Abstract
New‐onset refractory status epilepticus (NORSE) is associated with high mortality, therapy‐resistant epilepsy (TRE), and poor cognitive and functional outcomes. Some patients develop multifocal TRE, for whom surgery with a curative intention, is not an option. In these patients, vagus nerve stimulation (VNS) is performed as a palliative treatment. We report the long‐term outcomes regarding seizure frequency, functional and cognitive outcome, and effectiveness of VNS in two patients with TRE as a consequence of NORSE. In the first patient with cryptogenic NORSE, VNS implantation occurred during the acute stage, probably contributing to the cessation of her status epilepticus. However, in the long‐term follow‐up, the patient persisted with daily multifocal seizures. In the second patient, VNS implantation was delayed to manage his epilepsy when the NORSE, ultimately due to autoimmune encephalitis, had resolved. During long‐term follow‐up, no reduction in seizure frequency was achieved. This evidence supporting the use of VNS in patients with TRE after NORSE warrants further investigation.
Keywords: autoimmune epilepsy, encephalitis, status epilepticus, therapy‐resistant epilepsy, VNS
1. INTRODUCTION
New‐onset refractory status epilepticus (NORSE) is a clinical presentation of refractory status epilepticus (RSE) in previously healthy individuals, without an acute structural, toxic, or metabolic cause. 1 In approximately 50% of cases, the etiology is unknown, while in the other half, autoimmune encephalitis is the most common cause. 2 No specific therapy is currently available for those patients; therefore, combinations of antiseizure medications (ASMs), anesthetics, immunotherapy, and ketogenic diet are prescribed in the attempt to stop NORSE. 3 Furthermore, few cases of acute implantation of vagus nerve stimulator (VNS) have been described. 4 Most treatment recommendations are based on expert opinion.
NORSE is associated with high mortality and poor cognitive and functional outcomes. 2 Around 40% of patients with NORSE develop epilepsy afterward. 2 , 3 Some patients can develop bitemporal or multifocal therapy‐resistant epilepsy (TRE), for whom surgery with a curative intention is not an option. 5 In these patients, the implantation of VNS is performed as a palliative treatment. Although the general effectiveness of VNS has been described, 6 there are insufficient data regarding its long‐term outcomes in patients after NORSE.
We describe two patients who had NORSE and developed TRE epilepsy. The VNS was implanted in the acute and chronic phase, respectively. The long‐term outcomes will be reviewed.
2. CASES PRESENTATION
2.1. Patient 1
A 37‐year‐old woman, with an unremarkable medical history, was found unconscious after a week of mild flu‐like symptoms, including fever. She was brought to the emergency department where a bilateral tonic–clonic seizure was witnessed. Her disease progressed to multifocal RSE. Different ASMs and anesthetics were prescribed without success. The initial work‐up was done early and was negative for structural, toxic, metabolic, and infectious etiologies. Autoimmune encephalitis was proposed as a possible etiology, and she completed a course of corticosteroids, intravenous immunoglobulin (IVIG), and rituximab weekly, while she was admitted to intensive care unit (ICU). When the results arrived, her complete autoimmune panel was negative. After 30 days of NORSE, she had received two more doses of rituximab, but she persisted in super‐RSE (SRSE). At that time, VNS was implanted, and rapid upwards titration of output current was established, reaching 1.25 mA in 48 hours and 1.75 mA on the seventh day. On the same day, the SRSE was aborted. Additionally, on the seventh day, she completed three doses of rituximab without changes to her ASMs or anesthetics during the preceding days. Even though NORSE was aborted, focal seizures, some of which progressed to bilateral tonic–clonic seizures, persisted (Table 1). Six doses of rituximab were given, and immunotherapy was continued as an outpatient using azathioprine, without improvement of the seizure frequency. She never experienced a seizure‐free period, and daily seizures occurred despite different combinations of ASMs, VNS, and azathioprine. After 1.5 years, she was admitted to the epilepsy monitoring unit (EMU). Immune work‐up was repeated and was negative. Multifocal epilepsy due to NORSE of unknown etiology was the final diagnosis. At the last follow‐up, 2 years after onset of NORSE, she persisted with daily focal seizure with impairment of awareness despite four ASMs and high VNS parameters. Neuropsychological evaluation showed moderate global cognitive and mild emotional involvement, affecting her social life (Table 1).
TABLE 1.
Clinical features, diagnostic investigations, and treatment during NORSE and Epilepsy after NORSE
| PHASE 1: NORSE | Patient 1 | Patient 2 |
|---|---|---|
| Current Age/Age at onset (years) | 39/37 | 44/33 |
| Etiology | Unknown | Encephalitis anti‐NMDAR no tumor associated |
| NORSE duration | 37 days | 42 days |
| Serum and CSF studies | ||
| Infectious |
CSF(a): Encephalitis panel, bacterial and fungal culture: Negative Serum: Hepatitis, HIV, VDRL, CMV: Negative |
CSF (b): Encephalitis panel, bacterial and fungal culture, VDRL: Negative Serum: Hepatitis, HIV, VDRL, Lyme, CMV: Negative |
| Inflammatory/immune |
CSF: Autoimmune panel: negative Serum: Autoimmune panel, ANA, Anti dsDNA, anti‐nucleosomes, anti‐histones, anti DFS70, ANCA, anti‐tiroglobuline, Rheumatoid factor: Negative |
CSF: Autoimmune panel: anti‐NMDA positive Serum: ANA, anti dsDNA, anti‐ENA, ANCA, Rheumatoid factor: Negative |
| Paraneoplastic |
CSF (Neoplastic cytology): Negative CT (Thorax‐Abdomen‐Pelvis) and Ultrasound (abdomen, pelvis) were negative for tumor |
CSF (Neoplastic cytology): Negative CT (Thorax‐Abdomen‐Pelvis) and Ultrasound (scrotum, thyroid, abdomen) were negative for tumor |
| MRI findings |
At onset: Negative During NORSE: Hypersignal in FLAIR in the left mesial temporal lobe and left insular white matter 1.5 Years after, the abnormal signal disappeared |
At onset: Negative During NORSE: Hypersignal in FLAIR in bilateral mesial temporal lobe, left occipital and splenium At discharge from hospital: Subtle signal change in the left hippocampus and small residual signal in splenium |
| EEG findings | Continuous multifocal spikes and polyspikes (maximum right posterior temporal region) | Burst suppression pattern with multifocal spikes (left temporal, left frontal, right frontocentral and bifrontal) |
| Treatment | ||
| ASM | PHT, LEV, LCM, CLB, PB, VPA, PER, LZP | PHT, PB, LEV |
| Anesthetics | Midazolam, Ketamine, Propofol | Midazolam, Fentanyl, Ketamine, Propofol |
| Antimicrobials | Acyclovir, Ceftriaxone, Vancomycin, Piperacilin/tazobactam (partial treatment) | Acyclovir, Vancomycin, Piperacilin/tazobactam (Partial course) |
| Immunomodulatory agents | Methylprednisolone, IVIG, Rituximab (six doses‐weekly), azathioprine (maintenance therapy) | Methylprednisolone (followed by Prednisone), azathioprine (maintenance therapy) |
| VNS |
Cessation of NORSE was 7 days after VNS was implanted: Day 0: OC = 0.25 mA, F = 20 Hz, PW = 250 μs, ON = 30 s, OFF = 30 min Day 1: OC = 1.0 mA, F = 20 Hz, PW = 250 μs, ON = 30 s, OFF = 30 min Day 2: OC = 1.25 mA, F = 20 Hz, PW = 250 μs, ON = 30 s, OFF = 30 min Day 7: OC = 1.75 mA, F = 20 Hz, PW = 250 μs, ON = 30 s, OFF = 30 min |
Implanted after NORSE was controlled |
| PHASE II: Epilepsy after NORSE | Patient 1 | Patient 2 |
|---|---|---|
| Current Age/Age at onset (years) | 39/37 | 44/33 |
| Latent period after NORSE | No | 8 months |
| Seizure classification | Type 1: focal impair awareness nonmotor seizures to bilateral tonic clonic seizures: déjà vu/ hear voices and sounds ➔ behavioral arrest ➔ eyes deviation to the left ➔ left facial asymmetry ➔ left arm dystonia ➔ head and eyes deviation to the right ➔ sometimes bilateral clonic movements. Type 2: strange sensation ➔ touches her head ➔ smiling ➔ whistling ➔ speak some words with a bit delayed in reacting | Type 1: focal impair awareness nonmotor seizures: behavioral arrest ➔ staring ➔ loss of awareness ➔ right hand automatisms /dystonic posture of the left hand. (Type 1 sometime progress to bilateral tonic–clonic) |
| EEG findings | Interictal: Multifocal independent spikes augmented during sleep (almost 2 years after NORSE). Ictal: onset changes among seizures with the majority over right posterior temporal region and a few over left temporal region (almost 2 years after NORSE) | Inter Ictal: Independent bitemporal spikes, maximum right (8 years after NORSE) Ictal: Onset over left temporal region mostly and over right temporal region in a lesser degree (3 years after NORSE) |
| MRI findings | Slightly increased T2 signal is present in both hippocampi. The abnormal signal in left insular region disappeared (almost 2 years after NORSE) | Bilateral hippocampal atrophy and T2 hyperintense signal abnormality. Resolution of signal abnormality in splenium (9 years after NORSE) |
| Epilepsy classification | Multifocal Epilepsy | Independent Bi Temporal Epilepsy |
| Etiology | Unknown | Structural (Bilateral MTS)/“Autoimmune‐associated” |
| Past ASM | PHT, LEV, LCM, CLB, PB, VPA, PER, LZP | LCS, ESL, PER, PHT, BRV |
| Current ASM | LEV, LCM, PER, ESL | PB, VPA, TPM, LTG |
| Other treatment | Immunotherapy: Azathioprine 150 mg/d since NORSE | None |
| VNS | Implanted at 30 days after NORSE (cessation of SRSE occurred 7 days after VNS was implanted) | Implanted 9 years after NORSE |
| Frequency of seizures before VNS | N.A. | 12 per month |
| Frequency of seizures after VNS (Output current mA) |
1 month: Up to 5 (2.5) 3 months: 1‐2 per day (2) 6 months: 1‐2 per day (2) 12 months: 1‐3 per day (2) 18 months: 1‐3 per day (2.25) 22 months: 1‐3 per day (3) 24 months: 1‐3 per day (3) |
3 months: 15 per month (0.85) 6 months: 10 per month (1.75) 8 months: 2‐3 months (2) 12 months: 2 months (2) 18 months: 2‐3 month (2) 21 months: 12 per month (2.75) 24 months:8‐12 per month (3) |
| Cognitive outcome | Mild‐to‐severe generalized impairments across all cognitive domains (Neuropsychological evaluation) | Multifocal Cognitive Impairment. Probably nondominant (more likely right) mesial temporal impairment and frontal dysfunction (not clearly lateralized). Dominant temporal impairment (more likely left) not ruled out |
| Emotional outcome | Mild symptoms of depression and anxiety (She took antidepressants when symptoms were severe) | No evidence of depression or any other mood disturbance |
| Social outcome | She is unable to carry out all of her previous activities but is able to take care of her own affairs without help. She works from home as a writer of educational material | Not currently working as truck driver. He is on a disability support program |
Note: Cytochemical study in CSF showed mild pleocytosis: a:14 cel; b: 20 cel (lymphocyte predominance). Encephalitis panel: Herpes Simple Virus I‐II, varicella zoster, and enterovirus RNA. Autoimmune panel: Anti‐AMPA, anti‐CASPR, anti DPPX, anti‐GABA antibody, anti‐LGI1, anti‐NMDA, anti‐GAD65, anti IgLON5.
Abbreviations: ANA, Antinuclear antibodies; ANA, Nuclear Antigen Antibodies; ANCA, Antineutrophil cytoplasmic antibody; ASM, antiseizure medication; BRV, brivaracetam; CLB; clobazam; CMV, Cytomegalovirus; CSF, cerebrospinal fluid; CT, Computerized tomography; dsDNA, double stranded DNA; EEG, electroencephalogram; ESL, eslicarbazepine; HIV, human immunodeficiency virus; IVIG, Intravenous Immunoglobulin; LCM, lacosamide; LEV, Levetiracetam; LTG, lamotrigine; LZP, Lorazepam; MRI, magnetic resonance imaging; MTS, mesial temporal sclerosis; N.A, not applicable; NMDAR, N‐methyl‐D‐aspartate receptor; PB, phenobarbital; PER, perampanel; PHT, phenytoin; SRSE, super refractory status epilepticus; TPM, topiramate; VDRL, test for syphilis; VNS, Vagus nerve stimulator; VPA, valproic acid.
2.2. Patient 2
A previously healthy 33‐year‐old man presented with RSE that progressed to SRSE over a few days. His NORSE presentation was preceded by flu‐like symptoms, without fever, 1 week before the onset. The SRSE required management in ICU, and an extensive work‐up was completed after which structural, toxic, metabolic, and infections causes were ruled out. Seventy‐two hours later, the cause of SRSE had not yet been identified, and the presentation was consistent with NORSE. Simultaneously, seizure management was initiated using different ASMs and anesthetics. After 6 weeks, the SRSE was aborted. However, due to a persistent state of confusion, an autoimmune encephalitis was considered, and immunotherapy was started. At that time, the autoimmune panel revealed a positive anti‐N‐methyl‐D‐aspartate receptor (NMDAR) antibody in CSF. Imaging studies and serum investigations were negative for a paraneoplastic syndrome. The patient improved, and 1.5 months later, he was discharged home with three ASMs, prednisone, and azathioprine (Table 1). Eight months later, when a taper of ASMs was trialed, the patient developed focal nonmotor seizures with impairment of awareness, which sometimes progressed to bilateral tonic–clonic seizures. Despite different ASMs and immunosuppressive treatments, the frequency of seizures worsened over time. Three years later, the patient was investigated in the EMU, and multiple independent bitemporal seizures were captured. Brain magnetic resonance imaging (MRI) showed bilateral mesial temporal sclerosis (MTS) and cortical atrophy mainly in the left posterior quadrant (Figure 1). A repeat autoimmune panel was negative. Azathioprine was discontinued, and VNS was proposed, which the patient declined. Over the subsequent 9 years, seizure frequency increased to up to twelve per month. At that time, the patient reconsidered VNS, which was implemented with the parameters increasing over time. Eight months after the VNS implantation, the frequency of seizures improved reaching 2‐3 per month. However, this was not sustained. Two years after the implantation, the seizure frequency returned to 8‐12 seizures per month. His neuropsychological assessment showed multifocal cognitive impairment, as well as poor quality of life (Table 1).
FIGURE 1.
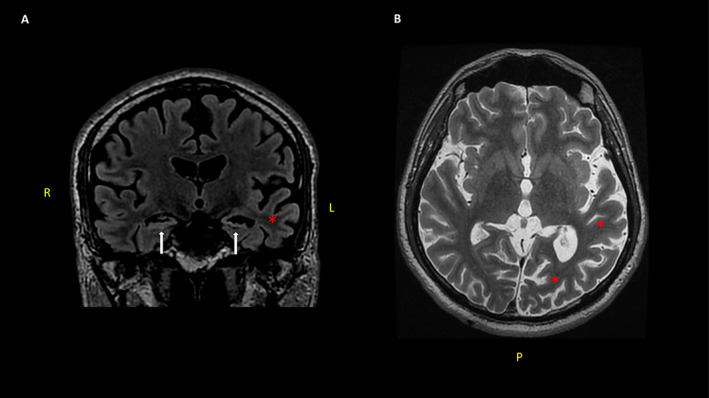
Bilateral mesial temporal sclerosis and left temporo‐occipital cortical atrophy were seen in Patient 2. Coronal FLAIR MRI showing bilateral atrophy and hyper signal in both hippocampi (white arrows) (A). Axial T2 MRI shows important cortical atrophy especially in the left posterior quadrant (red asterisks) (B). R (right); L (left); P (posterior)
3. DISCUSSION
We presented two patients with multifocal and bitemporal TRE, respectively, that developed as a consequence of NORSE. In Patient 1, the clinical presentation was compatible with febrile infection‐related epilepsy syndrome (a subtype of NORSE), and the etiology was unknown despite an extensive work‐up. In addition, there was no clear response to immune‐based therapies over time, which potentially argues against an autoimmune etiology. For this patient, VNS was implanted during the SRSE in an attempt to resolve the SRSE. In Patient 2, the NORSE was eventually found to be caused by an anti‐NMDAR antibody encephalitis. In this second patient, VNS was implanted in a delayed manner to manage escalating seizures, years after the NORSE had presented. In both patients, the NORSE led to prolonged admissions to ICU and resulting in TRE.
The effectiveness of VNS in patients during NORSE and with epilepsy after NORSE is unknown. A recent systematic review focused on the use of VNS in the acute management of RSE and included nine patients with NORSE. The analysis reported that for 66% (six of nine) of patients RSE ceased after the implantation of VNS. 4 The systematic review included only case reports, which were the highest level of available evidence for this rare condition. There was a clear relationship between VNS and cessation of RSE in only two cases. 7 , 8 A different report details a patient whose implantation was considered successful, seizures recurred, and the patient died. 9 In the other reports, it is unclear which of the multiple treatments or a combination thereof were responsible for ceasing the NORSE. 4 , 10 In Patient 1, about whom we report, a rapid upwards titration of output current was completed without side effects reaching a stimulation intensity of 1.75 mA by the seventh day, which coincided with cessation of status epilepticus. However, discrete seizures persisted daily, and the patient developed epilepsy without a latent period after NORSE. In this patient, no reduction in the frequency of seizures was achieved over time despite the combination of different ASMs, immunotherapy, and high parameters of VNS.
Even though in Patient 2, the NORSE was due to autoimmune encephalitis, the immunological study was negative in the post‐NORSE stage, which may explain the lack of response to chronic immunotherapy. At this stage, structural changes (such as MTS, focal, or diffuse cortical atrophy) would be the main mediators in seizure production. In this patient, the term “autoimmune‐associated epilepsy,” that was recently proposed by the International League Against Epilepsy (ILAE), is appropriate to highlight how other nonimmune factors may play a role in postautoimmune encephalitis epilepsy. 11 In this patient, the implantation of VNS was late (9 years after NORSE), and although he experienced a significant reduction (75%) in the frequency of seizures, this was not sustained overtime.
In our first patient, the VNS titration was fast, reaching 1.75 mA in 1 week; in the second patient it was slow, reaching 2 mA in 8 months. The safety of rapid titration is currently unknown, and several factors need to be considered to avoid complications and reduce adverse effects. Notably, in the acute stages of NORSE, patients are sedated and unconscious and therefore less likely to experience side effects.
The VNS implantation in TRE post‐NORSE has been described in three patients: two patients with FIRES 5 and one with acute encephalitis with refractory repetitive partial seizures (AERRPS), 12 and only the last patient experienced a decrease in the number of seizures of more than 50% in follow‐up. The mechanisms how VNS can improve NORSE are still unknown. An anti‐inflammatory theory has been proposed. Brain inflammation may play a role in increasing the hyperexcitability during NORSE, and VNS has shown to reduce levels in interleukins in patients with TRE. 4 , 5 The lack of response to VNS in the chronic stage for both of our patients could be explained by a lack of immunological role in the chronic stages of their disease course. However, we do not know whether our first patient would be worse without VNS.
Furthermore, we have described the poor cognitive, emotional, and social outcomes that developed our patients. Our findings show that the long‐term effect of NORSE leads to multifocal impairments across different cognitive domains, which is likely to have a direct impact on quality of life.
4. CONCLUSION
NORSE has significant long‐term morbidity, including therapy‐resistant epilepsy and cognitive, emotional, and social consequences. Available treatments lack effectiveness We describe two cases with TRE after NORSE, who underwent VNS implantation. Although the VNS implantation was done at different points in the illness trajectory (acute and chronic phase of NORSE), we show the long‐term outcomes after the implantation of VNS, which is still unknown and warrants further investigation. Our case description also adds to the literature with respect to the effect of VNS during the acute phase of NORSE. In fact, in the first case, VNS likely contributed controlling status epilepticus acutely; however, the current evidence supporting its use is poor.
CONFLICT OF INTEREST
Jorge G. Burneo is the Jack Cowin Endowed Chair in Epilepsy Research at Western Ontario. The remaining authors have no conflict of interest.
ETHICAL APPROVAL
We confirm that we have read the Journal´s position on issues involved in ethical publications and affirm that this report is consistent with those guidelines.
Espino PH, Burneo JG, Moscol G, Gofton T, MacDougall K, Suller Marti A. Long‐term outcomes after NORSE: Treatment with vagus nerve stimulation. Epilepsia Open. 2022;7:822–828. 10.1002/epi4.12654
REFERENCES
- 1. Hirsch LJ, Gaspard N, van Baalen A, Nabbout R, Demeret S, Loddenkemper T, et al. Proposed consensus definitions for new‐onset refractory status epilepticus (NORSE), febrile infection‐related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59(4):739–44. [DOI] [PubMed] [Google Scholar]
- 2. Gaspard N, Foreman BP, Alvarez V, Cabrera Kang C, Probasco JC, Jongeling AC, et al. New‐onset refractory status epilepticus: etiology, clinical features, and outcome. Neurology. 2015;85(18):1604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mantoan Ritter L, Nashef L. New‐onset refractory status epilepticus (NORSE). Pract Neurol. 2021;21(2):119–27. [DOI] [PubMed] [Google Scholar]
- 4. Dibué‐Adjei M, Brigo F, Yamamoto T, Vonck K, Trinka E. Vagus nerve stimulation in refractory and super‐refractory status epilepticus – a systematic review. Brain Stimul. 2019;12(5):1101–10. [DOI] [PubMed] [Google Scholar]
- 5. Howell KB, Katanyuwong K, MacKay MT, Bailey CA, Scheffer IE, Freeman JL, et al. Long‐term follow‐up of febrile infection‐related epilepsy syndrome. Epilepsia. 2012;53(1):101–10. [DOI] [PubMed] [Google Scholar]
- 6. Englot DJ, Rolston JD, Wright CW, Hassnain KH, Chang EF. Rates and predictors of seizure freedom with vagus nerve stimulation for intractable epilepsy. Neurosurgery. 2016;79(3):345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamazoe T, Okanishi T, Yamamoto A, Yamada T, Nishimura M, Fujimoto A, et al. New‐onset refractory status epilepticus treated with vagus nerve stimulation: a case report. Seizure. 2017;47:1–4. [DOI] [PubMed] [Google Scholar]
- 8. Alsaadi T, Shakra M, Turkawi L, Hamid J. VNS terminating refractory nonconvulsive SE secondary to anti‐NMDA encephalitis: a case report. Epilepsy Behav Case Rep. 2015;3:39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kurukumbi M, Leiphart J, Asif A, Wang J. Vagus Nerve Stimulation (VNS) in Super Refractory New Onset Refractory Status Epilepticus (NORSE). Case Rep Neurol Med. 2019;2019:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skaff PT, Labiner DM. Status epilepticus due to human parvovirus B19 encephalitis in an immunocompetent adult. Neurology. 2001;57(7):1336–7. [DOI] [PubMed] [Google Scholar]
- 11. Steriade C, Britton J, Dale RC, Gadoth A, Irani SR, Linnoila J, et al. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune‐associated epilepsy: conceptual definitions. Epilepsia. 2020;61(7):1341–51. [DOI] [PubMed] [Google Scholar]
- 12. Morita M, Fujimoto A, Okanishi T, Nishimura M, Sato K, Kanai S, et al. Vagus nerve stimulation therapy improved refractory epilepsy secondary to acute encephalitis with refractory, repetitive partial seizures (AERRPS). Interdiscip Neurosurg. 2017;9:76–9. 10.1016/j.inat.2017.03.007 [DOI] [Google Scholar]


