Abstract
Aims:
Aerobic exercise is an important component of rehabilitation after cardiovascular injuries including myocardial infarction (MI). In human studies, the beneficial effects of exercise after an MI are blunted in patients who are obese or glucose intolerant. Here, we investigated the effects of exercise on MI-induced cardiac dysfunction and remodeling in mice chronically fed a high-fat diet (HFD). Main methods: C57Bl/6 male mice were fed either a standard (Chow; 21% kcal/fat) or HFD (60% kcal/fat) for 36 weeks. After 24 weeks of diet, the HFD mice were randomly subjected to an MI (MI) or a sham surgery (Sham). Following the MI or sham surgery, a subset of mice were subjected to treadmill exercise.
Key findings:
HFD resulted in obesity and glucose intolerance, and this was not altered by exercise or MI. MI resulted in decreased ejection fraction, increased left ventricle mass, increased end systolic and diastolic diameters, increased cardiac fibrosis, and increased expression of genes involved in cardiac hypertrophy and heart failure in the MI-Sed and MI-Exe mice. Exercise prevented HFD-induced cardiac fibrosis in Sham mice (Sham-Exe) but not in MI-Exe mice. Exercise did, however, reduce post-MI mortality.
Significance:
These data indicate that exercise significantly increased survival after MI in a model of diet-induced obesity independent of effects on cardiac function. These data have important translational ramifications because they demonstrate that environmental interventions, including diet, need to be carefully evaluated and taken into consideration to support the effects of exercise in the cardiac rehabilitation of patients who are obese.
Keywords: Obesity, glucose intolerance, high-fat diet, myocardial infarction, exercise
Introduction
Obesity is a major public health epidemic that has reached alarming proportions worldwide. Over 650 million adults were categorized as clinically obese in 2020 (1), and it is expected that nearly half of the world’s population will be overweight or obese by 2030 (1, 2). In the US, the prevalence of obesity in the adult population is nearly 40%, imposing a tremendous financial burden on health care systems (3). The implications of this epidemic on the healthcare system are enormous as obesity increases the risk of several comorbidities including cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) (3). The mortality from CVD is three- to eight-fold higher in patients who are obese or suffer from T2DM when compared to patients who are normal weight or do not have diabetes (4, 5), making the management of both pathological conditions essential for an effective reduction of CVD mortality.
Myocardial infarction (MI) results from ischemic injury induced by sudden reduced blood flow to the heart. MI remains one of the most significant complications of CVD and is associated with substantial disability and mortality (6). Following an MI, cardiomyocytes in the ischemic zone die by both apoptosis and necrosis and are replaced by fibrotic tissue. This is accompanied by left ventricular (LV) remodeling and loss of cardiac function, both of which contribute to the progression of heart failure (HF) (7). While the survival rate of patients after an MI has increased over the last few decades (3), patients who are obese or have T2DM are more likely to have adverse LV remodeling and heart failure post-MI, resulting in further impairments to cardiovascular function(4–6). Thus, interventions to improve cardiovascular health and remodeling, in particular in patients who are obese or suffer from diabetes, is of utmost importance.
Exercise is a well-established component of cardiac rehabilitation after MI (8). Aerobic exercise training decreases resting heart rate, blood pressure, systemic inflammation, increases physiological cardiac hypertrophy (8), and has marked benefits on systemic metabolism (7). The beneficial effects of exercise on both cardiac and metabolic outcomes are well-established in clinical and pre-clinical models of MI. In humans, clinical studies have shown that exercise training post-MI improves LV function and attenuates pathological LV remodeling (9), increases exercise tolerance (10), reduces the incidence of subsequent cardiac events (8, 9), and improves quality of life (10). However, the beneficial effects of exercise after an MI are blunted in patients who are obese or have T2DM (11–16), and the reasons for this are not fully understood. Similarly in lean rodent models, exercise training performed after ischemic injury improves hemodynamic parameters, LV remodeling, and reduces inflammation and fibrosis (17–20), but the effects of exercise after an MI in obese mice have not been thoroughly investigated. Because the incidence of MI is greater among patients who are obese or have T2DM, determining if aerobic exercise is beneficial post-MI in the context of obesity and glucose intolerance is of significant translational relevance. Here, we investigated the effects of exercise on MI-induced cardiac dysfunction and remodeling in mice chronically fed a high-fat diet.
Experimental Methods
Mice and Myocardial Infarction Surgery
Male C57Bl/6 mice (6 weeks-old) (Charles River Laboratories) were placed on a high-fat diet (HFD) (Research Diets D12492; 60% kcal saturated fat from lard) for 24 weeks and then divided into 2 groups: Sham operated (Sham), or mice that underwent myocardial infarction surgery (MI) (Supplemental Table 1, Figure 1). Mice fed a Chow diet (PharmaServ 9F5020; 21% kcal from fat) were used as age-matched controls. For the MI procedure, a permanent MI surgery was performed by anaesthetizing mice with 2% isoflurane in 98% O2 and mechanical ventilation. After a left thoracotomy, the fourth intercostal space and the lungs were retracted. The LAD coronary artery was permanently ligated with an 8-0 silk low suture 0.6 mm distal to the atrioventricular junction, and a mild MI was induced by a low suture in the left anterior descending (LAD) coronary artery, as previously described (21–23). The mice were kept on heat pad until recovery of consciousness. All mice were observed for the next 72 h after surgery. The Sham groups were subjected to all the procedures except the LAD ligation.
Figure 1. Experimental design.

Six-week-old C57BL/6 mice were placed on a HFD for 24 weeks then subjected to Myocardial Infarction. Four weeks after MI, mice from the Exe groups were subjected to treadmill exercise training (EXE) for 8 weeks. Body composition assessment was performed 2 weeks before MI, 4 and 12 weeks post-MI. Glucose tolerance tests (GTT), and Insulin tolerance tests (ITT) were performed 4 and 12 weeks post-MI. Echocardiography was performed 2 weeks before MI, and at 12 weeks post MI. At 12 weeks post MI, mice were euthanized, and tissues were collected for assessment of gene expression.
Animals were housed at room temperature (22 °C), with free access to food and water, on a 12-h light/dark cycle. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health following protocols approved by the IACUC at The Ohio State University. Animals were euthanized using isoflurane and cervical dislocation followed by collection of tissues.
Exercise training
Four weeks after sham or MI surgery, mice were subdivided into sedentary (Sed) or exercise groups (mice undergoing exercise training, Exe): Sham-Sedentary (Sham-Sed), MI-Sedentary (MI-Sed), Sham-Exe and MI-Exe. The Exe groups were trained on a treadmill designed for mice with individual lanes (Columbus Instruments). Four weeks after the MI surgery, all mice were acclimated to the treadmill for three consecutive days. During acclimation, mice were placed in the treadmill for 3 min, after which the shock grid was activated (3 Hz and 1.5 mA). The treadmill was engaged to a walking speed of 6 m/min for 5 min and progressively increased up to 12 m/min for a total duration of 12 min of exercise (23). After 3 days, a subset of mice underwent an exercise training program for 8 weeks consisting of moderate aerobic exercise training started after acclimation and consisted of treadmill exercise 60 min/day, 0%−10% treadmill inclination, and speed 8–14 m/min, 5 days/week, for 8 weeks (Supplemental Table 2).
After the exercise training program, all groups underwent an exercise tolerance test (24). This consisted of placing the mice on the treadmill at 0° incline with the shock grid activated. The treadmill speeds were then increased until exhaustion as follows: (speed, duration, grade)—(0 m/min, 3 min, 0°), (6 m/min, 3 min, 0°), (9 m/min, 3 min, 5°), (12 m/min, 3 min, 10°), (15 m/min, 3 min, 15°), (18, 21, 23, 24 m/min, 3 min, 15°), and (+1 m/min, each 1 min thereafter, 15°). The endpoint for treadmill cessation was defined as the time at which mice maintained continuous contact with the shock grid for 5 s (24).
Metabolic Characterization
Body weight was measured weekly and body composition was determined using EchoMRI (EchoMRI™3-in-1) 2 weeks prior to MI and 4 and 12 weeks post-MI. Glucose tolerance tests (GTT) were performed at 4 and 12 weeks post-MI. Mice were fasted for 11 hours (22:00–9:00) with free access to drinking water (25). A baseline blood sample was collected from the tail of fully conscious mice, followed by i.p. injection of glucose (2.0 g/kg body weight), and blood was taken from the tail at 15, 30, 60, and 120 minutes after injection. Insulin tolerance tests (ITTs) were performed at 4 and 11 weeks post-MI. Mice were fasted for 2 hours (10:00–12:00), and baseline blood samples were collected from the tail of fully conscious mice. Insulin (1 U/kg body weight) (Humulin; Eli Lilly) was administered by i.p. injection, and blood samples were taken from the tail at 10, 15, 30, 45, and 60 minutes after injection (25). Glucose concentrations were determined using a OneTouch Ultra-portable glucometer (LifeScan).
Echocardiography
Echocardiography was performed prior to MI and 12 weeks post-MI. Mice were anesthetized with 1–2% isoflurane and echocardiography were performed using a Vevo 2100 Ultrasound (23), and ejection fraction, fractional shortening, and ventricular chamber dimensions were measured in M mode using the parasternal short axis view (21). Echocardiogram data was analyzed using VevoLab software to determine ejection fraction (EF), fraction shortening (FS), stroke volume, cardiac output, end systolic diameter (ESD), end diastolic diameter (EDD), end systolic volume (ESV), end diastolic volume (EDV), left ventricle (LV) mass, LV anterior wall (LVAW) and LV posterior wall (LVPW).
Histology and Biochemical Methods
Heart tissue processing and quantitative PCR (qPCR) were performed on tissue isolated from mice sacrificed at 42 weeks of age (12 weeks post-MI) as previously described (26). Tissue for qPCR was flash frozen and stored at −80°C until processing. mRNA was measured by qRT-PCR (Roche LightCycler 480II) using SYBR Green detection (QuantaBio). IDT custom primers were used for genes of interest with the sequences shown in Table S3 (27). Gene expression was normalized to the housekeeping gene RPL7a.
For histology, the fresh hearts (apex) were frozen in liquid nitrogen-cooled isopentane using OCT (Tissue-Tek) and cut sectioned serially at 5 microns. The sections were then stained using Masson’s trichrome to examine the amount of interstitial fibrosis. The percent area of cardiac fibrosis was obtained by an Automated Fibrosis Analysis Tool (AFAT). The amount of collagen relative to total tissue area in heart tissue sections processed with Masson’s Trichrome staining (collagen stained blue) was calculated using a custom-built MATLAB program (28).
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Statistical Analysis
The data are presented as means ± SEM. One-way and two-way ANOVA with Tukey post hoc analysis were performed using GraphPad Prism software 7.0 (GraphPad Prism Software Inc., San Diego-CA). Kaplan–Meyer survival curve was plotted to identify the survival rate post-MI, and survival curves were compared using Log-rank (Mantel-Cox) test and Logrank test for trend. Values of P<0.05 were considered statistically significant.
Results
Exercise training increased survival but did not improve diet-induced obesity and affect glucose metabolism post-MI.
MI induces critical impairments in cardiac function (28), and several studies have shown that the 1-year mortality rate after MI is higher in patients who are obese or have T2DM (13, 29). Exercise is an important component of cardiac rehabilitation in patients who suffered an MI, and clinical studies have shown that aerobic exercise reduces the mortality rate after an MI in patients who are not obese (30). However, the effects of exercise training post-MI in obese models is not fully understood. In this study, we investigated the effects of exercise after an MI in a model of chronic diet-induced obesity. Four weeks post-MI, the mice that survived were subdivided into Sed or Exe (Figure 1; Supplemental Table 1). Following the 8-week exercise-training protocol, there was no difference in time to exhaustion in an exercise tolerance test (Supplemental Figure 1B), but the survival rate of the group of the MI-Exe group (88%) was significantly higher than the MI-Sed group (61%) (Figure 2A). The Sham-Sed mice had a 92% survival rate, while the Sham-Exe mice had a 100% survival rate (Figure 2A). This indicates exercise has a protective effect on survival rate after MI in mice fed a chronic high-fat diet.
Figure 2. Exercise increased survival and did not affect body composition or glucose metabolism post-MI.

(A) Survival curve after the start of exercise training, at 4 weeks post MI with initial and final n in Chow-fed (n=11, n=11), Sham-Sed (n=9, n=8), MI-Sed (n=10, n=5), Sham-Exe (n=4, n=4), and MI-Exe (n=13, n=11). The body composition was assessed by Echo MRI. (B) Body weight, (C) % fat mass, and (D) % lean mass at 4 and 12 weeks post MI in Chow-fed (n=10), Sham-Sed (n=8), MI-Sed (n=5), Sham-Exe (n=4), and MI-Exe (n=10). Whole-body glucose homeostasis was assessed by insulin tolerance tests (ITT), and glucose tolerance test (GTT). (E) GTT excursion curve at 11 weeks post MI. (F) Glucose tolerance test (GTT) area under curve (AUC) at 4 and 11-weeks post MI in Chow-fed (n=5), Sham-Sed (n=8), MI-Sed (n=5), Sham-Exe (n=4, n=4), and MI-Exe (n=13, n=11). (G) ITT excursion curve at 12 weeks post MI. (H) AUC of Insulin tolerance test (ITT) at 4, and 12 weeks post MI. (I) Overnight fasting glucose at 4, and 12 weeks post MI. Data are presented as mean ± S.E.M. Survival curves were compared using Logrank test, and $ symbols represent significant difference vs all other groups. One-way ANOVA was used with Tukey’s multiple comparisons tests. Values of p<0.05 were considered statistically significant.; (a) Symbols represent difference vs. Chow-fed mice, (c) Symbols represent difference vs MI-Sed, and † symbols represent difference vs 4 weeks post MI.
High-fat fed mice had elevated body weight and % fat mass, and reduced % lean mass, compared to chow-fed mice, but this was not affected by exercise or MI (Figure 2B–D, Supplemental Table 5). There were also no differences in glucose tolerance test (GTT) or insulin tolerance test (ITT) among HFD mice, regardless of MI or exercise (Figure 2D–G, Supplemental Table 5). These data confirm that this model of HFD results in increased body fat accumulation and glucose intolerance and this was not affected by treadmill exercise training or MI.
Exercise training did not prevent cardiac dysfunction and remodeling after MI in obese mice.
To determine the effects of HFD, MI, and exercise on cardiovascular health, echocardiography was performed prior to MI and 12 weeks post-MI (Figures 3A–F, Supplemental Tables 4 and 5). There was no effect of 24 weeks of a high-fat diet on baseline ejection fraction (EF) and fractional shortening (FS) when compared to age-matched Chow-fed mice (Figure 3A,B). There was an overall effect of age on EF and FS in all groups (pre- to post-MI or Sham surgery) (Figure 3A,B), but EF and FS were not different among Chow-Sed, Sham-Sed, or Sham-Exe mice at either baseline or 12 weeks post-MI (Figure 3A,B). Myocardial infarction (MI) further impaired EF and FS compared to Sham mice, and there was no effect of exercise on EF or FS in the presence or absence of MI (Figure 3A,B, Supplemental Table 5). This indicates that MI impairs EF and FS after 12 weeks in mice fed a HFD, and exercise does not attenuate this effect.
Figure 3. Exercise training did not improve MI-induced cardiac dysfunction and remodeling in obese mice.

Cardiac function and structure were measured by (A) ejection fraction, (B) fraction shortening, (C) end systolic diameter, (D) end diastolic diameter, (E) end systolic volume, (F) end diastolic volume, and (G) left ventricle mass before MI in Chow-fed (n=11) and HFD (n=28), and 12 weeks post MI in Sham-Sed (n=8), MI-Sed (n=5), Sham-Exe (n=4), and MI-Exe (n=11). Data are presented as mean ± S.E.M. One-way ANOVA was used with Tukey’s multiple comparisons tests. Kaplan–Meyer survival curve was plotted to identify the survival rate post-MI. Values of p<0.05 were considered statistically significant, and *represent difference vs Chow-fed pre-MI; # represent difference vs HFD pre-MI; Symbols represent difference vs. Chow-fed mice (a), difference vs Sham-Sed (b), and difference vs Sham-Exe (d).
Pathological LV hypertrophy is associated with impaired cardiovascular health (31), and the development of cardiac hypertrophy is regarded as a critical event in the process of cardiac remodeling and heart failure after a MI (31). To determine if exercise-training protects against cardiac hypertrophy after MI in HFD mice, several cardiac parameters were measured by echocardiography (Figure 3C–F). A chronic HFD increased baseline end diastolic diameter, end diastolic volume, and left ventricle mass in mice when compared to age-matched Chow-fed mice (Figure 3D–G) indicating that a HFD induced adverse cardiac remodeling independent of MI. There was an effect of aging on ESD in both Chow-fed and Sham-Sed mice (Figure 3C). There was an additional effect of HFD on ESD and EDV in Sham-Sed compared to Chow-fed mice, and this was not affected by exercise (Figure 3E). These data indicate that there is an effect of both age and HFD on LV remodeling, and HFD mice develop pathological cardiac hypertrophy earlier than Chow-fed mice. MI results in impaired cardiac remodeling, and exercise does not diminish this adverse effect (Figure 3 C–G, Supplemental Table 5).
Exercise training did not protect against the development of MI-induced cardiac fibrosis.
Following an MI injury, the necrotic cells are replaced by fibrotic tissue in the myocardium. Increased fibrosis leads to progressive cardiac dysfunction and heart failure (32). Studies have shown that cardiac fibrosis is increased in patients who are obese or have insulin resistance one year post-MI (32, 33). Rodent studies have shown that a HFD increased LV fibrosis post-MI compared to chow-fed mice (34, 35), and studies in lean mice have shown that exercise-training reduces cardiac fibrosis after MI (18, 19). To determine the effects of exercise on cardiac fibrosis post-MI, Masson’s Trichrome staining was used 12 weeks post-MI. HFD increased fibrosis compared to Chow-fed mice, but cardiac fibrosis was not different among Sham-Sed, MI-Sed, and MI-Exe mice (Figure 4A). However, there was a significant effect of exercise to reduce or prevent the development of fibrosis in Sham mice (Sham-Exe) (Figure 4A, Supplemental Table 5). These data indicate that a chronic HFD increases cardiac fibrosis, and that exercise-training protects against the development of HFD-induced cardiac fibrosis in the absence of an MI.
Figure 4. Exercise training did not protect against the development of MI-induced cardiac fibrosis.

Masson’s Trichrome staining was used to measure fibrosis. (A) The % of fibrosis is represented in Chow-fed (n=10), Sham-Sed (n=3), MI (n=6), Sham-Exe (n=4), and MI-Exe (n=6). Representative heart images are shown below the bar graph. Data are presented as mean ± S.E.M. One-way ANOVA was used with Tukey’s multiple comparisons tests. Values of p<0.05 were considered statistically significant. Symbols represent difference vs. Chow-fed mice (a), difference vs Sham-Sed (b), and difference vs Sham-Exe (d).
Exercise did not prevent MI-induced increase in cardiac remodeling gene expression
To determine the effect of HFD, MI, and exercise on cardiac gene expression, we analyzed genes involved in heart contractile function, cardiac remodeling, inflammation, and fibrosis. In total, the expression of 51 genes were measured (Table S3). The mean differences of all groups were analyzed with one-way ANOVA (Figures 5 and 6), and the effects of both MI and Exe between the groups fed a HFD (Sham-Sed; MI-Sed; Sham-Exe; MI-Exe) were analyzed by two-way ANOVA. When all groups were compared, HFD did not affect the expression of genes involved in cardiac function and remodeling (Figure 5A,B), but Sham-Sed hearts had increased expression of genes involved in fibrosis (ColII, ColIII, Ddr2, Acta2, and Vim) and inflammation (Tnfa) (Figure 6 A,B). Both MI groups had significantly increased expression of genes involved in cardiac hypertrophy and heart failure. MI-Sed mice had increased expression of genes involved in contractile function (Myh6; Myh7βMHC; Serca2a) (Figure 5A), cardiac hypertrophy (Pi3k; Akt; Mtor; S6k; Erk2; Creb; Caln -Calcineurin; Nfat; FoxO1; Murf1) (Figure 5B), fibrosis (ColI; ColII; ColIII; Ddr2; Acta2; Mmp2; Vim) (Figure 6A), and inflammation (Tnfa; Tgfb; Il1b) (Figure 6B) compared to Chow-fed, Sham-Sed and Sham-Exe. There was a significant effect of MI when HFD groups were compared with two-way ANOVA (Supplemental Table 5). In MI-Exe mice, exercise negated the MI-induced increase in Myh7 and Serca2a genes (Figure 5A) and decreased the expression of Calcineurin compared to MI-Sed (Figure 5B, Supplemental Table 5). MI-Exe mice had increased expression of markers of heart failure including Bnp (Figure 5A) and Atrogin (Figure 5B), and the angiogenesis marker Vegfa (Figure 6A) compared to all other groups. These data suggest that exercise did not significantly protect the HFD mice from post-MI increases in expression of genes associated to cardiac remodeling, inflammation, and heart failure.
Figure 5. Exercise did not prevent MI-induced increase in cardiac remodeling gene expression.
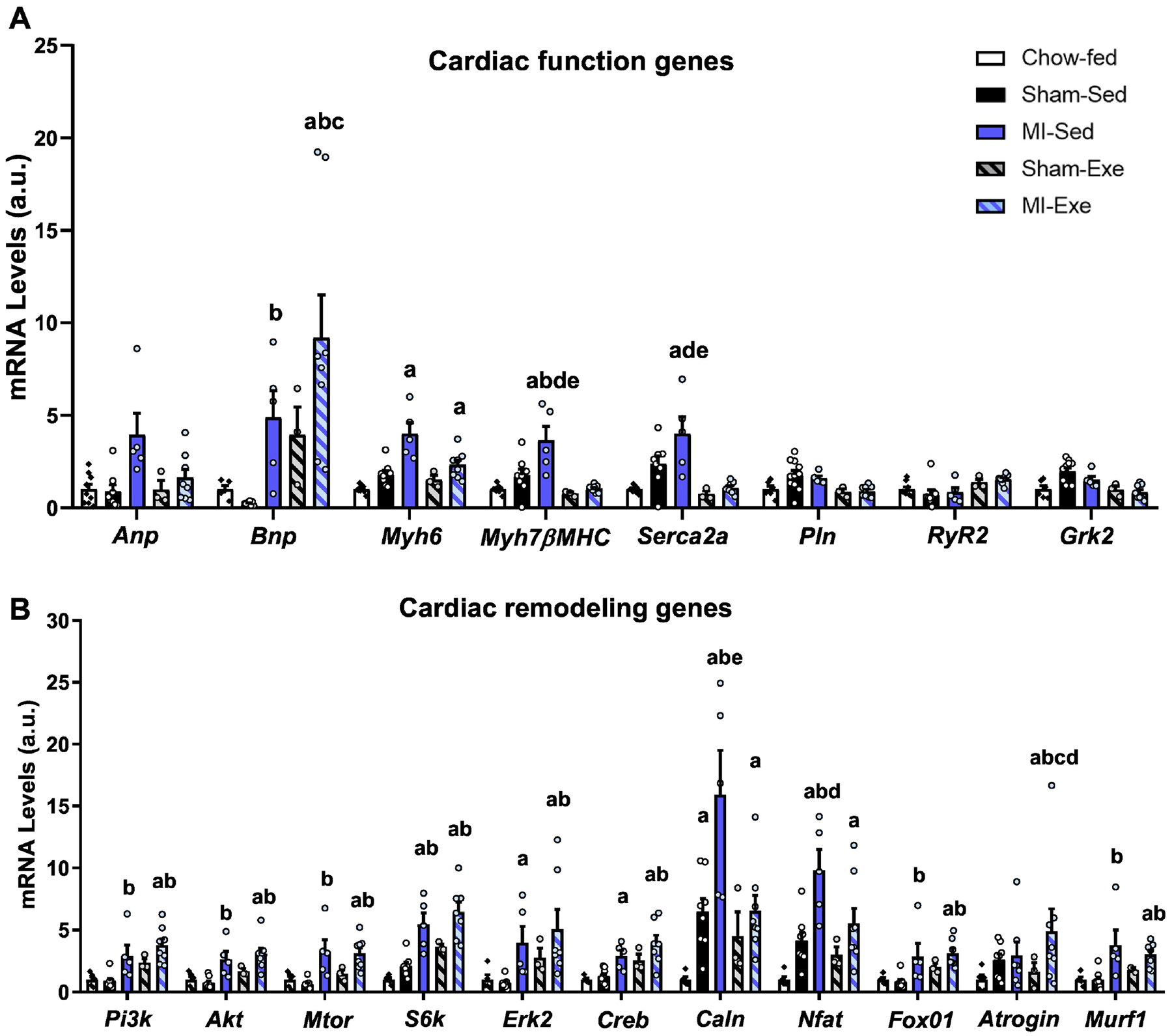
Quantitative PCR (qPCR) was performed on heart tissue isolated from mice that were sacrificed at 42 weeks of age (12 weeks post-MI). Genes (expressed as arbitrary units; A.U.) related to (A) cardiac function, and (B) remodeling were accessed in Chow-fed (n=5–11), Sham-Sed (n=8), MI-Sed (n=5), Sham-Exe (n=3), and MI-Exe (n=8). Data are presented as mean ± S.E.M. One-way ANOVA was used with Tukey’s multiple comparisons tests. Values of p<0.05 were considered statistically significant. Symbols represent difference vs. Chow-fed mice (a), difference vs Sham-Sed (b), difference vs MI-Sed (c), difference vs Sham-Exe (d), and difference vs. MI-Exe mice (e).
Figure 6. Exercise did not prevent MI-induced increase in cardiac inflammation gene expression and increased ER stress genes.

Quantitative PCR (qPCR) was performed on heart tissue isolated from mice that were sacrificed at 42 weeks of age (12 weeks post-MI). Genes (expressed as arbitrary units; A.U.) related to (A) fibrosis, (B) inflammation were accessed in Chow-fed (n=5–11), Sham-Sed (n=8), MI-Sed (n=5), Sham-Exe (n=4), and MI-Exe (n=11). Data are presented as mean ± S.E.M. One-way ANOVA was used with Tukey’s multiple comparisons tests. Values of p<0.05 were considered statistically significant. Symbols represent difference vs. Chow-fed mice (a), difference vs Sham-Sed (b), and difference vs Sham-Exe (d).
Genes involved in cardiac metabolism, including insulin signaling, glucose and lipid metabolism, mitochondrial biogenesis, and redox state, were also measured in HFD mice. HFD increased the expression of several metabolic and antioxidant genes in all groups compared to Chow-fed mice, including fatty acid binding protein (Fabp3) (Supplemental Figure 2B), Pgc1a (Supplemental Figure 2C), and the antioxidant enzymes Sod1 and Prdx1 (Supplemental Figure 3). Exercise had a minimal effect on expression of metabolic genes associated with a HFD, preventing HFD-induced increase only in the expression of the insulin receptor substrate Irs1 (Supplemental Figure 2A), and the mitochondrial biogenesis genes Nrf2 and Tfam (Supplemental Figure 2C), in the presence or absence of MI. Exercise also increased expression of the glucose metabolism genes Hk2 and Gpi1 Sham-Exe mice (Supplemental Figure 2A). These data indicate that HFD has a significant impact on the expression of genes related to cardiac metabolism, but there was no additive effect of MI. In addition, exercise has a minimal effect to alter the expression of metabolic genes to counteract the effects of an HFD, suggesting a limited contribution of exercise to alter expression of cardioprotective genes in HFD mice subjected to an MI.
Discussion
Cardiovascular diseases, including myocardial infarction, represent 31% of all deaths each year (36), and it is expected that the global burden of CVD and myocardial injury will grow exponentially over the next few years. Exercise is an important therapeutic tool to improve metabolic and cardiovascular health, but the beneficial effects of exercise on cardiovascular health of obese mice subjected to MI is not fully understood. In this study, mice were fed a high-fat diet composed mainly of saturated fats for 24 weeks, resulting in increased obesity, adiposity, impaired glucose tolerance, and LV hypertrophy. MI impaired cardiovascular health, and while aerobic exercise training increased the survival rate post-MI, it had a minimal effect on cardiovascular remodeling, fibrosis, obesity, or glucose metabolism.
In humans, the mortality post-MI is higher in patients who are overweight or have type 2 diabetes compared to patients who are normoglycemic (5, 29), and it is known that exercise after MI improves overall and cardiovascular-related mortality in patients with a BMI less than 28 (37, 38). However, since a large percentage of patients who have an MI are obese (BMI >30), investigating the role of exercise in a mouse model of obesity is of critical translational importance. In our mouse model of diet-induced obesity, exercise training increased the survival in obese mice subjected to MI. These data corroborate previous findings in rodents who are lean or have type 1 diabetes where exercise training after MI also decreased the mortality rate (39–41). The reasons for this are not clear, but increased survival post MI in exercised models have been associated with improved autonomic function, baroreflex sensitivity and heart rate variability (39, 41). It is possible that moderate intensity aerobic exercise training increased survival post-MI by preventing the development of severe loss of heart function and cardiac death. This finding is noteworthy because it shows that exercise prevented post-MI mortality in obese mice independent of improvements in glucose metabolism and or reductions in fat mass, which were not significantly altered by exercise training.
Exercise-based cardiac rehabilitation (CR) is recognized as an important strategy to improve the recovery of patients who suffer an MI (8, 38). Increased levels of physical activity are associated with lower fatal cardiac events (9, 11, 42), reduced infarct size, and preservation of cardiac function (9, 11, 42). However, patients who are obese or have type 2 diabetes have a blunted response to exercise compared to patients who are non-obese or normoglycemic (12–16). The reason for this lack of beneficial response is unknown, but several mechanisms have been hypothesized. It is known that obesity and T2DM are associated with several adverse cardiovascular conditions. It has also been suggested that high blood glucose impairs exercise-induced cardiovascular adaptations in patients with diabetes (15, 16, 43). Patients who are obese have excessive adipose tissue mass and increased blood volume and LV afterload to the ventricles, which can increase wall thickness and dilation of these chambers (44). This suggests that the presence of obesity-induced cardiovascular impairments is an important parameter to consider in patients who show decreased exercise-induced adaptations post-MI.
In pre-clinical models, the link between obesity, cardiovascular remodeling, cardiac fibrosis, and the protective role of exercise is well established (45–47), but the effects of HFD and exercise on cardiac remodeling and fibrosis after an MI have not been investigated in obese mice. In our study, we found that a HFD rich in saturated fats increased the cardiac fibrosis area, and the expression of several fibrosis genes, including ColI, ColII, ColII, Ddr2, Acta2, and Vim. Exercise training reduced cardiac fibrosis only in the absence of MI, independent of any effects on cardiovascular health or remodeling. This indicates that exercise protects the heart of HFD-fed mice from fibrosis, but it did not affect cardiac function. Our data correlates with previous studies that observed that low intensity exercise training did not improve post-MI cardiac remodeling in rats fed a HFD (43), and decreased cardiac fibrosis in a mouse model of metabolic syndrome, but cardiac performance was not improved (48). While there is data from chow-fed, lean mice, to indicate that aerobic exercise training reduces cardiac fibrosis and prevents pathological cardiac hypertrophy after an MI (19, 20), our data indicates that exercise alone is not an effective intervention to prevent the progression of cardiac fibrosis or adverse remodeling in the presence of chronic HFD and MI.
To investigate potential mechanisms for the role of exercise training on cardiac parameters of obese mice subjected to MI, we assessed the expression of more than 50 genes associated with cardiac remodeling and heart failure, including brain natriuretic peptide (Bnp) and atrial natriuretic peptide (Anp). Bnp expression was increased in the MI-Exe mice compared to all chow, Sham-Sed, and MI mice, but Anp was only increased in the MI mice. Importantly, ANP and BNP increase in response to cardiac stress after an MI and are important markers of development of heart failure (49). Previous studies have shown that in lean MI mice, exercise decreased BNP levels and prevented pathological cardiac remodeling (50, 51). In contrast, in mice fed a high-fat diet, exercise impaired diet-induced cardiac remodeling and increased cardiac expression of ANP and BNP (52). In our study, we see that exercise after MI increased BNP, but blunted the MI-induced increase in ANP. This indicates that the effects of exercise training in the expression of natriuretic peptides is affected by the consumption of a high-fat diet. Future studies are needed to fully elucidate the role of exercise on ANP and BNP after MI.
Exercise training prevented the mRNA upregulation of the heart failure marker β-Myosin heavy chain 7 (Myh7βMHC) after an MI (MI-Exe mice). This is important as Myh7βMHC has been identified as a definitive marker in diagnostic gene panels of cardiomyopathy (53) and associated with sudden cardiac death in patients with hypertrophic cardiomyopathy (54). A previous study showed that swimming decreased Myh7βMHC in a rodent model of heart failure, partially preventing pathological cardiac remodeling (55), suggesting that decreased Myh7βMHC expression may be involved in beneficial cardiac effects of exercise training in obese mice. These results should be interpreted with caution, since exercise training did not affect in vivo cardiac parameters in MI mice, and further mechanistic investigation is needed to explain the role exercise training in the cardiac health of HFD-fed mice subjected to MI.
Our findings contrast previous studies showing that treadmill aerobic exercise training performed prior to temporary coronary occlusion in ischemia-reperfusion (IR) were effective in overcoming the deleterious effects of MI in obese models (56–58). In that case, mice fed a HFD for 12 weeks and exercised for 4 weeks before IR (56–58) accelerated the recovery of myocardial tissue oxygenation, decreased inflammation and fibrosis accumulation, and improved cardiac contractile response (56–58). These divergent findings could be due to different onset of exercise training, cardiac ischemia techniques, and extent of diet-induced obesity. In the current study, MI was induced through permanent LAD occlusion, which results in complete blockade of blood flow and irreversible hypoxia, leading to tissue necrosis and a permanent scar (59). Permanent LAD closely recapitulates the human responses to acute cardiac injury, the repercussion on the organic systems, as well as the progression to heart failure (60). Moreover, HFD started 6 months before the induction of MI, and it was uninterrupted throughout the entire study. Thus, whereas exercise training performed before the onset of MI shows beneficial effects in preventing cardiac dysfunction and remodeling from temporary occlusion in mice fed a HFD for 12 weeks, its therapeutic benefits are limited in a model of permanent occlusion and sustained HFD. These findings highlight the parallel between our rodent model and patients that are chronically obese and sedentary, and fail to comply to important dietary changes after suffering an MI. In fact, increased sedentary time is significantly associated with higher cardiac dysfunction among MI survivors (61), and dietary noncompliance is implicated in nearly one-fourth of all 30-day post-MI hospital readmissions (62). Therefore, more studies are needed to develop clinically effective therapeutic approaches for these patients.
There are some limitations to the current study. Because the incidence of MI, obesity and type 2 diabetes are significantly higher in male (63–65), the current study was performed in male mice, thus it is not clear if these data would be applicable in females. Additionally, we did not alter the diet, perform reperfusion studies, or induce any pharmacological treatments after MI, thus it is not known if exercise would be more beneficial in the presence of multiple therapeutic interventions. Finally, the assessment of LV function and dimensions was done through M-Mode echocardiography. M-mode echo relies on geometric assumptions that can be overestimated in patients and rodent models with LV remodeling (66). Further studies are needed to access the post-MI cardiac function with other non-invasive methods, including 2D echo evaluated by Simpson’s biplane method and cardiovascular magnetic resonance.
In summary, this rodent investigation indicates that the beneficial effects of exercise on cardiovascular health after an MI are blunted in a mouse model of obesity or glucose intolerance. These data have significant translational relevance because suggests that the impact of exercise to restore cardiac function appears to be limited in previously sedentary subjects with irreversible cardiac damage following MI, and when poor dietary choices are not resolved. As the incidence of MI in patients younger than 40 years-old is less than 10% (67, 68), and ischemic heart disease and post-MI heart failure occurs predominantly in patients who are >45 years old with increased cardiometabolic risk factors, obesity and type 2 diabetes (67, 69), further experimental and clinical studies are needed to provide strategies that will optimize exercise training as a therapeutical approach for these populations.
Supplementary Material
Key Points.
Highlights
Exercise increases survival in high-fat fed mice after a myocardial infarction.
Exercise has a minimal effect on left ventricular remodeling in high-fat fed mice after a myocardial infarction.
As obesity increases the risk for development of heart failure development post-myocardial infarction, these data provide insight into the role of exercise as a therapeutic approach in patients who are obese after an MI.
Acknowledgements
This work was supported by National Institutes of Health (NIH) Grants R01-HL138738 to K.I.S.; R01-AG060542 to K.I.S. and M.T.Z.; K.M.P. was supported by NIH F31-HL152648-01A1 and K.M.P and S.L.S. were supported by NIH T32-HL134616. P.V. was supported by an American Heart Association predoctoral award AHAPRE903654. The authors thank Dr. Loren E. Wold for critical discussions regarding this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Declaration of Interests
The authors declare no conflicts of interest.
References
- 1.Cawley J, Biener A, Meyerhoefer C, Ding Y, Zvenyach T, Smolarz BG, et al. Direct medical costs of obesity in the United States and the most populous states. J Manag Care Spec Pharm. 2021;27(3):354–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief. 2020(360):1–8. [PubMed] [Google Scholar]
- 3.Kragelund C, Hassager C, Hildebrandt P, Torp-Pedersen C, Køber L. Impact of obesity on long-term prognosis following acute myocardial infarction. Int J Cardiol. 2005;98(1):123–31. [DOI] [PubMed] [Google Scholar]
- 4.Nirengi S, Peres Valgas da Silva C, Stanford KI. Disruption of energy utilization in diabetic cardiomyopathy; a mini review. Curr Opin Pharmacol. 2020;54:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–34. [DOI] [PubMed] [Google Scholar]
- 6.Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016;365(3):563–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–8. [DOI] [PubMed] [Google Scholar]
- 8.Pinckard K, Baskin KK, Stanford KI. Effects of Exercise to Improve Cardiovascular Health. Front Cardiovasc Med. 2019;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haykowsky M, Scott J, Esch B, Schopflocher D, Myers J, Paterson I, et al. A meta-analysis of the effects of exercise training on left ventricular remodeling following myocardial infarction: start early and go longer for greatest exercise benefits on remodeling. Trials. 2011;12:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannuzzi P, Temporelli PL, Corrà U, Gattone M, Giordano A, Tavazzi L. Attenuation of unfavorable remodeling by exercise training in postinfarction patients with left ventricular dysfunction: results of the Exercise in Left Ventricular Dysfunction (ELVD) trial. Circulation. 1997;96(6):1790–7. [DOI] [PubMed] [Google Scholar]
- 11.Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, et al. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2016;67(1):1–12. [DOI] [PubMed] [Google Scholar]
- 12.Izawa K, Tanabe K, Omiya K, Yamada S, Yokoyama Y, Ishiguro T, et al. Impaired chronotropic response to exercise in acute myocardial infarction patients with type 2 diabetes mellitus. Jpn Heart J. 2003;44(2):187–99. [DOI] [PubMed] [Google Scholar]
- 13.Banks AZ, Mentz RJ, Stebbins A, Mikus CR, Schulte PJ, Fleg JL, et al. Response to Exercise Training and Outcomes in Patients With Heart Failure and Diabetes Mellitus: Insights From the HF-ACTION Trial. J Card Fail. 2016;22(7):485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergès B, Patois-Vergès B, Cohen M, Lucas B, Galland-Jos C, Casillas JM. Effects of cardiac rehabilitation on exercise capacity in Type 2 diabetic patients with coronary artery disease. Diabet Med. 2004;21(8):889–95. [DOI] [PubMed] [Google Scholar]
- 15.Soleimani A, Nejatian M, Hajizaynali MA, Abbasi SH, Alidoosti M, Sheikhfathollahi M, et al. Effect of gender and type 2 diabetes mellitus on heart rate recovery in patients with coronary artery disease after cardiac rehabilitation. Endokrynol Pol. 2009;60(6):430–6. [PubMed] [Google Scholar]
- 16.Gadager BB, Tang LH, Ravn MB, Doherty P, Harrison A, Christensen J, et al. Benefits of cardiac rehabilitation following acute coronary syndrome for patients with and without diabetes: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2022;22(1):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Waard MC, van der Velden J, Bito V, Ozdemir S, Biesmans L, Boontje NM, et al. Early exercise training normalizes myofilament function and attenuates left ventricular pump dysfunction in mice with a large myocardial infarction. Circ Res. 2007;100(7):1079–88. [DOI] [PubMed] [Google Scholar]
- 18.van Deel ED, Octavia Y, de Waard MC, de Boer M, Duncker DJ. Exercise Training Has Contrasting Effects in Myocardial Infarction and Pressure Overload Due to Divergent Endothelial Nitric Oxide Synthase Regulation. Int J Mol Sci. 2018;19(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puhl SL, Müller A, Wagner M, Devaux Y, Böhm M, Wagner DR, et al. Exercise attenuates inflammation and limits scar thinning after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2015;309(2):H345–59. [DOI] [PubMed] [Google Scholar]
- 20.Liao Z, Li D, Chen Y, Li Y, Huang R, Zhu K, et al. Early moderate exercise benefits myocardial infarction healing via improvement of inflammation and ventricular remodelling in rats. J Cell Mol Med. 2019;23(12):8328–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shettigar V, Zhang B, Little SC, Salhi HE, Hansen BJ, Li N, et al. Rationally engineered Troponin C modulates in vivo cardiac function and performance in health and disease. Nat Commun. 2016;7:10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degabriele NM, Griesenbach U, Sato K, Post MJ, Zhu J, Williams J, et al. Critical appraisal of the mouse model of myocardial infarction. Exp Physiol. 2004;89(4):497–505. [DOI] [PubMed] [Google Scholar]
- 23.Peres Valgas da Silva C, Shettigar VK, Baer LA, Abay E, Madaris KL, Mehling MR, et al. Brown adipose tissue prevents glucose intolerance and cardiac remodeling in high-fat-fed mice after a mild myocardial infarction. Int J Obes (Lond). 2022;46(2):350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrosino JM, Heiss VJ, Maurya SK, Kalyanasundaram A, Periasamy M, LaFountain RA, et al. Graded Maximal Exercise Testing to Assess Mouse Cardio-Metabolic Phenotypes. PLoS One. 2016;11(2):e0148010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123(1):215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lessard SJ, Rivas DA, Alves-Wagner AB, Hirshman MF, Gallagher IJ, Constantin-Teodosiu D, et al. Resistance to aerobic exercise training causes metabolic dysfunction and reveals novel exercise-regulated signaling networks. Diabetes. 2013;62(8):2717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehnig AC, Dewal RS, Baer LA, Kitching KM, Munoz VR, Arts PJ, et al. Exercise Training Induces Depot-Specific Adaptations to White and Brown Adipose Tissue. iScience. 2019;11:425–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gratz D, Winkle AJ, Dalic A, Unudurthi SD, Hund TJ. Computational tools for automated histological image analysis and quantification in cardiac tissue. MethodsX. 2020;7:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhl J, Jörneskog G, Wemminger M, Bengtsson M, Lundman P, Kalani M. Long-term clinical outcome in patients with acute coronary syndrome and dysglycaemia. Cardiovasc Diabetol. 2015;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekblom O, Ek A, Cider Å, Hambraeus K, Börjesson M. Increased Physical Activity Post-Myocardial Infarction Is Related to Reduced Mortality: Results From the SWEDEHEART Registry. J Am Heart Assoc. 2018;7(24):e010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatt AS, Ambrosy AP, Velazquez EJ. Adverse Remodeling and Reverse Remodeling After Myocardial Infarction. Curr Cardiol Rep. 2017;19(8):71. [DOI] [PubMed] [Google Scholar]
- 32.Cahill TJ, Kharbanda RK. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: Mechanisms, incidence and identification of patients at risk. World J Cardiol. 2017;9(5):407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruzdeva O, Uchasova E, Dyleva Y, Borodkina D, Akbasheva O, Belik E, et al. Relationships between epicardial adipose tissue thickness and adipo-fibrokine indicator profiles post-myocardial infarction. Cardiovasc Diabetol. 2018;17(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Shan S, Lyu A, Wan Y, Zhang J. A novel model of myocardial infarction based on atherosclerosis in mice. Biochem Biophys Res Commun. 2021;576:100–7. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Liang J, Bin W, Luo H, Yang X. Anti-hyperlipidemic, Anti-inflammatory, and Ameliorative Effects of DRP1 Inhibition in Rats with Experimentally Induced Myocardial Infarction. Cardiovasc Toxicol. 2021;21(12):1000–11. [DOI] [PubMed] [Google Scholar]
- 36.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chow CK, Jolly S, Rao-Melacini P, Fox KA, Anand SS, Yusuf S. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation. 2010;121(6):750–8. [DOI] [PubMed] [Google Scholar]
- 38.Ekblom Ö, Cider Å, Hambraeus K, Bäck M, Leosdottir M, Lönn A, et al. Participation in exercise-based cardiac rehabilitation is related to reduced total mortality in both men and women: results from the SWEDEHEART registry. Eur J Prev Cardiol. 2022;29(3):485–92. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues B, Jorge L, Mostarda CT, Rosa KT, Medeiros A, Malfitano C, et al. Aerobic exercise training delays cardiac dysfunction and improves autonomic control of circulation in diabetic rats undergoing myocardial infarction. J Card Fail. 2012;18(9):734–44. [DOI] [PubMed] [Google Scholar]
- 40.Jorge L, Rodrigues B, Rosa KT, Malfitano C, Loureiro TC, Medeiros A, et al. Cardiac and peripheral adjustments induced by early exercise training intervention were associated with autonomic improvement in infarcted rats: role in functional capacity and mortality. Eur Heart J. 2011;32(7):904–12. [DOI] [PubMed] [Google Scholar]
- 41.Barboza CA, Rocha LY, Mostarda CT, Figueroa D, Caperuto EC, De Angelis K, et al. Ventricular and autonomic benefits of exercise training persist after detraining in infarcted rats. Eur J Appl Physiol. 2013;113(5):1137–46. [DOI] [PubMed] [Google Scholar]
- 42.Thijssen DHJ, Redington A, George KP, Hopman MTE, Jones H. Association of Exercise Preconditioning With Immediate Cardioprotection: A Review. JAMA Cardiol. 2018;3(2):169–76. [DOI] [PubMed] [Google Scholar]
- 43.Boudia D, Domergue V, Mateo P, Fazal L, Prud’homme M, Prigent H, et al. Beneficial effects of exercise training in heart failure are lost in male diabetic rats. J Appl Physiol (1985). 2017;123(6):1579–91. [DOI] [PubMed] [Google Scholar]
- 44.Ebong IA, Goff DC Jr., Rodriguez CJ, Chen H, Bertoni AG. Mechanisms of heart failure in obesity. Obes Res Clin Pract. 2014;8(6):e540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Li L, Zhao H, Peng S, Zuo Z. Chronic high fat diet induces cardiac hypertrophy and fibrosis in mice. Metabolism. 2015;64(8):917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lavie CJ, Johannsen N, Swift D, Sénéchal M, Earnest C, Church T, et al. Exercise is Medicine - The Importance of Physical Activity, Exercise Training, Cardiorespiratory Fitness and Obesity in the Prevention and Treatment of Type 2 Diabetes. Eur Endocrinol. 2014;10(1):18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leite RD, Durigan Rde C, de Souza Lino AD, de Souza Campos MV, Souza M, Selistre-de-Araújo HS, et al. Resistance training may concomitantly benefit body composition, blood pressure and muscle MMP-2 activity on the left ventricle of high-fat fed diet rats. Metabolism. 2013;62(10):1477–84. [DOI] [PubMed] [Google Scholar]
- 48.Tóth ME, Sárközy M, Szűcs G, Dukay B, Hajdu P, Zvara Á, et al. Exercise training worsens cardiac performance in males but does not change ejection fraction and improves hypertrophy in females in a mouse model of metabolic syndrome. Biol Sex Differ. 2022;13(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuwahara K The natriuretic peptide system in heart failure: Diagnostic and therapeutic implications. Pharmacol Ther. 2021;227:107863. [DOI] [PubMed] [Google Scholar]
- 50.Yoshinari K, Yaoita H, Maehara K, Maruyama Y. Different therapeutic responses to treadmill exercise of heart failure due to ischemia and infarction in rats. Cardiovasc Res. 2005;65(2):457–68. [DOI] [PubMed] [Google Scholar]
- 51.Wolff AM, Rasmussen TP, Wichern CR, Peterson MR, Stayton MM, Thomas DP. Effects of pericardiectomy on training- and myocardial infarction-induced left ventricular hypertrophy, chamber dimensions and gene expression. Int J Sports Med. 2017;38(1):27–34. [DOI] [PubMed] [Google Scholar]
- 52.Yan Z, Kronemberger A, Blomme J, Call JA, Caster HM, Pereira RO, et al. Exercise leads to unfavourable cardiac remodelling and enhanced metabolic homeostasis in obese mice with cardiac and skeletal muscle autophagy deficiency. Sci Rep. 2017;7(1):7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jordan E, Peterson L, Ai T, Asatryan B, Bronicki L, Brown E, et al. Evidence-Based Assessment of Genes in Dilated Cardiomyopathy. Circulation. 2021;144(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mori AA, Castro LR, Bortolin RH, Bastos GM, Oliveira VF, Ferreira GM, et al. Association of variants in MYH7, MYBPC3 and TNNT2 with sudden cardiac death-related risk factors in Brazilian patients with hypertrophic cardiomyopathy. Forensic Sci Int Genet. 2021;52:102478. [DOI] [PubMed] [Google Scholar]
- 55.Lin H, Zhu Y, Zheng C, Hu D, Ma S, Chen L, et al. Antihypertrophic Memory After Regression of Exercise-Induced Physiological Myocardial Hypertrophy Is Mediated by the Long Noncoding RNA Mhrt779. Circulation. 2021;143(23):2277–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Musman J, Pons S, Barau C, Caccia C, Leoni V, Berdeaux A, et al. Regular treadmill exercise inhibits mitochondrial accumulation of cholesterol and oxysterols during myocardial ischemia-reperfusion in wild-type and ob/ob mice. Free Radic Biol Med. 2016;101:317–24. [DOI] [PubMed] [Google Scholar]
- 57.Kleindienst A, Battault S, Belaidi E, Tanguy S, Rosselin M, Boulghobra D, et al. Exercise does not activate the β3 adrenergic receptor-eNOS pathway, but reduces inducible NOS expression to protect the heart of obese diabetic mice. Basic Res Cardiol. 2016;111(4):40. [DOI] [PubMed] [Google Scholar]
- 58.Lund J, Hafstad AD, Boardman NT, Rossvoll L, Rolim NP, Ahmed MS, et al. Exercise training promotes cardioprotection through oxygen-sparing action in high fat-fed mice. Am J Physiol Heart Circ Physiol. 2015;308(8):H823–9. [DOI] [PubMed] [Google Scholar]
- 59.Moraes-Silva IC, Rodrigues B, Coelho-Junior HJ, Feriani DJ, Irigoyen MC. Myocardial Infarction and Exercise Training: Evidence from Basic Science. Adv Exp Med Biol. 2017;999:139–53. [DOI] [PubMed] [Google Scholar]
- 60.De Villiers C, Riley PR. Mouse models of myocardial infarction: comparing permanent ligation and ischaemia-reperfusion. Dis Model Mech. 2020;13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Z, Huang Z, Wu Y, Huang S, Wang Y, Zhao H, et al. Sedentary time, metabolic abnormalities, and all-cause mortality after myocardial infarction: A mediation analysis. Eur J Prev Cardiol. 2019;26(1):96–104. [DOI] [PubMed] [Google Scholar]
- 62.Razavi AC, Monlezun DJ, Sapin A, Sarris L, Schlag E, Dyer A, et al. Etiological Role of Diet in 30-Day Readmissions for Heart Failure: Implications for Reducing Heart Failure-Associated Costs via Culinary Medicine. American journal of lifestyle medicine. 2020;14(4):351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.González KA, Stickel AM, Kaur SS, Ramos AR, González HM, Tarraf W. Serum Cystatin-C is linked to increased prevalence of diabetes and higher risk of mortality in diverse middle-aged and older adults. PLoS One. 2022;17(9):e0270289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nordström A, Hadrévi J, Olsson T, Franks PW, Nordström P. Higher Prevalence of Type 2 Diabetes in Men Than in Women Is Associated With Differences in Visceral Fat Mass. J Clin Endocrinol Metab. 2016;101(10):3740–6. [DOI] [PubMed] [Google Scholar]
- 65.Zhu Y, Sidell MA, Arterburn D, Daley MF, Desai J, Fitzpatrick SL, et al. Racial/Ethnic Disparities in the Prevalence of Diabetes and Prediabetes by BMI: Patient Outcomes Research To Advance Learning (PORTAL) Multisite Cohort of Adults in the U.S. Diabetes Care. 2019;42(12):2211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindsey ML, Kassiri Z, Virag JAI, de Castro Brás LE, Scherrer-Crosbie M. Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol. 2018;314(4):H733–h52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jortveit J, Pripp AH, Langørgen J, Halvorsen S. Incidence, risk factors and outcome of young patients with myocardial infarction. Heart (British Cardiac Society). 2020;106(18):1420–6. [DOI] [PubMed] [Google Scholar]
- 68.Doughty M, Mehta R, Bruckman D, Das S, Karavite D, Tsai T, et al. Acute myocardial infarction in the young--The University of Michigan experience. American heart journal. 2002;143(1):56–62. [DOI] [PubMed] [Google Scholar]
- 69.Abate SM, Mantefardo B, Nega S, Chekole YA, Basu B, Ali SA, et al. Global burden of acute myocardial injury associated with COVID-19: A systematic review, meta-analysis, and meta-regression. Annals of medicine and surgery (2012). 2021;68:102594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


