Abstract
Objective
This study aimed to investigate the efficacy and tolerability of perampanel (PER) therapy and to optimize a specific plasma reference range for PER in children. Another major aim was to evaluate the potential determinators of PER concentration.
Methods
Concentrations obtained from 80 children were analyzed for routine therapeutic drug monitoring (TDM) between 2021 and 2022. We retrospectively reviewed the clinical data of these patients and assessed the efficacy at 3 months after treatment initiation. Trough concentration‐to‐dose ratio (C 0/Dose ratio) of PER was compared among patients on various potential influencing factors.
Results
A 3‐month PER therapy produced a ≥50% reduction in seizure frequency in 58.8% of patients. Twelve patients reported at least one adverse effect (AE), mainly dizziness. The monitoring data showed that the median C 0 was 325.5 ng/mL. Under maintenance dosages, approximately 75% of the C 0 values were 180.0‐610.0 ng/mL. The C 0/Dose ratio in patients aged 1 to <4 was significantly lower by twofold than in those aged 4 to ≤12 years (P = 0.001). Enzyme‐inducing ASMs (EIASMs) decreased the C 0/Dose ratio of PER by 25.9% (P = 0.165). In addition, seizure frequency reduction in responders was achieved at a median PER C 0 value of 357 ng/mL, which was similar to the value of 314 ng/mL found in nonresponders (P = 0.288). No significant difference was found in PER C 0 values between patients with and without AEs (P = 0.082).
Significance
In this study, PER treatment showed acceptable efficacy and tolerance in Chinese children with epilepsy. Contributing factors like age to variable C 0/Dose ratios were identified, and complex PER‐ASMs interactions were observed. Notably, the reference range, that is, 180.0‐610.0 ng/mL, for routine PER monitoring may be more applicable for them. Routine TDM should be considered a positive attempt to manage the effectiveness and safety of PER.
Keywords: children, epilepsy, perampanel, plasma concentration range, therapeutic drug monitoring
Key Points.
Reports on clinical response, safety, and pharmacokinetics of perampanel (PER) in Chinese children with epilepsy are still lacking.
Therapeutic drug monitoring (TDM) is clinically useful, but the potential influencing factors on PER plasma concentration remain unknown.
PER monotherapy or add‐on therapy seemed to be effective and safe in Chinese children with epilepsy.
The variable plasma PER concentration was associated with age, but independent of body weight, sex and polytherapy.
The suggested reference range for plasma PER concentration is 180.0‐610.0 ng/mL for Chinese children.
1. INTRODUCTION
Perampanel (PER) is a third‐generation antiseizure medication (ASM) and was approved in 2021 to treat focal‐onset seizures, with or without secondarily generalized seizures, in people 4 years of age and older in China. 1 As a noncompetitive selective antagonist of glutamate α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole propionic acid receptors, PER may be essential in reducing seizure‐induced neuronal damage and potential for broad‐spectrum efficacy. 2
In pediatric epilepsy, previous studies found that adjunctive PER therapy appeared effective and tolerable. 3 , 4 , 5 A good efficacy was also seen in PER monotherapy, evidenced by a multicenter, retrospective, observational study. 6 However, daily clinical experiences have shown large interindividual variability in the relationship between plasma PER levels and its clinical effects. Phase III studies revealed a significant association between increases in plasma PER concentration and reduction in seizure frequency, as well as a higher risk of adverse effects (AEs). 7 , 8 But it was not true in another study. 9 Therefore, the inconclusive correlation between PER plasma concentrations and both tolerability and seizure control in children with epilepsy needs to be further evaluated.
Therapeutic drug monitoring (TDM) is essential for evaluating the efficacy and side effects and for providing robust confirmation of pharmacokinetic interactions. Moreover, it also helps identify unknown pharmacokinetic interactions. 10 , 11 Obviously, reliable measurements are mandatory for implementing TDM. In our laboratory, we have published an easy‐to‐use LC–MS/MS assay to monitor plasma PER in children with epilepsy for ordering pediatricians. 12 Of note, defining a specific reference range of PER is meaningful. Although several ranges, for example, 180‐980 ng/mL and 200‐600 ng/mL, have been recommended for those populations, the optimal therapeutic range is still inconclusive. 7 , 13 , 14 For Chinese counterparts, such a reference range has not even been reported. Moreover, the efficacy, tolerability, and pharmacokinetics of PER are still lacking in Chinese pediatric patients.
In fact, various factors may determine the real plasma PER concentration. PER potently interacts with albumin and α‐1‐acid glycoprotein, 15 which might lead to significant drug interactions for the patients receiving polytherapy, due to the potential displacement of concomitant drugs bound to plasma proteins and the potential increase in the free drug fractions. 16 Additionally, PER is metabolized by CYP450 enzyme, especially for CYP3A4, 7 , 17 , 18 which partly determines the efficacy and AEs related to its concentration. 9 It is important to note the effects of enzyme induction on plasma PER concentrations, particularly coadministration with enzyme‐inducing ASMs (EIASMs) (e.g., oxcarbazepine). 3 Unfortunately, there are few reports to evaluate possible factors affecting the systemic exposure to PER, such as daily PER dose, sex, age, body weight (BW), and concomitant ASMs.
Therefore, this retrospective study aimed to (1) review the efficacy and safety of PER as a monotherapy or an adjunctive treatment for Chinese children with epilepsy; (2) optimize a specific plasma reference range for PER according to previous reports; and (3) identify the potential factors determining its plasma concentrations.
2. PATIENTS AND METHODS
2.1. Patients
This retrospective study reviewed the clinical data from children who were diagnosed with epilepsy and received the PER treatment at the Children's Hospital of Nanjing Medical University from June 2021 to April 2022 (Figure 1). Diagnosis of epileptic seizures and syndromes followed the principles of the 2017 International League Against Epilepsy classification of epilepsies. 19 The Ethics Committee of the Children's Hospital of Nanjing Medical University approved the study (Protocol number 202207141‐1). Written consents were waivered due to the retrospective nature of the study.
FIGURE 1.
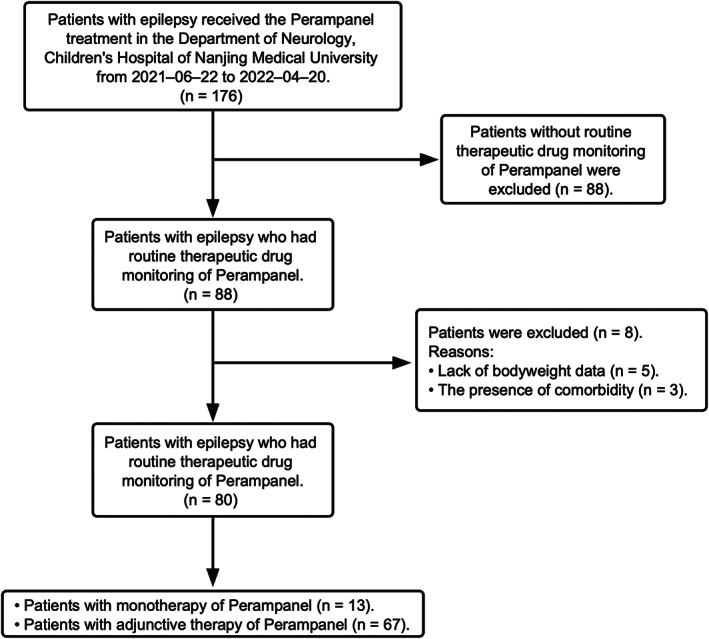
Numbers of patients who were eligible for the study.
2.2. Data collection
We collected various data on age, sex, BW, types of epilepsy, electroencephalography (EEG) findings, magnetic resonance imaging (MRI), duration of epilepsy before starting PER therapy, duration of PER treatment, numbers and types of previous ASMs treatment, concomitant ASMs, treatment response, reported AEs, and reasons for therapy interruption. Specific data on PER, including its initial and maximal dose and routine TDM, if possible, were also reviewed. The efficacy evaluation was based on the change in seizure frequency at least 3‐month follow‐up. AEs were recorded according to the observations of the parents and physicians.
2.3. Definitions of clinical response
Baseline seizure frequency was calculated over the 3 months prior to initiation of PER therapy. According to the change in seizure frequency, the patients were divided into the responder group and the nonresponder group 14 : (1) responders, that is, patients with a ≥50% reduction in seizure frequency or absence of seizures on unchanged medication; and (2) nonresponders, that is, patients with a <50% reduction in seizure frequency on unchanged medication.
2.4. Treatment protocol
All patients in this study received once‐daily oral administration of PER as monotherapy or add‐on therapy at bedtime. 20 The dose of PER was titrated according to the individual patient response aiming to balance the efficacy and tolerability.
Generally, the daily PER dose can be stratified according to the real BW. 21 For children aged 4‐12 years, weighing ≥30 kg, the starting dose was 2 mg/d. For children weighing ≥20 but <30 kg, the starting dose was 1 mg/d. For pediatric patients below the age of 4 years or weighing <20 kg, the starting dose was 0.5 mg/d. Thereafter, the dose was tailored to the maintenance dose of 2‐8 mg/d after 1‐2 weeks. Considering plasma PER concentrations potentially become lower in patients once receiving concomitant EIASMs (i.e., oxcarbazepine and topiramate) than in those taking non‐EIASMs (i.e., valproic acid, levetiracetam, lacosamide, zonisamide, and clonazepam), the dose is generally adjusted when coadministration with EIASMs.
2.5. Routine therapeutic monitoring of PER
Blood samples were collected at a steady‐state concentration of PER, which was defined as the patient took the same PER dosing schedule for at least 21 days. 12 , 22 The bioanalysis was performed on an LC–MS/MS system. In brief, the LC–MS/MS system consisted of a Triple Quad™ 4500MD mass spectrometer (AB Sciex Pte. Ltd) interfaced via a Turbo V™ ion source with a Jasper™ liquid chromatography system (AB Sciex Pte. Ltd), which comprised a binary pumps (Sciex Dx™), an online degasser (Sciex Dx™), an autosampler (Sciex Dx™), and a column oven (Sciex Dx™). To the best of our knowledge, commercial kits of PER are unavailable up till now in China. A published method was used for monitoring PER, which was developed and validated in our laboratory. 12 The inter‐batch precision (CV, %) and accuracy (Bias, %) results of plasma PER concentration based on three‐level quality control (QC) samples over the past 6 months were ≤ ±9% and within the acceptable criteria (≤ ±15%) 23 (Table S1).
2.6. Statistical analysis
All data were statistically analyzed using GraphPad Prism 9 (GraphPad Software) and SPSS version 26.0 software (IBM). Normality was assessed using Shapiro–Wilk tests. The frequency for categorical variables, means and standard deviations for normally distributed continuous variables, and median with an interquartile range for nonnormally distributed continuous variables were described for demographic data and clinical characteristics, respectively. Pearson's chi‐square test and Fisher's exact test were used to compare categorical variables. Mann–Whitney U test was used to compare continuous variables. Kruskal–Wallis test and Dunn's test were used to evaluate the differences between independent groups. An analysis of Spearman's correlation coefficient was conducted to test the correlations. A P value of <0.05 was considered statistically significant.
3. RESULTS
3.1. Patients' characteristics
A total of 80 children were in our final study cohort according to the inclusion and exclusion criterion. The characteristics of the patients are summarized in Table 1.
TABLE 1.
Clinical characteristics of patients
| Characteristics | Value |
|---|---|
| Age (y) | |
| Median | 7.8 |
| IQR | 6.5 |
| Sex | |
| M | 36 |
| Weight (kg) | |
| Median | 28 |
| IQR | 21 |
| Type of epilepsy, n (%) | |
| Focal | 53 (66.3) |
| Generalized | 6 (7.5) |
| Combined generalized and focal | 3 (3.8) |
| Unknown | 18 (22.4) |
| Dose (mg/kg) | |
| Median | 0.16 |
| IQR | 0.1 |
| Number of previous ASMs | |
| Median | 2 |
| IQR | 2 |
| Number of ASMs when PER initiated, n (%) | |
| 0 | 13 (16.3) |
| 1 | 25 (31.3) |
| 2 | 34 (42.5) |
| 3 | 9 (11.3) |
| 4 | 2 (2.5) |
| Concomitant ASMs, n (%) | |
| VPA | 32 (40.0) |
| LEV | 23 (28.8) |
| OXC | 19 (23.8) |
| CZP | 16 (20.0) |
| LMT | 18 (22.5) |
| TPM | 11 (13.8) |
| LCM | 2 (2.5) |
| ZNS | 3 (3.8) |
Abbreviations: ASM, antiseizure medicine; CZP, clonazepam; LCM, lacosamide; LEV, levetiracetam; LMT, lamotrigine; M, male; OXC, oxcarbazepine; PER, perampanel; TPM, topiramate; VPA, valproic acid; ZNS, zonisamide.
3.2. Efficacy
The clinical outcomes of patients are shown in Table 2. In total, 51 patients had at least a 3‐month follow‐up period. The response rate for these patients was 58.8%, and 31.4% (n = 16) of them achieved seizure freedom. In addition, as compared to patients with generalized as well as combined focal and generalized seizures, 65.9% of patients with focal seizures responded well to the PER therapy. Children with ≥4 years of age, particularly those more than 12 years old, had better clinical outcomes than patients with <4 years of age (P = 0.025). And 63.6% of males got seizure reduction ≥50%, but there was no statistical difference between males and females (P = 0.579). No difference between the subgroups with a BW of ≤20, 20‐40, or ≥40 kg (P = 0.293). Intriguingly, there was no statistical difference between monotherapy and add‐on therapy (P = 0.069), but PER monotherapy appeared to be more effective than adjunctive therapy.
TABLE 2.
Efficacy and tolerability at 3 mo after starting PER therapy
| Variable | Number | Percent (%) | Responders (n, %) | Nonresponders (n, %) | P‐value | Adverse effects (n, %) | P‐value |
|---|---|---|---|---|---|---|---|
| Total | 51 | 100.0 | 30 (58.8) | 21 (41.2) | 7 (13.7) | ||
| Age (y) | |||||||
| < 4 | 11 | 21.6 | 3 (27.3) | 8 (72.7) | 0.025* | 1 (9.1) | 0.577 |
| 4‐12 | 32 | 62.7 | 20 (62.5) | 12 (37.5) | 4 (12.5) | ||
| ≥12 | 8 | 15.7 | 7 (87.5) | 1 (12.5) | 2 (25.0) | ||
| Sex | |||||||
| M | 22 | 43.1 | 14 (63.6) | 8 (36.4) | 0.579 | 3 (13.6) | 0.999 |
| F | 29 | 56.9 | 16 (55.2) | 13 (44.8) | 4 (13.8) | ||
| Weight (kg) | |||||||
| ≤20 | 18 | 35.3 | 9 (50.0) | 9 (50.0) | 0.293 | 1 (5.6) | 0.108 |
| 20‐40 | 20 | 39.2 | 11 (55.0) | 9 (45.0) | 2 (10.0) | ||
| ≥40 | 13 | 25.5 | 10 (76.9) | 3 (23.1) | 4 (30.8) | ||
| Range of C 0 values (ng/mL) | |||||||
| <180 | 5 | 9.8 | 3 (60.0) | 2 (40.0) | 0.590 | 1 (20.0) | 0.609 |
| 180‐610 | 41 | 80.4 | 23 (56.1) | 18 (43.9) | 6 (15.0) | ||
| >610 | 5 | 9.8 | 4 (80.0) | 1 (20.0) | 0 | ||
| Adjunctive therapy | |||||||
| With 1 ASMs | 15 | 29.4 | 11 (73.3) | 4 (26.7) | 2 (13.3) | ||
| With 2 ASMs | 22 | 43.1 | 12 (54.5) | 10 (45.5) | 4 (18.2) | ||
| With 3 ASMs | 7 | 13.7 | 2 (28.6) | 5 (71.4) | 0 | ||
| With 4 ASMs | 2 | 3.9 | 0 | 2 (100.0) | 0 | ||
| With EIASMs | 7 | 13.7 | 5 (71.4) | 2 (28.6) | 0.999 | 2 (28.5) | 0.179 |
| With non‐EIASMs | 18 | 35.3 | 11 (61.1) | 7 (38.9) | 1 (5.6) | ||
| Total | 46 | 90.2 | 25 (54.3) | 21 (45.7) | 0.069 | 6 (13.0) | 0.537 |
| Monotherapy | 5 | 9.8 | 5 (100.0) | 0 | 1 (20.0) | ||
Abbreviations: ASM, antiseizure medicine; EIASM, enzyme‐inducing antiseizure medicine; F, female; M, male; PER, perampanel.
P < 0.05.
3.3. Tolerability
Mild or moderate AEs occurred in 12 patients (15%). The most frequently reported AEs were dizziness (n = 8), and 50% of them were accompanied by somnolence (n = 2), irritability (n = 1), and ataxia (n = 1). Five patients of them developed AEs within 2 months of PER treatment, and another seven patients experienced AEs after more than 3 months (Table 2). Children ≥12 years old had a higher AEs than younger children (P = 0.577). Patients with a BW of ≤20 kg had a lower AEs than patients with a BW of >20 kg (P = 0.108). No statistical difference was found between PER with EIASMs and non‐EIASMs (P = 0.179).
3.4. TDM of PER
In total, 91 measures were recorded for all 80 patients, with C 0 values found to be between 30.1 and 937.0 ng/mL (Figure 2A). Notably, approximately 75% of the monitored C 0 values ranged from 180.0 to 610.0 ng/mL. Moreover, in the range of 180.0‐610.0 ng/mL, 69.5% of patients were followed up for 3 months or more and 56.1% (n = 23) responded well to PER mono‐ or add‐on therapy (Figure 2A). Similarly, in PER add‐on therapy, 76 measures were conducted for 67 patients, and 54.3% (n = 25) of patients had seizures reduced by >50% (Figure 2B). Moreover, approximately 80.0% of the C 0 values were scattered at 180.0‐610.0 ng/mL (Figure 2C) in monotherapy.
FIGURE 2.
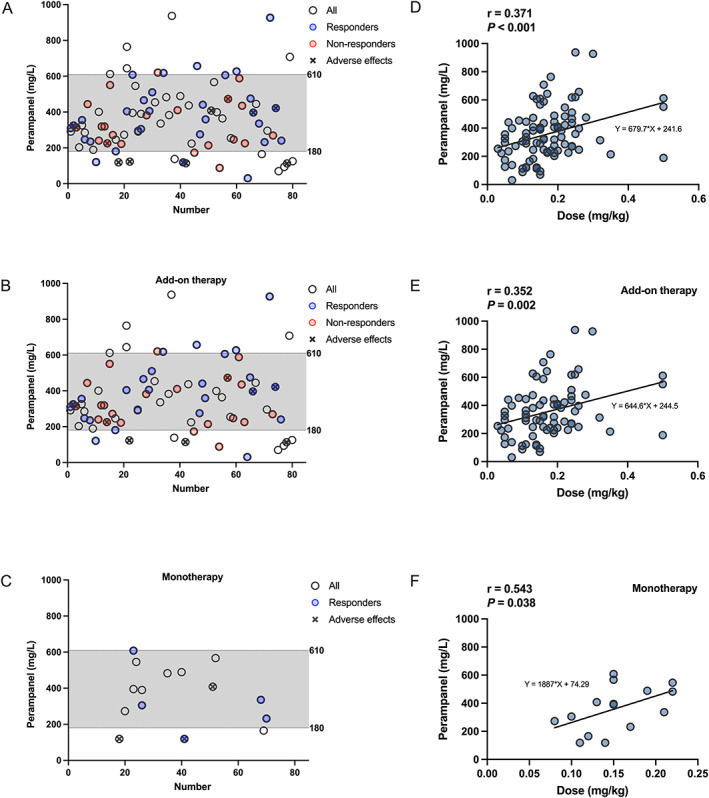
Plasma PER C 0 (ng/mL) measures of the maintenance dose in children with epilepsy. The x‐axis shows the number of patients. The hollow dots depict the measured C 0 values. Red circles denote C 0 measures of the nonresponder group, and blue circles denote C 0 measures of the responder group, and circles with × denote C 0 measures of the adverse‐effects group, respectively. (A) C 0 values in 80 children with epilepsy. (B) C 0 values in add‐on therapy. (C) C 0 values in monotherapy. (D‐F) show the correlation between C 0 (ng/mL) and dose (mg/kg). (D) In all 80 patients (n = 91); (E) in adjunctive therapy (n = 76); (F) in monotherapy (n = 15).
There was a weak but positive relationship between the plasma PER C 0 values and administration doses in both mono‐ or adjunctive therapy (r = 0.371; P < 0.001; Figure 2D). Similar results were obtained for PER monotherapy and add‐on therapy (Figure 2E,F; P < 0.05).
3.5. Age, BW, sex, and the C 0 /Dose ratio of PER
In patients with PER mono‐ or adjunctive therapy, we found no correlation between age and C 0/Dose ratio (r = 0.114; P = 0.283; Figure 3A), but the dose‐corrected C 0 values in children with 1 to <4 years of age were twofold lower than those in patients with 4 to <12 years of age (P = 0.001; Figure 3B). There was no correlation between BW and C 0/Dose ratio (r = 0.182; P = 0.085; Figure 3C) and no statistical difference among patients with a BW of ≤20 kg, between 20 and 40 kg, and ≥40 kg (P = 0.313; Figure 3D). Similarly, no significant difference was found between males and females in C 0/Dose ratio (P = 0.999).
FIGURE 3.
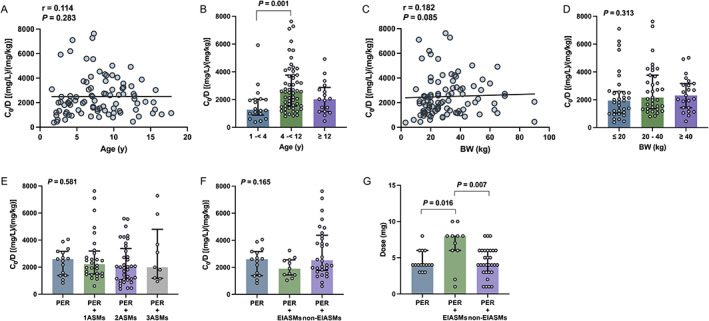
Association between C 0/Dose ratio [(ng/mL)/(mg/kg)] and various influencing factors. (A, B) Age; (C, D) BW; (E) coadministration with 1 ASM, 2 ASMs, and 3 ASMs; (F) coadministration with enzyme‐inducing ASMs (EIASMs) and non‐EIASMs. (G) A comparison of dose between monotherapy and coadministration with EIASMs and non‐EIASMs.
3.6. Concomitant drugs and the C 0 /Dose ratio of PER
Totally, no significant difference was found in the C 0/Dose ratio between PER monotherapy and add‐on therapy with different numbers of ASMs (P = 0.581; Figure 3E). The plasma concentration of PER as add‐on therapy with EIASMs was lower by 25.9% than that of PER monotherapy, but no significant difference among PER monotherapy, PER + EIASMs, or PER + non‐EIASMs (P = 0.165; Figure 3F).
Interestingly, there was a significant difference in dose levels in patients with different types of concomitant ASMs. The median dose in PER monotherapy was 4 mg daily and was twofold lower than add‐on therapy with EIASMs (P = 0.016, Figure 3G). Similarly, the median dose in adjunctive therapy with non‐EIASMs was half of the median dose in PER with EIASMs (P = 0.007, Figure 3G).
3.7. Other factors determining the C 0 /Dose ratio of PER
The duration of PER treatment, disease duration, MRI, and EEG readings in these pediatric patients were evaluated, and only negligible effects were observed.
3.8. Relationship between plasma concentrations and clinical outcomes
Twelve patients (15%) reported at least one AE, mainly dizziness and somnolence. No significant difference was found in median plasma PER concentrations (269.5 vs 331.0 ng/mL) (P = 0.082, Figure 4A) between patients with and without AEs.
FIGURE 4.
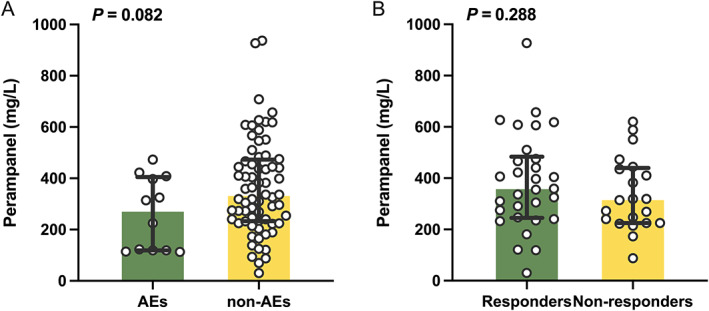
(A) Comparison of C 0 between patients with adverse effects (AEs) (n = 12) and non‐AEs (n = 68); (B) a comparison of C 0 between responders (n = 30) and nonresponders (n = 21).
In total, 51 patients had completed no <3 months of follow‐up (Table 2). The median plasma concentration was 325.0 ng/mL (range: 30.1‐927.0 ng/mL). Thirty patients (58.8%) were responders, with a median PER C 0 value of 357.0 ng/mL, which was similar to the value of 314.0 ng/mL observed in nonresponders (P = 0.288, Figure 4B).
4. DISCUSSION
Pediatric patients are particularly at risk of seizures, and the core of pharmacological treatment for epilepsy is to eliminate seizures completely while minimizing the adverse effects of ASMs. To achieve this, drug dosages need to be personalized based on a better understanding of its mechanism of action, efficacy, pharmacokinetics, and tolerability. This retrospective study assessed the efficacy and safety of PER as mono‐ and add‐on therapy in Chinese pediatric patients. Particularly, we optimized, for the first time, the TDM reference range of PER for those children and evaluated how the demographic and clinical variables determined the plasma PER concentration.
In terms of efficacy and tolerability, the PER seemed to be a good choice for Chinese children with epilepsy based on our findings. After 3‐month follow‐up, the response rate for 51 patients was 58.8%, and 31.4% of them even achieved seizure freedom. At the same time, AEs occurred in 13.7% (7/51) of patients yet, the most of which was dizziness. This was in line with a very recent report. 3
To further evaluate the influencing factors on the efficacy and tolerability, we divided patients by age, BW, sex, distribution of C 0 values, and drug combination. Notably, we found that children ≥4 years of age, particularly those more than 12 years old, had a better response than patients ages <4 years of old. But they experienced more PER‐associated AEs. These findings were like several previous reports. 5 , 24 , 25 Intriguingly, seizure freedom was achieved much easier when PER was used as a monotherapy, if not, an early add‐on (≤2 prior ASMs) was better than late (≥3 prior ASMs) (Table 2), just as previous reports. 6 , 26
As late add‐on therapy to epilepsy, PER treatment seemed to be less effective and safe than an early add‐on or monotherapy. However, the interpretation of this apparent finding should be more cautious. Late add‐on therapy or polytherapy is often performed in patients with refractory or drug‐resistant epilepsy. The progression of the disease itself and/or complex drug–drug interactions (DDIs) can occur simultaneously when PER is added to the therapy. The evaluation of efficacy and tolerability at this time point is not so much the result of the add‐on of the PER treatment only, but rather the result of the sum of all factors. The main reason for this situation is the retrospective nature, which cannot accurately stratify patients according to the concomitant ASMs, thereby losing control over the heterogeneity of enrolled cases. It may be imprecise for PER to simply attribute the non‐PER idiopathic AEs that occurred at this time to the PER therapy.
An impressive finding in this study was that a matched range of plasma PER levels was provided firstly for Chinese children with epilepsy. To be honest, the reference concentration ranges of PER are still open for discussion. 7 , 13 , 14 In our study, approximately 75% of the monitored C 0 values ranged from 180.0 to 610.0 ng/mL. Notably, in the range of 180.0‐610.0 ng/mL, 56.1% of patients responded well to PER mono‐ or adjunctive therapy, while AEs occurred only in several patients. Therefore, the data in our hands suggested that aiming at a C 0 (180.0‐610.0 ng/mL), by comparison, might be alternative and more suitable when PER was used as monotherapy or adjunctive therapy for Chinese children with epilepsy.
One of the major strengths of this study was the routine plasma PER monitoring for the first time for childhood epilepsy in China, so we have the chance to examine the impact of various variables on the C 0/Dose ratio of PER in our subjects. We found the C 0/Dose ratios in children with 1 to <4 years of age were twofold lower than those in patients with 4 to <12 years of age. An increase in C 0/Dose ratio with age appeared to be accompanied by a rapid total clearance, which was similar to the previous study. 27 One particular concern was the potential DDIs in this study. Surprisingly, on the whole, no significant differences were found in the C 0/Dose ratio between PER monotherapy and add‐on therapy with different numbers of ASMs (Figure 3E). However, compared with monotherapy, the plasma PER concentration as add‐on therapy with EIASMs was lower by 25.9%, but no statistical difference. Similar findings were observed in previous reports. 28 , 29 Therefore, oral use of PER in combination with EIASMs may thus require dose tailoring to achieve the same efficacy in seizure control.
Another question we asked in this study was whether there was a possible association between plasma PER levels and clinical outcomes. In this study, there was a trend toward higher plasma PER concentrations in seizure‐free patients, but not statistically different between responders and nonresponders, which was in line with previous research. 9 , 27 In addition, no significant difference was found either in median plasma PER concentrations (269.5 vs 331.0 ng/mL) between patients with and without the occurrence of AEs. 30
This study was retrospective in nature with several limitations. Firstly, the sample size was relatively small due to the new approval in China for pediatric patients. As such, our findings should be viewed cautiously as a reference. Secondly, the treatment periods of 80 children included were variable, and we had to rely on real‐world clinical reports rather than prospective seizure diaries. Even so, we still successfully reviewed the effectiveness data of PER, alone or in co‐therapy, over a variety of time periods with variable numbers of enrolled subjects. In addition, the plasma total drug levels, instead of the free PER concentrations, were measured in the current study, which appeared to sacrifice the informative and predictive role of PER concentration data generated by free drug assays. Nevertheless, the real‐world clinical findings from this study, particularly in the context of plasma PER monitoring in children, may be of great application to pediatricians and TDM pharmacists if they want to tailor its dosages for precision therapy.
In addition, the sampling method should also be considered when implementing TDM in children. Compared with conventional venipuncture sampling, the microsampling strategies, like dried blood spots (DBS) technique, are less invasive and seemed more appropriate when dealing with pediatric TDM. 31 , 32 , 33 In our laboratory, the “wet method” for plasma PER concentration was successful. Alternatively, the development of DBS‐based methods for PER and other ASMs should be established, and this is an ongoing work.
5. CONCLUSIONS
In this retrospective study, it was confirmed that the PER treatment was effective and tolerable in Chinese children with epilepsy. Of note, based on the data in our hands, we proposed an alternative reference range of plasma PER levels, that is, 180.0‐610.0 ng/mL, for pediatric patients in China. Determinators of plasma PER concentration were identified, and we observed complex DDIs when PER was taken with EIASMs. Considering the existing study limitations, future research is warranted.
AUTHOR CONTRIBUTIONS
Li had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Chen and Lu involved in concept, design, administrative, technical, or material support, and supervision. All authors involved in acquisition, analysis, or interpretation of data. Li and Chen involved in drafting of the manuscript. Li, Dong, and Chen involved in critical revision of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Table S1
ACKNOWLEDGMENTS
This research was supported by the Specially Appointed Medical Expert Project of the Jiangsu Commission of Health (2019).
Li Y, Dong N, Qin Y‐X, Dai H‐R, Hu Y‐H, Zhao Y‐T, et al. Therapeutic drug monitoring of perampanel in children diagnosed with epilepsy: Focus on influencing factors on the plasma concentration‐to‐dose ratio. Epilepsia Open. 2022;7:737–746. 10.1002/epi4.12653
Na Dong, Hao‐Ran Dai and Yue‐Tao Zhao are visiting graduate students from the China Pharmaceutical University.
Yu‐Xin Qin is a visiting undergraduate student from the Nanjing Medical University.
[Correction added on 26 October 2022, after first online publication: The units have been changed from mg/L to ng/ml throughout]
Contributor Information
Jing Chen, Email: chenjing5640042@163.com.
Xiao‐Peng Lu, Email: lxp20071113@sina.com.
Feng Chen, Email: cy.chen508@gmail.com.
REFERENCES
- 1. Anti‐epileptic drug fycompa® approved in China as monotherapy for partial‐onset seizures and pediatric indication for partial‐onset seizures.
- 2. Trinka E, Lattanzi S, Carpenter K, Corradetti T, Nucera B, Rinaldi F, et al. Exploring the evidence for broad‐spectrum effectiveness of perampanel: a systematic review of clinical data in generalised seizures. CNS Drugs. 2021;35:821–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ishikawa N, Tateishi Y, Tani H, Kobayashi Y, Kobayashi M. Clinical profiles associated with serum perampanel concentrations in children with refractory epilepsy. Epilepsy Behav. 2019;94:82–6. [DOI] [PubMed] [Google Scholar]
- 4. Qu R, Dai Y, Chen X, Li R, Liu M, Zhu Y. Effectiveness and safety of perampanel in Chinese paediatric patients (2‐14 years) with refractory epilepsy: a retrospective, observational study. Epileptic Disord. 2021;23:854–64. [DOI] [PubMed] [Google Scholar]
- 5. Biro A, Stephani U, Tarallo T, Bast T, Schlachter K, Fleger M, et al. Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies: first experiences. Neuropediatrics. 2015;46:110–6. [DOI] [PubMed] [Google Scholar]
- 6. Toledano Delgado R, Garcia‐Morales I, Parejo‐Carbonell B, Jimenez‐Huete A, Herrera‐Ramirez D, Gonzalez‐Hernandez A, et al. Effectiveness and safety of perampanel monotherapy for focal and generalized tonic‐clonic seizures: experience from a national multicenter registry. Epilepsia. 2020;61:1109–19. [DOI] [PubMed] [Google Scholar]
- 7. Gidal BE, Ferry J, Majid O, Hussein Z. Concentration‐effect relationships with perampanel in patients with pharmacoresistant partial‐onset seizures. Epilepsia. 2013;54:1490–7. [DOI] [PubMed] [Google Scholar]
- 8. Krauss GL, Serratosa JM, Villanueva V, Endziniene M, Hong Z, French J, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial‐onset seizures. Neurology. 2012;78:1408–15. [DOI] [PubMed] [Google Scholar]
- 9. Steinhoff BJ, Hubers E, Kurth C, Jurges K‐KU. Plasma concentration and clinical effects of perampanel‐the Kork experience. Seizure. 2019;67:18–22. [DOI] [PubMed] [Google Scholar]
- 10. Johannessen Landmark C, Patsalos PN. Methodologies used to identify and characterize interactions among antiepileptic drugs. Expert Rev Clin Pharmacol. 2012;5:281–92. [DOI] [PubMed] [Google Scholar]
- 11. Stepanova D, Beran RG. The benefits of antiepileptic drug (AED) blood level monitoring to complement clinical management of people with epilepsy. Epilepsy Behav. 2015;42:7–9. [DOI] [PubMed] [Google Scholar]
- 12. Dai H‐R, Hu Y‐H, Long J‐Y, Xia Y, Guo H‐L, Xu J, et al. LC‐MS/MS assay for the therapeutic drug monitoring of perampanel in children with drug‐resistant epilepsy. Acta Chromatogr. 2022. doi: 10.1556/1326.2022.01023 [DOI] [Google Scholar]
- 13. Patsalos PN. The clinical pharmacology profile of the new antiepileptic drug perampanel: a novel noncompetitive AMPA receptor antagonist. Epilepsia. 2015;56:12–27. [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto Y, Shiratani Y, Asai S, Usui N, Nishida T, Imai K, et al. Pharmacokinetics, tolerability, and clinical effectiveness of perampanel in Japanese patients with epilepsy. Seizure. 2020;83:181–6. [DOI] [PubMed] [Google Scholar]
- 15. Patsalos PN, Zugman M, Lake C, James A, Ratnaraj N, Sander JW. Serum protein binding of 25 antiepileptic drugs in a routine clinical setting: a comparison of free non‐protein‐bound concentrations. Epilepsia. 2017;58:1234–43. [DOI] [PubMed] [Google Scholar]
- 16. Charlier B, Coglianese A, De Rosa F, de Grazia U, Operto FF, Coppola G, et al. The effect of plasma protein binding on the therapeutic monitoring of antiseizure medications. Pharmaceutics. 2021;13:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacob S, Nair AB. An updated overview on therapeutic drug monitoring of recent antiepileptic drugs. Drugs R D. 2016;16:303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sabenca R, Bicker J, Silva R, Carona A, Silva A, Santana I, et al. Development and application of an HPLC‐DAD technique for human plasma concentration monitoring of perampanel and lamotrigine in drug‐resistant epileptic patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1162:122491. [DOI] [PubMed] [Google Scholar]
- 19. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Biase S, Nilo A, Bernardini A, Gigli GL, Valente M, Merlino G. Timing use of novel anti‐epileptic drugs: is earlier better? Expert Rev Neurother. 2019;19:945–54. [DOI] [PubMed] [Google Scholar]
- 21. Renfroe JB, Mintz M, Davis R, Ferreira J, Dispoto S, Ferry J, et al. Adjunctive perampanel oral suspension in pediatric patients from ≥2 to <12 years of age with epilepsy: pharmacokinetics, safety, tolerability, and efficacy. J Child Neurol. 2019;34:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamamoto Y, Usui N, Nishida T, Takahashi Y, Imai K, Kagawa Y, et al. Therapeutic drug monitoring for Perampanel in Japanese epilepsy patients: influence of concomitant antiepileptic drugs. Ther Drug Monit. 2017;39:446–9. [DOI] [PubMed] [Google Scholar]
- 23. Bioanalytical method validation guidance for industry. 2022.
- 24. Heyman E, Lahat E, Levin N, Epstein O, Lazinger M, Berkovitch M, et al. Tolerability and efficacy of perampanel in children with refractory epilepsy. Dev Med Child Neurol. 2017;59:441–4. [DOI] [PubMed] [Google Scholar]
- 25. Yun Y, Kim D, Lee YJ, Kwon S, Hwang SK. Efficacy and tolerability of adjunctive perampanel treatment in children under 12 years of age with refractory epilepsy Korean. J Pediatr. 2019;62:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villanueva V, Montoya J, Castillo A, Mauri‐Llerda JA, Giner P, Lopez‐Gonzalez FJ, et al. Perampanel in routine clinical use in idiopathic generalized epilepsy: the 12‐month GENERAL study. Epilepsia. 2018;59:1740–52. [DOI] [PubMed] [Google Scholar]
- 27. Ikemoto S, Hamano SI, Hirata Y, Matsuura R, Koichihara R. Efficacy and serum concentrations of perampanel for treatment of drug‐resistant epilepsy in children, adolescents, and young adults: comparison of patients younger and older than 12 years. Seizure. 2019;73:75–8. [DOI] [PubMed] [Google Scholar]
- 28. Contin M, Bisulli F, Santucci M, Riva R, Tonon F, Mohamed S, et al. Effect of valproic acid on perampanel pharmacokinetics in patients with epilepsy. Epilepsia. 2018;59:e103–8. [DOI] [PubMed] [Google Scholar]
- 29. Patsalos PN, Gougoulaki M, Sander JW. Perampanel serum concentrations in adults with epilepsy: effect of dose, age, sex, and concomitant anti‐epileptic drugs. Ther Drug Monit. 2016;38:358–64. [DOI] [PubMed] [Google Scholar]
- 30. Contin M, Pondrelli F, Muccioli L, Mohamed S, Santucci M, Ferri L, et al. Relationship between plasma concentrations and clinical effects of perampanel: a prospective observational study. Epilepsy Behav. 2020;112:107385. [DOI] [PubMed] [Google Scholar]
- 31. D'Urso A, Cangemi G, Barco S, Striano P, D'Avolio A, de Grazia U. LC‐MS/MS‐based quantification of 9 antiepileptic drugs from a dried sample spot device. Ther Drug Monit. 2019;41:331–9. [DOI] [PubMed] [Google Scholar]
- 32. Dubois S, Marchese F, Pigliasco F, Barco S, Tripodi G, Lomonaco T, et al. A volumetric absorptive microsampling technique to monitor cannabidiol levels in epilepsy patients. Front Pharmacol. 2020;11:582286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pigliasco F, Barco S, Dubois S, Marchese F, Striano P, Lomonaco T, et al. Cannabidiol determination on peripheral capillary blood using a microsampling method and ultra‐high‐performance liquid chromatography tandem mass spectrometry with on‐line sample preparation. Molecules. 2020;25:3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


