Abstract
Attenuated Salmonella enterica serovar Typhi live vector vaccine strains are highly immunogenic in mice following intranasal but not orogastric inoculation. To elucidate the relationship between organs within which vaccine organisms are found and the induction of specific serum immunoglobulin G (IgG) antibodies, we examined the in vivo distribution of serovar Typhi vaccine strain CVD 908-htrA following intranasal administration. Vaccine organisms were cultured from the nasal lymphoid tissue (NALT), lungs, and Peyer's patches 2 min after intranasal inoculation. Vaccine organisms persisted longer in NALT than in other organs. By decreasing the volume of intranasal inoculum containing 109 CFU (from a single 30- or 10-μl dose to four 2.5-μl doses given over the course of 1 h), we were able to significantly reduce the number of vaccine organisms isolated from the lungs (P < 0.05) without reducing the number of vaccine organisms in NALT. Reducing the number of vaccine organisms in the lungs resulted in a significant decrease in the serum tetanus antitoxin response elicited by CVD 908-htrA expressing tetanus toxin fragment C under the control of the redox-responsive nir15 promoter. In contrast, a similar construct expressing tetanus toxin fragment C under control of the constitutive lpp promoter stimulated a strong serum IgG tetanus antitoxin response with both inoculation regimens. The data suggest that following intranasal inoculation, NALT is a sufficient inductive site for elicitation of an immune response against both the live vector and heterologous antigen and, as occurs following oral inoculation of humans, attenuated serovar Typhi vaccine organisms elicit serum IgG responses.
Attenuated Salmonella enterica serovar Typhi strains can serve as live oral vaccines to prevent typhoid fever. These strains also show much promise as live vector vaccines to deliver foreign antigens to the human immune system because of their ability to elicit serum immunoglobulin G (IgG), mucosal IgA, and a variety of cell-mediated immune responses, including cytotoxic T lymphocytes (9, 12, 14, 20–23). For years an important obstacle to the development of attenuated serovar Typhi vaccines and live vectors was the lack of a small-animal model in which to reliably assess immunogenicity of candidate vaccine strains following mucosal inoculation (14, 15). Because of the narrow host restriction of serovar Typhi, oral inoculation of mice with potential serovar Typhi vaccine strains typically resulted in minimal, if any, immune responses that paled by comparison with the responses elicited by attenuated serovar Typhimurium strains administered to mice orally. A serendipitous breakthrough was made when Galen et al. (8) observed that attenuated serovar Typhi administered intranasally to mice were strongly immunogenic, particularly in eliciting a serum IgG response. This model has since been extensively used to assess the immunogenicity of attenuated serovar Typhi live vector constructs expressing foreign antigens (1, 17a, 18). Despite the established utility of this murine intranasal model (1, 8), almost nothing that explains the extraordinary immunogenicity of serovar Typhi vaccine strains administered by this route is known. Accordingly, herein we report the results of in vivo studies performed to characterize the murine intranasal model, including determination of the anatomic sites where attenuated serovar Typhi vaccine organisms are found following intranasal inoculation and the identification of some parameters that influence immunogenicity.
MATERIALS AND METHODS
Culture of vaccine strain.
The vaccine strain used was a ΔaroC ΔaroD ΔhtrA derivative of wild-type serovar Typhi strain Ty2, designated CVD 908-htrA (22, 23). Strains were streaked from a frozen glycerol stock onto rich agar medium (super agar; 32 g of tryptone, 20 g of yeast extract, 5 g of sodium chloride [Difco, Detroit, Mich.; Sigma Chemical, St. Louis, Mo.]) supplemented with 0.0001% dihydroxybenzoic acid (DHB; Sigma) and grown overnight at 37°C. Approximately 100 to 150 isolated colonies were suspended in 150 ml of Miller's Luria-Bertani broth (Gibco BRL, Gaithersburg, Md.) supplemented with 0.0001% DHB. This broth culture was incubated in a baffled flask at 37°C and 220 to 250 rpm in a rotary shaker/incubator. The culture was allowed to grow to an optical density at 600 nm of approximately 1.3, which corresponds to the late logarithmic phase of the CVD 908-htrA growth curve. The culture was pelleted and resuspended in 0.5 ml of sterile phosphate-buffered saline (PBS). The number of viable vaccine organisms was checked by plating serial dilutions of the inoculum onto salmonella-shigella (SS) agar (BBL, Becton Dickinson and Co., Cockeysville, Md.). We compared growth of CVD 908-htrA on Luria agar supplemented with DHB and on SS agar and determined that addition of DHB to this medium was unnecessary. SS agar, which is composed primarily of protease digests of animal tissue and nutrient and bile salts, provides the vaccine strain with the required aromatic compounds.
Inoculation of mice.
Five- to eight-week-old BALB/c female mice (Charles River Laboratories) were inoculated intranasally in the right naris with 1 × 109 to 4 × 109 CFU of a vaccine suspension by means of a pipette delivering either a single 30- or 10-μl volume or four fractional volumes of 2.5 μl each (spaced 15 min apart). Mice were inoculated via the orogastric route by placing 100 μl of a vaccine suspension containing 2 × 109 to 4 × 109 CFU into the lower esophagus, using a gavage needle. Mice were inoculated intravenously by injecting 100 μl of a vaccine suspension containing 2 × 108 CFU directly into the tail vein, using a tuberculin syringe fitted with a 25-gauge needle. Anesthesia was not used for any inoculation procedure. Booster doses were given 28 to 30 days following the primary inoculation and in the identical manner.
Harvesting of tissues.
Ten hours following inoculation, mice were sacrificed by CO2 asphyxiation. For kinetics experiments, animals were bled and sacrificed at 2 and 15 min and at 1, 10, 18, and 72 h. Organs were removed aseptically and placed in 0.5 ml of chilled, sterile PBS. The entire palate containing the nasal lymphoid tissue and neighboring epithelium (NALT) was removed by the method of Wu et al. (25). Blood, lungs, Peyer's patches (PP; seven patches per animal), and NALT were removed in all experiments. Additional organs and tissues removed for particular experiments included spleen, liver, cervical lymph nodes (CLN), mesenteric lymph nodes (MLN), gallbladder, and bone marrow. In an additional set of experiments, the entire gut was cultured 2 min or 1 h following inoculation of mice with 2 × 109 CFU in a volume of 10 μl. Mice were sacrificed by CO2 asphyxiation, and the alimentary tract was removed from mid-esophagus to rectum; this tissue will be referred to as whole gut.
Homogenization and plating of tissues.
Organs were aseptically homogenized in an electric homogenizer, and lungs, NALT, and PP were diluted 10- to 100-fold in sterile PBS. All organs removed from intravenously inoculated mice were similarly diluted. Neat and diluted tissue homogenates were spread onto fresh, dry SS agar, and the plates were incubated at 37°C overnight. In experiments where the entire gut was removed, this tissue was homogenized in sterile PBS in a total volume of 5 ml. This homogenate was diluted 10- to 1,000,000-fold in sterile PBS, and 100 μl of diluted homogenate was spread onto fresh, dry SS agar. Colonies were counted and scored as the sum of CFU per whole homogenized organ or, in the case of PP, colonies per seven individual PP.
Measurement of serum antibody.
Mice were bled from the retro-orbital sinus at 0, 2, 4, 6, and 8 weeks following inoculation to obtain serum. Serum anti-lipopolysaccharide (LPS) and tetanus antitoxin were measured by enzyme-linked immunosorbent assay (ELISA). Immulon2 polystyrene microtiter ELISA plates (Dynex Technologies, Inc., Chantilly, Va.) were coated with serovar Typhi LPS (0.5 μg/well; Difco) in carbonate buffer, pH 9.1. Similarly, to measure serum IgG tetanus antitoxin, plates were coated with tetanus toxoid (0.2 μg/well; Connaught Laboratories, Swiftwater, Pa.) in carbonate buffer, pH 9.1. Serum IgG specific for the listed antigens was detected with alkaline phosphatase-conjugated goat anti-mouse IgG and p-nitrophenylphosphate substrate (Kirkegaard & Perry Laboratories, Bethesda, Md.). Colorimetric reactions were stopped with 3 N NaOH, and optical density at 405 nm was measured. The endpoint titer was defined as the maximum serum dilution having an optical density greater than 0.1. The 0.1 optical density cutoff value was experimentally determined to be the mean plus 2 standard deviations of the optical density of 20 normal mouse serum samples measured at a dilution of 1:20.
Statistics.
The numbers of vaccine organisms isolated from murine organs in the localization experiments were compared by the Mann-Whitney U test. For immunogenicity experiments, endpoint titers determined by ELISA experimental groups were compared by Student's two-tailed t test. For all comparisons, P < 0.05 was considered significant.
RESULTS
Effect of inoculum volume on in vivo distribution of CVD 908-htrA.
In early experiments, the volume of the inoculum containing serovar Typhi was 30 μl (which approximates the volume of the murine nasal cavity) (10). Preliminary experiments performed to determine the in vivo distribution of vaccine organisms following intranasal inoculation of mice with a 30-μl inoculum containing 2 × 109 CFU of CVD 908-htrA showed that vaccine organisms were always isolated from the NALT, lungs, and PP but not from the spleen, lymph nodes, or blood. Recognizing that ingestion or aspiration of vaccine organisms could affect the distribution of attenuated serovar Typhi within the mouse, a much smaller inoculum volume (10 μl) was tested to begin to ascertain the effect of inoculum volume on the distribution pattern of attenuated serovar Typhi in the murine NALT, lungs, and PP. Mice were inoculated with 109 CFU in a volume of either 10 or 30 μl, as shown in Table 1, which summarizes the methods used for this and each subsequent experiment. The results of two separate experiments showed that there was no significant difference (P > 0.05) in the numbers of vaccine organisms cultured from the NALT, lungs, and PP of mice that received the 10 μl versus those that got the 30-μl inoculum (Fig. 1A).
TABLE 1.
Summary of design of experiments
| Expt | No. of doses | CFU/dosea | Inoculum vol (μl) | Related figure |
|---|---|---|---|---|
| Effect of inoculum volume on in vivo distribution of CVD 908-htrA | 1 | 109 | 10 | 1 |
| 1 | 109 | 30 | ||
| Effect of inoculum volume on immunogenicity of CVD 908-htrA(pTETnir15) | 2 | 109 | 10 | 1 |
| 2 | 109 | 30 | ||
| Determination of sites and kinetics of in vivo distribution of vaccine organisms | 1 | 109 | 10 | 2 |
| Effect of route of inoculation | 1 intranasal | 109 | 10 | |
| 1 orogastric | 109 | 100 | 3, 4 | |
| 1 intravenous | 108 | 100 | ||
| In vivo distribution of CVD 908-htrA administered by a fractional-dose regimen | 1 fractional | 109 | 2.5 × 4 | 5 |
| 1 full | 109 | 10 | ||
| Effect of fractional dosage on immunogenicity of: | ||||
| CVD 908-htrA(pTETnir15) | 2 fractional (days 0 and 30) | 109 | 2.5 × 4 | 6 |
| CVD 908-htrA(pTETlpp) | 2 full (days 0 and 30) | 109 | 10 |
Range = 1.3 × 109 to 3.99 × 109 CFU/dose.
FIG. 1.
(A) In vivo distribution of CVD 908-htrA in the NALT, lungs, or PP of mice 10 h after intranasal administration of 10 μl (n = 5) (●) or 30 μl (n = 6) (○) containing 109 CFU. Each plot point represents a single mouse; bars represent group geometric mean. (B) Serum IgG responses against tetanus toxin and serovar Typhi LPS in mice inoculated intranasally with 10 μl (n = 10) or 30 μl (n = 10) containing 109 CFU of CVD 908-htrA(pTETnir15). Arrowheads indicate the time of the administration of the booster dose; error bars represent standard error.
Effect of inoculum volume on immunogenicity of CVD 908-htrA.
In analogous experiments, we investigated the influence of inoculum volume on immunogenicity, using CVD 908-htrA(pTETnir15) administered intranasally. This live vector vaccine construct expresses tetanus toxin fragment C under the control of the redox-responsive nir15 promoter (4, 8, 19) and has been used extensively as a model heterologous antigen system. We chose this model system in order to assess serum immune responses both against the live vector and against the heterologous antigen. Mice were inoculated on day 0 and boosted on day 30 and then were bled at 2-week intervals, twice following the first inoculation and twice following the second inoculation. Mice inoculated intranasally on days 0 and 30 with 109 CFU of CVD 908-htrA(pTETnir15) in a volume of either 10 or 30 μl showed no significant difference in the magnitude of serum IgG responses against tetanus toxin fragment C (Fig. 1B) or serovar Typhi LPS (Fig. 1B) between the two groups. Based on the results of both the in vivo localization and the immunogenicity experiments, the volume of inoculum used in the remaining experiments was 10 μl unless otherwise noted. This smaller volume was also easier to deliver to the mice and appeared to be less stressful to the animals.
Determination of kinetics of in vivo distribution of vaccine organisms.
The kinetics of in vivo distribution of CVD 908-htrA in the organs of the mouse were examined at 2 and 15 min and at 1, 10, 18, and 72 h following intranasal inoculation with 109 CFU. For the kinetics experiment we examined nine mice (three mice, repeated three times) at 2 min, three mice at 15 min, nine mice (three mice, repeated three times) at 1 h, 15 mice (3 mice, repeated five times) at the 10-h time point, six mice (three mice, repeated two times) at 18 h and four mice at 72 h. CVD 908-htrA was used in this and the following in vivo distribution experiments because we wished to establish baseline characteristics of in vivo distribution and because in preliminary experiments we noted that CVD 908-htrA and CVD 908-htrA(pTETnir15) were isolated in equivalent numbers from the murine organs. As early as 2 min after intranasal inoculation of mice, attenuated serovar Typhi was cultured from the NALT and lungs. Notably, serovar Typhi was also isolated from the PP at this early time point (Fig. 2). At each time point thereafter, vaccine organisms were cultured from the NALT, lungs, and PP, albeit in progressively lower numbers. At 1 and 10 h after inoculation, the geometric mean numbers of vaccine organisms isolated from the NALT and PP were almost identical (approximately 4.5 and 4.0 logs, respectively). At the 72-h time point, 1 log of vaccine organisms could still be recovered from the NALT (Fig. 2A), whereas less than one organism, on average, was isolated from either the lungs (Fig. 2B) or PP (Fig. 2C).
FIG. 2.
Kinetics of in vivo distribution of CVD 908-htrA in murine NALT, lungs, and PP 2 min (n = 9) (●), 15 min (n = 3) (○), 1 h (n = 9) (▾), 10 h (n = 15) (▿), 18 h (n = 6) (■), and 72 h (n = 4) (□) after inoculation with 10 μl containing 109 CFU. Each plot point represents a single mouse; bars represent group geometric mean.
Culture of the whole gut.
It became clear from the kinetics experiments that the number of vaccine organisms recovered was around 1% of the total inoculum delivered. To determine the fate of the majority of organisms, 2 min and 1 h after inoculation we cultured the whole gut (esophagus to rectum) of mice inoculated intranasally with 2 × 109 CFU in a 10-μl volume. Two minutes after inoculation, we isolated 7 logs of vaccine organisms, i.e., approximately 3% of the total inoculum, from the whole gut. One hour following inoculation, we isolated from the whole gut 8 logs of vaccine organisms, equivalent to approximately 11% of the total inoculum and significantly more (P < 0.02) than the number isolated 2 min after inoculation (data not shown).
Route of inoculation (i) Intranasal versus orogastric.
We previously reported marked differences in the magnitude of the immune response elicited by attenuated serovar Typhi live vectors against serovar Typhi LPS and heterologous antigen when mice were inoculated via the orogastric versus the intranasal route (1, 8). To determine if this striking discrepancy is related to differences in the distribution of vaccine organisms in the various murine organs, mice were inoculated with CVD 908-htrA via these routes.
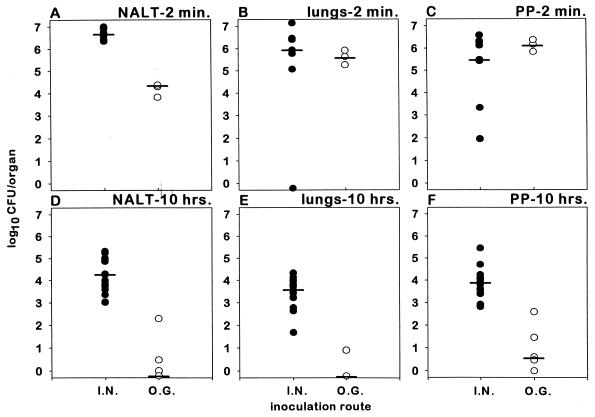
Two minutes after inoculation with 109 CFU, significantly more vaccine organisms were isolated from the NALT (n = 9, geometric mean = 4.4 × 106 CFU [Fig. 3A]) of mice inoculated intranasally than from the NALT of those inoculated orogastrically (n = 3, geometric mean = 1.4 × 104 CFU) (P < 0.05). In contrast, following either intranasal or orogastric inoculation, the numbers of vaccine organisms cultured from the lungs and PP were not significantly different (Fig. 3B and C) (P = 0.3 for comparison of each organ). Ten hours after inoculation, strikingly lower numbers of vaccine organisms (<1 log) were recovered from the NALT, lungs and PP of orogastrically inoculated mice (n = 6, repeated twice) versus mice (n = 15) inoculated by the intranasal route (3 to 5 logs) (P < 0.01 for comparison of each organ) (Fig. 3D, to F).
FIG. 3.
In vivo distribution of CVD 908-htrA in the NALT, lungs, and PP of mice 2 min (n = 9 for the intranasal [I.N.] group and n = 3 for the orogastric [O.G.] group) and 10 h (n = 15 for the intranasal group and n = 6 for the orogastric group) after intranasal (●) or orogastric (○) inoculation of mice with 10 μl containing 109 CFU. Each plot point represents a single mouse; bars represent group geometric mean.
(ii) Intranasal versus intravenous.
The organs from which vaccine organisms could not be isolated were also quite revealing. Attenuated serovar Typhi was not cultured from the spleen, CLN, or MLN at 3, 10, 18, or 72 h after intranasal inoculation. Furthermore, no vaccine organisms were isolated from peripheral blood removed via the tail vein or from cardiac blood at any time point. Whereas vaccine organisms were occasionally observed in the liver at 10 h, the numbers were inconsistent and less than 700 CFU/liver (geometric mean < 50 CFU/liver).
Intravenous inoculation gave a distinct pattern of widespread involvement of all organs of the reticuloendothelial system. Ten hours following intravenous inoculation, large numbers of vaccine organisms were cultured from the spleen, lungs, NALT, PP, MLN, and CLN (Fig. 4) as well as from liver, gallbladder, bone marrow, and peripheral blood removed from the tail vein (data not shown). In mice inoculated intranasally, vaccine organisms were consistently cultured only from the NALT, lungs, and PP. Ten hours following inoculation, the numbers of vaccine organisms recovered from lungs and PP (Fig. 4B and C) of intravenously inoculated mice were 4 and 1 log higher, respectively, than the number of vaccine organisms recovered from the same organs of intranasally inoculated mice. The opposite was true for cultures of NALT, as 1.5 logs more vaccine organisms were recovered from mice inoculated intranasally than from those inoculated intravenously (Fig. 4A). These observations agree with earlier experiments wherein vaccine organisms administered via the intravenous route were observed in spleen, liver, lungs, CLN, MLN, and blood at very high levels 24 h after inoculation (data not shown).
FIG. 4.
In vivo distribution of CVD 908-htrA in the NALT, lungs, PP, spleen, CLN, and MLN of mice 10 h (n = 3 for the intranasal [I.N.] group and n = 2 for the intravenous [I.V.] group) after intranasal inoculation of mice with 10 μl containing 109 CFU (●) or intravenous inoculation of mice with 100 μl containing 108 CFU (○). Each plot point represents a single mouse; bars represent group geometric mean.
Organ distribution of CVD 908-htrA administered by a fractional-dose regimen.
Having determined that vaccine organisms introduced by the intranasal route could be isolated from murine lungs, NALT, and PP, we attempted to manipulate the model in such a manner that vaccine organisms would be readily found in the NALT but few would reach the lungs. Only if this could be accomplished could we be confident in drawing conclusions about the role of NALT versus the lungs in eliciting serum IgG antibody responses. We assumed that the larger the volume of intranasal inoculum, the greater the chance that vaccine organisms might be aspirated into the lungs of the mice. Accordingly, to successfully deliver vaccine organisms to the NALT while diminishing aspiration into the lungs, we divided the standard full 10-μl dose of CVD 908-htrA into four 2.5-μl fractional intranasal doses, with one 2.5-μl dose being administered every 15 min over the course of 1 h. In this manner, at the end of a 1-h period, the total volume of inoculum and the total CFU of vaccine organisms administered were equal to those delivered with the single 10-μl dose full-dose regimen.
In preliminary experiments, the lungs of mice cultured 10 h after administration of the fractional-dose regimen failed to yield attenuated serovar Typhi. This finding, in stark contrast to the results for mice inoculated with the 10-μl full-dose regimen, supported the utility of the fractional-dose regimen. In a subsequent experiment (Fig. 5), we examined the kinetics of tissue localization in mice receiving the fractional-dose regimen by culturing organs harvested at 2 min and at 1, 5, 10, 18, and 72 h following the fourth 2.5-μl dose. These kinetics experiments in which specimens were obtained just 2 min after intranasal inoculation yielded surprising results. At the earliest time point (i.e., 2 min after inoculation), vaccine organisms were recovered from the lungs in numbers similar to what was found in mice that received a full 10-μl dose of vaccine. Only at subsequent time points were striking differences noted. In mice that received the fractional-dose regimen, counts of vaccine organisms plummeted such that by 10 h after inoculation, vaccine organisms could not be recovered from the lungs. In contrast, in mice that received the full 10-μl dose of vaccine, the fall in counts was not precipitous; approximately 4 logs of vaccine organisms were still recovered from the lung at 10 h after inoculation (P < 0.01) (Fig. 5B). After the 1-h time point, significant decreases were also observed in the numbers of vaccine organisms isolated from the NALT and PP (P < 0.05) (Fig. 5A and C). However, the number of vaccine organisms isolated from the NALT of mice inoculated via the fractional-dose regimen differed from that of mice receiving the full-dose regimen only at the 10-h time point. From the 18-h time point onward, the numbers of vaccine organisms isolated from the NALT of mice in each of these inoculation groups were nearly equal, remaining at approximately 1 log. In contrast, the number of vaccine organisms isolated from the lungs and PP dropped to less than one organism on average from the 10-h time point onward.
FIG. 5.
Comparison of the kinetics of in vivo distribution of CVD 908-htrA in the NALT, lungs, and PP of mice inoculated intranasally by either the full-dose regimen (●) or the fractional-dose regimen (○). The numbers of mice analyzed at each time point were as follows: nine at 2 min, six at 1 h, nine at 10 h, nine at 18 h, and seven at 72 h. Each plot point represents the geometric mean of the group. The full-dose regimen data are compiled from results depicted in Fig. 3.
Effect of fractional dosage on immunogenicity of serovar Typhi live vector strains.
We next undertook to determine whether the overall reduction in the number of attenuated serovar Typhi in the NALT, lungs, and PP at 10 h after intranasal inoculation with the fractional-dose regimen affects the serum IgG response. Two groups of mice were inoculated intranasally with CVD 908-htrA(pTETnir15); one group received a single 10-μl full dose, while the second group received the same inoculum in four 2.5-μl fractional doses. Mice were administered a booster dose on day 30. Whereas both groups of animals exhibited a serum IgG response against serovar Typhi LPS (Fig. 6B), serum IgG tetanus antitoxin titers were significantly decreased in the mice that received the fractional-dose regimen (P < 0.01) (Fig. 6A). Since the tetanus toxin fragment C gene in this construct is under control of the redox-regulated nir15 promoter, we surmised the most likely explanation for this result is that this promoter is induced when vaccine organisms reach the milieu of the lungs but not when they are in the environment of the NALT.
FIG. 6.
Comparison of the effects of fractional-dose and full-dose regimens on the elicitation of serum IgG against tetanus toxin and serovar Typhi LPS in mice inoculated intranasally with either CVD 908-htrA(pTETnir15) or CVD 908-htrA(pTETlpp), as indicated. Arrowheads indicate the time of administration of the booster dose; error bars represent the standard error.
To test this promoter hypothesis, we conducted a final experiment that was identical in design to the last experiment comparing the immunogenicity of one full 10-μl dose versus four 2.5-μl fractional doses except that in this experiment CVD 908-htrA carried a plasmid encoding tetanus toxin fragment C under control of a powerful constitutive lpp promoter (16). As in the previous experiment, both groups exhibited a serum anti-LPS IgG response (Fig. 6D). However, in this instance the tetanus antitoxin response was strong in both groups of mice (Fig. 6C).
DISCUSSION
The discovery that strong immune responses could be elicited in mice following intranasal but not oral inoculation has revolutionized serovar Typhi live vector vaccinology by providing a model for preclinical testing. However, the physiological basis for the effectiveness of intranasal inoculation was not known. We were unaware, for example, of the organ distribution of attenuated serovar Typhi after intranasal inoculation of mice and, by extension, of the sites of immune induction for the strong serum antibody response that follows intranasal inoculation. The results of the experiments reported herein have determined that depending on the volume of the intranasal inoculum administered, in addition to NALT, variable numbers of vaccine organisms are found in the lungs and PP of mice as early as 2 min after inoculation. To find large numbers of vaccine organisms in the PP of the distal ileum just 2 min after intranasal inoculation was startling. We reasoned that the most likely routes by which the vaccine organisms could reach this distant site so rapidly would be via (i) the blood or (ii) ingestion followed by intestinal motility. Three key observations argue against blood-borne spread of vaccine organisms. First, vaccine organisms were never isolated from cardiac blood or peripheral blood removed from the tail vein. Second, if a marked vaccine bacteremia was occurring, one would expect to detect comparable numbers of vaccine organisms in the spleen, liver, bone marrow, and other organs of the reticuloendothelial system, an observation not made in mice inoculated intranasally. Indeed, in mice inoculated intravenously with CVD 908-htrA, large numbers of vaccine organisms were cultured from these organs, in addition to the lungs and PP. Third, in cultures of the whole mouse gut, we were able to recover 3% of the total bacterial inoculum that had been administered intranasally 2 min earlier. Therefore, we conclude that an early substantial bacteremia was probably not responsible for vaccine organisms reaching the lungs and PP. Rather, it is more likely that vaccine organisms reached the lungs by aspiration from the nasopharynx and that they reached the PP by ingestion followed by rapid intestinal transit.
By 72 h after inoculation, vaccine organisms were no longer recoverable from the lungs and PP but were still isolated, albeit in small numbers, from the NALT. Thus, it appears that serovar Typhi vaccine strain CVD 908-htrA does not multiply in, and is rapidly cleared from, murine tissues. These findings concur with those of O'Brien (17), who concluded that following intravenous inoculation of mice with wild-type serovar Typhi, murine tissues do not support the growth of this organism. Similarly, Carter and Collins (2) determined that wild-type serovar Typhi strain Ty2 was largely cleared within 24 h of being administered intravenously to mice. An auxotroph such as CVD 908-htrA should be even less capable of in vivo multiplication in murine tissues (13).
Two minutes after a 109-CFU inoculum of serovar Typhi live vector was delivered intranasally in a volume of 10 μl, we were able to recover from the lungs, NALT, and PP combined approximately 1% of the total inoculum administered; an additional 3% could be accounted for from cultured of the whole gut. We hypothesize that most of the remaining intranasally administered inoculum entered the stomach from the nasopharynx, where the ingested vaccine organisms were then killed in the acidic gastric environment. In support of this hypothesis is the report of Carter and Collins on a similarly designed mouse experiment in which cultures of the whole gut yielded only 1% of the total inoculum 1 h after orogastric inoculation with mouse pathogen Salmonella serovar Enteriditis (3).
We surmised that a comparison of the distribution of vaccine organisms in murine organs after intranasal inoculation versus orogastric inoculation might provide important insights regarding the inductive sites responsible for the strong immunologic responses associated with intranasal inoculation. Notably, 2 min after inoculation, similar large numbers of vaccine organisms were detected in the lungs and PP of both intranasally and orogastrically inoculated mice, whereas counts in NALT were significantly higher in mice inoculated intranasally. Ten hours following inoculation, the number of vaccine organisms isolated from the NALT and lungs had dwindled to less than 1 log, whereas large numbers of vaccine organisms were still recoverable from these organs in mice inoculated intranasally. Interestingly, 10 h after inoculation, fewer than 10 vaccine organisms on average were cultured from the PP of orogastrically inoculated mice, despite the fact that orogastric inoculation places the majority of vaccine organisms directly into the gut. If vaccine organisms are taken up by the PP from the intestinal lumen, it is not clear why higher and more persistent counts in the intestinal lymphoid follicles are obtained following intranasal compared to orogastric inoculation. The significant reduction in the number of vaccine organisms isolated from the organs of orogastrically inoculated mice between the 2-min and 10-h time points suggests that for some reason attenuated serovar Typhi may be cleared more rapidly from the tissues of the latter mice. Therefore, it is the lack of persistence in murine tissues that likely accounts in part for the poor serum antibody responses against both the serovar Typhi vector and heterologous antigen observed in orogastrically inoculated mice (8). The impressive persistence of vaccine organisms in the NALT following intranasal inoculation may occur because the vaccine organisms encounter there an environment less hostile than that in either the lungs or the PP (7, 11, 24). Furthermore, as vaccine organisms are cleared from the lungs and PP, organisms persisting in the NALT may continuously reseed these organs at a very low level via aspiration and ingestion. This could account for our ability to isolate organisms from these tissues at 10 and 18 h following intranasal but not orogastric inoculation.
The use of an intranasal fractional-dose regimen (2.5 μl administered intranasally every 15 min) in which few vaccine organisms were recovered from the lungs 1 h after inoculation was extremely helpful in discerning the relative contributions of NALT and lungs to the induction of serum antibody responses. The persistence of vaccine organisms in the NALT but not the lungs implies involvement of the NALT in the elicitation of immune responses. We hypothesized that if the NALT is a sufficient inductive site for the elicitation of serum IgG, then mice receiving the fractional-dose regimen should exhibit both serum anti-serovar Typhi LPS and tetanus antitoxin responses. In contrast, if the NALT is an inadequate inductive site and if involvement of the lungs is required to induce a serum immune response, then mice receiving the fractional-dose regimen should not exhibit a serum IgG response against either serovar Typhi LPS or tetanus toxin. The observations of strong serum anti-serovar Typhi LPS and tetanus antitoxin responses in mice inoculated with CVD 908-htrA(pTETlpp) support our hypothesis that the murine NALT is sufficient to induce serum IgG against both the serovar Typhi vector and tetanus toxin.
The inability of the fractional-dose regimen of CVD 908-htrA(pTETnir15) to elicit serum tetanus antitoxin, even though anti-serovar Typhi responses were elicited, and the contrasting strong tetanus antitoxin responses that followed immunization with the same number of CFU administered in a larger (10-μl) volume indicate that the nir15 promoter is activated in the environment of the murine lungs but not in the NALT. The nir15 promoter is believed to be induced in the intracellular environment, in particular within macrophages (5, 6). We therefore hypothesize that shortly after intranasal administration of vaccine organisms in an inoculum volume of 10 μl, numerous vaccine organisms reached the lungs, where they were internalized by alveolar macrophages; the nir15 promoter was thereupon induced, leading to expression of a high level of tetanus toxin fragment C and stimulation of high titers of serum antitoxin. Conversely, in mice that received the fractional-dose regimen of this same construct (a regimen in which few vaccine organisms reach the lungs), only low expression of tetanus toxin fragment C apparently ensued, resulting in a markedly lower tetanus antitoxin response.
The purpose of placing heterologous antigens under the control of environmentally responsive promoters is to delay antigen expression until the live vector strain is in an in vivo site appropriate for optimal induction of an immune response. Therefore, it is not unexpected that an environmentally regulated promoter such as the nir15 promoter would be induced in one in vivo site but not another. It is intriguing that the intranasal murine model which has been successfully used to assess the immunogenicity of serovar Typhi vaccine and live vector candidates may also be able to discern the capabilities of different promoters to induce expression of heterologous antigens. A wider array of environmentally regulated promoters must be assessed before we can conclude how reliably the murine intranasal model can predict promoter effectiveness in humans. An important consideration is that the in vivo environments encountered by serovar Typhi vaccine strains in a human host (e.g., the lumen of the small intestine) may vary widely with those encountered in the murine host (i.e., the nasal cavity). Although careful and systematic analysis of promoter efficacy has been initiated in attenuated serovar Typhimurium live vectors (5), similar analysis must also be carried out with serovar Typhi live vectors. Indeed, any promoter determined to be induced in vivo in the murine intranasal model must be subject to further analysis in clinical studies to determine the relevance of the promoter induction in humans. Some instances of the apparent failure of immunogenicity of live vectors carrying plasmid constructs with heterologous antigen expression under the control of environmentally regulated promoters may be attributable to misconceptions about the in vivo sites where the live vectors reside following mucosal inoculation and the environmental milieu in those sites.
When these studies were initiated, almost nothing was known regarding the mechanism by which serovar Typhi vaccine and live vector strains elicited serum IgG responses when given to mice by the intranasal route. Studies with the fractional-dose regimen suggest that the persistence of vaccine organisms in the NALT is sufficient to induce specific serum IgG responses. This is an important finding, since it indicates that introduction of vaccine organisms at a mucosal site in mice can induce a systemic immune response. As more human clinical trial results become available with serovar Typhi live vector construct, it will be possible to compare how well the immune responses of intranasally immunized mice predict those of orally immunized humans.
ACKNOWLEDGMENTS
This work was supported by grants RO1 AI29471 and RO1 AI40297 (to M.M.L.) and RO1 AI36525 (to M.B.S.) and by research contract NO1-AI-45251 from the National Institute of Allergy and Infectious Diseases, NIH.
REFERENCES
- 1.Barry E M, Gomez-Duarte O G, Chatfield S, Rappuoli R, Pizza M, Losonsky G, Galen J E, Levine M M. Expression and immunogenicity of pertussis S1 subunit-tetanus toxin fragment C fusions in Salmonella typhi vaccine strain CVD 908. Infect Immun. 1996;64:4172–4181. doi: 10.1128/iai.64.10.4172-4181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter P B, Collins F M. Growth of typhoid and paratyphoid bacilli in intravenously infected mice. Infect Immun. 1974;10:816–822. doi: 10.1128/iai.10.4.816-822.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter P B, Collins F M. The route of infection in normal mice. J Exp Med. 1974;139:1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatfield S N, Charles I G, Makoff A J, Oxer M D, Dougan G, Pickard D, Slater D, Fairweather N F. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Bio/Technology. 1992;10:888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- 5.Everest P, Frankel G, Li J, Lund P, Chatfield S, Dougan G. Expression of LacZ from htrA, nirB, and groE promoters in a Salmonella vaccine strain: influence of growth in mammalian cells. FEMS Microbiol Lett. 1995;126:97–101. doi: 10.1111/j.1574-6968.1995.tb07398.x. [DOI] [PubMed] [Google Scholar]
- 6.Fairweather N F, Chatfield S N, Makoff A J, Strugnell R A, Bester J, Maskell D J, Dougan G. Oral vaccination of mice against tetanus by use of a live attenuated Salmonella carrier. Infect Immun. 1990;58:1323–1326. doi: 10.1128/iai.58.5.1323-1326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franke-Ullmann G, Pfortner C, Walter P, Steinmuller C, Lohmann-Matthes M, Kobzik L. Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages in vitro. J Immunol. 1996;157:3097–3104. [PubMed] [Google Scholar]
- 8.Galen J E, Gomez-Duarte O G, Losonsky G A, Halpern J L, Lauderbaugh C S, Kaintuck S, Reymann M K, Levine M M. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to foreign antigens. Vaccine. 1997;15:700–708. doi: 10.1016/s0264-410x(96)00227-7. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez C, Hone D, Noriega F R, Tacket C O, Davis J R, Losonsky G A, Nataro J P, Hoffman S, Malik A, Nardin E, Sztein M B, Heppner D G, Fouts T R, Isibasi A, Levine M M. Salmonella typhi vaccine strain CVD 908 expressing the circumsporozoite protein of Plasmodium falciparum: strain construction and safety and immunogenicity. J Infect Dis. 1994;169:927–931. doi: 10.1093/infdis/169.4.927. [DOI] [PubMed] [Google Scholar]
- 10.Gross E A, Swenberg J A, Fields S, Popp J A. Comparative morphometry of the nasal cavity in rats and mice. J Anat. 1982;135:83–88. [PMC free article] [PubMed] [Google Scholar]
- 11.Heritage P L, Underdown B J, Arsenault A L, Snider D P, McDermott M R. Comparison of murine nasal-associated lymphoid tissue and Peyer's patches. Am J Respir Crit Care Med. 1997;156:1256–1262. doi: 10.1164/ajrccm.156.4.97-03017. [DOI] [PubMed] [Google Scholar]
- 12.Hohmann E L, Oletta C A, Killeen K P, Miller S I. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect Dis. 1996;173:1408–1414. doi: 10.1093/infdis/173.6.1408. [DOI] [PubMed] [Google Scholar]
- 13.Hoiseth S, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and are effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 14.Hone D M, Attridge S R, Forrest B, Morona R, Daniels D, LaBrooy J T, Bartholomeusz R C, Shearman D J C, Hackett J. A galE via (Vi antigen-negative) mutant of Salmonella typhi Ty2 retains virulence in humans. Infect Immun. 1988;56:1326–1333. doi: 10.1128/iai.56.5.1326-1333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hone D M, Tacket C O, Harris A M, Kay B, Losonsky G A, Levine M M. Evaluation in volunteers of a candidate live oral attenuated Salmonella typhi vector vaccine. J Clin Investig. 1992;90:412–420. doi: 10.1172/JCI115876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masui Y, Mizuno T, Inouye M. Novel high-level expression cloning vehicles: 10,000-fold amplification of Escherichia coli minor protein. Bio/Technology. 1984;2:81–85. [Google Scholar]
- 17.O'Brien A D. Innate resistance of mice to Salmonella typhi infection. Infect Immun. 1982;38:948–952. doi: 10.1128/iai.38.3.948-952.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Orr N, Galen J E, Levine M M. Expression and immunogenicity of a mutant diphtheria toxin molecule, CRM197, and its fragment in Salmonella typhi vaccine strain CVD 908-htrA. Infect Immun. 1999;67:4290–4294. doi: 10.1128/iai.67.8.4290-4294.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasetti M F, Anderson R J, Noriega F R, Levine M M, Sztein M B. Attenuated ΔguaBA Salmonella typhi vaccine strain CVD 915 as a live vector utilizing prokaryotic or eukaryotic expression systems to deliver foreign antigens and elicit immune responses. Clin Immunol. 1999;92:76–89. doi: 10.1006/clim.1999.4733. [DOI] [PubMed] [Google Scholar]
- 19.Oxer M D, Bentley C M, Doyle J G, Peakman T C, Charles I G, Makoff A J. High level heterologous expression in E. coli using the anaerobically-activated nirB promoter. Nucleic Acids Res. 1991;19:2889–2892. doi: 10.1093/nar/19.11.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sztein M B, Tanner M K, Polotsky Y, Orenstein J M, Levine M M. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J Immunol. 1995;155:3987–3993. [PubMed] [Google Scholar]
- 21.Sztein M B, Wasserman S A, Tacket C O, Edelman R, Hone D, Lindberg A A, Levine M M. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. 1994;170:1508–1517. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 22.Tacket C O, Hone D M, Losonsky G A, Guers L, Edelman R, Levine M M. Clinical acceptability and immunogenicity of CVD 908 Salmonella typhi vaccine strain. Vaccine. 1992;10:443–446. doi: 10.1016/0264-410x(92)90392-w. [DOI] [PubMed] [Google Scholar]
- 23.Tacket C O, Sztein M B, Losonsky G A, Wasserman S A, Nataro J P, Edelman R, Pickard D, Chatfield S, Levine M M. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect Immun. 1997;65:452–456. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vladoianu I, Chang H R, Pechere J. Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin. Microb Pathog. 1990;8:83–90. doi: 10.1016/0882-4010(90)90072-x. [DOI] [PubMed] [Google Scholar]
- 25.Wu H-Y, Nguyen H H, Russell M W. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46:506–517. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]