Abstract
Objective:
To estimate the age-specific incidence of uterine leiomyomas identified by transvaginal ultrasound among participants in the Study of Environment, Lifestyle and Fibroids (SELF).
Methods:
SELF is a longitudinal cohort study of individuals aged 23–35 years who self-identified as Black. Participants were recruited from the Detroit, Michigan, area and underwent up to five transvaginal ultrasounds over a period of up to 10 years to identify uterine leiomyomas. We randomly imputed incidence dates between the last ultrasound date in which no leiomyomas were detected and the date of the ultrasound in which leiomyomas were first detected. We used Poisson regression to estimate age-specific incidence rates per 1,000 person-years with 95% confidence intervals (CIs). The rates were then compared with those of the Black Women’s Health Study (BWHS) and the Nurses’ Health Study II (NHS II) – two prospective cohort studies based on self-reported leiomyoma diagnoses.
Results:
In this cohort, 1,693 participants completed a baseline interview and ultrasound. We excluded 385 (22.7%) participants with a leiomyoma detected during baseline, 7 participants whose ultrasound scans were poor quality, and 60 participants with only a baseline ultrasound. Among the remaining 1241 participants, the overall incidence rate was 53.9 cases per 1,000 person-years (95% CI 48.6, 59.6). The age-specific incidence rates (cases per 1,000 person-years) were: <30 years (49.7, 95% CI 40.9, 59.9); 30–34 years (55.2, 95% CI 47.0, 64.3); and 35–39 years (58.2, 95% CI 47.3, 70.9). Among participants age <30 years, the incidence rate in SELF was more than double that of the BWHS or the NHS II.
Conclusion:
The high age-specific leiomyoma incidence rates in this prospective ultrasound-based study indicate that many young Black individuals with leiomyomas go undiagnosed. These data suggest individuals could benefit from ultrasound screening when they experience symptoms compatible with leiomyomas (e.g., heavy menstrual bleeding, anemia, pelvic pain).
Precis:
Age-specific rates of leiomyomas in pre-menopausal Black individuals significantly exceed prior estimated rates by as much as 2-fold among those under 30 years.
INTRODUCTION
Uterine leiomyomas, commonly known as leiomyomas, are benign neoplasms that can cause heavy menstrual bleeding, pelvic pain, bulk symptoms and infertility and are a leading cause of hysterectomy in the United States.1 However, there are no effective primary prevention strategies for leiomyomas.1 Black individuals are younger at diagnosis compared with White individuals and they tend to have more and larger leiomyomas when first diagnosed.2 Ultrasound is the most widely-used clinical technique for identifying leiomyomas,3 but imaging is largely only performed during pregnancy, when symptoms are present and reported to a physician or nurse, or in follow up to an abnormal bimanual exam. In a study with ultrasound leiomyoma screening of 35–49 year-olds, approximately 50% of individuals with leiomyomas reported no prior diagnosis, though some had symptoms.4 This variation in diagnosis and symptom presentation, along with the need for imaging to confirm leiomyomas, has led to the misclassification of non-cases and an underestimation of true leiomyoma incidence which is needed to understand the impact of disease on population health. If it is appreciated that leiomyomas often develop at young ages, individuals could have earlier intervention with minimally invasive treatments that could delay or eliminate the need for interventions such as hysterectomy.
Two large prospective epidemiologic cohort studies, the Nurses’ Health Study II (NHS II)5 and the Black Women’s Health Study (BWHS)6, estimated age-specific leiomyoma incidence rates. Both studies defined leiomyomas based on self-reported clinical diagnoses, documenting that self-reported leiomyoma incidence rates for Black individuals at hysterectomy were a fraction of the leiomyoma incidence rates estimated by hysterectomy or clinically indicated ultrasound. These results demonstrate the challenge of calculating age-specific incidence rates when the entire population is not screened with imaging.
The purpose of the present study was to estimate the age-specific leiomyoma incidence rates for up to 10 years of follow-up in the first longitudinal study with repeated transvaginal ultrasound screenings of a closed cohort of participants: the Study of Environment, Lifestyle and Fibroids (SELF). Participants self-identified as Black and were ages 23–35 years at time of enrollment (2010–2012). Participants were screened with ultrasound for the detection of leiomyomas at baseline and at four follow-up visits during 2010–2021. To understand how screening with ultrasound versus self-reported age at diagnosis may impact incidence rates, we compared the age-specific uterine leiomyoma incidence rates from SELF with those rates previously reported in the NHS II5 and BWHS6.
METHODS
The Study of Environment, Lifestyle and Fibroids (SELF) is an ongoing longitudinal cohort study specifically designed to study incidence and growth of uterine leiomyomas with standardized ultrasound assessments.7,8 Briefly, in 2010–2012, we enrolled 1,693 individuals who self-identified as Black or African American, were ages 23–35 years, had a uterus and did not report a prior diagnosis of uterine leiomyomas. We have previously published details of participant recruitment; briefly, we mailed letters to Henry Ford Health patients who were ages 23–34 years to invite study participation and patients 35–65 to ask them to share study information with those who may be eligible. Media advertisements and information booths at community events were also used to recruit possible participants. Participants were provided stipends for research activities. We did not collect data on sex assigned at birth or gender identity. Participants signed written informed consent for all research activities approved by the Institutional Review Boards (IRB) at Henry Ford Health (HFH) (HFH IRB), the National Institute of Environmental Health Sciences (NIEHS) (NIEHS IRB) and Boston University Medical Campus (BUMC IRB). Participants completed baseline visits and were asked to return at intervals to complete four follow-up clinic visits at HFH clinic locations in Detroit (median time between baseline and first follow-up visit = 1.6 years, median time between follow-up visit 1 and follow-up visit 2 = 1.6 years, median time between follow-up visit 2 and follow-up visit 3 = 1.6 years; and median time between follow-up visit 3 and follow-up visit 4 = 2.6 years).
At each study-specific clinic visit (baseline and four follow-up visits), participants completed self-administered questionnaires and telephone interviews about their behaviors and health history, including any uterine surgeries in which uterine leiomyomas could be detected or treated, such as hysterectomy and myomectomy. The ultrasound team performed a transvaginal ultrasound and participants provided blood and urine specimens and vaginal swabs and had their height and weight measured. Some participants were pregnant at the time of a scheduled study visit (<4% in each follow-up) and study visits were delayed for these participants until 3–6 months postpartum, when post-partum uterine changes would not interfere with ultrasound imaging.
In this cohort, 1,693 participants enrolled in the study and had a baseline interview and ultrasound. The 385 (22.7%) participants who had a leiomyoma detected during the baseline ultrasound were excluded from these analyses of incident leiomyomas and their age distribution is presented in Appendix 1, available online at http://links.lww.com/xxx. We further excluded 7 participants whose ultrasound scans were of poor quality and 60 participants who only had a baseline and no additional ultrasounds. We analyzed incidence in the remaining 1241 participants (Figure 1).
Figure 1.
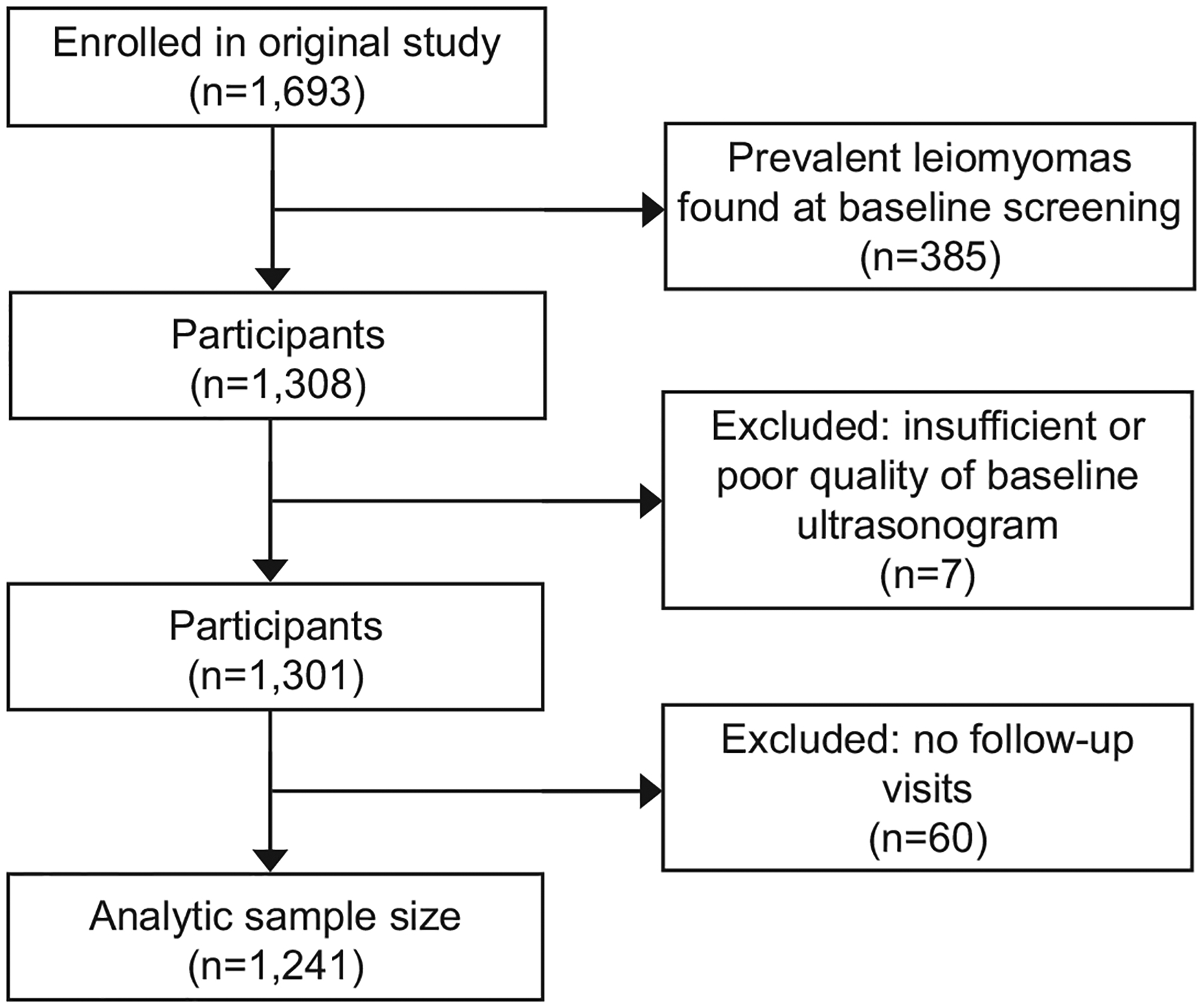
Study participant flow chart.
The details of the ultrasound procedures have been described previously.7,8 HFHS clinical staff performed all ultrasounds transvaginally, unless an additional abdominal ultrasound was needed for a complete assessment.8 A fixed cadre of experienced ultrasonographers (≥3 years of experience in gynecologic ultrasound) were trained to ensure high data quality. Study-specific training included an emphasis on distinguishing leiomyomas from other pathologic changes in the uterus (e.g., polyps).
We did not schedule ultrasounds to correspond with any phase of the menstrual cycle, and we asked participants to empty their bladder prior to imaging. We used a standardized data collection form and asked sonographers to map and number all leiomyomas on a diagram. Sonographers identified each leiomyoma ≥0.5 cm diameter and characterized the 6 largest leiomyomas in terms of size and location. The sonographers placed the ultrasound calipers from outer border to outer border to assess each diameter, which were measured in three perpendicular planes (longitudinal, anterior-posterior, transverse), and the sonographer repeated three separate passes through the uterus. They recorded any leiomyoma-like echo pattern that could not be visualized in all three planes as a “questionable fibroid” (referred to in the remainder of this article as “questionable leiomyoma”). Additional imaging was not performed.9,10
Together, each study sonographer and the lead sonographer (TC) reviewed the first 10 ultrasound examinations performed by each of the study sonographers. The lead sonographer provided feedback to the sonographers at that time and throughout the baseline visit and three follow-up visits as the lead sonographer reviewed 8% of all sonograms performed by each sonographer each month, with overrepresentation of sonograms showing leiomyomas. We did not record the agreement of each review (e.g., agreement, recommended revision, etc.). The lead sonographer reviewed all questionable leiomyomas. After this additional review, there were three participants with a questionable leiomyoma that were considered incident leiomyoma cases.
For participants reporting any treatment of their uterus during the study, we requested procedure details and medical records from the facility where they received the treatment. We reviewed the medical records for the presence of leiomyomas, including procedure notes and any pathology reports, and recorded the date of the procedure. For the one participant who had not attended all follow-up visits and a leiomyoma first identified by medical records rather than ultrasound, we considered the date of surgery the date of first leiomyoma detection.
We calculated person-years at risk from the start of follow-up (2010–2012) until the ultrasound detection of incident leiomyomas or a censoring event (i.e., hysterectomy without leiomyomas or loss to follow-up), whichever came first. Because leiomyomas were observed at irregular intervals (study visits), a random date imputation method was used rather than mid-point imputation, which has been shown to create biased estimates when participants begin to miss study visits, which is expected in a longitudinal cohort study.11 We randomly imputed incidence dates between the last ultrasound date in which no leiomyomas were detected and the date of the ultrasound in which leiomyomas were first detected. Person time was right-censored at either the date of the last ultrasound (if leiomyomas were not present) or the imputed date of leiomyoma detection. We then calculated age-specific incidence rates, per 1,000 person-years, as the number of participants with incident leiomyomas detected divided by the total person-time at risk in each age category (<30, 30–34, 35–39 years). We used Poisson regression to estimate age-group-specific incidence rates and 95% confidence intervals (CIs). Given the enrollment ages, few participants reached age 44 by end of follow-up, and only 188 person years were accrued during person ages 40–44 years. Therefore, we do not present the incidence rate for this age group.
We compared the age-specific incidence rates in SELF to those reported in NHS II and BWHS, both of which relied on self-reported clinical diagnosis. NHS II used data from 1,309 premenopausal Black women with intact uteri to estimate age-specific leiomyoma incidence during a 4-year period in which 140 incident cases were reported and confirmed.5 The BWHS is a prospective cohort study that examined incidence rates for self-reported leiomyoma diagnoses over a period of four years (1997–2001) among women who identified as “Black” or “African American”, were premenopausal, had intact uteri, and were 23–69 years of age at the start of follow up.6 In the BWHS, there were 76,711 woman-years of follow-up and 2,637 incident cases of leiomyomas reported by 22,895 participants.
RESULTS
At enrollment, the distribution of the participants was fairly evenly distributed across the age intervals (Table 1). A quarter of the participants had earned at least a college degree and most other participants had at least some college education. Almost half the participants had a total household income that was less than $20,000. While 21% of the participants had a body mass index (BMI) of 25–29 kg/m2, 58.9% had a BMI ≥30 kg/m2. Nearly three-quarters of the participants had never smoked. Approximately one-third (36.1%) of participants were nulliparous and 26.9% of those with at least one prior livebirth breastfed their children more than 6 months (cumulative across all their children). Among the parous participants, approximately one quarter of the participants had delivered in the two years prior to enrollment. Almost two-thirds of the participants reported menarche at ages 11–13 years with 17.3% of the participants reporting age at menarche as 10 years or younger. During the baseline interview, 11.7% of the participants were using oral contraceptives and 6.5% were using depot-medroxyprogesterone acetate. The participants enrolled in SELF were ages 33–45 years at the time of their most recent follow-up visit.
Table 1.
Descriptive information at the baseline clinic visit among 1,241 reproductive-aged Black participants in the Study Environment Lifestyle and Fibroids (SELF)
| Number (%) | |
|---|---|
| Sociodemographic variables | |
| Age (years) | |
| 23–25 | 315 (25.4) |
| 26–28 | 321 (25.9) |
| 29–31 | 327 (26.3) |
| 32–35 | 278 (22.4) |
| Education level * | |
| ≤High school/GED (12 years) | 286 (23.1) |
| Some college (13–15 years) | 638 (51.4) |
| ≥Bachelor’s degree (16 years) | 316 (25.5) |
| Annual household income† | |
| <$20,000 | 568 (46.2) |
| $20,000-$50,000 | 474 (38.5) |
| >$50,000 | 188 (15.3) |
| Anthropometric and lifestyle variables | |
| Body mass index (kg/m2) | |
| <25 | 249 (20.1) |
| 25–29 | 261 (21.0) |
| 30–34 | 236 (19.0) |
| 35–39 | 205 (16.5) |
| ≥40 | 290 (23.4) |
| Cigarette smoking | |
| Never | 908 (73.2) |
| Past | 91 (7.3) |
| Current <10 cigarettes/day | 185 (14.9) |
| Current ≥10 cigarettes/day | 57 (4.6) |
| Reproductive variables | |
| Parity (births) | |
| Nulliparous | 448 (36.1) |
| 1 | 331 (26.7) |
| 2 | 233 (18.8) |
| ≥3 | 229 (18.4) |
| Breastfeeding duration (months)‡ | |
| ≤6 | 580 (73.1) |
| >6 | 213 (26.9) |
| Time since last birth (years)‡ | |
| <2 | 209 (26.3) |
| 2–4 | 271 (34.2) |
| 5–9 | 229 (28.9) |
| ≥10 | 84 (10.6) |
| Age at menarche (years) | |
| ≤10 | 215 (17.3) |
| 11 | 255 (20.6) |
| 12 | 343 (27.6) |
| 13 | 203 (16.4) |
| ≥14 | 225 (18.1) |
| Current oral contraceptive use | |
| No | 1096 (88.3) |
| Yes | 145 (11.7) |
| Current DMPA use | |
| No | 1160 (93.5) |
| Yes | 81 (6.5) |
| Typical cycle length (days)║ | |
| <25 | 254 (20.7) |
| 25–27 | 158 (12.9) |
| 28–31 | 561 (45.7) |
| ≥32 | 98 (8.0) |
| Cycles too irregular to say | 57 (4.6) |
| No period within past year | 100 (8.1) |
DMPA = Depot medroxyprogesterone acetate.
n=1 missing education level;
n=11 missing income level;;
Among parous individuals, lifetime cumulative;
n=13 missing cycle length information
The 1,241 participants eligible for incidence analyses contributed 7,038 person-years to the analyses. There were 379 incident cases of leiomyomas of which 378 were identified through study ultrasound and one was identified through medical records; 371 of the 379 incident cases were in participants aged <40 years. (Appendix 2, available online at http://links.lww.com/xxx). The overall incidence rate was 53.9 cases per 1,000 person-years (95% CI 48.6, 59.6), or an average risk of 5.4% per year (95% CI 4.9, 6.0). The age-specific incidence rates (cases per 1,000 person-years) were: <30 years (49.7, 95% CI 40.9, 59.9); 30–34 years (55.2, 95% CI 47.0, 64.3); and 35–39 years (58.2, 95% CI 47.3, 70.9) (Table 2).
Table 2.
Age-specific incidence rates among 1,241 reproductive-aged Black participants in the Study Environment Lifestyle and Fibroids (SELF) using random date imputation
| Age | Person-years of follow-up | Number of Incident Leiomyomas | Age-specific Incidence Rate* (IR), (95% CI) |
|---|---|---|---|
| < 30 | 2212 | 110 | 49.7 (40.9, 59.9) |
| 30 – 34 | 2937 | 162 | 55.2 (47.0, 64.3) |
| 35 – 39 | 1701 | 99 | 58.2 (47.3, 70.9) |
Incidence rate defined as number of new cases per 1,000 person-years
Figure 2 presents a comparison of incidence rates between the SELF, BWHS and NHS II cohorts. In NHS II, overall, there were 140 cases confirmed by ultrasound or hysterectomy (30.6 cases per 1,000 person years, 95% CI 25.5, 35.7). The age-specific incidence rates of self-reported cases diagnosed via ultrasound or hysterectomy per 1,000 person years were the following for Black or African American women (n=1309): 26–29 years: 5.6; 30–34 years: 23.3; 35–39 years: 38.1; and 40–44 years: 34.5. In the BWHS, when the incident leiomyoma was confirmed by ultrasound or hysterectomy, the incidence age-specific rates (cases per 1,000 person-years) were: <30 years 17.8 (95% CI 16.0–19.8); 30–34 years, 28.2 (95% CI 25.9–30.7); 35–39 years, 34.6 (95% CI 31.9–37.5); 40–44 years, 39.8 (95% CI 36.5–43.4); and, 45–49 years, 35.8 (95% CI 31.6–40.5).6 Among participants younger than age 30 years, the incidence rate in SELF was more than double that of either the BWHS or the NHS II.
Figure 2.
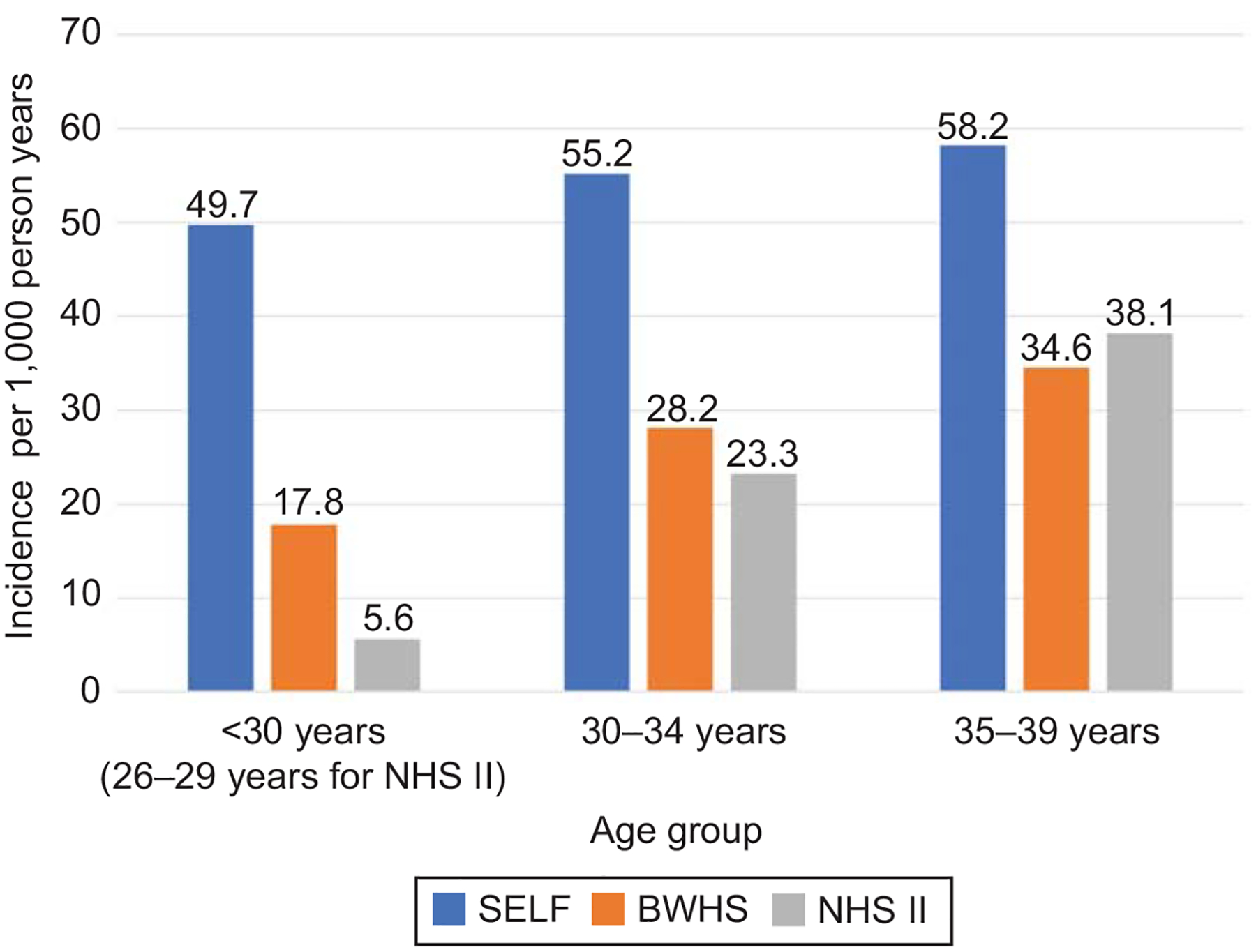
Incidence rate of leiomyoma cases identified by ultrasonography or hysterectomy per 1,000 person years. Study of Environment, Lifestyle and Fibroids (SELF) is the only study that screened all participants for leiomyomas with ultrasonography. BWHS, Black Women’s Health Study; NHS II, Nurses’ Health Study II.
DISCUSSION
In this prospective cohort study of premenopausal individuals who identify as Black or African American, the age-specific rates exceeded prior estimates reported in epidemiologic cohort studies that did not systematically screen all participants with ultrasound. For example, the overall age-standardized incidence rate was 30.6 per 1,000 person years in NHS II, similar to the BWHS estimate of 34.4 (95% CI 33.1–35.7) cases per 1,000 person years,6 but less than the 53.9 (95% CI 48.6. 59.6) cases per 1,000 person years in SELF. Prospective studies that directly query participants about clinical diagnoses of leiomyomas underestimate incidence rates. However, this underestimation based on self-report is less than underestimation of incidence in studies that only utilize medical record or claims data.6
The results from our prospective study of ultrasound imaging verify that leiomyoma incidence begins at a young age in Black individuals. This is consistent with prior reports using cross-sectional ultrasound data and statistical modelling to estimate age-specific cumulative incidence of leiomyomas. Those studies suggested that leiomyoma onset begins about a decade earlier for young Black individuals compared with White individuals.12 The incidence rate of 50 cases per 1,000 person-years for SELF participants in their 20s translates to a cumulative incidence of 30% by age 30 years and aligns with the early onset that was suggested by the prior work. Such age-specific data to verify modelled estimates for White individuals and those of other racial/ethnic groups are not yet available.
The young age of onset means that Black individuals have more years for premenopausal hormones to drive leiomyoma growth compared with White individuals. Those with the highest risk of developing major symptoms are probably those with the earliest onset. Screening ultrasounds at age 30 for individuals with leiomyoma symptoms (heavy menstrual bleeding, anemia, pelvic pain), followed by growth-limiting treatments for those with leiomyomas, could substantially reduce their high health burden from leiomyomas.
The results from our study also highlight the challenges of using data without widespread ultrasound screening to confirm leiomyoma status. In their study of medical record data and diagnoses codes from Kaiser Permanente in Washington, Yu et al. reported incidence rates for leiomyoma diagnoses were highest for the age group 45–49 years in 2014 with 24.0 cases per 1,000 person-years.13 They also reported that annual overall incidence rates (cases per 1,000 person-years) declined over time from 13.9 in 2005 to 10.14 in 2014. The overall incidence rates are substantially lower than those reported in SELF (53.9 cases per 1,000 person-years). The large difference in incidence rates between SELF and claims data suggest that claims data may better support studies of treatment frequency or relative treatment effectiveness rather than overall disease incidence. Furthermore, these differences between incidence rates defined by ultrasound and claims data suggest a need to better understand the way that individuals experience their leiomyomas and their treatment seeking behaviors, especially factors that could affect their ability to access care. It is critical to understand who receives a diagnosis and treatment and how they differ from those who are not diagnosed or treated.
The limitations of this work include the length of time between ultrasounds which averaged two years. Because an incident leiomyoma might have been detectable after only a few months, a shorter time between intervals would likely have resulted in slightly increased incidence estimates. However, the cost of conducting more ultrasounds within shorter intervals would have made the cost of the study prohibitive and increased participant burden. The participants in SELF are also all from one area of the country (southeast Michigan), which may limit generalizability to other Black individuals if leiomyoma risk is associated with geographically specific environmental risk factors.
The prospective design and leiomyoma screening by ultrasound are unique and key strengths of this work, thereby allowing us to document a greater frequency of uterine leiomyomas in young Black individuals when compared with prior cohort studies. Thus, ultrasound studies are useful for advancing our knowledge about the true incidence and etiology of leiomyomas. The high retention rate of this study (>80% over 10 years) is another strength.
In conclusion, this work highlights leiomyomas are a commonly detected neoplasm in Black individuals of reproductive age. Furthermore, our results, in comparison with prior epidemiologic studies, demonstrate the importance of imaging to identify both leiomyomas requiring surgical intervention as well as those for which individuals have not yet received a diagnosis. Knowledge of leiomyoma incidence rates can improve scientific understanding of the true prevalence of disease. The results from this study of Black individuals can also raise awareness of the elevated risk for young individuals who may benefit from ultrasound assessment when symptoms (heavy menstrual bleeding, anemia, pelvic pain) are compatible with leiomyomas as part of their clinical care. With continued follow-up of the SELF cohort, we will further assess leiomyomas in terms of their characteristics (growth, size, number, location), symptom burden, and their impact on quality of life.
Supplementary Material
Acknowledgement:
The authors thank Dr. Lynn Marshall for providing data from the Nurses’ Health Study II.
Funding:
This work was funded by R01 ES028235. This work was also supported, in part, by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences and funds from the American Recovery and Reinvestment Act funds designated for National Institute of Health research.
Footnotes
Financial Disclosure: Erica E. Marsh is a consultant for Myovant Sciences and Pzifer Inc. Lauren A. Wise is a consultant for AbbVie Inc. The other authors did not report any potential conflicts of interest.
References
- 1.Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG 2017;124:1501–12. doi: 10.1111/1471-0528.14640 [DOI] [PubMed] [Google Scholar]
- 2.Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med 1996;41:483–90. [PubMed] [Google Scholar]
- 3.Hurley V. Imaging techniques for fibroid detection. Baillieres Clin Obstet Gynaecol 1998;12:213–24. doi: 10.1016/s0950-3552(98)80062-x [DOI] [PubMed] [Google Scholar]
- 4.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–7. doi: 10.1067/mob.2003.99 [DOI] [PubMed] [Google Scholar]
- 5.Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 1997;90:967–73. doi: 10.1016/s0029-7844(97)00534-6 [DOI] [PubMed] [Google Scholar]
- 6.Wise LA, Palmer JR, Stewart EA, Rosenberg L. Age-specific incidence rates for self-reported uterine leiomyomata in the Black Women’s Health Study. Obstet Gynecol 2005;105:563–8. doi: 10.1097/01.AOG.0000154161.03418.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baird DD, Harmon QE, Upson K, et al. A Prospective, Ultrasound-Based Study to Evaluate Risk Factors for Uterine Fibroid Incidence and Growth: Methods and Results of Recruitment. J Womens Health (Larchmt) 2015;24:907–15. doi: 10.1089/jwh.2015.5277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baird DD, Patchel SA, Saldana TM, et al. Uterine fibroid incidence and growth in an ultrasound-based, prospective study of young African-Americans. Am J Obstet Gynecol 2020. doi: 10.1016/j.ajog.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of O, Gynecologists’ Committee on Practice B-G. Management of Symptomatic Uterine Leiomyomas: ACOG Practice Bulletin, Number 228. Obstet Gynecol 2021;137:e100–e15. [DOI] [PubMed] [Google Scholar]
- 10.Committee on Practice B-G. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol 2012;120:197–206. [DOI] [PubMed] [Google Scholar]
- 11.Vandormael A, Dobra A, Barnighausen T, de Oliveira T, Tanser F. Incidence rate estimation, periodic testing and the limitations of the mid-point imputation approach. Int J Epidemiol 2018;47:236–45. doi: 10.1093/ije/dyx134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laughlin SK, Schroeder JC, Baird DD. New directions in the epidemiology of uterine fibroids. Semin Reprod Med 2010;28:204–17. doi: 10.1055/s-0030-1251477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu O, Scholes D, Schulze-Rath R, Grafton J, Hansen K, Reed SD. A US population-based study of uterine fibroid diagnosis incidence, trends, and prevalence: 2005 through 2014. Am J Obstet Gynecol 2018;219:591 e1–e8. doi: 10.1016/j.ajog.2018.09.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


