Abstract
The Clostridioides difficile pathogen is responsible for nosocomial infections. Germination is an essential step for the establishment of C. difficile infection (CDI) because toxins that are secreted by vegetative cells are responsible for the symptoms of CDI. Germination can be stimulated by the combinatorial actions of certain amino acids and either conjugated or deconjugated cholic acid-derived bile salts. During synthesis in the liver, cholic acid- and chenodeoxycholic acid-class bile salts are conjugated with either taurine or glycine at the C24 carboxyl. During GI transit, these conjugated bile salts are deconjugated by microbes that express bile salt hydrolases (BSHs). Here, we surprisingly find that several C. difficile strains have BSH activity. We observed this activity in both C. difficile vegetative cells and in spores and that the observed BSH activity was specific to taurine-derived bile salts. Additionally, we find that this BSH activity can produce cholate for metabolic conversion to deoxycholate by C. scindens. The C. scindens-produced deoxycholate signals to C. difficile to initiate biofilm formation. Our results show that C. difficile BSH activity has the potential to influence the interactions between microbes, and this could extend to the GI setting.
Subject terms: Biofilms, Pathogens, Bacteriology
Introduction
Clostridioides difficile is a Gram-positive, spore-forming, pathogenic bacterium that is considered the main cause of antibiotic-associated diarrhea. The infectious agent of C. difficile is the spore form since dormant spores can persist in the environment for long periods of time and are resistant to commonly used disinfectants1,2. Nevertheless, CDI symptoms are a result of the TcdA and TcdB toxins that are secreted by C. difficile vegetative cells2–4. Thus, germination of the dormant C. difficile spores to the growing vegetative cells is essential for disease development. In C. difficile the germination process can be triggered by certain host-derived bile salts and certain amino acids5–11.
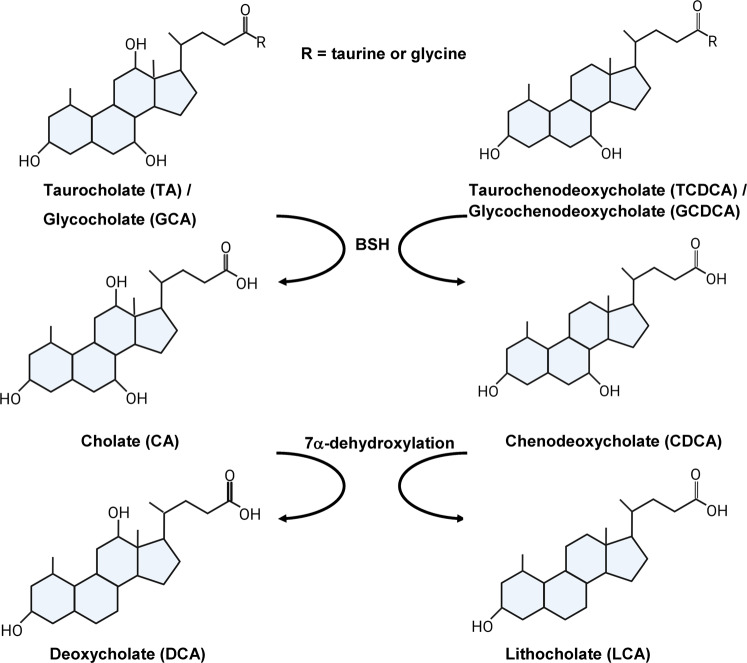
Bile salts are cholesterol-based molecules that are synthesized in the liver and, in humans, consist of the cholic acid (CA) and chenodeoxycholic acid (CDCA) base structures (Fig. 1). Subsequently, these base molecules are further modified by the addition of either taurine or glycine at C-24 [yielding taurocholic acid (TA)/taurochenodeoxycholic acid (TCDCA) and glycocholic acid (GCA)/glycochenodeoxycholic acid (GCDCA), respectively (Fig. 1)]. These conjugated bile salts are then secreted into the intestines where they aid in the absorption of fats and cholesterol12,13. Both the conjugated and deconjugated forms of the cholic acid-class bile salts promote C. difficile spore germination, whereas chenodeoxycholic acid, and its derivatives, inhibit germination5,9–11,14–19. Through an enterohepatic recirculation process, the majority of the intestinal bile salts are reabsorbed and sent back to the liver for other rounds of digestion. Bile salts that do not undergo enterohepatic recirculation can be deconjugated by bile salt hydrolases (BSHs), which remove the conjugated amino acid at the C-24 position20–22. Deconjugated primary bile salts can then undergo additional biotransformations, such as epimerization, oxidation, 7α/β-dehydroxylation, and dehydrogenation20,23. The 7α-dehydroxylation process, performed by a small subset of gut microbes, generates the secondary bile salts deoxycholic acid (DCA) from CA and lithocholic acid (LCA) from CDCA (Fig. 1). Secondary bile salts strongly correlate with, but may not result in, an environment that resists C. difficile colonization24–28. Because bile salt deconjugation is a requirement for downstream bile salt modifications, the role of BSH enzymes has been studied due to their effects on bile salt regulation and potential effects on lowering cholesterol levels, management of obesity, and the effects in gut inflammatory disorders (e.g., inflammatory bowel diseases and type 2 diabetes)29–33.
Fig. 1. Host bile salts and derivatives.
The two primary bile acids, cholate (CA) and chenodeoxycholate (CDCA), can be conjugated with taurine or glycine. Many bacteria in the gut can remove the conjugated amino acid (deconjugation) to generate the base bile salt. The deconjugated bile acids undergo 7α-dehydroxylation, performed by a small percentage of gut microbes, to generate the secondary bile salts deoxycholate (DCA) from CA and lithocholate (LCA) from CDCA.
Bile salt hydrolases (BSH) are part of the N-terminal nucleophilic (Ntn) hydrolase superfamily whose αββα-core structure is highly conserved34. The presence of BSH genes is widely distributed. A study recently reported 69 bacterial genera in the HMP database that showed the presence of BSH genes, with the Bacillus, Staphylococcus, Paenibacillus, Lysinibacillus, Clostridium, and Brevibacillus genera having the greatest abundance of BSH genes35. Although most bacteria encode only one BSH, there are some that encode up to four BSHs with the implication that multiple enzymes are expressed because of the well-established substrate specificity observed in BSH enzymes, thus allowing bacteria to deconjugate both taurine-conjugated and glycine-conjugated bile salts31,35,36.
Bile salt hydrolase characterization in Clostridia is limited. Although the C. perfringens BSH enzyme is well-studied, with knowledge of the enzyme-substrate specificity, cellular location, and crystal structure37–39, BSH enzymes in other Clostridia are less understood. This is despite the apparent high abundance of BSH genes present, based on taxonomic and metagenomic data31,35,40. To date, most experimental data on BSHs are derived from studies in Lactobacillus and Bifidobacterium species that are commonly used for probiotics32,33,35,36,41,42.
To our knowledge, no prior studies have tested BSH activity in C. difficile strains. Understandably, C. difficile does not encode homologs of known BSH enzymes. However, herein, we show that several C. difficile strains, both laboratory- and epidemic-type strains, have BSH activity, and that this activity is specific to taurine-conjugated bile salts. This activity is not restricted to vegetative cells as C. difficile spores also have a low amount of BSH activity, suggesting a potential role of the enzyme in C. difficile germination. Finally, we show that the identified BSH activity of C. difficile cells can generate a cholic acid source for C. scindens. C. scindens uses this CA source to generate DCA, thereby stimulating biofilm formation by C. difficile cells. Thus, our work shows that the identified BSH activity may provide C. difficile a mechanism to generate small amounts of CA for use by a competing microbe and stimulate its own biofilm formation and, thus, potential maintenance within a host.
Results
Different C. difficile ribotypes have substrate-specific bile salt hydrolase activity
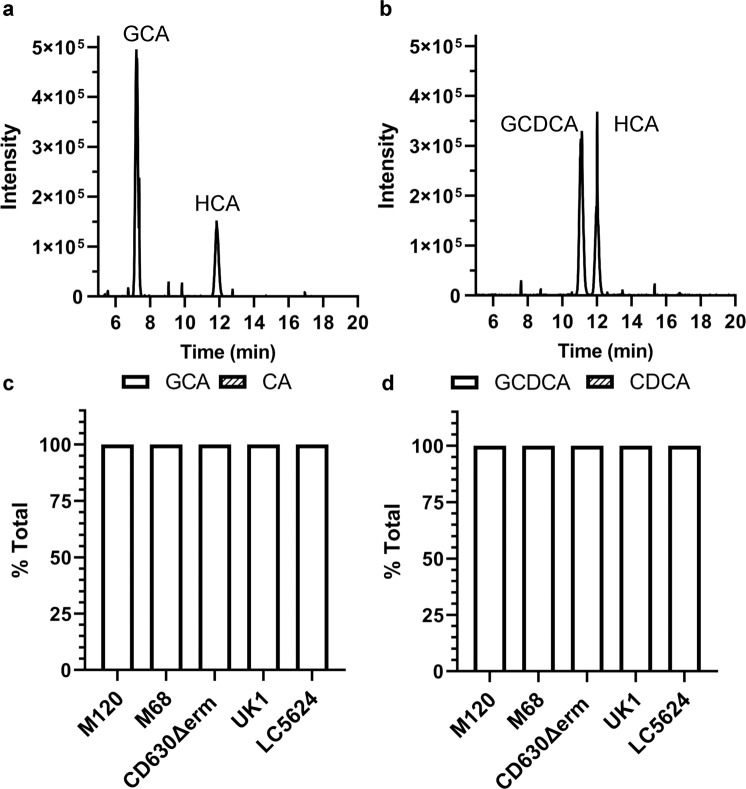
In prior work, we tested the BSH of C. scindens and C. hiranonis and found that C. scindens cannot deconjugate bile salts but that C. hiranonis could28. Surprisingly, when we included C. difficile as a supposed negative control, we found that the C. difficile CD630Δerm strain could remove the taurine from TA to generate CA. This was observed by incubating C. difficile vegetative cells overnight in a rich medium supplemented with 1 mM TA. Subsequently, the bile salts were extracted, separated by high-performance liquid chromatography (HPLC), and detected by evaporative light scattering (Supplementary Fig. 1). As shown in Fig. 2a, C. difficile CD630Δerm could produce CA during this incubation, hyodeoxycholate (HCA) was included as an internal standard.
Fig. 2. Several C. difficile strains can deconjugate taurocholate.
a C. difficile CD630Δerm was grown in rich medium supplemented with 1 mM taurocholate (TA) for 24 h. The bile salts were then analyzed by HPLC-coupled evaporative light scattering. Peak retention times for each bile acid were determined by using bile acid standards (Supplementary Fig. 1). b Deconjugation of TA by C. difficile strains M120, M68, CD630Δerm, UK1, and LC5624 were determined as in (a). Values were calculated by measuring the peak areas for TA and CA and expressed as a percentage of the total input. HDCA was included as an internal standard. Values shown are the average of three different independent experiments, and error bars represent the standard error of the mean.
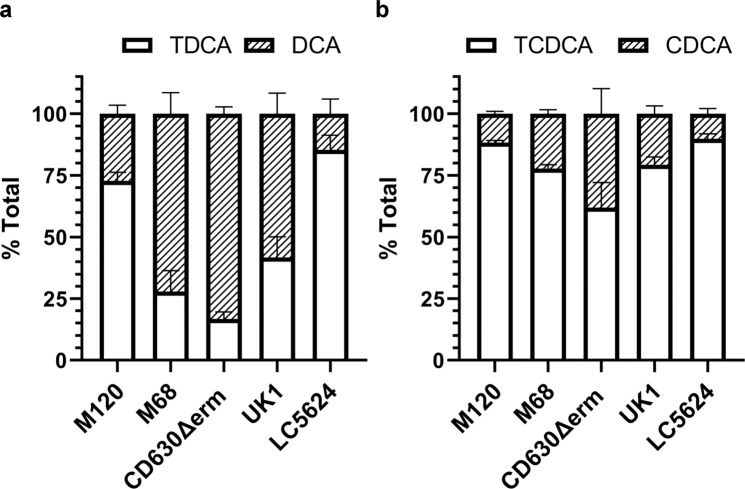
To understand if other C. difficile strains also had BSH activity, we grew strains of C. difficile derived from different ribotypes in the presence of taurocholate and quantified the amount of deconjugation. Surprisingly, in addition to C. difficile CD630Δerm (ribotype 014), we observed deconjugation of TA in 3 other strains [C. difficile M120 (ribotype 078), C. difficile M68 (ribotype 017), C. difficile UK1 (ribotype 027)] (Fig. 2b). The C. difficile LC5624 strain (ribotype 106) did not deconjugate TA (Fig. 2b). However, C. difficile M68 deconjugated approximately 70% of the TA to CA (Fig. 2b). C. difficile CD630Δerm and UK1 deconjugated approximately 50% of the TA to CA, and C. difficile M120 had the poorest activity with only ~10% (Fig. 2b).
To understand if this activity was specific to TA or if other taurine-derive bile salts could be deconjugated, we tested taurodeoxycholate (TDA) and taurochenodeoxycholate (TCDCA). The TDCA and TCDCA bile salts were deconjugated by all five C. difficile strains tested, including C. difficile LC5624 (Fig. 3a, b). Similar to what we observed for the deconjugation of TA, the levels of deconjugation for TDCA and TCDCA varied between strains. TCDCA showed the lowest deconjugation levels from the three tested taurine bile salts (Fig. 3b), and the C. difficile LC5624 strain, ribotype 106, and the predominant ribotype causing CDI infections in the US at present43 showed the lowest levels of BSH activity (Fig. 3).
Fig. 3. C. difficile deconjugates taurodeoxycholate and taurochenodeoxycholate.
Deconjugation of taurodeoxycholate (TDCA) (a) or taurochenodeoxycholate (TCDCA) (b) by C. difficile strains M120, M68, CD630Δerm, UK1, and LC5624 were determined as in Fig. 2. Values were calculated by measuring the peak areas for TDCA and DCA (a) or TCDCA and CDCA (b) and expressed as a percentage of total input. Hyodeoxycholate (HDCA) was included as an internal standard. Values shown are the average of three different independent experiments, and error bars represent the standard error of the mean.
It is possible that the removal of the taurine from the Tauro-conjugated bile salts could yield an amino acid that is important for C. difficile growth. To test this hypothesis, we grew C. difficile UK1 in rich medium (BHIS) alone or minimal medium (CDMM) alone or these media supplemented with 0.1% taurocholate. Prior work has shown that taurocholate does not enhance the growth in the rich medium for the C. difficile CD196 strain5. Indeed, the growth of the C. difficile UK1 strain is not affected by 0.1% taurocholate (Supplementary Fig. 2) in either BHIS (Supplementary Fig. 2a) or CDMM medium (Supplementary Fig. 2b).
C. difficile BSH activity is specific to taurine-derived bile salts
Because some, but not all, BSH enzymes have specificity to one conjugated form or another41,44,45, we next tested if C. difficile BSH activity is restricted to either the taurine-derived bile salts or if C. difficile can also deconjugate glycine-conjugated bile salts. C. difficile strains were grown in a rich medium supplemented with glycine-conjugated bile salt and analyzed as described above. C. difficile CD630Δerm could not deconjugate GCA (Fig. 4a) or GCDCA (Fig. 4b). Moreover, none of the tested strains had any BSH activity against the GCA (Fig. 4c) or GCDCA (Fig. 4d). Taken together, our data show the presence of BSH activity that is conserved throughout several C. difficile ribotypes and that this BSH activity is taurine-specific.
Fig. 4. C. difficile BSH activity is taurine-specific.
C. difficile CD630Δerm was grown in a rich medium supplemented with 1 mM glycocholate (GCA) (a) or glycochenodeoxycholate (GCDCA) (b) for 24 h. The bile salts were then analyzed for HPLC-coupled evaporative light scattering. Peak retention times for each bile acid were determined by using bile acid standards (Supplementary Fig. 1). Deconjugation of GCA (c) or GCDCA (d) by C. difficile strains M120, M68, CD630Δerm, UK1, and LC5624 were determined as in (a, b). Values were calculated by measuring the peak areas for GCA and CA or GCDCA and CDCA and expressed as a percentage of the total input. HDCA was included as an internal standard. Values shown are the average of three different independent experiments, and error bars represent the standard error of the mean.
Dormant C. difficile spores deconjugate taurine-derived bile salts
It is well established that the most effective germinants for C. difficile spores are TA and glycine5,6,18. Because we observed BSH activity in C. difficile vegetative cells, we next tested if C. difficile spores also have BSH activity. To test for the presence of BSH activity in dormant C. difficile spores, we purified spores and incubated them in buffer supplemented with 1 mM bile salts but no cogerminants (e.g., glycine; to prevent the spores from germinating). Though we observed BSH activity in spores, it was much lower when compared to the activity observed in vegetative cells (Fig. 5). However, we observed that spores deconjugated: TA (Fig. 5a), TDCA (Fig. 5b), and TCDCA (Fig. 5c).
Fig. 5. C. difficile spores show low levels of BSH activity toward taurine-derived bile acids.
Totally, 1.2 × 109 spores were incubated for 24 h in an aerobic environment at room temperature in phosphate buffer saline (PBS) supplemented with a 1 mM TA, b 1 mM TDCA, and c 1 mM TCDCA. Samples were centrifuged, and filter sterilized. Bile salts were measured using HPLC. Values were calculated by measuring the peak areas for the indicated bile salt and expressed as a percentage of the total input. HDCA was included as an internal standard. Values shown are the average of three different independent experiments, and error bars represent the standard error of the mean.
To test if the spore-derived BSH activity was a result of low levels of autogermination of spores to the vegetative form, which could then provide BSH activity, we tested BSH activity in spores derived from the C. difficile JSC10 strain. C. difficile JSC10 has a mutation in the gene coding for the bile salt germinant receptor, CspC, and as such, does not germinate in response to bile salts8. As we observed for spores derived from the wild-type strains, the C. difficile JSC10 strain also had BSH activity (Fig. 5a–c).
C. difficile can feed CA to C. scindens for DCA generation
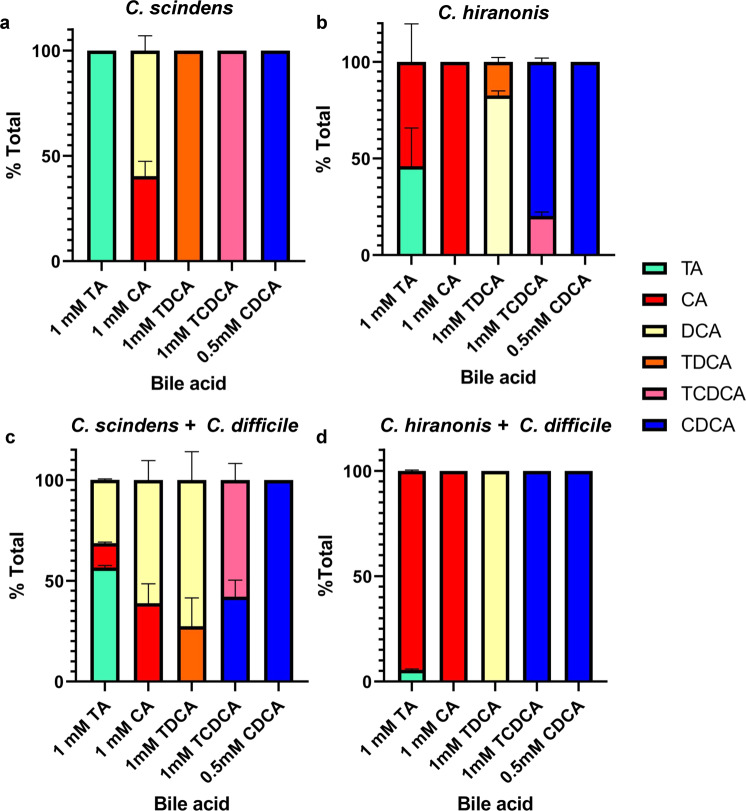
Bile salt-metabolizing bacteria (e.g., C. scindens or C. hiranonis) are well-documented to correlate with a colonic environment that resists C. difficile colonization26,46,47. Though the mechanism by which these bacteria protect against CDI may be through metabolic competition28,48–51, C. difficile must interact with these bile salt biotransforming bacteria. We decided to analyze potential coupling functions between C. difficile and other Clostridial species present in the GI tract. As we showed previously, C. scindens VPI12708 did not have BSH activity against TA, TDCA, or TCDCA (Fig. 6a). However, when grown in the presence of CA, C. scindens 7α-dehydroxylated CA to generate DCA (Fig. 6a) (we did not observe generation of LCA from CDCA in these conditions). When C. hiranonis was grown in the presence of TA, it was able to efficiently deconjugate TA to CA and deconjugated TDCA and TCDCA [Fig. 6b28]. However, unlike C. scindens, our C. hiranonis 10542 strain did not 7α-dehydroxylate CA to generate DCA (Fig. 6b).
Fig. 6. C. difficile BSH activity can feed CA to gut microbes.
C. scindens (a) or C. hiranonis (b) overnight cultures were back diluted to 108 CFU/mL and grown in the presence of the indicated bile salts for 24 h at 37 °C in an anaerobic environment. c C. scindens and C. difficile or d C. hiranonis and C. difficile overnight cultures were back diluted to 106 CFU/mL and grown in the presence of the indicated bile salts for 24 h at 37 °C in an anaerobic environment. Values were calculated by measuring the peak areas for the indicated bile salt and expressed as a percentage of the total input. HDCA was included as an internal standard. Values shown are the average of three different independent experiments, and error bars represent the standard error of the mean.
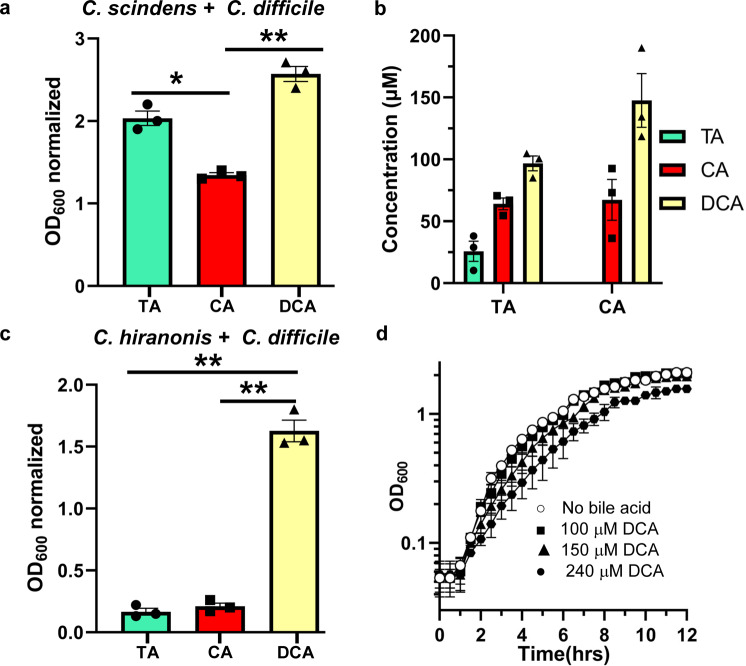
We next co-cultured C. difficile and C. scindens in the presence of different bile salts. We hypothesized that if C. difficile can deconjugate TA to CA, C. scindens could use this CA to produce DCA. Indeed, when C. difficile and C. scindens were grown in a rich medium supplemented with TA, we observed DCA production (Fig. 6c). Similar to when C. difficile was grown in the presence of TDCA and TCDCA, we still observed deconjugation of these molecules (Fig. 6c) but not the conversion of CDCA to LCA, similar to what we observed when C. scindens was grown in isolation (Fig. 6a). When C. difficile and C. hiranonis were co-cultured, the amount of TA deconjugated was greater than when either of these bacteria was grown alone (Fig. 2, Fig. 6b). However, and as expected because C. hiranonis did not generate DCA from CA when grown in isolation (Fig. 6b), we also did not observe DCA production in co-culture conditions. These results suggest C. scindens and C. difficile could interact, and that bile salts could mediate this interaction.
C. difficile can promote its own biofilm formation using BSH activity
In prior work, Dubois and colleagues found that 240 μM DCA promotes biofilm formation in C. difficile CD630Δerm52. Because C. difficile BSH activity can produce CA from TA, we hypothesized that C. difficile may indirectly stimulate its own biofilm formation by feeding CA to C. scindens. To test this hypothesis, biofilm assays were run in an anaerobic chamber for 72 h in the presence of TA or CA, or DCA, as described previously53. Subsequently, bile salt concentrations were determined, and biofilms were quantified using crystal violet staining. During co-culture of C. difficile and C. scindens, growth in the presence of TA yielded robust biofilm formation (Fig. 7a). As expected, and as a positive control for biofilm formation, growth in the presence of DCA yielded robust biofilm (Fig. 7a). However, we could not recapitulate biofilm levels observed in CA presence, despite the production of DCA in these conditions (Fig. 7b). Because our C. hiranonis strain either does not generate DCA or does so below the limit of detection for our instrument (0.2 nmol), we tested if merely growing C. difficile in the presence of another metabolic competitor28 induces biofilm formation by C. difficile cells (Fig. 7c). Despite co-culturing C. difficile and C. hiranonis in the presence of either TA or CA, we did not observe biofilm formation (Fig. 7c). However, growth in the presence of DCA, as expected, generated robust biofilm (Fig. 7c).
Fig. 7. C. difficile BSH activity contributes to biofilm formation in a C. difficile-C. scindens dual-species biofilm.
a C. difficile was co-cultured with C. scindens with specific bile salt concentrations (TA, CA, DCA), and the amount of biofilm formation was quantified using a crystal violet assay. Values shown are the average of three different independent experiments, and error bars represent the standard error of the mean. The values shown have the negative control conditions (growth without bile acid) subtracted to reflect the amount made in response to the indicated condition. b Measured concentrations of the indicated bile salts (TA, CA, DCA) in the C. difficile–C. scindens biofilm assay wells. Unwashed biofilms were used to measure bile salt concentrations in wells due to loss of supernatant when washing with PBS for crystal violet staining. c C. difficile was co-cultured with C. hiranonis with specific bile salt concentrations (TA, CA, DCA), and the amount of biofilm formation was quantified using a crystal violet assay. d Overnight cultures of C. difficile CD630Δerm were back diluted to an OD600 0.05 and monitored for 12 h in rich medium alone (○) or supplemented with 100 µM deoxycholate (DCA) (■), 150 µM DCA (▲), 240 µM DCA (•). Values shown are the average of three different independent experiments, and error bars represent the standard error of the mean. Statistical analysis was performed using an ordinary one-way ANOVA. *p < 0.005, **p < 0.001.
We next quantified the amount of bile salt present in the biofilm conditions. When C. difficile and C. scindens were co-cultured in the presence of TA, approximately 75 μM CA was generated, and approximately 100 μM DCA was generated (Fig. 7b). When grown with CA, approximately 150 μM DCA was produced. Secondary bile salts are well-documented to inhibit C. difficile growth. To understand if the DCA produced by C. scindens can inhibit C. difficile growth, we grew C. difficile in 100, 150, or 240 μM DCA. As shown in Fig. 7d, these concentrations of DCA did not significantly inhibit C. difficile growth, though there was a trend of less growth at 240 μM DCA. These results indicate that the amount of DCA produced by C. scindens in response to deconjugation of TA by C. difficile does not inhibit growth but that C. difficile responds to this by producing biofilm.
Discussion
BSH research has increased significantly since their discovery in the 1970s due to their potential to modulate bile salts in the gut environment, the health implications in bile salt modulation, as well as bile salt toxicity towards gut microbiota32,33,36,41,54. Nevertheless, most BSH research has focused on bacterial species that are used as probiotics, whereas studies aiming at the characterization and function of BSH in human pathogens is limited. Here, we provided an initial characterization of the BSH activity in the human pathogen C. difficile. Our results show that BSH activity is conserved across ribotypes and that deconjugation is specific to taurine-conjugated bile salts (Figs. 2 and 3). C. difficile does not encode orthologues of known BSH genes. Based upon recent work33,36, we attempted to bioinformatically determine if C. difficile had a weak homolog, but this proved fruitless. At its basis, BSH activity is the cleavage of an amide bond. We hypothesize that one of the many amidases or peptidases encoded by C. difficile is either a BSH or moonlights as a BSH. We are continuing our efforts in identifying this enzyme.
It is unclear why C. difficile BSH activity favors taurine-conjugated bile salts. One hypothesis is the use of taurine for Stickland fermentation. However, glycine is only one of three amino acids that can be used in the reductive branch of C. difficile Stickland metabolism. Thus, the ability to use glycine released by glycine-derived bile salts should also be beneficial to C. difficile48,55–57. On the other hand, recent discoveries of bile salts as modulators of C. difficile TcdB effectiveness showed the ability of the taurine-conjugated bile salt TCDCA, to inhibit toxin activity in a dose-dependent manner58. The BSH activity by different C. difficile isolates to deconjugate TCDCA (Fig. 3b), and other taurine bile salt derivatives may be a mechanism by the bacterium to modulate toxin activity depending on the surrounding conditions.
Germination by C. difficile spores is well-established to be influenced by and dependent on host bile salts5,7–9,11,14–19,59. Although all cholic acid-derived primary bile salts promote germination5, a study testing the germination of individual C. difficile spores found that TA and TDCA had the highest germination levels after one hour60. Because we observe low BSH activity in C. difficile spores, we hypothesize that the observed activity is due to the misincorporation of the enzyme responsible for this activity into the coating layer of the developing spore. Still, this could provide a means to modulate the efficacy of germination by converting TCDCA to CDCA, a competitive inhibitor of spore germination. This sort of mechanism is observed for germination in bacilli where L-alanine stimulates spore germination, but a spore-associated alanine racemase converts l-alanine to d-alanine, an inhibitor of spore germination61–64. BSH activity by C. difficile and the colonic microbiota could modulate the abundance of TA by converting this to CA. However, CA is also an effective germinant5. But the cells could convert TCDCA to CDCA, which is a potent inhibitor of spore germination14–16. Importantly, though, CDCA is quickly absorbed by the colonic epithelium65, thus the C. difficile BSH activity may be important for generating a local concentration within a biofilm to keep spores in a dormant state and, thus, potentially, contribute to disease recurrence.
During growth in a host, C. difficile encounters an environment that has a diverse repertoire of bile salts. The ability to use this information to affect changes to the community would provide an advantage for C. difficile in the host. Previously, we hypothesized that C. difficile competes with C. scindens, and related bacteria, for proline and/or glycine28. During a successful infection, C. difficile also consumes proline and glycine, which may exclude C. scindens from regaining a foothold to provide colonization resistance. However, should C. scindens begin to accumulate in the GI, this could signal to C. difficile that an environment that is non-conducive for optimum growth is being established. To resist these changes and maintain itself, the formation of a biofilm would be advantageous. We hypothesize that C. difficile-mediated deconjugation of TA results in the environment being ‘seeded’ with CA. As C. scindens becomes abundant in the GI, C. scindens converts the generated CA to DCA. The generated DCA signals back to C. difficile to initiate biofilm production and maintenance in the host (Fig. 8). Moreover, C. difficile could take advantage of high TA levels during the initial stages of infection not only for germination66, but for the generation of CA that can be used by other bacteria that do not encode BSH enzymes (e.g., C. scindens) to generate DCA and persist in the gut environment and, potentially, to cause recurring infections.
Fig. 8. Working model for DCA-mediated dual-species biofilm formation.
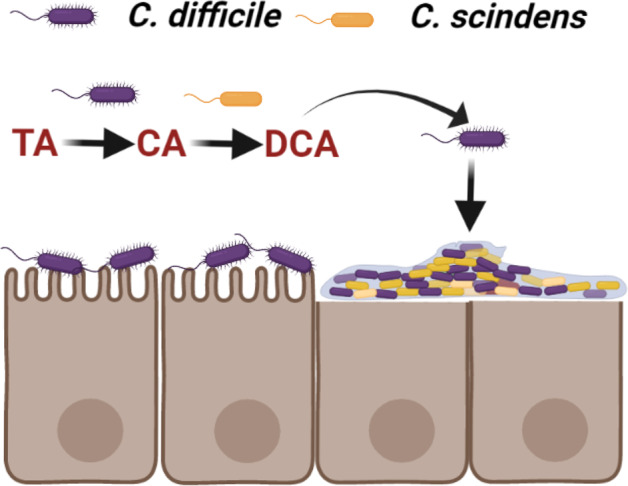
The ability of C. difficile to deconjugate TA during colonization “seeds” the gut environment with CA to be used by C. scindens during gut microbiome recovery. The presence of DCA signals a potentially non-conducive environment for C. difficile growth and the bacteria forms a DCA-mediated biofilm to persist in the gut. Created with BioRender.com.
Methods
Bacterial and strains
Clostridial strains were grown at 37 °C in an anaerobic chamber (Coy Laboratories; model B; >4% H2, 5% CO2, 85% N2) on brain heart infusion agar (BHI) and 0.1% l-cysteine, BHI supplemented with 5 g/l yeast extract and 0.1% l-cysteine (BHIS) or BHIS with 100 mM glucose and 0.1% l-cysteine (BHISG). Spores were generated on 70:30 agar medium [63 g/L Bacto peptone, 3.5 g/L protease peptone, 11.1 g/L BHI, 1.5 g/L yeast extract, 1.06 g/L tris base and 0.7 g/L ammonium sulfate (NH4SO4)].
BSH single and dual-species activity assays
Clostridial species were grown on BHI medium with 0.1 % l-cysteine for 16 h. Cultures were back diluted to 108 CFU into fresh BHI medium supplemented with 1 mM or 0.5 mM of specified bile salt. For dual species assays, overnight cultures were back diluted to 106 CFU, and added to BHI medium, in a 1:1 ratio, with specified bile salts. Cultures were grown for 24 h and then centrifuged for 10 min at 4000 × g. The pellet was suspended in 100% methanol, and the supernatant was lyophilized and resuspended in the already suspended pellet solution. The presence of specific bile salts in samples was measured as described below.
Bile salt separation
Bile salts were separated by reverse-phase HPLC using a Shimadzu Prominence HPLC system. Twenty-five microliter samples were separated using a Synchronis C18 column (4.6 by 250 mm; 5 μm particle size; ThermoFisher 97105–254630) using a mobile phase consisting of 53% methanol, 24% acetonitrile, 23% water and 30 mM ammonium acetate (pH 5.6). Bile salt peaks were detected using a Sedere Sedex model 80 LT- ELSD (low temperature-evaporative light scattering detector) using an air pressure of 50 psi of Zero Grade air at 94 °C. Different amounts of specific bile salts [taurocholic acid (Sigma Aldrich 86339-25G), glycocholic acid (Sigma Aldrich G7132-1G), taurochenodeoxycholic acid (Sigma Aldrich T6260-250MG), glycochenodeoxycholic acid (Sigma Aldrich G0759-500MG), hyodeoxycholic acid (Sigma Aldrich H3878-5G), chenodeoxycholic acid (Acros organics C9377-25G), cholic acid (Sigma Aldrich C1129-100G), deoxycholic acid (Sigma Aldrich D2510-100G), lithocholic acid (Acros organics L6250-5G), taurodeoxycholate (Sigma Aldrich T0875-5G)] were separated to generate standard curves. The area under each peak was calculated and plotted against the concentration of bile salt added, and a trend line was generated for each bile salt. The concentration of the bile salts in samples (nmol) was calculated using the standard curves of pure bile salts and normalized with the added internal standard (HDCA). The percent total was calculated by dividing the concentration of specific bile salt over the total bile salt presence.
C. difficile spores BSH assays
Spores of C. difficile strains were purified as described below on 70:30 sporulation media. Following purification, spores were counted, and 1.5 × 108 spores were used for each BSH assay. Purified spores were incubated in PBS supplemented with 1 mM TA, TDCA, or TCDCA at room temperature for 24 h in an aerobic environment. The presence of bile salts was measured by HPLC-ELSD as described above.
Spore purification
Spores were purified as previously described (10, 16, 36, 65). Briefly, strains were grown on a 70:30 sporulation medium. After 5 days, growth from 7 plates each were scraped into 1 mL distilled water (dH2O) in microcentrifuge tubes and left overnight at 4 °C. The cultures were then resuspended on dH2O in the same microcentrifuge tubes and centrifuged at >14,000 × g for 1 min, the top layer containing vegetative cells and cell debris was removed by pipetting, and the rest of the sediment resuspended in fresh dH2O. The tubes, again, were centrifuged for 1 min at >14,000 × g, the top layer removed, and the sediment resuspended. This was repeated five more times, combining the sediment from seven tubes into one. The spores were then separated from the cell debris by centrifugation through 50% sucrose for 20 min at 4 °C and 3500 × g. The resulting spore pellet was then washed 5 times with dH2O, resuspended in 1 mL dH2O, and stored at 4 °C until use.
Dual species biofilm assays
Biofilm assays were done as previously reported with some modifications. Briefly, overnight cultures of C. difficile, C. scindens, and C. hiranonis in BHIS were normalized to an OD600 nm of 1.00, and an equal volume of each (10 µL) was added to prepared wells (final volume 1 mL: 24-well plate). Filter sterilized bile salts were introduced into an equilibrated BHISG medium at 190–240 µM. Cultures were incubated in 24-well tissue culture-treated plates, and the plates were incubated for 72 h at 37 °C in an anaerobic chamber, as described previously53. After 72 h, plates were taken out of the anaerobic environment, and spent media was removed by washing individual wells twice with phosphate-buffered saline (PBS). Biofilms were air-dried and stained with crystal violet (CV; 0.2% w/v) for 20 min. CV was removed by inversion; wells were washed twice with PBS and then air-dried. Dye bound to the biofilm biomass was solubilized by adding 1 mL of a 75% ethanol solution, and the absorbance, corresponding to the biofilm biomass, was measured at 600 nm with a plate reader (Promega GloMax Explorer). When necessary, the solubilized dye was diluted for the reading to remain in the linear range of the spectrophotometer. Because of individual variations in negative control wells between rows, each row in the 24-well plates were normalized to their row negative control. C. difficile - C. scindens dual-species biofilm was normalized to the levels of biofilm found in a C. scindens-only control. Unwashed biofilms were used to measure the presence of primary and secondary bile salts using the above-described HPLC–ELSD method.
Growth curves
An overnight culture of C. difficile CD630∆erm in BHISG was back diluted to an OD600 0.05 into fresh BHISG alone or in BHISG supplemented with 150, 200, or 240 µM DCA. OD600 was recorded every half hour for a total of 12 h.
For growth in the presence of taurocholate, C. difficile UK1 was grown overnight in BHIS or CDMM medium and grown to an OD600 = ~0.7. The culture was then back diluted to an OD600 = 0.05 in 96-well plates containing either media alone or media supplemented with 0.1% taurocholate. Growth was monitored every 3 min for 18–24 h using a Cerillo Stratus plate reader (Cerillo, Charlottesville, VA). Data points represent the average of 3 independent experiments, and error bars represent the standard error of the mean.
Statistical analyses
Data represent results from at least three independent experiments, and the error bars represent standard errors of the means. One-way ANOVA analysis was performed using GraphPad Prism version 9.0.2 (161) for Windows (GraphPad Software, San Diego, California, USA). A two-way ANOVA with Tukey’s test for multiple comparisons was used to determine the significance of the growth curves. No statistical significance was detected.
Supplementary information
Acknowledgements
This project was supported by awards R01AI116895, U01AI124290, and R21AI144454 from the National Institute of Allergy and Infectious Diseases. Andrea Martinez Aguirre is a recipient of a CONACYT-COECYT fellowship 2017–2022 scholar/scholarship 625561/472087. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID. The funders had no role in study design, data collection, interpretation, or the decision to submit the work for publication.
Author contributions
A.M.A. conceived the project, designed and performed the experiments, analyzed the data, and wrote the article. A.O.A. completed the growth curves and analyzed the data. J.A.S. analyzed the data, provided direction for the project, secured funding, and wrote the article.
Data availability
All data used for this study appear in the illustrated figures, and the raw data will promptly be made available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41522-022-00358-0.
References
- 1.Jump RL, Pultz MJ, Donskey CJ. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob. Agents Chemother. 2007;51:2883–2887. doi: 10.1128/AAC.01443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat. Rev. Dis. Prim. 2016;2:16020. doi: 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol Rev. 2005;18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Just I, et al. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 5.Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 2008;190:2505–2512. doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrestha R, Sorg JA. Hierarchical recognition of amino acid co-germinants during Clostridioides difficile spore germination. Anaerobe. 2018;49:41–47. doi: 10.1016/j.anaerobe.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrestha R, Cochran AM, Sorg JA. The requirement for co-germinants during Clostridium difficile spore germination is influenced by mutations in yabG and cspA. PLoS Pathog. 2019;15:e1007681. doi: 10.1371/journal.ppat.1007681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis MB, Allen CA, Shrestha R, Sorg JA. Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog. 2013;9:e1003356. doi: 10.1371/journal.ppat.1003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharjee D, McAllister KN, Sorg JA. Germinants and their receptors in Clostridia. J. Bacteriol. 2016;198:2767–2775. doi: 10.1128/JB.00405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodatko T, Akoachere M, Jimenez N, Alvarez Z, Abel-Santos E. Dissecting interactions between nucleosides and germination receptors in Bacillus cereus 569 spores. Microbiology. 2010;156:1244–1255. doi: 10.1099/mic.0.030270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez N, Liggins M, Abel-Santos E. Kinetic evidence for the presence of putative germination receptors in Clostridium difficile spores. J. Bacteriol. 2010;192:4215–4222. doi: 10.1128/JB.00488-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J. Hepatol. 2004;40:539–551. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Molinaro A, Wahlstrom A, Marschall HU. Role of Bile Acids in Metabolic Control. Trends Endocrinol. Metab. 2018;29:31–41. doi: 10.1016/j.tem.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Sorg JA, Sonenshein AL. Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination. J. Bacteriol. 2009;191:1115–1117. doi: 10.1128/JB.01260-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis MB, Allen CA, Sorg JA. Muricholic acids inhibit Clostridium difficile spore germination and growth. PLoS One. 2013;8:e73653. doi: 10.1371/journal.pone.0073653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorg JA, Sonenshein AL. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J. Bacteriol. 2010;192:4983–4990. doi: 10.1128/JB.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howerton A, Patra M, Abel-Santos E. A new strategy for the prevention of Clostridium difficile infection. J. Infect. Dis. 2013;207:1498–1504. doi: 10.1093/infdis/jit068. [DOI] [PubMed] [Google Scholar]
- 18.Howerton A, Ramirez N, Abel-Santos E. Mapping interactions between germinants and Clostridium difficile spores. J. Bacteriol. 2011;193:274–282. doi: 10.1128/JB.00980-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howerton A, et al. Effect of the synthetic bile salt analog CamSA on the hamster model of Clostridium difficile infection. Antimicrob. Agents Chemother. 2018 doi: 10.1128/AAC.02251-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22–39. doi: 10.1080/19490976.2015.1127483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzior DV, Quinn RA. Review: microbial transformations of human bile acids. Microbiome. 2021;9:140. doi: 10.1186/s40168-021-01101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman JP, White WB, Lijewski M, Hylemon PB. Nucleotide sequence and regulation of a gene involved in bile acid 7-dehydroxylation by Eubacterium sp. strain VPI 12708. J. Bacteriol. 1988;170:2070–2077. doi: 10.1128/jb.170.5.2070-2077.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallonee DH, White WB, Hylemon PB. Cloning and sequencing of a bile acid-inducible operon from Eubacterium sp. strain VPI 12708. J. Bacteriol. 1990;172:7011–7019. doi: 10.1128/jb.172.12.7011-7019.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buffie CG, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theriot, C. M., Bowman, A. A. & Young, V. B. Antibiotic-induced alterations of the gut microbiota alter secondary bile acid production and allow for Clostridium difficile spore germination and outgrowth in the large intestine. mSphere1, 10.1128/mSphere.00045-15 (2016). [DOI] [PMC free article] [PubMed]
- 28.Aguirre AM, et al. Bile acid-independent protection against Clostridioides difficile infection. PLoS Pathog. 2021;17:e1010015. doi: 10.1371/journal.ppat.1010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogilvie LA, Jones BV. Dysbiosis modulates capacity for bile acid modification in the gut microbiomes of patients with inflammatory bowel disease: a mechanism and marker of disease? Gut. 2012;61:1642–1643. doi: 10.1136/gutjnl-2012-302137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labbe A, Ganopolsky JG, Martoni CJ, Prakash S, Jones ML. Bacterial bile metabolising gene abundance in Crohn’s, ulcerative colitis and type 2 diabetes metagenomes. PLoS One. 2014;9:e115175. doi: 10.1371/journal.pone.0115175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl Acad. Sci. USA. 2008;105:13580–13585. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourgin, M. et al. Bile Salt Hydrolases: At the Crossroads of Microbiota and Human Health. Microorganisms9, 10.3390/microorganisms9061122 (2021). [DOI] [PMC free article] [PubMed]
- 33.Foley MH, O’Flaherty S, Barrangou R, Theriot CM. Bile salt hydrolases: Gatekeepers of bile acid metabolism and host-microbiome crosstalk in the gastrointestinal tract. PLoS Pathog. 2019;15:e1007581. doi: 10.1371/journal.ppat.1007581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oinonen C, Rouvinen J. Structural comparison of Ntn-hydrolases. Protein Sci.: a Publ. Protein Soc. 2000;9:2329–2337. doi: 10.1110/ps.9.12.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song Z, et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome. 2019;7:9. doi: 10.1186/s40168-019-0628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foley, M. H. et al. Lactobacillus bile salt hydrolase substrate specificity governs bacterial fitness and host colonization. Proc. Natl. Acad. Sci USA118, 10.1073/pnas.2017709118 (2021). [DOI] [PMC free article] [PubMed]
- 37.Gopal-Srivastava R, Hylemon PB. Purification and characterization of bile salt hydrolase from Clostridium perfringens. J. Lipid Res. 1988;29:1079–1085. doi: 10.1016/S0022-2275(20)38464-9. [DOI] [PubMed] [Google Scholar]
- 38.Rossocha M, Schultz-Heienbrok R, vonMoeller H, Coleman JP, Saenger W. Conjugated bile acid hydrolase is a tetrameric N-terminal thiol hydrolase with specific recognition of its cholyl but not of its tauryl product. Biochemistry. 2005;44:5739–5748. doi: 10.1021/bi0473206. [DOI] [PubMed] [Google Scholar]
- 39.Coleman JP, Hudson LL. Cloning and characterization of a conjugated bile acid hydrolase gene from Clostridium perfringens. Appl Environ. Microbiol. 1995;61:2514–2520. doi: 10.1128/aem.61.7.2514-2520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia B, Park D, Hahn Y, Jeon CO. Metagenomic analysis of the human microbiome reveals the association between the abundance of gut bile salt hydrolases and host health. Gut Microbes. 2020;11:1300–1313. doi: 10.1080/19490976.2020.1748261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jarocki P, Podlesny M, Glibowski P, Targonski Z. A new insight into the physiological role of bile salt hydrolase among intestinal bacteria from the genus Bifidobacterium. PLoS One. 2014;9:e114379. doi: 10.1371/journal.pone.0114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cameron D, et al. Probiotics for gastrointestinal disorders: Proposed recommendations for children of the Asia-Pacific region. World J. Gastroenterol. 2017;23:7952–7964. doi: 10.3748/wjg.v23.i45.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kociolek LK, Gerding DN, Hecht DW, Ozer EA. Comparative genomics analysis of Clostridium difficile epidemic strain DH/NAP11/106. Microbes Infect. 2018;20:245–253. doi: 10.1016/j.micinf.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim GB, Miyamoto CM, Meighen EA, Lee BH. Cloning and characterization of the bile salt hydrolase genes (bsh) from Bifidobacterium bifidum strains. Appl Environ. Microbiol. 2004;70:5603–5612. doi: 10.1128/AEM.70.9.5603-5612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren J, Sun K, Wu Z, Yao J, Guo B. All 4 bile salt hydrolase proteins are responsible for the hydrolysis activity in Lactobacillus plantarum ST-III. J. Food Sci. 2011;76:M622–M628. doi: 10.1111/j.1750-3841.2011.02431.x. [DOI] [PubMed] [Google Scholar]
- 46.Kang JD, et al. Bile Acid 7alpha-Dehydroxylating Gut Bacteria Secrete Antibiotics that Inhibit Clostridium difficile: Role of Secondary Bile Acids. Cell Chem. Biol. 2019;26:27–34 e24. doi: 10.1016/j.chembiol.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thanissery R, Winston JA, Theriot CM. Inhibition of spore germination, growth, and toxin activity of clinically relevant C. difficile strains by gut microbiota derived secondary bile acids. Anaerobe. 2017;45:86–100. doi: 10.1016/j.anaerobe.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girinathan BP, et al. In vivo commensal control of Clostridioides difficile virulence. Cell Host Microbe. 2021;29:1693–1708.e1697. doi: 10.1016/j.chom.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Battaglioli, E. J. et al. Clostridioides difficile uses amino acids associated with gut microbial dysbiosis in a subset of patients with diarrhea. Science Translational Medicine10, 10.1126/scitranslmed.aam7019 (2018). [DOI] [PMC free article] [PubMed]
- 50.Jenior, M. L., Leslie, J. L., Young, V. B. & Schloss, P. D. Clostridium difficile Colonizes Alternative Nutrient Niches during Infection across Distinct Murine Gut Microbiomes. mSystems2 (2017). [DOI] [PMC free article] [PubMed]
- 51.Leslie, J. L. et al. Protection from lethal Clostridioides difficile infection via intraspecies competition for cogerminant. mBio12, 10.1128/mBio.00522-21 (2021). [DOI] [PMC free article] [PubMed]
- 52.Bouillaut L, et al. Role of the global regulator Rex in control of NAD(+) -regeneration in Clostridioides (Clostridium) difficile. Mol. Microbiol. 2019;111:1671–1688. doi: 10.1111/mmi.14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dubois T, et al. A microbiota-generated bile salt induces biofilm formation in Clostridium difficile. NPJ Biofilms Microbiomes. 2019;5:14. doi: 10.1038/s41522-019-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu F, et al. The complex structure of bile salt hydrolase from Lactobacillus salivarius reveals the structural basis of substrate specificity. Sci. Rep. 2019;9:12438. doi: 10.1038/s41598-019-48850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neumann-Schaal M, Jahn D, Schmidt-Hohagen K. Metabolism the Difficile Way: The Key to the Success of the Pathogen Clostridioides difficile. Front Microbiol. 2019;10:219. doi: 10.3389/fmicb.2019.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenior, M. L., Leslie, J. L., Young, V. B. & Schloss, P. D. Clostridium difficile Alters the Structure and Metabolism of Distinct Cecal Microbiomes during Initial Infection To Promote Sustained Colonization. mSphere3, 10.1128/mSphere.00261-18 (2018). [DOI] [PMC free article] [PubMed]
- 57.Huijghebaert SM, Eyssen HJ. Specificity of bile salt sulfatase activity from Clostridium sp. strains S1. Appl Environ. Microbiol. 1982;44:1030–1034. doi: 10.1128/aem.44.5.1030-1034.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tam J, et al. Intestinal bile acids directly modulate the structure and function of C. difficile TcdB toxin. Proc. Natl Acad. Sci. 2020;117:6792–6800. doi: 10.1073/pnas.1916965117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liggins M, Ramirez N, Magnuson N, Abel-Santos E. Progesterone analogs influence germination of Clostridium sordellii and Clostridium difficile spores in vitro. J. Bacteriol. 2011;193:2776–2783. doi: 10.1128/JB.00058-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang S, Shen A, Setlow P, Li YQ. Characterization of the dynamic germination of individual Clostridium difficile spores using Raman spectroscopy and differential interference contrast microscopy. J. Bacteriol. 2015;197:2361–2373. doi: 10.1128/JB.00200-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanodia S, et al. Biochemical characterization of alanine racemase-a spore protein produced by Bacillus anthracis. BMB Rep. 2009;42:47–52. doi: 10.5483/BMBRep.2009.42.1.047. [DOI] [PubMed] [Google Scholar]
- 62.Xiao Y, Angulo MT, Lao S, Weiss ST, Liu YY. An ecological framework to understand the efficacy of fecal microbiota transplantation. Nat. Commun. 2020;11:3329. doi: 10.1038/s41467-020-17180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venir E, et al. Involvement of alanine racemase in germination of Bacillus cereus spores lacking an intact exosporium. Arch. Microbiol. 2014;196:79–85. doi: 10.1007/s00203-013-0946-y. [DOI] [PubMed] [Google Scholar]
- 64.Chesnokova ON, McPherson SA, Steichen CT, Turnbough CL., Jr. The spore-specific alanine racemase of Bacillus anthracis and its role in suppressing germination during spore development. J. Bacteriol. 2009;191:1303–1310. doi: 10.1128/JB.01098-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mekhjian HS, Phillips SF, Hofmann AF. Colonic absorption of unconjugated bile acids: perfusion studies in man. Dig. Dis. Sci. 1979;24:545–550. doi: 10.1007/BF01489324. [DOI] [PubMed] [Google Scholar]
- 66.Wexler AG, et al. Clostridioides difficile infection induces a rapid influx of bile acids into the gut during colonization of the host. Cell Rep. 2021;36:109683. doi: 10.1016/j.celrep.2021.109683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used for this study appear in the illustrated figures, and the raw data will promptly be made available upon request.