Abstract
Patients with obsessive–compulsive disorder (OCD) exhibit abnormality in their subjective perception of internal sensation, a process known as interoceptive sensibility (IS), as well as altered functioning of the insula, a key neural structure for interoception. We investigated the multivariate structure of IS in 77 OCD patients and 53 controls and examined associations of IS with resting-state functional connectivity (FC) of the insula within the OCD group. For each group, principal component analysis was performed on 8 subscales of the Multidimensional Assessment of Interoceptive Awareness assessing putatively “adaptive” and “maladaptive” aspects of IS. Associations between IS components and insula FC in the OCD group were evaluated using seed regions placed in each of 3 subdivisions of the insula (posterior, anterior dorsal, and anterior ventral). Behaviorally, controls showed a 2-component solution broadly categorized into “adaptive” and “maladaptive” IS, while OCD patients exhibited a 3-component solution. The general tendency to notice or be aware of sensation loaded onto an “adaptive” IS component in controls but loaded onto both “adaptive” and “maladaptive” IS components in OCD. Within OCD, insula FC was differentially associated with distinct aspects of IS, identifying network connections that could serve as future targets for the modulation of IS in OCD.
Keywords: interoception, interoceptive sensibility, obsessive–compulsive disorder, resting-state functional connectivity, insula
Introduction
Patients with obsessive–compulsive disorder (OCD) experience obsessions (recurrent and distressing thoughts, impulses, or images) and/or compulsions (repetitive behaviors and mental acts performed to reduce anxiety or discomfort). OCD symptoms are highly heterogeneous, and approximately 30%–40% of patients with OCD report aversive or uncomfortable body sensations that immediately precede or accompany their compulsions (also known as “sensory phenomena”) (Lee et al. 2009; Ferrão et al. 2012; Shavitt et al. 2014). Additionally, some OCD patients fear anxiety-related body sensations (such as sweating and rapid heart rate), an experience known as “anxiety sensitivity” (Blakey et al. 2017; Blakey and Abramowitz 2018). The process by which individuals sense, interpret, and integrate signals from within the body is known as interoception (Tsakiris and Critchley 2016; Khalsa et al. 2018). We recently found that patients with OCD report differences from healthy controls in several aspects of interoception, including exhibiting a hyperawareness of body sensations, a greater tendency to distract themselves from and worry about aversive body sensations, and a lower tendency to experience their body as safe and trustworthy (Eng et al. 2020). Some of these effects, such as hyperawareness of sensation and worry about sensation, were associated with the severity of OCD symptoms. These findings indicate that patients with OCD subjectively experience and interpret body sensations differently than controls, which may contribute to their symptoms.
The neural basis of interoception has been fairly well delineated. Afferent fibers carry interoceptive signals from visceral (e.g. heart, lungs, gastrointestinal, urogenital), somatic (e.g. muscles, joints, skin), and homeostatic systems (e.g. temperature, mechanical stress, cellular activity) to the brain stem (Light and Perl 1979; Craig 2002; Critchley and Harrison 2013). These ascending interoceptive signals relay to midbrain regions and project to the posterior division of the insula cortex primarily through the thalamus (Flynn 1999; Craig 2002).
The insula is a key neural structure for interoception (Craig 2002, 2003, 2009). Several neuroimaging approaches have converged on a tripartite parcellation of the insula that includes posterior, dorsal anterior, and ventral anterior subdivisions (Mutschler et al. 2009; Deen et al. 2011; Chang et al. 2013; Nomi et al. 2017; Wager and Barrett 2017). It has been proposed that afferent fibers carrying interoceptive signals from within the body are initially represented at the sensory level in the posterior insula, which has functional connections to primary and secondary motor and somatosensory cortices (Craig 2002; Cauda et al. 2011; Deen et al. 2011; Vogt et al. 2016). Signals from the posterior insula are then relayed to the anterior insula for more complex processing (Craig 2003). The anterior insula itself has dissociative functional connectivity (FC) profiles within its ventral and dorsal subdivisions (Deen et al. 2011). The ventral anterior insula is functionally connected to limbic/paralimbic regions, such as the pregenual anterior cingulate, amygdala, lateral orbitofrontal, and ventral tegmental areas (Mutschler et al. 2009; Deen et al. 2011; Chang et al. 2013), and has a role in emotion processing (Touroutoglou et al. 2012; Wager and Barrett 2017). By contrast, the dorsal anterior insula is functionally connected to regions involved in cognitive control, such as the dorsal anterior cingulate (dACC) and lateral prefrontal cortex (Taylor et al. 2009; Deen et al. 2011; Chang et al. 2013), and is active in tasks requiring effortful attention and salience detection (Seeley et al. 2007; Sridharan et al. 2008; Menon and Uddin 2010). Through the integration of emotional and cognitive information (purportedly represented in subcortical, limbic, and executive control structures) with sensory information (purportedly represented in posterior insula), the anterior insula has been proposed to carry a higher-order re-representation of interoception (Craig 2003, 2009).
Prior work has implicated the insula in OCD. Studies assessing structural morphometry reported higher gray matter volumes in the anterior insula (Kim et al. 2001; Valente Jr et al. 2005; Nishida et al. 2011; Song et al. 2011) and lower gray matter volumes in the posterior insula (Pujol et al. 2004; Song et al. 2011) in OCD patients compared to controls. In addition, using functional magnetic resonance imaging (fMRI), studies reported insula alterations in OCD, primarily with increases in anterior (Shapira et al. 2003; Stern et al. 2012, 2020; Li et al. 2013; Beucke et al. 2014; Peng et al. 2014; Berlin et al. 2017; Fan et al. 2017; Posner et al. 2017) and posterior insula (Peng et al. 2014; Berlin et al. 2017; Stern et al. 2020) in measures of connectivity and task activation.
Building on our finding of altered interoception in OCD and prior work implicating the insula in OCD pathophysiology, the present study investigated the multivariate structure of interoception in OCD patients and matched controls by conducting a principal component analysis (PCA) on the subscales from the self-report Multidimensional Assessment of Interoceptive Awareness (MAIA; Mehling et al. 2012). To determine whether different aspects of interoception were associated with insula connectivity patterns in patients with OCD, we further examined the relationship between MAIA component scores and resting-state FC for the different insula subdivisions (dorsal anterior, ventral anterior, and posterior) within the patient sample.
Materials and methods
Subjects and procedure
Ninety patients with OCD and 54 healthy controls were recruited from 3 locations: Icahn School of Medicine at Mount Sinai (ISMMS), Nathan Kline Institute for Psychiatric Research (NKI), and New York University School of Medicine (NYUSoM) (Supplementary Information). The study protocol was approved by the Institutional Review Boards at each institution and all subjects provided written informed consent. Data from 13 OCD patients and 1 control were excluded (Supplementary Information) (Yan et al. 2013). Final data were analyzed from 77 OCD patients and 53 controls. Behavioral results comparing OCD and controls on self-reported interoception derived from subscales of the MAIA were recently published that included many of the same subjects (68 OCD patients and 47 controls from the present sample; Eng et al. 2020).
All patients met criteria according to The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) for OCD and were excluded for lifetime presence of bipolar disorder, psychotic disorder, or moderate/severe alcohol or substance use disorder. Healthy controls were excluded for any lifetime presence of Axis I diagnoses. Diagnoses were made by a trained rater using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al. 1998). All patients had OCD as their primary diagnosis as determined by the MINI, even if comorbid conditions were present.
Behavioral and clinical assessments
Interoception was assessed using the self-report MAIA (Mehling et al. 2012; Mehling 2016) to index the subjective experience of the body, a process referred to as interoceptive sensibility (IS) (Garfinkel et al. 2015). The MAIA includes 32 questions assessing 8 dimensions of IS previously identified through factor analysis: (1) tendency to notice or become aware of body sensations (``Noticing''), (2) tendency to not distract oneself from sensations of pain or discomfort (``Not-Distracting''), (3) tendency to not worry about or experience emotional distress in response to sensations of pain or discomfort (``Not-Worrying''), (4) ability to sustain and control attention to body sensations (``Attentional Control''), (5) awareness of the link between emotion and body sensations (``Emotional Awareness''), (6) ability to regulate negative emotion through attention to body sensations (``Self-Regulation''), (7) tendency to listen to body sensations for insight into emotion and guide behavior (``Listening''), and (8) tendency to experience the body as safe and trustworthy (``Trusting'').
The MAIA was designed to distinguish between “adaptive” and “maladaptive” forms of IS (Mehling 2016). Higher scores on Attentional Control, Self-Regulation, Listening, and Trusting subscales reflect a mindful style of attention and are considered “adaptive”, while higher distraction (i.e. lower scores on Not-Distracting) and worrying (i.e. lower scores on Not-Worrying) over unpleasant body sensations are considered “maladaptive” (Goubert et al. 2004; Mehling et al. 2009; Mehling 2016). The Noticing and Emotional Awareness subscales are considered ambiguous and can be classified as either “adaptive” or “maladaptive” depending on their associations with other subscales (Mehling et al. 2009; Mehling 2016). For example, regulating attention to the sensation is considered “adaptive” (Burns 2006; Mehling et al. 2009), while experiencing emotional distress over uncomfortable body sensation is “maladaptive” and is generally associated with anxiety, hypochondriasis, and somatization disorders (Porges 1993; Mehling 2016). The MAIA has construct validity with other measures of body awareness, mindfulness, and emotion regulation (Mehling et al. 2012; Mehling 2016), such as the Five Facet Mindfulness Questionnaire (Baer et al. 2006, 2008), Difficulties in Emotion Regulation Scale (Gratz and Roemer 2004), and Body Responsiveness Questionnaire (Spielberger et al. 1983; Daubenmier 2005).
OCD symptoms were measured using the clinician-administered Yale-Brown Obsessive Compulsive Scale (Y-BOCS; Goodman et al. 1989) and the self-report Dimensional Obsessive–Compulsive Scale (DOCS; Supplementary Information; Abramowitz et al. 2010), the latter of which assesses severity of 4 different dimensions of OCD symptoms. Sensory phenomena were measured using the clinician-administered University of Sao Paolo’s Sensory Phenomena Scale (SPS; Rosario et al. 2009; Sampaio et al. 2014). State anxiety, and depression were measured using the self-report Beck Anxiety Inventory (BAI; Beck et al. 1988) and Quick Inventory of Depressive Symptomatology (QIDS; Rush et al. 2003), respectively.
Neuroimaging data acquisition and preprocessing
All MRI scanning for patients occurred on Siemens 3T scanners (ISMMS-recruited patients were scanned on a MAGNETOM Skyra and NYUSoM- and NKI-recruited patients were scanned on a MAGNETOM TrioTim) using a 32-channel head coil, with sequences harmonized between the 2 scanning sites. Structural data were obtained using a T1-weighted MP-RAGE protocol at both ISSMS and NKI—repetition time (TR) = 2,400 ms, flip angle = 8°, field of view (FOV) = 256 mm, 0.80 mm isotropic voxels. The orientation of acquisition, echo time (TE), and number of slices were different between the 2 scanning sites (ISSMS: transverse acquisition, TE = 2.07 ms, 224 slices; NKI: sagittal acquisition, TE = 2.01 ms, 208 slices). Participants viewed a fixation cross in the center of the screen during resting-state acquisitions, which were acquired using a high-resolution multiband-accelerated echo-planar sequence for full-brain coverage (TR = 1,000 ms, flip angle = 60°, FOV = 228 mm, 72 slices, 2.1 mm isotropic voxels, no gap, acceleration factor = 6, ascending interleaved order). In order to match all other aspects of the sequences as closely as possible, the TEs were slightly different between the 2 scanning sites (TE = 25 ms at ISMMS and TE = 25.4 ms at NKI). The first 10 volumes were discarded to allow magnetization to reach equilibrium.
Preprocessing was performed using a combination of Statistical Parametric Mapping v.12, scripts taken from the Human Connectome Project preprocessing pipeline (Glasser et al. 2013, 2016), AFNI (v.10.6, “3dSkullStrip”), and FSL v.5.0.10. Structural images were skull-stripped, nonlinearly corrected for gradient field distortion, and normalized to a Montreal Neurological Institute (MNI) template (the “tissue probability map” [tpm] image in SPM v.12). Preprocessing for functional images included gradient nonlinearity distortion correction, realignment to the first volume of the run, normalization to MNI template, and spatial smoothing using a 6 mm kernel. Six rigid-body realignment parameters (3 for translation: X, Y, and Z; and 3 for rotation: pitch, roll, and yaw) were produced for each subject following the realignment step. Registrations of T1-weighted and blood oxygen level-dependent (BOLD) images to the MNI template were checked manually for each participant as part of our quality control procedures.
The preprocessed structural and functional data were entered into CONN-fMRI Functional Connectivity Toolbox for SPM (v17f; Whitfield-Gabrieli and Nieto-Castanon 2012) for further processing before conducting FC analyses. Motion scrubbing as implemented in the CONN tool utilizes the Artifact Rejection Toolbox (ART, www.nitrc.org/projects/artifact_detect/). Using stringent thresholds, volumes with framewise displacement >0.5 mm or with global-signal Z value >3 were included as regressors-of-no-interest (one regressor per outlier). Through this process, data of 4 patients were excluded for having greater than 180 (out of 480) high motion volumes, consistent with recommendations for the inclusion of at least 5 min of usable resting-state data (Yan et al. 2013). Physiological noise, motion, and other artifactual confounds were removed from BOLD data using component-based noise correction (CompCor) that regressed out the first 5 component timeseries of segmented and normalized cerebrospinal fluid and white matter, 12 motion regressors (6 realignment parameters and their first derivatives), and 2 regressors for the “effect of rest” (a function convolved with the hemodynamic response function and its first derivative, to account for potential ramping effects at the beginning of the scan). CompCor effectively corrects for physiological noise without regressing out the global signal, hence allowing for better interpretations of correlations and anticorrelations (Behzadi et al. 2007; Chai et al. 2012; Whitfield-Gabrieli and Nieto-Castanon 2012). BOLD data were band-pass filtered at 0.008 < f < 0.09 Hz.
Data analysis
Behavioral and clinical data
Analyses of variance (ANOVAs) examining group comparisons for each MAIA subscale (also previously reported in Eng et al. (2020) are presented in the Supplementary Information. To examine the component structure of IS, PCA with orthogonal rotation (quartimax) was applied on the 8 MAIA subscales for the patient and control groups separately (Supplementary Information).
To examine associations between component scores and clinical measures within the OCD patient group, we first determined whether any demographic variables were related to IS within the patient group by conducting Pearson’s correlations between age and education and component scores, as well as a t-test comparing males and female on component scores. We also performed a chi-square test to determine whether the proportion of males and females differed within the patient group. The results from these analyses determined whether any demographic variables were included as covariates in subsequent analyses. We performed correlations (or partial correlations) between component scores and OCD symptom dimensions (measured using the DOCS), severity of overall OCD symptoms, compulsions, and obsessions (measured using the Y-BOCS), state anxiety (measured using the BAI), severity of depression (measured using the QIDS), and severity of sensory phenomena (measured using the SPS), controlling for demographic variables, where appropriate, and corrected for multiple correlations using false discovery rate (FDR) < 0.05 (Benjamini and Hochberg 1995). We conducted three 2 × 2 between-subjects ANOVAs to evaluate the effects of Axis I comorbidities (yes/no) and current use of psychotropic medication (yes/no) within the OCD patient group on IS component scores.
Functional connectivity
Correlations between component scores and FC of the insula were conducted for both OCD and control groups. However, as the two PCAs were conducted separately for each group, the factor structure of the MAIA and associations with insula FC are not directly comparable between the groups. Although this paper primarily focuses on FC relationships with IS component scores in the OCD group only, for completeness we present connectivity results from the control group in the Supplementary Information (Appendix 1).
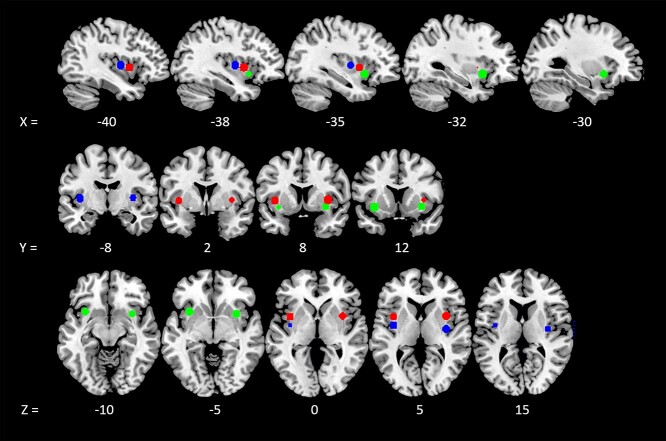
Seeds for FC analyses were generated using the SPM MarsBaR Toolbox (Brett et al. 2002) with six 6-mm radius spheres centered around MNI coordinates that represent the functional tripartite subdivision of the insula (Deen et al. 2011): bilateral dorsal anterior insula (x = −38, y = 6, z = 2; x = 35, y = 7, z = 3), ventral anterior insula (x = −33, y = 13, z = −7; x = 32, y = 10, z = −6), and posterior insula (x = −38, y = −6, z = 5; x = 35, y = −11, z = 6) (Fig. 1). At the individual subject level, timecourses representing the average within each sphere were correlated with all gray matter voxels, and the resultant correlation coefficients were z-transformed using Fisher’s transformation to generate seed-to-voxel FC maps. Group-level statistics evaluated the relationship between IS component scores and FC with the insula seeds within OCD patients by conducting a regression analysis with component scores as covariates-of-interest, controlling for demographic variables, where appropriate, and scan site (62 scanned at NKI, 15 at ISMMS). Despite sequence harmonization, we statistically controlled for residual differences in image quality between the 2 scanning sites by specifying scan site as a covariate for all group-level imaging analyses in order to account for variance related to potential site-based clustering effects, consistent with recommendations by McNeish and Kelley (2019) and approaches used by multi-center studies (Glover et al. 2012; Forsyth et al. 2014). All results were thresholded using a voxelwise threshold of P < 0.001 with whole-brain family-wise error (FWE) cluster correction P < 0.05.
Figure 1.
Insula seeds for FC. Seeds were 6 spheres of 6-mm radius centered around MNI coordinates as reported in Deen et al. 2011, corresponding to the dorsal anterior insula (red; coordinates: 35 7 3, −38 6 2), ventral anterior insula (green; coordinates: 32 10 –6, −33 13 –7), and posterior insula (blue; coordinates: 35 –11 6, −38 −6 5).
Results
Clinical and demographic information are shown in Table 1. There were no significant group differences in age, education, and sex, although there was a trend toward a greater frequency of females in the patient group than the control group (P = 0.07).
Table 1.
Demographics and clinical information.
| Controls (n = 53) | OCD (n = 77) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Group comparisons | ||
| Age (years) | 31.8 | 10.8 | 31.6 | 10.9 | t(128) = −0.10, P = 0.92 | |
| Education (years) | 15.8 | 2.2 | 15.6 | 2.1 | t(128) = −0.33, P = 0.74 | |
| Biological sex | 27 M/26 F | 27 M/50 F | χ2 (1) = 3.3, P = 0.07 | |||
| Y-BOCS Obsessions Severity | - | - | 11.7 | 3.0 | - | |
| Y-BOCS Compulsions Severity | - | - | 12.2 | 3.0 | - | |
| Y-BOCS total | - | - | 23.9 | 5.7 | - | |
| Beck Anxiety Score (average) | 0.1 | 0.1 | 0.8 | 0.6 | t(84.9) = 11.4a,b | |
| MAIA subscales (average score) | ||||||
| Noticing | 2.6 | 1.3 | 3.7 | 0.93 | F(1,89.9) = 29.0a,b | |
| Not-Distracting | 3.2 | 1.0 | 2.4 | 0.94 | F(1,128) = 18.2b | |
| Not-Worrying | 3.2 | 0.88 | 2.4 | 1.0 | F(1,128) = 22.9b | |
| Attention Control | 3.0 | 1.2 | 3.0 | 0.91 | F(1,128) = 0.11, P = 0.74 | |
| Emotional Awareness | 2.9 | 1.3 | 3.9 | 0.86 | F(1,81.2) = 22.7a,b | |
| Self-Regulation | 3.1 | 1.2 | 2.7 | 1.1 | F(1,128) = 3.40, P = 0.07 | |
| Listening | 2.1 | 1.4 | 2.5 | 1.4 | F(1,128) = 3.08, P = 0.08 | |
| Trusting | 3.8 | 0.91 | 3.1 | 1.3 | F(1,127.8) = 14.1a,b | |
Note: Group differences in each MAIA subscale (range of scores: 0–5) were computed and presented.
aLevene’s test of homogeneity of variance across the groups was not assumed, and degrees of freedom were adjusted using Satterthwaite’s approximation.
b P < 0.001.
Component structure of MAIA subscales
PCA revealed a 2-component solution that explained a total variance of 66.1% in controls and a 3-component solution that explained a total variance of 69.5% in OCD patients (Table 2). In controls, subscales that loaded onto Component 1 were Noticing, Attentional Control, Emotional Awareness, Self-Regulation, and Body Listening; subscales that loaded onto Component 2 were Distracting (i.e. negatively loaded for Not-Distracting) and Not-Worrying. The Trusting subscale cross-loaded on both Components 1 and 2 in the control group.
Table 2.
Component structure and loadings of MAIA in patients and controls.
| Controls (n = 53) | OCD (n = 77) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Component | Component | ||||||||
| MAIA Subscale | MSA | Communalities | 1 | 2 | MSA | Communalities | 1 | 2 | 3 |
| Noticing | 0.85 | 0.58 | 0.76 | 0.66 | 0.66 | 0.57 | −0.54 | ||
| Not-distracting | 0.63 | 0.40 | −0.63 | 0.37 | 0.94 | 0.97 | |||
| Not-worrying | 0.42 | 0.67 | 0.82 | 0.59 | 0.60 | 0.74 | |||
| Attention control | 0.87 | 0.68 | 0.82 | 0.83 | 0.68 | 0.75 | |||
| Emotional awareness | 0.77 | 0.89 | 0.95 | 0.74 | 0.67 | 0.67 | 0.46 | ||
| Self-regulation | 0.85 | 0.82 | 0.90 | 0.77 | 0.76 | 0.86 | |||
| Listening | 0.82 | 0.77 | 0.88 | 0.75 | 0.71 | 0.84 | |||
| Trusting | 0.79 | 0.47 | 0.44 | 0.53 | 0.86 | 0.55 | 0.70 | ||
| Cumulative Variance (%) | 48.99 | 66.08 | Cumulative Variance (%) | 41.25 | 55.96 | 69.48 | |||
Note: Loadings of absolute values <0.32 were not presented. MSA and communalities <0.50 are italicized.
In OCD patients, subscales that loaded onto Component 1 were very similar to that of controls, with the exception that Trusting preferentially loaded onto Component 1 in OCD patients but not controls. Subscales that loaded onto Component 2 in the patient group were Noticing (negatively loaded) and Not-Worrying. Emotional Awareness cross-loaded with Components 1 and 2, but preferentially loaded onto Component 1. Lastly, the Not-Distracting subscale loaded robustly onto its own component (Component 3).
Relationship between IS components and clinical measures
Within OCD patients, age, sex, and education were not associated with IS component scores (P > 0.05). Chi-square test showed significant differences in the proportion of males and females in the patient group, χ2 = 6.87, P = 0.009. Therefore, sex was used as a covariate in subsequent analyses within the patient sample.
Higher Component 1 scores (i.e. more noticing, control, emotional awareness, regulation, listening, and trusting of the body) were related to lower overall OCD symptom severity (total Y-BOCS score, rpartial = −.306, FDR-adjusted P = 0.036), compulsion severity (Y-BOCS compulsion subscale, rpartial = −.302, FDR-adjusted P = 0.036), and state anxiety (BAI; rpartial = −0.338, FDR-adjusted P = 0.019). Lower Component 2 scores (i.e. more noticing of sensations, more worrying about uncomfortable sensations, and less awareness of the link between emotion and the body) were associated with greater severity of OCD symptoms specifically relating to “responsibility for harm” as measured by the DOCS (rpartial = −.343, FDR-adjusted P = 0.019) and well as greater state anxiety (rpartial = −.373, FDR-adjusted P = 0.019). Lastly, greater Component 3 scores (i.e. less distracting/more focusing on uncomfortable sensation) were associated with greater severity of sensory phenomena (SPS; rpartial = 0.358, FDR-adjusted P = 0.019). Consistent with this finding, greater Component 3 scores were associated with higher severity of OCD symptoms relating to “symmetry and the need for things to be just right” as measured by the DOCS (rpartial = 0.284, FDR-adjusted P = 0.050). All correlations were controlled for sex.
Patients with at least one Axis I comorbidity had lower Component 2 scores compared to those without any comorbidity (P= 0.030) (Supplementary Information). There were no significant effects of psychotropic medication use on IS components scores.
Neuroimaging results
Correlations with component scores in the OCD group
Neuroimaging results examining the relationship between IS component scores and insula connectivity in the OCD group are presented in Table 3 and Figs 2 and 3. Scatterplots showing correlations between FC indices and components scores are presented in Supplementary Fig. S4.
Table 3.
Significant correlations between component scores and insula-to-whole-brain FC in OCD.
| Peak coordinates | Other regions included within cluster (>30 voxels) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BA | k | x | y | z | T | BA | Regions [aal labels/TD labels] | |||
| Higher Component 1 scores were associated with greater FC in: | ||||||||||
| Seed: Left dorsal anterior insula (−38 6 2) | ||||||||||
| L Precuneus | - | 799 | −18 | −46 | 2 | 5.76 | 19 | Posterior cingulate, R Lingual gyrus, R Fusiform gyrus, R Parahippocampal gyrus, R Calcarine, Cerebellar vermis IV–V | ||
| L Lingual gyrus | 30 | −12 | −40 | −4 | 5.36 | |||||
| L Calcarine | 30 | −16 | −54 | 4 | 5.31 | |||||
| L Cerebellum Crus I | - | 183 | −16 | −84 | −24 | 4.60 | 18 | Lingual gyrus | ||
| L Cerebellum Crus I | - | −6 | −82 | −18 | 4.16 | |||||
| L Cerebellum Crus I | - | −26 | −84 | −26 | 3.96 | |||||
| L Precuneus | - | 243 | −10 | −58 | 72 | 4.43 | 7 | Postcentral gyrus, superior parietal gyrus | ||
| L Precuneus | - | −6 | −48 | 74 | 4.13 | |||||
| L Precuneus | - | −6 | −66 | 66 | 4.08 | |||||
| R Precuneus | 7 | 340 | 12 | −70 | 58 | 4.13 | - | L Superior parietal gyrus | ||
| R Precuneus | - | 4 | −56 | 70 | 4.03 | |||||
| R Precuneus | - | 12 | −64 | 64 | 3.81 | |||||
| R Interior parietal gyrus | - | 165 | 34 | −50 | 40 | 3.97 | 40 | - | ||
| R Superior parietal gyrus | - | 40 | −50 | 56 | 3.96 | |||||
| R Postcentral gyrus | 2 | 38 | −42 | 64 | 3.56 | |||||
| Seed: Left posterior insula (−38 –6 5) | ||||||||||
| L Parahippocampal gyrus | - | 144 | −18 | −14 | −28 | 4.62 | - | - | ||
| Pons | - | −8 | −18 | −30 | 4.56 | |||||
| Higher Component 1 scores were associated with less FC in: | ||||||||||
| Seed: Right posterior insula (35 –11 6) | ||||||||||
| R Olfactory | 25 | 132 | 4 | 20 | −4 | 4.71 | - | - | ||
| R Subgenual anterior cingulate | - | 12 | 36 | 0 | 4.69 | |||||
| Lower Component 2 scores were associated with less FC in: | ||||||||||
| Seed: Right ventral anterior insula (32 10 –6) | ||||||||||
| R Anterior cingulate | - | 353 | 14 | 32 | 28 | 5.48 | - | Middle frontal gyrus, R Middle cingulate | ||
| L Anterior cingulate | 32 | −12 | 28 | 30 | 4.86 | |||||
| L Middle cingulate | - | 0 | 26 | 32 | 4.77 | |||||
| Lower Component 3 scores were associated with greater FC in: | ||||||||||
| Seed: Left dorsal anterior insula (−38 6 2) | ||||||||||
| L Interior frontal gyrus, triangularis | 46 | 779 | −50 | 30 | 18 | 5.79 | 45 | L Inferior frontal gyrus (opercularis), L Middle frontal gyrus | ||
| L Interior frontal gyrus, triangularis | - | −48 | 36 | 8 | 5.69 | |||||
| L Interior frontal gyrus, triangularis | 46 | −54 | 22 | 26 | 5.06 | |||||
| Seed: Right dorsal anterior insula (35 7 3) | ||||||||||
| L Interior frontal gyrus, triangularis | - | 370 | −48 | 34 | 8 | 5.18 | 46 | Middle frontal gyrus | ||
| L Interior frontal gyrus, triangularis | - | −52 | 34 | 20 | 4.47 | |||||
| L Interior frontal gyrus, triangularis | - | −48 | 24 | 20 | 4.32 | |||||
Note: Clusters significant at P < 0.001 voxelwise (uncorrected) with FWE < 0.05 clusterwise correction. Sex and scan site were included as covariates-of-no-interest. Coordinates are in MNI space.
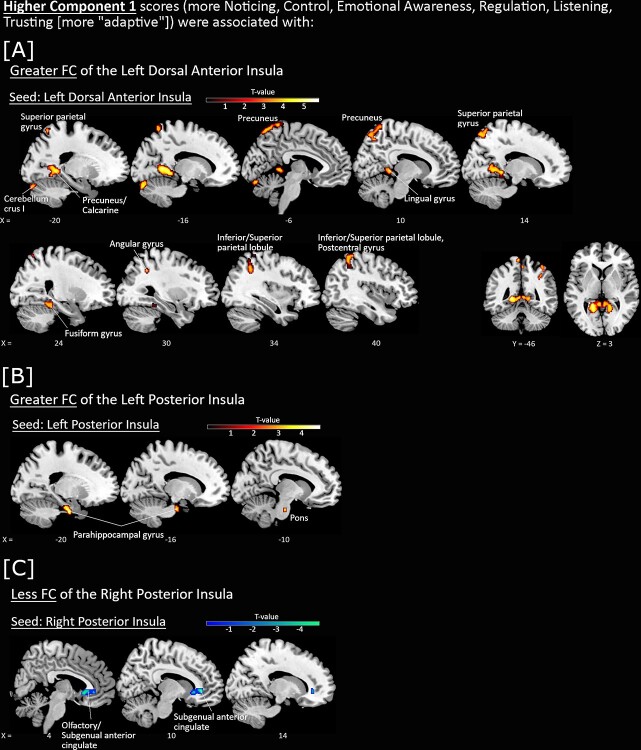
Figure 2.
Associations between FC of insula seeds with Component 1 scores, with sex and scan site included as covariates-of-no-interest. Higher Component 1 scores (more Noticing, Control, Emotional Awareness, Regulation, Listening, and Trusting of the body [more “adaptive”]) were associated with greater FC (red-yellow) of the A) left dorsal anterior insula and B) left posterior insula seeds, and C) less FC of the right posterior insula seed (blue-green). All P < 0.001 voxelwise (uncorrected) with FWE < 0.05 clusterwise correction.
Figure 3.
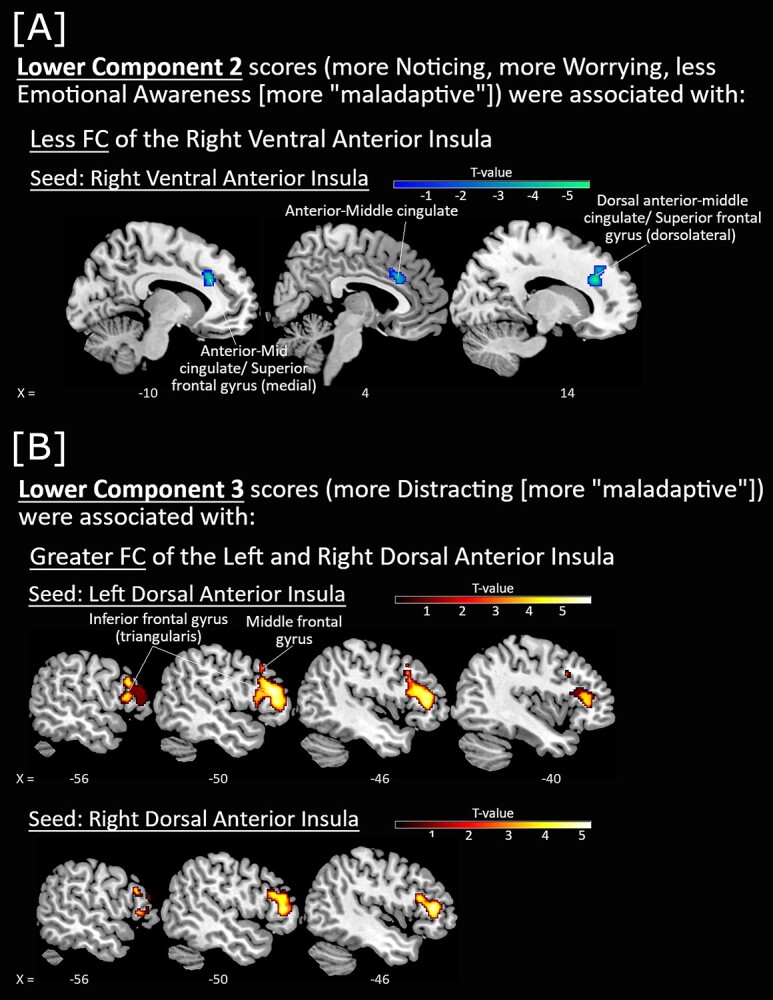
Associations between FC of insula seeds with Component 2 and Component 3 scores, with sex and scan site included as covariates-of-no-interest. A) Lower Component 2 scores (more Noticing, more Worrying, and less Emotional Awareness [more “maladaptive”]) were associated with less FC of the right ventral anterior insula seed (blue-green). B) Lower Component 3 scores (more Distracting [more “maladaptive”] were associated with greater FC of the left and right dorsal anterior insula seeds (red-yellow). All P < 0.001 voxelwise (uncorrected) with FWE < 0.05 clusterwise correction.
Higher Component 1 scores (i.e. more noticing, control, emotional awareness, regulation, listening, and trusting of the body) were associated with greater FC of the left dorsal anterior insula seed with occipital, parietal, retrosplenial, and cerebellar regions (Fig. 2A), as well as FC of the left posterior insula seed with left parahippocampal gyrus (Fig. 2B). Higher Component 1 scores were also associated with less FC of the right posterior insula seed with the right subgenual anterior cingulate (sgACC) (Fig. 2C).
Lower Component 2 scores (i.e. more general noticing of sensations, more worry about uncomfortable sensations, less awareness of the link between emotion and the body) were associated with less FC of the right ventral anterior insula seed with the dACC cluster overlapping the middle cingulate (Fig. 3A).
Lastly, lower Component 3 scores (i.e. more distracting from uncomfortable body sensations) were associated with greater FC of the left and right dorsal anterior insula with the left inferior frontal gyrus (pars triangularis) (Fig. 3B).
Discussion
This study evaluated the structure of IS in patients with OCD and examined the relationship between IS components and resting-state FC of insula subdivisions (dorsal anterior insula, ventral anterior insula, and posterior insula) within the OCD patient sample (Deen et al. 2011).
Component structure of IS
Healthy controls showed a 2-component solution of IS that broadly resembles the “adaptive”/“maladaptive” segregation of the MAIA (Mehling 2016). Interestingly, the first putatively ``adaptive'' component in controls also included the more ambiguous Noticing subscale, suggesting that, in the context of relative mental health, the general tendency to be aware of and notice body sensation may be linked to broader indices of psychological well-being. In contrast, patients with OCD showed a 3-component solution of IS. Although Component 1 in OCD patients identified similar “adaptive” tendencies as in controls, notably, the Noticing subscale loaded onto both the patients’ “adaptive” Component 1 and “maladaptive” Component 2, the latter of which also included subscales related to worrying about uncomfortable sensation and emotional awareness. These data indicate that interoception qualitatively differs between OCD patients and controls in that the general tendency to notice or be aware of sensations may serve both “maladaptive” and “adaptive” functions in patients only. In addition to the Noticing subscale, prior work has also considered the Emotional Awareness subscale to be ambiguous with regard to adaptiveness (Mehling et al. 2009; Mehling 2016). However, in our data, the Emotional Awareness subscale was positively related to “adaptive” scales (loaded in the same direction) for Component 1 for both OCD patients and controls and was negatively related to the ``maladaptive'' scale of Worrying about sensation in Component 2 for OCD patients. As such, the present work appears to link the Emotional Awareness subscale to ``adaptive'' processes only.
Higher “adaptive” IS is associated with lower OCD and anxiety severity
IS components were related to clinical symptoms within the patient sample. More “adaptive” IS (i.e. higher Component 1 scores) was associated with less severe state anxiety and overall OCD symptom severity, while more “maladaptive” IS (i.e. lower Component 2 scores) was associated with higher state anxiety and OCD symptoms related to responsibility for harm. These associations between IS components and indices of symptom severity highlight the relevance of IS to OCD psychopathology, suggesting that “adaptive” and “maladaptive” aspects of IS may be appropriate future targets for interventions seeking to reduce OCD symptoms.
“Adaptive” IS involves connectivity between multiple diverse brain areas
Higher Component 1 scores (more “adaptive” IS) were associated with greater FC of insula seeds with parahippocampal gyrus and retrosplenial cortex (Brodmann’s area [BA] 30), 2 regions of the medial temporal sub-system of the default-mode network (DMN; Andrews-Hanna 2012; Kaboodvand et al. 2018) that are involved in memory recollection, prospective thinking (Schacter et al. 1999; Buckner and Carroll 2007; Bar 2009; Andrews-Hanna, Reidler, Huang, et al. 2010a; Andrews-Hanna, Reidler, Sepulcre, et al. 2010b; Sheldon et al. 2019), emotional regulation, and self-referential processes (Schacter et al. 1999; Viard et al. 2011). Whereas the posterior insula may process the initial sensory representation of the body at a cortical level, the dorsal anterior insula is thought to represent a later stage of interoceptive processing (Craig 2003) and is activated during a variety of cognitive processes (Cauda et al. 2011; Deen et al. 2011). The fact that both the left posterior and dorsal anterior insula seeds were connected to medial temporal DMN regions suggests that “adaptive” IS may involve the integration of cognitive and sensory aspects of interoception with memory and other self-referential processes.
In addition to the above-described regions showing greater connectivity with more “adaptive” IS, higher “adaptive” IS in patients was also related to less FC between the right posterior insula and sgACC. Prior studies in nonhuman primates found strong reciprocal connections between the sgACC with amygdala (involved in conscious and unconscious emotional processing; Morris et al. 1998; Whalen et al. 1998), hypothalamus (involved in stress response; Porter 1952; Swanson 1991; DiMicco et al. 2002), and prefrontal cortex (Vogt and Pandya 1987; Carmichael and Price 1996; Joyce and Barbas 2018). In human studies, the sgACC is thought to act as a gateway between emotional and higher cognitive centers (Greicius et al. 2007; Johansen-Berg et al. 2008; Beckmann et al. 2009; Ramirez-Mahaluf et al. 2018; Scharnowski et al. 2020). Perhaps due to its role in sadness and negative emotional processing (George et al. 1995; Vogt 2005; Freed and Mann 2007; Ramirez-Mahaluf et al. 2018), the sgACC is implicated in anxiety and mood disorders (Gotlib et al. 2005; Drevets et al. 2008; Johansen-Berg et al. 2008; Connolly et al. 2013) and is one of the target regions for deep brain stimulation in treatment-resistant depression (Mayberg et al. 2005). This association between more “adaptive” IS and less connectivity between posterior insula and sgACC suggests that reduced communication between sensory-interoceptive and negative emotion processing regions may promote “adaptive” responses to the body among OCD patients.
“Maladaptive” IS may involve emotional and salience-detection processes
Higher “maladaptive” IS (i.e. lower Component 2 scores; more general noticing of sensations, more worry about uncomfortable sensations, less awareness of the link between emotion and the body) was associated with less FC of the right ventral anterior insula seed with dACC, an area involved in salience detection (Seeley et al. 2007). The salience network detects, identifies, and integrates salient information and acts as a “hub” for dynamic cognitive control by appropriately engaging and disengaging the executive control (task-positive) and default-mode (task-negative) networks (Sridharan et al. 2008; Menon and Uddin 2010; Menon 2011). Prior research in patients with OCD compared to controls reported less FC within the salience network (Chen et al. 2018; Gürsel et al. 2018), although existing work also reported greater connectivity within the same network (Fan et al. 2017). Altered connectivity involving regions of the salience network may influence effective switching between default-mode and executive control networks, thus contributing to difficulties in disengaging from self-referential thoughts to engage in adaptive cognitive control (Sridharan et al. 2008; Beucke et al. 2014; Chen et al. 2018). Considering that the ventral anterior insula seed is involved in emotion processing (Deen et al. 2011; Touroutoglou et al. 2012; Chang et al. 2013), our data suggest that “maladaptive” responses to IS in OCD (more general awareness of body sensations, more worrying, and less awareness of the link between emotions and body sensations) may involve emotional and salience detection-related processes.
Distracting from uncomfortable sensations involves connectivity of cognitive control regions
Finally, we observed that greater distracting from uncomfortable sensation (i.e. lower scores in Not-Distracting) was associated with higher FC between the left and right dorsal anterior insula and left inferior frontal gyrus (pars triangularis). The dorsal anterior insula and inferior frontal gyrus are both associated with cognitive control (Aron et al. 2004; Deen et al. 2011), and greater connectivity between these regions has been associated with interoceptive attention and arousal (Jarrahi et al. 2015; Wang et al. 2019). Prior research on pain suggest that distraction as a general coping strategy can be adaptive or maladaptive—while distracting reduces experimentally evoked pain (such as cold pressor or thermal stimulation; McCaul and Malott 1984; Verhoeven et al. 2010), distracting from severe/chronic pain or catastrophizing about pain is maladaptive (Rosenstiel and Keefe 1983; Keefe et al. 1990; Johnson 2005). Greater distracting from aversive body sensations was associated with worse psychiatric outcomes (Brown et al. 2017; Rogers et al. 2018), and this was consistent with cognitive behavioral therapy studies revealing that distracting from uncomfortable events impedes the reduction of distress over time (Wahl et al. 2013) and is detrimental to the therapeutic process (Gillihan et al. 2012; McKay et al. 2015; Blakey and Abramowitz 2018).
Interestingly, we found that greater tendency to focus (or “not-distract”) on uncomfortable body sensation was associated with greater severity of sensory phenomena as measured using the SPS. “Sensory phenomena” refers to symptoms involving sensations relating to symmetry, completeness, and need for things to be “just right” that drive repetitive behaviors (Cohen and Leckman 1992; Ferrão et al. 2012). Approximately 70% of patients experience some form of sensory phenomena, with 30%–40% of patients reporting aversive or uncomfortable body sensations that immediately precede or accompany the compulsions (Lee et al. 2009; Ferrão et al. 2012; Shavitt et al. 2014). Prior studies proposed that individuals with sensory phenomena tend to focus on internal feelings and sensations relating to their sensory phenomena to help determine when it is appropriate to terminate performing compulsions (Cougle et al. 2011, 2013). This is consistent with our prior published work showing a positive correlation between the general tendency to notice body sensation (as measured by the MAIA Noticing subscale) and severity of symptoms relating to symmetry, completeness, and need for things to be “just right” as measured by the DOCS (Eng et al. 2020). Overall, these findings support the view that certain aspects of IS, such as those related to focusing on and noticing sensation, might be related to sensory-based symptoms in OCD.
A note about the anterior insula
It is notable that we observed consistent involvement of the anterior insula in all 3 IS components. Our results are broadly in line with prior studies that observe activations preferentially in the left anterior insula during both positive and negative emotion feelings (Dal Monte et al. 2013; Gu et al. 2013) and the right anterior insula during negative feelings (Gu et al. 2013). The right anterior insula, in particular, is known to have an important role in interoceptive accuracy (typically measured using heartbeat detection tasks) (Critchley 2004; Haruki and Ogawa 2021) and generating subjective feelings of the present moment (Critchley 2004; Craig 2009). Compared to the left anterior insula, the right anterior insula also has greater activations during interoceptive self-referential tasks (Modinos et al. 2009; Murray et al. 2012; Scalabrini et al. 2021) and has more connections to DMN regions (Scalabrini et al. 2021). The present findings revealing relationships between IS components and anterior insula connectivity are generally consistent with previous work suggesting a potential lateralization of function within the insula, although further work is required to examine insula lateralization in IS.
Clinical implications
Our findings have implications for the treatment of OCD and future work. First, the finding that patients reporting more “adaptive” IS tended to have milder OCD symptoms is consistent with emerging evidence that mindfulness-based interventions can be efficacious (Wahl et al. 2013; Selchen et al. 2018). Mindfulness training is often body-focused (Lee 2009; Kerr et al. 2013; Gibson 2019) and aims to teach practitioners to observe distressing events or sensation without acting on or feeling controlled by them (Teasdale et al. 2002; Crowe and McKay 2016; Crane 2017), thereby modulating attentional control and emotional regulation (Coffey et al. 2010; Marchand 2013; Guendelman et al. 2017; Gibson 2019). Our study raises the possibility that mindfulness may be helpful in OCD in part by promoting “adaptive” IS responses. Future studies could seek to determine if mindfulness therapy outcomes in OCD are mediated by changes in IS and to identify neural changes associated with improvements in “adaptive” IS following mindfulness training.
Next, our finding that the “maladaptive” Component 2 scores correlated with severity of responsibility for harm symptoms in OCD suggests that therapies aimed at reducing sensitivity to and worry about body sensations and promoting understanding of emotion–body connection may be beneficial for patients specifically with these types of symptoms. The therapeutic approach of exposure to interoceptive signals, for example, involves inducing distressing sensations to enhance tolerance and emotional acceptance (Boswell et al. 2013; Blakey and Abramowitz 2018). Interoceptive exposure helps the individual regulate their arousal without controlling or changing the sensations using “maladaptive” behavioral responses that ultimately reinforce the undesirable experience (Blakey and Abramowitz 2018). Our data support the recommendations by Blakey and Abramowitz (2018) to integrate interoceptive exposure to uncomfortable body sensations into treatment for OCD (Blakey and Abramowitz 2018) and further suggest this may be particularly useful for patients with responsibility for harm symptoms. Additionally, as our data showed that lower state anxiety was related to both greater “adaptive” IS and less “maladaptive” IS, interventions aiming to enhance “adaptive” IS (e.g. mindfulness) and reduce “maladaptive” IS (e.g. interoceptive exposure) may both be generally anxiolytic (Frances et al. 2020), especially when therapy is carefully conceptualized for the patient (McKay et al. 2015, 2020).
Interestingly, however, while distracting (or not-focusing) from uncomfortable sensation is known to lead to worse outcomes in individuals, including those with eating disorders and pain (Rosenstiel and Keefe 1983; Keefe et al. 1990; Johnson 2005; Brown et al. 2017; Rogers et al. 2018), we found that greater tendency to focus on body sensation (less distracting) was related to more severe sensory phenomena among OCD patients. This suggests that therapies aimed at reducing a patient’s focus on uncomfortable body sensation may be beneficial for OCD patients with prominent sensory phenomena.
Lastly, higher “maladaptive” IS was related to less connectivity between anterior insula and dACC. Excitatory deep transcranial magnetic stimulation (dTMS) targeting the anterior cingulate is approved by the Food and Drug Administration (FDA) as a treatment of OCD (Carmi et al. 2018, 2019). Our results are consistent with the large body of literature supporting the importance of the ACC in OCD (Saxena et al. 2009; McGovern and Sheth 2017) and raise the possibility that modulating ACC using dTMS may impact OCD symptoms at least partially through alterations of “maladaptive” IS. Future efforts could evaluate this question directly and consider testing the effectiveness of anterior cingulate dTMS in combination with therapies that target “maladaptive” IS such as interoceptive exposure.
OCD is highly heterogeneous, and the current study reported differential associations of IS with symptoms related to harm avoidance and sensory phenomena in a sample of patients with OCD. However, these symptoms are transdiagnostic and prevalent in other psychiatric disorders. Harm avoidance is prominent in anxiety disorders, such as panic, and social anxiety disorders (Kampman et al. 2014). Sensory phenomena are associated with compulsions not only in patients with OCD (Lee et al. 2009; Ferrão et al. 2012; Shavitt et al. 2014) but also in tic disorders, skin picking, and trichotillomania (Prado et al. 2008; Ferrão et al. 2012). As such, clinical implications drawn from our results may therefore be applicable to individuals across several disorders. Future work should seek to examine these processes in additional patient populations to determine whether the IS component structure and relationships with FC identified in OCD would be similar across disorders.
Limitations
There are several limitations to our study. We were not able to compare OCD and healthy control groups on insula FC associated with IS components as the PCA analysis on the MAIA subscales was conducted separately in each patient and control group, yielding group-specific component solutions that are not directly comparable. Although we present results for the control group in Appendix 1 for completeness, it should be noted that our sample of healthy controls was smaller than that of the patients. Future work focusing on case–control comparisons would benefit from obtaining more even sample sizes. Next, the MAIA is a self-report questionnaire and may be susceptible to individual biases. Indeed, the current study only assessed the subjective experience of the body and did not examine patients’ ability to objectively detect sensation, as in prior work (Yoris et al. 2017; Schultchen et al. 2019) (known as interoceptive accuracy and frequently assessed using heartbeat detection paradigms; Critchley 2004; Garfinkel et al. 2015; Yoris et al. 2017; Schultchen et al. 2019) or the correspondence between subjective and objective measures of interoception (known as interoceptive awareness; Garfinkel and Critchley 2013; Garfinkel et al. 2015). As these dimensions of interoception are distinct and dissociable (Garfinkel et al. 2015), future studies should evaluate all 3 indices of interoception in the same patients to better decipher interoceptive alterations in OCD. Lastly, as we did not administer any measure of anxiety sensitivity, we were unable to directly examine associations between anxiety sensitivity, IS components, and OCD symptoms. However, prior work has associated anxiety sensitivity with symmetry/“just-right” symptoms in both patients with OCD (Poli et al. 2017) and nonclinical college students (Wheaton et al. 2012). Furthermore, anxiety sensitivity has similarities with the “worrying about sensation” subscale of the MAIA and is elevated in OCD as well as panic disorder, post-traumatic stress disorder, and generalized anxiety disorder (Naragon-Gainey 2010; Blakey and Abramowitz 2018). As such, future studies could directly examine associations between anxiety sensitivity, IS, and disorder-specific clinical symptoms across anxiety diagnoses in order to better elucidate the relationships between these constructs. Despite these limitations, we identified relationships between “adaptive” and “maladaptive” components of IS and symptom severity in OCD, suggesting that these processes are relevant for impairment in OCD. Further, differential insula FC was related to distinct aspects of IS, identifying multiple network connections that could serve as future targets for the modulation of IS in OCD.
Supplementary Material
Acknowledgments
Separate behavioral findings from a sample that included many of the same subjects (68 OCD patients and 47 controls from the present sample) were recently published in the Journal of Obsessive–Compulsive and Related Disorders, 27, pp. 100584.
Contributor Information
Goi Khia Eng, Department of Psychiatry, New York University School of Medicine, One Park Ave, 8th Floor, New York, NY 10016, United States; Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, NY 10962, United States.
Katherine A Collins, Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, NY 10962, United States.
Carina Brown, San Diego State University/University of California San Diego Joint Doctoral Program in Clinical Psychology, 6363 Alvarado Court, San Diego, CA 92120, United States.
Molly Ludlow, Ferkauf Graduate School of Psychology, 1165 Morris Park Ave, Bronx, NY 10461, United States.
Russell H Tobe, Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, NY 10962, United States.
Dan V Iosifescu, Department of Psychiatry, New York University School of Medicine, One Park Ave, 8th Floor, New York, NY 10016, United States; Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, NY 10962, United States.
Emily R Stern, Department of Psychiatry, New York University School of Medicine, One Park Ave, 8th Floor, New York, NY 10016, United States; Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, NY 10962, United States.
Funding
This work is supported by National Institute of Mental Health (NIH grants R21/R33MH107589 and R01MH111794 awarded to ERS). NIH had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Conflict of interest statement
Dr KAC is a paid independent rater for MedAvante-ProPhase. Dr RHT has received research support (through Nathan Kline Institute) from NIH, Roche Pharmaceuticals, and Janssen Pharmaceuticals; Nathan Kline Institute has received all honoraria for Dr Tobe’s consulting with Roche Pharmaceuticals. In the last 5 years, Dr DVI has received consulting honoraria from Alkermes, Axsome, Centers for Psychiatric Excellence, Jazz, Lundbeck, Otsuka, Precision Neuroscience, Sage, Sunovion; he has received research support (through his academic institution) from Alkermes, Astra Zeneca, Brainsway, Litecure, Neosync, Otsuka, Roche, Shire. All other authors declare that they have no conflicts of interest.
References
- Abramowitz JS, Deacon BJ, Olatunji BO, Wheaton MG, Berman NC, Losardo D, Timpano KR, McGrath PB, Riemann BC, Adams T, et al. Assessment of obsessive-compulsive symptom dimensions: development and evaluation of the dimensional obsessive-compulsive scale. Psychol Assess. 2010:22:180–198. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012:18:251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. J Neurophysiol. 2010a:104:322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010b:65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004:8:170–177. [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006:13:27–45. [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Lykins E, Button D, Krietemeyer J, Sauer S, Walsh E, Duggan D, Williams JMG. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008:15:329–342. [DOI] [PubMed] [Google Scholar]
- Bar M. The proactive brain: memory for predictions. Philos Trans R Soc Lond Ser B Biol Sci. 2009:364:1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988:56:893–897. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009:29:1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007:37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995:57:289–300. [Google Scholar]
- Berlin HA, Stern ER, Ng J, Zhang S, Rosenthal D, Turetzky R, Tang C, Goodman W. Altered olfactory processing and increased insula activity in patients with obsessive-compulsive disorder: an fMRI study. Psychiatry Res Neuroimaging. 2017:262:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beucke JC, Sepulcre J, Eldaief MC, Sebold M, Kathmann N, Kaufmann C. Default mode network subsystem alterations in obsessive-compulsive disorder. Br J Psychiatry. 2014:205:376–382. [DOI] [PubMed] [Google Scholar]
- Blakey SM, Abramowitz JS. Interoceptive exposure: an overlooked modality in the cognitive-behavioral treatment of OCD. Cogn Behav Pract. 2018:25:145–155. [Google Scholar]
- Blakey SM, Abramowitz JS, Reuman L, Leonard RC, Riemann BC. Anxiety sensitivity as a predictor of outcome in the treatment of obsessive-compulsive disorder. J Behav Ther Exp Psychiatry. 2017:57:113–117. [DOI] [PubMed] [Google Scholar]
- Boswell JF, Farchione TJ, Sauer-Zavala S, Murray HW, Fortune MR, Barlow DH. Anxiety sensitivity and interoceptive exposure: a transdiagnostic construct and change strategy. Behav Ther. 2013:44:417–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B editors. Region of interest analysis using an SPM toolbox [abstract], 8th International Conference on Functional Mapping of the Human Brain; 2002. June 2–6; Sendai, Japan: NeuroImage; (Vol 16, No. 2, p. 497). [Google Scholar]
- Brown TA, Berner LA, Jones MD, Reilly EE, Cusack A, Anderson LK, Kaye WH, Wierenga CE. Psychometric evaluation and norms for the multidimensional assessment of interoceptive awareness (MAIA) in a clinical eating disorders sample. Eur Eat Disord Rev. 2017:25:411–416. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007:11:49–57. [DOI] [PubMed] [Google Scholar]
- Burns JW. The role of attentional strategies in moderating links between acute pain induction and subsequent psychological stress: evidence for symptom-specific reactivity among patients with chronic pain versus healthy nonpatients. Emotion (Washington, DC). 2006:6:180–192. [DOI] [PubMed] [Google Scholar]
- Carmi L, Alyagon U, Barnea-Ygael N, Zohar J, Dar R, Zangen A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018:11:158–165. [DOI] [PubMed] [Google Scholar]
- Carmi L, Tendler A, Bystritsky A, Hollander E, Blumberger DM, Daskalakis J, Ward H, Lapidus K, Goodman W, Casuto L. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019:176:931–938. [DOI] [PubMed] [Google Scholar]
- Carmichael S, Price J. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996:371:179–207. [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. NeuroImage. 2011:55:8–23. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012:59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013:23:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-H, Li S-F, Lv D, Zhu G-D, Wang Y-H, Meng X, Hu Q, Li C-C, Zhang L-T, Chu X-P, et al. Decreased intrinsic functional connectivity of the salience network in drug-naïve patients with obsessive-compulsive disorder. Front Neurosci. 2018:12:889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey KA, Hartman M, Fredrickson BL. Deconstructing mindfulness and constructing mental health: understanding mindfulness and its mechanisms of action. Mind. 2010:1:235–253. [Google Scholar]
- Cohen AJ, Leckman JF. Sensory phenomena associated with Gilles de la Tourette's syndrome. J Clin Psychiatry. 1992:53:319–323. [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Frank G, Hendren R, Max JE, Paulus MP, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013:74:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougle JR, Goetz AR, Fitch KE, Hawkins KA. Termination of washing compulsions: a problem of internal reference criteria or 'not just right' experience? J Anxiety Disord. 2011:25:801–805. [DOI] [PubMed] [Google Scholar]
- Cougle JR, Fitch KE, Jacobson S, Lee H-J. A multi-method examination of the role of incompleteness in compulsive checking. J Anxiety Disord. 2013:27:231–239. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002:3:655. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003:13:500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009:10:59–70. [DOI] [PubMed] [Google Scholar]
- Crane R. Mindfulness-based cognitive therapy: distinctive features. London (UK): Routledge; 2017 [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci U S A. 2004:101:6333–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013:77:624–638. [DOI] [PubMed] [Google Scholar]
- Crowe K, McKay D. Mindfulness, obsessive–compulsive symptoms, and executive dysfunction. Cognit Ther Res. 2016:40:627–644. [Google Scholar]
- Dal Monte O, Krueger F, Solomon JM, Schintu S, Knutson KM, Strenziok M, Pardini M, Leopold A, Raymont V, Grafman J. A voxel-based lesion study on facial emotion recognition after penetrating brain injury. Soc Cogn Affect Neurosci. 2013:8:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier JJ. The relationship of yoga, body awareness, and body responsiveness to self-objectification and disordered eating. Psychol Women Q. 2005:29:207–219. [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011:21:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002:71:469–480. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008:13:663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng GK, Collins KA, Brown C, Ludlow M, Tobe RH, Iosifescu DV, Stern ER. Dimensions of interoception in obsessive-compulsive disorder. J Obsessive Compuls Relat Disord. 2020:27:100584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Zhong M, Zhu X, Gan J, Liu W, Niu C, Liao H, Zhang H, Yi J, Tan C. Resting-state functional connectivity between right anterior insula and right orbital frontal cortex correlate with insight level in obsessive-compulsive disorder. NeuroImage Clinical. 2017:15:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrão YA, Shavitt RG, Prado H, Fontenelle LF, Malavazzi DM, de Mathis MA, Hounie AG, Miguel EC, do Rosário MC. Sensory phenomena associated with repetitive behaviors in obsessive-compulsive disorder: an exploratory study of 1001 patients. Psychiatry Res. 2012:197:253–258. [DOI] [PubMed] [Google Scholar]
- Flynn FG. Anatomy of the insula functional and clinical correlates. Aphasiology. 1999:13:55–78. [Google Scholar]
- Forsyth JK, McEwen SC, Gee DG, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Olvet DM, et al. Reliability of functional magnetic resonance imaging activation during working memory in a multi-site study: analysis from the North American Prodrome Longitudinal Study. NeuroImage. 2014:97:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances S, Shawyer F, Cayoun B, Enticott J, Meadows G. Study protocol for a randomized control trial to investigate the effectiveness of an 8-week mindfulness-integrated cognitive behavior therapy (MiCBT) transdiagnostic group intervention for primary care patients. BMC Psychiatry. 2020:20:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed PJ, Mann JJ. Sadness and loss: toward a neurobiopsychosocial model. Am J Psychiatry. 2007:164:28–34. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Critchley HD. Interoception, emotion and brain: new insights link internal physiology to social behaviour. Commentary on: "Anterior insular cortex mediates bodily sensibility and social anxiety" by Terasawa et al. (2012). Soc Cogn Affect Neurosci. 2013:8:231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol Psychol. 2015:104:65–74. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995:152:341–351. [DOI] [PubMed] [Google Scholar]
- Gibson J. Mindfulness, interoception, and the body: a contemporary perspective. Front Psychol. 2019:10:2012–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillihan SJ, Williams MT, Malcoun E, Yadin E, Foa EB. Common pitfalls in exposure and response prevention (EX/RP) for OCD. J Obsessive Compuls Relat Disord. 2012:1:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013:80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Smith SM, Marcus DS, Andersson JLR, Auerbach EJ, Behrens TEJ, Coalson TS, Harms MP, Jenkinson M, Moeller S. The human connectome project's neuroimaging approach. Nat Neurosci. 2016:19:1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Mueller BA, Turner JA, van Erp TGM, Liu TT, Greve DN, Voyvodic JT, Rasmussen J, Brown GG, Keator DB, et al. Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. J Magn Reson Imaging. 2012:36:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989:46:1006–1011. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JD, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005:16:1731–1734. [DOI] [PubMed] [Google Scholar]
- Goubert L, Crombez G, Eccleston C, Devulder J. Distraction from chronic pain during a pain-inducing activity is associated with greater post-activity pain. Pain. 2004:110:220–227. [DOI] [PubMed] [Google Scholar]
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav Assess. 2004:26:41–54. [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007:62:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, Fan J. Anterior insular cortex and emotional awareness. J Comp Neurol. 2013:521:3371–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guendelman S, Medeiros S, Rampes H. Mindfulness and emotion regulation: insights from neurobiological, psychological, and clinical studies. Front Psychol. 2017:8:220–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürsel DA, Avram M, Sorg C, Brandl F, Koch K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev. 2018:87:151–160. [DOI] [PubMed] [Google Scholar]
- Haruki Y, Ogawa K. Role of anatomical insular subdivisions in interoception: interoceptive attention and accuracy have dissociable substrates. Eur J Neurosci. 2021:53:2669–2680. [DOI] [PubMed] [Google Scholar]
- Jarrahi B, Mantini D, Balsters JH, Michels L, Kessler TM, Mehnert U, Kollias SS. Differential functional brain network connectivity during visceral interoception as revealed by independent component analysis of fMRI time-series. Hum Brain Mapp. 2015:36:4438–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman D, Behrens T, Matthews P, Rushworth M, Katz E, Lozano A, Mayberg H. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008:18:1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. How does distraction work in the management of pain? Curr Pain Headache Rep. 2005:9:90–95. [DOI] [PubMed] [Google Scholar]
- Joyce MKP, Barbas H. Cortical connections position primate area 25 as a keystone for interoception, emotion, and memory. J Neurosci. 2018:38:1677–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaboodvand N, Bäckman L, Nyberg L, Salami A. The retrosplenial cortex: a memory gateway between the cortical default mode network and the medial temporal lobe. Hum Brain Mapp. 2018:39:2020–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman O, Viikki M, Järventausta K, Leinonen E. Meta-analysis of anxiety disorders and temperament. Neuropsychobiology. 2014:69:175–186. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Crisson J, Urban BJ, Williams DA. Analyzing chronic low back pain: the relative contribution of pain coping strategies. Pain. 1990:40:293–301. [DOI] [PubMed] [Google Scholar]
- Kerr CE, Sacchet MD, Lazar SW, Moore CI, Jones SR. Mindfulness starts with the body: somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation. Front Hum Neurosci. 2013:7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, Feusner JD, Garfinkel SN, Lane RD, Mehling WE, et al. Interoception and mental health: a roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018:3:501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Lee MC, Kim J, Kim IY, Kim SI, Han MH, Chang KH, Kwon JS. Grey matter abnormalities in obsessive-compulsive disorder: statistical parametric mapping of segmented magnetic resonance images. Br J Psychiatry. 2001:179:330–334. [DOI] [PubMed] [Google Scholar]
- Lee I. Brain wave vibration: getting back into the rhythm of a happy, healthy life. Sedona (AZ): Best Life Media; 2009 [Google Scholar]
- Lee JC, Prado HS, Diniz JB, Borcato S, da Silva CB, Hounie AG, Miguel EC, Leckman JF, do Rosario MC. Perfectionism and sensory phenomena: phenotypic components of obsessive-compulsive disorder. Compr Psychiatry. 2009:50:431–436. [DOI] [PubMed] [Google Scholar]
- Li P, Li S-F, Han H-Y, Dong Z-Y, Luo J, Guo Z-H, Xiong H-F, Zang Y-F, Li Z-J. Abnormal spontaneous neural activity in obsessive-compulsive disorder: a resting-state functional magnetic resonance imaging study. PLoS One. 2013:8:e67262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol. 1979:186:117–131. [DOI] [PubMed] [Google Scholar]
- Marchand WR. Mindfulness meditation practices as adjunctive treatments for psychiatric disorders. Psychiatr Clin North Am. 2013:36:141–152. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005:45:651–660. [DOI] [PubMed] [Google Scholar]
- McCaul KD, Malott JM. Distraction and coping with pain. Psychol Bull. 1984:95:516. [PubMed] [Google Scholar]
- McGovern RA, Sheth SA. Role of the dorsal anterior cingulate cortex in obsessive-compulsive disorder: converging evidence from cognitive neuroscience and psychiatric neurosurgery. J Neurosurg. 2017:126:132–147. [DOI] [PubMed] [Google Scholar]
- McKay D, Sookman D, Neziroglu F, Wilhelm S, Stein DJ, Kyrios M, Matthews K, Veale D. Efficacy of cognitive-behavioral therapy for obsessive-compulsive disorder. Psychiatry Res. 2015:225:236–246. [DOI] [PubMed] [Google Scholar]
- McKay D, Abramowitz JS, Storch EA. Mechanisms of harmful treatments for obsessive–compulsive disorder. Clin Psychol Sci Pract. 2020:00:e12337. [Google Scholar]
- McNeish D, Kelley K. Fixed effects models versus mixed effects models for clustered data: reviewing the approaches, disentangling the differences, and making recommendations. Psychol Methods. 2019:24:20–35. [DOI] [PubMed] [Google Scholar]
- Mehling WE. Differentiating attention styles and regulatory aspects of self-reported interoceptive sensibility. Philos Trans R Soc Lond Ser B Biol Sci. 2016:371:1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling WE, Gopisetty V, Daubenmier J, Price CJ, Hecht FM, Stewart A. Body awareness: construct and self-report measures. PLoS One. 2009:4:e5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, Stewart A. The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLoS One. 2012:7:e48230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011:15:483–506. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010:214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. Activation of anterior insula during self-reflection. PLoS One. 2009:4:e4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998:393:467–470. [DOI] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, Debbané M. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci Biobehav Rev. 2012:36:1043–1059. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, Ball T. Functional organization of the human anterior insular cortex. Neurosci Lett. 2009:457:66–70. [DOI] [PubMed] [Google Scholar]
- Naragon-Gainey K. Meta-analysis of the relations of anxiety sensitivity to the depressive and anxiety disorders. Psychol Bull. 2010:136:128. [DOI] [PubMed] [Google Scholar]
- Nishida S, Narumoto J, Sakai Y, Matsuoka T, Nakamae T, Yamada K, Nishimura T, Fukui K. Anterior insular volume is larger in patients with obsessive-compulsive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011:35:997–1001. [DOI] [PubMed] [Google Scholar]
- Nomi JS, Schettini E, Broce I, Dick AS, Uddin LQ. Structural connections of functionally defined human insular subdivisions. Cereb Cortex. 2017:28:3445–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Xu T, He QH, Shi CZ, Wei Z, Miao GD, Jing J, Lim KO, Zuo XN, Chan RCK. Default network connectivity as a vulnerability marker for obsessive compulsive disorder. Psychol Med. 2014:44:1475–1484. [DOI] [PubMed] [Google Scholar]
- Poli A, Melli G, Ghisi M, Bottesi G, Sica C. Anxiety sensitivity and obsessive-compulsive symptom dimensions: further evidence of specific relationships in a clinical sample. Personal Individ Differ. 2017:109:130–136. [Google Scholar]
- Porges S. Body perception questionnaire. University of Maryland (MD): Laboratory of Developmental Assessment; 1993 [Google Scholar]
- Porter R. Alterations in electrical activity of the hypothalamus induced by stress stimuli. Am J Phys. 1952:169:629–637. [DOI] [PubMed] [Google Scholar]
- Posner J, Song I, Lee S, Rodriguez CI, Moore H, Marsh R, Blair SH. Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2017:38:678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado HS, Rosário MC, Lee J, Hounie AG, Shavitt RG, Miguel EC. Sensory phenomena in obsessive-compulsive disorder and tic disorders: a review of the literature. CNS Spectr. 2008:13:425–432. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004:61:720–730. [DOI] [PubMed] [Google Scholar]
- Ramirez-Mahaluf JP, Perramon J, Otal B, Villoslada P, Compte A. Subgenual anterior cingulate cortex controls sadness-induced modulations of cognitive and emotional network hubs. Sci Rep. 2018:8:8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ML, Hagan CR, Joiner TE. Examination of interoception along the suicidality continuum. J Clin Psychol. 2018:74:1004–1016. [DOI] [PubMed] [Google Scholar]
- Rosario MC, Prado HS, Borcato S, Diniz JB, Shavitt RG, Hounie AG, Mathis ME, Mastrorosa RS, Velloso P, Perin EA, et al. Validation of the University of São Paulo Sensory Phenomena Scale: initial psychometric properties. CNS Spectr. 2009:14:315–323. [DOI] [PubMed] [Google Scholar]
- Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983:17:33–44. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003:54:573–583. [DOI] [PubMed] [Google Scholar]
- Sampaio AS, McCarthy KD, Mancuso E, Stewart SE, Geller DA. Validation of the University of São Paulo's Sensory Phenomena Scale -- English version. Compr Psychiatry. 2014:55:1330–1336. [DOI] [PubMed] [Google Scholar]
- Saxena S, O’Neill J, Rauch SL. The role of cingulate cortex dysfunction in obsessive-compulsive disorder. In: Vogt BA, editors. Cingulate neurobiology and disease. New York (NY): Oxford University Press; 2009. pp. 587–617 [Google Scholar]
- Scalabrini A, Wolman A, Northoff G. The self and its right insula—differential topography and dynamic of right vs. Left Insula Brain Sci. 2021:11:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Curran T, Reiman EM, Chen K, Bandy DJ, Frost JT. Medial temporal lobe activation during episodic encoding and retrieval: a PET study. Hippocampus. 1999:9:575–581. [DOI] [PubMed] [Google Scholar]
- Scharnowski F, Nicholson AA, Pichon S, Rosa MJ, Rey G, Eickhoff SB, Van De Ville D, Vuilleumier P, Koush Y. The role of the subgenual anterior cingulate cortex in dorsomedial prefrontal–amygdala neural circuitry during positive-social emotion regulation. Hum Brain Mapp. 2020:41:3100–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultchen D, Zaudig M, Krauseneck T, Berberich G, Pollatos O. Interoceptive deficits in patients with obsessive-compulsive disorder in the time course of cognitive-behavioral therapy. PLoS One. 2019:14:e0217237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007:27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selchen S, Hawley LL, Regev R, Richter P, Rector NA. Mindfulness-based cognitive therapy for OCD: stand-alone and Post-CBT augmentation approaches. Int J Cogn Ther. 2018:11:58–79. [Google Scholar]
- Shapira NA, Liu Y, He AG, Bradley MM, Lessig MC, James GA, Stein DJ, Lang PJ, Goodman WK. Brain activation by disgust-inducing pictures in obsessive-compulsive disorder. Biol Psychiatry. 2003:54:751–756. [DOI] [PubMed] [Google Scholar]
- Shavitt RG, de Mathis MA, Oki F, Ferrao YA, Fontenelle LF, Torres AR, Diniz JB, Costa DL, do Rosario MC, Hoexter MQ, et al. Phenomenology of OCD: lessons from a large multicenter study and implications for ICD-11. J Psychiatr Res. 2014:57:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998:59:22–33. [PubMed] [Google Scholar]
- Sheldon S, Fenerci C, Gurguryan L. A neurocognitive perspective on the forms and functions of autobiographical memory retrieval. Front Syst Neurosci. 2019:13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A, Jung WH, Jang JH, Kim E, Shim G, Park HY, Choi C-H, Kwon JS. Disproportionate alterations in the anterior and posterior insular cortices in obsessive-compulsive disorder. PLoS One. 2011:6:e22361–e22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory (Form Y). Palo Alto, CA: Consulting Psychologists Press; 1983 [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008:105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One. 2012:7:e36356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ER, Brown C, Ludlow M, Shahab R, Collins K, Lieval A, Tobe RH, Iosifescu DV, Burdick KE, Fleysher L. The buildup of an urge in obsessive–compulsive disorder: Behavioral and neuroimaging correlates. Hum Brain Mapp. 2020:41:1611–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. Biochemical switching in hypothalamic circuits mediating responses to stress. Prog Brain Res. 1991:87:181–200. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009:30:2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Moore RG, Hayhurst H, Pope M, Williams S, Segal ZV. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psychol. 2002:70:275–287. [DOI] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman BL. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. NeuroImage. 2012:60:1947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M, Critchley H. Interoception beyond homeostasis: affect, cognition and mental health. Philos Trans R Soc Lond Ser B Biol Sci. 2016:371:20160002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente AA Jr, Miguel EC, Castro CC, Amaro E Jr, Duran FL, Buchpiguel CA, Chitnis X, McGuire PK, Busatto GF. Regional gray matter abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Biol Psychiatry. 2005:58:479–487. [DOI] [PubMed] [Google Scholar]
- Verhoeven K, Crombez G, Eccleston C, Van Ryckeghem DM, Morley S, Van Damme S. The role of motivation in distracting attention away from pain: an experimental study. Pain. 2010:149:229–234. [DOI] [PubMed] [Google Scholar]
- Viard A, Chételat G, Lebreton K, Desgranges B, Landeau B, de La Sayette V, Eustache F, Piolino P. Mental time travel into the past and the future in healthy aged adults: an fMRI study. Brain Cogn. 2011:75:1–9. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005:6:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents J Comp Neurol. 1987:262:271–289. [DOI] [PubMed] [Google Scholar]
- Vogt KM, Becker CJ, Wasan AD, Ibinson JW. Human posterior insula functional connectivity differs between electrical pain and the resting state. Brain Connect. 2016:6:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Barrett LF. From affect to control: functional specialization of the insula in motivation and regulation. bioRxiv. 2017(Jan): 10.1101/102368. [DOI] [Google Scholar]
- Wahl K, Huelle JO, Zurowski B, Kordon A. Managing obsessive thoughts during brief exposure: an experimental study comparing mindfulness-based strategies and distraction in obsessive-compulsive disorder. Cognit Ther Res. 2013:37:752–761. [Google Scholar]
- Wang X, Wu Q, Egan L, Gu X, Liu P, Gu H, Yang Y, Luo J, Wu Y, Gao Z, et al. Anterior insular cortex plays a critical role in interoceptive attention. elife. 2019:8:e42265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998:18:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton MG, Mahaffey B, Timpano KR, Berman NC, Abramowitz JS. The relationship between anxiety sensitivity and obsessive-compulsive symptom dimensions. J Behav Ther Exp Psychiatry. 2012:43:891–896. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. CONN: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012:2:125–141. [DOI] [PubMed] [Google Scholar]
- Yan C-G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo X-N, Castellanos FX, Milham MP. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage. 2013:76:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoris A, García AM, Traiber L, Santamaría-García H, Martorell M, Alifano F, Kichic R, Moser JS, Cetkovich M, Manes F, et al. The inner world of overactive monitoring: neural markers of interoception in obsessive–compulsive disorder. Psychol Med. 2017:47:1957–1970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.