Abstract
Antibody (Ab) responses to polysaccharides (PSs) such as Neisseria meningitidis group C PS (MCPS) are characterized as being thymus independent (TI) and are restricted with regard to clonotype and isotype expression. PS conjugated to proteins, e.g., MCPS coupled to tetanus toxoid (MCPS-TT), elicits a thymus-dependent (TD) response. In order to understand the influence of the form of a vaccine (TI versus TD) on the Ab repertoire, we generated monoclonal antibody (MAb) panels from mice immunized and boosted with MCPS or MCPS-TT in different ways. The panels of MAbs were examined for isotype, fine specificity, affinity, and VH gene family usage. The use of MCPS-TT resulted in a shift in the isotype from immunoglobulin M (IgM) and IgG3 elicited in response to the MCPS to primarily IgG1. This isotype shift was accompanied by a change in the fine specificity of the response to the conjugate compared to that of PS. New fine specificities and increased affinity were observed in response to the TD antigen (Ag). Dot blot and Northern analyses of MCPS MAbs revealed that VH gene family usage is dominated by VHJ558, used by 23 of 39 MAbs. VH3609 was seen in three MAbs of restricted fine specificity. VHQ52, VH7183, and VHVGAM3-8 were seen in more than one MAb across these panels, while VH10 and VHX24 were detected only once in response to the TI-2 Ag. All MAbs in the panels utilized kappa light chains, and all functional Jκ genes were expressed.
The capsular polysaccharide (PS) constitutes the major virulence factor of many pathogenic bacteria that cause invasive diseases. Antibodies (Abs) against these PSs are protective (27, 28). PSs are classified as thymus-independent-2 (TI-2) antigens (Ags) because they do not require mature T cells to elicit a humoral response in vivo. These PS Ags are immunogenic in adults but are only poorly or nonimmunogenic in infants and young children who are highly susceptible to infection caused by encapsulated bacteria (28, 31, 44, 65).
The response to capsular PS is markedly different from the response to most protein Ags (thymus-dependent [TD] Ags). The Ab response to TI-2 Ag develops late in ontogeny (25, 44, 50) and in mice utilizes a particular late-developing subset of B cells that is defined by the expression of Lyb5 and other cell markers (39, 60). TI Ags also generally fail to elicit a memory response or show affinity maturation. In contrast, the ability to respond to a TD Ag is present at birth and results in the formation of memory cells, and the Ab response undergoes subsequent affinity maturation upon reimmunization (61). For TI Ag, immunoglobulin G3 (IgG3) and IgM are the major isotypes expressed in mice, even after secondary immunization (45), whereas for TD Ag, the ratio of IgG to IgM increases after secondary immunization, with IgG1 being the major subclass (52, 59, 60).
The majority of the anti-PS responses are oligoclonal and encoded by a few variable regions of the heavy chain (VH) gene families (10). The anti-α(1→3) dextran Ab, for example, expresses mainly the VHJ558 family (68); whereas anti-β(2→1) fructosan Ab predominantly expresses the VHJ606 gene family (11) and Abs to β(2→6) fructosan and β(1→6) galactan express the genes of the VHX24 family (42, 67). The anti-group A streptococcal carbohydrate Ab reflects a germline repertoire that includes at least two VH gene families, one of which belongs to the VHJ606 family paired with several Vκ gene families (46). The response to the glucuronoxylomannan component of the capsular PS of Cryptococcus neoformans serogroup D uses the VHX24 family (13), and immunized mice respond with VH7183 Ab specific for serogroup A (40), indicating the highly restricted usage of VH gene families in anti-PS responses and differences depending on the structure. However, some Ab responses, for example, in the anti-α(1→6) dextran response, were shown to be encoded by the VH genes of the VHJ606, VHJ558, and VH3660 families (3, 57).
The immunogenicity of TI Ag has been shown to be enhanced by covalently binding TI Ag to carrier proteins, thus converting the response to TD (5, 59, 60). Haemophilus influenzae type b (Hib) was once the most common cause of bacterial meningitis in children in the United States, but immunization with TD conjugate vaccines has been remarkably successful in decreasing the incidence of Hib disease (1, 15, 41). These conjugate vaccines have been particularly useful for prevention of Hib infection in high-risk infant populations (30, 53–55). The almost complete disappearance of Hib disease and the reduction in pharyngeal carriage of Hib (6) point out the importance of these conjugate vaccines (6, 15, 62).
Neisseria meningitidis remains one of the major causes of bacterial meningitis in children and young adults worldwide. N. meningitidis PS vaccines have been available for quite some time (29); however, the PS is a TI-2 Ag which is poorly immunogenic in infants and has a short duration of protection in young children (14, 24, 26, 35, 65). The capsular PS, N. meningitidis group C PS (MCPS), is a linear homopolymer of α(2→9)-linked sialic acid residues that are O acetylated at carbons 7 and/or 8 (9, 19). Early murine studies of meningococcal conjugate vaccines showed mainly IgG1 antibodies to PS and carrier after one dose (8) and increased IgG titers after a second dose (17). Our previous studies with mice confirmed and extended these observations (52). Several oligosaccharide-protein conjugate vaccines that elicit a TD response to protect young children against invasive meningococcal disease (17, 32) have been developed and are currently being evaluated in clinical trials (4, 17, 20, 34, 36, 37, 49, 66).
In order to look at the influence of the form of the vaccine (TI versus TD) on the Ab repertoire, we generated two new monoclonal Ab (MAb) panels after primary immunization with MCPS-tetanus toxoid (MCPS-TT) followed by a boost with MCPS or MCPS-TT. Data show that, compared to MCPS, the response to MCPS-TT results in isotype shift, a shift in fine specificity, and increased affinity. The data also show that the secondary Ab repertoire is determined by the primary immunization in that the response to an MCPS booster after MCPS-TT priming resembled the response to MCPS-TT rather than that to MCPS. The results of the isotype, fine specificity, affinity, and VH gene family analyses of the three MAb panels are presented here.
MATERIALS AND METHODS
Animals.
Four-week-old female BALB/cAnN (BALB/c) and pregnant female BALB/cAnN mice were purchased from Charles River Laboratories through the National Institutes of Health Small Animal Section and maintained under pathogen-free conditions in our animal rooms. All animal protocols were approved by the Center for Biologics Evaluation Research Animal Care and Use Committee.
PS.
The MCPS prepared from N. meningitidis C11 was obtained from Merck, Inc., West Point, Pa. (lot 1815T). The structures of the PSs used in these studies are as follows: native MCPS, a homopolymer of α(2→9)-linked sialic acid residues that are O acetylated at carbons 7 and/or 8 (9, 19); OAc−, a naturally occurring non-O-acetylated variant of MCPS; Escherichia coli K92, a homopolymer of alternating α(2→9)- and α(2→8)-linked sialic acid; and E. coli K1, a homopolymer of α(2→8)-linked sialic acid.
Conjugate vaccines.
A group C meningococcal oligosaccharide coupled to TT (also referred to as MCPS-TT) was prepared as previously described (32) and was used for all experiments as described in the work of Rubinstein et al. (52). The molecular mass of the MCPS in the conjugate is 10 kDa (52).
Immunization.
The MCPS MAbs were produced by immunizing 8- to 12-week-old BALB/c mice as indicated previously (51). For the anticonjugate-primed and -boosted (C2) MAb, mice were immunized intraperitoneally with 10 μg of MCPS-TT in 5% Maalox as an adjuvant (modified from reference 48) and then rested for a minimum of 8 weeks, after which they were boosted intravenously with 10 μg of MCPS-TT. Similarly, for the anticonjugate-primed and PS-boosted (CP) MAb, mice were immunized intraperitoneally with 10 μg of MCPS-TT in 5% Maalox and boosted with MCPS in saline or 108 CFU of fixed bacteria (see Table 2, footnote h, for identification).
TABLE 2.
Fine specificities and avidities of MAbs
| MAb | Specificity by FELISA | Specificity by PPTa
|
Concn at 50% bindingb
|
||||
|---|---|---|---|---|---|---|---|
| MCPS | OAc− | K92 | MCPS | OAc− | K92 | ||
| MCPSg | |||||||
| IgM(κ) | |||||||
| 3624.22 | MCPS | + | −c | − | 0.01 | − | −d |
| 1863.5 | MCPS | − | − | − | 0.05 | − | − |
| 2010.10 | OAc− ≥ MCPS >> K92 | + | + | + | 0.025 | 0.03 | >3.0 |
| 1702.10 | OAc− >> MCPS | + | + | − | >3.0 | 0.2 | − |
| 1922.2 | OAc− >> MCPS | − | − | − | 2.0 | 0.08 | − |
| IgG3(κ) | |||||||
| 1705.18 | MCPS | + | − | − | 0.7 | − | − |
| 1846.13 | MCPS | + | − | − | 0.35 | − | − |
| 2055.5 | MCPS | + | − | − | 0.2 | − | − |
| 2750.27 | MCPS | + | − | − | >3.0 | − | − |
| 3006.18 | MCPS | + | − | − | 2.1 | − | − |
| 3079.6 | MCPS > OAc− | + | + | − | 0.3 | 0.8 | − |
| 181.1 | OAc− >>> MCPS | + | + | − | >3.0 | 0.002 | − |
| 2016.3 | OAc− >> MCPS | + | + | − | >3.0 | 0.1 | − |
| IgG1(κ) | |||||||
| 1946.13 | MCPS >>> OAc− | + | − | − | 0.001 | >3.0 | − |
| 177.16 | OAc− > MCPS | + | + | − | 0.03 | 0.004 | − |
| IgG2b(κ) | |||||||
| 78.2 | MCPS | − | − | − | >3.0 | − | − |
| C2 | |||||||
| IgA(κ) | |||||||
| C2/273.7 | OAc− >> MCPSe | − | − | − | 0.02 | 0.015 | − |
| C2/969.3 | OAc− >> MCPSe | + | + | − | 0.09 | 0.015 | − |
| IgG1(κ) | |||||||
| C2/205.10 | MCPS >>> OAc− | + | − | − | 0.003 | 1.0 | − |
| C2/951.8 | MCPS > OAc− | + | − | − | 0.006 | 0.05 | − |
| C2/630.10 | MCPS > OAc− | + | + | − | 0.003 | 0.03 | − |
| C2/735.4 | MCPS > OAc− | + | − | − | 0.005 | 0.09 | − |
| C2/35.3 | MCPS ≈ OAc− | + | + | − | 0.004 | 0.005 | − |
| C2/233.2 | MCPS ≈ OAc− | − | − | − | 0.15 | 0.5 | − |
| C2/256.8 | MCPS ≈ OAc− | + | + | − | 0.005 | 0.003 | − |
| C2/998.3 | MCPS ≈ OAc− | + | + | − | 0.003 | 0.003 | − |
| C2/974.1 | MCPS ≈ OAc− >>> K92 | + | + | −f | 0.002 | 0.003 | 5.0 |
| C2/1076.10 | MCPS ≈ OAc− >>> K92 | + | + | −f | 0.005 | 0.002 | 1.5 |
| C2/655.7 | OAc− >> MCPS | + | + | − | 0.3 | 0.003 | − |
| C2/181.7 | OAc− > MCPS | + | + | − | 0.1 | 0.01 | − |
| C2/706.12 | OAc− > MCPS >>> K92 | + | + | −f | 0.02 | 0.003 | 3.0 |
| CP | |||||||
| IgG1(κ) | |||||||
| CP1049.19 | MCPS ≈ OAc− | + | − | − | 0.25 | 0.12 | − |
| CP875.2h | MCPS ≈ OAc− | + | + | − | 0.005 | 0.0015 | − |
| CP882.2h | MCPS ≈ OAc− | − | − | − | 0.002 | 0.0015 | − |
| CP947.6h | MCPS ≈ OAc− | + | + | − | 0.007 | 0.002 | − |
| CP1092.23h | MCPS ≈ OAc− | + | + | − | 0.007 | 0.003 | − |
| CP1160.12h | MCPS ≈ OAc− | + | + | − | 0.007 | 0.003 | − |
| CP163.6h | OAc− >> MCPS | + | + | − | 0.55 | 0.0015 | − |
| IgG2b(κ) | |||||||
| CP1050.20 | MCPS > OAc− | + | − | − | 0.0025 | 0.015 | − |
Determined by Ouchterlony precipitation (PPT). Proteins were tested at 1 mg/ml.
Proteins were tested by 12 threefold serial dilutions starting at 3 μg/ml. Values are the concentrations at 50% binding in micrograms per milliliter determined at the midpoint of the linear part of the titration curve on PS-coated plates.
Not detectable at highest concentration tested (1 mg/ml).
Not detectable at highest concentration tested (3 μg/ml).
Determined by ascites fluid diluted 1:100.
Negative with the purified protein, but undiluted ascites was positive on all three Ags and negative on K1.
The fine specificity and precipitation values with MCPS and OAc− (for 15 of 16) and the concentrations at 50% binding (for 7 of 16) MCPS MAbs were previously published (51) and are reproduced with the permission of The Journal of Immunology.
These hybridomas were from mice boosted with fixed bacteria. All other CP MAbs came from mice boosted with purified MCPS.
Hybridoma production and MAb purification.
Spleens were removed 3 days after the last injection. Fusions were performed with the nonsecreting myeloma cell line SP2/0, according to the protocol of Kennett (33) as modified by Rubinstein and Stein (51). Supernatants from wells containing growing hybrids were assayed by fluorescence enzyme-linked immunosorbent assay (FELISA) and selected on the basis of reactivity with MCPS and no reactivity on a chemically similar but non-cross-reactive PS, E. coli K1. Positive cells were expanded; tested on MCPS, OAc−, K92, and K1 to confirm specificity; and cloned by limiting dilution. All IgM MAbs were purified on an anti-mouse κ-Sepharose column (187.1 rat anti-mouse κ cell line [ATCC HB58]) from the nonbinding fraction of ascites that had been passed over a protein A-Sepharose column (Pharmacia Biotech Inc., Piscataway, N.J.). All IgG MAbs were purified from ascites fluid on a protein A-Sepharose column (Pharmacia) or a protein A/G or protein G column (Pierce, Rockford, Ill.). The IgA-secreting hybridomas were grown in Ultradoma protein-free medium (Biowhittaker, Walkersville, Md.) and purified from Amicon-filtered concentrates (Amicon, Beverly, Mass.) with a fast protein liquid chromatography Q-Sepharose column (Pharmacia, Uppsala, Sweden) followed by an anti-mouse κ-Sepharose column. All purified MAbs were shown to be >97% pure by immunoelectrophoresis. In addition, all IgG MAbs were clonally distinct by isoelectric focusing.
Characterization of the MAbs.
The MAbs were characterized by Ouchterlony precipitation (43) at a concentration of 1 mg/ml for both MAb and PS, isoelectric focusing (58), and a direct binding in FELISA. Isotypes were determined by FELISA (50) with supernatants from the clones assayed on Ag-coated plates and developed with alkaline phosphatase-labeled anti-isotype reagents purchased from Southern Biotechnology (Birmingham, Ala.).
FELISA.
The MCPS MAb FELISA has been described in detail elsewhere (50). Titers are expressed as reciprocal dilutions, determined by extrapolation to zero from the linear part of the titration curve. The proteins were tested by 12 threefold serial dilutions starting at an Ab concentration of 3 μg/ml. An ≈ symbol is defined as approximately equal reactivity on MCPS, OAc−, and K92. > is defined as an approximately 0.5- to 1-log-lower concentration for 50% binding, >> is defined as a 2-log-lower concentration for 50% binding, and >>> is defined as a 3-log-lower concentration for 50% binding. The FELISA is similar to a conventional ELISA; however, it has three unique features. The Microfluor “W” U plates (Dynatech Laboratories, Chantilly, Va.) are opaque, and PSs adhere well; the substrate, 4-methylumbelliferyl phosphate (Sigma Chemical Co., St. Louis, Mo.), is not hydrolyzed in water, resulting in a background that is stable over time; and the scale on the Microfluor reader (Dynatech) is 0 to 4 rather than 0 to 2.
RNA preparation, dot blot, and Northern blot analysis.
Total RNA isolated from hybridoma cells was prepared by guanidinium thiocyanate lysis and cesium chloride purification essentially as described by Chirgwin et al. (16). mRNAs were isolated from hybridoma cells by using the Fast Track mRNA isolation kit (Invitrogen, Carlsbad, Calif.) following the manufacturer's directions. All RNA solutions were made in diethyl pyrocarbonate-treated H2O (Research Genetics, Huntsville, Ala.). RNA dot blots of 40 μg of total RNA were performed as indicated by Boswell et al. (11) with nitrocellulose membranes (Schleicher & Schuell, Inc., Keene, N.H.) on a Minifold I apparatus (Schleicher & Schuell). The filters were UV cross-linked while damp, two times, with 1,200 μJ in a Stratalinker (Stratagene, La Jolla, Calif.). Membranes were prehybridized for 4 h at 68°C followed by hybridization for 20 h at 68°C in fresh prehybridization buffer with 32P-labeled DNA probes added to a final concentration of 2 × 106 to 5 × 106 cpm/ml as described by Boswell et al. (11).
Total RNAs (40 μg) for Northern blots were fractionated through 1.2% agarose gels containing formaldehyde, transferred to Nytran membranes (Schleicher & Schuell), and UV cross-linked as before. Blots were prehybridized in buffer containing 5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]), 2× Denhardt's solution, 10 μg of salmon sperm DNA per ml, 0.1% sodium dodecyl sulfate, and 50% formamide for 2 h. Hybridization was in fresh prehybridization buffer with 32P-labeled DNA probes for 18 h at 42°C. Blots were washed twice at 42°C in 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate for 10 min/wash and twice in 0.2× SSC at 65°C for 30 min. Filters were then exposed to X-ray film at −70°C with an intensifying screen (Sigma) for 24 h or placed on a PhosphorImager screen (Molecular Dynamics, Sunnyvale, Calif.) for 8 h.
Hybridization probes.
The hybridization probes for VH gene family assignments used in this study were agarose gel-purified DNA fragments passed through ELUTIP-d columns (Schleicher & Schuell) following the manufacturer's directions. CH probes were isolated as indicated for VH probes. All probes are the same as those used by S. H. Feng (22) and C. Boswell (11) in our laboratory. DNA probes were labeled (21) with [α-32P]dCTP with the Amersham Corp. random primer kit (Arlington Heights, Ill.) following the manufacturer's directions. Blots were stripped and rehybridized with CH region DNA probes after VH hybridization to confirm the presence of Ig RNA in all samples. The control myeloma and MAb cell lines for the different VH gene family probes have been described previously (11, 12).
Our approach to the molecular analysis of the VH and VL gene usage in the three panels of MAbs was to prepare total RNA from the cells and use specific V region probes to determine the V gene families by dot blot and Northern blot analysis (16). For the VH determination, this approach was quite successful, and VH families of all 39 MAbs were determined. This approach was not useful in determining Vκ family usage because, in general, Vκ families have more homology in sequence to each other than VH families, making Vκ family analyses by probe hybridization less reliable (64).
Primer extension of mRNA.
mRNA was used as a template for synthesis of full-length cDNA from transcribed VL genes as described by Shlomchik et al. (56). Briefly, the 5′ ends of oligonucleotide Jk primers were labeled with 32P by using polynucleotide kinase (New England Biolabs, Beverly, Mass.) and [γ-32P]dATP (Amersham). The labeled primers were mixed with 1.5 μg of mRNA, and cDNA was synthesized by avian myeloblastosis virus reverse transcriptase (Stratagene) at 42°C for 45 min in the presence of excess deoxyribonucleic triphosphates (Pharmacia), followed by an incubation with terminal deoxynucleotidyl transferase (Stratagene) at 37°C for 30 min, and run in a 5% polyacrylamide gel.
Statistical analysis.
Student's t test was used to compare the affinities of the three panels of MAbs to MCPS.
RESULTS
We previously described a panel of 15 MAbs generated against the TI form of MCPS (51). In order to investigate the influence of the form of the Ag (i.e., TI-2 versus TD) on the Ab response, we produced two additional panels of MAbs. The first new panel was generated by immunizing mice twice with MCPS-TT (referred to as C2 MAb, for conjugate twice), and the second panel, designed to mimic the situation in human infants who might encounter an encapsulated organism following a single immunization with a conjugate vaccine, was generated from mice immunized with MCPS-TT followed by immunization with MCPS (referred to as CP MAb for conjugate primed and PS or encapsulated fixed bacteria boosted).
Isotype distribution.
The MCPS MAbs are primarily of the IgG3 and IgM isotypes with a small percentage of IgG1 and IgG2b (51) whereas both the C2 MAbs and CP MAbs are mainly IgG1, 87% (13 of 15) and 88% (7 of 8), respectively. Two IgA MAbs, 13% (2 of 15), and one IgG2b MAb, 12% (1 of 8), were observed in the C2 and CP MAb responses, respectively. No IgM or IgG3 MAbs were recovered from the response to conjugate vaccine. No IgG2a responses were observed with any form of immunization, and all MAbs from each panel used a kappa light chain.
Analysis of MCPS and MCPS-TT MAb fine specificity.
IgA, IgG, and IgM MAbs were purified and tested for fine specificity by a quantitative measure of binding to purified MCPS, OAc−, or K92 PS in a FELISA. Table 1 summarizes the differences in the patterns of fine specificity seen among the panels of MAbs. The titer of each MAb was determined on MCPS-, OAc−-, and K92-coated plates, and the fine specificity listed is based on the relative titers with each of the Ags. Major fine specificity differences were seen in these panels. As reported earlier, the MCPS MAbs are predominantly of two specificities. Half of these are MCPS specific, with a majority of the others having a higher titer on OAc− than on MCPS (OAc− > MCPS) (51). The C2 MAbs do not exhibit a pronounced dominance of any one specificity, and interestingly, none are specific for native MCPS. Three of these also bound K92, suggesting that the C2 MAbs were primarily seeing the α(2→9)-linked sialic acid backbone. A new specificity not seen in the MCPS MAb panel was found in the C2 MAb, with approximately the same reactivity on MCPS and OAc− (MCPS ≈ OAc−). The CP MAb panel also lacked the specificity for native MCPS, but the MAbs were more limited in their fine specificity than the C2 panel, with six of eight showing equal reactivity with MCPS and OAc−. Most of the CP MAbs were of this fine specificity, suggesting that this panel derived from a population of cells primed by conjugate.
TABLE 1.
Summary of specificities for each MAb panel
| Specificity | No. of MAbsd
|
||
|---|---|---|---|
| MCPSa (n = 16) | C2b (n = 15) | CPc (n = 8) | |
| MCPS | 8 | 0 | 0 |
| MCPS > OAc− | 2 | 4 | 1 |
| MCPS ≈ OAc− | 0 | 4 | 6 |
| OAc− > MCPS | 5 | 4 | 1 |
| OAc− ≈ MCPS > K92 | 1 | 3 | 0 |
Hyperimmunized with PS-encapsulated bacteria.
Primed and boosted with MCPS-TT.
Primed with MCPS-TT and boosted with MCPS or PS-encapsulated bacteria.
n = total number of MAbs in panel.
Analysis of MAb affinity.
The purified proteins were also tested for affinity by a quantitative measure of binding to purified PS in the FELISA. The distribution of fine specificities among the three panels of MAbs, expressed as the concentration for 50% binding ([50%]), is shown in Table 2. Reported relative affinities are the average of nine values from triplicate readings per assay for each MAb, tested in three separate assays. As seen in Table 2, MAbs fell into two categories: low-avidity binders (arbitrarily defined as the [50%] binding to PS of ≥0.1 μg/ml) and high-avidity binders (defined as the [50%] binding to PS of <0.1 μg/ml). To assess the overall influence of the Ag used for immunization on the affinity of the purified Abs, the mean values of binding to MCPS and OAc− were determined for each panel. The mean [50%] binding by the MCPS MAb to MCPS was 1.29 μg/ml (range, 0.01 to >3 μg/ml) and to OAc− was 0.527 μg/ml (range, 0.002 to >3 μg/ml). In contrast, the mean [50%] binding by C2 MAb to MCPS was 0.048 μg/ml (range, 0.002 to 0.3 μg/ml) and to OAc− was 0.115 μg/ml (range, 0.002 to 1 μg/ml). The mean [50%] binding by CP MAb to MCPS was 0.104 μg/ml (range, 0.002 to 0.55 μg/ml) and to OAc− was 0.018 μg/ml (range, 0.0025 to 0.12 μg/ml). The MAbs of both the C2 and CP panels had 1- to 2-orders-of-magnitude-lower [50%] binding to MCPS and OAc−, indicating that the MAbs from mice primed with the TD Ag were of significantly higher affinity than the MAbs from mice primed with the TI Ag regardless of whether the booster was TD or TI (P < 0.002 for binding to MCPS for C2 versus MCPS MAb and P < 0.003 for binding to MCPS for CP versus MCPS MAb). Comparisons of C2 and CP MAb binding to OAc− versus MCPS MAb binding to OAc− were not significant.
Also shown in Table 2 is the ability of the MAb to precipitate PS in gel (Ouchterlony analysis), which correlated well with the specificity determined by quantitative FELISA. It is of note that of the four MAbs that bound K92, only the MCPS MAb IgM (2010.10) precipitated K92 PS. Thus, the Ouchterlony technique remains a useful method for determining the fine specificity of anti-PS MAbs. Only three MAbs of the C2 or CP MAb panels did not precipitate Ag. The inability to precipitate Ag was unrelated to the affinity or the Ig class of the MAb, as the three nonprecipitating Abs were of the IgG1 or IgA isotypes.
VH gene family usage.
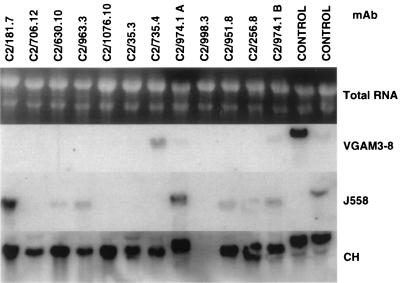
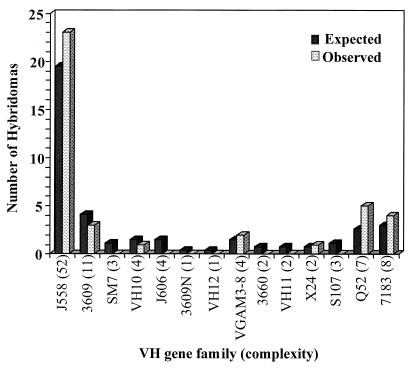
Because different fine specificities were generated against MCPS and MCPS-TT, it was of interest to explore the diversity of these Abs at the gene level. Panels of MAbs were analyzed first by dot blot or by Northern blot analysis, with 14 VH family DNA probes. All MAbs were assigned to VH families by independent testing with VH family probes and verification of the presence of Ig RNA with a C region probe. A typical example of Northern blot analysis is shown in Fig. 1. Dot blot and Northern blot analyses suggest that the VH gene family usage in three panels of MAbs stimulated by either MCPS or MCPS-TT is dominated by VHJ558, the largest VH gene family. Whether this reflects a random distribution or a restriction to VHJ558 cannot be determined without knowledge of individual gene usage. It is of note, however, that among the 39 MAbs, 7 of 14 VH families examined were not expressed. All families with four or more genes were expressed, except VHJ606, which was not expressed in any of the panels (Table 3 and Fig. 2). VHJ558 was found in 8 of 16 MCPS TI-2 MAbs and 15 of 23 MCPS-TT TD MAbs (both C2 and CP) (Table 3). VHJ558 is represented in all fine specificity groups (Table 3), although in only one (C2/974.1) of four MAbs (2010.10, C2/706.12, C2/974.1, and C2/1076.10) cross-reactive with K92 (Table 3). The VH3609 family was limited to the MCPS MAb panel and a single fine specificity while VH7183 and VHVGAM3-8 were limited to the C2 and CP MAb panels. Also presented in the table are the fusion number or letter and isoelectric point (pI) so that clonal relatedness can be ruled out, if possible. For example, two of the MAbs utilizing the VH7183 gene family cross-react with K92, but these MAbs were derived from different fusions; therefore, they arose independently (Table 3). Although MAbs 1702.10 and 1922.2 are from the same fusion and have the same fine specificity, sequence data indicate that these MAbs utilize different members of the VHQ52 gene family (P. A. García-Ojeda et al., unpublished data).
FIG. 1.
Examples of Northern blot hybridization of total RNA of the C2 MAb panel with VH probes for 2 of 15 murine VH gene families and CH probes for heavy chain constant region. Ethidium bromide staining of total RNA was included in the top scan followed by autoradiography of MAbs and positive controls under them.
TABLE 3.
Gene usage by fine specificities for each MAb panel
| MAb | Specificity by FELISA | Fusion | pIa | VH gene |
|---|---|---|---|---|
| 181.1 | OAc− >>> MCPS | 1 | 7.2–7.9 | VH10 |
| 1702.10 | OAc− >> MCPS | 2 | ND | Q52 |
| 1922.2 | OAc− >> MCPS | 2 | ND | Q52 |
| 2016.3 | OAc− >> MCPS | 3 | 8.3–8.6 | J558 |
| C2/273.7 | OAc− >> MCPS | B | ND | J558 |
| C2/655.7 | OAc− >> MCPS | C | 5.6–6.7 | J558 |
| C2/969.3 | OAc− >> MCPS | E | ND | J558 |
| CP163.6 | OAc− >> MCPS | 1B | 7.1–7.6 | J558 |
| 177.16 | OAc− > MCPS | 1 | 6.8–7.2 | J558 |
| C2/181.7 | OAc− > MCPS | B | 6.7–7.6 | J558 |
| 1946.13 | MCPS >>> OAc− | 2 | 6.7–7.2 | J558 |
| C2/205.10 | MCPS >>> OAc− | B | 6.7–7.0 | Q52 |
| 3079.6 | MCPS > OAc− | 6 | 8.2–8.3 | Q52 |
| C2/630.10 | MCPS > OAc− | C | 6.3–6.7 | J558 |
| C2/735.4 | MCPS > OAc− | D | 6.7–7.1 | VGAM3-8 |
| C2/951.8 | MCPS > OAc− | E | 6.7–7.3 | J558 |
| CP1050.20 | MCPS > OAc− | 3A | 6.9–7.3 | J558 |
| C2/35.3 | MCPS ≈ OAc− | A | 7.0–7.4 | 7183 |
| C2/233.2 | MCPS ≈ OAc− | B | 6.1–6.4 | Q52 |
| C2/256.8 | MCPS ≈ OAc− | B | 6.7–7.0 | J558 |
| C2/998.3 | MCPS ≈ OAc− | E | 6.2–6.5 | J558 |
| CP882.2 | MCPS ≈ OAc− | 2B | 6.8–7.1 | 7183 |
| CP875.2 | MCPS ≈ OAc− | 2B | 6.9–7.2 | J558 |
| CP947.6 | MCPS ≈ OAc− | 2B | 7.0–7.5 | J558 |
| CP1049.19 | MCPS ≈ OAc− | 3A | 6.1–7.2 | VGAM3-8 |
| CP1160.12 | MCPS ≈ OAc− | 4B | 6.8–7.5 | J558 |
| CP1092.23 | MCPS ≈ OAc− | 4B | 6.9–7.5 | J558 |
| 3624.22 | MCPS | 7 | ND | 3609 |
| 2750.27 | MCPS | 4 | 7.6–8.1 | 3609 |
| 78.2 | MCPS | 1 | 6.1–6.8 | 3609 |
| 1705.18 | MCPS | 2 | 7.9–8.3 | J558 |
| 1846.13 | MCPS | 2 | 8.1–8.3 | J558 |
| 1863.5 | MCPS | 2 | ND | J558 |
| 2055.5 | MCPS | 3 | 8.4–8.8 | J558 |
| 3006.18 | MCPS | 5 | 7.6–8.1 | J558 |
| 2010.10 | OAc− ≥ MCPS >> K92 | 3 | ND | X24 |
| C2/706.12 | OAc− > MCPS >>> K92 | D | 6.4–7.6 | 7183 |
| C2/974.1 | MCPS ≈ OAc− >>> K92 | E | 6.4–6.9 | J558 |
| C2/1076.10 | MCPS ≈ OAc− >>> K92 | E | 6.4–6.8 | 7183 |
pI data for the MCPS MAbs are from reference 51 and are reproduced with the permission of The Journal of Immunology. ND, not determined.
FIG. 2.
VH gene family usage in MAbs against MCPS and MCPS-TT in BALB/c mice. The expected frequencies were derived from the estimated number of VH genes per family (complexity) in the germ line (R. Riblet, presentation to the American Association of Immunologists, San Francisco, Calif., 1997, with permission).
VL gene family usage.
All MAbs in the three panels utilized κ light chains. Examination of Jκ region utilization indicates that, in 32 of 39 MAbs tested, all functional Jκ genes are expressed (data not shown). Preliminary analysis of VL genes from the MCPS MAbs revealed that the VκOx1 light chain of the Vκ4/5 family was the VL most commonly paired with the VHJ558 genes (P. A. García-Ojeda et al., unpublished data). Also, some VH-VL pairs are seen in this response in a restricted fashion. For example, a VH3609-Vκ23 combination (P. A. García-Ojeda et al., unpublished data) in MAbs 78.2, 2750.27, and 3624.22 correlates with reactivity to native MCPS. This fine specificity was seen only with the MCPS MAb panel, not with the C2 or CP MAb panels (Tables 1 and 3).
DISCUSSION
We have shown previously that the immune response to MCPS (a TI-2 Ag) in BALB/c mice provides a model system which closely parallels the response in humans (50). The primary response induced mostly Abs with IgG3 and IgM isotypes, and the secondary immunization with MCPS was similar to the primary immunization, typical of a TI-2 response (52, 61). In contrast, the response to MCPS-TT shifted the response to IgG1 Abs with bactericidal activity at least 10-fold higher than the response to MCPS. This response was maintained when boosted by either MCPS or MCPS-TT (52). IgG1 memory B cells against the capsular PS of meningococcal group C were observed in the MCPS-TT-primed mice as shown in adoptive transfer experiments, and this response could be boosted by either MCPS or MCPS-TT in the absence of T cells (52). The data demonstrated that the influence of the TD Ag during the primary immunization is to induce class switching and generate a memory B-cell population that can be boosted by either a TI-2 or a TD Ag (52).
Previously a panel of MAbs generated against the TI-2 form of the Ag, MCPS (51), was described. To expand these data to conjugate vaccines, we describe here two additional panels of MAbs generated against the TD form, MCPS-TT, and boosted with either MCPS-TT or MCPS. An analysis of these panels shows that increases in Ab diversity and affinity are additional features of the response to TD Ag priming.
Previously, we showed that MAbs generated in response to MCPS were largely restricted to two fine specificities (51) and were primarily of the IgG3 and IgM isotypes. Half of these MAbs are native MCPS specific, requiring the OAc groups, with most of the remaining MAbs reacting better with the OAc− Ag than with MCPS. The MCPS-TT MAbs are mainly IgG1, consistent with the serum Ab data (52). The conjugate response (C2 and CP panels) generated a new fine specificity (MCPS ≈ OAc−) without a pronounced dominance of any one specificity, and none of the MCPS-TT MAbs were native MCPS specific. In the CP MAb panel, there was a predominance of the MCPS ≈ OAc−, a specificity seen in the C2 but not the MCPS panel, reinforcing the concept that the secondary Ab repertoire is determined by the primary immunization.
The native MCPS has very few non-O-acetylated sialic acid residues, with an average of 1.16 equivalents of O-acetyl per sialic acid (9). In the MCPS MAb panel, 7 of 16 MAbs bound to both MCPS and OAc−, and of these, five bound 1 to 2 orders of magnitude better to OAc− than to MCPS (Table 2) (51). All the C2 and CP MAbs also bound both Ags; however, most reacted equally well on both MCPS and OAc−. These data are consistent with the finding of Glode et al. (23) that immunization with native MCPS elicits Abs that are bactericidal for both C11 (native O-acetylated) and MC19 (OAc−) strains of N. meningitidis and that MC19 PS could absorb a large part of the bactericidal activity for strain C11.
The affinity of the MCPS-TT Abs is 10- to 100-fold higher than that of the MCPS Abs. Whether this increase in Ab affinity is due to somatic mutations (7) is currently under investigation (P. A. García-Ojeda et al., unpublished data). The data show, moreover, that, on the whole, the IgG3 Abs were of lower affinity than were the IgG1 Abs. Ab affinity may be an important determinant of host defense and should be considered as important as Ab concentration in evaluating Ab response to vaccination (63). Although we have not correlated the Ab affinity of our MAb with in vivo protection, Ahlstedt et al. (2) demonstrated that high-avidity Ab against E. coli O Ag was more protective against intraperitoneal infection in mice than was Ab of low avidity.
We examined the VH gene families utilized by MAbs derived from the various immunization schemes in order to determine the relationship between the type of antigenic stimulation and fine specificities with VH gene family usage. The primary Ab response in fetal mice is biased toward VH family members that lie proximal to the DH locus (47, 69), while in adult mice this bias disappears and the naive repertoire correlates with the size of the VH gene family (18). The majority of anti-PS responses are encoded by a few VH gene families, and neither chromosomal position nor family size seems important (10). The anti-α(1→3) dextran Abs draw their specificity from restricted VH gene family usage, mainly the VHJ558 family (68), while the α(1→6) dextran Abs are encoded by a variety of VH and VL genes (3, 57). Examining the diversity of the MAbs at the gene level indicates that the response to α(2→9)-linked sialic acid is dominated by VHJ558, the largest VH gene family. Among 39 MAbs, more than half (58%) are VHJ558. Nearly half of the MCPS MAbs used VHQ52 and VH3609. VH3609 is the second largest VH gene family, as recently reported by R. Riblet (presentation to the American Association of Immunologists, 1997; R. Riblet, personal communication).
The MCPS-TT MAb panel utilizes two VH genes, VH7183 and VHVGAM3-8, not utilized by the MCPS MAb panel. The shift in gene usage could be a consequence of the recruitment of T cells by the MCPS-TT MAb-producing cells or by the TD Ag. Stein et al. (59) showed that IM6-keyhole limpet hemocyanin stimulated additional anti-α(1→6) dextran clones that were not seen after immunization with dextran B512. Similar results have been observed by Matsuda and Kabat (38), where the immune response to the TI form of the Ag used different V genes than those responding to the TD form (38).
Two of the MAbs utilizing the VH7183 gene family cross-react with K92. This fine specificity was also observed for one MCPS MAb. It is of interest that three different gene families (VHX24, VHJ558, and VH7183) are used among four MAbs that cross-react with K92 (Table 3). These data suggest that there may be a variety of combining sites that can accommodate K92; however, definitive conclusions cannot be reached until the sequencing and molecular modeling studies of these MAbs are completed (P. A. García-Ojeda et al., unpublished data).
All MAbs in the three panels utilized members of kappa light chains. Although both the VH and Vκ families are made up of multiple gene families, anti-PS responses typically are characterized by a limited usage of V genes and particularly by restricted pairing of VH genes with Vκ or Vλ genes (10). With the exception of VH3609, no other correlation of VH gene usage and fine specificity was observed in our panels. Akolkar et al. (3) reported that even when MAbs specific for α(1→6) dextran having very similar properties (in terms of size, shape, fine structure, and binding constants in their combining sites) were examined, six different combinations of VH and VL genes were found.
In conclusion, the data show that the use of a TD form of MCPS, compared to the TI form, results in an isotype shift, a change in fine specificity, and an increase in affinity. The MCPS MAbs are primarily of the IgG3 and IgM isotypes, whereas the MCPS-TT MAbs are mainly IgG1, consistent with the serum Ab data (52). The responses to both the TI MCPS and the TD MCPS-TT are dominated by VHJ558, and the affinity of the MCPS-TT Abs is 10- to 100-fold higher than that of the MCPS Abs. Sequencing studies are being conducted to examine the specific VH genes within the VHJ558 family and to determine whether specific gene usage correlates with fine specificity and affinity.
ACKNOWLEDGMENTS
We thank Kurt Brorson and Lucio Miele for technical advice in the isolation of the IgA MAbs, Datsen George Wei and Anita Cywinski for technical help, and Leslie Shelly for materials supplied. We also thank Marjorie Shapiro and Carolyn Deal for critical review of the manuscript.
REFERENCES
- 1.Adams W G, Deaver K A, Cochi S L, Plikaytis B D, Zell E R, Broome C V, Wenger J D. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA. 1993;269:221–226. [PubMed] [Google Scholar]
- 2.Ahlstedt S, Holmgren J, Hanson L A. Protective capacity of antibodies against E. coli O antigen with special reference to the avidity. Int Arch Allergy Appl Immunol. 1974;46:470–480. doi: 10.1159/000231150. [DOI] [PubMed] [Google Scholar]
- 3.Akolkar P N, Sikder S K, Bhattacharya S B, Liao J, Gruezo F, Morrison S L, Kabat E A. Different VL and VH germ-line genes are used to produce similar combining sites with specificity for α(1→6) dextrans. J Immunol. 1987;138:4472–4479. [PubMed] [Google Scholar]
- 4.Anderson E L, Bowers T, Mink C M, Kennedy D J, Belshe R B, Harakeh H, Pais L, Holder P, Carlone G M. Safety and immunogenicity of meningococcal A and C polysaccharide conjugate vaccine in adults. Infect Immun. 1994;62:3391–3395. doi: 10.1128/iai.62.8.3391-3395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avery O T, Goebel W F. Chemo-immunological studies on conjugated carbohydrate-proteins. V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of type III pneumococcus with foreign protein. J Exp Med. 1931;54:437–447. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour M L. Conjugate vaccines and the carriage of Haemophilus influenzae type b. Emerg Infect Dis. 1996;2:176–182. doi: 10.3201/eid0203.960303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berek C, Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 8.Beuvery E C, van Delft R W, Miedema F, Kanhai V, Nagel J. Immunological evaluation of meningococcal group C polysaccharide-tetanus toxoid conjugate in mice. Infect Immun. 1983;41:609–617. doi: 10.1128/iai.41.2.609-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharjee A K, Jennings H J, Kenny C P, Martin A, Smith I C P. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroup B and C with carbon 13 nuclear magnetic resonance. J Biol Chem. 1975;250:1926–1932. [PubMed] [Google Scholar]
- 10.Bona C. Molecular characteristics of anti-polysaccharide antibodies. Semin Immunopathol. 1993;15:103–118. doi: 10.1007/BF00201095. [DOI] [PubMed] [Google Scholar]
- 11.Boswell C M, Irwin D C, Goodnight J, Stein K E. Strain-dependent restricted VH and VL usage by anti-bacterial levan monoclonal antibodies. J Immunol. 1992;148:3864–3872. [PubMed] [Google Scholar]
- 12.Boswell C M, Stein K E. Avidity maturation, repertoire shift, and strain differences in antibodies to bacterial levan, a type 2 thymus-independent polysaccharide antigen. J Immunol. 1996;157:1996–2005. [PubMed] [Google Scholar]
- 13.Casadevall A, Scharff M D. The mouse antibody response to infection with Cryptococcus neoformans: VH and VL usage in polysaccharide binding antibodies. J Exp Med. 1991;174:151–160. doi: 10.1084/jem.174.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceesay S J, Allen S J, Menon A, Todd J E, Cham K, Carlone G M, Turner S H, Gheesling L L, DeWitt W, Plikaytis B D, Greenwood B. Decline in meningococcal antibody levels in African children 5 years after vaccination and the lack of an effect of booster immunization. J Infect Dis. 1993;167:1212–1216. doi: 10.1093/infdis/167.5.1212. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Achievements in public health, 1900–1999: impact of vaccines universally recommended for children—United States, 1900–1998. Morbid Mortal Weekly Rep. 1999;48:243–248. [PubMed] [Google Scholar]
- 16.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 17.Costantino P, Viti S, Podda A, Velmonte M A, Nencioni L, Rappuoli R. Development and phase 1 clinical testing of a conjugate vaccine against meningococcus A and C. Vaccine. 1992;10:691–698. doi: 10.1016/0264-410x(92)90091-w. [DOI] [PubMed] [Google Scholar]
- 18.Dildrop R, Krawinkel U, Winter E, Rajewsky K. VH gene expression in murine lipopolysaccharide blasts distributes over the nine known VH gene groups and may be random. Eur J Immunol. 1985;15:1154–1156. doi: 10.1002/eji.1830151117. [DOI] [PubMed] [Google Scholar]
- 19.Egan W. Structure of the capsular polysaccharide antigens from Haemophilus influenzae and Neisseria meningitidis by 13C NMR spectroscopy. In: Cohen J S, editor. magnetic resonance in biology. Vol. 1. New York, N.Y: John Wiley & Sons; 1980. pp. 197–258. [Google Scholar]
- 20.Fairley C K, Begg N, Borrow R, Fox A J, Jones D M, Cartwright K. Conjugate meningococcal serogroup A and C vaccine: reactogenicity and immunogenicity in United Kingdom infants. J Infect Dis. 1996;174:1360–1363. doi: 10.1093/infdis/174.6.1360. [DOI] [PubMed] [Google Scholar]
- 21.Feinberg A P, Volgerstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 22.Feng S H, Stein K E. VH gene family expression in mice with the xid defect. J Exp Med. 1991;174:45–51. doi: 10.1084/jem.174.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glode M P, Lewin E B, Sutton A, Le C T, Gotschlich E C, Robbins J B. Comparative immunogenicity of vaccines prepared from capsular polysaccharides of group C Neisseria meningitidis O-acetyl-positive and O-acetyl-negative variants and Escherichia coli K92 in adult volunteers. J Infect Dis. 1979;139:52–59. doi: 10.1093/infdis/139.1.52. [DOI] [PubMed] [Google Scholar]
- 24.Gold R, Lepow M L, Goldschneider I, Draper T L, Gotschlich E C. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J Clin Investig. 1975;56:1536–1547. doi: 10.1172/JCI108235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold R, Lepow M L, Goldschneider I, Gotschlich E C. Immune response of human infants to polysaccharide vaccines of group A and C Neisseria meningitidis. J Infect Dis. 1977;136(Suppl.):S31–S35. doi: 10.1093/infdis/136.supplement.s31. [DOI] [PubMed] [Google Scholar]
- 26.Gold R, Lepow M L, Goldschneider I, Draper T L, Gotschlich E C. Kinetics of antibody production to group A and group C meningococcal polysaccharide vaccines administered during the first six years of life: prospects for routine immunization of infants and children. J Infect Dis. 1979;140:690–697. doi: 10.1093/infdis/140.5.690. [DOI] [PubMed] [Google Scholar]
- 27.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gotschlich E C, Goldschneider I, Artenstein M S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969;129:1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison L H, Tajkowski C, Croll J, Reid R, Hu D, Brenneman G, Weatherholtz R C, Santosham M. Postlicensure effectiveness of Haemophilus influenzae type b polysaccharide-Neisseria meningitidis outer-membrane protein complex conjugate vaccine among Navajo children. J Pediatr. 1994;125:571–576. doi: 10.1016/s0022-3476(94)70009-5. [DOI] [PubMed] [Google Scholar]
- 31.Hutchins W A, Carlone G M, Westerink M A J. Elderly immune response to a TI-2 antigen: heavy and light chain use and bactericidal activity to Neisseria meningitidis serogroup C polysaccharide. J Infect Dis. 1999;179:1433–1440. doi: 10.1086/314750. [DOI] [PubMed] [Google Scholar]
- 32.Jennings H J, Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J Immunol. 1981;127:1011–1018. [PubMed] [Google Scholar]
- 33.Kennett R H. Fusion protocols. Fusion by centrifugation of cells suspended in polyethylene glycol. In: Kennett R H, McKearn T J, Bechtol K B, editors. Monoclonal antibodies. Hybridomas: A new dimension in biological analyses. New York, N.Y: Plenum Press; 1980. pp. 365–367. [Google Scholar]
- 34.Leach A, Twumasi P A, Kumah S, Banya W S, Jaffar S, Forrest B D, Granoff D M, LiButti D E, Carlone G M, Pais L B, Broome C V, Greenwood B M. Induction of immunologic memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal polysaccharide-protein conjugate vaccine. J Infect Dis. 1997;175:200–204. doi: 10.1093/infdis/175.1.200. [DOI] [PubMed] [Google Scholar]
- 35.Lepow M L, Goldschneider I, Gold R, Randolph M, Gotschlich E C. Persistence of antibody following immunization of children with groups A and C meningococcal polysaccharide vaccines. Pediatrics. 1977;60:673–680. [PubMed] [Google Scholar]
- 36.Lieberman J M, Chiu S S, Wong V K, Partridge S, Chang S-J, Chiu C-Y, Gheesling L L, Carlone G M, Ward J I. Safety and immunogenicity of serogroup A/C Neisseria meningitidis oligosaccharide-protein conjugate vaccine in young children. A randomized controlled trial. JAMA. 1996;275:1499–1503. [PubMed] [Google Scholar]
- 37.MacDonald N E, Halperin S A, Law B J, Forrest B, Danzig L E, Granoff D M. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers. A randomized controlled trial. JAMA. 1998;280:1685–1689. doi: 10.1001/jama.280.19.1685. [DOI] [PubMed] [Google Scholar]
- 38.Matsuda T, Kabat E A. Variable region cDNA sequences and antigen binding specificity of mouse monoclonal antibodies to isomaltosyl oligosaccharides coupled to proteins. T-dependent analogues of α(1→6) dextran. J Immunol. 1989;142:863–870. [PubMed] [Google Scholar]
- 39.Mosier D E, Zitron I M, Mond J J, Ahmed A, Scher I, Paul W E. Surface immunoglobulin D as a functional receptor for a subclass of B lymphocytes. Immunol Rev. 1977;37:89–104. doi: 10.1111/j.1600-065x.1977.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee J, Casadevall A, Scharff M D. Molecular characterization of the humoral responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J Exp Med. 1993;177:1105–1116. doi: 10.1084/jem.177.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy T V, White K E, Pastor P, Gabriel L, Medley F, Granoff D M, Osterholm M T. Declining incidence of Haemophilus influenzae type b disease since introduction of vaccination. JAMA. 1993;269:246–248. [PubMed] [Google Scholar]
- 42.Ollo R, Auffray C, Sikorav J L, Rougeon F. Mouse heavy chain variable regions: nucleotide sequence of a germ-line VH gene segment. Nucleic Acids Res. 1981;9:4099–4109. doi: 10.1093/nar/9.16.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouchterlony O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;51:1–78. [PubMed] [Google Scholar]
- 44.Peltola H, Käyhty H, Sivonen A, Makela P H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977;60:730–737. [PubMed] [Google Scholar]
- 45.Perlmutter R M, Hansburg D, Briles D E, Nicolotti R A, Davie J M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978;121:566–572. [PubMed] [Google Scholar]
- 46.Perlmutter R M, Klotz J L, Bond M W, Nahm M, Davie J M, Hood L. Multiple VH gene segments encode murine antistreptococcal antibodies. J Exp Med. 1984;159:179–192. doi: 10.1084/jem.159.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perlmutter R M, Kearney J F, Chang S P, Hood L E. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985;227:1596–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- 48.Pierres M, Germain R N, Dorf M E, Benacerraf B. In vivo effects of anti-Ia alloantisera. I. Elimination of specific suppression by in vivo administration of antisera specific for I-J controlled determinants. J Exp Med. 1978;147:656–666. doi: 10.1084/jem.147.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richmond P, Borrow R, Miller E, Clark S, Sadler F, Fox A, Begg N, Morris R, Cartwright K. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J Infect Dis. 1999;179:1569–1572. doi: 10.1086/314753. [DOI] [PubMed] [Google Scholar]
- 50.Rubinstein L J, Stein K E. Murine immune response to the Neisseria meningitidis group C capsular polysaccharide. I Ontogeny J Immunol. 1988;141:4352–4356. [PubMed] [Google Scholar]
- 51.Rubinstein L J, Stein K E. Murine immune response to the Neisseria meningitidis group C capsular polysaccharide. II Specificity J Immunol. 1988;141:4357–4362. [PubMed] [Google Scholar]
- 52.Rubinstein L J, García-Ojeda P A, Michon F, Jennings H J, Stein K E. Murine immune responses to Neisseria meningitidis group C capsular polysaccharide and a thymus-dependent toxoid conjugate vaccine. Infect Immun. 1998;66:5450–5456. doi: 10.1128/iai.66.11.5450-5456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santosham M, Hill J, Wolff M, Reid R, Lukacs L, Ahonkhai V. Safety and immunogenicity of a Haemophilus influenzae type b conjugate vaccine in a high risk American Indian population. Pediatr Infect Dis J. 1991;10:113–117. doi: 10.1097/00006454-199102000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Santosham M, Wolff M, Reid R, Hohenboken M, et al. The efficacy in Navajo infants of a conjugate vaccine consisting of Haemophilus influenzae type b polysaccharide and Neisseria meningitidis outer-membrane protein complex. N Engl J Med. 1991;324:1767–1772. doi: 10.1056/NEJM199106203242503. [DOI] [PubMed] [Google Scholar]
- 55.Santosham M, Rivin B, Wolff M, Reid R, Newcomer W, Letson G W, Almeido-Hill J, Thompson C, Siber G R. Prevention of Haemophilus influenzae type b infection in Apache and Navajo children. J Infect Dis. 1992;165(Suppl. 1):S144–S151. doi: 10.1093/infdis/165-supplement_1-s144. [DOI] [PubMed] [Google Scholar]
- 56.Shlomchik M J, Nemazee D A, Sato V L, Van Snick J, Carson D A, Weigert M G. Variable region sequences of murine IgM anti-IgG monoclonal autoantibodies (rheumatoid factors). A structural explanation for the high frequency of IgM anti-IgG B cells. J Exp Med. 1986;164:407–427. doi: 10.1084/jem.164.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sikder S K, Akolkar P N, Kaladas P M, Morrison S L, Kabat E A. Sequences of variable regions of hybridoma antibodies to α(1→6) dextran in BALB/c and C57BL/6 mice. J Immunol. 1985;135:4215–4221. [PubMed] [Google Scholar]
- 58.Stein K E, Bona C, Lieberman R, Chien C C, Paul W E. Regulation of the anti-inulin antibody response by a nonallotype-linked gene. J Exp Med. 1980;151:1088–1102. doi: 10.1084/jem.151.5.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stein K E, Zopf D A, Johnson B M, Miller C B, Paul W E. The immune response to an isomaltohexosyl-protein conjugate, a thymus-dependent analogue of α(1→6) dextran. J Immunol. 1982;128:1350–1354. [PubMed] [Google Scholar]
- 60.Stein K E, Zopf D A, Miller C B, Johnson B M, Mongini P K A, Ahmed A, Paul W E. The immune response to a thymus-dependent form of B512 dextran requires the presence of Lyb5+ lymphocytes. J Exp Med. 1983;157:657–666. doi: 10.1084/jem.157.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stein K E. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992;165(Suppl. 1):S49–S52. doi: 10.1093/infdis/165-supplement_1-s49. [DOI] [PubMed] [Google Scholar]
- 62.Steinhoff M C. Haemophilus influenzae type b infections are preventable everywhere. Lancet. 1997;349:1186–1187. doi: 10.1016/S0140-6736(97)22017-9. [DOI] [PubMed] [Google Scholar]
- 63.Steward M W, Lew A M. The importance of antibody affinity in the performance of immunoassays for antibody. J Immunol Methods. 1985;78:173–190. doi: 10.1016/0022-1759(85)90074-2. [DOI] [PubMed] [Google Scholar]
- 64.Strohal R, Helmberg A, Kroemer G, Kofler R. Mouse Vk gene classification by nucleic acid sequence similarity. Immunogenetics. 1989;30:475–493. doi: 10.1007/BF02421180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taunay A E, Feldman R A, Bastos C O, Galvão P A A, Morais J S, Castro I O. Avaliação do efeito protetor de vacina polissacarídica antimeningocócica do grupo C, em crianças de 6 a 36 meses. Rev Inst Adolfo Lutz. 1978;38:77–82. [Google Scholar]
- 66.Twumasi P A, Jr, Kumah S, Leach A, O'Dempsey T J D, Ceesay S J, Todd J, Broome C V, Carlone G M, Pais L B, Holder P K, Plikaytis B D, Greenwood B M. A trial of a group A plus group C meningococcal polysaccharide-protein conjugate vaccine in African infants. J Infect Dis. 1995;171:632–638. doi: 10.1093/infdis/171.3.632. [DOI] [PubMed] [Google Scholar]
- 67.Victor-Kobrin C, Bonilla F A, Bellon B, Bona C A. Immunochemical and molecular characterization of the regulatory idiotopes expressed by monoclonal antibodies exhibiting or lacking β2-6 fructosan binding activity. J Exp Med. 1985;162:647–662. doi: 10.1084/jem.162.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ward R E, Kearney J F, Köhler H. Light chain isotypes selectively associate with heavy chain idiotypes in T-dependent and T-independent dextran-specific precursors. Nature. 1981;292:629–631. doi: 10.1038/292629a0. [DOI] [PubMed] [Google Scholar]
- 69.Yancopoulus G D, Desiderio S V, Paskind M, Kearney J F, Baltimore D, Alt F W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984;311:727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]