Abstract
A notorious issue of task-based functional magnetic resonance imaging (fMRI) is its large cross-trial variability. To quantitatively characterize this variability, the blood oxygenation level-dependent (BOLD) signal can be modeled as a linear summation of a stimulation-relevant and an ongoing (i.e. stimulation-irrelevant) component. However, systematic investigation on the spatiotemporal features of the ongoing BOLD component and how these features affect the BOLD response is still lacking. Here we measured fMRI responses to light onsets and light offsets in awake rats. The neuronal response was simultaneously recorded with calcium-based fiber photometry. We established that between-region BOLD signals were highly correlated brain-wide at zero time lag, including regions that did not respond to visual stimulation, suggesting that the ongoing activity co-fluctuates across the brain. Removing this ongoing activity reduced cross-trial variability of the BOLD response by ~30% and increased its coherence with the Ca2+ signal. Additionally, the negative ongoing BOLD activity sometimes dominated over the stimulation-driven response and contributed to the post-stimulation BOLD undershoot. These results suggest that brain-wide ongoing activity is responsible for significant cross-trial BOLD variability, and this component can be reliably quantified and removed to improve the reliability of fMRI response. Importantly, this method can be generalized to virtually all fMRI experiments without changing stimulation paradigms.
Keywords: ongoing brain activity, high-dimensional linear model, fiber photometry, fMRI, awake rat
Introduction
Since its inception, the blood oxygenation level-dependent (BOLD)-based functional magnetic resonance imaging (fMRI) has quickly become a prominent neuroimaging method for noninvasively monitoring brain-wide neural activity (Kwong et al. 1992; Ogawa et al. 1992; Bandettini et al. 1993). Despite the versatile applicability, a troubling issue of fMRI is its relatively large cross-trial variability. The BOLD response to the identical stimulus can be considerably different from trial to trial. This problem is detrimental to the detection sensitivity of fMRI, making the fMRI measurement at the individual level less reliable and thereby limiting its potential in clinical applications.
The cross-trial variability of BOLD responses could have contributions from the underlying neural activity, physiological fluctuations, or even instrumentation noise. Neuronal responses (both local field potentials [LFPs] and firing rates) to the identical stimulus can vary significantly across trials. This can be caused by the ongoing neural activity prior to stimulation (Arieli et al. 1996; Azouz and Gray 1999) and fluctuated excitability over time and along the signal pathway (Goris et al. 2014). Behavioral state (e.g. attention, task-engagement, perceptual learning) differences across trials can affect neural variability at the single-neuron level (Mitchell et al. 2007; Hussar and Pasternak 2010), as well as the correlations across groups of neurons (Cohen and Maunsell 2009; Mitchell et al. 2009; Ni et al. 2018). Other, nonexclusive sources of trial-to-trial variability in the BOLD signal are slow variations in physiological signals (e.g. respiration, cardiac rate, vasomotion) (Raj et al. 2001; Liu et al. 2017; Winder et al. 2017; Das et al. 2021) and instrumentation noise such as subject motion and scanner instability (Power et al. 2014; Liu 2016).
In order to quantitatively characterize the cross-trial BOLD variability, the BOLD response has been modeled as a linear superposition of a task-irrelevant (i.e. ongoing) component and a task-relevant component. For example, Fox et al. (2006) showed that the right-hand button-press-related BOLD response in the left somatomotor cortex became more reliable after subtracting a scaled BOLD activity of the unresponsive right somatomotor cortex. This study has demonstrated that the ongoing BOLD activity in a nonresponding region can account for significant cross-trial variability in the BOLD response of an activated region. Nonetheless, this previous study only examined the ongoing activity in local brain regions pertinent to the specific stimulation involved, while the spatiotemporal features of ongoing BOLD activity across the entire brain and its impact on the BOLD variability that can be generalized to any stimulation paradigm remain elusive.
To systematically investigate how brain-wide ongoing activity affects the measured BOLD sensory response, we collected the BOLD signal with fMRI during visual stimulation in awake rats. The local neuronal response was simultaneously measured with calcium-based fiber photometry. Awake rodent fMRI has the capability to control the confounding effects of anesthesia (Gao et al. 2017) and can be translated to awake imaging studies in humans and other primates. The BOLD responses to both the visual stimulation onset and offset were quantified for the first time in awake rats. Between-region BOLD signals across the brain were highly correlated at zero time lag, providing a means to estimate ongoing brain activity during virtually any type of stimulation. To generalize the previous linear model, we designed a high-dimensional linear model to estimate the measured BOLD response of primary visual regions across different stimulation conditions, with the ongoing activity being derived from both stimulation-responding and nonresponding regions. This linear model suggests that the ongoing activity explained a large amount of the variance in the measured BOLD visual response, and removing this shared ongoing component improved the reliability of BOLD responses across trials by ~30%. Finally, in a substantial number of trials, despite positive Ca2+ response in neurons, a widespread negative ongoing BOLD activity dominated over the stimulation-relevant response and contributed to the post-stimulation undershoot of BOLD responses averaged across all trials.
Methods and materials
Animals
All procedures were carried out under the protocol approved by the Pennsylvania State University Institutional Animal Care and Use Committee (IACUC). Adult male rats (Long Evans, Charles River, Wilmington, MA) were used in this study, including 7 for the fiber photometry-fMRI experiment (3-channel coil), 4 for the optogenetic-fMRI experiments (3-channel coil), 11 for the fMRI only experiment using the 3-channel coil, and 13 for the fMRI only experiment using the 4-channel coil. All rats were housed in home cages for at least 7–10 days before surgery. Food and water were provided ad libitum. Room temperature was kept at 22–24 °C with a 12 h light, 12 h dark cycle.
Surgery
Before surgery, animals were anesthetized by ketamine (40 mg/kg) and xylazine (12 mg/kg). Dexamethasone (0.5 mg/kg) was injected to prevent brain tissue swelling. The rats were intubated and ventilated with oxygen and isoflurane (0.5–2%). The physiological parameters including the heart rate, respiration rate, and SpO2 were monitored (MouseSTAT Jr, Kent Scientific Corporation). The body temperature was monitored and maintained at 35–36 °C by a warming pad (PhysioSuite, Kent Scientific Corporation). For the fiber photometry experiment, AAV9.Syn.GCaMP6s (1,000 nL, Addgene) was injected into the superior colliculus (sSC) at 3 depths (6.3–6.5 mm posterior to bregma, 0.8–1.2 mm right to the midline, 3.2–2.8 mm below the brain surface). An optical fiber (0.4 mm diameter, 0.5 NA, Thorlabs, Newton, NJ) was implanted at the injection site (2.9 mm below the brain surface). For optogenetic targeting at the sSC, AAV9.CaMKIIα.ChR2 (350 nL, Addgene) was injected into the sSC (6.3–6.5 mm posterior to bregma, 0.8–1.2 mm right to the midline, 3.0 mm below the brain surface) and an optical fiber (0.4 mm diameter, 0.39 NA, Thorlabs, Newton, NJ) was implanted 200–300 μm above the injection site. For optogenetic targeting at the intermediate/deep layer of the SC (SCid), AAV9.CaMKIIα.ChR2 (400 nL per depth, Addgene) was injected into the SCid at 3 depths (6.3–6.5 mm posterior to bregma, 1.8 mm right to the midline, 4.1–5.1 mm below the brain surface) and an optical fiber (0.4 mm diameter, 0.39 NA, Thorlabs, Newton, NJ) was implanted in the intermediate layer of the SC (3.7–3.9 mm below the brain surface).
Visual stimulation
Blue laser (473TB-300FC, Shanghai Laser & Optics Century Co., Ltd) light was calibrated to 25–50 μW at the patchcord tip (Doric lenses, Quebec, Canada), which was placed 1–2 cm away from the left eye of the rat, whose right eye was covered with a black patch. We used relatively dim light to avoid irritating the animal. A TTL pulse was sent out from the scanner after each brain volume collection, which was used to trigger the light on/off. For example, if the on duration was 20 s, the light stayed on until 20 TTL pulses were collected from the scanner (as the repetition time [TR] = 1 s). By doing so, we synchronized the light onset and offset with a specific phase of the fMRI recording, and as a result, across trials brain volumes were collected at exactly the same time after each stimulation transition, which avoids the jittering of BOLD time series. The actual light onset/offset was detected by a photodiode and the timestamps were recorded by a DAQ device (USB-6211, National Instruments, Austin, TX) to estimate the delay induced by the software and the laser’s reaction time. The total delay in the current setup was <10 ms.
Optogenetics
A blue laser (473TB-300FC, Shanghai Laser & Optics Century Co., Ltd) triggered by a DAQ device (USB-6211, National Instruments, Austin, TX) was used for optogenetic stimulation. The rats’ bilateral eyes were covered with black patches. The stimulation was delivered at 20 Hz, 15 ms pulse width (30% duty cycle), 10 pulses per trial, and 25 s intertrial interval. The intertrial interval was counted the same way as in the visual stimulation by using the number of TTL pulses collected from the scanner. The light intensity at the fiber tip before going into the brain was 2–4 mW (time-averaged light power density: 4.8–9.5 mW/mm2). In 2 control animals that were ChR2 negative, we gradually increased the light power to ~10 mW at the fiber tip and did not observe any suspected activation/deactivation around the SC. According to the literature (Christie et al. 2013; Duffy et al. 2015), time-averaged light power density 4.8–9.5 mW/mm2 is safe from heating-related artifacts.
Fiber photometry calcium signal recording
The setup was modified from Liang et al. (2017). The GCaMP and calcium-independent signals were excited by a 465 and 405 nm LED (Doric lenses, Quebec, Canada), respectively. A mini cube (Doric lenses, Quebec, Canada) was used to combine the 465 and 405 nm light into a patchcord (Doric lenses, Quebec, Canada) for excitation. The 465 and 405 nm LEDs were toggled at 400 Hz (40% duty cycle), with interleaved 465 and 405 nm excitations. The light intensity at the patchcord tip was 10–20 μW. The excited fluorescence was split in the same mini cube, sent through the same patchcord and collected by a silicon photomultiplier (MiniSM 30035, SensL, Ireland), and then amplified through a lock-in amplifier (SR810, Stanford Research Systems, Sunnyvale, CA). The amplified signal was recorded by a DAQ device (USB-6211, National Instruments, Austin, TX) sampled at 10 kHz.
fMRI experiment
Before the fMRI experiment, animals were acclimated 7 days outside of the scanner to reduce the motion and the stress level (Dopfel and Zhang 2018). fMRI scanning was conducted on a 7T MRI system interfaced with a Bruker console (Billerica, MA). During imaging, anatomical T1-weighted images were collected first, and then gradient-echo images were acquired using echo-planar imaging with the parameters: TR = 1 s, echo time (TE) = 15 ms, matrix size = 64 × 64, FOV = 3.2 × 3.2 cm2, slice number = 20, and slice thickness = 1 mm. Right after each volume acquisition, the scanner sent out a TTL signal, which was recorded by the DAQ device (USB-6211, National Instruments, Austin, TX) to synchronize the fMRI signal with the fiber photometry recording/optogenetic/visual stimulation events.
Each animal was imaged once per week with 3 fMRI runs. For the visual stimulation experiment, each run was 15 min. The first 5 min was resting-state fMRI acquisition followed by 10-min visual stimulations. For the fixed stimulation paradigm, the on and off duration was both 25 s per trial; for the randomized stimulation paradigm, the on and off durations were drawn from a uniform distribution of 10–30 s per trial. In the fixed stimulation paradigm, we randomly skipped 2–3 light-on trials in each run to examine the possible anticipatory effect, and the data were used for another study. For the optogenetic fMRI experiment, each run was 15 min. The first 5 min was resting-state fMRI acquisition without optogenetic stimulation. In the following 10 min, 10 light pulses were delivered per trial (20 Hz, 15 ms pulse width, 30% duty cycle). The intertrial interval was 25 s. For the fiber photometry fMRI experiment, each run was 10 min. The first run was resting-state fMRI recording without visual stimulation. In the other 2 fMRI runs, visual stimulations were delivered with repeated light on for 20 s followed by light off for 20 or 30 s. For both visual and optogenetic stimulations, a ~5-min interval was allowed between consecutive runs. For each ON–OFF cycle in all visual stimulation experiments, the “ON-trial” response was defined as the BOLD response from the start of light on to the end of light on period, and the “OFF-trial” response was defined as the BOLD response from the start of light offset to the end of light off period.
To ensure our results were not biased by the B1 profile of the radiofrequency (RF) coil used, in this study, we used 2 coils (3-channel and 4-channel) with different sensitivity profiles and showed the result from each coil separately (3-channel coil: n = 11 rats, 63 fMRI runs; 4-channel coil: n = 10 rats, 83 fMRI runs). The 4-channel coil had an overall higher signal-to-noise ratio than the 3-channel coil, resulting in slightly higher contrast in correlation maps (Fig. 1B, right).
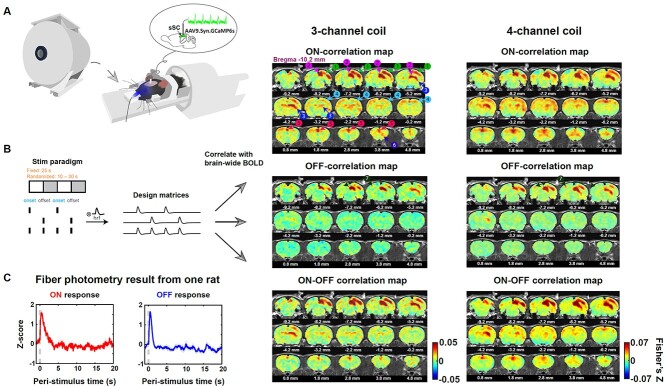
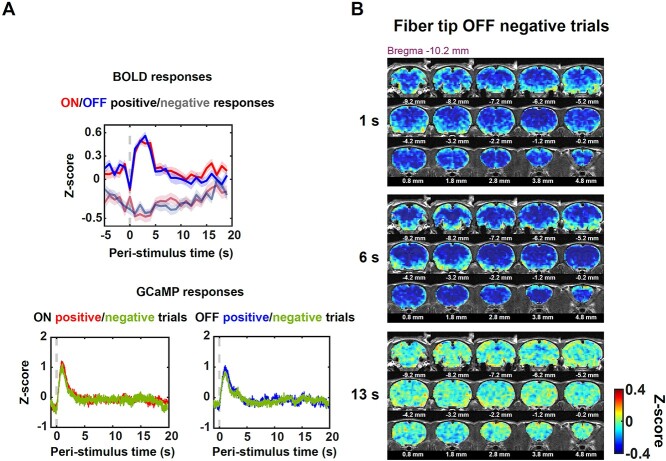
Fig. 1.
Measuring evoked visual responses in awake rats. A) Setup of simultaneous fMRI-GCaMP experiment with visual stimulation in awake rats. Animal was restrained with a 3D printed restrainer. During visual stimulation, the left eye was illuminated by blue light with the right eye being covered with a black patch. B) Two visual stimulation paradigms were used: fixed (on/off duration 25 s) and randomized stimulation (on/off duration randomly drawn from a uniform distribution of 10–30 s). The stimulation paradigm vectors with (i) the light onsets only, (ii) the light offsets only, and (iii) both the light onsets and offsets being setting as 1 s and the rest being set as 0 s were convolved with a canonical HRF to generate the ON/OFF/ON–OFF design matrix. The BOLD response of each voxel was correlated with these 3 design matrices for each fMRI run. The Pearson correlation values were Fisher-Z transformed and averaged across runs and animals to obtain the corresponding correlation maps. Three-channel coil: n = 11 rats, 63 runs; 4-channel coil: n = 10 rats, 83 runs. C) Trial-averaged Ca2+ responses to the light onset and offset from an example animal. Shaded areas denote SEM. Dashed lines indicate the light onset/offset.
fMRI preprocessing
The fMRI signal was preprocessed following the pipeline developed in the lab (data preprocessing steps 1–5 in Liu et al. (2020)) with several changes highlighted below. First, the framewise displacement (FD) was calculated, and frames with FD > 0.25 mm were labeled as motion frames. Motion frames and their neighboring frames were removed from the corresponding runs. The first 10 frames of each run were also removed to ensure the stabilization of MR signal. Runs with >20% frames removed were discarded for future analysis. Second, each brain was registered to a template with Medical Image Visualization and Analysis (MIVA, http://ccni.wpi.edu/). Third, motion correction was conducted with SPM12 (http://www.fil.ion.ucl.ac.uk/spm/). Fourth, spatial smoothing was performed using a Gaussian filter (full-width at half-maximum = 0.75 mm). For visual stimulation and optogenetic fMRI runs, 6 motion parameters were regressed out and the signal was detrended (MATLAB function: detrend, order 2) to remove the potential signal drifting during scanning. For resting-state data, motion parameters and mean response from the white matter (WM) and ventricles were regressed out, and the signal was temporally filtered to 0.01–0.1 Hz with a fourth-order bandpass Butterworth filter.
For the visual stimulation and optogenetic fMRI runs, the fMRI signal from the first 5 min without visual/optogenetic stimulation was defined as the baseline. The BOLD time course of each voxel was normalized by subtracting the baseline mean and dividing the baseline standard deviation of the voxel. For the fiber photometry fMRI run, when identifying the positive and negative trials, BOLD time series from the last 4 s of each OFF trial were concatenated and used as the baseline for that run; when calculating the coherence between the BOLD and Ca2+ signal, the BOLD signal was normalized to the whole run (in the same fashion as the Ca2+ signal).
GCaMP signal preprocessing
The Ca2+ signal preprocessing was modified from (Martianova et al. 2019). First, the fluorescent signal sampled at 10 kHz was separated into 465 and 405 nm excited signals, given that they were temporally interleaved. Each signal was low pass filtered to <20 Hz. Second, baseline drifting and low-frequency fluctuations in each signal were removed using the adaptive iteratively reweighted Penalized Least Square (airPLS) algorithm (Zhang et al. 2010). We noticed that the slow fluctuations in the 465 nm signal, which were unlikely the neuronal responses due to the slow temporal profile, could not be captured by the 405 nm signal occasionally. The airPLS algorithm can estimate and remove certain slow fluctuations to reduce this contamination. The cleaned signal was then standardized by subtracting its median and dividing its standard deviation. The standardized 405 nm signal was regressed out from the standardized 465 nm signal through the Ordinary Least-Squares regression (Python package: sklearn.linear_model.LinearRegression).
Definition of functional brain masks for the right SC, right visual area, and cingulate
For each animal, according to the cross-run averaged ON/OFF correlation maps, we employed a liberal threshold of 80% confidence interval of the Fisher-Z score (without correction) as the threshold to extract “activated” voxels to the stimulation onset/offset. Those “activated” voxels were then intersected with the anatomically defined brain masks for right SC (rSC), right visual area (rVA), and bilateral cingulate (Cg) to obtain the corresponding functional masks. This approach preserved high-responsive voxels within the anatomical structure. As Cg did not show robust OFF response, we used the anatomical Cg mask to represent the Cg OFF mask.
Definition of functional brain masks for the left SC and left VA
For each animal, resting-state BOLD time courses (i.e. data from the first 5 min of each fMRI run) for voxels within the anatomically defined left SC (lSC) (or left VA [lVA]) were correlated with the mean BOLD signal from the functionally defined masks of rSC (or rVA). Voxels with a significant correlation coefficient (P < 0.0001, uncorrected) were identified as the functional masks for the lSC and lVA.
Definition of the mask of “nonresponding voxels”
We first ran an individual-level linear regression on each fMRI run. The independent variable in this linear regression was the ON–OFF design matrix, which was defined as the stimulation paradigm vector (with the light onsets and offsets being set as 1 s, and the rest being set as 0 s) convolved with a canonical HRF (SPM function: spm_hrf, with P = [3 6 1 2 2 0 32]). The dependent variable was the BOLD response of each voxel. The estimated regression coefficients derived from each animal were pooled together and went into the group-level linear mixed effects (LME) model fitting (MATLAB function: fitlme). We used the threshold P > 0.3 (without correction) to extract nonresponding voxels. We further masked the voxels identified above with a manually defined brain mask to remove voxels at the brain boundary, given that these voxels had lower signal-to-noise ratio. This procedure resulted in 897 and 536 “nonresponding voxels” for the fMRI data from the 3- and 4-channel coils, respectively.
Extracting BOLD responses from the rSC, rVA, and Cg
The BOLD time course of each region was obtained by averaging BOLD time courses of voxels within the region. Values of motion frames were set as NaNs. The total number of trials for each region from each coil under each stimulation condition is shown in Table 1. The Cg had less ON trials in the 4-channel coil data as one animal did not show robust Cg ON response (i.e. the Cg ON mask was missed in that animal).
Table 1.
Number of trials for each region under on/off stimulation.
| Three-channel coil fixed stimulation | Four-channel coil fixed stimulation | Four-channel coil randomized stimulation | ||||
|---|---|---|---|---|---|---|
| Region | ON-trial number | OFF-trial number | ON-trial number | OFF-trial number | ON-trial number | OFF-trial number |
| rSC | 759 | 759 | 918 | 918 | 577 | 561 |
| rVA | 759 | 759 | 918 | 918 | 577 | 561 |
| Cg | 759 | 759 | 818 | 918 | 505 | 561 |
BOLD correlation maps
The stimulation design matrices were defined by the stimulation paradigm vectors, with either the light onsets or offsets or both being set as 1 s and the rest being set as 0 s, convolved with a canonical HRF (SPM function: spm_hrf, with P = [3 6 1 2 2 0 32]). For each fMRI fun, we calculated the Pearson correlation values between the normalized BOLD time course of each voxel and the corresponding stimulation design matrix. The Pearson correlation values were Fisher-Z transformed and averaged across runs and animals.
A similar procedure was used for the optogenetic fMRI data. The optogenetic stimulation on period was 500 ms for each trial, which was shorter than the TR (1 s). The stimulation paradigm vector, with the volumes when the optogenetic stimulation was on being set as 1 s and the rest being set as 0 s, was convolved with a canonical HRF (SPM function: spm_hrf, with P = [3 6 1 2 2 0 32]) to obtain the stimulation design matrix. The BOLD response was voxelwise correlated with the design matrix. The Pearson correlation values were Fisher-Z transformed and averaged across runs and animals.
Extracting positive/negative BOLD responsive trials
We combined all trials from the 3- and 4-channel coils. The voxelwise BOLD response was z-scored by the baseline. Trials with any motion frames 0–4 s post-stimulation were excluded. Positive trials were defined as trials that met the criteria: (i) the averaged BOLD amplitude of 1–4 s post-stimulation was greater than zero; (ii) the integral of the time course (area under the curve) during 1–4 s after subtracting the BOLD response at 0 s was greater than zero; negative trials were defined as trials that met the criterion: the averaged BOLD amplitude of 1–4 s post-stimulation was less than zero. The numbers of positive and negative trials for each region under each stimulation condition are shown in Table 2.
Table 2.
Number of positive and negative trials for each region under on/off stimulation.
| Positive-trial number | Negative-trial number | |
|---|---|---|
| rSC ON | 1,221 | 493 |
| rSC OFF | 1,012 | 606 |
| rVA ON | 1,113 | 559 |
| rVA OFF | 998 | 653 |
Notably, positive and negative trials commonly existed across subjects. For example, for the onset response in the rVA, the percentage of negative trials averaged across animals was 34.5%, with a minimal percentage of 25.7% and a maximal percentage of 47.1%.
BOLD linear model
We modeled the measured BOLD response as the linear summation of ongoing activity and stereotyped impulse response. Consider responses to light onset/offset: each trial was 20 s long with N trials in total Equation (1).
 |
(1) |
Here, 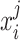 denotes the BOLD response of trial j, at the time i, extracted from a region of interest (ROI), which was used to estimate the ongoing response;
denotes the BOLD response of trial j, at the time i, extracted from a region of interest (ROI), which was used to estimate the ongoing response; 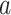 is a scaling factor; the vector
is a scaling factor; the vector 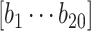 constrains the shape of the evoked response, which remains the same across all trials;
constrains the shape of the evoked response, which remains the same across all trials; 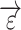 denotes the estimation error;
denotes the estimation error; 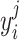 denotes the measured BOLD response of trial j, at the time i from a visual responsive region.
denotes the measured BOLD response of trial j, at the time i from a visual responsive region.
The above equation is an overdetermined system. To estimate  and
and 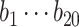 , the mean squared error is given by:
, the mean squared error is given by: 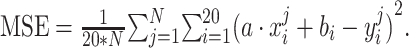 To get the global minimum of the loss function, we took the derivatives of
To get the global minimum of the loss function, we took the derivatives of 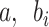 and set them all to 0:
and set them all to 0: 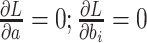 . The equivalent matrix form is as follows Equation (2):
. The equivalent matrix form is as follows Equation (2):
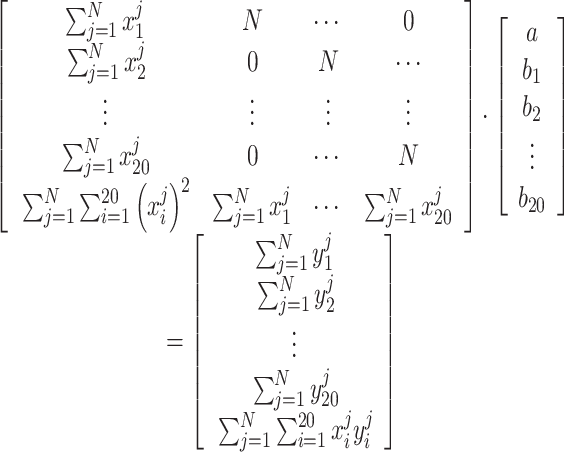 |
(2) |
 can be solved by the matrix left division. We first fit the ON and OFF trials separately, which resulted in a similar scaling factor
can be solved by the matrix left division. We first fit the ON and OFF trials separately, which resulted in a similar scaling factor 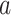 across the 2 conditions for a given ROI. To get a general relationship between the ongoing and measured BOLD activity, we used the same scaling factor across different stimulation conditions (i.e. light onset and light offset). However, the evoked stereotyped response can be different under distinct stimulation conditions. We pooled the ON and OFF trials together to estimate the scaling factor and corresponding stereotyped responses Equation (3).
across the 2 conditions for a given ROI. To get a general relationship between the ongoing and measured BOLD activity, we used the same scaling factor across different stimulation conditions (i.e. light onset and light offset). However, the evoked stereotyped response can be different under distinct stimulation conditions. We pooled the ON and OFF trials together to estimate the scaling factor and corresponding stereotyped responses Equation (3).
 |
(3) |
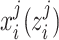 denotes the ON (OFF) response (of trial j, at the time i) extracted from the region, which was used to estimate the ongoing response;
denotes the ON (OFF) response (of trial j, at the time i) extracted from the region, which was used to estimate the ongoing response; 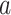 is a scaling factor; the vector
is a scaling factor; the vector 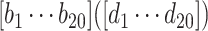 constrains the shape of the evoked ON (OFF) response, which remains the same across all ON (OFF) trials;
constrains the shape of the evoked ON (OFF) response, which remains the same across all ON (OFF) trials; 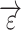 denotes the estimation error;
denotes the estimation error; 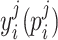 denotes the ON (OFF) response (of trial j, at the time i) from the visual responsive region.
denotes the ON (OFF) response (of trial j, at the time i) from the visual responsive region.
Similarly, the global minimum of 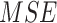 is given by Equation (4):
is given by Equation (4):
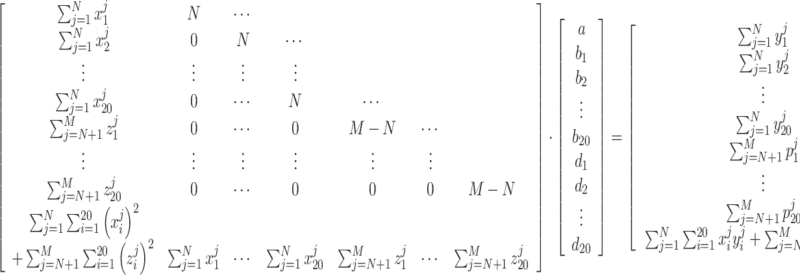 |
(4) |
Fitting performance of the BOLD linear model
Responses of all ROIs (either within the left or right hemisphere, Table 3) were utilized to estimate the ongoing activity. Each trial was truncated to 0–19 s post-stimulation transition. To reduce the artifacts of animal motion in this fitting process, trials with any motion frames (0–19 s) were discarded. In total, we had 614 ON trials and 630 OFF trials for the fixed stimulation paradigm acquired with the 3-channel coil; 803 ON trials and 825 OFF trials for the fixed stimulation paradigm with the 4-channel coil; 447 ON trials and 444 OFF trials for the randomized stimulation paradigm with the 4-channel coil. To estimate a general linear coefficient between the ongoing and measured activities, we pooled the ON and OFF trials and split them into 10 partitions. Each partition was utilized to test the explained variance of the linear model, which was estimated from the other 9 partitions of the data. We reported the performance from each fold (each dot in Fig. 3C) and the average across 10 folds (the height of each bar in Fig. 3C). For the optogenetic data, 249 trials from 2 animals with sSC stimulation were included. Due to the smaller dataset, the fitting process was done over 5 partitions.
Table 3.
Abbreviation of ROIs.
| CA1 | CA1 |
| CA3 | CA3 |
| Dentate gyrus | DG |
| Entorhinal area | ENT |
| Subiculum | SUB |
| Ventral posteromedial nucleus thalamus | VPM |
| Medial dorsal thalamus | MD |
| Zona incerta | ZI |
| Lateral hypothalamic area | LHA |
| Preoptic area | POA |
| Lateral septum complex | LSC |
| Caudoputamen | CP |
| Accumbens | ACB |
| Olfactory tubercle | OT |
| Globus pallidus | GP |
| Ventral pallidum | VP |
| Anterior cingulate cortex | ACA |
| Prelimbic area | PL |
| Orbital area | ORB |
| Agranular insula | AI |
| Retrosplenial complex | RSP |
| Ventral temporal association areas | TEv |
| Ectorhinal area | ECT |
| Main olfactory bulb | MOB |
| Piriform area | PIR |
| Gustatory area | GU |
| Visceral area | VISC |
| Primary motor cortex | Mop |
| Secondary motor cortex | Mos |
| Primary somatosensory cortex | SSp |
| Supplemental somatosensory cortex | SSs |
| Auditory cortex | AUD |
| Visual cortex | VIS |
| Periaqueductal gray | PAG |
| Superior colliculus | SC |
| Mesencephalic reticular nucleus | MRN |
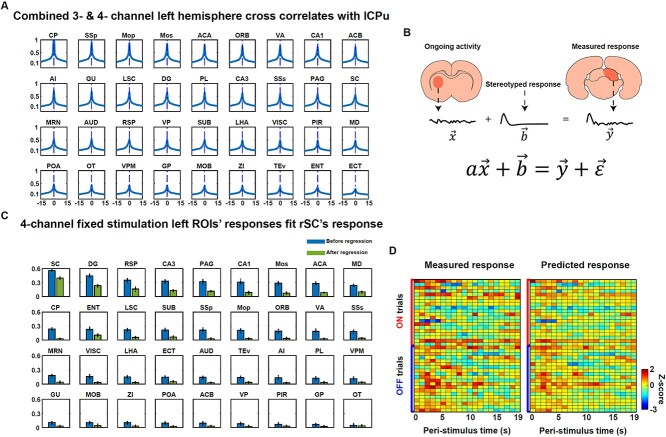
Fig. 3.
Brain-wide BOLD activities are highly correlated during visual stimulation. A) Cross-correlation of BOLD activities of different ROIs in the left hemisphere with the “target region” within the lCPu. Results shown were averaged across the 3- and 4-channel coil data. Maximum correlations all occurred at zero time lag with the majority of peak correlations >0.5. B) An illustration of the linear model to predict the evoked response in an activated region by the summation of the ongoing activity estimated from a ROI (e.g. lCPu) and a stereotyped stimulation-driven impulse response. C) The fitting performance of the linear model in B with the 4-channel data during the fixed stimulation paradigm. The ongoing response was estimated from ROIs in the left hemisphere. The predicted response was the measured response of the rSC. The height of each bar indicates the variance explained averaged across 10 folds (blue bars: before the ongoing activity regression; green bars: after the ongoing activity regression). Each dot indicates the variance explained from 1-fold. ROIs were sorted in a descending order based on the averaged variance explained before regression. D) Examples of measured responses and predicted responses from randomly picked ON and OFF trials, with the ongoing activity derived from the lSC.
Coherence between the BOLD and Ca2+ signal
The BOLD signal was normalized to the entire fMRI run. First, the “gaps” in the BOLD time courses due to motion were filled with an autoregressive process (MATLAB function: fillgaps, maxlen = 30, the order was determined by minimizing the “AIC” information). Second, the preprocessed Ca2+ signal sampled at 400 Hz was downsampled to 1 Hz by forward and backward resampling to avoid the phase shift. Finally, the magnitude-squared coherence between the BOLD and Ca2+ signal was calculated with the Chronux toolbox (function: coherencyc, with the tapers = [3,5], pad = 0).
Statistics
Repeated measure ANOVA was carried out in SPSS (IBM SPSS Statistics 27), with BOLD responses before and after regression modeled as the within-subject variables, and the rat identity modeled as the between-subject factor. Generalized linear mixed-effect model was performed with the MATLAB function: fitglme. The FD was treated as a fixed effect, and the rat identity was treated as a random effect.
Histochemistry
Brain slices were cut into 60 micro meter thickness. Washed with 1× PBS once and left in 1× PBS with 0.3% Triton at room temperature for 1–2 h on a shaker. Slices were blocked with 5% normal donkey serum (Jackson ImmunoResearch Laboratories) in 0.3% Triton and 1× PBS at room temperature for 1 h on a shaker. Subsequently, slices were incubated with the primary antibody, rabbit anti-Calbindin D-28k (Swant, CB-38a (new batch), 1:1,000 dilution), which was used to label the superficial gray layers of the SC (Lee et al. 2020), in a humid box in 4 °C overnight. The prepared slices were washed with 0.05% Triton in 1× PBS 3 times (10 min each), then incubated with the secondary antibody (Alexa Fluor 488 Donkey anti Rabbit IgG (H + L), Invitrogen) in a humid box at room temperature for 1 h on a shaker. Next, slices were washed with 0.3% Triton in 1× PBS 3 times (10 min each) and mounted with the Prolong Diamond antifade mountant with DAPI (Invitrogen). Lastly, slices were imaged with a lab-built wide-field microscope.
Results
Light stimulation onset and offset evoke different spatiotemporal dynamics of BOLD activation
First, we characterized the spatiotemporal dynamics of BOLD activation to visual stimulation. Given that primary visual neurons respond to both the stimulation onset and offset, we specifically mapped the spatiotemporal BOLD responses to light onset and offset, by using sustained on–off light stimulation (Fig. 1B). The awake-rat fMRI was conducted using the setup shown in Fig. 1A, where the animal was restrained in a 3D-printed restrainer while a whole-field visual stimulus was delivered unilaterally to the (left) eye via an optical fiber. The contralateral (right) eye was covered with a black patch. In a subset of animals (n = 7), we performed simultaneous calcium-based fiber photometry during fMRI to record the neuronal responses in a primary visual area—the superficial layer of the SC. Before awake animal imaging, the rat was acclimated to the restrainer and scanner noise for 7 days to reduce its motion and stress level (Dopfel and Zhang 2018). We used 2 stimulation paradigms: fixed and randomized stimulation. In the fixed stimulation paradigm, the duration of stimulus on and off per cycle was fixed (25 s) in each fMRI run (i.e. one fMRI scan). In the randomized stimulation paradigm, the durations of stimulus on and off per cycle were randomly drawn from a uniform distribution of 10–30 s. Three types of design matrices were used here: (i) The ON–OFF design matrix: a canonical HRF was convolved with the stimulation paradigm vector, where the light onsets and offsets were set as 1 s and the rest were set as 0 s. (ii) The ON-design matrix: a canonical HRF was convolved with the vector, where only the light onsets were set as 1 s. (iii) The OFF-design matrix: a canonical HRF was convolved with the vector, where only the light offsets were set as 1 s. Only transitions of light stimulation (i.e. onsets and offsets) were included in these design matrices, given that the primary visual system inherited the light detection properties from the retina which is more responsive to light changes rather than sustained illumination. Our trial-averaged BOLD time courses of the primary sensory regions (shown below) verified this as well. The design matrices for each fMRI run were then correlated with the normalized BOLD time course of each voxel. The Pearson correlation values were Fisher-Z transformed and averaged across runs and animals to generate the brain-wide responsive patterns: the ON–OFF correlation map, ON-correlation map, and OFF-correlation map. Correlation maps from the fixed stimulation paradigm are shown in Fig. 1B, right. To avoid any potential bias in our results caused by the B1 profile of the RF coil used, we used 2 coils with different sensitivity profiles and showed the result from each coil separately.
The spatial response patterns to the light onset and offset were consistent across 2 coils. Generally the ON-correlation map displayed more positive correlative regions compared to the OFF-correlation map, and this was true for both coils. Specifically, the contralateral visual areas (labeled “1,” including the retrosplenial cortex [RS], primary visual cortex [V1], and secondary visual cortex [V2]), contralateral SC (labeled “2”), thalamic nuclei (labeled “3,” including the lateral posterior thalamic nucleus and geniculate nucleus), sensorimotor areas (labeled “4,” including the primary and secondary motor cortices [M1 and M2], as well as somatosensory cortices), cingulate (labeled “5”), and frontal area (labeled “6,” including the orbital cortex) showed prominent positive correlations with the ON-design matrix. In contrast, fewer regions, including contralateral visual areas, contralateral SC, thalamus, and some voxels in the ipsilateral visual areas (labeled “7”), were activated in the OFF-correlation map. Simultaneously recorded Ca2+ signal in the sSC during fMRI showed robust evoked neuronal responses to light onset and offset (Fig. 1C), confirming the neuronal basis of BOLD responses we observed. Notably, this difference between ON and OFF BOLD responses was not caused by different head-motion levels between the 2 stimulation conditions, as there was no significant difference in the FD between ON and OFF trials (GLME, P = 0.10, Fig. 2 supplementary 1B).
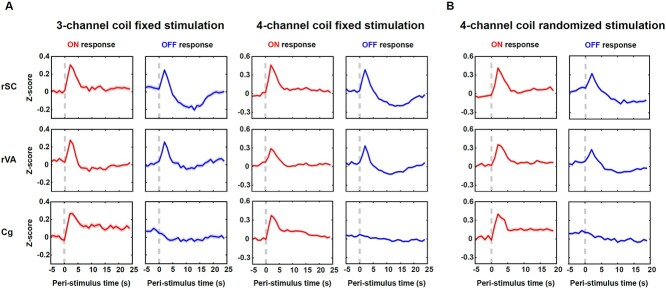
To examine the temporal profiles of the evoked BOLD signal, we showed the BOLD responses of the rSC, combined responses of nearby rVA which included the right RS, V1, and V2, and responses of the bilateral Cg. Due to the limited spatial resolution of fMRI, we did not separate responses from adjacent small regions of RS, V1, and V2. Time courses from the 2 coils showed similar temporal profiles (Fig. 2A). First, both the rSC and rVA showed evoked responses following stimulation transitions, while the Cg only responded to the stimulation onset, not the offset. All evoked responses reached the peak around 2 s after stimulation transitions, consistent with the measured sensory BOLD response in awake mice (Chen et al. 2020). Second, the OFF response of the rSC had a large undershoot following BOLD peaks (10–15 s). Correspondingly, a moderate undershoot was observed in the rVA’s OFF response and a mild, sustained undershoot in the rVA’s ON response was recorded by the 3-channel coil. Third, the ON response in the Cg did not return to baseline after BOLD peaks but remained positive during the on period. It has been suggested that the Cg is critical for attention (Wu et al. 2017) and arousal regulation (Ebitz and Platt 2015). The animal might be more engaged during the on period compared to the off period, despite this being a passive visual stimulation task.
Fig. 2.
BOLD responses in activated brain areas. A) Averaged time courses of the rSC, rVA, and Cg to the light onset and offset for the fixed stimulation paradigm collected with the 3- and 4-channel coils. B) Averaged time courses of the rSC, rVA, and Cg to the light onset and offset for the randomized stimulation paradigm collected with the 4-channel coil. Shaded areas denote SEM. Dashed lines indicate the light onset/offset.
To confirm that the BOLD undershoot in the off period was not entrained by the fixed stimulation paradigm, we plotted data from the randomized stimulation paradigm in rats (n = 13) before they were exposed to the fixed stimulation paradigm. Large undershoot still existed in the rSC’s OFF response, while a moderate undershoot was in the rVA’s OFF response (Fig. 2B). Time courses from these ROIs were highly consistent with those obtained from the fixed stimulation, suggesting this BOLD temporal feature was not entrained by the periodic stimulation. We next tested whether the undershoot was caused by the respiration rate change during the off period, as respiration rate can impact arterial oxygenation (Zhang et al. 2019). On average, there was no appreciable variation in the respiration rate during on and off periods (Fig. 2 supplementary 1A). We observed that although in some animals the respiration rate was increased after the light onset, this increase returned to baseline before the light was turned off and remained constant across the entire off period (Fig. 2 supplementary 1A). Besides, the undershoot occurred in the late off period (10–15 s) was spatially localized within the rSC and rVA (Fig. 5 supplementary 1, left), arguing against the possibility that the BOLD undershoot was dominated by respiration changes.
In conclusion, light onset and offset activated different brain-wide spatial patterns, and the responsive regions (rSC, rVA, Cg) contained characteristic temporal profiles to light onset and offset.
BOLD activities are brain-wide correlated with zero time lag
Next, we tested the hypothesis that the observed BOLD response can be modeled as a linear sum of a stimulation-driven, deterministic component and a stimulation-independent (i.e. ongoing) component. Before applying this linear model, we first asked how we could extract stimulation-irrelevant ongoing activity for brain regions exhibiting evoked BOLD responses (e.g. rSC, rVA). To do this, we quantified how BOLD activities from different brain regions were correlated with various time delays. We manually labeled a nonresponding subregion within the left caudate putamen (lCPu, including 86 continuous voxels with minimal activation) as the “target” region according to the correlation maps in Fig. 1B and cross-correlated its time course with BOLD signals of individual regions from the rest of the brain. All regions were defined anatomically except for the lSC, lVA, rSC, rVA, which were functionally defined (see Section 2 for details). Given the visual stimulation was unilateral, we considered the left- and right-hemisphere ROIs separately. Our data showed that the BOLD signal from each region within the left hemisphere was maximally correlated with the response of the “target” at zero time lag, with the majority of ROIs showing a peak correlation of Fisher’s Z > 0.5 (Fig. 3A). To further rule out the possibility that these BOLD signal correlations resulted from systematic transit time delays in the circulation through the cerebral vascular tree (which has been shown to contribute to delayed correlations of slow BOLD signals (0.005–0.1 Hz) from different regions in humans (Tong et al. 2019)), we further low pass filtered the BOLD signal <0.1 Hz, and the peak correlation still occurred at zero time lag (Fig. 3 supplementary 2). Cross-correlations of the BOLD activity of the “target” with ROIs in the right hemisphere were similar (Fig. 3 supplementary 1). This result suggests that we can use nonresponding regions to estimate the ongoing activity without shifting the time courses.
Subsequently, we modeled the measured response during visual stimulation as a linear summation of an ongoing and a deterministic stimulus-driven component, as illustrated in Fig. 3B. For each trial, the BOLD signal 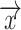 from one nonresponding ROI (e.g. lCPu) scaled by a constant “
from one nonresponding ROI (e.g. lCPu) scaled by a constant “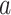 ” was used to estimate the ongoing activity of the rSC/rVA. The sum of this estimated ongoing activity and a deterministic stimulus-driven response
” was used to estimate the ongoing activity of the rSC/rVA. The sum of this estimated ongoing activity and a deterministic stimulus-driven response 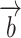 was used to predict the measured response in the rSC/rVA (i.e.
was used to predict the measured response in the rSC/rVA (i.e. 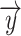 ) for that trial. In addition to nonresponding ROIs, this linear model can also be generalized to using signals of activated ROIs to estimate the ongoing activity of the rSC/rVA, as the measured responses of these ROIs can also be expressed as the summation of the ongoing and the deterministic stimulus-driven component
) for that trial. In addition to nonresponding ROIs, this linear model can also be generalized to using signals of activated ROIs to estimate the ongoing activity of the rSC/rVA, as the measured responses of these ROIs can also be expressed as the summation of the ongoing and the deterministic stimulus-driven component 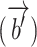 , and the vector
, and the vector 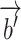 is independent of the ongoing component in these ROIs and does not contribute to BOLD variability.
is independent of the ongoing component in these ROIs and does not contribute to BOLD variability.
To examine whether the scaling factor “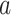 ” can be generalized across different stimulation conditions (i.e. light onset and offset), we first used ON and OFF trials separately to estimate this linear model and yielded similar results for “
” can be generalized across different stimulation conditions (i.e. light onset and offset), we first used ON and OFF trials separately to estimate this linear model and yielded similar results for “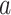 ” for a given ROI (Fig. 3 supplementary 4). Therefore, we pooled ON and OFF trials to fit this linear model, and let ON and OFF trials share the same scaler “
” for a given ROI (Fig. 3 supplementary 4). Therefore, we pooled ON and OFF trials to fit this linear model, and let ON and OFF trials share the same scaler “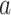 .” In addition, given that the rSC/rVA responded differently to light onset and offset (Fig. 2), we let each region have 2 stereotyped responses for light onset and offset, respectively, in our model (see Section 2 for a detailed matrix expression).
.” In addition, given that the rSC/rVA responded differently to light onset and offset (Fig. 2), we let each region have 2 stereotyped responses for light onset and offset, respectively, in our model (see Section 2 for a detailed matrix expression).
We next quantified how much cross-trial variance of the measured response in the rSC/rVA during visual stimulation can be explained by this linear model. Each trial was cut to a 0–19 s post-stimulation epoch, and thereby each trial was represented by a 20-dimension vector. The fitting performance of applying this linear model with the ongoing response derived from individual left-hemisphere ROIs for the 4-channel data during the fixed stimulation is shown in Fig. 3C. ON and OFF trials were split into 10 partitions. Each partition was used to test the performance of the 20-dimension linear model, which was estimated with the other 9 partitions. The fitting performance for each ROI was the average across 10-folds (the height of each blue bar, with each dot representing the performance of 1-fold). All left ROIs were sorted in a descending order based on the averaged variance explained. The activity of the left homologous region, lSC explained the highest amount of variance of the measured response in the rSC. Consistent results were found in the 3-channel data (Fig. 3 supplementary 3A). The fitting results for the rVA’s response provided the same pattern: the activity of the left homologous region, lVA, explained the highest amount of variance of the measured response in the rVA (Fig. 3 supplementary 3A). For the vast majority of ROIs, their activity explained over 20% of the variance of the measured response in the rSC/rVA for data from both coils.
One possible cause of the observed coactivation across ROIs could be the repetitive nature of the fixed stimulation paradigm. Certain physiological states (e.g. heartbeat, respiration, arousal levels) can be entrained by periodic stimulation, which can lead to synchronized BOLD activities across regions. However, our data from the randomized stimulation paradigm showed similar results as the fixed stimulation (Fig. 3 supplementary 3A). To further eliminate any systematic bias induced by the visual stimulation itself, we performed optogenetic stimulation to a group of animals during fMRI (opto-fMRI) with the 3-channel coil, activating the right sSC or the SCid. To avoid the confounding effect of light leakage from the head implant, the animal’s eyes were covered during optogenetic stimulations. Stimulating sSC induced a spatial responsive pattern mainly localized within the unilateral SC, entorhinal cortex, frontal region, whereas stimulating SCid induced a spatial responsive pattern that was more bilateral, also heavily involving the motor, auditory systems (Fig. 3 supplementary 5A). We repeated the linear model fitting procedure using the opto-fMRI data with sSC stimulation (n = 2) to estimate the measured response in the rSC with the ROIs from the rest of the brain. The fitting performance was comparable to, if not higher than what we obtained from the visual stimulation data (Fig. 3 supplementary 3B). These results together suggest that the brain-wide ongoing activity, which explained a significant amount of cross-trial variance in the measured responses, was not entrained by the fixed visual stimulation paradigm. Notably, although we confirmed that the ongoing activity did not result from systematic bias induced by the visual stimulation itself, this synchronized ongoing activity could still have a physiological origin. All in all, these results suggest that the ongoing BOLD activity is synchronized brain-wide, irrelevant to external stimulations, and contributes to a large amount of variance of the measured responses in excited regions.
Removing ongoing activity reduced the cross-trial variability of the BOLD response
Since the stimulation-irrelevant ongoing activity contributed to a large amount of variance of the observed BOLD responses during visual stimulation, it is conceivable that removing this ongoing activity can increase the reliability of measured BOLD responses across trials. To examine this notion, we next quantified the reduction of variance in the measured response of the rSC/rVA explained by the activity of each ROI after removing this brain-wide shared ongoing component. To identify brain voxels that showed the least stimulation evoked response (i.e. “nonresponding voxels”), we ran a LME model on the responses of brain-wide voxels to the ON–OFF stimulation paradigm. We set P > 0.3 (no correction) on the group-level LME as the threshold for nonresponding voxels. Because the BOLD signal of voxels along the brain boundary had low signal-to-noise ratio, the voxels identified above were further masked with a brain mask, which removed voxels at the brain boundary. This step can also eliminate the potential contamination of the BOLD signal from large superficial veins. This process yielded 897 nonresponding voxels for the 3-channel data and 536 nonresponding voxels for the 4-channel data (Fig. 3 supplementary 7). The shared ongoing activity across the brain was removed by regressing out the mean activity of nonresponding voxels from each voxels’ BOLD signal. We next repeated the linear fitting process on the resulting brain responses. The variances explained were considerably reduced across all ROIs (Fig. 3C, green bars), while the left homologous region (lSC) still explained the highest amount of variance of the rSC’s responses. The same effect was shown in the rVA’s responses, as well as the 3-channel data (Fig. 3 supplementary 3).
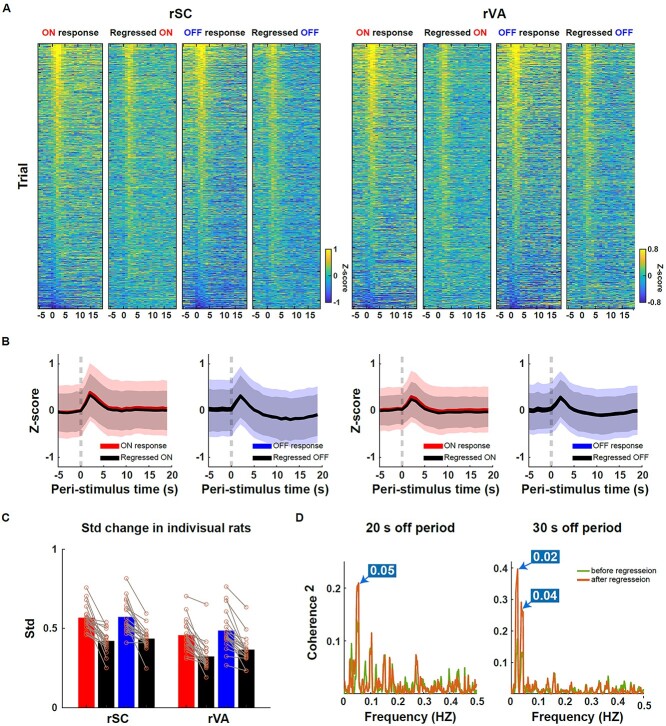
Figure 4A shows trial-by-trial, ON and OFF responses of rSC and rVA, before and after regressing out the mean activity of nonresponding voxels. Trials were sorted in a descending order based on the mean amplitude of 1–3 s post-stimulation BOLD signal before regression. Trials from both 3- and 4-channel data, fixed and randomized stimulation paradigms were included in this analysis. After the regression, extremely positive and negative responses were reduced. Ongoing activity removal uniformly reduced the trial-by-trial variability at different time points of the entire trial (−5 to 19 s), while the peak amplitude of the evoked response remained consistent before and after regression (Fig. 4B, Fig. 3 supplementary 5B). When averaging across time points, ongoing activity removal reduced 28%, 26%, 32%, and 27% of the variance for rSC ON trials, rSC OFF trials, rVA ON trials, and rVA OFF trials, respectively. The changed variance from individual animals is shown in Fig. 4C.
Fig. 4.
Ongoing response regression increased the reliability of the measured BOLD response. A) The trial-by-trial ON and OFF responses in the rSC (left)/rVA (right) before and after regressing out the ongoing activity. Trials were sorted in a descending order according to the averaged response of 1–3 s post-stimulation transition before regression. ON: 2,254 trials; OFF: 2,238 trials. B) Averaged time courses of ON and OFF responses before and after the ongoing activity regression. Shaded area: SD. C) Variance change before and after regression averaged across the entire stimulation duration (−5 to 19 s). Each circle denotes the result from one rat. D) The BOLD signal became more coherent with the Ca2+ signal at the stimulation frequencies after the ongoing BOLD activity regression. The on duration was always 20 s, while the off duration was in either 20 s (n = 16 runs) or 30 s (n = 18 runs). The frequencies where the highest peaks of the magnitude-squared coherence occurred across the 2 paradigms well matched the visual stimulation frequencies.
Ongoing activity removal increased the coherence between measured BOLD and Ca2+ responses at the stimulation frequencies
To further test the effect of the ongoing activity removal on the measured BOLD response, we examined the magnitude-squared coherence between measured BOLD and Ca2+ responses at the stimulation frequencies before and after the regression. Coherence calculation can accommodate different impulse response functions between the Ca2+ and BOLD signals and the magnitude-squared coherence is an approximation of R2 (Drew et al. 2020). During the simultaneous fiber photometry and fMRI experiment (with the 3-channel coil), we used either a 20s-on, 20s-off or 20s-on, 30s-off visual stimulation paradigm. The Ca2+ and BOLD signals from voxels around the fiber tip were used. For 20s-on, 20s-off fMRI runs (run number = 16), ongoing activity removal increased the magnitude-squared coherence between the BOLD and Ca2+ signal at 0.05 Hz (20-s period) (P = 0.010, repeated-measure ANOVA, Fig. 4D). Similarly, for 20s-on, 30s-off runs (run number = 18), ongoing activity removal increased the magnitude-squared coherence between the BOLD and Ca2+ signal at 0.04 Hz (25-s period) (P = 0.023, repeated-measure ANOVA, Fig. 4D). The magnitude-squared coherence also increased at 0.02 Hz (50-s period), although statistically not significant (P = 0.189, repeated-measure ANOVA, Fig. 4D). This result again suggests that the ongoing component contributes to the BOLD variability and removing this component increases the reliability of the measured BOLD response.
The undershoot following evoked peak response is contributed by negative brain-wide BOLD activity
A noticeable feature for the BOLD responses in the visual regions is a prominent undershoot in the late period (10–15 s) followed the light offset (Figs 2 and 4A and B). Several mechanisms have been proposed to explain BOLD undershoots including blood stealing (i.e. “blood flowing to other regions”), decreased neuronal response (Smith et al. 2004; Devor et al. 2007; Boorman et al. 2015), and reversed neurovascular coupling (Shih et al. 2009). Since the brain-wide ongoing activity explained a large portion of the variances in the measured responses of the visual regions, we asked whether the negative ongoing activity (i.e. ongoing activity below the resting-state baseline) contributed to BOLD post-stimulation undershoot. We hypothesized that in certain trials strong negative ongoing activity can dominate over the evoked, stimulation-relevant portion of the BOLD signal.
To test this hypothesis, we first identified trials that showed unambiguous sign of BOLD responses to the stimulus, defined as “positive” and “negative” responsive trials, respectively, based on 2 metrics: (i) the mean BOLD amplitude 1–4 s post-stimulation transitions; (2) the relative area under the curve of 1–4 s post-stimulation transition. If both metrics were positive, that trial was defined as a “positive trial.” If the first metric was negative, that trial was defined as a “negative trial.” The criteria for positive trials were used to constrain both the mean response being positive and the BOLD time series moving upward after the stimulation transitions. We pooled all trials from 3- and 4-channel data, fixed and randomized stimulation paradigms, and obtained 1,221 positive trials and 493 negative trials in the rSC following stimulation onset; 1,012 positive trials and 606 negative trials in the rSC following stimulation offset; 1,113 positive trials and 559 negative trials in the rVA following stimulation onset; 998 positive trials and 653 negative trials in the rVA following stimulation offset. Figure 5A shows the averaged ON and OFF responses in the rSC and rVA (the first 2 columns), as well as the averaged ON and OFF responses from positive and negative trials (the third column). For negative trials, on average, the negative response started before the stimulation transition and was maintained until the end of those trials (Fig. 5A, the third column). However, there was a clear difference in the late phase of BOLD time series (10–15 s) between rSC ON and OFF negative trials: In the ON response, the BOLD signal nearly returned to the baseline, but it remained appreciably negative in the OFF response. In addition, the OFF positive trials (blue) showed lower BOLD responses compared to the ON positive trials (red) during the late period (10–15 s), especially in the rSC (Fig. 5A, the third column). As a result, the sustained negative response in the late OFF period (10–15 s) of negative trials could not be compensated by the positive response from other trials, which contributed to the undershoot in the averaged OFF responses.
Fig. 5.
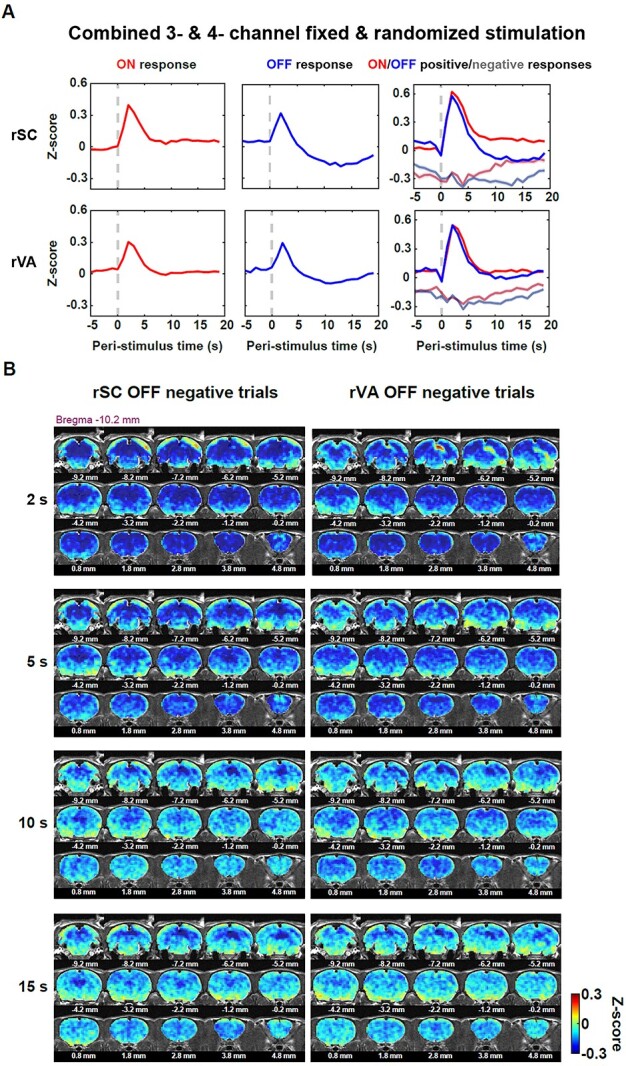
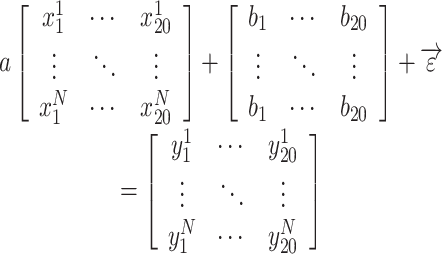
Negative trials contribute to the undershoot observed after the evoked OFF response. A) ON/OFF trials of the rSC and rVA were separated into positive and negative trials based on the BOLD response during 1–4 s post-stimulation transition. The first 2 columns: the averaged ON (red) and OFF (blue) time courses for the rSC and rVA, respectively. The third column: averaged BOLD time courses for ON positive (red)/negative (gray-red) and OFF positive (blue)/negative (gray-blue) trials for the rSC and rVA. Shaded area: SEM. B) The pattern of brain-wide BOLD activity of the rSC/rVA negative trials. Most of the regions were negative right after the stimulation offset (2 s) for both the rSC and rVA’s OFF negative trials, except that positive activation occurred around the rVA in the rSC negative trial response pattern, and positive activation occurred around the rSC in the rVA negative trial response pattern. The late negative response (10–15 s) was stronger in the rSC and rVA, than the rest of the brain.
To test whether the negative BOLD activity observed in negative trials resulted from reduced neuronal activity in these trials, we examined simultaneous calcium data and the BOLD signal from voxels around the fiber tip. The positive and negative BOLD responsive trials were identified with the same criteria (Fig. 6A, top panel, ON positive: 143 trials, ON negative: 111 trials, OFF positive: 133 trials, OFF negative: 132 trials). Interestingly, the averaged Ca2+ responses were consistent for positive and negative trials for both the stimulation onset and offset, suggesting that the negative BOLD activity in negative trials was not caused by differences in neuronal firing (Fig. 6A, bottom panel).
Fig. 6.
Consistent calcium responses for positive and negative BOLD trials. A) Evoked neuronal Ca2+ response was similar regardless of whether the BOLD response was positive or negative after stimulation transitions. The BOLD responses were separated into positive and negative responsive trials (top panel), and consistent Ca2+ response peaks were observed for positive and negative BOLD trials (bottom panel). B) The brain-wide BOLD pattern of the rSC (voxels around the fiber tip) negative trials from the data acquired by simultaneous fMRI and fiber photometry. The whole-brain BOLD activity was largely negative within 6 s post light offset. At 13 s, the negative BOLD activity was still observed around the rSC.
The whole-brain coverage of fMRI allowed us to visualize the spatial patterns of these OFF negative trials. Spatial patterns at different time points at 2, 5, 10, 15 s post-stimulation offset are shown in Fig. 5B. For both rSC and rVA OFF negative trials, the negative response that occurred right after the stimulation offset (at 2 s) was widely spread across the whole brain. The only exceptions were the positive rVA OFF response in rSC negative trials as well as the positive rSC OFF response in rVA negative trials (Fig. 5B). During 10–15 s post-stimulation offset, rSC and rVA still showed a largely negative response and the rest of the brain was in negative as well. Since the largely negative response was more localized within the visual responsive regions in the late off period (10–15 s), removing the shared ongoing component across the brain could not completely remove this undershoot, suggesting that post-stimulation BOLD undershoot also had a local source. Taken together, these data demonstrate that a sizable portion of trials showed persistent negative BOLD activity across the whole brain which lasted much longer than the evoked peak responses in the rSC despite similar neuronal firing, and the negative BOLD activity during the late phase of these trials contributed to the undershoot observed in the averaged OFF responses (Figs 5B and6B).
Discussion
To gain more insight into large cross-trial BOLD variability, here we showed that stimulation-irrelevant, ongoing BOLD activity co-fluctuated across the entire brain and is responsible for significant cross-trial BOLD variability. Regressing out the ongoing activity reduced cross-trial variability of the measured BOLD response by ~30% and increased its coherence with the underlying neuronal activity. Importantly, this method established here is generalizable to virtually all fMRI experiments and paradigms.
Mapping both ON and OFF visual responses in awake rats
To our knowledge, this is the first study mapping brain-wide BOLD responses to the light onset and offset in awake rodents. Although it has been well known that primary visual regions (e.g. VA, SC, and thalamic nuclei) responded to both light onset and offset, brain activation to light offset has been reported in very few fMRI studies in anesthetized rodents. Bailey et al. (2013) observed V1 activation induced by the light offset (at the end of flashing-train stimuli) at various flashing frequencies (1, 5, and 10 Hz), but SC offset activation only at high flashing frequencies (5 and 10 Hz) in urethane anesthetized rats. In addition, Niranjan et al. (2016) found weaker BOLD response to black flashes on a bright background (equivalent to light offset) in the V1 relative to the response to bright flashes on a dark background (equivalent to light onset), and almost no detectable response to the light offset in the sSC and dorsal lateral geniculate nucleus of the thalamus (LGd) in isoflurane and medetomidine anesthetized mice. Notably, our data showed a more robust OFF response compared to these previous studies, likely due to the sustained stimulation paradigm we used. Because of the refractory period and neuroadaptation, the retinal ganglion cells and downstream neurons cannot follow extremely fast stimulation (Lee et al. 2020). As a result, a sustained stimulation paradigm might be more suitable to measure the onset and offset responsive features in the BOLD signal compared to flashing stimulation.
The establishment of robust BOLD onset and offset activations has important implication in the design and interpretation to rodent fMRI data involving visual stimulation, as the BOLD response to stimulus offset has been often overlooked. In visual fMRI studies, on–off or checkboard flashing stimuli were widely used across species. A common approach to extract activated brain voxels is to apply a general linear model (GLM) using the stimulation design matrix as a predictor to estimate the observed BOLD signal. According to neuronal recordings, on and off pathways have asymmetric transfer speeds in the first retinal synapse owing to ionotropic and metabolic glutamate receptors that respectively operate in the on- and off-bipolar cells (Nakajima et al. 1993; Yang 2004; Burkhardt 2011). Furthermore, on/off retinal ganglion cells and downstream neurons typically have varied response delays and firing rates to the stimulus onset and offset within the receptive field (Chichilnisky and Kalmar 2002; van Wyk et al. 2009; Yeh et al. 2009; Jin et al. 2011; Ravi et al. 2018; Williams et al. 2021). Theoretically, the conventional GLM is an acceptable approach if the stimulus flashing frequency is higher than the fMRI volume collection frequency (1/TR), because each collected volume sampled the overall effect from both stimulation onset and offset. Otherwise, as the recorded BOLD signal is a mixture of responses to the 2 stimulation phases (i.e. light onset and light offset), the way they are mixed and interacted may confound the interpretation of the BOLD spatiotemporal responsive features. This notion is consistent with previous reports that the evoked BOLD amplitude (Bailey et al. 2013; Niranjan et al. 2016), polarity (positive vs. negative) (Niranjan et al. 2016), and linearity (Liu et al. 2010) were tuned by the stimulation frequency.
Our BOLD correlation maps indicate that frontal regions (Cg, OFC, and other frontal areas) responded to the stimulation onset. In mice, anatomically the visual cortex has a reciprocal connection with the anterior cingulate area (ACA) (Zhang et al. 2016). Functionally, high-density neuronal recordings showed that frontal regions (ACA, medial orbital cortex, prelimbic cortex) responded to the visual stimulation onset (Steinmetz et al. 2019), consistent with the BOLD activation in frontal regions observed in our study. Notably, compared to the response in the rVA and rSC, the Cg BOLD time course showed sustained activation after the light onset and remained positive during the on period. Previous studies in mice showed that top-down projections from the Cg to V1 (Zhang et al. 2014), SC and pulvinar (Hu et al. 2019) modulate visual processing in the V1 and animal’s behavior. In rats, the sustained activity of the ACA correlates with sustained attention (Wu et al. 2017). It is possible that the light on condition increased the animal’s arousal (or attention) level during the on period, which caused the sustained activation in the BOLD signal of the Cg.
Brain-wide ongoing activity explains significant cross-trial variance of measured BOLD responses
Both neuronal and BOLD responses to sensory stimulation can be modeled as a linear summation of the ongoing and task-relevant response (Arieli et al. 1996; Fox et al. 2006, 2007; Shimaoka et al. 2019), which suggests a linear summation can be a generalized rule for the interaction between the ongoing and task-relevant response across signal modalities. To quantitatively characterize how brain-wide ongoing BOLD activity affects the measured BOLD sensory response, we first established that between-region BOLD signals across the brain were highly correlated at zero time lag, suggesting that the ongoing activity during stimulation co-fluctuates across the brain at the awake state. This result is consistent with early studies reporting globally coherent BOLD fluctuations in an anesthesia depth-dependent manner in the rat brain (Liu et al. 2011, 2013). This finding also agrees with the human fMRI study that the evoked sensory responses are correlated with the signal of the whole-brain gray matter (Mayhew et al. 2016). This brain-wide BOLD signal co-fluctuations are not caused by the fixed periodic stimulation or the light stimulation per se, though the underlying mechanisms can still be physiological such as that discussed in the global brain signal (Liu et al. 2017; Murphy and Fox 2017). To further examine how this shared ongoing signal affects cross-trial variability of measured BOLD responses, we designed a high-dimensional linear model to estimate the measured response in the rSC/rVA with the ongoing activity derived from both stimulation-responding and nonresponding regions. Signals of most brain regions explained >20% of the variance of the measured BOLD response. This result is consistent with previous work showing that the ongoing activity estimated from the contralateral homologous region explained a large amount of cross-trial variability in the measured sensory response for both the neuronal and BOLD signals and that ongoing hemodynamics signals additively interacts with sensory-evoked components (Fox et al. 2006; Winder et al. 2017; Shimaoka et al. 2019). However, our study goes beyond the previous finding and provides a comprehensive view of the spatiotemporal characteristics of ongoing brain activity and how it impacts evoked BOLD responses.
It has to be noted that cross-trial variability is not necessarily nuisance noise. Previous theoretical studies have indicated that cross-trial variability in the neuronal response affects sensory information coding (Knill and Pouget 2004; Ma et al. 2006) and perception (Haefner et al. 2016). Human fMRI studies showed that subjects’ behaviors are correlated with the cross-trial variability (Fox et al. 2007; He 2013). Understanding the specific mechanism and functional roles of ongoing activity goes beyond the scope of this study. However, as it is irrelevant to the stimulation paradigm, regressing it out will improve the detection sensitivity of task fMRI. Indeed, brain-wide ongoing activity removal considerably decreased cross-trial variability in the measured BOLD response by ~30% and increased its coherence with the measured Ca2+ signal recorded simultaneously at the stimulation frequencies. More importantly, this method can be applied to all other fMRI experiments without needing to adjust stimulation paradigms. Responses of the left homologous region still explained the highest amount of variance of the response in the rSC/rVA after regression, suggesting that the ongoing activity is more coherent within the same sensory network (Fox et al. 2006; Mayhew et al. 2016), which well agrees with neuronal recording data (Shimaoka et al. 2019). The functional role of this more coherent fluctuation within the same neuroanatomical system requires further exploration.
Undershoot following the peak response of the BOLD signal in the off period is partially contributed by negative brain-wide BOLD activity
After separating the BOLD response into “positive” and “negative trials,” our data showed that a number of negative trials, which initiated with the brain-wide negative responses and were sustained during the late off period, partially contributed to the undershoot. Consistently, after removing these negative OFF trials, the undershoot in the late off period was largely reduced in both the rSC and rVA responses (Fig. 5 supplementary 1). Simultaneous fMRI and fiber photometry recordings showed that the neuronal firing to visual stimulation remained consistent across the positive and negative trials. This result suggests that in rSC OFF negative trials, the BOLD signal did not faithfully reflect the underlying neuronal firing, as the measured neuronal spiking activity was positive, but the measured BOLD signal was negative, which could indicate a possible neurovascular decoupling. The discrepancy between neuronal and BOLD signals might result from animals’ behavioral states such as sleep, which can differentially modulate the cortical neuronal and hemodynamic fluctuations (Turner et al. 2020). A recent study in mice reveals that during NREM and REM sleep, the total hemoglobin change and arteriole diameters increased 2–5 times compared to the awake resting state, while the arteriole constricted rapidly when the animal wakes up (Fig. 4 in Turner et al. 2020). This fast arteriole constriction (within a few seconds) can cause a decrease of the total hemoglobin (Fig. 1—figure supplement 8 in Turner et al. 2020), which can lead to a prolonged negative BOLD signal. However, to further understand the potential linkage of the brain-wide negative BOLD activity and animals’ behavioral states, physiological parameters (e.g. pupil size, heartbeats, respiration, facial motion, EMG, and hippocampal/cortical LFPs) should be measured in future fMRI studies, so the BOLD signal can be processed within well-defined behavioral states. In addition to the behavioral state, other non-neuronal factors in physiological signals (e.g. respiration, cardiac rate, vasomotion) could contribute to the difference in neuronal and BOLD responses. Lastly, although we found brain-wide negative BOLD signal partially contributed to the post-stimulation undershoot, there is also a local component that contributed to this undershoot (Uhlirova et al. 2016), given that regressing out the global ongoing component from nonresponding voxels cannot eliminate the undershoot.
Implications to other task-fMRI studies
Without losing its generality, the method in the present study can be applied to virtually all task-fMRI experiments without changing stimulation paradigms. For a given fMRI paradigm, the “ongoing activity” can be conveniently extracted from nonresponding brain regions, defined by model fitting approaches in the corresponding hypothesis-based analysis, and this activity can be regressed out to improve the reliability of evoked BOLD responses.
Some model-based denoising methods can also help reduce the impact of ongoing activity on cross-trial variability. For instance, the RETROICOR method (Glover et al. 2000) models the BOLD response based on monitored physiological parameters (e.g. heartbeat, respiration) and removes non-neuronal physiological components from the measured BOLD signal. When the measurement of different physiological parameters is not available, regressing out the signals from the WM and ventricles can also potentially reduce the impact of the ongoing activity (particularly the non-neuronal components), although it needs to be cautious that the regressors should not be contaminated by the partial volume effect from nearby brain areas, particularly when these brain areas are activated by the fMRI paradigm. At last, in addition to regressing out the mean BOLD activity of nonresponding voxels, removing separate components derived from the signals of nonresponding voxels like the method of CompCor (Behzadi et al. 2007) could generate a more reliable BOLD response.
Supplementary Material
Contributor Information
Qingqing Zhang, Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA 16802, United States; Center for Neural Engineering, The Pennsylvania State University, University Park, PA 16802, United States.
Samuel R Cramer, Center for Neural Engineering, The Pennsylvania State University, University Park, PA 16802, United States; The Neuroscience Graduate Program, The Huck Institutes of the Life Sciences, The Pennsylvania State University, University Park, PA 16802, United States.
Zilu Ma, Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA 16802, United States; Center for Neural Engineering, The Pennsylvania State University, University Park, PA 16802, United States.
Kevin L Turner, Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA 16802, United States; Center for Neural Engineering, The Pennsylvania State University, University Park, PA 16802, United States.
Kyle W Gheres, Center for Neural Engineering, The Pennsylvania State University, University Park, PA 16802, United States; Graduate Program in Molecular, Cellular, and Integrative Biosciences, The Pennsylvania State University, University Park, PA 16802, United States.
Yikang Liu, Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA 16802, United States; Center for Neural Engineering, The Pennsylvania State University, University Park, PA 16802, United States.
Patrick J Drew, Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA 16802, United States; Center for Neural Engineering, The Pennsylvania State University, University Park, PA 16802, United States; The Neuroscience Graduate Program, The Huck Institutes of the Life Sciences, The Pennsylvania State University, University Park, PA 16802, United States; Graduate Program in Molecular, Cellular, and Integrative Biosciences, The Pennsylvania State University, University Park, PA 16802, United States; Department of Engineering Science and Mechanics, The Pennsylvania State University, University Park, PA 16802, United States; Department of Neurosurgery, The Pennsylvania State University, Hershey, PA 17033, United States.
Nanyin Zhang, Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA 16802, United States; Center for Neural Engineering, The Pennsylvania State University, University Park, PA 16802, United States; The Neuroscience Graduate Program, The Huck Institutes of the Life Sciences, The Pennsylvania State University, University Park, PA 16802, United States; Graduate Program in Molecular, Cellular, and Integrative Biosciences, The Pennsylvania State University, University Park, PA 16802, United States.
Funding
The present study was partially supported by National Institute of Neurological Disorders and Stroke (R01NS085200), National Institute of Mental Health (RF1MH114224), and National Institute of General Medical Sciences (R01GM141792). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement. None.
Author contributions
Q. Z. & N. Z. designed the research; Q.Z. performed the imaging experiment; Q.Z. & Z.M. performed the fiber photometry experiment, S. R. C. designed the restrainers; K.L.T. & K.W.G. performed the histology experiment; Q.Z. performed data analysis; Y.L. wrote the fMRI preprocessing code; Q.Z. & N.Z. wrote the paper; Q.Z., S.R.C., P.J.D. and N.Z. edited the paper; P.J.D and N.Z. provided funding.
References
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996:273:1868–1871. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Cellular mechanisms contributing to response variability of cortical neurons in vivo. J Neurosci. 1999:19:2209–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CJ, Sanganahalli BG, Herman P, Blumenfeld H, Gjedde A, Hyder F. Analysis of time and space invariance of BOLD responses in the rat visual system. Cereb Cortex. 2013:23:210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993:30:161–173. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007:37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman L, Harris S, Bruyns-Haylett M, Kennerley A, Zheng Y, Martin C, Jones M, Redgrave P, Berwick J. Long-latency reductions in gamma power predict hemodynamic changes that underlie the negative BOLD signal. J Neurosci. 2015:35:4641–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt DA. Contrast processing by ON and OFF bipolar cells. Vis Neurosci. 2011:28:69–75. [DOI] [PubMed] [Google Scholar]
- Chen X, Tong C, Han Z, Zhang K, Bo B, Feng Y, Liang Z. Sensory evoked fMRI paradigms in awake mice. NeuroImage. 2020:204:116242. [DOI] [PubMed] [Google Scholar]
- Chichilnisky EJ, Kalmar RS. Functional asymmetries in ON and OFF ganglion cells of primate retina. J Neurosci. 2002:22:2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie IN, Wells JA, Southern P, Marina N, Kasparov S, Gourine AV, Lythgoe MF. fMRI response to blue light delivery in the naive brain: implications for combined optogenetic fMRI studies. NeuroImage. 2013:66:634–641. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009:12:1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Murphy K, Drew PJ. Rude mechanicals in brain haemodynamics: non-neural actors that influence blood flow. Philos Trans R Soc Lond Ser B Biol Sci. 2021:376:20190635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007:27:4452–4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopfel D, Zhang N. Mapping stress networks using functional magnetic resonance imaging in awake animals. Neurobiol Stress. 2018:9:251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PJ, Mateo C, Turner KL, Yu X, Kleinfeld D. Ultra-slow oscillations in fMRI and resting-state connectivity: neuronal and vascular contributions and technical confounds. Neuron. 2020:107:782–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy BA, Choy M, Chuapoco MR, Madsen M, Lee JH. MRI compatible optrodes for simultaneous LFP and optogenetic fMRI investigation of seizure-like afterdischarges. NeuroImage. 2015:123:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebitz RB, Platt ML. Neuronal activity in primate dorsal anterior cingulate cortex signals task conflict and predicts adjustments in pupil-linked arousal. Neuron. 2015:85:628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006:9:23–25. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007:56:171–184. [DOI] [PubMed] [Google Scholar]
- Gao YR, Ma Y, Zhang Q, Winder AT, Liang Z, Antinori L, Drew PJ, Zhang N. Time to wake up: studying neurovascular coupling and brain-wide circuit function in the un-anesthetized animal. NeuroImage. 2017:153:382–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000:44:162–167. [DOI] [PubMed] [Google Scholar]
- Goris RL, Movshon JA, Simoncelli EP. Partitioning neuronal variability. Nat Neurosci. 2014:17:858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefner RM, Berkes P, Fiser J. Perceptual decision-making as probabilistic inference by neural sampling. Neuron. 2016:90:649–660. [DOI] [PubMed] [Google Scholar]
- He BJ. Spontaneous and task-evoked brain activity negatively interact. J Neurosci. 2013:33:4672–4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Kamigaki T, Zhang Z, Zhang S, Dan U, Dan Y. Prefrontal corticotectal neurons enhance visual processing through the superior colliculus and Pulvinar thalamus. Neuron. 2019:104:1141–1152.e4. [DOI] [PubMed] [Google Scholar]
- Hussar C, Pasternak T. Trial-to-trial variability of the prefrontal neurons reveals the nature of their engagement in a motion discrimination task. Proc Natl Acad Sci U S A. 2010:107:21842–21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Wang Y, Lashgari R, Swadlow HA, Alonso JM. Faster thalamocortical processing for dark than light visual targets. J Neurosci. 2011:31:17471–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knill DC, Pouget A. The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci. 2004:27:712–719. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992:89:5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Tran A, Turan Z, Meister M. The sifting of visual information in the superior colliculus. elife. 2020:9:e50678. 10.7554/eLife.50678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Ma Y, Watson GDR, Zhang N. Simultaneous GCaMP6-based fiber photometry and fMRI in rats. J Neurosci Methods. 2017:289:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT. Noise contributions to the fMRI signal: an overview. NeuroImage. 2016:143:141–151. [DOI] [PubMed] [Google Scholar]
- Liu Z, Rios C, Zhang N, Yang L, Chen W, He B. Linear and nonlinear relationships between visual stimuli, EEG and BOLD fMRI signals. NeuroImage. 2010:50:1054–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu XH, Zhang Y, Chen W. Neural origin of spontaneous hemodynamic fluctuations in rats under burst-suppression anesthesia condition. Cereb Cortex. 2011:21:374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu XH, Zhang Y, Chen W. The change of functional connectivity specificity in rats under various anesthesia levels and its neural origin. Brain Topogr. 2013:26:363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Nalci A, Falahpour M. The global signal in fMRI: nuisance or information? NeuroImage. 2017:150:213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Perez PD, Ma Z, Ma Z, Dopfel D, Cramer S, Tu W, Zhang N. An open database of resting-state fMRI in awake rats. NeuroImage. 2020:220:117094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WJ, Beck JM, Latham PE, Pouget A. Bayesian inference with probabilistic population codes. Nat Neurosci. 2006:9:1432–1438. [DOI] [PubMed] [Google Scholar]
- Martianova E, Aronson S, Proulx CD. Multi-Fiber photometry to record neural activity in freely-moving animals. J Vis Exp. 2019:e60278. 10.3791/60278. [DOI] [PubMed] [Google Scholar]
- Mayhew SD, Mullinger KJ, Ostwald D, Porcaro C, Bowtell R, Bagshaw AP, Francis ST. Global signal modulation of single-trial fMRI response variability: effect on positive vs negative BOLD response relationship. NeuroImage. 2016:133:62–74. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007:55:131–141. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009:63:879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Fox MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. NeuroImage. 2017:154:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993:268:11868–11873. [PubMed] [Google Scholar]
- Ni AM, Ruff DA, Alberts JJ, Symmonds J, Cohen MR. Learning and attention reveal a general relationship between population activity and behavior. Science. 2018:359:463–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan A, Christie IN, Solomon SG, Wells JA, Lythgoe MF. fMRI mapping of the visual system in the mouse brain with interleaved snapshot GE-EPI. NeuroImage. 2016:139:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992:89:5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014:84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj D, Anderson AW, Gore JC. Respiratory effects in human functional magnetic resonance imaging due to bulk susceptibility changes. Phys Med Biol. 2001:46:3331–3340. [DOI] [PubMed] [Google Scholar]
- Ravi S, Ahn D, Greschner M, Chichilnisky EJ, Field GD. Pathway-specific asymmetries between ON and OFF visual signals. J Neurosci. 2018:38:9728–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Chen CC, Shyu BC, Lin ZJ, Chiang YC, Jaw FS, Chen YY, Chang C. A new scenario for negative functional magnetic resonance imaging signals: endogenous neurotransmission. J Neurosci. 2009:29:3036–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka D, Steinmetz NA, Harris KD, Carandini M. The impact of bilateral ongoing activity on evoked responses in mouse cortex. elife. 2019:8:e43533. 10.7554/eLife.43533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AT, Williams AL, Singh KD. Negative BOLD in the visual cortex: evidence against blood stealing. Hum Brain Mapp. 2004:21:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NA, Zatka-Haas P, Carandini M, Harris KD. Distributed coding of choice, action and engagement across the mouse brain. Nature. 2019:576:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Yao JF, Chen JJ, Frederick BD. The resting-state fMRI arterial signal predicts differential blood transit time through the brain. J Cereb Blood Flow Metab. 2019:39:1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KL, Gheres KW, Proctor EA, Drew PJ. Neurovascular coupling and bilateral connectivity during NREM and REM sleep. elife. 2020:9:e62071. 10.7554/eLife.62071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlirova H, Kilic K, Tian P, Thunemann M, Desjardins M, Saisan PA, Sakadzic S, Ness TV, Mateo C, Cheng Q, et al. Cell type specificity of neurovascular coupling in cerebral cortex. elife. 2016:5:e14315. 10.7554/eLife.14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B, Del Rosario J, Muzzu T, Peelman K, Coletta S, Bichler EK, Speed A, Meyer-Baese L, Saleem AB, Haider B. Spatial modulation of dark versus bright stimulus responses in the mouse visual system. Curr Biol. 2021:31:4172–4179.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder AT, Echagarruga C, Zhang Q, Drew PJ. Weak correlations between hemodynamic signals and ongoing neural activity during the resting state. Nat Neurosci. 2017:20:1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Deng H, Xiao X, Zuo Y, Sun J, Wang Z. Persistent neuronal activity in anterior cingulate cortex correlates with sustained attention in rats regardless of sensory modality. Sci Rep. 2017:7:43101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk M, Wassle H, Taylor WR. Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Vis Neurosci. 2009:26:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL. Characterization of receptors for glutamate and GABA in retinal neurons. Prog Neurobiol. 2004:73:127–150. [DOI] [PubMed] [Google Scholar]
- Yeh CI, Xing D, Shapley RM. "Black" responses dominate macaque primary visual cortex v1. J Neurosci. 2009:29:11753–11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZM, Chen S, Liang YZ. Baseline correction using adaptive iteratively reweighted penalized least squares. Analyst. 2010:135:1138–1146. [DOI] [PubMed] [Google Scholar]
- Zhang S, Xu M, Kamigaki T, Do JPH, Chang W-C, Jenvay S, Miyamichi K, Luo L, Dan Y. Long-range and local circuits for top-down modulation of visual cortex processing. Science. 2014:345:660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xu M, Chang WC, Ma C, Hoang Do JP, Jeong D, Lei T, Fan JL, Dan Y. Organization of long-range inputs and outputs of frontal cortex for top-down control. Nat Neurosci. 2016:19:1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Roche M, Gheres KW, Chaigneau E, Kedarasetti RT, Haselden WD, Charpak S, Drew PJ. Cerebral oxygenation during locomotion is modulated by respiration. Nat Commun. 2019:10:5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.