Abstract
Although sensory input is continuous, information must be combined over time to guide action and cognition, leading to the proposal of temporal sampling windows. A number of studies have suggested that a 10-Hz sampling window might be involved in the “frame rate” of visual processing. To investigate this, we tested the ability of participants to localize and enumerate 1 or 2 visual flashes presented either at near-threshold or full-contrast intensities, while recording magnetoencephalography. The inter-stimulus interval (ISI) between the 2 flashes was varied across trials. Performance in distinguishing between 1 and 2 flashes was linked to the alpha frequency, both at the individual level and trial-by-trial. Participants with a higher resting-state alpha peak frequency showed the greatest improvement in performance as a function of ISI within a 100-ms time window, while those with slower alpha improved more when ISI exceeded 100 ms. On each trial, correct enumeration (1 vs. 2) performance was paired with faster pre-stimulus instantaneous alpha frequency. Our results suggest that visual sampling/processing speed, linked to peak alpha frequency, is both an individual trait and can vary in a state-dependent manner.
Keywords: individual alpha frequency, instantaneous alpha frequency, temporal integration, temporal segregation, visual processing speed
1. Introduction
Sensory information arrives continuously to the brain, raising the question of how cognitive processing, which takes time, is able to act upon the analog flow of information. It is clear that sensory information is combined over time to support tasks such as visual motion processing, multisensory integration, or parsing spoken language, as well as to simply improve the accuracy of our perception of stable features of the world. This suggests that perceptual systems process continuous sensory input in discrete “temporal integration windows” (TIWs: Pöppel 1997, 2009; Hasson et al. 2008), which may be embedded in sampling rhythms (VanRullen and Koch 2003; Dehaene 2016). Specifically, it has been argued that the alpha rhythm provides a natural time frame for temporal integration in visual processing (Varela et al. 1981; Gho and Varela 1988).
A typical approach to measure TIWs is to present 2 stimuli in succession, separated by a varying inter-stimulus interval (ISI). When the duration of the ISI is relatively short, participants typically perceive only a single, unified percept. In contrast, when the ISI exceeds a certain duration, participants are able to correctly report 2 unique events (VanRullen and Koch 2003; Wutz, Weisz, et al. 2014; Cecere et al. 2015; Samaha and Postle 2015; Wutz et al. 2018). In a recent study using functional magnetic resonance imaging, for example, 2 visual flashes separated by a varying ISI were presented (Zhou et al. 2018). In voxels localized in early visual cortex, the temporal window of interaction between the 2 pulses was relatively short (about 100 ms), while higher level regions showed interactions even when the signals were separated by a full second. Similar results were found with human intracranial recordings, with the first flash attenuating the evoked response to the second stimulus when both flashes fell within a certain temporal window (Zhou et al. 2018). More generally, this basic “dual-pulse” of presenting 2 stimuli separated by an ISI has been used to measure temporal windows in visual processing (Gottlieb et al. 1985; Samaha and Postle 2015; Ronconi et al. 2017), audio–visual processing (Kawabe 2009), auditory processing (Robin and Royer 1987), and tactile processing (Wühle et al. 2010, 2011).
In the case of tactile perception, a neural measure of TIWs has been estimated in an magnetoencephalography (MEG) paradigm by quantifying the influence of a near-threshold (NT) tactile stimulus on the evoked response to a second, full intensity stimulus (Wühle et al. 2010, 2011). This is a modified version of the dual-pulse paradigm in which the first stimulus (near threshold) is weaker and thus less likely to evoke a strong response on its own, allowing its impact on the second stimulus to be measured with less temporal smearing of the 2 evoked responses. Wühle et al. reasoned that if the ISI between the 2 stimuli exceeded the temporal integration window, then the evoked response to the full stimulus would no longer be affected/attenuated by the NT stimulus. Across 2 studies (Wühle et al. 2010, 2011), they found attenuation of the MEG evoked response to the second (full-threshold) stimulus in the primary somatosensory (SI) cortex for ISI values up to 60 ms, while the attenuation in secondary somatosensory (SII) cortex occurred even for ISIs of several hundred milliseconds. In other words, the temporal resolution of area SI was relatively short (within 60 ms), while temporal integration within area SII lasted over hundreds of milliseconds. This finding was partially replicated in another MEG study, which also found TIWs for tactile stimuli in the order of 50–75 ms (Yamashiro et al. 2011).
The use of paired NT + full stimulation, as used by Wühle et al. in the somatosensory domain, could be used to measure the neural correlates of visual TIWs. Importantly, in terms of links to visual sampling and a potential role for alpha oscillations, a NT stimulus is less likely to induce a stimulus-evoked oscillation or traveling wave (Sato et al. 2012), or to align separate endogenous oscillatory phases temporarily due to a phase reset (for a similar logic, see Ronconi and Melcher 2017). It is theoretically useful to distinguish between an endogenous oscillation and other oscillatory-like activity that does not reflect a true endogenous oscillation (see, for example Capilla et al. 2011; Zoefel et al. 2018; Doelling et al. 2019; Lakatos et al. 2019). The presentation of a stimulus might induce a short-lived oscillatory pattern due to recurrent processing and/or traveling waves (Pfurtscheller and Lopes da Silva 1999; Sato et al. 2012). Alternatively, the stimulus might temporarily align separate endogenous rhythms for a brief time before they desynchronize into independent rhythms. It is well known that the presentation of a strong visual stimulus influences alpha coherence (for review: Pfurtscheller and Lopes da Silva (1999)). It has been shown that a strong phase reset to the first stimulus can influence processing of the second stimulus (Wutz, Muschter, et al. 2014), but such a result does not necessarily reflect an endogenous sampling rhythm. Measuring temporal effects without inducing a new traveling wave or phase-aligning separate oscillations would provide stronger evidence for a role of endogenous oscillations. The disadvantage of such an approach, however, is that the phase of the ongoing oscillation will vary across trials, reducing precision in the timing of the behavioral effect.
The main focus of this current study was to investigate the duration of the visual TIW and the role of sampling rhythms in visual perception by testing whether alpha peak frequency predicted performance in this NT + Full dual-pulse stimulation paradigm. The idea of an endogenous visual sampling rhythm at the alpha oscillatory frequency, although relatively old (Bishop 1932; Lansing 1957; Harter 1967; VanRullen 2013), remains controversial. One open question is which alpha rhythm is involved and whether it is linked to individual differences or to variations within individuals over time. On the one hand, it has been argued that peak alpha frequency (PAF) is an individual trait, with some people showing consistently higher or lower alpha peak frequency across testing sessions (Salinsky et al. 1991). Indeed, a number of studies have linked individual differences in PAF to speed of visual processing (Walsh 1952; Varela et al. 1981; Samaha and Postle 2015). However, recent studies have investigated variation in this peak frequency by measuring rapid alterations in the instantaneous PAF (Haegens et al. 2014). Specifically, performance in single trials, in rapid visual tasks, has been found to be related to the instantaneous alpha peak frequency (IAF) on that trial (Wutz, Weisz, et al. 2014; Cecere et al. 2015; Samaha and Postle 2015; Wutz et al. 2018) with evidence that IAF might be to some degree under top–down control (Wutz et al. 2018).
We measured both individual PAF, determined for each individual in separate trials, and instantaneous alpha frequency within each trial. We hypothesized that individuals with a higher PAF would have shorter TIWs and that the instantaneous measure of alpha peak would predict, on single trials, whether participants reported seeing separate flashes or a single fused percept.
2. Methods
2.1 Study design
We tested 3 main predictions. First, based on the previous studies with tactile stimulation, we predicted that the processing of a full-contrast stimulus would be influenced by a preceding NT stimulus when the ISI was within the TIW of around 100 ms (one alpha cycle).
Second, we predicted that individual participant performance in the perceptual segregation task should be better for participants with higher temporal resolution. In a general TIW context, 2 stimuli might be fused into one perceived event if they fall into the same TIW, but they might be resolved into 2 perceived events if they fall into different (possibly consecutive) TIWs (VanRullen and Koch 2003; Wutz, Weisz, et al. 2014; Cecere et al. 2015; Samaha and Postle 2015; Wutz et al. 2018). Under the assumption that the individual resting-state PAF is an indicator of the speed of a particular participant’s visual system, one may assume that the minimal duration of a TIW, and thereby of the ability to resolve brief temporal events, is linked to the PAF (Cecere et al. 2015; Samaha and Postle 2015; Wutz et al. 2018). Temporal segregation should therefore be more often successful with higher PAF. We thus hypothesized that those participants with higher individual alpha peak frequency would improve their performance from baseline (i.e. the performance at the shortest ISI) at shorter ISI values compared to participants with lower alpha peak frequency (who would have longer TIWs). Participants with high individual alpha frequency should therefore improve more in the first ISI step (the interval from 33 to 67 ms) than those with low individual alpha frequency, while participants with low individual alpha frequency should improve more towards the later ISIs (100–400 ms). This provided a between-subjects test of the role of PAF.
Third, based on recent work linking performance in rapid visual tasks with the IAF on that trial (Wutz, Weisz, et al. 2014b; Cecere et al. 2015; Samaha and Postle 2015; Wutz et al. 2018), we hypothesized that we would also find higher IAF on trials in which participants correctly discriminated between 1 and 2 stimuli. A higher IAF should be accompanied by a higher chance to correctly separate 2 consecutive flashes on a trial-by-trial basis within subjects, in addition to any hypothesized PAF effect in general. This provided a within-subjects test of the role of PAF in dual-pulse segregation.
2.2 Experimental procedure
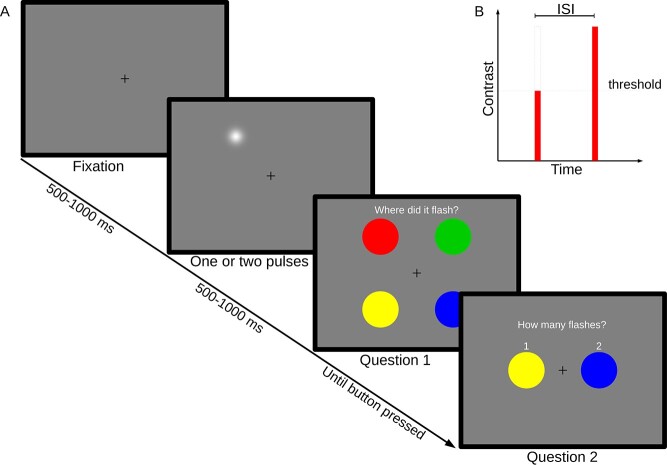
The task consisted of detecting, localizing, and enumerating the number of flashes presented on the display. On each trial, 1 or 2 localized visual flashes were presented on a medium (50%) gray background (see Fig. 1). Flashes were 2D Gaussian luminance distributions with an approximate total diameter of 1 degree visual field, although the perceived size of the Gaussian blob may have been smaller depending on participant and adjusted contrast. Flashes were shown at 2 contrast levels, full contrast and NT contrast. The duration of each flash was set to 8.3 ms (one screen refresh, 120 Hz). In full-contrast flashes, the peak of the blob was white (maximum luminance of the display system), giving maximum luminance contrast against the background. Prior to the main experimental sessions, NT contrast flashes were adjusted by a Quest procedure (Watson and Pelli 1983) until participants were just above chance performance (57% correct) at detecting a single threshold flash. To reduce the number of chance hits included in the analysis, flashes (or pairs of flashes) were shown in 1 of 4 quadrants, symmetrically arranged around the point of fixation, and randomly chosen for each trial (see Fig. 1). Participants were then asked to identify the quadrant where they had seen the flash, rather than whether they had seen it. Flash eccentricity was 6 degrees from point of fixation. Stimuli were generated on a UNIX computer using Matlab 8.0 (The MathWorks) and the PsychophysicsToolbox Version 3 (Brainard 1997; Pelli 1997).
Fig. 1.
Experimental paradigm. A) One or 2 brief flashes of light are shown in 1 of 4 quadrants. Participants pressed colored buttons to indicate their responses. B) Stimulus design. Flashes were either of threshold intensity or full intensity, in the case of dual pulses with a variable ISI.
Each trial was chosen from 1 of 6 experimental conditions in a randomized fashion. Four NT—full dual-flash conditions with different ISIs (33, 67, 100, and 400 ms) were intended to probe the temporal integration behavior of the participants. A single-threshold pulse and 2 full-contrast pulses with an ISI of 33 ms served as baseline conditions. After each trial, participants were prompted to make 2 responses (see Fig. 1). The first response was to indicate the screen quadrant in which the flashes were perceived. The second question asked the number of flashes perceived (1 or 2). Responses were made by means of an MEG-compatible button box, with matching color coding between buttons and displayed questions (see Fig. 1). After completion of the main experiment, participants were directed to look at a fixation cross at the center of the screen, without further tasks. During this time period, 5 min of MEG data was recorded for the purpose of resting-state analysis.
2.3 MEG data acquisition
MEG was recorded during a visual stimulation paradigm. Participants were seated in an Elekta Neuromag 306 in vertical position, placed inside a magnetically shielded chamber. The display system was a DLP projector (Panasonic PT-D7700E) running at a refresh rate of 120 Hz, aimed at a translucent back-projection screen located in the dimly lit chamber.
2.4 Participants
Twenty participants (9 females, mean age 25.6 years, SD = 2.39 years, all right-handed) participated in the experiment. All participants provided written informed consent; the study was approved by the Ethical Committee of the University of Trento and was conducted in accordance with the Declaration of Helsinki. Two participants were excluded from analysis due to magnetic interference, suspected to be due to unreported dental work, and 1 participant aborted the experiment prematurely.
2.5 Data processing
2.5.1 Behavior/general
Participants identified the quadrant in which they perceived the visual stimuli; trials on which the wrong quadrant was identified were treated as misses and thus excluded from further analysis (13% of trials, see Section 3).
There were limits to the extent to which behavioral data could be used to estimate the TIW for each participant. The first issue, raised in Section 1, is that the lack of a strong phase reset by the first stimulus would mean that the interaction between the 2 stimuli might depend on the phase of the ongoing oscillation, which would be randomly distributed across trials. In principle, this should “flatten” the psychophysical curve of performance as a function of ISI. In addition, there is a question of fitting the minimum to the psychophysical curve. One may argue that at zero ISI, the number of trials classified as 2 flashes should be zero as well, since there would truly be only one flash. However, 2 flashes, even at zero ISI, would be longer than a single flash, which might lead to judgments indicating the perception of 2 flashes rather than 1. Thus, it is not immediately clear how the psychometric fits should be constrained in terms of a minimum. Additionally, the shortest and longest ISIs may be close to the tails of the psychometric curves, making the fitting procedure susceptible to noise.
Therefore, in addition to psychometric fits for individual participants, we also performed a correlation analysis between the individual resting-state PAF of our participants and their respective performance improvements between the 4 ISIs of the Threshold-Full condition (see Section 3).
2.5.2 Event-related fields
Data were recorded at 1 kHz, then downsampled to 250 Hz. The continuous data were then visually inspected and noisy channels were excluded without interpolation. For the trial data analysis, environmental noise was removed and the data were co-registered in order to remove small head movements across the separate measurement runs through signal space separation with spatiotemporal extension (Taulu et al. 2005; Taulu and Kajola 2005) implemented via the MaxFilter software version 2.2.15 (Elektra-Neuromag Ltd, Helsinki, Finland). Data were then analyzed in Matlab using the Fieldtrip toolbox (Oostenveld et al. 2010) for general MEG data treatment and the CoSMoMVPA toolbox for multivariate cluster statistics (Oosterhof et al. 2016). Epochs of 4 s were centered on the stimulus onset (in case of 2 pulses, on the second pulse). Trials were visually inspected for artifacts and contaminated trials were removed. Of the 1,160 trials recorded from each participant, an average of 1,016 trials remained. No further filtering was performed, unless otherwise mentioned below.
2.5.3 Resting state
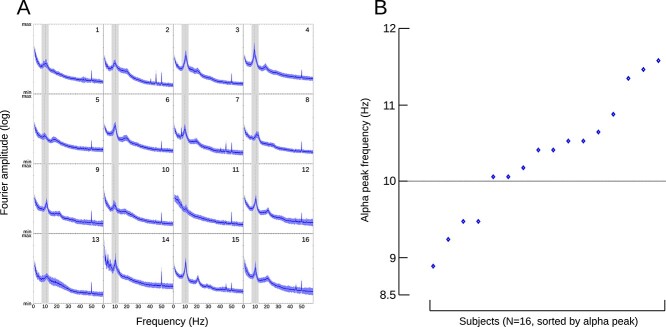
For the resting-state analysis, a 10-s long hamming window was moved over the whole 5-min resting-state recording in 1-s steps, and the Fourier amplitude spectra of each window were averaged to form the resting-state spectrum of each participant, which was averaged over all channels. The alpha peak of each participant was then identified as the location of the highest amplitude in the alpha frequency range, defined generally as 7–13 Hz.
Of the 17 participants, 1 participant had to be excluded, as resting-state data were unavailable due to technical failure. The remaining 16 participants all had unambiguous alpha peaks (see Fig. 2A) in their resting-state data and were entered into the following analysis. On average, individual PAF was 10.48 Hz (median: 10.6 Hz, min: 9.1 Hz, max 11.6 Hz, see Fig. 2B). These results are comparable to the values reported in previous studies (Klimesch 1999; Aurlien et al. 2004; Haegens et al. 2014; Cecere et al. 2015; Wutz et al. 2018) and also confirm that a wider or narrower limitation of the definition of the alpha band was not required with these participants.
Fig. 2.
Analysis of individual alpha peak frequency from resting state data. A) Subject number indicated in the top right corner. Blue line and shade indicate mean and SD of a 10-s sliding window moving over a 5-min recording (see Section 2). The alpha band (7–13 Hz) has been highlighted, and the determined peak in the alpha band marked with a dashed line. B) Individual alpha peak frequency for 16 participants, plotted in ascending order.
2.5.4 Instantaneous alpha frequency
As detailed in previously published work (Cohen 2014; Samaha and Postle 2015; Wutz et al. 2018), the instantaneous frequency provides information regarding the internal organization of the signal and is defined as the time rate of change of the instantaneous phase angle. Thus, informed by previous studies, experimental data were first band-pass filtered with a zero-phase, plateau-shaped FIR filter in the alpha frequency band between 7 and 13 Hz. Then, we computed the instantaneous phase angle over time with a Hilbert transformation. The temporal derivate of the instantaneous Hilbert phase corresponds to the instantaneous frequency in Hertz (scaled by sampling rate and 2π). In order to account for potential sharp artifacts in the resulting phase angle time series, we applied a 10-fold median filter (10 equally spaced window sizes between 10 and 400 ms, see Wutz et al. 2018). Across those resulting median filter windows, we calculated the median instantaneous frequency estimates. As task performance was comparable at ISIs of 67 and 100 ms, we pooled these data together and focused our analysis on participants that reached at least 20% of accuracy (N = 14) in order to obtain adequate statistical power. For each perceptual outcome and for each participant, an equal number of trials (in which participants had first correctly identified the correct stimulus location) were randomly selected to prevent any bias across conditions.
We then statistically compared the difference in IAF between consciously perceiving 1 or 2 flashes. Following the methodology of Samaha and Postle (2015), we selected the sensors with the highest group-level pre-stimulus power (right occipital: MEG2511 and MEG2541) and calculated the difference between perceptual outcomes in the pre-stimulus time period from −500 to 0 ms (onset of the first flash stimulus) with a dependent samples t-test with correction for multiple comparison by means of a nonparametric cluster-based permutation procedure (Oosterhof et al. 2016).
2.5.5 Phase locking
The NT stimuli were specifically intended to minimize the strength of a possible induced phase reset. To quantify any residual induced phase reset, we computed the inter-trial phase coherence (ITPC) across subjects (VanRullen et al. 2011) for the NT-only condition. The 2 channels with the highest pre-stimulus alpha power were pooled (MEG2511 and MEG2541, see above), and random permutation across time (N = 100k) was used to compute the P-values of the resulting ITPC values in the range from 3 to 30 Hz.
3. Results
3.1 Behavioral data
On average, across all conditions, participants detected the stimuli (i.e. identified the correct quadrant) on approximately 87% of all trials (range: 67.2–95.8%). Single NT pulses were located correctly in 48.5% of trials, while NT + Full pulses were correctly localized on 95% of all trials. This confirms both that the NT stimuli were truly near threshold and that the participants were clearly able to see the full-contrast stimuli. Detection performance was distributed homogenously across quadrants (SD of quadrant means 4.0%). A repeated measures analysis of variance on the quadrant localization correctness showed a significant effect of the factor stimulus type (F(5,95) = 430, GGe = 0.286, P < 0.001) but not the factor quadrant (F(3,57) = 0.99, GGe = 0.561, P = 0.369) or the interaction thereof (F(15,285) = 2.44, GGe = 0.198, P = 0.074).
As expected, correct enumeration performance (1 vs. 2 flashes) varied as a function of ISI (Fig. 3). On average, performance correct (identifying the number of flashes shown) for the NT + Full condition ranged from 22% at 33 ms ISI to just less than 50% at 400 ms ISI and was therefore within expectation, given that the probability of detecting the threshold pulse was by design adjusted to 57%. This difference in percentage correct enumeration between individual ISIs for the NT + Full conditions was significant (F(3,57) = 28.71, GGe = 0.54, P < 0.001). Performance on the Full + Full condition (33 ms) was around 34% and therefore higher than the NT + Full condition with the same ISI (paired t-test, t = 5.16, df = 19, P < 0.001). Correct performance with only a single NT pulse was just below 90%, showing that participants predominantly reported seeing only a single flash (rather than two) when the single NT stimulus was presented and correctly localized.
Fig. 3.
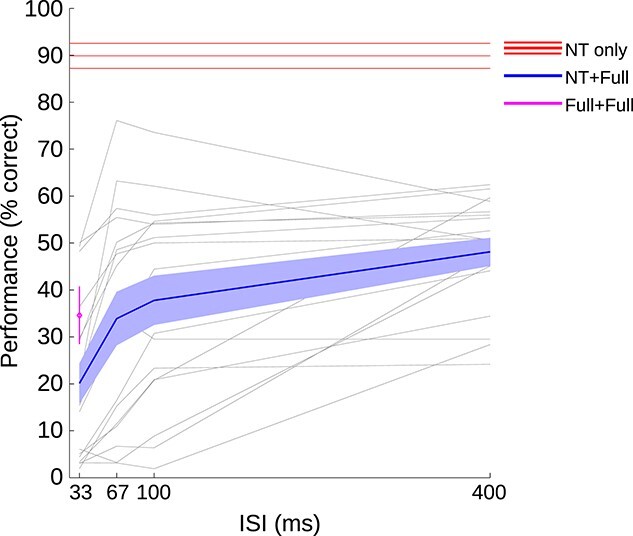
Percent correct responses in enumerating the number of flashes (only including trials in which the flash was correctly localized in spatial location). Blue lines indicate performance in the main experimental condition, in which 1 NT flash and 1 full contrast stimulus were presented (NT + Full). The solid line shows the mean and the shaded area indicates the standard error of the mean across participants. The red line shows performance in the NT-only condition (mean and SEM across participants). The magenta symbol, at 33 ms, shows performance for 2 full pulses separated by a 33 ms ISI (mean and SEM across participants). The faint gray lines represent performance of individual participants in the NT + Full condition.
The main focus of the analysis was on the improvement in performance in the main experimental condition (dual pulse NT + Full), as a function of ISI (Fig. 3, blue line). As expected, participants improved their performance in the NT + Full condition when the temporal separation between flashes was increased. This improvement leveled off between 100 and 400 ms ISI, on average. However, there were large individual differences. Some participants improved to levels near their individual maximum performance already by 67 ms, while other participants appeared to require longer ISIs to reach maximum performance (individual participant data shown as individual gray lines in Fig. 3).
3.2 MEG data
The analysis of the MEG data was focused on 3 main aspects of the time series data. First, we analyzed stimulus-evoked (ERF) signatures in order to relate the magnitude of the evoked response to behavioral performance. Second, we determined the individual PAF for each participant and tested whether this was correlated with average behavioral performance of the participant. Finally, we measured the trial-by-trial instantaneous alpha frequency (IAF) to see whether trials in which the alpha peak frequency was higher led to better performance in the 2-flash task.
3.3 Event-related fields
We first confirmed that the NT and full strength stimuli were processed differently, with greater response to the full strength flash. As expected, posterior/occipital and parietal sensor clusters responded based on stimulus strength, with weakest responses to the NT stimulus and strongest response to 2 full-contrast stimuli, with the ERF for the combination of a NT and a full-contrast stimulus falling somewhere in between, often a reduced version of the Full + Full condition. Locally averaged magnetometer ERFs (Supplementary Fig. S1) as well as general topographic time course (Supplementary Fig. S2, left) suggest the presence of spatially large, lateral dipoles involved in visual processing. Gradiometers showed strong earlier activations mostly in posterior (occipital) regions, while later activity appears to transition to more central locations (Fig. S2, right). To further identify regions that were sensitive to the stimulation strength, the time course of the pooled NT + Full conditions was subtracted from the Full + Full condition (see Supplementary Fig. S3).
3.4 Phase locking
To investigate the amount of phase reset induced by the NT stimuli, we computed the ITPC across subjects (see Fig. 4), and random permutation (N = 100 k) across time was used to compute P-values of the resulting ITPC values. After FDR correction at the 0.05 level (Genovese et al. 2002), no statistically significant P-values remained.
Fig. 4.
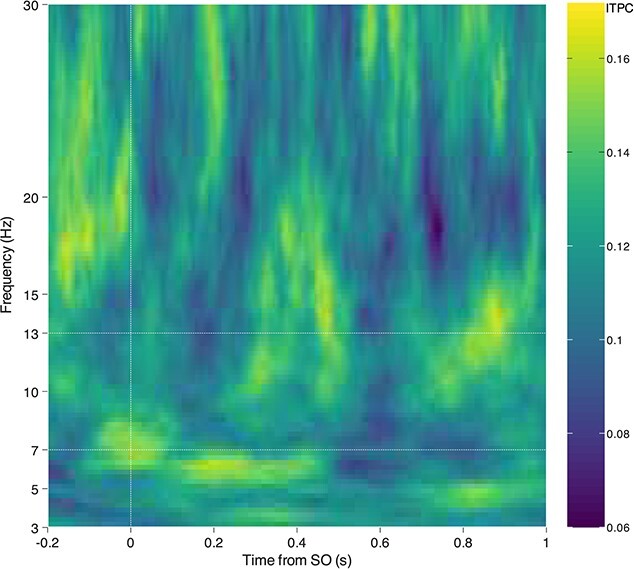
Inter-trial phase locking analysis. For the NT condition, the 2 channels with the highest pre-stimulus alpha power were averaged, and the ITPC was computed across subjects in time–frequency space. No significant phase locking was discovered.
3.5 Correlation analysis: individual peak alpha frequency
The behavioral data showed a common pattern: all participants exhibited relatively poor performance at an ISI of 33 ms but also relatively high performance at 400 ms ISI. The distribution of the improvement from the shortest to the longest timing varied between participants; some participants showed a strong increase in the earliest interval (33–67 ms), while other participants showed the largest improvement in the intermediate (67–100 ms) or even the latest interval (100–400 ms). This pattern is consistent with some participants integrating the 2 signals over a relatively short (less than 100 ms) or relatively long (over 100 ms) temporal window, as previously shown in other paradigms using 2 suprathreshold stimuli (Samaha and Postle 2015; Ronconi et al. 2017).
As hypothesized, individual participant performance in this perceptual segregation task should be better for participants with faster temporal resolution. Under the assumption that the individual resting-state alpha peak frequency is an indicator of the individual speed of a participants’ visual system and thereby of the ability to resolve brief temporal events (Cecere et al. 2015; Samaha and Postle 2015; Wutz et al. 2018), we hypothesize that those participants with higher PAF will improve their performance in earlier ISIs compared to participants with slower PAF. Participants with high individual PAF might therefore improve their correct detection rate (relative to 33 ms baseline) already in the first step (from 33 to 67 ms), while participants with low PAF should improve mostly in the later steps (67–100, 100–400 ms).
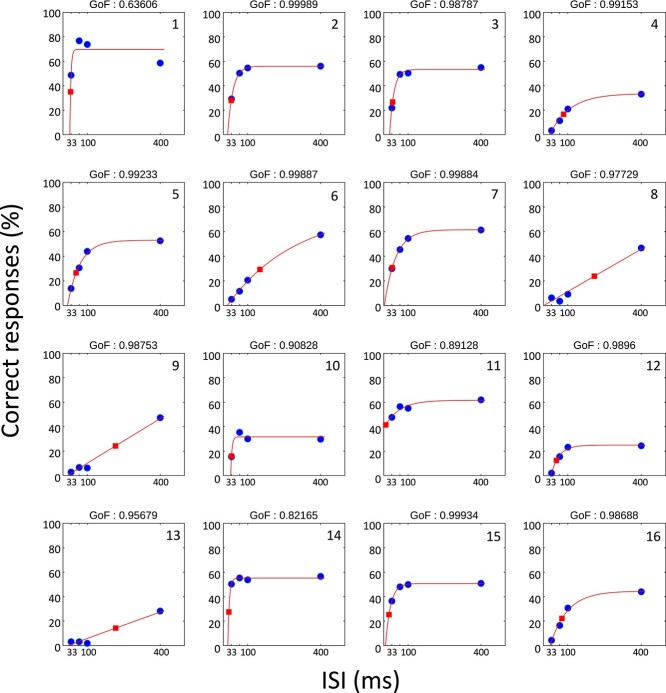
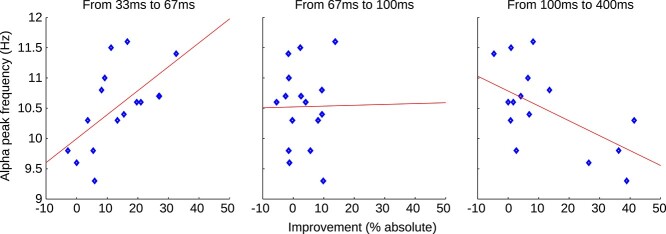
Psychometric function fitting (see Section 2) and the resulting individual thresholds (50% of the individual maximum performance) can be seen in Fig. 5. As described above (see Section 2), there were several practical issues with fitting the behavioral data given that the minimum value was not necessarily zero and the steepness of the curve was likely to be artificially “flattened” by differences in the phase of the alpha oscillation across trials. Ideally, to be considered a psychophysical curve measuring performance as a function of ISI, the fit to the data should start at a low value and asymptote (stop increasing) at 50% or higher, with a goodness of fit of 0.90 or better. The data of only 6 out of 16 participants would meet these minimal criteria. The identified thresholds from the existing fits are located at ISIs between 10 and 260 ms, corresponding to frequencies between 3.84 and 100 Hz, with only 2 out of 16 values located in the alpha band. Due to the lack of curve fits that “look like” a psychophysical curve and the wide spread of the estimated thresholds, we believe that the results from the fitting procedure do not successfully represent the subjects TIWs. Not surprisingly, there was no significant correlation between the identified thresholds and the individual PAF (Pearson’s R = 0.076, P = 0.779). The more robust results from directly correlating the improvement between ISIs with the PAF (see Section 2) are shown in Fig. 6. We found a significant positive correlation between the performance improvement and individual PAF in the first interval (33–67 ms, Pearson’s R = 0.60, P = 0.014). No correlation was observed in the intermediate interval (67–100 ms, R = 0.01, P = 0.966), whereas a significant negative correlation was found in the late interval (100–400 ms, R = −0.60, P = 0.013). Note that reported P-values are uncorrected but remain significant after Bonferroni correction (N = 3, threshold ≤0.05).
Fig. 5.
Psychometric fits (unconstrained) for 16 participants. Participant IDs are noted in the top-right corners. Blue dots indicate the performance means of the respective individual (percentage of trials correctly identified as 2 stimuli, see also Fig. 3). Red lines represent the fitted psychometric functions. Red squares indicate the location of the individual 50% relative performance thresholds.
Fig. 6.
Peak alpha frequency versus behavioral performance. Blue markers represent individual participants; red lines are fitted to the data for better visualization. Results indicate a positive correlation between individual PAF and performance improvement in the early interval (33–67 ms, Pearson’s R = 0.60, P = 0.014). No correlation was observed in the intermediate interval (67–100 ms, R = 0.01, P = 0.966), whereas a negative correlation was found in the late interval (100–400 ms, R = −0.60, P = 0.013).
3.6 Correlation analysis: pre-stimulus instantaneous alpha frequency
As mentioned in Section 1, alpha frequency is generally considered to be a long-term stable feature (Salinsky et al. 1991). However, on a shorter time scale, peak oscillatory frequency (in the alpha band) varies within an individual during visual perception (Cohen 2014;Samaha and Postle 2015 ; Wutz et al. 2018). It has further been shown that just before stimulus onset, correctly discriminated (segregated) visual stimuli in a temporal fusion task on average exhibit slightly higher IAF compared to incorrectly discriminated (fused) stimuli (Samaha and Postle 2015; Wutz et al. 2018).
Consistent with this prediction, IAF in our participants was higher on average for trials in which both flashes were reported, compared to those trials where only one flash was reported (Fig. 7). Permutation testing revealed a significant difference between correctly and incorrectly reported stimuli over an interval from 344 to 296 ms before stimulus onset (P = 0.013, cluster corrected).
Fig. 7.
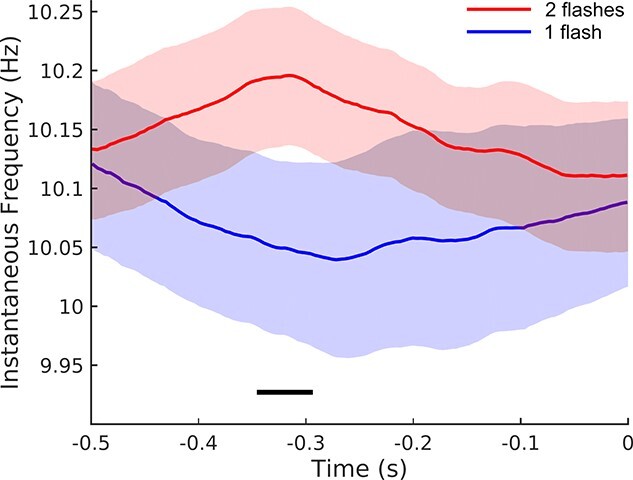
Pre-stimulus instantaneous alpha frequency. Within-participants analysis comparing NT + Full trials in which participants correctly reported 2 flashes (red) versus those trials in which participants reported only one flash (blue), mean across participants and within-participant SEM. Time zero indicates the location of the first flash. The black line indicates the temporal location of a significant difference (cluster-corrected permutation test; P < 0.05). The legend indicates the perceptual outcome as reported by the participants, since all trials were in fact 2-flash presentations (NT + Full).
4. Discussion
Using a modified version of the 2-flash fusion task, in which we also included pairs made up of 1 NT stimulus and 1 suprathreshold stimulus, with a varied ISI (based on Wühle et al. 2010, 2011), we investigated the link between alpha frequency and the processing of 2 rapidly presented stimuli. Consistent with the aim of our experimental design, the initial NT stimulus did not generate a strong event-related signal, meaning that the NT + full paradigm can indeed serve as a useful tool for investigating temporal integration without the risk of the first stimulus causing a strong phase-reset (Brandt 1997; Wutz, Weisz, et al. 2014) or artificially generating an “oscillation-like” activity (VanRullen and Macdonald 2012).
It is possible that the detection likelihood of the NT stimuli was influenced by the phase of the ongoing brain activity around the time of stimulus onset. However, as the NT + Full pulses were localized correctly in 95% of the trials, we may conclude that localization performance in these conditions depended mostly on the second, full intensity pulse. Still, the enumeration performance may have been affected, as the likelihood of the detection of the first NT pulse may have altered with the phase of ongoing oscillatory activity. As the phase of ongoing oscillations is generally distributed randomly with respect to the stimulus onset timing, any such effect would have occurred in all subjects and conditions equally, effectively being canceled out of the results.
Temporal integration is usually not sharp-edged or absolute (Drewes and VanRullen 2011; Drewes et al. 2014, 2015; Wutz et al. 2016). Stimuli falling near the temporal borders of a TIW may be only partially merged or be merged according to some stochastic principle. Also, the TIWs in our study are most likely not phase-reset at the time of the threshold stimulus, meaning the TIWs do not always start at or near the first flash but are in their respective phase resultant from ongoing activity and thus randomly aligned with the presentation times during each trial. Therefore, depending on the temporal width of each individual TIW, which in turn is dependent on both the individual PAF and trial-by-trial IAF, the chance for 2 stimuli to be merged at a given ISI will be overall larger if the window width is larger, and overall smaller if the window is narrower. Only when the temporal distance between 2 stimuli is entirely out of the range of 1 single window (as in the 400 ms condition here) a segregation of the stimuli may be considered reliable.
The results presented here indicate that 2 stimuli presented at ISIs of 33 ms (and, by trivial assumption, faster than that) are most likely to be merged into one percept, whereas ISIs of 400 ms (and slower than that) are likely segregated into 2 percepts.
For ISIs in between these 2 extremes, the likelihood at which they will be integrated or segregated will depend on the size (and in a random fashion, the phase) of the individual TIWs. For the shortest TIWs, the increase in ISI from 33 to 67 ms may (and apparently does, as our data suggests) already suffice to cause a pronounced increase in segregation likelihood, whereas the longest TIWs may require times longer than 100 ms to reach the same level.
Further studies with this type of stimulus, using a more parametric mapping of ISIs, could be used (as in the Wühle et al.’s studies) to map the effective TIW of different areas in MEG source space.
Our first hypothesis was that individual differences in the PAF, measured during a separate resting-state period, would be related to individual differences in temporal resolution. We used the degree to which performance increased rapidly as a function of ISI (showing that the participant had a high temporal resolution) as a proxy measure for the speed of visual sampling. Participants who improved when ISI was increased to 67 ms were considered to have a TIW of less than 100 ms, while those who improved only when ISI increased from 100 to 400 ms showed performance consistent with a longer TIW (>100 ms). Consistent with our hypothesis, participants with a faster PAF showed the most rapid improvement in performance: They improved as ISI increased up to around 100 ms, but then no longer benefitted from the longer ISI. Conversely, those with slower PAF did not benefit much from the initial increase in ISI but did improve more when ISI was over 100 ms. This pattern of results provides further evidence for a link between resting-state PAF and visual temporal resolution (Walsh 1952; Varela et al. 1981; VanRullen 2013; Wutz, Weisz, et al. 2014).
The second main finding was that the instantaneous measure of peak alpha frequency (IAF), estimated on each trial for the pre-stimulus period, was higher/faster when participants correctly reported 2 flashes on that trial compared to when they only reported 1 flash. This provides a replication of a previous finding with the more traditional 2-flash fusion task (Samaha and Postle 2015), but without the possible confound of the first flash resetting the alpha phase. It was also interesting to show this effect in the current study given the other finding, described above, linking performance to individual differences. In terms of the question of whether the link between visual temporal resolution and alpha rhythms reflects a “trait” or a “state,” our findings suggest that both may be the case.
Due to uncertainty in both time and frequency, this identified location of the IAF effect is only approximate. Still, the effect is found in the interval from 344 to 296 ms before stimulus onset, which is significantly longer than one alpha period. In previous studies (e.g. Drewes and VanRullen 2011; Wutz, Weisz, et al. 2014; Samaha and Postle 2015), the phase/frequency effects predictive of trial outcome were also located before time zero (e.g. stimulus onset), sometimes by comparable temporal distances. For example, Busch et al. (2009) found alpha power to be most predictive of trial outcome at around 490 ms before stimulus onset, and alpha phase in a time window ranging from 300 to 50 ms before stimulus onset. The latter is particularly relevant to our analysis, as instantaneous alpha frequency and alpha phase are closely related, and the critical time interval identified by our permutation testing overlaps with the interval of the mentioned study.
One possible reason for the temporal distance between measured effect and stimulus onset may be an indirect coupling between measurement and effect: The IAF difference we were able to identify may not be the direct cause of the trial-by-trial performance difference; instead, there may be an intermediate link in the visual processing chain, dependent on the identified IAF effect, but in itself not detectable with the current methodology.
The overall pattern of results found here provides converging evidence for the claim that the “frame rate” of visual processing is linked to a specific visual temporal integration window that depends on the temporal sampling rate, at around 10 Hz. This is not to say that every aspect of visual (or multisensory) processing operates at this same frame rate (see, for example: Ronconi et al. 2017). Some forms of flicker, for example, are visible at faster rates (Landis 1953; Brindley et al. 1966; Campos and Bedell 1978; Capilla and Aguilar 1993). One possible interpretation of the current set of findings is that rapid individuation of potential objects as distinct entities in space and time involves this 10 Hz rhythm, while processing of more complex objects and events may involve slower rhythms, in the theta range (Drewes et al. 2015; Wutz et al. 2016; Zhu et al. 2016; Ronconi et al. 2017). Moreover, there is converging evidence that selective attention may also alternate, in terms of its function or spatial locus, in the theta to low-alpha range (Landau and Fries 2012; Fiebelkorn et al. 2013; VanRullen 2013; Song et al. 2014; Dugué et al. 2015).
In conclusion, our results addressed the question of whether the individual PAF, linked to a visual sampling rhythm, is an individual trait (PAF) or state-dependent and subject to change via top–down control (Samaha and Postle 2015; Wutz et al. 2018) or other factors. The term “instantaneous alpha frequency” (IAF) suggests variability over time. Here, we found that overall performance was indeed linked to an individual trait (PAF), but also that variations in the IAF predicted performance on individual trials. In other words, peak alpha may be both a trait and a state.
Supplementary Material
Acknowledgments
We would like to thank Poppy Sharp for her assistance with data collection, and Andreas Wutz for his help with data analysis.
Contributor Information
Jan Drewes, Institute of Brain and Psychological Sciences, Sichuan Normal University, 610066 Chengdu, China; Department of Psychology and Center for Mind/Brain Sciences, University of Trento, 38068 Rovereto, Italy.
Evelyn Muschter, Department of Psychology and Center for Mind/Brain Sciences, University of Trento, 38068 Rovereto, Italy; Centre for Tactile Internet with Human-in-the-Loop (CeTI), Technische Universität Dresden, 01069 Dresden, Germany.
Weina Zhu, Department of Psychology and Center for Mind/Brain Sciences, University of Trento, 38068 Rovereto, Italy; School of Information Science, Yunnan University, 650091 Kunming, China.
David Melcher, Department of Psychology and Center for Mind/Brain Sciences, University of Trento, 38068 Rovereto, Italy; Psychology Program, Division of Science, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates.
Funding
JD, EM, and DM were supported by a European Research Council (ERC) grant (Grant Agreement No. 313658) and High-level Foreign Expert Grant (GDT20155300084). WZ was supported by the National Natural Science Foundation of China (61263042, 61563056).
Conflict of interest statement.. None declared.
References
- Aurlien H, Gjerde IO, Aarseth JH, Eldøen G, Karlsen B, Skeidsvoll H, Gilhus NE. EEG background activity described by a large computerized database. Clin Neurophysiol. 2004:115:665–673. [DOI] [PubMed] [Google Scholar]
- Bishop GH. Cyclic changes in excitability of the optic pathway of the rabbit. Am J Physiol. 1932:103:213–224. [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis. 1997:10:433–436. [PubMed] [Google Scholar]
- Brandt ME. Visual and auditory evoked phase resetting of the alpha EEG. Int J Psychophysiol. 1997:26:285–298. [DOI] [PubMed] [Google Scholar]
- Brindley GS, Du Croz JJ, Rushton WAH. The flicker fusion frequency of the blue-sensitive mechanism of colour vision. J Physiol. 1966:183:497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J Neurosci. 2009:29:7869–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos EC, Bedell HE. Critical flicker-fusion frequency as an indicator of human receptive field-like properties. Invest Ophthalmol Vis Sci. 1978:17:533–538. [PubMed] [Google Scholar]
- Capilla P, Aguilar M. Red-green flicker resolution as a function of heterochromatic luminous modulation. Ophthalmic Physiol Opt. 1993:13:183–185. [DOI] [PubMed] [Google Scholar]
- Capilla A, Pazo-Alvarez P, Darriba A, Campo P, Gross J. Steady-state visual evoked potentials can be explained by temporal superposition of transient event-related responses. PLoS One. 2011:6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecere R, Rees G, Romei V. Individual differences in alpha frequency drive crossmodal illusory perception. Curr Biol. 2015:25:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. Fluctuations in oscillation frequency control spike timing and coordinate neural networks. J Neurosci. 2014:34:8988–8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S. Temporal oscillations in human perception. Psychol Sci. 2016:4(4):264–270. [Google Scholar]
- Doelling KB, Assaneo MF, Bevilacqua D, Pesaran B, Poeppel D. An oscillator model better predicts cortical entrainment to music. Proc Natl Acad Sci. 2019:116:10113–10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes J, VanRullen R. This is the rhythm of your eyes: the phase of ongoing electroencephalogram oscillations modulates saccadic reaction time. J Neurosci. 2011:31:4698–4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes J, Zhu W, Melcher D. Dissociation between spatial and temporal integration mechanisms in Vernier fusion. Vis Res. 2014:105:21–28. [DOI] [PubMed] [Google Scholar]
- Drewes J, Zhu W, Wutz A, Melcher D. Dense sampling reveals behavioral oscillations in rapid visual categorization. Sci Rep. 2015:5:16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué L, Marque P, VanRullen R. Theta oscillations modulate attentional search performance periodically. J Cogn Neurosci. 2015:27:945–958. [DOI] [PubMed] [Google Scholar]
- Fiebelkorn IC, Saalmann YB, Kastner S. Rhythmic sampling within and between objects despite sustained attention at a cued location. Curr Biol. 2013:23:2553–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002:15:870–878. [DOI] [PubMed] [Google Scholar]
- Gho M, Varela FJ. A quantitative assessment of the dependency of the visual temporal frame upon the cortical rhythm. J Physiol Paris. 1988:83:95–101. [PubMed] [Google Scholar]
- Gottlieb MD, Kietzman ML, Berenhaus IJ. Two-pulse measures of temporal integration in the fovea and peripheral retina. Percept Psychophys. 1985:37:135–138. [DOI] [PubMed] [Google Scholar]
- Haegens S, Cousijn H, Wallis G, Harrison PJ, Nobre AC. Inter- and intra-individual variability in alpha peak frequency. NeuroImage. 2014:92:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter MR. Excitability cycles and cortical scanning: a review of two hypotheses of central intermittency in perception. Psychol Bull. 1967:68:47–58. [DOI] [PubMed] [Google Scholar]
- Hasson U, Yang E, Vallines I, Heeger DJ, Rubin N. A hierarchy of temporal receptive windows in human cortex. J Neurosci. 2008:28:2539–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T. Audiovisual temporal capture underlies flash fusion. Exp Brain Res. 2009:198:195–208. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999:29:169–195. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Gross J, Thut G. A new unifying account of the roles of neuronal entrainment. Curr Biol. 2019:29:R890–R905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau AN, Fries P. Attention samples stimuli rhythmically. Curr Biol. 2012:22:1000–1004. [DOI] [PubMed] [Google Scholar]
- Landis C. An annotated bibliography of flicker fusion phenomena covering the period 1740–1952. National Academies; 1953. [Google Scholar]
- Lansing RW. Relation of brain and tremor rhythms to visual reaction time. Electroencephalogr Clin Neurophysiol. 1957:9:497–504. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2010:2011:e156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhof NN, Connolly AC, Haxby JV. CoSMoMVPA: multi-modal multivariate pattern analysis of neuroimaging data in Matlab/GNU octave. Front Neuroinformatics. 2016:10:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997:10:437–442. [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999:110:1842–1857. [DOI] [PubMed] [Google Scholar]
- Pöppel E. A hierarchical model of temporal perception. Trends Cogn Sci. 1997:1:56–61. [DOI] [PubMed] [Google Scholar]
- Pöppel E. Pre-semantically defined temporal windows for cognitive processing. Philos Trans R Soc B Biol Sci. 2009:364:1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin DA, Royer FL. Auditory temporal processing: two-tone flutter fusion and a model of temporal integration. J Acoust Soc Am. 1987:82:1207–1217. [DOI] [PubMed] [Google Scholar]
- Ronconi L, Melcher D. The role of oscillatory phase in determining the temporal organization of perception: evidence from sensory entrainment. J Neurosci. 2017:37:10636–10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronconi L, Oosterhof NN, Bonmassar C, Melcher D. Multiple oscillatory rhythms determine the temporal organization of perception. Proc Natl Acad Sci U S A. 2017:114:13435–13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinsky MC, Oken BS, Morehead L. Test-retest reliability in EEG frequency analysis. Electroencephalogr Clin Neurophysiol. 1991:79:382–392. [DOI] [PubMed] [Google Scholar]
- Samaha J, Postle BR. The speed of alpha-band oscillations predicts the temporal resolution of visual perception. Curr Biol. 2015:25:2985–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TK, Nauhaus I, Carandini M. Traveling waves in visual cortex. Neuron. 2012:75:218–229. [DOI] [PubMed] [Google Scholar]
- Song K, Meng M, Chen L, Zhou K, Luo H. Behavioral oscillations in attention: rhythmic α pulses mediated through θ band. J Neurosci. 2014:34:4837–4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Kajola M. Presentation of electromagnetic multichannel data: the signal space separation method. J Appl Phys. 2005:97:124905. [Google Scholar]
- Taulu S, Simola J, Kajola M. Applications of the signal space separation method. IEEE Trans Signal Process. 2005:53:3359–3372. [Google Scholar]
- VanRullen R. Visual attention: a rhythmic process? Curr Biol. 2013:23:R1110–R1112. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Koch C. Is perception discrete or continuous? Trends Cogn Sci. 2003:7:207–213. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Macdonald JSP. Perceptual echoes at 10 Hz in the human brain. Curr Biol. 2012:22:995–999. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Busch N, Drewes J, Dubois J. Ongoing EEG phase as a trial-by-trial predictor of perceptual and attentional variability. Front Percept Sci. 2011:2:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela FJ, Toro A, John ER, Schwartz EL. Perceptual framing and cortical alpha rhythm. Neuropsychologia. 1981:19:675–686. [DOI] [PubMed] [Google Scholar]
- Walsh EG. Visual reaction time and the alpha-rhythm, an investigation of a scanning hypothesis. J Physiol. 1952:118:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983:33:113–120. [DOI] [PubMed] [Google Scholar]
- Wühle A, Mertiens L, Rüter J, Ostwald D, Braun C. Cortical processing of near-threshold tactile stimuli: an MEG study. Psychophysiology. 2010:47:523–534. [DOI] [PubMed] [Google Scholar]
- Wühle A, Preissl H, Braun C. Cortical processing of near-threshold tactile stimuli in a paired-stimulus paradigm--an MEG study. Eur J Neurosci. 2011:34:641–651. [DOI] [PubMed] [Google Scholar]
- Wutz A, Muschter E, van Koningsbruggen M, Melcher D. Saccades reset temporal integration windows. J Vis. 2014:14:584–584. [Google Scholar]
- Wutz A, Weisz N, Braun C, Melcher D. Temporal windows in visual processing: “Prestimulus Brain State” and “Poststimulus Phase Reset” segregate visual transients on different temporal scales. J Neurosci. 2014:34:1554–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz A, Muschter E, van Koningsbruggen MG, Weisz N, Melcher D. Temporal integration windows in neural processing and perception aligned to saccadic eye movements. Curr Biol. 2016:26:1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz A, Melcher D, Samaha J. Frequency modulation of neural oscillations according to visual task demands. Proc Natl Acad Sci. 2018:115:1346–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro K, Inui K, Otsuru N, Urakawa T, Kakigi R. Temporal window of integration in the somatosensory modality: an MEG study. Clin Neurophysiol. 2011:122:2276–2281. [DOI] [PubMed] [Google Scholar]
- Zhou J, Benson NC, Kay KN, Winawer J. Compressive temporal summation in human visual cortex. J Neurosci. 2018:38:691–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Drewes J, Melcher D. Time for awareness: the influence of temporal properties of the mask on continuous flash suppression effectiveness. PLoS One. 2016:11:e0159206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoefel B, ten Oever S, Sack AT. The involvement of endogenous neural oscillations in the processing of rhythmic input: more than a regular repetition of evoked neural responses. Front Neurosci. 2018:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.