Abstract
Traumatic brain injury is one of the main causes of death and disability worldwide, and results in multi-system complications. However, the mechanism of mild traumatic brain injury (MTBI) on lung injury remains unclear. In this study, we used a murine model of MTBI and pneumonia (Pseudomonas aeruginosa; PA) to explore the relationship between these conditions and the underlying mechanism.
Methods:
Mice (n=104) were divided into Control, MTBI, Pneumonia, and MTBI + Pneumonia groups. MTBI was induced by weight-drop method. Pneumonia was induced by intratracheal injection with PA Xen5 strain. Animals were sacrificed 24 hours after bacterial challenging. Histological, cellular, and molecular indices of brain and lung injury were assessed using various methods.
Results:
Mice in both MTBI and Pneumonia groups had more FJC-positive neurons than controls (P<0.01), but mice in the MTBI + Pneumonia group had fewer FJC-positive cells than the Pneumonia group (P<0.01). The MTBI + Pneumonia mice showed decreased bacterial load (P<0.05), reduced lung injury score and pulmonary permeability (P<0.01), less inflammatory cells, and lower levels of proinflammatory cytokines (tumor necrosis factor [TNF]-α and interleukin [IL]-1β) (P<0.01) when compared to the Pneumonia group. Molecular analysis indicated lower levels of phosphorylated NF-κB (p-NF-κB) in the lung of MTBI + Pneumonia mice compared with pneumonia group (P<0.01). Furthermore, alveolar macrophages from MTBI mice exhibited enhanced bactericidal capacity compared to those from controls (P<0.01). Moreover, MTBI + Pneumonia mice exhibited less CD86-positive M1 macrophages than the Pneumonia group (P<0.01).
Conclusions:
MTBI attenuates pneumonia-induced ALI through the modulation of alveolar macrophage bactericidal capacity and M1 polarization in bacterial pneumonia model.
Keywords: Alveolar macrophage, Brain injury, Lung injury, M1 polarization, Bacterial Pneumonia
Introduction
Acute respiratory distress syndrome (ARDS) involves diffuse inflammatory acute lung injury (ALI) that leads to decreased lung tissue ventilation, increased pulmonary vascular permeability, and decreased lung function (1). The incidence of ARDS is about 86.2 per 100,000 person-years, with pneumonia being the most common trigger (2). Although there are many treatments for ARDS, such as mechanical ventilation, prone position ventilation, manual lung inflation and nitric oxide inhalation, the mortality rate of patients remains at around 30% (3). This study therefore attempts to identify a more effective treatment for ALI/ARDS.
Traumatic brain injury (TBI) is a serious global issue that results in about 2.5 million emergency hospital visits every year (4). More than 90% of TBI cases are classified as mild traumatic brain injury (MTBI) (5). MTBI is especially common in the context of sports and military activities (3). Athletes using bicycles or participating in contact sports frequently suffer from MTBI (6). A survey of 3,952 US Army soldiers returning from Iraq showed that 14.9% of them had experienced MTBI (7).
Moderate and severe brain injury have been shown to be associated with ALI/ARDS (8). Interestingly, previous studies have demonstrated that MTBI alleviates lung injury(9, 10). Understanding the underlying mechanism why MTBI exerts protective effects on ALI/ARDS may provide novel perspectives for the treatment of ALI/ARDS, especially in veterans and athletes. Pseudomonas aeruginosa (PA), a Gram-negative bacterium, is one common causes of nosocomial pneumonia, especially in cases of brain injury-associated ventilator-associated pneumonia (VAP) (11, 12). In this study, we hypothesized that MTBI plays a role in modulating PA-induced ARDS/ALI. We have used the weight-drop method and PA pneumonia to induce MTBI and ALI/ARDS, respectively. We then studied the histological, cellular and molecular mechanisms of MTBI on ALI/ARDS; we found that MTBI attenuates pneumonia-induced lung inflammation and ALI by modulating alveolar macrophage bactericidal capacity and M1 polarization in bacterial pneumonia model.
MATERIALS AND METHODS
Animals
Original FVB/N mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Adequate food and water were supplied throughout the entire study. Mice were maintained on a diurnal 12-hour light/dark cycle under controlled temperature and humidity conditions. All animals were housed at the Animal Core Facility of SUNY Upstate Medical University under pathogen-free conditions. The mice were 8–12 weeks old and weighed about 25 g. All the related animal protocols were strictly adhered to according to ARRRIVE guidelines and approved by the Institutional Animal Care and Use Committee of SUNY Upstate Medical University (IACUC #380). All mice (n=104) including both male and female were assigned randomly to four groups: Control, Brain injury, Pneumonia, and MTBI + Pneumonia groups.
Mild brain injury and pneumonia model
Mild brain injury was induced via weight-drop method and the mice were then intratracheally injected with PA Xen5 to induce pneumonia. The mice were anesthetized by intraperitoneal injection of ketamine/xylazine (90 mg/kg ketamine, 10 mg/kg xylazine). Then, the mice were placed prone on a foam bed and fitted with a steel helmet. This helmet is a metal disk that is used to prevent skull fracture when the mice are hit. An 82-g rod was dropped from a height of 10 cm through a vertical pipe onto the helmet (13). In our pilot experiments, we did not find significant changes of brain in the histopathologic H/E staining as well as by Evan blue test (Data not shown) in this mild model. Thirty minutes later, one 0.5-cm incision was made to expose the trachea and 50 μl of bioluminescent PA Xen5 solution (~2 × 104 colony forming units [CFU]/mouse) was injected into the trachea. In this study, 2 × 104 CFU/mouse of PA Xen5 was deemed appropriate for in vivo imaging analysis and about 50% of mice were still alive 72 h after bacterial challenge (14).
In vivo imaging analysis
PA Xen5 produces bioluminescence signals that can be detected in vivo and is positively associated with the bacterial load as described previously (14). Bioluminescence was assessed at 24 hours after bacterial challenge to determine bacterial quantity using an in vivo imaging system (IVIS-200; Caliper Life Science, Hopkinton, MA, USA), as described previously (14). A cooled charge-coupled device (CCD) camera (Xenogen-200; Caliper Life Sciences) was used to take photographs. Mice were anesthetized with isoflurane (2% in oxygen) in the induction chamber and then transferred into the imaging chamber of the IVIS-200 with continuous isoflurane anesthesia. The bioluminescent signals were spatially localized by merging pseudo-colored images of photon emissions and gray scale images of the mice.
Specimen collection
Lung tissues and bronchoalveolar lavage fluid (BALF) were collected as described previously (14). To collect the specimens, mice were anesthetized by intraperitoneal injection of the ketamine/xylazine cocktail described above, and then exsanguinated to collect or lavage lungs. BALF was obtained by lavaging the lung three times with 0.5 ml saline.
Histopathological analysis
Lung tissues were fixed with 10% formalin for at least 24 h and embedded in paraffin. 5μm lung sections were prepared for hematoxylin-eosin (HE) staining using standard procedures. Two investigators then examined multiple randomly chosen microscopic fields from 5 mice in each group, in a blinded manner. Twenty high power fields (HPF) were chosen for each slide. A lung injury scoring system was used to estimate the severity of lung injury (15).
Fluoro-Jade C (FJC) staining
FJC staining was performed using the Fluoro-Jade C Staining Kit (Biosensis, Thebarton, Australia) in accordance with the manufacturer’s instructions. Paraffin-embedded brain tissue sections were dewaxed and rehydrated by xylene and graded alcohol solutions. The sections were then incubated in 0.06% KMnO4 for 10 minutes, transferred into 0.0001% FJC in 0.1% acetic for a further 10 minutes, washed, dried, and mounted using DXP. Sections from 5 mice in each group were observed using the TE2000-U microscope (Nikon, Tokyo, Japan) and the number of FJC-positive neuron axons from 20 HPF were analyzed statistically among groups.
Evans blue dye
To evaluate the pulmonary permeability, the extravasation of Evans blue (EB) dye was assessed as described previously (16). EB dye binds with albumin and other plasma proteins immediately after injection into the vein and serves as a marker for plasmexhidrosis. EB dye (2% in phosphate-buffered saline [PBS]; Sigma, St. Louis, MO, USA) was injected into the mice through the tail vein (4 ml/kg). One and a half hours after injection, mice were exsanguinated and perfused through the left ventricle with 30 ml of PBS. The lungs from 5 or 6 mice in each group were collected for analysis. To evaluate EB dye extravasation, each lung was homogenized with 1 mL of formamide. The solutions were incubated at 55°C for 24 h. The homogenates were centrifuged at 21,130 x g for 25 min, and the supernatants were transferred into a new tube and detected spectrophotometrically at OD 620 nm.
Inflammatory cells in BALF
To assess the inflammatory response within the lungs, we quantified macrophages and neutrophils in BALF as described previously (14). Five or six mice from each group were killed at 24 hours after bacterial challenge and the tracheas were cannulated; 0.5 mL of saline was injected into the lungs, aspirated, and collected. Alveolar lavage was repeated three times and about 1.2 ml of BALF was collected. Cell pellets were collected by centrifugation and cells were sedimented by cyto-spin onto glass slides. The cells were stained using a Hema-3 staining kit (Thermo Fisher Scientific, Waltham, MA). Cells on the slides were identified and quantified using the TE2000-U microscope (Nikon).
Concentrations of cytokines in lung tissues
Proinflammatory mediators reflect the intensity of pulmonary inflammation. As shown in our previous publication (14), lung tissues from 5 mice in each group were homogenized to measure the concentrations of interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α using enzyme-linked immunosorbent assay (ELISA) kits following the manufacturer’s instructions (Super Array Bioscience, Frederick, MD, USA).
Western blotting analysis
The transcription factors of the nuclear factor (NF)-κB family play an important role in inflammatory responses. NF-κB is found in the cytoplasm, but is activated by phosphorylation in the nucleus to regulate inflammatory (and other) responses. We assessed the expression of phosphorylated NF-κB (p-NF-κB) by Western blotting as described previously (14). Lung tissues from 5 mice in each group were ground into tissue homogenates with phosphatase inhibitor and protease. Homogenates were centrifuged at 13,000 x g for 10 min to collect supernatants. The protein concentrations of the supernatants were determined using Micro-BCA protein assay kits (Thermo Scientific, Rockford, IL, USA). A total of 50 μg of protein was loaded onto reducing 12% sodium dodecyl sulfate-polyacrylamide gel for electrophoresis and then transferred onto a PVDF membrane (Bio-Rad, Hercules, CA, USA). The membrane was blocked in 3% skim milk in Tris-buffered saline, incubated with a primary antibody against p-NF-κB p65 (#3033; Cell Signaling Technology, Danvers, MA, USA), and subsequently incubated with a secondary HRP-conjugated antibody (Bio-Rad). The bands were detected using Pierce ECL Western Blotting detection reagent (Thermo Scientific) and developed by X-film. The membrane was stripped and incubated with β-actin antibody (ab16039; Abcam, Cambridge, UK). The bands on the films were analyzed using ImageJ software (version 1.48; NIH, Bethesda, MD, USA).
Macrophage killing of bacteria
Alveolar macrophages recognize and eliminate pathogens in airways. To evaluate the bactericidal capacity of alveolar macrophages, bacteria were phagocytosed and, after a certain period, the residual bacteria were sprayed onto agar plates. The bacterial colonies were counted after incubation (17). Thirty minutes after challenge with weight drop, the macrophages of 5 mice from the Brain injury group were collected. Macrophages from the Brain injury and Control groups were co-cultured with PA Xen5 for 30 min; the ratio of macrophages to PA Xen5 was 1:10. The PA Xen5 on the surface of the macrophages was killed and removed using gentamicin (50 μg/mL). The gentamicin was washed off using saline, and macrophages containing PA were resuspended in distilled water to release the swallowed bacteria. Distilled water containing the released bacteria was sprayed onto agar plates. The plates were incubated at 37°C overnight to count the bacterial colonies.
Immunofluorescence analysis
Immunofluorescence analysis of specific M1 macrophage biomarker CD86 was performed as described previously (14). In brief, paraffin-embedded sections with lung tissues were deparaffinized with xylene and graded ethanol after incubation at 60°C for 1 hour. Antigen were retrieved over 10 minutes with boiling sodium citrate buffer (pH 6.0). Sections were permeabilized with 0.2% Triton X-100 and then blocked with 10% donkey serum (ab7475; Abcam). Sections from 5 mice in each group were incubated with CD86 antibodies (14086282; eBioscience, San Diego, CA, USA) or CD16 antibodies (sc-53376, Satnta Cruz biotech. Dallas, Texas), and then incubated with secondary antibodies (ab150073; Abcam). The nuclei were counterstained with DAPI (ab104139; Abcam). Sections were examined using the TE2000-U microscope (Nikon).
Statistical analysis
All data are expressed as mean ± SEM. The normality of the data was first examined using Kolmogorov-Simirnov test. Statistical analyses including one-way analysis of variance (ANOVA) followed by the Student-Newman-Keul test or student t test for one pair were performed using SPSS software (ver. 20.0; SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
RESULTS
Reduced brain injury in MTBI + pneumonia mice compared to either MTBI or pneumonia mice
FJC, a polyanionic fluorescein, was used to examine neuronal changes/injury in MTBI (18). FJC-positive myelin/cells were found in both the Brain injury and Pneumonia groups (Fig. 1a–d). However, the MTBI + Pneumonia group had significantly fewer positive myelin/cells (Fig. 1e, P<0.01). Hence, the combined effects of MTBI and pneumonia seems to reduce the brain damage and injury compared to only either MTBI or PA pneumonia.
Figure 1. Mild traumatic brain injury (MTBI) attenuated neuronal changes caused by pneumonia of Pseudomonas aeruginosa (PA) Xen5.
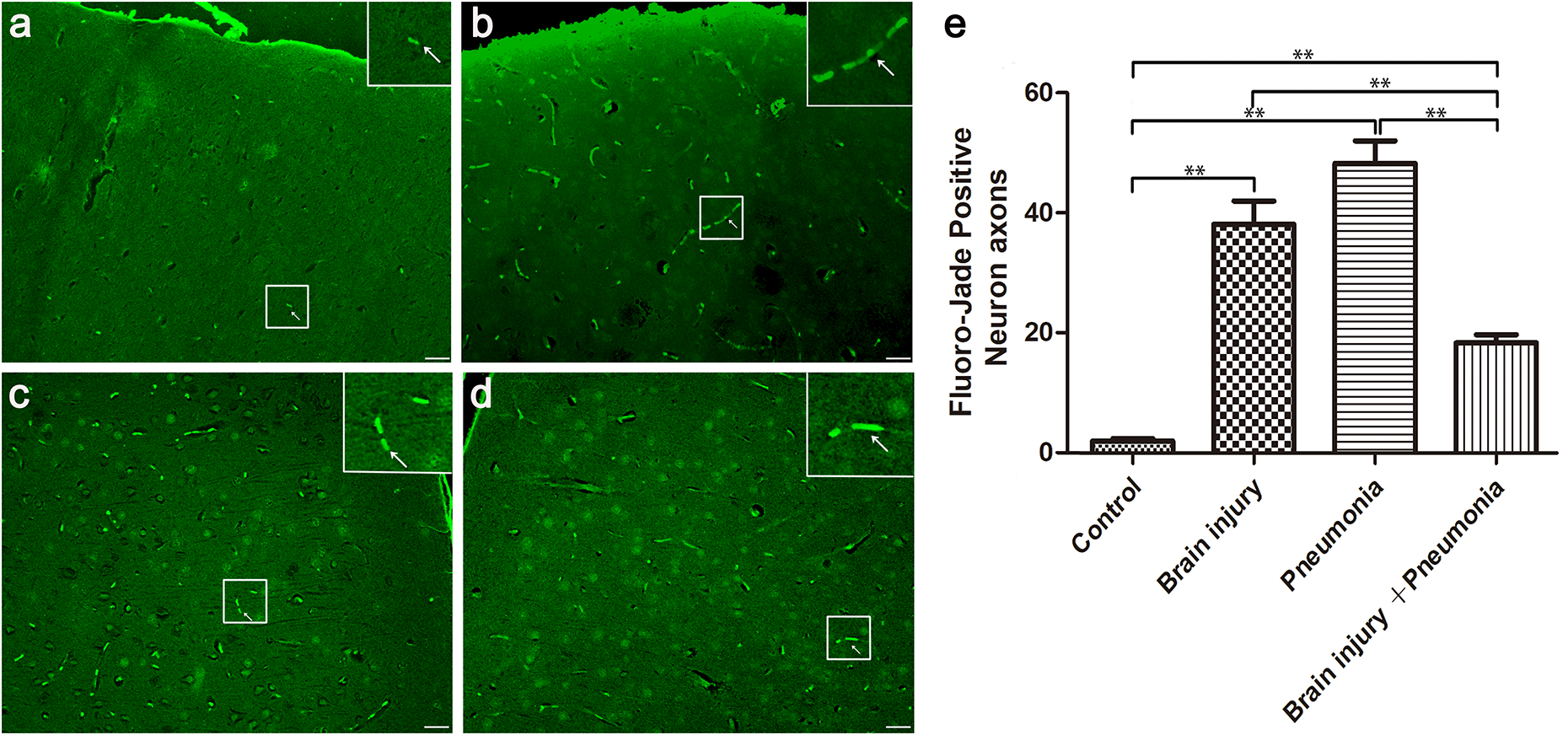
Mice were separated to four groups: Control, Brain injury, Pneumonia, and Brain injury + pneumonia. The brain tissue was embedded by paraffin and the section of brain tissue was stained by Fluoro-Jade stain C(FJC) staining kit. Damaged myelin was detected by Fluoro-Jade C (FJC) staining. (a) – (d) show FJC-positive neurons from Control, Brain injury, Pneumonia, and MTBI + Pneumonia group mice, respectively. (e) shows the statistical data for the FJC-positive neuron axons. Both MTBI and PA Xen5 infection increased FJC-positive neuron axons in the brains of the mice. Interestingly, MTBI + pneumonia together reduced FJC-positive neurons compared to MTBI or pneumonia alone. At least Three independent experiments were performed for this study. Graphs show the mean ± SEM. N = 5 mice/group. ** P < 0.01. Scale bar =10 μm.
Decreased bacterial load in the lung of MTBI + Pneumonia mice compared to pneumonia mice
To study the effect of MTBI on bacterial growth, the bacterial load of the mice was determined by in vivo imaging analysis (Fig. 2a). As expected, no signal was detected in the control and MTBI groups. The bioluminescence signal of the MTBI + Pneumonia group was significantly lower than that of the pneumonia groups (Fig. 2b; P<0.05).
Figure 2. MTBI damped bacterial growth of PA Xen5 in the lung of infected mice.
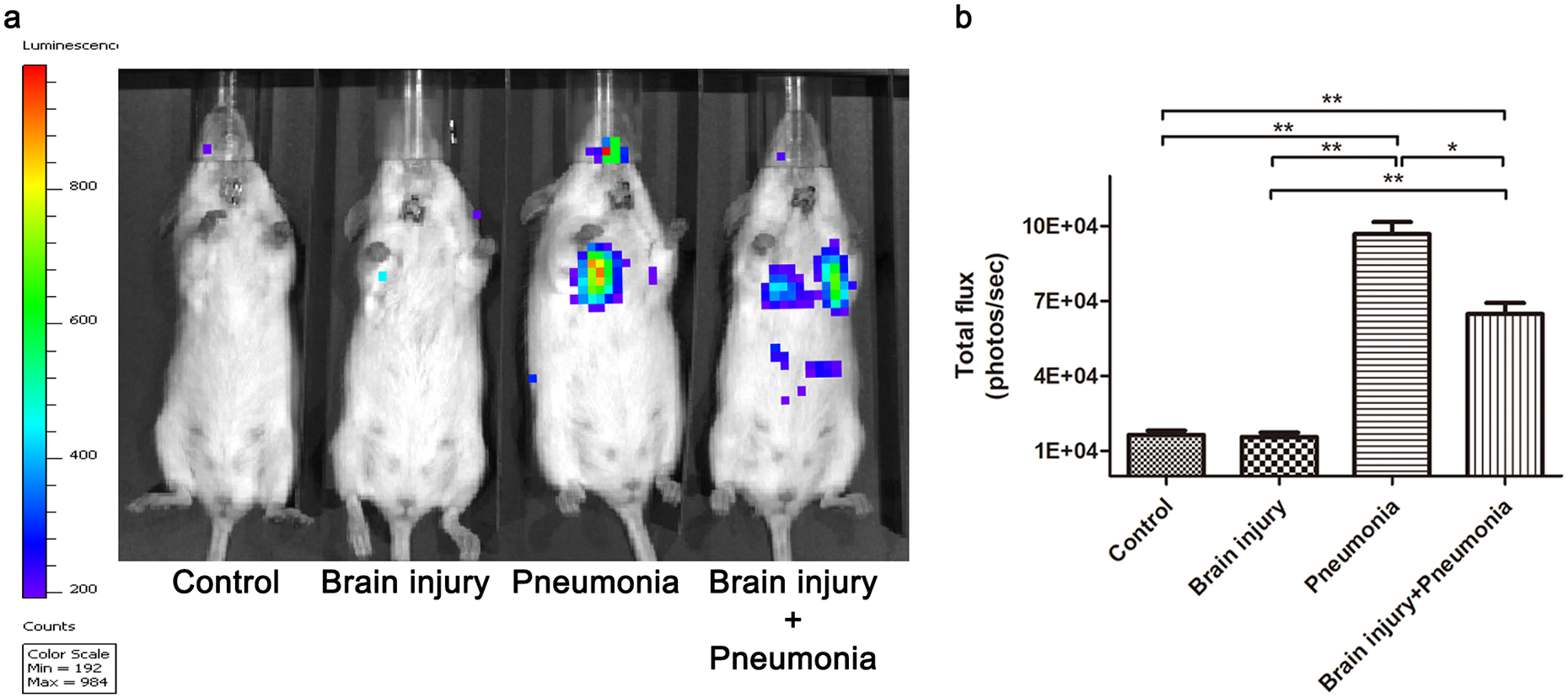
The growth of PA Xen5 in the lung was assessed by its bioluminescence with in vivo imaging system. (a) shows a representative image of bacterial proliferation in the infected mice. As expected, no bacteria exist in the control group and the Brain injury group. (b) is the statistical data for bioluminescent signal levels of the four groups. The MTBI + Pneumonia group shows lower level of bacteria compared to the pneumonia group (P<0.05). At least Three independent experiments were performed for this study. Graphs represent the mean ± SEM. N = 5 mice/group. * P < 0.05, ** P < 0.01.
Decreased pulmonary microvascular permeability in the lung of MTBI + Pneumonia mice compared to pneumonia mice
The inflammation associated with pneumonia induced pulmonary microvascular permeability (19). The amount of EB dye in the lung was taken to reflect pulmonary microvascular permeability. As expected, there was no increase of lung microvascular permeability in and MTBI group compared to the controls (Fig. 3a and b). The Pneumonia group showed greater leakage of EB dye than the MTBI + Pneumonia group (Fig. 3c and d). The EB dye was quantified using a spectrophotometer method, and was significantly higher in the Pneumonia and MTBI + Pneumonia groups compared to the Control and MTBI groups (P<0.01). There was also a statistical difference between the Pneumonia and MTBI + Pneumonia groups (Fig. 3b; P<0.01).
Figure 3. MTBI reduced lung vascular permeability in mice infected with PA Xen5.

Evan blue (EB) dye was used to assess pulmonary vascular permeability. Mice were injected with EB, exsanguinated, and perfused through the left ventricle 1.5 hours after injection. Lungs in each group were collected for analysis. (a)–(d) show gross views of the lungs. (e) shows the results of quantitative examination of EB in the four groups. The lungs of the Pneumonia group contained significantly more EB compared to the other groups (P<0.01). The EB content of the MTBI + Pneumonia group was lower compared to the Pneumonia group, but higher than in the Control and Brain injury groups (P<0.01). At least Three independent experiments were performed for this study. (graphs represent the mean ± SEM. N = 5–6 mice/group. * P < 0.05, ** P < 0.01).
Reduced lung injury in MTBI + Pneumonia mice compared to pneumonia mice
To evaluate the effects of MTBI on PA Xen5 induced pneumonia, we examined the lung injury using HE stained sections. Infection with PA Xen5 caused significantly pulmonary histopathological changes in the Pneumonia group, while the Brain injury group showed only mild-moderate histopathological changes in the lungs (Fig. 4a–d). Of note, quantitative analysis indicated that the MTBI + Pneumonia group showed less lung injury than the Pneumonia group (Fig. 4e; P<0.01).
Figure 4. MTBI attenuated lung injury caused by infection of PA Xen5.

Lungs were fixed with 10% Formalin solution and then embed with paraffin. The paraffin-embedded sections were prepared and stained with HE staining. Histological changes in the lung were evaluated by HE staining. (a)–(d) show changes in lung histopathology in the four groups. (e) shows the lung injury scores of the groups. The lungs of Pneumonia group mice exhibited obvious inflammatory cell infiltration and tissue edema. Tissue edema and inflammatory cell infiltration were also apparent in mice in the MTBI + Pneumonia group, but were less severe than in the Pneumonia group (P<0.01). At least three independent experiments were performed for this study. Graphs show the mean ± SEM. N = 5 mice/group. ** P < 0.01. Scale bar =10 μm.
Reduced inflammatory cells in BALF of MTBI + Pneumonia mice compared to pneumonia mice
The number of inflammatory cells in BALF is known to accurately reflect pathological changes in cases of acute lung injury. We determined inflammatory cells (macrophages and neutrophils) in BALF from the four groups. As shown in Fig. 5, the cells in BALF from Control and MTBI mice were almost alveolar macrophages (Fig. 5a and b). Bacterial infection significantly increased neutrophils in the lung of infected mice (Fig. 5f; P<0.01). However, the MTBI + pneumonia mice had less abundant macrophages and neutrophils when compared to the mice in the Pneumonia group (Fig. 5e and f; P<0.05).
Figure 5. MTBI changed inflammatory cells in BALF of infected mice.
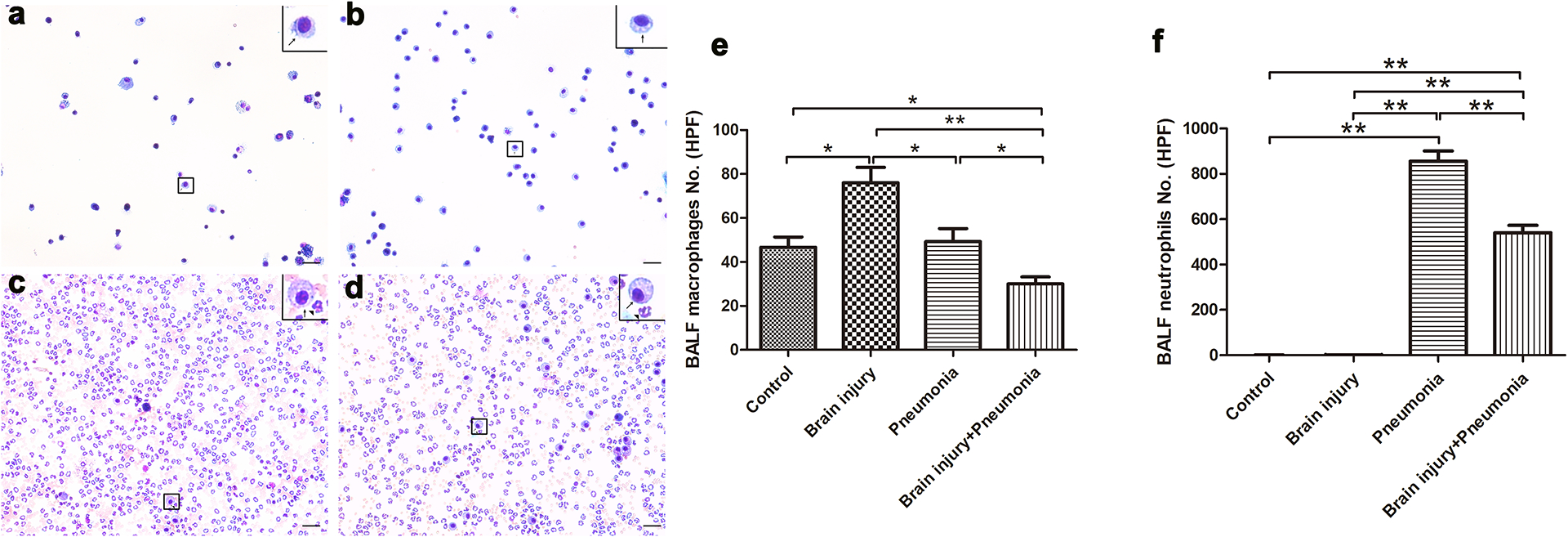
The cells in BALF were mounted on slides by cytospin centrifugation. The slides were stained using the Hema-3 staining kit. (a)–(d) show that inflammatory cells were greatly increased in the Pneumonia group, of which the great majority were neutrophils. (e and f) show the results of the quantitative analyses of BALF macrophages (e) and neutrophils (f) in all four groups. At least Three independent experiments were performed for this study. Graphs show the mean ± SEM. N = 5–6 mice/group. ** P < 0.01. Scale bar =10 μm.
Decreased proinflammatory cytokines in the lungs of MTBI + Pneumonia mice compared to pneumonia mice
The proinflammatory cytokines (IL-1β, IL-6, and TNF-α) were measured in lung tissues via ELISA. There was no statistical difference in the concentrations of IL-1, IL-6, and TNF-α between the Control and Brain injury groups (Fig. 6). The levels of all three cytokines were higher in the Pneumonia than Control and Brain injury groups (P<0.01). Of note, the concentrations of IL-1β and TNF-α were significantly lower in the MTBI + Pneumonia mice when compared to Pneumonia group (P<0.05). However, no statistical difference of the concentration of IL-6 was found between the MTBI + Pneumonia and Pneumonia groups (Fig. 6b).
Figure 6. MTBI inhibited the elevation of inflammatory cytokines in mice infected with PA Xen5.

Lung tissues were homogenized after being harvested at 24 hours post-infection to measure the concentrations of interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α. The ratios between cytokine levels and total lung tissue weight were calculated. (a) shows the IL-β levels of the four groups. The levels of IL-β in the Control and Brain injury groups were similarly low. The Pneumonia group had a significantly higher level of IL-β than the other groups. The MTBI + Pneumonia groups showed lower levels of IL-β than the Pneumonia group but higher levels than the Control and Brain injury groups. (b) and (c) show the levels of IL-6 and TNF-α, respectively. The levels of these cytokines were similar among the four groups. Three independent experiments were performed for this study. Graphs show the mean ± SEM. N = 5 mice/group. ** P < 0.01.
Reduced phosphorylated NF-κB level in the lungs of MTBI + Pneumonia mice compared to pneumonia mice
Phosphorylation of NF-κB activates the expression of a series of proinflammatory cytokines and is emerging as an important determinant of inflammation. Western blotting analysis with p-NF-κB specific antibody showed increased p-NF-κB level in the lung tissue of three treated groups but not in controls (Fig. 7a). The level of pNF-κB 65 in the lung tissue of MTBI + Pneumonia group was significantly lower than that of Pneumonia group (Fig. 7b; P<0.01). But MTBI mice had significantly lower level of p-NF-κB than that of either MTBI + Pneumonia or Pneumonia groups (Fig. 7b; P<0.01).
Figure 7. MTBI reduced the NF-κB activation in the lung of mice infected with PA Xen5.

Lung tissues were homogenized after being harvested at 24 hours post-infection. Western blots of phosphorylated NF-κB (p-NF-κB) and β-actin are shown in (a). Quantitative analysis of p-NF-κB expression was performed as shown in panel (b). Both MTBI and infection by PA Xen5 increased the expression of p-NF-κB in the lungs of the mice. However, the combination of MTBI and infection by PA Xen5 attenuated the expression of p-NF-κB compared to only pneumonia (P<0.01). Three independent experiments were performed for this study. Graphs represented as the mean ± SEM; N=5 mice/group. *P < 0.05, ** P < 0.01.
Enhanced bactericidal capacity of alveolar macrophages from MTBI mice
We collected alveolar macrophages to evaluate their bactericidal capacity. Alveolar macrophages were collected from mice with or without MTBI challenge. After mixing macrophages and PA xen5 bacteria (ratio: 1:10) and co-culturing for 30 min, bacteria that were associated with the surface of the cells were killed by gentamicin; bacteria inside the cells were released and cultured onto LB-agar plates. Bacteria colonies on the LB-agar plates were counted. The results showed significantly less CFUs in the macrophages from MTBI mice compared to those from Control mice (Fig. 8, P<0.01), suggesting that the macrophages from MTBI mice had increased bactericidal capacity compared to those from control mice without MTBI challenge.
Figure 8. MTBI enhanced the bactericidal capacity of alveolar macrophages.

Alveolar macrophages were obtained from the lungs of brain injury mice and controls. The macrophages were then co-cultured with PA Xen5 for 30 min. Co-culture with macrophages and PA Xen5 was performed at a ratio of 1:10 for 30 min. Gentamicin (50 μg/mL) was used to kill outside bacteria around the macrophages, and then washed off using saline. Macrophages containing PA were re-suspended in distilled water to release bacteria. Bacteria in distilled water were sprayed onto agar plates and incubated at 37°C overnight to count the bacterial colonies. The colony-forming units (CFUs) per 50 macrophages of mice with brain injury were significantly less abundant compared to the Control group (P<0.01), suggesting that MTBI enhanced the bactericidal capacity of alveolar macrophages in the lung. Three independent experiments were performed for this study. N=5 mice/group. ** P < 0.01.
Reduced M1 macrophages in the lungs of MTBI + Pneumonia mice compared to pneumonia mice
Macrophages play an important role in pathogen clearance and inflammatory modulation in the lung. Alveolar macrophages are activated by many stimuli, a process known as polarization. M1 macrophages are known to be proinflammatory and cytotoxic, while M2 macrophages are anti-inflammatory “pro-repair” macrophages (20). CD86 expression is a specific biomarker for the identification of M1 macrophages (21). We found increased expression of CD86-positive cells in the lung of both MTBI and Pneumonia groups compared to Control group (Fig. 9a–d); whereas expression of CD86 was decreased significantly in the MTBI + Pneumonia group compared to the Pneumonia group (Fig. 9e). Additionally, similar pattern of the changes of CD16 biomarker was observed in the lung of this model. These results indicate that M1 polarization was induced by bacterial infection, but that this process may be inhibited in MTBI mice. The inhibition of M1 polarization of macrophages in MTBI mice may explain the protective effects of MTBI in pneumonia-induced ALI/ARDS.
Figure 9. MTBI modulated M1 polarization of alveolar macrophages in the lung of mice infected with PA Xen5, thus alleviating excessive inflammation.

M1 macrophages are known to be proinflammatory and cytotoxic macrophages. CD86 was as a specific biomarker of M1 macrophages. Lung tissue sections from the four groups were deparaffinized and incubated with CD86 antibody (a – d). Nuclei were counterstained with DAPI. The results of quantitative analysis of CD86 positive cells are shown in the Pane (e). CD86-positive cells were higher in the Pneumonia group compared to the Brain injury group or the MTBI + Pneumonia group (P<0.01), indicating that MTBI influenced M1 polarization after PA Xen5 infection. At least three independent experiments were performed for this study. Graphs represented as the mean ± SEM; N=5 mice/group. *P < 0.05, ** P < 0.01.
DISCUSSION
Lung infection following severe TBI is common and severe TBI is one of major risk factors to cause other organ injuries, including ALI/ARDS (12). The relationship between lung infection and MTBI, however, has not been explored extensively. In this study, our results revealed that MTBI can attenuate inflammation and acute lung injury in one murine model with PA Xen5 lung infection, which has been found to be associated with the enhanced bactericidal capacity of alveolar macrophages and the inhibition of M1 macrophage polarization after MTBI.
The lung is involved in complex cross-talk with many other organs, including the brain, gut, and kidney (22–24). The brain can affect the lung via complex pathways (25), such that in many cases brain damage/injury is often associated with lung injury. This interrelation is linked to impaired airway protection, cytokine release via neuronal activation, pulmonary venoconstriction, and higher capillary permeability due to increased sympathetic activity (22). Similarly to previous studies, this study revealed that MTBI mice also suffered from acute, albeit mild, lung injury. Paradoxically, we found that MTBI attenuated the neurological damage and pulmonary acute injury caused by bacterial infection with PA Xen5. Altered macrophage phagocytosis and polarization may be major reasons underlying the MBTI-related protective effect against bacterial pneumonia. These observations in this study are consistent with the recent findings by Hsieh et al. (10), in which increased substance P expression was found in MTBI mice. Further understanding of the cellular and molecular mechanisms related to protective effects of MTBI against pneumonia may facilitate the treatment of bacterial induced ALI/ARDS.
For clinical diagnosis of MTBI, one or more of the following criteria must be met: confusion or disorientation, loss of consciousness for up to 30 minutes, post-traumatic amnesia for less than 24 hours, and/or other transient neurological abnormalities such as focal signs, seizure, and intracranial lesions not requiring surgery; and a Glasgow Coma Scale score of 13–15 at least 30 minutes after injury upon presentation to a healthcare facility (26). In general, MTBI is not associated with obvious pathological changes in routine radiological tests, or based on H/E staining. Although many animal models have been developed to replicate human TBI(13), we adopted the weight drop mouse model because it is closer to the clinical condition of human TBI and been proven to be consistent by Vaickus et al (9, 27). The general view and H/E staining of the brain between mice with weight drop injury and the normal mice showed no significant difference. We also examined the leakage of Evans blue dye in the brain of mice with and without weight drop, and no difference was found (Data not shown). The damage degree was mild as previously described (27). Although there was no obvious histological change in the brain of mice belonging to the MTBI group, we found positive signals in the MTBI mice by FJC staining, which suggested more testing methods should be adopted to access the MTBI. Functional magnetic resonance imaging (fMRI) detected myelin in MTBI and may imply that more research is required in the study of MTBI(28).
In this study, both FJC-positive cells and lung injury were found in the MTBI group mice, consistent with previous studies (22, 29). MTBI attenuated the myelin injury associated with pneumonia, which may be via the expression and regulation of glucocorticoids. Previous studies demonstrated that serum glucocorticoid levels increased immediately after MTBI (30). Hormone secretions, including those of adrenocorticotropin (ACTH), among others, are altered in the acute phase of brain injury. These hormones are important in trauma and bacterial infection (31). Clinical studies showed that plasma cortisol levels were markedly elevated in the acute phase of brain injury. In one pilot study we also observed increased level of serum cortisol 30 min post MTBI (data not shown). Therefore, it is warranted to examine the change of glucocorticoid level in the blood of MTBI/pneumonia mice in the future. Previous studies have demonstrated that glucocorticoids have been shown to attenuate lung injury by modulating macrophage polarization. Tu et al. found that methylprednisolone induced M2 polarization of macrophages to ameliorate acute liver injury (ALI) (32). We found that MTBI enhanced bacterial clearance by alveolar macrophages in lung tissue of mice and reduced CD86-positive cells in the lungs. MTBI may regulate macrophage polarization through cortisone secretion, resulting in protection against pneumonia. Although the use of cortisol to prevent lung injury in patients suffering from brain injures has been controversial, it seems that the results from this study support the use of cortisol in MTBI cases. However, further studies are necessary to assess the role of cortisol and the protective effects of MTBI against bacterial pneumonia.
The bacterial PA Xen5 used in this study is high toxicity due to its specific type III secretion system and the bacterial dose used in this study could cause severe lung injury and ARDS based on our previous observation (14, 33). Of interesting, MTBI mice exhibited reduced bacterial load and pulmonary permeability and decreased proinflammatory cytokine expression in the lung after bacterial infection compared to without MTBI infected mice. Further analysis of ex vivo alveolar macrophages revealed enhanced bactericidal capacity of the alveolar macrophages from MTBI mice compared to those from control mice. Altered activation/status of alveolar macrophages that play a critical role in the lung innate immunity and host defense could influence outcome of bacterial pneumonia, which has been observed in other conditions (34). Furthermore, it has been found that NF-κB signaling pathway is involved in the pathogenesis of ALI/ARDS induced by bacterial infection or other conditions (33, 35). Decreased NF-κB activation and less lung injury was observed in the MTBI + pneumonia group in comparison of only pneumonia group, which is consistent with the published observations (33, 35).
Although the findings in this study are exciting there are several limitations. First, all experiments were performed with an FVB/N mouse strain that are relatively T helper-2 prone and anti-inflammatory property in their immune response to infection, this property may have a degree of impact on the response of bacterial infection after MTBI. Second, there are limited mouse number of each group to analyze sex difference for the output measures although both male and female animals were used in the study. Third, this design is to address the situation of MTBI/lung infection simultaneously so animals were infected with P. aeruginosa 30 min after MTBI treatment, which is not related to clinically ventilated-induced pneumonia of MTBI. Fourth, we examined a few biomarkers of alveolar macrophages and used a classic M1/M2 model to explain the effect of MTBI-induced changes of alveolar macrophage phenotypes. It should be helpful to analyze the profile of many gene expression in alveolar macrophages with modern sc-RNA sequencing method or other techniques because there exists more complex profile of gene expression in alveolar macrophages after MTBI and pneumonia.
In summary, this study used a mouse model of weight-drop induced MTBI combined with bacterial induced pneumonia to explore the relationships of MTBI and pneumonia-induce ALI/ARDS. We found that MTBI attenuates pneumonia-induced ALI, the mechanism may involve the modulation of alveolar macrophage bactericidal activity and M1 polarization in the lung after MTBI and bacterial challenges.
ACKNOWLEDGMENTS
The authors thank Dr. Jennifer F. Moffat of the Department of Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, NY, for kindly providing the bioluminescent bacterial strain of P. aeruginosa Xen5 and in vivo imaging system.
Funding
This study is supported by NIH R01HL136706 and in part by the NSF research award (1722630) (to GW) and Zhejiang Provincial Natural Science Foundation (No. Y15H150006) (RF).
Footnotes
Conflicts of interest
The authors confirm no competing interests.
The authors have no relevant conflicts of interest.
REFERENCES:
- 1.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J and Matthay MA: Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med 187(7):751–60, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and outcomes of acute lung injury. N Engl J Med 353(16):1685–93, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Fesharaki-Zadeh A: Chronic Traumatic Encephalopathy: A Brief Overview. Front Neurol 10:713, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daugherty J, Waltzman D, Sarmiento K and Xu L: Traumatic Brain Injury-Related Deaths by Race/Ethnicity, Sex, Intent, and Mechanism of Injury - United States, 2000–2017. MMWR Morb Mortal Wkly Rep 68(46):1050–1056, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feigin VL, Theadom A, Barker-Collo S, Starkey NJ, McPherson K, Kahan M, Dowell A, Brown P, Parag V, Kydd R, et al. : Incidence of traumatic brain injury in New Zealand: a population-based study. Lancet Neurol 12(1):53–64, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Bazarian JJ, McClung J, Shah MN, Cheng YT, Flesher W and Kraus J: Mild traumatic brain injury in the United States, 1998−−2000. Brain Inj 19(2):85–91, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Wilk JE, Thomas JL, McGurk DM, Riviere LA, Castro CA and Hoge CW: Mild traumatic brain injury (concussion) during combat: lack of association of blast mechanism with persistent postconcussive symptoms. J Head Trauma Rehabil 25(1):9–14, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Hu PJ, Pittet JF, Kerby JD, Bosarge PL and Wagener BM: Acute brain trauma, lung injury, and pneumonia: more than just altered mental status and decreased airway protection. Am J Physiol Lung Cell Mol Physiol 313(1):L1–l15, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, Stepien D, Hanseman D, Robinson B, Goodman MD, Pritts TA, Caldwell CC, Remick DG and Lentsch AB: Substance P mediates reduced pneumonia rates after traumatic brain injury. Crit Care Med 42(9):2092–100, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh T, Vaickus MH, Stein TD, Lussier BL, Kim J, Stepien DM, Duffy ER, Chiswick EL and Remick DG: The Role of Substance P in Pulmonary Clearance of Bacteria in Comparative Injury Models. Am J Pathol 186(12):3236–3245, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moradali MF, Ghods S and Rehm BHA: Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Frontiers in cellular and infection microbiology 7:39–39, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang KW, Chen HJ, Lu K, Liliang PC, Huang CK, Tang PL, Tsai YD, Wang HK and Liang CL: Pneumonia in patients with severe head injury: incidence, risk factors, and outcomes. J Neurosurg 118(2):358–63, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Xiong Y, Mahmood A and Chopp M: Animal models of traumatic brain injury. Nat Rev Neurosci 14(2):128–42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang F, Zhang J, Yang Y, Ruan F, Chen X, Guo J, Abdel-Razek O, Zuo YY and Wang G: Regulatory Roles of Human Surfactant Protein B Variants on Genetic Susceptibility to Pseudomonas Aeruginosa Pneumonia-Induced Sepsis. Shock 54(4):507–519, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM and Acute Lung Injury in Animals Study G: An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 44(5):725–38, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Zhao Z, Chai Y, Luo L, Jiang R, Dong J and Zhang J: Stress-dose hydrocortisone reduces critical illness-related corticosteroid insufficiency associated with severe traumatic brain injury in rats. Crit Care 17(5):R241, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drevets DA, Canono BP and Campbell PA: Measurement of bacterial ingestion and killing by macrophages. Curr Protoc Immunol 109:14.6.1–17, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Anderson KJ, Miller KM, Fugaccia I and Scheff SW: Regional distribution of fluoro-jade B staining in the hippocampus following traumatic brain injury. Exp Neurol 193(1):125–30, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Zarbock A, Distasi MR, Smith E, Sanders JM, Kronke G, Harry BL, von Vietinghoff S, Buscher K, Nadler JL and Ley K: Improved survival and reduced vascular permeability by eliminating or blocking 12/15-lipoxygenase in mouse models of acute lung injury (ALI). Journal of immunology (Baltimore, Md. : 1950) 183(7):4715–4722, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M and David S: TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83(5):1098–116, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Jaiswal A, Reddy SS, Maurya M, Maurya P and Barthwal MK: MicroRNA-99a mimics inhibit M1 macrophage phenotype and adipose tissue inflammation by targeting TNFalpha. Cell Mol Immunol 16(5):495–507, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelosi P and Rocco PR: The lung and the brain: a dangerous cross-talk. Crit Care 15(3):168, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tulic MK, Piche T and Verhasselt V: Lung-gut cross-talk: evidence, mechanisms and implications for the mucosal inflammatory diseases. Clin Exp Allergy 46(4):519–28, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Husain-Syed F, Slutsky AS and Ronco C: Lung-Kidney Cross-Talk in the Critically Ill Patient. Am J Respir Crit Care Med 194(4):402–14, 2016. [DOI] [PubMed] [Google Scholar]
- 25.Mrozek S, Constantin JM and Geeraerts T: Brain-lung crosstalk: Implications for neurocritical care patients. World J Crit Care Med 4(3):163–78, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll LJ, Cassidy JD, Holm L, Kraus J and Coronado VG: Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med (43 Suppl):113–25, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Vaickus M, Hsieh T, Kintsurashvili E, Kim J, Kirsch D, Kasotakis G and Remick DG: Mild Traumatic Brain Injury in Mice Beneficially Alters Lung NK1R and Structural Protein Expression to Enhance Survival after Pseudomonas aeruginosa Infection. Am J Pathol 189(2):295–307, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flierl MA, Stahel PF, Beauchamp KM, Morgan SJ, Smith WR and Shohami E: Mouse closed head injury model induced by a weight-drop device. Nat Protoc 4(9):1328–37, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Dettman RW and Dizon MLV: How lung injury and therapeutic oxygen could alter white matter development. J Neurosci Res, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuckerman A, Ram O, Ifergane G, Matar MA, Kaplan Z, Hoffman JR, Sadot O and Cohen H: Role of Endogenous and Exogenous Corticosterone on Behavioral and Cognitive Responses to Low-Pressure Blast Wave Exposure. J Neurotrauma 36(2):380–394, 2019. [DOI] [PubMed] [Google Scholar]
- 31.Zaben M, El Ghoul W and Belli A: Post-traumatic head injury pituitary dysfunction. Disabil Rehabil 35(6):522–5, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Tu GW, Shi Y, Zheng YJ, Ju MJ, He HY, Ma GG, Hao GW and Luo Z: Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J Transl Med 15(1):181, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du J, Abdel-Razek O, Shi Q, Hu F, Ding G, Cooney RN and Wang G: Surfactant protein D attenuates acute lung and kidney injuries in pneumonia-induced sepsis through modulating apoptosis, inflammation and NF-kappaB signaling. Sci Rep 8(1):15393, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorenoor N, Kawasawa YI, Gandhi CK and Floros J: Sex-Specific Regulation of Gene Expression Networks by Surfactant Protein A (SP-A) Variants in Alveolar Macrophages in Response to Klebsiella pneumoniae. Front Immunol 11:1290, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J, Ni L, Zhang X, Zhang J, Abdel-Razek O and Wang G: Surfactant Protein D Dampens Lung Injury by Suppressing NLRP3 Inflammasome Activation and NF-kappaB Signaling in Acute Pancreatitis. Shock 51(5):557–568, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


