INTRODUCTION
Despite ongoing study into chronic rhinosinusitis (CRS) disease mechanisms, the origins of chronic inflammation are somewhat elusive.1 CRS is defined by 3 months of symptoms with evidence of disease, either radiographically or endoscopically.2 Interestingly, pathologic correlates of inflammation are not required for diagnosis, but are well described. Some of these findings include classic acute inflammatory pathologic hallmarks (ie, edema and neutrophilic infiltrate), and some are characteristic of chronic inflammation (ie, lymphocytic infiltrate, basement membrane thickening, and fibrosis). Even in well-defined subgroups,3 it appears that pathologic and molecular CRS research findings support the presence of mixed acute and chronic inflammation. The role of microbes in CRS remains unclear, as current models describe CRS as a disease of inflammation.
Herein we have we applied systems biology and transcriptome network approaches to identify de-novo pathways and putative etiologic factors for inflammatory activation in CRS tissues. Recent works have utilized cluster analyses to delineate CRS inflammatory endotypes types using targeted transcript profiling and bulk tissue or single-cell transcriptomics.3–6 Notably, this approach has not been performed across CRS phenotypes and controls.
PATIENTS AND METHODS
Twenty-seven subjects (7 controls, 20 with CRS) were enrolled into a prospective study, wherein ethmoid sinus tissues were interrogated using the Affymetrix Human Transcriptome Array 2.0 (Thermo Fisher Scientific, Waltham, MA). Data were analyzed for differential gene expression, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment, and gene ontology (GO) enrichment (Supplementary Information). CRS subjects refractory to attempted medical therapies underwent endoscopic sinus surgery according to clinical guidlines2 (Table S1). Control subjects had no clinical or radiographic evidence of rhinosinusitis. Control participants had normal ethmoid mucosa obtained during endoscopic dacryocystorhinostomy or transnasal pituitary surgery, where preoperative serum hormone panels revealed no abnormality.
RESULTS
As anticipated, global transcriptome profiles clearly delineated controls from CRS subjects (Figs. 1 and S1). Of the 25,476 annotated genes detected by the Affymetrix platform, 211 exhibited a |log2 fold change| >1 with a false discovery rate <0.1 when comparing CRS with controls (121 genes downregulated and 90 upregulated in CRS). Surprisingly, few differences were noted between nonpolyp CRS (CRSsNP) vs polypoid CRS (CRSwNP). In fact, no genes reached significance using these statistical criteria.
FIGURE 1.
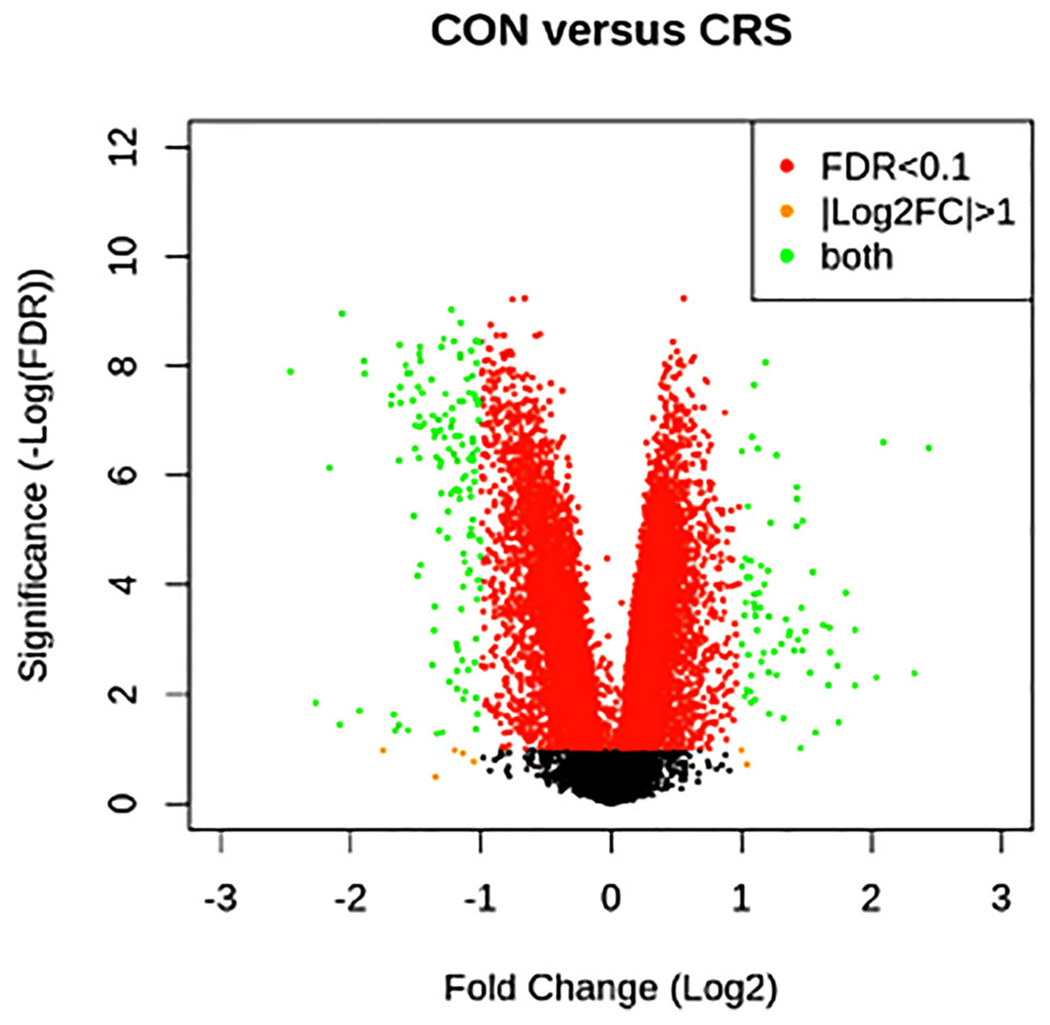
Transcriptome array. Volcano plot of genes identified in CRS vs CON subjects using the Affymetrix 2.0 Transcriptome Array. FC is shown with respect to CRS.
Abbreviations: CON = control; CRS = chronic rhinosinusitis; FC = fold change; FDR = false discovery rate.
Transcriptome variability was attributable primarily to the subject, then to diagnosis (CRS), and then presence of asthma or purulence, before polyp condition (Fig. S2). Exploratory principal components analysis supported these findings, where CRS and controls clustered separately, but no distinction based on these features was evident within CRS subjects (Fig. S1). Consideration of a third phenotypic group of CRS asthmatics7—in addition to CRSsNP and CRSwNP—may clarify variance that otherwise obscures the delineation between polyp and nonpolyp cohorts (Figs. S2–S4). It is currently unclear whether asthmatic CRS represents an intermediate group bridging these categories with shared inflammatory mechanisms, or whether it exists as a function of time (ie, asthmatic CRSsNP may eventually become CRSwNP).
Pathway analyses were then performed. KEGG analysis identified the major differentially regulated pathways to be those involved in immune system regulation, including activation by both infectious and allergic stimuli (Table 1 and Fig. S5). These include antigen processing and presentation, major histocompatibility complex (MHC) class I and II signaling, classical and alternative complement pathways, conventional opsonization, and toll-like receptor (TLR)-mediated phagocystosis, as well as the cytokine activation often associated with infection (eg, tumor necrosis factor-alpha [TNF-α], interleukin-1 [IL-1], and interferon-gamma [IFN-γ]). GO informatics also implicated immune activation, as CRS was enriched in innate and adaptive inflammatory responses, regulation of leukocyte differentiation and migration, antigen processing, viral and fungal response, neutrophil degranulation, and IFN (type I and II) cytokine production. Control samples had enriched RNA processing GO pathways but no KEGG pathways reaching statistical significance.
TABLE 1.
Top 20 GO and KEGG pathway analysis dysregulated pathways in CRS relative to controls
| Enrichment | Significancea | |
|---|---|---|
| GO | ||
| GO term | ||
| GO:0006955 immune response | 8.936 | <0.001 |
|
| ||
| GO:0006952 defense response | 8.374 | <0.001 |
|
| ||
| GO:0002376 immune system process | 7.691 | <0.001 |
|
| ||
| GO:0050776 regulation of immune response | 7.105 | <0.001 |
|
| ||
| GO:0002252 immune effector process | 6.69 | <0.001 |
|
| ||
| GO:0006954 inflammatory response | 6.168 | <0.001 |
|
| ||
| GO:0045087 innate immune response | 6.062 | <0.001 |
|
| ||
| GO:0045321 leukocyte activation | 5.932 | <0.001 |
|
| ||
| GO:0019724 B-cell–mediated immunity | 5.85 | <0.001 |
|
| ||
| GO:0002684 positive regulation of immune system process | 5.811 | <0.001 |
|
| ||
| GO:0016070 RNA metabolic process | −4.526 | <0.01 |
|
| ||
| GO:0090304 nucleic acid metabolic process | −4.195 | <0.01 |
|
| ||
| GO:0006397 mRNA processing | −4.308 | <0.01 |
|
| ||
| GO:0006351 transcription, DNA-dependent | −4.091 | <0.01 |
|
| ||
| GO:0010467 gene expression | −4.04 | <0.01 |
|
| ||
| GO:0008380 RNA splicing | −4.199 | <0.01 |
|
| ||
| GO:0006396 RNA processing | −4.057 | <0.01 |
|
| ||
| GO:0034645 cellular macromolecule biosynthetic process | −3.914 | <0.01 |
|
| ||
| GO:0051252 regulation of RNA metabolic process | −3.867 | <0.01 |
|
| ||
| GO:0032774 RNA biosynthetic process | −3.849 | <0.01 |
| KEGG | ||
| KEGG pathway | ||
|
| ||
| hsa04640 hematopoietic cell lineage | 5.829 | <0.001 |
|
| ||
| hsa04514 CAMs | 4.616 | <0.001 |
|
| ||
| hsa04145 phagosome | 4.296 | <0.001 |
|
| ||
| hsa04672 intestinal immune network for IgA production | 5.138 | <0.001 |
|
| ||
| hsa04612 antigen processing and presentation | 4.075 | <0.01 |
|
| ||
| hsa04380 osteoclast differentiation | 3.288 | <0.01 |
|
| ||
| hsa04062 chemokine-signaling pathway | 2.786 | <0.1 |
|
| ||
| hsa04142 lysosome | 2.469 | <0.1 |
|
| ||
| hsa04610 complement and coagulation cascades | 2.304 | <0.1 |
|
| ||
| hsa00190 oxidative phosphorylation | 2.235 | <0.1 |
|
| ||
| hsa03040 spliceosome | −2.317 | NS |
|
| ||
| hsa04070 phosphatidylinositol-signaling system | −1.677 | NS |
|
| ||
| hsa04742 taste transduction | −1.54 | NS |
|
| ||
| hsa04012 ErbB-signaling pathway | −1.422 | NS |
|
| ||
| hsa04912 GnRH-signaling pathway | −1.36 | NS |
|
| ||
| hsa04270 vascular smooth muscle contraction | −1.324 | NS |
|
| ||
| hsa03013 RNA transport | −1.275 | NS |
|
| ||
| hsa04730 long-term depression | −1.248 | NS |
|
| ||
| hsa04510 focal adhesion | −1.14 | NS |
|
| ||
| hsa04150 mTOR-signaling pathway | −1.107 | NS |
Note: Components of these pathways can be viewed at http://geneontology.org or https://www.genome.jp/kegg; for example, the most enriched KEGG pathway (hematopoietic cell lineage) is displayed in Figure S5.
Significance levels of p values are adjusted for FDR.
CAM = cell adhesion molecule; CRS = chronic rhinosinusitis; FDR = false discovery rate; GO = gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; IgA = immunoglobulin A; mTOR = mammalian target of rapamycin; NS = not significant.
DISCUSSION
We observed broadly similar biologic processes in CRS irrespective of phenotype or endotype, with pathway analyses revealing chronic inflammation as a common disease endpoint resulting from infectious exposures and/or allergic or autoimmune contributions. Consistent activation of positive acute-phase proteins (eg, TNF-α and IL-1) and IFN-γ signaling pathways across the CRS spectrum, taken together with pathway analyses implicating responses to microbial exposures, suggest that repeated responses to acute pro-inflammatory microbial stimuli may be a key component of the disease process. Consistent with classic pathologic descriptions, these molecular analyses support the concept that chronic inflammatory and acute infectious processes occur concurrently in CRS tissues, and that repeated acute insults occur in the disease and perhaps even sustain the condition.
Several limitations should be acknowledged. Although the RNA-based array includes all known transcript isoforms, analyses rely on existing databases and cannot account for translational or posttranslational regulation. We purposely sought a real-world disease cohort for this study, and our analytical approach helped to mitigate concern for cross-sectional sampling and disease heterogeneity. However, it is possible that inclusion of more subjects, curation by endotype or cell type, or stratification by coincidental observation of purulence, would induce segregation within CRS groups. For instance, a recent study utilizing single-cell RNAseq was able to elucidate transcriptional differences in individual cell types in CRSwNP compared with CRSsNP.8 However, the study did not include a surgical control group, and assessed CRSwNP subjects with aspirin-exacerbated respiratory disease and varied medication usage, reiterating the challenges of enrolling clean cohorts in human CRS studies.
The etiopathogenesis of CRS continues to engender significant debate, with erstwhile prevailing thought attributing an infectious origin to the disease, and a modern transition in belief toward a chronic inflammatory disease process.1,9 Herein we used tissue transcriptome pathway analysis to demonstrate that these viewpoints are not mutually exclusive—inflammatory processes responsible for CRS chronicity ostensibly include repeated acute inflammatory responses to microbial pathogens.
Supplementary Material
ACKNOWLEDGMENTS
V.R.R. is supported by a grant for this investigation from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD., USA (K23DC014747); D.N.F. and V.R.R. are supported by grants from the Flight Attendant Medical Research Institute (CIA130066 and CIA160014). These funding organizations did not contribute to the design or conduct of this study, preparation, review, approval, or decision to submit this manuscript for publication.
Footnotes
Potential conflict of interest: None provided.
Presented orally in abstract form at the American Rhinologic Society meeting at Rhinoworld in Chicago, IL, in June 2019.
View this article online at wileyonlinelibrary.com.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICAL PUBLICATION STATEMENT
This study was performed in compliance with the Declaration of Helsinki for medical research involving human subjects, and accordingly was approved by the Colorado Multi-Institutional Review Board (COMIRB #HS-11-1442), with all patients providing informed consent.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Hamilos DL. Drivers of chronic rhinosinusitis: inflammation versus infection. J Allergy Clin Immunol. 2015;136(6):1454–1459. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1–S39. [DOI] [PubMed] [Google Scholar]
- 3.Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137(5):1449–1456. [DOI] [PubMed] [Google Scholar]
- 4.Tyler MA, Russel CB, Smith DE, et al. Large-scale gene expression profiling reveals distinct type 2 inflammatory patterns in chronic rhinosinusitis subtypes. J Allergy Clin Immunol. 2017;139(3):1061–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, Gao Z, Wang H, et al. Transcriptome analysis reveals distinct gene expression profiles in eosinophilic and noneosinophilic chronic rhinosinusitis with nasal polyps. Sci Rep. 2016;6:26604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okada N, Nakayama T, Asaka D, et al. Distinct gene expression profiles and regulation networks of nasal polyps in eosinophilic and non-eosinophilic chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(5):592–604ww. [DOI] [PubMed] [Google Scholar]
- 7.Han JK. Subclassification of chronic rhinosinusitis. Laryngo-scope. 2013;123(suppl 2):S15–S27. [DOI] [PubMed] [Google Scholar]
- 8.Ordovas-Montanes J, Dwyer DF, Nyquist SK, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560(7720):649–654. doi: 10.1038/s41586-018-0449-8. Epub 2018 Aug 22.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens WW, Lee RJ, Schleimer RP, Cohen NA. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol. 2015;136(6):1442–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


