Abstract
Many end-stage liver disease etiologies are attributed to robust inflammatory cell recruitment. Neutrophils play an important role in inflammatory infiltration and neutrophil phagocytosis, oxidative burst, and degranulation. It has also been suggested that neutrophils may release neutrophil extracellular traps (NETs) to kill pathogens. It has been proven that neutrophil infiltration within the liver contributes to an inflammatory microenvironment and immune cell activation. Growing evidence implies that NETs are involved in the progression of numerous complications of liver transplantation, including ischemia-reperfusion injury, acute rejection, thrombosis, and hepatocellular carcinoma recurrence. NETs are discussed in this comprehensive review, focusing on their effects on liver transplantation complications. Furthermore, we discuss NETs as potential targets for liver transplantation therapy.
Keywords: neutrophil extracellular traps, liver transplantation, ischemia-reperfusion injury, acute rejection, thrombosis, hepatocellular carcinoma recurrence, therapeutic targets
Introduction
Neutrophils play a major role in the innate immune response (1), and have a wide range of immune functions, including phagocytosis, reactive oxygen species (ROS) production, lytic enzyme activation, and neutrophil extracellular traps (NETs) production through a process called NETosis (2, 3). NETs comprise chromatin, DNA fibers, and granule proteins. Additionally, NETs are important in treating non-infectious diseases, such as cancer, diabetes, thrombosis, and autoimmune illnesses (4–6). Recent evidence suggests that NETs may contribute to pathological changes after liver transplantation, including liver ischemia-reperfusion injury (IRI), acute rejection, and recurrence of hepatocellular carcinoma (7–9). However, there is little knowledge of the relationship between NET formation and complications of liver transplantation. Herein, we summarize the latest findings that associate NETs with liver IRI, acute rejection, thrombosis, and hepatocellular carcinoma recurrence. We also discuss the potential of NET as a potential therapeutic target in patients following liver transplantation. NET targeting and degradation could be novel promising therapeutic interventions in end-stage liver disease and complications of liver transplantation.
NET formation
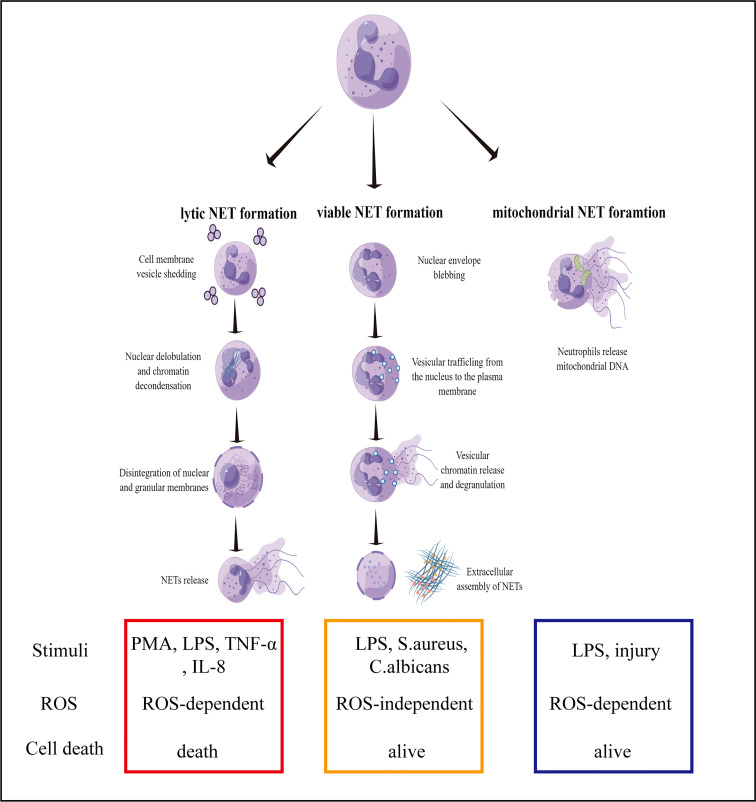
A novel immune defense mechanism known as NETs was discovered in 2004. However, it is difficult to clearly define the specific function of NETs in immune defense (10, 11). With regards to neutrophil pathogenic stimulation, the activation of the signaling pathways, and membrane integrity, the formation of NETs can be classified into three types, namely, lytic, viable, and mitochondrial NET formation (12). Lytic NETs are formed within ten minutes from neutrophil stimulation with phorbol myristate acetate (PMA), lipopolysaccharide (LPS), or IL-8 (13). Several pathways lead to the formation of lytic NETs, including ROS generation in neutrophils that lead to the activation of the enzyme, peptidylarginine deiminase 4 (PAD4). Subsequently, PAD4 converts arginine residues on histones into citrulline, which results in chromatin decondensation (14, 15). In addition, neutrophil elastase (NE) and myeloperoxidase (MPO) are activated and translocated to the nucleus. NE and MPO are also synergistically involved in chromatin decondensation. Likewise, NE can also degrade actin filaments, and block the phagocytosis pathway (16). Single-stranded DNAs and histones are released in the cytoplasm, and form early NETs with antibacterial proteins (e.g., MPO, citH3, NE, and cathepsin G) (17). NET formation requires NADPH oxidase activity and downstream ROS formation (18). Lytic NET formation can be induced by bacteria, fungi and especially chemical stimuli, such as LPS, TNF-α and IL-8 (19). Several in vitro studies showed neutrophils formed NET-like structures in response to PMA, LPS, TNF-α and IL-8. In these cells, pretreatment with CI-amidine or use of a PAD4-deficinet line reduced citrullination of histones and NET formation (20, 21).
In viable NET formation, PAD4 is activated by TLR-2 and TLR-4 receptors on neutrophils under different stimuli. For example, bacterial LPS results in the entry of PAD4 into the nucleus to citrullinate the histones, H3 and H4, and unwind DNA strands (22, 23). In contrast to the formation of lytic NET, the PAD4 gene is activated without ROS and does not rupture the cell or nuclear membrane. During the process, the neutrophils are not destroyed, and unwound DNA strands enter the cytoplasm to form early NETs with bacteriostatic proteins. As fascicles, they are exocytosed and released from the cell. Despite the absence of nuclear DNA, neutrophils are still capable of phagocytosing bacteria and killing them (24, 25).
The third mechanism describes the formation of NETs with mitochondrial DNA. A previous study demonstrated that eosinophils release mitochondrial DNA after the initial priming with IL-5 or INF-γ, and subsequent LPS stimulation (26). DNA release is ROS-dependent and independent of eosinophil apoptosis. In a subsequent study, mitochondrial NETs were reported to be damaged after the neutrophils were primed with GM-CSF for 20 minutes and then stimulated with LPS for another 15 minutes, which resulted in the release of DNA into the extracellular matrix (27). Neutrophil granular proteins, such as MPO and NE, were also detected in the extracellular matrix with the DNA, but nuclear proteins were not found. It was later reported that NETs contained mitochondrial DNA rather than nuclear DNA sequences (28). Unlike viable NETs, the formation of mitochondrial NETs is ROS-dependent, because the effects of ROS inhibitors and neutrophil deficiency inhibit the release of NETs (29). Recent studies further elucidated the importance of the post-translational modifications of histones, which has a biphasic impact on NETs formation (30). Another study found that the pre-forming protein gasdermin D (GSDMD) plays a key role in neutrophil membrane lysis, nuclear membrane development, and NETs formation (31). However, still unclear about the involvement of PAD4 activation and the presence of mitochondrial DNA in the formation and structure of NETs. To conclude, the mechanism behind the generation and release of NETs requires further investigation ( Figure 1 ).
Figure 1.
Mechanisms of NET formation. Three mechanisms of NET formation have been described: lytic NET formation, viable NET formation, and mitochondrial NET formation.
NETs function
Researchers have demonstrated that NETs have a wide range of efficacy against bacteria, viruses, fungi, and parasites. Several components in NETs, including histones, contain bactericidal and antimicrobial properties (32). NE, a granular protein, can also degrade certain bacterial virulence factors (33). According to prior studies, a fibrous NET structure enhances its bactericidal activity, by either concentrating the antimicrobial molecules into a small area or even serving as a physical barrier against microorganisms (34). Despite NETs’ ability to fight infections, it was soon realized that they were also detrimental to gastrointestinal, liver, and lung inflammations (35, 36). Activated neutrophils co-cultured with enterocyte-like Caco-2 cells revealed that NETs might damage epithelial cells by directly binding to their proteases (37). The researchers also proposed that NETs could facilitate the attachment of enteropathogenic E. coli to the mucosa by causeing damage to the intestinal mucosal barrier (38). Inflammation-associated lung damage and fibrosis are linked to NETs. A recent study revealed postmortem that the four patients who died of COVID-19, each had NETs in their lungs. Airway compartments and neutrophil-rich inflammatory areas of the interstitium contained NETs, while the arteriolar microthrombi contained NET-prone primed neutrophils (39). Murao A, et al. reported that the extracellular cold inducible RNA-binding protein (eCIRP)/TREM-1 interaction and Rho activation are expected to support the development of novel therapeutic molecules able to mitigate inflammation and sepsis by comtrolling NET formation (40).
NETs are considered to be double-edged swords in innate immunity. Because NETs play both an antibacterial and anti-infective role in the early stages of pathogenic microorganism invasion. However, excessive deposition and clearance disorder can lead to inflammation and immune damage to target organs (4, 16, 18, 41). Surgical stress, including liver resection and liver resection and liver transplantation that lead to NET formation. Yazdani et al. found that NET formation was decreased in IL-33 KO mice. IL-33 deficiency protected livers from I/R injury, whereas rIL-33 administration during I/R exacerbated hepatotoxicity and systemic inflammation. In vitro, IL-33 mainly released from liver sinusoidal endothelial cells, causes excessive sterile inflammation after hepatic I/R by inducing NET formation (42).
Thrombosis is the formation of a blood clot from the actions of platelets and coagulation factors in the events of vascular damage. A thrombus is formed when coagulation is activated, and fibrinolytic activity is decreased, thereby causing vasculitides to block and disrupt the blood supply to the tissues (43–45). Recent studies have reported the presence of neutrophils and NETs in the thrombus of humans and mice (46). In addition, NETs have been found to stimulate both internal and external coagulation pathways that promote thrombosisby providing a scaffold for the deposition of fibrinogen, platelets, von Willebrand factors, and erythrocytes (47). NETs also promote the deposition of thrombogenic substances. As platelets aggregate and become activated in NETs, the histones interact with fibrinogen, TLR2, and TLR4, to generate thrombin (48). In mouse models, DNase I can effectively prevent intravascular microthrombosis, which suggests a key role of NETs in thrombosis (49). However, another study argued that NETs promote thrombosis through their DNA and histone components instead of the deposition approach (50). That said, further studies are required to fully understand the promoter role of NETs in thrombosis.
Over the past few years, NETs have attracted increasing attention due to their essential role in innate immunology and thrombosis. However, there is also evidence that NETs play a pro-tumorigenic role in cancer (51). A growing number of studies are looking into the potential diagnostic and prognostic values of circulating NETs (52). The deposition of NETs promotes tumor cell proliferation, immunosuppression, and cancer-associated thrombosis. In addition, NETs can accelerate metastasis by contributing to epithelial-to-mesenchymal transition. NETs collect and multiply circulating tumor cells, resulting in tumor cell intravasation and micrometastases (53). At the same time, post-operative infections can increase NETs deposition, which exacerbates the recurrence and progression of post-surgical cancer (54). Considering their integral role in cancer, NETs could be potential therapeutic targets to inhibit tumor cell proliferation, metastasis, and thrombosis.
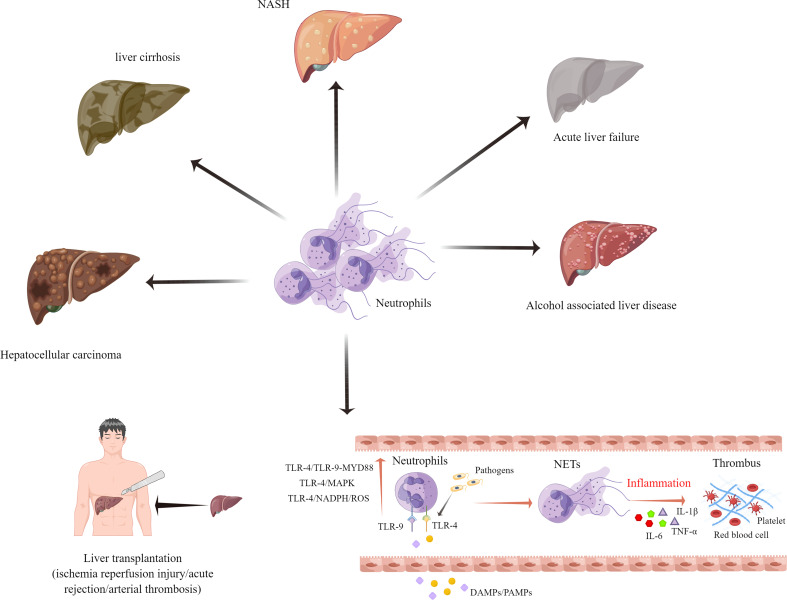
With deepening research in the field of NETs in liver transplantation, multiple studies discovered that DAMPs, including HMGB1 and histones or superoxide released during liver IRI, related in NETs formation. TLR-4 and/or TLR-9-myeloid differentiation primary response protein signaling pathways stimulated by HMGB1 and histones, respectively, are thought to exacerbate liver IRI (7, 55, 56). Our study founded that NETs promote kupffer cell M1 polarization and intracellular translocation of HMGB1 aggravating liver IRI even cause acute graft rejection following liver transplantation (57).
NETs detection
Whilst the importance of NETs has been highlighted in innate immunity, it is a challenge to detect NETs due to their heterogenous and acellular structure (58). Moreover, primary human neutrophils cannot be transfected for mechanistic interrelation studies, further complicating NET-related studies (59). Besides that, NETs must be distinguished from cell-free DNA (cfDNA), which originates independently of necrosis and apoptosis (60). Hence, it is crucial to discover NETosis markers and develop quantitative detection strategies that are sensitive and specific, particularly towards lytic NETs. Immunoconfocal microscopes are commonly used to detect NETs via immunocytochemistry and immunohistochemistry. Several groups have recommended co-localizing at least three key NET components (i.e., extracellular DNA, NE, and histones) for the accurate detection of NETs. This co-localization helps to differentiate NETs from dead or dying cells that release DNA (61). SYTOX Green dye stain is more specific than DAPI for the detection of NETs in a mixture with extracellular DNA (62, 63). Despite the simple concept, the methodology is not well-developed. There are several challenges to this, such as the need for researchers to manually evaluate the presence of neutrophil-derived proteins and DNA, difficulty in quantifying the formation of NETs, and controversial reported analytical techniques (64). H3cit, MPO and NE are considered as NETs-specific biomarker. Thus, those markers can be used for the ELISA-based detection of NETs (65).
To improve the detection of NETs in vitro, two types of flow cytometry methods (i.e., image-based and cell-appendant) have been developed using antibodies against major NET components (66). For example, Zhao et al. used multispectral imaging flow cytometry to identify the swelling of the nuclei in NET-neutrophils as a potential marker for NETosis (67). According to Gavillet et al., NETs simultaneously express both MPO and citrullinated histones on their surface, and these molecules can be detected by flow cytometry (68). In another study, Cichon et al. introduced a novel method to detect NET formation in vivo via intravital microscopy (69). Recently, it was reported that CDr15 dye stain was impermeable to cell membranes and emitted strong fluorescent signals when bound to the extracellular DNA of NETs. When compared to SYTOX Green, CDr15 showed lower background fluorescence and higher specificity towards NETs. This was supported by the successful detection of NETs stained with CDr15 in formaldehyde-fixed tumor specimens (70). These novel approaches highlight the promising future developments of NET detection technologies. With advancing technology in NETs detection, accumulating evidence demonstrated that NETs may be a potential biomarker of inflammation and autoimmune diseases to reflect the degree of tissue damage and inflammatory conditions (71). A study reported that the serum levels of NETs changed dynamically during severe fever with thrombocytopenia syndrome (SFTS) progression. High NETs levels were strongly associated with multiple pathological processes and predicted severe illness in patients with SFTS (72). Another study found that serum levels of NETs can provide a picture of systemic inflammatory state and thereby estimate risk for HCC recurrence after surgery. The research of NETs detection technology have important clinical implications for both treatment and biomarker discovery (73).
NETs and end-stage liver disease
NETs can also play a pivotal role in liver diseases such as acute liver failure, alcohol-associated liver disease, non-alcoholic steatohepatitis (NASH), liver cirrhosis, and hepatocellular carcinoma (HCC) (59, 74, 75). Globally, liver cirrhosis ranks among the top ten leading causes of death (76). A recent study by Zenlander et al. suggests that the level of plasma markers for NET formation correlates to the severity of liver dysfunction in patients with liver cirrhosis and HCC. A comparison between patients with liver cirrhosis and HCC showed that there was no significant increase in the plasma NET markers (77). Another study demonstrated that a higher rate of complications such as recurrent infections, may occur in liver cirrhosis patients with deficient NETs (78). NASH is becoming the most prevalent chronic liver disease in Western society due to its increasing prevalence (79). According to findings by Van der Windt et al., NETs may be involved in the protumorigenic inflammatory environment in NASH. The strategies used to eliminate NETs may also reduce the risk of HCC in NASH (78). Another study proved that NETs promote regulatory T cell activity through metabolic reprogramming in NASH. In other words, therapeutic targeting of NETs and regulatory T cells can prevent the development of HCC in NASH patients (80).
Acute liver failure (ALF) is a life-threatening condition, that is caused by a variety of factors, including viral infections and drug-induced liver damage (81). A clinical study involving 676 patients with ALF reported that patients with ALF had 7.1 folds higher levels of cfDNA and 2.5 folds higher levels of MPO-DNA complexes, as compared to healthy controls. The levels of cfDNA did not correlate with the 21-day transplant-free survival, but they were higher in more severe cases. This finding suggests that NET formation is a contributing factor to disease development (82). Another study of ALF in mice reported the pathogenic role of NETs in ALF. The management of ALF could be improved by regulating the levels of miR-223/NE and NETs (83).
There is also growing evidence that the presence of NETs in a cancer inflammatory microenvironment promotes HCC cell proliferation (53). Researchers found that neutrophils isolated from patients with HCC produced more NETs in vitro. The presence of elevated MPO-DNA was associated with increased mortality after liver surgery in HCC patients (84, 85). The oncogenic role of NETs in HCC has been preliminary confirmed. However, the specific mechanisms of NETs in portal vein tumor thrombus and HCC recurrence after surgical resection require further investigations ( Figure 2 ).
Figure 2.
Neutrophil extracellular traps have been implicated in the pathophysiology of several different end-stage liver diseases.
NETs and IRI
End-stage liver disease patients benefit from liver transplantation. However, liver IRI is a major cause of liver failure and graft loss associated with liver transplantation (86, 87). In clinical studies, ischemia-reperfusion-related tissue injury accounts for approximately 10% of early graft failures and is a major contributor to both acute and chronic rejection (88). Ischemia livers produce lesser ATPs due to lower oxygen levels. As ATP is low, ROS cytokinesis, vasoactive agents, and adhesion molecules are produced, which can aggravate the damage (89). As a result of ROS generation, the concentration of intracellular calcium increases, and the pH changes, leading to apoptosis and necrosis (90). An important component of liver IRI is inflammation, and neutrophils play an important role in the events leading to liver injury after reperfusion. The excessive activation and recruitment of neutrophils during reperfusion contribute significantly to the pathogenesis of IRI. A neutrophil induces liver injury through a multistep process that involves neutrophil activation, vasculature transport, and migration across the endothelium (91–93).
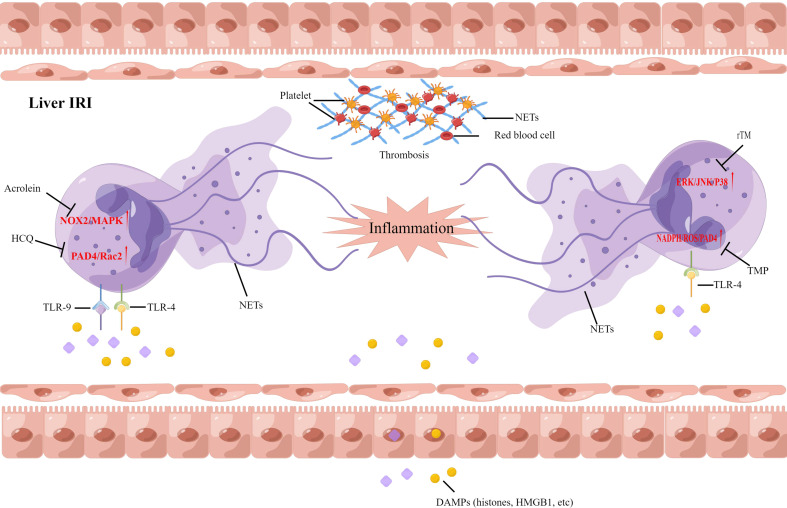
As more studies on the functions of NETs emerge, it is implicated that NETs may contribute to the pathogenesis of hepatic IRI. Histones and high mobility group box 1 (HMGB1) proteins, commonly associated with tissue damage, are released from damaged hepatocytes, and this activates TLR4 and TLR9 to induce NETs formation. A recent study suggested that NADPH-mediated superoxide production initiates NETs formation after IRI. Pretreatment with allopurinol and N-acetylcysteine was found to decrease NETs formation and liver injury after ischemia injury in mice (94). Neutrophils and NETs were found in the liver from ischemia-reperfusion mice models, and both were negatively correlated with histidine-rich glycoprotein (HRG) expression. Supplemental HRG treatment inhibited neutrophil infiltration and NETs formation in livers to alleviate liver IRI (95). Zhang et al. also correlated the presence of NETs with hepatic IRI, and hydroxychloroquine could alleviate hepatic IRI by inhibiting NETs formation in hepatic ischemia-reperfusion mice models (55). One study suggested that acrolein can cause the release of NETs in the liver during IRI and slow the recovery rate of a post-operative liver. In patients with chronic hepatic disorders, targeting NOX2 and P38MAPK signaling could inhibit the formation of NETs, and improve the survival and function of the post-operative liver (56). In our study, we found that tetramethylpyrazine (TMP), a compound extracted from Ligusticum wallichii Franchat, has the potential to improve liver functions and alleviate hepatic IRI. Furthermore, TMP inhibited NADPH oxidase activity, thus inhibiting the formation of NETs in rats after liver transplantation. We provide the first evidence of a synergistic effect between TMP and diphenyleneiodonium to alleviate hepatic IRI (96). We further examined the effect of recombinant human thrombomodulin (rTM) on liver transplantation in a rat model, focusing on the TLR-4/MAPK axis. Our data illustrated that NETs independently contribute to hepatic IRI, and rTM treatment mitigated neutrophil infiltration and suppressed NETs formation after liver transplantation (97). Although these results suggest that antioxidant treatment can protect against liver IRI via the attenuation of NETs formation, the therapeutic benefits of NETs inhibition should also take into account the possible complications in immunocompromised individuals after transplantation ( Figure 3 ).
Figure 3.
Neutrophil extracellular traps have been implicated in the pathophysiology of liver ischemia-reperfusion injury following liver transplantation. DAMPs, damage-associated molecular patterns; HCQ, Hydroxychloroquine; TMP; Tetramethylpyrazine, rTM; recombinant human thrombomodulin.
NETs and acute rejection
Many individuals with end-stage liver disease around the world benefit from liver transplantation (98). According to a recent publication, the 5-year survival rate of grafts and patients after a liver transplant was 72.8 and 76.1%, respectively. Acute rejection (AR) is a common complication after liver transplantation, that affects about 25 to 50% of patients (99). There is evidence that immunosuppressive agents can reduce the rates of acute rejection, but immunosuppressive treatments can also decrease the survival rate of patients (100, 101). Total bilirubin, alanine aminotransferase, and aspartate aminotransferase are commonly used clinically to monitor the liver function of liver grafts (102). Furthermore, the levels of immunosuppressive drugs in the blood can be monitored to predict the risk of AR (103). However, standard laboratory tests are inefficient for detecting AR, in terms of time and specificity (104, 105). Hence, the identification of therapeutic targets and early diagnostic strategies for AR should be the focus of future research (106). Recently, donor-derived cell-free DNA (dd-cfDNA) in AR is attracting increasing attention as a diagnostic biomarker (107). Allograft injury releases dd-cfDNA into the patient’s serum, which makes it a good biomarker to evaluate the condition of allografts and the possibility of rejection (108). A study was conducted by Schutz et al. that measured the levels of dd-cfDNA in 107 patients with liver transplantation. Patients with AR had the highest percentage (29.6%) compared with those healthy controls (3.3%) (109). A more recent study suggested that dd-cfDNA is even more sensitive than conventional transaminases to detect AR (110).
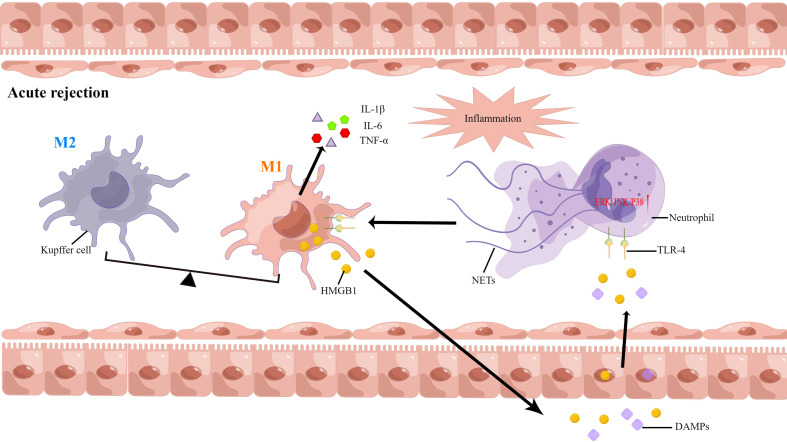
Extracellular DNA is the most important component of NETs, and neutrophils are generally activated in the AR. However, there are insufficient studies to assess the correlation between NETs formation and AR after liver transplantation. As such, we have conducted some studies in this area (8, 15). Serum samples obtained from 13 liver transplant individuals were analyzed, and we found that the levels of NETs were elevated. During recovery, the levels of NETs decreased gradually and then stabilized. The levels of NETs increased following AR diagnosis, and decreased following treatment with oral rapamycin, in three patients. Next, the serum NETs were measured in liver transplant patients with AR. The levels of NETs in patients undergoing liver transplantation were positively correlated with liver enzymes and the incidence of AR. Our findings revealed that AR is influenced independently by NETs and that NETs subsequently induced kupffer cell M1 polarization and intracellular translocation of HMGB1. On the other hand, HMGB1 activates the TLR-4/MAPK signaling pathway, which causes NETs formation. This positive feedback loop between neutrophils and kupffer cells further amplifies the inflammatory signals and graft injury. Additionally, NET inhibitors combined with immunosuppressive agents may offer a novel treatment option for AR (57). NETs are a potential novel target for AR diagnosis and treatment ( Figure 4 ).
Figure 4.
Neutrophil extracellular traps have been implicated in the pathophysiology of acute rejection following liver transplantation.
NETs and arterial thrombosis
Hepatic artery thrombosis is the most common vascular complication, that may lead to non-functional liver graft and acute liver failure, following liver transplantation (111, 112). A significant proportion of this is seen in patients with recurrent biliary tract infection or asymptomatic biliary leakage with liver dysfunction (113). Thrombosis after liver transplantation has a high incidence rate and poor prognosis in children undergoing liver transplantation because it is difficult to diagnose in the early stages (114). It is well-recognized that neutrophils and platelets act as first responders to injuries and infections (115). As part of their host defense mechanisms, neutrophils promote blood coagulation by increasing fibrin deposition and limiting the spread of infections (32). NET-fibrin interactions prevent bacterial invasion into the surrounding tissues of the liver microvasculature, while their disruption promotes bacterial dissemination throughout the body (116). A dysregulation or excessive stimulation of the vasculature may lead to pathological thrombosis. Therefore, neutrophils play a pivotal role in regulating thrombosis through several mechanisms (117).
NETs have been recently identified as new DNA-based components involved in the formation of blood clots and thrombosis (118). Platelets, red blood cells, and platelet adhesion molecules adhere to NETs via a scaffold, which promotes thrombosis (119). Additionally, many of the scaffold components can also stimulate platelet activation and blood coagulation (6). In addition, NETs can stimulate both intrinsic and extrinsic coagulation, primarily through the serine proteases in neutrophils. Endothelial cells are highly cytotoxic to the histones, H3 and H4, and that platelets can aggregate because of these histones (5). In comparison to venous thrombosis, arterial thrombosis is more common in acute events, as a result of thrombus shedding in acute myocardial infarction (AMI), ischemic stroke, and acute arterial embolism (120). According to Riegger et al., NETs were present in the thrombus of stroke patients and atherosclerotic plaques of patients with atrial fibrillation (121). Another study found that NETs were more prevalent in newly formed coronary thrombi than in older ones and that both myocardial infarction and ST-segment elevation were positively associated with the level of NETs in the coronary thrombi (122). The surgical stress response from liver resection and transplantation can aggravate the deposition of NETs in the liver, and platelets activated with NETs can produce a systemic procoagulant state, leading to immunothrombosis and remote organ injury (123). A mouse model with liver IRI was found to significantly increase both circulating platelet activation and platelet-neutrophil aggregation. NETs and platelet-rich microthrombi were found in the microvasculature of injured organs after liver surgery, and the inhibition of NETs with DNase reduced immune thrombosis and organ damage (124). Although the key role of NETs in immune thrombosis and its related mechanisms have been reported by a large number of studies, the findings on the regulation of coagulation and immune thrombosis after liver transplantation are still lacking. Hence, it is necessary to explore the role and specific mechanisms of NETs in immune thrombosis after liver transplantation, for better management of the condition.
NETs and hepatocellular carcinoma recurrence
Hepatocellular carcinoma (HCC) and end-stage liver diseases have widely benefited from liver transplantation (125, 126). However, HCC recurrence is one of the main causes of mortality in HCC patients who undergo liver transplantation (127, 128). It has been established that certain tumor characteristics can lead to the recurrence of HCC, including the concentration of alpha fetoproteins, tumor diameter, macrovascular invasion, and extended orthotopic liver transplantation criteria (129). A recent study found that both pre-operative serum hepatitis B viral DNA and pre-operative prognostic nutritional index can potentially be used to predict HCC recurrence after liver transplantation (130, 131). However, these studies all had small sample sizes or were conducted retrospectively and lack of molecular-biological investigations. Despite these advances, the mechanism behind the high HCC recurrence rates remains a mystery, and that these biomarkers have clear limitations.
Recently, NETs have been detected in various cancer samples (i.e., breast, liver, and gastric cancers) and metastatic tumors. In tumor development, NETs play an important role in cancer immunoediting and immune-cell interactions (132–134). Research suggests that NETs activate dormant cancer cells, which causes tumor recurrence (135). Furthermore, HMGB1 is also involved in NETs formation in TME by interacting with TLR4, and this releases excessive inflammatory cytokines (136). NETs also promote cancer invasion and migration, which exacerbates tumor aggressiveness (137). It is well known that the degradation of matrix proteins inhibits the immune system of the host, which is one of the mechanisms of tumor evasion (53). NETs-associated proteinases activate matrix metalloproteinases to induce tumor-associated macrophages, which stimulate the release of pro-inflammatory factors (i.e., IL-8, IL-1β, and TNF-α), eventually leading to immune escape and tumor metastasis (138).
Tumor metastasis is the main cause of cancer mortality, and neutrophils are involved in this process (139). Multiple studies reported that NETs trap circulating cancer cells and release proteases, which results in tumor metastasis and proliferation (35, 140). Najmeh et al. demonstrated that β1-integrin can induce NET-related entrapment of circulating lung carcinoma cells, resulting in cancer development and metastasis (141). These results were supported by Cools-Lartigue et al., where circulating lung carcinoma cells were found to be encapsulated in NET DNA conglomerates in a murine model. It was also shown that circulating “NETs-cancer cells packages” seeded in the liver, produced micrometastases within 48 hours and secondary hepatocellular carcinoma after two weeks (51). A retrospective analysis found that high level of NETs predicted shorter recurrence-free survival and overall survival. Serum levels of NETs as a biomarker pre-surgery can help identify patients with a higher risk for HCC recurrence (73). Another study showed that HCC is capable to stimulate NETs enriched in oxidized mitochondrial-DNA, which are highly pro-inflammatory and pro-metastatic (142). Cheng Y et al. demonstrated that combination of NK cell adoptive therapy and hydrogel-based delivery system can destruct NETs and prevent post-resection and post liver transplantation HCC recurrence (143). NETs play an integral role in cancer invasion, transport, and transendothelial migration according to a multilevel model, especially in HCC recurrence should be further studied.
Potential therapeutic targets for NETs
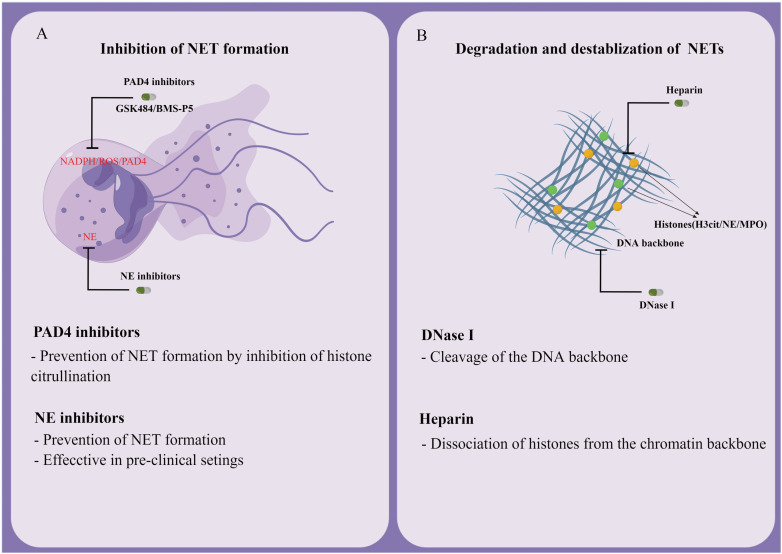
In various diseases, NETs play the role of pathogenic drivers, thus making them attractive therapeutic targets. Studies have found that the levels of NETs correlated with the survival of cancer patients (142, 144). However, the risks of using NETs as therapeutic targets should also be evaluated. Targeting NETs would increase infection susceptibility, considering the protective role of NETs against severe infectious diseases (58). A study reported that mice with deletions of PAD4 were more vulnerable to bacterial infections (145). According to another study, PAD4 knockout may protect mice from polymicrobial sepsis-induced septic shock (146). Therefore, the potential risk of targeting NET formation may be determined by the type of disease and immune status of the organism. Another major risk of NETs degradation is the release of NETs-derived DNA and histones, which may trigger inflammation. Currently, therapies targeting NETs can be segmented into two categories: degradation/destabilization of NETs, and the inhibition of NETs formation.
The degradation of NETs has already been extensively studied. Research found that DNase I was capable of partially lysing NETs, and that tPA and DNase I could synergistically initiate thrombolysis (122). The use of DNase I as a treatment in mice suffering from thrombosis was also effective at preventing recurrent stroke, myocardial infarction, and deep vein thrombosis (49). However, further research is required to determine whether DNase I degradation of NETs would increase inflammation and risk for thrombosis. It has also been suggested that treatment with low molecular weight heparins (LMWH) can reduce NETs formation (5). Some researchers reported that histones could be dissociated from the chromatin backbone of NETs via heparin therapy and that LMWH can inhibit PMA-induced NETs formation (147). According to a study, the therapeutic use of heparin to treat NET-associated pathologies reported the opposite effect. Lelliott, et al. showed that heparin induced NETs formation in vitro, in the absence of PAD4 (148). Two independent studies reported that heparin-induced thrombocytopenia-related thrombosis was caused by NETs (149, 150). These contradictory results, as well as the potential side effects and risks, suggest the need for further investigations.
Another strategy to target already formed NETs is to interfere with their formation. Cloamidine, a pan PAD inhibitor, was found to inhibit the expression of PAD4, which subsequently prevents NETosis (151). Another potential advantage is that PAD4 deficiency in mice does not affect bacteremia during polymicrobial sepsis. The efficacy of GSK484 (developed by Glaxo Smith Kline) and BMS-P5 (developed by Bristol-Myers Squibb) in inhibiting NET development and suppressing associated diseases have now been confirmed by several in vitro and in vivo studies (152, 153). As a potential therapeutic target, NE inhibitors have proven effective in inhibiting NETs formation (51). For example, it was demonstrated that Sivelestat (an inhibitor of NE) inhibited NETs growth in mice with lung carcinoma (154). Antibodies are also known to prevent the formation and release of NETs in several inflammatory conditions, owing to their action toward citrullinated proteins ( Figure 5 ) (155).
Figure 5.
Potential therapeutic targets for NETs. (A) Inhibition of NET formation. (B) Degradation and destabilization of already formed NETs.
Conclusion and future perspectives
There is increasing evidence showing that NETs contribute to ischemia-reperfusion injury, acute rejection, thrombosis, and the recurrence of hepatocellular carcinoma. There is also potential for NET-related molecules as biomarkers and as targets for therapeutic intervention in complications of live transplantation. With further study, NETs is promising to provide a vast number of innovative applications in liver transplantation. There is an urgent need for the development of new methodologies to accurately detect NETs formation, considering the limitations of current methods. In addition, NETs detection should be standardized to ensure consistent results from comparative studies by different research groups. Thus far, strong evidence has shown that NETs might induce inflammation and tumor immune escape in ischemia-reperfusion injury and recurring hepatocellular carcinoma. Further research is required to understand the pathogenicity of NETs in liver transplantation. Neutrophils and NETs play a pivotal role in immune defense, and their potential as therapeutic targets warrants further study. A long-term safety assessment is also needed to assess the benefits and risks of NET-inhibition treatment. As an emerging field within liver transplantation, the relationship between NETs and the postoperative complication of liver transplantation also requires further investigation.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81873592 and No.82170666).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Brinkmann V. Neutrophil extracellular traps in the second decade. J Innate Immun (2018) 10(5-6):414–21. doi: 10.1159/000489829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu L, Gao X, Guo Q, Li J, Yao J, Yan K, et al. The role of neutrophils in innate immunity−driven nonalcoholic steatohepatitis: Lessons learned and future promise. Hepatol Int (2020) 14(5):652–66. doi: 10.1007/s12072-020-10081-7 [DOI] [PubMed] [Google Scholar]
- 3. Bartneck M, Wang J. Therapeutic targeting of neutrophil granulocytes in inflammatory liver disease. Front Immunol (2019) 10:2257. doi: 10.3389/fimmu.2019.02257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herre M, Cedervall J, Mackman N, Olsson AK. Neutrophil extracellular traps in the pathology of cancer and other inflammatory diseases. Physiol Rev (2023) 103 (1): 277–312. doi: 10.1152/physrev.00062.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Jr DDM, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA (2010) 107(36):15880–5. doi: 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou Y, Tao W, Shen F, Du W, Xu Z, LIu Z. The emerging role of neutrophil extracellular traps in arterial, venous, and cancer-associated thrombosis. Front Cardiovasc Med (2021) 2(8):786387. doi: 10.3389/fcvm.2021.786387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang H, Tohme S, AI-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology (2015) 62(2):600–14. doi: 10.1002/hep.27841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Qin X, Lei Z, Chai W, Wu Z. Diphenyleneiodonium ameliorates acute liver rejection during transplantation by inhibiting neutrophil extracellular traps formation in vivo . Transpl Immunol (2021) 68:101434. doi: 10.1016/j.trim.2021.101434 [DOI] [PubMed] [Google Scholar]
- 9. Erpenbeck L, Schön MP. Neutrophil extracellular traps: Protagonists of cancer progression? Oncogene (2017) 36(18):2483–90. doi: 10.1038/onc.2016.406 [DOI] [PubMed] [Google Scholar]
- 10. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science (2004) 303(5663):1532–5. doi: 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 11. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol (2018) 18(02):134–47. doi: 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- 12. Thiam HR, Wong SL, Qiu R, Kittisopikul M, Vahabikashi A, Goldman AE, et al. NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proc Natl Acad Sci USA (2020) 117(13):7326–37. doi: 10.1073/pnas.1909546117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol (2007) 176(2):231–41. doi: 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kapoor S, Opneja A, Nayak L. The role of neutrophils in thrombosis. Thromb Res (2018) 170:87–96. doi: 10.1016/j.thromres.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Avondt KV, Hartl D. Mechanisms and disease relevance of neutrophil extracellular trap formation. Eur J Clin Invest. (2018) 48Suppl2:e12919. doi: 10.1111/eci.12919 [DOI] [PubMed] [Google Scholar]
- 16. Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med (2017) 23(3):279–87. doi: 10.1038/nm.4294 [DOI] [PubMed] [Google Scholar]
- 17. Kenny EF, Herzig A, Krüger R, Muth A, Mondal S, Thompson PR, et al. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife (2017) 6:e24437. doi: 10.7554/eLife.24437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Denning NL, Aziz M, Gurien SD, Wang P. DAMPs and NETs in sepsis. Front Immunol (2019) 10:2536. doi: 10.3389/fimmu.2019.02536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Li M, Stadler S, Li P, Wang D, Hayama R, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol (2009) 184:205–13. doi: 10.1083/jcb.200806072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi L, Aymonnier K, Wagner DD. Neutrophil stimulation with citrullinate histone H4 slows down calcium influx and reduces NET formation compared with native histone H4. PloS One (2021) 16:e0251726. doi: 10.1371/journal.pone.0251726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eghbalzadeh K, Georgi L, Louis T, Zhao H, Keser U, Weber C, et al. Compromised anti-inflammatory action of neutrophil extracellular traps in PAD4-deficient mice contributes to aggravated acute inflammation after myocardial infarction. Front Immunol (2019) 10:2313. doi: 10.3389/fimmu.2019.02313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. Neutrophils sense microbe size and selectively ReleaseNeutrophil extracellular traps in response to Large pathogens. Nat Immunol (2014) 15(11):1017–25. doi: 10.1038/ni.2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thålin C, Hisada Y, Lundström S, Mackman N, Wallén H. Neutrophil extracellular traps: Villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler Thromb Vasc Biol (2019) 39(9):1724–38. doi: 10.1161/ATVBAHA.119.312463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adrover JM, Aroca-Crevillén A, Crainiciuc G, Ostos F, Rojas-Vega Y, Rubio-Ponce A, et al. Programmed ‘disarming’ of the neutrophil proteome reduces the magnitude of inflammation. Nat Immunol (2020) 21(2):135–44. doi: 10.1038/s41590-019-0571-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Altznauer F, Martinelli S, Yousefi S, Thürig C, Schmid, Conway EM, et al. Inflammation-associated cell cycle-independent block of apoptosis by survivin in terminally differentiated neutrophils. J Exp Med (2004) 199(10):1343–54. doi: 10.1084/jem.20032033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med (2008) 14(9):949–53. doi: 10.1038/nm.1855 [DOI] [PubMed] [Google Scholar]
- 27. Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ (2009) 16(11):1438–44. doi: 10.1038/cdd.2009.96 [DOI] [PubMed] [Google Scholar]
- 28. Lood C, Blanco LP, Purmalek M, Carmona-Rivera C, Ravin SSD, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med (2016) 22(2):146–53. doi: 10.1038/nm.4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Claushuis TAM, van der Donk LEH, Luitse AL, van Veen HA, van der Wel NN, van Vught AL, et al. Role of peptidylarginine deiminase 4 in neutrophil extracellular trap formation and host defense during klebsiella pneumoniae-induced pneumonia-derived sepsis. J Immunol (2018) 201(4):1241–52. doi: 10.4049/jimmunol.1800314 [DOI] [PubMed] [Google Scholar]
- 30. Hamam HJ, Palaniyar N. Post-translational modifications in NETosis and NETs-mediated diseases. Biomolecules (2019) 9(8):369. doi: 10.3390/biom9080369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sollberger G, Choidas A, Burn GL, Habenberger P, Lucrezia RD, Kordes S, et al. Gasdermin d plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol (2018) 3(26):eaar6689. doi: 10.1126/sciimmunol.aar6689 [DOI] [PubMed] [Google Scholar]
- 32. McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the blood-stream during sepsis. Cell Host Microbe (2012) 12(3):324–33. doi: 10.1016/j.chom.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 33. Weinrauch Y, Drujan D, Shapiro SD, Weiss J, Zychlinsky A. Neutrophil elastase targets virulence factors of enterobacteria. Nature (2002) 417(6884):91–4. doi: 10.1038/417091a [DOI] [PubMed] [Google Scholar]
- 34. Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol (2007) 5(08):577–82. doi: 10.1038/nrmicro1710 [DOI] [PubMed] [Google Scholar]
- 35. Kolaczkowska E, Jenne CN, Surewaard BGJ, Thanabalasuriar A, Lee WY, Sanz MJ, et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun (2015) 6:6673. doi: 10.1038/ncomms7673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology (2012) 143(5):1158–72. doi: 10.1053/j.gastro.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 37. Marin-Esteban V, Turbica I, Dufour G, Semiramoth N, Gleizes A, Gorges R, et al. Afa/Dr diffusely adhering escherichia coli strain C1845 induces neutrophil extracellular traps that kill bacteria and damage human enterocyte-like cells. Infect Immun (2012) 80(5):1891–9. doi: 10.1128/IAI.00050-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crane JK, Broome JE, Lis A. Biological activities of uric acid in infection due to enteropathogenic and shiga-toxigenic escherichia coli. Infect Immun (2016) 84(4):976–88. doi: 10.1128/IAI.01389-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d''mal C, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med (2020) 217(12):e20201012. doi: 10.1084/jem.20201012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murao A, A1 A, Brenner M, Denning NL, Jin H, Takizawa S, et al. Extracellular CIRP and TREM-1 axis promotes ICAM-1-Rho-mediated NETosis in sepsis. FASEB J (2020) 34(7):9771–86. doi: 10.1096/fj.202000482R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonaventura A, Liberale L, Carbone F, Vecchié A, Diaz-Cañestro C, Camici GG, et al. The pathophysiological role of neutrophil extracellular traps in inflammatory diseases. Thromb Haemost. (2018) 118(1):6–27. doi: 10.1160/TH17-09-0630 [DOI] [PubMed] [Google Scholar]
- 42. Yazdani HO, Chen HW, Tohme S, Tai S, van der Windt DJ, Loughran P, et al. IL-33 exacerbates liver sterile inflammation by amplifying neutrophil extracellular trap formation. J Hepatol (2017) S0168-8278(17):32291–2. doi: 10.1016/j.jhep.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou Y, Xu Z, Liu Z. Impact of neutrophil extracellular traps on thrombosis formation: New findings and future perspective. Front Cell Infect Microbiol (2022) 12:910908. doi: 10.3389/fcimb.2022.910908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kimball AS, Obi AT, Diaz JA, Henke PK. The emerging role of NETs in venous thrombosis and immunothrombosis. Front Immunol (2016) 7:236. doi: 10.3389/fimmu.2016.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol (2013) 13:34–45. doi: 10.1038/nri3345 [DOI] [PubMed] [Google Scholar]
- 46. Kambas K, Mitroulis I, Apostolidou E, Girod A, Chrysanthopoulou A, Pneumatikos I, et al. Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis. PloS One (2012) 7(9):e45427. doi: 10.1371/journal.pone.0045427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Noubouossie DF, Reeves BN, Strahl BD, Key NS. Neutrophils: Back in the thrombosis spotlight. Blood (2019) 133(20):2186–97. doi: 10.1182/blood-2018-10-862243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang X, Li L, Liu J, Lv B, Chen F. Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-kb and AP-1. Thromb Res (2016) 137:211–8. doi: 10.1016/j.thromres.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 49. Ducroux C, Meglio LD, Loyau S, Delbosc S, Boisseau W, Deschildre C, et al. Thrombus neutrophil extracellular traps content impair tPA induced thrombolysis in acute ischemic stroke. Stroke (2018) 49(3):754–7. doi: 10.1161/STROKEAHA.117.019896 [DOI] [PubMed] [Google Scholar]
- 50. Noubouossie DF, Whelihan MF, Yu YB, Sparkenbaugh E, Pawlinski R, Monroe DM, et al. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood (2017) 129(8):1021–9. doi: 10.1182/blood-2016-06-722298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. (2013) 123(8):3446–58. doi: 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Y, Hu Y, Ma C, Sun H, Wei X, Li M, et al. Diagnostic, therapeutic predictive, and prognostic value of neutrophil extracellular traps in patients with gastric adenocarcinoma. Front Oncol (2020) 10:1036. doi: 10.3389/fonc.2020.01036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meo MLD, Spicer JD. The role of neutrophil extracellular traps in cancer progression and metastasis. Semin Immunol (2021) 57:101595. doi: 10.1016/j.smim.2022.101595 [DOI] [PubMed] [Google Scholar]
- 54. Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res (2016) 76(6):1367–80. doi: 10.1158/0008-5472.CAN-15-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang S, Zhang Q, Wang F, Guo X, Liu T, Zhao Y, et al. Hydroxychloroquine inhibiting neutrophil extracellular trap formation alleviates hepatic ischemia/reperfusion injury by blocking TLR9 in mice. Clin Immunol (2020) 216:108461. doi: 10.1016/j.clim.2020.108461 [DOI] [PubMed] [Google Scholar]
- 56. Arumugam S, Subbiah KG, Kemparaju K, Thirunavukkarasu C. Neutrophil extracellular traps in acrolein promoted hepatic ischemia-reperfusion injury: Therapeutic potential of NOX2 and p38MAPK inhibitors. J Cell Physiol (2018) 233(4):3244–61. doi: 10.1002/jcp.26167 [DOI] [PubMed] [Google Scholar]
- 57. Liu Y, Pu X, Qin X, Gong J, Huang Z, Lou Y, et al. Neutrophil extracellular traps regulate HMGB1 translocation and kupffer cell M1 polarization during acute liver transplantation rejection. Front Immunol (2022) 13:823511. doi: 10.3389/fimmu.2022.823511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boeltz S, Amini P, Anders HJ, Andrade F, Billy R, Chatfield S, et al. To NET or not to NET: Current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ (2019) 26(3):395–408. doi: 10.1038/s41418-018-0261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Honda M, Kubes P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat Rev Gastroenterol Hepatol (2018) 15(4):206–21. doi: 10.1038/nrgastro.2017.183 [DOI] [PubMed] [Google Scholar]
- 60. Breda SV, Vokalova L, Neugebauer C, Rossi SW, Hahn S, Hasler P, et al. Computational methodologies for the in vitro and in situ quantification of neutrophil extracellular traps. Front Immunol (2019) 10:1562. doi: 10.3389/fimmu.2019.01562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Seper A, Hosseinzadeh A, Gorkiewicz G, Lichtenegger S, Roier S, Lietner DR, et al. Vibrio cholerae evades neutrophil extracellular traps by the activity of two extracellular nucleases. PloS Pathog (2013) 9(9):e1003614. doi: 10.1371/journal.ppat.1003614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood (2011) 117(3):953–9. doi: 10.1182/blood-2010-06-290171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol (2010) 191(3):677–91. doi: 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Carmona-Rivera C, Kaplan MJ. Induction and quantification of NETosis. Curr Protoc Immunol (2016) 115:14.41.1–14. doi: 10.1002/cpim.16 [DOI] [PubMed] [Google Scholar]
- 65. Tan C, Aziz M, Wang P. The vitals of NETs. J Leukoc Biol (2021) 110(4):797–808. doi: 10.1002/JLB.3RU0620-375R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. de Buhr N, von Kockritz-Blickwede M. How neutrophil extracellular traps become visible. J Immunol Res (2016) 2016:4604713. doi: 10.1155/2016/4604713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhao W, Fogg DK, Kaplan MJ. A novel image-based quantitative method for the characterization of NETosis. J Immunol Methods (2015) 423:104–10. doi: 10.1016/j.jim.2015.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gavillet M, Martinod K, Renella R, Harris C, Shapiro NI, Wagner DD, et al. Flow cytometric assay for direct quantification of neutrophil extracellular traps in blood samples. Am J Hematol (2015) 90(12):1155–8. doi: 10.1002/ajh.24185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cichon I, Santocki M, Ortmann W, Kolaczkowska E, et al. Imaging of neutrophils and neutrophil extracellular traps (NETs) with intravital (In vivo) microscopy. Methods Mol Biol (2020) 2087:443–66. doi: 10.1007/978-1-0716-0154-9_26 [DOI] [PubMed] [Google Scholar]
- 70. Kim SJ, Kim J, Kim B, Lee WW, Liu X, Chang YT, et al. Validation of CDr15 as a new dye for detecting neutrophil extracellular trap. Biochem Biophys Res Commun (2020) 527(3):646–53. doi: 10.1016/j.bbrc.2020.04.153 [DOI] [PubMed] [Google Scholar]
- 71. Domerecka W, Homa-Mlak I, Mlak R, et al. Indicator of inflammation and NETosis-Low-Density granulocytes as a biomarker of autoimmune hepatitis. J Clin Med (2022) 11(8):2174. doi: 10.3390/jcm11082174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang Y, Song R, Shen Y, Zhao Y, Zhao Z, Fan T, et al. High levels of circulating cell-free DNA are associated with a poor prognosis in patients with severe fever with thrombocytopenia syndrome. Clin Infect Dis (2020) 70(9):1941–9. doi: 10.1093/cid/ciz553 [DOI] [PubMed] [Google Scholar]
- 73. Kaltenmeier CT, Yazdani H, van der Windt D, Nolinari M, Geller D, Tsung A, et al. Neutrophil extracellular traps as a novel biomarker to predict recurrence-free and overall survival in patients with primary hepatic malignancies. HPB (Oxford). (2021) 23(2):309–20. doi: 10.1016/j.hpb.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hilscher MB, Shah VH. Neutrophil extracellular traps and liver disease. Semin Liver Dis (2020) 40(2):171–9. doi: 10.1055/s-0039-3399562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Meijenfeldt FA, Jenne CN. Netting liver disease: Neutrophil extracellular traps in the initiation and exacerbation of liver pathology. Semin Thromb Hemost. (2020) 46(6):724–34. doi: 10.1055/s-0040-1715474 [DOI] [PubMed] [Google Scholar]
- 76. Bosetti C, Levi F, Lucchini F, Zatonski WA, Negri E, Vecchia CL. Worldwide mortality from cirrhosis: An update to 2002. J Hepatol (2007) 46:827–39. doi: 10.1016/j.jhep.2007.01.025 [DOI] [PubMed] [Google Scholar]
- 77. Zenlander R, Havervall S, Magnusson M, Engstrand J, Åren A, Thålin C, et al. Neutrophil extracellular traps in patients with liver cirrhosis and hepatocellular carcinoma. Sci Rep (2021) 11(1):18025. doi: 10.1038/s41598-021-97233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Van der Windt D, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology (2018) 68(4):1347–60. doi: 10.1002/hep.29914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol (2013) 10(11):686–90. doi: 10.1038/nrgastro.2013.171 [DOI] [PubMed] [Google Scholar]
- 80. Wang H, Zhang H, Wang Y, Brown ZJ, Xia Y, Huang Z, et al. Regulatory T cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol (2021) 75(6):1271–83. doi: 10.1016/j.jhep.2021.07.032 [DOI] [PubMed] [Google Scholar]
- 81. Bernal W, Wendon J. Acute liver failure. N Engl J Med (2013) 369(26):2525–34. doi: 10.1056/NEJMra1208937 [DOI] [PubMed] [Google Scholar]
- 82. Meijenfeldt FA, Stravitz RT, Zhang J, Adelmeijer J, Zen Y, Durkalski V, et al. Generation of neutrophil extracellular traps in patients with acute liver failure is associated with poor outcome. Hepatology (2022) 75(3):623–33. doi: 10.1002/hep.32174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ye D, Yao J, Du W, Chen C, Yang Y, Yan K, et al. Neutrophil extracellular traps mediate acute liver failure in regulation of miR-223/Neutrophil elastase signaling in mice. Cell Mol Gastroenterol Hepatol (2022) 14(3):587–607. doi: 10.1016/j.jcmgh.2022.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei R, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol (2020) 13(1):3. doi: 10.1186/s13045-019-0836-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Velliou R, Mitroulis I, Chatzigeorgiou A. Neutrophil extracellular traps contribute to the development of hepatocellular carcinoma in NASH by promoting treg differentiation. Hepatobiliary Surg Nutr (2022) 11(3):415–8. doi: 10.21037/hbsn-21-557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Starzl TE, Fung JJ. Themes of liver transplantation. Hepatology (2010) 51(6):1869–84. doi: 10.1002/hep.23595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu Y, Lu T, Zhang C, Xu J, Xue Z, Busuttil RW, et al. Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury. J Hepatol (2019) 71(4):719–30. doi: 10.1016/j.jhep.2019.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Uchida Y, Ke B, Freitas MCS, Yagita H, Akiba H, Busuttil RW, et al. T-Cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology (2010) 139(6):2195–206. doi: 10.1053/j.gastro.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Selzner M, Selzner N, Jochum W, Graf R, Clavien PA. Increased ischemic injury in old mouse liver: An ATP-dependent mechanism. Liver Transpl (2007) 13(3):382–90. doi: 10.1002/lt.21100 [DOI] [PubMed] [Google Scholar]
- 90. Guan LY, Fu PY, Li PD, Li ZN, Li HY, Xin MG, et al. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg (2014) 6(7):122–8. doi: 10.4240/wjgs.v6.i7.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Oliveira THC, Marques PE, Proost P, Teixeira MMM. Neutrophils: A cornerstone of liver ischemia and reperfusion injury. Lab Invest. (2018) 98(1):51–62. doi: 10.1038/labinvest.2017.90 [DOI] [PubMed] [Google Scholar]
- 92. Nakamura K, Kageyama S, Kupiec-Weglinski JW. The evolving role of neutrophils in liver transplant ischemia-reperfusion injury. Curr Transplant Rep (2019) 6(1):78–89. doi: 10.1007/s40472-019-0230-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. (2009) 119(2):305–14. doi: 10.1172/JCI35958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Al-Khafaji AB, Tohme S, Yazdani HO, Miller D, Huang H, Tsung A. Superoxide induces neutrophil extracellular trap formation in a TLR-4 and NOX-dependent mechanism. Mol Med (2016) 22:621–31. doi: 10.2119/molmed.2016.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Guo J, Akahoshi T, MizutaAl Y, Murata M, Narahara S, Kawano T, et al. Histidine-rich glycoprotein alleviates liver Ischemia/Reperfusion injury in mice with nonalcoholic steatohepatitis. Liver Transpl. (2021) 27(6):840–53. doi: 10.1002/lt.25960 [DOI] [PubMed] [Google Scholar]
- 96. Liu Y, Qin X, Lei Z, Chai H, Huang Z, Wu Z. Tetramethylpyrazine inhibits neutrophil extracellular traps formation and alleviates hepatic ischemia/reperfusion injury in rat liver transplantation. Exp Cell Res (2021) 406(1):112719. doi: 10.1016/j.yexcr.2021.112719 [DOI] [PubMed] [Google Scholar]
- 97. Liu Y, Lei Z, Chai H, Xiang S, Wang Y, Yan P, et al. Thrombomodulin-mediated inhibition of neutrophil extracellular trap formation alleviates hepatic ischemia-reperfusion injury by blocking TLR4 in rats subjected to liver transplantation. Transplantation (2022) 106(2):e126–40. doi: 10.1097/TP.0000000000003954 [DOI] [PubMed] [Google Scholar]
- 98. Martinelli J, Habes D, Majed L, Guettier C, Gonzalès E, Linglart A, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant. (2018) 18(7):1680–9. doi: 10.1111/ajt.14626 [DOI] [PubMed] [Google Scholar]
- 99. Craig E, Heller M. Complications of liver transplant. Abdom Radiol(NY) (2021) 46(1):43–67. doi: 10.1007/s00261-019-02340-5 [DOI] [PubMed] [Google Scholar]
- 100. Dogan N, Husing-Kabar A, Schmidt HH, Cicinnati VR, Beckebaum S, Kabar I, et al. Acute allograft rejection in liver transplant recipients: Incidence, risk factors, treatment success, and impact on graft failure. J Int Med Res (2018) 46(9):3979–90. doi: 10.1177/0300060518785543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Levitsky J, Goldberg D, Smith A, Mansfield SA, Gillespie BW, Merion RM, et al. Acute rejection increases risk of graft failure and death in recent liver transplant recipients. Clin Gastroenterol Hepatol (2017) 15(4):584–93. doi: 10.1016/j.cgh.2016.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Florez MC, Bruner J, Zarrinpar A. Progress and challenges in diagnosis and treatment of rejection following liver transplantation. Curr Opin Organ Transplant. (2021) 26(6):669–74. doi: 10.1097/MOT.0000000000000924 [DOI] [PubMed] [Google Scholar]
- 103. Kawahara T, Asthana S, Kneteman NM. mTOR inhibitors: What role in liver transplantation? J Hepatol (2011) 55(6):1441–51. doi: 10.1016/j.jhep.2011.06.015 [DOI] [PubMed] [Google Scholar]
- 104. Perottino G, Harrington C, Levitsky J. Biomarkers of rejection in liver transplantation. Curr Opin Organ Transplant. (2022) 27(2):154–8. doi: 10.1097/MOT.0000000000000959 [DOI] [PubMed] [Google Scholar]
- 105. Sánchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology (2011) 140(1):51–64. doi: 10.1053/j.gastro.2010.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Krenzien F, Keshi E, Splith K, Griesel S, Kamali K, Sauer IM, et al. Diagnostic biomarkers to diagnose acute allograft rejection after liver transplantation: Systematic review and meta-analysis of diagnostic accuracy studies. Front Immunol (2019) 10:758. doi: 10.3389/fimmu.2019.00758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Taner T, Bruner J, Emamaullee J, Bonaccorsi-Riani E, Zarrinpar A. New approaches to the diagnosis of rejection and prediction of tolerance in liver transplantation. Transplantation (2022) 106(10):1952–62. doi: 10.1097/TP.0000000000004160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Grskovic M, Hiller DJ, Eubank LA, Sninsky JJ, Christopherson C, Collins JP, et al. Validation of a clinical-grade assay to measure donor-derived cell-free DNA in solid organ transplant recipients. J Mol Diagn. (2016) 18(6):890–902. doi: 10.1016/j.jmoldx.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 109. Schütz E, Fischer A, Beck J, Harden M, Koch M, Wuensch T, et al. Graft-derived cell-free DNA, a noninvasive early rejection and graft damage marker in liver transplantation: A prospective, observational, multicenter cohort study. PloS Med (2017) 14(4):e1002286. doi: 10.1371/journal.pmed.1002286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Levitsky J, Kandpal M, Guo K, Kleiboeker S, Sinha R, Abecassis M, et al. Donor-derived cell-free DNA levels predict graft injury in liver transplant recipients. Am J Transplant. (2022) 22(2):532–40. doi: 10.1111/ajt.16835 [DOI] [PubMed] [Google Scholar]
- 111. Abdelaziz O, Osman AMA, Hosny KA, Emad-Eldin S, Serour 1DK, Mostafa M. Management of early hepatic artery thrombosis following living-donor liver transplantation: feasibility, efficacy and potential risks of endovascular therapy in the first 48 hours post-transplant-a retrospective cohort study. Transpl Int (2021) 34(6):1134–49. doi: 10.1111/tri.13839 [DOI] [PubMed] [Google Scholar]
- 112. Park J, Kim SH, Park SJ. Hepatic artery thrombosis following living donor liver transplantation: A 14-year experience at a single center. J Hepatobiliary Pancreat Sci (2020) 27(8):548–54. doi: 10.1002/jhbp.771 [DOI] [PubMed] [Google Scholar]
- 113. Duffy JP, Hong JC, Farmer DG, Ghobrial RM, Yersiz H, Hiatt JR, et al. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am Coll Surg (2009) 208(5):896–903. doi: 10.1016/j.jamcollsurg.2008.12.032 [DOI] [PubMed] [Google Scholar]
- 114. Xu M, Dong C, Sun C, Wang K, Zhang W, Wu D, et al. Management and outcome of hepatic artery thrombosis with whole-liver transplantation using donors less than one year of age. J Pediatr Surg (2022), 54(11):656–65. doi: 10.1016/j.jpedsurg.2022.05.009 [DOI] [PubMed] [Google Scholar]
- 115. Massberg S, Grahl L, von Bruehl M-L, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med (2010) 16(8):887–96. doi: 10.1038/nm.2184 [DOI] [PubMed] [Google Scholar]
- 116. Shi C, Yang L, Braun A, Anders H. Extracellular DNA-a danger signal triggering immunothrombosis. Front Immunol (2020) 11:568513. doi: 10.3389/fimmu.2020.568513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Carminita E, Crescence L, Panicot-Dubois L, Dubois C. Role of neutrophils and NETs in animal models of thrombosis. Int J Mol Sci (2022) 23(3):1411. doi: 10.3390/ijms23031411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gould TJ, Lysov Z, Liaw PC. Extracellular DNA and histones: double-edged swords in immunothrombosis. J Thromb Haemost. (2015) 13 Suppl 1:S82–91. doi: 10.1111/jth.12977 [DOI] [PubMed] [Google Scholar]
- 119. Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med (2009) 15(11):1318–21. doi: 10.1038/nm.2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wendelboe AM, Raskob GE. Global burden of thrombosis: Epidemiologic aspects. Circ Res (2016) 118(9):1340–7. doi: 10.1161/CIRCRESAHA.115.306841 [DOI] [PubMed] [Google Scholar]
- 121. Riegger J, Byrne RA, Joner M, Chandraratne S, Gershlick AH, Ten Berg JM, et al. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: A report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur Heart J (2016) 37(19):1538–49. doi: 10.1093/eurheartj/ehv419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenböck A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res (2015) 116(7):1182–92. doi: 10.1161/CIRCRESAHA.116.304944 [DOI] [PubMed] [Google Scholar]
- 123. Arshad F, Lisman T and Porte RJ. Hypercoagulability as a contributor to thrombotic complications in the liver transplant recipient. Liver Int (2013) 33(6):820–7. doi: 10.1111/liv.12140 [DOI] [PubMed] [Google Scholar]
- 124. Zhang H, Goswami J, Varley P, van der Windt DJ, Ren J, Loughran P, et al. Hepatic surgical stress promotes systemic immunothrombosis that results in distant organ injury. Front Immunol (2020) 11:987. doi: 10.3389/fimmu.2020.00987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol (2017) 14(4):203–17. doi: 10.1038/nrgastro.2016.193 [DOI] [PubMed] [Google Scholar]
- 126. Costentin CE, Bababekov YJ, Zhu AX, Yeh H. Is it time to reconsider the Milan criteria for selecting patients with hepatocellular carcinoma for deceased-donor liver transplantation? Hepatology (2019) 69(3):1324–36. doi: 10.1002/hep.30278 [DOI] [PubMed] [Google Scholar]
- 127. Straś WA, Wasiak D, Łągiewska B, Tronina O, Hreńczuk M, Gotlib J, et al. Recurrence of hepatocellular carcinoma after liver transplantation: Risk factors and predictive models. Ann Transplant. (2022) 27:e934924. doi: 10.12659/AOT.934924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kim SJ, Kim JM. Prediction models of hepatocellular carcinoma recurrence after liver transplantation: A comprehensive review. Clin Mol Hepatol (2022) 28(4): 739–753. doi: 10.3350/cmh.2022.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. European Association for the study of the liver. EASL clinical practice guidelines:Management of hepatocellular carcinoma. J Hepatol (2022) 77(2):479–502. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 130. Kornberg A, Kaschny L, Kornberg J, Friess H. Preoperative prognostic nutritional index may be a strong predictor of hepatocellular carcinoma recurrence following liver transplantation. J Hepatocell Carcinoma. (2022) 9:649–60. doi: 10.2147/JHC.S366107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang D, Feng D, Ren M, Bai Y, Lui Z, Wang H, et al. Preoperative serum hepatitis b virus DNA was a risk factor for hepatocellular carcinoma recurrence after liver transplantation. Ann Med (2022) 54(1):2213–21. doi: 10.1080/07853890.2022.2107233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, et al. DNA Of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature (2020) 583(7814):133–8. doi: 10.1038/s41586-020-2394-6 [DOI] [PubMed] [Google Scholar]
- 133. Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorn J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med (2016) 8(361):361ra138. doi: 10.1126/scitranslmed.aag1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Snoderly HT, Boone BA, Bennewitz MF. Neutrophil extracellular traps in breast cancer and beyond: current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res (2019) 21(1):145. doi: 10.1186/s13058-019-1237-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science (2018) 361(6409):eaao4227. doi: 10.1126/science.aao4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tadie JM, Bae HB, Jiang S, Park DW, Bell CP, Yang H, et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with toll-like receptor 4. Am J Physiol Lung Cell Mol Physiol (2013) 304(5):L342–9. doi: 10.1152/ajplung.00151.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Leach J, Morton JP, Sansom OJ. Neutrophils: Homing in on the myeloid mechanisms of metastasis. Mol Immunol (2019) 11:69–76. doi: 10.1016/j.molimm.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Leal AC, Mizurini DM, Gomes T, Rochael NC, Saraiva EM, Dias MS, et al. Tumor-derived exosomes induce the formation of neutrophil extracellular traps: Implications for the establishment of cancer-associated thrombosis. Sci Rep (2017) 7(1):6438. doi: 10.1038/s41598-017-06893-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature (2019) 566(7745):553–7. doi: 10.1038/s41586-019-0915-y [DOI] [PubMed] [Google Scholar]
- 140. Wang H, Zhang Y, Wang Q, Wei X, Wang H, Gu K. The regulatory mechanism of neutrophil extracellular traps in cancer biological behavior. Cell Biosci (2021) 11(1):193. doi: 10.1186/s13578-021-00708-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin-mediated interactions. Int J Cancer. (2017) 140(10):2321–30. doi: 10.1002/ijc.30635 [DOI] [PubMed] [Google Scholar]
- 142. Yang LY, Shen XT, Sun HT, Zhu WW, Zhang JB, Lu L. Neutrophil extracellular traps in hepatocellular carcinoma are enriched in oxidized mitochondrial DNA which is highly pro-inflammatory and pro-metastatic. J Cancer. (2022) 13(4):1261–71. doi: 10.7150/jca.64170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cheng Y, Gong Y, Chen X, Zhang Q, Zhang X, He Y, et al. Injectable adhesive hemostatic gel with tumor acidity neutralizer and neutrophil extracellular traps lyase for enhancing adoptive NK cell therapy prevents post-resection recurrence of hepatocellular carcinoma. Biomaterials (2022) 284:121506. doi: 10.1016/j.biomaterials.2022.121506 [DOI] [PubMed] [Google Scholar]
- 144. Rosell A, Aguilera K, Hisada Y, Schmedes Y, Mackman N, Wallén H, et al. Prognostic value of circulating markers of neutrophil activation, neutrophil extracellular traps, coagulation and fibrinolysis in patients with terminal cancer. Sci Rep (2021) 11(1):5074. doi: 10.1038/s41598-021-84476-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med (2010) 207(9):1853–62. doi: 10.1084/jem.20100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, Gallant M, et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood (2015) 125(12):1948–56. doi: 10.1182/blood-2014-07-587709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Manfredi AA, Rovere-Querini P, D’Angelo A, Maugeri N. Low molecular weight heparins prevent the induction of autophagy of activated neutrophils and the formation of neutrophil extracellular traps. Pharmacol Res (2017) 123:146–56. doi: 10.1016/j.phrs.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 148. Lelliott PM, Momota M, Shibahara T, Lee MSJ, Smith NI, Ishii KJ, et al. Heparin induces neutrophil elastase-dependent vital and lytic NET formation. Int Immunol (2020) 32(5):359–68. doi: 10.1093/intimm/dxz084 [DOI] [PubMed] [Google Scholar]
- 149. Gollomp K, Kim M, Johnston I, Hayes V, Welsh J, Arepally GM, et al. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight (2018) 3(18):e99445. doi: 10.1172/jci.insight.99445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Perdomo J, Leung HHL, Ahmadi Z, Yan F, Chong JJH, Passam FH, et al. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat Commun (2019) 10(1):1322. doi: 10.1038/s41467-019-09160-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Luo Y, Arita K, Bhatia M, Knuckley B, Lee YH, Stallcup MR, et al. Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization. Biochemistry (2006) 45(39):11727–36. doi: 10.1021/bi061180d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol (2015) 11(3):189–91. doi: 10.1038/nchembio.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Li M, Lin C, Deng H, Strnad J, Bernabei L, Vogl DT, et al. A novel peptidylarginine deiminase 4 (PAD4) inhibitor BMS-P5 blocks formation of neutrophil extracellular traps and delays progression of multiple myeloma. Mol Cancer Ther (2020) 19(7):1530–8. doi: 10.1158/1535-7163.MCT-19-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Rayes RF, Mouhanna JG, Nicolau I, Bourdeau F, Giannias B, Rousseau S, et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight (2019) 5(16):e128008. doi: 10.1172/jci.insight.128008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Chirivi RGS, van Rosmalen JWG, van der Linden M, Euler M, Schmets G, Bogatkevich G, et al. Therapeutic ACPA inhibits NET formation: A potential therapy for neutrophil-mediated inflammatory diseases. Cell Mol Immunol (2021) 18(6):1528–44. doi: 10.1038/s41423-020-0381-3 [DOI] [PMC free article] [PubMed] [Google Scholar]