Abstract
Dopamine is a key neuromodulator of neuroplasticity and an important neuronal substrate of learning, and memory formation, which critically involves glutamatergic N-methyl-D-aspartate (NMDA) receptors. Dopamine modulates NMDA receptor activity via dopamine D1 and D2 receptor subtypes. It is hypothesized that dopamine focuses on long-term potentiation (LTP)-like plasticity, i.e. reduces diffuse widespread but enhances locally restricted plasticity via a D2 receptor-dependent NMDA receptor activity reduction. Here, we explored NMDA receptor–dependent mechanisms underlying dopaminergic modulation of LTP-like plasticity induced by transcranial direct current stimulation (tDCS). Eleven healthy, right-handed volunteers received anodal tDCS (1 mA, 13 min) over the left motor cortex combined with dopaminergic agents (the D2 receptor agonist bromocriptine, levodopa for general dopamine enhancement, or placebo) and the partial NMDA receptor agonist D-cycloserine (dosages of 50, 100, and 200 mg, or placebo). Cortical excitability was monitored by transcranial magnetic stimulation-induced motor-evoked potentials. We found that LTP-like plasticity was abolished or converted into LTD-like plasticity via dopaminergic activation, but reestablished under medium-dose D-cycloserine. These results suggest that diffuse LTP-like plasticity is counteracted upon via D2 receptor-dependent reduction of NMDA receptor activity.
Keywords: dopamine, NMDA receptor, plasticity, transcranial direct current stimulation
Introduction
Dopamine (DA) plays an important role in a variety of cognitive processes (Robbins 2003; Seamans and Yang 2004; Bäckman et al. 2006). The neurophysiological basis of DAergic effects is related to its modulatory impact on neuroplasticity, including long-term potentiation (LTP) and depression (LTD) (Otani et al. 1998; Jay 2003; Cooke and Bliss 2006). In accordance, DA antagonists prevent both excitatory and inhibitory plasticity induced in the motor cortex via noninvasive brain stimulation (NIBS) (Nitsche et al. 2006; Monte-Silva et al. 2011). Both transcranial direct current stimulation (tDCS) and paired associative stimulation (PAS) have been used to investigate DAergic modulation of plasticity (Kuo et al. 2008; Nitsche et al. 2009; Monte-Silva et al. 2010). In tDCS, a diffuse weak electrical field is applied over the target region, which modulates spontaneous neuronal activity via “subthreshold” neuronal membrane polarization and induces neuroplasticity with longer stimulation durations (Nitsche and Paulus 2000; Nitsche, Nitsche, et al. 2003; Stagg and Nitsche 2011). In contrast, PAS-induced plasticity is “suprathreshold” and involves a more focal activation of specific sensorimotor synapses (Stefan et al. 2002; Carson and Kennedy 2013). Despite differences in the focality of stimulation, both tDCS and PAS induce calcium (Ca2+) and NMDA receptor–dependent plasticity (Nitsche and Paulus 2000; Stefan et al. 2000; Liebetanz et al. 2002; Nitsche, Nitsche, et al. 2003). Interestingly, 100 mg L-Dopa, which improves learning in healthy humans (Flöel et al. 2005), has differential effects on tDCS- and PAS-induced plasticity. While L-Dopa stabilizes PAS-induced focal plasticity and nonfocal cathodal tDCS-induced LTD-like plasticity, it abolishes and/or converts nonfocal excitability-enhancing anodal tDCS effects into excitability-diminishing plasticity (Kuo et al. 2008; Thirugnanasambandam et al. 2011), suggesting a focusing effect of DA on LTP-like plasticity (see Fig. 1 for a schematic summary).
Fig. 1.
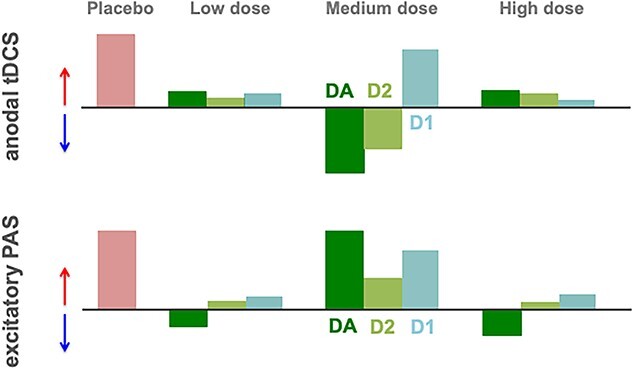
Schematic overview of DAergic modulation on LTP-like plasticity in the human motor cortex. This figure summarizes our previous findings about how activation of DA and its receptor subtypes, D1 and D2, modulate facilitatory, LTP-like plasticity induced by anodal tDCS (upper figure) or excitatory PAS (lower figure) in a dosage-dependent manner. tDCS hereby induces a more diffuse, while PAS results in a more focally restricted kind of plasticity. Red and blue arrows indicate the direction of plasticity; light red color bars represent the extend of plasticity under placebo medication, which is compared with plasticity under low, medium, and high dosage of global DA (dark green) as well as D1 (light blue) and D2 activation (light green) (Nitsche et al. 2006; Kuo et al. 2008; Monte-Silva et al. 2009; Nitsche et al. 2009; Monte-Silva et al. 2010; Thirugnanasambandam et al. 2011; Fresnoza, Paulus, et al. 2014; Fresnoza, Stiksrud, et al. 2014).
The focusing effect of DA can be described as enhancement of signal-to-noise ratio, as DA improves relevant neural activation while suppressing irrelevant background activity. In cognition, this would reinforce task-relevant information and inhibit task-irrelevant information (Seamans and Yang 2004). The mechanisms underlying this focusing effect of DA are yet to be clarified. Similar effects are observed with acetylcholine, a neuromodulator with prominent effects on arousal and cognition (Kuo et al. 2007), which are driven by calcium dynamics via nicotinic receptors (Grundey et al. 2018). The respective conversion effect of nicotine on tDCS-induced LTP-like plasticity seems to be caused by calcium overflow, as a reduction of neuronal calcium influx via an NMDA receptor blocker reestablished the LTP-like aftereffects (Lugon et al. 2017).
While calcium dynamics might also be relevant for DA effects on plasticity, the specific cholinergic and dopaminergic mechanisms might however differ. The impact of global dopaminergic activation via L-Dopa on LTP-like plasticity is relatively similar to that of D2 receptor activation (Monte-Silva et al. 2010; Thirugnanasambandam et al. 2011; Fresnoza, Stiksrud, et al. 2014). Since D2 receptors reduce NMDA receptor activity, and neuronal calcium influx (Higley and Sabatini 2010), it is hypothesized that the focusing effect of DA on LTP-like plasticity is due to a reduction of the diffuse and moderate NMDA receptor activity enhancement and calcium influx accomplished by anodal tDCS via D2 receptors. For the stronger activity enhancement and calcium influx accomplished by suprathreshold PAS, which is assumed to induce action potentials at the targeted synapses, such a gradual decline of NMDA receptor activity might not suffice to abolish LTP-like plasticity.
In the present study, the causal contribution of NMDA receptor mechanisms was examined by D-cycloserine (CYC), a partial NMDA receptor agonist (Thomas et al. 1988), which enhances the efficacy of anodal tDCS-generated LTP-like plasticity (Nitsche et al. 2004). Medium dosages of the DA precursor L-Dopa or the D2 receptor agonist bromocriptine, which converted LTP-like effects of anodal tDCS into LTD-like effects in previous studies, were combined with different dosages of CYC. We hypothesized that dose-dependent enhancement of NMDA receptor activity via CYC restores LTP-like plasticity induced by anodal tDCS under global DA activation with L-Dopa and the selective D2 receptor agonist bromocriptine.
Materials and methods
Participants
Thirteen subjects were initially recruited for the study, and two of them discontinued participation due to private reasons after 1 and 4 sessions, respectively. The remaining eleven volunteers (5 females, mean age = 29.90 ± 6.15 years; all healthy, right-handed, and nonsmoking) completed all experimental sessions. Sample size was selected based on previous pharmacological studies (Nitsche et al. 2006; Wischnewski et al. 2019) as well as a power analysis. The power calculation resulted in 10 subjects required for critical alpha and beta errors of 0.05 and a medium effect size of f = 0.35, based on the suggested medium power for tDCS studies (Minarik et al. 2016), and the primary statistical test, a repeated-measures ANOVA (for details, see below). Participants underwent a medical screening to verify that there were no history of neurological, psychiatric, or medical disease and no metal implants or intake of acute or chronic CNS-active medication and to exclude current pregnancy. The study was approved by the local Ethics Committee to the Declaration of Helsinki. Participants gave informed consent before participation and received monetary compensation.
Measurement of corticospinal excitability
To monitor excitability changes of the motor cortex, motor-evoked potentials (MEPs) of the right abductor digiti minimi muscle (ADM) were recorded. Single TMS pulses were applied by a PowerMag magnetic 20 stimulator (Mag&More, Munich, Germany), with a figure-of-eight coil (diameter of one winding: 70 mm; peak magnetic field: 2 T) at a frequency of 0.25 Hz with 10% jitter. The coil was held tangentially to the skull, over the primary motor cortex, with the handle targeting backwards and laterally at 45° from the midline. The optimal coil position on the head was defined as the site where stimulation resulted consistently in the largest MEPs with a given medium TMS intensity. A waterproof pen was used to mark the position of TMS coil on the scalp. The intensity of the TMS pulses was adjusted to elicit MEPs with an average peak-to-peak amplitude of 1 mV (SI1mV) for baseline recordings. Baseline and post-intervention cortical excitability was determined by measuring 25 MEPs at the respective time points. Surface EMG was recorded from the right ADM with gold cup electrodes in a belly-tendon montage. The signals were amplified and filtered (1,000; 3 Hz–3 kHz) using D440–2 (Digitimer, Welwyn Garden City, UK) with a time constant of 10 ms and a low-pass filter of 2.5 kHz. Subsequently, signals were digitized with a micro 1401 AD converter (Cambridge Electronic Design, Cambridge, UK) at an analogue-to-digital rate of 5 kHz, controlled and recorded by Signal Software (Cambridge Electronic Design, v. 2.13).
Neuroplasticity induction by tDCS
Electrical current was delivered by a battery-driven constant current stimulator (neuroConn GmbH, Ilmenau, Germany) with a maximum output of 3 mA, via a pair of saline-soaked surface sponge electrodes (7 × 5 cm). The anodal electrode was positioned over the motor-cortical position of the right ADM as identified by TMS, and the reference electrode was placed over the contralateral supraorbital area. The distance on the scalp between the edges of the electrodes was kept at a minimum of 6 cm to reduce current shunting through the scalp (Nitsche et al. 2007). Direct currents with 1 mA intensity and anodal polarity were applied for 13 min with 15 s ramping up/down at the beginning/end of stimulation.
Pharmacological interventions
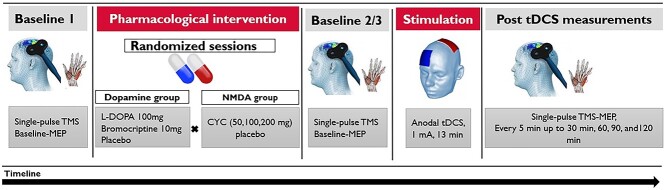
To determine whether the excitability-diminishing effect of DAergic agents on anodal tDCS, which causes LTP-like plasticity without pharmacological intervention, is caused by D2 receptor-generated NMDA activity reduction, we explored the combination of two groups of medication: (i) dopamine group: 100 mg L-Dopa (Levodopa/Benserazid—neuraxpharm) for general dopaminergic receptor activation, based on previous work showing converting effects of medium dosage L-Dopa on plasticity (Kuo et al. 2008; Monte-Silva et al. 2010), 10 mg of the D2 agonist bromocriptine (bromocriptine—ratiopharm), which had similar conversion effects on anodal tDCS-induced plasticity as L-Dopa at this dosage (Fresnoza, Stiksrud, et al. 2014), or equivalent placebo medication (P-Tabletten weiss 8 mm—Zentiva Pharma GmbH) and (ii) NMDA group: the NMDA agonist CYC (Cycloserine capsules—MEIJI) in three dosages (50, 100, and 200 mg) or placebo. The combination of both groups resulted in 12 pharmacological conditions (Fig. 2). The application of these specific dosages of CYC was based on previous works that have shown a dosage-dependent effect of CYC on NMDA receptors, in which the medium dosage (100 mg) improved anodal tDCS-induced aftereffects (Nitsche et al. 2004). The medications were taken orally by the participants 1.5 h before the start of tDCS application to ensure that tDCS was conducted during peak plasma concentration of the substances (van Berckel et al. 1997; Flöel et al. 2005; Fresnoza, Stiksrud, et al. 2014). About 20 mg domperidone was administered three times per day for 2 days and also 2 h before each session, to minimize systemic side effects of the DAergic agents. It has been shown that domperidone alone does not change motor cortical excitability (Grundey et al. 2013).
Fig. 2.
Study protocol. First, 25 MEP were elicited by single-pulse TMS over the representational area of the right ADM to measure baseline cortical excitability. Afterwards, combination of two groups of medication: (i) L-Dopa 100 mg, bromocriptine 10 mg, or equivalent placebo and (ii) CYC in three dosages (50, 100, and 200 mg), or placebo, was administered in 12 randomized sessions. Two hours later, a second baseline was recorded to explore the effect of medication on corticospinal excitability, and stimulation intensity was adjusted, if necessary. Then anodal tDCS was applied for 13 min with 1 mA intensity. Immediately after tDCS, aftereffects were monitored by TMS-induced MEPs every 5 min for up to 30 min and the following time points of 60, 90, and 120 min.
Experimental design
The experiment was conducted in a double-blinded, randomized, and placebo-controlled design. Session orders were arranged using the randomization function of excel and manually adjusted to ensure an evenly distributed order of interventions also within each condition. Each subject participated in 12 sessions with at least one-week interval between each session to avoid interference effects. At the beginning of each session, participants were seated in a comfortable chair, and the left motor cortical representational area of the right ADM was identified by TMS with the stimulation intensity adjusted to elicit MEPs with a peak-to-peak amplitude of about 1 mV. Twenty-five MEPs were recorded as the first baseline (BL1) before medication was taken by participants. Two hours after medication, the second baseline (BL2) with the same TMS intensity was recorded. If the second baseline measure MEP amplitude deviated more than 20% from the first baseline, TMS intensity was adjusted to obtain a baseline MEP amplitude of about 1 mV, and baseline 3 (BL3) was recorded (Kuo et al. 2017). TMS intensity was then kept constant during all post-tDCS measurements. Afterwards, anodal tDCS was applied for 13 min. Immediately after tDCS, MEPs were recorded every 5 min for half an hour and then every 30 min until 2 h after intervention. At the end of each session, participants completed a questionnaire for evaluation of tDCS side effects (Fig. 2).
Statistical analyses
Data analyses were conducted with the statistical package SPSS (IBM Corp. version 26.0). The individual MEP amplitude means were calculated for the baselines and all time points after neuroplasticity induction. To control for potential differences of baseline measurements between sessions, BL1 and BL2/3 MEP amplitudes (absolute values) and %MSO (maximal stimulator output) were analyzed with one-way ANOVAs with condition as the within-subject factor. To test if medication affected cortical excitability, two 2-factorial ANOVAs were calculated with “condition” (11 levels) and “time” (2 levels, before and after medication) as within-subject factor for “baseline MEP” (BL1 vs BL2) and “SI1mV” values (BL1 vs BL3) as dependent variables. For the effect of medication on tDCS-induced neuroplasticity, a repeated-measures ANOVA was performed, with time course (11 levels), DA (PLC, L-Dopa, bromocriptine), and CYC (PLC, CYC50, 100, 200 mg) as the within-subject factors, and the MEP amplitudes normalized to the corresponding BL2 (or BL3 when readjusted) as the dependent variable. In case of significant ANOVA results (P < 0.05), respective post hoc comparisons of MEP amplitudes at each time point were performed using Student t-tests (paired, two-tailed, P < 0.05).
Results
Six subjects experienced dizziness, nausea, or vomiting at around 4–5 h after drug intake (bromocriptine or L-Dopa, with or without CYC) (Table 3a); one of them experienced milder symptoms during the second exposition, and another participant also in the third exposition. The adverse effects were prominent in two subjects and their sessions were canceled and later repeated. All side-effects occurred after tDCS and the following main aftereffects measurement (0–30 min) and faded away within few hours. Therefore, the blinding of medication was considered to be effective, although these symptoms were not quantified with a questionnaire since these were scarce, also according to our previous observations (Nitsche et al. 2006, 2009; Kuo et al. 2008; Monte-Silva et al. 2009, 2010; Thirugnanasambandam et al. 2011; Fresnoza, Paulus, et al. 2014; Fresnoza, Stiksrud, et al. 2014). In addition, the average reported side effects related to tDCS are summarized in Table 3b. No significant difference was observed between the side effects associated with tDCS reported in each condition (Table 4).
Table 3.
Reported side effects associated with a) medication given in the session, and b) tDCS during stimulation in each session.
| a) | ||||
|---|---|---|---|---|
| Subject | Medication given in the respective session (symptoms) | |||
| A | BROMO + CYC50 (nausea) | |||
| B | BROMO + CYC100(nausea) | BROMO + CYC200 (nausea) | ||
| C | BROMO + CYC50 (nausea) | BROMO + PLC (nausea, vomiting) | L-Dopa + CYC100 (nausea) | |
| D | BROMO + CYC100 (nausea) | |||
| E | BROMO + CYC200 (nausea, vomiting) | |||
| F | L-Dopa + CYC200 (nausea) | |||
| b) | ||||
| Side effect | Placebo | |||
| PLC + PLC | PLC + CYC200 | PLC + CYC100 | PLC + CYC50 | |
| Visual | 0.00 | 0.090 ± 0.301 | 0.00 | 0.00 |
| Itching | 1.727 ± 1.103 | 1.363 ± 1.120 | 1.545 ± 1.128 | 1.272 ± 1.103 |
| Tingling | 1.454 ± 0.687 | 1.000 ± 0.447 | 1.272 ± 0.904 | 1.545 ± 1.128 |
| Burning | 0.636 ± 0.674 | 0.909 ± 1.044 | 1.000 ± 1.000 | 1.181 ± 1.328 |
| Pain | 0.272 ± 0.904 | 0.00 | 0.454 ± 0.820 | 0.272 ± 0.467 |
| BROMO | ||||
| PLC + BROMO | BROMO + CYC200 | BROMO + CYC100 | BROMO + CYC50 | |
| Visual | 0.00 | 0.00 | 0.00 | 0.00 |
| Itching | 1.000 ± 0.894 | 1.636 ± 1.026 | 1.181 ± 1.167 | 1.363 ± 0.924 |
| Tingling | 1.000 ± 0.632 | 1.363 ± 0.924 | 1.000 ± 1.264 | 1.363 ± 0.504 |
| Burning | 0.909 ± 1.044 | 1.454 ± 1.035 | 1.000 ± 1.000 | 1.363 ± 0.809 |
| Pain | 0.181 ± 0.603 | 0.181 ± 0.404 | 0.363 ± 0.504 | 0.454 ± 0.687 |
| L-Dopa | ||||
| PLC + L-Dopa | L-Dopa + CYC200 | L-Dopa + CYC100 | L-Dopa + CYC50 | |
| Visual | 0.00 | 0.00 | 0.00 | 0.00 |
| Itching | 1.090 ± 0.700 | 1.818 ± 1.328 | 1.727 ± 1.4206 | 1.545 ± 0.934 |
| Tingling | 1.454 ± 0.820 | 1.000 ± 1.095 | 1.181 ± 0.750 | 1.454 ± 0.820 |
| Burning | 0.909 ± 0.700 | 1.181 ± 0.981 | 1.181 ± 0.750 | 1.090 ± 0.943 |
| Pain | 0.454 ± 0.522 | 0.272 ± 0.646 | 0.363 ± 0.504 | 0.272 ± 0.467 |
Note: a) Occurrence and quality of side effects in single subjects under different medication conditions. b) The presence and intensity of tDCS-related side effects were rated on a numerical scale ranging from 0 to 5, 0 representing no and 5 extremely strong sensations. Data are presented as mean ± SD. tDCS = transcranial direct current stimulation, PLC = Placebo; CYC = D-cycloserine; BROMO = Bromocriptine.
Table 4.
Repeated-measures ANOVA results for the presence and intensity of reported tDCS side effects during experimental conditions.
| Side effects | Factors | df | F value | P-value |
|
|---|---|---|---|---|---|
| Visual | Condition | 1 | 1 | 0.341 | 0.091 |
| Itching | Condition | 4.620 | 0.679 | 0.629 | 0.064 |
| Tingling | Condition | 4.483 | 0.663 | 0.637 | 0.062 |
| Burning | Condition | 5.718 | 0.550 | 0.759 | 0.052 |
| Pain | Condition | 5.014 | 0.572 | 0.722 | 0.054 |
Note: The presence and intensity of reported side effects during anodal tDCS were analyzed by repeated-measures ANOVAs with tDCS condition (12 values) as the within-subject factor.
No differences of baseline MEP amplitudes and %MSO between conditions
Baseline measurements including the MEP amplitudes and corresponding SI1mVs are summarized in Table 1. The results of the one-way ANOVAs showed no significant main effect of condition for BL1 (df = 5.46, F = 1.115, P = 0.365) and BL3 (df = 4.70, F = 0.183, P = 0.962) with respect to absolute MEPs as well as for BL1 (df = 4.56, F = 0.741, P = 0.586) and BL3 (df = 4.58, F = 0.551, P = 0.722) for %MSO. For BL1 vs BL2 MEP amplitude values, the results of the respective ANOVA showed no main effects of time (BL1–2: df = 1, F = 0.451, P = 0.517) and condition (BL 1–2: df = 4.40, F = 0.623, P = 0.664) or time × condition interaction (BL 1–2: df = 4.56, F = 0.813, P = 0.537), implying that medication did not significantly affect corticospinal excitability. In addition, the repeated measures two-way ANOVA conducted for SI1mV revealed no significant main effects of time (df = 1, F = 0.497, P = 0.497), and condition (df = 5.67, F = 0.644, P = 0.657), or time × condition interaction for %MSO between BL1 and 3 (df = 3.55, F = 0.739, P = 0.569).
Table 1.
TMS stimulation intensities and baseline measurements.
| Experimental session | SI 1mv (%MSO) | Baseline MEP (mV) | |||
|---|---|---|---|---|---|
| BL1 | BL3 | BL1 | BL2 | BL3 | |
| PLC+ PLC | 48.68 ± 10.55 | 48.90 ± 10.63 | 1.04 ± 0.06 | 0.98 ± 0.12 | 1.01 ± 0.07 |
| 50 mg CYC + PLC | 48.22 ± 9.02 | 48.5 ± 9.03 | 1.02 ± 0.08 | 1.00 ± 0.09 | 1.00 ± 0.05 |
| 100 mg CYC + PLC | 48.59 ± 9.55 | 48.59 ± 9.58 | 1.03 ± 0.07 | 1.00 ± 0.08 | 1.00 ± 0.06 |
| 200 mg CYC + PLC | 48.59 ± 9.26 | 48.40 ± 9.05 | 0.99 ± 0.06 | 1.06 ± 0.19 | 1.00 ± 0.07 |
| PLC + bromocriptine | 48.22 ± 9.18 | 47.95 ± 8.91 | 1.00 ± 0.01 | 1.02 ± 0.17 | 0.99 ± 0.05 |
| 50 mg CYC + bromocriptine | 48.59 ± 9.98 | 48.59 ± 9.60 | 1.03 ± 0.06 | 1.03 ± 0.17 | 1.01 ± 0.07 |
| 100 mg CYC + bromocriptine | 48.86 ± 9.37 | 48.59 ± 9.48 | 1.03 ± 0.04 | 1.07 ± 0.13 | 1.02 ± 0.02 |
| 200 mg CYC + bromocriptine | 48.36 ± 10.27 | 48.5 ± 10.538 | 0.98 ± 0.06 | 0.99 ± 0.17 | 1.01 ± 0.03 |
| PLC + L-Dopa | 47.59 ± 9.86 | 47.59 ± 9.85 | 1.05 ± 0.07 | 1.02 ± 0.06 | 1.02 ± 0.05 |
| 50 mg CYC + L-Dopa | 47.63 ± 9.54 | 48.04 ± 10.46 | 1.00 ± 1.00 | 1.01 ± 0.17 | 1.02 ± 0.05 |
| 100 mg CYC + L-Dopa | 47.40 ± 7.83 | 47.86 ± 8.43 | 1.00 ± 0.04 | 0.94 ± 0.11 | 1.00 ± 0.07 |
| 200 mg CYC + L-Dopa | 47.63 ± 8.51 | 47.72 ± 8.59 | 1.03 ± 0.06 | 0.99 ± 0.13 | 1.00 ± 0.05 |
SI1mV refers to the maximal stimulator output (%MSO) that was required for generating ~1 mV MEP. BL1 refers to the baseline MEPs measured at the beginning of each session, BL2 refers to the baseline MEPs measured 2 h after medication, and BL3 refers to the last baseline measurement, including required TMS intensity adjustments. The results of the ANOVAs indicate no significant differences between baseline MEP and SI1mV before and after medication between sessions. Data are presented as mean ± SD.
NMDA receptor dependency of dopaminergic modulation of tDCS-induced plasticity
The results of the repeated-measures ANOVA revealed significant main effects of DA (df = 1.900, F = 24.230, P = 0.001, ηp2 = 0.708), CYC (df = 2.341, F = 19.362, P = 0.001, ηp2 = 0.659), and time (df = 4.412, F = 6.370, P = 0.001, ηp2 = 0.389) (Table 2). The interactions of DA× CYC (df = 3.834, F = 3.814, P = 0.011, ηp2 = 0.276), DA × time (df = 5.949, F = 4.458, P = 0.001, ηp2 = 0.308), and CYC × time (df = 6.395, F = 4.167, P = 0.001, ηp2 = 0.294) were significant as well (Table 2). This indicates that the observed effects were medication- and dosage-specific, as described in more detail below.
Table 2.
Results of the repeated-measures ANOVA conducted for two types of medication (DA andCYC).
| df | F | P | ηp2 | |
|---|---|---|---|---|
| DA | 1.900 | 24.230 | <.001* | .708 |
| CYC | 2.341 | 19.362 | <.001* | .659 |
| time | 4.412 | 6.370 | <.001* | .389 |
| DA × CYC | 3.834 | 3.814 | .011* | .276 |
| DA × time | 5.949 | 4.458 | .001* | .308 |
| CYC × time | 6.395 | 4.167 | .001* | .294 |
| DA × CYC × time | 8.149 | 1.919 | .067 | .161 |
Note: DA = Dopaminergic medication (L-Dopa 100 mg, bromocriptine 10 mg, placebo); CYC = D-cycloserine (50, 100, and 200 mg, or placebo); *P < 0.05.
Dosage-dependent effect of the partial NMDA receptor agonist D-cycloserine on tDCS-induced LTP-like plasticity
As shown by the post hoc t-tests (Fig. 3a), anodal tDCS under placebo medication significantly increased cortical excitability for up to 1 h after stimulation, as compared to baseline. Medium-dose CYC significantly prolonged this LTP-like plasticity. The respective excitability enhancement remained significant at the 60-min (P = 0.019), 90-min (P < 0.001), and 120-min (P = 0.015) time points post tDCS, as compared to baseline, and the placebo condition. Low-dose CYC resulted in an MEP enhancement lasting for 60 min after tDCS, but this enhancement was slightly reduced, as compared to the effect of anodal tDCS under placebo medication, and this reduction was significant 10 and 30 min after stimulation. High-dose CYC prevented aftereffects of anodal tDCS when compared to placebo medication and baseline excitability.
Fig. 3.
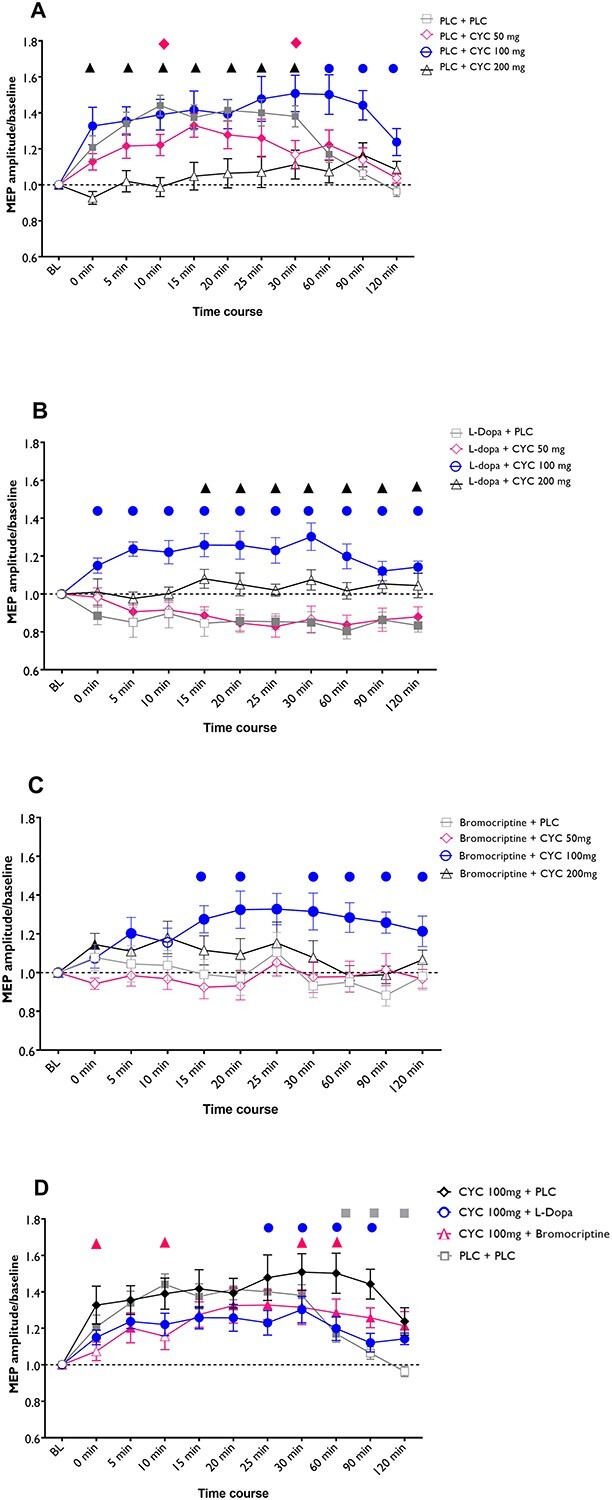
Dose-dependent effect of NMDA receptor activation on dopaminergic modulation of LTP-like plasticity induced by anodal tDCS. The x-axis displays the time points (in minutes) of baseline (BL) and after measurements during the experiment. MEP amplitudes standardized to the corresponding baseline values are plotted on the y-axis. a) Low-dose CYC alone did slightly reduce anodal tDCS-generated LTP-like plasticity, while the medium dosage prolonged and high-dosage CYC diminished the aftereffects. b) L-Dopa alone and L-Dopa combined with low-dosage CYC reversed anodal tDCS-induced excitatory plasticity, which was reestablished by medium-dose CYC. Adding high-dosage CYC to L-Dopa abolished tDCS-induced aftereffects. c) Bromocriptine alone abolished anodal tDCS-generated aftereffects, and medium-dose CYC reestablished LTP-like plasticity under bromocriptine, while low- and high-dose CYC did not change the effects of bromocriptine. d) Medium-dose CYC alone resulted in a higher excitability enhancement following anodal tDCS compared with L-Dopa or bromocriptine plus medium-dose CYC. Data were extracted from Fig. 3a–c with the conditions CYC 100 mg + PLC, CYC 100 mg + bromocriptine, and CYC 100 mg + L-Dopa, respectively. Filled symbols indicate a significant difference of cortical excitability against the respective baselines. In (a–c), floating symbols indicate a significant difference between each medication combined with CYC dosages with its respective PLC condition at a given time point. In (d), the floating symbols indicate significant differences between the medium-dose CYC alone condition and the respective medium-dose CYC+ L-dopa and medium-dose CYC+ bromocriptine conditions. Error bars represent SEM.
Dosage-dependent effect of D-cycloserine on tDCS-induced LTP-like plasticity under L-Dopa
Results of the post hoc t-tests show that L-Dopa alone reversed LTP-like MEP enhancements to LTD-like MEP amplitude reductions, as compared to baseline MEPs, and this effect lasted for up to 120 min following stimulation. Low-dose CYC did not significantly alter this effect. Under combination of medium-dose CYC and L-Dopa, MEPs were significantly enhanced compared to baseline, and the L-Dopa alone medication condition, until 120 min after stimulation. High-dose CYC combined with L-Dopa prevented aftereffects of anodal tDCS at all time points compared to baseline and resulted in larger MEP amplitudes as compared to the L-Dopa alone condition between 15 and 120 min after intervention (Fig. 3b).
Dosage-dependent effect of D-cycloserine on tDCS-induced LTP-like plasticity under D2 receptor activation
As revealed by the post hoc t-tests, bromocriptine prevented an MEP amplitude enhancement after anodal tDCS, as compared to baseline (Fig. 3c). When combined with low-dosage CYC, the same result was observed, and in this condition, MEP amplitudes also did not differ from those under bromocriptine alone. Under medium-dose CYC combined with bromocriptine, however, excitability was significantly enhanced by anodal tDCS at all time points except 0, and 10 min after stimulation, as compared to baseline, and 15, 20, as well as 30–120 min vs the bromocriptine alone condition. High-dose CYC combined with bromocriptine prevented tDCS-generated excitability enhancements at all time points except 0 min after stimulation, where it significantly enhanced MEP amplitudes vs baseline.
Comparison of tDCS-induced plasticity under medium-dose CYC alone vs CYC combined with L-Dopa or bromocriptine
Finally, tDCS-induced LTP-like plasticity under medium-dose CYC alone was compared with the combination of L-Dopa or bromocriptine added to CYC. Results of the post hoc t-tests show that excitability enhancements are significantly higher under medium-dose CYC alone for the time points of 25, 30, 60, and 90 min as compared to the combination with L-Dopa, for the time points of 0, 10, 30, and 60 min as compared to its combination with bromocriptine, and for the time points of 60, 90, and 120 min as compared to the pure placebo condition (Fig. 3d).
Discussion
In this study, we investigated the contribution of NMDA receptors on the focusing effect of DA on tDCS-induced neuroplasticity. Our results revealed that the partial NMDA receptor agonist CYC alone exerted a nonlinear dosage-dependent effect on tDCS-induced aftereffects. Medium-dose CYC most efficiently prolonged tDCS-generated LTP-like plasticity. L-Dopa and the D2 agonist bromocriptine abolished or reversed anodal tDCS-induced facilitatory plasticity, which was restored via co-application of medium-dose CYC. These findings suggest that the abolishing or converting effect of general DA activation on tDCS-induced LTP-like plasticity is accomplished by enhanced D2 activity via a reduction of NMDA receptor activity, as CYC reestablished dopamine-dependently abolished or converted LTP-like plasticity induced by anodaltDCS.
Effect of D-cycloserine on tDCS-induced LTP-like plasticity
Our results revealed a nonlinear modulation of cortical plasticity via CYC. While low-dose CYC slightly reduced excitatory plasticity, the medium dose significantly prolonged anodal tDCS-induced LTP-like effects, as shown in previous experiments (Nitsche et al. 2004). High-dose CYC abolished LTP-like plasticity. CYC is a partial agonist acting at the glycine-binding site of the NMDA receptor, which plays a key role in plasticity induction (Thomas et al. 1988). The medium dose of CYC used in our study has been demonstrated to facilitate neuroplasticity in both motor and visual cortices and to improve memory in humans (Nitsche et al. 2004; Forsyth et al. 2015; Brown et al. 2020). Low-dose CYC slightly diminished LTP-like plasticity. In vivo microdialysis in rats revealed that systematic administration of D-cycloserine at lower range, but not higher dosages, significantly decreased glutamate levels (Fujihira et al. 2007; Lehner et al. 2010), which provides an explanation for this effect. The plasticity-diminishing effect accomplished by high-dose CYC suggests an antagonist-like action at this dosage level, supported by behavioral studies in humans, where 250-mg CYC failed to facilitate motor learning (Cherry et al. 2014; Günthner et al. 2016). A similar dose-dependent modification of plasticity by CYC was observed in animal experiments, where LTP was significantly facilitated when the CYC dosage was increased from low to the medium range (Zhang et al. 2008). Similarly, it was shown that medium-dose CYC promoted both working memory and long-term episodic memory in mice (Zlomuzica et al. 2007; Bado et al. 2011), while higher dose CYC slightly depressed motor behavior (Polc et al. 1986). As supported by the above-mentioned studies, our results indicate that optimal NMDA receptor activation is critical for the induction of LTP-like plasticity by anodal tDCS and that optimizing activity of NMDA receptors with medium-dose CYC consolidates plasticity.
DAergic modulation of LTP-like plasticity induced by anodal tDCS
The results here showed a negative impact of L-Dopa and bromocriptine, as L-Dopa reversed anodal tDCS-induced LTP-like plasticity into LTD-like plasticity, and bromocriptine abolished it. This finding is in general accordance with previous results (Kuo et al. 2008; Monte-Silva et al. 2010; Fresnoza, Stiksrud, et al. 2014), although the inhibitory effect of the D2 agonist in the present study was less pronounced, possibly due to heterogeneities between participants. The effect of bromocriptine has been shown to depend on individual baseline DA activity associated with DA synthesis capacity as well as D2 receptor availability (Cools et al. 2009; Lövdén et al. 2018; Papenberg et al. 2020), which may explain gradual interstudy variabilities. On the other hand, L-Dopa clearly reversed LTP- into LTD-like plasticity, which replicates our previous findings. It cannot be completely excluded that the focusing effect of DA requires synergistic actions from different receptor subtypes, including not only D2- but also D1-like receptors (Seamans and Yang 2004). Moreover, methodologically, the specific dosages of the substances might not be exactly bioequivalent regarding their effects on D2-like receptors, since respective data are approximations. This could partially explain the reduced susceptibility to individual variability as well as the more homogenous excitability-diminishing effect under L-Dopa.
DAergic effects on cognitive functions are assumed to be accomplished by strengthening task-relevant neural activity and suppressing irrelevant network activity, thereby enhancing the signal-to-noise ratio. tDCS-induced cortical activity and excitability alterations might be more closely related to the latter, due to the moderate and diffuse tDCS effects. The neurophysiological mechanism of these effects has been explored in animal experiments. DA application to the frontal cortex in vivo results in reduction of neuronal excitability (Bernardi et al. 1982; Gulledge and Jaffe 1998; Gorelova and Yang 2000), which was accomplished by D2 activation (Gulledge and Jaffe 2001). In accordance, D2 agonists also diminish plasticity in the hippocampus as well as in corticostriatal regions (Huang and Kandel 1995; Manahan-Vaughan and Kulla 2003; Higley and Sabatini 2010). Furthermore, enhancing D2 receptor activity converted striatal LTP into LTD (Shen et al. 2008). Similarly, both bromocriptine and L-Dopa abolished and reversed anodal tDCS-induced excitatory plasticity in humans (Kuo et al. 2008; Monte-Silva et al. 2010; Fresnoza, Stiksrud, et al. 2014). A comparable effect was also described at the behavioral level, where L-Dopa or D2 activation alone impaired associative learning in primates and humans (Mehta et al. 2001; Gallant et al. 2016; Vo et al. 2017; Marino and Levy 2019).
Proposed mechanisms of action
The results of the present study indicate a common mechanism of L-Dopa and bromocriptine underlying the modulation of anodal tDCS-generated LTP-like plasticity, since both substances altered plasticity in the same direction, and this effect was affected in a similar way by CYC. The contribution of the D2 receptor to the respective LTP-like plasticity abolishment accomplished by L-Dopa is likely associated with a respective reduction of NMDA receptor activity (Zheng et al. 1999; Kotecha et al. 2002; Liu et al. 2006). Furthermore, D2 activation diminished the NMDA receptor–associated excitatory effect on prefrontal pyramidal neuron activity (Tseng and O’Donnell 2004) and reversed LTP into LTD in the striatum (Shen et al. 2008). Moreover, the plasticity diminution accomplished by D2 activation is due to reduced NMDA-mediated calcium influx (Higley and Sabatini 2010). Calcium concentration is a critical factor for plasticity direction, with a moderate calcium influx required for LTP induction (Yang et al. 1999; Lisman 2001; Misonou et al. 2004). The D2 receptor regulates intracellular calcium concentration via several downstream signaling cascades associated with the NMDA receptor (Hernandez-Lopez et al. 2000; Li et al. 2009; Higley and Sabatini 2010; de Bartolomeis and Tomasetti 2012). It is therefore conceivable that fine-tuning of calcium dynamics by D2 receptor activation exerts a critical influence on NMDA receptor–dependent plasticity. Consistently, our results demonstrated that medium-dose CYC, which facilitates anodal tDCS-induced excitatory plasticity, restored the abolished or converted LTP-like plasticity under L-Dopa and bromocriptine, presumably via increase of intracellular calcium level. While the missing effect of 50-mg CYC could be explained by insufficient calcium concentration caused by minor NMDA receptor activation, it might also be due to the decreased glutamate level as observed in an animal study under low-dosage CYC (Lehner et al. 2010). At the right end of inverted U-shaped curve, high-dose CYC resulted in a significant decrease of LTP-like plasticity, possibly via its antagonistic effect on NMDA receptor activity, as demonstrated by in vivo models (Anthony and Nevins 1993; Lanthorn 1994).
Functional implications
It is proposed that DA modulates cognitive functions via its bidirectional influence on underlying neuronal activity and excitability alterations, including plasticity, and that the precise control of the direction of respective alterations is determined by the amount of DA receptor activation. The results from the current study, in line with others, confirm that DA suppresses diffuse LTP-like plasticity in neural networks and that this effect is provided by D2 receptor activation (Kuo et al. 2008; Fresnoza, Stiksrud, et al. 2014). Studies in which catechol-O-methyltransferase (COMT) polymorphisms and D2 receptor availability were assessed via positron emission tomography showed a positive association between DA and D2 activity and memory functions, suggesting a critical role of inhibitory D2 functions for cognitive performance (Papenberg et al. 2020). Both L-Dopa and bromocriptine have been shown to impair probabilistic reversal learning in humans, suggesting moreover that the directionality of these effects depends critically on task characteristics, in accordance with the assumed focusing effect of DA and D2 receptor activation (Mehta et al. 2001; Cools and D’Esposito 2011). Pathophysiologically, an imbalance of DA receptor activation might be relevant for various neuropsychiatric diseases. In schizophrenia, specific cognitive symptoms such as impaired recognition and working memory are suggested to be partially related to D2 receptor overactivation (Durstewitz and Seamans 2008). Nevertheless, cognitive impairments in schizophrenia have also been shown to be associated with other receptors and neurotransmitters/modulators, such as NMDA and the cholinergic system (Goff et al. 2011). Given that the contribution of D2-like receptors to the global dopaminergic modulation of tDCS-induced plasticity is evident as revealed in the present study, it cannot be completely ruled out that other receptor subtypes are involved in the focusing effect of L-Dopa. For example, D1 activation has been shown to be associated with reduction of NMDA transmission (Law-Tho et al. 1994). In fact, bidirectional effects of D1 on NMDA receptors have been observed and suggested to be dependent of multiple factors such as dosage as well as level of neural network activation, and for higher network activation states and NMDA receptor activity–enhancing effect was observed (for details see Seamans and Yang 2004). Moreover, in our previous studies, D1-like receptor activation via combined application of L-Dopa and the D2 receptor antagonist sulpiride did not abolish LTP-like plasticity (Nitsche et al. 2009; Fresnoza, Paulus, et al. 2014). Further studies are nevertheless required for a more detailed clarification of the specific involvement of dopamine receptor subtypes in the modulatory effects of dopamine on plasticity.
In summary, the results of the present study help to clarify mechanistic aspects of DA receptor–dependent modulation of neuroplasticity with respect to NMDA receptor activation. The suppressive effect of both L-Dopa and bromocriptine on diffuse LTP-like plasticity induced by anodal tDCS is likely caused by negative modulation of NMDA receptors, which is suggested by the reestablishment of LTP-like plasticity via medium-dosage CYC. Since NMDA receptors have calcium channel functions, and tDCS-induced plasticity is calcium-dependent, it can furthermore be assumed that this effect is obtained via alterations of intracellular calcium concentration.
One of the limitations of the current study is the plasticity model applied in our experiment. Neuroplasticity in the human motor cortex has been explored in the majority of neurophysiology studies using noninvasive brain stimulation techniques because of the convenience of MEP recordings as index of cortical excitability. For neurological disorders such as stroke associated with motor dysfunction, DA has indeed been applied to augment poststroke motor recovery, although the outcomes revealed no benefit in a recent study (Ford et al. 2019). This could be partially explained by the LTP-reducing effects of L-Dopa most likely via NMDA receptor downregulation, as shown in the current study. Following this line, D1 enhancement might evolve as a better candidate for stroke rehabilitation as suggested by facilitation of visuomotor/consolidation under D1 activation in healthy humans in a recent study (Chen et al. 2020). On the other hand, reduction of LTP-like plasticity has also been shown in schizophrenia (Hasan et al. 2011). It is nevertheless crucial to transfer these findings to other areas, such as the prefrontal cortex, which plays a pivotal role in cognitive functions involving a focusing effect of dopamine. The recently introduced TMS-EEG approach allows a noninvasive physiological readout of plasticity in this area via TMS-evoked cortical potentials and will thereby enable respective studies in the future.
It should also be noted that serum concentration of medication was not acquired in the present study, and direct effects of glutamate levels associated with NMDA receptors were not determined. Nevertheless, our results revealed significant patterns of plasticity modulation by DA and NMDA receptors. Furthermore, DA effects on cognition have been shown to vary between individuals, presumably due to differences of baseline DA levels as well as the extent of receptor subtype activities, caused by genetic variation (Cools et al. 2009; Lövdén et al. 2018). Future experiments combining genetic classification as well as neuroimaging for quantification of the activity of specific DA receptor subtypes will provide more detailed information to elucidate further the mechanisms of DA modulation on cerebral physiology.
Contributor Information
Elham Ghanavati, Department of Psychology and Neurosciences, Leibniz Research Centre for Working Environment and Human Factors, Ardeystr. 67, 44139 Dortmund, Germany.
Mohammad Ali Salehinejad, Department of Psychology and Neurosciences, Leibniz Research Centre for Working Environment and Human Factors, Ardeystr. 67, 44139 Dortmund, Germany.
Lorena De Melo, Department of Psychology and Neurosciences, Leibniz Research Centre for Working Environment and Human Factors, Ardeystr. 67, 44139 Dortmund, Germany; International Graduate School of Neuroscience, Ruhr University Bochum, Universitätsstr. 150, 44801 Bochum, Germany.
Michael A Nitsche, Department of Psychology and Neurosciences, Leibniz Research Centre for Working Environment and Human Factors, Ardeystr. 67, 44139 Dortmund, Germany; Department of Neurology, University Medical Hospital Bergmannsheil, Bürkle de la Camp-Platz 1, 44789 Bochum, Germany.
Min-Fang Kuo, Department of Psychology and Neurosciences, Leibniz Research Centre for Working Environment and Human Factors, Ardeystr. 67, 44139 Dortmund, Germany.
Funding
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG NI683-13-1 to MAN, MFK, and EGH).
Conflict of interest statement. MAN is member of the Scientific Advisory Boards of Neuroelectrics and NeuroDevice.
References
- Anthony EW, Nevins ME. Anxiolytic-like effects of N-methyl-D-aspartate-associated glycine receptor ligands in the rat potentiated startle test. Eur J Pharmacol. 1993:250(2):317–324. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006:30(6):791–807. [DOI] [PubMed] [Google Scholar]
- Bado P, Madeira C, Vargas-Lopes C, Moulin TC, Wasilewska-Sampaio AP, Maretti L, de Oliveira RV, Amaral OB, Panizzutti R. Effects of low-dose D-serine on recognition and working memory in mice. Psychopharmacology. 2011:218(3):461–470. [DOI] [PubMed] [Google Scholar]
- Bernardi G, Cherubini E, Marciani MG, Mercuri N, Stanzione P. Responses of intracellularly recorded cortical neurons to the iontophoretic application of dopamine. Brain Res. 1982:245(2):267–274. [DOI] [PubMed] [Google Scholar]
- Brown JC, DeVries WH, Korte JE, Sahlem GL, Bonilha L, Short EB, George MS. NMDA receptor partial agonist, d-cycloserine, enhances 10 Hz rTMS-induced motor plasticity, suggesting long-term potentiation (LTP) as underlying mechanism. Brain Stimul. 2020:13(3):530–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RG, Kennedy NC. Modulation of human corticospinal excitability by paired associative stimulation. Front Hum Neurosci. 2013:7:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS, Jamil A, Liu LC, Wei SY, Tseng HH, Nitsche MA, Kuo MF. Nonlinear effects of dopamine D1 receptor activation on Visuomotor coordination task performance. Cereb Cortex. 2020:30(10):5346–5355. [DOI] [PubMed] [Google Scholar]
- Cherry KM, Lenze EJ, Lang CE. Combining d-cycloserine with motor training does not result in improved general motor learning in neurologically intact people or in people with stroke. J Neurophysiol. 2014:111(12):2516–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006:129(Pt 7):1659–1673. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011:69(12):e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009:29(5):1538–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bartolomeis A, Tomasetti C. Calcium-dependent networks in dopamine-glutamate interaction: the role of postsynaptic scaffolding proteins. Mol Neurobiol. 2012:46(2):275–296. [DOI] [PubMed] [Google Scholar]
- Flöel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, Knecht S, Cohen LG. Dopaminergic influences on formation of a motor memory. Ann Neurol. 2005:58(1):121–130. [DOI] [PubMed] [Google Scholar]
- Ford GA, Bhakta BB, Cozens A, Hartley S, Holloway I, Meads D, Pearn J, Ruddock S, Sackley CM, Saloniki EC, et al. Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2019:18(6):530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth JK, Bachman P, Mathalon DH, Roach BJ, Asarnow RF. Augmenting NMDA receptor signaling boosts experience-dependent neuroplasticity in the adult human brain. Proc Natl Acad Sci U S A. 2015:112(50):15331–15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresnoza S, Paulus W, Nitsche MA, Kuo MF. Nonlinear dose-dependent impact of D1 receptor activation on motor cortex plasticity in humans. J Neurosci. 2014:34(7)::2744–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresnoza S, Stiksrud E, Klinker F, Liebetanz D, Paulus W, Kuo MF, Nitsche MA. Dosage-dependent effect of dopamine D2 receptor activation on motor cortex plasticity in humans. J Neurosci. 2014:34(32):10701–10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihira T, Kanematsu S, Umino A, Yamamoto N, Nishikawa T. Selective increase in the extracellular D-serine contents by D-cycloserine in the rat medial frontal cortex. Neurochem Int. 2007:51(2–4):233–236. [DOI] [PubMed] [Google Scholar]
- Gallant H, Vo A, Seergobin KN, MacDonald PA. Pramipexole impairs stimulus-response learning in healthy young adults. Front Neurosci. 2016:10:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011:99(2):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova NA, Yang CR. Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons in vitro. J Neurophysiol. 2000:84(1):75–87. [DOI] [PubMed] [Google Scholar]
- Grundey J, Freznosa S, Klinker F, Lang N, Paulus W, Nitsche MA. Cortical excitability in smoking and not smoking individuals with and without nicotine. Psychopharmacology. 2013:229(4):653–664. [DOI] [PubMed] [Google Scholar]
- Grundey J, Barlay J, Batsikadze G, Kuo MF, Paulus W, Nitsche M. Nicotine modulates human brain plasticity via calcium-dependent mechanisms. J Physiol. 2018:18(21):9139–9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci. 1998:18:9139–9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. Multiple effects of dopamine on layer V pyramidal cell excitability in rat prefrontal cortex. J Neurophysiol. 2001:86(2):586–595. [DOI] [PubMed] [Google Scholar]
- Günthner J, Scholl J, Favaron E, Harmer CJ, Johansen-Berg H, Reinecke A. The NMDA receptor partial agonist d-cycloserine does not enhance motor learning. J Psychopharmacol. 2016:30(10):994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A, Nitsche MA, Rein B, Schneider-Axmann T, Guse B, Gruber O, Falkai P, Wobrock T. Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behav Brain Res. 2011:224(1):15–22. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci. 2000:20(24):8987–8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010:13(8):958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1995:92(7):2446–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003:69(6):375–390. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, MacDonald JF. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002:35(6):1111–1122. [DOI] [PubMed] [Google Scholar]
- Kuo MF, Grosch J, Fregni F, Paulus W, Nitsche MA. Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J Neurosci. 2007:27(52):14442–14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MF, Paulus W, Nitsche MA. Boosting focally-induced brain plasticity by dopamine. Cereb Cortex. 2008:18(3):648–651. [DOI] [PubMed] [Google Scholar]
- Kuo HI, Paulus W, Batsikadze G, Jamil A, Kuo MF, Nitsche MA. Acute and chronic noradrenergic effects on cortical excitability in healthy humans. Int J Neuropsychopharmacol. 2017:20:634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanthorn TH. D-Cycloserine: agonist turned antagonist. Amino Acids. 1994:6(3):247–260. [DOI] [PubMed] [Google Scholar]
- Law-Tho D, Hirsch JC, Crepel F. Dopamine modulation of synaptic transmission in rat prefrontal cortex: an in vitro electrophysiological study. Neurosci Res. 1994:21(2):151–160. [DOI] [PubMed] [Google Scholar]
- Lehner M, Wisłowska-Stanek A, Taracha E, Maciejak P, Szyndler J, Skórzewska A, Turzyńska D, Sobolewska A, Hamed A, Bidziński A, et al. The effects of midazolam and D-cycloserine on the release of glutamate and GABA in the basolateral amygdala of low and high anxiety rats during extinction trial of a conditioned fear test. Neurobiol Learn Mem. 2010:94(4):468–480. [DOI] [PubMed] [Google Scholar]
- Li YC, Xi D, Roman J, Huang YQ, Gao WJ. Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. J Neurosci. 2009:29(49):15551–15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002:125(Pt 10):2238–2247. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Three Ca2+ levels affect plasticity differently: the LTP zone, the LTD zone and no man’s land. J Physiol. 2001:532(Pt 2):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, et al. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006:52(5):897–909. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Karalija N, Andersson M, Wåhlin A, Axelsson J, Köhncke Y, Jonasson LS, Rieckman A, Papenberg G, Garrett DD, et al. Latent-profile analysis reveals behavioral and brain correlates of dopamine-cognition associations. Cereb Cortex. 2018:28(11):3894–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugon MD, Batsikadze G, Fresnoza S, Grundey J, Kuo MF, Paulus W, Nakamura-Palacios EM, Nitsche MA. Mechanisms of nicotinic modulation of glutamatergic neuroplasticity in humans. Cereb Cortex. 2017:27(1):544–553. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A. Regulation of depotentiation and long-term potentiation in the dentate gyrus of freely moving rats by dopamine D2-like receptors. Cereb Cortex. 2003:13(2):123–135. [DOI] [PubMed] [Google Scholar]
- Marino RA, Levy R. Differential effects of D1 and D2 dopamine agonists on memory, motivation, learning and response time in non-human primates. Eur J Neurosci. 2019:49(2):199–214. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Swainson R, Ogilvie AD, Sahakian J, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D(2) agonist bromocriptine in human volunteers. Psychopharmacology. 2001:159(1):10–20. [DOI] [PubMed] [Google Scholar]
- Minarik T, Berger B, Althaus L, Bader V, Biebl B, Brotzeller F, Fusban T, Hegemann J, Jesteadt L, Kalweit L, et al. The importance of sample size for reproducibility of tDCS effects. Front Hum Neurosci. 2016:10:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004:7:711–718. [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo MF, Thirugnanasambandam N, Liebetanz D, Paulus W, Nitsche MA. Dose-dependent inverted U-shaped effect of dopamine (D2-like) receptor activation on focal and nonfocal plasticity in humans. J Neurosci. 2009:29(1):6124–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte-Silva K, Liebetanz D, Grundey J, Paulus W, Nitsche MA. Dosage-dependent non-linear effect of L-dopa on human motor cortex plasticity. J Physiol. 2010:588(Pt 18):3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte-Silva K, Ruge D, Teo JT, Paulus W, Rothwell JC, Nitsche MA. D2 receptor block abolishes θ burst stimulation-induced neuroplasticity in the human motor cortex. Neuropsychopharmacology. 2011:36(10):2097–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000:527(Pt 3):633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003:553:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003:114:600–604. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology. 2004:29(8):1573–1578. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Lampe C, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W. Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur J Neurosci. 2006:23(6):1651–1657. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Doemkes S, Karaköse T, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007:97:3109–3117. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Kuo MF, Grosch J, Bergner C, Monte-Silva K, Paulus W. D1-receptor impact on neuroplasticity in humans. J Neurosci. 2009:29:2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S, Blond O, Desce JM, Crépel F. Dopamine facilitates long-term depression of glutamatergic transmission in rat prefrontal cortex. Neuroscience. 1998:85(3):669–676. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Karalija N, Salami A, Rieckmann A, Andersson M, Axelsson J, Riklund K, Lindenberger U, Lövdén M, Nyberg L, et al. Balance between transmitter availability and dopamine D2 receptors in prefrontal cortex influences memory functioning. Cereb Cortex. 2020:30(3):989–1000. [DOI] [PubMed] [Google Scholar]
- Polc P, Pieri L, Bonetti EP, Scherschlicht R, Moehler H, Kettler R, Burkard W, Haefely W. L-cycloserine: behavioural and biochemical effects after single and repeated administration to mice, rats and cats. Neuropharmacology. 1986:25(4):411–418. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Dopamine and cognition. Curr Opin Neurol. 2003:16(Suppl 2):S1–S2. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004:74(1):1–58. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008:321(5890):848–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011:17(1):37–53. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000:123(Pt 3):572–584. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002:543(Pt 2):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirugnanasambandam N, Grundey J, Paulus W, Nitsche MA. Dose-dependent nonlinear effect of L-DOPA on paired associative stimulation-induced neuroplasticity in humans. J Neurosci. 2011:31(14):5294–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JW, Hood WF, Monahan JB, Contreras PC, O’Donohue TL. Glycine modulation of the phencyclidine binding site in mammalian brain. Brain Res. 1988:442(2):396–398. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004:24(22):5131–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Lipsch C, Timp S, Gispen-de Wied C, Wynne H, van Ree JM, Kahn RS. Behavioral and neuroendocrine effects of the partial NMDA agonist D-cycloserine in healthy subjects. Neuropsychopharmacology. 1997:16:317–324. [DOI] [PubMed] [Google Scholar]
- Vo A, Seergobin KN, MacDonald PA. Effects of levodopa on stimulus-response learning versus response selection in healthy young adults. Behav Brain Res. 2017:317:553–561. [DOI] [PubMed] [Google Scholar]
- Wischnewski M, Engelhardt M, Salehinejad MA, Schutter DJLG, Kuo MF, Nitsche MA. NMDA receptor-mediated motor cortex plasticity after 20 Hz transcranial alternating current stimulation. Cereb Cortex. 2018. [DOI] [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol. 1999:81:781–787. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Sullivan JA, Moskal JR, Stanton PK. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology. 2008:55(7):1238–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zhang XX, Bunney BS, Shi WX. Opposite modulation of cortical N-methyl-D-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience. 1999:91:527–535. [DOI] [PubMed] [Google Scholar]
- Zlomuzica A, De Souza Silva MA, Huston JP, Dere E. NMDA receptor modulation by D-cycloserine promotes episodic-like memory in mice. Psychopharmacology. 2007:193(2):503–509. [DOI] [PubMed] [Google Scholar]