Abstract
We sought to assess the anti‐seizure efficacy of carbamazepine (CBZ) and retention rate (RR) in randomized, controlled trials (RCTs) in epilepsy. Our analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. Inclusion criteria were monotherapy of CBZ in adequate dosage for epilepsy treatment and RCT duration of ≥3 months. Outcome measures were seizure freedom rate (SFR) and RR. Random‐effects meta‐analyses were performed to allow for comparison with other anti‐seizure medications (ASMs). Thirty RCTs of 734 were included. SFR at last follow‐up ranged from 11% at 36 months to 85% at 3 months. The aggregated SFR at 6 months was 58% (CI 49–66%) and 48% (CI 40–57%) at 12 months. The 6‐month SFR among blinded studies was 55% (CI 43–66%), compared with 61% (CI 50–71%) in unblinded studies. The 12‐month SFR was not significantly linked to the age of study participants. RR varied from 36% at 24 months to 81% at 6 months. When adjusting for blinding, the aggregated 6‐month RR in blinded studies was 59% (CI 52–66%) vs 76% (CI 71–81%) in unblinded studies. The point estimates of SFR of all RCTs showed an upward time trend, with an increase of approximately 15% between the years 1981 and 2018. In conclusion, the SFR and RR of CBZ were highly variable in RCTs and especially affected by study duration and blinding. These results underscore the impact of the design of RCTs investigating ASM and may challenge the wide use of CBZ as a comparator.
Keywords: antiepileptic drug, antiseizure medication, clinical trial, focal epilepsy, monotherapy, study design
Key points.
Seizure freedom and retention rates of CBZ greatly varied in randomized controlled monotherapy trials for focal epilepsy.
The outcomes were especially affected by the study duration and blinded vs unblinded design.
These results should be considered when defining CBZ as a comparator in RCT for focal epilepsy.
1. INTRODUCTION
The choice of pharmacological treatment in epilepsy can be challenging. In a long‐term observational study, about 50% of patients with epilepsy achieved prolonged seizure freedom with the first anti‐seizure medication (ASM) and a further 12% with the second. 1 The clinical criteria for drug‐resistant epilepsy (DRE) are fulfilled once appropriate administration of two ASMs does not result in seizure freedom. 2 Reliable data on the seizure efficacy and tolerability of ASMs, especially first‐choice drugs, are of great importance. Intriguingly, published findings suggest that overall efficacy has not increased significantly, 3 despite the approval of more than 10 new ASMs since the 1980s. Nevertheless, some authors suggest newer ASMs may be more tolerable and may have fewer interactions overall, 4 , 5 , 6 , 7 , 8 with several notable exceptions.
Current European Medicines Agency (EMA) guidelines for monotherapy studies in epilepsy state that “monotherapy studies should be randomized, double‐blind active‐controlled trials aiming to demonstrate at least a similar benefit/risk balance of the test product as compared to an acknowledged standard product at its optimal dose”. 9 In 2013, Glauser et al 10 published the updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy and found that out of 39 published randomized clinical trials (RCTs) of ASMs, 23 included treatment with carbamazepine (CBZ) as the active control condition, making it the most studied ASM, followed by phenytoin (PHT) and valproate (VPA), both with half as many published works (12 and 11, respectively).
After its synthesis in 1953 11 and the first trial in 1963, 12 CBZ had not only become the drug of the first choice in focal seizures but also had been adopted as a comparator in many RCTs 13 because it is arguably supported by the best evidence base 10 of efficacy and safety as monotherapy in RCTs with class I evidence, 10 , 14 , 15 , 16 with seizure freedom rates (SFR) of >80% at different study time points. 5 , 17 , 18 However, the factors that may influence the outcome measures of these studies are not fully understood. We performed a meta‐analysis to document the anti‐seizure efficacy of CBZ in published RCTs, and to identify study‐related factors that contribute to its apparent efficacy.
2. METHODS
2.1. Information sources and search strategies
We carried out two systematic searches, conducted by two independent investigators (KO, CH), of the literature according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement, via PubMed (National Library of Medicine), Cochrane Controlled Trials Register (JohnWiley & Sons), and EMBASE. The last search was conducted in December 2019. Studies in English, German, Spanish, and French were eligible. Two search strategies were applied.
Search strategy 1: Identification of all clinical studies involving CBZ and epilepsy.
Pubmed search terms: ((epilepsy [MeSH Terms]) AND carbamazepine [Title/Abstract]) AND ((Meta‐Analysis[ptyp] OR Randomized Controlled Trial[ptyp] OR systematic[sb] OR Clinical Trial[ptyp]))
Embase search terms: (“epilepsy”/exp/dm_dt AND ([cochrane review]/lim OR [systematic review]/lim OR [meta‐analysis]/lim OR [randomized controlled trial]/lim)) AND (“epilepsy”/exp AND ([Cochrane review]/lim OR [systematic review]/lim OR [meta‐analysis]/lim OR [randomized controlled trial]/lim)) AND “carbamazepine”:ab,ti
Cochrane search terms: (epilepsy): ti,ab,kw AND (carbamazepine): ti,ab,kw in Cochrane Reviews, Trials with “Epilepsy” in Cochrane Groups (Word variations have been searched)
Search strategy 2: Identification of randomized, controlled clinical trials comparing CBZ to placebo or other ASMs.
Pubmed search terms: (carbamazepin[tiab] OR carbamazepine[tiab]) AND (epilepsy[tiab] OR seizure[tiab]) AND (randomized[tiab] OR randomized[tiab]) AND Clinical Trial[ptyp]
Both searches were compared, and duplicates were removed. The initial screening process determined whether studies were relevant to the meta‐analysis. Full‐text records were then assessed according to the established eligibility criteria. Additionally, eligible studies' references were screened to obtain further relevant works. Unclear cases were resolved in consensus with two other independent investigators (RS, LW).
2.2. Inclusion criteria
2.2.1. Population
We included studies with patients of all ages who fulfilled diagnostic criteria for epilepsy at the time of the study, with any seizure type.
2.2.2. Outcomes
Randomized, controlled trials in newly diagnosed epilepsy have had limited success in finding differences in efficacy among ASMs, whereas differences in tolerability have been easier to document. 13 , 19 , 20 , 21 The best outcome measure in this context appears to be effectiveness, defined as a combination of efficacy and tolerability. Several authors suggest the best way to measure effectiveness is to record the proportion of seizure‐free patients on an ASM as compared to an “established treatment” (frequently CBZ) for a period as long as to assume good tolerability (12 months or longer). 13 , 19 , 20 , 21 The following efficacy outcomes were analyzed:
Proportion of patients who remained on the allocated treatment (Retention rate, RR). Under the assumption that patients will leave a study in case of low seizure control or adverse side effects, RR reflects both drug effectiveness, ie, seizure efficacy and tolerability. The Commission on Antiepileptic Drugs of the International League against Epilepsy (ILAE) recommended RR as the primary outcome measure in RCTs. 22
Proportion of seizure‐free patients (seizure freedom rate, SFR) at specific time points (eg, 3, 6, 12 months). This represents a direct measure of anti‐seizure efficacy and is easily comparable among eligible studies. The European Medicines Agency (EMA) recommended SFR as the primary endpoint for monotherapy studies in epilepsy with a follow‐up of at least 1 year. 9
2.2.3. Intervention
The administration of CBZ for the treatment of epilepsy, for at least 3 months, in monotherapy, for any type of seizure.
2.2.4. Design of primary studies
Inclusion criteria for the design of primary studies were as follows: Randomized, blinded or unblinded, parallel group or crossover monotherapy studies comparing any ASM or placebo to CBZ, including RR and/or SFR as outcome measures. The minimum follow‐up was 3 months. There were no restrictions in the year of publication or sample size.
2.2.5. Statistical analysis
In the case of SFR, we included per‐protocol (PP) analyses where reported and intention‐to‐treat (ITT) analysis for RR. Some of the included RCTs did not specify, which analysis was used, their methods nevertheless corresponded to ITT or PP analyses (these studies are identified as (PP) or (ITT) in Table 1, Results). Others ran ITT or PP analyses for both variables. Where possible, we extracted the remaining study populations at the relevant time points and performed PP analysis for SFR. When this was not possible, we opted for the ITT analysis.
TABLE 1.
RCTs by age group and number of subjects included
| Age group | Elderly only | Adults (>18) | Children and adults | Children (<18) |
|---|---|---|---|---|
|
Studies (included ages)*, (n) *where applicable |
Werhahn et al 2015 (>60), (n = 55) Saetre et al 2007 (>65), (n = 91) Rowan et al 2005 (>60), (n = 70) Brodie et al (1999) (>65), (n = 20) Consoli et al (2012) (>65), (n = 54) |
Brodie et al 2007 (n = 224) Baulac et al 2012, 2014 (n = 300) Bittencourt et al 1993 (n = 20) Mikkelsen et al 1981 (n = 19) Tanganelli et al 1996 (n = 39) Ramsay et al 1983 (n = 35) Suresh et al 1995 (n = 30) |
Marson et al 2007b (>4), (n = 156) Steinhoff et al 2005 (≥12), (n = 88) Reunanen et al 1996 (≥12), (n = 76) Heller et al 1995 (≥16), (n = 61) Brodie et al 1995 (≥13), (n = 129) Kälviäinen et al 1995 (≥15), (n = 50) Trinka et al 2018 (≥16), (n = 412) Privitera et al 2003 (≥6), (n = 63) Lee et al 2018 (≥16), (n = 90) Baulac et al 2017 (≥16), (n = 442) Chadwick et al 1998 (≥12), (n = 74) Trinka et al 2012 (≥16), (n = 480) Nieto‐Barrera et al 2001 (>2), (n = 201) |
Camfield et al 1998 (CSGCE) (n = 379) de Silva et al 1996 (n = 54) Reséndiz‐Aparicio et al 2004 (n = 32) Wheless et al 2004 (n = 23) |
| Combined study population (Total n = 3767) | n = 290 | n = 667 | n = 2322 | n = 488 |
2.3. Quality assessment and data extraction
The following data were extracted from included publications by two investigators (KO, CH): Study number (in order of inclusion in our database), initial sample size (CBZ), specified outcome (SFR/RR/both), outcome proportions (%), range of outcome proportion, etiology of epilepsy (%), head‐to‐head OR placebo‐controlled, publication year, study design, comparator drug if head‐to‐head, proportion of males and females, age at epilepsy onset, family history of epilepsy (%), (SD), duration of epilepsy, time since last seizure at beginning of the study in days (d), BMI in kg/m2, dosage of CBZ (mg/d), seizure onset type, previously treated/untreated.
This information was systematically entered into a previously designed database. In the event of incomplete data, the authors were contacted to request necessary data for analysis. When data were not retrievable, the trial in question was excluded. A third investigator (LW) reviewed the database for possible errors, duplicates, omissions, or other inconsistencies. We used Cochrane's revised Risk of Bias Tool (RoB 2.0) to assess the quality of the evidence provided in each study. 23 The tool was applied independently by two investigators (KO, LW). It includes the assessment of six domains in each study: sequence generation, allocation concealment, blinding of personnel and outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. Discrepancies were resolved in discussion with a fourth investigator (RS).
2.4. Quantitative synthesis
Random‐effects meta‐analysis was performed to compare the administration of CBZ with other ASMs or placebo for epilepsy. Heterogeneity between studies was assessed by the heterogeneity variance and the statistic. 24 It was considered elevated when values were >75%. 24 Funnel plots were used to investigate small‐study bias (eg, publication bias).
As the most frequent reported follow‐up time point was SFR at 12 months, we carried out a meta‐analysis of SFR at this time point for best comparison. Additionally, a meta‐regression was performed on all available studies adjusted for the follow‐up time. SFR and RR were logit‐transformed prior to the meta‐analysis. Results are presented in terms of (back‐transformed) RR and SFR at specific time points (eg, 3, 6, and 12 months). Additionally, funnel and forest plots showing the primary study estimates and confidence intervals are presented.
As a sensitivity analysis, and to investigate a potential bias in the unblinded primary studies, the meta‐analysis was additionally performed on the blinded studies only. Furthermore, we carried out meta‐regression to estimate the RR and SFR including blinding (yes/no) and age group as covariates.
Trends over time were calculated for SFR and RR based on the values in each RCT and publication year. We calculated the meaningfulness of the trend using the open‐access tool by Bryhn and Dimberg. 25 This feature divides the time series of the trend into several separate time intervals (in our case, yearly intervals) and provides mean values, a correlation coefficient (R 2), and P‐values for each interval as well as the entire series. The time trend is considered statistically meaningful if the P‐value <0.05 and r 2 ≥ 0.65. 25
The present meta‐analysis is registered in PROSPERO for accountability and dissemination (ID CRD42020190181, submitted 8 June 2020, edited 28 September 2021). It is published as a preprint with supporting data on medRxiv.org and Researchgate.org.
3. RESULTS
3.1. Search results and characteristics of the included studies
Out of the total of 734 screened works, 30 randomized clinical trials were included in the data analysis (Figure 1). The main reasons for exclusion of studies were as follows: Outcomes did not include SFR or RR, imprecise or missing data, study population did not meet the set criteria (eg, diagnostic criteria for epilepsy, patients who have undergone epilepsy surgery, etc.), and study design did not meet the set criteria (nonrandomized or noncontrolled trials, polytherapy, etc.). Included studies were published between 1981 and 2018. Study populations ranged from 19 to 480 participants and were comprised children only, adults only, adults ≥60 years only, or children and adults (Table 1). Combined trial populations totaled 3467 patients allocated to CBZ. Included studies are listed in the (Table S1). Five of the included studies used standard‐release CBZ, 12 used slow‐release CBZ, two used both, and 11 did not explicitly state which formulation was used. The geographical distribution of recruiting sites varied, with 11 multicenter international studies across continents. Nevertheless, European and North American studies were the majority.
FIGURE 1.
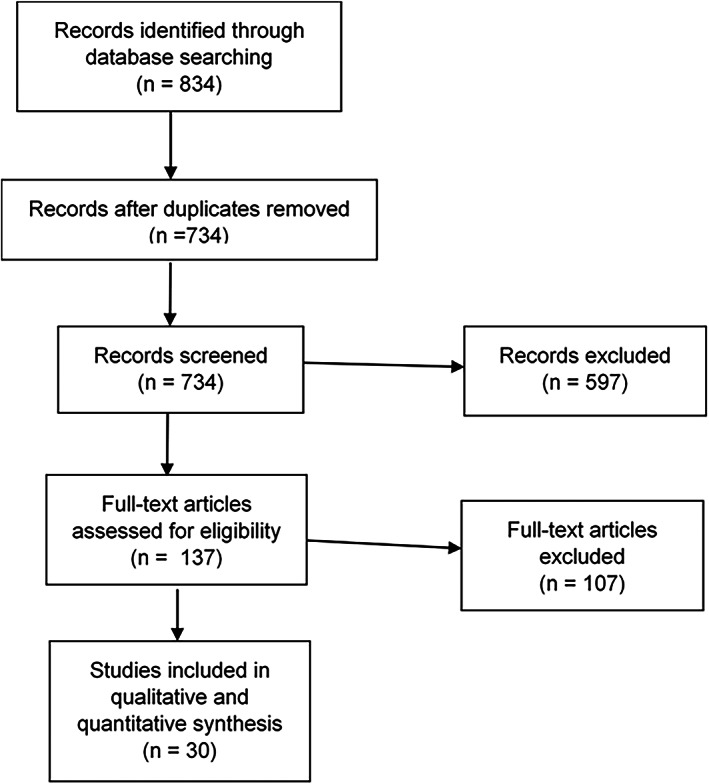
Flow diagram (adapted from PRISMA guidelines 25 )
3.2. Comparators
Comparators for CBZ were blinded or unblinded alternative ASMs licensed for the treatment of epilepsy. These included eslicarbazepine‐acetate (ESL), gabapentin (GBP), lacosamide (LCM), lamotrigine (LTG), levetiracetam (LEV), oxcarbazepine (OXC), phenobarbitone (PB), phenytoin (PHT), topiramate (TPM), valproic acid (VPA), vigabatrin (VGB), and zonisamide (ZNS). LTG was the most frequent comparator (seven RCTs), followed by LEV (four RCTs) and TPM (three RCTs). Trial durations ranged from 3 months to several years, though SFR and RR were not always among the variables examined for the whole duration of the study. The longest time point defined among the included studies was 36 months. 26
There was no relevant heterogeneity in baseline patient characteristics among trials (except in age group), but data were missing in some RCTs. Among the 13 RCTs that reported the type of seizure onset (focal/generalized), 10 evaluated the efficacy of CBZ specifically on focal seizures (15 RCTs did not provide this information). Age of epilepsy onset and duration of epilepsy were reported in six RCTs, respectively. Data on the etiology of epilepsy, baseline seizure frequency, and previous treatment were limited, and the type of the variable used in reporting these features varied among studies. Etiology is of clinical importance in the choice of ASM and was among our main objectives in this meta‐analysis. Nevertheless, data on etiology was available in less than half of the SFR studies and roughly a third of RR studies (Table 2) and was expressed as different variables. Information on etiology ranged from nuanced subgroups to subdivision into “structural/not structural”. This precluded a systematic comparison and pooled statistical analysis. Due to this heterogeneity, subgroup analyses based on patient demographics were not possible (Table 2). We decided to conduct separate meta‐regressions by age and study characteristics (eg, blinding, follow‐up interval) instead.
TABLE 2.
Reporting of characteristics across included RCTs
| Characteristic | Seizure freedom (n = 29 studies) | Retention rate (n = 14 studies) |
|---|---|---|
| Sex (mean and range of percentage) a | ||
| Male | mean = 53.1 (min = 35.0, max = 69.0) | mean = 52.6 (min = 41.0, max = 61.5) |
| Female | mean = 46.0 (min = 31.0, max = 65.0) | mean = 47.4, (min = 38.5, max = 59.0) |
| Seizure onset (Focal/generalized, absolute frequency) | ||
| 100% focal | 11 | 4 |
| 74% focal, 22% generalized | 1 | 0 |
| 90.5% focal | 1 | 1 |
| Focal and generalized, percentage not specified | 1 | 0 |
| no information on onset | 15 | 9 |
| Etiology of epilepsy (absolute frequency (%)) | ||
| Information on etiology | 13 (44.8%) | 6 (42.9%) |
| Study drug | ||
| Clobazam | 1 | 1 |
| Clonazepam | 1 | 0 |
| ESLI | 1 | 0 |
| GBP, LTG | 1 | 0 |
| GBP, LTG, OXC, TPM | 1 | 1 |
| LCS | 1 | 2 |
| LEV | 3 | 1 |
| LEV, LTG | 2 | 1 |
| LTG | 6 | 4 |
| LTG + VPA | 1 | 0 |
| PB, PHT, VPA | 1 | 1 |
| PHT | 2 | 0 |
| TPM | 3 | 0 |
| VGB | 2 | 2 |
| ZNS | 1 | 1 |
Abbreviations: ESLI—eslicarbazepine‐acetate; GBP—gabapentin; LCS—lacosamide; LTG—lamotrigine; LEV—levetiracetam; OXC—oxcarbazepine; PB—phenobarbitone; PHT—phenytoin; TPM—topiramate; VPA—valproate; VGB—vigabatrin; ZNS—zonisamide
All included RCTs reported the proportion of male and female participants.
We also aimed to examine dosage effects on seizure‐free outcomes. However, this information was provided as different variables (mean, median, range), rendering a nuanced cross‐referenced analysis uninformative. Publication year did not seem to have a relevant effect on RR (R 2 = 0.0067), though the trend (calculated using regression) ticked slightly upwards (trend not statistically meaningful, 24 P = 0.780; Figure 2A). SFR, on the other hand, showed an upward trend with an increase of ≈15% between the years 1981 and 2018 (Figure 2B). However, the high variance and dispersion of these data made such interpretations challenging, and the trend was not statistically meaningful (P = 0.322, R 2 = 0.038).
FIGURE 2.
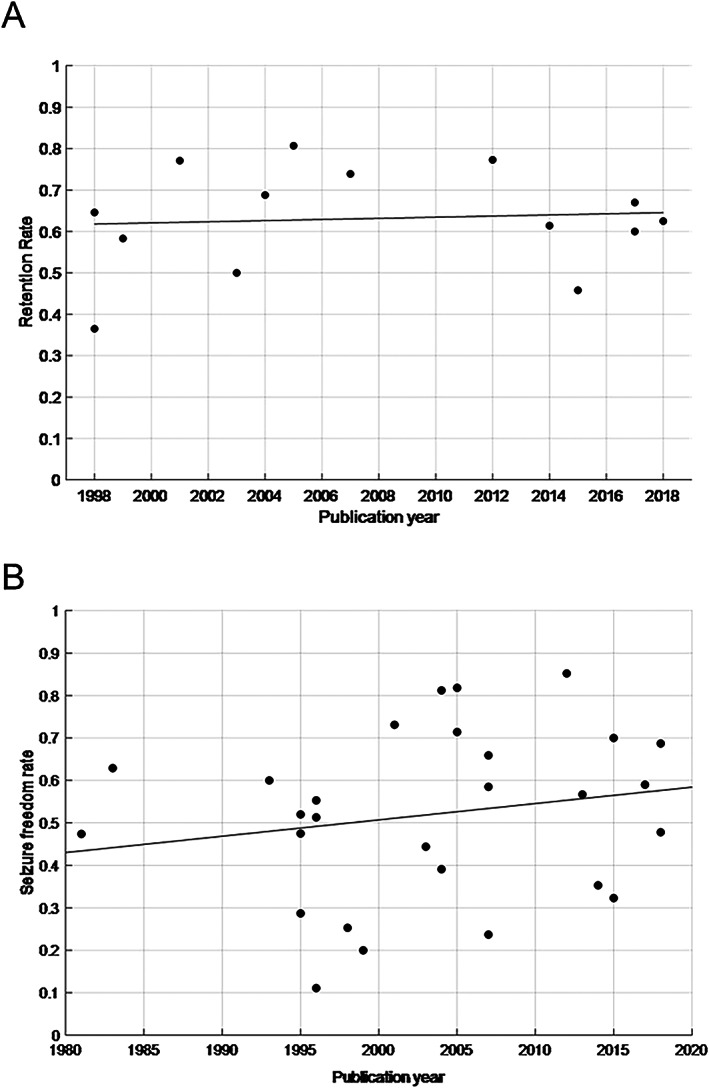
Distribution of included RCTs by RR and publication year, including trend line A, and distribution of included RCTs by SFR and publication year, including trend line B
3.3. Risk of bias
The bias risk of the included publications was assessed using RoB 2.0, according to ITT or PP analysis. Of the 30 included publications, five were assessed as “low risk of bias” and two as “high risk of bias”, the rest were classified as “some concerns” (Figure 3). The traffic light plots show the individual assessment by domains (Figure S1). One study is shown in a separate plot, as its design (crossover) required a modified version of the RoB tool (Figure S2). Funnel plots were used to investigate small study bias and heterogeneity among studies, using 12‐month SFR among all studies (Figure S3), and separately among blinded studies (Figure S4). Both plots showed significant asymmetry, suggesting the presence of severe study heterogeneity, publication bias, and small‐study bias.
FIGURE 3.
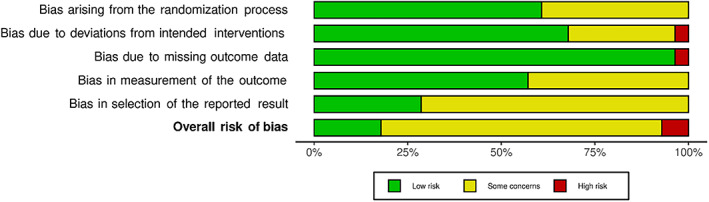
Risk of bias, summarized as RoB 2.0 assessment of all included RCTs
3.4. Quantitative Meta‐Analyses
3.4.1. SFR
12‐month SFR
To establish a better comparison of studies regarding SFR, we first performed a meta‐analysis including only the most frequently reported follow‐up time point among studies (12 months, bold in Figure 4). In this subset of 13 studies, SFR ranged from 25.32% 27 to 81.2% 18 Study populations varied between 32 and 480 patients. The pooled SFR in this subset was 53.0% (CI 42.9%–62.9%; Table 3, Model 1). Heterogeneity was very elevated (I 2 = 95.5%).
FIGURE 4.
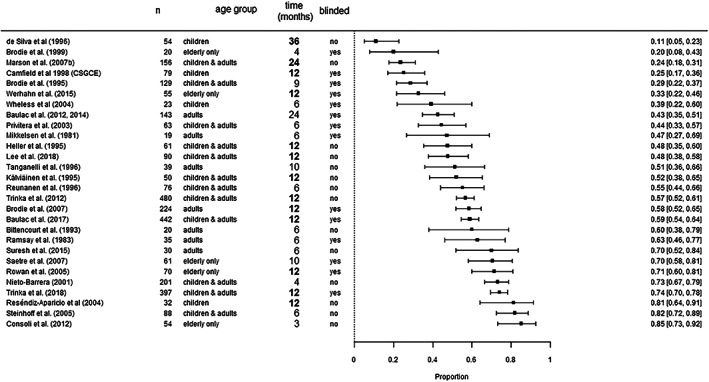
Distribution of reported SFR across included RCTs. Studies that reported 12‐month SFR are printed bold. The studies from de Silva et al. (1996) and Marson et al. (2007) reported 12‐month SFR and SFR at additional time points (36 and 24 months, respectively) 26 , 30
TABLE 3.
Meta‐analysis and regression of seizure freedom rate (SFR)
| Seizure freedom rate (12 months) | Seizure freedom rate (all studies included) | ||
|---|---|---|---|
| Model 1 | Model 4 | ||
| Variables | Estimate (95%‐CI) | Variables | Estimate (95%‐CI) |
| Intercept | 0.530 (0.429–0.629) | 6 months | 0.581 (0.494–0.664) |
| 12 months | 0.480 (0.395–0.566) | ||
| N = 15, τ 2 = 0.551, I 2 = 95.5% | N =29, τ 2 = 0.566, I 2 = 93.0% | ||
| Model 2 | Model 5 | ||
| Variables | Estimate (95%‐CI) | Variables | Estimate (95%‐CI) |
| Blinded | 0.575 (0.440–0.700) | 6 months | |
| blinded | 0.548 (0.430–0.660) | ||
| unblinded | 0.611 (0.500–0.710) | ||
| Unblinded | 0.483 (0.348–0.620) | 12 months | |
| blinded | 0.548 (0.430–0.660) | ||
| unblinded | 0.611 (0.500–0.710) | ||
| N = 15, τ 2 = 0.551, I 2 = 95.5% | N =29, τ 2 = 0.557, I 2 = 92.4% | ||
| Model 3 | |||
| Variables | Estimate (95%‐CI) | ||
| Children | 0.429 (0.245–0.635) | ||
| All ages | 0526 (0.399–0.650) | ||
| Adults | 0.671 (0.441–0.841) | ||
| Elderly only | 0.527 (0.290–0.752) | ||
| N =15, τ 2 = 0.448, I 2 = 94.9% | |||
Note: Model 1 refers to a meta‐analysis for studies that reported 12‐month results. Model 2 is a meta‐regression of 12‐month SFR adjusted for blinding status. Model 3 is a meta‐regression of 12‐month SFR adjusted for age groups. Model 4 is a meta‐regression of SFR adjusted for follow‐up time. Model 5 is a meta‐regression of SFR adjusted for follow‐up time and blinded status of the primary studies.
Effect of blinding on 12‐month SFR
To isolate the effect of blinding among the studies that reported 12‐month SFR, we carried out a meta‐regression with blinding as a covariate (Figure 4 ). The aggregated 12‐month SFR among the blinded RCTs was 57.5% (CI 44.0%–70.0%), whereas among the unblinded RCTs it was lower (48.3%; CI 34.8%–62.0%, P = 0.350; Model 2 in Table 3). Heterogeneity remained similarly high (I 2 = 94.7%).
Effect of age of study participants on 12‐month SFR
We carried out an additional meta‐regression using the age group of the study population as a covariate to examine its effect on 12‐month SFR. 12‐month SFR was not found to differ significantly between age groups (P = 0.493; Model 3 in Table 3) with average values of 67.1% in adults (CI 44.1%–84.1%), 52.6% in studies including patients of all ages (CI 39.9%–65.0%), 52.7% in elderly only studies (CI 29.0%–75.2%), and 42.9% in children (CI 24.5%–63.5%).
Effect of study duration on SFR at last follow‐up
Twenty‐nine studies included SFR as an outcome measure (Table 1 ). We carried out a meta‐regression of SFR at the last follow‐up point reported in each study, adjusted for the follow‐up time (Figure 4 ). These time points varied from 3 to 36 months. Study populations ranged from 19 to 480 patients. SFR was very variable, ranging from 11.1 16 to 85.2%. 17 Based on our model, the estimated SFR while taking CBZ is 58.1% (CI 49.4–66.4%) after 6 and 48.0% (CI 39.5–56.6%) after 12 months (P = 0.026; Table 3, Model 4). However, heterogeneity among studies was very large (I 2 = 93%).
Combined effect of blinding and study duration on SFR at last follow‐up
To examine the effect of blinding on SFR, we carried out a meta‐regression, adjusted for blinded status (Figure 4 ). Among the blinded studies, the estimated SFR at 6 months was 54.8% (CI 43.0%–66.0%) and 45.1% at 12 months (CI 34.5%–56.0%). The estimated SFR among unblinded studies was higher (P = 0.396; Model 5 in Table 3): 61.1% (CI 50.0%–71.0%) at 6 and 51.5% (CI 39.9%–63.0%) at 12 months. Again, heterogeneity among studies was very high (I 2 = 92.4%).
3.4.2. RR
Effect of study duration on RR
We carried out a separate meta‐regression including the studies that reported the RR during treatment with CBZ (Figure 5 ). Reported values ranged from 36.5% 28 to 80.7%, 29 and follow‐up times varied from 4 to 24 months. We included the time points that were most similar among studies to increase comparability, eg, in the case of Marson et al, 2007 30 and Baulac et al, 2012, 5 2014 6 we chose 12 months instead of the last follow‐up point (24 months, respectively). Aggregated average RR at 6 months was 68.0% (CI 60%–76%) and 61% at 12 months (CI 54%–68%; Model 6 in Table 4). Heterogeneity was lower in this instance (I 2 = 87.6%).
FIGURE 5.
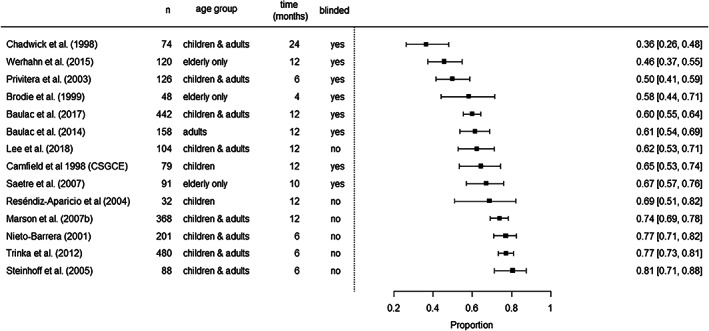
Distribution of reported RR in included RCTs
TABLE 4.
Meta‐analyses and regression of retention rate.
| Model 6 | |
| Time | Estimate (95%‐CI) |
| 6 months | 0.683 (0.599–0.757) |
| 12 months | 0.611 (0.535–0.683) |
| N =14, τ 2 = 0.212, I 2 = 87.6% | |
| Model 7 | |
| Time | Estimate (95%‐CI) |
| 6 months | |
| Blinded | 0.594 (0.519–0.664) |
| Unblinded | 0.761 (0.705–0.809) |
| 12 months | |
| Blinded | 0.544 (0.485–0.601) |
| Unblinded | 0.722 (0.658–0.778) |
| N =14, τ 2 = 0.063, I 2 = 66.9% | |
Note: Model 6 refers to a meta‐regression adjusted for follow‐up time. Model 7 refers to a meta‐regression adjusted for follow‐up time and blinding status.
Effect of blinding on RR
Lastly, we carried out a meta‐analysis with blinding as a covariate in RR. Estimated RR values were lower among the blinded studies (54% at 12 months, CI 48%–60%) compared with unblinded studies (72% at 12 months, CI 66%–78%, P < 0.001, Model 7 in Table 4). Heterogeneity was lowest in this analysis (I 2 = 66.6%). In this instance, we opted not to carry out an analysis by age group, due to the low number of studies including children and elderly patients (two studies for each age group).
4. DISCUSSION
We have found that SFR and RR greatly varied in RCTs with CBZ in monotherapy for epilepsy, and that longer study duration and blinded drug administration were linked to lower SFR and RR. Previous works have investigated the statistical efficiency of RCTs in epilepsy and found that the choice of seizure outcome measure, drug effect size, patients' baseline characteristics, and trial duration has a critical impact on the statistical power of RCTs in epilepsy. 31 , 32 , 33 , 34 Due to lacking data and small sample sizes of some RCTs, our meta‐analysis only addressed trial duration, blinded/unblinded drug administration, and age of included participants.
4.1. Impact of statistical analysis
The most adequate analysis for SFR is a per‐protocol approach, whereas for RR, the best approach is an intention‐to‐treat analysis. Some of the 15 RCTs reporting RR and SFR carried out separate analyses using the best approach for each variable. However, some of the included trials carried out an intention‐to‐treat analysis of both variables. This may be a factor behind the variability of the reported outcome measures throughout the years.
4.2. Age groups
Differences in the reported effectiveness of CBZ among age groups may be due to several factors. The largest group was patients of all ages (n = 2322). Elderly patients comprised only 290 of the total of 3654 study participants. Among elderly patients, there may be many causes for variable results, 35 , 36 , 37 , 38 , 39 increased drug interactions, 40 and adverse reactions 41 due to polytherapy, comorbidities, and age‐related changes in pharmacokinetics and pharmacodynamics. 42 , 43 , 44 , 45 Other causes for differences in published data on the effectiveness of CBZ in elderly patients are research‐related: elderly patients are underrepresented in study populations due to a variety of reasons, from research discrimination to challenges in recruitment. 35 , 46 In children, concerns over age‐related differences in metabolization are equally relevant. 47 Polypharmacy is less frequently an issue in children compared with elderly adults. 48 One of the main considerations that may influence treatment efficacy is age‐specific etiology, 49 such as perinatal and epileptic encephalopathies. Furthermore, as in elderly patients, children are underrepresented in trial populations for similar reasons.
4.3. Impact of trial characteristics on study outcomes
There were no placebo‐controlled RCTs among the included studies, most likely due to concerns over equipoise. Heterogeneity among included studies was very high for SFR, which made comparison among studies difficult and prevented in‐depth analysis of important factors (eg, daily dose of CBZ). Heterogeneity was markedly lower for RR than for SFR. This may be because RR studies usually report a single value for this variable, whereas SFR is frequently measured at several time points, resulting in multiple values for the variable in each study. The reasons behind the variability of efficacy and tolerability are unclear, and may include study design, pharmacological characteristics of CBZ, and its efficacy in certain types of seizures and/or etiologies. The effect of blinding on both variables is perhaps unsurprising: both SFR and RR were higher in unblinded studies. However, blinding had a more pronounced effect on RR. Unblinded studies tend to have larger treatment effects than blinded studies, and this effect is stronger in self‐reported outcomes such as epileptic seizures. 29 , 50 , 51 Perhaps dropout rates due to patients' doubts about their blinded treatment have a disproportionate effect on RR. Furthermore, the decision to maintain a patient on anti‐seizure therapy in unblinded studies may also be influenced by the epileptologist's background, practice volume, and seizure frequency where seizure freedom cannot be expected.
A previous meta‐analysis examined the factors determining the response to different ASMs in RCTs 52 and drew three major conclusions from their findings: responder rates (i) correlated positively with the duration of the study (including titration); (ii) increased throughout the years both for ASMs and for placebo; and (iii) there were large differences in efficacy related to dose selection and type of analysis. By contrast, our results, adjusted for follow‐up time, suggested that SFR decreases with longer study duration. The same was true for RR. Though we did not evaluate the responder rate as a variable, SFR and responder rate are both measures of seizure reduction. These opposing findings may be due to differences in patient characteristics and seizure types. The aforementioned meta‐analysis included studies in refractory epilepsy with higher seizure frequencies at baseline, and trial drugs were administered as an add‐on to an existing polytherapy. It also included many different ASMs with variable mechanisms of action, which further increases heterogeneity. By contrast, our study considered only people with focal epilepsy on monotherapy, commonly in the early disease course. In addition, RCTs with adjunctive ASMs commonly last only 3–6 months, whereas we have considered studies with longer durations. Perhaps study outcomes display a more complex time‐dependence with stronger and possibly inverse effects in trials that last longer than 6 months (in contrast to trial durations between 3 and 6 months). Regarding the relationship with the year of publication, our results showed a nonmeaningful 24 upward trend in SFR between 1981 and 2018, consistent with the findings in the aforementioned study.
It is important to note that most of the trials included in our study are designed to assess safety and efficacy by licensing bodies. 13 These tend to be double‐blind, noninferiority, short‐term RCTs with small patient populations and restricted dosage regimens. 7 These studies are necessary but only partially informative for daily clinical practice. Clinically useful RCTs are long‐term, superiority studies with diverse study populations including, among others, women of childbearing age, who are routinely excluded from many trials due to concerns over teratogenic effects of trial drugs. These concerns are important but can be addressed with safety measures. In the 2013 ILAE evidence review of antiepileptic drugs, 10 the authors classified only two RCTs on focal epilepsies as Class 1 evidence. 21 , 28 One of our conclusions is the need for reliable, generalizable, useful, long‐term data on ASMs in epilepsy. 7 , 10 In order to achieve this goal, it is important to examine the reliance of the clinical community on the industry for the performance of trials, and the responsibility to generate RCTs that are independent of industry or licensing concerns. 53 Nevertheless, we must recognize the trade‐offs that are sometimes necessary to generate good evidence that is applicable in clinical practice. The SANAD I and II trials (Standard and New Antiepileptic Drugs) are multi‐centric RCTs comparing CBZ, GBP, LEV, LTG, OXC, TPM, and ZNS in focal epilepsy. 30 , 50 They have been influential for treatment decisions in epilepsy. Nevertheless, because it is unblinded, SANAD I is not among the RCTs considered Class I Evidence by ILAE guidelines. Long‐term head‐to‐head studies with large patient populations are arguably the most useful sources of information in clinical research on ASMs, yet these studies are the most challenging to run in a double‐blind manner: the costs, logistics, and practicalities of long‐term masking become unsurmountable when considering study durations exceeding several months. 53
An additional factor of study design that may influence RR is the choice of standard or slow‐release CBZ. Slow‐release CBZ has been available since the mid‐eighties of the last century, and over time became the default form of CBZ. Only five of the RCTs explicitly included standard‐release CBZ. RR was an outcome variable in only one of these studies, which did not allow for a meaningful statistical analysis of a possible correlation.
The geographical distribution of recruiting sites has been shown to have a complex influence on study outcomes. 54 Challenging recruitment and high costs in Europe and North America have led to increasing numbers of multicenter RCTs with sites across continents. Though the effects of this on study outcomes are not fully understood, one issue is the parallel increase of RR and recruiting sites needed to include enough patients for phase 3 RCTs.
4.4. Limitations of this meta‐analysis
The main obstacle to drawing clinically relevant conclusions from published works, apart from the aforementioned factors, is the lack of uniformity among them. This is true of the group of RCTs we analyzed. Heterogeneity measures were high, and consequently, P‐values were elevated. This forces us to relativize our findings due to the challenging comparability among studies and may mean that many other factors affect the anti‐seizure efficacy of CBZ but are obscured by heterogeneous study design. An especially important factor may be the elevated number of studies that did not specify which formulation of CBZ had been used (slow‐ or standard‐release). Slow‐release CBZ has been shown to have higher retention rates than standard‐release CBZ, which may have directly affected RR. This may be a possible line of future work.
We acknowledge this may preclude a meta‐analysis of the data such as this one. 53 We nevertheless believe there is value in the systematic evaluation of the available data for the current gold standard in focal epilepsies. A way to circumvent this lack of uniformity would be a meta‐analysis of individual patient data. Another limitation of our work is the limited number of covariate analyses due to the aforementioned factors. Furthermore, generalizability and representativeness may be skewed by the recruitment of insufficiently diverse populations into the clinical trials we included.
4.5. Conclusions
In conclusion, our meta‐analysis suggests that seizure freedom and retention rates of CBZ greatly vary in randomized controlled monotherapy trials for focal epilepsy, and this may be essentially explained by characteristics of study design, among other factors. The degree to which CBZ as a substance is responsible for these results remains unclear due to the heterogeneous methodology of the studies. These findings should be considered when defining CBZ as a comparator in RCTs for focal epilepsy. Our observations also contribute to the discussion about CBZ as the first‐choice ASM in the treatment of focal epilepsies. Furthermore, our results reiterate the need for reliable, generalizable, useful, long‐term data on ASMs in epilepsy and a more standardized study design.
AUTHOR CONTRIBUTIONS
Christian Hoppe: Data curation (equal), writing – review and editing (equal), Karmele Olaciregui‐Dague: Methodology (equal), investigation (lead), formal analysis (supporting), data curation (lead), visualization (equal), writing – original draft preparation (lead), writing – review and editing (equal). Matthias Schmid: methodology (supporting), formal analysis (equal), supervision (equal), writing – review and editing (equal). Rainer Surges: Conceptualization (lead), supervision (lead), writing – review and editing (equal), investigation (equal). Leonie Weinhold: Methodology (equal), data curation (equal), formal analysis (lead), visualization (equal), writing – original draft preparation (supporting), writing – review and editing (equal).
CONFLICTS OF INTEREST
KOD has received speaker fees from Eisai, unrelated to this work. RS has received fees as speaker or for serving on the advisory board from Angelini, Arvelle, Bial, Desitin, Eisai, Janssen‐Cilag GmbH, LivaNova, Novartis, Precisis GmbH, UCB Pharma, UnEEG and Zogenix. These activities were not related to the content of this manuscript. C. Hoppe received software license fees and honoraria from Eisai Inc. unrelated to this work. The remaining authors have no conflicts of interest.
DATA SHARING STATEMENT
The data that support the findings of this study are openly available in MedRxiv.
Supporting information
Appendix S1
Olaciregui‐Dague K, Weinhold L, Hoppe C, Schmid M, Surges R. Anti‐seizure efficacy and retention rate of carbamazepine is highly variable in randomized controlled trials: A meta‐analysis. Epilepsia Open. 2022;7:556–569. 10.1002/epi4.12644
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30‐year longitudinal cohort study [published correction appears in JAMA neurol. 2018 mar 1;75(3):384]. JAMA Neurol. 2018;75(3):279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies [published correction appears in Epilepsia. 2010 Sep;51(9):1922]. Epilepsia. 2010;51(6):1069–77. 10.1111/j.1528-1167.2009.02397.x [DOI] [PubMed] [Google Scholar]
- 3. Brodie MJ, Sills GJ. Combining antiepileptic drugs‐‐rational polytherapy? Seizure. 2011;20(5):369–75. 10.1016/j.seizure.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 4. Brodie MJ, Richens A, Yuen AW. Double‐blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK lamotrigine/carbamazepine monotherapy trial group [published correction appears in lancet 1995 mar 11;345(8950):662]. Lancet. 1995;345(8948):476–9. 10.1016/s0140-6736(95)90581-2 [DOI] [PubMed] [Google Scholar]
- 5. Baulac M, Brodie MJ, Patten A, Segieth J, Giorgi L. Efficacy and tolerability of zonisamide versus controlled‐release carbamazepine for newly diagnosed partial epilepsy: a phase 3, randomised, double‐blind, non‐inferiority trial. Lancet Neurol. 2012;11(7):579–88. 10.1016/S1474-4422(12)70105-9 [DOI] [PubMed] [Google Scholar]
- 6. Baulac M, Patten A, Giorgi L. Long‐term safety and efficacy of zonisamide versus carbamazepine monotherapy for treatment of partial seizures in adults with newly diagnosed epilepsy: results of a phase III, randomized, double‐blind study. Epilepsia. 2014;55(10):1534–43. 10.1111/epi.12749 [DOI] [PubMed] [Google Scholar]
- 7. Trinka E, Marson AG, Van Paesschen W, et al. KOMET: an unblinded, randomised, two parallel‐group, stratified trial comparing the effectiveness of levetiracetam with controlled‐release carbamazepine and extended‐release sodium valproate as monotherapy in patients with newly diagnosed epilepsy. J Neurol Neurosurg Psychiatry. 2013;84(10):1138–47. 10.1136/jnnp-2011-300376 [DOI] [PubMed] [Google Scholar]
- 8. Saetre E, Perucca E, Isojärvi J, Gjerstad L, LAM 40089 Study Group . An international multicenter randomized double‐blind controlled trial of lamotrigine and sustained‐release carbamazepine in the treatment of newly diagnosed epilepsy in the elderly. Epilepsia. 2007;48(7):1292–302. 10.1111/j.1528-1167.2007.01128.x [DOI] [PubMed] [Google Scholar]
- 9. European Medicines Agency (EMA) Committee for medicinal products for human use . Guideline on clinical investigation of medicinal products in the treatment of epileptic disorders, Revision 2. Neuropsychopharmacol. 2010; CHMP/EWP/566/98 Rev.2/Corr.
- 10. Glauser T, Ben‐Menachem E, Bourgeois B, Cnaan A, Guerreiro C, Kälviäinen R, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54(3):551–63. 10.1111/epi.12074 [DOI] [PubMed] [Google Scholar]
- 11. Schindler W, Häfliger F. Über Derivate des Iminodibenzyls. Helv Chim Acta. 1954;37(2):472–83. [Google Scholar]
- 12. Brodie MJ. Antiepileptic drug therapy the story so far. Seizure. 2010;19(10):650–5. 10.1016/j.seizure.2010.10.027 [DOI] [PubMed] [Google Scholar]
- 13. Brodie MJ, Whitehead J. Active control comparisons: the ideal trial design. Epilepsy Res. 2006;68(1):69–73. 10.1016/j.eplepsyres.2005.09.025 [DOI] [PubMed] [Google Scholar]
- 14. Nolan SJ, Marson AG, Weston J, Tudur SC. Carbamazepine versus phenobarbitone monotherapy for epilepsy: an individual participant data review. Cochrane Database Syst Rev. 2015;(7):CD001904. 10.1002/14651858.CD001904.pub2 [DOI] [PubMed] [Google Scholar]
- 15. Nolan SJ, Marson AG, Weston J, Tudur SC. Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review. Cochrane Database Syst Rev. 2015;(8):CD001911. 10.1002/14651858.CD001911.pub2 [DOI] [PubMed] [Google Scholar]
- 16. Nolan SJ, Tudur Smith C, Weston J, Marson AG. Lamotrigine versus carbamazepine monotherapy for epilepsy: an individual participant data review. Cochrane Database Syst Rev. 2016;11(11):CD001031. 10.1002/14651858.CD001031.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Consoli D, Bosco D, Postorino P, Galati F, Plastino M, Perticoni GF, et al. Levetiracetam versus carbamazepine in patients with late poststroke seizures: a multicenter prospective randomized open‐label study (EpIC project). Cerebrovasc Dis. 2012;34(4):282–9. 10.1159/000342669 [DOI] [PubMed] [Google Scholar]
- 18. Reséndiz‐Aparicio JC, Rodríguez‐Rodríguez E, Contreras‐Bernal J, Ceja‐ Moreno H, Barragán‐Pérez E, Garza‐Morales S, et al. Estudio abierto aleatorio y comparativo de monoterapia con topiramato frente a carbamacepina en el tratamiento de pacientes pediátricos con epilepsia de reciente diagnóstico [a randomised open trial comparing monotherapy with topiramate versus carbamazepine in the treatment of paediatric patients with recently diagnosed epilepsy]. Rev Neurol. 2004. Aug 1‐15;39(3):201‐4. Spanish. PMID: 15284957;39:201–4. [PubMed] [Google Scholar]
- 19. Kwan P, Brodie MJ. Clinical trials of antiepileptic medications in newly diagnosed patients with epilepsy. Neurology. 2003;60(11 Suppl 4):S2–S12. 10.1212/wnl.60.11_suppl_4.s2 [DOI] [PubMed] [Google Scholar]
- 20. Mattson RH, Cramer JA, Collins JF, Smith DB, Delgado‐Escueta AV, Browne TR, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic‐clonic seizures. N Engl J Med. 1985;313(3):145–51. 10.1056/NEJM198507183130303 [DOI] [PubMed] [Google Scholar]
- 21. Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic‐clonic seizures in adults. The Department of Veterans Affairs Epilepsy Cooperative Study no. 264 group. N Engl J Med. 1992;327(11):765–71. 10.1056/NEJM199209103271104 [DOI] [PubMed] [Google Scholar]
- 22. Chadwick D, Beghi E, Callaghan N, de Bittencourt P, Dulac O, Gram L, et al. Considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia. 1998;39(7):799–803. 10.1111/j.1528-1157.1998.tb01167.x [DOI] [PubMed] [Google Scholar]
- 23. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 25. Bryhn AC, Dimberg PH. An operational definition of a statistically meaningful trend. PLoS One. 2011;6(4):e19241. 10.1371/journal.pone.0019241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Silva M, MacArdle B, McGowan M, Hughes E, Stewart J, Reynolds EH, et al. Randomised comparative monotherapy trial of phenobarbitone, phenytoin, carbamazepine, or sodium valproate for newly diagnosed childhood epilepsy. Lancet. 1996;347(9003):709–13. 10.1016/s0140-6736(96)90074-4 [DOI] [PubMed] [Google Scholar]
- 27. (Camfield et al 1998) Canadian Study Group for Childhood Epilepsy . Clobazam has equivalent efficacy to carbamazepine and phenytoin as monotherapy for childhood epilepsy. Epilepsia. 1998;39(9):952–9. 10.1111/j.1528-1157.1998.tb01444.x [DOI] [PubMed] [Google Scholar]
- 28. Chadwick DW, Anhut H, Greiner MJ, Alexander J, Murray GH, Garofalo EA, et al. A double‐blind trial of gabapentin monotherapy for newly diagnosed partial seizures. International gabapentin monotherapy study group 945‐77. Neurology. 1998;51(5):1282–8. [DOI] [PubMed] [Google Scholar]
- 29. Savović J, Jones H, Altman D, Harris RJ, Jűni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technol Assess. 2012;16(35):1–82. 10.3310/hta16350 [DOI] [PubMed] [Google Scholar]
- 30. Marson AG, Al‐Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369(9566):1000–15. 10.1016/S0140-6736(07)60460-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldenholz DM, Tharayil J, Moss R, Myers E, Theodore WH. Monte Carlo simulations of randomized clinical trials in epilepsy. Ann Clin Transl Neurol. 2017;4(8):544–52. 10.1002/acn3.426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karoly PJ, Romero J, Cook MJ, Freestone DR, Goldenholz DM. When can we trust responders? Serious concerns when using 50% response rate to assess clinical trials. Epilepsia. 2019;60(9):e99–e103. 10.1111/epi.16321 [DOI] [PubMed] [Google Scholar]
- 33. Romero J, Goldenholz DM. Statistical efficiency of patient data in randomized clinical trials of epilepsy treatments. Epilepsia. 2020;61(8):1659–67. 10.1111/epi.16609 [DOI] [PubMed] [Google Scholar]
- 34. Romero J, Larimer P, Chang B, Goldenholz SR, Goldenholz DM. Natural variability in seizure frequency: implications for trials and placebo. Epilepsy Res. 2020;162:106306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pohlmann‐Eden B, Marson AG, Noack‐Rink M, et al. Comparative effectiveness of levetiracetam, valproate and carbamazepine among elderly patients with newly diagnosed epilepsy: subgroup analysis of the randomized, unblinded KOMET study. BMC Neurol. 2016;16(1):149. 10.1186/s12883-016-0663-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arain AM, Abou‐Khalil BW. Management of new‐onset epilepsy in the elderly. Nat Rev Neurol. 2009;5(7):363–71. 10.1038/nrneurol.2009.74 [DOI] [PubMed] [Google Scholar]
- 37. Pohlmann‐Eden B. Issues when treating epilepsy in the elderly. Acta Neurol Scand Suppl. 2005;181:40–6. 10.1111/j.1600-0404.2005.00508.x [DOI] [PubMed] [Google Scholar]
- 38. Bergey G, Birnbaum AK, Caserta FM, Ferrendelli JA, French JA, Leppik I. Diagnosis and treatment selection in elderly patients with epilepsy. Adv Stud Med. 2006;6(3C):S195–209. [Google Scholar]
- 39. Leppik IE. Epilepsy in the elderly. Epilepsia. 2006;47(Suppl 1):65–70. 10.1111/j.1528-1167.2006.00664.x [DOI] [PubMed] [Google Scholar]
- 40. Patsalos PN, Fröscher W, Pisani F, van Rijn CM. The importance of drug interactions in epilepsy therapy. Epilepsia. 2002;43(4):365–85. 10.1046/j.1528-1157.2002.13001.x [DOI] [PubMed] [Google Scholar]
- 41. Routledge PA, O’Mahony MS, Woodhouse KW. Adverse drug reactions in elderly patients. Br J Clin Pharmacol. 2004;57(2):121–6. 10.1046/j.1365-2125.2003.01875.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bourdet SV, Gidal BE, Alldredge BK. Pharmacologic management of epilepsy in the elderly. J Am Pharm Assoc (Wash). 2001;41(3):421–36. 10.1016/s1086-5802(16)31256-6 [DOI] [PubMed] [Google Scholar]
- 43. McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2004;56(2):163–84. 10.1124/pr.56.2.4 [DOI] [PubMed] [Google Scholar]
- 44. Gidal BE, French JA, Grossman P, Le Teuff G. Assessment of potential drug interactions in patients with epilepsy: impact of age and sex. Neurology. 2009;72(5):419–25. 10.1212/01.wnl.0000341789.77291.8d [DOI] [PubMed] [Google Scholar]
- 45. Pugh MJ, Vancott AC, Steinman MA, et al. Choice of initial antiepileptic drug for older veterans: possible pharmacokinetic drug interactions with existing medications. J Am Geriatr Soc. 2010;58(3):465–71. 10.1111/j.1532-5415.2010.02732.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology. 2004;62(5 Suppl 2):S24–9. 10.1212/wnl.62.5_suppl_2.s24 [DOI] [PubMed] [Google Scholar]
- 47. Mitchell WG, Chavez JM. Carbamazepine versus phenobarbital for partial onset seizures in children. Epilepsia. 1987;28(1):56–60. 10.1111/j.1528-1157.1987.tb03623.x [DOI] [PubMed] [Google Scholar]
- 48. Sullivan JE 3rd, Dlugos DJ. Antiepileptic drug monotherapy: pediatric concerns. Semin Pediatr Neurol. 2005;12(2):88–96. 10.1016/j.spen.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 49. Guerrini R. Epilepsy in children. Lancet. 2006;367(9509):499–524. 10.1016/S0140-6736(06)68182-8 [DOI] [PubMed] [Google Scholar]
- 50. Marson A, Burnside G, Appleton R, Smith D, Leach JP, Sills G, et al. The SANAD II study of the effectiveness and cost‐effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open‐label, non‐inferiority, multicentre, phase 4, randomised controlled trial [published correction appears in lancet. May 15, 2021;397(10287):1808]. Lancet. 2021;397(10282):1363–74. 10.1016/S0140-6736(21)00247-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–12. 10.1001/jama.273.5.408 [DOI] [PubMed] [Google Scholar]
- 52. Rheims S, Perucca E, Cucherat M, Ryvlin P. Factors determining response to antiepileptic drugs in randomized controlled trials. A systematic review and meta‐analysis. Epilepsia. 2011;52(2):219–33. 10.1111/j.1528-1167.2010.02915.x [DOI] [PubMed] [Google Scholar]
- 53. Chadwick D, Marson T. Choosing a first drug treatment for epilepsy after SANAD: randomized controlled trials, systematic reviews, guidelines and treating patients. Epilepsia. 2007;48(7):1259–63. 10.1111/j.1528-1167.2007.01086.x [DOI] [PubMed] [Google Scholar]
- 54. Friedman D, French JA. Clinical trials for therapeutic assessment of antiepileptic drugs in the 21st century: obstacles and solutions. Lancet Neurol. 2012;11(9):827–34. 10.1016/S1474-4422(12)70177-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


