Abstract
Introduction
Research comparing levodopa/carbidopa intestinal gel (LCIG), deep brain stimulation (DBS), and continuous subcutaneous apomorphine infusion (CSAI) for advanced Parkinson’s disease (PD) is lacking. This network meta-analysis (NMA) assessed the comparative effectiveness of LCIG, DBS, CSAI and best medical therapy (BMT) in reducing off-time and improving quality of life (QoL) in patients with advanced PD.
Methods
A systematic literature review was conducted for randomized controlled trials (RCTs), observational and interventional studies from January 2003 to September 2019. Data extracted at baseline and 6 months were off-time, as reported by diary or Unified Parkinson’s Disease Rating Scale Part IV item 39, and QoL, as reported by Parkinson’s Disease Questionnaire (PDQ-39/PDQ-8). Bayesian NMA was performed to estimate pooled treatment effect sizes and to rank treatments in order of effectiveness.
Results
A total of 22 studies fulfilled the inclusion criteria (n = 2063 patients): four RCTs, and 16 single-armed, one 2-armed and one 3-armed prospective studies. Baseline mean age was between 55.5–70.9 years, duration of PD was 9.1–15.3 years, off-time ranged from 5.4 to 8.7 h/day in 9 studies, and PDQ scores ranged from 28.8 to 67.0 in 19 studies. Levodopa/carbidopa intestinal gel and DBS demonstrated significantly greater improvement in off-time and QoL at 6 months compared with CSAI and BMT (p < 0.05). There was no significant difference in the effects of LCIG and DBS, but DBS was ranked first for reduction in off-time, and LCIG was ranked first for improvement in QoL.
Conclusions
This NMA found that LCIG and DBS were associated with superior improvement in off-time and PD-related QoL compared with CSAI and BMT at 6 months after treatment initiation. This comparative effectiveness research may assist providers, patients, and caregivers in the selection of the optimal device-aided therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40263-022-00963-9.
Key Points
| As Parkinson’s disease (PD) progresses and optimized treatment with oral therapies fails to control symptoms, device-aided therapies such as levodopa/carbidopa intestinal gel (LCIG), deep brain stimulation (DBS), and continuous subcutaneous apomorphine infusion (CSAI) may improve symptoms such as off-time. |
| Comparative randomized controlled trials evaluating device-aided therapies for advanced PD are not available. |
| This network meta-analysis was conducted to compare the three device-aided therapies and best medical therapy (BMT), with a focus on changes in off-time and quality of life (QoL). |
| In this analysis, LCIG and DBS were associated with superior improvement in off-time and PD-related QoL compared with CSAI and BMT at 6 months after treatment initiation. |
| There was no significant difference in the effects of LCIG and DBS, but DBS was ranked first for reduction in off-time, and LCIG was ranked first for improvement in QoL. |
| These results could help inform treatment choices for people whose PD symptoms are not well controlled with optimized oral therapy. |
Introduction
As Parkinson’s disease (PD) progresses, motor complications can arise and worsen over time; for example, an increase in off-time frequently occurs. This, along with other symptoms, has a negative impact on patient’s quality of life (QoL) and activities of daily living (ADL) [1, 2]. The burden of advancing disease also affects caregivers of people with PD [3, 4]. In practice, when motor fluctuations cannot be improved with optimized oral therapy (or best medical therapy [BMT]), patients may be classified as having advanced PD [5–7]. For these patients, device-aided therapy may be considered as an alternative treatment option to BMT.
Device-aided therapies for PD include levodopa/carbidopa intestinal gel (LCIG), deep brain stimulation (DBS), and continuous subcutaneous apomorphine infusion (CSAI). Levodopa/carbidopa intestinal gel is a gel formulation of levodopa/carbidopa that is administered continuously via a percutaneous endoscopic gastrostomy (PEG) into the small intestine using a portable pump [8]. Levodopa/carbidopa intestinal gel circumvents the impact of erratic gastric emptying on oral levodopa/carbidopa and provides more consistent plasma levels of levodopa [9], which is a metabolic precursor of dopamine that is depleted in PD. Deep brain stimulation involves the surgical insertion of electrodes to deliver controlled electrical impulses to the subthalamic nucleus, the internal globus pallidus, or the ventral intermediate nucleus. The electrodes deliver high frequency stimulation to the targeted area, which overcomes the abnormal activity associated with some PD symptoms. Continuous subcutaneous apomorphine infusion is the continuous subcutaneous delivery of apomorphine solution via a pump [10]. This approach provides a continuous plasma delivery of apomorphine, which mimics the effect of dopamine in the striatum. These device-aided therapies have all been shown to reduce off-time, and in some studies they have also improved QoL in patients with advanced PD [11–13]. These improvements have also been observed in longer term follow-up and observational studies [14–16]. Comparative effectiveness of some device-aided therapies in patients with PD has been reported [17, 18]. A recent systematic review compared data from studies of LCIG, DBS, and CSAI and presented the results in a format aimed at patients [19], and expert guidance on such therapies has been published [20]. However, there have been no comprehensive clinical trials comparing the three device-aided therapies for advanced PD. Individual patient and disease factors may influence the selection of patients for each device-aided therapy [6], and with limited published data, information on the comparative effectiveness of device-aided therapies for reducing off-time and improving QoL is still needed to aid the decision-making process for patients, caregivers, and providers.
When single-arm studies constitute the majority of the evidence, traditional network meta-analysis is a sub-optimal approach due to the lack of common comparators. An unanchored matching-adjusted indirect comparison (MAIC) to connect single-arm studies to the evidence network is an alternative approach. Matching-adjusted indirect comparison is an indirect comparison method frequently used by health technology assessment agencies [21, 22]. It is used to compare one therapy with individual patient-level data (IPD) with another therapy with published aggregate data (AD). It reweights the IPD to match the AD population in baseline characteristics, estimates the outcome in the weighted IPD, and compares it to the outcome in the AD study as if the outcomes were assessed within the same study.
The primary objective of this analysis was to assess the effectiveness of LCIG, DBS, CSAI, and BMT in reducing off-time and improving QoL in patients with advanced PD, using MAIC and a Bayesian network meta-analysis (NMA) of clinical trial and observational study data.
Methods
Data Source and Search Strategy
A systematic literature review was conducted in Medline, Embase, and the Cochrane Library within the date range of January 2003 (the earliest date when all three device-aided therapies were widely available) to September 2019 to identify clinical studies of CSAI, DBS, and LCIG for the management of patients with advanced PD (the methodology of this literature review was not registered). Individual patient-level data were extracted from four AbbVie-sponsored studies [11, 23–25]. Predefined search terms were based on the Patient Intervention Comparator Outcome Study (PICOS) design criteria [26] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA [27]). Search terms included ‘Parkinson disease’ in combination with variations of the device-aided therapy names including ‘deep brain stimulation’, ‘CSAI’, ‘LCIG’, and ‘infusion’ (see Online Resource Table 1 for a detailed list of the search terms).
Selection of Studies, Screening, and Data Extraction
Studies were included in the analysis if they were published in English, reported randomized clinical trials (RCTs), retrospective and prospective observational studies and other interventional studies and had a sample size of ≥ 20 people with advanced PD treated with either LCIG, DBS, CSAI, or BMT. Studies were included if off-time was assessed by diary or Unified Parkinson's Disease Rating Scale (UPDRS) part IV item 39 and/or QoL was assessed by Parkinson’s Disease Questionnaire (PDQ-39/PDQ-8). The UPDRS item 39 records the proportion of the waking day spent in off-time on a scale from 0 (none) to 4 (76–100%). Parkinson’s Disease Questionnaire scores range from 0 to 100, with higher scores indicating worse health. Data were included if the outcomes were reported at baseline and 6 months or as change at 6 months (± 3 months where the 6-month follow-up was not available). Data had to be reported as mean values with standard error and the number of patients assessed—or if not reported, then this needed to be calculable from standard deviations (SDs) or confidence intervals (CIs) from the published data. Studies were excluded if they included patients with early-stage PD, Parkin mutations, and PD-related dementia, and if they were comparisons of subgroups (e.g., female vs male; overweight vs normal weight; mutation-positive vs mutation-negative).
Following the removal of duplicate studies, each article was screened by two independent reviewers (ET, MLE, SK, FD, SP, BW, or HW; see Acknowledgments for details of reviewers) based on its title and abstract. Full-text publications of studies that passed the first round of screening were then reviewed by two independent reviewers. Data extracted from each selected publication for the NMA were amount of off-time/day and QoL scores at baseline and 6 months. In addition, information on study design, patient characteristics, treatments, and last reported follow-up were extracted. Information was independently entered into a collection form by two reviewers. For the IPD extraction, internal access to the original trial data was permitted. Study investigators (LW and PLK) used the trial protocols and data dictionary to extract individual patient characteristics (age, gender, PD duration, daily levodopa dose, and daily levodopa equivalent dose), outcomes of interest (off-time and PDQ scores), as well as trial-level information from the original datasets. To ensure accuracy of the data collected, each reviewer audited the other reviewer’s collection form. Disputes and any inconsistencies identified were resolved through discussion between the first two reviewers or adjudication by a third reviewer.
Network Meta-analytical Approach
Single-arm studies were incorporated by unanchored MAIC [21]. To simulate RCTs, studies with IPD were matched to AD from published literature with similar distributions of baseline patient characteristics (e.g., baseline age, sex, years since PD diagnosis, and levodopa daily dose, off-time and PD-related QoL). Matching-adjusted indirect comparison was performed to match each single-arm trial or single treatment observational study with AD to one of three studies with IPD [11, 23, 25]. The fourth study from which IPD were available was not used as only 37 patients were included and this study did not report daily levodopa dose at baseline or off-time at 6 months [24]. Matching-adjusted indirect comparison reweighted the IPD study population such that it had baseline characteristics (mean and percentage composition) similar to the AD study population. The weight assigned to any individual in an IPD study was equal to the odds of being enrolled in an AD trial [21, 22]. Because several AD studies were matched to the same IPD study, correlation between relative effects was accounted for by multinormal distributions with variance-covariance matrix [28]. For off-time, two RCTs were simulated by MAIC, and for QoL, three RCTs were simulated by MAIC (networks shown in Online Resource Figure 1). A sensitivity analysis by type of study (e.g., excluding single-arm studies) is not reported as the findings were considered unmeaningful.
Network meta-analysis was performed on RCTs, simulated RCTs and comparative observational studies. A Bayesian hierarchical model with Markov Chain Monte Carlo (MCMC) simulation was used to estimate the relative effect of different treatments. Treatment parameters were estimated using normal likelihood with identity link. Three parallel Markov chains, with 50,000 iterations each discarding the first 5000 burnt-in, were used. The convergence of MCMC chains was checked by trace plots and Gelman–Rubin diagnostic statistics [28]. Fixed and random effects models were assessed and compared using overall residual deviance and the deviance information [28]. To check for consistency of the results, an inconsistency model was used to fit to the data and compared with a standard consistency model [29]. The inconsistency model assumes unrelated mean relative effects with no consistency, and is approximately equivalent to performing separate pairwise meta‐analyses, while the random effects models allow shared variance parameters to be estimated [29]. These direct estimates can be used to calculate inconsistency between studies. League tables of pairwise comparisons for all treatments are reported. Relative treatment effects of all treatment comparisons are reported as median differences and 95% credible intervals (CrI). Treatments are ranked as the percentage of time ranked 1st, 2nd, 3rd, or 4th from Bayesian iterations. A fixed-effect model was chosen for the analysis because most pairwise treatment comparisons involved only one underlying study rather than multiple studies. In addition, results using the fixed and random effect models were found to be similar, with the only difference being that estimations had wider credible intervals using the random effects model.
Study bias was analyzed using the Cochrane Risk of Bias (RoB) tool to assess RCTs, ROBINS-I tool for non-randomized comparative studies, and the National Institutes of Health (NIH) Quality Assessment Tool for before-after (pre-post) studies with no control group or single-arm studies [30–32].
Results
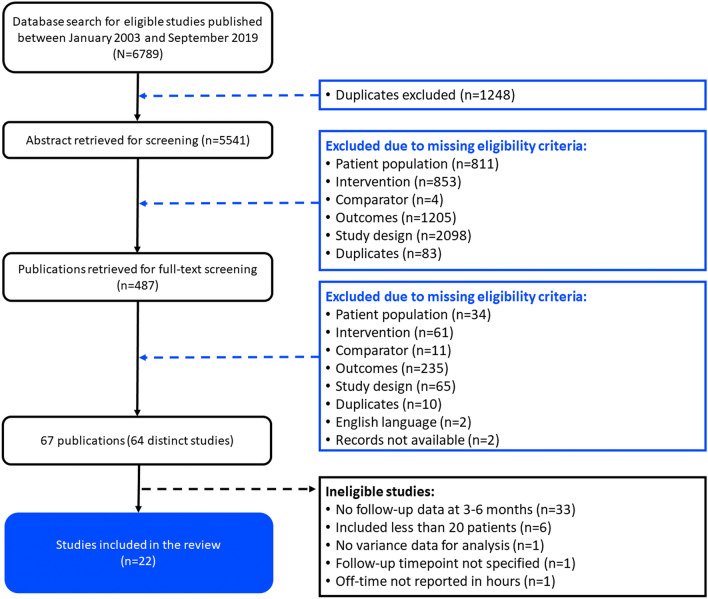
The literature review identified 64 publications; of these, 22 with data from 2063 patients fulfilled the inclusion criteria and were included in the analysis (Fig. 1; Table 1) [11–13, 23–25, 33–48]. In these studies, 908 patients were assigned to treatment with LCIG, 705 to DBS, and 322 to CSAI. Of the 22 selected studies, 16 were single-arm studies (seven of LCIG, two of CSAI and seven of DBS), two compared DBS with BMT, and four studies separately compared LCIG with BMT, LCIG with CSAI, CSAI with BMT, and DBS versus LCIG versus CSAI. In two of the RCTs [11, 12], follow-up was 3 months and so data were carried forward to create the 6-month datapoint.
Fig. 1.
PRISMA flow chart showing identification and selection of studies
Table 1.
Summary of the 22 studies included in the network meta-analysis
| Author/Reference | Study design | Countries | Number of patients included in analysis | Data source | Trial interventions |
|---|---|---|---|---|---|
| Antonini et al. (2017) [25] | Single-arm, prospectivea | Australia, Austria, Belgium, Bulgaria, Czech Republic, Denmark, France, Germany, Greece, Ireland, Italy, Netherlands, Norway, Romania, Slovenia, Spain, Switzerland, UK | 375 | Patient-level | LCIG |
| Fernandez et al. (2015) [23] | Single-arm, prospective | Australia, Canada, Czech Republic, Finland, Germany, Israel, Italy, Netherlands, New Zealand, Poland, Portugal, Russian Federation, Spain, Thailand, United Kingdom, USA | 272 | Patient-level | LCIG |
| Palhagen et al. (2016) [24]b | Single-arm, prospective | Norway, Sweden | 37 | Patient-level | LCIG |
| De Fabregues et al. (2017) [36] | Single-arm, prospective | Spain | 37 | Aggregate level | LCIG |
| Honig et al. (2009) [40] | Single-arm, prospective | Germany, Italy, UK | 22 | Aggregate level | LCIG |
| Vijiaratanam et al. (2018) [47] | Single-arm, prospective | Australia | 22 | Aggregate level | LCIG |
| Murata et al. (2016) [44] | Single-arm, prospective | Japan, South Korea, Taiwan | 31 | Aggregate level | LCIG |
| Floden et al. (2015) [39] | Single-arm, retrospective | USA | 106 | Aggregate level | DBS |
| Dafsari et al. (2016) [48] | Single-arm, retrospective | Germany, UK | 67 | Aggregate level | DBS |
| Dafsari et al. (2018) [34] | Single-arm, prospective | Germany, UK | 67 | Aggregate level | DBS |
| Kubu et al. (2017) [41] | Single-arm, prospective | USA | 52 | Aggregate level | DBS |
| Soulas et al. (2011) [45] | Single-arm, prospective | France | 41 | Aggregate level | DBS |
| Timmermann et al. (2015) [46] | Single-arm, prospective | Austria, France, Germany, Italy, Spain, UK | 40 | Aggregate level | DBS |
| Li et al. (2017) [42]c | Single-arm, prospective | China | 32 | Aggregate level | DBS |
| Drapier et al. (2016) [38] | Single-arm, prospective | France | 142 | Aggregate level | CSAI |
| Borgemeester and van Laar (2017) [33] | Single-arm, retrospective | Netherlands | 45 | Aggregate level | CSAI |
| Martinez-Martin et al. (2015) [43] |
Two-arm prospective |
Austria, Denmark, Germany, Italy, Slovenia, Spain, Sweden, UK | 87 | Aggregate level | LCIG vs CSAI |
| Dafsari et al. (2019) [35] |
Three-arm prospective |
Brazil, Germany, Italy, Slovenia, UK | 173 | Aggregate level | DBS vs LCIG vs CSAI |
| Weaver et al. (2009) [13] | Randomized, controlled trial | USA | 255 | Aggregate level | DBS vs BMT |
| Deuschl et al. (2006) [37] | Randomized, controlled trial | Austria, Germany | 156 | Aggregate level | DBS vs BMT |
| Katzenschlager et al. (2018) [12] | Randomized, controlled trial | Austria, Denmark, France, Germany, Spain, Netherlands, UK | 106 | Aggregate level | CSAI vs BMT |
| Olanow et al. (2014) [11] | Randomized, controlled trial | Germany, New Zealand, USA | 66 | Patient-level | LCIG vs BMT |
BMT best medical treatment, CSAI continuous subcutaneous apomorphine infusion, DBS deep brain stimulation, LCIG levodopa/carbidopa intestinal gel
aIncluded both prospective and retrospective data
bOnly LCIG naïve patients included
cOnly 1 month group included
Patient Characteristics
Men outnumbered women in 18 of the 22 studies (Table 2). The mean age at baseline was between 55.5 and 70.9 years, the mean duration of PD was between 9.1 and 15.3 years, and daily levodopa dose at baseline ranged from 905.5 to 1080.3 mg/day (Table 2).
Table 2.
Selected baseline characteristics in individual studies
| Author/Reference | Male,% | Age, years, mean (SD) | PD duration, years mean (SD) |
Trial interventions | Daily levodopa dose in mg, mean (SD) | Daily levodopa equivalent dose in mg, mean (SD) |
|---|---|---|---|---|---|---|
| Antonini et al. (2017) [25] | 59 | 66.4 (8.8) | 12.7 (6.3) | LCIG | 905.5 (443.5) | 1061.3 (555.5) |
| Fernandez et al. (2015) [23] | 59 | 64.0 (9.1) | 12.2 (5.6) | LCIG | 1080.3 (546.7) | NA |
| Palhagen et al. (2016) [24] | 62 | 63.6 (7.6) | 11 (4.4) | LCIG | NA | NA |
| De Fabregues et al. (2017) [36] | 59 | 68.2 (6.8) | 13.5 (5.6) | LCIG | NA | NA |
| Honig et al. (2009) [40] | 73 | 58.6 (9.1) | 15.3 (5.9) | LCIG | NA | NA |
| Vijiaratanam et al. (2018) [47] | 55 | 70.7 (6.9) | 15.2 (6.8) | LCIG | NA | 1655 (546) |
| Murata et al. (2016) [44] | 39 | 61.6 (10.5) | 12.4 (5.1) | LCIG | 1011.7 (629.7) | NA |
| Floden et al. (2015) [39] | 75 | 62.4 (7.9) | 10.6 (5.1) | DBS | NA | 958.5 (517.7) |
| Dafsari et al. (2016) [48] | 58 | 61.6 (7.8) | 10.5 (4.2) | DBS | NA | 1073.55 (475.93) |
| Dafsari et al. (2018) [34] | 75 | 62.3 (7.8) | 10.9 (4.8) | DBS | NA | 1121.6 (515.2) |
| Kubu et al. (2017) [41] | 75 | 61.3 (9.3) | 9.1 (4.1) | DBS | NA | NA |
| Soulas et al. (2011) [45] | NA | 62.0 (8.0) | 14.5 (5.7) | DBS | NA | 1504 (667.32) |
| Timmermann et al. (2015) [46] | 33 | 60.2 (7.8) | 11.7 (4.6) | DBS | NA | 1399.1 (726.1) |
| Li et al. (2017) [42] | 53 | 55.5 (9.1) | 9.8 (4.5) | DBS | NA | 953.20 (398.02) |
| Drapier et al. (2016) [38] | 58 | 66.7 (10.8) | 11.6 (5.4) | CSAI | NA | 1154.1 (758.9) |
| Borgemeester and van Laar (2017) [33] | 58 | 70.9 (8.1) | 10.8 (4.8) | CSAI | 1061 (NA) | NA |
| Martinez-Martin et al. (2015) [43] | 53 | 62.5 (9.9) | 15.1 (5.7) | LCIG vs CSAI | NA | NA |
| Dafsari et al. (2019) [35] | 48 | 63.6 (8.9) | 13.3 (4.8) | DBS vs LCIG vs CSAI | NA | 1300.0 (564.4) |
| Weaver et al. (2009) [13] | 82 | 62.3 (8.9) | 11.7 (5.5) | DBS vs BMT | NA | 1285.2 (534.2) |
| Deuschl et al. (2006) [37] | 64 | 60.7 (7.6) | 13.4 (5.7) | DBS vs BMT | NA | 1175.5 (489.8) |
| Katzenschlager et al. (2018) [12] | 62 | 63.3 (8.8) | 11.2 (5.0) | CSAI vs BMT | 954.7 (490.9) | 1479.1 (638.8) |
| Olanow et al. (2014) [11] | 64 | 60.7 (7.6) | 13.4 (5.7) | LCIG vs BMT | NA | NA |
BMT best medical treatment, CSAI continuous subcutaneous apomorphine infusion, DBS deep brain stimulation, LCIG levodopa/carbidopa intestinal gel, NA not available, SD standard deviation
Off-time
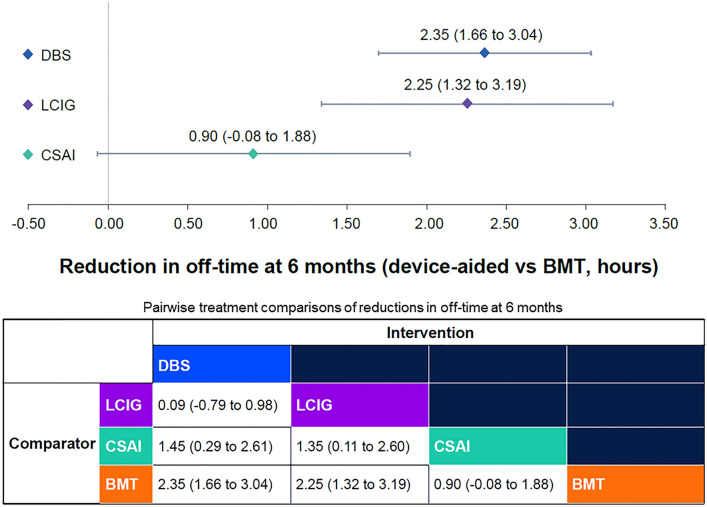
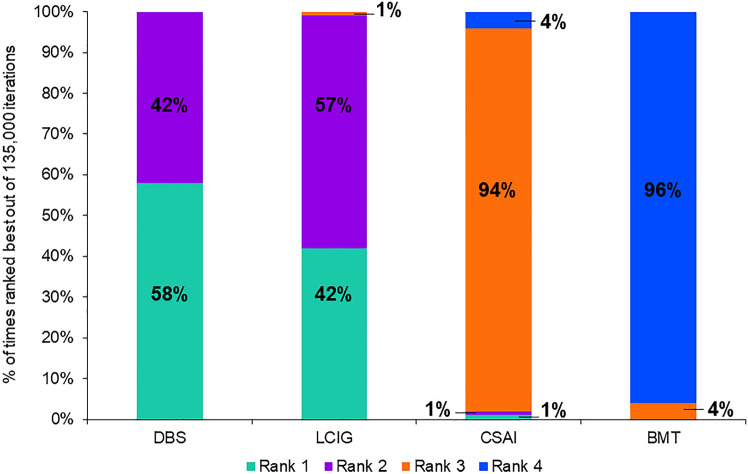
Of the selected studies, nine reported off-time at baseline (ranging from 5.4 to 8.7 h/day) and at 6 months, and one reported change from baseline at 6 months (Table 3). Nine studies used patient diaries to assess daily off-time, and one study used UPDRS part IV item 39. Deep brain stimulation (2.35 h/day; 95% CI 1.66, 3.04), and LCIG (2.25 h/day; 95% CI 1.32, 3.19) but not CSAI (0.90 h/day; 95% CI − 0.08, 1.88) produced significantly greater reductions in off-time compared with BMT (Fig. 2). Pairwise comparisons indicated that DBS (1.45 h/day; 95% CI 0.29, 2.61) and LCIG (1.35 h/day; 95% CI 0.11, 2.60) resulted in greater improvements in off-time at 6 months compared with CSAI. This was not statistically different between DBS and LCIG (Fig. 2). Based on 135,000 Bayesian iterations, DBS ranked highest (ranked 1 for 58% of iterations), and LCIG ranked second highest (ranked 1 for 42% of iterations) for off-time reduction (Fig. 3).
Table 3.
Summary of off-time changes from the ten studies that reported off-time at 6 months
| Author/Reference | Trial interventions | Baseline off-time in h/day, mean (SD)a | Reduction in off-time at 6 months in h/day, mean (SD) |
|---|---|---|---|
| Antonini et al. (2017) [25] | LCIG | 5.9 (3.1) | 4.2 (3.2) |
| Fernandez et al. (2015) [23] | LCIG | 6.8 (2.4) | 4.3 (3.2) |
| Olanow et al. (2014) [11] | LCIG | 6.3 (1.7) | 3.3 (3.1)b |
| BMT | 6.9 (2.1) | 2.0 (2.3)b | |
| Borgemeester and van Laar (2017) [33] | CSAI | NR | 1.7 (3.6) |
| De Fabregues et al. (2017) [36] | LCIG | 6.0 (1.4) | 4.9 (1.1)b |
| Katzenschlager et al. (2018) [12] | CSAI | 6.7 (2.2) | 2.5 (3.7)b |
| BMT | 6.7 (2.5) | 0.6 (2.8)b | |
| Li et al. (2017) [42] | DBS | 8.7 (2.9) | 4.4 (4.7)b |
| Murata et al. (2016) [44] | LCIG | 7.4 (2.3) | 4.6 (3.0)b |
| Timmermann et al. (2015) [46] | DBS | 5.4 (3.1) | 3.2 (3.9) |
| Weaver et al. (2009) [13] | DBS | 5.9 (2.6) | 2.4 (3.7) |
| BMT | 5.6 (2.9) | 0.0 (1.5) |
BMT best medical treatment, CSAI continuous subcutaneous apomorphine infusion, DBS deep brain stimulation, LCIG levodopa/carbidopa intestinal gel, NR not reported, SD standard deviation
aMeasured by patient diary except Antonini et al. (2017)
bData at 3 months carried forward
Fig. 2.
Mean (95% CI) off-time reduction (h/day) with the three device-aided therapies versus best medical treatment. The analysis adjusted for baseline age, sex, disease duration, levodopa daily dose, and off-time. BMT best medical therapy, CSAI continuous subcutaneous apomorphine infusion, DBS deep brain stimulation, LCIG levodopa/carbidopa intestinal gel
Fig. 3.
Ranking of device-aided therapy and best medical therapy based on improvements in off-time at 6 months
Although the inconsistency model had a lower posterior mean of the residual difference in off-time and hence a better fit to the data, the difference in deviance information criterion (DIC) between the two models was < 5 (Online Resource Table 2A). A difference in DIC > 5 is important to choose one model over the other [49]. In addition, the 95% CrI from the two models overlapped for all comparisons. Thus, no inconsistency was observed for this parameter.
Quality of Life
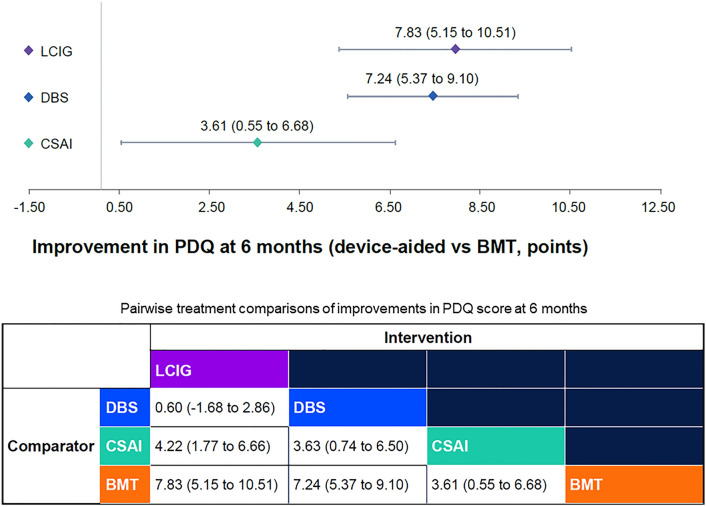
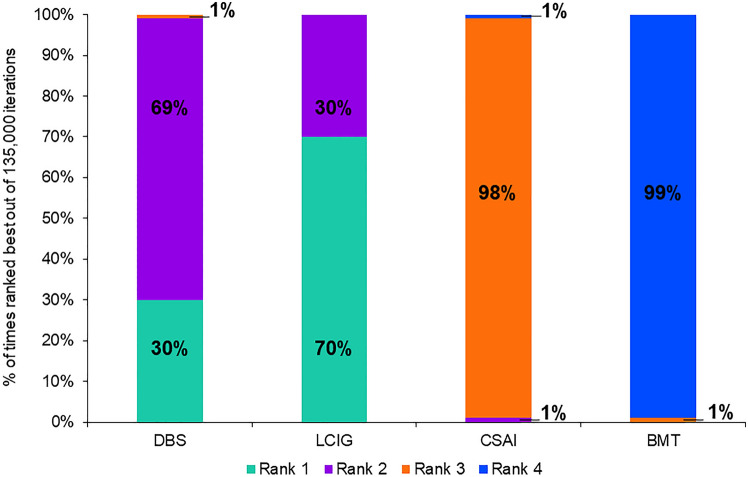
Baseline PDQ scores were reported in 19 studies with a range from 28.8 to 67.0 (Table 4). All device-aided therapies demonstrated greater improvements in PD-specific QoL than BMT at 6 months, but with smaller estimated improvements for CSAI (3.61; 95% CI 0.55, 6.68) than for LCIG (7.83; 95% CI 5.15, 10.51) and DBS (7.24; 95% CI 5.37, 9.10) (Fig. 4). Pairwise comparisons indicated that both LCIG (4.22, 95% CI 1.77, 6.66) and DBS (3.63, 95% CI 0.74, 6.50) resulted in significantly greater improvements in QoL at 6 months compared with CSAI. Improvement in QoL was not statistically different between LCIG and DBS (Fig. 4). Based on 135,000 Bayesian iterations, LCIG ranked highest (ranked 1 for 70% of iterations), and DBS ranked second highest (ranked 1 for 30% of iterations) for improvement in QoL (Fig. 5).
Table 4.
Summary of Parkinson’s disease-related quality of life changes from the 19 studies that reported PDQ scores at 6 months
| Author/Reference | Trial interventions | Mean (SD) PDQ score at baseline | Mean PDQ score at 6 months | Mean (SD) reduction in PDQ score at 6 months |
|---|---|---|---|---|
| Antonini et al. (2017) [25] | LCIG | 47.1 (19.0) | – | 9.8 (19.9) |
| Fernandez et al. (2015) [23] | LCIG | 43.2 (15.0) | – | 8.8 (14.7) |
| Olanow et al. (2014) [11] | LCIG | 34.3 (17.6) | – | 10.0 (12.1)a |
| BMT | 38.3 (18.4) | 5.7 (11.8)a | ||
| Palhagen et al. (2016) [24] | LCIG | 32.8 (11.0) | – | 5.9 (11.2) |
| Dafsari et al. (2016) [48] | DBS | 33.2 (18.0) | 24.7 (16.0) | – |
| Dafsari et al. (2018) [34] | DBS | 33.3 (17.4) | 23.3 (14.4) | – |
| Dafsari et al. (2019) [35] | LCIG | 45.3 (18.3) | 37.4 (13.7) | – |
| DBS | 46.9 (12.0) | 37.5 (15.9) | ||
| CSAI | 45.8 (15.4) | 32.2 (16.9) | ||
| Deuschl et al. (2006) [37] | DBS | 41.8 (13.9) | – | 9.5 (15.3) |
| BMT | 39.6 (16.0) | 0.2 (11.2) | ||
| Drapier et al. (2016) [38] | CSAI | 41.2 (15.5) | 36.5 (13.9) | – |
| Floden et al. (2015) [39] | DBS | 28.8 (14.2) | – | 9.1 (11.5) |
| Honig et al. (2009) [40] | LCIG | 44.2 (18.4) | 20.7 (12.0) | – |
| Katzenschlager et al. (2018) [12] | CSAI | 32.7 (15.0) | – | 0.1 (14.7)a |
| BMT | 31.0 (12.7) | − 2.4 (11.8)a | ||
| Kubu et al. (2017) [41] | DBS | 47.9 (23.8) | 25.1 (15.4) | |
| Martinez-Martin et al. (2015) [43] | LCIG | 48.6 (14.6) | 32.0 (14.9) | – |
| CSAI | 49.9 (16.6) | 35.0 (18.0) | ||
| Murata et al. (2016) [44] | LCIG | 35.5 (13.8) | – | 12.0 (11.5)a |
| Soulas et al. (2011) [45] | DBS | 49.9 (11.1) | 41.7 (13.7) | – |
| Timmermann et al. (2015) [46] | DBS | 30.5 (11.0) | – | 9.6 (13.4) |
| Vijiaratanam et al. (2018) [47] | LCIG | 67.0 (18.0) | – | 14.0 (18.9) |
| Weaver et al. (2009) [13] | DBS | 44.9 (13.2) | – | 7.7 (11.5) |
| BMT | 44.3 (13.1) | − 0.4 (2.4) |
BMT best medical treatment, CSAI continuous subcutaneous apomorphine infusion, DBS deep brain stimulation, LCIG levodopa/carbidopa intestinal gel, NR not reported, PDQ Parkinson’s Disease Questionnaire, SD standard deviation
aData at 3 months carried forward
Fig. 4.
Mean (95% CI) Parkinson’s disease-specific quality of life improvement (according to PDQ-39/PDQ-8 score) with the three device-aided therapies versus best medical treatment. The analysis adjusted for baseline age, sex, disease duration, levodopa daily dose, and off-time. BMT best medical therapy, CSAI continuous subcutaneous apomorphine infusion, DBS deep brain stimulation, LCIG levodopa/carbidopa intestinal gel, PDQ Parkinson’s Disease Questionnaire
Fig. 5.
Ranking of device-aided therapy and best medical therapy based on improvement in Parkinson’s disease-related quality of life at 6 months
Although the inconsistency model had a lower posterior mean of the residual difference in PDQ scores and hence a better fit to the data, the difference in DIC between the two models was <1 (Online Resource Table 2B), and no inconsistency was observed for this parameter.
Risk of Bias Assessment
Of the four RCTs assessed using the Cochrane Risk of Bias Tool [30], three studies demonstrated a high risk of bias in at least one domain, and one study demonstrated some concerns of bias due to unclear risk of bias in one domain (see Online Resource Figs. 2 and 5). The two non-randomized cohort studies, assessed using the ROBINS-I Tool, demonstrated a low-to-moderate risk of bias (see Online Resource Figs. 3 and 5). One study had a moderate risk of bias due to confounding. The 16 single-arm studies assessed using the NIH Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Groups, each demonstrated a fair risk of bias due to lack of blinding, low sample size, loss to follow-up, and/or unclear eligibility or selection criteria (see Online Resource Figs. 4 and 5).
Discussion
To our knowledge, this is the first study to evaluate the relative effectiveness of all three currently available device-aided therapies using a Bayesian NMA. Results showed that LCIG and DBS were associated with superior improvement in off-time and PD-related QoL compared with CSAI and BMT at 6 months after treatment initiation. While this analysis suggested that reduction in off-time with CSAI was not significant compared with BMT, the pivotal trials of the three device-aided therapies demonstrated that off-time significantly improved from baseline [11–13]. A reduction of ≥ 1-h/day of off-time is considered clinically meaningful [50], and this was observed for DBS and LCIG, but not for CSAI. While the reduction in off-time was similar for LCIG and DBS, other aspects of these treatments that impact QoL are also considered by patients, caregivers and providers when selecting individualized treatment options. A clinically meaningful improvement in PDQ-8 score is − 5.94 and in PQD-39 is − 4.72 [51], as with off-time such improvements were demonstrated with both LCIG and DBS, but not for CSAI. Patient preference is essential to consider, as some patients may be unwilling to undergo invasive brain surgery for DBS or PEG surgery for LCIG, and would prefer the less invasive procedure of CSAI.
The selection of a device-aided therapy for a patient with advanced PD is a complex process that involves multiple specialties as well as the patient and their caregivers—a shared decision approach is, therefore, essential [52, 53]. While treatment decisions need to be individualized, the choice of device-aided therapy can be guided by some general principles based upon the patient’s age, cognitive function, dyskinesia, and frailty [6]. For example, in patients aged > 70 years, DBS may be suitable in a smaller proportion of patients than infusion therapies. Although older patients can undergo brain surgery if cognitive function is preserved and magnetic resonance imaging (MRI) excludes significant atrophy and vascular lesions, LCIG, or CSAI may be better and safer options [6, 54]. Additionally, there have been suggestions that discontinuation rates with CSAI are relatively high in the long-term due to troublesome adverse events related to the medication (i.e., nausea) or to the route of administration (i.e., subcutaneous infusion-site reactions) [15, 55–57]. A clinical situation in which DBS may be preferred over the infusion therapies is when the patient has severe dyskinesia [58]. Despite the guidance offered by these general principles, comparative data are still needed to assess the benefits of each device-aided therapy. In the absence of head-to-head trials, this NMA provides important information to add to the comprehensive evaluation of patients’ clinical status and preference that is warranted to ensure optimal symptom control and QoL. Despite the apparent benefits of LCIG and DBS versus CSAI and BMT shown in this analysis, identification of patients who may benefit more from one device-aided therapy over another depends on individual patient choice as well as hospital resource availability and healthcare professional experience.
Given the magnitude of benefit achieved with all device-aided therapies, the main take-home message is the need to improve the timely referral and identification of patients whose symptoms are poorly controlled by optimized oral treatment. Patients with advanced PD have a higher disease burden in terms of symptoms and negative effects on ADL and QoL [1], and this may also have a negative impact on the caregivers’ burden [59, 60]. Validated selection criteria and easy-to-use tools for identification of advanced PD (e.g., 5-2-1 criteria [61] and MANAGE-PD [62]) may help to improve the timely introduction of device-aided therapies. Research to identify suitable validated clinical indicators is ongoing [1, 5, 61, 63–65].
Since the literature search was conducted in 2019, a number of studies have been published. No direct comparisons of device-aided therapies with off-time or QoL as the main endpoints were identified in the last 3 years (a comparison of LCIG and DBS on QoL outcomes is ongoing [66]). Likewise, recently published RCTs of individual device-aided therapies have focused on different aspects of treatment such as dyskinesia [67], night-time treatment and sleep disturbances [68], and management of axial features [69]. A host of single-arm and/or observational studies on these three device-aided therapies have been published and some report off-time or QoL as the main outcomes [70–76]; however, the results of these studies would have no clear impact on the current analysis. In the future, a follow-up analysis may help refine the results of the current analysis.
The strengths of the approach taken in our analysis include the simultaneous comparison of the three most widely used device-aided therapies, the use of all evidence by the inclusion of single-arm studies, and the development of an analytical framework that could potentially be used to include future treatments. This analysis also has some limitations. Individual patient-level data were extracted from studies of LCIG only, but with the inclusion of several AD studies of LCIG, we do not believe this results in any bias in either direction. In the absence of sufficient randomized evidence to allow for direct comparisons, the use of MAICs to simulate RCTs and adjust for between-trial differences was conducted to reduce this potential confounding factor [21], and has been accepted as an appropriate methodology by decision-making bodies such as the National Institute for Health and Care Excellence (NICE) [22]. The inclusion of single-arm studies and non-randomized studies was deemed necessary as the number of RCTs was limited (three studies for off-time analysis and four studies for QoL analysis), particularly for LCIG and CSAI (one RCT each). Two RCTs were for DBS [13, 37], which may contribute to biases in favor of DBS, especially as the RCTs for DBS had larger patient samples. Furthermore, the DBS RCT outcomes were available at 6 months, while RCTs for LCIG and CSAI were available at 3 months. To maximize the available data and permit similar timepoint comparisons, the Month 3 data were carried forward to Month 6 for the LCIG and CSAI outcomes; however, greater efficacy benefits could have been experienced by patients on LCIG and CSAI if 6-month data were available. Fewer patients were assessed with CSAI (N = 322) than with LCIG (N = 908) and DBS (N = 705), which is evidenced by the wider CIs for CSAI in off-time reduction and QoL improvement. However, the point estimates for CSAI fall outside of CIs of both DBS and LCIG, suggesting a statistically inferior efficacy of CSAI to DBS and LCIG.
The studies included in the NMA were heterogenous, and therefore, there is the potential to introduce confounding factors. The definition of BMT differed between studies, but considering that adjustment and optimization of medications were permitted across studies, BMT is likely to be similar. Furthermore, only a small proportion of studies (three studies in the off-time analysis and four studies in the QoL analysis) included a BMT arm, so this heterogeneity is unlikely to have had a major influence on the findings. While baseline characteristics differed between studies, matching of studies by age, sex, years since PD diagnosis, daily levodopa dose, off-time and PD-related QoL, and a focus on changes in off-time and QoL rather than their absolute values at 6 months, should have limited confounding effects. Ideally, PD severity would have been a useful addition factor for matching of studies, but this was not commonly reported across studies and daily levodopa dose and baseline off-time may serve as proxies. Specific aspects of QoL may be important in patient/physician preference for a given device-aided therapy for each individual, but sub-domain scores for PDQ-8/PDQ-39 were not consistently available across the studies included in this analysis and so a more detailed analysis of QoL was not possible. Due to these described limitations of the included studies and the varying levels of risk of bias across the RCTs, this limited the meaningfullness of conducting a systematic array of sensitivity analyses (such as conducting the analysis with single-arm studies excluded). Therefore, a robust network meta-analysis was performed, using all available evidence in the published literature with the MAIC approach on the available IPD studies to minimize confounding factors.
In the absence of sufficient randomized evidence to allow for direct comparisons the use of MAICs to simulate RCTs and adjust for between-trial differences was conducted to reduce this potential confounding factor [21], and has been accepted as an appropriate methodology by decision-making bodies such as the NICE. [22].
Conclusions
In conclusion, given the absence of head-to-dead direct comparisons of the three currently available device-aided therapies for advanced PD, this Bayesian network meta-analysis provides comparative data on the clinical and humanistic value of these treatments. Levodopa/carbidopa intestinal gel and DBS demonstrated superior reductions in off-time and improvement in PD-related QoL compared with CSAI and BMT at 6 months after treatment initiation. Understanding the comparative benefits of each treatment provides additional information that can help the patient, caregiver, and provider in the selection of the most appropriate therapy to ensure optimal symptom control and improved QoL. Patients’ treatment preferences must be part of the shared decision approach, and this aspect has been also highlighted by the recent European Guidelines on invasive therapies for PD [20]. Future efforts should focus on the earlier detection of patients who are candidates for device-aided therapy, increasing appropriate referral of these patients, and to broaden the availability of these therapies globally for patients with advanced PD including the potential to increase access to costly treatments for patients in the developing world. Patient preference studies may also inform treatment and reimbursement decision-making.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Yash J Jalundhwala (who is an employee of AbbVie and may own stocks/shares in the company) for his input into the study planning and analysis of data reported in this manuscript. Emi Terasawa (ET) assisted with adjudication of the article screening and data extraction results. Marie Louise Edwards (MLE), Sneha Kelkar (SK) and Francesca Devine (FD) assisted with article screening and data extraction. In later updates of the systematic literature review, they assisted with adjudication of the literature screening and data extraction results. Selina Pi (SP), Benjamin Westermeyer (BW), and Helen Wang (HW) assisted with the article screening and data extraction. Priscilla Lopez (PL) and SP assisted with the RoB assessments. Viviana Garcia Horton (VGH) and ET assisted with adjudications. We acknowledge medical writing support in the drafting of the manuscript and assistance in the submission process from Martin Gilmour of Empowering Strategic Performance (ESP) Ltd, Crowthorne, UK, which was funded by AbbVie in accordance with Good Publication Practice guidelines.
Declarations
Funding Statement
This study and its open access publication was supported and funded by AbbVie Inc. AbbVie participated in study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication.
Conflicts of Interests
Angelo Antonini has received compensation for consultancy and speaker-related activities from UCB, Boehringer Ingelheim, Britannia, AbbVie, Kyowa Kirin, Zambon, Bial, Neuroderm, Theravance Biopharma, Roche. He receives research support from Chiesi Pharmaceuticals, Lundbeck, Bial, Horizon2020 Grant 825785, Horizon2020 Grant 101016902, Ministry of Education University and Research (MIUR) Grant ARS01_01081, Cariparo Foundation. He serves as consultant for Boehringer–Ingelheim for legal cases on pathological gambling. Rajesh Pahwa has received consulting fees from AbbVie, ACADIA, Acorda, Adamas, Cynapsus, Global Kinetics, Lundbeck, Neurocrine, Pfizer, Sage, Sunovion, Teva Neuroscience and US World Meds. He has received research grants from AbbVie, Adamas, Avid, Biotie, Boston Scientific, Civitas, Cynapsus, Kyowa, National Parkinson Foundation, NIH/NINDS, Parkinson Study Group. Per Odin has received compensations for consultancy and speaker-related activities from AbbVie, Bial, Britannia, Ever Pharma, Lobsor, Nordic Infucare, Stada, and Zambon. He has received royalties from Uni-Med Verlag. Stuart Isaacson has received honoraria for CME, consultant, research grants, and/or promotional speaker on behalf of AbbVie, Acadia, Acorda, Adamas, Addex, Affiris, Alexva, Allergan, Amarantus, Amneal, Aptinyx, Axial, Axovant, Benevolent, Biogen, Britannia, Cadent, Cala, Cerecor, Cerevel, Cipla, Eli Lilly, Enterin, GE Healthcare, Global Kinetics, Impax, Impel, Intec Pharma, Ipsen, Jazz, Kyowa, Lundbeck, Merz, Michael J. Fox Foundation, Mitsubishi Tanabe, Neuralys, Neurocrine, Neuroderm, Parkinson Study Group, Pharma2B, Prilenia, Promentis, Revance, Roche, Sanofi, Sunovion, Sun Pharma, Supernus, Teva, Theravance, UCB. Aristide Merola has received grant support from the NIH (KL2 TR001426). He has received speaker honoraria or advisory board honoraria from CSL Behring, AbbVie, Abbott, Medtronic, Theravance, and Cynapsus Therapeutics. He has received grant support from Lundbeck and AbbVie. Lin Wang, Lakshmi P. Kandukuri, Ali Alobaidi, Yanjun Bao, Cindy Zadikoff, Juan Carlos Parra, Lars Bergmann, and Connie H. Yan are employees of AbbVie and may own stocks/shares in the company. K. Ray Chaudhuri has received educational funding from UCB, and honoraria for sponsored symposia from UCB, AbbVie, Britannia, US Worldmeds, Otsuka, Medtronic, Zambon and acted as a consultant for AbbVie, UCB, Britannia, Medtronic, and Mundipharma.
Ethics Approval
As this is a retrospective study with anonymous data, no Ethics Committee or Institutional Review Board approvals were needed.
Consent to Participate
As this is a retrospective study with anonymous data, no patient consent was needed.
Consent to Publish
Not applicable.
Code Availability
Not applicable.
Availability of Data and Material
All data included in this analysis are in the public domain in the individual published studies.
Author Contributions
All authors meet the ICMJE criteria for authorship, and have contributed to the development of the manuscript, and read and approved the final version for submission and agree to be accountable for the work. The concept of the article was provided by Angelo Antonini, Yash J. Jalundhwala and Lin Wang. Lin Wang and Prasanna L. Kandukuri provided the data analysis.
References
- 1.Fasano A, Fung VSC, Lopiano L, Elibol B, Smolentseva IG, Seppi K, et al. Characterizing advanced Parkinson's disease: OBSERVE-PD observational study results of 2615 patients. BMC Neurol. 2019;19(1):50. doi: 10.1186/s12883-019-1276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skorvanek M, Martinez-Martin P, Kovacs N, Zezula I, Rodriguez-Violante M, Corvol JC, et al. Relationship between the MDS-UPDRS and Quality of Life: a large multicenter study of 3206 patients. Parkinsonism Relat Disord. 2018;52:83–89. doi: 10.1016/j.parkreldis.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Martin P, Arroyo S, Rojo-Abuin JM, Rodriguez-Blazquez C, Frades B, de Pedro CJ, et al. Burden, perceived health status, and mood among caregivers of Parkinson's disease patients. Mov Disord. 2008;23(12):1673–1680. doi: 10.1002/mds.22106. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Martin P, Benito-Leon J, Alonso F, Catalan MJ, Pondal M, Zamarbide I, et al. Quality of life of caregivers in Parkinson's disease. Qual Life Res. 2005;14(2):463–472. doi: 10.1007/s11136-004-6253-y. [DOI] [PubMed] [Google Scholar]
- 5.Antonini A, Stoessl AJ, Kleinman LS, Skalicky AM, Marshall TS, Sail KR, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson's disease: a multi-country Delphi-panel approach. Curr Med Res Opin. 2018;34(12):2063–2073. doi: 10.1080/03007995.2018.1502165. [DOI] [PubMed] [Google Scholar]
- 6.Odin P, Ray Chaudhuri K, Slevin JT, Volkmann J, Dietrichs E, Martinez-Martin P, et al. Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson's disease: consensus from an international survey and discussion program. Parkinsonism Relat Disord. 2015;21(10):1133–1144. doi: 10.1016/j.parkreldis.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Luquin MR, Kulisevsky J, Martinez-Martin P, Mir P, Tolosa ES. Consensus on the definition of advanced Parkinson's disease: a neurologists-based Delphi study (CEPA study). Parkinsons Dis. 2017;2017:4047392. [DOI] [PMC free article] [PubMed]
- 8.AbbVie. Duodopa Intestinal Gel summary of product characteristics. 2014 26 June 2014 [cited 12 May 2015]; http://www.medicines.org.uk/emc/medicine/20786. Accessed: Dec 2021.
- 9.Nyholm D, Odin P, Johansson A, Chatamra K, Locke C, Dutta S, et al. Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson's disease patients. AAPS J. 2013;15(2):316–323. doi: 10.1208/s12248-012-9439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.APO-go PFS 5 mg/ml solution for infusion in pre-filled syringe summary of product characteristics. July 2018 [cited; Available from: https://www.medicines.org.uk/emc/product/3908/smpc. Accessed: Dec 2021.
- 11.Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141–149. doi: 10.1016/S1474-4422(13)70293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, et al. Apomorphine subcutaneous infusion in patients with Parkinson's disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2018;17(9):749–759. doi: 10.1016/S1474-4422(18)30239-4. [DOI] [PubMed] [Google Scholar]
- 13.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansouri A, Taslimi S, Badhiwala JH, Witiw CD, Nassiri F, Odekerken VJJ, et al. Deep brain stimulation for Parkinson's disease: meta-analysis of results of randomized trials at varying lengths of follow-up. J Neurosurg. 2018;128(4):1199–1213. doi: 10.3171/2016.11.JNS16715. [DOI] [PubMed] [Google Scholar]
- 15.Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, et al. Long-term safety and efficacy of apomorphine infusion in Parkinson's disease patients with persistent motor fluctuations: results of the open-label phase of the TOLEDO study. Parkinsonism Relat Disord. 2021;83:79–85. doi: 10.1016/j.parkreldis.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Antonini A, Odin P, Pahwa R, Aldred J, Alobaidi A, Jalundhwala YJ, et al. The long-term impact of levodopa/carbidopa intestinal gel on 'off'-time in patients with advanced Parkinson's disease: a systematic review. Adv Ther. 2021;38(6):2854–2890. doi: 10.1007/s12325-021-01747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merola A, Zibetti M, Angrisano S, Rizzi L, Lanotte M, Lopiano L. Comparison of subthalamic nucleus deep brain stimulation and Duodopa in the treatment of advanced Parkinson's disease. Mov Disord. 2011;26(4):664–670. doi: 10.1002/mds.23524. [DOI] [PubMed] [Google Scholar]
- 18.Antonini A, Isaias IU, Rodolfi G, Landi A, Natuzzi F, Siri C, et al. A 5-year prospective assessment of advanced Parkinson disease patients treated with subcutaneous apomorphine infusion or deep brain stimulation. J Neurol. 2011;258(4):579–585. doi: 10.1007/s00415-010-5793-z. [DOI] [PubMed] [Google Scholar]
- 19.Nijhuis FAP, Esselink R, de Bie RMA, Groenewoud H, Bloem BR, Post B, et al. Translating evidence to advanced Parkinson's disease patients: a systematic review and meta-analysis. Mov Disord. 2021;36(6):1293–1307. doi: 10.1002/mds.28599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deuschl G, Antonini A, Costa J, Smilowska K, Berg D, Corvol JC, et al. European Academy of Neurology/Movement Disorder Society-European Section Guideline on the treatment of Parkinson's disease: i. invasive therapies. Mov Disord. 2022;37:1360–1374. doi: 10.1002/mds.29066. [DOI] [PubMed] [Google Scholar]
- 21.Phillipo D, Ades A, Dias S, Palmer S, Abrams K, Welton N. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–211. doi: 10.1177/0272989X17725740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. NICE DSU technical support document 18: Methods for population-adjusted indirect comparisons in submission to NICE. 2016 [cited; Available from: http://nicedsu.org.uk/wp-content/uploads/2017/05/Population-adjustment-TSD-FINAL.pdf. Accessed: Dec 2021.
- 23.Fernandez HH, Standaert DG, Hauser RA, Lang AE, Fung VS, Klostermann F, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson's disease: final 12-month, open-label results. Mov Disord. 2015;30(4):500–509. doi: 10.1002/mds.26123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palhagen SE, Sydow O, Johansson A, Nyholm D, Holmberg B, Widner H, et al. Levodopa-carbidopa intestinal gel (LCIG) treatment in routine care of patients with advanced Parkinson's disease: An open-label prospective observational study of effectiveness, tolerability and healthcare costs. Parkinsonism Relat Disord. 2016;29:17–23. doi: 10.1016/j.parkreldis.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Antonini A, Poewe W, Chaudhuri KR, Jech R, Pickut B, Pirtosek Z, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson's: final results of the GLORIA registry. Parkinsonism Relat Disord. 2017;45:13–20. doi: 10.1016/j.parkreldis.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Centre for Reviews and Dissemination . Systematic reviews: CRD's guidance for undertaking reviews in healthcare. University of York; 2009. [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Network meta-analysis for decision making. Wiley; 2018. [Google Scholar]
- 29. Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ (editors). Chapter 7. Checking for Inconsistency. In: Network meta-analysis for decisionmaking, 1st ed. Wiley; 2018.
- 30.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sterne JAC, Hernan MA, McAleenan A, Reeves BC, Higgins JPT. Assessing risk of bias in a non-randomized study. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available fromhttps://training.cochrane.org/handbook
- 32.NIH. Study Quality Assessment Tools; 2021.[cited 7 November 2022]; Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed: 7 Nov 2022.
- 33.Borgemeester RWK, van Laar T. Continuous subcutaneous apomorphine infusion in Parkinson's disease patients with cognitive dysfunction: a retrospective long-term follow-up study. Parkinsonism Relat Disord. 2017;45:33–38. doi: 10.1016/j.parkreldis.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 34.Dafsari HS, Silverdale M, Strack M, Rizos A, Ashkan K, Mahlstedt P, et al. Nonmotor symptoms evolution during 24 months of bilateral subthalamic stimulation in Parkinson's disease. Mov Disord. 2018;33(3):421–430. doi: 10.1002/mds.27283. [DOI] [PubMed] [Google Scholar]
- 35.Dafsari HS, Martinez-Martin P, Rizos A, Trost M, Dos Santos Ghilardi MG, Reddy P, et al. EuroInf 2: subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson's disease. Mov Disord. 2019;34(3):353–365. doi: 10.1002/mds.27626. [DOI] [PubMed] [Google Scholar]
- 36.De Fabregues O, Dot J, Abu-Suboh M, Hernandez-Vara J, Ferre A, Romero O, et al. Long-term safety and effectiveness of levodopa-carbidopa intestinal gel infusion. Brain Behav. 2017;7(8):e00758. doi: 10.1002/brb3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355(9):896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 38.Drapier S, Eusebio A, Degos B, Verin M, Durif F, Azulay JP, et al. Quality of life in Parkinson's disease improved by apomorphine pump: the OPTIPUMP cohort study. J Neurol. 2016;263(6):1111–1119. doi: 10.1007/s00415-016-8106-3. [DOI] [PubMed] [Google Scholar]
- 39.Floden D, Busch RM, Cooper SE, Kubu CS, Machado AG. Global cognitive scores do not predict outcome after subthalamic nucleus deep brain stimulation. Mov Disord. 2015;30(9):1279–1283. doi: 10.1002/mds.26292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honig H, Antonini A, Martinez-Martin P, Forgacs I, Faye GC, Fox T, et al. Intrajejunal levodopa infusion in Parkinson's disease: a pilot multicenter study of effects on nonmotor symptoms and quality of life. Mov Disord. 2009;24(10):1468–1474. doi: 10.1002/mds.22596. [DOI] [PubMed] [Google Scholar]
- 41.Kubu CS, Cooper SE, Machado A, Frazier T, Vitek J, Ford PJ. Insights gleaned by measuring patients' stated goals for DBS: More than tremor. Neurology. 2017;88(2):124–130. doi: 10.1212/WNL.0000000000003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li D, Zhang C, Gault J, Wang W, Liu J, Shao M, et al. Remotely programmed deep brain stimulation of the bilateral subthalamic nucleus for the treatment of primary Parkinson disease: a randomized controlled trial investigating the safety and efficacy of a novel deep brain stimulation system. Stereotact Funct Neurosurg. 2017;95(3):174–182. doi: 10.1159/000475765. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Martin P, Reddy P, Katzenschlager R, Antonini A, Todorova A, Odin P, et al. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson's disease. Mov Disord. 2015;30(4):510–516. doi: 10.1002/mds.26067. [DOI] [PubMed] [Google Scholar]
- 44. Murata M, Mihara M, Hasegawa K, Beomseok J, Chon-Haw T, Nishikawa N, et al. Efficacy and safety of levodopa-carbidopa intestinal gel from a study in Japanese, Taiwanese, and Korean advanced Parkinson's disease patients. NPJ Parkinsons Dis. 2016;2:16020. [DOI] [PMC free article] [PubMed]
- 45.Soulas T, Sultan S, Gurruchaga JM, Palfi S, Fenelon G. Depression and coping as predictors of change after deep brain stimulation in Parkinson's disease. World Neurosurg. 2011;75(3–4):525–532. doi: 10.1016/j.wneu.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Timmermann L, Jain R, Chen L, Maarouf M, Barbe MT, Allert N, et al. Multiple-source current steering in subthalamic nucleus deep brain stimulation for Parkinson's disease (the VANTAGE study): a non-randomised, prospective, multicentre, open-label study. Lancet Neurol. 2015;14(7):693–701. doi: 10.1016/S1474-4422(15)00087-3. [DOI] [PubMed] [Google Scholar]
- 47.Vijiaratnam N, Hewer S, Varley S, Paul E, Bertram KL, Lee W, et al. Levodopa-carbidopa intestinal gel: is the naso-jejunal phase a redundant convention? Intern Med J. 2018;48(4):469–471. doi: 10.1111/imj.13754. [DOI] [PubMed] [Google Scholar]
- 48.Dafsari HS, Reddy P, Herchenbach C, Wawro S, Petry-Schmelzer JN, Visser-Vandewalle V, et al. Beneficial effects of bilateral subthalamic stimulation on non-motor symptoms in Parkinson's disease. Brain Stimul. 2016;9(1):78–85. doi: 10.1016/j.brs.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 49. MRC Biostatistics Unit, University of Cambridge. DIC: Deviance Information Critera. [cited 7 November 2022]; Available from: https://www.mrc-bsu.cam.ac.uk/software/bugs/the-bugs-project-dic/ . Accessed: 7 Nov 2022.
- 50.Hauser RA, Auinger P, Parkinson Study G Determination of minimal clinically important change in early and advanced Parkinson's disease. Mov Disord. 2011;26(5):813–818. doi: 10.1002/mds.23638. [DOI] [PubMed] [Google Scholar]
- 51.Horvath K, Aschermann Z, Kovacs M, Makkos A, Harmat M, Janszky J, et al. Changes in quality of life in Parkinson's disease: how large must they be to be relevant? Neuroepidemiology. 2017;48(1–2):1–8. doi: 10.1159/000455863. [DOI] [PubMed] [Google Scholar]
- 52.van der Marck MA, Bloem BR. How to organize multispecialty care for patients with Parkinson's disease. Parkinsonism Relat Disord. 2014;20(Suppl 1):S167–S173. doi: 10.1016/S1353-8020(13)70040-3. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen SW, Clausen J, Gregerslund MM. Practical guidance on how to handle levodopa/carbidopa intestinal gel therapy of advanced PD in a Movement Disorder Clinic. Open Neurol J. 2012;6:37–50. doi: 10.2174/1874205X01206010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antonini A, Moro E, Godeiro C, Reichmann H. Medical and surgical management of advanced Parkinson's disease. Mov Disord. 2018;33(6):900–908. doi: 10.1002/mds.27340. [DOI] [PubMed] [Google Scholar]
- 55.Olivola E, Fasano A, Varanese S, Lena F, Santilli M, Femiano C, et al. Continuous subcutaneous apomorphine infusion in Parkinson's disease: causes of discontinuation and subsequent treatment strategies. Neurol Sci. 2019;40(9):1917–1923. doi: 10.1007/s10072-019-03920-5. [DOI] [PubMed] [Google Scholar]
- 56.Stocchi F, Vacca L, De Pandis MF, Barbato L, Valente M, Ruggieri S. Subcutaneous continuous apomorphine infusion in fluctuating patients with Parkinson's disease: long-term results. Neurol Sci. 2001;22(1):93–94. doi: 10.1007/s100720170062. [DOI] [PubMed] [Google Scholar]
- 57.Sesar A, Fernandez-Pajarin G, Ares B, Rivas MT, Castro A. Continuous subcutaneous apomorphine infusion in advanced Parkinson's disease: 10-year experience with 230 patients. J Neurol. 2017;264(5):946–954. doi: 10.1007/s00415-017-8477-0. [DOI] [PubMed] [Google Scholar]
- 58.Antonini A, Tolosa E. Apomorphine and levodopa infusion therapies for advanced Parkinson's disease: selection criteria and patient management. Expert Rev Neurother. 2009;9(6):859–867. doi: 10.1586/ern.09.48. [DOI] [PubMed] [Google Scholar]
- 59.Tessitore A, Marano P, Modugno N, Pontieri FE, Tambasco N, Canesi M, et al. Caregiver burden and its related factors in advanced Parkinson's disease: data from the PREDICT study. J Neurol. 2018;265(5):1124–1137. doi: 10.1007/s00415-018-8816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Timpka J, Nitu B, Datieva V, Odin P, Antonini A. Device-aided treatment strategies in advanced Parkinson's disease. Int Rev Neurobiol. 2017;132:453–474. doi: 10.1016/bs.irn.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Santos-Garcia D, de Deus Fonticoba T, Suarez Castro E, Aneiros Diaz A, McAfee D. 5-2-1 Criteria: a simple screening tool for identifying advanced PD patients who need an optimization of Parkinson’s treatment. Parkinsons Dis. 2020;2020:7537924. [DOI] [PMC free article] [PubMed]
- 62.Antonini A, Odin P, Schmidt P, Cubillos F, Standaert DG, Henriksen T, et al. Validation and clinical value of the MANAGE-PD tool: a clinician-reported tool to identify Parkinson's disease patients inadequately controlled on oral medications. Parkinsonism Relat Disord. 2021;92:59–66. doi: 10.1016/j.parkreldis.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 63.Santos D, de Deus T, Suárez Castro E, Aneiros A. Motor and non-motor symptoms burden, autonomy, quality of life and principal caregiver status is worse in patients with 5-2-1 positive criteria: a simple screening tool for identifying PD patients who need an optimization of Parkinson´s treatment. Mov Disord. 2019;34(suppl. 2):A628. [Google Scholar]
- 64.Santos D, de Deus T, Suárez E. EPR2071.Quality of life is worse in Parkinson’s disease (PD) patients with 5-2-1 positive criteria: a simple screening tool for identifying advance PD cases who need an optimization of Parkinson’s treatment. Eur J Neurol. 2019;26 (suppl.1):238.
- 65. Tsuboi Y, Nakagawa R, Ishido M, Yoshinaga Y, Hashimoto T, Mishima T, et al. EPR3072. The quality of life burden in advanced Parkinson's disease applying "5-2-1" diagnosing criteria: subgroup analyses of the JAQPAD (Japanese QOL survey of Parkinson's disease) study. Eur J Neurol. 2019;26 (suppl. 1):316.
- 66.van Poppelen D, Sisodia V, de Haan RJ, Dijkgraaf MGW, Schuurman PR, Geurtsen GJ, et al. Protocol of a randomized open label multicentre trial comparing continuous intrajejunal levodopa infusion with deep brain stimulation in Parkinson's disease—the INfusion VErsus STimulation (INVEST) study. BMC Neurol. 2020;20(1):40. doi: 10.1186/s12883-020-1621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freire-Alvarez E, Kurča E, Lopez Manzanares L, Pekkonen E, Spanaki C, Vanni P, et al. Levodopa-carbidopa intestinal gel reduces dyskinesia in Parkinson's disease in a randomized trial. Mov Disord. 2021;36(11):2615–2623. doi: 10.1002/mds.28703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Cock VC, Dodet P, Leu-Semenescu S, Aerts C, Castelnovo G, Abril B, et al. Safety and efficacy of subcutaneous night-time only apomorphine infusion to treat insomnia in patients with Parkinson's disease (APOMORPHEE): a multicentre, randomised, controlled, double-blind crossover study. Lancet Neurol. 2022;21(5):428–437. doi: 10.1016/S1474-4422(22)00085-0. [DOI] [PubMed] [Google Scholar]
- 69.Karl JA, Ouyang B, Goetz S, Metman LV. A novel DBS paradigm for axial features in Parkinson's disease: a randomized crossover study. Mov Disord. 2020;35(8):1369–1378. doi: 10.1002/mds.28048. [DOI] [PubMed] [Google Scholar]
- 70.Meira B, Degos B, Corsetti E, Doulazmi M, Berthelot E, Virbel-Fleischman C, et al. Long-term effect of apomorphine infusion in advanced Parkinson's disease: a real-life study. NPJ Parkinsons Dis. 2021;7(1):50. doi: 10.1038/s41531-021-00194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Todt I, Al-Fatly B, Granert O, Kuhn AA, Krack P, Rau J, et al. The contribution of Subthalamic Nucleus Deep Brain Stimulation to the improvement in motor functions and quality of life. Mov Disord. 2022;37(2):291–301. doi: 10.1002/mds.28952. [DOI] [PubMed] [Google Scholar]
- 72.Bove F, Mulas D, Cavallieri F, Castrioto A, Chabardes S, Meoni S, et al. Long-term outcomes (15 Years) after Subthalamic Nucleus Deep Brain Stimulation in patients with Parkinson disease. Neurology. 2021;97:e254–62. doi: 10.1212/WNL.0000000000012246. [DOI] [PubMed] [Google Scholar]
- 73.Volonte MA, Clarizio G, Galantucci S, Scamarcia PG, Cardamone R, Barzaghi LR, et al. Long term follow-up in advanced Parkinson's disease treated with DBS of the subthalamic nucleus. J Neurol. 2021;268(8):2821–2830. doi: 10.1007/s00415-021-10430-y. [DOI] [PubMed] [Google Scholar]
- 74.Antonini A, Abbruzzese G, Berardelli A, Modugno N, Stroppa I, Tamma F, et al. The TANDEM investigation: efficacy and tolerability of levodopa-carbidopa intestinal gel in (LCIG) advanced Parkinson's disease patients. J Neural Transm. 2020;127(6):881–891. doi: 10.1007/s00702-020-02175-1. [DOI] [PubMed] [Google Scholar]
- 75.Standaert DG, Aldred J, Anca-Herschkovitsch M, Bourgeois P, Cubo E, Davis TL, et al. DUOGLOBE: one-year outcomes in a real-world study of levodopa carbidopa intestinal gel for Parkinson's disease. Mov Disord Clin Pract. 2021;8(7):1061–1074. doi: 10.1002/mdc3.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kovacs N, Bergmann L, Anca-Herschkovitsch M, Cubo E, Davis TL, Iansek R, et al. Outcomes impacting quality of life in advanced Parkinson's disease patients treated with levodopa-carbidopa intestinal gel. J Parkinsons Dis. 2022;12(3):917–926. doi: 10.3233/JPD-212979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.