Abstract
Mitochondria are cellular organelles that perform various functions within cells. They are responsible for ATP production, cell-signal regulation, autophagy, and cell apoptosis. Because the mitochondrial proteins that perform these functions need Ca2+ ions for their activity, mitochondria have ion channels to selectively uptake Ca2+ ions from the cytoplasm. The ion channel known to play the most important role in the Ca2+ uptake in mitochondria is the mitochondrial calcium uniporter (MCU) holo-complex located in the inner mitochondrial membrane (IMM). This ion channel complex exists in the form of a complex consisting of the pore-forming protein through which the Ca2+ ions are transported into the mitochondrial matrix, and the auxiliary protein involved in regulating the activity of the Ca2+ uptake by the MCU holo-complex. Studies of this MCU holo-complex have long been conducted, but we didn’t know in detail how mitochondria uptake Ca2+ ions through this ion channel complex or how the activity of this ion channel complex is regulated. Recently, the protein structure of the MCU holo-complex was identified, enabling the mechanism of Ca2+ uptake and its regulation by the MCU holo-complex to be confirmed. In this review, I will introduce the mechanism of action of the MCU holo-complex at the molecular level based on the Cryo-EM structure of the MCU holo-complex to help understand how mitochondria uptake the necessary Ca2+ ions through the MCU holo-complex and how these Ca2+ uptake mechanisms are regulated.
Keywords: Calcium channel, Ion channel, MCU, Mitochondria, Mitochondrial calcium uniporter complex
INTRODUCTION
All cells have a cell membrane, which separates the outside from the inside of the cell. By numerous metabolic processes, cells must take in the materials they need, and they must also expel the substances they make that are undesired, often by means of the proteins in the cell membrane that surrounds the cell.
Ion channels are typical proteins that are involved in the transport of substances in the cell membrane (1). Since many ions are needed for the various metabolic processes taking place inside the cell, the cell must take in the requisite ions from the environment and release them to the environment if there are too many ions inside. However, ions cannot traverse hydrophobic cell membranes by themselves, because of their high hydrophilicity. In order to have only the right number of ions in the cell, cells use ion channels in the cell membrane to absorb the required ions and release ions that are no longer required or that are present in excess.
Ion channels in the cell membrane allow Ca2+ ions, which are representative ions, to pass across the membrane. Ca2+ ions are a type of second messenger; that is, they are a typical signal-transmission material that carries signals generated in cells (2-5). For example, Ca2+ ions in nerve cells serve as neurotransmitters (6), and Ca2+ ions in muscle cells are responsible for transmitting signals that cause muscles to contract. Ca2+ ions also play a significant role in the regulation of the activity of enzymes. This is accomplished by the Ca2+ ion’s role as cofactors in a variety of enzymes that are responsible for mediating different metabolic events inside cells. Ca2+ ions are needed for clotting, and a variety of enzymes that mediate clotting events require Ca2+ ions as key coenzymes in order to cause clotting reactions (7). Ca2+ ions are found in bones and teeth, also play a role in the construction of the organs in the body, and are necessary for the production of bone and tooth. Most of the Ca2+ present in the body resides as calcium hydroxyapatite in the bones and teeth (8).
Most eukaryotic cells contain mitochondria, which are essential parts of a cell. The energy needed for cell growth is produced by mitochondria during cellular respiration in the form of adenosine triphosphate (ATP) (9, 10). And mitochondria also affect programmed cell death and the production of reactive oxygen species (ROS), which in turn regulate cell signaling (11-14). Finally, mitochondria and endoplasmic reticulum (ER) are in charge of controlling the level of Ca2+ ions in cells by storing Ca2+ ions (15-19).
Ca2+ ions are needed for the functions of mitochondria as cell organelles. In mitochondria, for example, ATP is synthesized by means of the TCA cycle (also known as the citric-acid cycle or the Krebs cycle), which consists of a chain reaction of several enzymes. The efficiency of ATP synthesis is affected by Ca2+ concentrations in the mitochondria because some of the enzymes in the TCA cycle require Ca2+ ions to be active (9, 10). On the other hand, excessive Ca2+ concentration in the mitochondria activates the pro-apoptotic factor, ultimately causing apoptosis (11, 20-22). These examples indicate that Ca2+ ions in mitochondria are very important for their functions in cells, and ultimately reflect that maintaining the Ca2+ concentration in mitochondria is essential for normal cell metabolism.
Since it is important to maintain Ca2+ concentrations in mitochondria, many researchers have wondered how mitochondria absorb and release Ca2+ ions to control their concentrations. Because mitochondria have a double membrane structure, ion channels and transporters for Ca2+ migration are required in the mitochondrial membrane. Therefore, many researches have long focused on finding proteins involved in Ca2+ migration across the mitochondrial membrane. Mitochondrial sodium-calcium exchangers (mNCLX) and mitochondrial proton-calcium exchangers (mHCX) are proteins that have been identified by these studies (23-25). They are transporters located in the IMM and play a role in releasing Ca2+ ions accumulated in the mitochondrial matrix into the mitochondrial intermembrane space (IMS) mediated by Na+ and H+. In contrast, proteins that uptake Ca2+ ions from IMS into the mitochondrial matrix are also known. One such is, like mHCX and mNCLX, in the IMM and is known to act very selectively on Ca2+ ions. Unlike mHCX and mNCLX, however, the protein acts as a uniporter, transferring Ca2+ ions only to the mitochondrial matrix, and is selectively inhibited by inhibitors such as Ruthenium red (RuR) (26). This protein was later identified as a mitochondrial calcium uniporter (MCU) holo-complex, which consists of the MCU, an ion-conducting pore protein with a Ca2+-selective filter and a luminal gate to regulate Ca2+ entry into the mitochondrial matrix, MICU1 and MICU2 that sense Ca2+ concentrations in the IMS, and EMRE (essential MCU regulator), which simultaneously binds to the MCU and MICU1 to regulate the activity of the MCU holo-complex (27-31). In this review, I will explain how mitochondria selectively uptake Ca2+ ions through the MCU holo-complex and how this Ca2+ uptake mechanism is regulated based on the structure of the MCU holo-complex.
MCU, ACTS AS A Ca2+ CHANNEL THAT CAN CONTROL THEIR ACTIVITY BY THEMSELVES
The MCU is the pore-forming part in the MCU holo-complex and is responsible for transferring Ca2+ ions from the IMS to the mitochondrial matrix across the IMM (32). According to genome sequence analysis, the MCU holo-complex of all metazoans, including humans, contains MCUs, which also exist in non-metazoan organisms, such as fungi (32, 33). That is, the MCU is the most essential subunit in the MCU holo-complex of all living things.
To date, the MCU structure of four fungal MCUs and one C. elegans MCU has been identified through Cryo-EM, X-ray crystallography, and NMR, and these structures help us understand how mitochondria uptake Ca2+ ions through MCUs at the molecular level (34-38).
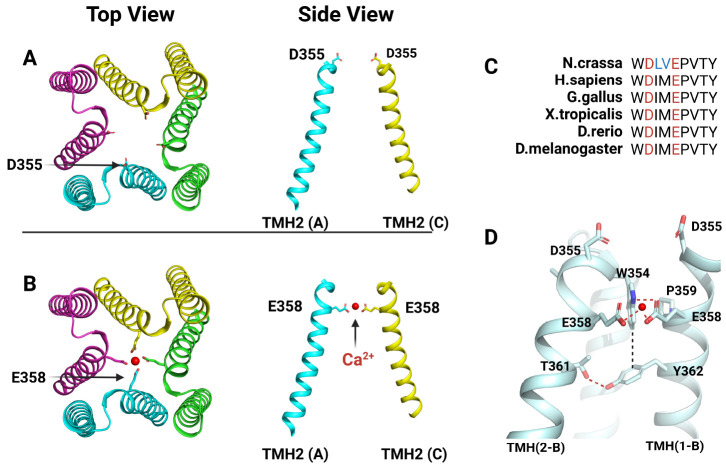
The MCU consists of three parts: N-terminal domain (NTD), coiled-coil domain (CCD), and transmembrane domain (TMD) (Fig. 1A) (34-37). In the MCU, the NTD is exposed to the mitochondrial matrix, the TMD is present in the IMM, and the CCD is located between these two domains. The MCU has a tetrameric structure in which pores are formed in the TMD to create a path through which Ca2+ ions can move. In the MCU, the TMD represents a four-fold symmetry, which is effective in transferring Ca2+ ions through a pore made by a tetrameric conformation of TMD (Fig. 1B) (34-37). The TMD of the MCU protomer consists of two α-helix (TMH: transmembrane helix). In the tetrameric structure of the MCU, TMH2 enters inward to form a pore, whereas TMH1 is exposed to the outside to contact the membrane (Fig. 1B). In the TMD, a DxxE motif (x are hydrophobic residues randomly) is located at the entrance side of the pore into which Ca2+ ions are introduced (Fig. 2A, B) (34-37). This DxxE motif is the most representative motif that characterizes MCUs and is conserved in the MCUs of all species as found by genome sequence analysis (Fig. 2C) (27, 28). That is, this DxxE motif plays a very important role in the function of the MCU. In the MCU structure, the function of this DxxE motif can be understood in more detail as a selective filter. The DxxE motif is located at TMH2 (Fig. 2A, B) (34-37). This motif has two acidic amino acids (Asp and Glu), which allow the MCU to have high selectivity for Ca2+ ions as well as uptake of Ca2+. Glu plays a key role in making the MCU highly selective for Ca2+ ions. Because of the tetrameric arrangement of the MCU protomer, Glu is also arranged in four-fold symmetry, and Ca2+ ions coordinate with each Glu at the center of the arrangement, allowing the MCU to transport Ca2+ ions (Fig. 2B) (34-37). What is most characteristic here is the distance between Glu and Glu, which represents the minimum distance that can make an ionic interaction with Ca2+ ions only; so Ca2+ ions can pass selectively through the Glu ring composed of four Glu (Fig. 2B). On the other hand, the Asp of the DxxE motif is exposed to the IMS and forms a ring wider than the Glu ring (Fig. 2A) (34-37). Therefore, rather than acting like Glu as a filter for Ca2+ ions, Asp plays a role in increasing the Ca2+ uptake efficiency of mitochondria through the MCU by recruiting Ca2+ ions from the IMS. Interestingly, RuR and Ru360—inhibitors that selectively act on the MCU—bind to the MCU via Asp (39). Considering the position of Asp in the MCU structure, RuR or Ru360 binds to the Asp ring and physically blocks the access of the Ca2+ ions to the entrance of the pore of the MCU, thereby inhibiting this activity of the MCU. In addition, MICU1 competes with RuR and Ru360 to inhibit their activity; hence the Asp of the DxxE motif plays an important role in binding to MICU1 (40).
Fig. 1.
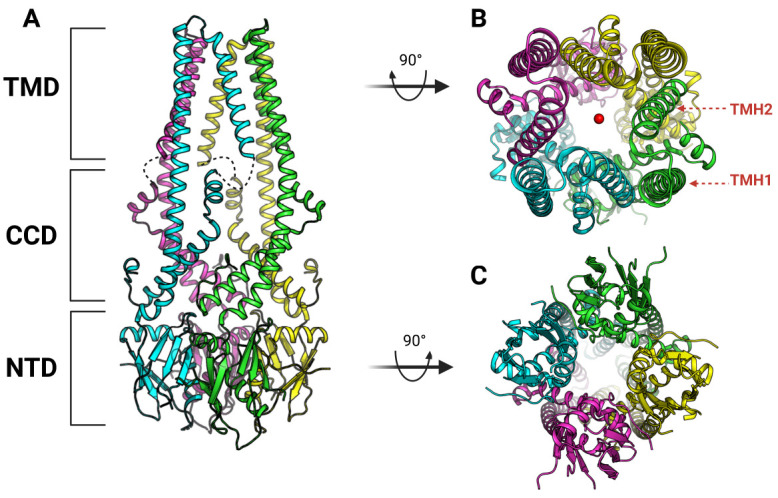
Overall architecture of Fungal MCU of N.crassa (37). (A) Each domains of N.crassa MCU are indicated (PDB code: 6DT0). Each protomer are colored separately. Missing part of N.crassa MCU structure are presented by black dashes. (B) Top view of N.crassa MCU structure. Ca2+ are represented by red spheres. Each TMH is marked in red letters at single protomer. (C) Bottom view of N.crassa MCU structure.
Fig. 2.
Ca2+ Selective filter in N.crassa MCU. (A) Top and side view of Asp (D355) ring in the pore of N.crassa MCU. It’s shown that side chain of D355 is in the shape of facing IMS at a side view. (B) Top and side view of Glu (E358) ring in the pore of N.crassa MCU. At top view, it’s shown clearly that side chain of E358 is toward to Ca2+ (red circle) to coordinate them. (C) Sequence alignment of DxxE motif from human to fungi. The two acidic residues in DxxE motif are represented in red and the two residues in cyan are those that exist only in the N.crassa MCU in DxxE motif. (D) Interactions made between DxxE motif and neighboring residues; hydrogen bonds are marked with red dashes and van der Waals interaction is marked with black dash.
Although residues consisting of the DxxE motif are important residues for the Ca2+ uptake of the MCU, the residue around the DxxE motif has also significant role. In fact, in all MCUs, not only the DxxE motif but also all the residues around this motif are conserved (Fig. 2C). Their roles are shown in Fig. 2D in detail. Thr of TMH2 makes hydrogen bonds with Tyr in TMH1 of the neighboring protomer. Trp of TMH2 interacts with this Tyr, and at the same time has a stacking interaction with the Pro of TMH1 in the neighboring protomer. Trp can thus interact with Glu in the DxxE motif. This interaction directs the side chain of Glu toward the center of the pore, allowing it to bind with Ca2+ ions entering the pore. Therefore, residues around the DxxE motif facilitate the binding of Glu in the DxxE motif with Ca2+ ions, ultimately helping Glu to act as a Ca2+ filter and uptake Ca2+ ions.
In addition to being a selectivity filter, the MCU also has a luminal gate between the TMD and the CCD. This luminal gate consists of a juxtamembrane loop (JML), through which Ca2+ ions exit from the TMD and move to the mitochondrial matrix (41). However, this luminal gate does not appear in the four fungal MCU structures (34-37). There are two possibilities for this: the JML, which makes up this luminal gate, is so flexible that it cannot be seen on an electron density map, or the luminal gate exists only in metazoans like humans (41), not in non-metazoans like fungi. Although luminal gates are not seen in the four fungal MCUs, the presence of luminal gates means that the Ca2+ uptake by mitochondria through the MCUs is highly regulated.
The NTD of the MCU is also involved in controlling the Ca2+ uptake of the MCU (42). There is a negatively charged patch in the NTD of the MCU, which is advantageous for divalent ion binding, such as with Ca2+ ions. Therefore, it can be assumed that the NTD is involved in controlling the activity of the MCU by binding to Ca2+ ions through the negatively charged patch. The NTD of the MCU is exposed to the mitochondrial matrix, which means that the concentration of Ca2+ ions in the mitochondrial matrix can be sensed by means of the Ca2+ ions that bind to the negatively charged patch of the NTD. If the sensing by the NTD shows that the Ca2+ concentration in the mitochondrial matrix is too high, the activity of the MCU should be suppressed (42). To this end, it is believed that the NTD can suppress the activity of the MCU by changing the oligomerization of the MCU (42, 43).
Unlike the TMD, the NTD of fungal MCU represents the dimer of dimer, which represents the structural flexibility of the NTD (Fig. 1C) (34-37). In addition, in the MCU structure of C. elegans, the structure was identified in the form of NTD removal, which showed a pentameric structure unlike that of fungal MCU (38). These examples indicate that the NTD affects the oligomerization of MCUs, suggesting that the NTD may regulate the activity of MCUs by regulating their oligomerization. Although further research is needed, it seems clear that the NTD affects the activity of the MCU, which means that the regulation mechanism of the MCU, along with the regulation of the MCU by MICU1-MICU2, is very sophisticated.
EMRE, THE ESSENTIAL PIECE FOR Ca2+ ION PERMEATION BY THE MCU
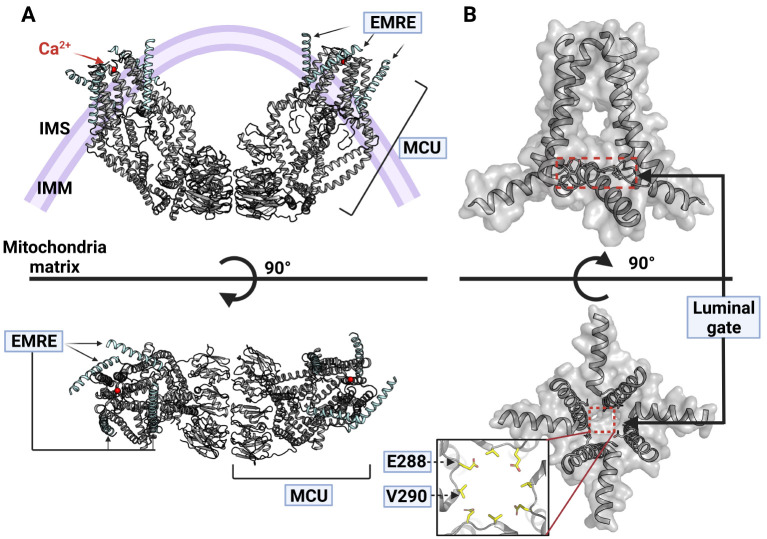
EMRE is a small protein that consists of about 100 amino acids and is essential for the activity of metazoan MCU (31). Therefore, in order to identify the Ca2+ uptake mechanism of metazoan MCU at the molecular level, efforts have recently been made to identify the complex structure of the MCU and EMRE. In 2019, the structure of the human MCU and EMRE complex was identified by means of Cryo-EM, and the role of EMRE in the MCU holo-complex uptake of Ca2+ ions could be identified in detail (Fig. 3A) (41).
Fig. 3.
Human MCU-EMRE complex structure. (A) Top and side view of hMCU-EMRE complex structure (PDB code: 6O58) (41). The hMCU is marked as a gray and the hEMRE is colored by a cyan, and the red ball is Ca2+. (B) Side and bottom view of the pore region of hMCU-EMRE complex. Luminal gates are represented by red dashed box at side and bottom view. There is an enlarged figure of a luminal gate with labels of two resides (E288 and V290) which consists of luminal gate is at bottom view.
In EMRE, the N-terminus faces the matrix of mitochondria, has a β-hairpin structure, and shows a single α-helix structure toward its C-terminus, which is embedded in the IMM (41). In the MCU and EMRE complex structure, four EMREs are bound to the tetrameric MCU, which means that the MCU and EMRE are bound at a ratio of 1:1 (Fig. 3A) (41).
The most dramatic structural feature of the MCU-EMRE complex is the dimerization of the MCU-EMRE by the interaction between the NTDs in each protomer (Fig. 3A) (41). The NTD of the MCU-EMRE induces dimerization by means of strong interaction with the NTD of another MCU-EMRE (Fig. 3A). The MCU-EMRE dimer formed in this way has a V-shape as a whole. If this structure is actually formed in the IMM, the MCU-EMRE dimer will also induce the IMM to have a V-shape (Fig. 3A). However, further research is needed to understand why EMRE changes the NTD structure of the MCU to transform the overall MCU-EMRE complex into a V-shape, and how these dramatic structural changes are related to Ca2+ uptake in mitochondria.
Another feature observed in the MCU-EMRE complex structure is a luminal gate that was not observed in the fungal MCU (Fig. 3B) (41). The MCU-EMRE complex structure has a β-hairpin in the N-terminus of EMRE dug into the JML direction of the MCU. This binding of EMRE causes TMH2 of the MCU and CC2 of the CCD to take place, and this structural change is thought to be accompanied by a structural change of JML constituting the luminal gate that creates an open structure for the movement of Ca2+ ions (Fig. 3B) (41, 44). Therefore, EMRE is essential for the activation of the MCU, because EMRE structurally opens the luminal gate for Ca2+ ions to pass through.
THE MCU HOLO-COMPLEX CONTROLS Ca2+ UPTAKE SOPHISTICATEDLY THROUGH ITS SUBUNITS
In the IMM, the MCU holo-complex has several subunit proteins, which transport Ca2+ ions into the mitochondrial matrix from the IMS. MCUb, MCUR1, and MICU3 are the subunit proteins that make up the MCU holo-complex, as do MCU, EMRE, MICU1, and MICU2 (30, 45-48). However, MCUb, MCUR1, and MICU3 are not considered to be essential subunit proteins for the MCU holo-complex, because of differences in a protein function and tissue specificity for their expression (30, 45-49). In general, the essential subunit proteins that make up the MCU holo-complex are MCU, EMRE, MICU1, and MICU2 (29-31, 50-55). Therefore, in order to study the mitochondrial Ca2+ uptake by the MCU holo-complex and its regulatory mechanism at the molecular level, we need a MCU holo-complex structure in which the MCU, EMRE, MICU1, and MICU2 are complexed.
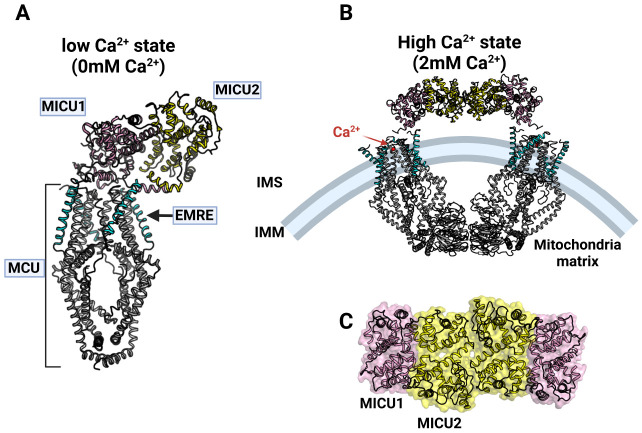
Recently, a MCU holo-complex with exactly such a structure has been identified (Fig. 4A) (56). MICU1 and MICU2 appear to be heterodimers in the MCU holo-complex structure that binds to the MCU-EMRE on the same side as IMS. MICU1 and MICU2 form heterodimers that are face to face and antiparallel. This heterodimer forms numerous interactions on the binding surface, indicating that these two proteins are very strongly associated (56-58). Like the previous MCU-EMRE complex structure, four molecules of the MCU and four molecules of EMRE make a complex in a 1:1 ratio in the MCU holo-complex structure. However, for MICU1 and MICU2, only one molecule of the MICU1-MICU2 heterodimer exists in the MCU holo-complex (Fig. 4A). Thus, the stoichiometry of each protein in the MCU holo-complex is 4:4:1:1 (MCU:EMRE:MICU1:MICU2) (56). In the MICU1-MICU2 heterodimer, MICU1 has many interactions with MCU and EMRE, whereas in MICU2, there is only a slight bond between the C-terminus and EMRE, and little direct interaction with the MCU. Therefore, interactions between the MCU-EMRE and MICU1 play an important role in forming the MCU holo-complex (56).
Fig. 4.
Human MCU holo-complex structure. (A) Side view of hMCU holo-complex at low Ca2+ state (0mM experimentally) (56). MCU (gray), EMRE (cyan), MICU1 (pink) and MICU2 (yellow) are colored (PDB code: 6WDN). (B) Side view of hMCU holo-complex at high Ca2+ state (2 mM experimentally, PDB code: 6WDO) (56). Colors shows each component of hMCU holo-complex are described as well as (A). Ca2+ is represented by a red circle. (C) Dimer of MICU1-MICU2 heterodimer in hMCU holo-complex at high Ca2+ state.
The most noticeable feature of the MCU holo-complex structure shown in low Ca2+ concentrations is that the MICU1-MICU2 heterodimer is located above the pore part of the MCU, so that the MCU seems to be covered with a lid consisting of the MICU1-MICU2 heterodimer (Fig. 4A) (56). In this structure, MICU1 is bound to the Asp ring formed by the Asp of the DxxE motif in the MCU through ionic interactions, so that MICU1 physically makes a closed form of the MCU holo-complex. Therefore, if there are few Ca2+ ions in the IMS, the MCU holo-complex exhibits a closed structure and does not uptake Ca2+. In addition, RuR and Ru360 compete with MICU1, which can also be confirmed in the MCU holo-complex structure of low Ca2+ concentrations (40). RuR and Ru360 bind to the MCU’s Asp ring to prevent Ca2+ ions from approaching the MCU’s pores, thereby inhibiting MCU activity (39). Since MICU1 also binds to the Asp ring, RuR, Ru360, and MICU1 have no choice but to compete with each other in the MCU holo-complex.
Another characteristic is that MICU1 binds in a form that blocks the entrance of the MCU when Ca2+ ions are few; so even if EMRE binds, the activity of the MCU holo-complex can be inhibited (Fig. 4A). EMRE is a protein that is important for the activation of metazoan MCU, and, as can be seen from the MCU-EMRE structure, the MCU can be permeable to Ca2+ ions, because the binding of EMRE opens the luminal gate (Fig. 3B). However, when Ca2+ ions are few in the IMM, even if EMRE binds to the MCU to open the luminal gate, MICU1 blocks the entrance of the MCU to inhibit access of the Ca2+ ions; so Ca2+ ions cannot pass through the MCU holo-complex. This implies that there is more than one mechanism that controls the activity of the MCU holo-complex, but also suggests that the activity of the MCU holo-complex is finely controlled by the Ca2+ ions present outside the MCU holo-complex by means of the MICU1-MICU2 heterodimer.
On the other hand, when Ca2+ concentration is high, the MCU holo-complex shows a significant structural change (Fig. 4B). When Ca2+ ions bind to the EF-handed motif of the MICU1-MICU2 heterodimer, the heterodimer of the MCU holo-complex interacts with the neighboring MICU1-MICU2 heterodimer, forming an O-shape overall (Fig. 4B, C) (40). The MCU-EMRE structure shown in this O-shaped MCU holo-complex structure is very similar to the V shape shown by the MCU-EMRE (Fig. 3A and 4B) although the NTD part is slightly turned (41, 56). In addition, in this structure, the luminal gate is also opened by the binding of EMRE as well as the case of low Ca2+ ion concentration (Fig. 4A, B). Therefore, the opening and closing of the luminal gate is entirely determined by the binding of EMRE (41, 56).
The most distinctive feature of the O-shaped MCU holo-complex structure is that, because most of the coupling between MICU1 and the MCU disappears, MICU1 no longer covers the pore part of the MCU, and the MICU1-MICU2 heterodimer moves to edge of the MCU, thereby opening the pore completely in the MCU (Fig. 4B) (56). Accordingly, Ca2+ ions can access the pore of the MCU and can move to the mitochondrial matrix through the MCU. Here, the N-terminus of MICU1 interacts with the MCU and the acid C-terminus tail of EMRE binds to the basic region of MICU1, which seems to be the minimum binding needed to maintain the binding between MICU1 and MCU (56). These results allow us to understand how the MCU holo-complex in resting conditions is activated by Ca2+ ions and transformed into a structure that can transfer Ca2+ ions to the mitochondrial matrix from IMS. That is, the Ca2+ uptake mechanism of the mitochondria is very sophisticated and would be controlled in several stages.
DISCUSSION
In this paper, I review how Ca2+ ions are transported preferentially from the IMS to the mitochondrial matrix via the MCU holo-complex in mitochondria and how the activity of these MCU holo-complexes is regulated based on their protein structure. The MCU, which is the pore-forming component of the MCU holo-complex, forms an ion pore with a tetrameric structure. The DxxE motif, the motif conserved from all MCU types, configures the selective filter of the MCU such that the MCU complex can preferentially uptake Ca2+ ions. Through their EF-handed motif, MICU1 and MICU2 regulate the activity of the MCU holo-complex according to the concentration of Ca2+ ions in the IMS. Despite its diminutive size, EMRE serves as a luminal gatekeeper by means of interactions with the MCU. Simultaneously, EMRE attaches to MICU1 to mediate the binding between the MCU and the MICU1-MICU2 heterodimer in order to regulate the MCU holo-complex’s ability to uptake Ca2+ ions based on their concentration at the IMS. Surprisingly, the binding of EMRE to the MCU causes the MCU-EMRE complex to form a V-shaped dimer, resulting in a substantial structural alteration in the MCU holo-complex.
The structure of the MCU holo-complex and each component helped us to comprehend the mechanism of Ca2+ uptake by the mitochondria via the MCU holo-complex in more detail. Nevertheless, there are still unanswered questions. It is unclear why the binding of EMRE generates the development of a V-shaped dimer of the MCU holo-complex and how this relates to the Ca2+ uptake mechanism. In addition, it also remains unclear if Ca2+ ions attach randomly to the MICU1-MICU2 heterodimer to activate the MCU complex or whether there is a sequential process in which the MICI1-MICU2 heterodimer interacts with Ca2+ ions. If we can discover answers to these questions, we will be able to comprehend not only the mechanism of Ca2+ uptake in mitochondria, but also the cellular Ca2+ homeostasis through mitochondria.
The modulation of mitochondrial Ca2+ ions has lately been linked to human disorders, such as heart disease and neurological disease. It is particularly intriguing that the MCU holo-complex is intimately associated with these disorders. Therefore, a comprehension of the process of Ca2+ uptake in mitochondria via the structure of the MCU holo-complex will significantly aid the investigation of treatments for these disorders.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1C1C1012076) and by the Chung-Ang University Research Grants in 2020.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Di Resta C, Becchetti A. Introduction to ion channels. Adv Exp Med Biol. 2010;674:9–21. doi: 10.1007/978-1-4419-6066-5_2. [DOI] [PubMed] [Google Scholar]
- 2.Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 3.Hetherington AM, Brownlee C. The generation of Ca(2+) signals in plants. Annu Rev Plant Biol. 2004;55:401–427. doi: 10.1146/annurev.arplant.55.031903.141624. [DOI] [PubMed] [Google Scholar]
- 4.Pandey GK, Cheong YH, Kim KN, et al. The calcium sensor calcineurin B-like 9 modulates abscisic acid sensitivity and biosynthesis in Arabidopsis. Plant Cell. 2004;16:1912–1924. doi: 10.1105/tpc.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu Rev Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- 6.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Dodt J, Volkers P, et al. Structure functional insights into calcium binding during the activation of coagulation factor XIII A. Sci Rep. 2019;9:11324. doi: 10.1038/s41598-019-47815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cashman KD. Calcium intake, calcium bioavailability and bone health. Br J Nutr. 2002;87 Suppl 2:S169–177. doi: 10.1079/BJN/2002534. [DOI] [PubMed] [Google Scholar]
- 9.Balaban RS. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim Biophys Acta. 2009;1787:1334–1341. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 12.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scorrano L, Oakes SA, Opferman JT, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 15.Chernorudskiy AL, Zito E. Regulation of calcium homeostasis by ER redox: a close-up of the ER/mitochondria connection. J Mol Biol. 2017;429:620–632. doi: 10.1016/j.jmb.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Bustos G, Ahumada-Castro U, Silva-Pavez E, Puebla A, Lovy A, Cesar Cardenas J. The ER-mitochondria Ca2+ signaling in cancer progression: Fueling the monster. Int Rev Cell Mol Biol. 2021;363:49–121. doi: 10.1016/bs.ircmb.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Ahumada-Castro U, Puebla-Huerta A, Cuevas-Espinoza V, Lovy A, Cardenas JC. Keeping zombies alive: The ER-mitochondria Ca2+ transfer in cellular senescence. Biochim Biophys Acta Mol Cell Res. 2021;1868:119099. doi: 10.1016/j.bbamcr.2021.119099. [DOI] [PubMed] [Google Scholar]
- 18.Smaili SS, Pereira GJ, Costa MM, et al. The role of calcium stores in apoptosis and autophagy. Curr Mol Med. 2013;13:252–265. doi: 10.2174/156652413804810772. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman RJ, Malhotra JD. Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics. Biochim Biophys Acta. 2014;1843:2233–2239. doi: 10.1016/j.bbamcr.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008;44:1–5. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 21.East DA, Campanella M. Ca2+ in quality control: an unresolved riddle critical to autophagy and mitophagy. Autophagy. 2013;9:1710–1719. doi: 10.4161/auto.25367. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb RA, Bernstein D. Mitochondrial remodeling: Rearranging, recycling, and reprogramming. Cell Calcium. 2016;60:88–101. doi: 10.1016/j.ceca.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crompton M, Kunzi M, Carafoli E. The calcium-induced and sodium-induced effluxes of calcium from heart mitochondria. Evidence for a sodium-calcium carrier. Eur J Biochem. 1977;79:549–558. doi: 10.1111/j.1432-1033.1977.tb11839.x. [DOI] [PubMed] [Google Scholar]
- 24.Palty R, Silverman WF, Hershfinkel M, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 27.Baughman JM, Perocchi F, Girgis HS, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perocchi F, Gohil VM, Girgis HS, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plovanich M, Bogorad RL, Sancak Y, et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One. 2013;8:e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sancak Y, Markhard AL, Kitami T, et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bick AG, Calvo SE, Mootha VK. Evolutionary diversity of the mitochondrial calcium uniporter. Science. 2012;336:886. doi: 10.1126/science.1214977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamer KJ, Mootha VK. The molecular era of the mitochondrial calcium uniporter. Nat Rev Mol Cell Biol. 2015;16:545–553. doi: 10.1038/nrm4039. [DOI] [PubMed] [Google Scholar]
- 34.Baradaran R, Wang C, Siliciano AF, Long SB. Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature. 2018;559:580–584. doi: 10.1038/s41586-018-0331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan C, Fan M, Orlando BJ, et al. X-ray and cryo-EM structures of the mitochondrial calcium uniporter. Nature. 2018;559:575–579. doi: 10.1038/s41586-018-0330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen NX, Armache JP, Lee C, et al. Cryo-EM structure of a fungal mitochondrial calcium uniporter. Nature. 2018;559:570–574. doi: 10.1038/s41586-018-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoo J, Wu M, Yin Y, Herzik MA, Jr, Lander GC, Lee SY. Cryo-EM structure of a mitochondrial calcium uniporter. Science. 2018;361:506–511. doi: 10.1126/science.aar4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oxenoid K, Dong Y, Cao C, et al. Architecture of the mitochondrial calcium uniporter. Nature. 2016;533:269–273. doi: 10.1038/nature17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao C, Wang S, Cui T, Su XC, Chou JJ. Ion and inhibitor binding of the double-ring ion selectivity filter of the mitochondrial calcium uniporter. Proc Natl Acad Sci U S A. 2017;114:E2846–E2851. doi: 10.1073/pnas.1620316114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paillard M, Csordas G, Huang KT, Varnai P, Joseph SK, Hajnoczky G. MICU1 interacts with the D-ring of the MCU pore to control its Ca2+ flux and sensitivity to Ru360. Mol Cell. 2018;72:778–785. doi: 10.1016/j.molcel.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Nguyen NX, She J, et al. Structural Mechanism of EMRE-Dependent Gating of the Human Mitochondrial Calcium Uniporter. Cell. 2019;177:1252–1261. doi: 10.1016/j.cell.2019.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SK, Shanmughapriya S, Mok MCY, et al. Structural insights into mitochondrial calcium uniporter regulation by divalent cations. Cell Chem Biol. 2016;23:1157–1169. doi: 10.1016/j.chembiol.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan Y, Cao C, Wen M, et al. Structural characterization of the N-terminal domain of the Dictyostelium discoideum mitochondrial calcium uniporter. ACS Omega. 2020;5:6452–6460. doi: 10.1021/acsomega.9b04045.f6232be39cdf4db485e6ebc55ecabe40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Keuren AM, Tsai CW, Balderas E, Rodriguez MX, Chaudhuri D, Tsai MF. Mechanisms of EMRE-dependent MCU opening in the mitochondrial calcium uniporter complex. Cell Rep. 2020;33:108486. doi: 10.1016/j.celrep.2020.108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raffaello A, De Stefani D, Sabbadin D, et al. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert JP, Luongo TS, Tomar D, et al. MCUB regulates the molecular composition of the mitochondrial calcium uniporter channel to limit mitochondrial calcium overload during stress. Circulation. 2019;140:1720–1733. doi: 10.1161/CIRCULATIONAHA.118.037968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mallilankaraman K, Cardenas C, Doonan PJ, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patron M, Granatiero V, Espino J, Rizzuto R, De Stefani D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death Differ. 2019;26:179–195. doi: 10.1038/s41418-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paupe V, Prudent J, Dassa EP, Rendon OZ, Shoubridge EA. CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metab. 2015;21:109–116. doi: 10.1016/j.cmet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Csordas G, Golenar T, Seifert EL, et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab. 2013;17:976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mallilankaraman K, Doonan P, Cardenas C, et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell. 2012;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai MF, Phillips CB, Ranaghan M, et al. Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex. Elife. 2016;5:e15545. doi: 10.7554/eLife.15545.77bd01778d114c698b8a3ab4108223cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pittis AA, Goh V, Cebrian-Serrano A, Wettmarshausen J, Perocchi F, Gabaldon T. Discovery of EMRE in fungi resolves the true evolutionary history of the mitochondrial calcium uniporter. Nat Commun. 2020;11:4031. doi: 10.1038/s41467-020-17705-4.be276a83a522423db2f562d273d11d58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamer KJ, Mootha VK. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep. 2014;15:299–307. doi: 10.1002/embr.201337946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patron M, Checchetto V, Raffaello A, et al. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell. 2014;53:726–737. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan M, Zhang J, Tsai CW, et al. Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex. Nature. 2020;582:129–133. doi: 10.1038/s41586-020-2309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu W, Shen Q, Zhang R, et al. The structure of the MICU1-MICU2 complex unveils the regulation of the mitochondrial calcium uniporter. EMBO J. 2020;39:e104285. doi: 10.15252/embj.2019104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang C, Jacewicz A, Delgado BD, Baradaran R, Long SB. Structures reveal gatekeeping of the mitochondrial Ca2+ uniporter by MICU1-MICU2. Elife. 2020;9:e59991. doi: 10.7554/eLife.59991.babbf9f640f54b3e8fb3f9f3f6888c17 [DOI] [PMC free article] [PubMed] [Google Scholar]