Abstract
Objective:
Adverse pregnancy outcomes (APOs) and early menopause are each associated with increased risk of cardiovascular disease (CVD); whether APOs are associated with age at menopause is unclear. We examined the association of gestational diabetes (GDM), hypertensive disorders of pregnancy (HDP), preterm birth, and multiple gestation with age at natural menopause.
Study design:
Observational, prospective study within the Nurses’ Health Study II cohort (1989–2019).
Main outcomes measures:
Risk of early natural menopause, defined as occurring before the age of 45 years, and age at onset of natural menopause (hazard ratio (HR) >1 indicates younger age at menopause).
Results:
The mean [SD] baseline age of 69,880 parous participants was 34.5 [4.7] years. Compared with participants who had a term singleton first birth, those with a term multiple-gestation first birth had higher risk of early menopause (HR: 1.65, 95% CI: 1.05, 2.60) and younger age at natural menopause (HR: 1.46, 95% CI: 1.31, 1.63). Estimates for preterm multiple gestation were of similar magnitude. Menopause occurred at a younger age for those with a preterm birth with spontaneous labor (HR: 1.08, 95% CI: 1.03, 1.14) compared to those with a term birth with spontaneous labor. Conversely, estimates for GDM (HR: 0.95, 95% CI: 0.89, 1.02) and HDP (preeclampsia, HR: 0.93, 95% CI: 0.89, 0.97) suggested an association with older age at menopause.
Conclusions:
In this large cohort study, several statistically significant associations between APOs and age at natural menopause were observed. A deeper understanding of the relationships among APOs, menopause, and CVD is needed to help identify people at higher risk for early menopause and later CVD.
Keywords: gestational diabetes, hypertensive disorders of pregnancy, menopause, multiple gestation, preterm birth, women’s health
1. Introduction
Early menopause, the cessation of ovarian function before the age of 45 years, affects approximately 10% of women [1]. Women who experience early menopause are at increased risk of a nonfatal cardiovascular disease (CVD) event before the age of 60 years, CVD mortality, and all-cause mortality [2, 3].
Adverse pregnancy outcomes (APOs) are prevalent in the United States with gestational diabetes (GDM) diagnosed in 6% to 15% of pregnancies [4], hypertensive disorders of pregnancy (HDP), including gestational hypertension and preeclampsia, diagnosed in 4% to 10% of pregnancies [5], and approximately 10% of pregnancies resulting in a preterm birth [4]. Mothers and infants experiencing these APOs are at increased risk for myriad health issues and premature death [6]. Furthermore, multiple-gestation pregnancies are at greater risk of APOs [7].
Research examining APOs and their relation to CVD has demonstrated a strong positive association [8]. In meta-analyses, GDM was associated with a 1.5 to 2-fold risk of future cardiovascular events [9], preterm birth with a 2-fold increase in deaths caused by coronary heart disease [10], and preeclampsia with a 4-fold increase in future incident heart failure [11]. Guidelines for the primary prevention of CVD specifically highlight premature menopause and a history of APOs as female-specific risk factors that enhance CVD risk [12, 13]. While the underlying mechanisms that contribute to APOs are not well understood, predisposition to cardiometabolic conditions and the interplay of these conditions during pregnancy may be contributory factors [14]. Consequently, pregnancy may be considered a “stress test” that reveals or accelerates premature vascular aging and shared underlying pathology may contribute to ovarian aging [14, 15]. Alternatively, factors related to APOs and multiple gestation (e.g., postpartum hemorrhage) may impact the ovarian vascular blood supply and have negative implications for ovarian function and reserve [16, 17].
A link between APOs and menopausal vasomotor symptoms has been demonstrated [18–22], yet, research examining the association of APOs with menopause timing is scarce [23]. We hypothesized that APOs and multiple gestation are associated with earlier natural menopause, and we evaluated the association of GDM, HDP, preterm birth, and multiple gestation with early natural menopause in a population-based cohort.
2. Methods
The Nurses’ Health Study II (NHS2) is a prospective cohort established in 1989 when 517,000 female registered nurses from 14 populous US states were mailed an invitation to participate; 123,000 (24%) completed a baseline questionnaire and 116,429 nurses aged 25 to 42 years were enrolled in the cohort [24, 25]. Participants complete paper or web-based questionnaires every 2 years and response rates have been 85% to 90% for each cycle.
2.1. Assessment of APOs
Parity was reported at baseline and every 2 years through 2009 by asking participants if they had been pregnant within the past 2 years and the number of pregnancies ≥6 months during this time period. In 2001, approximately 91,000 participants were invited to complete a supplemental questionnaire where they provided information on their first 5 pregnancies lasting ≥12 weeks. In 2009, the biennial questionnaire completed by all participants included a reproductive grid where participants provided detailed information for all pregnancies, including the calendar year each pregnancy ended. Complete pregnancy history for each participant was derived from the 2009 grid with backfill of data from the 2001 grid and biennial questionnaires when data were missing or incomplete; these derived data were the primary source of exposure data for this study. On the 2009 grid, participants indicated if they experienced GDM, pregnancy-related high blood pressure, or preeclampsia/toxemia during pregnancies lasting ≥20 weeks. Completed weeks of each pregnancy were captured via 9 categories on the 2009 grid (<8, 8–11, 12–19, 20–27, 28–31, 32–36, 37–39, 40–42 or 43+ weeks) and 7 categories on the 2001 grid (12-<20, 20-<24, 24-<28, 28-<32, 32-<37, 37–42 or 43+ weeks). Both grids asked participants to indicate whether each pregnancy resulted in a “single live birth,” “twins/triplets+,” or “miscarriage/stillbirth.” Delivery type was captured on the 2009 grid as spontaneous and/or induced labor.
2.2. Analytical cohort
The analytical sample was limited to parous (births at ≥20 weeks gestation) participants who completed the 2001 and/or 2009 reproductive grids, including live and stillbirths (n=71,923) (Fig. 1). Additional exclusion criteria included: first birth age ≤18 (n=905) or ≥45 years (n=92); missing pregnancy outcome, gestational age, or year of first birth (n=517); conflicting data between age at menopause with age at first birth (n=145); report of cancer, hysterectomy, or oophorectomy before first birth (n=134); and missing pregnancy data for subsequent births (n=250). This yielded a final sample of 69,880 participants. Since GDM and HDP data were not captured on the 2001 grid, these data were not available for 8,106 participants and were excluded from corresponding analyses.
Fig. 1. Flowchart of participant selection, Nurses’ Health Study II (NHS2).
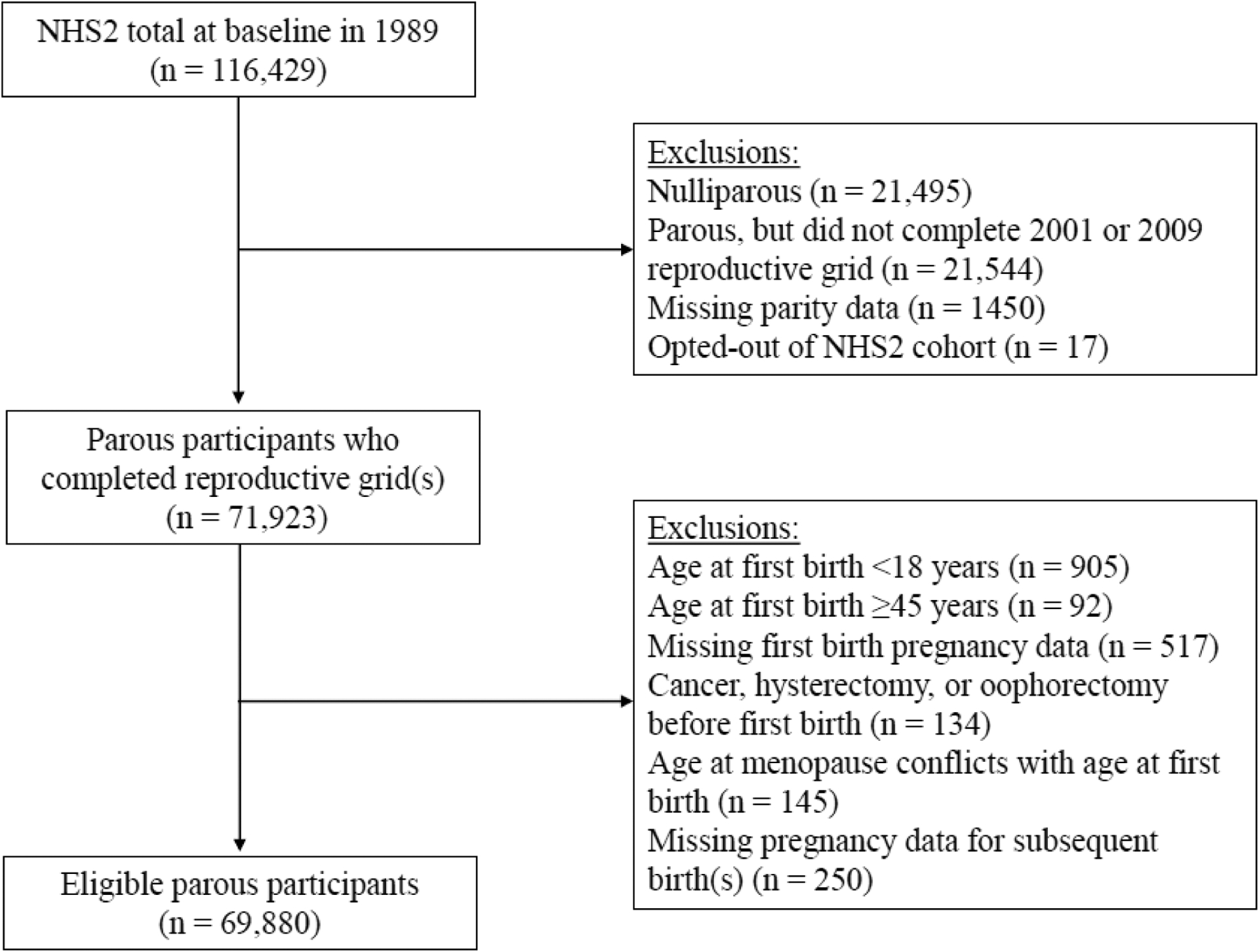
Of the 116,429 participants enrolled in the NHS2 cohort in 1989, a total of 69,880 who completed a reproductive grid in 2001 and/or 2009 and reported their menopause status from 1989 through 2019 were included in the analytical sample. Participants were followed from the year of their first birth (1964–2008) to 2019 for occurrence of natural menopause.
2.3. Assessment of menopause
Every 2 years, participants reported whether their menstrual periods had ceased (no: premenopausal, yes: no menstrual periods, yes: had menopause but now have periods induced by hormones, or not sure.) If yes, participants were asked, “Age periods ceased?” with an open response field, and “For what reason did your periods cease?”, with options of surgery, radiation or chemotherapy, and natural. Participants also reported use of menopausal hormone therapy (HT). Age at menopause was defined as age at last menstrual period followed by 12 consecutive months of amenorrhea. Cases of early menopause were classified as natural menopause before age 45 years.
2.4. Assessment of covariates
At baseline, participants were allowed multiple responses to report major ancestry, however, only one derived race/ethnicity was assigned in the following order: African American, Hispanic, Asian, Caucasian, or other. Pre-enrollment factors were captured at baseline when participants reported their age at menarche, weight at age 18, participation in strenuous physical activity/sports at least twice per week during ages 18 to 22, average number of cigarettes smoked per day during 6 age intervals (<15, 15–19, 20–24, 25–29, 30–35 years, and current), usual number of alcoholic drinks consumed during 4 age intervals (15–17, 18–22, 23–30, and 31–40 years), oral contraceptive (OC) use for each year of age from 13 to 42 years, and the age when they first tried to become pregnant for more than 1 year without success. Current lifestyle factors were collected on baseline, biennial, and supplemental questionnaires. Age, weight, smoking, current OC use, and duration of OC use were assessed every 2 years. Infertility was assessed on biennial questionnaires to 2001 and twice more through 2009 by asking participants if they experienced infertility since the last questionnaire and, if so, the cause (e.g., ovulatory disorder). Alcohol consumption was assessed in 1991 and every 4 years thereafter via semiquantitative food frequency questionnaires. Parental education was assessed in 2005 by separately asking how many years of education the participant’s mother and father had completed when the participant was an infant. Cumulative breastfeeding was measured 3 times by asking participants how many months in total they breastfed for all their births combined.
2.5. Statistical analysis
Pre-pregnancy characteristics were examined according to report of GDM, HDP, preterm birth, or multiple gestation during participants’ first pregnancy. For our primary analyses, we used Cox proportional hazards regression with time since first birth as the time scale to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association of each APO from participants’ first birth with risk of early menopause. We also estimated onset of natural menopause, where HRs greater than 1 indicate the APO is associated with younger menopause age, and HRs less than 1 indicate an association with older menopause age. Participants contributed follow-up time from year of their first birth (1964–2008) to: onset of menopause; report of a hysterectomy, bilateral oophorectomy, or unilateral oophorectomy; cancer (not including nonmelanoma skin cancer); death; loss to follow-up; or end of follow-up on June 30, 2019, whichever came first. Analyses of early menopause were additionally censored at age 45 years. Linear trends for gestational age were assessed by modeling midpoints of preterm birth categories as a continuous variable.
Data from first births were used to create exposure categories for our primary analyses. GDM was modeled as a binary variable. HDP was categorized as preeclampsia, gestational hypertension, or normotensive. Births were categorized by term (≥37 weeks) or preterm (<37 weeks), gestation type (singleton or multiple), and type of labor (spontaneous, induced, or missing). Preterm births were further categorized as moderate preterm (≥32 to <37 weeks) or very preterm (≥20 to <32 weeks). Birth history was classified by first birth (term or preterm) and all later births (all term, at least one preterm, no future birth).
We created two multivariable models to account for potential confounding. In our first model, we adjusted for covariates identified a priori from examinations of the association of APOs with risk of CVD in the NHS2 cohort [26–28]. Thus, model 1 was adjusted for a set of demographic variables (age at first birth, age at baseline, race/ethnicity, and parental education) and pre-pregnancy factors (body mass index (BMI), smoking status, alcohol consumption, physical activity at 18 years of age, and years of OC use). Age at menarche and pre-pregnancy infertility due to ovulatory disorder were also included as they were previously associated with menopause in the cohort. The measurement of early-life factors at baseline, along with continued assessment on supplemental and biennial questionnaires, allowed for derivation of covariate exposure history for each year of age from 18 through end of follow-up or for specific age timeframes (e.g., alcohol consumption during ages 23 to 30 years). Thus, pre-pregnancy covariate values were assigned using the information closest to, but preceding, a participant’s first birth. In model 2, we adjusted for post-first birth, time-varying lifestyle factors also associated with menopause in the cohort (i.e., BMI, alcohol, smoking, parity, breastfeeding, and ovulatory disorder infertility); these factors were also assigned from the applicable age-derived variables. Missing indicators were used for the small amount of missing data.
In supplemental analyses, we examined the APOs in several ways: 1) for each APO, we compared participants who experienced the APO in any of their pregnancies to those who never experienced an APO; 2) with stillbirths excluded; 3) among a subset of premenopausal participants who reported menopause status prospectively; 4) adjusted for HT use; 5) censored at first report of HT use; 6) within strata of first birth outcome (singleton, multiple gestation, or stillbirth); and 7) with APOs mutually adjusted for each other. Since multiple gestation may be associated with exposure to assisted reproductive technologies (ART) [29] and since these data were not available, we also stratified by participants’ use of ovarian stimulation hormones as a proxy for ART. Lastly, we calculated years to natural menopause from first birth and age at natural menopause by strata of each APO.
All statistical analyses were conducted with SAS version 9.4 (SAS Institute).
3. Results
Pre-pregnancy characteristics by each APO are shown in Table 1. Notably, among those who reported GDM or multiple gestation age at first birth (29.4 years) was approximately 2 years higher than birth age for all participants (27.0 years) and for those reporting HDP (27.5 years) or preterm birth (27.7 years). Infertility prior to the first birth was less common overall (4.6%) compared to those who reported an APO (HDP, 5.8%; preterm birth, 7.4%; GDM, 8.7%; multiple gestation, 18.5%).
Table 1.
Pre-pregnancy characteristics of 69,880 parous participants by adverse pregnancy outcomes of first birth, Nurses’ Health Study II, 1989–2019.
| Pre-pregnancy characteristica | All participants (n = 69,880) | Gestational diabetes (n = 1806)b | Hypertensive disorders of pregnancy (n = 6243)b | Preterm birth (n = 6133)c | Multiple gestation (n = 1006) |
|---|---|---|---|---|---|
| Age at baseline, mean (SD) years | 34.5 (4.7) | 33.2 (4.7) | 34.0 (4.6) | 34.3 (4.8) | 33.4 (4.7) |
| Age at menarche, mean (SD) years | 12.4 (1.4) | 12.3 (1.5) | 12.2 (1.4) | 12.3 (1.4) | 12.3 (1.4) |
| Age at first birth, mean (SD) years | 27.0 (4.7) | 29.4 (5.1) | 27.5 (4.9) | 27.7 (5.1) | 29.4 (5.5) |
| Body mass index, mean (SD)d | 22.0 (3.7) | 23.7 (5.2) | 23.3 (4.5) | 22.2 (4.0) | 22.6 (4.6) |
| Alcohol consumption, mean (SD) g/day | 2.7 (1.5) | 2.6 (1.5) | 2.6 (1.5) | 2.7 (1.6) | 2.7 (1.5) |
| Non-Hispanic, white | 64,825 (92.8) | 1643 (91.0) | 5831 (93.4) | 5576 (90.9) | 942 (93.6) |
| Maternal education, > high school | 19,103 (27.3) | 510 (28.2) | 1744 (27.9) | 1599 (26.1) | 280 (27.8) |
| Paternal education, > high school | 22,098 (31.6) | 590 (32.7) | 1972 (31.6) | 1887 (30.8) | 338 (33.6) |
| Strenuous physical activity at ages 18–22, months per year | |||||
| Never | 19,494 (27.9) | 467 (25.9) | 1704 (27.3) | 1651 (26.9) | 258 (25.7) |
| 1–3 | 22,011 (31.5) | 587 (32.5) | 1950 (31.2) | 1917 (31.3) | 321 (31.9) |
| 4–6 | 11,976 (17.1) | 317 (17.6) | 1159 (18.6) | 1085 (17.7) | 178 (17.7) |
| 7–9 | 8121 (11.6) | 231 (12.8) | 715 (11.5) | 703 (11.5) | 97 (9.6) |
| 10–12 | 7861 (11.3) | 189 (10.5) | 678 (10.9) | 735 (12.0) | 148 (14.7) |
| Missing | 417 (0.6) | 15 (0.8) | 37 (0.6) | 42 (0.7) | 4 (0.4) |
| Oral contraceptive use, years | |||||
| Never | 13,973 (20.0) | 323 (17.9) | 1161 (18.6) | 1112 (18.1) | 166 (16.5) |
| <2 | 17,115 (24.5) | 349 (19.3) | 1569 (25.1) | 1407 (22.9) | 238 (23.7) |
| ≥2 to <4 | 15,132 (21.7) | 390 (21.6) | 1305 (20.9) | 1228 (20.0) | 220 (21.9) |
| ≥4 | 21,875 (31.3) | 705 (39.0) | 2089 (33.5) | 2144 (35.0) | 360 (35.8) |
| Missing | 1785 (2.6) | 39 (2.2) | 119 (1.9) | 242 (4.0) | 22 (2.2) |
| Smoking status | |||||
| Never | 47,243 (67.6) | 1219 (67.5) | 4251 (68.1) | 4160 (67.8) | 685 (68.1) |
| Past | 6911 (9.9) | 232 (12.9) | 668 (10.7) | 616 (10.0) | 139 (13.8) |
| Current | 15,306 (21.9) | 350 (19.4) | 1290 (20.7) | 1317 (21.5) | 177 (17.6) |
| Missing | 420 (0.6) | 5 (0.3) | 34 (0.5) | 40 (0.7) | 5 (0.5) |
| Infertility due to ovulatory disordere | 3219 (4.6) | 157 (8.7) | 362 (5.8) | 451 (7.4) | 186 (18.5) |
| Final parityf | |||||
| 1 | 11,745 (16.8) | 463 (25.6) | 1,456 (23.3) | 1461 (23.8) | 521 (51.8) |
| 2 | 34,021 (48.7) | 896 (49.6) | 3019 (48.4) | 2658 (43.3) | 340 (33.8) |
| 3 | 17,844 (25.5) | 362 (20.0) | 1361 (21.8) | 1441 (23.5) | 114 (11.3) |
| ≥4 | 6270 (9.0) | 85 (4.7) | 407 (6.5) | 573 (9.3) | 31 (3.1) |
| Final breastfeeding duration, median (IQR), monthsg | 11.5 (3.0, 22.0) | 9.5 (2.0, 20.0) | 9.0 (1.5, 20.0) | 9.0 (1.5, 19.0) | 5.0 (0.0, 14.5) |
| Time from 1st birth to menopause, mean (SD) years | |||||
| Age at menopause, mean (SD) years |
Abbreviations: IQR, interquartile range; multiple gestation, twins, triplets, or higher-order multiples; SD, standard deviation.
Pre-pregnancy measurements were extracted from the biennial questionnaire preceding a participant’s first birth or from baseline and supplemental questionnaires for first births occurring prior to baseline. Frequency (%) is presented unless otherwise specified.
Gestational diabetes and hypertensive disorders of pregnancy data were not available for participants who only completed the 2001 reproductive grid (n = 8106).
Gestational age <37 completed weeks.
Calculated as weight in kilograms divided by height in meters squared.
Infertility diagnosed prior to first birth.
Cumulative parity through 2009 (last assessment in cohort).
Cumulative total breastfeeding through 2003 (last assessment in cohort).
Participants were followed for up to 50 years (median, 24; range, 1–50). During 1.6 million person-years of follow-up, 1,763 participants experienced early menopause. GDM and HDP during the first pregnancy were not associated with risk of early menopause, however, estimates for onset of natural menopause suggested an association with older age at menopause (Table 2). In the fully adjusted model and compared to no report of GDM, the hazard ratio (HR) for GDM was 0.95 (model 2: 95% CI: 0.89, 1.02). Participants with gestational hypertension (HR: 0.95, 95% CI: 0.90, 1.01) and those with preeclampsia (HR: 0.93, 95% CI: 0.89, 0.97) also experienced an older age at menopause when compared with participants who were normotensive.
Table 2.
Multivariable associations of gestational diabetes and hypertensive disorders of pregnancy during the first birth with natural menopause among 61,774 parous participants in the Nurses’ Health Study II, 1989–2019.
| Adverse pregnancy outcome | Early natural menopause (<45 years) | Natural menopausea | |||
|---|---|---|---|---|---|
| Model 1b | Model 2c | Model 2c | |||
| Casesd | Person-years | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Gestational diabetes | |||||
| No | 1514 | 1,078,516 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 40 | 28,029 | 0.92 (0.67, 1.27) | 0.90 (0.65, 1.23) | 0.95 (0.89, 1.02) |
| Hypertensive disorders of pregnancy | |||||
| Normotensive | 1394 | 999,574 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Gestational hypertension | 44 | 33,423 | 0.89 (0.66, 1.21) | 0.85 (0.63, 1.15) | 0.95 (0.90, 1.01) |
| Preeclampsia | 116 | 73,548 | 1.17 (0.97, 1.42) | 1.11 (0.92, 1.35) | 0.93 (0.89, 0.97) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Onset of natural menopause: HR>1 indicates younger age at menopause; HR<1 indicates older age menopause.
Adjusted for age at first birth (continuous), age at baseline (continuous), race/ethnicity (African American, Hispanic, Asian, Caucasian, or other), maternal and paternal education (<9, 9–11, 12, 13–15, or ≥16 years), and pre-pregnancy factors of age at menarche (≤9, 10, 11, 12, 13, 14, 15, 16, or ≥17 years), physical activity at 18–22 years of age (never, 1–3, 4–6, 7–9, or 10–12 months per year), body mass index (weight (kg)/height (m)2; 15.0-<18.5, 18.5-<25.0, 25.0-<30, or 30.0-<50.0), alcohol consumption (none, ≤1/week, 2–6/week, or ≥1/day), smoking status (never, past, or current smoker), oral contraceptive use (none, <2, ≥2-<4, or ≥4 years), and infertility due to ovulatory disorder (no or yes).
Additionally adjusted for post-first birth factors of body mass index (weight (kg)/height (m)2; <18.5, 18.5-<25.0, 25.0-<30.0, ≥30.0), alcohol consumption (none, ≤1/week, 2–6/week, or ≥1/day), smoking status (never, past, or current smoker), infertility due to ovulatory disorder (no or yes), parity (continuous), and breastfeeding (<1, 1–3, >3–6, >6–12, >12–18, >18–24, >24–36, or >36 months).
Cases do not sum to 1763 because gestational diabetes and hypertensive disorders of pregnancy data were not collected for 8106 participants.
Preterm first birth was associated with higher risk of early menopause in our model adjusted for pre-pregnancy factors (model 1: HR: 1.18, 95% CI: 1.01, 1.38), but this association was slightly attenuated when time-varying factors were included (model 2: HR: 1.14, 95% CI: 0.98, 1.34) (Table 3). We did not observe an association of preterm birth with onset of natural menopause (HR: 1.00, 95% CI: 0.97, 1.04). Results were similar for moderate and very preterm birth categories. Compared with participants experiencing a term singleton first birth, those with a multiple-gestation first birth had higher risk of early menopause regardless of gestational age (term, HR: 1.65, 95% CI: 1.05, 2.60; preterm, HR: 2.38, 95% CI: 1.57, 3.61) and onset of menopause at a younger age (term, HR: 1.46, 95% CI: 1.31, 1.63; preterm, HR: 1.21, 95% CI: 1.07, 1.36). No clear patterns emerged in our examination of risk of early menopause by labor type, however, we observed onset of natural menopause at a younger age for participants who reported preterm birth with spontaneous labor compared to term birth with spontaneous labor (HR: 1.08, 95% CI: 1.03, 1.14).
Table 3.
Multivariable associations of preterm birth and multiple gestation of the first birth with natural menopause among 69,880 parous participants in the Nurses’ Health Study II, 1989–2019.
| Adverse pregnancy outcome | Early natural menopause (<45 years) | Natural menopausea | |||
|---|---|---|---|---|---|
| Model 1b | Model 2c | Model 2c | |||
| Cases | Person-years | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Timing of birth in 2 categories (weeks) | |||||
| Term (≥37) | 1588 | 1,144,708 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Preterm (<37) | 175 | 104,971 | 1.18 (1.01, 1.38) | 1.14 (0.98, 1.34) | 1.00 (0.97, 1.04) |
| Timing of birth in 3 categories (weeks) | |||||
| Term (≥37) | 1588 | 1,144,708 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Moderate preterm (>32 to <37) | 133 | 79,998 | 1.17 (0.98, 1.39) | 1.16 (0.97, 1.39) | 1.01 (0.97, 1.05) |
| Very preterm (≥20 to <32) | 42 | 24,973 | 1.23 (0.91, 1.68) | 1.09 (0.80, 1.48) | 0.98 (0.91, 1.05) |
| P value for trendd | 0.04 | 0.18 | 0.78 | ||
| Per 1 week increase in gestational age | 0.98 (0.96, 1.00) | 0.99 (0.97, 1.01) | 1.00 (1.00, 1.01) | ||
| Preterm birth by gestation type | |||||
| Term singleton | 1569 | 1,136,209 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Term multiple gestation | 19 | 8499 | 1.48 (0.94, 2.34) | 1.65 (1.05, 2.60) | 1.46 (1.31, 1.63) |
| Preterm singleton | 152 | 97,880 | 1.11 (0.94, 1.31) | 1.07 (0.90, 1.26) | 0.99 (0.96, 1.03) |
| Preterm multiple gestation | 23 | 7091 | 2.23 (1.47, 3.37) | 2.38 (1.57, 3.61) | 1.21 (1.07, 1.36) |
| Preterm birth by labor type | |||||
| Term, spontaneous | 830 | 625,901 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Preterm, spontaneous | 76 | 48,761 | 1.14 (0.90, 1.44) | 1.11 (0.88, 1.41) | 1.08 (1.03, 1.14) |
| Term, induced | 224 | 158,914 | 1.02 (0.88, 1.18) | 1.00 (0.86, 1.16) | 0.94 (0.91, 0.97) |
| Preterm, induced | 19 | 11,415 | 1.17 (0.74, 1.85) | 1.10 (0.70, 1.74) | 0.91 (0.82, 1.01) |
| Term, not specified | 534 | 359,893 | 1.06 (0.95, 1.19) | 1.08 (0.96, 1.20) | 1.01 (0.98, 1.03) |
| Preterm, not specified | 80 | 44,795 | 1.29 (1.03, 1.63) | 1.25 (0.99, 1.58) | 0.93 (0.88, 0.98) |
Abbreviations: CI, confidence interval; HR, hazard ratio; multiple gestation, twins, triplets, or higher-order multiples; preterm, gestational age <37 completed weeks; term, gestational age ≥37 completed weeks.
Onset of natural menopause: HR>1 indicates younger age at menopause; HR<1 indicates older age menopause.
Adjusted for age at first birth (continuous), age at baseline (continuous), race/ethnicity (African American, Hispanic, Asian, Caucasian, or other), maternal and paternal education (<9, 9–11, 12, 13–15 or ≥16 years), and pre-pregnancy factors of age at menarche (≤9, 10, 11, 12, 13, 14, 15, 16, or ≥17 years), physical activity at 18 years of age (never, 1–3, 4–6, 7–9, or 10–12 months per year), body mass index (weight (kg)/height (m)2; 15.0-<18.5, 18.5-<25.0, 25.0-<30.0, or 30.0-<50.0), alcohol consumption (none, ≤1/week, 2–6/week, or ≥1/day), smoking (never, past, or current smoker), oral contraceptive use (none, <2, ≥2-<4, or ≥4 years), and infertility due to ovulatory disorder (no or yes).
Additionally adjusted for post-first birth factors of body mass index (weight (kg)/height (m)2; <18.5, 18.5-<25.0, 25.0-<30.0, or ≥30.0 kg/m2), alcohol consumption (none, ≤1/week, 2–6/week, or ≥1/day), smoking status (never, past, or current smoker), infertility due to ovulatory disorder (no or yes), parity (continuous), and breastfeeding (<1, 1–3, >3–6, >6–12, >12–18, >18–24, >24–36, or >36 months).
Linear trends were assessed by modeling category midpoints as a continuous variable.
Using a history of more than one term birth as the reference group, participants reporting only one birth over the course of their reproductive history experienced a 2-fold increased risk of early menopause irrespective of the birth being term (HR: 2.20, 95% CI: 1.89, 2.55) or preterm (HR: 2.25, 95% CI: 1.65, 3.06) (Table 4). Estimates for onset of natural menopause at a younger age were also elevated (one term birth, HR: 1.52, 95% CI: 1.47, 1.58; one preterm birth, HR: 1.49, 95% CI: 1.38, 1.60).
Table 4.
Multivariable associations of birth history with natural menopause among 69,880 parous participants in the Nurses’ Health Study II, 1989–2019.
| Birth historyb | Early natural menopause (<45 years) | Natural menopausea | |||
|---|---|---|---|---|---|
| Model 1c | Model 2d | Model 2d | |||
| Cases | Person-years | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Term 1st birth, term subsequent birth(s) | 1161 | 916,872 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Term 1st birth, preterm subsequent birth(s) | 101 | 70,269 | 1.19 (0.97, 1.46) | 1.01 (0.82, 1.24) | 0.95 (0.90, 0.99) |
| Term 1st birth, no subsequent birth(s) | 326 | 157,567 | 1.48 (1.30, 1.68) | 2.20 (1.89, 2.55) | 1.52 (1.47, 1.58) |
| Preterm 1st birth, term subsequent birth(s) | 70 | 48,876 | 1.14 (0.89, 1.45) | 1.08 (0.85, 1.37) | 0.95 (0.90, 1.00) |
| Preterm 1st birth, preterm subsequent birth(s) | 59 | 35,328 | 1.33 (1.02, 1.73) | 1.21 (0.93, 1.57) | 1.04 (0.98, 1.11) |
| Preterm 1st birth, no subsequent birth(s) | 46 | 20,767 | 1.53 (1.13, 2.06) | 2.25 (1.65, 3.06) | 1.49 (1.38, 1.60) |
Abbreviations: CI, confidence interval; HR, hazard ratio; preterm, gestational age <37 completed weeks; term, gestational age ≥37 completed weeks.
Onset of natural menopause: HR>1 indicates younger age at menopause; HR<1 indicates older age menopause.
Exposure variables for early natural menopause only include births that occurred prior to age 45.
Adjusted for age at first birth (continuous), age at baseline (continuous), race/ethnicity (African American, Hispanic, Asian, Caucasian, or other), maternal and paternal education (<9, 9–11, 12, 13–15, or ≥16 years), and pre-pregnancy factors of age at menarche (≤9, 10, 11, 12, 13, 14, 15, 16, or ≥17 years), physical activity at 18 years of age (never, 1–3, 4–6, 7–9, or 10–12 months per year), body mass index (weight (kg)/height (m)2; 15.0-<18.5, 18.5-<25.0, 25.0-<30.0, or 30.0-<50.0 kg/m2), alcohol consumption (none, ≤1/week, 2–6/week, or ≥1/day), smoking status (never, past, or current smoker), oral contraceptive use (none, <2, ≥2-<4, or ≥4 years), and infertility due to ovulatory disorder (no or yes).
Additionally adjusted for post-first pregnancy factors of body mass index (weight (kg)/height (m)2; <18.5, 18.5-<25.0, 25.0-<30.0, or ≥30.0 kg/m2), alcohol consumption (none, ≤1/week, 2–6/week, or ≥1/day), smoking status (never, past, or current smoker), infertility due to ovulatory disorder (no or yes), parity (continuous), and breastfeeding (<1, 1–3, >3–6, >6–12, >12–18, >18–24, >24–36, or >36 months).
In analyses that examined APOs across any pregnancy, estimates for onset of natural menopause were slightly strengthened for GDM and HDP and slightly attenuated for multiple gestation (eTable 1). Results from analyses that excluded stillbirths (eTable 2), examined risk based on prospective reporting of menopause (eTable 3), accounted for HT use (eTable 4), within strata of pregnancy outcome (eTable 5), considered use of ovarian stimulation hormones (eTable 5), and mutually adjusted for each APO (eTable 6) were essentially identical to our main findings. Estimates for time to natural menopause from first birth and age at natural menopause according to strata of each APO followed patterns similar to our observed estimates of risk (eTable 7).
4. Discussion
To the best of our knowledge, this is the first study examining the association of various APOs with menopause timing. We observed positive associations of multiple gestation with risk of early menopause and onset of menopause at a younger age. Risk was higher for multiple gestation occurring in the first or subsequent pregnancy, and whether the pregnancy was term or preterm. Overall, preterm birth was not associated with menopause timing, however, preterm birth with spontaneous labor was associated with younger age at natural menopause. Conversely, our findings suggest that GDM and HDP may be associated with older age at menopause. Only one other epidemiological study has examined the association of an APO with menopause timing. Li et al. [23] examined 13 common diseases, including preeclampsia, and timing of menopause among 61,936 Swedish women. In a model limited to participants who never had cervical or ovarian surgery and adjusted for reproductive and lifestyle factors plus the common diseases examined, preeclampsia was associated with a younger age at menopause (HR: 0.93, 95% CI: 0.86, 1.00). Our findings are similar for preeclampsia and extend the literature by examining multiple APOs with the association of both onset of natural menopause and risk of early menopause.
Our observation of risk of earlier menopause for participants with only one birth is consistent with the inverse association of parity with early menopause we observed previously in the NHS2 cohort [30]. In contrast, the strong association of multiple gestation with earlier menopause timing, regardless of gestational age, appears to be a novel finding. Only one small study investigated this association by comparing onset of menopause in mothers of natural dizygotic twins (n=16) to mothers of singletons (n=14) [31]. Although more mothers of twins had entered menopause 15 years after study entry compared to controls (8 vs. 1, p = 0.07), none of the mothers had entered menopause prior to the age of 45 years.
APOs are associated with CVD risk factors and metabolic conditions [14]. Premenopausal cardiometabolic risk factors may lead to vascular dysfunction that impairs ovarian blood flow leading to diminished levels of circulating estrogen and thus, earlier ovarian aging [32–34]. APOs may be an early manifestation of these conditions among individuals who will ultimately develop vascular damage that leads to earlier menopause [15]. Yet, this hypothesis does not fully account for the association of multiple gestation and earlier menopause observed in our data. While multiple-gestation pregnancies are associated with increased risk of APOs, the underlying causes of APOs in singleton versus multiple-gestation pregnancies may differ due to the increased physiologic demands on the mother from increased placental mass in multiple-gestation pregnancies [7]. In fact, in the few studies that examined the association of multiple gestation with CVD risk, a history of multiple gestation did not increase long-term CVD risk [35–38]; as such, questions regarding potential overlapping pathways remain.
Other mechanisms may be involved. Multiple gestation may be the result of ART, which has been associated with certain APOs [39] and can be indicative of underlying subfertility/infertility [29], a condition associated with earlier menopause [40] and CVD risk [41]. APOs and multiple gestation are known risk factors for postpartum hemorrhage [16, 17] and treatment of this condition may disrupt blood flow to the ovaries [42, 43]. Finally, there may be faster depletion of the ovarian follicle pool in women with multiple-gestation pregnancies due to a higher likelihood of hyper ovulation [44, 45]. It is conceivable that these factors contributed to the increased risk of earlier menopause observed in our study.
The potential mechanisms contributing to later onset of menopause among participants with a history of GDM or HDP in our study are likewise unclear. One possible explanation is that women with a history of polycystic ovary syndrome (PCOS) are at increased risk for developing GDM and HDP [46] and PCOS may be associated with older age at menopause [23, 47]. A reliable measure of PCOS was not available to appropriately assess the impact of PCOS on our findings.
Our study has several limitations but also important strengths. We relied on self-report of menopause, which might contribute to some misclassification [48]; yet, we collected menopause data prospectively for most participants in our study and a prior validation study in the comparable Nurses’ Health Study found that menopausal status can be self-reported with a high degree of reproducibility and accuracy [49]. Approximately 80% of participants experienced their first birth prior to NHS2 enrollment, as such, the majority of exposure data were reported retrospectively. However, previous NHS2 validation studies comparing self-reported APOs to medical records resulted in 81% sensitivity and 92% specificity for preterm birth [27], an 89% positive predictive value for HDP [26], and 94% of GDM cases confirmed [50]. APOs in our study were not mutually exclusive, therefore, future exploration of multiple APOs and menopause timing may be warranted [51]. Some of our exposure categories had low numbers which may have resulted in low precision; thus, we emphasize caution in interpreting results. Lastly, our study population was homogenous with respect to race and ethnicity. While the menopausal transition varies among different racial/ethnic groups [52], age at natural menopause does not [53]. Yet, Black women have consistently higher risk of APOs compared to non-Hispanic White women [54] and exploration of these associations in more diverse populations is warranted.
5. Conclusions
In this first study examining the association of various APOs and natural menopause timing several statistically significant associations were observed. APOs and early menopause are each associated with increased CVD risk; however, it is unclear whether early menopause shares an overlapping etiology with APOs, if early menopause is on the biological pathway of APOs and risk of CVD, or if APOs contribute directly to menopause timing. Expert panels recommend consideration of APOs in evaluating CVD risk [55]. Further research is needed to clarify the biological relationships among APOs, early natural menopause, and CVD; deeper understanding may help identify people at higher risk for early menopause and later CVD who might benefit from increased surveillance or preventative lifestyle modifications.
Supplementary Material
Highlights.
In this study, multiple gestation was associated with increased risk of early natural menopause.
Preterm birth with spontaneous labor was associated with younger age at natural menopause.
Gestational diabetes and hypertensive disorders of pregnancy were associated with older age at natural menopause.
Acknowledgements
The authors thank Drs. Anita Subramanian and Jennifer Woo for their helpful review of a prior draft of this manuscript.
Funding
The NHS2 is supported by the National Institutes of Health (grants U01CA176726 and R01HD078517). This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. The funding sources had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Abbreviations:
- APO
adverse pregnancy outcome
- ART
assisted reproductive technologies
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- GDM
gestational diabetes
- HDP
hypertensive disorders of pregnancy
- HR
hazard ratio
- HT
hormone therapy
- NHS2
Nurses’ Health Study II cohort
- OC
oral contraceptive
- PCOS
polycystic ovary syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical approval
The study protocol was approved by the institutional review boards at Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health, and participants provided written informed consent.
Declaration of competing interest
The authors declare that they have no competing interest.
Research data (data sharing and collaboration)
Procedures for obtaining and accessing data from the Nurses’ Health Studies are described on the studies’ website (https://www.nurseshealthstudy.org/researchers) (e-mail: nhsaccess@channing.harvard.edu).
References
- [1].Giri R, Vincent AJ, Prevalence and risk factors of premature ovarian insufficiency/early menopause, Semin Reprod Med 38(4–05) (2020) 237–246. 10.1055/s-0040-1722317 [DOI] [PubMed] [Google Scholar]
- [2].Zhu D, Chung H-F, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE, Gold EB, Derby CA, Matthews KA, Cade JE, Greenwood DC, Demakakos P, Brown DE, Sievert LL, Anderson D, Hayashi K, Lee JS, Mizunuma H, Tillin T, Simonsen MK, Adami H-O, Weiderpass E, Mishra GD, Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data, The Lancet Public Health 4(11) (2019) e553–e564. 10.1016/s2468-2667(19)30155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, Kavousi M, Franco OH, Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis, JAMA Cardiol 1(7) (2016) 767–776. 10.1001/jamacardio.2016.2415 [DOI] [PubMed] [Google Scholar]
- [4].Osterman MJK, Hamilton BE, Martin JA, Discoll AK, Valenzuela CP, Births: Final Data for 2020, National Vital Statistics Reports 70 (2022). 10.15620/cdc:112078 [DOI] [PubMed] [Google Scholar]
- [5].Butwick AJ, Druzin ML, Shaw GM, Guo N, Evaluation of US state-level variation in hypertensive disorders of pregnancy, JAMA Netw Open 3(10) (2020) e2018741. 10.1001/jamanetworkopen.2020.18741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Arabin B, Baschat AA, Pregnancy: an underutilized window of opportunity to improve long-term maternal and infant health - an appeal for continuous family care and interdisciplinary communication, Front Pediatr 5 (2017) 69. 10.3389/fped.2017.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].SMFM Research Committee, Grantz KL, Kawakita T, Lu YL, Newman R, Berghella V, Caughey A, SMFM Special Statement: State of the science on multifetal gestations: unique considerations and importance, Am J Obstet Gynecol 221(2) (2019) B2–B12. 10.1016/j.ajog.2019.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].O’Kelly AC, Michos ED, Shufelt CL, Vermunt JV, Minissian MB, Quesada O, Smith GN, Rich-Edwards JW, Garovic VD, El Khoudary SR, Honigberg MC, Pregnancy and reproductive risk factors for cardiovascular disease in women, Circ Res 130(4) (2022) 652–672. 10.1161/CIRCRESAHA.121.319895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kramer CK, Campbell S, Retnakaran R, Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis, Diabetologia 62(6) (2019) 905–914. 10.1007/s00125-019-4840-2 [DOI] [PubMed] [Google Scholar]
- [10].Wu P, Gulati M, Kwok CS, Wong CW, Narain A, O’Brien S, Chew-Graham CA, Verma G, Kadam UT, Mamas MA, Preterm delivery and future risk of maternal cardiovascular disease: a systematic review and meta-analysis, J Am Heart Assoc 7(2) (2018). 10.1161/JAHA.117.007809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA, Preeclampsia and future cardiovascular health: a systematic review and meta-analysis, Circ Cardiovasc Qual Outcomes 10(2) (2017). 10.1161/CIRCOUTCOMES.116.003497 [DOI] [PubMed] [Google Scholar]
- [12].Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC Jr., Virani SS, Williams KA Sr., Yeboah J, Ziaeian B, 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, J Am Coll Cardiol 74(10) (2019) 1376–1414. 10.1016/j.jacc.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maas A, Rosano G, Cifkova R, Chieffo A, van Dijken D, Hamoda H, Kunadian V, Laan E, Lambrinoudaki I, Maclaran K, Panay N, Stevenson JC, van Trotsenburg M, Collins P, Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists, Eur Heart J 42(10) (2021) 967–984. 10.1093/eurheartj/ehaa1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Agarwala A, Michos ED, Samad Z, Ballantyne CM, Virani SS, The use of sex-specific factors in the assessment of women’s cardiovascular risk, Circulation 141(7) (2020) 592–599. 10.1161/CIRCULATIONAHA.119.043429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yarde F, Maas AH, Franx A, Eijkemans MJ, Drost JT, van Rijn BB, van Eyck J, van der Schouw YT, Broekmans FJ, Serum AMH levels in women with a history of preeclampsia suggest a role for vascular factors in ovarian aging, J Clin Endocrinol Metab 99(2) (2014) 579–86. 10.1210/jc.2013-2902 [DOI] [PubMed] [Google Scholar]
- [16].Ende HB, Lozada MJ, Chestnut DH, Osmundson SS, Walden RL, Shotwell MS, Bauchat JR, Risk factors for atonic postpartum hemorrhage: a systematic review and meta-analysis, Obstet Gynecol 137(2) (2021) 305–323. 10.1097/AOG.0000000000004228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, Tita AT, Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery, Am J Obstet Gynecol 209(1) (2013) 51 e1–6. 10.1016/j.ajog.2013.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ri M, Hayashi K, Kurabayashi T, Lee JS, Ideno Y, Nagai K, Yasui T, Kubota T, Takamatsu K, Hypertensive disorders of pregnancy increase the risk of future menopausal hot flashes in Japanese women: Results from the Japan Nurses’ Health Study, Menopause (2021). 10.1097/GME.0000000000001889 [DOI] [PubMed] [Google Scholar]
- [19].Drost JT, van der Schouw YT, Herber-Gast GC, Maas AH, More vasomotor symptoms in menopause among women with a history of hypertensive pregnancy diseases compared with women with normotensive pregnancies, Menopause 20(10) (2013) 1006–11. 10.1097/GME.0b013e3182886093 [DOI] [PubMed] [Google Scholar]
- [20].Faubion SS, King A, Kattah AG, Kuhle CL, Sood R, Kling JM, Mara KC, Kapoor E, Hypertensive disorders of pregnancy and menopausal symptoms: a cross-sectional study from the data registry on experiences of aging, menopause, and sexuality, Menopause 28(1) (2020) 25–31. 10.1097/GME.0000000000001638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cortes YI, Conant R, Catov JM, Matthews KA, Crawford SL, Hedderson MM, Thurston RC, Impact of nulliparity, hypertensive disorders of pregnancy, and gestational diabetes on vasomotor symptoms in midlife women, Menopause 27(12) (2020) 1363–1370. 10.1097/GME.0000000000001628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lane-Cordova AD, Gunderson EP, Greenland P, Catov JM, Lewis CE, Pettee Gabriel K, Wellons MF, Carnethon MR, Life-course reproductive history and cardiovascular risk profile in late mid-life: The CARDIA Study, J Am Heart Assoc 9(10) (2020) e014859. 10.1161/JAHA.119.014859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li J, Eriksson M, Czene K, Hall P, Rodriguez-Wallberg KA, Common diseases as determinants of menopausal age, Hum Reprod 31(12) (2016) 2856–2864. 10.1093/humrep/dew264 [DOI] [PubMed] [Google Scholar]
- [24].Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, Chavarro JE, Origin, methods, and evolution of the three Nurses’ Health Studies, Am J Public Health 106(9) (2016) 1573–81. 10.2105/AJPH.2016.303338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].H.M.S. Brigham and Women’s Hospital, and Harvard T.H. Chan School of Public Health, Nurses' Health Study History, 2016. https://nurseshealthstudy.org/about-nhs/history. (Accessed July 29, 2022). [Google Scholar]
- [26].Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James-Todd TM, Rich-Edwards JW, Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study, Ann Intern Med 169(4) (2018) 224–232. 10.7326/M17-2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tanz LJ, Stuart JJ, Williams PL, Rimm EB, Missmer SA, Rexrode KM, Mukamal KJ, Rich-Edwards JW, Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women, Circulation 135(6) (2017) 578–589. 10.1161/CIRCULATIONAHA.116.025954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, Hu FB, Manson JE, Zhang C, Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women, JAMA Intern Med 177(12) (2017) 1735–1742. 10.1001/jamainternmed.2017.2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fauser BCJM, Devroey P, Macklon NS, Multiple birth resulting from ovarian stimulation for subfertility treatment, The Lancet 365(9473) (2005) 1807–1816. 10.1016/s0140-6736(05)66478-1 [DOI] [PubMed] [Google Scholar]
- [30].Langton CR, Whitcomb BW, Purdue-Smithe AC, Sievert LL, Hankinson SE, Manson JE, Rosner BA, Bertone-Johnson ER, Association of parity and breastfeeding with risk of early natural menopause, JAMA Netw Open 3(1) (2020) e1919615. 10.1001/jamanetworkopen.2019.19615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Van der Stroom EM, Konig TE, Vink JM, Boomsma DI, Lambalk CB, Ovarian reserve and anti-Mullerian hormone (AMH) in mothers of dizygotic twins, Twin Res Hum Genet 16(2) (2013) 634–8. 10.1017/thg.2013.4 [DOI] [PubMed] [Google Scholar]
- [32].Zhu D, Chung HF, Pandeya N, Dobson AJ, Hardy R, Kuh D, Brunner EJ, Bruinsma F, Giles GG, Demakakos P, Lee JS, Mizunuma H, Hayashi K, Adami HO, Weiderpass E, Mishra GD, Premenopausal cardiovascular disease and age at natural menopause: a pooled analysis of over 170,000 women, Eur J Epidemiol 34(3) (2019) 235–246. 10.1007/s10654-019-00490-w [DOI] [PubMed] [Google Scholar]
- [33].Bleil ME, Gregorich SE, McConnell D, Rosen MP, Cedars MI, Does accelerated reproductive aging underlie premenopausal risk for cardiovascular disease?, Menopause 20(11) (2013) 1139–46. 10.1097/GME.0b013e31828950fa [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kok HS, van Asselt KM, van der Schouw YT, van der Tweel I, Peeters PH, Wilson PW, Pearson PL, Grobbee DE, Heart disease risk determines menopausal age rather than the reverse, J Am Coll Cardiol 47(10) (2006) 1976–83. 10.1016/j.jacc.2005.12.066 [DOI] [PubMed] [Google Scholar]
- [35].Okby R, Shoham-Vardi I, Sergienko R, Sheiner E, Twin pregnancy: is it a risk factor for long-term cardiovascular disease?, J Matern Fetal Neonatal Med 29(10) (2016) 1626–30. 10.3109/14767058.2015.1057491 [DOI] [PubMed] [Google Scholar]
- [36].van Baar PM, Welters SM, Ravelli ACJ, de Boer MA, de Groot CJM, Cardiovascular mortality risk a decade after twin and singleton pregnancies complicated by hypertensive disorders of pregnancy, Pregnancy Hypertens 28 (2022) 9–14. 10.1016/j.preghy.2022.01.009 [DOI] [PubMed] [Google Scholar]
- [37].Hiersch L, Ray JG, Barrett J, Berger H, Geary M, McDonald SD, Diong C, Gandhi S, Guan J, Murray-Davis B, Melamed N, Doh NET, Maternal cardiovascular disease after twin pregnancies complicated by hypertensive disorders of pregnancy: a population-based cohort study, CMAJ 193(37) (2021) E1448–E1458. 10.1503/cmaj.202837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bergman L, Nordlof-Callbo P, Wikstrom AK, Snowden JM, Hesselman S, Edstedt Bonamy AK, Sandstrom A, Multi-fetal pregnancy, preeclampsia, and long-term cardiovascular disease, Hypertension 76(1) (2020) 167–175. 10.1161/HYPERTENSIONAHA.120.14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chih HJ, Elias FTS, Gaudet L, Velez MP, Assisted reproductive technology and hypertensive disorders of pregnancy: systematic review and meta-analyses, BMC Pregnancy Childbirth 21(1) (2021) 449. 10.1186/s12884-021-03938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rosato E, Perrone G, Capri O, Galoppi P, Candelieri M, Marcoccia E, Schiavi MC, Zannini I, Brunelli R, Hypertension and early menopause after the use of assisted reproductive technologies in women aged 43 years or older: Long-term follow-up study, J Obstet Gynaecol Res 42(12) (2016) 1782–1788. 10.1111/jog.13141 [DOI] [PubMed] [Google Scholar]
- [41].Murugappan G, Leonard SA, Farland LV, Lau ES, Shadyab AH, Wild RA, Schnatz P, Carmichael SL, Stefanick ML, Parikh NI, Association of infertility with atherosclerotic cardiovascular disease among postmenopausal participants in the Women’s Health Initiative, Fertil Steril 117(5) (2022) 1038–1046. 10.1016/j.fertnstert.2022.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Raba G, Effect of internal iliac artery ligation on ovarian blood supply and ovarian reserve, Climacteric 14(1) (2011) 54–7. 10.3109/13697130903548916 [DOI] [PubMed] [Google Scholar]
- [43].Rasheed SM, Amin MM, Abd Ellah AH, Abo Elhassan AM, El Zahry MA, Wahab HA, Reproductive performance after conservative surgical treatment of postpartum hemorrhage, Int J Gynaecol Obstet 124(3) (2014) 248–52. 10.1016/j.ijgo.2013.08.018 [DOI] [PubMed] [Google Scholar]
- [44].Martin NG, Shanley S, Butt K, Osborne J, O’Brien G, Excessive follicular recruitment and growth in mothers of spontaneous dizygotic twins, Acta Genet Med Gemellol (Roma) 40(3–4) (1991) 291–301. 10.1017/s0001566000003470 [DOI] [PubMed] [Google Scholar]
- [45].Beemsterboer SN, Homburg R, Gorter NA, Schats R, Hompes PG, Lambalk CB, The paradox of declining fertility but increasing twinning rates with advancing maternal age, Hum Reprod 21(6) (2006) 1531–2. 10.1093/humrep/del009 [DOI] [PubMed] [Google Scholar]
- [46].Fornes R, Simin J, Nguyen MH, Cruz G, Crisosto N, van der Schaaf M, Engstrand L, Brusselaers N, Pregnancy, perinatal and childhood outcomes in women with and without polycystic ovary syndrome and metformin during pregnancy: a nationwide population-based study, Reprod Biol Endocrinol 20(1) (2022) 30. 10.1186/s12958-022-00905-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ramezani Tehrani F, Amiri M, The association between chronic diseases and the age at natural menopause: a systematic review, Women Health 61(10) (2021) 917–936. 10.1080/03630242.2021.1992067 [DOI] [PubMed] [Google Scholar]
- [48].den Tonkelaar I, Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project, Maturitas 27 (1997) 117–123. 10.106/s0378-5122(97)01122-5 [DOI] [PubMed] [Google Scholar]
- [49].Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE, Reproducibility and validity of self-reported menopausal status in a prospective cohort study, Am J Epidemiol 126(2) (1987) 319–325. 10.1093/aje/126.2.319 [DOI] [PubMed] [Google Scholar]
- [50].Solomon CG, Willett WC, Rich-Edwards J, Hunter DJ, Stampfer MJ, Colditz GA, Manson JE, Variability in diagnostic evaluation and criteria for gestational diabetes, Diabetes Care 19(1) (1996) 12–16. 10.2337/diacare.19.1.12 [DOI] [PubMed] [Google Scholar]
- [51].Sondergaard MM, Hlatky MA, Stefanick ML, Vittinghoff E, Nah G, Allison M, Gemmill A, Van Horn L, Park K, Salmoirago-Blotcher E, Sattari M, Sealy-Jefferson S, Shadyab AH, Valdiviezo C, Manson JE, Parikh NI, Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women, JAMA Cardiol 5(12) (2020) 1390–1398. 10.1001/jamacardio.2020.4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].El Khoudary SR, Greendale G, Crawford SL, Avis NE, Brooks MM, Thurston RC, Karvonen-Gutierrez C, Waetjen LE, Matthews K, The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN), Menopause 26(10) (2019) 1213–1227. 10.1097/GME.0000000000001424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, Lee JS, Thurston R, Vuga M, Harlow SD, Factors related to age at natural menopause: longitudinal analyses from SWAN, Am J Epidemiol 178(1) (2013) 70–83. 10.1093/aje/kws421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Grobman WA, Parker CB, Willinger M, Wing DA, Silver RM, Wapner RJ, Simhan HN, Parry S, Mercer BM, Haas DM, Peaceman AM, Hunter S, Wadhwa P, Elovitz MA, Foroud T, Saade G, Reddy UM, Eunice H Kennedy Shriver National Institute of Child, N. Human Development Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, Racial disparities in adverse pregnancy outcomes and psychosocial stress, Obstet Gynecol 131(2) (2018) 328–335. 10.1097/AOG.0000000000002441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, Gunderson EP, Stuart JJ, Vaidya D, E. American Heart Association Council on, Prevention, T. Council on Arteriosclerosis, B. Vascular, C. Council on, N. Stroke, C. the Stroke, Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association, Circulation 143(18) (2021) e902–e916. 10.1161/CIR.0000000000000961 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Procedures for obtaining and accessing data from the Nurses’ Health Studies are described on the studies’ website (https://www.nurseshealthstudy.org/researchers) (e-mail: nhsaccess@channing.harvard.edu).


