Abstract
Macrophage activation has long been implicated in a myriad of human pathophysiology, particularly in the context of the dysregulated capacities of an unleashing intracellular or/and extracellular inflammatory response. A growing number of studies have functionally coupled the macrophages’ inflammatory capacities with dynamic metabolic reprogramming which occurs during activation, albeit the results have been mostly interpreted through classic metabolism point of view; macrophages take advantage of the rewired metabolism as a source of energy and for biosynthetic precursors. However, a specific subset of metabolic products, namely immune-modulatory metabolites, has recently emerged as significant regulatory signals which control inflammatory responses in macrophages and the relevant extracellular milieu. In this review, we introduce recently highlighted immuno-modulatory metabolites, with the aim of understanding their physiological and pathological relevance in the macrophage inflammatory response.
Keywords: Immuno-modulatory metabolites, Inflammatory diseases, Macrophages
INTRODUCTION
Inflammation is characterized by a process through which various immune subpopulations and the relevant biological components coordinate in order to eliminate harmful substances/stimuli, including damaged/infected tissues, pathogens, and toxic compounds (1-3). Throughout this process, macrophages play central roles in shaping a range of systemic/local inflammatory responses via their multiple functions, including antigen presentation, phagocytosis, and immunomodulation (4, 5). Such functional responses are expressed differentially through deliciated regulatory mechanisms in a tired manner, which has been shown to be largely modulated by various intracellular signaling pathways (e.g., NF-κB, AP-1, and STAT-1) with the relevant cytokines and growth factors secretion (e.g., interleukin family, tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β) (3, 6). Although the majority of studies have focused mainly on the physiological and pathological relevance of the aforementioned protein molecules in inflammatory response, and the mechanisms of the proteins as functional linkers between intracellular and extracellular immunomodulation, growing evidence in vitro and in vivo have recently suggested that the protein components alone may not be sufficient in recapitulating the macrophages-regulated inflammatory response, opening up the possibility that other cellular components should be on board, such as metabolites (7-9).
Macrophage metabolism is rapidly rewired throughout the process of pathogen-stimulated macrophage polarization, tipping toward aerobic glycolysis, a process referred to as the “Warburg effect”; Increased glucose uptake, glycolytic flux, pyruvate conversion to lactate, and decreased oxygen consumption even under oxygen-rich environments, which results in energy production without the loss of carbon, an important building block required for rapid proliferation (10-12).
Such dynamic metabolic changes have provided key biosynthetic precursors required for the functioning of macrophages during inflammation. For example, the pentose phosphate pathway (PPP) downstream of glycolysis, which is activated in lipopolysaccharide (LPS)-stimulated macrophages, provides NADPH, which serves as a substrate for NADPH oxidase during the killing of pathogens, as well as the build-ups of reductive powers for glutathione-mediated antioxidant defense (13, 14). Amino acids have also been emphasized for their capacity to fuel biosynthetic precursors during inflammatory responses. One of the most evident observations within this context is the metabolic regulation of arginine and citrulline, biosynthetic precursors of nitric oxide, which plays a critical role in determining the extent of macrophages’ inflammatory response (15, 16).
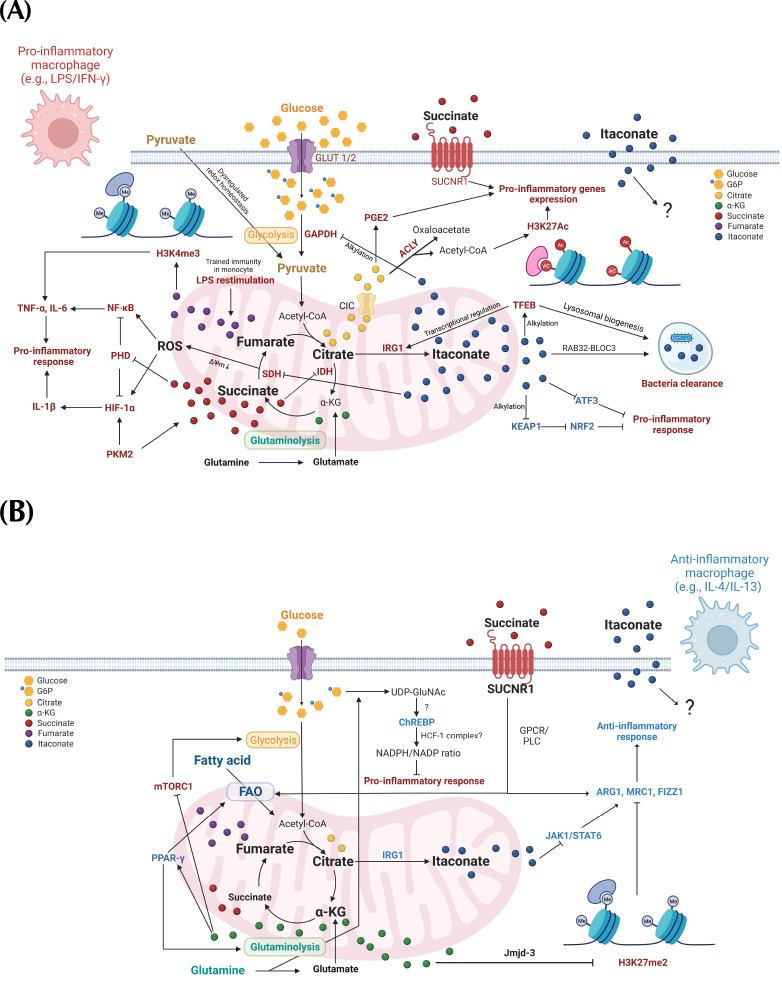
A growing number of studies have recently revealed the atypical mechanisms by which a specific subset of metabolites in macrophages serve as regulatory signals to attune immunomodulation, which is distinct from the classic function of metabolism including energy production and biosynthetic precursors. Such metabolites serve as immunomodulatory metabolites which display unique physio-chemical properties, and the mechanisms of action including transcriptional and epigenetic regulation, and post-translational modification (PTM) (17). In this review, we discuss the recent advances in the understanding of the functional implication of 5 metabolites, including glucose and four tricarboxylic acid (TCA) cycle intermediates in macrophages’ inflammatory response, as well as the underlying biochemical mechanisms by which those metabolites coordinately shape the inflammatory responses of macrophages (Fig. 1).
Fig. 1.
The schematics of immunomodulatory metabolites roles in shaping the inflammatory response of the macrophage. (A) The immunoregulatory functions of metabolites in macrophages upon pro-inflammatory stimulation (e.g., LPS/IFN-γ). LPS, lipopolysaccharide; IFN-γ, interferon-γ; NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IL-1β, interleukin-1β; PHD, prolyl hydroxylase; HIF-1α, hypoxia-inducible factor-1α; SDH, succinate dehydrogenase; IDH, isocitrate dehydrogenase; IRG1, immune-responsive gene 1; ACLY, ATP-citrate Lyase; PGE2, prostaglandin 2; H3K4me3, histone 3 lysine 4 tri-methylation; H3K27Ac, histone 3 lysine 27 acetylation; SUCNR1, succinate receptor 1; TFEB, transcription factor EB; Keap1, kelch-like ECH-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; ATF3, activating transcription factor 3; Rab32/BLOC3, Rab32/member RAS oncogene family 32/biogenesis of lysosomal organelles complex 1 subunit 3. (B) The immunoregulatory functions of metabolites in macrophages upon anti-inflammatory stimulation (e.g., IL-4/IL-13). IL-4, interleukin-4; IL-13, interleukin-13; FAO, fatty acid oxidation; PPAR-γ, peroxisome proliferator activated receptor-gamma; UDP-GluNAc, uridine diphosphate N-acetylglucosamine; ChREBP, carbohydrate response element binding protein; NADPH/NADP, nicotinamide-adenine dinucleotide phosphate reduced/nicotinamide-adenine dinucleotide phosphate oxidized; GPCR/PLC, G protein-coupled receptor/phospholipase C; H3K27me2, histone 3 lysine 4 di-methylation; JAK1/STAT6, janus kinase 1/Signal transducer and activator of transcription 6; ARG1, arginase 1; FIZZ1, found in inflammatory zone 1; MRC1, mannose receptor C-type 1. The red and blue color-coded components within the figures respectively represent the constituents of anti- and pro-inflammatory responses.
GLUCOSE
Cells have evolved in multiple ways to utilize glucose as the main fuel for maintaining metabolic homeostasis. In particular, glucose and the glycolytic intermediates provide biologically relevant energy and reducing currents (e.g., ATP and NADH), and various biosynthetic precursors, all of which are essentially critical to support proliferation and maintain cellular physiology (18). In this context, the physiological relevance of glucose metabolism in the inflammatory responses of macrophages has initially been evident, considering observation that macrophages undergo rapid proliferation and dramatic functional changes during activation. For example, glucose transporter 1 and 2 (GLUT1, 2) and glucose-utilizing enzymes are transcriptionally upregulated upon LPS stimulation, which leads to a rapidly increased glycolytic flux for the provision of cellular building blocks and a source of energy (Fig. 1A) (19, 20).
The roles of glucose as a regulatory signal has been proposed from the discovery of ChREBP, a glucose-stimulated transcription factor which turns on the transcriptional program of hepatic de novo lipogenesis (21, 22). Several biochemical mechanisms were also suggested, including allosteric regulation, UDP-GlcNAc-mediated PTM, and the activation of a nutrient sensitive complex including HCF-1 (23). Although the very initial findings have only focused on the hepatic relevance of the glucose-ChREBP connection, this axis has recently been expanded towards other cells/tissues physiology, including pancreatic beta cells, adipose tissues, and immune cells (24, 25).
Sarrazy et al. showed that ChREBP activity is reduced upon LPS-stimulated macrophages, while the activated ChREBP in IL-4-treated macrophages tones down pro-inflammatory responses (26). The phenotype may attribute to the effects of ChREBP activity on the cellular redox status, including changes in the NADP/NADPH ratio, which is critical for shaping macrophages’ inflammatory response. Such observation, together with the increased glucose flux into the hexosamine synthetic pathway following IL-4 stimulation (27), may lead to a possibility where the glucose function in macrophages with anti-inflammatory features may be rerouted from the biosynthetic precursors/energy sources to a regulatory signal for the UDP-GlcNAc activation of ChREBP-HCF1 complex (Fig. 1B) (28).
ALPHA-KETOGLUTARATE (α-KG)
Amino acids are metabolically integrated into the central carbon metabolism through the chemical reactions of nitrogen removal/addition, referred to as transamination. Alpha-ketoglutarate (α-KG) is a key substrate/product of the reaction through which several amino acids, including alanine, aspartate, glutamine, and glutamate are synthesized or TCA cycle-incorporated, thereby recapitulating the carbon and nitrogen homeostasis (29).
Several studies have suggested the immunomodulatory roles of α-KG in dynamic transcriptional and epigenetic reprogramming during the macrophages’ inflammatory response, thereby pushing the macrophages phenotype towards ‘anti-inflammatory’ (Fig. 1B) (30). One essential aspect of α-KG in terms of its functions, may be that α-KG serves as a key cofactor in many cellular biochemical reactions. For example, the enzymatic activity of prolyl hydroxylase domain enzyme (PHD), an inhibitor of the hypoxia-inducible factor-a (HIF-1α) and NF-κB, requires α-KG as a cofactor for the substrates hydroxylation recognized by the von hippel lindau (VHL) E3 ligase for subsequent proteasomal degradation (31-33). Such biochemical mechanisms may be physiologically relevant in macrophages’ inflammatory response, as the in vivo treatment of dimethyl-KG (DKG), a cell permeable α-KG derivative, significantly decreases the expression of HIF-1α and NF--κB target pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, as well as the serum levels of IL-6 and IL-12 (34). Other α-KG-regulated mechanisms have been shown in a study of an LPS-induced acute lung injury (ALI) model, where DKG treatment triggered the transcriptional activation of peroxisome proliferator-activated receptor-gamma (PPAR-γ), while the mTOR-activated transcriptional program of proinflammatory genes was diminished (35). Although the mechanisms underlying the α-KG regulation of mTOR and PPAR-γ are still unclear, one study suggested a potential mechanism of α-KG inhibition of mTOR in vitro, where the α-KG directly binds and inhibits the beta-subunit of ATP synthase, followed by decreased ATP leading to mTOR inhibition (36).
The α-KG-induced anti-inflammatory phenotypes may not be limited to the transcriptional regulations; specifically, the phenotypes may also significantly attribute to its role in epigenetic reprogramming through the regulation of epigenetic modifiers, including Jumonji domain-containing protein-3 (Jmjd-3), a histone demethylase enzyme (Fig. 1B) (37, 38). This concept has been proposed by Liu and colleagues; They showed that the glutaminolysis-derived α-KG regulation of Jmjd-3 is functionally relevant in macrophages for endotoxin tolerance, specifically through the provoking of epigenetic modification in promoter of anti-inflammatory genes which leads to NF-κB pathway suppression (38).
Taken together, α-KG shapes the transcriptional and epigenetic landscapes in macrophages, thereby exhibiting its anti-inflammatory function in macrophages.
SUCCINATE
Although the accumulation of succinate, a four carbon TCA cycle metabolite, has long been proposed as a key metabolic signature of macrophages which display pro-inflammatory tones (39, 40), many efforts have recently been made for a more precise interpretation of the physiological relevance of the accumulation in macrophages’ inflammatory response (Fig. 1). A very initial study of the succinate function in macrophages has well-recapitulated the functional relevance of succinate in pro-inflammatory response of macrophages (Fig. 1A) (39). In this study, the authors showed that succinate, which displays a high synthetic flux that is derived from glutamine and GABA shunt upon LPS stimulation, triggers the HIF-1α-mediated proinflammatory response in macrophages, including the expression of IL-1β. This phenomenon, namely, ‘pseudohypoxia,’ attributes towards a regulatory signal role of succinate for the HIF-1α activation through PHD inhibition following competition with α-KG, which is a cofactor of PHD (41). Such observation has been further elaborated by a study where pyruvate kinase M2 (PKM2) function coordinates with the succinate/HIF-1α axis (42); LPS stimulation evokes the conformational change of PKM2 into a state of monomer/dimers, which then acts as a transcriptional co-activator of HIF-1α in exchange for its enzymatic activity, resulting in glucose metabolism shunted for succinate accumulation subsequently followed by stabilization and transcriptional activation of HIF-1α.
Recent studies in the genetic model of succinate dehydrogenase (SDH), the succinate oxidative enzyme, have provided in vivo and in vitro evidence which allows us to better conceptualize succinate contribution to the inflammatory response (43); Mills et al. have shown that the succinate accumulation following SDH inhibition suppresses the pro-inflammatory gene expression including IL-1β while also triggering a subset of anti-inflammatory gene expression that is epitomized by IL-1RA and IL-10, thereby suggesting that succinate oxidation is a significant prerequisite for the induction of pro-inflammatory responses. They also suggested that the mechanisms may involve an increase of ROS production following the SDH oxidation of succinate, thereby leading to an elevation of the mitochondrial membrane potential.
Extracellular metabolites have recently emerged as key regulatory signals for the metabolic crosstalk between cells within the microenvironment (44). Succinate has also been studied in this context (40, 45-47), thereby providing the unexpected evidence where extracellular succinate provokes the anti-inflammatory response (Fig. 1B). For example, succinate receptor 1 (SUCNR1) activated by extracellular succinate triggers GPCR/PLC signaling, which in turn activates the transcriptional program for the expression of anti-inflammatory genes, including Arg1, Mrc1, Fizz1, and fatty acid oxidation (FAO)-relevant genes (40, 46, 47). The proinflammatory effects of succinate that is derived from microbiota via the SUCNR1, which exacerbates the proinflammatory response in the mouse model of inflammatory bowel disease, has also been further elucidated (Fig. 1A) (45).
The last add-up in this section, which may also serve as a significant mechanism of succinate function in the shaping of the macrophages’ inflammatory response, is protein succinylation, a non-enzymatic PTM on lysine residues of substrates (48-50). Such a notion may be relevant, as the increased protein succinylation upon LPS stimulation has been widely observed (39). This phenotype can be explained by both the accumulation of succinate, whose cellular level has been tightly associated with the level of succinylated protein, and the decreased gene expression Sirt5, a known de-succinylating enzyme (48, 51). This observation, together with the significant effects of succinylation on protein functionalities, thereby justifies future investigation on the spectrum of protein succinylation and its functional relevance in inflammatory responses (48, 49).
Taken together, succinate serves as a key immunomodulatory metabolite with differential functional outcomes which attributes to its context-dependent roles in augmenting pro- or anti-inflammatory response of macrophages.
FUMARATE
The metabolic origin of fumarate as a TCA cycle metabolite is differentially regulated; naïve macrophages or anti-inflammatory macrophages runs TCA cycle to synthesize fumarate while it is synthesized from glutaminolysis in pro-inflammatory macrophages where succinate dehydrogenase (SDH) activity is significantly blunted (27). Fumarate accumulation in macrophages and its physiological relevance have been particularly emphasized from a study by Arts et al., where the accumulated fumarate led to epigenetic reprogramming that is characterized by increased H3K4me3 and H3K27Ac in promoter of LPS-stimulated pro-inflammatory genes, which significantly contributes to beta-glucan-induced innate immune memory (Fig. 1A) (52). Although the underlying biochemical mechanisms were not clearly stated, some evidence does point out the structural similarity with α-KG, which allows for the fumarate inhibition of the enzyme activity of epigenetic modifiers including lysine demethylase 5 (KDM5). The epigenetic roles of fumarate in other contexts remain to be further explored given the significance of epigenetic regulation in shaping macrophages’ inflammatory capacities (53).
Fumarate also inhibits the enzymatic activities of others through the ‘α-KG competition’ mechanism that is relevant to the KDM5 inhibition. One of the most relevant targets in the context of macrophages’ inflammatory response is supposedly PHD. For example, the genetic inactivation of fumarate hydratase, a metabolic enzyme with the activity of fumarate oxidation, which also leads to fumarate accumulation, results in the inhibition of PHD activity and subsequent HIF-1α activation, thereby driving renal tumorigenesis (54). The fumarate regulation of HIF-1α, together with the key roles of activated HIF-1α in the provoking of a proinflammatory response during macrophages activation (55), easily led to a speculation whereby fumarate may serve as a proinflammatory metabolite in macrophages. However, this may be not as simple as expected. For example, a study using dimethyl fumarate (DMF), a cell permeable fumarate derivative, shows that itaconate may rather trigger anti-inflammatory responses in macrophages including IL-4 and IL-10 production through mechanism(s) related to nuclear factor erythroid-2-related factor 2 (Nrf2) activation (Fig. 1B) (56). This result, taken together with the above-mentioned epigenetic roles of fumarate in enhancing pro-inflammatory gene expression upon LPS restimulation, suggest that fumarate may display different physiological target(s) in macrophages inflammatory response. However, we also cannot rule out the possibility that the phenotypes may be derived from differential chemical properties of DMF versus fumarate; DMF has been shown to display a very high electrophilic property (57, 58).
Fumarate has recently been shown to serve as a modifier in tissues such as brain muscle, and adipose tissues for protein succination, an irreversible, non-enzymatic protein modification (Of note, the succination is chemically distinct from succinylation described in the previous section) (59-62). Although a spectrum of succinated proteins have been characterized, the physiological relevance remains still elusive. One study has shown that a feedback mechanism regarding metabolic homeostasis where hepatic fumarate accumulation upon excessive cellular glucose uptake can induce GAPDH succination which regulates glycolytic flux (59). Given the high glucose flux and utilization during macrophages activation, this mechanism may be physiologically or pathologically relevant in the context of inflammatory response of macrophages.
ITACONATE
Expression of immune-responsive gene 1 (IRG1), a metabolic enzyme responsible for decarboxylation of cis-aconitate, is rapidly induced upon bacterial infection or toxin stimulation including LPS, which leads to accumulation of the metabolic product, namely itaconate (Fig. 1A) (63). Itaconate has first been discovered as an antimicrobial metabolite allowing for efficient killing of phagocytosed bacteria in macrophages (e.g., Staphylococcus aureus and Pseudomonas aeruginosa) (64). Mechanistically, Rab32-BLOC3 complex serves as scaffold to coordinate for itaconate delivery into the bacteria-containing vacuole where this metabolite exerts the bactericidal effects which mainly rely on 1) itaconate inhibition of iso-citrate lyase in glyoxylate shunt pathway, a key metabolic pathway for bacterial growth, and 2) itaconate-derived itaconyl-CoA suppression of methyl-malonyl-CoA mutase leading to inefficient carbon source supply into bacterial TCA cycle (65-67).
Besides the antimicrobial function, the versatile immunomodulatory roles of itaconate have initially been proposed and further elaborated on by Viki and colleagues, who provided biochemical evidence on the itaconate-induced alkylation of SDH followed by enzymatic activity loss and subsequent succinate accumulation (Fig. 1A) (68). The functional relevance of the metabolic phenotype in inflammatory response has been subsequently shown in the study; Dimethyl-itaconate (DI), a derivative of itaconate, leads to a compromised pro-inflammatory gene expression, such as IL-6, suggesting itaconate as an anti-inflammatory metabolite. This observation is also recapitulated by the IRG KO macrophages which displays a more robust proinflammatory response upon LPS stimulation due to reduced PPP, which is then characterized by increased IL-1β, IL-6 and TNF-α expression (69). Of note, the anti-inflammatory roles of itaconate can also be well-supported by the aforementioned study of functional outcomes upon the genetic deletion of SDH in macrophages (43).
In 2018, Mills and colleagues discovered the itaconate-regulation of Nrf2, a master transcription factor of antioxidant response, which is another layer of mechanistic insight into itaconate’s role as an immunomodulatory metabolite (Fig. 1A) (70). The authors observed that DI treatment leads to the alkylation-induced inhibition of Keap1, an E3 ligase which targets Nrf2 for proteasomal degradation, thereby exerting an anti-inflammatory response triggered by Nrf2 activation. A spectrum of alkylation targets has further been identified in the context of itaconate’s capacity of triggering anti-inflammatory gene expression; Atf3 is alkylated upon DI treatment, thereby leading to an Nrf2-independent increase in IL-6 gene expression. In addition, the treatment of 4-octyl itaconate (4-OI), a different class of itaconate derivative also induces GAPDH alkylation, thereby suppressing the glycolytic flux which is indispensable for pro-inflammatory phenotype (71). Itaconate also exerts its anti-inflammatory function through the alleviation of inflammasome cascade via NLRP3 alkylation on cysteine 548, a residue that is functionally relevant for the interaction with NEK7 (72).
Such an anti-inflammatory mechanism of action may also serve as a critical host-defense mechanism within peripheral tissues. For example, neuron-infected zika virus induces the expression of IRG1 downstream of pro-inflammatory signaling components, including receptor-interacting protein kinases 1 and 3 (RIPK1 and 3), and interferon regulatory factor 1 (IRF1), which in turn triggers Nrf2-dependent antioxidant response and the subsequent inhibition of zika virus that takes advantage of exacerbated neuronal inflammatory response for its replication (73).
The anti-inflammatory functions of itaconate have thus led to recent studies where the therapeutic potentials of itaconate have been tested in various contexts of human pathophysiology, specifically in regard to aberrant pro-inflammatory response including sepsis, HDM-induced asthma, pulmonary fibrosis, and influenza A infection (74-78). However, the majority of the studies have implemented DI or 4-OI, which are cell-permeable itaconate derivatives with unique physiochemical properties, which may lead to biologically irrelevant functional outcomes following artificial protein alkylation (79). For example, DI displays a somewhat different spectrum of cellular protein alkylation, which is presumably due to its much higher electrophilicity as compared to that of itaconate. Moreover, stable tracing analysis using C13-DI revealed that DI fails to be metabolized to itaconate, and that it rather induces endogenous itaconate accumulation, pointing towards the possibility of significant differences in functional outcomes following the treatment of DI versus itaconate (79, 80). 4-OI has been shown to efficiently hydrolyze to itaconate in cells, suggesting a more relevant itaconate derivative when compared to DI, although there are caveats of using 4-OI which have been reported upon; 1) LPS stimulation required for intercellular 4-OI conversion into itaconate, and 2) the differential alkylation targets of 4-OI compared to itaconate (70, 79).
The cell permeability of itaconate has also been controversial, which has made DI and 4-OI as alternative chemical tools for exploring itaconate function (79). However, studies using C13-labelled itaconate have recently shown that pH-adjusted itaconate is macrophage-permeable, although its level of efficacy seems to be cell-type and context dependent (80). This observation, together with a comparative study in mouse primary macrophages where the differential regulation of cytokine expression by itaconate vs derivatives was observed, suggests that previous conclusions on the physiological and functional relevance of itaconate, which exclusively relied on 4-OI or DI treatment, may need to be more carefully interpreted (79, 81).
Although the aforementioned observation has already provided a sufficient amount of evidence on itaconate as an anti-inflammatory metabolite, a growing number of studies are expanding the spectrum of immunomodulatory roles for itaconate. For example, the IRG-itaconate-SDH axis plays a central role in the suppression of an exacerbated inflammatory response during immune tolerance, which is a key target of beta-glucan-induced immune training (82). Of particular note, is the fact that itaconate’s effects on suppressing macrophage phenotypes may also be relevant to an anti-inflammatory response, as well as a pro-inflammatory response, which is evident from a study where the response of macrophages with anti-inflammatory phenotype was significantly limited by the treatment of itaconate or the derivatives through the targeting of the JAK-STAT pathway, which is a key transcriptional nodule to activate anti-inflammatory genes expression (Fig. 1B) (83). This study provided with a deeper insight of the itaconate’s immunomodulatory roles; they are not limited to simply alleviating pro-inflammatory response, rather play key roles in restricting ‘exacerbated inflammatory responses’ in both pro- and anti-inflammatory context.
On the other hand, recently, a study has proposed a previously unappreciated role for itaconate in triggering phagocytotic capacities of macrophages via alkylation dependent activation of transcription factor EB (TFEB), a transcription factor for lysosomal biogenesis, suggesting that itaconate may also boost macrophage phenotypes (Fig. 1A) (84).
Although physiological and functional aspects of itaconate have been main interests in this field, the importance of regulatory mechanisms of itaconate accumulation has recently been appreciated as well (Fig. 1A). For example, Schuster et al. reported TFEB as a transcription factor for IRG expression, which is critical for removing intravacuolar pathogen Salmonella Enterica Serovar Typhimurium (85). Physiologically relevant carbon source for itaconate synthesis has also been explored in a comprehensive tracing analysis showing glucose and glutamine as major fuels for itaconate production (27). In addition, our group has recently observed that extracellular pyruvate may be a pathologically relevant fuel source for itaconate synthesis in a situation where protein redox homeostasis is dysregulated upon depletion of MsrB1, a reducing enzyme for oxidation on protein methionine residues (86).
Finally, handful amount of very recent evidence suggested that itaconate may be extracellularly secreted in pathological conditions such as lung fibrosis and infection (75, 87), although the precise physiological relevance and the mechanisms of action (e.g., Which types of immune cells are responsible for the secretion or serve as recipient cells? are the immunomodulatory effects on effector cells either suppressive or activating?) have yet to be elusive.
CONCLUSION AND PERSPECTIVE
The metabolic signatures of macrophages have recently been well-characterized. For example, macrophages upon pro-inflammatory stimulation display an increased glycolysis, and reduced capacity of oxidative phosphorylation, while mitochondrial fuel metabolisms are active in macrophages upon anti-inflammatory cues such as anti-inflammatory cytokines, as well as some pathogenic bacterial proteins (20). LPS and Interferon-γ (IFN-γ) are respectively known as the pro-inflammatory cytokines and the gram-negative bacteria-derived toxic compound, which serve as the key ligands for triggering the metabolic reprogramming (4, 6); LPS and IFN-γ are both required for the full activation of glycolysis, while the differential downstream targets of IFN-γ suppress the oxidative phosphorylation in order to complete the shaping of the pro-inflammatory metabolic signature (88).
An initial impression where metabolism is simply one of the effectors of the classically important components in the stimulation of macrophages, is mainly due to the supposedly limited functions as the supplier of biosynthetic precursors and energy power. However, a growing number of studies have provided significant evidence whereby some metabolites derived from the rewired metabolism in turn function as critical ‘regulatory signals’ to essentially attune the classic key components of macrophages stimulation (12). Such metabolites are referred to as immunomodulatory metabolites, and, herein we discussed the recent advances in understanding the functional and physiological relevance of these metabolites within the macrophages’ inflammatory response.
Although in this review we only focused on 5 key metabolites which serve directly as regulatory signals for the immunomodulatory function, recent studies have proposed that some metabolites also indirectly exert immunomodulatory function; namely, their metabolic product/substrate provide regulatory signals through immunomodulation. For example, citrate participates in the regulating of epigenetic and transcriptional reprogramming during LPS stimulation, as evidently shown in studies where 1) LPS employs the mechanism of citrate transporter (CIC)-dependent mitochondrial citrate export to cytosol where it serves as a biosynthetic precursor for prostaglandin E2, a critical regulatory signal for amplifying the pro-IL-1β-producing pathway, and 2) ATP-citrate lyase functions as a crucial component of the macrophages’ inflammatory response through its enzymatic activity, which breaks down cytosolic citrate to produce acetyl-CoA, a key regulatory signal that links metabolism with the epigenetic regulations required for proinflammatory gene expression, such as IL-6 production (89-91).
Besides the classic roles of glutamine and glutamate in serving as significant energy sources and integrating amino acids and carbon metabolism, they also display indirect immunomodulatory functions; their metabolic fate in macrophages is rapidly rewired upon stimulation, thereby producing the metabolic products which serve as the main biosynthetic precursors for α-KG, succinate, fumarate, and itaconates, the key immunomodulatory metabolites discussed in this review (27, 39, 52, 92).
The field of immuno-metabolism has led to a previously unrecognized insight of how intracellular metabolism, a cellular physiology, can shape and remodel the systemic inflammatory response within macrophages as well as the relevant environmental milieu. The classic roles of metabolism as biological building blocks and powerplants have been challenged to fully recapitulate the metabolic regulation of macrophages’ inflammatory response, thereby leading to a discovery of immunomodulatory metabolites with unique regulatory signal functions. Although numerous efforts have been made in order to understand the spectrum of the functional relevance of immunomodulatory metabolites, the mechanistic details of the intracellular and extracellular function and the precise physiological/pathological relevance in macrophages’ inflammatory response still remain elusive, which is to be explored in the near future.
ACKNOWLEDGEMENTS
This study was supported by a research funding of Chungnam National University.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551–567. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- 2.Netea MG, Balkwill F, Chonchol M, et al. A guiding map for inflammation. Nat Immunol. 2017;18:826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe S, Alexander M, Misharin AV, Budinger GRS. The role of macrophages in the resolution of inflammation. J Clin Invest. 2019;129:2619–2628. doi: 10.1172/JCI124615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic instruction of immunity. Cell. 2017;169:570–586. doi: 10.1016/j.cell.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makowski L, Chaib M, Rathmell JC. Immunometabolism: from basic mechanisms to translation. Immunol Rev. 2020;295:5–14. doi: 10.1111/imr.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 11.Liberti MV, Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly B, O'Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagg D, Englund MC, Jernas M, et al. Oxidized LDL induces a coordinated up-regulation of the glutathione and thioredoxin systems in human macrophages. Atherosclerosis. 2006;185:282–289. doi: 10.1016/j.atherosclerosis.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 15.Hibbs JB, Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/S0006-291X(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 16.Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anders CB, Lawton TMW, Ammons MCB. Metabolic immunomodulation of macrophage functional plasticity in nonhealing wounds. Curr Opin Infect Dis. 2019;32:204–209. doi: 10.1097/QCO.0000000000000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakrani MN, Wineland RH, Anjum F. Physiology, glucose metabolism. statpearls; treasure island (FL): 2022. Bookshelf ID: NBK560599. [DOI] [PubMed] [Google Scholar]
- 19.Freemerman AJ, Johnson AR, Sacks GN, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289:7884–7896. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blagih J, Jones RG. Polarizing macrophages through reprogramming of glucose metabolism. Cell Metab. 2012;15:793–795. doi: 10.1016/j.cmet.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita H, Takenoshita M, Sakurai M, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci U S A. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci U S A. 2001;98:13710–13715. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdul-Wahed A, Guilmeau S, Postic C. Sweet sixteenth for ChREBP: established roles and future goals. Cell Metab. 2017;26:324–341. doi: 10.1016/j.cmet.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Iizuka K, Takao K, Yabe D. ChREBP-mediated regulation of lipid metabolism: involvement of the gut microbiota, liver, and adipose tissue. Front Endocrinol (Lausanne) 2020;11:587189. doi: 10.3389/fendo.2020.587189.35f3d5af8ab0480d97199b2d5bee4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortega-Prieto P, Postic C. Carbohydrate sensing through the transcription factor ChREBP. Front Genet. 2019;10:472. doi: 10.3389/fgene.2019.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarrazy V, Sore S, Viaud M, et al. Maintenance of macrophage redox status by ChREBP limits inflammation and apoptosis and protects against advanced atherosclerotic lesion formation. Cell Rep. 2015;13:132–144. doi: 10.1016/j.celrep.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 27.Jha AK, Huang SC, Sergushichev A, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Lane EA, Choi DW, Garcia-Haro L, et al. HCF-1 regulates de novo lipogenesis through a nutrient-sensitive complex with ChREBP. Mol Cell. 2019;75:357–371. doi: 10.1016/j.molcel.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao D, Zeng L, Yao K, Kong X, Wu G, Yin Y. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids. 2016;48:2067–2080. doi: 10.1007/s00726-016-2254-8. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Yang J, Wu Z. The regulatory role of alpha-ketoglutarate metabolism in macrophages. Mediators Inflamm. 2021;2021:5577577. doi: 10.1155/2021/5577577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schofield CJ, Zhang Z. Structural and mechanistic studies on 2-oxoglutarate-dependent oxygenases and related enzymes. Curr Opin Struct Biol. 1999;9:722–731. doi: 10.1016/S0959-440X(99)00036-6. [DOI] [PubMed] [Google Scholar]
- 32.Kaelin WG., Jr Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–682. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- 33.Cummins EP, Berra E, Comerford KM, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng MX, Cao D, Chen Y, Li JZ, Tu B, Gong JP. Alpha-ketoglutarate attenuates ischemia-reperfusion injury of liver graft in rats. Biomed Pharmacother. 2019;111:1141–1146. doi: 10.1016/j.biopha.2018.12.149. [DOI] [PubMed] [Google Scholar]
- 35.Wilson MG, Lin MS. Prenatal diagnosis of mosaicism for del (18) (q12.2q21.1) and a normal cell line. J Med Genet. 1988;25:635–636. doi: 10.1136/jmg.25.9.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chin RM, Fu X, Pai MY, et al. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510:397–401. doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran KA, Dillingham CM, idharan R., Sr The role of alpha-ketoglutarate-dependent proteins in pluripotency acquisition and maintenance. J Biol Chem. 2019;294:5408–5419. doi: 10.1074/jbc.TM118.000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu PS, Wang H, Li X, et al. Alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18:985–994. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- 39.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harber KJ, de Goede KE, Verberk SGS, et al. Succinate is an inflammation-induced immunoregulatory metabolite in macrophages. Metabolites. 2020;10:372. doi: 10.3390/metabo10090372.362e1315cbf4439eacd33d59b4a64102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 42.Palsson-McDermott EM, Curtis AM, Goel G, et al. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21:65–80. doi: 10.1016/j.cmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills EL, Kelly B, Logan A, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–470. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elia I, Haigis MC. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat Metab. 2021;3:21–32. doi: 10.1038/s42255-020-00317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fremder M, Kim SW, Khamaysi A, et al. A transepithelial pathway delivers succinate to macrophages, thus perpetuating their pro-inflammatory metabolic state. Cell Rep. 2021;36:109521. doi: 10.1016/j.celrep.2021.109521. [DOI] [PubMed] [Google Scholar]
- 46.Keiran N, Ceperuelo-Mallafre V, Calvo E, et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat Immunol. 2019;20:581–592. doi: 10.1038/s41590-019-0372-7. [DOI] [PubMed] [Google Scholar]
- 47.Trauelsen M, Hiron TK, Lin D, et al. Extracellular succinate hyperpolarizes M2 macrophages through SUCNR1/GPR91-mediated Gq signaling. Cell Rep. 2021;35:109246. doi: 10.1016/j.celrep.2021.109246. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Gibson GE. Succinylation links metabolism to protein functions. Neurochem Res. 2019;44:2346–2359. doi: 10.1007/s11064-019-02780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alleyn M, Breitzig M, Lockey R, Kolliputi N. The dawn of succinylation: a posttranslational modification. Am J Physiol Cell Physiol. 2018;314:C228–C232. doi: 10.1152/ajpcell.00148.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7:58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rardin MJ, He W, Nishida Y, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18:920–933. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arts RJ, Novakovic B, Ter Horst R, et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016;24:807–819. doi: 10.1016/j.cmet.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34:216–223. doi: 10.1016/j.it.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tong WH, Sourbier C, Kovtunovych G, et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell. 2011;20:315–327. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cummins EP, Keogh CE, Crean D, Taylor CT. The role of HIF in immunity and inflammation. Mol Aspects Med -48. 2016;47:24–34. doi: 10.1016/j.mam.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Han R, Xiao J, Zhai H, Hao J. Dimethyl fumarate attenuates experimental autoimmune neuritis through the nuclear factor erythroid-derived 2-related factor 2/hemoxygenase-1 pathway by altering the balance of M1/M2 macrophages. J Neuroinflammation. 2016;13:97. doi: 10.1186/s12974-016-0559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Majkutewicz I. Dimethyl fumarate: a review of preclinical efficacy in models of neurodegenerative diseases. Eur J Pharmacol. 2022;926:175025. doi: 10.1016/j.ejphar.2022.175025. [DOI] [PubMed] [Google Scholar]
- 58.Brennan MS, Matos MF, Li B, et al. Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS One. 2015;10:e0120254. doi: 10.1371/journal.pone.0120254.91b25af29668434ab4aa49d5b7aec570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blatnik M, Thorpe SR, Baynes JW. Succination of proteins by fumarate: mechanism of inactivation of glyceraldehyde-3-phosphate dehydrogenase in diabetes. Ann N Y Acad Sci. 2008;1126:272–275. doi: 10.1196/annals.1433.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jove M, Pradas I, Mota-Martorell N, et al. Succination of protein thiols in human brain aging. Front Aging Neurosci. 2020;12:52. doi: 10.3389/fnagi.2020.00052.e8b834370b12414a8b30a10a01a61d16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagai R, Brock JW, Blatnik M, et al. Succination of protein thiols during adipocyte maturation: a biomarker of mitochondrial stress. J Biol Chem. 2007;282:34219–34228. doi: 10.1074/jbc.M703551200. [DOI] [PubMed] [Google Scholar]
- 62.Ruecker N, Jansen R, Trujillo C, et al. Fumarase deficiency causes protein and metabolite succination and intoxicates mycobacterium tuberculosis. Cell Chem Biol. 2017;24:306–315. doi: 10.1016/j.chembiol.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Neill LAJ, Artyomov MN. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat Rev Immunol. 2019;19:273–281. doi: 10.1038/s41577-019-0128-5. [DOI] [PubMed] [Google Scholar]
- 64.Michelucci A, Cordes T, Ghelfi J, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci U S A. 2013;110:7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peace CG, O'Neill LA. The role of itaconate in host defense and inflammation. J Clin Invest. 2022;132:e148548. doi: 10.1172/JCI148548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen M, Sun H, Boot M, et al. Itaconate is an effector of a Rab GTPase cell-autonomous host defense pathway against Salmonella. Science. 2020;369:450–455. doi: 10.1126/science.aaz1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruetz M, Campanello GC, Purchal M, et al. Itaconyl-CoA forms a stable biradical in methylmalonyl-CoA mutase and derails its activity and repair. Science. 2019;366:589–593. doi: 10.1126/science.aay0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lampropoulou V, Sergushichev A, Bambouskova M, et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016;24:158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu X, Guo Y, Liu Z, Yang J, Tang H, Wang Y. Itaconic acid exerts anti-inflammatory and antibacterial effects via promoting pentose phosphate pathway to produce ROS. Sci Rep. 2021;11:18173. doi: 10.1038/s41598-021-97352-x.959d8c87bc274311acd4d3ae86aa21eb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mills EL, Ryan DG, Prag HA, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556:113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao ST, Han C, Xu DQ, Fu XW, Wang JS, Kong LY. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat Commun. 2019;10:5091. doi: 10.1038/s41467-019-13078-5.2b66b3e7c6704dc1a4818c1e7d684e87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hooftman A, Angiari S, Hester S, et al. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab. 2020;32:468–478. doi: 10.1016/j.cmet.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daniels BP, Kofman SB, Smith JR, et al. The nucleotide sensor ZBP1 and kinase RIPK3 induce the enzyme IRG1 to promote an antiviral metabolic state in neurons. Immunity. 2019;50:64–76. doi: 10.1016/j.immuni.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He R, Liu B, Xiong R, et al. Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury. Cell Death Discov. 2022;8:43. doi: 10.1038/s41420-021-00807-3.2f0a49725c994bff8826064c8c59cfa5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ogger PP, Albers GJ, Hewitt RJ, et al. Itaconate controls the severity of pulmonary fibrosis. Sci Immunol. 2020;5:eabc1884. doi: 10.1126/sciimmunol.abc1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sohail A, Iqbal AA, Sahini N, et al. Itaconate and derivatives reduce interferon responses and inflammation in influenza A virus infection. PLoS Pathog. 2022;18:e1010219. doi: 10.1371/journal.ppat.1010219.566eee10fa3944c78bd62fa5ef5ffdb5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jaiswal AK, Yadav J, Makhija S, et al. Irg1/itaconate metabolic pathway is a crucial determinant of dendritic cells immune-priming function and contributes to resolute allergen-induced airway inflammation. Mucosal Immunol. 2022;15:301–313. doi: 10.1038/s41385-021-00462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang S, Jiao Y, Li C, et al. Dimethyl itaconate alleviates the inflammatory responses of macrophages in sepsis. Inflammation. 2021;44:549–557. doi: 10.1007/s10753-020-01352-4. [DOI] [PubMed] [Google Scholar]
- 79.Hooftman A, O'Neill LAJ. The immunomodulatory potential of the metabolite itaconate. Trends Immunol. 2019;40:687–698. doi: 10.1016/j.it.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 80.ElAzzouny M, Tom CT, Evans CR, et al. Dimethyl itaconate is not metabolized into itaconate intracellularly. J Biol Chem. 2017;292:4766–4769. doi: 10.1074/jbc.C117.775270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swain A, Bambouskova M, Kim H, et al. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat Metab. 2020;2:594–602. doi: 10.1038/s42255-020-0210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dominguez-Andres J, Novakovic B, Li Y, et al. The itaconate pathway is a central regulatory node linking innate immune tolerance and trained immunity. Cell Metab. 2019;29:211–220. doi: 10.1016/j.cmet.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 83.Runtsch MC, Angiari S, Hooftman A, et al. Itaconate and itaconate derivatives target JAK1 to suppress alternative activation of macrophages. Cell Metab. 2022;34:487–501. doi: 10.1016/j.cmet.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Z, Chen C, Yang F, et al. Itaconate is a lysosomal inducer that promotes antibacterial innate immunity. Mol Cell. 2022;82:2844–2857. doi: 10.1016/j.molcel.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 85.Schuster EM, Epple MW, Glaser KM, et al. TFEB induces mitochondrial itaconate synthesis to suppress bacterial growth in macrophages. Nat Metab. 2022;4:856–866. doi: 10.1038/s42255-022-00605-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoo HJ, Choi DW, Roh YJ, et al. MsrB1-regulated GAPDH oxidation plays programmatic roles in shaping metabolic and inflammatory signatures during macrophage activation. Cell Rep. 2022;41:111598. doi: 10.1016/j.celrep.2022.111598. [DOI] [PubMed] [Google Scholar]
- 87.Wong Fok Lung T, Charytonowicz D, Beaumont KG, et al. Klebsiella pneumoniae induces host metabolic stress that promotes tolerance to pulmonary infection. Cell Metab. 2022;34:761–774. doi: 10.1016/j.cmet.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cameron AM, Castoldi A, Sanin DE, et al. Inflammatory macrophage dependence on NAD(+) salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat Immunol. 2019;20:420–432. doi: 10.1038/s41590-019-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Infantino V, Iacobazzi V, Palmieri F, Menga A. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem Biophys Res Commun. 2013;440:105–111. doi: 10.1016/j.bbrc.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 91.Hu L, Yu Y, Huang H, et al. Epigenetic regulation of interleukin 6 by histone acetylation in macrophages and its role in paraquat-induced pulmonary fibrosis. Front Immunol. 2016;7:696. doi: 10.3389/fimmu.2016.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palmieri EM, Menga A, Martin-Perez R, et al. Pharmacologic or genetic targeting of glutamine synthetase skews macrophages toward an M1-like phenotype and inhibits tumor metastasis. Cell Rep. 2017;20:1654–1666. doi: 10.1016/j.celrep.2017.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]