Abstract
Objectives
To provide our oncology-specific adult abdominal-pelvic CT reference levels for image noise and radiation dose from a high-volume, oncologic, tertiary referral center.
Methods
The portal venous phase abdomen-pelvis acquisition was assessed for image noise and radiation dose in 13,320 contrast-enhanced CT examinations. Patient size (effective diameter) and radiation dose (CTDIvol) were recorded using a commercial software system, and image noise (Global Noise metric) was quantified using a custom processing system. The reference level and range for dose and noise were calculated for the full dataset, and for examinations grouped by CT scanner model. Dose and noise reference levels were also calculated for exams grouped by five different patient size categories.
Results
The noise reference level was 11.25 HU with a reference range of 10.25 – 12.25 HU. The dose reference level at a median effective diameter of 30.7 cm was 26.7 mGy with a reference range of 19.6 – 37.0 mGy. Dose increased with patient size; however, image noise remained approximately constant within the noise reference range. The doses were 2.1 – 2.5 times than the doses in the ACR DIR registry for corresponding patient sizes. The image noise was 0.63 – 0.75 times the previously published reference level in abdominal-pelvic CT examinations.
Conclusions
Our oncology-specific abdominal-pelvic CT dose reference levels are higher than in the ACR dose index registry and our oncology-specific image noise reference levels are lower than previously proposed image noise reference levels.
Advances in Knowledge
This study reports reference image noise and radiation dose levels appropriate for the indication of abdomen-pelvis CT examination for cancer diagnosis and staging. The difference in these reference levels from non oncology-specific CT examinations highlight a need for indication-specific, dose index and image quality reference registries.
Keywords: Diagnostic Reference Levels, radiation reduction, oncology, CT, image noise
Introduction
Radiation dose index registries provide an important frame of reference for imaging practices to build and optimize safe, effective CT protocols. Through high-volume, multicenter reporting [1], achievable radiation doses (50th percentile) and diagnostic reference levels (DRL) (75 percentile) have been created, such as from the ACR dose index registry (ACR DIR) [2]. DRLs were first mentioned by the International Commission of Radiological Protection (ICRP) in 1990 and the concept of achievable doses was introduced in 1999. Use of these concepts have been refined over the years and the ACR DIR recently expanded this through the additional reporting of data by patient size, which provided another level of detail for practice optimization [3]. However, diagnostic quality is only inferred through this approach which assumes that practices have generally arrived at appropriate imaging presumably using radiation doses which are as low as reasonably achievable (ALARA) and yet still diagnostic for the clinical task. The latter point is critical to inform the use of such registries when building and optimizing CT protocols. In the abdomen, when submitted and available radiation dose data are categorized based only on anatomical segments and the presence or absence of IV contrast, a critical limitation is introduced; for instance, CT protocols used for appendicitis evaluation are grouped with patients undergoing presurgical liver evaluation for colorectal metastatic disease. These two different clinical indications may require very different levels of image quality, yet their dose levels are considered together in one group when a user references the DIR. A few studies have assessed DRLs for certain clinical indications in the abdomen, but no broad comparison of oncologic radiation doses has been performed [4-6]. This issue with DRLs that are irrespective of clinical indication has been recognized such as described by the ICRP in publication 135 [7] and some investigation has begun associating image quality metrics with DRLs [8].
At present, registries provide indirect, inferred diagnostic CT image quality. However, image quality depends not only on radiation dose, but also on other factors including image reconstruction algorithm and filter (kernel), X-ray beam collimation, filtration and modulation, as well as vendor-specific X-ray detector implementation. To normalize for these varying factors, image quality reference levels are needed in addition to dose reference levels. The challenge in establishing image quality reference levels is apparent. Whereas dose reference levels use standardized CT dose index data (CTDIvol) outputted automatically by the CT scanner for each examination, there is no corresponding automatic quality index output. Standard metrics of image quality such as pixel standard deviation (noise), noise power spectrum (noise texture), contrast-to-noise ratio (contrast resolution) and modulation transfer function (spatial resolution), are traditionally measured only in phantom images.
Work, such as by Ria and colleagues, is beginning to connect reported dose levels with image quality metrics [8]. Their study provided reference levels from a single practice for chest and abdominopelvic CT examinations, not only for radiation doses, but they expanded the concept to also include image noise. This automated, direct measure of an image quality metric provides yet another level of detail for practices when refining protocols and they report further objective automated measures that are feasible for reporting in the future such as noise texture and spatial resolution. Such efforts have been extended across large cohorts of facilities [9], extended to the likelihood of lesion detection [10], and validated to be reflective of radiologists preference [11] and, more importantly, diagnostic accuracy [12, 13].
The purpose of our study was to provide oncology-specific adult abdominal-pelvic CT reference levels for radiation dose and image noise from a high-volume, oncologic, tertiary referral center.
Materials and Methods
This retrospective study was approved by our institutional review board as Health Insurance Portability and Accountability Act compliant, and written informed consent was waived. A total of 18762 consecutive adult chest-abdomen-pelvis (CAP) CT examinations were performed between November 1, 2020 and June 30, 2021. Analysis of images for collection of image noise data started on different dates for different scanners. As a result, 13,320 out of the 18,762 available examinations were analyzed for noise, and only these examinations were included in this study.
The patient cohort demographics are included in Table I.
Table I -.
Patient demographics
| Total number of exams | 13320 | |
| Percentage of exams with male patient | 49.8% | |
| Percentage of exams with female patient | 50.2% | |
| Patient Age (y) | Range | 18.0 – 93.4 |
| Mean | 61.2 | |
| Median | 63.1 | |
| Patient Body Mass Index (BMI) (kg/m2) | Range | 12.9 – 70.4 |
| Mean | 28.6 | |
| Median | 27.9 | |
| BMI <= 19 | 264 | |
| BMI 19 – 25 | 3631 | |
| Number of exams | BMI 25 – 30 | 4533 |
| BMI 30 – 35 | 2951 | |
| BMI 35 – 40 | 1364 | |
| BMI > 40 | 577 | |
| Patient Effective Diameter (ED) (cm) | Range | 19.2 – 43.4 |
| Mean | 30.8 | |
| Median | 30.7 | |
| ED <= 21 | 7 | |
| ED 21 – 25 | 669 | |
| ED 25 – 29 | 3573 | |
| Number of exams | ED 29 – 33 | 5431 |
| ED 33 – 37 | 2939 | |
| ED 37 – 41 | 679 | |
| ED > 41 | 22 | |
Only the abdomen-pelvis portion of the exam was considered in this study given that this region was implicated in the authors’ prior work [14]. Chest-abdomen-pelvis examinations were chosen for inclusion in this study instead of abdomen-only or abdomen-pelvis exams because of the relatively larger volume of these examinations at the authors’ institution.
The CT examinations were performed across six CT scanner models. The list of scanner models, the number of examinations performed, and the scan protocol parameters are listed in Table II. Two of the scanner models (GE Discovery750 HD and GE Revolution HD) are essentially the same in terms of acquisition and image reconstruction parameters; the data for these two scanner models have been combined into a single scanner model named “Discovery750/Revolution HD”. The number of examinations by scanner model and tube voltage/kV is listed in Table III.
Table II -.
CT scanner models and protocol parameters
| CT Scanner Model | Detector Configuration (mm) |
Tube Voltage (kV) |
Automatic dose prescription control parameter |
Ref. kV and CarekV setting |
Rotation time (s) |
Spiral pitch factor |
Slice Thickness (mm) |
Reconstruction algorithm parameters |
|---|---|---|---|---|---|---|---|---|
| GE Discovery750/Revolution HD | 40 | 120 | 11 (NI)¥# | (n/a) | 0.5# | 0.516 | 2.5 | Standard*, E1, ASIR-V 30%# |
| Siemens SOMATOM Force | 57.6 | Variable | 300 (Qref) | 120/7 | 0.5 | 0.6 | 3 | Br40 ADMIRE 2 |
| Siemens SOMATOM Definition Flash | 38.4 | Variable | 400 (Qref) | 120/7 | 0.5 | 0.6 | 3 | I40f SAFIRE 2 |
| Siemens SOMATOM Edge Plus | 38.4 | Variable | 400 (Qref) | 120/7 | 0.5 | 0.6 | 3 | Br40 ADMIRE 2 |
| GE Revolution CT | 80 | 120 | 9/10.4 (NI)¥^ | (n/a) | 0.5/0.6^ | 0.507 | 2.5 | Standard, E1, DLIR medium strength |
Note—NI-noise index, Qref-quality reference mAs
a small percentage (< 5%) of these exams used the “Detail” reconstruction filter, instead of “Standard” due to a software difference on 1 scanner out of the 13 scanners of the same model) [29].
Based upon 5mm primary reconstruction series
5.6% of exams on this scanner model used a NI of 12, rotation time of 0.7 s, and ASIR-V 40% reconstruction setting, necessitated by large patient size
approximately 40% and 60% of exams on this scanner model used 9.0 and 10.4 NI; and 0.5 and 0.6 s rotation time respectively, according to two different patient size protocols (“small” and “large”), with threshold approximately BMI 27.
Table III-.
Number of examinations by scanner model and tube voltage used
| Scanner Model | 100 kV | 110 kV | 120 kV | 130 kV |
|---|---|---|---|---|
| GE Discovery750/Revolution HD | - | - | 8400 | - |
| Siemens SOMATOM Force | 1288 | 167 | 159 | 505 |
| Siemens SOMATOM Definition Flash | 729 | - | 792 | - |
| Siemens SOMATOM Edge Plus | 348 | 157 | 47 | 342 |
| GE Revolution CT | - | - | 386 | - |
The radiation dosimetry information from these exams, in the form of a radiation structured dose report, was processed and recorded using a commercial dose monitoring system, Radimetrics (Bayer Healthcare, Whippany, NJ, USA). The chest-abdomen-pelvis exam contains several irradiation events: scout/topogram radiographs, bolus tracking scans, a pre-contrast scan (when indicated), a post-contrast scan in the portal-venous phase, and a delayed phase scan (when indicated). For the purpose of this work, only the post-contrast portal-venous phase scan was considered. The commercial system recorded both the radiation dose during this scan (CTDIvol) and patient effective diameter in the scanned region. Patient size can be measured in a variety of different quantities; in this work, effective diameter [15] was used (as opposed to lateral, anterior-posterior dimensions or water equivalent diameter) to facilitate comparison to the diagnostic reference level of Ria and colleagues [8]. The patient effective diameter was measured automatically by the dose monitoring system using the scout/topogram radiographs acquired as part of the examination.
A custom system was created to automatically measure the noise of the axial post-contrast image series, using the global noise (GN) method introduced by Christianson and colleagues [16] and subsequently validated in different settings [17]. Essentially, the algorithm measures noise globally across the image series by scanning regions-of-interest (ROIs) across all soft-tissues regions in the, finding the most homogeneous regions, and summarizing noise across those ROIs. A computer program implementing the GN algorithm was hosted on a computing server. The computer program receives relevant reconstructed image series immediately upon creation and transmission from the scanner. The system measures the GN on each image slice within an image series, and the median GN value in the image series is recorded in a database along with exam identifying information.
Only the 2.5 or 3 mm thick (depending on the scanner vendor) axial portal-venous phase image series of the abdomen-pelvis were analyzed for noise. Unlike the study by Ria et al., the measured image noise here was not normalized to a 5-mm slice thickness. The rationale was that slice thickness values fell within a narrow range, and the study aimed to be reflective of the reader-preferred noise magnitude in the clinically-used slice thickness.
The reference level and reference range were calculated for dose and image noise. The reference level was calculated as the median value of the distribution, and the reference range was calculated as the 25th - 75th inter-quantile range. The dose reference levels were compared to ACR DIR and to that of Ria and colleagues.
A prediction model of dose over different patient sizes was created for each of the scanner models and for all examinations overall. The model consists of a smoothed fit of dose as a function of patient size. The smoothed fit used locally estimated scatterplot smoothing (LOESS)[18]. A subgroup analysis was performed on groups divided into the following patient size (effective diameter) categories to parallel prior publications: 21-25 cm, 25-29 cm, 29-33 cm, 33-37 cm, and 37-41 cm. Most exams were included in one of these categories; only 29 out of 13320 exams were excluded in this analysis. Within each patient category, the reference level and range were calculated for dose and image noise. To test for any potential patient size selection differences across scanners, the proportion of examinations in any scanner-patient size subgroup was tested for a difference from the overall proportion of patient size groups. Tests for difference were performed using a z-test for proportions.
Results
Table IV shows number of examinations by scanner model and patient size. Some patient size groups were found to be statistically under- or over- represented. In particular, patient selection on the Siemens Definition Edge scanner appeared to favor small patients over large patients. When correcting for multiple testing using the Bonferroni correction, only the proportion of > 37 cm patients on the Siemens Edge Plus scanner remains significantly different. Apart from these exceptions, patient size groups are equally represented across scanner models.
Table IV-.
Number of examinations by scanner model and patient size
| N exams (% on scanner model) | Effective Diameter (cm) | |||||
|---|---|---|---|---|---|---|
| CT Scanner Model | < 25 | 25 – 29 | 29 – 33 | 33 – 37 | > 37 | Total |
| GE Discovery750/Revolution HD | 407 (4.8%) | 2229 (26.5%) | 3398 (40.5%) | 1879 (22.4%) | 487 (5.8%)* | 8400 (100%) |
| Siemens SOMATOM Force | 121 (5.7%) | 568 (26.8%) | 851 (40.2%) | 468 (22.1%) | 111 (5.2%) | 2119 (100%) |
| Siemens SOMATOM Definition Flash | 94 (6.2%)* | 414 (27.2%) | 617 (40.6%) | 330 (21.7%) | 66 (4.3%) | 1521 (100%) |
| Siemens SOMATOM Edge Plus | 44 (4.9%) | 267 (29.9%)* | 402 (45.0%)* | 166 (18.6%)* | 15 (1.7%)*** | 894 (100%) |
| GE Revolution CT | 10 (2.6%) | 95 (24.6%) | 163 (42.2%) | 96 (24.9%) | 22 (5.7%) | 386 (100%) |
| Total | 676 (5.1%) | 3573 (26.8%) | 5431 (40.8%) | 2939 (22.1%) | 701 (5.3%) | 13320 (100%) |
p < 0.05
p < 0.001
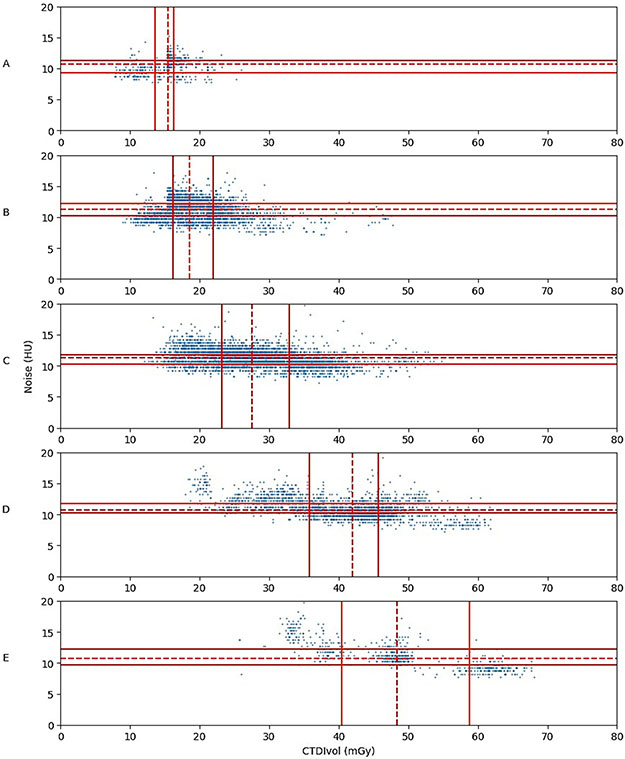
Table V presents summary statistics for dose and image noise for the examinations included in this study. The statistics are further grouped by scanner model. Table VI presents the dose and noise reference levels in this study grouped by the five different patient size categories and includes comparable reference levels from Ria et al. Table VII presents the comparable dose reference from the ACR DIR survey. Overall, the mean dose was 2.1 – 2.5 times the achievable dose (50th percentile) in the ACR DIR registry and 2.8 – 3.5 times the dose reference level of Ria et al., across the patient size groups. The image noise in this study was 25% – 37% lower than the noise reference level in the study by Ria et al. A view of the patient size, dose, and noise data is also presented in Figure 1 in the same format as in the article by Ria et al. The noise and dose data are plotted separately for five different patient size categories. The dose reference level and range changed with patient size; however, the image noise was approximately constant with a reference level of 11.25 HU and a reference range of 10.25 – 12.25 HU. The undesirable clustering of data into three clusters in Figure 1E was due to differences in dose vs. size curves and in maximum radiation output limits for different scanner manufacturers.
Table V -.
Noise and dose reference levels by scanner model
| CT Scanner Model | Noise Reference Level (HU)¥ |
Noise Reference range (HU)¥ |
Dose Ref Level (mGy)* |
Dose Reference range (mGy)* |
|---|---|---|---|---|
| All scanner models | 11.25 | 10.25 – 12.25 | 26.7 | 19.6 – 37.0 |
| GE Discovery750/Revolution HD | 11.25 | 10.25 – 12.25 | 29.0 | 21.5 – 40.6 |
| Siemens SOMATOM Force | 10.25 | 9.75 – 11.25 | 23.0 | 16.2 – 37.7 |
| Siemens SOMATOM Definition Flash | 11.75 | 10.75 – 12.75 | 22.1 | 16.5 – 28.1 |
| Siemens SOMATOM Edge Plus | 10.25 | 9.25 – 10.75 | 26.5 | 20.9 – 35.0 |
| GE Revolution CT | 10.25 | 9.75 – 11.25 | 23.1 | 19.8 – 30.7 |
Dose reference levels refer to the 50th percentile of dose data (Range, 25th-75th percentile)
Noise reference levels refer to the 50th percentile of noise data (Range, 25th-75th percentile)
Table VI -.
Noise and dose reference levels in the abdomen-pelvis by patient size groups in present study in comparison to cited study.
| Reference size range (cm) |
No. of exams |
Noise Reference Level (HU)¥ |
Noise Reference range (HU)¥ |
Dose Ref Level (mGy)* |
Dose Reference range (mGy)* |
Cited Noise Reference Level (HU)¥^ |
Cited Noise Reference range (HU)¥^ |
Cited Dose Ref Level (mGy)*^ |
Cited Dose Reference range (mGy)*^ |
|---|---|---|---|---|---|---|---|---|---|
| 21-25 | 669 | 10.75 | 9.25 – 11.25 | 15.4 | 13.6 – 16.3 | 14.3 | 13.3 – 16.7 | 5.0 | 4.4 – 5.6 |
| 25-29 | 3573 | 11.25 | 10.25 – 12.25 | 18.5 | 16.2 – 21.9 | 16.4 | 14.8 – 17.8 | 6.5 | 5.7 – 7.8 |
| 29-33 | 5431 | 11.25 | 10.25 – 11.75 | 27.6 | 23.2 – 32.9 | 16.4 | 14.6 – 18.1 | 9.2 | 7.9 – 11.1 |
| 33-37 | 2939 | 10.75 | 10.25 – 11.75 | 41.9 | 35.7 – 45.7 | 16.0 | 13.6 – 18.2 | 12.1 | 9.6 – 17.3 |
| 37-41 | 679 | 10.75 | 9.75 – 12.25 | 48.3 | 40.4 – 58.8 | 17.1 | 15.1 – 19.4 | 16.7 | 12.6 – 20.5 |
Dose reference levels refer to the 50th percentile of dose data (Range, 25th-75th percentile)
Noise reference levels refer to the 50th percentile of noise data (Range, 25th-75th percentile)
Data collated from Ria and colleagues [8]. Noise normalized to a 2.5 mm image slice thickness.
Table VII -.
ACR DIR dose reference levels
| Reference size range (cm) | No. of patients | Achieveable dose (mGy) | Diagnostic reference level (mGy) |
|---|---|---|---|
| 21-25 | 29691 | 7 | 9 |
| 25-29 | 82822 | 9 | 11 |
| 29-33 | 108921 | 12 | 15 |
| 33-37 | 76681 | 17 | 21 |
| 37-41 | 30640 | 21 | 24 |
Data collated from Kanal and colleagues [3]
Note- Size was measured as water-equivalent-diameter. Achievable dose is the 50th percentile among surveyed facilities. Diagnostic reference level is the 75th percentile among surveyed facilities.
Figure 1.
Scatter plots of image noise and dose distributions for the different patient size groups in the study subgroup analysis. A: 21-25 cm (n = 56); B: 25-29 cm (n = 238); C: 29-33 cm (n = 322); D: 33-37 cm (n = 266); E: 37-41 cm (n = 134). Solid lines indicate 25th and 75th percentiles; dashed lines, median values.
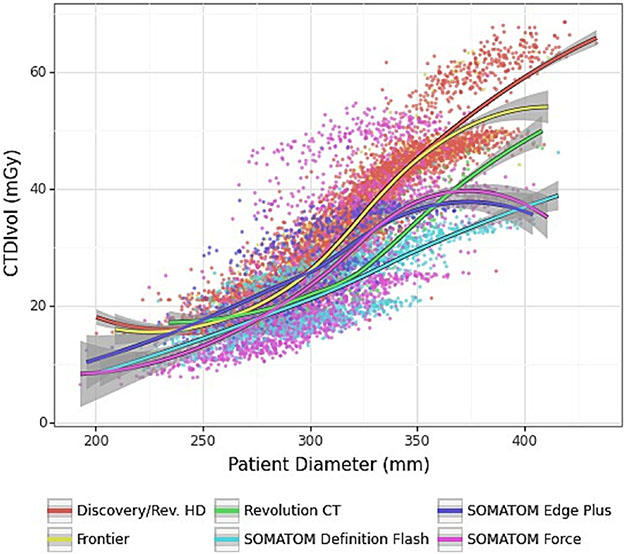
A plot of scan dose versus patient size is shown in Figure 2. As designed, the scan dose increased with patient size. The plot reveals some differences in dose across scanner models; however, at the average patient size of 30.8 cm, the mean dose for each scanner model was within 23 – 30 mGy. Greater variation was observed with large patient sizes.
Figure 2.
Plot of dose as a function of patient size. Data are grouped by scanner model. Note: 95% confidence intervals are shown in gray.
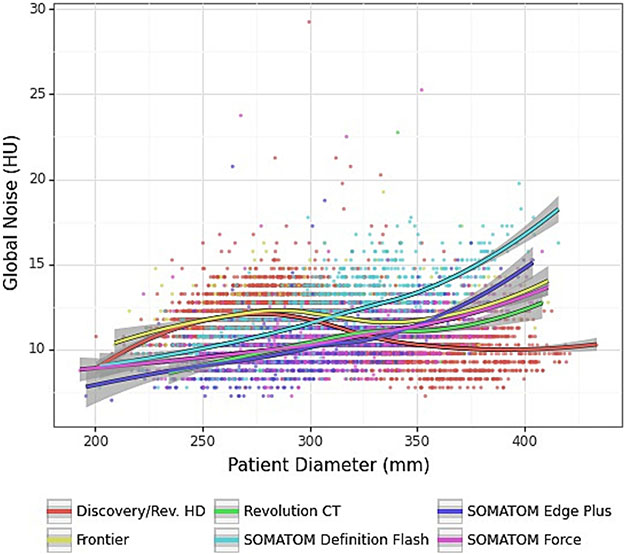
A plot of image noise versus patient size is shown in Figure 3. Over a broad range of patient sizes (24 – 37.5 cm), the mean image noise per scanner model was within 9 – 15 HU. The examinations on the Siemens scanners showed a consistent increase in noise with patient size, unlike the exams performed on the GE scanners. This difference may be attributed to the relatively moderate dose modulation used on Siemens examinations compared to GE examinations, which achieve a flatter (more constant) noise vs. size profile. The horizontal banding of the data in this plot was due to the coarse, discrete precision (0.5 HU) of the GN measurement method.
Figure 3.
Plot of image noise as a function of patient size. Data are grouped by scanner model. Note: 95% confidence intervals are shown in gray.
Two groups of outliers were observed in the data: 1) the Siemens Definition Flash model scanner had higher noise and lower dose for large patients; and 2) the GE Discovery 750 / Revolution HD scanner had higher dose and lower noise for large patients – see Table V and Fig. 3. In the case of the Definition Flash model, it was found that exam doses for large patients were being constrained by the maximum tube current. In the case of the GE Discovery750/Revolution HD model, the NI control parameter was programmed too low. After these findings, the protocol programming on these scanners were adjusted to make dose and noise across these sub-groups. The tube current limitation on the Flash scanner model was addressed by reducing the scan pitch from 0.6 to 0.5 to slow down the scan speed and achieve the required mA. These findings are an example of the utility of DRLs for practice improvement.
Discussion
Our oncology-specific reference levels for radiation doses are higher than doses listed in the ACR DIR and our reference levels for image noise are lower than those reported for general CT abdominopelvic examinations by Ria and colleagues [8]. These findings are in-line with expectations based on prior studies demonstrating the need for higher radiation doses in oncology given the often important clinical task of low contrast liver lesion evaluation [14, 19, 20]. While many findings can be made at lower radiation doses, the most difficult task of low contrast lesion evaluation becomes the limiting factor when determining appropriate radiation dose levels in oncology. Viry and colleagues reported limited task-based image quality for a 5-mm low contrast lesion phantom evaluation even when assessed with radiation doses above reported DRLs [21]. This correlates with findings by Jensen et al. demonstrating inferior low-contrast liver lesion evaluation even when performed in the 25th to 75th percentile range of radiation dose levels reported in the ACR DIR [14]. Taking these past studies, and our present result of dose and noise reference levels differing from the ACR DIR, we propose expansion of the dose index registries to collect oncology-specific dose data and to consider the addition of image noise reference levels.
Our single-center, oncology-specific data can be used to further the discussion and collection of oncologic CT reference levels. Some differences between scanner models are to be expected especially due to different image reconstruction algorithms with varying noise texture and resolution. Therefore, it is likely appropriate to set different dose and image noise levels on different scanner models. At large patient sizes, the deviation in dose across scanner models became more apparent, most likely due to differences between the vendors’ differing approaches to dose customization to the patient size. In particular, the examinations on the Siemens scanners used the ‘CarekV’ function which performs automatic tube voltage selection based on patient size. The examinations on the GE scanners did not use a similar feature.
This initial work providing single-center, oncology-specific reference levels provides practices additional granularity to aid in their CT protocol optimization. Apart from the granular noise and dose reference levels, this work also provides a novel quality control methodology. Even if a practice operates at different image quality and dose levels, the methodology presented here can be used by a practice to monitor both its image quality and radiation dose. As a tertiary referral center, we frequently review outside hospital scans that are overly noisy for oncologic evaluation thereby limiting interpretation. Reference levels such as these can be used for quality improvement and assurance. Outlier CT examinations in terms of radiation dose and/or image noise may be identified and optimization can then be undertaken such as to improve image quality in the case of low dose/high noise examinations or, conversely, examinations that exceed reference levels for dose may be lowered for patient safety to ALARA. Furthermore, if image noise appears too low or too high while doses are in an acceptable range, the degree of denoising techniques should be reassessed. As more image quality metrics are added for reference in the various registries, this process will allow for greater insights [12]. For example, a certain scanner platform may be within radiation dose and image noise reference levels, yet could be resulting in low spatial resolution compared to the remainder of the scanner fleet and/or reference registry.
There were several limitations of this study. First, this study presents the current image noise and doses in our practice and are not necessarily ideal. We have chosen one imaging examination performed using our routine imaging protocol. Our institution’s practice includes further specialized exams; for example, follow-up exams in treated leukemia, lymphoma, and testicular cancer in patients under 40 years old are indicated for a low-dose exam, due to long term follow-up which may raise radiation exposure concern. Also, some exams may be indicated primarily for complications from treatment or unrelated acute etiologies, and not solely for cancer evaluation. It should be noted, however, that while exams may be indicated for general abdominal pain, there is still a need for reassessment of metastatic burden and the differential diagnosis is often broader than in a general population. The routine protocol is still broadly indicated in our practice. We have presented the routine imaging protocol from our tertiary oncologic practice; importantly, there is quite a difference of required image quality for follow-up of a patient with treated renal cell carcinoma who is without evidence of disease for many years compared to presurgical patients in whom identification of subtle potential liver lesions is of critical importance [14, 21]. Thus, our presented data should be considered as a general reference of oncologic radiation doses and noise, but further work is needed to present cancer-specific and indication-specific reference levels across many centers. These reference levels are specific to our practice, and it is unclear how they compare to other oncology centers without more specific national registries. This work provides an initial baseline toward that objective.
Second, we chose to focus on a single image series in the examination. The examinations contain multiple acquisitions, each of which may be used to produce multiple reconstructions or multiplanar reformat series. Image series of the delayed phase, or in some cases, the pre-contrast phase, were not included in the analysis, even though these images add additional diagnostic information. Overall study quality was not confirmed by the standards of subjective assessment, and the inclusion of other phases may impact overall study quality.
Third, It should be noted that we are adopting the use of deep learning reconstructions as they become available on a few of our scanners; therefore, it is likely that many of our dose levels will be reduced in the near future [22, 23]. However, our recently published data using deep learning reconstructions suggests that dose levels must still be closely maintained for small, low contrast liver lesion evaluation [24].
Fourth, our data only represents two vendors and five scanner models, thus some caution is advised regarding extrapolation to other vendors; Vanaudenhove and colleagues demonstrated 10-20% variability between device-specific DRLs [25]. In this regard, it would be desirable to have scanner specific DRLs. A benefit of our highly standardized data is the avoidance of inter-practice variability (e.g., variable use of iterative reconstruction strengths) [6].
Fifth, the automatic global noise measurement method is limited in that it measures an index of noise in soft tissue throughout the entire image set and is insensitive to local variation of noise in the image. This is analogous to CTDI, which represents a spatially averaged absorption of radiation energy and does not account for spatial dose variations.
Finally, there are many other aspects of image quality such as IV contrast injection parameters that are not considered in this work [26-28]. For example, a practice using higher IV contrast injection rates and volumes might be able to operate at slightly lower radiation doses given improved lesion contrast and vice versa. All studied exams were intended and assumed to be during the portal venous phase in our highly standardized practice; however, the exams were not reviewed for timing accuracy. Other aspects of image quality not considered in this work are spatial resolution and noise texture, which may vary from patient-to-patient due to differences in kernels and reconstructed field-of-view
In summary, our oncology-specific adult abdominal CT dose levels are higher than in the ACR dose index registry and our oncology-specific noise levels are lower than previously reported noise levels. Some unintended differences between scanners were discovered after compiling these data, highlighting the approach as a useful practice improvement tool. To define CT reference levels for oncology, we propose the creation of an oncology-specific category withing dose index registries and consideration for the future inclusion of image quality metrics.
Highlights:
Oncology-specific adult abdominal-pelvic CT image noise reference levels are lower than previously proposed reference levels for undifferentiated abdominal CT examinations.
Oncology-specific adult abdominal-pelvic CT dose reference levels are higher than those in the ACR dose index registry for undifferentiated abdominal CT examinations.
There is a need for indication-specific, multi-center dose index and image quality reference level registries.
Registry and Funding:
Supported by institutional CCSG (cancer center support grant) from the NIH/National Cancer Institute under award number P30CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statements and Declarations: The authors report no relevant disclosures. C.T.J. and X.L. received an in-kind grant from General Electric for the evaluation of deep learning image reconstruction in CT. E.S. discloses a relationship with the following entities unrelated to the present publication: GE, Siemens, Imalogix, 12Sigma, SunNuclear, Nanox, Metis Health Analytics, Cambridge University Press, and Wiley and Sons.
References
- 1.Shrimpton PC, Jansen JT, and Harrison JD, Updated estimates of typical effective doses for common CT examinations in the UK following the 2011 national review. Br J Radiol, 2016. 89(1057): p. 20150346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morin RL, Coombs LP, and Chatfield MB, ACR Dose Index Registry. J Am Coll Radiol, 2011. 8(4): p. 288–91. [DOI] [PubMed] [Google Scholar]
- 3.Kanal KM, et al. , U.S. Diagnostic Reference Levels and Achievable Doses for 10 Adult CT Examinations. Radiology, 2017. 284(1): p. 120–133. [DOI] [PubMed] [Google Scholar]
- 4.Brat H, et al. , Local clinical diagnostic reference levels for chest and abdomen CT examinations in adults as a function of body mass index and clinical indication: a prospective multicenter study. Eur Radiol, 2019. 29(12): p. 6794–6804. [DOI] [PubMed] [Google Scholar]
- 5.Paulo G, et al. , Diagnostic Reference Levels based on clinical indications in computed tomography: a literature review. Insights Imaging, 2020. 11(1): p. 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsapaki V, et al. , CT diagnostic reference levels based on clinical indications: results of a large-scale European survey. Eur Radiol, 2021. 31(7): p. 4459–4469. [DOI] [PubMed] [Google Scholar]
- 7.Vano E, et al. , ICRP Publication 135: Diagnostic Reference Levels in Medical Imaging. Ann ICRP, 2017. 46(1): p. 1–144. [DOI] [PubMed] [Google Scholar]
- 8.Ria F, et al. , Expanding the Concept of Diagnostic Reference Levels to Noise and Dose Reference Levels in CT. AJR Am J Roentgenol, 2019. 213(4): p. 889–894. [DOI] [PubMed] [Google Scholar]
- 9.Smith TB, et al. , Variability in image quality and radiation dose within and across 97 medical facilities. J Med Imaging (Bellingham), 2021. 8(5): p. 052105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacy T, et al. , Patient-based Performance Assessment for Pediatric Abdominal CT: An Automated Monitoring System Based on Lesion Detectability and Radiation Dose. Acad Radiol, 2021. 28(2): p. 217–224. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y, et al. , Validation of algorithmic CT image quality metrics with preferences of radiologists. Med Phys, 2019. 46(11): p. 4837–4846. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Y, et al. , Correlation of Algorithmic and Visual Assessment of Lesion Detection in Clinical Images. Acad Radiol, 2020. 27(6): p. 847–855. [DOI] [PubMed] [Google Scholar]
- 13.Smith TB, Solomon J, and Samei E, Estimating detectability index in vivo: development and validation of an automated methodology. J Med Imaging (Bellingham), 2018. 5(3): p. 031403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen CT, et al. , Detection of Colorectal Hepatic Metastases Is Superior at Standard Radiation Dose CT versus Reduced Dose CT. Radiology, 2019. 290(2): p. 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCollough C, et al. , Use of Water Equivalent Diameter for Calculating Patient Size and Size-Specific Dose Estimates (SSDE) in CT: The Report of AAPM Task Group 220. AAPM Rep, 2014. 2014: p. 6–23. [PMC free article] [PubMed] [Google Scholar]
- 16.Christianson O, et al. , Automated Technique to Measure Noise in Clinical CT Examinations. AJR Am J Roentgenol, 2015. 205(1): p. W93–9. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad M, Tan D, and Marisetty S, Assessment of the global noise algorithm for automatic noise measurement in head CT examinations. Med Phys, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox J, Nonparametric regression. Appendix to: An R and S-PLUS Companion to Applied Regression. 2002: p. 1–7. [Google Scholar]
- 19.Solomon J, et al. , Effect of Radiation Dose Reduction and Reconstruction Algorithm on Image Noise, Contrast, Resolution, and Detectability of Subtle Hypoattenuating Liver Lesions at Multidetector CT: Filtered Back Projection versus a Commercial Model-based Iterative Reconstruction Algorithm. Radiology, 2017. 284(3): p. 777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher JG, et al. , Observer Performance in the Detection and Classification of Malignant Hepatic Nodules and Masses with CT Image-Space Denoising and Iterative Reconstruction. Radiology, 2015. 276(2): p. 465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viry A, et al. , Assessment of task-based image quality for abdominal CT protocols linked with national diagnostic reference levels. Eur Radiol, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen CT, et al. , Image Quality Assessment of Abdominal CT by Use of New Deep Learning Image Reconstruction: Initial Experience. AJR Am J Roentgenol, 2020. 215(1): p. 50–57. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadinejad P, et al. , CT Noise-Reduction Methods for Lower-Dose Scanning: Strengths and Weaknesses of Iterative Reconstruction Algorithms and New Techniques. Radiographics, 2021. 41(5): p. 1493–1508. [DOI] [PubMed] [Google Scholar]
- 24.Jensen CT, et al. , Reduced-Dose Deep Learning Reconstruction for Abdominal CT of Liver Metastases. Radiology, 2022: p. 211838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanaudenhove T, et al. , CT diagnostic reference levels: are they appropriately computed? Eur Radiol, 2019. 29(10): p. 5264–5271. [DOI] [PubMed] [Google Scholar]
- 26.Jensen CT, et al. , Comparison of Abdominal Computed Tomographic Enhancement and Organ Lesion Depiction Between Weight-Based Scanner Software Contrast Dosing and a Fixed-Dose Protocol in a Tertiary Care Oncologic Center. J Comput Assist Tomogr, 2019. 43(1): p. 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen CT, et al. , Delayed bolus-tracking trigger at CT correlates with cardiac dysfunction and suboptimal portovenous contrast phase. Abdom Radiol (NY), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fielding JC, et al. , Improved Computed Tomography Contrast Injection Rates Through Implantable Chest Power Ports. J Comput Assist Tomogr, 2020. 44(6): p. 911–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Einstein SA, et al. , Quantification and homogenization of image noise between two CT scanner models. J Appl Clin Med Phys, 2020. 21(1): p. 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]