Abstract
Post-transcriptional modifications in RNAs regulate their biological behaviors and functions. N1-methyladenosine (m1A), which is dynamically regulated by writers, erasers and readers, has been found as a reversible modification in tRNA, mRNA, rRNA and long non-coding RNA (lncRNA). m1A modification has impacts on the RNA processing, structure and functions of targets. Increasing studies reveal the critical roles of m1A modification and its regulators in tumorigenesis. Due to the positive relevance between m1A and cancer development, targeting m1A modification and m1A-related regulators has been of attention. In this review, we summarized the current understanding of m1A in RNAs, covering the modulation of m1A modification in cancer biology, as well as the possibility of targeting m1A modification as a potential target for cancer diagnosis and therapy.
Abbreviations: m1I, 1-methylinosine; UTR, 5′ untranslated region; hm5C, 5-hydroxymethylcytosine; m5C, 5-methylcytidine; AAA, Abdominal aortic aneurysm; ALKBH1, Alkb homologue 1; ALKBH3, Alkb homologue 3; ALKBH7, Alkb homologue 7; AML, Acute myeloid leukemia; BC, Breast cancer; BLCA, Bladder urothelial carcinoma; CC, Colon cancer; CRC, Colorectal cancer; cyt-tRNA, Cytoplasmic tRNA; FTO, Fat mass and obesity-associated protein; GBM, Glioblastoma multiforme; GI, Gastrointestinal cancer; HCC, Hepatocellular carcinoma; lncRNA, Long non-coding RNA; MA2, Meclofenamic acid 2; mt-tRNA, Mitochondrial tRNA; m1A, N1-methyladenosine; m6Am, N6,2′-O-dimethyladenosine; m6A, N6-methyladenosine; m7G, N7-methylguanosine; NSAID, Nonsteroidal anti-inflammatory drug; NSCLC, Non-small-cell lung cancer; NML, Nucleomethylin; OSCC, Oral squamous cell carcinoma; OC, Ovarian cancer; PAAD, Pancreatic cancer; PC, Prostate cancer; R-2HG, R-2-hydroxyglutarate; Nm, Ribose-methylation; SAM, S-adenosyl-l-methionine; tRFs, tRNA fragments; TRMT10C, tRNA methyltransferase 10C; TRMT6, tRNA methyltransferase 6; TRMT61A, tRNA methyltransferase 61A; TRMT61B, tRNA methyltransferase 61B; tDRs, tRNA-derived small RNAs; TME, Tumor microenvironment; YTH, YT521-B homology; YTHDC1, YTH domain containing 1; YTHDF1, YTH domain family protein 1; YTHDF2, YTH domain family protein 2; YTHDF3, YTH domain family protein 3
Keywords: M1A, RNA, Cancer biology, Diagnosis, Therapy
1. Introduction
Over 170 distinct chemical modifications have been identified in RNA, which post-transcriptionally and extensively regulate the behaviors and biological functions of RNAs. Methylation is the most frequent internal modification in RNA, including N6-methyladenosine (m6A), N1-methyladenosine (m1A), N6,2′-O-dimethyladenosine (m6Am), 5-methylcytidine (m5C), 5-hydroxymethylcytosine (hm5C), N7-methylguanosine (m7G), and ribose-methylation (Nm). Among them, m1A is a critical internal RNA modification that controls gene expression.
1) Discovery of m1A methylation.
m1A, which occurs on the first nitrogen atom of adenosine in RNA, is a ubiquitous RNA modification (Fig. 1). m1A was first reported in 1961 [1]. Later, m1A was found to exist in tRNAs [2], rRNAs [3], [4], mRNAs [5], [6], and long non-coding RNAs (lncRNAs) [7]. Among them, tRNA is the most heavy-modified class of RNA. In tRNA, N1-methylation can occur on both purines (adenine and guanine), with m1A occurring at a greater number of positions than m1G [8]. In mRNA, m1A is less prevalent compared with m6A. The occurrence of m1A in mRNA is about 6-fold less than that of m6A in mRNA [9]. Interestingly, it was reported that the nitrogen atom could transfer from m1A to m6A after the Dimroth rearrangement under an alkaline environment [10], suggesting the potential joint regulation of RNA via the dynamic interaction between m1A and m6A.
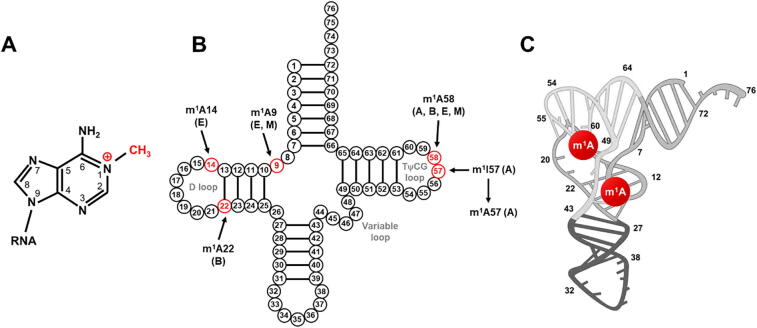
Fig. 1.
M1A methylation and its positions in tRNAs. (A) Chemical structure of the m1A modification; (B) The m1A methylation shown on a tRNA at reported modification sites (red). The domain in which the modification has been identified is indicated as A: archaea, E: eukaryotes, and B: bacteria. M indicates m1A modifications found in mitochondria; (C) Location of m1A modifications in human tRNA with tertiary structure. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2) Characterizations of m1A methylation in RNAs.
m1A was found to be an ancient RNA modification across Bacteria, Archaea and Eukarya. It has been identified in various RNA transcripts including Table 1: i) tRNA: m1A modification occurs at positions 9, 14, 22, 57, and 58 of cytoplasmic tRNAs (cyt-tRNAs), and positions 9 and 58 in mitochondrial tRNAs (mt-tRNAs) [8], [11], [12]. The m1A at position 58 (m1A58) is the first m1A modification of the initial transcripts of tRNA in the nucleus [13], and it is the most conserved and common in bacteria, archaea, and eukaryotes [14], especially that of eukaryotic initiator tRNAiMet [15]; m1A14 is only found in a limited number of cytoplasmic cyt-tRNAPhe of mammals [16]; m1A22 occurs only in bacterial tRNAs [17]; m1A57 exists as an intermediate to 1-methylinosine (m1I) by hydrolytic deamination in archaea [18]; and m1A9 has been found in cyto-tRNA from archaea and mammalian mt-tRNAs such as mt-tRNALys and mt-tRNAAsp [19], [20]. ii) rRNA: m1A modification occurs in the bacterial and mitochondrial 16S rRNA, yeast 25S rRNA, and 28S rRNA in humans and mice [3], [21]. Among them, m1A is conserved in position 947 of the mitochondrial 16S rRNA of vertebrates [22]; m1A645 in 25S rRNA of budding yeast and m1A1322 in 28S rRNA of mammals are conserved 3. iii) mRNA: over 900 transcripts containing m1A modification were identified in eukaryotic cells [5]. The deposition of m1A can occur along the mRNA, but most heavy-modified in the first splice site and the highly structured regions in the 5′ untranslated regions (UTRs) of mRNAs such as GC-rich regions [5]. Current reports suggest that each modified transcript contains on average one m1A, and the ratio of m1A/A is approximately 0.02 % in humans, which is relatively low compared to m6A/A in mRNA [5], [9], [23]. In mitochondria, one single m1A site in ND5 mRNA is identified [24]. vi) lncRNA: recent study reveals that each m1A methylated lncRNA had an average of 1.04 m1A modification sites, where HGGAGRA and WGGANGA (H = A, U, or C, R = G or A) might be the m1A motifs in lncRNA [7]. Nevertheless, m1A in position 8398 of MALAT1 has been the only validated m1A modification in lncRNA [24].
Table 1.
Overview of m1A modifications in human RNAs.
| RNA | Position | Functions |
|---|---|---|
| tRNA | 9 | Formation of cloverleaf structure; Stabilization of tRNA structure [35] |
| mt-tRNA | 9 | Formation of cloverleaf structure; Stabilization of tRNA structure [19], [36] |
| tRNAPhe | 14 | Unknown [16] |
| tRNA | 58 | Promotion of translation; Stabilization of tRNAiMet[15], [29] |
| mt-tRNA | 58 | Unknown [12] |
| mt-16S rRNA | 947 | Stabilization of mitochondrial ribosome [22] |
| 28S rRNA | 1322 | Formation of ribosome 60S subunit [37] |
| mRNA | All segments | Promotion of translation [9] |
| ATP5D mRNA | A71 at Exon 1 | Suppression of translation elongation [38] |
| mt-mRNA | CDS | Prevent the effective translation of modified codons [6] |
| ND5 mt-mRNA | 1374 | Unknown [24] |
| MALAT1 lncRNA | 8398 | Unknown [24] |
3) Regulators of m1A methylation in mammalian RNAs.
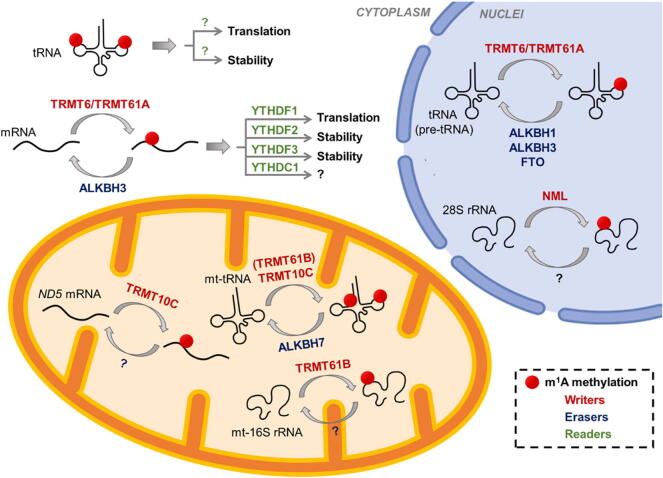
Similar to the dynamic modification of m6A, m1A is installed by methyl-transferases called “writer” (TRMT6, TRMT61A, TRMT61B, TRMT10C, and NML), removed by demethylases called “eraser” (ALKBH1, ALKBH3, ALKBH7, FTO), and recognized by m1A-binding proteins called “reader” (YTHDF1, YTHDF2, YTHDF3, YTHDC1) [21], [24], [25], [26], [27]. Here, m1A-related enzymes in human are focused on (Fig. 2). i) Writer: TRMT61A contains a methyl donor (S-adenosyl-l-methionine, SAM) binding pocket and functions as the catalytic subunit. TRMT61A forms a functional heterotetramer complex with TRMT6, which lacks SAM binding motif and is essential for tRNA-binding [28], [29]. TRMT6/61A complex locates in the cytosol and seems to recognize targets in a structure-dependent manner. TRMT6/61A can methylate both cyto-tRNA m1A58 (T-loop) and mRNAs with a T-loop-like structure, where with a GUUCRA tRNA-like motif6. TRMT61B methylases mitochondrial 16S rRNA [22], whilst recombinant TRMT10B can weakly methylate the A9 of tRNAAsp(GTC) in vitro [30]. TRMT61B likely recognizes its tRNA and rRNA targets by a similar mechanism, where a weak consensus sequence (YMRAW) is revealed surrounding targets [22]. TRMT10C installed mt-tRNA m1A and/or m1G at position 9 coupling with SDR5C1 [31]. NML, also known as RRP8, locates in nuclei and methylases the m1A on 28S rRNA [21]. ii) Eraser: ALKBH1 demethylates most m1A in cyto-tRNAs, except tRNAPhe(GAA) and tRNASec(UCA), and m1A58 in tRNAs is a major substrate of ALKBH1 [15]. ALKBH3 performs demethylation of both m1A and m3C in tRNAs, as well as the m1A demethylation in mRNA [5], [27]. ALKBH7, which locates in mitochondria, can demethylate the m1A and m22G in mitochondrial pre-tRNALeu1 and pre-tRNAIle in the nascent polycistronic mitochondrial RNA [32]. FTO, the first discovered RNA m6A demethylase, is found to bind multiple RNA species and perform demethylation including m6A and m6Am in mRNA and snRNA, and m1A in tRNA [33]. iii) Reader: Four YT521-B homology (YTH) domain-containing proteins including YTHDF1, YTHDF2, YTHDF3, and YTHDC1 are recently identified as readers for m1A modification, in spite of their roles of m6A readers [24], [26]. Their binding affinity with m1A sites is weaker than that of m6A. YTH domain is presented in 174 different proteins and is evolutionarily conserved across the eukaryotic species [34], suggesting that the YTH domain is one of the sufficient structures for the recognition of methyl-group in RNAs. Research on their functions acting as m1A readers remained limited.
Fig. 2.
M1A modification of RNAs in mammalian cells.
2. Functions of m1A in RNAs
The methyl group in m1A carries a positive electrostatic charge under physiological conditions [39], which can disrupt the Watson-Crick base pairing with uridine. The electro-chemical interaction of m1A-modified RNAs may have impacts on the processing, structural formation, and biological functions of RNAs, as well as their interactions with potential partner proteins.
1) Alteration of pre-RNA processing: m1A58 can be found in both mt-tRNA and mt-dsRNA. Within the mitochondrial Leu1 pre-tRNA region, m1A58 is partially methylated and can be demethylated by ALKBH7, whilst the m1A9 level is not affected. Knockdown of ALKBH7 reduces the total mt-tRNA level through degradation of pre-tRNA and nascent mt-mRNA (mt-dsRNA) [32], suggesting that m1A in mt-RNA regulates the nascent polycistronic mt-RNA processing.
2) Alteration of RNA structure: The distribution of m1A in RNAs shows marked features, commonly around the GC-rich regions, such as the T-loop of tRNAs, within sequence GUUCNANNC in mRNAs [6], [9], and around sequences HGGAGRA and/or WGGANGA (H = A, U, or C, R = G or A) in lncRNAs [7]. m1A is considered to alter the secondary and tertiary structures of RNA via its positive charge [7], [9]. It is worth noting that the GC-rich regions tend to form T-loop-like or hairpin structures within RNAs, which is almost invariable for the m1A deposition. In addition, m1A writer TRMT6/TRMT61A catalyzes m1A at T-loop-like elements of RNA [24]. Therefore, m1A is likely to play role in maintaining or stabilizing the T-loop-like structure, instead of changing the linear structure into a T-loop-like structure. The “loop” structure could be further strengthened by the presence of m1A through its positive charge to impair Watson-Crick base pairing. In some cases, m1A induces the correct folding of tRNAs. For instance, in mt-tRNALys, m1A9 shifts the dynamic equilibrium in favor of the cloverleaf structure and contributes to the correct formation of DHU loop [19], [36]; in the initiator methionine tRNA (tRNAiMet), m1A58 maintains the tRNA-like structure for tRNAiMet through the unique A54–A58 interaction [29], [40]. m1A modification is likely an early maturation event for tRNA.
3) Regulation of translation: The m1A can affect both the initiation and elongation process of translation via modifications in tRNA, mRNA and rRNA. The regulatory effects on translation depend on the deposition of its targets. i) tRNA: m1A modification in either tRNA or mt-tRNA shows a positive role in translation. For instance, overexpression of m1A erasers ALKBH1, ALKBH3 or FTO significantly suppresses the translation initiation and translation efficiency [15], [33], [41], [42]. On one hand, m1A58 stabilizes tRNAiMet to enhance the translation initiation, and ALKBH1-catalyzed demethylation attenuated the translation initiation [15], [29]. On the other hand, the m1A-modified tRNAs are preferentially recruited to active polysomes to promote translation [15]. In mt-tRNA, m1A modifications in mt-tRNAs promote the translation of mitochondrial transcripts [43]. For instance, the level of m1A58 in mt-tRNALys strongly increases protein synthesis [44]. ii) mRNA: The modifications of m1A have diversified effects on protein synthesis. m1A located in 5′UTR of mRNA is associated with increased translation initiation and translation efficiency [9]. m1A can disrupt RNA base-pairing and induce local RNA duplex melting, which destabilizes the secondary structure in the 5′ UTR to promote the initiation step [45]. In contrast, m1A in the CDS of both mRNA and mt-mRNA interferes with translation [6], [24], [32]. The inhibitory effect of m1A within the CDS is probably through a mechanism involving ribosomal scanning or the binding of releasing factor to modulate translation [45]. Our recent study revealed that knockdown of ALKBH3 can increase the binding between ATP5D mRNA and eRF1, which resulted in enhanced termination efficiency [38]. iii) rRNA: m1A in rRNA is likely associated with the proper formation of the 80S initiation complex. Lacking yeast m1A methylase Rrp8 causes the incompetent loading of the 60S on the 43S-preinitiation complex, which may be due to the conformational alteration of 25S rRNA in the absence of m1A645 [37].
4) Regulation of RNA stability: The presence of m1A might be involved in the structural thermostability of tRNAs. In yeast and mammals, tRNA m1A58 is a major modification and is critical for the stability of tRNAiMet. ALKBH1 deficiency improves the cellular level of tRNAiMet by stabilizing tRNAiMet [15]; hypomodified tRNAiMet can be polyadenylated and then degraded by exonuclease Rex1p and exosome in yeast [46]. On the contrary, deficiency of m1A writers has the possibility of inducing thermo-sensitivity of tRNAs [8]; knockdown of ALKBH1 fails to rescue tRNA from cleavage [47]; overexpression of ALKBH3 induces the formation of tRNA fragments (tRFs) [27]. It is reported that m1A together with other post-transcriptional modifications can enhance the melting temperature of tRNAs [48]. Interestingly, certain nucleotide modifications including m1A can be induced at high temperatures, and the biosynthesis of these modifications confers significant thermal stabilization to thus modified tRNAs [48]. However, m1A58 in the mt-tRNALeu(UUR) does not appear to affect its stability [44]. In mitochondrial 16S rRNA, m1A947 is likely to stabilize it [22]. Besides, m1A is associated with the mRNA destabilization mediated by reader proteins YTHDF2 and YTHDF3 [49], [50], [51]. Together, the regulation of m1A in the stability of RNA is far better studied. It is likely that m1A plays a critical role in the stabilization of tRNAs, but its effect on the stabilization of other RNAs such as mt-tRNA and mRNA remains to be elucidated.
In the nucleus, m1A modifications in pre-tRNA (as well as tRNA) and 28S rRNA are installed by m1A writers TRMT6/TRMT61A and NML, respectively. m1A-modified tRNA can be removed by erasers ALKBH1, ALKBH3 and FTO. In the mitochondrion, mt-tRNA and a subset of mt-mRNA are methylated by TRMT10C. TRMT61B methylates mt-16S rRNA and possibly mt-tRNA. m1A in mt-tRNA is erased by ALKBH7. mt, mitochondrial. In the cytoplasm, mRNA is methylated by TRMT6/TRMT61A and demethylated by ALKBH3. m1A readers recognize m1A and execute specific functions.
3. Modulation of m1A modification in cancer biology
m1A is associated with diverse cellular functions. Dysregulation of m1A leads to diseases such as tumorigenesis, cardiovascular diseases, pulmonary diseases and Alzheimer’s disease [52], [53], [54]. Herein, the roles of m1A in cancer biology are summarized Table 2.
Table 2.
Regulation of m1A modifications in cancer biology.
| Biological function | m1A regulator | RNA target | Effect | Cancer/Cell type | Mechanism |
|---|---|---|---|---|---|
| Proliferation | TRMT6, TRMT61A, ALKBH3 | mRNA | Promotion | GI, HCC, Glioma | Modulation of PI3K/AKT pathway [56], [57], [61] |
| TRMT6, TRMT61A | tRNAiMet | Promotion | Glioma | Upregulation of m [1]A58 in tRNAiMet[58] | |
| ALKBH3 | tRNA | Promotion | PAAD, OC | Enhancing protein synthesis by demethylating tRNA [41]Generation of tRNA-derived small RNAs by demethylating tRNA [27] | |
| Invasion | ALKBH3 | mRNA | Promotion | BC, OC | Promoting translation of m1A-modified CSF-1 mRNA [51] |
| ALKBH3 | tRNA | Promotion | PAAD, OC | Generation of tRNA-derived small RNAs by demethylating tRNA [27] | |
| YTHDF3 | mRNA | Suppression | Trophoblast | Promoting the mRNA decay of IGF1R mRNA [50] | |
| Cancer metabolism | TRMT6, TRMT61A | tRNA | Promotion | HCC | Increasing PPARδ translation to stimulate cholesterol synthesis [62] |
| ALKBH3, YTHDF1 | mRNA | Promotion | OC | Increasing expression of ATP5D mRNA to promote the glycolysis [38] | |
| Senescence, cell death | ALKBH3 | mRNA | Promotion | NSCLC, BLCA | Increasing the expression of p27 and p21 [65], [66]. |
| Tumor microenvironment | YTHDF3 | mRNA | Promotion | RAW264.7 | Regulating the macrophage M1 and M2 polarization [70], |
1) Proliferation: m1A regulators including TRMT6, TRMT61A and ALKBH3 have been found to promote the proliferation of cancer cells from gastrointestinal cancer (GI) [55], [56], hepatocellular carcinoma (HCC) [57], glioma [58], prostate cancer [41], [59], and colorectal cancer (CRC) [60]. In GI, HCC and glioma, both m1A regulators and m1A-modified mRNAs can modulate the PI3K/AKT pathway to promote the proliferation of cancer cells [56], [57], [61]. In addition to mRNA, m1A can promote proliferation via the regulation of tRNAs. For instance, TRMT6/TRMT61A-elevated m1A58 tRNAiMet promotes the proliferation and malignant transformation of glioma [58]; ALKBH3 promotes cell proliferation through enhancing protein synthesis by demethylation of tRNA in prostate cancer [41]. Remarkably, our recent study shows that ALKBH3 can generate tRNA-derived small RNAs (tDRs) via demethylation of m1A-tRNA, which further promotes cancer cell proliferation and invasion [27].
2) Invasion: Only a few studies have reported the regulation of m1A on cell invasion. In ovarian and breast cancers, ALKBH3 induces the increased m1A-modified CSF-1 mRNA to improve its translation initiation and cancer cell invasiveness [51]; ALKBH3 can also promote cancer cell invasion via destabilizing tRNAs [27]. YTHDF3, acting as m1A reader, is found to suppress the invasion and migration of trophoblast, via promoting the mRNA decay of IGF1R [50].
3) Cell metabolism: In HCC, TRMT6/TRMT61A elevates a subset of m1A-tRNA to increase PPARδ translation, resulting in the stimulation of cholesterol synthesis to promote the oncogenesis of HCC [62]. Our recent study reveals that ALKBH3 promotes the glycolysis of cancer cells by modulating the expression of m1A-modified ATP5D mRNA [38]. In addition, ALKBH7 is found to regulate obesity by facilitating the utilization of short-chain fatty acids [63]. However, whether m1A modification is involved in the ALKBH7-mediated regulation of obesity remains to be elucidated.
4) Senescence and cell death: m1A might likely play role in the regulation of cellular senescence and cancer cell death, since ALKBH3 has been reported to regulate the cell cycle [64]. Knockdown of ALKBH3 results in the senescence induction and cell cycle arrest in lung cancer and urothelial carcinomas, by increasing the expression of cell cycle arrest proteins p27 and p21, and modulating NADPH oxidase and tweak/Fn14/VEGF signals [65], [66]. However, whether ALKBH3 mediates this function as an RNA or DNA methyltransferase was not elucidated.
5) Tumor microenvironment (TME): Studies shows that m1A modification is critical to the shaping of immune microenvironment and the formation of TME complexity. In ovarian cancer (OC), colon cancer (CC), HCC and oral squamous cell carcinoma (OSCC), m1A regulators and m1A modification patterns correspond to the ever-changing immune microenvironment during cancer development [60], [67], [68], [69]. The m1A-related phenotypes are correlated to the immune cell infiltration in TME, where different m1A patterns are identified in immune-desert, immune-inflamed and immune-excluded phenotypes [60], [67]. In addition, eight m1A regulators are positively correlated with activated mast cells, plasma cells and M1 macrophages in abdominal aortic aneurysm (AAA). Among them, YTHDF3 promotes macrophage M1 polarization but inhibits macrophage M2 polarization [70].
Increasing evidence highlights the important role of m1A modification in the tumorigenesis of various cancers. Remarkably, some of these correlations might be due to the downstream effects or “side effects” of the global manipulation of m1A-related regulators. To avoid such interference, site-specific manipulation of m1A meets the urgent needs of m1A methylation study. However, only two programmable m1A tools have been reported [38], [71] and their usage in functional study remains to be popularized.
4. m1A modification as a potential diagnostic and therapeutic target
The relevance between m1A modifications and cancer progression and prognosis has been of attention recently. Increasing reports suggest that the levels of m1A methylation, m1A-related regulators, as well as the m1A-related RNAs might be novel biomarkers for cancer prognosis. In addition, modulating the m1A-related regulators and/or m1A modification on transcripts is likely becoming a breakthrough in cancer therapies.
1) Targeting m1A methylation for cancer prognosis:
i) Total m1A level: Level of m1A can be detected in spontaneous urine samples. Zheng et al., first reported that the m1A content in CRC patients is higher than that in healthy controls. And m1A levels are positively associated with the Duke stage for CRC [72]. Later, m1AScore is generated to quantify the individual patient’s m1A modification pattern. In CC and OSCC, patients with a high m1AScore show more lymphatic invasion, shorten survival and worse response after immunotherapy [60], [68]. However, a high m1AScore in OC patients is usually accompanied by a better survival advantage, a lower mutational load and marked therapeutic benefits from chemotherapy and immunotherapy [67]. The m1A level shows the potential predictive ability for the prognosis of cancers, but the relevance between m1A level and cancer progression and/or prognosis is likely related to tumor species.
ii) m1A-related regulators: m1A-related regulators are commonly high expressed in multiple cancers. Among them, m1A writer TRMT6, TRMT61A and eraser ALKBH3 are associated with poorer prognosis in a variety of cancers [41], [51], [65], [66], [73], [74], [56], [57], [59], [60], [61]. Expression of TRMT61B and TRMT10C, which are m1A writers for mitochondrial RNAs, could be used as an independent risk factor for the prognosis of gastric cancer and HCC, respectively [55], [57], [75]. ALKBH7, which is high expression in 17 cancers and low expression in 5 cancers compared to paired normal tissues, might be a potential biomarker for pan-cancer prognosis and prediction of therapeutic outcomes [76]. Remarkably, ALKBH1 is the only m1A regulator that is related to the good prognosis of patients with pancreatic cancer (PAAD) [77]. In glioblastoma, ALKBH1 expression of patients is not associated with overall survival rates [78]. For FTO, YTHDF1, YTHDF2, YTHDF3 and YTHDC1, high expression of them is closely associated with poor clinical outcomes in both CRC and HCC [57], [60], [79]. However, all of them are not restricted in the regulation of m1A, but also m6A and other modifications. Therefore, their prognostic potential may be associated with m6A as well.
iii) m1A-related RNAs: m1A-related RNAs might become effective prognostic predictors for cancer diagnosis. On one hand, Zheng et al., reveal that m1A-regulating genes are correlated with advanced clinical stages and poor prognosis of PAAD [77]. On the other hand, tRFs derived from m1A-regulated tRNAs are correlated with the dysregulation of TRMT6/61A expression, and long 3′ tRFs are highly enriched in bladder cancer [80]. Our study further confirms that the plasma level of 5′-tRF-GlyGCC acts as a novel biomarker for colorectal cancer diagnosis [81].
2) m1A modification for cancer therapy.
i) Inhibitors targeting m1A-related regulators: Due to the positive roles of m1A-related regulators in tumorigenesis, developing selective inhibitors targeting m1A-related regulators is one of the efficient ways for cancer therapy. So far, inhibitors targeting TRMT6/TRMT61A, ALBKH3 and FTO are reported (Table 3). For TRMT6/TRMT61A, thiram (tetramethyl thiuram disulfide) is recently identified as a potential candidate compound that selectively inhibits the interaction between TRMT6 and TRMT61A. Thiram treatment suppresses the self-renewal of HCC cells in vitro and also decreases tumor growth in vivo [62]. However, thiram was approached by FDA as an antimicrobial drug and shows liver damage in chickens at high doses [82]. The pre-clinical investigation of HCC therapy needs further evaluation. For ALKBH3, HUHS015 and its derivative Compound 7I (1-(5-fluoro-1H-benzimidazol-2-yl)-3-methyl-4-phenyl-1H-pyrazol-5-ol) are evaluated as prostate cancer antigen-1 (PCA-1/ALKBH3) inhibitors. Compound 7I shows potent antiproliferative effects on DU145 cells in vivo [83], [84]. For FTO, as the first discovered RNA methylase, over ten FTO-targeted small molecule inhibitors are developed, including Rhein, CHTB, N-CDPCB, Meclofenamic acid 2 (MA2), R-2-hydroxyglutarate (R-2HG), FB23-2, CS1, CS2, 18,077 and 18,097 [85], [86]. Among them, seven FTO inhibitors, MA2, R-2HG, FB23-2, CS1, CS2, 18,077 and 18,097 show anti-cancer effects both in vitro and in vivo. MA2, which can bind at the surface area of the FTO active site, is an FDA-approved nonsteroidal anti-inflammatory drug (NSAID) [87]. MA2 shows an inhibitory effect on tumor growth in glioblastoma-bearing mice [88], which likely becomes the most potent small molecule FTO inhibitor for anti-tumor therapy.
Table 3.
Inhibitors targeting m1A-related regulators.
| Target | Compound | Mechanism | Effect on cancer |
|---|---|---|---|
| TRMT6/TRMT61A | Thiram | Inhibits TRMT6-TRMT61A interaction | HCC: Suppress self-renewal and tumor growth [62] |
| ALKBH3 | HUHS015/Compound 7I | Suppress ALKBH3 activity | PC: Inhibit proliferation of DU145 cells [83], [84] |
| FTO | 18077/18097 | Bind to the active site of FTO | BC: Inhibited cell cycle process and migration of cancer cells [86] |
| CHTB | Bind to a novel site of FTO and inhibit demethylase activity [89] | − | |
| CS1/CS2 | Block the catalytic pocket of FTO | AML: Inhibits cancer cell proliferation, cancer stem cell self-renewal and immune evasion [90] | |
| Dac51 | Suppress FTO activity | Melanoma: Promotes T cell response and enhances the anti-PD-1 therapy [91] | |
| Entacapone | Compete with the catalytic site of FTO | HCC: Interfere with gluconeogenesis through FOXO1 [92] | |
| FB23/FB23-2 | Directly bind to FTO and inhibit demethylase activity | AML: Suppress proliferation and promote differentiation/apoptosis [93]. | |
| FTO-04 | Block the catalytic activity of FTO | GBM: Prevents neurosphere formation and self-Renewal [94]. | |
| N-CDPCB | Competitive bind to a novel site of FTO and inhibit demethylase activity [95] | − | |
| MA/MA2 | Inhibition of the catalytic activity of FTO | GBM: Inhibit GSC cell growth and self-renewal [88] | |
| MO-I-500 | Inhibition of the catalytic activity of FTO | BC: Inhibit cell survival via decreasing FTO and IRX3 proteins [96] | |
| R-2HG | Suppress FTO activity | AML, GBM: Inhibit proliferation/survival of FTO-high cancer cells [97] | |
| Rhein | Competitive bind to FTO catalytic domain | AML: Prevent or override tyrosine kinase inhibitor resistance [98] BC: Decrease tumor growth [99] |
|
| Saikosaponin-d | Occupy the substrate-binding site of FTO | AML: suppress cell proliferation, promote apoptosis/cell-cycle arrest [100] |
ii) Editing m1A-modified RNAs: m1A modification on target RNA regulates its expression. Therefore, targeted editing of m1A in specific RNA becomes a possible approach for cancer therapy. CRISPR proteins recognizing RNA as substrates, such as Cas13 and CasRx, are powerful tools to establish editing systems targeting RNA modification. Xie et al., developed a targeted m1A demethylation tool called “REMOVER” via combining the RNA-targeting capability of dCasRx (catalytically inactive RNA-targeting RfxCas13d enzyme) and m1A eraser ALKBH3. “REMOVER” can demethylate m1A of specific RNA such as MALAT1 and modulate their stability71. However, no further application of “REMOVER” has been reported yet. Recently, we establish a targeted m1A demethylation system “dm1ACRISPR” by fusing PspCas13b with ALKBH3 [38], which was based on our previous method to demethylase m6A [101]. Targeted demethylation of m1A in ATP5D mRNA, which is a key regulator for glycolysis, by “dm1ACRISPR” significantly suppresses the energy metabolism and tumorigenesis of cancer cells [38], indicating the possibility of novel cancer therapy via modulating specific m1A modification. Similar to CRISPR/Cas9 which has shown great progress and potential in tumor therapy, site-specific manipulation strategy on m1A exhibits anticipated potential as well. In addition, the m1A modification pattern is closely associated with the immune cell infiltration in TME. Therefore, combining the targeting m1A-editing and immune response therapy such as PD-L1, might be another promising approach for cancer therapy in the future.
5. Challenges and perspectives
m1A methylation is one of the most prevalent modifications that are highly conserved in both eukaryotic and prokaryotic RNAs, suggesting its critical role in living body. Maintaining the structure/folding of RNA is considered to be the most important function of m1A, especially in tRNAs that regulate protein biosynthesis. There are over 90 modifications in tRNAs, and about 13 modifications per tRNA are presented on average [8], [102]. Modifications in tRNA greatly limit the functional study of one particular modification in tRNAs. Therefore, whether m1A would cooperate with other modifications in tRNA to shape the tRNA folding, whether there are other unrevealed m1A sites in tRNA, and what the exact function of m1A in tRNAs are questions that need to be elucidated. In addition to tRNA, m1A is presented in a low number of mRNAs, typically at low stoichiometries. The low stoichiometry of m1A has important implications for how m1A could affect mRNA. It is commonly believed that m1A regulates the expression of targets, and it acts as a gene expression regulation mechanism in response to changes in the external environment. However, this is worth thinking about the impact of m1A, which with low stoichiometry in mRNAs, on the regulation of target expression.
Despite the to-be-elucidated function of m1A on RNAs, the regulatory effects of m1A in cancer development have been confirmed in the past decade. It is confirmed that the m1A level, m1A modification pattern, and the expression of m1A-related regulators are closely associated with tumorigenesis. Therefore, it is promising to target m1A and its regulators for cancer diagnosis and therapies. Targeting epigenetic modification is a new and challenging development direction for cancer diagnosis and therapy. For instance, there is no epigenetic drug targeting RNA modification has been approached currently, although thousands of candidates are under clinical evaluation. So far, there are twelve potential chemicals targeting m1A-related regulators that show suppressive effects on cancer cells, meaning that the anti-cancer drugs targeting m1A are still in the initial stage of research. In addition to small molecule inhibitors, targeted m1A editing is a novel approach to cancer therapy. CRISPR/Cas9 system is a powerful tool for genetic disease treatments. Similar to CRISPR/Cas9, RNA-targeting CRISPR shows great capability in transcript editing, with further advantages such as no DNA mutation inheritance caused by the off-target effect. However, challenges about CRISPR delivery and its editing effect in solid tumors remain to be solved.
6. Conclusions
In this review, we summarized the functions and the modulation of m1A modification in cancer biology. Acting as one of the most abundant internal modifications of RNAs, the specific biological functions of m1A modification in RNAs, including tRNA, rRNA, mRNA and lncRNA, remains largely unknown. Increasing evidence suggests a critical role of m1A modification in cancer development. On one hand, m1A-related regulators can modulate the progression of cancers; on the other hand, m1A level and/or m1A-related regulators might be novel biomarkers or therapeutic targets for cancers. A full understanding of the mechanism underlying m1A modification is distant. However, with the in-depth study of m1A modification, including its functions and association with cancer progression, targeting m1A modification might provide new potential options for both cancer diagnosis and treatment.
Funding
The authors’ research on RNA modifications is currently funded by the National Key Research and Development Program of China (No. 2022YFC2601800), the National Natural Science Foundation of China (Grant Nos. 32161143017, 82173833, 82173126, and 81973343), The International Cooperation Project of the Science and Technology Planning Project of Guangdong Province, China (No. 2021A0505030029), the Open Program of Shenzhen Bay Laboratory (No. SZBL202009051006), the Fundamental Research Funds for the Central Universities (Sun Yat-sen University) (Nos. 19ykzd24, 19ykpy130), the Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery (2019B030301005), the Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515010290), and Shenzhen Bay Scholars Program.
CRediT authorship contribution statement
Jiexin Li: Conceptualization, Investigation, Writing – original draft. Haisheng Zhang: Investigation, Writing – original draft. Hongsheng Wang: Conceptualization, Investigation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Dunn D.B. The occurrence of 1-methyladenine in ribonucleic acid. Biochim Biophys Acta. 1961;46:198–200. doi: 10.1016/0006-3002(61)90668-0. [DOI] [PubMed] [Google Scholar]
- 2.RajBhandary U.L., Stuart A., Faulkner R.D., Chang S.H., Khorana H.G. Nucleotide sequence studies on yeast phenylalanine sRNA. Cold Spring Harb Symp Quant Biol. 1966;31:425–434. doi: 10.1101/sqb.1966.031.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Peifer C., et al. Yeast Rrp8p, a novel methyltransferase responsible for m1A 645 base modification of 25S rRNA. Nucleic Acids Res. 2013;41:1151–1163. doi: 10.1093/nar/gks1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma S., Watzinger P., Kotter P., Entian K.D. Identification of a novel methyltransferase, Bmt2, responsible for the N-1-methyl-adenosine base modification of 25S rRNA in Saccharomyces cerevisiae. Nucleic Acids Res. 2013;41:5428–5443. doi: 10.1093/nar/gkt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X., et al. Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat Chem Biol. 2016;12:311–316. doi: 10.1038/nchembio.2040. [DOI] [PubMed] [Google Scholar]
- 6.Li X., et al. Base-Resolution Mapping Reveals Distinct m(1)A Methylome in Nuclear- and Mitochondrial-Encoded Transcripts. Mol Cell. 2017;68:993–1005 e1009. doi: 10.1016/j.molcel.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi L., Chen W., Zhang Z., Chen J., Xue M. N1-methyladenosine profiling of long non-coding RNA in colorectal cancer. IUBMB Life. 2021;73:1235–1243. doi: 10.1002/iub.2534. [DOI] [PubMed] [Google Scholar]
- 8.Oerum S., Degut C., Barraud P., Tisne C. m1A Post-Transcriptional Modification in tRNAs. Biomolecules. 2017;7 doi: 10.3390/biom7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominissini D., et al. The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530:441–446. doi: 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macon J.B., Wolfenden R. 1-Methyladenosine. Dimroth rearrangement and reversible reduction. Biochemistry. 1968;7:3453–3458. doi: 10.1021/bi00850a021. [DOI] [PubMed] [Google Scholar]
- 11.Juhling F., et al. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki T., Nagao A., Suzuki T. Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu Rev Genet. 2011;45:299–329. doi: 10.1146/annurev-genet-110410-132531. [DOI] [PubMed] [Google Scholar]
- 13.Hopper A.K. Transfer RNA post-transcriptional processing, turnover, and subcellular dynamics in the yeast Saccharomyces cerevisiae. Genetics. 2013;194:43–67. doi: 10.1534/genetics.112.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker C.G. Transfer RNA and transfer RNA modification in differentiation and neoplasia. Introductory remarks Cancer Res. 1971;31:598. [PubMed] [Google Scholar]
- 15.Liu F., et al. ALKBH1-Mediated tRNA Demethylation Regulates Translation. Cell. 2016;167:816–828 e816. doi: 10.1016/j.cell.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sprinzl M., Vassilenko K.S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 2005;33:D139–D140. doi: 10.1093/nar/gki012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roovers M., et al. The YqfN protein of Bacillus subtilis is the tRNA: m1A22 methyltransferase (TrmK) Nucleic Acids Res. 2008;36:3252–3262. doi: 10.1093/nar/gkn169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grosjean H., et al. Enzymatic conversion of adenosine to inosine and to N1-methylinosine in transfer RNAs: a review. Biochimie. 1996;78:488–501. doi: 10.1016/0300-9084(96)84755-9. [DOI] [PubMed] [Google Scholar]
- 19.Helm M., et al. The presence of modified nucleotides is required for cloverleaf folding of a human mitochondrial tRNA. Nucleic Acids Res. 1998;26:1636–1643. doi: 10.1093/nar/26.7.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark W.C., Evans M.E., Dominissini D., Zheng G., Pan T. tRNA base methylation identification and quantification via high-throughput sequencing. RNA. 2016;22:1771–1784. doi: 10.1261/rna.056531.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waku T., et al. NML-mediated rRNA base methylation links ribosomal subunit formation to cell proliferation in a p53-dependent manner. J Cell Sci. 2016;129:2382–2393. doi: 10.1242/jcs.183723. [DOI] [PubMed] [Google Scholar]
- 22.Bar-Yaacov D., et al. Mitochondrial 16S rRNA Is Methylated by tRNA Methyltransferase TRMT61B in All Vertebrates. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.1002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang W., et al. The N(1)-Methyladenosine Methylome of Petunia mRNA. Plant Physiol. 2020;183:1710–1724. doi: 10.1104/pp.20.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safra M., et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551:251–255. doi: 10.1038/nature24456. [DOI] [PubMed] [Google Scholar]
- 25.Chujo T., Suzuki T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA. 2012;18:2269–2276. doi: 10.1261/rna.035600.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai X., Wang T., Gonzalez G., Wang Y. Identification of YTH Domain-Containing Proteins as the Readers for N1-Methyladenosine in RNA. Anal Chem. 2018;90:6380–6384. doi: 10.1021/acs.analchem.8b01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z., et al. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47:2533–2545. doi: 10.1093/nar/gky1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M., et al. Crystal structure of the two-subunit tRNA m(1)A58 methyltransferase TRM6-TRM61 from Saccharomyces cerevisiae. Sci Rep. 2016;6:32562. doi: 10.1038/srep32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson J., Phan L., Hinnebusch A.G. The Gcd10p/Gcd14p complex is the essential two-subunit tRNA(1-methyladenosine) methyltransferase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2000;97:5173–5178. doi: 10.1073/pnas.090102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howell N.W., Jora M., Jepson B.F., Limbach P.A., Jackman J.E. Distinct substrate specificities of the human tRNA methyltransferases TRMT10A and TRMT10B. RNA. 2019;25:1366–1376. doi: 10.1261/rna.072090.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilardo E., et al. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase–extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. 2012;40:11583–11593. doi: 10.1093/nar/gks910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L.S., et al. ALKBH7-mediated demethylation regulates mitochondrial polycistronic RNA processing. Nat Cell Biol. 2021;23:684–691. doi: 10.1038/s41556-021-00709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei J., et al. Differential m(6)A, m(6)Am, and m(1)A Demethylation Mediated by FTO in the Cell Nucleus and Cytoplasm. Mol Cell. 2018;71:973–985 e975. doi: 10.1016/j.molcel.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao S., Sun H., Xu C. YTH Domain: A Family of N(6)-methyladenosine (m(6)A) Readers. Genomics Proteomics Bioinformatics. 2018;16:99–107. doi: 10.1016/j.gpb.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobitski A.Y., Hengesbach M., Helm M., Nienhaus G.U. Sculpting an RNA conformational energy landscape by a methyl group modification–a single-molecule FRET study. Angew Chem Int Ed Engl. 2008;47:4326–4330. doi: 10.1002/anie.200705675. [DOI] [PubMed] [Google Scholar]
- 36.Voigts-Hoffmann F., et al. A methyl group controls conformational equilibrium in human mitochondrial tRNA(Lys) J Am Chem Soc. 2007;129:13382–13383. doi: 10.1021/ja075520+. [DOI] [PubMed] [Google Scholar]
- 37.Sharma S., et al. A single N(1)-methyladenosine on the large ribosomal subunit rRNA impacts locally its structure and the translation of key metabolic enzymes. Sci Rep. 2018;8:11904. doi: 10.1038/s41598-018-30383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Y.C.Z., Xie G., Zhang H., Wang Z., Zhou J., Chen F., Li J., Chen L., Niu H., Wang H. RNA m1A methylation regulates glycolysis of cancer cells through modulating ATP5D. Proc Natl Acad Sci U S A. 2022 doi: 10.1073/pnas.2119038119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agris P.F. The importance of being modified: roles of modified nucleosides and Mg2+ in RNA structure and function. Prog Nucleic Acid Res Mol Biol. 1996;53:79–129. doi: 10.1016/s0079-6603(08)60143-9. [DOI] [PubMed] [Google Scholar]
- 40.Schevitz R.W., et al. Crystal structure of a eukaryotic initiator tRNA. Nature. 1979;278:188–190. doi: 10.1038/278188a0. [DOI] [PubMed] [Google Scholar]
- 41.Ueda Y., et al. AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci Rep. 2017;7:42271. doi: 10.1038/srep42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ougland R., et al. AlkB restores the biological function of mRNA and tRNA inactivated by chemical methylation. Mol Cell. 2004;16:107–116. doi: 10.1016/j.molcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Kawarada L., et al. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017;45:7401–7415. doi: 10.1093/nar/gkx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richter U., et al. RNA modification landscape of the human mitochondrial tRNA(Lys) regulates protein synthesis. Nat Commun. 2018;9:3966. doi: 10.1038/s41467-018-06471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou H., et al. m(1)A and m(1)G disrupt A-RNA structure through the intrinsic instability of Hoogsteen base pairs. Nat Struct Mol Biol. 2016;23:803–810. doi: 10.1038/nsmb.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozanick S.G., et al. Rex1p deficiency leads to accumulation of precursor initiator tRNAMet and polyadenylation of substrate RNAs in Saccharomyces cerevisiae. Nucleic Acids Res. 2009;37:298–308. doi: 10.1093/nar/gkn925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rashad S., et al. The stress specific impact of ALKBH1 on tRNA cleavage and tiRNA generation. RNA Biol. 2020;17:1092–1103. doi: 10.1080/15476286.2020.1779492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motorin Y., Helm M. tRNA stabilization by modified nucleotides. Biochemistry. 2010;49:4934–4944. doi: 10.1021/bi100408z. [DOI] [PubMed] [Google Scholar]
- 49.Seo K.W., Kleiner R.E. YTHDF2 Recognition of N(1)-Methyladenosine (m(1)A)-Modified RNA Is Associated with Transcript Destabilization. ACS Chem Biol. 2020;15:132–139. doi: 10.1021/acschembio.9b00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Q., et al. Cytoplasmic m(1)A reader YTHDF3 inhibits trophoblast invasion by downregulation of m(1)A-methylated IGF1R. Cell Discov. 2020;6:12. doi: 10.1038/s41421-020-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woo H.H., Chambers S.K. Human ALKBH3-induced m(1)A demethylation increases the CSF-1 mRNA stability in breast and ovarian cancer cells. Biochim Biophys Acta Gene Regul Mech. 2019;1862:35–46. doi: 10.1016/j.bbagrm.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Shafik A.M., Zhou H., Lim J., Dickinson B., Jin P. Dysregulated mitochondrial and cytosolic tRNA m1A methylation in Alzheimer's disease. Hum Mol Genet. 2022;31:1673–1680. doi: 10.1093/hmg/ddab357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Y., Zhan S., Xu Y., Gao X. RNA modifications in cardiovascular diseases, the potential therapeutic targets. Life Sci. 2021;278 doi: 10.1016/j.lfs.2021.119565. [DOI] [PubMed] [Google Scholar]
- 54.Teng P.C., et al. RNA Modifications and Epigenetics in Modulation of Lung Cancer and Pulmonary Diseases. Int J Mol Sci. 2021;22 doi: 10.3390/ijms221910592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J., et al. Differential analysis of RNA methylation regulators in gastric cancer based on TCGA data set and construction of a prognostic model. J Gastrointest Oncol. 2021;12:1384–1397. doi: 10.21037/jgo-21-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y., et al. m1A Regulated Genes Modulate PI3K/AKT/mTOR and ErbB Pathways in Gastrointestinal Cancer. Transl Oncol. 2019;12:1323–1333. doi: 10.1016/j.tranon.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Q., Xue C., Yuan X., He Y., Yu Z. Gene signatures and prognostic values of m1A-related regulatory genes in hepatocellular carcinoma. Sci Rep. 2020;10:15083. doi: 10.1038/s41598-020-72178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macari F., et al. TRM6/61 connects PKCalpha with translational control through tRNAi(Met) stabilization: impact on tumorigenesis. Oncogene. 2016;35:1785–1796. doi: 10.1038/onc.2015.244. [DOI] [PubMed] [Google Scholar]
- 59.Konishi N., et al. High expression of a new marker PCA-1 in human prostate carcinoma. Clin Cancer Res. 2005;11:5090–5097. doi: 10.1158/1078-0432.CCR-05-0195. [DOI] [PubMed] [Google Scholar]
- 60.Gao Y., et al. Integrated analyses of m(1)A regulator-mediated modification patterns in tumor microenvironment-infiltrating immune cells in colon cancer. Oncoimmunology. 2021;10:1936758. doi: 10.1080/2162402X.2021.1936758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang B., Niu L., Wang Z., Zhao Z. RNA m1A Methyltransferase TRMT6 Predicts Poorer Prognosis and Promotes Malignant Behavior in Glioma. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.692130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., et al. N(1)-methyladenosine methylation in tRNA drives liver tumourigenesis by regulating cholesterol metabolism. Nat Commun. 2021;12:6314. doi: 10.1038/s41467-021-26718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solberg A., et al. Deletion of mouse Alkbh7 leads to obesity. J Mol Cell Biol. 2013;5:194–203. doi: 10.1093/jmcb/mjt012. [DOI] [PubMed] [Google Scholar]
- 64.Kogaki T., et al. TP53 gene status is a critical determinant of phenotypes induced by ALKBH3 knockdown in non-small cell lung cancers. Biochem Biophys Res Commun. 2017;488:285–290. doi: 10.1016/j.bbrc.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 65.Tasaki M., Shimada K., Kimura H., Tsujikawa K., Konishi N. ALKBH3, a human AlkB homologue, contributes to cell survival in human non-small-cell lung cancer. Br J Cancer. 2011;104:700–706. doi: 10.1038/sj.bjc.6606012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimada K., et al. ALKBH3 contributes to survival and angiogenesis of human urothelial carcinoma cells through NADPH oxidase and tweak/Fn14/VEGF signals. Clin Cancer Res. 2012;18:5247–5255. doi: 10.1158/1078-0432.CCR-12-0955. [DOI] [PubMed] [Google Scholar]
- 67.Liu J., et al. Comprehensive of N1-Methyladenosine Modifications Patterns and Immunological Characteristics in Ovarian Cancer. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.746647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao L., et al. The Impact of m1A Methylation Modification Patterns on Tumor Immune Microenvironment and Prognosis in Oral Squamous Cell Carcinoma. Int J Mol Sci. 2021;22 doi: 10.3390/ijms221910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao M., Shen S., Xue C. A Novel m1A-Score Model Correlated With the Immune Microenvironment Predicts Prognosis in Hepatocellular Carcinoma. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.805967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu Y., et al. N1-Methyladenosine (m1A) Regulation Associated With the Pathogenesis of Abdominal Aortic Aneurysm Through YTHDF3 Modulating Macrophage Polarization. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.883155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie S., et al. Programmable RNA N(1) -Methyladenosine Demethylation by a Cas13d-Directed Demethylase. Angew Chem Int Ed Engl. 2021;60:19592–19597. doi: 10.1002/anie.202105253. [DOI] [PubMed] [Google Scholar]
- 72.Zheng Y.F., et al. Urinary nucleosides as biological markers for patients with colorectal cancer. World J Gastroenterol. 2005;11:3871–3876. doi: 10.3748/wjg.v11.i25.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi L., et al. Expression and significance of m1A transmethylase, hTrm6p/hTrm61p and its related gene hTrm6/hTrm61 in bladder urothelial carcinoma. Am J Cancer Res. 2015;5:2169–2179. [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y., Huang Q., Deng T., Li B.H., Ren X.Q. Clinical Significance of TRMT6 in Hepatocellular Carcinoma: A Bioinformatics-Based Study. Med Sci Monit. 2019;25:3894–3901. doi: 10.12659/MSM.913556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Y., et al. Role of Main RNA Methylation in Hepatocellular Carcinoma: N6-Methyladenosine, 5-Methylcytosine, and N1-Methyladenosine. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.767668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen K., et al. A Pan-Cancer Analysis Reveals the Prognostic and Immunotherapeutic Value of ALKBH7. Front Genet. 2022;13 doi: 10.3389/fgene.2022.822261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng Q., Yu X., Zhang Q., He Y., Guo W. Genetic characteristics and prognostic implications of m1A regulators in pancreatic cancer. Biosci Rep. 2021;41 doi: 10.1042/BSR20210337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lyu C., et al. Rare and misincorporated DNA N(6)-methyladenine is a hallmark of cytotoxic stresses for selectively stimulating the stemness and proliferation of glioblastoma cells. Cell Discov. 2022;8:39. doi: 10.1038/s41421-022-00399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J., et al. m6A demethylase FTO promotes hepatocellular carcinoma tumorigenesis via mediating PKM2 demethylation. Am J Transl Res. 2019;11:6084–6092. [PMC free article] [PubMed] [Google Scholar]
- 80.Su Z., et al. TRMT6/61A-dependent base methylation of tRNA-derived fragments regulates gene-silencing activity and the unfolded protein response in bladder cancer. Nat Commun. 2022;13:2165. doi: 10.1038/s41467-022-29790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu Y., et al. 5'-tRF-GlyGCC: a tRNA-derived small RNA as a novel biomarker for colorectal cancer diagnosis. Genome Med. 2021;13:20. doi: 10.1186/s13073-021-00833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kong A., et al. The fungicide thiram perturbs gut microbiota community and causes lipid metabolism disorder in chickens. Ecotoxicol Environ Saf. 2020;206 doi: 10.1016/j.ecoenv.2020.111400. [DOI] [PubMed] [Google Scholar]
- 83.Nakao S., et al. Design and synthesis of prostate cancer antigen-1 (PCA-1/ALKBH3) inhibitors as anti-prostate cancer drugs. Bioorg Med Chem Lett. 2014;24:1071–1074. doi: 10.1016/j.bmcl.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 84.Ueda M., et al. Novel Metabolically Stable PCA-1/ALKBH3 Inhibitor Has Potent Antiproliferative Effects on DU145 Cells In Vivo. Anticancer Res. 2018;38:211–218. doi: 10.21873/anticanres.12210. [DOI] [PubMed] [Google Scholar]
- 85.Lan Q., et al. The Emerging Roles of RNA m(6)A Methylation and Demethylation as Critical Regulators of Tumorigenesis, Drug Sensitivity, and Resistance. Cancer Res. 2021;81:3431–3440. doi: 10.1158/0008-5472.CAN-20-4107. [DOI] [PubMed] [Google Scholar]
- 86.Xie G., et al. A novel inhibitor of N (6)-methyladenosine demethylase FTO induces mRNA methylation and shows anti-cancer activities. Acta Pharm Sin B. 2022;12:853–866. doi: 10.1016/j.apsb.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang Y., et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43:373–384. doi: 10.1093/nar/gku1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui Q., et al. m(6)A RNA Methylation Regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell Rep. 2017;18:2622–2634. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qiao Y., et al. A Novel Inhibitor of the Obesity-Related Protein FTO. Biochemistry. 2016;55:1516–1522. doi: 10.1021/acs.biochem.6b00023. [DOI] [PubMed] [Google Scholar]
- 90.Su R., et al. Targeting FTO Suppresses Cancer Stem Cell Maintenance and Immune Evasion. Cancer Cell. 2020;38:79–96 e11. doi: 10.1016/j.ccell.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Y., et al. Tumors exploit FTO-mediated regulation of glycolytic metabolism to evade immune surveillance. Cell Metab. 2021;33:1221–1233. doi: 10.1016/j.cmet.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 92.Peng S., et al. Identification of entacapone as a chemical inhibitor of FTO mediating metabolic regulation through FOXO1. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau7116. [DOI] [PubMed] [Google Scholar]
- 93.Huang Y., et al. Small-Molecule Targeting of Oncogenic FTO Demethylase in Acute Myeloid Leukemia. Cancer Cell. 2019;35:677–691 e610. doi: 10.1016/j.ccell.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huff S., Tiwari S.K., Gonzalez G.M., Wang Y., Rana T.M. m(6)A-RNA Demethylase FTO Inhibitors Impair Self-Renewal in Glioblastoma Stem Cells. ACS Chem Biol. 2021;16:324–333. doi: 10.1021/acschembio.0c00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He W., et al. Identification of A Novel Small-Molecule Binding Site of the Fat Mass and Obesity Associated Protein (FTO) J Med Chem. 2015;58:7341–7348. doi: 10.1021/acs.jmedchem.5b00702. [DOI] [PubMed] [Google Scholar]
- 96.Singh B., et al. Important Role of FTO in the Survival of Rare Panresistant Triple-Negative Inflammatory Breast Cancer Cells Facing a Severe Metabolic Challenge. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Su R., et al. R-2HG Exhibits Anti-tumor Activity by Targeting FTO/m(6)A/MYC/CEBPA Signaling. Cell. 2018;172:90–105 e123. doi: 10.1016/j.cell.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan F., et al. A dynamic N(6)-methyladenosine methylome regulates intrinsic and acquired resistance to tyrosine kinase inhibitors. Cell Res. 2018;28:1062–1076. doi: 10.1038/s41422-018-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Niu Y., et al. RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol Cancer. 2019;18:46. doi: 10.1186/s12943-019-1004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun K., et al. Saikosaponin D exhibits anti-leukemic activity by targeting FTO/m(6)A signaling. Theranostics. 2021;11:5831–5846. doi: 10.7150/thno.55574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li J., et al. Targeted mRNA demethylation using an engineered dCas13b-ALKBH5 fusion protein. Nucleic Acids Res. 2020;48:5684–5694. doi: 10.1093/nar/gkaa269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pan T. Modifications and functional genomics of human transfer RNA. Cell Res. 2018;28:395–404. doi: 10.1038/s41422-018-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]