Graphical abstract
Keywords: Pollen, Protein, Ultrasound, Hydrolysis, Freeze-thawing
Highlights
-
•
Different methods were used to disrupt cell wall for extraction of camellia bee pollen protein isolates (CBPPI).
-
•
The yield of CBPPI was affected by wall disruption methods.
-
•
The disruption methods also affected the functional properties of CBPPI.
-
•
Enzymatic hydrolysis improved the yield and functional properties of CBPPI.
-
•
Combination with ultrasound further improved the yield and functional properties.
Abstract
Camellia bee pollen protein isolates were extracted by cell wall disruption using ultrasonication, freeze-thawing, enzymatic hydrolysis, and their combinations. The effects of these methods on microstructure of cell wall, protein release, protein yield, physiochemical properties and structure of proteins were investigated. As compared with physical treatments (ultrasonication, freeze-thawing and their combination), the enzymatic hydrolysis significantly improved the yield of proteins, because it not only promoted the release of proteins from the inside of pollen, but also released proteins in pollen wall. The proteins extracted by enzymatic hydrolysis method also exhibited better solubility, emulsifying and gelation properties due to the partial hydrolysis of proteins by protease. In addition, when ultrasound was combined with freeze-thawing or enzymatic hydrolysis, it could further improve the yield of proteins and the functional properties of proteins, which was mainly related to the changes of protein structure induced by cavitation effect of ultrasound.
1. Introduction
Bee pollen has been used as traditional food or traditional medicine since ancient time. The ancient Egyptians, Greeks, Romans, Americans, Indians and Chinese recognized the health benefits of bee pollen [1]. Recently, bee pollen has once again become an important part of the functional food, due to its high nutritional profile. It has been reported that bee pollen contains a large number of nutrients, including essential amino acids, polyunsaturated fatty acids, minerals, vitamins, nucleic acids, enzymes, carotenoids, phenolics, flavonoids and anthocyanin [2], [3], [4], [5]. Therefore, it has been proven that the consumption of bee pollen has positive effects on prostate problem, cancer, immunity, cardiovascular diseases, intestinal functions and neurodegenerative diseases [6], [7], [8].
In fact, bee pollen is rich in protein and the content of protein is ranging from 15 % to 20 %, depending on botanical sources [9]. In particular, eighteen essential and non-essential amino acids were found in bee pollen. However, the researches mainly focused on the allergens and hydrolysates of proteins from bee pollen [10], [11], the physicochemical properties of which have been less noticed.
Extraction is the first crucial step for the protein isolation from bee pollen. Unfortunately, the protein recovery may be restricted due to three domains in the pollen wall. The outer layer (known as exine) is mostly composed of sporopollenin, which is very resistant to acid, alkali and corrosion [12]. The inner layer (known as intine) is similar to a plant cell wall in chemical compositions [13]. In addition, the domain extending from exine to intine was known as pollen coat. These three domains with complex structure can protect the intracellular compounds of pollen from releasing. In general, physical, chemical and biological methods have been used to break the cell of bee pollen [5], [14]. Compared to single method used, combination methods have more effects on breaking the cell wall of the pollen. For example, enzymatic hydrolysis and sonication treatment were combined to both disintegrate intine and crack the pollen exine, rupturing bee pollen into fragments [15]. However, the effects of breaking methods on the physicochemical properties of pollen proteins still remains unclear.
Camellia is a traditional oil plant species in China and the camellia oil is also known as four largest woody plant oils in the world due to high content of unsaturated fatty acids (>80 %) [16]. Therefore, it has been widely cultivated in the southern part of China [17], which could be available for production of camellia bee pollen. At present, camellia pollen has become one of the most important bee pollens that are extensively consumed in China [6] and its price is about 5 $ per kilogram. However, little information has been provided about the physicochemical properties of proteins in camellia bee pollen. The aim of this study is to evaluate the effects of wall breaking technologies on physicochemical properties and structure of bee pollen proteins. Physical methods (including ultrasonication and freeze-thawing treatment), biological methods (enzymatic hydrolysis) and their combination were used to disrupt the cell wall of camellia bee pollen. The wall breaking technologies on yield, particle size, secondary and tertiary structure, solubility, emulsifying properties, digestibility and rheological properties of proteins were investigated. Our study could provide theoretical foundation and technical support for the preparation of novel proteins from bee pollen and its application in food and pharmaceutical industry.
2. Materials and methods
2.1. Materials and chemicals
Camellia bee pollen was purchased from local supermarket (Nanjing, China). The 1, 8-anilinonaphthalenesulfonate (ANS) was purchased from Sigma (St. Louis, MO, USA). The polyacrylamide gel and pre-stained protein marker were purchased from Guge Biotechnology Co., ltd (Wuhan, China). Other reagents were purchased from Sinopharm Chemical Reagent Co., ltd (Shanghai, China).
2.2. Cell wall disruption of pollen
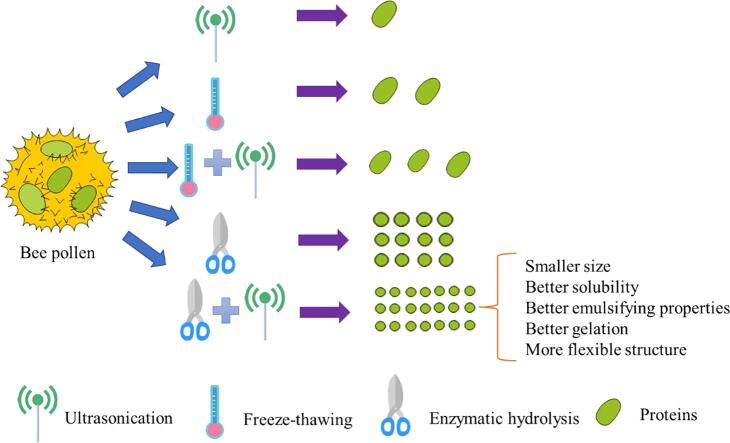
The procedure for cell wall disruption was provided in Fig. 1 and the details for every step and each method were described as follows. The bee pollen was grounded with a grinder (Breville, BCG 300, Australia) for 2 mins and passed through a 150-mesh sieve. Then 100 g grounded pollen was dispersed in 1200 mL petroleum ether for oil extraction. Ultrasound wall-broken (U): 10 g defatted pollen was added into 120 mL distilled water and the dispersion was treated at 400 W for 20 min by ultrasound equipment (NingBo Scientz Biotechnology Co. ltd., China) at 20 kHz using a 0.636 cm diameter titanium probe. Ultrasound energy was calculated according to previous study [18] and ultrasound treatment at power output of 400 W for 20 min had the intensity of 103–106 (W.cm−2). Temperature change (freeze-thawing) wall-broken (T): 10 g defatted pollen was stored at −20 °C for 24 h and then was added into 120 mL water (80 °C), then the solution was kept at 45 °C for 7 h. Enzyme hydrolysis wall-broken (E): 10 g defatted pollen was added into 120 mL distilled water and pH was adjusted to 4.0, and then hydrolyzed by 0.12 g enzymes (cellulase: pectinase: xylanase: papain, 4:2:1:3) at 45 °C for 6 h. The combined temperature change and ultrasound wall-broken (T + U): defatted pollen was first treated by temperature change, and then by ultrasound. The combined enzyme hydrolysis and ultrasound wall-broken (E + U): defatted pollen was first treated by enzyme hydrolysis, and then by ultrasound.
Fig. 1.
The procedure for cell wall disruption of bee pollen.
2.3. Preparation of camellia bee pollen protein isolates (CBPPI)
The defatted pollen after wall-broken treatment was dispersed in 380 mL distilled water and the pH was adjusted to 10.0 with stirring for 80 min, and centrifuged at 2950g for 10 min. The supernatant was then adjusted to 4.0 for protein precipitation and centrifuged at 2950g for 10 min. The pellet was dispersed in distilled water and neutralized to pH 7.0, and then dried using FD-2B freezing dryer (Beijing Boyikang Experimental Instrument Co. ltd., China). The defatted pollen without wall-broken treatment was also prepared with the above method and labeled as control.
2.4. Microstructure of pollen after wall-broken treatment
The morphology of pollen after wall-broken treatment was acquired using a scanning electron microscope (SEM) system (ZEISS, Germany). The pollen was freezing-dried and sputter coated with gold–palladium before acquiring the SEM micrographs. The observation was performed at 20 kV acceleration voltage under vacuum (1.0 × 10-3 Pa).
2.5. Release of CBPPI after wall-broken treatment
The proteins release from pollen was observed using a fluorescence microscope (DM2500, Leica, Germany). Fluorescein isothiocyanate of 100 μL (0.1 mg/mL in dimethyl sulfoxide) was added into 10 mL pollen dispersion to identify the proteins.
2.6. Yield of CBPPI
The protein extraction yield was calculated according to following equation.
2.7. Physiochemical properties of CBPPI
The 1 g lyophilized proteins were dispersed in 99 mL distilled water and the particle size was measured by dynamic light scattering (Malvern Instruments ltd., UK). The 2 g lyophilized proteins were dispersed in 98 mL distilled water and the pH values were adjusted from 2 to 10 by using 1 N HCl or 1 N NaOH. The protein dispersion was centrifuged at 12,000×g for 30 min at 4 °C. The proteins content in the supernatant was measured by Lowry method [19] and the bovine serum albumin was used as standard. The 80 mL protein dispersion (2 mg/mL) was mixed with 20 mL corn oil and homogenized at 20,000 rpm for 1 min. The 50 μL emulsion was taken from the bottom before and after 10 mins and diluted with 5 mL sodium dodecyl sulfate solution. The absorbance was measured at 500 nm to calculated emulsifying activity index (EAI) and emulsion stability index (ESI) according to previous study [20].The interfacial tension between protein solution (1 %, m/v) and the oil phase was measured based on the Due Nouy ring method using tensiometer (DSA25, KRUSS, Germany) according to previous study [21]. The lyophilized proteins were dispersed in phosphate buffer (10 mM. pH 7.0) to abstain the concentration of 0.05–2.0 mg/mL. The ANS (8.0 mM) was added into the dispersion and fluorescence intensity was recorded at 390 nm (excitation) and 470 nm (emission). The surface hydrophobicity of proteins was calculated from the slope of fluorescence intensity versus protein concentration plot according to previous study [22]. In vitro simulation of gastrointestinal digestion was performed by incubating protein with gastric and intestinal juice, respectively [23]. In brief, proteins were treated with gastric fluid at 1:20 (w/v) for 4 h and then the pH was adjusted to 6.8. The pancreatin was added to mimic intestinal fluid and the mixture was hydrolyzed for 6 h. The hydrolysates were collected and freeze-dried. The digestibility of protein was calculated by determining the release of free amino groups [24]. The temperature sweep measurements of proteins (10 %, m/v) were performed with a rheometer (HR-1, TA Instruments, Leatherhead, UK). The protein solutions were heated from 25 to 85 °C at a heating rate of 10 °C/min, kept at 85 °C for 15 min and cooled to 25 °C at the same rate as of heating. A constant frequency of 1 Hz and strain of 0.05 % were applied during these tests.
2.8. Structure of CBPPI
The proteins were scanned using IS5 spectroscope (Thermal Fisher Scientific, USA) to obtain Fourier transform infrared spectra (FTIR) in the wavenumber range of 500–4000 cm −1. In order to obtain the intrinsic tryptophan (Trp) fluorescence, protein solution (1 mg/mL) were excited at 285 nm and emission spectra were recorded from 300 to 600 nm by using Spark 10 M spectrophotometer (Tecan, Switzerland) [25]. The measurement for secondary structure of proteins samples (0.2 mg/mL) were performed using a Mos-450CD spectropolarimeter (Biologic, Claix, France) [26] and secondary structure was calculated by computer program CDPro (http://lamar.colostate.edu/sreeram/CDPro/main.html). For submits analysis, the sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was carried out on a Bio-rad Mini-Protein Tetra Electrophoresis System (Hercules, USA) with a 12 % separating gel and a 5 % stacking gel according to previous study [27]. Freshly prepared loading buffer with 4 mg/mL protein content was used in SDS-PAGE test.
2.9. Statistical analysis
All of the tests mentioned above were performed in triplicate and the data obtained were analyzed by one-way analysis of variance using SPSS for Windows version 17.0 (IBM, USA). Values are expressed as mean ± standard deviation. Duncan’s multiple range test was used to identify significant difference between two mean values at 95 % confidence level (p < 0.05).
3. Results and discussion
3.1. Microstructure of pollen after wall-broken treatment
According to Fig. 2, the control sample without wall-broken treatment exhibited entirely cell wall with smooth spheroidal shape. After ultrasound treatment, the pollen showed a crack (as marked by red rectangle) in the exine with roughly spheroidal shape. After freeze-thawing treatment, the pollen showed morphological change that the gap appeared in the inner layer. After treated with enzymatic hydrolysis, both the exine and the intine of pollen was entirely disrupted and pollen was completely unfolded. Under combination of freeze-thawing and ultrasound treatment, the pollen was broken into two parts. Under combination of enzymatic hydrolysis and ultrasound treatment, the pollen wall was broken into fragments. Our result indicated that the method of enzymatic hydrolysis seemed to be more effective for disruption of cell wall as compared with physical methods (ultrasonication, freeze-thawing and their combination). This can be attributed to the decompositions of pollen cell wall. The pollen wall is mainly composed of cellulose, pectin, proteins and lipids [28]. When pollen was treated with enzymes, the polysaccharides and protein in exine and intine were hydrolyzed, which eventually led to the disintegration of pollen wall. This technology is also widely used for disruption of plant cell wall in order to improve extraction efficiency of bioactive compounds [29]. Furthermore, with the assistant of ultrasound, the disruption of pollen wall was reinforced. This can be attributed to the cavitation phenomenon created by ultrasound, which could lead to breakage of cell wall by mechanisms of fragmentation, erosion and detexturation [30].
Fig. 2.
Scanning electron micrographs of pollen sample under different treatment. U: pollen sample treated with ultrasonication at 400 W for 20 min; T: pollen sample treated with freeze (−20 °C for 24 h) and thawing (45 °C for 7 h); E: pollen sample treated with enzymatic hydrolysis at 45 °C for 6 h; T + U: pollen sample treated with combination of freeze-thawing and ultrasonication; E + U: pollen sample treated with combination of enzymatic hydrolysis and ultrasonication.
3.2. Release of CBPPI from pollen after wall-broken treatment
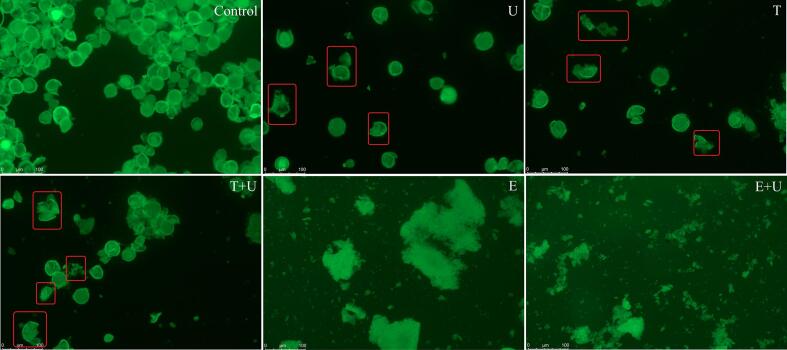
According to Fig. 3, the proteins mainly existed on the pollen cell wall and inside the pollen. For the untreated sample, the pollen aggregated with each other and this phenomenon disappeared after wall-broken treatment. After ultrasound, freeze-thawing or combination of freeze-thawing and ultrasound treatment, the pollen presented a morphological change that some pollen tried to crack (as marked by red rectangle), which led to release of proteins from pollen. Under enzymatic hydrolysis treatment, most of pollen tended to be degraded and the background turned to green, which indicated that proteins began to dissolve into the solution. Furthermore, the pollen was cracked into smaller fragments after combination of enzymatic hydrolysis and ultrasonication treatment. This could be attributed to the shearing force of ultrasound causing attrition in mass and break the aggregates into smaller particles [31]. The results were consistent with the observation in SEM that the release of proteins from pollen increased with the destruction of pollen wall.
Fig. 3.
Release of proteins from pollen sample under different treatment. U: pollen sample treated with ultrasonication at 400 W for 20 min; T: pollen sample treated with freeze (−20 °C for 24 h) and thawing (45 °C for 7 h); E: pollen sample treated with enzymatic hydrolysis at 45 °C for 6 h; T + U: pollen sample treated with combination of freeze-thawing and ultrasonication; E + U: pollen sample treated with combination of enzymatic hydrolysis and ultrasonication.
3.3. Yield of CBPPI
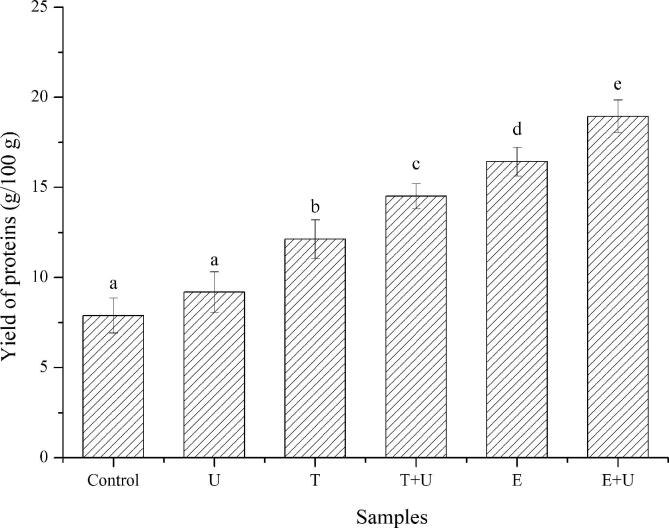
According to Fig. 4, the yield of proteins from pollen was not significantly (p < 0.05) improved by ultrasonication treatment. This is probably due to the fact that most of proteins was enveloped by pollen wall and the ultrasound only break the exine of pollen, which can be proved in Fig. 1. The yield of proteins was significantly (p < 0.05) improved by freeze-thawing treatment, which could be related to the disruption of intine (as proved in Fig. 1). The formation of gap helped to release the proteins from pollen. With the assistant of ultrasonication, the yield of proteins was further improved. This could be due to the synergistic effect of freeze-thawing and ultrasound, which resulted in breaking down of pollen wall. As compared with physical treatments (ultrasonication, freeze-thawing and their combination), the yield of proteins was much higher by using enzymatic hydrolysis. This could be attributed to the application of cell wall polysaccharide-degrading enzymes to enhance protein extraction efficiency [32]. Therefore, enzymatic hydrolysis could not only promote the release of proteins inside the pollen, but also promote the release of proteins in pollen wall. In addition, the higher yield of proteins was obtained by combination of enzymatic hydrolysis and ultrasonication. This result further confirmed that the treatment assisted with ultrasound was helpful to improve the release of proteins from pollen. The improvement could be attributed to the decomposition and fragmentation of cell wall. As shown in Fig. 2 and Fig. 3, the cell wall was totally decomposed and broken into smaller particle after treated by combination of enzymatic hydrolysis and ultrasonication. Previous studies have proved that the ultrasound-assisted treatment improved the extraction efficacy of bioactive compounds from Psidium guajava leaves [33], rice bran [34], makiang seed [35], bitter melon seeds [36] and grapes [37]. The authors attributed the improvement of yield to the cavitation caused by ultrasonic waves, which led to faster transfer of mass out of the cell, increasing of solubility ratio, disruption of cell wall and increasing contact between the solvent and the bioactive compounds.
Fig. 4.
Yield of proteins from pollen sample under different treatment. U: pollen sample treated with ultrasonication at 400 W for 20 min; T: pollen sample treated with freeze (−20 °C for 24 h) and thawing (45 °C for 7 h); E: pollen sample treated with enzymatic hydrolysis at 45 °C for 6 h; T + U: pollen sample treated with combination of freeze-thawing and ultrasonication; E + U: pollen sample treated with combination of enzymatic hydrolysis and ultrasonication. Results having different letters in same pattern are significantly different (p < 0.05).
3.4. Physicochemical properties of CBPPI
3.4.1. Particle size distribution of CBPPI
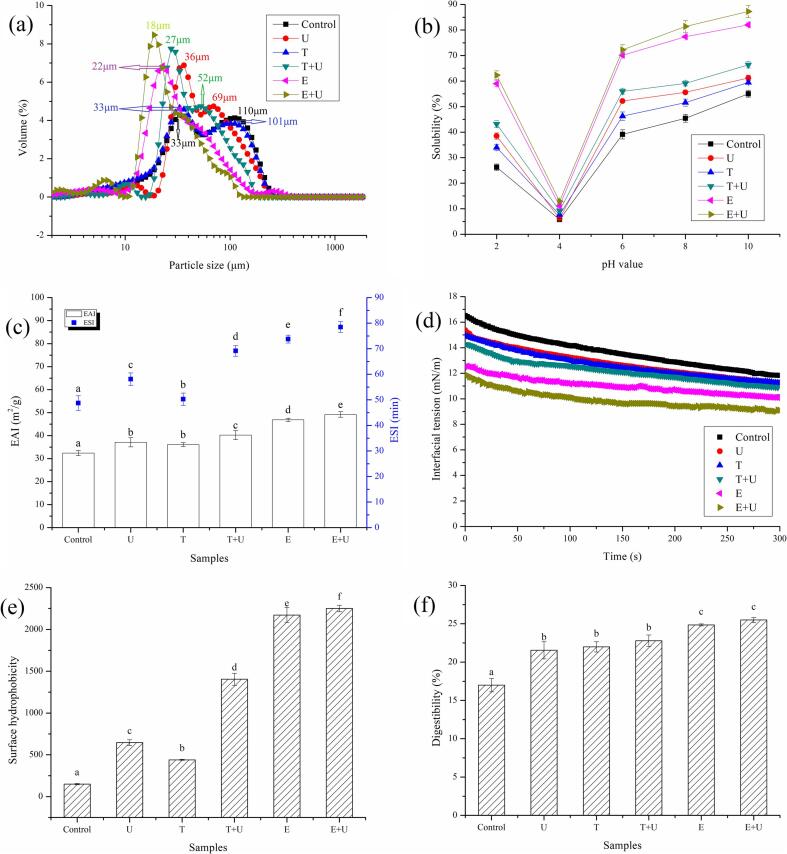
According to Fig. 5a, two major peaks at 110 μm and 33 μm were observed in control sample. The sample from freeze-thawing treatment exhibited similar pattern of size distribution with two peaks at 101 μm and 33 μm. The ultrasonication provoked a shift in the particle size distribution to lower diameters (with the peak at 69 μm and 36 μm). The reduction in particle size was also observed in sample from combination of freeze-thawing and ultrasonication (with the peak at 52 μm and 27 μm). The reduction of particle size could be attributed to the shearing forces from collapse of cavitation bubbles and high-velocity microjets during acoustic cavitation [38]. Numerous studies have also shown that ultrasound treatment led to a decrease in particle size of whey proteins [39], soy proteins [40] and lupin proteins [41]. It is obvious that a major peak at 22 μm was observed in sample from enzymatic hydrolysis treatment, indicating a shift in the particle size distribution to much lower diameters. This is probably due to the existence of protease in enzymatic hydrolysis, which not only helped to destroy the pollen wall, but also might partially hydrolyze the proteins in pollen. Earlier studies have proved that proteases treatment decreased particle size of mung bean proteins [42] and rice derived proteins [43]. In addition, the proteins from combination of enzymatic hydrolysis and ultrasonication exhibited much lower size (with the peak at 18 μm) than others. The decrease in particle size could be attributed to the synergistic effects between proteins hydrolysis induced by enzyme and the breaking down of molecular interactions induced by ultrasound.
Fig. 5.
Particle size distribution (a), solubility (b), emulsifying properties (c), interfacial tension (d), surface hydrophobicity (e) and digestibility (f) of proteins from pollen sample under different treatment. U: pollen sample treated with ultrasonication at 400 W for 20 min; T: pollen sample treated with freeze (−20 °C for 24 h) and thawing (45 °C for 7 h); E: pollen sample treated with enzymatic hydrolysis at 45 °C for 6 h; T + U: pollen sample treated with combination of freeze-thawing and ultrasonication; E + U: pollen sample treated with combination of enzymatic hydrolysis and ultrasonication. Results having different letters in same pattern are significantly different (p < 0.05).
3.4.2. Solubility of CBPPI
According to Fig. 5b, all samples showed the lowest solubility at pH 4.0, indicating that isoelectric point for CBPPI is about 4.0. As compared with control sample, all treatments could improve the solubility of proteins. The proteins from enzymatic hydrolysis possessed better solubility than those from physical treatments (ultrasonication, freeze-thawing and their combination). This is probably due to the partially hydrolysis of CBPPI induced by protease, which could break specific peptide bonds in CBPPI and resulted in smaller molecular size and less secondary structure than the native proteins [44]. Previous studies also showed that the hydrolysis could improve the solubility of faba bean proteins [45] and pumpkin seed proteins [46]. In addition, the proteins from methods assisted with ultrasonication exhibited higher solubility than those from same method without ultrasonication. Numerous studies also showed that the ultrasound treatment could improve the solubility of pea proteins [47], soy proteins [48] and black bean proteins [49]. The improvement in solubility of proteins could be attributed to the ultrasonic cavitation which led to the breaking of hydrogen bonds and hydrophobic bonds, exposure of hydrophilic groups in proteins, reducing the particle size, enhancing the contact between proteins and water [50].
3.4.3. Emulsifying properties of CBPPI
According to Fig. 5c, the samples from all treatments exhibited higher ESI and EAI than control. As compared with physical treatments (ultrasonication, freeze-thawing and their combination), the sample from enzymatic hydrolysis treatment showed higher ESI and EAI. The improvement in emulsifying properties could be attributed to the generation of more flexible amphiphilic hydrolysates and exposure of free amino groups with ability to adsorb rapidly at the oil–water interface and reduce the interfacial tension [51]. Enzymatic hydrolysis has also been reported to enhance the emulsifying properties of rice bran proteins [52] and soy proteins [53]. In addition, the proteins from methods assisted with ultrasonication exhibited better emulsifying properties than those from same method without ultrasonication. The improvement in emulsifying properties could be attributed to acoustic streaming and high-energy shear waves generated by ultrasound, which led to conformational changes of protein molecules and exposure of hydrophobic groups of proteins [54]. Ultrasonication has also been used to improve the emulsifying properties of pea proteins [55] and soybean proteins [54].
3.4.4. Interfacial tension of CBPPI
According to Fig. 5d, the samples from all treatments exhibited lower interfacial tension than control, which helped to explain why proteins from all treatments showed better emulsifying properties than control. As compared with physical treatments (ultrasonication, freeze-thawing and their combination), the proteins from enzymatic hydrolysis treatment showed lower interfacial tension. The decrease in interfacial tension could be attributed to the faster diffusion of hydrolysates to the interface due to the reduction of size and more exposure of hydrophobic groups in hydrolysates due to the conformational changes [56]. In addition, the proteins from methods assisted with ultrasonication exhibited lower interfacial tension than those from same method without ultrasonication. The decrease in interfacial tension could be attributed to the unfolding of compact structure, higher molecular flexibility and surface hydrophobicity, smaller particle size induced by strong shear force of ultrasound, which helped proteins to adsorb rapidly at the oil–water interface and subsequently reduce the interfacial tension [21]. Previous studies suggested that ultrasonication has also been used to reduce the interfacial tension of grass pea proteins [21] and sodium caseinate [57].
3.4.5. Surface hydrophobicity of CBPPI
According to Fig. 5e, surface hydrophobicity of CBPPI was significantly (p < 0.05) affected by extraction methods and the samples from all treatments possessed higher surface hydrophobicity than control. The higher surface hydrophobicity could decrease the time needed to absorb at the interface, which helped to explain why those samples showed lower interfacial tension. As compared with physical treatments (ultrasonication, freeze-thawing and their combination), the proteins from enzymatic hydrolysis treatment showed much higher surface hydrophobicity. This is probably due to the partial hydrolysis of proteins induced by protease, which led to the exposure of non-polar amino acids [58]. As expected, the proteins from methods assisted with ultrasonication exhibited higher surface hydrophobicity than those from same method without ultrasonication. The increase in surface hydrophobicity helped to explain why those proteins exhibited better emulsifying properties.
3.4.6. Digestibility of CBPPI
According to Fig. 5f, the proteins from physical treatments (ultrasonication, freeze-thawing and their combination) exhibited higher digestibility than control. This could be attributed to the partial denaturation of proteins induce by freeze-thawing or ultrasonication, which led to the increase of pepsin and trypsin accessibility of CBPPI. Furthermore, the digestibility of CBPPI was further improved when enzymatic hydrolysis was applied to disrupt the pollen wall. This result also confirmed that enzymatic hydrolysis led to exposure of more groups and regions which could support the pepsin and trypsin hydrolysis.
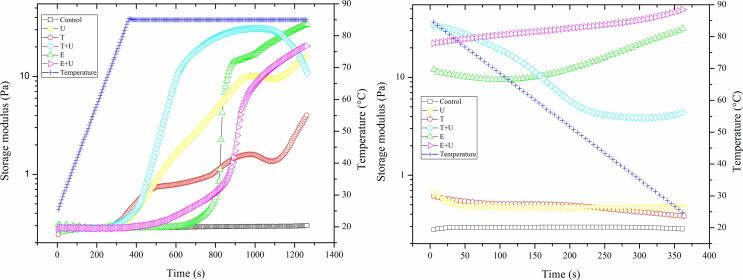
3.4.7. Rheological properties of CBPPI
According to Fig. 6, no obvious changes were observed on the G′ values of control sample during the heating–cooling cycle, which indicated that heating treatment cannot induce the unfolding of CBPPI and the devolvement of intermolecular network among molecules. In contrast, samples from the all treatments showed an increase in G′, when heated to 85 °C, suggesting the partial denaturation of CBPPI induced by ultrasound, freeze-thawing, enzymatic or their combination treatment. However, subsequent cooling to 25 °C led to decrease of G′ in proteins from physical treatments (ultrasonication, freeze-thawing and their combination), which indicated that the intermolecular network was weaken and reversible. The proteins from enzymatic hydrolysis exhibited an increase in G′, when cooled to 25 °C, suggesting the formation of a gelled structure. This could be attributed to the partial hydrolysis induced by protease. Previous study has proved that limited enzymatic hydrolysis could induce the gelation of proteins with lower concentration and temperature due to the promotion of more cross-links than native proteins [59]. Furthermore, the proteins from combination of enzymatic hydrolysis and ultrasonication exhibited higher G′ after heating–cooling cycle than those from enzymatic hydrolysis treatment, indicating the synergistic effect between enzymatic and ultrasonic treatment. This could be attributed to the decrease in particle size, increase in solubility and exposure of more active groups induce by ultrasonic treatment [60].
Fig. 6.
Temperature sweep tests of proteins from pollen sample under different treatment. U: pollen sample treated with ultrasonication at 400 W for 20 min; T: pollen sample treated with freeze (−20 °C for 24 h) and thawing (45 °C for 7 h); E: pollen sample treated with enzymatic hydrolysis at 45 °C for 6 h; T + U: pollen sample treated with combination of freeze-thawing and ultrasonication; E + U: pollen sample treated with combination of enzymatic hydrolysis and ultrasonication.
3.5. Structure of CBPPI
3.5.1. Fourier transform infrared spectra (FTIR) of CBPPI
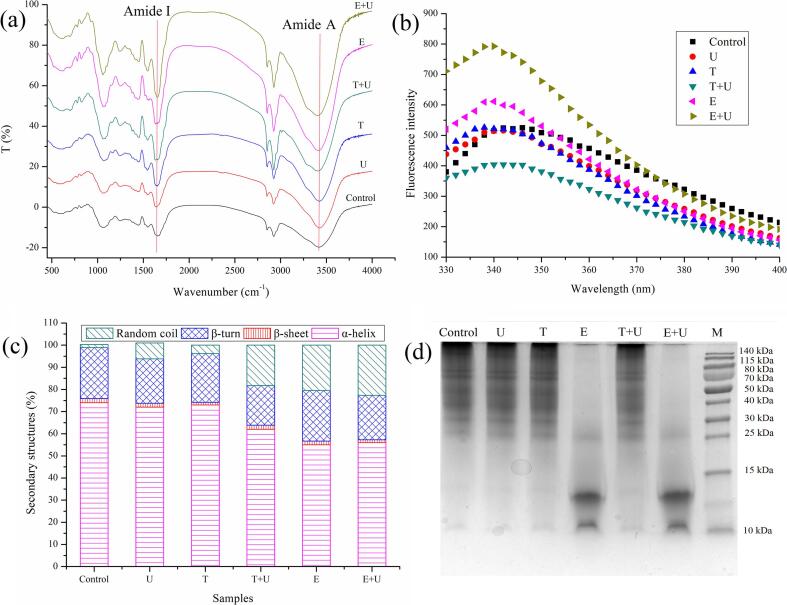
According to Fig. 7a, all treatments increased the intensity of band at 3300–3450 cm−1 (Amide A), indicating the enhancing of hydrogen bonds [61]. The enhancement could be attributed to the conformational changes of proteins, which led to exposure of hydrophilic groups. As compares with physical treatments (ultrasonication, freeze-thawing and their combination), the proteins from enzymatic hydrolysis treatment showed higher band intensity at 3300–3450 cm−1, suggesting the stronger H-bond interactions. The enhancement of H-bond interactions helped to explain why those proteins were easier to form gelled structure. Furthermore, the changes in 1600–1700 cm−1 (Amide I) were similar with Amide A, indicating that all treatments led to the changes of secondary structure. Therefore, the analysis about tertiary and secondary structure was performed as follows.
Fig. 7.
Fourier transform infrared spectra (a), emission fluorescence spectroscopy (b), secondary structure content (c) and subunits (d) of proteins from pollen sample under different treatment. U: pollen sample treated with ultrasonication at 400 W for 20 min; T: pollen sample treated with freeze (−20 °C for 24 h) and thawing (45 °C for 7 h); E: pollen sample treated with enzymatic hydrolysis at 45 °C for 6 h; T + U: pollen sample treated with combination of freeze-thawing and ultrasonication; E + U: pollen sample treated with combination of enzymatic hydrolysis and ultrasonication. M: markers.
3.5.2. Emission fluorescence spectroscopy of CBPPI
According to Fig. 7b, a blue-shifting of λmax was observed in the proteins from ultrasonication or freeze-thawing treatment as compared with control, indicating microenvironment changes of tryptophan residues. The fluorescence intensity decreased in proteins from combination of freeze-thawing and ultrasonication, indicating quenching of the indole ring in tryptophan induced by conformational disruption of combination treatment [55]. As compared with physical treatments (ultrasonication, freeze-thawing and their combination), an increasing of fluorescence intensity was observed in proteins from enzymatic hydrolysis, indicating the local tertiary structures of CBPPI has been changed. The similar study also reported that enzymatic hydrolysis increased the fluorescence intensity of rice proteins due to formation of peptides with shorter chains and increase of exposed hydrophobic groups [62]. In addition, the fluorescence intensity was further increased by combination of enzymatic hydrolysis and ultrasonication, indicating their synergistic effect led to more extended tertiary structures of CBPPI. The increase in fluorescence intensity could also be attributed to sonication effect which led to decrease in particle size [61] and burying of tryptophan residues into hydrophobicity area [63].
3.5.3. Secondary structure of CBPPI
According to Fig. 7c, it was obvious that all treatments led to an increase of random coil content, especially for the proteins from enzymatic hydrolysis which possessed much higher content of random coil. The changes in secondary structure content were consistent with the intensity changes of Amide I from FTIR result. The higher content of random coil could be attributed to the partial denaturation which resulted in more disordered structures. In addition, with the assistant of ultrasonication, the proteins exhibited higher content of random coil. This is consistent with previous study that cavitation effect caused by ultrasound led to decomposition of α-helix, which is one of main structures for protein stable and folding [22]. The unfolding of proteins might increase their interactions with water molecules or enzyme, adsorption capacity at the water/oil interface and intermolecular interactions, which helped to explain why CBPPI from ultrasound assisted method exhibited better solubility, emulsifying properties, digestibility and gelation property.
3.5.4. Subunits of CBPPI
According to Fig. 7d, the molecule weight of protein subunits from control or physical treatments (ultrasonication, freeze-thawing and their combination) were in the range of 25–140 kDa and the molecule weight of protein subunits from enzymatic treatment was in the range of 10–25 kDa. This result indicated that proteins from enzymatic treatment possessed much lower molecular weight than other treatments. Furthermore, certain amounts of polymers on the top of gel were observed in samples from control or physical treatments (ultrasonication, freeze-thawing and their combination). However, all polymers disappeared and new subunits with molecular weight between 10 and 15 kDa were observed when enzymatic hydrolysis was applied. This result confirmed that protease (papain) can not only destroy the pollen wall, but also hydrolyze the CBPPI released from pollen. The hydrolysis led to the decrease in particle size, unfolding of molecules and exposure of hydrophobic groups, which helped to improve the solubility, emulsifying properties, digestibility and gelation property of CBPPI. In addition, a few antinutrients (such as allergens, pyrrolizidine alkaloids and potentially toxic elements) were found in bee pollen, which might confer foodborne allergenicity and limit their applications [64]. However, the enzymes (such as cellulase, pectase and papain) possessed the ability to induce the degradation of allergens in bee pollen [10]. Therefore, the enzymatic hydrolysis applied in this study might also help to reduce the allergenicity of camellia bee pollen, which also needs to be confirmed by future study.
4. Conclusion
The wall disruption not only affected the release of proteins from pollen, but also affected the physiochemical properties and structure of proteins. As compared with physical wall disruption (ultrasonication, freeze-thawing and their combination), enzymatic hydrolysis could significantly improve the yield of proteins, and proteins extracted by which also exhibited better solubility, emulsifying properties, digestibility and gelation property due to the partial hydrolysis of proteins induced by protease. Furthermore, as compared with single treatment, the combination methods assisted with ultrasound significantly improved the yield of proteins, and the proteins extracted by combination methods also exhibited better functional properties due to smaller size and more flexible structure caused by ultrasound cavitation effect. In addition, it can be found that when the combination of enzymatic hydrolysis and ultrasound was used to treat bee pollen, the cell wall was completely decomposed and fragmented (as shown in Fig. 2 and Fig. 3), which indicated that this method could also help to improve the extraction rate of other bioactive compounds in bee pollen. Therefore, the future research will be carried out to study the effects of this method on yield, structure and activities of carotenoids, vitamins and lipids in bee pollen.
CRediT authorship contribution statement
Feng Xue: Writing – review & editing. Chen Li: Conceptualization, Methodology, Software, Investigation, Formal analysis, Writing – original draft, Funding acquisition, Resources, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by The Open Project of Chinese Materia Medica First-Class Discipline of Nanjing University of Chinese Medicine (2020YLXK021), Jiangsu Agriculture Science and Technology Innovation Fund (CX(21)3110).
References
- 1.Lu P., Takiguchi S., Honda Y., Lu Y., Mitsui T., Kato S., Kodera R., Furihata K., Zhang M., Okamoto K., Itoh H., Suzuki M., Kono H., Nagata K. NMR and HPLC profiling of bee pollen products from different countries. Food Chem.: Mol. Sci. 2022;5 doi: 10.1016/j.fochms.2022.100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ares A.M., Valverde S., Bernal J.L., Nozal M.J., Bernal J. Extraction and determination of bioactive compounds from bee pollen. J. Pharm. Biomed. Anal. 2018;147:110–124. doi: 10.1016/j.jpba.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Kieliszek M., Piwowarek K., Kot A.M., Błażejak S., Chlebowska-Śmigiel A., Wolska I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018;71:170–180. [Google Scholar]
- 4.Feás X., Vázquez-Tato M.P., Estevinho L., Seijas J.A., Iglesias A. Organic Bee Pollen: Botanical Origin, Nutritional Value, Bioactive Compounds, Antioxidant Activity and Microbiological Quality. Molecules. 2012;17:8359–8377. doi: 10.3390/molecules17078359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X., Sun L., Dong J., Zhang H. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innov. Food Sci. Emerg. Technol. 2009;10:42–46. [Google Scholar]
- 6.Yang Y.F., Lai X.Y., Lai G.Y., Jiang Z.D., Ni H., Chen F. Purification and characterization of a tyrosinase inhibitor from camellia pollen. J. Funct. Foods. 2016;27:140–149. [Google Scholar]
- 7.Cornara L., Biagi M., Xiao J., Burlando B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang K., Wu D., Ye X., Liu D., Chen J., Sun P. Characterization of Chemical Composition of Bee Pollen in China. J. Agric. Food Chem. 2013;61:708–718. doi: 10.1021/jf304056b. [DOI] [PubMed] [Google Scholar]
- 9.Taha E.-K.-A., Al-Kahtani S., Taha R. Protein content and amino acids composition of bee-pollens from major floral sources in Al-Ahsa, eastern Saudi Arabia. Saudi J. Biol. Sci. 2019;26:232–237. doi: 10.1016/j.sjbs.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tao Y., Yin S., Fu L., Wang M., Meng L., Li F., Xue X., Wu L., Li Q. Identification of allergens and allergen hydrolysates by proteomics and metabolomics: A comparative study of natural and enzymolytic bee pollen. Food Res. Int. 2022;158 doi: 10.1016/j.foodres.2022.111572. [DOI] [PubMed] [Google Scholar]
- 11.Maqsoudlou A., Sadeghi Mahoonak A., Mohebodini H., Koushki V. Stability and structural properties of bee pollen protein hydrolysate microencapsulated using maltodextrin and whey protein concentrate. Heliyon. 2020;6:e03731. doi: 10.1016/j.heliyon.2020.e03731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackmore S., Wortley A.H., Skvarla J.J., Rowley J.R. Pollen wall development in flowering plants. New Phytol. 2007;174:483–498. doi: 10.1111/j.1469-8137.2007.02060.x. [DOI] [PubMed] [Google Scholar]
- 13.Franchi G.G., Franchi G., Corti P., Pompella A. Microspectrophotometric evaluation of digestibility of pollen grains. Plant Foods Hum. Nutr. 1997;50:115–126. doi: 10.1007/BF02436031. [DOI] [PubMed] [Google Scholar]
- 14.Liu X.-D., Zhang F.-B., Zhou B., Shan H., Chen P.-Y. Effect of sonication on different quality parameters of Pinus massoniana pollen. Ultrason. Sonochem. 2015;22:174–181. doi: 10.1016/j.ultsonch.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Dong J., Gao K., Wang K., Xu X., Zhang H. Cell Wall Disruption of Rape Bee Pollen Treated with Combination of Protamex Hydrolysis and Ultrasonication. Food Res. Int. 2015;75:123–130. doi: 10.1016/j.foodres.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 16.Yang J., Qin L., Zhu Y., He C. The regularity of heat-induced free radicals generation and transition of camellia oil. Food Res. Int. 2022;157 doi: 10.1016/j.foodres.2022.111295. [DOI] [PubMed] [Google Scholar]
- 17.Xiang Z., Xia C., Feng S., Chen T., Zhou L., Liu L., Kong Q., Yang H., Ding C. Assessment of free and bound phenolics in the flowers and floral organs of two Camellia species flower and their antioxidant activities. Food Biosci. 2022;49 [Google Scholar]
- 18.Jambrak A.R., Lelas V., Mason T.J., Krešić G., Badanjak M. Physical properties of ultrasound treated soy proteins. J. Food Eng. 2009;93:386–393. [Google Scholar]
- 19.Xue F., Li C., Zhu X., Wang L., Pan S. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res. Int. 2013;51:490–495. [Google Scholar]
- 20.Xue F., Zhu C., Liu F., Wang S., Liu H., Li C. Effects of high-intensity ultrasound treatment on functional properties of plum (Pruni domesticae semen) seed protein isolate. J. Sci. Food Agric. 2018;98:5690–5699. doi: 10.1002/jsfa.9116. [DOI] [PubMed] [Google Scholar]
- 21.Mozafarpour R., Koocheki A., Nicolai T. Modification of grass pea protein isolate (Lathyrus sativus L.) using high intensity ultrasound treatment: Structure and functional properties. Food Res. Int. 2022;158 doi: 10.1016/j.foodres.2022.111520. [DOI] [PubMed] [Google Scholar]
- 22.Li X., Da S., Li C., Xue F., Zang T. Effects of high-intensity ultrasound pretreatment with different levels of power output on the antioxidant properties of alcalase hydrolyzates from Quinoa (Chenopodium quinoa Willd.) protein isolate. Cereal Chem. 2018;95:518–526. [Google Scholar]
- 23.Liang Q., Chalamaiah M., Ren X., Ma H., Wu J. Identification of New Anti-inflammatory Peptides from Zein Hydrolysate after Simulated Gastrointestinal Digestion and Transport in Caco-2 Cells. J. Agric. Food Chem. 2018;66:1114–1120. doi: 10.1021/acs.jafc.7b04562. [DOI] [PubMed] [Google Scholar]
- 24.Connolly A., Piggott C.O., FitzGerald R.J. Technofunctional properties of a brewers' spent grain protein-enriched isolate and its associated enzymatic hydrolysates. LWT Food Sci. Technol. 2014;59:1061–1067. [Google Scholar]
- 25.Xue F., Wu Z., Tong J., Zheng J., Li C. Effect of combination of high-intensity ultrasound treatment and dextran glycosylation on structural and interfacial properties of buckwheat protein isolates. Biosci. Biotechnol. Biochem. 2017;81:1891–1898. doi: 10.1080/09168451.2017.1361805. [DOI] [PubMed] [Google Scholar]
- 26.Li C., Huang X., Peng Q., Shan Y., Xue F. Physicochemical properties of peanut protein isolate–glucomannan conjugates prepared by ultrasonic treatment. Ultrason. Sonochem. 2014;21:1722–1727. doi: 10.1016/j.ultsonch.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Wang S., Wang J., Xue F., Li C. Effects of heating or ultrasound treatment on the enzymolysis and the structure characterization of hempseed protein isolates. J. Food Sci. Technol. 2019;56:3337–3346. doi: 10.1007/s13197-019-03815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vizcay-Barrena G., Wilson Z.A. Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. J. Exp. Bot. 2006;57:2709–2717. doi: 10.1093/jxb/erl032. [DOI] [PubMed] [Google Scholar]
- 29.Das S., Nadar S.S., Rathod V.K. Integrated strategies for enzyme assisted extraction of bioactive molecules: A review. Int. J. Biol. Macromol. 2021;191:899–917. doi: 10.1016/j.ijbiomac.2021.09.060. [DOI] [PubMed] [Google Scholar]
- 30.Yusoff I.M., Mat Taher Z., Rahmat Z., Chua L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022;157 doi: 10.1016/j.foodres.2022.111268. [DOI] [PubMed] [Google Scholar]
- 31.Alavi F., Chen L., Emam-Djomeh Z. Effect of ultrasound-assisted alkaline treatment on functional property modifications of faba bean protein. Food Chem. 2021;354 doi: 10.1016/j.foodchem.2021.129494. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y., Zhou X., Zheng Y., Wang D., Deng Y., Zhao Y. Impact of ultrasonication/shear emulsifying/microwave-assisted enzymatic extraction on rheological, structural, and functional properties of Akebia trifoliata (Thunb.) Koidz. seed protein isolates. Food Hydrocoll. 2021;112 [Google Scholar]
- 33.Wani K.M., Uppaluri R.V.S. Efficacy of ultrasound-assisted extraction of bioactive constituents from Psidium guajava leaves. Appl. Food Res. 2022;2 [Google Scholar]
- 34.Fraterrigo Garofalo S., Demichelis F., Mancini G., Tommasi T., Fino D. Conventional and ultrasound-assisted extraction of rice bran oil with isopropanol as solvent. Sustain. Chem. Pharm. 2022;29 [Google Scholar]
- 35.Sirichan T., Kijpatanasilp I., Asadatorn N., Assatarakul K. Optimization of ultrasound extraction of functional compound from makiang seed by response surface methodology and antimicrobial activity of optimized extract with its application in orange juice. Ultrason. Sonochem. 2022;83 doi: 10.1016/j.ultsonch.2022.105916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naik M., Natarajan V., Modupalli N., Thangaraj S., Rawson A. Pulsed ultrasound assisted extraction of protein from defatted Bitter melon seeds (Momardica charantia L.) meal: Kinetics and quality measurements. LWT. 2022;155 [Google Scholar]
- 37.Watrelot A.A., Bouska L. Optimization of the ultrasound-assisted extraction of polyphenols from Aronia and grapes. Food Chem. 2022;386 doi: 10.1016/j.foodchem.2022.132703. [DOI] [PubMed] [Google Scholar]
- 38.Csiszar E., Szabo Z., Balogh O., Fekete E., Koczka K. The role of the particle size reduction and morphological changes of solid substrate in the ultrasound-aided enzymatic hydrolysis of cellulose. Ultrason. Sonochem. 2021;78 doi: 10.1016/j.ultsonch.2021.105711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jambrak A.R., Mason T.J., Lelas V., Paniwnyk L., Herceg Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014;121:15–23. [Google Scholar]
- 40.Morales R., Martínez K.D., Pizones Ruiz-Henestrosa V.M., Pilosof A.M.R. Modification of foaming properties of soy protein isolate by high ultrasound intensity: Particle size effect. Ultrason. Sonochem. 2015;26:48–55. doi: 10.1016/j.ultsonch.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Lo B., Kasapis S., Farahnaky A. Effect of low frequency ultrasound on the functional characteristics of isolated lupin protein. Food Hydrocoll. 2022;124 [Google Scholar]
- 42.Liu F.-F., Li Y.-Q., Wang C.-Y., Liang Y., Zhao X.-Z., He J.-X., Mo H.-Z. Physicochemical, functional and antioxidant properties of mung bean protein enzymatic hydrolysates. Food Chem. 2022;393 doi: 10.1016/j.foodchem.2022.133397. [DOI] [PubMed] [Google Scholar]
- 43.Singh J., Karmakar S., Banerjee R. An integrated study using ultrasonic-assisted enzymatic extraction of hydrolysates from rice based distillery byproduct and its characterization. Process Biochem. 2022;119:128–139. [Google Scholar]
- 44.Yalçın E., Çelik S. Solubility properties of barley flour, protein isolates and hydrolysates. Food Chem. 2007;104:1641–1647. [Google Scholar]
- 45.Nawaz M.A., Buckow R., Jegasothy H., Stockmann R. Enzymatic hydrolysis improves the stability of UHT treated faba bean protein emulsions. Food Bioprod. Process. 2022;132:200–210. [Google Scholar]
- 46.Bučko S., Katona J., Popović L., Petrović L., Milinković J. Influence of enzymatic hydrolysis on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate. Food Hydrocoll. 2016;60:271–278. [Google Scholar]
- 47.Gao K., Zha F., Yang Z., Rao J., Chen B. Structure characteristics and functionality of water-soluble fraction from high-intensity ultrasound treated pea protein isolate. Food Hydrocoll. 2022;125 [Google Scholar]
- 48.Hu H., Wu J., Li-Chan E.C.Y., Zhu L., Zhang F., Xu X., Fan G., Wang L., Huang X., Pan S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013;30:647–655. [Google Scholar]
- 49.Jiang L., Wang J., Li Y., Wang Z., Liang J., Wang R., Chen Y., Ma W., Qi B., Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014;62:595–601. [Google Scholar]
- 50.Gao K., Rao J., Chen B. Unraveling the mechanism by which high intensity ultrasound improves the solubility of commercial pea protein isolates. Food Hydrocoll. 2022;131 [Google Scholar]
- 51.Falade E.O., Mu T.-H., Zhang M. Improvement of ultrasound microwave-assisted enzymatic production and high hydrostatic pressure on emulsifying, rheological and interfacial characteristics of sweet potato protein hydrolysates. Food Hydrocoll. 2021;117 [Google Scholar]
- 52.Zang X., Yue C., Wang Y., Shao M., Yu G. Effect of limited enzymatic hydrolysis on the structure and emulsifying properties of rice bran protein. J. Cereal Sci. 2019;85:168–174. [Google Scholar]
- 53.Chen L., Chen J., Ren J., Zhao M. Modifications of soy protein isolates using combined extrusion pre-treatment and controlled enzymatic hydrolysis for improved emulsifying properties. Food Hydrocoll. 2011;25:887–897. [Google Scholar]
- 54.Wang Y., Li B., Guo Y., Liu C., Liu J., Tan B., Guo Z., Wang Z., Jiang L. Effects of ultrasound on the structural and emulsifying properties and interfacial properties of oxidized soybean protein aggregates. Ultrason. Sonochem. 2022;87 doi: 10.1016/j.ultsonch.2022.106046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sha L., Xiong Y.L. Comparative structural and emulsifying properties of ultrasound-treated pea (Pisum sativum L.) protein isolate and the legumin and vicilin fractions. Food Res. Int. 2022;156 doi: 10.1016/j.foodres.2022.111179. [DOI] [PubMed] [Google Scholar]
- 56.Ruiz-Álvarez J.M., del Castillo-Santaella T., Maldonado-Valderrama J., Guadix A., Guadix E.M., García-Moreno P.j. pH influences the interfacial properties of blue whiting (M. poutassou) and whey protein hydrolysates determining the physical stability of fish oil-in-water emulsions. Food Hydrocoll. 2022;122 [Google Scholar]
- 57.Li K., Li Y., Liu C.-L., Fu L., Zhao Y.-Y., Zhang Y.-Y., Wang Y.-T., Bai Y.-H. Improving interfacial properties, structure and oxidative stability by ultrasound application to sodium caseinate prepared pre-emulsified soybean oil. LWT. 2020;131 [Google Scholar]
- 58.Xu X., Qiao Y., Shi B., Dia V.P. Alcalase and bromelain hydrolysis affected physicochemical and functional properties and biological activities of legume proteins. Food Struct. 2021;27 [Google Scholar]
- 59.Chen D., Campanella O.H. Limited enzymatic hydrolysis induced pea protein gelation at low protein concentration with less heat requirement. Food Hydrocoll. 2022;128 [Google Scholar]
- 60.Ma Z., Li L., Wu C., Huang Y., Teng F., Li Y. Effects of combined enzymatic and ultrasonic treatments on the structure and gel properties of soybean protein isolate. LWT. 2022;158 [Google Scholar]
- 61.Xue F., Li C., Adhikari B. Physicochemical properties of soy protein isolates-cyanidin-3-galactoside conjugates produced using free radicals induced by ultrasound. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.104990. [DOI] [PubMed] [Google Scholar]
- 62.Liu N., Lin P., Zhang K., Yao X., Li D., Yang L., Zhao M. Combined effects of limited enzymatic hydrolysis and high hydrostatic pressure on the structural and emulsifying properties of rice proteins. Innov. Food Sci. Emerg. Technol. 2022;77 [Google Scholar]
- 63.Zhao R., Liu X., Liu W., Liu Q., Zhang L., Hu H. Effect of high-intensity ultrasound on the structural, rheological, emulsifying and gelling properties of insoluble potato protein isolates. Ultrason. Sonochem. 2022;85 doi: 10.1016/j.ultsonch.2022.105969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kostić A.Ž., Milinčić D.D., Barać M.B., Ali Shariati M., Tešić Ž.L., Pešić M.B. The Application of Pollen as a Functional Food and Feed Ingredient—The Present and Perspectives. Biomolecules. 2020;10:84. doi: 10.3390/biom10010084. [DOI] [PMC free article] [PubMed] [Google Scholar]