Abstract
The blood brain barrier (BBB) plays a critically important role in the regulation of central nervous system (CNS) homeostasis, but also represents a major limitation to treatments of brain pathologies. In recent years, focused ultrasound (FUS) in conjunction with gas-filled microbubble contrast agents has emerged as a powerful tool for transiently and non-invasively disrupting the BBB in a targeted and image-guided manner, allowing for localized delivery of drugs, genes, or other therapeutic agents. Beyond the delivery of known therapeutics, FUS-mediated BBB opening also demonstrates the potential for use in neuromodulation and the stimulation of a range of cell- and tissue-level physiological responses that may prove beneficial in disease contexts. Clinical trials investigating the safety and efficacy of FUS-mediated BBB opening are well underway, and offer promising non-surgical approaches to treatment of devastating pathologies. This article reviews a range of pre-clinical and clinical studies demonstrating the tremendous potential of FUS to fundamentally change the paradigm of treatment for CNS diseases.
1. Introduction
The blood brain barrier (BBB) is the product of distinct physiologic characteristics of the microvasculature within the central nervous system (CNS) [1-5]. The defining features of the BBB include tight junctions that seal the extracellular space between adjacent endothelial cells (ECs), expression of efflux transporters on the luminal membrane of ECs, dense pericyte coverage, downregulation of transcytosis and transcytosis-associated proteins, and ensheathment of the capillaries by astrocyte end feet [1,3-10] (Fig. 1). Altogether, these features produce a dynamic and highly selective physiological barrier, which exists to maintain homeostasis of the neural microenvironment by tightly regulating bidirectional flux of blood-borne neuroactive solutes from the systemic circulation to the CNS and vice versa [1-3,5-7].
Fig. 1.
The physiology of the blood brain barrier, and the permeabilization of the blood brain barrier with microbubbles and focused ultrasound. (A) Focused ultrasound induced volumetric oscillations of microbubbles. (B) Transcytosis of a therapeutic through an endothelial cell. (C) Sonoporation of an endothelial cell. (D) Disruption of tight junctions between adjacent endothelial cells.
Movement of substances across the BBB occurs via several highly controlled mechanisms: paracellular transport, passive diffusion, solute carriers, and adsorptive or receptor mediated transcytosis [2,5]. These transport avenues serve to shield the CNS from potentially harmful endogenous and exogenous species, while simultaneously permitting entry of required metabolites. However, in the context of treatment of CNS disease, the BBB also poses a significant challenge to drug and gene delivery, as it blocks transport of 100 % of large and 98 % of small systemically administered neurotherapeutics [7]. Thus, many of the current treatment options for CNS diseases require invasive procedures that are often associated with substantial risks and long recovery times.
Focused ultrasound (FUS) applied in conjunction with intravenously administered micron-scale microbubbles (MBs) has emerged as an effective modality to non-invasively and reversibly circumvent the transport limitations of the BBB in a targeted and localized manner [10-14]. The technique was first pioneered by Hynynen et al. in 2001, where the combination of FUS and MBs was shown to transiently permeabilize the cerebral vasculature of rabbits [10-13,15]. Mechanistically, ultrasound is a sinusoidal pressure wave, and FUS is the convergence of many ultrasound waves to a small volume, producing an amplified effect [11]. At low acoustic pressures, at its focal point, FUS induces consistent volumetric oscillations of the gas-filled MBs, known as stable cavitation, within the capillary lumen (Fig. 1A). The alternating expansion and contraction of the MBs generates mechanical forces on the surrounding capillary walls [10,12,14,16,17]. The resultant stresses lead to the local permeabilization of the BBB through disruption of the tight junctions between ECs (Fig. 1D), sonoporation of ECs (Fig. 1C), and upregulation of transcytosis (Fig. 1B) [10,11,15]. These impacts are transient and typically reverse themselves within 24 h [10,11,15,18].
Increases in acoustic pressure are correlated with increases in BBB permeability. While this can be beneficial, high acoustic pressures also lead to larger volumetric oscillations of the MBs and eventual MB collapse [16,19]. This phenomenon, known as inertial cavitation, produces additional mechanical stresses on the capillaries in the form of micro-jets that can damage the surrounding capillary and brain parenchyma [14,16]. Under certain circumstances, such as the delivery of therapeutics in the size range of 500–2000 kDa or within a tumor, the increased permeability of inertial cavitation may outweigh its damaging consequences [14]. Regardless of the cavitation specifics, FUS and MBs have provided a method to safely permeabilize the BBB in a localized and transient manner, offering the possibility of noninvasive delivery of molecular species to targeted regions of the CNS [7,11,15,18] (Fig. 1C).
Henceforth, we will refer to FUS-mediated BBB opening with MBs as “BBBO”. While BBBO presents a new way to deliver therapeutics to the CNS, there are also several novel safety considerations, such as the need for accurate targeting, the risk of tissue heating, and the ability to monitor in vivo MB cavitation [11,15,18]. Minimizing off-target application of FUS is commonly addressed by integrating high resolution three-dimensional magnetic resonance imaging (MRI) with the FUS system. This approach, denoted as magnetic resonance guided focused ultrasound (MRgFUS), ensures accurate placement of the focal spot at the intended treatment site [11,13,15]. An alternative approach is neuronavigation-guided FUS, whereby treatment planning on MR images is used to co-localize FUS delivery with a single-element transducer, aided by surface-attached fiducial markers [20,21]. Heating occurs from the temporal summing of energy deposited into the tissue by the ultrasonic waves [17]. This phenomenon has been exploited for procedures such as thermal ablation, where FUS is applied continuously to induce large temperature changes in the targeted tissue [15,17]. In the context of BBB opening, however, large temperature changes are undesirable due to the risk of causing damage to non-diseased neural tissue. Therefore, FUS is applied with lower pressure intensities and in a pulsed manner to reduce energy deposition while still allowing for MB modulation [11,15,17].
Lastly, MB cavitation must be monitored to ascertain whether stable or inertial cavitation is occurring. Traditionally, this is done by monitoring the acoustic emissions of the oscillating MBs in real-time. The presence of harmonics, subharmonics, or ultraharmonics of the original FUS wave in the acoustic emission profiles provides feedback about the in vivo behavior of the MBs [14-16]. Therefore, cavitation monitoring allows for real-time adjustments of MB activity and can be used to set FUS pressure thresholds to avoid inertial cavitation [14,16,17].
This review seeks to discuss the current applications of FUS and MB-mediated BBB opening (BBBO) and potential future applications. This includes the delivery of therapeutic agents to treat neurological disorders (Alzheimer’s disease, Parkinson’s disease, and glioblastoma), the secondary effects of BBB opening/permeabilization, pre-clinical neuroscience tools and a review of current clinical trials. In contrast to other reviews on similar topics, we take advantage of the Advanced Drug and Delivery Reviews format to provide a very comprehensive summary of clinical and preclinical studies within the field, as well as offering our forward-looking thoughts on emerging new applications and future possibilities for the field.
2. Pre-Clinical delivery of therapeutic agents
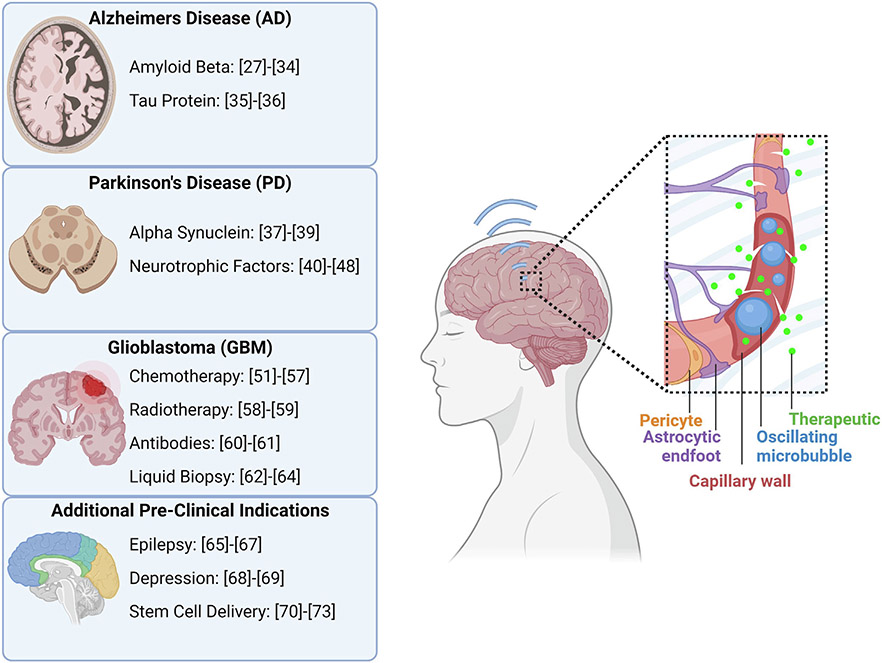
As described previously, the physiology of the brain causes many challenges to the delivery of therapeutic agents for neurological pathologies. Promising candidates such as genes, peptides, or other pharmacological agents are frequently stymied by the structure of the BBB. Some of these physiological challenges, which have slowed the progression of clinical options for CNS diseases, may be overcome with FUS-mediated BBBO [22-24]. These CNS diseases, including neurodegenerative conditions, primary and secondary brain tumors, and psychiatric disorders, affect many millions of people worldwide and place a tremendous strain on healthcare systems [25]. For these reasons, there have been many preclinical studies on the efficacy of FUS-mediated BBBO to facilitate the delivery of therapeutics in animal models of CNS diseases, some of which will be highlighted below. The molecular and cellular targets of the various therapies delivered are shown in Fig. 2. A summary of the FUS parameters for each study is provided in Table 1.
Fig. 2.
A summary of the pre-clinical therapeutic targets for which drugs and genes are being delivered with FUS to the brain for various disease applications. References correspond to studies investigating that therapeutic target with FUS BBBO. Created with Biorender.com.
Table 1.
Summary of FUS parameters utilized and therapeutic payload delivered in the studies covered in the Pre-Clinical Delivery of Therapeutic Agents section. It should be noted that the studies provide different levels of detail in their methodology, including FUS pulsing parameters and MB dosage, composition, and size distribution. Here, we have highlighted as many FUS parameters as could be determined from the studies. MBs can be formulated with a wide variety of shell materials, gas cores, charges, functional group attachments, size distributions, and concentrations. These features may differentially impact the bioeffects of the MBs upon FUS application. It is difficult to draw definitive conclusions on their relative contributions due to a lack of standardization within the field with regards to reporting and controlling for these characteristics.
| Disease | Ref. | Animal model | Delivered agent | FUS Parameters f = frequency; dc = duty cycle; BL = burst length; PRF = pulse repetition frequency; PNP = peak negative pressure; I = Intensity t = time |
MB type |
|---|---|---|---|---|---|
| Alzheimer’s Disease (AD) | [27] | APPswe/PSENl-dE9 transgenic mice | GSK-3 inhibitor | f = 0.4 MHz; BL = 10 ms; PRF = 1 Hz; PNP = 0.41 MPa; t = 60 s | SonoVue® SF6-filled |
| [28] | TgCRND8 transgenic mice | TrkA-agonist | f = 1.68 MHz; BL = 10 ms; PRF = 1 Hz; PNP = 1.29 MPa; t = 120 s | Definity | |
| [29] | TgCRND8 transgenic mice | TrkA-agonist | f = 1.68 MHz; BL = 10 ms; PRF = 1 Hz; PNP = 1.29 MPa; 120 s | Definity | |
| [30] | TgCRND8 Transgenic mice | Intravenous immunoglobulin (IVIg) | f = 1.68 MHz; BL = 10 ms; PRF = 1 Hz; t = 120 s | Definity | |
| [31] | APPswe/PSddE9 transgenic mice | anti-pGlu3 Aβ mAb | f = 0.835 MHz; BL = 10 ms; PRF = 2 Hz; PNP = 0.33 MPa; t = 100 s | Optison™ | |
| [32] | APP/PS1 transgenic mice | Quercetin-modified sulfur nanoparticles | PNP = 1 MPa; t = 600 s | Poly(α-cyanoacrylate nbutyl acrylate) | |
| [33] | TgCRND8 Transgenic mice | rAAV1/2 | f = 1.68 MHz; BL = 10 ms; PRF = 1 Hz; t = 120 s | Definity | |
| [34] | TgCRND8 transgenic mice | rAAV9 | f = 1.68–1.78 MHz; BL = 10 ms; PRF = 1 Hz; t = 120 s | Definity | |
| [35] | pR5 tau transgenic mice | Single chain antibody fragment RN2N | f = 1 MHz; dc = 10 %; BL = 10 ms; PRF = 10 Hz; PNP = 0.7 MPa; t = 6 s/spot | Phospholipid shell, octafluoropropane gas core | |
| [36] | pR5 tau transgenic mice | RN2N IgG | f = 1 MHz; dc = 10 %; BL = 10 ms; PRF = 10 Hz; PNP = 0.6 MPa; t = 60 s | Phospholipid shell, octafluoropropane gas core | |
| Parkinson’s Disease (PD) | [37] | Human α-syn expressing transgenic mice | rAAV9 | f = 1.68 MHz; BL = 10 ms; PRF = 1 Hz; PNP = 1.0–1.1 MPa; t = 120 s | Definity |
| [38] | C57BL/6 mice | α-syn plasmid | f = 0.4 MHz; BL = 10 ms; PRF = 1 Hz; PNP = 0.3 MPa; t = 60 s | SonoVue® SF6-filled | |
| [39] | MPTP mice | Curcumin | f = 1 MHz; BL = 200 ms; PRF = 1 Hz; PNP = 0.24–0.45 MPa; t = 60 s | Lipid-PLGA nanobubbles | |
| [40] | MPTP mice | GDNF plasmid liposome | f = 0.5 MHz; BL = 10 ms; PRF = 1 Hz; PNP = 0.3–0.8 MPa; t = 60 s | Avidinylated MBs conjugated with LpDNA | |
| [41] | 6-OHDA rats | GDNF plasmid | f = 1 MHz; cycle number = 5,000; PRF = 1 Hz; PNP = 0.7 MPa | GDNFp-loaded cationic microbubbles | |
| [42] | 6-OHDA rats | Nrf2 | f = 0.5 MHz; t = 30 s | Nrf2-loaded cationic nanomicrobubbles | |
| [43] | 6-OHDA rats | GDNF-loaded BPNs | f = 1.15 MHz; BL = 10 ms; PRF = 0.5 Hz; PNP = 0.6 MPa; t = 120 s | Albumin-shelled, octofluoropropane gas core | |
| [44] | 6-OHDA rats | GDNF-loaded PEGylated liposome | f = 1 MHz; dc = 20 %; I = 2 W/cm2 | Avidinylated biotinylated lipid-shelled | |
| [45] | MitoPark mice | polyethylenimine superparamagnetic iron oxide plasmid DNA | f = 1 MHz; cycle number = 5000; PRF = 1 Hz; PNP = 0.3 MPa; t = 60 s | Lipid-shelled | |
| [46] | MPTP mice | AAV-GDNF and neurturin (NTN) | f = 1.5 MHz; PRF = 10 Hz; PNP = 0.45 MPa; t = 60 s | In-house polydisperse | |
| [47] | MPTP mice | BDNF | f = 1.5 MHz; BL = 6.7 ms; PRF = 10 Hz; PNP = 0.45 MPa; t = 60 s | Size-isolated lipid-shelled | |
| [48] | C57BL/6 mice | BDNF | f = 1.5 MHz; BL = 6.7 ms; PRF = 5 Hz; PNP = 0.45 MPa; t = 60 s | Lipid-shelled, perfluorobutane core | |
| Glioblastoma Multiforme (GBM) | [51] | U87-luc and patient-derived 6240-luc Mice | Carboplatin | f = 1.05 MHz; BL = 23.8 ms; PRF = 1 Hz; PNP = 0.3 MPa; t = 120 s | SonoVue® |
| [52] | MGPP3 (Pdgf+/Pten−/−/P53−/−) Mice | Etoposide | f = 1.5 MHz; BL = 1 ms; PRF = 5 Hz; PNP = 0.7 MPa; t = 30 s | In-house polydisperse, lipid-shelled | |
| [54] | C6 Rats | Liposomal temozolomide nanoparticles | f = 1 MHz; cycle number = 10,000; PRF = 1 Hz; PNP = 0.8 MPa; t = 60 s | In-house lipid-shelled, perfluoropropane gas core | |
| [55] | Patient-derived glioma Mice | Paclitaxel | f = 1 MHz; BL = 12 ms; PRF = 1 Hz; PNP = 0.3 MPa; t = 120 s | Lumason | |
| [56] | U87 and B16F1ova Mice | ZsGreen BPNs and Luc-BPNs | f = 1.1 MHz; BL= 10 ms; PRF = 0.5 Hz; PNP = 0.55 MPa; t = 120 s | Albumin-shelled | |
| [57] | T98G Mice | pCas9/MGMT-loaded cRGD Lipid-polymer hybrid nanoparticles (LPHNs) | I = 1.84 W | In-house avidinylated biotinylated lipid-shelled conjugated to LPHNs-cRGD | |
| [58] | GL261 Mice | PEG-b-PMBSH nanoparticles | f = 1 MHz; dc = 0.5 %; cycle number = 5,000; PRF = 1 Hz; PNP = 0.3–0.7 MPa; t = 60 s | In-house cationic lipid-shelled | |
| [59] | 3-Gy irradiated Mice | Trypan blue dye solution | f 0.69 MHz; BL = 10 ms; PRF = 2 Hz; PNP = 0.26–0.27 MPa; t = 95 s | Optison | |
| [60] | GL261 Mice | mCD47 | f = 1.1 MHz; dc = 0.5 %; PNP = 0.4 MPa; t = 120 s | Albumin-shelled | |
| [61] | Patient-derived glioma Mice | 89Zr-radiolabelled antibody targeting EphA2 receptors | f = 1.1 MHz; BL = 10 ms; PRF = 0.4 Hz; PNP = 0.85 MPa; t = 120 s | Definity | |
| [62] | eGFP-transduced U87 and GL261 Mice | eGFP mRNA | f = 1.44 MHz; dc =1%; BL = 10 ms; PRF = 1 Hz; PNP = 3.82 MPa; t = 120 s | In-house lipid-shelled, perfluorobutane core | |
| [63] | eGFP-transduced U87 and GL261 Mice | eGFP mRNA | f = 1.44 MHz; dc = 1 %; BL = 10 ms; PRF = 1 Hz; PNP = 3.82 MPa (U87) and 1.52, 2.74, 3.53 MPa (GL261); t = 120 s | In-house lipid-shelled, perfluorobutane core | |
| [64] | Porcine model Normal brain tissue | GFAP and myelin basic protein release | f = 0.65 MHz; BL = 10 ms; PRF = 1 Hz; PNP = 1.5 MPa; t = 180 s | Definity | |
| Temporal Lobe Epilepsy | [65] | Rat model pilocarpine | N/A | f = 0.65 MHz; dc = 2 %; BL = 20 ms; PRF = 1 Hz; PNP = 0.4 MPa; t = 90 s | Definity |
| [66] | CD-1 mice | Quinolinic acid | f = 1.5 MHz; dc = 2 %; BL = 20 ms; PRF = 1 Hz; PNP = 0.33, 0.5, 0.67 MPa; t = 120 s | Definity | |
| [67] | Sprague Dawley rats | Quinolinic acid | f = 1.5 MHz; dc = 2 %; BL = 20 ms; PRF = 1 HZ; PNP = 0.69 MPa; t = 120 s | In-house PEG lipid-shelled, decafluorobutane gas core | |
| Stem Cell Delivery for CNS Disorders | [70] | Sprague Dawley rats | Iron labeled GFP neural stem cells | f = 0.558 MHz; BL = 10 ms; PRF = 1 Hz; PNP = 0.24 MPa; t = 120 s | Definity |
| [72] | Sprague-Dawley rats | SPION-loaded hNPCs | f = 1.5 MHz; BL = 10 ms; PRF = 10 Hz; PNP = 0.45 MPa; t = 60 s | monodisperse microbubbles (SIMB4-5) | |
| [73] | Sprague–Dawley rats | Mesenchymal stem cells | f = 0.515 MHz; BL = 10 ms; PRF = 1 Hz; PNP = 0.25 MPa; t = 300 s | Definity |
2.1. Alzheimer’s disease
FUS-mediated BBBO has been used to deliver a wide variety of therapeutic agents (i.e. drugs, antibodies, nanoparticles, gene therapies) for the treatment of Alzheimer’s Disease (AD) in preclinical studies. The studies to date have investigated the effect of FUS delivery of these therapeutics on amyloid and tau burden, behavioral impairments, inflammatory responses, and neuronal health. It should be noted that a multitude of studies have indicated that FUS-mediated BBBO alone (i.e. without therapeutic delivery) is sufficient to reduce plaque burden and rescue behavioral impairments; however, this phenomenon will be discussed in detail in the later section entitled “Pre-Clinical Secondary Effects of BBB Opening”.
2.1.1. Amyloid beta
Several investigations have been conducted using FUS-mediated BBBO specifically in amyloidosis models of AD. Delivery of an inhibitor to glycogen synthase kinase 3 (GSK3), which is thought be dysregulated in the neurons of the AD brain [26], has been investigated to reduce synthesis of amyloid-β plaque [27]. In this study, the GSK3 inhibitor AR-A014418 was delivered unilaterally to the hippocampus in APPswe/PSEN1-de9 transgenic mice for 5 weekly treatments. Not only was GSK3 activity reduced by over 60 % following their treatment regime, but amyloid-β plaque was found to decrease significantly by over 30 % as well. FUS-mediated drug delivery in amyloidosis models has also sought to combat cholinergic neuron degeneration, which is a known consequence of AD contributing to decreased cognition. Xhima et al. explored FUS-mediated BBBO and delivery of the TrkA selective agonist D3 to rescue neurotrophin signaling in an amyloidosis model [28]. With a single treatment targeting the bilateral basal forebrain in TgCRND8 mice, the selective TrkA agonist enhanced cholinergic transmission and rescued neurotrophin signaling. The group went on to investigate 3 repeat treatments, performed weekly, targeting the bilateral forebrain, hippocampus, and cortex [29]. This treatment regime not only attenuated cholinergic degeneration but also reduced amyloid plaque burden and increased hippocampal neurogenesis.
Several studies have also investigated antibody delivery for amyloid-β reduction. One group investigated FUS-mediated delivery of intravenous immunoglobulin (IVIg) containing human polyclonal antibodies in TgCRND8 mice [30]. FUS + IVIg treatment targeted towards the bilateral dorsal hippocampus resulted in reduced amyloid plaque pathology, increased neurogenesis, and decreased TNFα in the hippocampus. Another study investigated delivery of an anti-pGlu3 amyloid-β monoclonal antibody, which targets the pathogenic form of amyloid-β [31]. In APPswe/PS1dE9 mice, FUS was applied to the hippocampus for 3 weekly treatments, resulting in reduced hippocampal plaque burden, increased hippocampal synaptic proteins, and improved spatial learning and memory. Additionally, monocyte infiltration and recruitment to plaque was augmented with this treatment.
To our knowledge, just one group has utilized FUS-mediated BBBO to deliver nanoparticles for AD. This group used microbubble-embedded sulfur nanoparticles, which have been shown to reduce neurotoxicity and ROS production, modified with quercetin to increase stability [32]. Their delivery to APPswe/PS1dE9 mice via FUS BBBO targeted to the right hemisphere of the brain for 5 weekly treatments resulted in reduced amyloid-β deposition and neuron loss, as well as improved learning and memory. In vitro studies indicated that these nanoparticles reduced neuronal apoptosis, inflammatory response, calcium homeostasis imbalance, and oxidative stress.
FUS-mediated gene therapy has also been demonstrated in mouse models of amyloidosis. One study outlines a strategy for increasing transgene expression near amyloid plaques by using the astrocytic GFAP promoter to drive recombinant adeno-associated virus mosaic serotype 1/2 (rAAV1/2) expression of green fluorescent protein (GFP) [33]. Targeted delivery to the cortex and hippocampus in TgCRND8 mice augmented GFP expression near amyloid-β plaques, though expression was also significant in the liver. FUS-mediated delivery of newer generations of rAAVs in amyloidosis mice has also been explored. One study compared FUS-mediated delivery of the older generation rAAVs (rAAV9) to the newer generation (rAAV-PHP.B) [34]. The same dose was needed for both generations to achieve delivery. However, unlike rAAV9, FUS and MBs did not decrease the rAAV-PHP.B dosage needed to transduce brain cells in TgCRND8 mice. FUS-mediated delivery of both generations induced major histocompatibility complex class II expression, complement system and microglial activation, and T cell infiltration.
2.1.2. Tau protein
Fewer studies have been conducted in tau-based models of AD. Nevertheless, promising studies have been conducted with FUS-mediated antibody delivery regimens. Nisbet et al. investigated the delivery of a single chain antibody fragment against the 2 N tau isoform (RN2N) [35]. Scanning focused ultrasound (with MBs) over the entire forebrain in pR5 mice expressing 2N4R tau, in conjunction with RN2N delivery, increased uptake by neurons in the targeted brain region, reduced phosphorylated tau levels, and reduced anxiety-like behaviors. This group went on to investigate the fragment antigen-binding versus full-sized immunoglobulin format of RN2N with scanning focused ultrasound [36]. The study indicates that full-sized RN2N is better retained and has higher concentrations in the brain compared to the smaller formats; meanwhile, uptake and distribution remain similar between the two groups. Overall, several studies have now robustly indicated the utility of FUS BBBO to enhance local therapeutic delivery to the brain in AD animal models. A multitude of therapeutic agents are effectively delivered and demonstrate improved cognitive effects and reduced amyloid and tau burdens.
2.2. Parkinson’s disease
Parkinson’s disease (PD) pathology is largely marked by overexpression of alpha-synuclein (α-syn) and neuron degeneration leading to impaired motor function and memory [37,38]. Thus, many PD therapies focus on delivering molecules to repair these dysregulations.
2.2.1. Alpha-Synuclein
α-syn is a neuronal protein that is misfolded and overexpressed in PD. This overexpression and misfolding allows for permissive templating of neighboring, healthy α-syn, leading to spreading of diseased tissue to multiple brain regions [37]. Xhima et al. used FUS-mediated BBBO to deliver recombinant adeno-associated virus serotype 9 (AAV9) expressing human α-syn shRNA to silence α-syn expression in transgenic mice expressing wild-type human α-syn. The construct was delivered to the hippocampus, substantia nigra (SN), olfactory bulbs, and dorsal motor nucleus of the vagus, all of which are brain regions in which α-syn aggregates. One month following treatment, α-syn expression, measured by α-syn immunoreactivity, was suppressed ~ 60 % across all brain regions [37]. However, behavioral changes post-treatment were not observed in this study. Another study performed by Yan et al. investigated the therapeutic impact of delivering curcumin, a neuroprotective agent that has been shown to inhibit and remove α-syn proteins [39]. An early stage MTPT PD model was exposed to FUS-mediated BBBO and curcumin treatments targeting deep-seated diseased brain regions [39]. Behavioral tests indicated improved neuromuscular coordination and motor abilities. While biological changes in α-syn expression were not observed, it can be postulated that the improved motor abilities result from a reduction of α-syn, given the anti-α-syn nature of curcumin [39], although further tests will need to be conducted to confirm this. This treatment regimen is a promising therapy for early-stage PD. However, most cases of PD are not diagnosed in the early stages due to late onset of symptoms [38]. It would be interesting to note the efficacy of this therapy on later-stage PD models.
In addition to using FUS for PD therapeutic developments, BBBO has also been used to create murine PD models more closely mimicking human PD pathology. Lin et al. used FUS to deliver α-syn plasmids to C57BL/6 mice resulting in increased α-syn expression, increased distribution of plasmid DNA in vivo, and human PD-like aggregation patterns of α-syn as compared to direct injection of the same plasmid [38]. Not only does this technique demonstrate the potential of BBBO to generate more accurate PD models, but it can also be used as a platform to enhance plasmid DNA delivery to brain tissue for therapeutic applications, such as knocking down α-syn protein expression or targeting other PD pathological implications.
2.2.2. Neurotrophic factors
Another component of PD pathology is loss of dopaminergic (DA) neurons and depletion of dopamine, which leads to motor activity degeneration [40-45]. Delivery of neurotrophic factors has been proven to elicit neuroprotective and neurotrophic effects [40,41,44,45], which may slow or reverse the effects of the disease.
The delivery of genetic material through viral vectors with BBBO provides strong and long-lasting transgene expression, minimal to no off-target effects, and a large reduction in therapeutic viral dose [46]. Karakatsani et al. used AAV1 to deliver glial-derived neurotropic factor (GDNF), which can promote DA neuron regeneration and prevent disease progression [40,41,43,45,46], targeting the SN and striatum. This treatment resulted in increased optical densities of dopaminergic dendrites coupled with improved behavioral responses [46]. While the viral dose was significantly decreased with BBBO, viral dosage may be further reduced by engineering AAVs to possess cell-specific capsids or cell-specific gene promoters, limiting delivery to non-target organs [46].
Although efficient at delivering gene therapies to tissue, viral vectors can elicit an immune response depending on the frequency and dosage of administration [45]. To overcome challenges associated with viral vectors, several non-viral methods of delivering genetic materials have been attempted for PD therapies. Many groups have exploited the positive charge of cationic MBs and negative charge of plasmid DNA to electrostatically load plasmids onto MBs to protect the DNA from enzyme-mediated degradation and enhance localization of the therapeutic to the treatment site [41,42,45]. GDNF plasmid (pGDNF)-MB complexes delivered to the SN and striatum of a 6-OHDA rat PD model with FUS BBBO resulted in enhanced transfection efficiency and duration as opposed to delivery of free plasmid DNA and MBs [41]. Furthermore, pGDNF-MB complexes displayed neuroprotective effects in PD, as evidenced by restoration of GDNF concentration, increased DA, and improved motor skills in rats [41].
Electrostatic interactions between DNA and cationic MBs can be transient, so alternative methods of conjugation between plasmids and MBs may ensure better colocalization at the FUS-targeted region [40,44]. Liposomes containing GDNF plasmids were complexed to MBs via biotin-avidin linkages and delivered to the SN of two different PD models after BBBO: an MPTP-induced murine model [44] and a 6-OHDA rat model [40]. In both models, DA levels increased after FUS-mediated treatment with the MB complexes, indicated by increased levels of GDNF, DAT (dopamine transporter) and TH-positive neurons (a marker for dopaminergic activity) [40,44]. Additionally, both models exhibited improvements in behavior and motor skills. While neither PD model exactly recapitulates human PD, it is promising that two different PD models respond to the therapy similarly.
Although the BBB markedly hinders the passage of therapeutics to brain tissue, once across the BBB, the transport of such therapeutics is further limited by the dense, negatively charged extracellular matrix (ECM) [43]. Recently, brain penetrating nanoparticles (BPNs), which are coated with a dense layer of neutral, hydrophilic PEG, and are less that 114 nm in size, have been shown to overcome the limitations of the ECM [43]. pGDNF encapsulating BPNs were delivered to the striatum of a 6-OHDA rat PD model after BBBO, yielding increased GDNF expression, increased DA levels, and increased TH-positive neurons up to 12 weeks post-treatment, indicating the regimen’s neuroprotective abilities. In addition, motor skills were significantly improved in treated rats. While nanoparticle-facilitated delivery of therapeutics to the brain helps overcome therapeutic limitations resulting from the brain microenvironment, the nanoparticles can accumulate in off-target tissue, thus resulting in off-target effects [45]. To further improve gene delivery and enhance therapeutic efficacy of neurotrophic factors, nanoparticles can be formulated with magnetic materials to allow for magnetic navigation to target tissues in addition to BBBO with FUS [45].
As an alternative to manipulating neurotrophic factor expression with gene therapy, some groups used FUS-mediated BBBO to facilitate localized delivery of intranasally administered neurotrophic factor proteins [47,48]. Brain-derived neurotrophic factor (BDNF), a key regulator of neuron growth and function [47], was successfully delivered to the striatum and SN, marked by on-target BDNF accumulation [48] and upregulation of TH expression [47], indicating the therapy’s potential to improve PD treatments.
While this section highlights therapies focusing on common PD targets, like α-syn and neurotrophic factors, other targets have also shown promising anti-PD results. One group delivered nuclear factor E2-related factor 2 (Nrf2) plasmids to the SN of a 6-OHDA rat PD model to regulate ROS, which play a role in PD [42]. Delivery of Nrf2 following BBBO significantly improved ROS regulation and increased expression of DAT and TH, suggesting neuroprotection [42]. In summary, FUS-induced BBBO is a promising supplement to current PD treatments as it improves localized therapeutic accumulation and treatment efficacy.
2.3. Glioblastoma Multiforme (GBM)
Glioblastoma Multiforme (GBM) is the most commonly diagnosed primary brain neoplasm, often treated with surgical resection of the tumor and concurrent chemoradiation therapy (CRT). However, even with intervention, patients are faced with the grim survival expectancy of only ~ 15 months post-diagnosis [49]. The low survival outcome is attributed in part to the BBB, as it limits the delivery of therapeutics to tumors and their surrounding infiltrating rim. As such, infiltrating tumor cells that are undetectable via conventional imaging modalities and unresectable with surgery are protected from cytotoxic therapies, allowing for tumor recurrence. Within the tumor, the BBB is often heterogeneously disrupted due to aberrant angiogenic signals, creating a ‘leaky’ blood-tumor barrier (BTB) [49,50]. Despite the irregular leakiness of these blood vessels, delivery of therapeutics is limited and would benefit from FUS BBBO to create a more evenly permeable network of capillaries.
2.3.1. Chemotherapy
FUS is a safe, noninvasive tool that can potentiate targeted chemotherapy in pre-clinical animal models of GBM. It is worth noting that, due to inherent physiological differences between the tumor tissue and healthy brain, such as higher cerebral blood volume in high grade GBM and a baseline irregularity and leakiness of the tumor capillaries, effort should be taken to tune safe FUS parameters for GBM applications. Recently, studies investigating systemic carboplatin and etoposide administration with FUS BBBO have been conducted. In one study, BBBO increased carboplatin brain penetration 4.2-fold and significantly increased survival and growth control in U87 xenograft and patient derived (PDX) orthotopic GBM models [51]. In another, FUS BBBO enhanced etoposide penetration and therapeutic efficacy in an orthotopic MGPP3 (Pdgf+, Pten−/−, P53−/−) mouse model of GBM [52]. These results showcase the therapeutic promise of FUS-mediated BBBO in conjunction with chemotherapies to control tumor growth and increase survival; however, systemic administration of chemotherapy can cause adverse effects [53], and FUS-increased penetrance of cytotoxic agents into the brain causes concern for increased neurotoxicity [52]. Recent studies have aimed to address side effects and neurotoxicity concerns. One such study leveraged liposome-encapsulated TMZ (TMZ-lipo) with FUS BBBO in an orthotopic C6 GBM rat model to address the side effects caused by high dosage requirements of systemic temozolomide (TMZ) [54]. TMZ-lipo + FUS enhanced local therapeutic delivery and significantly increased survival and tumor growth control. Another study assessed distribution, toxicity, and efficacy of FUS-mediated delivery of two clinically approved formulations of paclitaxel (PTX), albumin-bound PTX (ABX) and PTX dissolved in cremophor (CrEL-PTX) [55]. ABX increased survival in a murine PDX model when compared to untreated control and CrEL-PTX treated mice. Further, while repeated FUS administration of ABX was well tolerated, ultrasound-mediated delivery of CrEL-PTX and CrEL alone were both lethal in > 35 % of mice treated. Together, these results emphasize that, while FUS is clearly able to enhance chemotherapeutic drug delivery to GBM, therapeutic formulation (and the clinical approval status of novel drug formulations) and toxicities will remain a key consideration when developing novel chemotherapeutic strategies against GBM.
In addition to increasing the therapeutic delivery of packaged chemotherapies, FUS BBBO has been used to enhance the delivery of gene-bearing nanoparticles for gene therapy. FUS with MBs has been shown to modulate intratumoral interstitial fluid flow and convective transport within the tumor that results in enhanced nanoparticle dispersion and tumor cell transfection in murine orthotopic models of GBM and melanoma [56]. Additionally, MB-conjugated lipid-polymer hybrid nanoparticles loaded with CRISPR/Cas9 plasmids targeting the MGMT gene, which confers TMZ resistance, enhanced the efficacy of TMZ, inhibited tumor growth, and increased survival in a murine T98G xenograft model of GBM upon activation with FUS [57].
2.3.2. Radiotherapy
While FUS BBBO is widely used to increase chemotherapy delivery to GBM, there have been fewer pre-clinical studies on the use of FUS in conjunction with radiotherapy (RT). One study explored FUS BBBO to increase the penetration of sodium borocaptate and BSH to potentiate boron neuron capture therapy (BNCT)[58]. Their therapeutic strategy coupled self-assembled boron-containing polyanion nanoparticles with cationic microbubble (B-MB) mediated FUS treatment of orthotopic murine GL261 tumor bearing mice. When coupled with FUS, the B-MB group had increased boron uptake by the tumor directly after sonication, suggesting that B-MBs increased delivery efficiency. However, future studies are needed to optimize this strategy, as the tumor-to-blood boron ratio did not reach a therapeutic threshold. Another study stressed the need to consider the impact of prior therapy on FUS BBBO, as GBM patients will likely have had previous RT, chemotherapy, and surgery prior to FUS sonication [59]. White et al. assessed the impact of full brain 3 Gy irradiation for 10 days on trypan blue dye penetration into mouse brains treated with FUS alone or FUS with prior irradiation. FUS + prior irradiation resulted in increased trypan blue when compared to irradiation or FUS alone, suggesting that radiation may be a sensitizing factor for FUS BBBO. While the brains of irradiated mice had hemorrhagic petechiae, they were outside of the sonication region, indicating that they are likely a direct result of radiation therapy and not FUS BBBO [59]. Together, these studies demonstrate that there is much left to investigate in how FUS BBBO can be used to potentiate RT and how previous RT may impact the safety and efficacy of FUS BBBO.
2.3.3. Antibody delivery
In addition to chemotherapy, FUS has been widely used to increase the delivery of immunotherapeutics. A recent study aimed to address intratumoral delivery of an anti-CD47 (mCD47) monoclonal antibody, assessing both dosing strategy and therapeutic efficacy [60]. Zirconium 89 ([89Zr]) labeled mCD47 was used to leverage positron emission tomography (immuno-PET) to non-invasively monitor mCD47 kinetics in an orthotopic Gl261 murine GBM model. Two dosing strategies were used; IV administration directly prior to FUS BBBO (FUS-pre) or IV administration 15 min post-FUS BBBO (FUS-post). FUS-post administration significantly increased intratumoral antibody penetration and enhanced animal survival, suggesting that timing of administration is a key parameter to consider. Another study employed immuno-PET and contrast-enhanced MRI to monitor the [89Zr] anti-EphA2 receptor kinetics in a PDX WK1 xenograft model of non-enhancing high-grade glioma (HGG) [61]. This study investigated FUS BBBO in an infiltrating tumor model characterized by high tumor burden protected by intact BBB. FUS significantly increased antibody uptake in previously non-enhancing tumor regions. Under experimental conditions, FUS BBBO was not sufficient to increase antibody delivery across already-disrupted BTB but was able to improve delivery to infiltrating tumor cells. Both studies underscore the value of using MRI and PET imaging in pre-clinical FUS BBBO studies and punctuate the need to probe how therapeutic administration, dosing strategy, FUS parameters, and model impact therapeutic efficacy and antibody delivery.
2.3.4. Liquid biopsy
In addition to FUS BBBO for drug delivery, groups are evaluating how FUS BBBO opens “two-way trafficking” from blood to brain and vice versa [62]. Two recent studies from Zhu and colleagues underscore the promise of FUS-mediated liquid biopsy (FUS-LBx), by assessing release of eGFP mRNA from the tumor into the bloodstream at different FUS pressures [62,63]. Peak negative pressures ranging from 0.59 MPa to 1.58 MPa were sufficient to enable FUS-LBx detection of eGFP mRNA in the plasma. Another study investigated FUS-LBx to detect brain-specific biomarkers in a non-tumor bearing porcine model after FUS-LBx [64]. Both studies, however, have some limitations. For example, the first only detected eGFP, which is not natively expressed by tumor cells. Further, this study lacked investigation on how eGFP signal changes over time. Meanwhile, the second study did not aim to detect tumor or disease specific biomarkers. The addition of such elements to future studies will further support the promise of FUS-mediated liquid biopsy for GBM.
2.4. Other applications
As demonstrated in the studies summarized above, FUS-mediated BBBO shows tremendous promise in pre-clinical studies to facilitate delivery of known therapeutic agents, including antibodies, proteins, gene therapy constructs, and small molecule drugs, into specific diseased structures of the brain. While we have primarily focused on the areas of Alzheimer’s disease, Parkinson’s disease, and GBM, it should be noted that FUS for BBBO and drug delivery is an active area of pre-clinical research in other neurological conditions as well. For example, FUS has been used in animal studies of temporal lobe epilepsy to deliver a neurotoxin called quinolinic acid to specific regions of excess electrical activity, to offer a non-invasive “surgical” option to resolve seizures [65-67]. There is also significant interest in using FUS to help ease the symptoms of psychiatric disorders, including depression [68,69]. In addition to delivering small molecules and proteins, there is ongoing research investigating the use of FUS-mediated BBBO to deliver stem cells to the brain, for a variety of CNS applications [70-73]. As pre-clinical research into FUS-mediated BBBO for therapeutic delivery advances, so too does advancement in the safety of such approaches, including with the use of chemical agents like vasculotide to restore BBB function more quickly after FUS [74], and via acoustic cavitation monitoring to assess microbubble behavior in real time [14].
3. Pre-Clinical secondary effects of BBB opening
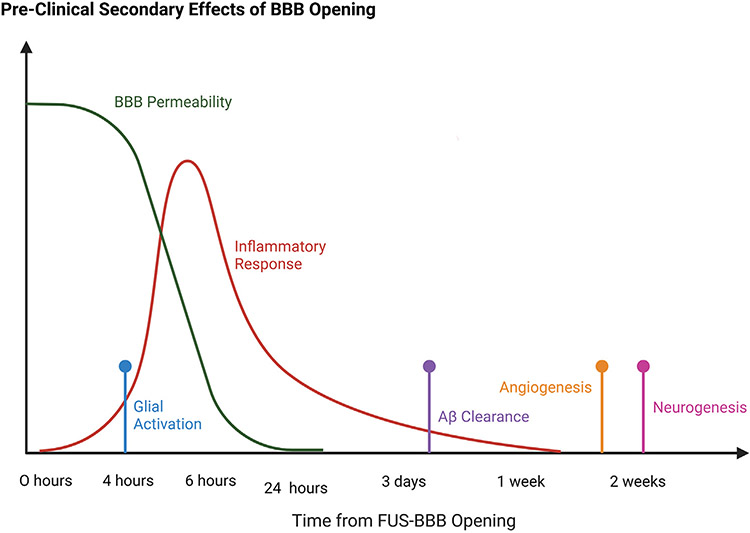
Beyond the increased delivery of therapeutic molecules facilitated by FUS-mediated BBBO, there is increasing evidence for several secondary effects of BBB disruption with FUS, including activation of inflammatory pathways, stimulation of neurogenesis and angiogenesis, increased clearance of amyloid beta plaques and tau protein tangles, and cell-specific activation. The timeline of these secondary effects is summarized in Fig. 3.
Fig. 3.
A summary of the timeline of various secondary physiological effects of FUS-mediated BBBO. Adapted from a figure in Todd et al., 2020 [91]. Made with Biorender.com.
3.1. Neurogenesis and angiogenesis
3.1.1. Neurogenesis
Interesting data has supported a role for FUS-mediated BBBO in facilitating the development of new neurons, known as neurogenesis. There has been a major focus on inducing neurogenesis in the dentate gyrus (DG) of the hippocampus since this is the region of the brain involved in learning and memory [75]. If FUS could increase neurogenesis in the hippocampus of patients with Alzheimer’s disease or other neurological diseases, this could represent a therapeutic strategy for slowing progression of the disease and improving cognition. Gene set enrichment analyses have suggested that neurogenesis, by way of extracellular matrix organization and regulation of ERK signaling cascades, is upregulated shortly after FUS BBBO [76]. In mouse models, FUS results in an increase in proliferation of neurons in the hippocampus as evidenced by increased BrdU staining in NeuN + cells [75]. Other cell types, including astrocytes, were unaffected by the treatment, suggesting that FUS may elicit neuron-specific growth responses in the hippocampus.
More recently, a study was conducted to elucidate the FUS parameters necessary to induce neurogenesis. Only when FUS was applied at 1 Hz burst repetition frequency in 10-ms bursts for 120-s at 0.78 MPa alongside MBs, did BrdU + NeuN + cells increase in the DG [77]. A pressure of 0.39 MPa with MBs, or 1.56–3 MPa without MBs, did not result in BBBO and therefore did not show increased neurogenesis in the hippocampus. Taken together, these data suggest that BBBO and thus exchange of components from the vascular environment are required for neurogenesis to occur.
Traditionally, cholinergic neurons release acetylcholinesterase (AChE) and choline acetyltransferase (ChAT) which aid in adult neurogenesis in the hippocampus. However, in Alzheimer’s disease, these cholinergic neurons can be dysfunctional [78]. While FUS BBBO has been shown to increase adult neurogenesis in Alzheimer’s models in which these cholinergic neurons are functional, the effect of FUS on neurogenesis in cholinergic-deficient models remained elusive. Cholinergic degeneration rat models were generated by administering a selective immunotoxin to induce lesions in the basal forebrain cholinergic neurons. Following FUS BBBO, AChE activity was increased significantly by 18 days post-treatment. BDNF protein expression, which is necessary for neurogenesis, also increased significantly as soon as 24 h post-treatment. Neuronal plasticity marker EGR1 was also upregulated in the cholinergic-deficient model following FUS BBBO treatment, while gliosis remained unaffected [78]. These data suggest that FUS with MBs may be an effective form of treatment for patients with dysfunctional cholinergic neurons in Alzheimer’s by promoting neurogenesis.
3.1.2. Angiogenesis
While FUS-mediated BBBO transiently increases the permeability of the cerebral microvasculature, there is evidence that BBBO can remodel brain vasculature long-term via stimulation of angiogenesis. Gene set enrichment analyses following FUS show upregulated expression of genes associated with endothelial development, regulation of vasculature development, and regulation of angiogenesis [76]. In rat models using MRgFUS, the density of new endothelial cells increased, suggesting that FUS results in increased generation of new blood vessels within the brain [79]. Additionally, BBBO limits the neurovascular response by impairing vessel dilation for increased blood flow in the region of sonication, though the underlying mechanism of how this occurs still needs to be investigated [80]. Thus, while many effects of FUS BBBO for drug delivery are transient, long-term secondary effects on the brain vasculature may also exist.
3.2. Amyloid-β
3.2.1. Plaque reduction
The pathological hallmark of Alzheimer’s disease (AD) is the presence of amyloid-β (Aβ) peptide aggregates that form amyloid plaques, which result from the inability to clear the peptide [81,82]. Recent treatment advances for AD make use of FUS BBBO [81]. In clinical AD cases, amyloid plaques are frequently found in small arteries, known as cerebral amyloid angiopathy [83]. Cerebral amyloid angiopathy results in reduced stability of the BBB because tight junctions are reduced. As a result, there exist concerns with using FUS BBBO in this pathology due to the risk of permanent vessel damage. To address these concerns, TgCRND8 mice were treated with MRgFUS and BBB dysfunction was evaluated for leakage probability using a fluorescently labeled dextran [83]. As acoustic pressure increased, as expected, the probability of BBB dysfunction increased as well. A peak negative pressure of 0.4 MPa in AD mice showed a 60 % probability of BBB dysfunction. While this study suggests that FUS does not worsen the dysfunction of amyloid-plaque containing vessels, future studies need to be conducted to establish if amyloid plaques impact the closure kinetics of the BBB[84,85].
Scanning ultrasound (SUS) with lipid-shelled MBs has proven effective in reducing Aβ plaques in APP23 Aβ-plaque forming transgenic mice [81]. However, in the absence of MBs, ultrasound alone is insufficient to clear Aβ deposits [86]. In TgCRND8 AD mice treated with FUS and commercially available MBs, Aβ plaques were reduced in size and surface area [87]. Data-driven approaches have also supported Aβ plaque clearance via gene set enrichment analyses [76].
3.2.2. Glial activation
In addition to plaque reduction, studies have shown that microglia can be activated as a result of BBBO in AD models. SUS-treated APP23 AD mice have shown increased Aβ present within the lysosomal compartment of CD68 positive microglia, suggesting that SUS is driving phagocytosis of Aβ by microglia and subsequent clearance of plaques [81]. 5xFAD mice treated with MBs and FUS for 1 h per day for 5 days show increased microglial activation that colocalized with Aβ relative to sham treated mice [88]. Iba1 staining marking microglia increased in TgCRND8 AD mice as soon as 4 h post-FUS [89]. Astrocytes, marked by GFAP, also increased starting 4 days post-treatment. Though staining increased, this was not a result of a greater number of Iba1 and GFAP-positive cells, but rather an increase in the volume and surface area of the cells themselves. Confocal imaging revealed a significant increase in the internalization of Aβ peptide within microglia and astrocytes [89].
3.2.3. Improved cognition
Reduced plaque burden has been frequently shown to improve memory and cognition in AD. In the APP23 AD mouse model, FUS BBBO treatment led to improved spatial learning as evaluated by the active place avoidance (APA) test and the novel object recognition (NOR) test [81]. Even clearance of Aβ from the brain parenchyma into the CSF led to improved cognition in AD mouse models [90].
3.3. Tau
3.3.1. Clearance
Tau, a microtubule-associated protein, can get hyperphosphory-lated at particular serine and threonine residues which can cause the normally unfolded highly soluble protein to form aggregates. While Aβ deposits are the primary pathology associated with Alzheimer’s disease, tau pathology is also frequently detected. In Alzheimer’s disease, tau forms what are known as neurofibrillary tangles (NFTs) within neurons. SUS-mediated BBBO of K3691 tau transgenic mice has shown promise in reducing the phosphorylation of tau proteins as well as NFTs [91]. Tau is traditionally cleared by the proteasome, though in its aggregated form, some studies have suggested that clearance is mediated by the autophagosome [91,92]. Increased LC3 and decreased mTOR activity supported the role of autophagy in the clearance of tau aggregates in the neurons of SUS-treated tau transgenic mice.
Increased phosphorylation of tau is correlated with impaired neuronal activity and memory. In rTg4510 mice, which depict early stages of tau pathology, FUS with MBswas able to reduce the levels of phosphorylated tau in the hippocampus relative to non-treated controls [93]. FUS BBBO had no effect on the density of NeuN + neuronal cells nor the density of neuronal processes, suggesting that the FUS regimen does not result in neuronal death or hyperproliferation. CD68 + activated immune cells were greatly increased following treatment as was colocalization of microglia with tau deposits.
3.3.2. Improved cognition
Following SUS-mediated BBBO of K3691 tau transgenic mice, reduction in NFTs within neurons also improved motor function. This was assessed with the Rotarod performance test, in which mice are placed on a rotating cylinder for as long as they can balance. Mice were significantly less likely to fall from the Rotarod and had better grip strength in both their fore and hindlegs [91]. Additionally, the K3691 mice treated with SUS showed improved spatial memory as evaluated by the Y-maze and general memory function according to the NOR test.
4. Pre-Clinical neuroscience tools
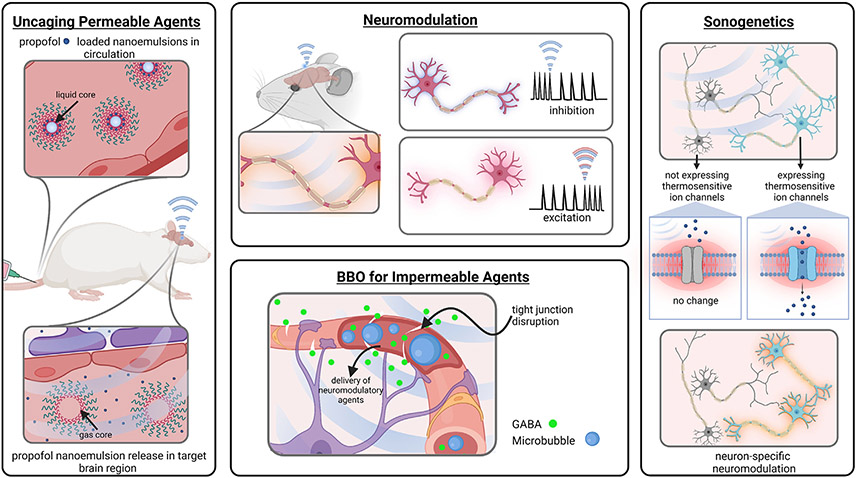
The tunability of FUS parameters and the ability to induce different bioeffects in conjunction with different co-administered agents has resulted in the development of a number of tools for neuroscience research at the pre-clinical level. These tools, including neuromodulation and sonogenetics, are summarized in Fig. 4.
Fig. 4.
A summary of the neuroscience tools being studied at the pre-clinical level which utilize FUS for various neurological applications. Created with Biorender.com.
4.1. Neuromodulation
Neuromodulation is the alteration or regulation of nerve activity via electrical or pharmacological agents. Many brain diseases affect neurotransmitter communication, thus neuromodulation via deep brain stimulation (DBS) and drug therapy are commonly employed. However, DBS is an invasive procedure and is limited by critical brain structures, and drug therapy can result in cytotoxicity from off-target effects [94]. FUS is a non-invasive alternative to neuromodulation. Herein, we discuss the use of neuromodulation using FUS for (1) BBBO to allow delivery of non-permeable pharmaceutics and (2) spatiotemporal uncaging of brain permeable agents.
4.1.1. BBBO for impermeable agents
As described previously in Section 2, extensive pre-clinical studies have examined FUS-mediated BBBO to facilitate drug and gene delivery in a variety of disease contexts. This approach to non-invasive delivery can also be used to allow for delivery of otherwise impermeable agents that permit neuromodulation of specific brain regions. In one such study, FUS was targeted to the right hindlimb somatosensory cortex in conjunction with intravenous gas-filled microbubbles. After FUS BBBO, animals received a bolus of the inhibitory neurotransmitter GABA. This elicited a reduced response to electrical stimulation of the right hind paw (but not the left), with a greater degree of inhibition than was observed with FUS BBBO alone or GABA alone [95]. A similar study was conducted to deliver GABA across the BBB in non-human primates, but targeting the visual cortex. The combination of FUS-mediated BBBO and GABA injection resulted in a significant inhibition in neuronal response to a visual stimulus [96]. FUS-mediated BBBO has also been used to permit the delivery of intravenously-administered glutamate-loaded nanoclusters to a specific structure in the brain. These nanoclusters can then be later activated by FUS to release their glutamate payload, stimulating regional neuronal activation [97].
4.1.2. Uncaging for permeable agents
Another strategy is to use chemical “caging” of BBB-permeable agents to conceal their effects systemically (i.e. periphery and whole brain) and FUS-induced “uncaging” to reveal their effect only to the targeted brain region. One group has demonstrated this through the caging of the readily BBB-permeable anesthetic agent propofol. Through nanoemulsion formulation and loading of propofol, the molecule is only released upon FUS sonication which transforms the nanoemulsion’s liquid core to its gaseous state, effectively ejecting the anesthetic agent. This technique has been demonstrated useful for the silencing of seizures in a rat model [98] as well as non-invasive mapping of brain network connectivity [99]. This strategy can prove useful for a variety of applications where it is desirable for only select brain areas to receive BBB-permeable agents.
4.1.3. FUS BBBO alone
It has been noted that FUS-induced BBBO alone is sufficient to elicit neuromodulation and the intensity and duration of its effects correlate with the magnitude of BBB disruption [100]. Changes induced by FUS neuromodulation were analyzed by measuring somatosensory evoked potentials (SSEPs) and blood-oxygen-level-dependent (BOLD) responses, which are commonly measured neuromodulation parameters [96,100]. Importantly, effects from repetitive BBBO treatments did not compound, suggesting the safety of repetitive treatments [100]. Although significant, one study found that BBBO-induced neuromodulation was found to be ~ 8.7 times less than effects from GABA inhibitor delivery [96]. Still, neuromodulatory effects resulting from BBBO should be a consideration in all treatment designs. Importantly, clinical trials are currently underway to test repetitive BBBO in humans indicating that BBBO-neuromodulation may be an option for near-future therapies [101].
4.2. Sonogenetics
Through the use of gas-filled MBs or vesicles, the mechanical bioeffects of FUS can be amplified for targeted, non-invasive modulation of neurons deep within the brain. An emerging and elegant technique for neuromodulation is sonogenetics, an approach deriving from opto- and chemogenetics, that targets specific neuronal populations through the use of sound waves activating genetically overexpressed mechanically or thermally sensitive ion channels. The first application of sonogenetic neuromodulation identified TRP-4 as a low-pressure ultrasound-sensitive subunit of a mechanosensitive ion channel. In a transgenic Caenorhabditis elegans model, FUS and MB activation of TRP-4 induced reversible behavioral outputs [102]. Other mechanosensitive channels have been identified in in vitro studies [17] and recent preliminary studies have highlighted the promise of sonogenetics in in vivo neuromodulation applications. One such study identified mPrestin, a transmembrane mechanosensitive protein, as a target to induce ultrasound sensitivity in an in vivo transgenic mouse model [103]. FUS stimulation (without MBs) of ventral tegmental area neurons overexpressing modified ultrasound-sensitive mPrestin induced a significant increase in c-Fos expression and calcium influx. Most preliminary studies leverage mechanical FUS; however, with recent advances in MR thermometry and image-guided FUS, additional sonogenetic platforms can be explored. One study developed sonothermogenetics using the thermosensitive ion channel TRPV1 to induce non-invasive FUS heating for neuron-specific neuromodulation in vivo [104]. Virally transfected TRPVI neurons in the somatosensory cortex of wild-type mice were activated with FUS stimulation in the absence of MBs, while TRPVI-negative neurons were not– emphasizing the utility of sonothermogenetics to target specific deep brain neurons. Additionally, FUS stimulation induced locomotor behaviors consistent with previous studies using other established methods (i.e., optogenetics and chemogenetics) to activate similar brain regions. Going forward, future studies will have to be conducted to optimize and identify ultrasound-sensitive proteins and investigate their utility in vivo.
4.3. Acoustic control of biomolecular therapeutics
As the therapeutic ultrasound domain becomes increasingly interdisciplinary, approaches resting at the nexus of synthetic biology and FUS are showing increasing promise for the utility of FUS as a remote, localizable acoustic switch for activation of engineered materials and genetic circuits [105].
4.3.1. Liposomes
Liposomes have been explored as drug delivery vehicles across multiple facets of nanomedicine. However, they can be limited in their capacity to release payloads in a controlled manner [106,107]. Recent work has demonstrated that low intensity FUS (LIFU) can achieve protein-membrane disruption and pore formation, leading to efflux of payloads. Specifically, acoustic intensity and duration of LIFU stimulation appear to impact protein-membrane channel disruption [107]. One study demonstrated the therapeutic potential of liposome stimulation via FUS mechanical disruption of the lipid bilayer. The liposomes generated had either NAK2K F92A potassium channel, KvAP potassium channel, mechanosensitive channel of large conductance (MscL), or no channel present. Encapsulated in the liposome was a self-quenching calcein dye, which limits fluorescent intensity when enclosed in the liposome. Through an in-vitro proteoliposome model, it was suggested that MscL, when stimulated with LIFU, can cause an increase in calcein efflux. This study also found that LIFU stimulation can even cause efflux of calcein with voltagegated channels (i.e. NaK2K F92A and KvAP) - suggesting that the mechanism underpinning this efflux is lipid bilayer perturbations rather than channel disruption [107].
4.3.2. Gene vectors
Many neurological and psychiatric pathologies are underscored by the dysfunction of neuronal networks. Acoustically targeted chemogenetics (ATAC) is an emerging neuromodulation technique that seeks to enable non-invasive spatial, cell-specific, and temporal control of neural networks via combination of FUS BBBO, adeno-associated virus (AAV) vector delivery, and engineered chemogenetic receptors [108-110]. In a transgenic Cre murine model, this strategy was effective in targeting and inhibiting neurons in the hippocampus. AAVs encoding chemogenetic designer receptor activated exclusively by designer drugs (DREADDs) were delivered across the BBB with FUS and MBs, resulting in the selective inhibition or excitement of neurons following introduction of clozapine-N-oxide (CNO). DREADDS are a commonly used mechanism that confer targeted neuronal control and are discriminatingly responsive to CNO. The behavioral impact of this approach was demonstrated via a fear conditioning protocol, wherein CNO-recipient mice saw a reduction in fear memory formation [108-111].
4.3.3. Engineered T cells
Genetically engineered T cells, such as chimeric antigen receptor (CAR)-expressing T cells, hold tremendous promise for cancer immunotherapy. However, off-tumor, on-target toxicities remain a critical challenge for these therapies [105,112-114]. It has been demonstrated that the remote acoustic control of engineered T cells with FUS may serve to curb these off-tumor toxicities by enabling localized control of thermally responsive T cells [112-115]. One recent in vitro study highlighting the development of genetically engineered thermally activatable T cells, through the incorporation of heat shock promoter (pHSP), showed that FUS-induced hyperthermia (up to 42°C) enabled regulation of key T cell functions, such as targeting of cancer cells through CAR recognition, as well as the production and efflux of cytokines [112]. A separate study extended this work to the in vivo setting, showcasing the ability of these acoustically responsive CAR-T cells to target solid tumors (Nalm6 leukemia and PC3 prostate cancer bilateral tumor models) with reduced non-specific off-tumor toxicity. Results demonstrated that FUS hyperthermia (43°C) enabled activation of CAR-T cells locally within the targeted tumor [113]. Akin to the aforementioned in vitro study, another also investigated exploiting Piezo1 ion channel through FUS as a mechanism for selective and modular activation of the CAR in engineered T cells. Specifically, this study utilized the Piezo1 ion channel as a mechanical sensor through which sonification could lead to transcriptional activation of anti-CD19 CAR expression, leading to recognition and eradication of tumor cells [114]. Through the advancement of acoustogenetic technology, CAR-T cells can be remotely controlled to exert their effects within confined target regions of solid tumors. This strategy will vastly aid in the reduction of broad off-target toxicities and further the advancement of this promising branch of immunotherapy. CAR-T cell delivery across the BBB with FUS is in its nascency, but we expect that future studies will aim to deliver acoustogenetically controllable CAR-T cells to brain tumors with FUS.
5. Clinical trials of FUS-Mediated BBB disruption
With rapid advances seen in the pre-clinical setting, more recent focus has been placed on translating FUS-mediated BBBO into the clinic. Given the brain’s vital role in cognition, motor control and maintaining homeostasis, even a slight disturbance to its function can have devastating consequences on an individual’s physical and mental wellbeing. Globally, between 1990 and 2019, neurological disorders have been associated with an increase of over 70 percent in disability-adjusted life years (DALYs) and more than double the proportion of deaths [25]. Moreover, by 2030, it is projected that neurological disorders will contribute to 103 million DALYs [116]. Therefore, non-invasive tools that can be precisely targeted to open the BBB, such as transcranial FUS, will accelerate new systemic treatment options for a range of neurological disorders.
5.1. Glioma and brain metastases
To date, the majority of clinical studies of FUS-mediated BBBO have been targeted to the treatment of malignant brain tumors, particularly glioblastoma (GBM). This group of diseases is ideal for study due to the availability of multiple active chemotherapeutic agents. It is known that malignant brain tumors alter the BBB as they infiltrate the brain, forming a blood-tumor barrier (BTB). Despite the loss of the protective BBB, the BTB has been shown to be heterogeneously permeable and still restricts drug ingress, leading to poor therapeutic efficacy [117,118].
One of the earliest published studies investigating FUS-mediated BTB disruption in recurrent GBM was performed using an implantable ultrasound device system, SonoCloud-1 (Carthera, France). In this dose escalating phase I/2a study, Carpentier et al. showed that repeated sonications of MBs at up to 1.1 MPa acoustic pressure could be applied safely in conjunction with a systemically administered cytotoxic chemotherapy without long-lasting adverse effects in any patient [119]. In their follow-up publication, Ibdaih et al. reported that dose-limiting toxicity was not reached with escalating sonication pressures and that a trend towards longer progression-free survival and overall survival was observed in sonicated patients who had clear BTB opening compared to those without BTB opening [120].
Mainprize et al. performed a window of opportunity study in five patients with confirmed or suspected high grade glioma. These patients received MBs and transcranial FUS delivered by a helmet-shaped transducer array, ExAblate Neuro (InSightec Tirat Carmel, Israel), coupled with real-time MR imaging guidance. Cytotoxic chemotherapy was systemically delivered concomitantly with MRgFUS-induced BTB opening, before tumor resection. Liquid chromatography-mass spectrometry analysis of peritumor tissue samples showed a trend towards elevated concentration in the “sonicated” versus “unsonicated” tissue [121]. However, the small number of quantifiable samples and the use of a chemotherapy (Temozolomide) that is known to penetrate the BBB precluded any solid conclusions from being drawn on the extent to which FUS-mediated BTB disruption impacts on drug delivery to a brain tumor.
More recently, a study in patients with Her2-positive breast cancer brain metastases has provided more definitive evidence of the therapeutic potential of FUS-mediated BTB opening with MBs. In a pilot study, Meng et al. selectively targeted intracranial lesions with MRgFUS before systemic administration of an anti-Her2 monoclonal antibody, Trastuzumab [122]. Using radiolabeled Trastuzumab and single-photon emission computed tomography imaging, the study group showed that drug penetrance into the tumor was enhanced following MRgFUS activation of MBs, particularly at a delayed (48 h) timepoint. Moreover, volumetric assessment of the treated tumors demonstrated that they were stable or reduced on size on MRI follow up compared to baseline measurements [122].
Considerable efforts remain in establishing the parameters for safe FUS-mediated BTB disruption. Anastasiadis et al. showed, in a phase 0 clinical study, that the acoustic energy required for sonication can greatly vary between patients, highlighting the importance of individual patient monitoring and dose titration. Furthermore, they observed that radiological and histological changes associated with BTB opening were positively correlated with the MB acoustic emissions, or harmonic dose, opening the possibility of tailoring the treatment regimen according to the desired effect [123]. Since chemotherapy treatment of brain tumors typically depends on multi-dose regimens, it is vital that repeated FUS and MB application does not cause any major toxicities. To that end, Park et al. demonstrated that, in patients with resected GBM on adjuvant chemotherapy, repeated and multiple treatments with MRgFUS and MBs on a four week cycle led to transient BTB opening that was well-tolerated [124]. In their follow up study, Park et al. found that none of the enrolled patients suffered from lasting sequelae arising from BTB opening [101].
Although much of the focus on FUS-mediated BTB opening has been on drug delivery, other novel applications have also been tested. One exciting development has been the use of FUS-induced opening of the BTB to promote release of brain-derived biomarkers from GBM, providing proof-of-concept in its potential utility as an aid for liquid biopsy in neuro-oncology [125]. Another potential application in the field of brain tumors is to stimulate an anti-tumor immune response, which is particularly relevant with the growing interest in cancer immunotherapy. Chen et al. investigated whether a neuronavigation-guided single element transducer, NaviFUS (NaviFUS Inc), for FUS delivery with MBs to open the BTB in recurrent GBM patients might lead to an enhanced immunological response. Although it was found that transient BTB opening was induced in a dose-dependent manner, no histological changes were seen pointing to Increased immune infiltration into the GBM or surrounding brain parenchyma [21]. Subsequent data in a rat model confirmed that Increased tumor lymphocyte infiltration could be achieved with a mechanical index of 0.81, raising the possibility that this effect could be replicated in humans, albeit with higher acoustic energy deposition levels [21].
5.2. Alzheimer’s disease
As previously discussed in Section 2, much excitement has been generated from pre-clinical models demonstrating the potential for MRgFUS-mediated BBBO for beta-amyloid clearance. In the first published study investigating this clinical effect in humans, Lipsman et al. conducted a phase I study in five patients with early to moderate AD. No significant reduction in Aβ was detected by [18F]-Florbetaben positron emission tomography (FBB PET) following BBBO [126]. Nonetheless, this pilot study yielded important information regarding the safety of transient BBBO in amyloid-positive AD subjects with no associated serious adverse events or deleterious effects on cognitive function. Similarly, Rezai et al. showed that focal BBBO by MRgFUS targeted to the hippocampus and entorhinal cortex in patients with AD was safe [18]. In a follow up study, the same group showed that there was an average reduction in Aβ plaque of roughly 5 percent following FUS-mediated BBBO [87].
To assess the impact of focal BBBO on fluid flow in early AD patients, Mehta et al. conducted a prospective, phase II clinical study exploring multiple MR imaging parameters after FUS BBBO. Besides parenchymal enhancement seen at the site of FUS targeting post-gadolinium contrast, indicating selective BBBO, the authors also observed enhancement along the draining venous structures immediately after sonication and 24 h later, after radiological signs of BBBO had resolved. This observation has been interpreted as blood-meningeal barrier permeabilization as a side effect of BBBO, which may have an additional role in immune cell trafficking [127].
Since the neuropathological changes associated with AD can be extensive, Park et al. investigated whether large volume (>20 cm3) FUS treatment could be delivered repeatedly in the brain. This proof-of-concept study demonstrated that extensive BBBO in the frontal lobe of patients, with AD and significant brain Aβ deposits, was well-tolerated. Secondarily, it was found on FBB PET that there was a significant reduction in Aβ deposits at targeted regions post-sonication; however, there was no overall improvement in neurocognitive assessments within the timeframe of the study [82].
5.3. Parkinson’s disease and amyotrophic lateral sclerosis
Though the bulk of published clinical studies has been targeted at brain tumors and AD, work is underway to establish FUS-mediated BBBO as a therapy across a much wider range of neurological disorders. In Parkinson’s disease (PD), FUS and MBs have already been shown to be effective at managing the motor manifestations of PD through targeted thermal ablation of deep brain structures, such as the subthalamus [128]. In the context of BBBO, FUS has been tested in patients with evidence of PD-associated dementia. Gasca-Salas et al. showed that repeated transient BBBO in the right parieto-occipito-temporal cortex was safe, though there was no significant change in radiological signs of Aβ volume or metabolic function in the posterior cortex [129]. In another example, Abrahao et al. investigated the safety of FUS-induced BBBO in patients with amyotrophic lateral sclerosis. Uniquely, this study targeted sonication to the primary motor cortex using functional MRI mapping and demonstrated that BBBO could be safely applied with minimal side effects [130].
5.4. Conclusions
In conclusion, the recent stream of published clinical studies is a positive sign that FUS-mediated BBB disruption is becoming a viable treatment tool for patients with neurological disorders. Given the possibility of delivering drugs previously excluded by the BBB, FUS has the potential to dramatically alter the treatment options for these challenging diseases. Though meaningful conclusions regarding therapeutic efficacy cannot be answered due to the small group sizes in most published studies, they all point to FUS-mediated BBBO as being safe, with the capability to treat eloquent regions of the cortex, to deliver treatment over repeated sessions and over a large brain volume. Therefore, these trials provide strong justification for further study in larger cohorts of patients. Table 2 outlines all currently registered clinical trials that have been completed or are in progress.
Table 2.
Summary of all currently-registered clinical trials of FUS-mediated BBB opening in human patients, separated by disease condition.
| Disease / condition | Study ID | No. of patients | Outcome measures | FUS device | Complete? |
|---|---|---|---|---|---|
| Alzheimer’s disease | NCT04118764 | 6 | Efficacy of BBBO No. of safety events Change in amyloid uptake Change in cognitive function |
Neuronavigation-guided system | No |
| NCT04526262 | 6 | Adverse events Efficacy of BBBO |
ExAblate 4000 Type 2 | No | |
| NCT02986932 | 6 | Efficacy of BBBO Change in amyloid uptake Adverse events Change in cognitive function |
ExAblate 4000 Type 2 | Yes | |
| NCT03739905 | 30 | Adverse events Efficacy of BBBO Change in cognitive function Change in amyloid uptake |
ExAblate 4000 Type 2 | No | |
| NCT04526262 | 6 | Adverse events Efficacy of BBBO |
ExAblate 4000 Type 2 | Yes | |
| Parkinson’s disease | NCT03608553 | 10 | Adverse events Feasibility of BBBO |
ExAblate 4000 Type 2 | No |
| NCT04370665 | 4 | Efficacy of BBBO Adverse events Efficacy of Cerezyme delivery |
ExAblate 4000 Type 2 | No | |
| Brain tumors | NCT02343991 | 10 | Adverse events | ExAblate 4000 Type 2 | Yes |
| NCT03551249 | 20 | Adverse events Feasibility of repeated BBBO |
ExAblate 4000 Type 2 | No | |
| NCT04063514 | 15 | Adverse events Functional magnetic resonance imaging |
DWL or Brainsonix | No | |
| NCT04804709 | 3 | Adverse events Survival Efficacy of BBBO |
Neuronavigation-guided system | No | |
| NCT03616860 | 20 | Adverse events Feasibility of repeated BBBO Efficacy of BBBO |
ExAblate 4000 Type 2 | No | |
| NCT04998864 | 5 | Adverse events Feasibility of repeated BBBO |
ExAblate 4000 Type 2 | No | |
| NCT03714243 | 10 | Adverse events Efficacy of BBBO |
ExAblate 4000 Type 2 | No | |
| NCT04440358 | 50 | Adverse events Efficacy of BBBO |
ExAblate 4000 Type 2 | No | |
| NCT04417088 | 30 | Adverse events Efficacy of BBBO |
ExAblate 4000 Type 2 | No | |
| NCT03712293 | 10 | Adverse events Efficacy of BBBO |
ExAblate 4000 Type 2 | No | |
| NCT03626896 | 6 | Adverse events Dose limiting toxicity Efficacy of BBBO |
NaviFUS | Yes | |
| NCT04446416 | 10 | Adverse events Survival Clinical response Efficacy of BBBO |
NaviFUS | No | |
| NCT03744026 | 33 | Dose limiting toxicity Efficacy of BBBO |
SonoCloud-9 | Yes | |
| Amyotrophic lateral sclerosis | NCT03321487 | 8 | Adverse events Efficacy of BBBO |
ExAblate 4000 Type 2 | Yes |
A number of unresolved issues remain on the path to translating FUS-mediated BBBO into new clinical treatment paradigms. Since many neurological diseases follow a chronic or indolent course, consideration will need to be made regarding frequency of sonications, the total number that can be safely administered, and the long-term effects/risk of repeated BBB disruption. Continued close collaboration between the bench and bedside will be necessary to select rational drug candidates for combining with FUS-mediated BBBO. Several competing FUS systems have been described and it will be interesting to see whether a dominant system will emerge or if unique niches will develop dependent on clinical indication. Finally, it is evident that FUS is a versatile tool that can be safely titrated to achieve a range of effects on the BBB. Therefore, careful consideration of the desired outcomes according to the clinical indication and personalization of treatment protocols to the patient will be necessary in future studies.
6. Future approaches and indications of focused ultrasound in the CNS
Thus far, this review has covered previous studies that emphasize the use of FUS for modulation of BBB permeability. Most preclinical and clinical applications have centered on deploying this approach for neuromodulation and/or image-guided drug and gene delivery to treat many common CNS pathologies. In this final section, we take a more forward-looking stance, offering perspectives on how, in the future, focused ultrasound might be rationally tuned to (1) further augment therapeutic efficacy via modulation of interstitial fluid transport, (2) achieve transfection of the BBB itself as a tool for neuroscience discovery and therapy, and (3) treat pathologies of the CNS wherein FUS-mediated BBBO has not yet been considered an option, but could still be quite promising.
6.1. Modulation of interstitial and glymphatic fluid flow
The ability of pulsed ultrasonic energy to augment interstitial tumor pressure, leading to the enhanced delivery of therapeutics from the bloodstream via enhanced convective transport, is known and fairly well-studied. Recently, an excellent review of the literature in this space was provided by Keller and Averkiou [131], and we refer the interested reader to that article for more detailed information. Here, we submit that this literature, as well as other key sources, suggest that FUS modulation of interstitial fluid flow could be deployed for therapeutic purposes in the CNS, both with and without concomitant BBBO. Indeed, consistent with generation of augmented interstitial fluid flow and studies performed in extracranial tumors, opening the BBB with FUS and MBs has been shown to enhance the dispersion of therapeutic agents through tissue [132-134]. In further support, a recent study used an MRI-based approach to demonstrate that BTB opening with FUS and MBs causes an approximate doubling of interstitial flow and marked alterations in fluid flow directions, which led to the enhanced dispersion of nanoparticles throughout the tumor [56]. These concepts have also been extended to consideration of changes in glymphatic transport due to FUS-mediated BBBO. Indeed, recent observations from clinical trials wherein the BBB was opened with FUS and MBs in Alzheimer’s disease and ALS patients provide compelling evidence that, at least in some patients, enhanced glymphatic transport of contrast agent from the brain to subarachnoid spaces occurs [135].
In all, these studies raise the intriguing possibility of developing FUS sequences that, in conjunction with BBBO for enhanced therapeutic delivery, may be able to augment and/or guide the transport of delivered payloads through other mechanisms. For example, “pre-conditioning” rat brain with pulsed FUS in the absence of MBs before seeding of subsequent BBBO with i.v.-injected MBs has been shown to augment brain tissue transfection magnitude with non-viral nanoparticles [136]. Although the mechanism of this effect is unknown, it appears to be primarily mechanical in nature and is likely related to the aforementioned effect of pulsed FUS on interstitial tissue structure and/or hemodynamics. Moreover, broadly along these same lines, important new research has shown that the penetration of intrathecally-injected agents into the brain can be augmented by scanning [137] or pulsed [138] ultrasound (without MBs or BBBO). The exciting potential to modulate glymphatic flow with FUS opens the door to the development of immunotherapeutic approaches that control the exposure of immune cells in the meninges and draining lymphatics to antigen and/or delivered immune-stimulatory molecules. Indeed, there is emerging evidence that immune cells in the meninges do respond, albeit modestly, to gentle BTB opening with FUS [139], indicating that such communication exists. Going forward, we are hopeful that acquiring a more mechanistic understanding of fluid interactions between the FUS-treated brain, meninges, and lymphatics will lead to exciting new methods to control such responses.
6.2. Selective transfection of Blood-Brain barrier endothelium with focused ultrasound
Another exciting new direction for FUS, in the context of both neuroscience research and therapeutic development for CNS pathologies, is the targeted genetic modulation of the endothelial layer of the BBB [76,140]. In essence, it has been determined that, when relatively low peak-negative pressure FUS is applied to plasmid-bearing cationic MBs, gene transfer to the endothelium in the BBB may be achieved without opening the BBB and without the need for an endothelial cell-specific promoter (Fig. 5). In this so-called “sonoselective” low peak-negative pressure FUS regime, consistent acoustic harmonic emissions signatures are generated and may be leveraged for control of transfection in the future. Because the integrity of the BBB is retained during transfection, this approach could be especially useful for conditions wherein even the modest elicitation of sterile inflammation could be too detrimental. Further, given the flexibility inherent in this system, manipulations centered on genome editing the BBB, as well as those facilitating controlled BBBO through chemogenetics (or similar approaches), may be operative. Indications potentially benefiting from (1) the recruitment of specific immune and/or progenitor cells from the circulation to FUS-targeted pathologies, (2) the expansion of the microvasculature via stimulation of angiogenesis, and/or (3) the long-term, spatially-controlled, modulation of BBB-function may all be germane to this sonoselective transfection strategy.
Fig. 5.
FUS peak-negative acoustic pressure (PNP) may be tuned to yield sonoselective cerebrovascular endothelial transfection. (A and B) Confocal images of FUS+ (0.1 MPa) and contralateral FUS− brain tissue showing expression of mCherry reporter gene (red) with respect to endothelial cells (BS-I lectin, green). Arrows denote mCherry colocalization with endothelium. (C) Bar graphs of fraction of mCherry expression in cerebrovascular endothelium as a function of PNP. Highly selective endothelial transfection is observed at low PNPs (i.e., 0.1 MPa and 0.2 MPa). One-way ANOVAs followed by Dunnett’s multiple comparisons tests. [140].
6.3. Potential future clinical applications
Understandably, most pre-clinical and clinical research to date in the field of FUS-mediated BBBO has revolved around a handful of pathological indications that are pervasive, generate high mortality and morbidity, and are generally agreed upon to have a sufficient safety profile for FUS-mediated BBBO. In particular, as previously highlighted in this review, investigations on brain tumors (primary and metastatic) and neurodegenerative diseases (Parkinson’s disease, Alzheimer’s disease, and ALS) have dominated the field to date. Nonetheless, given the great success and safety enjoyed by the field, as well as our improved understanding of the side effects of FUS-mediated BBBO, it may be time to consider the potential use of FUS-mediated BBBO for conditions that are either more challenging from a safety perspective and/or less common. We close this review by highlighting two such applications, ischemic stroke and cerebral cavernous malformations (CCMs), that we submit could benefit markedly from the development of FUS-based therapies, despite limited (ischemic stroke) or no (CCMs) progress to date with regard to the development of therapies deploying FUS-mediated BBB modulation.
Broadly speaking, FUS treatment options for ischemic stroke have been under investigation for some time; however, most approaches have centered on the use of FUS for clot dissolution (i.e. “sonothrombolysis”) [141]. Relatively fewer studies have tested the use of FUS-mediated BBBO for therapeutic delivery. This is certainly understandable given the delicate state of ischemic neural tissue, as well as the likelihood of limited microbubble access due to poor perfusion. Nonetheless, a couple of preclinical studies offer promise for the use of FUS in treating stroke beyond sonothrombolysis. In one such investigation, the neuroprotective agent erythropoietin was delivered to the infarct zone of rats exposed to middle cerebral artery occlusion, eliciting reduced infarct volume and neuronal loss, as well as improved long-term behavior [142]. And, more recently, successful subcortical stroke treatment, as assessed by improved functional recovery and fiber tract connectivity, was achieved by delivering brain-derived neurotrophic factor (BDNF) to the brain with FUS and MBs [143]. Notably, despite the already inflamed tissue microenvironment, neither study reported significant adverse effects.
In a similar vein, we posit that CCMs could also represent an attractive application for drug and gene delivery via FUS-mediated BBB modulation. CCMs are hemorrhagic vascular lesions in the central nervous system, affecting ~ 0.5 % of the population [144-146]. CCM patients can experience weakness, numbness, severe headaches, vision changes, difficulty speaking and understanding, stroke, seizures, and paralysis [147], and in some patients, neurological issues progressively worsen due to recurrent hemorrhages [148]. The primary treatment for symptomatic CCM is surgical removal, which presents high risk. Notably, given that CCMs are characterized by endothelial cell proliferation and neuroin-flammation, many existing drug and gene therapies (i.e. anti-angiogenesis and anti-inflammatory agents) could be successfully deployed to control CCMs and their sequelae. Because CCMs present as discrete lesions on MRI, it is reasonable to hypothesize that they may be successfully targeted with FUS under MR image guidance. Furthermore, vascular access to the CCM microenvironment exists via surrounding microvasculature, the permeability of which could be enhanced via FUS and MBs to facilitate drug and gene delivery. Of course, given the altered endothelial cell phenotypes that are present in organisms harboring CCMs, BBB-opening FUS thresholds may be different than in brains without lesions. Additionally, excessive iron deposition within the lesions could result in altered acoustic properties and potentially increased heating upon application of FUS. For these reasons, careful safety studies would need to be performed, including MR thermometry. However, given the field’s increasing refinement of acoustic emissions-based feedback control for BBBO with FUS, we are hopeful it may eventually be possible to safely control BBB opening in this challenging microenvironment to provide new treatment options for patients suffering from CCMs.
Acknowledgments
Supported by NIH R01EB030409, R01EB030744, R01NS111102, and R21NS118278 to R.J.P. and NIH DP5OD031846 to N.D.S.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Daneman R, Prat A, The Blood-Brain Barrier, Cold Spring Harb. Perspect. Biol 7 (1) (2015) a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fu BM, Transport Across the Blood-Brain Barrier, in: 2018: pp. 235–259. 10.1007/978-3-319-96445-4_13. [DOI] [PubMed] [Google Scholar]
- [3].Wolburg H, Lippoldt A, Tight junctions of the blood–brain barrier, Vascul. Pharmacol 38 (2002) 323–337, 10.1016/S1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- [4].Segarra M, Aburto MR, Acker-Palmer A, Blood-Brain Barrier Dynamics to Maintain Brain Homeostasis, Trends Neurosci. 44 (2021) 393–405, 10.1016/j.tins.2020.12.002. [DOI] [PubMed] [Google Scholar]
- [5].Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ, Structure and function of the blood–brain barrier, Neurobiol. Dis 37 (2010) 13–25, 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- [6].Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C, Pericytes regulate the blood–brain barrier, Nature. 468 (2010) 557–561, 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- [7].Pardridge WM, The blood-brain barrier: Bottleneck in brain drug development, NeuroRX. 2 (2005) 3–14, 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Löscher W, Potschka H, Blood-brain barrier active efflux transporters: ATP-binding cassette gene family, NeuroRX. 2 (2005) 86–98, 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bosma EK, van Noorden CJF, Schlingemann RO, Klaassen I, The role of plasmalemma vesicle-associated protein in pathological breakdown of blood–brain and blood–retinal barriers: potential novel therapeutic target for cerebral edema and diabetic macular edema, Fluids Barriers CNS. 15 (2018) 24, 10.1186/s12987-018-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K, Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles, Ultrasound Med. Biol 30 (2004) 979–989, 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- [11].Timbie KF, Mead BP, Price RJ, Drug and gene delivery across the blood–brain barrier with focused ultrasound, J. Control. Release 219 (2015) 61–75, 10.1016/j.jconrel.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Song K-H, Harvey BK, Borden MA, State-of-the-art of microbubble-assisted blood-brain barrier disruption, Theranostics. 8 (2018) 4393–4408, 10.7150/thno.26869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA, Noninvasive MR Imaging–guided Focal Opening of the Blood-Brain Barrier in Rabbits, Radiology. 220 (2001) 640–646, 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- [14].Çavuşoğlu M, Zhang J, Ielacqua GD, Pellegrini G, Signorell RD, Papachristodoulou A, Brambilla D, Roth P, Weller M, Rudin M, Martin E, Leroux JC, Werner B, Closed-loop cavitation control for focused ultrasound-mediated blood–brain barrier opening by long-circulating microbubbles, Phys. Med. Biol, 64 (2019), 10.1088/1361-6560/aafaa5. [DOI] [PubMed] [Google Scholar]
- [15].McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS, Temporary Disruption of the Blood–Brain Barrier by Use of Ultrasound and Microbubbles: Safety and Efficacy Evaluation in Rhesus Macaques, Cancer Res. 72 (2012) 3652–3663. 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gray M, Vasilyeva AV, Brans V, Stride E, Studying Cavitation Enhanced Therapy, J. Vis. Exp (170) (2021), 10.3791/61989. [DOI] [PubMed] [Google Scholar]
- [17].Rabut C, Yoo S, Hurt RC, Jin Z, Li H, Guo H, Ling B, Shapiro MG, Ultrasound Technologies for Imaging and Modulating Neural Activity, Neuron. 108 (2020) 93–110, 10.1016/j.neuron.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rezai AR, Ranjan M, D’Haese P-F, Haut MW, Carpenter J, Najib U, Mehta RI, Chazen JL, Zibly Z, Yates JR, Hodder SL, Kaplitt M, Noninvasive hippocampal blood–brain barrier opening in Alzheimer’s disease with focused ultrasound, Proc. Natl. Acad. Sci 117 (2020) 9180–9182, 10.1073/pnas.2002571117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wu S-Y, Sanchez CS, Samiotaki G, Buch A, Ferrera VP, Konofagou EE, Characterizing Focused-Ultrasound Mediated Drug Delivery to the Heterogeneous Primate Brain In Vivo with Acoustic Monitoring, Sci. Rep 6 (2016) 37094, 10.1038/srep37094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pouliopoulos AN, Kwon N, Jensen G, Meaney A, Niimi Y, Burgess MT, Ji R, McLuckie AJ, Munoz FA, Kamimura HAS, Teich AF, Ferrera VP, Konofagou EE, Safety evaluation of a clinical focused ultrasound system for neuronavigation guided blood-brain barrier opening in non-human primates, Sci. Rep 11 (2021) 15043, 10.1038/s41598-021-94188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen KT, Chai WY, Lin YJ, Lin CJ, Chen PY, Tsai HC, Huang CY, Kuo JS, Liu HL, Wei KC, Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors, Sci. Adv 7 (2021), 10.1126/SCIADV.ABD0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Konofagou EE, Tunga Y-S, Choia J, Deffieuxa T, Baseria B, Vlachosa F, Ultrasound-Induced Blood-Brain Barrier Opening, Curr. Pharm. Biotechnol 13 (2012) 1332, 10.2174/138920112800624364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen W, Hu Y, Ju D, Gene therapy for neurodegenerative disorders: advances, insights and prospects, Acta Pharm. Sin. B 10 (8) (2020) 1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ebrahimi Z, Talaei S, Aghamiri S, Goradel NH, Jafarpour A, Negahdari B, Overcoming the blood–brain barrier in neurodegenerative disorders and brain tumours, IET Nanobiotechnology. 14 (2020) 441–448, 10.1049/IET-NBT.2019.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Global Burden of Disease Collaborative Network, Global Burden of Disease Study 2019 (GBD 2019) Results, Inst. Heal. Metrics Eval (2020). http://ghdx.healthdata.org/gbd-results-tool (accessed February 3, 2022). [Google Scholar]
- [26].Hooper C, Killick R, Lovestone S, The GSK3 hypothesis of Alzheimer’s disease, J. Neurochem 104 (2008) 1433, 10.1111/J.1471-4159.2007.05194.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hsu P-H, Lin Y-T, Chung Y-H, Lin K-J, Yang L-Y, Yen T-C, Liu H-L, Focused Ultrasound-Induced Blood-Brain Barrier Opening Enhances GSK-3 Inhibitor Delivery for Amyloid-Beta Plaque Reduction, Sci Rep 8 (1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xhima K, Markham-Coultes K, Nedev H, Heinen S, Saragovi HU, Hynynen K, Aubert I, Focused ultrasound delivery of a selective TrkA agonist rescues cholinergic function in a mouse model of Alzheimer’s disease, Sci. Adv 6 (2020), 10.1126/SCIADV.AAX6646/SUPPL_FILE/AAX6646_SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].K X, K M-C, RH K, HU S, K H, I A, Ultrasound delivery of a TrkA agonist confers neuroprotection to Alzheimer-associated pathologies, Brain. 139 (2021) 16–17. 10.1093/BRAIN/AWAB460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dubey S, Heinen S, Krantic S, JoAnne McLaurin DR, Branch K, Hynynen IA, Clinically approved IVIg delivered to the hippocampus with focused ultrasound promotes neurogenesis in a model of Alzheimer’s disease, Proc. Natl. Acad. Sci. U. S. A 117 (2020) 32691–32700, 10.1073/PNAS.1908658117/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sun T, Shi Q, Zhang Y, Power C, Hoesch C, Antonelli S, Schroeder MK, Caldarone BJ, Taudte N, Schenk M, Hettmann T, Schilling S, McDannold NJ, Lemere CA, Focused ultrasound with anti-pGlu3 Aβ enhances efficacy in Alzheimer’s disease-like mice via recruitment of peripheral immune cells, J. Control. Release 336 (2021) 443–456, 10.1016/J.JCONREL.2021.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu Y, Gong Y, Xie W, Huang A, Yuan X, Zhou H, Zhu X, Chen X, Liu J, Liu J, Qin X, Microbubbles in combination with focused ultrasound for the delivery of quercetin-modified sulfur nanoparticles through the blood brain barrier into the brain parenchyma and relief of endoplasmic reticulum stress to treat Alzheimer’s disease, Nanoscale. 12 (2020) 6498–6511, 10.1039/C9NR09713A. [DOI] [PubMed] [Google Scholar]
- [33].Weber-Adrian D, Kofoed RH, Chan JWY, Silburt J, Noroozian Z, Kügler S, Hynynen K, Aubert I, Strategy to enhance transgene expression in proximity of amyloid plaques in a mouse model of Alzheimer’s disease, Theranostics. 9 (2019) 8127–8137, 10.7150/THNO.36718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kofoed RH, Heinen S, Silburt J, Dubey S, Dibia CL, Maes M, Simpson EM, Hynynen K, Aubert I, Transgene distribution and immune response after ultrasound delivery of rAAV9 and PHP.B to the brain in a mouse model of amyloidosis, Mol. Ther. - Methods Clin. Dev 23 (2021) 390–405, 10.1016/J.OMTM.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nisbet RM, Van Der Jeugd A, Leinenga G, Evans HT, Janowicz PW, Götz J, Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model, Brain. 140 (2017) 1220–1230, 10.1093/BRAIN/AWX052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Janowicz PW, Leinenga G, Götz J, Nisbet RM, Ultrasound-mediated blood-brain barrier opening enhances delivery of therapeutically relevant formats of a tau-specific antibody, Sci. Rep 9 (2019), 10.1038/s41598-019-45577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xhima K, Nabbouh F, Hynynen K, Aubert I, Tandon A, Noninvasive delivery of an α-synuclein gene silencing vector with magnetic resonance-guided focused ultrasound, Mov. Disord 33 (2018) 1567–1579, 10.1002/mds.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lin CJ, Lin CY, Lin YT, Huang CY, Wei KC, Chen JC, Lee-Chen GJ, Liu HL, Microbubble-facilitated ultrasound pulsation promotes direct α-synuclein gene delivery, Biochem. Biophys. Res. Commun 517 (2019) 77–83, 10.1016/J.BBRC.2019.07.017. [DOI] [PubMed] [Google Scholar]
- [39].Yan Y, Chen Y, Liu Z, Cai F, Niu W, Song L, Liang H, Su Z, Yu B, Yan F, Brain Delivery of Curcumin Through Low-Intensity Ultrasound-Induced Blood-Brain Barrier Opening via Lipid-PLGA Nanobubbles, Int. J. Nanomedicine 16 (2021) 7433–7447, 10.2147/IJN.S327737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lin CY, Hsieh HY, Chen CM, Wu SR, Tsai CH, Huang CY, Hua MY, Wei KC, Yeh CK, Liu HL, Non-invasive, neuron-specific gene therapy by focused ultrasound-induced blood-brain barrier opening in Parkinson’s disease mouse model, J. Control. Release 235 (2016) 72–81, 10.1016/j.jconrel.2016.05.052. [DOI] [PubMed] [Google Scholar]
- [41].Fan C-H, Ting C-Y, Lin C-Y, Chan H-L, Chang Y-C, Chen Y-Y, Liu H-L, Yeh CK, Noninvasive, Targeted and Non-Viral Ultrasound-Mediated GDNF-Plasmid Delivery for Treatment of Parkinson’s Disease, Sci Rep 6 (1) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Long L, Cai X, Guo R, Wang P, Wu L, Yin T, Liao S, Lu Z, Treatment of Parkinson’s disease in rats by Nrf2 transfection using MRI-guided focused ultrasound delivery of nanomicrobubbles, Biochem. Biophys. Res. Commun 482 (2017) 75–80, 10.1016/J.BBRC.2016.10.141. [DOI] [PubMed] [Google Scholar]
- [43].Mead BP, Kim N, Miller GW, Hodges D, Mastorakos P, Klibanov AL, Mandell JW, Hirsh J, Suk JS, Hanes J, Price RJ, Novel Focused Ultrasound Gene Therapy Approach Noninvasively Restores Dopaminergic Neuron Function in a Rat Parkinson’s Disease Model, Nano Lett. 17 (2017) 3533–3542, 10.1021/ACS.NANOLETT.7B00616/SUPPL_FILE/NL7B00616_SI_001.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yue P, Miao W, Gao L, Zhao X, Teng J, Ultrasound-triggered effects of the microbubbles coupled to GDNF plasmid-loaded PEGylated liposomes in a rat model of Parkinson’s disease, Front. Neurosci 12 (2018) 222, 10.3389/FNINS.2018.00222/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wu CY, Huang RY, Liao EC, Lin YC, Ho YJ, Chang CW, Chan HL, Huang YZ, Hsieh TH, Fan CH, Yeh CK, A preliminary study of Parkinson’s gene therapy via sono-magnetic sensing gene vector for conquering extra/intracellular barriers in mice, Brain Stimul. 13 (2020) 786–799, 10.1016/J.BRS.2020.02.024. [DOI] [PubMed] [Google Scholar]
- [46].Karakatsani ME, Wang S, Samiotaki G, Kugelman T, Olumolade OO, Acosta C, Sun T, Han Y, Kamimura HAS, Jackson-Lewis V, Przedborski S, Konofagou E, Amelioration of the nigrostriatal pathway facilitated by ultrasound-mediated neurotrophic delivery in early Parkinson’s disease, J. Control. Release 303 (2019) 289–301, 10.1016/J.JCONREL.2019.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ji R, Smith M, Niimi Y, Karakatsani ME, Murillo MF, Jackson-Lewis V, Przedborski S, Konofagou EE, Focused ultrasound enhanced intranasal delivery of brain derived neurotrophic factor produces neurorestorative effects in a Parkinson’s disease mouse model, Sci Rep 9 (1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chen H, Yang GZX, Getachew H, Acosta C, Sierra Sanchez C, Konofagou EE, Focused ultrasound-enhanced intranasal brain delivery of brain-derived neurotrophic factor, Sci Rep 6 (1) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bunevicius A, McDannold NJ, Golby AJ, Focused Ultrasound Strategies for Brain Tumor Therapy, Oper. Neurosurg Operative Surg 19 (1) (2020) 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wu SK, Tsai CL, Huang Y, Hynynen K, Focused Ultrasound and Microbubbles-Mediated Drug Delivery to Brain Tumor, Pharmaceutics. 13 (2020) 1–15, 10.3390/PHARMACEUTICS13010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dréan A, Lemaire N, Bouchoux G, Goldwirt L, Canney M, Goli L, Bouzidi A, Schmitt C, Guehennec J, Verreault M, Sanson M, Delattre JY, Mokhtari K, Sottilini F, Carpentier A, Idbaih A, Temporary blood-brain barrier disruption by low intensity pulsed ultrasound increases carboplatin delivery and efficacy in preclinical models of glioblastoma, J. Neurooncol 144 (2019) 33–41, 10.1007/S11060-019-03204-0. [DOI] [PubMed] [Google Scholar]
- [52].Wei H-J, Upadhyayula PS, Pouliopoulos AN, Englander ZK, Zhang X, Jan C-I, Guo J, Mela A, Zhang Z, Wang TJC, Bruce JN, Canoll PD, Feldstein NA, Zacharoulis S, Konofagou EE, Wu C-C, Focused Ultrasound-Mediated Blood-Brain Barrier Opening Increases Delivery and Efficacy of Etoposide for Glioblastoma Treatment, Int. J. Radiat. Oncol 110 (2021) 539–550, 10.1016/j.ijrobp.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rallis KS, Yau THL, Sideris M, Chemoradiotherapy in Cancer Treatment: Rationale and Clinical Applications, Anticancer Res. 41 (2021) 1–7, 10.21873/ANTICANRES.14746. [DOI] [PubMed] [Google Scholar]
- [54].Song Z, Huang X, Wang J, Cai F, Zhao P, Yan F, Targeted Delivery of Liposomal Temozolomide Enhanced Anti-Glioblastoma Efficacy through Ultrasound-Mediated Blood-Brain Barrier Opening, Pharmaceutics. 13 (2021) 1270, 10.3390/pharmaceutics13081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang DY, Dmello C, Chen L, Arrieta VA, Gonzalez-Buendia E, Robert Kane J, Magnusson LP, Baran A, David James C, Horbinski C, Carpentier A, Desseaux C, Canney M, Muzzio M, Stupp R, Sonabend AM, Ultrasound-mediated Delivery of Paclitaxel for Glioma: A Comparative Study of Distribution, Toxicity, and Efficacy of Albumin-bound Versus Cremophor Formulations, Clin. Cancer Res 26 (2020) 477–486. 10.1158/1078-0432.CCR-19-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Curley CT, Mead BP, Negron K, Kim N, Garrison WJ, Wilson Miller G, Kingsmore KM, Andrew Thim E, Song J, Munson JM, Klibanov AL, Suk JS, Hanes J, Price RJ, Augmentation of brain tumor interstitial flow via focused ultrasound promotes brain-penetrating nanoparticle dispersion and transfection, Sci. Adv 6 (2020) aay1344, 10.1126/sciadv.aay1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yang Q, Zhou Y, Chen J, Huang N, Wang Z, Cheng Y, Gene Therapy for Drug- Resistant Glioblastoma via Lipid-Polymer Hybrid Nanoparticles Combined with Focused Ultrasound, Int. J. Nanomedicine 16 (2021) 185–199, 10.2147/IJN.S286221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fan C-H, Wang T-W, Hsieh Y-K, Wang C-F, Gao Z, Kim A, Nagasaki Y, Yeh C-K, Enhancing Boron Uptake in Brain Glioma by a Boron-Polymer/Microbubble Complex with Focused Ultrasound, ACS Appl. Mater. Interfaces 11 (2019) 11144–11156, 10.1021/acsami.8b22468. [DOI] [PubMed] [Google Scholar]
- [59].White PJ, Zhang YZ, Power C, Vykhodtseva N, McDannold N, Observed Effects of Whole-Brain Radiation Therapy on Focused Ultrasound Blood-Brain Barrier Disruption, Ultrasound Med. Biol 46 (2020) 1998–2006, 10.1016/J.ULTRASMEDBIO.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sheybani ND, Breza VR, Paul S, McCauley KS, Berr SS, Miller GW, Neumann KD, Price RJ, ImmunoPET-informed sequence for focused ultrasound-targeted mCD47 blockade controls glioma, J. Control. Release 331 (2021) 19–29, 10.1016/j.jconrel.2021.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Brighi C, Reid L, White AL, Genovesi LA, Kojic M, Millar A, Bruce Z, Day BW, Rose S, Whittaker AK, Puttick S, MR-guided focused ultrasound increases antibody delivery to nonenhancing high-grade glioma, Neuro-Oncology Adv. 2 (2020), 10.1093/noajnl/vdaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhu L, Nazeri A, Pacia CP, Yue Y, Chen H, Sherman JH, Focused ultrasound for safe and effective release of brain tumor biomarkers into the peripheral circulation, PLoS One. 15 (6) (2020) e0234182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhu L, Cheng G, Ye D, Nazeri A, Yue Y, Liu W, Wang X, Dunn GP, Petti AA, Leuthardt EC, Chen H, Focused Ultrasound-enabled Brain Tumor Liquid Biopsy, Sci. Rep 8 (2018) 6553, 10.1038/s41598-018-24516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pacia CP, Zhu L, Yang Y, Yue Y, Nazeri A, Michael Gach H, Talcott MR, Leuthardt EC, Chen H, Feasibility and safety of focused ultrasound-enabled liquid biopsy in the brain of a porcine model, Sci Rep 10 (1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhang Y, Buckmaster PS, Qiu L, Wang J, Keunen O, Ghobadi SN, Huang A, Hou Q, Li N, Narang S, Habte FG, Bertram EH, Lee KS, Wintermark M, Non-invasive, neurotoxic surgery reduces seizures in a rat model of temporal lobe epilepsy, Exp. Neurol, 343(2021), 10.1016/J.EXPNEUROL.2021.113761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang Y, Liao C, Qu H, Huang S, Jiang H, Zhou H, Abrams E, Habte FG, Yuan L, Bertram EH, Lee KS, Pauly KB, Buckmaster PS, Wintermark M, Testing Different Combinations of Acoustic Pressure and Doses of Quinolinic Acid for Induction of Focal Neuron Loss in Mice Using Transcranial Low-Intensity Focused Ultrasound, Ultrasound Med. Biol 45 (2019) 129–136, 10.1016/J.ULTRASMEDBIO.2018.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang Y, Anzivino MJ, Zhang Y, Bertram EH, Woznak J, Klibanov AL, Dumont E, Wintermark M, Lee KS, Noninvasive disconnection of targeted neuronal circuitry sparing axons of passage and nonneuronal cells, J. Neurosurg (2021) 1–11, 10.3171/2021.7.JNS21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wang F, Li N, Wei X, Jia X, Liu H, Wang Y, Shi Y, Pan M, Wang Y, Yin Y, Yan F, Chen Y, MRI-Guided Focused Ultrasound-Induced Blood Brain Barrier Disruption to Deliver Glial Cell Line Derived Neurotropic Factor Proteins into Brain to Treat Rat Depression, J. Biomed. Nanotechnol 16 (2020) 626–639, 10.1166/JBN.2020.2914. [DOI] [PubMed] [Google Scholar]
- [69].Tsai SJ, Transcranial focused ultrasound as a possible treatment for major depression, Med. Hypotheses 84 (2015) 381–383, 10.1016/J.MEHY.2015.01.030. [DOI] [PubMed] [Google Scholar]
- [70].Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K, Jordã JF, Aubert I, Hynynen K, Jord??o JF, Aubert I, Hynynen K, Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier, PLoS One. 6 (2011) e27877-. 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ahmed N, Gandhi D, Melhem ER, Frenkel V, MRI Guided Focused Ultrasound-Mediated Delivery of Therapeutic Cells to the Brain: A Review of the State-of-the-Art Methodology and Future Applications, Front. Neurol 12. (2021), 10.3389/FNEUR.2021.669449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bin Shen W, Anastasiadis P, Nguyen B, Yarnell D, Yarowsky PJ, Frenkel V, Fishman PS, Magnetic Enhancement of Stem Cell-Targeted Delivery into the Brain Following MR-Guided Focused Ultrasound for Opening the Blood-Brain Barrier, Cell Transplant. 26 (2017) 1235–1246, 10.1177/0963689717715824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lee J, Chang WS, Shin J, Seo Y, Kong C, Song B-W, Na YC, Kim BS, Chang JW, Non-invasively enhanced intracranial transplantation of mesenchymal stem cells using focused ultrasound mediated by overexpression of cell-adhesion molecules, Stem Cell Res. 43 (2020) 101726. [DOI] [PubMed] [Google Scholar]
- [74].Lynch M, Heinen S, Markham-Coultes K, O’reilly M, Van Slyke P, Dumont DJ, Hynynen K, Aubert I, Vasculotide restores the blood-brain barrier after focused ultrasound-induced permeability in a mouse model of alzheimer’s disease, Int. J. Med. Sci 18 (2021) 482–493, 10.7150/IJMS.36775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Scarcelli T, Jordao JF, O’Reilly MA, Ellens N, Hynynen K, Aubert I, Stimulation of Hippocampal Neurogenesis by Transcranial Focused Ultrasound and Microbubbles in Adult Mice, Brain Stimulation 7 (2) (2014) 304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mathew AS, Gorick CM, Price RJ, Single-cell mapping of focused ultrasound-transfected brain, Gene Ther. (2021) 1–9. 10.1038/s41434-021-00226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mooney SJ, Shah K, Yeung S, Burgess A, Aubert I, Hynynen K, Deli MA, Focused ultrasound-induced neurogenesis requires an increase in blood-brain barrier permeability, PLoS One. 11 (7) (2016) e0159892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Shin J, Kong C, Lee J, Choi BY, Sim J, Koh CS, Park M, Na YC, Suh SW, Chang WS, Chang JW, Focused ultrasound-induced blood-brain barrier opening improves adult hippocampal neurogenesis and cognitive function in a cholinergic degeneration dementia rat model, Alzheimer’s Res. Ther 11 (2019) 1–15, 10.1186/s13195-019-0569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].McMahon D, Mah E, Hynynen K, Angiogenic response of rat hippocampal vasculature to focused ultrasound-mediated increases in blood-brain barrier permeability, Sci. Rep 8 (2018), 10.1038/S41598-018-30825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Todd N, Zhang Y, Livingstone M, Borsook D, McDannold N, The neurovascular response is attenuated by focused ultrasound-mediated disruption of the blood-brain barrier, Neuroimage. 201 (2019), 10.1016/j.neuroimage.2019.116010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Leinenga G, Götz J, Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model, Sci. Transl. Med 7 (2015) 278ra33, 10.1126/scitranslmed.aaa2512. [DOI] [PubMed] [Google Scholar]
- [82].Park SH, Baik K, Jeon S, Chang WS, Ye BS, Chang JW, Extensive frontal focused ultrasound mediated blood–brain barrier opening for the treatment of Alzheimer’s disease: a proof-of-concept study, Transl. Neurodegener 10 (2021) 1–11, 10.1186/S40035-021-00269-8/TABLES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Burgess A, Nhan T, Moffatt C, Klibanov AL, Hynynen K, Analysis of focused ultrasound-induced blood-brain barrier permeability in a mouse model of Alzheimer’s disease using two-photon microscopy, J. Control. Release 192 (2014) 243–248, 10.1016/j.jconrel.2014.07.051. [DOI] [PubMed] [Google Scholar]
- [84].Marty B, Larrat B, Van Landeghem M, Robic C, Robert P, Port M, Le Bihan D, Pernot M, Tanter M, Lethimonnier F, Mériaux S, Dynamic study of blood-brain barrier closure after its disruption using ultrasound: a quantitative analysis, J. Cereb. Blood Flow Metab 32 (2012) 1948–1958, 10.1038/JCBFM.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].O’Reilly MA, Hough O, Hynynen K, Blood-Brain Barrier Closure Time After Controlled Ultrasound-Induced Opening Is Independent of Opening Volume, J. Ultrasound Med 36 (2017) 475–483, 10.7863/ULTRA.16.02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Leinenga G, Koh WK, Götz J, Scanning ultrasound in the absence of blood-brain barrier opening is not sufficient to clear β-amyloid plaques in the APP23 mouse model of Alzheimer’s disease, Brain Res. Bull 153 (2019) 8–14, 10.1016/J.BRAINRESBULL.2019.08.002. [DOI] [PubMed] [Google Scholar]
- [87].D’Haese PF, Ranjan M, Song A, Haut MW, Carpenter J, Dieb G, Najib U, Wang P, Mehta RI, Chazen JL, Hodder S, Claassen D, Kaplitt M, Rezai AR, β-Amyloid Plaque Reduction in the Hippocampus After Focused Ultrasound-Induced Blood-Brain Barrier Opening in Alzheimer’s Disease, Front. Hum. Neurosci 14 (2020), 10.3389/fnhum.2020.593672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Jordão JF, Thévenot E, Markham-Coultes K, Scarcelli T, Weng YQ, Xhima K, O’Reilly M, Huang Y, McLaurin JA, Hynynen K, Aubert I, Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound, Exp. Neurol 248 (2013) 16–29, 10.1016/J.EXPNEUROL.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lee Y, Choi Y, Park E-J, Kwon S, Kim H, Lee JY, Lee DS, Improvement of glymphatic-lymphatic drainage of beta-amyloid by focused ultrasound in Alzheimer’s disease model, Sci Rep 10 (1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Todd N, Angolano C, Ferran C, Devor A, Borsook D, McDannold N, Secondary effects on brain physiology caused by focused ultrasound-mediated disruption of the blood–brain barrier, J. Control. Release 324 (2020) 450–459, 10.1016/j.jconrel.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wang Y, Martinez-Vicente M, Krüger U, Kaushik S, Wong E, Mandelkow EM, Cuervo AM, Mandelkow E, Synergy and antagonism of macroautophagy and chaperone-mediated autophagy in a cell model of pathological tau aggregation, Autophagy. 6 (2010) 182–183, 10.4161/auto.6.1.10815. [DOI] [PubMed] [Google Scholar]
- [92].Schaeffer V, Lavenir I, Ozcelik S, Tolnay M, Winkler DT, Goedert M, Stimulation of autophagy reduces neurodegeneration in a mouse model of human tauopathy, Brain. 135 (2012) 2169–2177, 10.1093/brain/aws143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Karakatsani ME, Kugelman T, Ji R, Murillo M, Wang S, Niimi Y, Small SA, Duff KE, Konofagou EE, Unilateral focused ultrasound-induced blood-brain barrier opening reduces phosphorylated Tau from the rTg4510 mouse model, Theranostics. 9 (2019) 5396–5411, 10.7150/thno.28717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wang JB, Di Ianni T, Vyas DB, Huang Z, Park S, Hosseini-Nassab N, Aryal M, Airan RD, Focused Ultrasound for Noninvasive, Focal Pharmacologic Neurointervention, Front. Neurosci 14 (2020) 675, 10.3389/FNINS.2020.00675/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Todd N, Zhang Y, Power C, Becerra L, Borsook D, Livingstone M, McDannold N, Modulation of brain function by targeted delivery of GABA through the disrupted blood-brain barrier, Neuroimage. 189 (2019) 267–275, 10.1016/J.NEUROIMAGE.2019.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Constans C, Ahnine H, Santin M, Lehericy S, Tanter M, Pouget P, Aubry JF, Non-invasive ultrasonic modulation of visual evoked response by GABA delivery through the blood brain barrier, J. Control. Release 318 (2020) 223–231, 10.1016/J.JCONREL.2019.12.006. [DOI] [PubMed] [Google Scholar]
- [97].Rich MC, Sherwood J, Bartley AF, Whitsitt QA, Lee M, Willoughby WR, Dobrunz LE, Bao Y, Lubin FD, Bolding M, Focused ultrasound blood brain barrier opening mediated delivery of MRI-visible albumin nanoclusters to the rat brain for localized drug delivery with temporal control, J. Control. Release 324 (2020) 172–180, 10.1016/J.JCONREL.2020.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Airan RD, Meyer RA, Ellens NPK, Rhodes KR, Farahani K, Pomper MG, Kadam SD, Green JJ, Noninvasive Targeted Transcranial Neuromodulation via Focused Ultrasound Gated Drug Release from Nanoemulsions, Nano Lett. 17 (2017) 652–659, 10.1021/ACS.NANOLETT.6B03517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wang JB, Aryal M, Zhong Q, Vyas DB, Airan RD, Noninvasive Ultrasonic Drug Uncaging Maps Whole-Brain Functional Networks, Neuron. 100 (2018) 728–738.e7, 10.1016/J.NEURON.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chu P-C, Liu H-L, Lai H-Y, Lin C-Y, Tsai H-C, Pei Y-C, Neuromodulation accompanying focused ultrasound-induced blood-brain barrier opening, Sci Rep 5 (1) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Park SH, Kim MJ, Jung HH, Chang WS, Choi HS, Rachmilevitch I, Zadicario E, Chang JW, One-Year Outcome of Multiple Blood-Brain Barrier Disruptions With Temozolomide for the Treatment of Glioblastoma, Front. Oncol 10 (2020), 10.3389/FONC.2020.01663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ibsen S, Tong A, Schutt C, Esener S, Chalasani SH, Sonogenetics is a non- invasive approach to activating neurons in Caenorhabditis elegans, Nat. Commun 6 (2015) 8264, 10.1038/ncomms9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Huang Y-S, Fan C-H, Hsu N, Chiu N-H, Wu C-Y, Chang C-Y, Wu B-H, Hong S-R, Chang Y-C, Yan-Tang Wu A, Guo V, Chiang Y-C, Hsu W-C, Chen L, Pin-Kuang Lai C, Yeh C-K, Lin Y-C, Sonogenetic Modulation of Cellular Activities Using an Engineered Auditory-Sensing Protein, Nano Lett. 20 (2020) 1089–1100, 10.1021/acs.nanolett.9b04373. [DOI] [PubMed] [Google Scholar]
- [104].Yang Y, Pacia CP, Ye D, Zhu L, Baek H, Yue Y, Yuan J, Miller MJ, Cui J, Culver JP, Bruchas MR, Chen H, Sonothermogenetics for noninvasive and cell-type specific deep brain neuromodulation, Brain Stimul. 14 (2021) 790–800, 10.1016/j.brs.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Cubillos-Ruiz A, Guo T, Sokolovska A, Miller PF, Collins JJ, Lu TK, Lora JM, Engineering living therapeutics with synthetic biology, Nat. Rev. Drug Discov 20 (2021) 941–960, 10.1038/s41573-021-00285-3. [DOI] [PubMed] [Google Scholar]
- [106].Amrahli M, Centelles M, Cressey P, Prusevicius M, Gedroyc W, Xu XY, So PW, Wright M, Thanou M, Mr-labelled liposomes and focused ultrasound for spatiotemporally controlled drug release in triple negative breast cancers in mice, Nanotheranostics. 5 (2021) 125–142, 10.7150/ntno.52168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Babakhanian M, Yang L, Nowroozi B, Saddik G, Boodaghians L, Blount P, Grundfest W, Effects of Low Intensity Focused Ultrasound on Liposomes Containing Channel proteins, Sci. Rep 8 (2018) 17250, 10.1038/s41598-018-35486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Szablowski JO, Harb M, Focused Ultrasound Induced Blood-Brain Barrier Opening for Targeting Brain Structures and Evaluating Chemogenetic Neuromodulation, J. Vis. Exp (2020), 10.3791/61352. [DOI] [PubMed] [Google Scholar]
- [109].Szablowski JO, Bar-Zion A, Shapiro MG, Achieving Spatial and Molecular Specificity with Ultrasound-Targeted Biomolecular Nanotherapeutics, Acc. Chem. Res 52 (2019) 2427–2434, 10.1021/acs.accounts.9b00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Menard C, Russo SJ, Non-invasive chemogenetics, Nat. Biomed. Eng 2 (2018) 467–468, 10.1038/s41551-018-0269-z. [DOI] [PubMed] [Google Scholar]
- [111].Szablowski JO, Lee-Gosselin A, Lue B, Malounda D, Shapiro MG, Acoustically targeted chemogenetics for the non-invasive control of neural circuits, Nat. Biomed. Eng 2 (2018) 475–484, 10.1038/s41551-018-0258-2. [DOI] [PubMed] [Google Scholar]
- [112].Abedi MH, Lee J, Piraner DI, Shapiro MG, Thermal Control of Engineered T-cells, ACS Synth. Biol 9 (2020) 1941–1950, 10.1021/acssynbio.0c00238. [DOI] [PubMed] [Google Scholar]
- [113].Wu Y, Liu Y, Huang Z, Wang X, Jin Z, Li J, Limsakul P, Zhu L, Allen M, Pan Y, Bussell R, Jacobson A, Liu T, Chien S, Wang Y, Control of the activity of CAR-T cells within tumours via focused ultrasound, Nat. Biomed. Eng 5 (2021) 1336–1347, 10.1038/s41551-021-00779-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Pan Y, Yoon S, Sun J, Huang Z, Lee C, Allen M, Wu Y, Chang Y-J, Sadelain M, Shung KK, Chien S, Wang Y, Mechanogenetics for the remote and noninvasive control of cancer immunotherapy, Proc. Natl. Acad. Sci 115 (2018) 992–997, 10.1073/pnas.1714900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wu Y, Huang Z, Harrison R, Liu L, Zhu L, Situ Y, Wang Y, Engineering CART cells for enhanced efficacy and safety, APL Bioeng. 6 (1) (2022) 011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].World Health Organisation, Neurological Disorders Public Health Challenges, 2006. https://www.who.int/mental_health/neurology/neurological_disorders_report_web.pdf (accessed February 3, 2022). [Google Scholar]
- [117].Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, Huang S, Palmieri D, Steeg PS, Smith QR, Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer, Clin. Cancer Res 16 (2010) 5664–5678. 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Juillerat-Jeanneret L, The targeted delivery of cancer drugs across the blood–brain barrier: chemical modifications of drugs or drug-nanoparticles?, Drug Discov Today. 13 (2008) 1099–1106, 10.1016/J.DRUDIS.2008.09.005. [DOI] [PubMed] [Google Scholar]
- [119].Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, Karachi C, Leclercq D, Lafon C, Chapelon JY, Capelle L, Cornu P, Sanson M, Hoang-Xuan K, Delattre JY, Idbaih A, Clinical trial of blood-brain barrier disruption by pulsed ultrasound, Sci. Transl. Med 8 (2016), 10.1126/SCITRANSLMED.AAF6086. [DOI] [PubMed] [Google Scholar]
- [120].Idbaih A, Canney M, Belin L, Desseaux C, Vignot A, Bouchoux G, Asquier N, Law-Ye B, Leclercq D, Bissery A, De Rycke Y, Trosch C, Capelle L, Sanson M, Hoang-Xuan K, Dehais C, Houillier C, Laigle-Donadey F, Mathon B, Andre A, Lafon C, Chapelon JY, Delattre JY, Carpentier A, Safety and Feasibility of Repeated and Transient Blood–Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma, Clin. Cancer Res 25 (2019) 3793–3801. 10.1158/1078-0432.CCR-18-3643. [DOI] [PubMed] [Google Scholar]
- [121].Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, Heyn C, Alkins R, Trudeau M, Sahgal A, Perry J, Hynynen K, Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study, Sci. Rep 9 (2019), 10.1038/S41598-018-36340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Meng Y, Reilly RM, Pezo RC, Trudeau M, Sahgal A, Singnurkar A, Perry J, Myrehaug S, Pople CB, Davidson B, Llinas M, Hyen C, Huang Y, Hamani C, Suppiah S, Hynynen K, Lipsman N, MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases, Sci. Transl. Med 13 (2021), 10.1126/SCITRANSLMED.ABJ4011/SUPPL_FILE/SCITRANSLMED.ABJ4011_SM.PDF. [DOI] [PubMed] [Google Scholar]
- [123].Anastasiadis P, Gandhi D, Guo Y, Ahmed AK, Bentzen SM, Arvanitis C, Woodworth GF, Localized blood-brain barrier opening in infiltrating gliomas with MRI-guided acoustic emissions-controlled focused ultrasound, Proc. Natl. Acad. Sci. U. S. A 118 (2021), 10.1073/PNAS.2103280118/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Park SH, Kim MJ, Jung HH, Chang WS, Choi HS, Rachmilevitch I, Zadicario E, Chang JW, Safety and feasibility of multiple blood-brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy, J. Neurosurg 134 (2020) 475–483, 10.3171/2019.10.JNS192206. [DOI] [PubMed] [Google Scholar]
- [125].Meng Y, Pople CB, Suppiah S, Llinas M, Huang Y, Sahgal A, Perry J, Keith J, Davidson B, Hamani C, Amemiya Y, Seth A, Leong H, Heyn CC, Aubert I, Hynynen K, Lipsman N, MR-guided focused ultrasound liquid biopsy enriches circulating biomarkers in patients with brain tumors, Neuro Oncol. 23 (2021) 1789–1797, 10.1093/NEUONC/NOAB057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, Herrmann N, Heyn C, Aubert I, Boutet A, Smith GS, Hynynen K, Black SE, Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound, Nat. Commun 91 (9) (2018) 1–8, 10.1038/s41467-018-04529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Mehta RI, Carpenter JS, Mehta RI, Haut MW, Ranjan M, Najib U, Lockman P, Wang P, D’Haese PF, Rezai AR, Blood-Brain Barrier Opening with MRI-guided Focused Ultrasound Elicits Meningeal Venous Permeability in Humans with Early Alzheimer Disease, Radiology. 298 (2021) 654–662, 10.1148/RADIOL.2021200643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Martínez-Fernández R, Máñez-Miró JU, Rodríguez-Rojas R, del Álamo M, Shah BB, Hernández-Fernández F, Pineda-Pardo JA, Monje MHG, Fernández-Rodríguez B, Sperling SA, Mata-Marín D, Guida P, Alonso-Frech F, Obeso I, Gasca-Salas C, Vela-Desojo L, Elias WJ, Obeso JA, Randomized Trial of Focused Ultrasound Subthalamotomy for Parkinson’s Disease, N. Engl. J. Med 383 (2020) 2501–2513, 10.1056/NEJMOA2016311/SUPPL_FILE/NEJMOA2016311_DATA-SHARING.PDF. [DOI] [PubMed] [Google Scholar]
- [129].Gasca-Salas C, Fernández-Rodríguez B, Pineda-Pardo JA, Rodríguez-Rojas R, Obeso I, Hernández-Fernández F, del Álamo M, Mata D, Guida P, Ordás-Bandera C, Montero-Roblas JI, Martínez-Fernández R, Foffani G, Rachmilevitch I, Obeso JA, Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia, Nat. Commun, 12 (2021), 10.1038/S41467-021-21022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Abrahao A, Meng Y, Llinas M, Huang Y, Hamani C, Mainprize T, Aubert I, Heyn C, Black SE, Hynynen K, Lipsman N, Zinman L, First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound, Nat. Commun 10 (2019), 10.1038/S41467-019-12426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Keller SB, Averkiou MA, The Role of Ultrasound in Modulating Interstitial Fluid Pressure in Solid Tumors for Improved Drug Delivery, Bioconjug. Chem 33 (6) (2022) 1049–1056. [DOI] [PubMed] [Google Scholar]
- [132].Hersh DS, Nguyen BA, Dancy JG, Adapa AR, Winkles JA, Woodworth GF, Kim AJ, Frenkel V, Pulsed ultrasound expands the extracellular and perivascular spaces of the brain, Brain Res. 1646 (2016) 543–550, 10.1016/j.brainres.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Hersh DS, Anastasiadis P, Mohammadabadi A, Nguyen BA, Guo S, Winkles JA, Kim AJ, Gullapalli R, Keller A, Frenkel V, Woodworth GF, Ceña V, MR-guided transcranial focused ultrasound safely enhances interstitial dispersion of large polymeric nanoparticles in the living brain, PLoS One. 13 (2) (2018) e0192240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wang S, Karakatsani ME, Fung C, Sun T, Acosta C, Konofagou E, Direct brain infusion can be enhanced with focused ultrasound and microbubbles, J. Cereb. Blood Flow Metab 37 (2017) 706–714, 10.1177/0271678X16637881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Meng Y, Abrahao A, Heyn CC, Bethune AJ, Huang Y, Pople CB, Aubert I, Hamani C, Zinman L, Hynynen K, Black SE, Lipsman N, Glymphatics Visualization after Focused Ultrasound-Induced Blood-Brain Barrier Opening in Humans, Ann. Neurol 86 (2019) 975–980, 10.1002/ana.25604. [DOI] [PubMed] [Google Scholar]
- [136].Mead BP, Curley CT, Kim N, Negron K, Garrison WJ, Song JI, Rao D, Miller GW, Mandell JW, Purow BW, Suk JS, Hanes J, Price RJ, Focused Ultrasound Preconditioning for Augmented Nanoparticle Penetration and Efficacy in the Central Nervous System, Small. 15(49) (2019) 1903460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Aryal M, Azadian MM, Hart AR, Macedo N, Zhou Q, Rosenthal EL, Airan RD, Noninvasive ultrasonic induction of cerebrospinal fluid flow enhances intrathecal drug delivery, J. Control. Release 349 (2022) 434–442, 10.1016/J.JCONREL.2022.06.067. [DOI] [PubMed] [Google Scholar]
- [138].Yoo S-S, Kim H-C, Kim J, Kim E, Kowsari K, Van Reet J, Yoon K, Enhancement of cerebrospinal fluid tracer movement by the application of pulsed transcranial focused ultrasound, Sci. Reports 121 (12) (2022) 1–13, 10.1038/s41598-022-17314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Curley CT, Stevens AD, Mathew AS, Stasiak K, Garrison WJ, Wilson Miller G, Sheybani ND, Engelhard VH, Bullock TNJ, Price RJ, Immunomodulation of intracranial melanoma in response to blood-tumor barrier opening with focused ultrasound, Theranostics. 10 (2020) 8821–8833, 10.7150/thno.47983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Gorick CM, Mathew AS, Garrison WJ, Thim EA, Fisher DG, Copeland CA, Song JI, Klibanov AL, Miller GW, Price RJ, Sonoselective transfection of cerebral vasculature without blood–brain barrier disruption, Proc. Natl. Acad. Sci. U.S.A 117(11) (2020) 5644–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zafar A, Quadri SA, Farooqui M, Ortega-Gutierrez S, Hariri OR, Zulfiqar M, Ikram A, Khan MA, Suriya SS, Nunez-Gonzalez JR, Posse S, Mortazavi MM, Yonas H, MRI-Guided High-Intensity Focused Ultrasound as an Emerging Therapy for Stroke: A Review, J. Neuroimaging 29 (2019) 5–13, 10.1111/JON.12568. [DOI] [PubMed] [Google Scholar]
- [142].Wu S-K, Yang M-T, Kang K-H, Liou H-C, Lu D-H, Fu W-M, Lin W-L, Karamyan V, Targeted Delivery of Erythropoietin by Transcranial Focused Ultrasound for Neuroprotection against Ischemia/Reperfusion-Induced Neuronal Injury: A Long-Term and Short-Term Study, PLoS One. 9 (2) (2014) e90107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Rodríguez-Frutos B, Otero-Ortega L, Ramos-Cejudo J, Martínez-Sánchez P, Barahona-Sanz I, Navarro-Hernanz T, Gómez-de Frutos MDC, Díez-Tejedor E, Gutiérrez-Fernández M, Enhanced brain-derived neurotrophic factor delivery by ultrasound and microbubbles promotes white matter repair after stroke, Biomaterials. 100 (2016) 41–52. [DOI] [PubMed] [Google Scholar]
- [144].Vernooij MW, Ikram MA, Tanghe HL, Vincent AJPE, Hofman A, Krestin GP, Niessen WJ, Breteler MMB, van der Lugt A, Incidental Findings on Brain MRI in the General Population, N. Engl. J. Med 357 (2007) 1821–1828, 10.1056/nejmoa070972. [DOI] [PubMed] [Google Scholar]
- [145].Del Curling O, Kelly DL, Elster AD, Craven TE, An analysis of the natural history of cavernous angiomas, J. Neurosurg 75 (1991) 702–708, 10.3171/jns.1991.75.5.0702. [DOI] [PubMed] [Google Scholar]
- [146].Ene C, Kaul A, Kim L, Natural history of cerebral cavernous malformations, in: Handb. Clin. Neurol., Handb Clin Neurol, 2017: pp. 227–232. 10.1016/B978-0-444-63640-9.00021-7. [DOI] [PubMed] [Google Scholar]
- [147].Haasdijk RA, Cheng C, Maat-Kievit AJ, Duckers HJ, Cerebral cavernous malformations: From molecular pathogenesis to genetic counselling and clinical management, Eur.J. Hum. Genet 20 (2012) 134–140, 10.1038/ejhg.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Snellings DA, Hong CC, Ren AA, Lopez-Ramirez MA, Girard R, Srinath A, Marchuk DA, Ginsberg MH, Awad IA, Kahn ML, Cerebral Cavernous Malformation: From Mechanism to Therapy, Circ. Res 129 (2021) 195–215, 10.1161/CIRCRESAHA.121.318174. [DOI] [PMC free article] [PubMed] [Google Scholar]