Abstract
Ribosomes, acting as the cellular factories for protein production, are essential for all living organisms. Ribosomes are composed of both proteins and RNAs and are established through the coordination of several steps, including transcription, maturation of ribosomal RNA (rRNA), and assembly of ribosomal proteins. In particular, diverse factors required for ribosome biogenesis, such as transcription factors, small nucleolar RNA (snoRNA)-associated proteins, and assembly factors, are tightly regulated by various post-translational modifications. Among these modifications, small ubiquitin-related modifier (SUMO) targets lots of proteins required for gene expression of ribosomal proteins, rRNA, and snoRNAs, rRNA processing, and ribosome assembly. The tight control of SUMOylation affects functions and locations of substrates. This review summarizes current studies and recent progress of SUMOylation-mediated regulation of ribosome biogenesis.
Keywords: Ribosome assembly factor, Ribosome biogenesis, Ribosomal protein, rRNA, snoRNA, SUMO
INTRODUCTION
Ribosomes are macromolecular complexes consisted of both proteins and RNAs. They are cellular factories for the translation of mRNAs into corresponding proteins (1, 2). In most eukaryotes, individual ribosomal proteins synthesized in the cytoplasm are reimported into the nucleus, while ribosomal RNA (rRNA) is synthesized in a nucleolus, a discrete compartment within the nucleus (3). The coordination between more than 200 assembly factors and many small nucleolar RNAs (snoRNAs) is essential for ribosome biogenesis and leads to the build-up of the 40S and 60S pre-ribosomal particles. The 40S particle contains distinct ribosomal proteins with 18S rRNA, whereas the 60S particle contains 28S, 5.8S, and 5S rRNA. Both subunits are exported to the cytoplasm for final maturation, and the 80S ribosome is eventually assembled from the 40S and 60S subunits to initiate protein synthesis (4). The ribosome biogenesis pathway requires the precise regulation of multiple steps, including transcription as well as protein assembly and transport. The transcriptional control of rRNA and ribosomal protein-encoding genes is the first rate-limiting step in biosynthesis. It is regulated by diverse pathways, such as PI3K/AKT/mTOR, RAS/RAF/MEK, p53, and Myc, which act as master regulators of cell proliferation (5-8). Subsequently, several regulatory factors engage in various steps during the successful maturation of pre-rRNA transcripts and ribosome assembly and export; these processes are tightly regulated by various post-translational modifications, including the small ubiquitin-related modifier (SUMO) pathway (9). Recent investigations utilizing biochemical and genome-wide analyses have significantly contributed toward our understanding of SUMO modification in ribosome synthesis and maturation; therefore, in the present review, we provide an overview of the newly discovered functions of SUMO in ribosome biogenesis.
THE SUMO PATHWAY
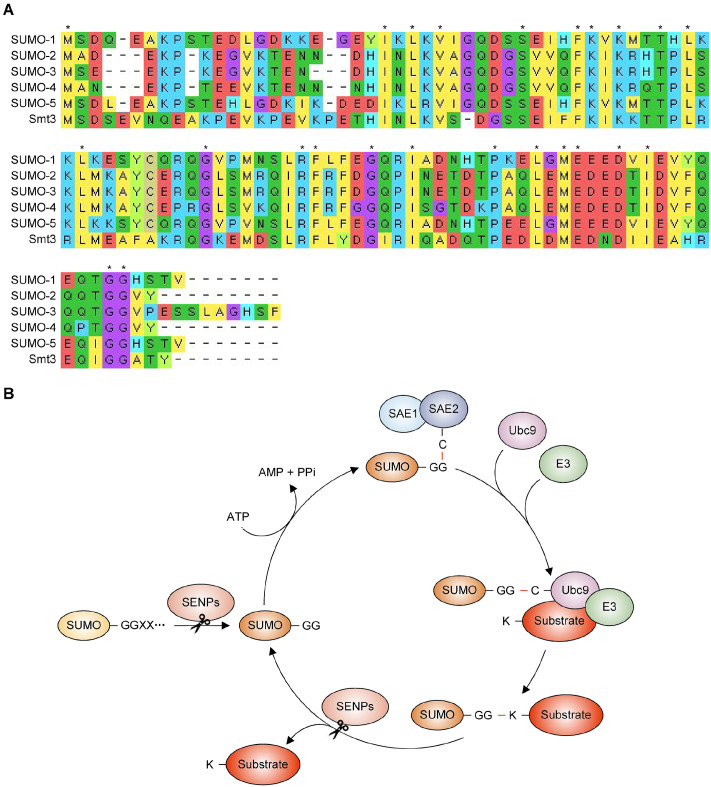
SUMO is an evolutionarily conserved protein that plays important roles in diverse cellular processes, including DNA replication, transcription, translation, viral infection, stress response, and ribosome biogenesis (10-14). Humans have five genes that encode SUMO paralogs, SUMO-1 to SUMO-5, while yeast Saccharomyces cerevisiae expresses a single SUMO ortholog, Smt3, sharing 48% identity and 75% similarity with SUMO-1 (15, 16) (Fig. 1A). SUMO-2 and SUMO-3 are almost identical, while SUMO-2/3 shares about 50% similarity with SUMO-1 (17). SUMO proteins are initially translated as precursors with C-terminal extension, and their C-terminal tails are then cleaved by SUMO-specific proteases to yield mature proteins with a pair of Gly residues (18). However, there is no in vivo experimental evidence for the ligation capacity of SUMO-4 or SUMO-5 (16, 19). The mature form of SUMO protein activated by SUMO-activating enzyme unit 1SAE1/SAE2 (Aos1/Uba2 in S. cerevisiae) is transferred to a cysteine in the Ubc9 SUMO-conjugating E2 enzyme, followed by the transfer of SUMO by SUMO E3 ligases from E2 to lysine residue(s) on substrate proteins (Fig. 1B) (20). In addition, SUMO can be assembled into polymeric chains on substrates, and such SUMO chains are disassembled by SUMO-specific proteases (SENPs) (18). Six SENPs (Ulp1 and Ulp2 in S. cerevisiae) have been studied for their functions and localizations in humans, but the recently discovered three SENPs have not been extensively investigated. SUMO conjugation can alter the interaction between its substrate and binding partner proteins, which possess one or more SUMO-interaction motifs (SIMs) for recognizing SUMO-conjugated proteins, maintaining protein stability, or bringing conformational changes in target proteins (21).
Fig. 1.
Alignment of SUMO protein sequences and SUMO pathway diagram. (A) Sequence alignment of human SUMO-1 through SUMO-5 with yeast SUMO (Smt3) using Molecular Evolutionary Genetic Analysis software (MEGA, https://www.megasoftware.net/). Different letters and colors indicate different amino acids, and asterisks denote amino acids conserved among all SUMO proteins. (B) Diagram of the SUMOylation cycle. The precursor form of SUMO is processed by SENP proteases to create a mature form with a C-terminal Gly-Gly (GG) motif. Mature SUMO is ATP-dependent and activated by heterodimeric E1 SUMO-activating enzymes, SAE1 and SAE2, through a catalytic cysteine (C) residue in SAE2. Next, SUMO is transferred to the C residue of the E2 SUMO-conjugating enzyme (Ubc9), resulting in the conjugation of SUMO to the lysine (K) residues of the substrate protein with the aid of an E3 SUMO ligase. Finally, SENPs can deconjugate SUMO from the substrate or edit SUMO chains, after which SUMO is recycled through the conjugation event.
SUMO REGULATES RIBOSOME BIOGENESIS
Ribosomal proteins are general targets of SUMO
Recent advances in molecular techniques and quantitative proteomics have revealed several interesting SUMO target proteins. Pioneer studies have been conducted in S. cerevisiae (22-26). Although SUMO substrates have been found in all cellular compartments, amounts of SUMO-conjugated proteins are much higher in the nucleus than in other regions. This finding is consistent with the extremely concentrated SUMO and SUMO pathway enzymes in the nucleus. Interestingly, all yeast studies have revealed proportionally high numbers of ribosomal proteins and assembly factors as SUMO targets, suggesting that SUMO is closely linked to ribosome biogenesis and remodeling. Furthermore, the SUMOylated ribosome itself and its regulators are also observed in proteomic analyses for the detection of targets of all three active SUMO isoforms (SUMO-1 to -3) in human cells or stem cells (27-34). Although factors involved in ribosome biogenesis are major targets of the SUMO pathway, how such SUMO modifications affect ribosome development and the mechanism underlying the regulation of SUMO conjugation levels of ribosomes are yet unknown.
SUMO promotes expression of ribosomal genes and rRNA
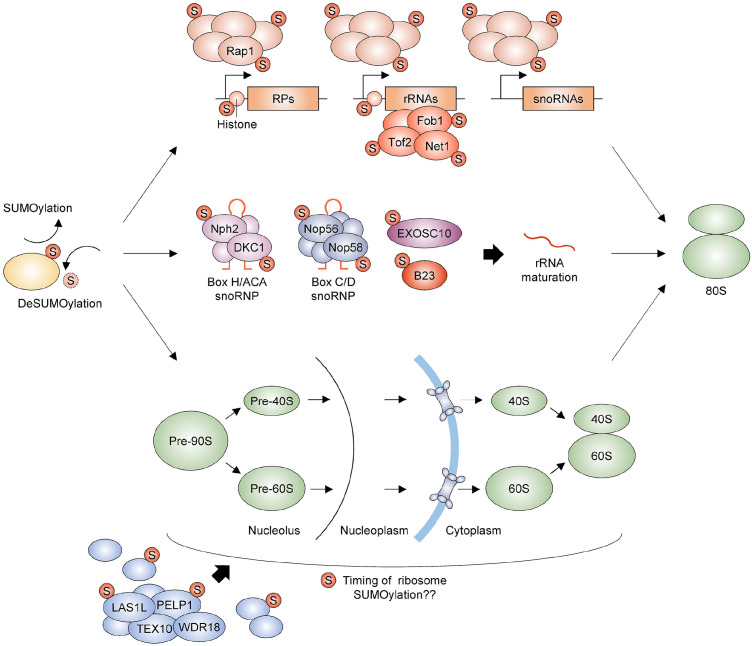
Primary target proteins of SUMO are transcription factors and chromatin-associated proteins in eukaryotes (35, 36). It was initially thought that SUMO mainly would suppress gene transcription because it either blocked the function of transcription activators or facilitated the function of transcription repressors (37). However, recent investigations have uncovered its more diverse roles in co-transcriptional processes, including transcription activation and chromatin remodeling (35). In particular, SUMO is highly enriched in genes encoding ribosomal proteins and rRNA in human cells, and inhibition of SUMOylation leads to expression upregulation of these genes, implying that SUMO normally plays a role in limiting their expression (Fig. 2) (38). Human PIAS SUMO E3 ligases are indirectly involved in the repression of rRNA transcription by suppressing the expression of upstream binding factor and c-Myc, which are required for rRNA transcription (39). On the other hand, a study in S. cerevisiae has reported conflicting results on the correlation between SUMO and ribosomal protein expression (40). SUMO most prominently occurs at ribosomal protein genes and positively regulates their expression in S. cerevisiae. In particular, SUMOylation of the transcription factor Rap1 leads to the recruitment of basal transcription machinery transcription factor IID (TFIID) for promoting transcription of ribosomal protein genes. In addition, SUMO protease Ulp2 is strongly associated with genes encoding ribosomal protein snoRNA as well as rRNA, and loss of Ulp2 expression leads to upregulation of their expression. This finding is consistent with the function of SUMO as a transcription activator (41, 42). Ulp2 can govern the level of SUMO conjugation and rDNA binding of Net1, Tof2, and Fob1, which are required for rDNA silencing (43). Histone SUMOylation has diverse roles in transcriptional regulation and is highly enriched at ribosomal protein and rDNA loci (44, 45). However, its function in ribosome biogenesis has not been reported yet.
Fig. 2.
SUMOylation regulates ribosome biogenesis. SUMOylation and deSUMOylation are highly dynamic cellular processes, and their versatile control is essential for ribosome biogenesis. First, several transcription factors, including yeast Rap1, are major substrates of the SUMO pathway. Their SUMOylation regulates the expression of genes encoding ribosomal proteins (RPs) and rRNAs (components of ribosomes) and snoRNAs (required for rRNA maturation). While the association of Net1, Tof2, and Fob1, which are required for rDNA silencing, with rRNA-encoding rDNA locus is regulated by their SUMOylation, SUMOylated histones are substantially located at RP genes and rDNA regions. However, the role of histone SUMOylation in these regions remains unelucidated. Second, the control of SUMOylation of snoRNP proteins, Nph2, DKC1, Nop56, and Nop58, the RNA exosome EXOSC10, and B23/nucleophosmin is required for rRNA maturation. Finally, ribosomal proteins and their assembly factors, including LAS1L and PELP1, are common targets of SUMOylation. However, the timing of SUMOylation required for successful ribosome assembly and export remains unknown. The illustration presents relevant components but not precise physical interactions between proteins or event orders.
SUMO affects rRNA processing
rRNA processing is essential for ribosome biogenesis. It is mediated by small nucleolar ribonucleoprotein complexes (snoRNPs) composed of snoRNAs and nucleolar proteins (46). snoRNAs are classified into two groups, box C/D snoRNAs responsible for 2’-O-ribose methylation and box H/ACA snoRNAs for mediating pseudouridylation. SUMOylation of the core box C/D snoRNP protein Nop58 is imperative for its association with snoRNAs, nucleolar positioning of snoRNAs, and proper snoRNP assembly, and blockade of SUMO conjugation to Nop58 facilitates proteasome-dependent protein degradation in the nucleoplasm (47). SUMOylation of the box H/ACA snoRNP protein Nhp2 is also involved in snoRNP biogenesis in the nucleoplasm (47). SENP3 can directly interact with B23/nucleophosmin, involved in ribosome biogenesis and export, and its loss leads to a defect in 28S rRNA maturation (48). Depletion of SENP5 has similar but milder effects on rRNA processing (49). In addition, USP36 deubiquitinase can physically interact with SUMO-2 and Ubc9 and promote SUMO conjugation with Nop56, Nop58, Nph2, and DKC1, eventually facilitating rRNA processing and translation (50). Cellular level of RNA exosome EXOSC10, required for 3’ pre-ribosomal RNA processing, is regulated by SUMO-1 conjugation to EXOSC10 (51). Taken together, these findings suggest that dynamic SUMO modification of nucleolar proteins is one of the critical factors for snoRNP-mediated rRNA modifications (Fig. 2).
SUMO guides ribosome assembly
During ribosome biogenesis, 90S pre-ribosomal particles are established in the nucleolus and then split into 60S and 40S pre-ribosomes. These pre-ribosomal subunits are transported into the cytoplasm for final maturation (52). Human SENP3 is co-purified with PELP1, TEX10, WDR18, and LAS1L. SENP3-mediated control of SUMO conjugation level of PELP1 and LAS1L is essential for the maturation of rRNA and nucleolar export of 60S pre-ribosomal particles (53-55). SUMO can negatively affect conjugation of NEDD8, another ubiquitin-like protein, to human Rpl11, and facilitate the translocation of Rpl11 from nucleoli (56). Rps3, a DNA repair endonuclease, is also a substrate of the SUMO pathway that increases the stability of Rps3 protein (57). SUMOylation of Rpl22e is important for nucleoplasmic distribution of Rpl22 in Drosophila meiotic spermatocytes (58). SUMO protease SMT7-mediated control of SUMO levels on Rpl30 might affect various cellular processes, including cell division in Chlamydomonas reinhardtii (59). In S. cerevisiae, an additional copy of the UBA2 gene complements abnormal nucleolar accumulation of the ribosomal 60S subunit Rpl25 in a rix16-1 mutant strain, in which the export of the pre-60S ribosomal subunit is impaired and mutations in ubc9, ulp1, and smt3 causes export defects of pre-60S particles (60). In particular, Ulp1 genetically interacts with nuclear export factor Mtr2 in the pre-60S export pathway. However, their exact correlation has not been reported yet. Taken together, these findings indicate that the SUMO pathway ensures the fidelity of pre-ribosome import into the cytoplasm and routes ribosome maturation via successful assembly of ribosome subsets (Fig. 2).
CONCLUSION
SUMOylation is known to play critical roles in ribosome biogenesis, and regulation of this modification is associated with gene expression, nuclear import, and assembly of ribosomal subunits. However, the ultimate and detailed functions of the SUMO pathway in ribosome establishment have remained unclear until recently. Here, we briefly summarize recent observations of how the SUMO pathway is involved in ribosome biogenesis. Several ribosomal proteins themselves and various factors required for ribosome assembly are substrates of SUMOylation. These SUMO modifications are tightly regulated by SUMO-specific proteases, leading to regulation of gene expression, localization, and function as well as proteolytic control of target proteins during ribosome maturation. Functionally healthy ribosomes are vital for cell survival, and several mutations in ribosomes or ribosome assembly factors have been found to be lethal (61, 62). Especially, specific defects in ribosome biogenesis or function could cause various clinical abnormalities, including skin and bone marrow failure syndromes such as X-linked dyskeratosis congenita and Schwachman-Diamond syndrome (63, 64). Thus, studying SUMO functions in ribosome biogenesis and activities might provide clue to develop new therapies and drug targets for human disorders of ribosome dysfunction.
ACKNOWLEDGEMENTS
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the South Korean government (MSIT) (nos. 2020R1C1C1009367 and 2020R1A4A1018280) and Korean Environment Industry & Technology Institute (KEITI) through Core Technology Development Project for Environmental Diseases Prevention and Management funded by Korean Ministry of Environment (MOE) (no. 2022003310001).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Thomson E, Ferreira-Cerca S, Hurt E. Eukaryotic ribosome biogenesis at a glance. J Cell Sci. 2013;126:4815–4821. doi: 10.1242/jcs.111948. [DOI] [PubMed] [Google Scholar]
- 2.Bassler J, Hurt E. Eukaryotic ribosome assembly. Annu Rev Biochem. 2019;88:281–306. doi: 10.1146/annurev-biochem-013118-110817. [DOI] [PubMed] [Google Scholar]
- 3.Kressler D, Hurt E, Bassler J. Driving ribosome assembly. Biochim Biophys Acta. 2010;1803:673–683. doi: 10.1016/j.bbamcr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Henras AK, Soudet J, Gerus M, et al. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell Mol Life Sci. 2008;65:2334–2359. doi: 10.1007/s00018-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diwakarla C, Hannan K, Hein N, Yip D. Advanced pancreatic ductal adenocarcinoma - complexities of treatment and emerging therapeutic options. World J Gastroenterol. 2017;23:2276–2285. doi: 10.3748/wjg.v23.i13.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nosrati N, Kapoor NR, Kumar V. Combinatorial action of transcription factors orchestrates cell cycle-dependent expression of the ribosomal protein genes and ribosome biogenesis. FEBS J. 2014;281:2339–2352. doi: 10.1111/febs.12786. [DOI] [PubMed] [Google Scholar]
- 7.Martin DE, Soulard A, Hall MN. TOR regulates ribosomal protein gene expression via PKA and the forkhead transcription factor FHL1. Cell. 2004;119:969–979. doi: 10.1016/j.cell.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 8.Neumansilberberg FS, Bhattacharya S, Broach JR. Nutrient availability and the ras/cyclic amp pathway both induce expression of ribosomal-protein genes in saccharomyces-cerevisiae but by different mechanisms. Mol Cell Biol. 1995;15:3187–3196. doi: 10.1128/MCB.15.6.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simsek D, Barna M. An emerging role for the ribosome as a nexus for post-translational modifications. Curr Opin Cell Biol. 2017;45:92–101. doi: 10.1016/j.ceb.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 2013;82:357–385. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- 11.Ryu HY. SUMO: a novel target for anti-coronavirus therapy. Pathog Glob Health. 2021;115:292–299. doi: 10.1080/20477724.2021.1906562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu HY, Ahn SH, Hochstrasser M. SUMO and cellular adaptive mechanisms. Exp Mol Med. 2020;52:931–939. doi: 10.1038/s12276-020-0457-2.e284bab50fa64d9ab5b52117e9c0a65e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaikam V, Karlson DT. Response and transcriptional regulation of rice SUMOylation system during development and stress conditions. BMB Rep. 2010;43:103–109. doi: 10.5483/BMBRep.2010.43.2.103. [DOI] [PubMed] [Google Scholar]
- 14.Ryu HY, Wilson NR, Mehta S, Hwang SS, Hochstrasser M. Loss of the SUMO protease Ulp2 triggers a specific multichromosome aneuploidy. Genes Dev. 2016;30:1881–1894. doi: 10.1101/gad.282194.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang WC, Ko TP, Li SS, Wang AH. Crystal structures of the human SUMO-2 protein at 1.6 A and 1.2 A resolution: implication on the functional differences of SUMO proteins. Eur J Biochem. 2004;271:4114–4122. doi: 10.1111/j.1432-1033.2004.04349.x. [DOI] [PubMed] [Google Scholar]
- 16.Liang YC, Lee CC, Yao YL, Lai CC, Schmitz ML, Yang WM. SUMO5, a novel poly-SUMO isoform, regulates PML nuclear bodies. Sci Rep. 2016;6:26509. doi: 10.1038/srep26509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller S, Hoege C, Pyrowolakis G, Jentsch S. SUMO, ubiquitin's mysterious cousin. Nat Rev Mol Cell Biol. 2001;2:202–210. doi: 10.1038/35056591. [DOI] [PubMed] [Google Scholar]
- 18.Hickey CM, Wilson NR, Hochstrasser M. Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol. 2012;13:755–766. doi: 10.1038/nrm3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owerbach D, McKay EM, Yeh ETH, Gabbay KH, Bohren KM. A proline-90 residue unique to SUMO-4 prevents maturation and sumoylation. Biochem Biophys Res Commun. 2005;337:517–520. doi: 10.1016/j.bbrc.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 20.Hendriks IA, Vertegaal AC. A comprehensive compilation of SUMO proteomics. Nat Rev Mol Cell Biol. 2016;17:581–595. doi: 10.1038/nrm.2016.81. [DOI] [PubMed] [Google Scholar]
- 21.Song J, Durrin LK, Wilkinson TA, Krontiris TG, Chen YA. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc Natl Acad Sci U S A. 2004;101:14373–14378. doi: 10.1073/pnas.0403498101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wohlschlegel JA, Johnson ES, Reed SI, Yates JR. Global analysis of protein sumoylation in Saccharomyces cerevisiae. J Biol Chem. 2004;279:45662–45668. doi: 10.1074/jbc.M409203200. [DOI] [PubMed] [Google Scholar]
- 23.Hannich JT, Lewis A, Kroetz MB, et al. Defining the SUMO-modified proteome by multiple approaches in Saccharomyces cerevisiae. J Biol Chem. 2005;280:4102–4110. doi: 10.1074/jbc.M413209200. [DOI] [PubMed] [Google Scholar]
- 24.Zhou WD, Ryan JJ, Zhou HL. Global analyses of sumoylated proteins in Saccharomyces cerevisiae - induction of protein sumoylation by cellular stresses. J Biol Chem. 2004;279:32262–32268. doi: 10.1074/jbc.M404173200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esteras M, Liu IC, Snijders AP, Jarmuz A, Aragon L. Identification of SUMO conjugation sites in the budding yeast proteome. Microb Cell. 2017;4:331–341. doi: 10.15698/mic2017.10.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wykoff DD, O'Shea EK. Identification of sumoylated proteins by systematic immunoprecipitation of the budding yeast proteome. Mol Cell Proteomics. 2005;4:73–83. doi: 10.1074/mcp.M400166-MCP200. [DOI] [PubMed] [Google Scholar]
- 27.Mojsa B, Tatham MH, Davidson L, Liczmanska M, Branigan E, Hay RT. Identification of SUMO targets associated with the pluripotent state in human stem cells. Mol Cell Proteomics. 2021;20:100164. doi: 10.1016/j.mcpro.2021.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendriks IA, D'Souza RC, Yang B, Verlaan-de Vries M, Mann M, Vertegaal AC. Uncovering global SUMOylation signaling networks in a site-specific manner. Nat Struct Mol Biol. 2014;21:927–936. doi: 10.1038/nsmb.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tammsalu T, Matic I, Jaffray EG, Ibrahim AFM, Tatham MH, Hay RT. Proteome-wide identification of SUMO2 modification sites. Sci Signal. 2014;7:rs2. doi: 10.1126/scisignal.2005146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao Z, Chang JG, Hendriks IA, Sigurethsson JO, Olsen JV, Vertegaal AC. System-wide analysis of SUMOylation dynamics in response to replication stress reveals novel small ubiquitin-like modified target proteins and acceptor lysines relevant for genome stability. Mol Cell Proteomics. 2015;14:1419–1434. doi: 10.1074/mcp.O114.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendriks IA, Treffers LW, Verlaan-de Vries M, Olsen JV, Vertegaal ACO. SUMO-2 orchestrates chromatin modifiers in response to DNA damage. Cell Rep. 2015;10:1778–1791. doi: 10.1016/j.celrep.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Impens F, Radoshevich L, Cossart P, Ribet D. Mapping of SUMO sites and analysis of SUMOylation changes induced by external stimuli. Proc Natl Acad Sci U S A. 2014;111:12432–12437. doi: 10.1073/pnas.1413825111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamoliatte F, Caron D, Durette C, et al. Large-scale analysis of lysine SUMOylation by SUMO remnant immunoaffinity profiling. Nat Commun. 2014;5:5409. doi: 10.1038/ncomms6409. [DOI] [PubMed] [Google Scholar]
- 34.Hendriks IA, D'Souza RC, Chang JG, Mann M, Vertegaal AC. System-wide identification of wild-type SUMO-2 conjugation sites. Nat Commun. 2015;6:7289. doi: 10.1038/ncomms8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verger A, Perdomo J, Crossley M. Modification with SUMO. A role in transcriptional regulation. EMBO Rep. 2003;4:137–142. doi: 10.1038/sj.embor.embor738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi J, Ryoo ZY, Cho DH, Lee HS, Ryu HY. Trans-tail regulation-mediated suppression of cryptic transcription. Exp Mol Med. 2021;53:1683–1688. doi: 10.1038/s12276-021-00711-x.b7cf57632e8a48a381b88dd44b26e808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouyang J, Gill G. SUMO engages multiple corepressors to regulate chromatin structure and transcription. Epigenetics. 2009;4:440–444. doi: 10.4161/epi.4.7.9807. [DOI] [PubMed] [Google Scholar]
- 38.Neyret-Kahn H, Benhamed M, Ye T, et al. Sumoylation at chromatin governs coordinated repression of a transcriptional program essential for cell growth and proliferation. Genome Res. 2013;23:1563–1579. doi: 10.1101/gr.154872.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng Y, Wang Z, Wang Z, Yu F, Li J, Wong J. SUMOylation down-regulates rDNA transcription by repressing expression of upstream-binding factor and proto-oncogene c-Myc. J Biol Chem. 2019;294:19155–19166. doi: 10.1074/jbc.RA119.010624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chymkowitch P, Nguea AP, Aanes H, et al. Sumoylation of Rap1 mediates the recruitment of TFIID to promote transcription of ribosomal protein genes. Genome Res. 2015;25:897–906. doi: 10.1101/gr.185793.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryu HY, Su D, Wilson-Eisele NR, Zhao DJ, Lopez-Giraldez F, Hochstrasser M. The Ulp2 SUMO protease promotes transcription elongation through regulation of histone sumoylation. Embo J. 2019;38:e102003. doi: 10.15252/embj.2019102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryu HY, Lopez-Giraldez F, Knight J, et al. Distinct adaptive mechanisms drive recovery from aneuploidy caused by loss of the Ulp2 SUMO protease. Nat Commun. 2018;9:5417. doi: 10.1038/s41467-018-07836-0.fa07ec2c498048edae01417a7f9d5564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillies J, Hickey CM, Su D, Wu Z, Peng J, Hochstrasser M. SUMO pathway modulation of regulatory protein binding at the ribosomal DNA locus in Saccharomyces cerevisiae. Genetics. 2016;202:1377–1394. doi: 10.1534/genetics.116.187252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryu HY, Hochstrasser M. Histone sumoylation and chromatin dynamics. Nucleic Acids Res. 2021;49:6043–6052. doi: 10.1093/nar/gkab280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryu HY, Zhao D, Li J, Su D, Hochstrasser M. Histone sumoylation promotes Set3 histone-deacetylase complex-mediated transcriptional regulation. Nucleic Acids Res. 2020;48:12151–12168. doi: 10.1093/nar/gkaa1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watkins NJ, Bohnsack MT. The box C/D and H/ACA snoRNPs: key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip Rev RNA. 2012;3:397–414. doi: 10.1002/wrna.117. [DOI] [PubMed] [Google Scholar]
- 47.Westman BJ, Verheggen C, Hutten S, Lam YW, Bertrand E, Lamond AI. A proteomic screen for nucleolar SUMO targets shows SUMOylation modulates the function of Nop5/Nop58. Mol Cell. 2010;39:618–631. doi: 10.1016/j.molcel.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haindl M, Harasim T, Eick D, Muller S. The nucleolar SUMO-specific protease SENP3 reverses SUMO modification of nucleophosmin and is required for rRNA processing. EMBO Rep. 2008;9:273–279. doi: 10.1038/embor.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yun C, Wang Y, Mukhopadhyay D, et al. Nucleolar protein B23/nucleophosmin regulates the vertebrate SUMO pathway through SENP3 and SENP5 proteases. J Cell Biol. 2008;183:589–595. doi: 10.1083/jcb.200807185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryu H, Sun XX, Chen Y, et al. The deubiquitinase USP36 promotes snoRNP group SUMOylation and is essential for ribosome biogenesis. EMBO Rep. 2021;22:e50684. doi: 10.15252/embr.202050684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knight JR, Bastide A, Peretti D, et al. Cooling-induced SUMOylation of EXOSC10 down-regulates ribosome biogenesis. RNA. 2016;22:623–635. doi: 10.1261/rna.054411.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson AW, Lund E, Dahlberg J. Nuclear export of ribosomal subunits. Trends Biochem Sci. 2002;27:580–585. doi: 10.1016/S0968-0004(02)02208-9. [DOI] [PubMed] [Google Scholar]
- 53.Finkbeiner E, Haindl M, Muller S. The SUMO system controls nucleolar partitioning of a novel mammalian ribosome biogenesis complex. EMBO J. 2011;30:1067–1078. doi: 10.1038/emboj.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castle CD, Cassimere EK, Denicourt C. LAS1L interacts with the mammalian Rix1 complex to regulate ribosome biogenesis. Mol Biol Cell. 2012;23:716–728. doi: 10.1091/mbc.e11-06-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finkbeiner E, Haindl M, Raman N, Muller S. SUMO routes ribosome maturation. Nucleus. 2011;2:527–532. doi: 10.4161/nucl.2.6.17604. [DOI] [PubMed] [Google Scholar]
- 56.El Motiam A, Vidal S, de la Cruz-Herrera CF, et al. Interplay between SUMOylation and NEDDylation regulates RPL11 localization and function. FASEB J. 2019;33:643–651. doi: 10.1096/fj.201800341RR. [DOI] [PubMed] [Google Scholar]
- 57.Jang CY, Shin HS, Kim HD, Kim JW, Choi SY, Kim J. Ribosomal protein S3 is stabilized by sumoylation. Biochem Biophys Res Commun. 2011;414:523–527. doi: 10.1016/j.bbrc.2011.09.099. [DOI] [PubMed] [Google Scholar]
- 58.Kearse MG, Ireland JA, Prem SM, Chen AS, Ware VC. RpL22e, but not RpL22e-like-PA, is SUMOylated and localizes to the nucleoplasm of Drosophila meiotic spermatocytes. Nucleus. 2013;4:241–258. doi: 10.4161/nucl.25261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin YL, Chung CL, Chen MH, Chen CH, Fang SC. SUMO protease SMT7 modulates ribosomal protein L30 and regulates cell-size checkpoint function. Plant Cell. 2020;32:1285–1307. doi: 10.1105/tpc.19.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panse VG, Kressler D, Pauli A, et al. Formation and nuclear export of preribosomes are functionally linked to the small-ubiquitin-related modifier pathway. Traffic. 2006;7:1311–1321. doi: 10.1111/j.1600-0854.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venturi G, Montanaro L. How altered ribosome production can cause or contribute to human disease: the spectrum of ribosomopathies. Cells. 2020;9:2300. doi: 10.3390/cells9102300.c15715893688468bb15e6a4926f1a797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park J, Park J, Lee J, Lim C. The trinity of ribosome-associated quality control and stress signaling for proteostasis and neuronal physiology. BMB Rep. 2021;54:439–450. doi: 10.5483/BMBRep.2021.54.9.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heiss NS, Knight SW, Vulliamy TJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 64.Boocock GR, Morrison JA, Popovic M, et al. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat Genet. 2003;33:97–101. doi: 10.1038/ng1062. [DOI] [PubMed] [Google Scholar]