Abstract
There is an increasing demand for more healthy and sustainable diets, which led to an interest in replacing synthetic colors with natural plant-based ones. Phycocyanin, which is commonly extracted from Spirulina platensis, has been explored as a natural blue pigment for application in the food industry. It is also used as a nutraceutical in food, cosmetic, and pharmaceutical products because of its potentially beneficial biological properties, such as radical scavenging, immune modulating, and lipid peroxidase activities. The biggest challenges to the widespread application of phycocyanin for this purpose are its high sensitivity to chemical degradation when exposed to heat, light, acids, high pressure, heavy metal cations, and denaturants. Consequently, it is of considerable importance to improve its chemical stability, which requires a thorough knowledge of the relationship between the structure, environment, and chemical reactivity of phycocyanin. To increase the application of this natural pigment and nutraceutical within foods and other products, the structure, biological activities, and factors affecting its stability are reviewed, as well as strategies that have been developed to improve its stability. The information contained in this article is intended to stimulate further studies on the development of effective strategies to improve phycocyanin stability and performance.
Keywords: Phycocyanin, Stability, Encapsulation, Food matrix effects, Bioactivity
Graphical abstract
Highlights
-
•
Brilliant blue phycocyanin extracted from spirulina belongs to the blue foods.
-
•
Important factors affected the stabilities of phycocyanin were reviewed.
-
•
Strategies methods to improve the stability and bioactivities of phycocyanin.
1. Introduction
The appearance of foods is one of the most important sensory characteristics impacting consumer acceptance (Luo et al., 2021; Martelli et al., 2014). Food colorants are therefore often added to foods to improve their visual appeal, which may be natural or synthetic. The US Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) limit the addition of many synthetic colorants in foods and beverages due to their potential toxic effects on humans, which has led to an interest in identifying natural colors to replace synthetic ones. Compared to red, yellow, orange, and green natural colorants, the development of blue natural colorants are still in its infancy (Kaderides et al., 2021; Landim Neves, Silva and Meireles, 2021). Blue pigments isolated from certain kinds of algae have been shown to have potential applications in foods (Fratelli et al., 2021). The utilization of this renewable source of natural pigments in foods may therefore improve the sustainability of the modern food supply (Gephart et al., 2021; Naylor et al., 2021; Tigchelaar et al., 2021; Short et al., 2021) (see Table 1).
Table 1.
The Strategy Methods of improving the stability of phycocyanin.
| Methods | Process conditions | Results | Mechanism | Characteristics | Disadvantages | References |
|---|---|---|---|---|---|---|
| Addition of biopolymers | ||||||
| ι-Carrageenan (combined with high-pressure processing) | Mixed polysaccharide solution and phycocyanin solution, then subjected the samples to high-pressure processing treatment (450 Mpa or 600 Mpa) | The light stability of phycocyanin-carrageenan at pH 7.0 is improved and prevent the attack of oxidizing radicals after high-pressure processing | ι-Carrageenan with highly negatively charged stretch phycocyanin's tertiary structure and maintain secondary structure, which may benefit the stability of phycocyanin | Less damage to its nutrient composition and improve the glycation reaction | Although high pressure can't influence the structure of small molecules (chromophore), phycocyanin aggregates with changes in secondary structures and forms a more compact protein structure |
https://www.sciencedirect.com/science/article/pii/S0268005X20305385 Zhang et al. (2021) |
| Whey protein (combined high-pressure treatment) | Mixed phycocyanin solution and whey protein solution, then subjected the samples to high-pressure processing treatment (450 Mpa or 600 Mpa) | The light stability of phycocyanin-whey improved at pH 5.0 after high pressure treatment | Phycocyanin-whey protein mixture with high-pressure progress may encapsulate the chromophore inside the apoprotein network and prevented the attack from oxidizing radicals | Less damage to its nutrient composition and improve the glycation reaction | Phycocyanin may form a more compact protein structure with the changes in secondary structures |
https://www.sciencedirect.com/science/article/pii/S0268005X20305385 Zhang et al. (2021) |
| Low concentration of whey (0.05–0.1%) | Whey protein was dissolved in phycocyanin solution in 100 mL tubes to obtain whey | Delay the color degradation of phycocyanin at pH 3.0 by light over 5 days | A low concentration of whey protein may help unfold chain tetrapyrroles of phycocyanin through transformation from α-helix to β-sheet and protected its secondary structure from being destroyed by light |
https://www.sciencedirect.com/science/article/pii/S0308814619317406 Zheng et al. (2020) |
||
| Preservatives (citric acid, sucrose and calcium chloride) | Added preservatives into the phycocyanin solution | Maintaining the stability of phycocyanin in aqueous phase at 35 ± 5 °C for 45 days | Increased the stability of the phycocyanin structure and disrupted the structure of water and decrease protein solubility (salting out) | Adjusted the sensory characteristics of food and extended shelf life | Preservative may damage the human health |
https://www.sciencedirect.com/science/article/pii/S1359511307003455 Mishra et al. (2008) |
| DSP [dithiobis (succinimidyl propionate)] | To 19 ml of phycocyanin (3.0 mg/ml) in 50 mM phosphate buffer saline (PBS) (pH 7.4) was added DSP (4.1, 8.2 and 16.4 mg) dissolved in 1.0 ml of dimethyl sulfoxide (DMSO) | The absorbance at 614 nm of cross-linked phycocyanin (Curve b) was retained at 70% of the original color development in 4 M urea for 8 h | DSP modified the amino groups of lysine to protect the high order structure of phycocyanin | Convenient to handling | Exist potential risks |
https://www.sciencedirect.com/science/article/pii/S0143720803002730 Fukui et al. (2004) |
| Formaldehyde | Added the formaldehyde into phycocyanin solution and dialyzed overnight | Phycocyanin-formaldehyde which may keep stabilize at 100% over 120 min and improve the stability up to 1.53-folds under the light | Covalent crosslinking between the formaldehyde and phycocyanin may prevent the dissociation of phycocyanin into subunits | Convenience in ease of handling, extreme adaptability and broadest reaction specificity | Not suitable for food industry |
https://www.sciencedirect.com/science/article/pii/S1359511319317131 Munawaroh et al. (2020) |
| Oil | Incorporated of EVO oil or sunflower oil at the concentration of 10 g oil/100 g of dough | After cooking, extra virgin olive-phycocyanin mixture maintained about 90% of the originally cyanobacterial biomass. | Tocopherol contained in EVO and sunflower oils was the main responsible for the protective action against phycocyanin degradation | Suitable for all bakery products | Not suitable for other food processing |
https://www.sciencedirect.com/science/article/pii/S0023643820317655 Niccolai et al. (2021) |
| Micelles | ||||||
| SDS micelles | Prepared the aqueous solutions of phycocyanin and SDS | Stabilized phycocyanin against pH-dependent color variation | Attributed to the SDS micelles entrapment of the molecule in the interior and stabilized by hydrophobic interactions, it has the capability of stabilizing a folded conformation at low pH | Don't need a substantial amount of encapsulation material. It can be used in transparent products |
SDS may confer an unacceptable detergent taste in food products |
https://www.sciencedirect.com/science/article/pii/S0308814617311548 Falkeborg et al. (2018) |
| Microencapsulation | ||||||
| Electrospraying technique | The capillary diameter was 0.45 mm and a distance between the capillary and the collector was 18 cm; 11% PVA and 2% PC, a feed rate of 50 μLh−1 and an electric potential of 20 kV; |
The polymer showed well thermal resistance up to 216 °C | Phycocyanin was surrounded by a barrier (PVA) to protect against light, oxygen, pH, moisture, heat, shear or other extreme conditions | Smaller droplet size with a narrow distribution; High encapsulation efficiency; Without using high temperature or pressure |
Low throughput |
https://www.sciencedirect.com/science/article/pii/S0960308519301762 Schmatz et al. (2020) |
| Extrusion technique | Alginate and chitosan were used as coating materials; Under the high pressure, the matrix dispersion through a single or a plurality of pathways directly into the continuous extraction phase |
The microcapsules could resistant to the light (light for 40 days), temperature (50 °C), acidic (SGF pH 1.2) and humid (31% relative humidity) environment | Attributed to the hygroscopicity of chitosan, the compact structure of microencapsulation was formed by the interaction of alginate and chitosan and prevented the entry of water | Simple and convenient; Better-controlled microsphere sizes |
Involved high pressure |
https://www.sciencedirect.com/science/article/pii/S0960308513000734 Yan et al. (2014) |
| Entrapped into silica matrixes | Encapsulated phycocyanin into hydrogel and controlled the gelation time to homogeneously dispersing it in silica matrix | The photodamage rate constant of phycocyanin in silica is 25 times slower than the phycocyanin in buffer solution | Phycocyanin encaged by Si–O bands, which could restrict the unfolding of phycobilin and protect the linear conformation |
https://link.springer.com/article/10.1007%2Fs11164-009-0061-5 Li et al. (2009) |
||
| Coated with STMP/STPP cross-linked starches | Sodium trimetaphosphate/sodium tripolyphosphate cross-linked potato, banana, corn, cassava, and breadfruit starches were as wall materials for C-phycocyanin encapsulation | Prolonged antihyperalgesic effects of phycocyanin in vivo | The C-phycocyanin was encapsulated within amorphous chains of cross-linked starches | High availability; Low cost |
https://www.sciencedirect.com/science/article/pii/S0141813020332633 Lemos et al. (2020) |
|
Researchers have reported that blue pigments isolated from Spirulina platensis, a kind of seaweed, have a desirable color profile, good sustainability, and potential health benefits (Golden et al., 2021). Spirulina platensis belongs to the cyanobacterium of high economic and nutritional value (Belay et al., 1996; Zhou et al., 1991). The pigments of Spirulina platensis primarily originate from the light-harvesting protein complex named phycobiliprotein, which consists of phycocyanin, phycoerythrin, and allophycocyanin. Structurally, these pigments are found around the core (allophycocyanin) of the phycobiliprotein and the periphery (phycoerythrin and phycocyanin) of the photosystem complex (Kannaujiya et al., 2019). The seaweed extracts are characterized by a bright blue color because of the presence of the phycocyanin.
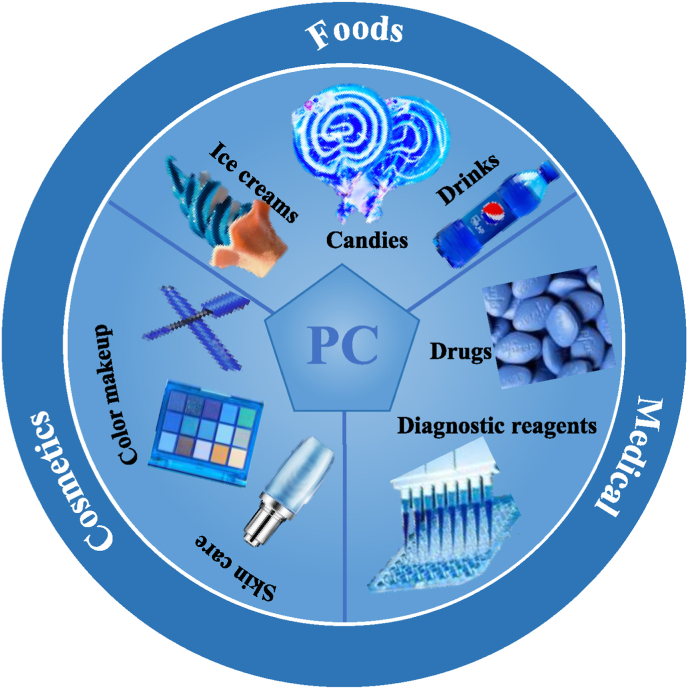
Commercially, phycocyanin is a supramolecular protein-chromophore complex, which is usually extracted from Spirulina platensis, and is currently valued at around $360 per kg (Chaiklahan et al., 2012; Li et al., 2021). Compared to other proteins, the phycobiliproteins of phycocyanin possesses high fluorescence quantum yields and the apo-protein has the ability to bind other molecules (such as immunoglobins and avidin) to form bonds, which may act as fluorescent probes that are used in fluorescence microscopy, immunoassays, and histochemistry (Eriksen, 2008; Khan et al., 2005; Romay et al., 1998; Romay et al., 2000). Also, phycocyanin has been reported to exhibit radical scavenging, immune modulation, and lipid peroxidase activities, which mean that it may also be used as a nutraceutical ingredient in foods, cosmetics, and pharmaceuticals (Fernandez-Rojas et al., 2014; Mishra et al., 2008; Patel et al., 2004). As a result of its desirable color profile and nutritional effects, the popularity of phycocyanin as a natural blue pigment and nutraceutical ingredient is growing in food industry.
In recent years, methods to extract and improve the stability of phycocyanin have been reviewed. Jaeschke et al. mainly reviewed phycocyanin extraction technologies, and reported that a pulsed electric field method was particularly suitable for obtaining high yields and purities (Jaeschke et al., 2021). Fratelli et al. also summarized the technologies available to extract phycocyanin, and reported that solvent extraction can better protect the antioxidant capacity of phycocyanin (Fratelli et al., 2021). Adjali et al. recently reported that the stability of phycocyanin can be improved by adding preservatives or by using packaging materials (Adjali et al., 2022). These authors reviewed the instability of phycocyanin under different light, temperature, and pH conditions, and did not explore the underlying mechanisms of phycocyanin instability. Therefore, we have carried out a thorough search of the literature and found that the various factors affecting phycocyanin stability have not been comprehensive reviewed, as well as the molecular and physicochemical origins of its instability. Therefore, this manuscript reviews the effects of temperature, light, pH, pressure, heavy metal ions, and denaturants on phycocyanin stability. Compared to previous review articles, we summarize the main factors promoting phycocyanin instability, explain the main reasons for this instability, and focus on potential degradation mechanisms. In addition, the different strategies developed to improve phycocyanin stability have been discussed. The knowledge presented in this article may facilitate the utilization of phycocyanin in the food industry by increasing the understanding of the main factors causing its degradation.
2. The structure and biological activities of the phycocyanin
Phycobilisome, the supramolecular protein complex, harvests light and delivers energy to the reaction centers of eukaryotic red algae and prokaryotic cyanobacteria. It therefore plays an important role in photosynthesis. It is composed of a central core called allophycocyanin and several rods that surround the core called phycocyanins (Ferraro et al., 2020) (see Fig. 1).
Fig. 1.
The industry applications of the phycocyanins.
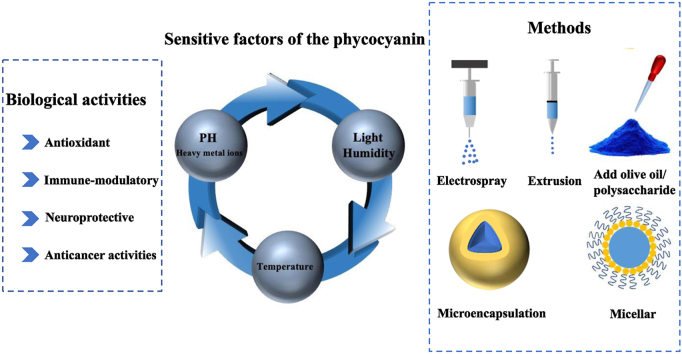
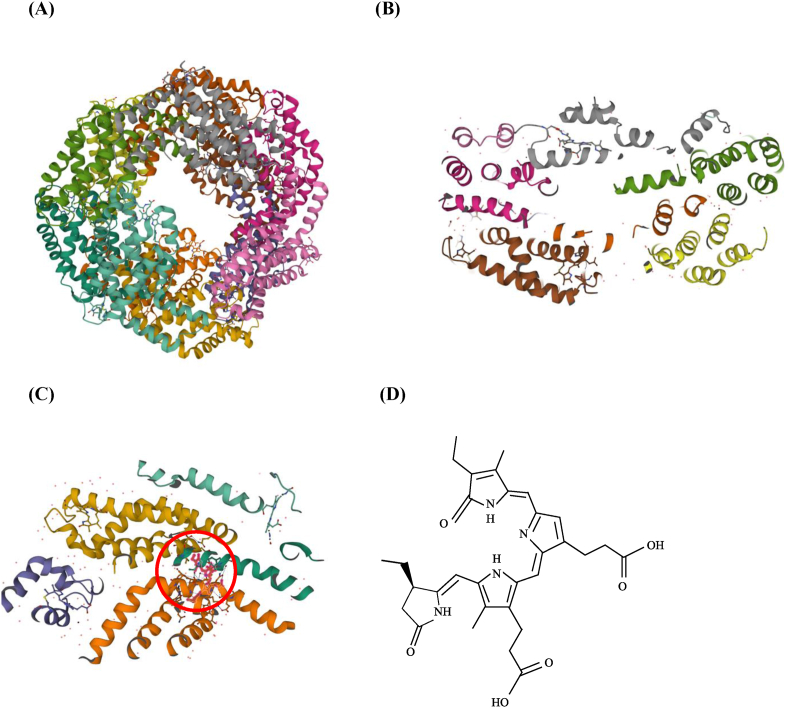
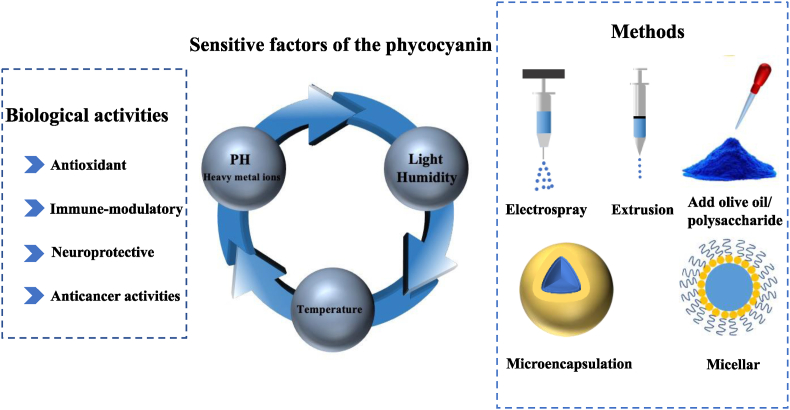
Phycobiliproteins have three main classes of pigments, each with its own spectral features: phycocyanin (phycocyanin; blue, λmax = 617 nm); phycoerythrin (red, λmax = 565 nm); and allophycocyanin (bluish green, λmax = 650 nm) (Ferraro et al., 2020). Of these, phycocyanin forms the major part of phycobiliproteins (Ma et al., 2020). The blue color of phycocyanin is due to a chromophore called phycocyanobilin, which binds proteins through thioether bonds (Pez et al., 2021). As shown in Fig. 2C and D, phycocyanin contains two homologous subunits: the α-subunit with one phycocyanobilin attached at cysteine 84 and the β-subunit with two phycocyanobilins attached at cysteines 84 and 155 (Tang et al., 2012; (Scheer and Zhao, 2008)). The phycocyanobilins are open-chain tetrapyrrole chromophores with a specific arrangement of conjugated double bonds, which are responsible for the unique radical scavenging and antioxidant properties of the phycocyanin, as well as its color. Phycocyanin has been reported to prevent or reduce hepatorenal and hematological damage because it protects the tissues via γ-glutamyl-cysteinyl-glycine, which can scavenge reactive oxygen species and free radicals through enzymatic reactions (Belay, 2002; Falkeborg et al., 2018; Sayed et al., 2022). In addition, phycocyanin has been reported to inhibit the growth of cancer cells, reduce inflammation, inhibit bleeding, prevent cataracts, and protect the heart, liver, lung, and kidneys (Fernandez-Rojas et al., 2014; Yu et al., 2017) (see Fig. 3).
Fig. 2.
Structure of phycocyanin from Spirulina platensis. (A) Three-dimensional structure of phycocyanin from cyanobacterium S. platensis in form of hexamer, image from PDB (https://www.rcsb.org/structure/1GH0). (B) Phycocyanin exists as (αβ)6 hexamer and both chains have the α-helix structure. (Green, brown and pink represent α subunit. Grey, yellow and dark brown represent β subunit). (C) (D) Three-dimensional and secondary structure of phycocyanobilin. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
The biological activities, sensitive factors of the phycocyanins and the methods of protecting the phycocyanins.
In solution, phycocyanin forms αβ units that assemble into multimeric structures (αβ)n (n = 1–6), whose molecular weights range from around 44 to 260 kDa (Ma et al., 2020; Zhang et al., 2021, Zhang et al., 2021). As shown in Fig. 2A and B, phycocyanin typically exists as an (αβ)3 trimer or (αβ)6 hexamer, and both subunits with molecular weight around 20 kDa have conserved α-helix regions (Jaouen et al., 1999; Munawaroh et al., 2020).
However, when the hexamers disassemble into trimers and monomers, the extinction coefficients of the phycobiliproteins decrease. The high order structure of phycocyanin is a major factor influencing its biological stability (Fukui et al., 2004). Also, the intensity and position of the absorption maximum of phycocyanin have been shown to depend on its state of aggregation (Hu et al., 2021). The aggregation and structure of phycocyanin are influenced by temperature, pH, light, pressure, heavy metal cations, and denaturants, which may therefore impact its biological activity and color profile. In addition, light absorbance and fluorescence polarization are influenced by the structure of the phycocyanin molecule (Moreira et al., 2019). Collectively, it can be concluded that the color and bioactivities of the phycocyanin are maintained through the high order structure of the phycocyanin protein.
3. Factors affecting the stabilities of phycocyanin
Numerous factors are considered to affect the stability of phycocyanin, including temperature, light, pH, pressure, heavy metal cations, and denaturants (Berns and MacColl, 1989; Kupka and Scheer, 2008; Sarada et al., 1999). Moreover, studies have highlighted that the water activity, glass transition temperature, and rheology of the system can also influence the stability of phycocyanin (Neri et al., 2011; Neri et al., 2014). By elucidating the impact of these factors on phycocyanin stability, it is possible to improve its stability in food and other applications.
3.1. Effect of temperature
Temperature significantly affects the conformation of phycocyanin. Studies have shown that a critical temperature for phycocyanin stability is 47 °C, as evidenced by an appreciable reduction in the half-life of phycocyanin in solution when the temperature exceeded this value (Chaiklahan et al., 2012). Similarly, in another analysis of the thermal stability, it was reported that the antioxidant potential of phycocyanin decreased more than 50% after 21 and 35 days of exposition at the temperature of 40 and 50 °C, respectively. A decrease in blue hue has also been reported when the phycocyanin is heated (Colla et al., 2017). Further, researchers found that the phycocyanin lost color when heated to 60 °C for 30 min (Rahman, Sarian, van Wijk, Martinez-Garcia and van der Maarel, 2017; Zhang et al., 2021). This phenomenon may be due to the conformational changes of the apoprotein disturbing the coupling and energy transfer pathways between the embedded chromophores (Li et al., 2021, Li et al., 2021). Heating caused the phycocyanin to partially unfold, thereby exposing hydrophobic groups that were normally located in the interior of the molecular. Furthermore, phycocyanin subunits aggregate during heat treatment, which can also cause degradation of phycocyanin and reduce the blue color. Other researchers reported that the aggregation and degradation of phycocyanin subunits during heating could be modeled using the Weibull model (Faieta et al., 2022).
3.2. Effect of light
Phycocyanin is unstable in the presence of light with the degradation rate depending on the intensity and wavelength of light used. Researchers have reported that the antioxidant potential of phycocyanin decreased under fluorescent (20 W, 0.16 A) and ultraviolet (15 W, λ = 265 nm) light (Colla et al., 2017). When exposed to light a cyclohelical conformation was adopted, it caused nonfluorescent, deactivation, and photo-degradation due to intramolecular energy transfer or radical reactions (Li et al., 2009). Other researchers found that adding epigallocatechin gallate can stabilize the phycocyanin when exposed to light (Yang et al., 2022).
3.3. Effect of pH
Phycocyanin is found to be unstable at the pH values between 4.0 and 4.8, which is fairly close to its isoelectric point (pH 3.4) (Li et al., 2021). Some researchers reported that phycocyanin was insoluble under acidic conditions (pH 3.0) but highly soluble under neutral conditions (pH 7.0) (Antelo et al., 2008). The net charge of phycocyanin at its isoelectric point is zero, resulting in a reduced electrostatic repulsion between the molecules, thereby promoting the aggregation and precipitation. Research have reported that phycocyanin exists in a monomer-trimer system at pH 4.5 to 6.0 (Mishra et al., 2008). However, it shifted towards the monomer when the system is diluted. Phycocyanin has also been reported to be unstable to aggregation at low pH values and high ionic strengths due to a reduction in the electrostatic repulsion between the protein molecules (Hattori et al., 1965; Newsome et al., 2014).
3.4. Effect of high-pressure processing
Recently, high-pressure processing has been applied to pasteurize beverages at reduced temperatures. This technique needs high hydraulic pressures (400–600 MPa) and can extend the shelf life and improve the safety of foods (Li et al., 2021; Xia et al., 2020). However, high-pressure processing can promote phycocyanin denaturation and aggregation, thereby altering its color (Zheng et al., 2020, Zheng et al., 2020).
3.5. Effect of heavy metal cations
The existence of excess cysteinyl residues and anionic amino acid residues in phycocyanin means it can coordinate with metal cations (Han et al., 2018). When heavy metal cations bind to phycocyanin, the fluorescence tends to be quenched due to a decrease in α-helix and β-turn structures and increase in random coil and β-sheet structures (Bhayani et al., 2016). Other researchers have reported that ion the type and concentration of metal influenced the light absorption of phycocyanin (Chi et al., 2020). These effects have been attributed to the binding of cationic ions to anionic groups on the protein surfaces, thereby altering the structure of phycocyanin. Other researchers reported that addition of Cu2+ and Zn2+ ions reduced the color of phycocyanin solutions significantly, which was attributed to the ability of the metal ions to inhibit electron transfer and photosynthetic oxygen (Memije-Lazaro et al., 2018). Heavy metal ions reduced the color of phycocyanin solutions may attribute to the formation of supramolecular fibrous networks (Han et al., 2018). Taken together, these studies indicated that metal ions disrupted the structure and aggregation of phycocyanin, thereby altering its color and biological activity.
3.6. Effect of denaturants
Phycocyanin may also lose its color and biological activities when exposed to certain kinds of protein denaturants. For instance, the ability of urea to decrease these activities was attributed to its ability to bind to the surface of the phycocyanin, thereby disrupting the molecular interactions and destabilizing the native state, and the color and biological activities of the protein molecules were reduced (Mishra et al., 2008).
4. Strategies to improve the stability of phycocyanin
The chemical stability of phycocyanin depends on its molecular characteristics, as well as solution and environmental conditions. Some of the methods used to protect phycocyanin are unsuitable for application in food products because they involve chemicals that are not safe for consumption. Therefore, alternative approaches are being developed to protect phycocyanin in foods, such as controlling food matrix composition and encapsulation.
4.1. Addition of biopolymers
In the food industry, it is common practice to add biopolymers, such as polysaccharides and proteins, to phycocyanin formulations to inhibit its aggregation and improve its stability.
4.1.1. Addition of polysaccharides
The stability of food proteins can be enhanced by physically complexing or chemically conjugating them to polysaccharides or other carbohydrates (Faieta et al., 2020a, Faieta et al., 2020b). Protein glycation is a common modification method used to optimize the properties of food proteins, which involves forming covalent crosslinks between the carbonyl groups of reducing sugars and amino groups of proteins. For example, glycation of phycocyanin has been reported to improve its functionality (Liu et al., 2020; Zheng et al., 2020).
Anionic food polysaccharides have been used to protect the biological activities and color of phycocyanin because they can stabilize the protein structure and inhibit protein aggregation. The negatively charge groups on the polysaccharides form electrostatic links to the positively charged groups on the proteins, thereby improving the stability and functionality of the phycocyanin (Barbiroli et al., 2017). As an example, forming ι-carrageenan-phycocyanin complexes was shown to improve the colloidal and color stabilities of the protein (Zhang et al., 2021). Moreover, the light stability of the phycocyanin and ι-carrageenan complexes at pH 7.0 was enhanced after a high-pressure treatment. Similarly, researchers reported that the blue color of phycocyanin remained stable after heat treatment at 90 °C at pH 2.5 and 3.0 after adding λ-carrageenan (Buecker, Grossmann, Loeffler, Leeb and Weiss, 2022b). The same research team showed that carrageenan-phycocyanin complexes were more stable to color fading at pH 2.5 than 4.5 (Buecker, Grossmann, Loeffler, Leeb and Weiss, 2022a). Presumably, the addition of ι-carrageenan altered the molecular structure of phycocyanin in a way that made it more resistant to degradation.
Also, sugars have been used as stabilizing agents for phycocyanin in both high and low moisture environments. For instance, researchers reported that the half-life of phycocyanin increased from 19 min to around 30–44 min at pH 7 after heating at 60 °C for 15 min when 20–40% glucose or sucrose was added (Faieta et al., 2020). Interestingly, the viscosity, water activity, and glass transition temperature of sugars have been reported to influence the stability of phycocyanin. Besides that, coating the polysaccharides on the surface of phycocyanin can prevent the protein aggregation of phycocyanin.
4.1.2. Addition of proteins
Numerous researchers have investigated the effects of proteins on the stability of phycocyanin. For example, Zhang et al. reported that phycocyanin-whey protein complexes formed using high pressure (450 MPa) treatment can encapsulate the chromophore and decrease the damage caused by oxidizing radicals (Zhang et al., 2021). Other researchers found that the whey protein blocked the aggregation of phycocyanin and stabilized it at pH 3.0 (Zhang et al., 2020). Also, it has been reported that adding a low concentration (around 0.05–0.1%) of whey protein could delay the photo-degradation of phycocyanin at pH 3.0 (Zhang et al., 2021). This effect may be due to that whey proteins protected the phycocyanin structure from being disrupted (Newsome et al., 2014). In general, these studies showed that certain kinds of proteins can prevent conformational changes, aggregation, and discoloration of phycocyanin, which may be beneficial for some food applications.
4.1.3. Addition of edible preservatives
Preservatives are used to ensure that foods remain safe and unspoiled throughout their shelf lives. Dithiothreitol and sodium azide have been shown to protect phycocyanin from degradation, however, these are toxic substances that are not suitable for food applications (Mishra et al., 2008). Thus, it is important to identify other kinds of food-grade preservatives that can protect the color and biological activities of phycocyanin.
Citric acid has been shown to protect phycocyanin from degradation in aqueous solutions when stored at 35 ± 5 °C for 45 days (Fabre et al., 2022; Mishra et al., 2008). The apoprotein part of the phycocyanin can be stabilized due to strong molecular interactions with the citrate anion, as this ion disrupted the water structure in phycocyanin solutions (Resch et al., 2005). Excessive use of preservatives may have negative effects on human health and so they should only be used in relatively small amounts.
4.1.4. Addition of crosslinkers
Glutaraldehyde and formaldehyde are the most commonly used crosslinkers for proteins because of their low price and strong reactivity (Hernandez-Munoz et al., 2005). Dithiobis (succinimidyl propionate) and formaldehyde have been used to prevent the deactivation of phycocyanin by crosslinking the protein. The dithiobis (succinimidyl propionate) is convenient and easy to handle, with extreme adaptability and the broadest reaction specificity. Previous research has found that dithiobis (succinimidyl propionate) could modify the amino groups of lysine and protect the high order structure of the phycocyanin. The absorbance at 614 nm of formaldehyde-crosslinked phycocyanin was retained at 70% of the original value when the proteins were incubated in a urea solution (4 M) for 8 h (Fukui et al., 2004). Similarly, phycocyanin-formaldehyde complexes were reported to maintain 100% stability over 120 min when exposed to light (Munawaroh et al., 2020). However, some of these crosslinking agents are toxic and unsuitable for food processing. Consequently, it is important to identify food-grade crosslinking agents that can be used to protect the color and activity of phycocyanin, such as some enzymes.
4.1.5. Addition of edible oil
Studies have shown that the stability of phycocyanin in some food products can be improved by incorporating oils. For instance, adding sunflower oil or extra virgin olive oil (10 g oil/100 g bakery dough) protected phycocyanin from thermal degradation during cooking (Niccolai et al., 2021). These researchers reported that the tocopherol contained in the oils was responsible for protecting the phycocyanin from degradation.
4.2. Encapsulation strategy
Encapsulation technologies can be used to protect sensitive bioactive molecules by packaging them within particles with micrometer- or nanometer-scale dimensions (Tie et al., 2022; Wang et al., 2022; Yu et al., 2022).
4.2.1. Formation of micelles
Encapsulation in sodium dodecyl sulfate (SDS) surfactant micelles has been shown to protect phycocyanin from degradation (Falkeborg et al., 2018), which was mainly attributed to the ability of the micelles to stabilize the blue circular helical regions of the phycocyanobilin via hydrophobic interactions. Moreover, the SDS-micelles coat the phycocyanin and prevent it from aggregating. Other researchers reported that anthocyanins could also be stabilized through their interactions with SDS (Liu et al., 2014), which in this case was attributed to electrostatic attraction between cationic protein groups and anionic charged SDS-micelles. Even though SDS can protect phycocyanin in acidic environments, this anionic surfactant is unsuitable for food applications. Therefore, it is still necessary to identify food-grade surfactants to prepare micelles for this purpose.
4.2.2. Formation of microencapsulation
Microencapsulation involves converting a fluid form of a material into a powdered form, which can protect them (Li et al., 2019). There has been some research on the protection of phycocyanins using microencapsulation (Pan et al., 2019). Methods such as extrusion, electro-spraying, spray drying, freeze drying, and fluid bed coating have been used for this purpose (Yan et al., 2014).
Electro-spraying produces ultrafine particles by applying an electric potential to a polymer solution under ambient temperature and pressure. When polyvinyl alcohol was used for the encapsulation of phycocyanin, the diameter of the particles produced by electro-spraying was 395 ± 71 nm and the encapsulation efficiency was 75.1 ± 0.2% (Schmatz et al., 2020). The encapsulated phycocyanin exhibited good thermal resistance up to 216 °C.
The extrusion method has been used to microencapsulate phycocyanin in a biopolymer matrix consisting of chitosan and alginate (Yan et al., 2014). The diameters of the microcapsules produced using this method were about 1.03 mm. Encapsulation was shown to improve the resistance of the phycocyanin to light exposure (40 days), heating (50 °C), acids (simulated gastric fluids, pH 1.2) and relative humidity (31% RH). This phenomenon was related to the dense structure of the phycocyanin/alginate/chitosan microcapsules. In situ mineralization of calcium phosphate (CAP) on the surfaces of phycocyanin was used to prepare the phycocyanin@Mg-CAP particles, which significantly improved the thermal stability of the encapsulated phycocyanin (Li et al., 2022).
Researchers have reported that entrapping phycocyanin within silica matrices reduced their rate of degradation when irradiated by ultraviolet rays, with the photodamage rate constant of phycocyanin in silica being 25 times less than in buffer solution (Li et al., 2009). The origin of this effect was attributed to the phycocyanin being encaged by Si–O bands, which restricted the unfolding of phycobilin, so that tetrapyrroles were able to protect the linear conformation, which is more photo-stable. Similarly, researchers showed that encapsulating phycocyanin in crosslinked starch matrixes improved its stability (Lemos et al., 2020). Taken together, these studies suggested that microencapsulation technologies can be used to improve the stability of phycocyanin.
5. Conclusion and perspectives
Phycocyanin extracted from spirulina is a natural blue pigment and nutraceutical that is finding widespread utilization in the food industry because of the trend towards healthier and more sustainable foods. The biological activities and color of phycocyanin depend on its high order structure. This article reviewed recent studies on the factors affecting the chemical degradation of phycocyanin and the strategies developed for improving its stability. The structure of phycocyanin was reviewed, the factors affecting its stability were discussed, and different approaches to protect it were highlighted. Moreover, we analyzed the relationship between the structure and biological activities of phycocyanin. This knowledge could be used by the food industry to improve the color stability and biological activities of phycocyanin-containing foods and beverages. Nevertheless, further research is still required to develop cost-effective and label-friendly approaches to stabilize phycocyanin in different kinds of food products, which may be fluid, semi-solid, or solid.
CRediT authorship contribution statement
Biao Yuan: Conceptualization, Methodology, Data curation, Resources, Writing – original draft, Funding acquisition. Zhuxin Li: Conceptualization, Methodology, Data curation, Resources, Writing – original draft, Funding acquisition. Honghong Shan: Writing – review & editing, Conceptualization. Badamkhand Dashnyam: Writing – review & editing, Conceptualization. Xiao Xu: Conceptualization, Supervision. David Julian McClements: Conceptualization, Supervision. Bingquan Zhang: Conceptualization, Supervision. Mingqian Tan: Supervision, Writing – review & editing, Funding acquisition. Zhixiang Wang: Supervision, Writing – review & editing, Funding acquisition. Chongjiang Cao: Supervision, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Key Research and Development Program of China (2018YFD0901106), the National Natural Science Foundation of China (No. 31901699), Scientific Research Start-up Fund of Shaoxing University (No. 20195015), the Fundamental Research Funds for the Central Universities (No. 2632021ZD01), China Postdoctoral Science Foundation (No. 2020T130138ZX), Jiangsu Planned Projects for Postdoctoral Research Funds.
References
- Antelo F.S., Costa J.A.V., Kalil S.J. Thermal degradation kinetics of the phycocyanin from Spirulina platensis. Biochem. Eng. J. 2008;41(1):43–47. doi: 10.1016/j.bej.2008.03.012. [DOI] [Google Scholar]
- Adjali A., Clarot I., Chen Z., Marchioni E., Boudier A. Physicochemical degradation of phycocyanin and means to improve its stability: a short review. J. Pharmaceut. Anal. 2022;12(3):406–414. doi: 10.1016/j.jpha.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbiroli A., Marengo M., Fessas D., Ragg E., Renzetti S., Bonomi F., Iametti S. Stabilization of beta-lactoglobulin by polyols and sugars against temperature-induced denaturation involves diverse and specific structural regions of the protein. Food Chem. 2017;234:155–162. doi: 10.1016/j.foodchem.2017.04.132. [DOI] [PubMed] [Google Scholar]
- Belay A., Kato T., Ota Y. Spirulina (Arthrospira): potential application as an animal feed supplement. J. Appl. Phycol. 1996;8(4–5):303–311. doi: 10.1007/BF02178573. [DOI] [Google Scholar]
- Belay A. The potential application of Spirulina (Arthrospira) as a nutritional and. therapeutic supplement in health management. JANA. 2002;5(2):27–48. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0041518445&partnerID=40&md5=6d411940da360aad7e0b2bc27fda4207 [Google Scholar]
- Berns D.S., MacColl R. Phycocyanin in physical chemical studies. Chem. Rev. 1989;89(4):807–825. doi: 10.1021/cr00094a005. [DOI] [Google Scholar]
- Bhayani K., Mitra M., Ghosh T., Mishra S. C-Phycocyanin as a potential biosensor for heavy metals like Hg2+ in aquatic systems. RSC Adv. 2016;6(112):111599–111605. doi: 10.1039/c6ra22753h. [DOI] [Google Scholar]
- Buecker S., Grossmann L., Loeffler M., Leeb E., Weiss J. Influence of storage temperature on the stability of heat treated phycocyanin-λ-carrageenan complexes in liquid formulations. Green Chem. 2022;24(10):4174–4185. doi: 10.1039/d2gc00809b. [DOI] [Google Scholar]
- Buecker S., Grossmann L., Loeffler M., Leeb E., Weiss J. Thermal and acidic denaturation of phycocyanin from Arthrospira platensis: effects of complexation with λ-carrageenan on blue color stability. Food Chem. 2022;380 doi: 10.1016/j.foodchem.2022.132157. [DOI] [PubMed] [Google Scholar]
- Chaiklahan R., Chirasuwan N., Bunnag B. Stability of phycocyanin extracted from Spirulina sp.: influence of temperature, pH and preservatives. Process Biochem.y. 2012;47(4):659–664. doi: 10.1016/j.procbio.2012.01.010. [DOI] [Google Scholar]
- Chi Z., Hong B., Tan S., Wu Y., Li H., Lu C.-H., Li W. Impact Assessment of heavy metal cations to the characteristics of photosynthetic phycocyanin. J. Hazard Mater. 2020;391 doi: 10.1016/j.jhazmat.2020.122225. [DOI] [PubMed] [Google Scholar]
- Colla L.M., Bertol C.D., Ferreira D.J., Bavaresco J., Costa J.A.V., Bertolin T.E. Thermal and photo-stability of the antioxidant potential of Spirulina platensis powder. Braz. J. Biol. 2017;77(2):332–339. doi: 10.1590/1519-6984.14315. [DOI] [PubMed] [Google Scholar]
- Eriksen N.T. Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008;80(1):1–14. doi: 10.1007/s00253-008-1542-y. [DOI] [PubMed] [Google Scholar]
- Fabre J.F., Niangoran N.U.F., Gaignard C., Buso D., Mouloungui Z., Valentin R. Extraction, purification and stability of C-phycocyanin from Arthrospira platensis. Eur. Food Res. Technol. 2022;248(6):1583–1599. doi: 10.1007/s00217-022-03987-z. [DOI] [Google Scholar]
- Faieta M., Corradini M.G., Di Michele A., Ludescher R.D., Pittia P. Effect of encapsulation process on technological functionality and stability of spirulina platensis extract. Food Biophys. 2020;15(1):50–63. doi: 10.1007/s11483-019-09602-1. [DOI] [Google Scholar]
- Faieta M., Neri L., Sacchetti G., Di Michele A., Pittia P. Role of saccharides on thermal stability of phycocyanin in aqueous solutions. Food Res. Int. 2020;132 doi: 10.1016/j.foodres.2020.109093. [DOI] [PubMed] [Google Scholar]
- Faieta M., Toong C., Corradini M.G., Ludescher R.D., Pittia P. Degradation kinetics of C-Phycocyanin under isothermal and dynamic thermal treatments. Food Chem. 2022;382 doi: 10.1016/j.foodchem.2022.132266. [DOI] [PubMed] [Google Scholar]
- Falkeborg M.F., Roda-Serrat M.C., Burnæs K.L., Nielsen A.L.D. Stabilising phycocyanin by anionic micelles. Food Chem. 2018;239:771–780. doi: 10.1016/j.foodchem.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Fernandez-Rojas B., Hernandez-Juarez J., Pedraza-Chaverri J. Nutraceutical properties of phycocyanin. J. Funct.Foods. 2014;11:375–392. doi: 10.1016/j.jff.2014.10.011. [DOI] [Google Scholar]
- Ferraro G., Imbimbo P., Marseglia A., Illiano A., Fontanarosa C., Amoresano A., Olivieri G., Pollio A., Monti D.M., Merlino A. A thermophilic C-phycocyanin with unprecedented biophysical and biochemical properties. Int. J. Biol. Macromol. 2020;150:38–51. doi: 10.1016/j.ijbiomac.2020.02.045. [DOI] [PubMed] [Google Scholar]
- Fratelli C., Burck M., Amarante M.C.A., Braga A.R.C. Antioxidant potential of nature's “something blue”: something new in the marriage of biological activity and extraction methods applied to C-phycocyanin. Trends Food Sci. Technol. 2021;107:309–323. doi: 10.1016/j.tifs.2020.10.043. [DOI] [Google Scholar]
- Fukui K., Saito T., Noguchi Y., Kodera Y., Matsushima A., Nishimura H., Inada Y. Relationship between color development and protein conformation in the phycocyanin molecule. Dyes Pigments. 2004;63(1):89–94. doi: 10.1016/j.dyepig.2003.12.016. [DOI] [Google Scholar]
- Gephart J.A., Henriksson P.J.G., Parker R.W.R., Shepon A., Gorospe K.D., Bergman K., Eshel G., Golden C.D., Halpern B.S., Hornborg S., Jonell M., Metian M., Mifflin K., Newton R., Tyedmers P., Zhang W., Ziegler F., Troell M. Environmental performance of blue foods. Nature. 2021;597(7876):360–365. doi: 10.1038/s41586-021-03889-2. [DOI] [PubMed] [Google Scholar]
- Golden C.D., Koehn J.Z., Shepon A., Passarelli S., Free C.M., Viana D.F., Matthey H., Eurich J.G., Gephart J.A., Fluet-Chouinard E., Nyboer E.A., Lynch A.J., Kjellevold M., Bromage S., Charlebois P., Barange M., Vannuccini S., Cao L., Kleisner K.M., Rimm E.B., Danaei G., DeSisto C., Kelahan H., Fiorella K.J., Little D.C., Allison E.H., Fanzo J., Thilsted S.H. Aquatic foods to nourish nations. Nature. 2021;598:315–320. doi: 10.1038/s41586-021-03917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Lv L., Yu D., Wu X., Li C. Coordination induced supramolecular assembly of fluorescent C-Phycocyanin for biologic discrimination of metal ions. Mater. Lett. 2018;215:238–241. doi: 10.1016/j.matlet.2017.12.116. [DOI] [Google Scholar]
- Hattori A., Crespi H.L., Katz J.J. Association and dissociation of phycocyanin and the effects of deuterium substitution on the processes. Biochem. 1965;4(7):1225–1238. doi: 10.1021/bi00883a003. [DOI] [Google Scholar]
- Hernandez-Munoz P., Kanavouras A., Lagaron J.M., Gavara R. Development and characterization of films based on chemically cross-linked gliadins. J. Agric. Food Chem. 2005;53(21):8216–8223. doi: 10.1021/jf050952u. [DOI] [PubMed] [Google Scholar]
- Hu D., Zhang Z., Yuan L., Li W., Guo Y., Zhang R., Yang X., Peng H. Load phycocyanin to achieve in vivo imaging of casein-porous starch microgels induced by ultra-high-pressure homogenization. Int. J. Biol. Macromol. 2021;193:127–136. doi: 10.1016/j.ijbiomac.2021.10.127. [DOI] [PubMed] [Google Scholar]
- Jaouen P., Lepine B., Rossignol N., Royer R., Quemeneur F. Clarification and concentration with membrane technology of a phycocyanin solution extracted from Spirulina platensis. Biotechnol. Tech. 1999;13(12):877–881. doi: 10.1023/A:1008980424219. [DOI] [Google Scholar]
- Kaderides K., Kyriakoudi A., Mourtzinos I., Goula A.M. Potential of pomegranate peel extract as a natural additive in foods. Trends Food Sci. Technol. 2021;115:380–390. doi: 10.1016/j.tifs.2021.06.050. [DOI] [Google Scholar]
- Kannaujiya V.K., Kumar D., Pathak J., Sinha R.P. In: Cyanobacteria. Mishra A.K., Tiwari D.N., Rai A.N., editors. Academic Press; 2019. Chapter 10 - phycobiliproteins and their commercial significance; pp. 207–216. [Google Scholar]
- Khan Z., Bhadouria P., Bisen P.S. Nutritional and therapeutic potential of spirulina. Curr. Pharmaceut. Biotechnol. 2005;6(5):373–379. doi: 10.2174/138920105774370607. [DOI] [PubMed] [Google Scholar]
- Kupka M., Scheer H. Unfolding of C-phycocyanin followed by loss of non-covalent chromophore-protein interactions - 1. Equilibrium experiments. BBA-Bioenergetics. 2008;1777(1):94–103. doi: 10.1016/j.bbabio.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Landim Neves M.I., Silva E.K., Meireles M.A.A. Natural blue food colorants: consumer acceptance, current alternatives, trends, challenges, and future strategies. Trends Food Sci. Technol. 2021;112:163–173. doi: 10.1016/j.tifs.2021.03.023. [DOI] [Google Scholar]
- Lemos P.V.F., Opretzka L.C.F., Almeida L.S., Cardoso L.G., Silva J.B. A.d., Souza C.O.d., Villarreal C.F., Druzian J.I. Preparation and characterization of C-phycocyanin coated with STMP/STPP cross-linked starches from different botanical sources. Int. J. Biol. Macromol. 2020;159:739–750. doi: 10.1016/j.ijbiomac.2020.05.111. [DOI] [PubMed] [Google Scholar]
- Li Q., Dong P., Li L. Preparation and characterization of Mg-doped calcium phosphate-coated phycocyanin nanoparticles for improving the thermal stability of phycocyanin. Foods. 2022;11(4):503. doi: 10.3390/foods11040503. https://www.mdpi.com/2304-8158/11/4/503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhang R., Lei D., Huang Y., Cheng S., Zhu Z., Wu Z., Cravotto G. Impact of ultrasound, microwaves and high-pressure processing on food components and their interactions. Trends Food Sci. Technol. 2021;109:1–15. doi: 10.1016/j.tifs.2021.01.017. [DOI] [Google Scholar]
- Li X.M., Wu Z.Z., Zhang B., Pan Y., Meng R., Chen H.Q. Fabrication of chitosan hydrochloride and carboxymethyl starch complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2019;293:197–203. doi: 10.1016/j.foodchem.2019.04.096. [DOI] [PubMed] [Google Scholar]
- Li Y., Yang H., Cao F.M. Effect of ultraviolet irradiation on photostability of C-phycocyanin in a silica matrix. Res. Chem. Intermed. 2009;35(5):607–613. doi: 10.1007/s11164-009-0061-5. [DOI] [Google Scholar]
- Li Y., Zhang Z., Abbaspourrad A. Improved thermal stability of phycocyanin under acidic conditions by forming soluble complexes with polysaccharides. Food Hydrocolloids. 2021;119 doi: 10.1016/j.foodhyd.2021.106852. [DOI] [Google Scholar]
- Liu G., Li W., Qin X., Zhong Q. Pickering emulsions stabilized by amphiphilic anisotropic nanofibrils of glycated whey proteins. Food Hydrocolloids. 2020;101 doi: 10.1016/j.foodhyd.2019.105503. [DOI] [Google Scholar]
- Liu S.B., Fu Y.Q., Nian S. Buffering colour fluctuation of purple sweet potato anthocyanins to acidity variation by surfactants. Food Chem. 2014;162:16–21. doi: 10.1016/j.foodchem.2014.04.029. [DOI] [PubMed] [Google Scholar]
- Luo M., Zhou D.-D., Shang A., Gan R.-Y., Li H.-B. Influences of food contaminants and additives on gut microbiota as well as protective effects of dietary bioactive compounds. Trends Food Sci. Technol. 2021;113:180–192. doi: 10.1016/j.tifs.2021.05.006. [DOI] [Google Scholar]
- Ma J., You X., Sun S., Wang X., Qin S., Sui S.-F. Structural basis of energy transfer in Porphyridium purpureum phycobilisome. Nature. 2020;579(7797):146–151. doi: 10.1038/s41586-020-2020-7. [DOI] [PubMed] [Google Scholar]
- Martelli G., Folli C., Visai L., Daglia M., Ferrari D. Thermal stability improvement of blue colorant C-Phycocyanin from Spirulina platensis for food industry applications. Process Biochem. 2014;49(1):154–159. doi: 10.1016/j.procbio.2013.10.008. [DOI] [Google Scholar]
- Memije-Lazaro I.N., Blas-Valdivia V., Franco-Colín M., Cano-Europa E. Arthrospira maxima (Spirulina) and C-phycocyanin prevent the progression of chronic kidney disease and its cardiovascular complications. J. Funct.Foods. 2018;43:37–43. doi: 10.1016/j.jff.2018.01.013. [DOI] [Google Scholar]
- Mishra S.K., Shrivastav A., Mishra S. Effect of preservatives for food grade C-PC from Spirulina platensis. Process Biochem. 2008;43(4):339–345. doi: 10.1016/j.procbio.2007.12.012. [DOI] [Google Scholar]
- Moreira J.B., Lim L.-T., Zavareze E.d.R., Dias A.R.G., Costa J.A.V., Morais M.G.d. Antioxidant ultrafine fibers developed with microalga compounds using a free surface electrospinning. Food Hydrocolloids. 2019;93:131–136. doi: 10.1016/j.foodhyd.2019.02.015. [DOI] [Google Scholar]
- Munawaroh H.S.H., Gumilar G.G., Alifia C.R., Marthania M., Stellasary B., Yuliani G., Wulandari A.P., Kurniawan I., Hidayat R., Ningrum A., Koyande A.K., Show P.-L. Photostabilization of phycocyanin from Spirulina platensis modified by formaldehyde. Process Biochem. 2020;94:297–304. doi: 10.1016/j.procbio.2020.04.021. [DOI] [Google Scholar]
- Naylor R.L., Kishore A., Sumaila U.R., Issifu I., Hunter B.P., Belton B., Bush S.R., Cao L., Gelcich S., Gephart J.A., Golden C.D., Jonell M., Koehn J.Z., Little D.C., Thilsted S.H., Tigchelaar M., Crona B. Blue food demand across geographic and temporal scales. Nat. Commun. 2021;12(1):5413. doi: 10.1038/s41467-021-26063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri L., Pittia P., Bertolo G., Torreggiani D., Sacchetti G. Influence of water activity and system mobility on peroxidase activity in maltodextrin solutions. Food Biophys. 2011;6(2):281–287. doi: 10.1007/s11483-011-9218-z. [DOI] [Google Scholar]
- Neri L., Pittia P., Di Mattia C.D., Bertolo G., Mastrocola D., Sacchetti G. Multiple effects of viscosity, water activity and glass transition temperature on peroxidase activity in binary and ternary carbohydrate solutions. Food Biophys. 2014;9(3):260–266. doi: 10.1007/s11483-014-9348-1. [DOI] [Google Scholar]
- Newsome A.G., Culver C.A., van Breemen R.B. Nature's palette: the search for natural blue colorants. J. Agric. Food Chem. 2014;62(28):6498–6511. doi: 10.1021/jf501419q. [DOI] [PubMed] [Google Scholar]
- Niccolai A., Venturi M., Galli V., Pini N., Rodolfi L., Biondi N., Granchi L., Tredici M.R. Vegetable oils protect phycocyanin from thermal degradation during cooking of spirulina-based “crostini”. LWT. 2021;138 doi: 10.1016/j.lwt.2020.110776. [DOI] [Google Scholar]
- Pan Y., Wu Z.Z., Zhang B., Li X.M., Meng R., Chen H.Q., Jin Z.Y. Preparation and characterization of emulsion stabilized by octenyl succinic anhydride-modified dextrin for improving storage stability and curcumin encapsulation. Food Chem. 2019;294:326–332. doi: 10.1016/j.foodchem.2019.05.053. [DOI] [PubMed] [Google Scholar]
- Patel A., Pawar R., Mishra S., Sonawane S., Ghosh P.K. Kinetic studies on thermal denaturation of C-phycocyanin. Indian J. Biochem. Biophys. 2004;41(5):254–257. doi: 10.1111/j.1440-1711.2004.01276.x. [DOI] [PubMed] [Google Scholar]
- Pez D., Rocha I., Damasceno L., Domeneghini G. Phycocyanin from Spirulina: a review of extraction methods and stability. Food Res. Int. 2021;143 doi: 10.1016/j.foodres.2021.110314. [DOI] [PubMed] [Google Scholar]
- Rahman D.Y., Sarian F.D., van Wijk A., Martinez-Garcia M., van der Maarel M. Thermostable phycocyanin from the red microalga Cyanidioschyzon merolae, a new natural blue food colorant. J. Appl. Phycool. 2017;29(3):1233–1239. doi: 10.1007/s10811-016-1007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch J.J., Daubert C.R., Foegeding E.A. The effects of acidulant type on the rheological properties of beta-lactoglobulin gels and powders derived from these gels. Food Hydrocolloids. 2005;19(5):851–860. doi: 10.1016/j.foodhyd.2004.10.034. [DOI] [Google Scholar]
- Romay C., Armesto J., Remirez D., Gonzalez R., Ledon N., Garcia I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998;47(1):36–41. doi: 10.1007/s000110050256. [DOI] [PubMed] [Google Scholar]
- Romay C., Ledon N., Gonzalez R. Effects of phycocyanin extract on prostaglandin E-2 levels in mouse ear inflammation test. Arzneim. Forsch. 2000;50(12):1106–1109. doi: 10.1055/s-0031-1300340. [DOI] [PubMed] [Google Scholar]
- Sarada R., Pillai M.G., Ravishankar G.A. Phycocyanin from Spirulina sp: influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem. 1999;34(8):795–801. doi: 10.1016/S0032-9592(98)00153-8. [DOI] [Google Scholar]
- Sayed A.A., Soliman A.M., Taha M.A., Sadek S.A. Spirulina and C-phycocyanin mitigate titanium dioxide nanoparticle-induced hematobiochemical and hepatorenal disorders through antioxidative pathway. Food Chem. Adv. 2022;1 doi: 10.1016/j.focha.2022.100035. [DOI] [Google Scholar]
- Scheer H., Zhao K.H. Biliprotein maturation: the chromophore attachment. Mol. Microbiol. 2008;68(2):263–276. doi: 10.1111/j.1365-2958.2008.06160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmatz D.A., Mastrantonio D.J.D., Costa J.A.V., de Morais M.G. Encapsulation of phycocyanin by electrospraying: a promising approach for the protection of sensitive compounds. Food Bioprod. Process. 2020;119:206–215. doi: 10.1016/j.fbp.2019.07.008. [DOI] [Google Scholar]
- Short R.E., Gelcich S., Little D.C., Micheli F., Allison E.H., Basurto X., Belton B., Brugere C., Bush S.R., Cao L., Crona B., Cohen P.J., Defeo O., Edwards P., Ferguson C.E., Franz N., Golden C.D., Halpern B.S., Hazen L., Hicks C., Johnson D., Kaminski A.M., Mangubhai S., Naylor R.L., Reantaso M., Sumaila U.R., Thilsted S.H., Tigchelaar M., Wabnitz C.C.C., Zhang W. Harnessing the diversity of small-scale actors is key to the future of aquatic food systems. Nat. Food. 2021;2(9):733–741. doi: 10.1038/s43016-021-00396-5. [DOI] [PubMed] [Google Scholar]
- Tang K., Zeng X.L., Yang Y., Wang Z.B., Wu X.J., Zhou M.…Zhao K.H. A minimal phycobilisome: fusion and chromophorylation of the truncated core-membrane linker and phycocyanin. Biochim. Biophys. Acta Bioenerg. 2012;1817(7):1030–1036. doi: 10.1016/j.bbabio.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Tie S., Xiang S., Chen Y., Qiao F., Cui W., Su W., Tan M. Facile synthesis of food-grade and size-controlled nanocarriers based on self-assembly of procyanidins and phycocyanin. Food Funct. 2022;13:4023–4031. doi: 10.1039/D1FO04222J. [DOI] [PubMed] [Google Scholar]
- Tigchelaar M., Cheung W.W.L., Mohammed E.Y., Phillips M.J., Payne H.J., Selig E.R., Wabnitz C.C.C., Oyinlola M.A., Frölicher T.L., Gephart J.A., Golden C.D., Allison E.H., Bennett A., Cao L., Fanzo J., Halpern B.S., Lam V.W.Y., Micheli F., Naylor R.L., Sumaila U.R., Tagliabue A., Troell M. Compound climate risks threaten aquatic food system benefits. Nat. Food. 2021;2(9):673–682. doi: 10.1038/s43016-021-00368-9. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhu M., Wang K., He S., Shi X., Yuan B., Dong B., Wang Z. Preparation of core-shell microcapsules based on microfluidic technology for the encapsulation, protection and controlled delivery of phycocyanin. J. Drug Deliv. Sci. Technol. 2022;72 doi: 10.1016/j.jddst.2022.103361. [DOI] [Google Scholar]
- Xia Q., Zheng Y., Liu Z., Cao J., Chen X., Liu L., Yu H., Barba F.J., Pan D. Nonthermally driven volatilome evolution of food matrices: the case of high pressure processing. Trends Food Sci. Technol. 2020;106:365–381. doi: 10.1016/j.tifs.2020.10.026. [DOI] [Google Scholar]
- Yan M.Y., Liu B., Jiao X.D., Qin S. Preparation of phycocyanin microcapsules and its properties. Food Bioprod. Process. 2014;92(C1):89–97. doi: 10.1016/j.fbp.2013.07.008. [DOI] [Google Scholar]
- Yang R., Ma T., Shi L., Wang Q., Zhang L., Zhang F., Wang Z., Zhou Z. The formation of phycocyanin-EGCG complex for improving the color protection stability exposing to light. Food Chem. 2022;370 doi: 10.1016/j.foodchem.2021.130985. [DOI] [PubMed] [Google Scholar]
- Yu H., Wang H., Su W., Song Y., Zaky A.A., Abd El-Aty A.M., Tan M. Co-delivery of hydrophobic astaxanthin and hydrophilic phycocyanin by a pH-sensitive water-in-oil-in-water double emulsion-filled gellan gum hydrogel. Food Hydrocolloids. 2022;131 doi: 10.1016/j.foodhyd.2022.107810. [DOI] [Google Scholar]
- Yu P., Wu Y., Wang G., Jia T., Zhang Y. Purification and bioactivities of phycocyanin. Crit. Rev. Food Sci. 2017;57(18):3840–3849. doi: 10.1080/10408398.2016.1167668. [DOI] [PubMed] [Google Scholar]
- Zhang S., Zhang Z., Dadmohammadi Y., Li Y., Jaiswal A., Abbaspourrad A. Whey protein improves the stability of C-phycocyanin in acidified conditions during light storage. Food Chem. 2021;344 doi: 10.1016/j.foodchem.2020.128642. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Cho S., Dadmohammadi Y., Li Y., Abbaspourrad A. Improvement of the storage stability of C-phycocyanin in beverages by high-pressure processing. Food Hydrocolloids. 2021;110 doi: 10.1016/j.foodhyd.2020.106055. [DOI] [Google Scholar]
- Zhang Z., Li Y., Abbaspourrad A. Improvement of the colloidal stability of phycocyanin in acidified conditions using whey protein-phycocyanin interactions. Food Hydrocolloids. 2020;105 doi: 10.1016/j.foodhyd.2020.105747. [DOI] [Google Scholar]
- Zheng J.-X., Yin H., Shen C.-C., Zhang L., Ren D.-F., Lu J. Functional and structural properties of spirulina phycocyanin modified by ultra-high-pressure composite glycation. Food Chem. 2020;306 doi: 10.1016/j.foodchem.2019.125615. [DOI] [PubMed] [Google Scholar]
- Zheng J.X., Yin H., Shen C.C., Zhang L., Ren D.F., Lu J. Functional and structural properties of spirulina phycocyanin modified by ultra-high-pressure composite glycation. Food Chem. 2020;306 doi: 10.1016/j.foodchem.2019.125615. [DOI] [PubMed] [Google Scholar]
- Zhou B., Liu W., Qu W., Tseng C.K. Application of spirulina mixed feed in the breeding of bay scallop. Bioresour. Technol. 1991;38(2):229–232. doi: 10.1016/0960-8524(91)90159-H. [DOI] [Google Scholar]