Abstract
We have previously shown that more prominent immune responses are induced to antigens expressed from multicopy plasmids in live attenuated vaccine vector strains of Vibrio cholerae than to antigens expressed from single-copy genes on the V. cholerae chromosome. Here, we report the construction of a ΔglnA derivative of V. cholerae vaccine strain Peru2. This mutant strain, Peru2ΔglnA, is unable to grow on medium that does not contain glutamine; this growth deficiency is complemented by pKEK71-NotI, a plasmid containing a complete copy of the Salmonella typhimurium glnA gene, or by pTIC5, a derivative of pKEK71-NotI containing a 1.8-kbp fragment that directs expression of CtxB with a 12-amino-acid epitope of the serine-rich Entamoeba histolytica protein fused to the amino terminus. Strain Peru2ΔglnA(pTIC5) produced 10-fold more SREHP-12-CtxB in supernatants than did ETR3, a Peru2-derivative strain containing the same fragment inserted on the chromosome. To assess immune responses to antigens expressed by this balanced lethal system in vivo, we inoculated germfree mice on days 0, 14, 28, and 42 with Peru2ΔglnA, Peru2ΔglnA(pKEK71-NotI), Peru2(pTIC5), Peru2ΔglnA(pTIC5), or ETR3. All V. cholerae strains were recoverable from stool for 8 to 12 days after primary inoculation, including Peru2ΔglnA; strains containing plasmids continued to harbor pKEK71-NotI or pTIC5 for 8 to 10 days after primary inoculation. Animals were sacrificed on day 56, and serum, stool and biliary samples were analyzed for immune responses. Vibriocidal antibody responses, reflective of in vivo colonization, were equivalent in all groups of animals. However, specific anti-CtxB immune responses in serum (P ≤ 0.05) and bile (P ≤ 0.001) were significantly higher in animals that received Peru2ΔglnA(pTIC5) than in those that received ETR3, confirming the advantage of higher-level antigen expression in vivo. The development of this balanced lethal system thus permits construction and maintenance of vaccine and vector strains of V. cholerae that express high levels of immunogenic antigens from plasmid vectors without the need for antibiotic selection pressure.
Vibrio cholerae has a number of attributes that make it an attractive candidate for use as a vaccine vector for inducing mucosal immunity against heterologous antigens. V. cholerae is a well-studied noninvasive organism that induces long-lasting mucosal and systemic immune responses (11, 18). Attenuated strains of V. cholerae have already been developed that have been shown to be both safe and immunogenic in humans (2, 12, 13, 16, 26, 28); moreover, vaccine strains of V. cholerae have been developed that are able to secrete large heterologous antigens through the use of the Escherichia coli hemolysin A protein export system (21). Attenuated vaccine strains of V. cholerae have also recently been developed that are able to express immunoadjuvants in vivo, such as LT(R192G), a nonenterotoxic mutant of E. coli heat-labile enterotoxin that retains immunoadjuvant activity (23). Previously, we have shown that the magnitude of immune responses induced against antigens expressed by attenuated vaccine strains of V. cholerae is directly related to the quantity of antigen produced, with more prominent immune responses induced to antigens expressed from multicopy plasmids than to antigens expressed from single-copy genes on the chromosome (22).
In enteric bacteria, glutamine and glutamate serve as the primary nitrogen donors for cellular metabolism (8, 19). Glutamine synthetase, encoded by glnA, is an enzyme required for synthesis of glutamine and is responsible for assimilation of ammonia when extracellular nitrogen concentrations are low (8, 25). The activity and synthesis of glutamine synthetase are regulated by availability of nitrogen (8, 17). Strains of V. cholerae have already been developed that are deficient in glutamine synthetase; these strains are unable to grow on minimal medium lacking glutamine (8–10).
Here we report whether complementation of a glnA chromosomal deletion with a plasmid expressing GlnA could be used as a balanced lethal system for in vivo expression of an antigen from a multicopy plasmid in vaccine and vector strains of V. cholerae.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains and plasmids used in this study are described in Table 1. All strains were maintained at −70°C in Luria-Bertani (LB) broth medium (24) containing 15% glycerol. Streptomycin (100 μg/ml), ampicillin (100 μg/ml), and chloramphenicol (15 μg/ml) were added as appropriate. Cultures were grown at 37°C with aeration in either LB medium, LB medium supplemented with 2 mM glutamine if growing noncomplemented glutamine auxotrophic strains, M9 minimal medium supplemented with 0.05 mM thiamine (Sigma Chemical Co., St. Louis, Mo.) and 0.3 mM cysteine (Sigma), or M9 minimal medium supplemented with 10 mM glutamine (Sigma), 10 mM NH4NO3 (Sigma), 0.05 mM thiamine, and 0.3 mM cysteine if growing noncomplemented glutamine auxotrophic strains. Culturing of stool was done on LB agar plates containing appropriate antibiotics and supplemented with 2 mM glutamine; isolates were confirmed as V. cholerae on thiosulfate-citrate-bile salts-sucrose plates. LB agar plates, made without NaCl and supplemented with 10% sucrose, were used to select for double homologous recombinants lacking the sacB gene during construction of V. cholerae vaccine strains containing the deletion in the chromosomal glnA gene (5, 9, 10, 14).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotypea | Source or reference |
|---|---|---|
| V. cholerae | ||
| C6709 | El Tor, Inaba, wild type; Smr | 27 |
| Peru2 | C6709 ΔattRS1; Smr | 27 |
| ETR3 | Peru2 ΔlacZ lacZ::lppP lacPO→ompA2::SREHP-12::ctxB Smr | 22 |
| Peru2ΔglnA | Peru2 with internal in-frame deletion in glnA of 354 bp (corresponding to amino acids phenylalanine-134 to glycine-251); Smr | This study |
| E. coli SM10λpir | Expresses pir gene product for maintenance of pGP704 derivative vectors containing R6K origin of replication | 14 |
| Plasmids | ||
| pETR5 | pBR322-based derivative expressing SREHP-12–CtxB from 1.8-kbp BglII-PvuI fragment (lppP lacPO→ompA2::SREHP-12::ctxB) Ampr | 22 |
| pCVD442 | pGP704 derivative containing 2.6-kbp fragment with sacB gene of Bacillus subtilis from pUM24; Ampr | 5, 15 |
| pKEK70 | pCVD442 derivative with approximately 720-bp fragment of V. cholerae classical O1 strain O395 glnA with internal in-frame 354-bp deletion (corresponding to amino acids phenylalanine-134 to glycine-251); Ampr | 10 |
| pKEK71 | pACYC184 derivative containing approximately 6-kbp fragment encoding Salmonella typhimurium glnA under control of high-level sigma 54-independent promoter; Tetr, Chloror | 10 |
| pKEK71-NotI | pKEK71 derivative containing BamHI-NotI adapter within tetracycline resistance gene; Chloror | This study |
| pTIC5 | pKEK71-NotI derivative expressing SREHP-12–CtxB from 1.8-kbp BglII-PvuI fragment of pETR5 (lppP lacPO→ompA2::SREHP-12::ctxB) blunt end ligated into BamHI within tetracycline resistance gene; Chloror | This study |
Ampr, ampicillin resistant; Smr, streptomycin resistant; Chloror, chloramphenicol resistant; Tetr, tetracycline resistant.
Genetic methods.
Isolation of plasmid DNA, restriction enzyme digestion, and agarose gel electrophoresis were performed by standard molecular biological techniques (24).
Genetic constructs.
A mutant of V. cholerae Peru2 deficient in glutamine synthesis, Peru2ΔglnA, was constructed by using a derivative of the suicide vector pCVD442 (5, 15). This derivative, pKEK70, contains a copy of the V. cholerae O395 glnA gene with an internal 354-bp deletion (corresponding to amino acids phenylalanine-134 to glycine-251) (10). Plasmid pKEK70 was mobilized from E. coli SM10 λpir into V. cholerae Peru2 by conjugation; recombinants were selected for by ampicillin resistance. Recombinants were grown to turbidity in the absence of selection pressure and plated on LB agar lacking NaCl but containing 10% sucrose (1, 5, 9, 10, 14, 20). Colonies of Peru2ΔglnA that had undergone allelic exchange to introduce the expected internal 354-bp deletion within glnA were confirmed by PCR amplification; isolates were confirmed as being nutritionally auxotrophic on M9 minimal medium lacking glutamine.
Construction of pKEK71 and pETR5 has been previously described (10, 22). Plasmid pKEK71-NotI is a pKEK71 derivative that contains a NotI adapter in BamHI within the tetracycline resistance gene. A 1.8-kbp BglII-PvuI fragment from pETR5 (encoding lppP lacPO→ompA2::SREHP-12::ctxB) was blunt end ligated into the BamHI site of pKEK71-NotI (replacing the NotI adapter), creating plasmid pTIC5. The lppP lacPO→ompA2::SREHP-12::ctxB fragment is identical to that inserted as a single copy into the lacZ gene of Peru2 to create vaccine strain ETR3 (22).
Quantitation of in vitro expression of SREHP-12–CtxB.
Overnight cultures of Peru2ΔglnA(pTIC5) and ETR3 grown in LB and M9 minimal media were divided into supernatant, periplasmic, enriched outer-membrane, and whole-cell fractions as previously described (3, 6, 7). In vitro expression of SREHP-12–CtxB was assayed in a quantitative enzyme-linked immunosorbent assay for CtxB (22, 23). Briefly, serial dilutions of cellular fractions in phosphate-buffered saline–0.05% Tween 20 (PBS-T; Sigma) were applied to 96-well microtiter plates previously coated with type III ganglioside (Sigma). Detection of CtxB was performed by using a 1:2,000 dilution of goat anti-CtxB antibody (List Biological Laboratories, Inc., Campbell, Calif.) in PBS-T, followed by a 1:2,000 dilution of rabbit anti-goat immunoglobulin G (IgG)-horseradish peroxidase conjugate (Southern Biotechnology Associates, Inc., Birmingham, Ala.). Plates were developed with a 1-mg/ml solution of 2,2-azinobis(3-ethylbenzthiazolenesulfonic acid) (ABTS; Sigma) with 0.1% H2O2, and the optical density at 405 nm (OD405) was read in a Vmax microplate reader (Molecular Devices Corp., Sunnyvale, Calif.). Measured optical densities were compared to a standard curve provided by dilutions of purified CtxB (List).
Inoculation and colonization of germfree mice.
Immediately upon removal of mice from the shipping container, five groups of 6 to 12 germfree female Swiss mice, 3 to 4 weeks old (Taconic Farms, Inc., Germantown, N.Y.), were orally inoculated via gastric intubation with 250-μl inocula containing approximately 108 organisms of V. cholerae strains resuspended in 0.5 M NaHCO3 (pH 8.0) (4). Prior to inoculation, Peru2ΔglnA was grown in M9 minimal medium supplemented with glutamine, NH4NO3, thiamine, and cysteine. Peru2ΔglnA(pKEK71-NotI) and Peru2ΔglnA(pTIC5) were grown in M9 minimal medium supplemented with thiamine and cysteine but not containing glutamine. Peru2(pTIC5) was grown in M9 medium containing chloramphenicol and supplemented with thiamine and cysteine. ETR3 was grown in M9 medium containing streptomycin and supplemented with thiamine and cysteine. Mice were subsequently housed under non-germfree conditions. No antibiotic selection pressure or specific nutritional supplementation was continued in vivo. All mice received repeat inocula on days 14, 28, and 42. To assess colonization, fresh stool samples were collected immediately upon passage from all mice every 24 h for the first 96 h after the day 0, 14, and 28 inoculations; pellets were also collected every 48 h from day 4 to day 14 after primary inoculation. Collected pellets were immediately resuspended in 500 μl of M9 medium, vortexed, and allowed to settle. One-hundred-microliter aliquots were plated on LB medium containing streptomycin and supplemented with 2 mM glutamine. Colonies were then replica plated onto thiosulfate-citrate-bile salts-sucrose medium and LB medium containing chloramphenicol and supplemented with 2 mM glutamine to assess the intestinal passage of V. cholerae strains of interest (22).
Immunological sampling.
Mice were sacrificed on day 56, at which time blood, stool, and bile were collected and processed as previously described (23). Processed samples were divided into aliquots and stored in −70°C for subsequent analysis.
Detection of vibriocidal and anti-CtxB antibodies.
Serum vibriocidal antibody titers were measured by a microassay as previously described (22, 23). To detect specific anti-CtxB IgG and IgA antibodies in sera, 100-μl duplicate samples of 1:200 dilutions of sera in PBS-T were placed in wells of microtiter plates previously coated with ganglioside and CtxB (22, 23). Plates were incubated at room temperature overnight and washed, and a 1:2,000 dilution in PBS-T of goat anti-mouse IgG or IgA conjugated to biotin (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was applied to each well. After 1 h of incubation at 37°C, the plates were again washed and a 1:4,000 dilution of streptavidin-horseradish peroxidase conjugate (Zymed Laboratories, Inc., South San Francisco, Calif.) was applied to each well. The plates were then incubated at 37°C for 1 h, washed, and developed with ABTS and 0.1% H2O2; the OD405 was detected kinetically with a Vmax microplate reader as previously described (21, 23). Plates were read for 5 min at 19-s intervals, and the maximum slope for an OD change of 0.2 U was reported as milliunits of OD per minute (21, 23).
To detect specific IgA antibody responses in stool and bile, measurements of total stool and bile IgA were first taken. Duplicate serial twofold dilutions of stool (1:100 to 1:800) and bile (1:800 to 1:102,400) samples in PBS-T were added to wells previously coated with rat monoclonal anti-mouse IgA antibody R5-140 (PharMingen, San Diego, Calif.) (23). After incubation of plates, a 1:2,000 goat anti-mouse IgA-horseradish peroxidase conjugate (Southern Biotechnology Associates) in PBS-T was added to each well and the plates were subsequently developed for horseradish peroxidase activity as described above (23). Comparisons were made to a mouse IgA standard (Kappa TEPC 15; Sigma) (23). To detect specific anti-CtxB IgA antibody in stool and bile, duplicate 200-μl samples of bile or stool containing 200 ng of total IgA in PBS-T were added to wells previously coated with ganglioside-CtxB. After incubation of plates, a 1:2,000 dilution of goat anti-mouse IgA-biotin conjugate (Kirkegaard & Perry) in PBS-T was added. The plates were assayed for horseradish peroxidase activity, and the OD405 was determined kinetically as described above.
Statistics and graphics.
Statistical analysis for the comparison of geometric means was performed for normally distributed data with the independent-sample Student t test or with the Mann-Whitney U test for nonparametric data by use of SPSS for Windows 8.0 (23). Data were plotted with Microsoft Excel 7.0a and GraphPad Prism 3.0.
RESULTS AND DISCUSSION
Construction of glutamine-deficient vaccine strains.
Peru2ΔglnA was constructed by in vivo marker exchange; this strain contains an internal in-frame 354-bp deletion of the glnA gene. Peru2ΔglnA is deficient in glutamine synthetase and is unable to grow on M9 minimal medium lacking glutamine but is able to grow on LB medium or on M9 medium supplemented with glutamine. Introduction of plasmid pKEK71-NotI containing a copy of the Salmonella typhimurium glnA gene under the control of a high-level sigma 54-independent mutant S. typhimurium glnA promoter (glnAp356) (10) complements this deficiency in glutamine synthesis in Peru2ΔglnA and allows Peru2ΔglnA(pKEK71-NotI) to grow on M9 minimal medium lacking glutamine. Plasmid pKEK71-NotI is maintained in glnA-deficient V. cholerae strains grown on M9 minimal medium without the need for antibiotic selection pressure.
A 1.8-kbp fragment (lppP lacPO→ompA2::SREHP-12::ctxB) expressing CtxB with a 12-amino-acid epitope of the serine-rich Entamoeba histolytica protein fused at the amino terminus (SREHP-12–CtxB) was inserted into plasmid pKEK71-NotI to make plasmid pTIC5. Plasmid pTIC5 complements Peru2ΔglnA and allows strain Peru2ΔglnA(pTIC5) to grow on M9 minimal medium lacking glutamine. As is the case for pKEK71-NotI, pTIC5 is maintained in glnA-deficient V. cholerae strains grown on M9 minimal medium without the need for antibiotic selection pressure.
Expression and localization of SREHP-12–CtxB by vaccine strains.
In vitro analysis of cellular fractions for the presence of SREHP-12–CtxB in vaccine strains Peru2(pTIC5) (a vaccine strain containing a wild-type V. cholerae glnA gene on the chromosome), Peru2ΔglnA(pTIC5), and ETR3 showed that essentially all of SREHP-12–CtxB localized to the supernatant fractions. In LB medium containing chloramphenicol, Peru2(pTIC5) expressed 2,339 ± 652 ng/ml/OD600 (geometric mean ± standard error of the mean) of SREHP-12–CtxB in the supernatant; in LB medium containing chloramphenicol, Peru2ΔglnA(pTIC5) expressed 954 ± 445 ng/ml/OD600, and in LB medium supplemented with streptomycin, ETR3 expressed 35 ± 4 ng/ml/OD600. In M9 minimal medium lacking both glutamine and antibiotics, Peru2ΔglnA(pTIC5) expressed SREHP-12–CtxB at 174 ± 52 ng/ml/OD600 and ETR3 expressed 12 ± 21 ng/ml/OD600. In summary, Peru2ΔglnA(pTIC5) expressed approximately 10-fold more SREHP-12–CtxB than did ETR3; this ratio was the same when strains were grown in medium containing antibiotics and when strains were grown in minimal medium lacking glutamine.
Intestinal colonization in mice following oral inoculation of vaccine constructs.
No antibiotic selection pressure or nutritional supplementation was maintained after oral inoculation of any group of mice. Surprisingly, despite complete auxotrophy of Peru2ΔglnA in M9 minimal medium lacking glutamine in vitro, Peru2ΔglnA was recoverable from the stool of mice for 8 days after primary inoculation (Fig. 1). Strains recovered on day 8 after oral inoculation were confirmed to contain the mutant glnA gene by PCR analysis; these isolates were confirmed to be auxotrophic on M9 minimal medium lacking glutamine in vitro. These results suggest that glutamine is present in sufficient quantities in the intestinal lumen of mice to overcome the deficiency in glutamine synthetase in the ΔglnA V. cholerae vaccine strains described in this paper.
FIG. 1.
Aggregate percentages of mice (solid bars) with V. cholerae recovered from stool cultures following oral inoculation with various vaccine strains. Arrows denote dates of oral inoculations. Mice were inoculated on days 0, 14, 28, and 42. Stool was not assayed for V. cholerae strains following the day 42 inoculation. Hatched bars represent aggregate percentages of mice from whom V. cholerae strains containing plasmid pKEK71-NotI or pTIC5 were recovered from stool following oral inoculation.
Compared to the 8-day recovery of Peru2ΔglnA after primary inoculation, V. cholerae vector strains were recoverable for 10 to 12 days after primary inoculation of mice with Peru2ΔglnA(pKEK71-NotI) or Peru2ΔglnA(pTIC5), suggesting a slight in vivo survival advantage for ΔglnA vaccine strains complemented for the mutant glnA gene. Plasmids pTIC5 and pKEK71-NotI were themselves recoverable for 8 to 10 days after primary inoculation of mice, and in vivo stability of pTIC5 was confirmed by restriction digestion of plasmid DNA isolated from strains recovered 6 days after primary inoculation. Corroborating previous results, nonauxotrophic strain ETR3 (containing wild-type glnA on the chromosome) was recoverable from stool for 8 days after primary inoculation (22).
In order to judge in vivo retention of plasmid pTIC5 by a vaccine strain of V. cholerae not requiring complementation of a mutant glnA gene, a cohort of mice were inoculated with Peru2(pTIC5) (containing wild-type glnA on the chromosome). Compared with that of mice that received Peru2ΔglnA(pTIC5), qualitative intestinal colonization of mice that received Peru2(pTIC5) was not appreciably different. V. cholerae vector strains were recoverable for 10 days after oral inoculation, and pTIC5 was maintained by Peru2 in vivo for 8 days after primary inoculation.
These results suggest that Peru2ΔglnA is a glutamine auxotroph in vitro but is not impaired for growth in vivo. Plasmids that complement the introduced glnA mutation do, however, appear to confer a slight in vivo survival advantage on ΔglnA V. cholerae vaccine strains. Colonization of the intestines of mice by ΔglnA strains containing plasmids that complement the glnA mutation was equivalent to that of strains containing wild-type glnA on the chromosome [ETR3 or Peru2(pTIC5)], and the retention of complementing plasmids by strains deficient in glnA was similar to the in vivo retention of plasmids by the parental strain Peru2. Quantitative intestinal colonization studies to assess whether complemented strains are more efficient at surviving in vivo were not performed.
As demonstrated in a previous study (22), V. cholerae vaccine strains were recoverable from stool for only 1 to 3 days following day 14 and later inoculations of mice, presumably due to increased competition from normal intestinal flora of mice housed under non-germfree conditions. Inoculations performed on days 14, 28, and 42 were, however, associated with boosting of the immune responses (data not shown).
Measurement of serum vibriocidal antibody responses.
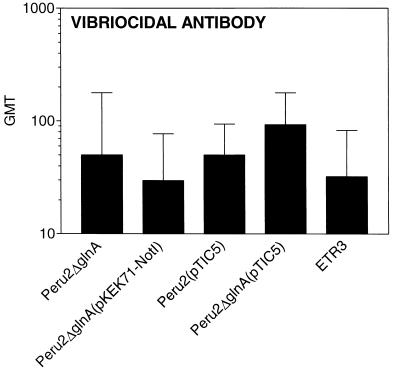
Vibriocidal antibodies were measured on day 56 samples (Fig. 2). Vibriocidal antibodies are a measure of immune responses against V. cholerae organisms themselves and reflect the ability of V. cholerae strains to colonize the intestine. In our experiment, all groups of mice developed vibriocidal antibody responses of comparable magnitudes, confirming the roughly equivalent abilities of the various V. cholerae vaccine strains to colonize the intestines of mice.
FIG. 2.
Geometric mean vibriocidal antibody titers (GMT) on day 56 following oral inoculation of mice on days 0, 14, 28, and 42 with various V. cholerae vaccine strains. An error bar depicts the standard error of the mean for each group. Vibriocidal responses were comparable in all groups of animals.
Measurement of anti-CtxB antibodies.
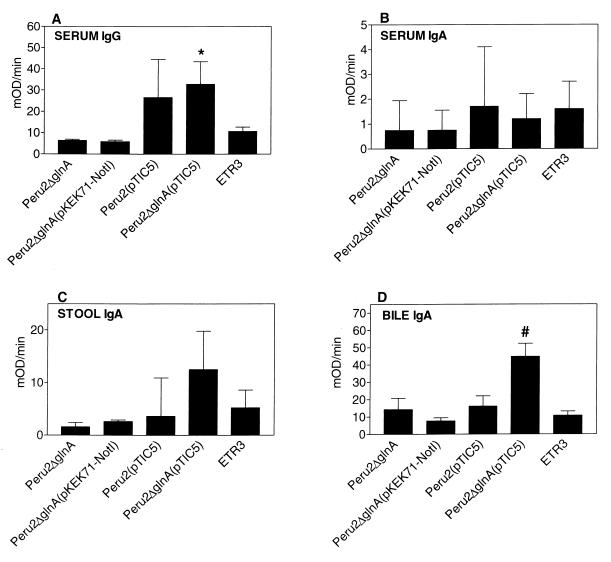
Anti-CtxB antibody responses were measured in day 56 samples of serum, stool, and bile (Fig. 3). As seen previously, a poor but statistically significant anti-CtxB antibody response was measurable in mice that received ETR3 [P ≤ 0.01; serum anti-CtxB IgG compared to responses in mice that received Peru2ΔglnA or Peru2ΔglnA(pKEK71-NotI)] (22). The most prominent anti-CtxB responses, however, were measurable in mice that received Peru2ΔglnA(pTIC5). Compared to the response in mice that received ETR3 expressing SREHP-12–CtxB from a single chromosomal copy, mice that were inoculated with Peru2ΔglnA(pTIC5) expressing SREHP-12–CtxB from the multicopy plasmid had significantly more anti-CtxB IgG antibody in serum (P ≤ 0.05) and mucosal anti-CtxB IgA in bile (P ≤ 0.001). The anti-CtxB IgA response in stool was also most prominent in mice that received Peru2ΔglnA(pTIC5) and approached but did not achieve statistical significance compared to the response measured in mice inoculated with ETR3. It is worth noting that mice that received Peru2ΔglnA(pTIC5) had a significantly higher anti-CtxB response in bile than did mice that received Peru2(pTIC5) (P ≤ 0.02), perhaps reflecting increased mucosal immune responses related to improved retention of plasmid pTIC5 in a ΔglnA V. cholerae strain compared to a V. cholerae strain containing wild-type glnA.
FIG. 3.
Serum IgG (A) and serum (B), stool (C), and bile (D) IgA anti-CtxB enzyme-linked immunosorbent assay results of day 56 samples from mice inoculated with various V. cholerae vaccine-strains. The geometric mean plus the standard error of the mean is reported for each group. mOD/min, milliunits of OD per minute. Symbols: ∗, P ≤ 0.05; #, ≤ 0.001 (compared to groups of animals receiving ETR3).
In summary, this report describes the development of a balanced lethal plasmid system that allows high-level expression of immunogenic antigens in vaccine and vector strains of V. cholerae without requiring antibiotic selection pressure. We have shown that this antigen expression system can induce specific systemic and mucosal immune responses that are more prominent than those elicited by a vaccine strain expressing the same antigen from a single-copy gene on the chromosome. Even though the described ΔglnA V. cholerae strains are auxotrophic for glutamine, their ability to survive in vivo appears to be equivalent to that of vaccine strains of V. cholerae containing wild-type glnA on the chromosome. The development of this balanced lethal plasmid system thus removes the need for antibiotic selection pressure in the construction of V. cholerae vaccine strains expressing immunogenic antigens from plasmid-based systems and removes the need to grow oral inocula of such strains in medium containing antibiotics. The development of this balanced lethal plasmid system should, therefore, assist in the development of improved vaccine and vector strains of V. cholerae.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants KO8 AI01332 (to E.T.R.) and AI40725 (to S.B.C.), both from the National Institute of Allergy and Infectious Diseases.
We are extremely grateful to Samuel L. Stanley, Jr.; Tonghai Zhang; and Lynne Foster for their assistance with CTB-SREHP–12 and to John J. Mekalanos for providing helpful input and V. cholerae Peru2.
REFERENCES
- 1.Blomfield I C, Vaughn V, Rest R F, Eisenstein B I. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol Microbiol. 1991;5:1447–1457. doi: 10.1111/j.1365-2958.1991.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 2.Butterton J R, Boyko S A, Calderwood S B. Use of the Vibrio cholerae irgA gene as a locus for insertion and expression of heterologous antigens in cholera vaccine strains. Vaccine. 1993;11:1327–1335. doi: 10.1016/0264-410x(93)90103-5. [DOI] [PubMed] [Google Scholar]
- 3.Butterton J R, Ryan E T, Acheson D W, Calderwood S B. Coexpression of the B subunit of Shiga toxin 1 and EaeA from enterohemorrhagic Escherichia coli in Vibrio cholerae vaccine strains. Infect Immun. 1997;65:2127–2135. doi: 10.1128/iai.65.6.2127-2135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butterton J R, Ryan E T, Shahin R A, Calderwood S B. Development of a germfree mouse model of Vibrio cholerae infection. Infect Immun. 1996;64:4373–4377. doi: 10.1128/iai.64.10.4373-4377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg M B, DiRita V J, Calderwood S B. Identification of an iron-regulated virulence determinant in Vibrio cholerae, using TnphoA mutagenesis. Infect Immun. 1990;58:55–60. doi: 10.1128/iai.58.1.55-60.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hovde C J, Calderwood S B, Mekalanos J J, Collier R J. Evidence that glutamic acid 167 is an active-site residue of Shiga-like toxin I. Proc Natl Acad Sci USA. 1988;85:2568–2572. doi: 10.1073/pnas.85.8.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klose K E, Mekalanos J J. Simultaneous prevention of glutamine synthesis and high-affinity transport attenuates Salmonella typhimurium virulence. Infect Immun. 1997;65:587–596. doi: 10.1128/iai.65.2.587-596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klose K E, Mekalanos J J. Differential regulation of multiple flagellins in Vibrio cholerae. J Bacteriol. 1998;180:303–316. doi: 10.1128/jb.180.2.303-316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klose K E, Mekalanos J J. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol Microbiol. 1998;28:501–520. doi: 10.1046/j.1365-2958.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 11.Levine M M, Black R E, Clements M L, Cisneros L, Nalin D R, Young C R. Duration of infection-derived immunity to cholera. J Infect Dis. 1981;143:818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- 12.Levine M M, Kaper J B, Herrington D, Ketley J, Losonsky G, Tacket C O, Tall B, Cryz S. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988;ii:467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- 13.Mekalanos J J, Swartz D J, Pearson G D, Harford N, Groyne F, de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 14.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mobley H L, Jarvis K G, Elwood J P, Whittle D I, Lockatell C V, Russell R G, Johnson D E, Donnenberg M S, Warren J W. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of alpha Gal(1-4) beta Gal binding in virulence of a wild-type strain. Mol Microbiol. 1993;10:143–155. doi: 10.1111/j.1365-2958.1993.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 16.Pearson G D, Woods A, Chiang S L, Mekalanos J J. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc Natl Acad Sci USA. 1993;90:3750–3754. doi: 10.1073/pnas.90.8.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter S C, North A K, Kustu S. Mechanism of transcriptional activation by NTRC. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 147–158. [Google Scholar]
- 18.Quiding M, Nordstrom I, Kilander A, Andersson G, Hanson L A, Holmgren J, Czerkinsky C. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-gamma production and evokes local immunological memory. J Clin Investig. 1991;88:143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reitzer L J. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine, and d-alanine. In: Neidhardt F C, Curtiss R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 391–407. [Google Scholar]
- 20.Ried J L, Collmer A. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene. 1987;57:239–246. doi: 10.1016/0378-1119(87)90127-2. [DOI] [PubMed] [Google Scholar]
- 21.Ryan E T, Butterton J R, Smith R N, Carroll P A, Crean T I, Calderwood S B. Protective immunity against Clostridium difficile toxin A induced by oral immunization with a live, attenuated Vibrio cholerae vector strain. Infect Immun. 1997;65:2941–2949. doi: 10.1128/iai.65.7.2941-2949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan E T, Butterton J R, Zhang T, Baker M A, Stanley S L J, Calderwood S B. Oral immunization with attenuated vaccine strains of Vibrio cholerae expressing a dodecapeptide repeat of the serine-rich Entamoeba histolytica protein fused to the cholera toxin B subunit induces systemic and mucosal antiamebic and anti-V. cholerae antibody responses in mice. Infect Immun. 1997;65:3118–3125. doi: 10.1128/iai.65.8.3118-3125.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan E T, Crean T I, John M, Butterton J R, Clements J D, Calderwood S B. In vivo expression and immunoadjuvancy of a mutant of heat-labile enterotoxin of Escherichia coli in vaccine and vector strains of Vibrio cholerae. Infect Immun. 1999;67:1694–1701. doi: 10.1128/iai.67.4.1694-1701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Stadtman E R, Mura U, Chock P B, Rhee S G. The interconvertible enzyme cascade that regulates glutamine synthetase activity. In: Mora J, Palacios R, editors. Glutamine: metabolism, enzymology, and regulation. New York, N.Y: Academic Press, Inc.; 1980. pp. 41–59. [Google Scholar]
- 26.Tacket C O, Losonsky G, Nataro J P, Comstock L, Michalski J, Edelman R, Kaper J B, Levine M M. Initial clinical studies of CVD112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J Infect Dis. 1995;172:883–886. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 27.Taylor D N, Killeen K P, Hack D C, Kenner J R, Coster T S, Beattie D T, Ezzell J, Hyman T, Trofa A, Sjogren M H. Development of a live, oral, attenuated vaccine against El Tor cholera. J Infect Dis. 1994;170:1518–1523. doi: 10.1093/infdis/170.6.1518. [DOI] [PubMed] [Google Scholar]
- 28.Waldor M K, Mekalanos J J. Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J Infect Dis. 1994;170:278–283. doi: 10.1093/infdis/170.2.278. [DOI] [PubMed] [Google Scholar]