Abstract
A major obstacle to development of subunit vaccines and diagnostic reagents for tuberculosis is the inability to produce large quantities of these proteins. To test the hypothesis that poor expression of some mycobacterial genes in Escherichia coli is due, in part, to the presence of low-usage E. coli codons, we used site-directed mutagenesis to convert low-usage codons to high-usage codons for the same amino acid in the Mycobacterium tuberculosis genes for antigens 85A and 85B and superoxide dismutase. Replacement of five codons in the wild-type gene for antigen 85B increased recombinant protein production in E. coli 54-fold. The recombinant antigen elicited proliferation and gamma interferon production by lymphocytes from healthy tuberculin reactors and was recognized by monoclonal antibodies to native antigen 85, indicating that the recombinant antigen contained T-cell and B-cell epitopes. Northern blotting demonstrated only a 1.7- to 2.5-fold increase in antigen 85B mRNA, suggesting that the enhanced protein production was due primarily to enhanced efficiency of translation. Codon replacement in the genes encoding antigen 85A and superoxide dismutase yielded four- to sixfold increases in recombinant protein production, suggesting that this strategy may be generally applicable to overexpression of mycobacterial genes in E. coli.
Despite widespread administration of bacillus Calmette-Guérin vaccine throughout the world, tuberculosis remains the leading cause of death from a single pathogen (3). Development of subunit tuberculosis vaccines has been spurred by findings that partial immunity is conferred by vaccination of animals with culture filtrate proteins (1, 18), purified antigen 85B (12), and naked DNA encoding mycobacterial antigens (13, 23). A major problem with developing a subunit vaccine is that Escherichia coli overexpression systems do not provide good yields of some Mycobacterium tuberculosis proteins, even when mycobacterial genes are placed behind strong E. coli promoters (15). Because the GC content of E. coli genes is only 50%, E. coli may lack the transcriptional and translational machinery needed to efficiently produce proteins from mycobacterial genes, which have a GC content of 65 to 70% (7). Difficulties in overexpressing mycobacterial genes in E. coli have led investigators to produce mycobacterial proteins in baculovirus expression systems (2) or to use bacteria that are phylogenetically closer to mycobacteria, such as Streptomyces lividans, Corynebacterium spp., and Mycobacterium smegmatis (7, 10, 16, 26).
When the gene encoding antigen 85B was first sequenced and cloned behind a trc promoter in E. coli, less than 0.5 mg of recombinant antigen 85B per liter was produced (15). Whereas this yield can be improved to 10 mg/liter by using a stronger promoter such as T7 (9), problems with achieving higher-level expression and solubility of antigen 85B have remained. Therefore, investigators studying antigen 85B and many other secreted M. tuberculosis antigens have generally purified them from M. tuberculosis (9, 12). This is extremely inefficient, since growth of M. tuberculosis for 2 to 3 weeks in 150 liters of broth culture was required to produce 100 mg of antigen 85B (9, 12). The yield of recombinant antigen 85B per liter can be improved 5- to 10-fold and the time until cultures are harvested can be shortened from weeks to days by overexpression in rapidly growing, nonpathogenic mycobacterial species such as M. smegmatis and Mycobacterium vaccae (10). However, for mycobacterial proteins to be used for large-scale immunization, more efficient means to produce large amounts of these proteins must be developed.
Although several codons can encode the same amino acid, E. coli contains more tRNA for certain high-usage codons than for other low-usage codons. Observations while working with antigens 85A, 85B, and 85C led us to consider the possibility that part of the problem with overexpressing mycobacterial genes in E. coli might derive from problems with translation rather than transcription. In this study, we tested the hypothesis that selective replacement of low-usage E. coli codons in mycobacterial genes by high-usage E. coli codons might enhance production of recombinant mycobacterial proteins. We find that this strategy has a dramatic effect on the yield of antigen 85B, and our experience with other mycobacterial genes suggests that selective codon replacement can enhance the overexpression of a wide variety of mycobacterial proteins in E. coli.
MATERIALS AND METHODS
Bacterial strains and DNA vectors.
M. tuberculosis H37Rv (ATCC 25618) was obtained from the American Type Culture Collection, Rockville, Md. E. coli TOP 10 and plasmids pTrcHisB and pRSETB were purchased from Invitrogen (Carlsbad, Calif.). The plasmids pTrcHisB and pRSETB are E. coli expression vectors containing the ampicillin resistance gene, the trc and T7 promoters, respectively, an ATG start codon, the sequence for a N-terminal fusion tag encoding six histidines and a monoclonal antibody (Anti-Xpress; Invitrogen) epitope, and a multiple-cloning site. E. coli JM109 DE3 was obtained from Promega (Madison, Wis.), and phagemid pBCSK+ was purchased from Stratagene (La Jolla, Calif.).
Cloning mycobacterial genes.
Mycobacterial chromosomal DNA was isolated from M. tuberculosis by the freeze-boil method (20) and used as a template for amplification by PCR in a Perkin-Elmer DNA thermal cycler, using oligonucleotide primers based on the DNA sequences of the antigen 85 genes (6, 15) (Table 1), 10% formamide, and vent DNA polymerase (New England Biolabs, Beverly, Mass.). PCR was performed with the following settings: 94°C for 1.5 min, followed by 40 cycles of 94°C for 1 min plus 50°C for 2 min and 72°C for 3 min, and ending with 72°C for 10 min. The PCR products were cloned into the phagemid pBCSK+ and transformed into E. coli DH5α, which served as an intermediate vector and host, respectively.
TABLE 1.
Primers used for cloning and site-directed mutagenesis
| Gene | Primer | Nucleotide sequencea |
|---|---|---|
| 85A | 85A left | GAT GAA TTC GCG GAA ATG CCA CCT TCA G |
| 85A right | GAT GGA TCC GCT AGA TGT TGT GTC TGT TCG GAG | |
| 85A left 2 | GATGGATCCA T(1)TT TCC CGG CCG GGC TTG CCG GT G(24) | |
| 85 A right 2 | GAT GAA TTC CTA G(885)GC GCC CTG CGG CGG GCC CGG(865) | |
| 85AL mut 9 | GATGGATCCA T(1)TT TCC CGT CCG GGC TTG CCG GTG GAG(27) | |
| 85AL mut 339 | C(337)GT CAC GTT AAG CCG ACC GGT AGC GCC GTC GTC GGT CTT (375) | |
| 85AR mut 357 | A (357)CC GGT CGG CTT AAC GTG ACG GTT GGC CTG CAG CCA CCC(319) | |
| 85B | 85 B left | GAT GAA TTC ACG ACT TTC GCC CGA ATC GAC ATT TGG |
| 85B right | GAT GGA TCC TCG CGA GGT ATA TCT CAC GTG GAC | |
| 85B left 2 | GATGGATCCA T(1)TC TCC CGG CCG GGG CTG CCG GTC(24) | |
| 85B right 2 | GAT GAA TTC T(852)CA GCC GGC GCC TAA CGA ACT CTG CAG (826) | |
| 85B L mut 9/15 | GATGGATCCA T(1)TC TCC CGT CCG GGT CTG CCG(21) | |
| 85 R 627 mut | A(627)AC CCA CAG ACG CGT GTT GTT TGC GAC CAG CTT GGG GAT CTG(585) | |
| 85 BL613 mut | A(613)CG CGT CTG TGG GTT TAT TGC GGG AAC GGC ACC CC (647) | |
| 85 B probe L | C(16)TGCCGGTCGAGTACCTGCAGGTGCCGTCG (46) | |
| 85B probe R | G(612)TTGTTTGCGACCAGCTTGGGGATCTGCTG(582) | |
| 85C | 85C left | GAT GAA TTC GTT GGG ATT GGT AGT AGC TAT GAC |
| 85 C right | GAT GGA TCC GGC TCA GGC GGC CGG CGC AGC AGG GGC | |
| 85C left 2 | GATGGATCCA T(1)TC TCT AGG CCC GGT CTT CCA GTG(24) | |
| 85C right 2 | GAT GAA TTC TCA G(882)GC GGC CGG CGC AGC AGG GGC(862) | |
| SOD | SOD left | GATGGATCCA G(1)CC GAA TAC ACC TTG CCA GAC(21) |
| SOD right | GATGAATTCCTA G(618)CC GAA TAT CAA CCC CTT GGT(598) | |
| SOD Lmut 192 | G(184)AA AAG AAC CTG GCT TTC AAC CTC GCC GGC(213) | |
| SOD Rmut 192 | G(210)GC GAG GTT GAA AGC CAG GTT CTT TTC GTT CAG CAA(175) | |
| SOD Lmut 414 | C(406)TG CTG ATC TTC CAG GTT TAC GAC(429) | |
| SOD Rmut 414 | G(426)TA AAC CTG GAA GAT CAG CAG CTT(403) |
Nucleotides are numbered beginning with the first base in the portion of the gene encoding the mature exoprotein. Bases corresponding to nucleotide substitutions introduced by site-directed mutagenesis are underlined.
Separate primers were constructed to amplify the gene encoding each mature exoprotein, without the promoter or leader sequence, from pBCSK+ into the E. coli expression vector pTrcHisB. PCR was performed under the same conditions noted above, except that the annealing temperature was 55°C. The 5′ and 3′ primers for the antigen 85A, 85B, and 85C genes were the corresponding left 2 and right 2 primers shown in Table 1. These PCR products were cloned into pTrcHisB and transformed into E. coli TOP 10.
The superoxide dismutase (SOD) gene encoding the mature exoprotein, without the promoter or leader sequence, was amplified with SOD left and SOD right primers (Table 1) from freshly isolated M. tuberculosis chromosomal DNA, by using the PCR conditions above, and cloned directly into pTrcHisB and E. coli TOP 10.
Codon replacement by site-directed mutagenesis.
Site-directed mutagenesis was performed by a PCR-based system as described by Ho et al. (11), using the 85A, 85B, or SOD gene in the pTrcHisB expression vector as the template. The primers used are shown in Table 1. The PCR products were ligated into the BamHI and EcoRI restriction sites of plasmids pTrcHisB and pRSETB and transformed into the E. coli strains TOP 10 and JM109 DE3, respectively.
Overexpression, purification, and resolubilization of recombinant proteins.
Flasks containing 50 ml of SOB broth and 200 μg of ampicillin per ml were inoculated with 300 μl of an overnight culture of the transformed TOP 10 or JM109 DE3 strain of E. coli and grown at 37°C with shaking to an absorbance at 600 nm (A600) of 0.6. Cultures were induced by adding 1 mM isopropyl-β-thiogalactopyranoside (IPTG; Promega) and grown for an additional 5 h. The E. coli cells were harvested by centrifugation and solubilized in 6 M guanidine hydrochloride, 500 mM sodium chloride, and 20 mM sodium phosphate solution (pH 7.8). The lysate was sonicated once for 5 s at medium intensity with a VirSonic 60 sonicator (VirTis Inc., Gardiner, N.Y.). Insoluble debris was removed by centrifugation at 3,000 × g, and the lysate was transferred to a nickel affinity column. After allowing the lysate to bind to the resin, the column was washed with increasingly acidic 8 M urea–500 mM sodium chloride–20 mM sodium phosphate solutions. The recombinant protein was eluted by lowering the pH to 3.8. Recombinant protein production was quantified spectrophotometrically with A280 readings, by using an approximate extinction coefficient of A280 of 1.0 for a 1.0-mg/ml protein solution.
For the Western blotting with antibodies to antigen 85 and for the studies evaluating lymphocyte proliferation and gamma interferon production, the recombinant proteins were resolubilized. Cell pellets from 50-ml cultures following the 5-h IPTG induction were resuspended and lysed in 10 ml of 25 mM Tris–500 mM sodium chloride–1% Triton X-100–0.1 mg of lysozyme per ml (pH 7.8) on ice for 30 min. This lysate was sonicated as described above, frozen in liquid nitrogen, and thawed at 37°C. The inclusion bodies were harvested by centrifugation at 7,000 rpm (Sorvall RC2-B) for 15 min and solubilized in 6 M guanidine-HCl–30 mM Tris–500 mM sodium chloride–10 mM dithiothreitol–2 mM EDTA (pH 7.8). The solubilized inclusion bodies were filtered through a 0.4-μm-pore-size filter (Millipore, Bedford, Mass.) and slowly diluted into 100 ml of 4 M urea–30 mM Tris–500 mM sodium chloride–6 mM reduced glutathione (Sigma)–0.6 mM oxidized glutathione (Sigma) (pH 7.8). After a 1-h incubation at room temperature, insoluble protein was removed by filtration. The filtrate was slowly added to 1 liter of 100 mM Tris–1 M sodium chloride–6 mM reduced glutathione–0.6 mM oxidized glutathione (pH 7.8). This solution was concentrated down to 30 ml with a stirred cell (Millipore), filtered through a 0.22-μm-pore-size filter, and dialyzed against 100 mM Tris–1 M sodium chloride (pH 7.8). After this resolubilization process, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of 85A and 85B demonstrated a single band of the expected molecular weight. Resolubilized antigens 85A and 85B were freed of endotoxin by passage over Detoxigel affinity columns (Pierce Chemical, Rockford, Ill.), after which endotoxin contamination was less than 3 pg per mg of protein (Limulus amebocyte assay; BioWhittaker, Walkersville, Md.).
Northern blotting.
The 85B probe was a 596-bp PCR product amplified from the nonmodified region of the wild-type antigen 85B gene by using the primers 85B probe left and 85B probe right (Table 1) and the PCR conditions outlined above. Ten nanograms of PCR product was end-labeled with [γ-32P]dATP (Du Pont NEN Research Products, Boston, Mass.) by using T4 polynucleotide kinase (New England Biolabs) for 90 min at 37°C. The labeled probe was purified on a Select G-25 column (5 Prime→3 Prime, Inc., Boulder, Colo.).
Recombinant antigen 85B protein expression was induced as described above. At several time points after addition of IPTG, a 1-ml aliquot of each induced culture was harvested by centrifugation and frozen at −70°C. Total cellular RNA was isolated and purified with the RNeasy column (Qiagen, Inc., Chatsworth, Calif.). Samples from each aliquot were applied to lanes of a gel, after adjusting the RNA content to 5 μg by A260 measurements. The samples were electrophoresed through a 1.5% agarose-formaldehyde gel containing ethidium bromide and transferred to a Hybond-N nylon membrane (Amersham Life Science Products, Arlington Heights, Ill.) with capillary methods, using 20× SSC (1× SSC is 0.15 M sodium chloride plus 0.015 M sodium citrate, pH 7.0). After drying the membrane, the RNAs were cross-linked by UV irradiation. The membrane was prehybridized for 4 h at 42°C in a 0.15-ml/cm2 buffer solution containing 1× Denhardt's solution, 6× SSC, 100 μg of salmon sperm per ml, and 0.5% SDS in 20 mM sodium phosphate (pH 7.4). Hybridization was performed for 18 h at 42°C in the same buffer containing 2 ng of the 32P-labeled probe. The membrane was washed, and DNA-RNA hybridization was detected with X-Omat AR film (Eastman Kodak, Rochester, N.Y.) and quantified with a Personal Densitometer SI (Molecular Dynamics, Sunnyvale, Calif.).
Western blotting.
Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes with a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad, Hercules, Calif.) by standard methods (8). After blocking with a solution containing 10 mM Tris (pH 8.0), 0.5 M sodium chloride, and 0.5% Tween 20, the nitrocellulose was incubated with the monoclonal antibodies HYT27 (1:100 dilution; provided by Thomas Shinnick, Centers for Disease Control and Prevention, Atlanta, Ga.), a 1:500 dilution of TBC-27 (21), or a 1:1,500 dilution of Anti-Express (Invitrogen). After a 1-h incubation, the membrane was washed three times in the Tween-containing solution described above and reincubated with a 1:3,000 dilution of goat anti-mouse polyclonal antibody conjugated to alkaline phosphatase (Bio-Rad). After more washing, the membrane was developed with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Bio-Rad), as directed by the manufacturer.
Lymphocyte proliferative responses and gamma interferon production.
Peripheral blood mononuclear cells (PBMC) from four healthy subjects with tuberculin reactions were isolated and cultured by standard methods (24) and plated in triplicate 200-μl wells at 2 × 105 cells/well in medium alone, with heat-killed M. tuberculosis Erdman (10 μg/ml), or with increasing concentrations of recombinant antigens 85A or 85B. Proliferative responses were determined by measurement of [3H]thymidine incorporation (24). Standard errors of the mean were <20% of mean values in all cases.
Previous studies have shown that gamma interferon concentrations produced by PBMC in response to mycobacterial antigens are maximal after 96 h (24), so supernatants from cultured cells were harvested at this time point and stored at −70°C. Gamma interferon concentrations were measured by enzyme-linked immunosorbent assay, using antibody pairs (Pharmingen, San Diego, Calif.).
RESULTS
Isolation of genes encoding antigens 85A, 85B, and 85C.
Although the genes encoding antigens 85A, 85B, and 85C are 70 to 78% identical (6), the differences outside the open reading frames allowed specific primers to be used to individually PCR amplify and clone these three genes from the chromosomal DNA of M. tuberculosis. The identity of each cloned gene was confirmed by endonuclease restriction and DNA sequencing (data not shown).
Overexpression of wild-type antigen 85 genes in E. coli.
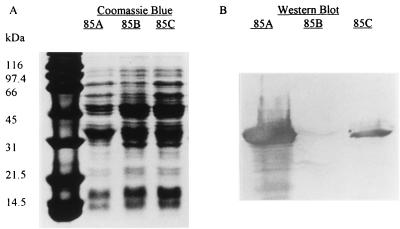
The M. tuberculosis genes encoding antigens 85A, 85B, and 85C were cloned into an E. coli expression vector, pTrcHisB. After transformation of the mycobacterial genes into E. coli host strain TOP 10, induction with IPTG of 50 ml of broth containing the transformant, lysis of the E. coli cells, solubilization of the inclusions using a denaturing protocol, and nickel affinity chromatography, the yields of antigens 85A, 85B, and 85C were 16, <0.5, and 7 mg/liter, respectively. Western blotting with an antibody to the N-terminal polyhistidine tag demonstrated significant quantities of antigens 85A and 85C but minimal amounts of antigen 85B (Fig. 1). The additional band of approximately 31 kDa in the lysates producing antigens 85B and 85C probably result from the larger amount of protein loaded in these lanes.
FIG. 1.
Coomassie blue-stained PAGE gel (A) and Western blot (B) of total cellular protein produced by E. coli expression vectors containing wild-type antigen 85A, 85B, and 85C genes. Western blotting was performed with a monoclonal antibody to the N-terminal polyhistidine tag.
Enhanced expression of antigens 85A and 85B by codon replacement.
Differential production of antigens 85A, 85B, and 85C occurred despite the high degree of homology of the antigen 85 genes. Because the growth curves of the E. coli transformants producing each antigen were indistinguishable, poor expression of antigen 85B was unlikely to be due to its toxic effects on E. coli. Furthermore, since each gene was cloned downstream of the trc promoter, transcription of each gene should be comparable. Therefore, the most likely reason for the observed differences in production of recombinant protein was differential mRNA stability or differential efficiency of mRNA translation.
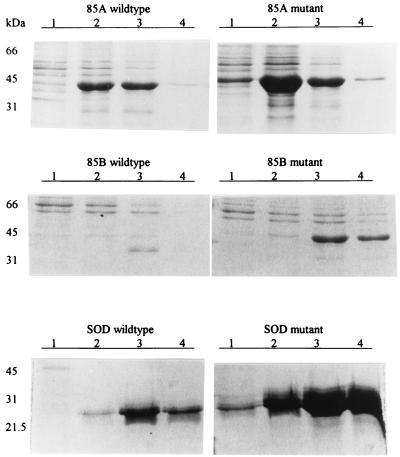
Examination of the antigen 85 genes revealed that the gene for 85B contains more low-usage codons that are inefficiently translated in E. coli than the antigen 85A gene (Table 2) (25). We hypothesized that the differences in recombinant protein yields were due to these differences in the distribution of low-usage codons. To test our hypothesis, we first identified two adjacent low-usage codons in the antigen 85B gene, CGG and CTA at nucleotides 616 to 621, coding for arginine and leucine, respectively. Using PCR-based site-directed mutagenesis (11), we changed these low-usage codons to the high-usage codons CGT and CTG, which encode the same amino acids. We produced a silent mutation in the preceding threonine codon (ACC to ACG) to add a MluI endonuclease restriction site, which produced a rapid means to screen for desired mutants. Furthermore, we changed nucleotides 7 to 15 from CGG-CCG-GGG, which codes for arginine-proline-glycine, to CGT-CCG-GGT, which codes for the same amino acids. By substituting these five high-usage codons for low-usage codons in the wild-type 85B gene in a manner that did not alter the amino acid sequence, the expression of recombinant antigen 85B improved more than 50-fold from less than 0.5 to 27 mg/liter (Fig. 2).
TABLE 2.
Number of low-usage codons in individual 85 antigen genes
| Usage in E. colia | Codons | No. of codonsb
|
||
|---|---|---|---|---|
| 85A | 85B | 85C | ||
| Least-used codons (<8%) | AGA, AGG, CGA, CGG, ATA, CTA, CCC, TCG | 23 | 24 | 25 |
| Adjacent least-used codons | 0 | 2 pairs | 0 | |
| 9 to 12% | GGA, CTC, CTT, TTG, TTA, ACA, TCA | 10 | 11 | 18 |
| 13 to 15% | GGG, CCT, TCG | 4 | 12 | 4 |
| Total low-usage codons | 37 | 47 | 47 | |
Usage in E. coli taken from work by Zhang et al. (25). Percent values refer to the percent occurrence of the codon relative to its synonymous codon family.
Total number of codons within the open reading frame of the exoprotein.
FIG. 2.
Coomassie blue-stained PAGE gels of recombinant antigens recovered from E. coli containing the wild-type and mutant genes for antigen 85A (top), 85B (middle), and SOD genes (bottom). Lane numbers represent the fractions that were eluted from the nickel resin affinity column. The protein on the gel was harvested from 0.5 ml of the original broth culture.
To confirm the effects of codon replacement on expression of a second mycobacterial gene, we substituted high-usage codons for low-usage codons in the antigen 85A gene as follows: (i) CGT (arginine) was substituted for CGG (arginine) at nucleotides 7 to 9; (ii) CGT (arginine) was substituted for AGG (arginine) at nucleotides 337 to 339; (iii) GTT (valine) was substituted for GTC (valine) at nucleotides 343 to 345; (iv) CCG (proline) was substituted for CCC (proline) at nucleotides 349 to 351; (v) GGT (glycine) was substituted for GGA (glycine) at nucleotides 355 to 358. These substitutions increased the yield of antigen 85A from 20 to 80 mg/liter (Fig. 2).
Codon replacement in the SOD gene.
The SOD gene (26) was amplified by PCR from H37Rv genomic DNA, sequenced, and cloned behind the trc promoter. Using the induction methods outlined above, recombinant SOD protein was undetectable. When the SOD gene was cloned behind the T7 promoter, only 8 mg of recombinant protein per liter was produced. The low-usage asparagine and leucine codons AAT and CTA at nucleotides 190 to 195 and the isoleucine codon ATA at positions 412 to 414 were exchanged for the high-usage codons AAC, CTG, and ATC, respectively. When this synthetic gene was cloned behind the T7 promoter and transformed into E. coli JM109 DE3, recombinant protein production increased sixfold to 50 mg/liter (Fig. 2).
Production of recombinant antigen 85 behind the T7 promoter.
The experiments outlined above were performed with the synthetic antigen 85 genes ligated behind the trc promoter. To characterize protein production with another promoter, the synthetic genes were ligated behind the T7 promoter in the plasmid pRSETB, which was transformed into E. coli JM109 DE3 and induced to overexpress by adding IPTG. This expression system yielded over 250 mg of antigen 85B per liter, which was approximately 50% of the total protein produced by the transformant. Similar yields were obtained with the synthetic antigen 85A gene behind the T7 promoter (data not shown).
Recognition of recombinant antigens by monoclonal antibodies.
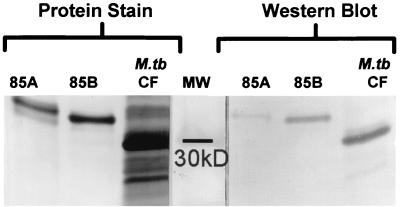
To confirm the identity of the proteins produced by the synthetic antigen 85A and 85B genes, Western blotting was performed with the TBC-27 antibody, which was raised against antigen 85B (21) and reacts more strongly with native antigen 85B than with antigen 85A (Robert Wallis, unpublished data). TBC-27 reacted with recombinant antigens 85A and 85B in a manner parallel to that seen with the respective native antigens (Fig. 3). A second anti-antigen 85 monoclonal antibody, HYT27, also reacted with both recombinant antigens 85A and 85B (data not shown).
FIG. 3.
Protein stain (left) and Western blot (right) of resolubilized recombinant 85A and 85B antigens and M. tuberculosis culture filtrate. Protein stain was performed with colloidal gold. Western blotting was performed with the anti-antigen 85 monoclonal antibody TBC-27.
Effect of codon replacement on mRNA expression.
We hypothesized that replacement of low-usage codons enhanced translation of mRNA of the mycobacterial genes. To evaluate the alternative possibility that mRNA production or stability was increased by codon replacement, Northern hybridization was performed. Cultures of the E. coli TOP 10 expression vectors containing either the wild-type or synthetic antigen 85B gene cloned into pTrcHisB were grown to an A600 of 0.6 and induced with IPTG. Total cellular RNA was isolated from 1-ml aliquots from each culture harvested at 0 min, 30 min, 1 h, and 5 h, postinduction. Northern hybridization, using a 594-bp radiolabeled probe amplified from the nonmodified region of the wild-type antigen 85B gene, demonstrated a 1.7- to 2.5-fold increase in antigen 85B mRNA concentration at all time points postinduction for the expression system containing the synthetic gene (Table 3). Thus, the 50-fold increase in recombinant protein production seen by replacing low-usage codons is not explained by increased mRNA production or stability.
TABLE 3.
Relative antigen 85B mRNA concentration in expression systems containing synthetic or wild-type antigen 85B genes
| Time (min)a | 85B mRNA concnb
|
Synthetic/wild-type ratio | |
|---|---|---|---|
| Wild type | Synthetic | ||
| 0 | 1.0c | 0.8 | 0.8 |
| 30 | 5.1 | 12.7 | 2.5 |
| 60 | 6.2 | 12.2 | 2.0 |
| 300 | 7.0 | 12.1 | 1.7 |
Minutes postinduction of expression vector with IPTG.
Based on densitometry measurements.
Value set at 1.
T-cell responses elicited by recombinant antigens 85A and 85B.
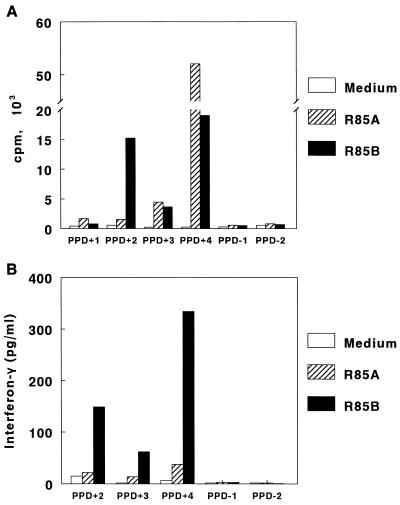
To determine if the recombinant antigens 85A and 85B contained epitopes recognized by human T cells, we cultured PBMC from four healthy subjects with tuberculin reactions with 1 to 100 μg of recombinant antigens 85A and 85B per ml from which endotoxin had been removed. Cells were also cultured in medium alone or with 10 μg of heat-killed M. tuberculosis per ml as controls. The concentration of recombinant antigen that yielded optimal proliferation varied widely in different donors, and the maximal responses are shown in Fig. 4A. Significant responses to antigen 85A were found in two subjects (delta cpm of 4,248 and 51,806, with stimulation indices of 21 and 193, respectively). Proliferative responses to antigen 85B were observed in three subjects (delta cpm of 3,457 to 18,782, with stimulation indices of 17 to 70). PBMC from all subjects proliferated in response to M. tuberculosis, with a delta cpm of 20,854 to 135,319 and stimulation indices of 46 to 497. No significant proliferation was observed in response to antigens 85A and 85B in two healthy tuberculin-negative donors (Fig. 4A).
FIG. 4.
Proliferation (A) and gamma interferon production (B) by PBMC in response to recombinant (R) antigens 85A and 85B. Proliferative responses were assessed in four tuberculin-positive donors (PPD+) and in two tuberculin-negative (PPD−) donors. Gamma interferon concentrations were measured in supernatants of PBMC from three tuberculin-positive donors and two tuberculin-negative donors.
Gamma interferon is critical in protective immunity against tuberculosis. To determine if recombinant antigens 85A and 85B elicited production of gamma interferon by human cells, we measured gamma interferon production by PBMC from healthy tuberculin reactors in response to these recombinant antigens (Fig. 4B). Recombinant antigen 85B elicited production of 62 to 334 pg of gamma interferon per ml. Recombinant antigen 85A induced lower concentrations of gamma interferon (14 to 38 pg/ml). No antigen-induced gamma interferon production was observed in two healthy tuberculin-negative donors.
DISCUSSION
Striking advances have been made in our understanding of the basic biology and immunology of tuberculosis in the past decade, capped by sequencing of the genome of M. tuberculosis (5). These advances will lead to identification of mycobacterial proteins that may be useful vaccine components or diagnostic reagents. However, a critical obstacle is the inability to produce large quantities of these proteins, using a laboratory organism such as E. coli, that does not require stringent containment precautions. We found that selective replacement of E. coli low-usage codons by high-usage codons in mycobacterial genes markedly enhanced production of recombinant mycobacterial proteins in E. coli. These recombinant proteins stimulated proliferation and gamma interferon production by human mononuclear cells and were recognized by monoclonal antibodies to the native mycobacterial proteins, indicating that the recombinant proteins contained T-cell and B-cell epitopes and are likely to be immunogenic. The strategy of selective codon replacement has the potential to greatly facilitate production of mycobacterial proteins for development of vaccines and diagnostic reagents.
Although codon usage preference is a recognized problem in expressing eukaryotic proteins in prokaryotic cells, the utility of codon exchange in expressing prokaryotic proteins in other prokaryotic bacteria has not been explored very well. To our knowledge, the only prokaryotic gene in which codon replacement has been reported to increase recombinant protein yield is the tetanus toxin C fragment (14). In this case, substitution of 280 out of 452 codons (62%) in the open reading frame of tetanus toxin C resulted in a threefold increase in recombinant protein yield. Our finding that only very small amounts of recombinant antigen 85B are produced when its gene is expressed behind trc in E. coli confirms the earlier report of Matsuo et al., who estimated a yield of 0.2 to 0.4 mg/liter (15). Observing that the trc-driven expression of the 85B gene was much less than that of the highly homologous 85A and 85C genes, we hypothesized that the poor expression of 85B might be due to the presence of codons in antigen 85B that are inefficiently translated in E. coli. Two pairs of adjacent least-used codons were identified in the antigen 85B gene, whereas none were observed in either the antigen 85A or 85C genes. Substituting one of them (CGG-CTA) along with three other low-usage codons in the portion of the antigen 85B gene corresponding to the mature extracellular protein increased the yield of recombinant antigen 85B 54-fold, compared to that of the wild-type gene. Furthermore, when this synthetic antigen 85B gene was cloned behind the T7 promoter, more than 250 mg of recombinant protein per liter was produced; this comprised about 50% of the total protein produced by the bacterial culture. Codon substitution also increased production of antigen 85A and SOD four- to sixfold, suggesting that this strategy may be generally applicable to overexpression of mycobacterial genes in E. coli.
Although replacement of low-usage codons increased expression of antigen 85B markedly, it was produced primarily as insoluble inclusions. A urea-based resolubilization protocol, similar to methods used in refolding other recombinant proteins, resulted in production of soluble recombinant antigen 85, which elicited proliferation and gamma interferon production by PBMC from healthy subjects with tuberculin reactions.
It has been postulated that low-usage codons inhibit protein production through ribosome pausing and resultant exposure of RNA to RNA endonuclease activity or rho-dependent RNA polymerase termination (4, 17, 19, 22). In E. coli, as in all prokaryotic organisms, transcription and translation are coupled. Therefore, when a ribosome pauses to find a rare tRNA, the length of exposed mRNA between it and the preceding ribosome or transcribing RNA polymerase lengthens, decreasing stability and production of mRNA. In contrast, our data indicate that increased production of recombinant protein by codon replacement was not due to increased mRNA production or stability, as mRNA concentrations were increased a maximum of 2.5-fold, whereas protein production increased over 50-fold. This suggests that the major detrimental effect of low-usage codons is decreased translational efficiency. When low-usage codons are encountered, the corresponding tRNA is difficult to locate, and the resultant prolonged ribosomal pause may cause ribosomal instability, disassociation of the ribosome-mRNA complex, and termination of protein production.
Codon substitution increased production of recombinant antigen 85B 54-fold but increased production of 85A only 4-fold, even though the same numbers of low-usage codons were replaced. As the antigen 85A and 85B genes contained DNA encoding the same 3-kb fusion protein at the 5′ end just behind the promoter, this marked difference was not due to ribosomal binding or synthesis initiation. The low-usage codons in the antigen 85B gene were adjacent, whereas those in the 85A gene were staggered or isolated, suggesting that adjacent low-usage codons more drastically reduced translational efficiency.
Secreted mycobacterial proteins are believed to be important in engendering protective immune responses, in comparison to cytoplasmic proteins. Harth and colleagues described an overexpression system in which M. smegmatis produced and secreted M. tuberculosis proteins which conferred protective immunity against tuberculosis (10). Our overexpression system yielded proteins that were not exported, and it may be advantageous to develop systems that yield proteins secreted by E. coli, since such proteins may be more likely to mimic the native mycobacterial antigens.
In summary, we demonstrated that selective codon replacement of mycobacterial genes can markedly enhance production of recombinant proteins in E. coli. Widespread application of this strategy has the potential to facilitate production of many mycobacterial proteins for basic research, as well as for development of vaccines and diagnostic reagents.
ACKNOWLEDGMENTS
D.L.L. was supported by the Pediatric Infectious Disease Society through a grant sponsored by Merck, by NIH training grant T 32 AI 07474, and by the Cain Foundation Endowment for Infectious Disease Research. D.S.K. was supported by NIH grant AI35250 and a grant from the Research Service of the Department of Veterans Affairs. K.M.E. was supported by NIH grant AI37871. P.F.B. was supported by NIH grant AI27285 and holds the Margaret E. Byers Cain Chair for Tuberculosis Research.
We thank Thomas Shinnick for providing the HYT27 monoclonal antibody.
REFERENCES
- 1.Anderson P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkins D, al-Ghusein H, Prehaund C, Coates A R. Overproduction and purification of Mycobacterium tuberculosis chaperonin 10. Gene. 1994;150:145–148. doi: 10.1016/0378-1119(94)90874-5. [DOI] [PubMed] [Google Scholar]
- 3.Bloom B R, Fine P E M. The BCG experience: implications for the future vaccines against tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. pp. 531–557. [Google Scholar]
- 4.Cannistrara V J, Subbarao M N, Kennell D. Specific endonucleolytic cleavage sites for decay of Escherichia coli mRNA. J Mol Biol. 1986;192:257–274. doi: 10.1016/0022-2836(86)90363-3. [DOI] [PubMed] [Google Scholar]
- 5.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 6.Content J, de la Cuvellerie A, De Wit L, Vincent-Levy-Frebault V, Ooms J, De Bruyn J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85-C of M. tuberculosis. Infect Immun. 1991;59:3205–3212. doi: 10.1128/iai.59.9.3205-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale J W, Patki A. Mycobacterial gene expression and regulation. In: McFadden J, editor. Molecular biology of the mycobacteria. London, United Kingdom: Surrey University Press; 1990. pp. 173–198. [Google Scholar]
- 8.Gallagher S, Winston S E, Fuller S A, Hurrell J G R. Immunoblotting and immunodetection. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1997. pp. 10.8.1–10.8.21. [Google Scholar]
- 9.Harth G, Lee B-Y, Wang J, Clemens D L, Horwitz M A. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect Immun. 1996;64:3038–3047. doi: 10.1128/iai.64.8.3038-3047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harth G, Lee B Y, Horwitz M A. High-level heterologous expression and secretion in rapidly growing nonpathogenic mycobacteria of four major Mycobacterium tuberculosis extracellular proteins considered to be leading vaccine candidates and drug targets. Infect Immun. 1997;65:2321–2328. doi: 10.1128/iai.65.6.2321-2328.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site- directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz M A, Lee B W E, Dillion B J, Harth G. Protection immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandendussche P, Van Vooren J P, Liu M, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 14.Makoff A J, Oxer M D, Romanos M A, Fairweather N F, Ballantine S. Expression of tetanus toxin fragment C in E. coli: high level expression by removing rare codons. Nucleic Acids Res. 1989;17:10191–10202. doi: 10.1093/nar/17.24.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo K, Yamaguchi R, Yamazaki A, Tasaka H, Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular a antigen. J Bacteriol. 1988;170:3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menéndez M C, Domenech P, Prieto J, Garcia M J. Cloning and expression of the Mycobacterium fortuitum superoxide dismutase gene. FEMS Microbiol Lett. 1995;134:273–278. doi: 10.1111/j.1574-6968.1995.tb07950.x. [DOI] [PubMed] [Google Scholar]
- 17.Nehrke K W, Platt T. A quaternary transcription termination complex: reciprocal stabilization by Rho factor and NusG protein. J Mol Biol. 1994;243:830–893. doi: 10.1006/jmbi.1994.1685. [DOI] [PubMed] [Google Scholar]
- 18.Pal P G, Horwitz M A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platt T. Rho and RNA: models for recognition and response. Mol Microbiol. 1994;11:983–990. doi: 10.1111/j.1365-2958.1994.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 20.Reischl U, Pulz M, Ehret W, Wolf H. PCR-based detection of mycobacteria in sputum samples using a simple and reliable DNA extraction protocol. Biotechniques. 1994;17:844–855. [PubMed] [Google Scholar]
- 21.Salata R A, Sanson A J, Malhotra I J, Wiker H G, Harboe M, Phillips N B, Daniel T M. Purification and characterization of the 30,000 dalton native antigen of Mycobacterium tuberculosis and characterization of six monoclonal antibodies reactive with a major epitope of this antigen. J Lab Clin Med. 1991;118:589–598. [PubMed] [Google Scholar]
- 22.Stanssens P, Remaut E, Fiers W. Inefficient translation causes premature transcription termination in the lacZ gene. Cell. 1986;44:711–718. doi: 10.1016/0092-8674(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 23.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Gong J, Iyer D V, Jones B E, Modlin R L, Barnes P F. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94:2435–2442. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Zubay G, Goldman E. Low-usage codons in Escherichia coli, yeast, fruit fly and primates. Gene. 1991;105:61–72. doi: 10.1016/0378-1119(91)90514-c. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Lathigra R, Garbe T, Catty D, Young D. Genetic analysis of superoxide dismutase, the 23 kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991;5:381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]