viruses. 2021;13:379. doi: 10.3390/v13030379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stabenow J., Buller R.M., Schriewer J., West C., Sagartz J.E., Parker S. A mouse model of lethal infection for evaluating prophylactics and therapeutics against monkeypox virus. J. Virol. 2010;84:3909–3920. doi: 10.1128/jvi.02012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Earl P.L., Americo J.L., Moss B. Lethal monkeypox virus infection of CAST/EiJ mice is associated with a deficient gamma interferon response. J. Virol. 2012;86:9105–9112. doi: 10.1128/jvi.00162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marennikova S.S., Seluhina E.M. Susceptibility of some rodent species to monkeypox virus, and course of the infection. Bull. World Health Organ. 1976;53:13–20. [PMC free article] [PubMed] [Google Scholar]
- 49.Osorio J.E., Iams K.P., Meteyer C.U., Rocke T.E. Comparison of monkeypox viruses pathogenesis in mice by in vivo imaging. PLoS One. 2009;4:e6592. doi: 10.1371/journal.pone.0006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Earl P.L., Americo J.L., Cotter C.A., Moss B. Comparative live bioluminescence imaging of monkeypox virus dissemination in a wild-derived inbred mouse (Mus musculus castaneus) and outbred African dormouse (Graphiurus kelleni) Virology. 2015;475:150–158. doi: 10.1016/j.virol.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sergeev A.A., Kabanov A.S., Bulychev L.E., Sergeev A.A., Pyankov O.V., Bodnev S.A., Galahova D.O., Zamedyanskaya A.S., Titova K.A., Glotov A.G., et al. The possibility of using the ICR mouse as an animal model to assess antimonkeypox drug efficacy. Transbound. Emerg. Dis. 2016;63:e419–e430. doi: 10.1111/tbed.12323. [DOI] [PubMed] [Google Scholar]
- 52.Americo J.L., Moss B., Earl P.L. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J. Virol. 2010;84:8172–8180. doi: 10.1128/jvi.00621-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Earl P.L., Americo J.L., Moss B. Natural killer cells expanded in vivo or ex vivo with IL-15 overcomes the inherent susceptibility of CAST mice to lethal infection with orthopoxviruses. PLoS Pathog. 2020;16:e1008505. doi: 10.1371/journal.ppat.1008505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Earl P.L., Americo J.L., Moss B. Genetic studies of the susceptibility of classical and wild-derived inbred mouse strains to monkeypox virus. Virology. 2015;481:161–165. doi: 10.1016/j.virol.2015.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warner B.M., Klassen L., Sloan A., Deschambault Y., Soule G., Banadyga L., Cao J., Strong J.E., Kobasa D., Safronetz D. In vitro and in vivo efficacy of Tecovirimat against a recently emerged 2022 Monkeypox virus isolate. Sci. Transl. Med. 2022;14:eade7646. doi: 10.1126/scitranslmed.ade7646. [DOI] [PubMed] [Google Scholar]
- 56.Prier J.E., Sauer R.M. A pox disease of monkeys. Ann. N. Y. Acad. Sci. 1960;85:951–959. doi: 10.1111/j.1749-6632.1960.tb50015.x. [DOI] [PubMed] [Google Scholar]
- 57.Prier J.E., Sauer R.M., Malsberger R.G., Sillaman J.M. Studies on a pox disease of monkeys. II. Isolation of the etiologic agent. Am. J. Vet. Res. 1960;21:381–384. [PubMed] [Google Scholar]
- 58.Wenner H.A., Bolano C.R., Cho C.T., Kamitsuka P.S. Studies on the pathogenesis of monkey pox - III. Histopathological lesions and sites of immunofluorescence. Arch. Gesamte Virusforsch. 1969;27:179–197. doi: 10.1007/BF01249642. [DOI] [PubMed] [Google Scholar]
- 59.Wenner H.A., Macasaet F.D., Kamitsuka P.S., Kidd P. Monkey pox. I. Clinical, virologic and immunologic studies. Am. J. Epidemiol. 1968;87:551–566. doi: 10.1093/oxfordjournals.aje.a120846. [DOI] [PubMed] [Google Scholar]
- 60.Wenner H.A., Bolano C.R., Cho C.T., Kamitsuka P.S. Studies on the pathogenesis of monkey pox - II. Dose-Response and Virus Dispersion. Arch. Gesamte Virusforsch. 1969;27:179–197. doi: 10.1007/BF01249642. [DOI] [PubMed] [Google Scholar]
- 61.Huggins J., Goff A., Hensley L., Mucker E., Shamblin J., Wlazlowski C., Johnson W., Chapman J., Larsen T., Twenhafel N., et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob. Agents Chemother. 2009;53:2620–2625. doi: 10.1128/AAC.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei H., Huang D., Fortman J., Wang R., Shao L., Chen Z.W. Coadministration of cidofovir and smallpox vaccine reduced vaccination side effects but interfered with vaccine-elicited immune responses and immunity to monkeypox. J. Virol. 2009;83:1115–1125. doi: 10.1128/jvi.00984-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jordan R., Goff A., Frimm A., Corrado M.L., Hensley L.E., Byrd C.M., Mucker E., Shamblin J., Bolken T.C., Wlazlowski C., et al. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob. Agents Chemother. 2009;53:1817–1822. doi: 10.1128/AAC.01596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., Eisenberg R.J., Hartmann C.J., Jackson D.L., Kulesh D.A., et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 65.Earl P.L., Americo J.L., Wyatt L.S., Espenshade O., Bassler J., Gong K., Lin S., Peters E., Rhodes L., Spano Y.E., et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl. Acad. Sci. USA. 2008;105:10889–10894. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buchman G.W., Cohen M.E., Xiao Y., Richardson-Harman N., Silvera P., DeTolla L.J., Davis H.L., Eisenberg R.J., Cohen G.H., Isaacs S.N. A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge. Vaccine. 2010;28:6627–6636. doi: 10.1016/j.vaccine.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Denzler K.L., Babas T., Rippeon A., Huynh T., Fukushima N., Rhodes L., Silvera P.M., Jacobs B.L. Attenuated NYCBH vaccinia virus deleted for the E3L gene confers partial protection against lethal monkeypox virus disease in cynomolgus macaques. Vaccine. 2011;29:9684–9690. doi: 10.1016/j.vaccine.2011.09.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Russo A.T., Berhanu A., Bigger C.B., Prigge J., Silvera P.M., Grosenbach D.W., Hruby D. Co-administration of tecovirimat and ACAM2000TM in non-human primates: effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge. Vaccine. 2020;38:644–654. doi: 10.1016/j.vaccine.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hahon N., McGavran M.H. Air-borne infectivity of the variola-vaccinia group of poxviruses for the cynomolgus monkey, macaca irus. J. Infect. Dis. 1961;109:294–298. doi: 10.1093/INFDIS/109.3.294. [DOI] [PubMed] [Google Scholar]
- 70.Hatch G.J., Graham V.A., Bewley K.R., Tree J.A., Dennis M., Taylor I., Funnell S.G.P., Bate S.R., Steeds K., Tipton T., et al. Assessment of the protective effect of imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J. Virol. 2013;87:7805–7815. doi: 10.1128/jvi.03481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaucha G.M., Jahrling P.B., Geisbert T.W., Swearengen J.R., Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab. Invest. 2001;81:1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goff A.J., Chapman J., Foster C., Wlazlowski C., Shamblin J., Lin K., Kreiselmeier N., Mucker E., Paragas J., Lawler J., et al. A novel respiratory model of infection with monkeypox virus in cynomolgus macaques. J. Virol. 2011;85:4898–4909. doi: 10.1128/jvi.02525-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stittelaar K.J., Neyts J., Naesens L., Van Amerongen G., Van Lavieren R.F., Holý A., De Clercq E., Niesters H.G.M., Fries E., Maas C., et al. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature. 2006;439:745–748. doi: 10.1038/nature04295. [DOI] [PubMed] [Google Scholar]
- 74.Barnewall R.E., Fisher D.A., Robertson A.B., Vales P.A., Knostman K.A., Bigger J.E. Inhalational monkeypox virus infection in cynomolgus macaques. Front. Cell. Infect. Microbiol. 2012;2:117. doi: 10.3389/fcimb.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nalca A., Livingston V.A., Garza N.L., Zumbrun E.E., Frick O.M., Chapman J.L., Hartings J.M. Experimental infection of cynomolgus macaques (Macaca fascicularis) with aerosolized monkeypox virus. PLoS One. 2010;5:e12880. doi: 10.1371/journal.pone.0012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Russo A.T., Grosenbach D.W., Brasel T.L., Baker R.O., Cawthon A.G., Reynolds E., Bailey T., Kuehl P.J., Sugita V., Agans K., et al. Effects of treatment delay on efficacy of tecovirimat following lethal aerosol monkeypox virus challenge in cynomolgus macaques. J. Infect. Dis. 2018;218:1490–1499. doi: 10.1093/infdis/jiy326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tree J.A., Hall G., Pearson G., Rayner E., Graham V.A., Steeds K., Bewley K.R., Hatch G.J., Dennis M., Taylor I., et al. Sequence of pathogenic events in cynomolgus macaques infected with aerosolized monkeypox virus. J. Virol. 2015;89:4335–4344. doi: 10.1128/jvi.03029-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson R.F., Dyall J., Ragland D.R., Huzella L., Byrum R., Jett C., St Claire M., Smith A.L., Paragas J., Blaney J.E., et al. Comparative analysis of monkeypox virus infection of cynomolgus macaques by the intravenous or intrabronchial inoculation route. J. Virol. 2011;85:2112–2125. doi: 10.1128/jvi.01931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Estep R.D., Messaoudi I., O’Connor M.A., Li H., Sprague J., Barron A., Engelmann F., Yen B., Powers M.F., Jones J.M., et al. Deletion of the monkeypox virus inhibitor of complement enzymes locus impacts the adaptive immune response to monkeypox virus in a nonhuman primate model of infection. J. Virol. 2011;85:9527–9542. doi: 10.1128/jvi.00199-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mucker E.M., Chapman J., Huzella L.M., Huggins J.W., Shamblin J., Robinson C.G., Hensley L.E. Susceptibility of marmosets (Callithrix jacchus) to monkeypox virus: a low dose prospective model for monkeypox and smallpox disease. PLoS One. 2015;10:e0131742. doi: 10.1371/journal.pone.0131742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mucker E.M., Wollen-Roberts S.E., Kimmel A., Shamblin J., Sampey D., Hooper J.W. Intranasal monkeypox marmoset model: prophylactic antibody treatment provides benefit against severe monkeypox virus disease. PLoS Negl. Trop. Dis. 2018;12:e0006581. doi: 10.1371/journal.pntd.0006581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao S.Y., Sbrana E., Watts D.M., Siirin M., da Rosa A.P.A.T., Tesh R.B. Experimental infection of prairie dogs with monkeypox virus. Emerg. Infect. Dis. 2005;11:539–545. doi: 10.3201/eid1104.040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hutson C.L., Carroll D.S., Self J., Weiss S., Hughes C.M., Braden Z., Olson V.A., Smith S.K., Karem K.L., Regnery R.L., et al. Dosage comparison of Congo Basin and West African strains of monkeypox virus using a prairie dog animal model of systemic orthopoxvirus disease. Virology. 2010;402:72–82. doi: 10.1016/j.virol.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hutson C.L., Olson V.A., Carroll D.S., Abel J.A., Hughes C.M., Braden Z.H., Weiss S., Self J., Osorio J.E., Hudson P.N., et al. A prairie dog animal model of systemic orthopoxvirus disease using west African and Congo Basin strains of Monkeypox virus. J. Gen. Virol. 2009;90:323–333. doi: 10.1099/vir.0.005108-0. [DOI] [PubMed] [Google Scholar]
- 85.Hutson C.L., Kondas A.V., Mauldin M.R., Doty J.B., Grossi I.M., Morgan C.N., Ostergaard S.D., Hughes C.M., Nakazawa Y., Kling C., et al. Pharmacokinetics and efficacy of a potential smallpox therapeutic, Brincidofovir, in a lethal monkeypox virus animal model. mSphere. 2021;6:009277-20. doi: 10.1128/msphere.00927-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weiner Z.P., Salzer J.S., LeMasters E., Ellison J.A., Kondas A.V., Morgan C.N., Doty J.B., Martin B.E., Satheshkumar P.S., Olson V.A., et al. Characterization of Monkeypox virus dissemination in the black-tailed prairie dog (Cynomys ludovicianus) through in vivo bioluminescent imaging. PLoS One. 2019;14:e0222612. doi: 10.1371/journal.pone.0222612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hutson C.L., Carroll D.S., Gallardo-Romero N., Weiss S., Clemmons C., Hughes C.M., Salzer J.S., Olson V.A., Abel J., Karem K.L., et al. Monkeypox disease transmission in an experimental setting: prairie dog animal model. PLoS One. 2011;6:e28295. doi: 10.1371/journal.pone.0028295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hutson C.L., Gallardo-Romero N., Carroll D.S., Clemmons C., Salzer J.S., Nagy T., Hughes C.M., Olson V.A., Karem K.L., Damon I.K. Transmissibility of the monkeypox virus clades via respiratory transmission: investigation using the prairie dog-monkeypox virus challenge system. PLoS One. 2013;8:e55488. doi: 10.1371/journal.pone.0055488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hutson C.L., Carroll D.S., Gallardo-Romero N., Drew C., Zaki S.R., Nagy T., Hughes C., Olson V.A., Sanders J., Patel N., et al. Comparison of monkeypox virus clade kinetics and pathology within the prairie dog animal model using a serial sacrifice study design. Biomed Res. Int. 2015;2015:965710. doi: 10.1155/2015/965710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keckler M.S., Salzer J.S., Patel N., Townsend M.B., Nakazawa Y.J., Doty J.B., Gallardo-Romero N.F., Satheshkumar P.S., Carroll D.S., Karem K.L., et al. Imvamune® and acam2000® provide different protection against disease when administered postexposure in an intranasal monkeypox challenge prairie dog model. Vaccines. 2020;8:396. doi: 10.3390/vaccines8030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tesh R.B., Watts D.M., Sbrana E., Siirin M., Popov V.L., Xiao S.Y. Experimental infection of ground squirrels with monkeypox virus. Emerg. Infect. Dis. 2004;10:1563–1567. doi: 10.3201/eid1009.040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sbrana E., Jordan R., Hruby D.E., Mateo R.I., Xiao S.Y., Siirin M., Newman P.C., da Rosa A.P.A.T., Tesh R.B. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am. J. Trop. Med. Hyg. 2007;76:768–773. doi: 10.4269/ajtmh.2007.76.768. [DOI] [PubMed] [Google Scholar]
- 93.Sbrana E., Xiao S.Y., Newman P.C., Tesh R.B. Comparative pathology of North American and Central African strains of monkeypox virus in a ground squirrel model of the disease. Am. J. Trop. Med. Hyg. 2007;76:155–164. doi: 10.4269/ajtmh.2007.76.155. [DOI] [PubMed] [Google Scholar]
- 94.Sergeev A.A., Kabanov A.S., Bulychev L.E., Sergeev A.A., Pyankov O.V., Bodnev S.A., Galahova D.O., Zamedyanskaya A.S., Titova K.A., Glotova T.I., et al. Using the ground squirrel (Marmota bobak) as an animal model to assess monkeypox drug efficacy. Transbound. Emerg. Dis. 2017;64:226–236. doi: 10.1111/tbed.12364. [DOI] [PubMed] [Google Scholar]
- 95.Falendysz E.A., Lopera J.G., Doty J.B., Nakazawa Y., Crill C., Lorenzsonn F., Kalemba L.N., Ronderos M.D., Mejia A., Malekani J.M., et al. Characterization of Monkeypox virus infection in African rope squirrels (Funisciurus sp.) PLoS Negl. Trop. Dis. 2017;11:e0005809. doi: 10.1371/journal.pntd.0005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gispen R., Brand-Saathof B. White” poxvirus strains from monkeys. Bull. World Health Organ. 1972;46:585–592. [PMC free article] [PubMed] [Google Scholar]
- 97.Gispen R., Verlinde J.D., Zwart P. Histopathological and virological studies on monkeypox. Arch. Gesamte Virusforsch. 1967;21:205–216. doi: 10.1007/BF01241445. [DOI] [PubMed] [Google Scholar]
- 98.Parker S., Buller R.M. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8:129–157. doi: 10.2217/fvl.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kozlov M. How deadly is monkeypox? What scientists know. Nature. 2022;609:663. doi: 10.1038/d41586-022-02931-1. [DOI] [PubMed] [Google Scholar]
- 100.Pereira V.R.A., Lorena V.M.B., Nakazawa M., Luna C.F., Silva E.D., Ferreira A.G.P., Krieger M.A., Goldenberg S., Soares M.B.P., Coutinho E.M., et al. Humoral and cellular immune responses in BALB/c and C57BL/6 mice immunized with cytoplasmic (CRA) and flagellar (FRA) recombinant repetitive antigens, in acute experimental Trypanosoma cruzi infection. Parasitol. Res. 2005;96:154–161. doi: 10.1007/s00436-005-1336-4. [DOI] [PubMed] [Google Scholar]
- 101.Trunova G.V., Makarova O.V., Diatroptov M.E., Bogdanova I.M., Mikchailova L.P., Abdulaeva S.O. Morphofunctional characteristic of the immune system in BALB/c and C57Bl/6 mice. Bull. Exp. Biol. Med. 2011;151:99–102. doi: 10.1007/s10517-011-1268-1. [DOI] [PubMed] [Google Scholar]
- 102.Duggal N.K., Ritter J.M., Pestorius S.E., Zaki S.R., Davis B.S., Chang G.J.J., Bowen R.A., Brault A.C. Frequent Zika virus sexual transmission and prolonged viral RNA shedding in an immunodeficient mouse model. Cell Rep. 2017;18:1751–1760. doi: 10.1016/j.celrep.2017.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hutson C.L., Abel J.A., Carroll D.S., Olson V.A., Braden Z.H., Hughes C.M., Dillon M., Hopkins C., Karem K.L., Damon I.K., et al. Comparison of West African and Congo Basin monkeypox viruses in BALB/c and C57BL/6 mice. PLoS One. 2010;5:e8912. doi: 10.1371/journal.pone.0008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hahon N. Smallpox and related poxvirus infections in the simian host. Bacteriol. Rev. 1961;25:459–476. doi: 10.1128/mmbr.25.4.459-476.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sauer R.M., Prier J.E., Buchanan R.S., Creamer A.A., Fegley H.C. Studies on a pox disease of monkeys. I. Pathology. Am. J. Vet. Res. 1960;21:377–380. [PubMed] [Google Scholar]
- 106.Sehgal C.L., Ray S.N. Laboratory studies on monkeypox virus. J. Commun. Dis. 1982;14:26–35. [PubMed] [Google Scholar]
- 107.Shchelukhina E.M., Marennikova S.S. [Generalized monkeypox in orally infected rabbits and white mice] Vopr. Virusol. 1975:703–705. [PubMed] [Google Scholar]
- 108.Marennikova S., Shelukhina E.M., Shenkman L.S. Twelfth International Poxvirus Symposium. St. Thomas US Virgin Islands; 1998. Cotton rats (Sigmodon hispidus) as an experimental model of monkeypox infection; pp. 6–10. [Google Scholar]
- 109.Schultz D.A., Sagartz J.E., Huso D.L., Buller R.M.L. Experimental infection of an African dormouse (Graphiurus kelleni) with monkeypox virus. Virology. 2009;383:86–92. doi: 10.1016/J.VIROL.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seang S., Burrel S., Todesco E., Leducq V., Monsel G., Le Pluart D., Cordevant C., Pourcher V., Palich R. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658–659. doi: 10.1016/S0140-6736(22)01487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]