Abstract
Rheumatoid arthritis (RA) is a chronic, progressive inflammatory and systemic autoimmune disease resulting in severe joint destruction, lifelong suffering and considerable disability. Diverse prescriptions of traditional Chinese medicine (TCM) containing Epimedii Herba (EH) achieve greatly curative effects against RA. The present review aims to systemically summarize the therapeutic effect, pharmacological mechanism, bioavailability and safety assessment of EH to provide a novel insight for subsequent studies. The search terms included were “Epimedii Herba”, “yinyanghuo”, “arthritis, rheumatoid” and “Rheumatoid Arthritis”, and relevant literatures were collected on the database such as Google Scholar, Pubmed, Web of Science and CNKI. In this review, 15 compounds from EH for the treatment of RA were summarized from the aspects of anti-inflammatory, immunoregulatory, cartilage and bone protective, antiangiogenic and antioxidant activities. Although EH has been frequently used to treat RA in clinical practice, studies on mechanisms of these activities are still scarce. Various compounds of EH have the multifunctional traits in the treatment of RA, so EH may be a great complementary medicine option and it is necessary to pay more attention to further research and development.
Keywords: Epimedii Herba, rheumatoid arthritis, pharmacology, bioavailability, toxicity
Introduction
Rheumatoid arthritis (RA) is classified as a chronic, progressive inflammatory and systemic autoimmune disease that primarily manifests as a symmetric poly-arthritis in hands and feet (Cush, 2021), leading to severe joint destruction, lifelong suffering and considerable disability (Burmester and Pope, 2017). The global prevalence of RA is estimated at approximately 1% (van der Woude and van der Helm-van Mil, 2018). In case of inadequate treatment, RA can result in permanent cartilage degradation, bone abrasions, joint impairment, impaired movement, and even irreversible disability (Chakraborty et al., 2021). Consequently, the issue of prompt and targeted RA treatment has received considerable attention. Currently, the main classes of therapeutic medications against RA are glucocorticoid, nonsteroidal anti-inflammatory drugs and disease-modifying antirheumatic drugs (DMARDs). Without doubt, those medications have greatly therapeutic effects, but they are related to harmful side effects, such as gastrointestinal bleeding, osteoporosis, stomatitis, fatigue and hepatotoxicity (Lin et al., 2020). Extensive research has shown that traditional Chinese medicine (TCM) has remarkable advantages of alleviation effectively of symptoms of RA and lowered side effects. Therefore, TCM is necessary to be seen as a complementary medicine strategy.
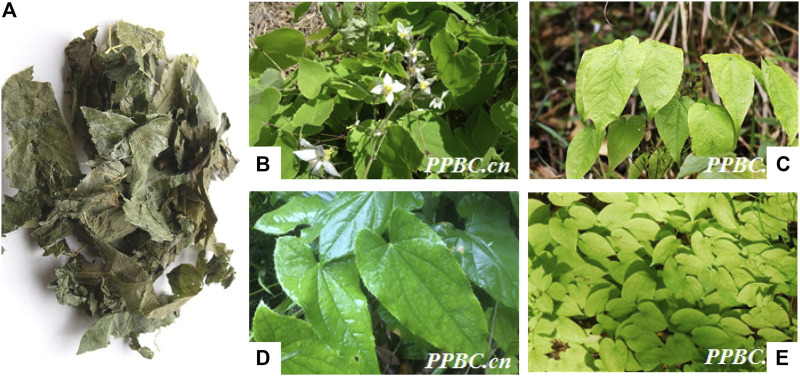
Epimedii Herba (EH), a classical herbal medicine, is the dried leaves originated from several plants of the genus Epimedium (Cho et al., 2017) including Epimedium brevicornu Maxim., Epimedium sagittatum (Sieb. EtZucc.) Maxim., Epimedium pubescens Maxim. and Epimedium koreanum Nakaias according to Chinese Pharmacopoeia (Figure 1). EH exerts osteoprotective effects by strengthening bones and muscle, dispelling wind chill and tonifying kidney in the Chinese medicine classics Shen Nong Ben Cao Jing (Yang et al., 2018a). EH alone, or combined within the TCM prescriptions has been used extensively to treat various disease including osteoporosis (Wang et al., 2016), cancer (Chen et al., 2016), chronic fatigue syndrome (Chi et al., 2017) and sexual dysfunction (Niu, 1989). Furthermore, in clinical practice, EH is one of the most frequently used herbs in a variety of traditional Chinese decoction for the treatment of RA, such as Bushen Quhan Zhiwang decoction (Wang G et al., 2021), Yishen Qubi Tongluo decoction (Luo. et al., 2019) and Bushen Jiedu Tongluo decoction (Yuan. et al., 2019). Additionally, EH is also a component of Chinese patent medicine for treating RA, including Wangbi tablet (Chen. et al., 2021), Kunxian capsule (Sun. and Chen., 2021) and Fugui Gutong capsule (Zhang. et al., 2020b). The prescriptions of TCM containing EH used by physicians for management of RA were shown in Table 1. EH had drawn increased attention and pharmacological research to explore material foundation and pharmacological mechanism for treating RA.
FIGURE 1.
Epimedii Herba (A) (https://www.daquan.com/) is the dried leaves originated from several plants of the genus Epimedium, including Epimedium brevicornu Maxim (B), Epimedium sagittatum (Sieb. EtZucc.) Maxim (C), Epimedium pubescens Maxim (D) and Epimedium koreanum Nakaias (E) (Cited from plant photo bank of China at http://ppbc.iplant.cn/).
TABLE 1.
The prescriptions of TCM containing EH used by physicians for management of RA.
| Prescription name | Components | Effects | References |
|---|---|---|---|
| Kunxian capsule | Epimrdii Herba , Cuscutae Semen, Lycii Fructus, Tripterygium hypoglaucum | ↓ESR, CRP, number of joint swelling, number of joint tenderness, morning stiffness time, VAS score | Sun. and Chen., 2021 |
| Bushen Quhan Zhiwang decoction | Psoraleae Fructus 10g, Dipsaci Radix 15 g, Rehmanniae Radix Praeparata 15g, Aconiti Lateralis Radix Praeparata 6g, Epimrdii Herba 10 g, Drynariae Rhizoma 10 g, Cinnamomi Ramulus 9 g, Angelicae Pubescentis Radix 20 g, Clematidis Radix Et Rhizoma 15 g, Paeoniae Radix Alba 20 g, Saposhnikoviae Radix 10 g, Atractylodis Rhizoma 15 g, Ephedra Herba 6 g, Paeoniae Radix Rubra 10 g, Anemarrhenae Rhizoma 10g, Lycopodii Herba 15 g, Eupolyphaga Steleophaga 10 g, Achyranthis Bidentatae Radix 10 g | ↓ESR, CRP, RF, number of joint swelling, number of joint tenderness, morning stiffness time, HAQ | Wang Y et al. (2021) |
| Wangbi tablet | Rehmanniae Radix Praeparata, Rehmanniae Radix, Anemarrhenae Rhizoma, Epimrdii Herba, Dipsaci Radix, Cibotii Rhizoma, Lycopodii Herba, Carthami Flos, Paeoniae Radix Alba, Cinnamomi Ramulus, Angelicae Pubescentis Radix, Saposhnikoviae Radix, Clematidis Radix Et Rhizoma, Aconiti Lateralis Radix Praeparata | ↓DAS28 score, TCM symptom score↑ACR20, ACR50 | Chen. et al. (2021) |
| Bushen Tongluo recipe | Epimrdii Herba 30 g, Eucommiae Cortex 9 g, Dipsaci Radix 9 g, Clematidis Radix Et Rhizoma 27 g, Radix Rhodomyrti 30 g | ↓TCM symptom score | Shu. and Cai., 2021 |
| Wanbi Xinglei Yin | Astragali Radix30 g, Angelicae Sinensis Radix20 g, Polygoni Multiflori Radix 30 g, Atractylodis Macrocephalae Rhizoma 15 g, Salviae Miltiorrhizae Radix Et Rhizoma 20g, Taxilli Herba 30 g, Epimrdii Herba 15 g, Acanthopanacis Cortex 15g , Manis pentadactyla 10g, Zaocys 12g, Speranskia tuberculate 30g, Glycyrrhizae Radix Et Rhizoma 9g | ↓VAS score, HAQ, number of joint swelling, number of joint tenderness, morning stiffness time | Zhang. et al. (2020a) |
| Fugui Gutong capsules | Aconiti Lateralis Radix Praeparata, Aconiti Radix Cocta, Cinnanmomi Cortex, Codonopsis Radix, Angelicae Sinensis Radix, Paeoniae Radix Alba, Epimrdii Herba, Olibanum | ↓ESR, CRP, number of joint swelling, number of joint tenderness, morning stiffness time, IL-1, IL-6, TNF-α | Zhang. et al. (2020b) |
| Yishen Qubi tongluo decoction | Epimrdii Herba 10g, Psoraleae Fructus 10 g, Dipsaci Radix 12 g, Rehmanniae Radix Praeparata 10g, Notopterygii Rhizoma Et Radix 1 5g, Angelicae Pubescentis Radix 15 g, Cinnamomi Ramulus 10g, Sinomenii Caulis 15 g, Olibanum 5 g, Myrrha 5 g, Angelicae Dahuricae Radix 10 g, Clematidis Radix Et Rhizoma 10 g, Astragali Radix 15 g, Angelicae Sinensis Radix 15 g | ↓TCM symptom score | Luo. et al. (2019) |
| Bushen Jiedu Tongluo decoction | Scorpio 3 g, Glycyrrhizae Radix Et Rhizoma 6g, Notopterygii Rhizoma Et Radix 9g, Moutan Cortex 9 g, Paeoniae Radix Alba 9 g, Zaocys 9 g, Curculiginis Rhizoma 15 g, Epimrdii Herba 15g, Dipsaci Radix 15 g, Sinomenii Caulis 15 g, Rehmanniae Radix 15 g, Curcumae Longae Rhizoma 15 g, Lonicerae Japonicae Caulis 30 g, Sarcandrae Herba 30g | ↓ESR, CRP, RF, number of joint swelling, number of joint tenderness, VAS score, HAQ, IL-1, DAS28 | Yuan. et al. (2019) |
| Tonifying Liver and Kidney decoction | Lycopodii Herba 20 g, Dipsaci Radix 20 g, Epimrdii Herba 15 g, Drynariae Rhizoma 15 g, Psoraleae Fructus 15 g, Spatholobi Caulis 15 g, Paeoniae Radix Alba 1 2g, Cinnamomi Ramulus 12 g, Angelicae Pubescentis Radix12g, Achyranthis Bidentatae Radix 12 g | ↓number of joint swelling, number of joint tenderness, morning stiffness time, hs-CRP, ESR, RF, ADL score, QOL score | Liang. et al. (2019) |
| Kidney-tonifying arthralgia-eliminating decoction | Aconiti lateralis Radix Praeparaia 6 g, Dipsaci Radix 10 g, Eucommiae Cortex 10g, Achyranthis Bidentatae Radix 10 g, Manis pentadactyla 9 g, Epimrdii Herba 8g, Hirudo 6g, Speranskia tuberculate 20 g, Rhizoma Seu Herba Aristolochiae Mollissimae 10g, Pyritum 6g | ↓number of joint swelling, number of joint tenderness, morning stiffness time, CRP, ESR, RF, FG, VEGF | Liu and Li (2018) |
| Xianzhi Fengsui decoction | Corni Fructus30 g, Epimrdii Herba 15 g, Psoraleae Fructus 15 g, Phellodendri Chinrnsis Cortex 10 g, Amomi Fructus 6 g, Glycyrrhizae Radix Et Rhizoma 6 g | ↓number of joint tenderness, morning stiffness time, ESR, RF, BMD, OPG | Zheng. et al. (2017) |
TCM, traditional Chinese medicine; VAS, visual analogue scale; HAQ, health assessment questionnaire; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; RF, rheumatoid factor; DAS28, Disease Activity Score for 28 joints; ACR20, American College of Rheumatology 20%; ADL, daily living ability; QOL, life therapy scale; FG, fibrinogen; VEGF, vascular endothelial growth factor; BMD, bone mineral density; OPG, osteoprotegerin.
Sze et al. have reviewed anti-oxidative properties of EH (Sze et al., 2010). However, the study did not review anti-inflammatory, immunoregulatory, osteoprotective and antiangiogenic activities. Consequently, the information of EH was searched to review the current research status. In detail, the literatures on compounds from EH were obtained using Web of Science, Google Scholar, Pubmed, and CNKI. Subsequently, the literatures on compounds involved in RA treatment were further filtered (Figure 2). Based on various pathological mechanism of RA including inflammation of the synovial membrane, oxidation, angiogenesis and bone destruction, this article systematically summarizes anti-RA activities of EH and further explores material basis and mechanism of these activities, so as to provide novel insight in the treatment of RA.
FIGURE 2.
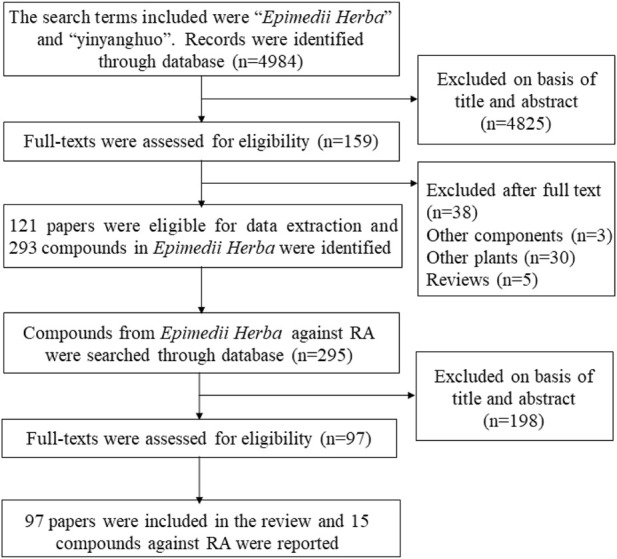
Articles were identified and screened for eligibility.
Effects of EH on RA
EH and its total flavonoids
In vivo studies were performed on adjuvant-induced arthritis (AIA) mice to investigate the anti-RA effects after an oral administration of EH and the results showed that compared with Tripterygii Radix, paw thickness was lower after oral administration of EH combined with Tripterygii Radix (Du. et al., 2019). It was reported that total flavonoids of EH can inhibit differentiation and bone resorption of osteoclasts (Zhang et al., 2012). Furthermore, total flavonoids of EH were found to promote osteogenic differentiation via the bone morphogenetic protein and Wnt/β-catenin signaling pathways (Zhang et al., 2010).
Components of EH
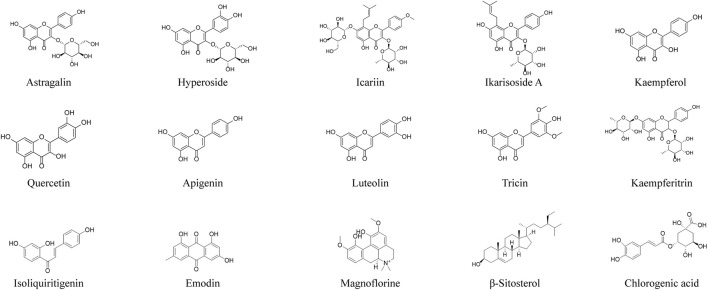
293 compounds from EH were searched in different database (Ma et al., 2011; Jin et al., 2014; Li F. et al., 2017; Pang et al., 2018; Ren et al., 2018; Su et al., 2018; Zhang et al., 2020; Hu. et al., 2021). Then, the studies of the compounds against RA were further searched in the database and 15 compounds for the treatment of RA have been reported, including icariin, quercetin, kaempferol, apigenin, luteolin, kaempferitrin, astragalin, hyperoside, ikarisoside A, tricin, isoliquiritigenin, emodin, β-sitosterol, magnoflorine and chlorogenic acid (Figure 3). Icariin is the most abundant constituent in EH and one of chemical markers for quality control of EH. Other compounds extracted from EH may have positive effects for the treatment of RA. The overview of the compounds for the treatment of RA was shown in Table 2.
FIGURE 3.
Structure of compounds from EH against RA.
TABLE 2.
Compounds from EH for the treatment of RA.
| NO. | Compounds | Subgroup | Formula | Molecular weight | Sources | Content (mg/g) | References |
|---|---|---|---|---|---|---|---|
| 1 | Magnoflorine | Alkaloids | C20H24NO4 | 341.1695 | a | 1.85 ± 1.16 | Ma. et al. (2019); Cheng. et al. (2022) |
| 2 | Emodin | Anthraquinone | C15H10O5 | 270.9787 | a,b | — | Cui. et al. (2010); Ma. et al. (2019) |
| 3 | Astragalin | Flavonoids | C21H20O11 | 448.0956 | a, d | — | Cheng. et al. (2006) |
| 4 | Hyperoside | Flavonoids | C21H20O12 | 464.0907 | a, b, c, d | 0.55–2.25 | Zheng. and Kong., 2002; Sun. et al. (2018); Ma. et al. (2019); Han., 2022 |
| 5 | Icariin | Flavonoids | C33H40O15 | 676.1256 | a, b, c, d | 7.86 ± 3.74 | Wang et al. (2007); Kim and Shim, (2019); Ma. et al. (2019); Yang et al. (2020); Cheng. et al. (2022) |
| 6 | Ikarisoside A | Flavonoids | C26H28O10 | 500.1623 | a, c, d | — | Li et al. (1995a); Zhang. et al. (2013); Ma. et al. (2019) |
| 7 | Kaempferol | Flavonoids | C15H10O6 | 286 | b, c | — | Wang et al. (2007); Zhang. et al. (2013) |
| 8 | Quercetin | Flavonoids | C15H10O7 | 302.7 | b, d | — | Li. et al. (1995b); Wang et al. (2007); Hu. et al. (2021) |
| 9 | Apigenin | Flavonoids | C15H10O5 | - | b | — | Wang et al. (2007) |
| 10 | Luteolin | Flavonoids | C15H10O6 | 286.0408 | a, b, d | — | Wang et al. (2007); Ma. et al. (2019) |
| 11 | Tricin | Flavonoids | C17H14O7 | 330 | b, c | — | Cui. et al. (2010); Zhang. et al. (2013) |
| 12 | Kaempferitrin | Flavonoids | C27H30O14 | 578.1590 | a | — | Ma. et al. (2019) |
| 13 | Isoliquiritigenin | Chalcone | C15H12O4 | - | d | — | Li. et al. (1994) |
| 14 | β-Sitosterol | Phytosterol | C29H50O | - | b | — | Cui. et al. (2010) |
| 15 | Chlorogenic acid | polyphenol | C16H18O9 | 354.0882 | a | 0.47 ± 0.38 | Ma. et al. (2019); Cheng. et al. (2022) |
a: Epimedium brevicornu Maxim. b: Epimedium sagittatum (Sieb. EtZucc.) Maxim. c: Epimedium pubescens Maxim d: Epimedium koreanum Nakaias.
Anti-inflammatory activities
RA is an inflammatory disease and pro-inflammatory cytokines and chemokines are overproduced in synovial fluid and serum of RA patients (Shrivastava and Pandey, 2013; Jiang et al., 2021). It was reported that astragalin, hyperoside, kaempferol, icariin, apigenin and kaempferitrin treatment decreased arthritic score and incidence of arthritis in animal models (Chi et al., 2014; Li et al., 2016; Jin et al., 2017; Lee et al., 2018; Jia et al., 2019a; Wang and Zhao, 2019; Lei. et al., 2020; Jin. et al., 2021). Furthermore, arthritis symptoms including paw volume and paw thickness were reduced significantly with quercetin, astragalin, hyperoside, icariin, luteolin, kaempferol and kaempferitrin treatment in animal models of arthritis (Haleagrahara et al., 2017; Pan et al., 2018; Jia et al., 2019b; Wang and Zhao, 2019; Lei. et al., 2020; Ahmed and Abd Elkarim, 2021; Jin. et al., 2021; Liu Y et al., 2021). The anti-arthritis effect of compounds from EH in the animal models was shown in Table 3. Anti-arthritis mechanism of compounds extracted from EH may be related to inhibiting inflammatory mediators, such as cytokines, chemokines, Prostaglandin E2 (PGE2), cyclooxygenase (COX)-2, nitric oxide (NO) and inducible nitric oxide synthase (iNOS).
TABLE 3.
The anti-arthritis effect of compounds from EH for the treatment of RA in the animal models.
| Compounds | Model | Dose (mg/kg) | Action | References |
|---|---|---|---|---|
| Apigenin | CAIA mice | 16 | ↓paw volume, paw thickness, arthritic score | Jin et al. (2017) |
| CIA mice | 20 | ↓arthritis incidence, joint swelling, clinical scores | Li et al. (2016) | |
| Astragalin | CIA mice | 5 | ↓arthritis index, swollen joints count, paw thickness | Jia et al. (2019a) |
| Chlorogenic acid | CIA mice | 60 | ↓arthritis index, paw thickness | Fu et al. (2019) |
| Emodin | AIA mice | 30 | ↓paw edema, arthritis scores | Zhu et al. (2019) |
| CIA mice | 20 | ↓paw thickness, arthritis index, incidence, clinical scores | Hwang et al. (2013); Zhu et al. (2013) | |
| Hyperoside | CIA mice | 50 | ↓paw thickness, arthritis index | Jin. et al. (2021) |
| Icariin | AIA rabbits | 60 | ↓mankin score | Wei et al. (2016) |
| CIA mice | 40 | ↓arthritic score, swollen joints | Lei. et al. (2020) | |
| CIA mice | 25 | ↓arthritis incidence | Chi et al. (2014) | |
| Kaempferitrin | CIA mice | 20 | ↓paw thickness, arthritis scores | Wang and Zhao, (2019) |
| Kaempferol | CIA mice | 200 | ↓disease severity, joint swelling | Pan et al. (2018) |
| CIA mice | 2 | ↓arthritis severity, arthritis incidence | Lee et al. (2018) | |
| AIA mice | 25 | ↓paw volume | Ahmed and Abd Elkarim, (2021) | |
| Luteolin | CIA mice | 100 | ↓paw volume | Liu Y. et al. (2021) |
| Magnoflorine | AIA mice | 10 | ↓arthritis scores, paw swelling. ↑ body weight | Shen et al. (2022) |
| Quercetin | CIA mice | 30 | ↓paw oedema | Haleagrahara et al. (2017) |
| β-Sitosterol | CIA mice | 100 | ↓arthritis index, ankle swelling, paw thickness | Liu et al. (2019); Qian et al. (2021) |
CIA, Collagen-induced arthritis; CAIA , Collagen antibody-induced arthritis., AIA, antigen-induced arthritis.
Effects on inflammatory cytokines
Proinflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-1β are responsible for the induction and maintenance of inflammatory milieu in the synovial tissue and articular destruction (Mateen et al., 2016b). Especially TNF-α, a central cytokine in RA pathophysiology (Feldmann et al., 1996), stimulates activation of leukocyte, endothelial, stromal-cell and chondrocyte, as well as expression of angiogenesis, nociception, chemokine (McInnes et al., 2016). Additionally, TNF-α directly increases osteocyte receptor activator of NF-κB ligand (RANKL) expression and induces formation of osteoclasts (Marahleh et al., 2019). Blocking bioactivity of TNF-α reduces production of IL-1, IL-1β, IL-6 and IL-8 (Brennan and McInnes, 2008). Consequently, TNF blockers are one of the most valuable agents to prevent bone erosion and loss in RA.
In vivo and in vitro studies showed that icariin, quercetin and kaempferitrin reduced the expression of IL-1β, IL-6 and TNF-α via PI3K/AKT/Mtor (Wang and Zhao, 2019; Xiang. et al., 2020), miR-223-3p/NLRP3 (Lei. et al., 2020; Wu et al., 2020), and NF-κB signaling pathway (Jia. et al., 2019a; Wang Z et al., 2019). Moreover, luteolin, tricin, kaempferol, isoliquiritigenin, emodin, astragalin and hyperoside inhibited proinflammatory cytokines including IL-1β, IL-6, TNF-α, IL-8, IL-17 and IL-1 (Kim et al., 2008; Hwang et al., 2013; Jin et al., 2016; Jia et al., 2019a; Zhu et al., 2019; Ji et al., 2021; Lin et al., 2021; Ling et al., 2021; Zhou et al., 2021). Apigenin suppressed bone marrow-derived dendritic cells (DCs) to produce cytokines including TNF-α, IL-12 p70 and IL-10, while increased the secretion of IL-1β (Li et al., 2016). Haque et al. reported that magnoflorine enhanced pro-inflammatory responses through producing of TNF-α and IL-1β (Haque et al., 2018). However, Shen et al. showed that magnoflorine attenuated inflammatory responses by reducing inflammatory cytokines, such as IL-6 and IL-8 (Shen et al., 2022). Further research is needed to confirm this opposite conclusion.
Effects on the production of chemokines
Previous research has confirmed that various chemokines are up-regulated in serum, synovial fluid and synovial tissue of patients with RA, compared with healthy controls (Miyabe et al., 2020). Furthermore, chemokines including CXCL13 and CXCL10 can be considered as biomarkers of RA disease activity (Meeuwisse et al., 2011; Pandya et al., 2017). In RA, chemokines boost neutrophils, T cells and the B cells recruitment into the joint (Lee et al., 2017; Miyabe et al., 2017; Armas-Gonzalez et al., 2018). Targeting chemokines might be a promising direction for RA therapies.
It was reported that quercetin suppressed expression of monocyte chemoattractant protein (MCP)-1 in the collagen-induced arthritis (CIA) and AIA mice (Gardi et al., 2015; Haleagrahara et al., 2017). Apigenin inhibited arthritis development through reducing the migration of DCs, which may be related to down-regulating the expression of chemokine receptor 4 (CXCR4) (Li et al., 2016).
Effects on the production of PGE2 and COX
PGE2, a main mediator of inflammation in RA, contributes to the pathogenesis of RA. It can be produced by diverse immune cells upon the activation of COX enzymes and PGE synthases (Park et al., 2006). PGE2 increased IL-17 production and promoted the migration and antigen-presenting function of DCs (Okano et al., 2006; Sheibanie et al., 2007). Furthermore, PGE2 suppressed regulatory T (Treg) cells differentiation via the EP2-cAMP/PKA signaling pathway (Li H. et al., 2017).
Quercetin and kaempferol remarkably suppressed production of PGE2 in human rheumatoid arthritis fibroblast-like synoviocytes (RAFLS), and the mechanism might be related to the inhibition of COX1 and COX2 expression (Lee and Kim, 2010; Sung et al., 2012; Yoon et al., 2013). PGE2 and COX2 were suppressed by quercetin, emodin and icariin in RA mice (Zhu et al., 2013; Yang et al., 2018b; Guazelli et al., 2018; Liu T et al., 2021). Apigenin, isoliquiritigenin and luteolin treatment had an inhibitory effect on the expression of COX2 in lipopolysaccharide (LPS) induced RAW 264.7 macrophage cells (Kim et al., 2008; Lee and Kim, 2010).
Effects on the production of NO and iNOS
Overexpression of iNOS increases the levels of NO, which is an important mediator of synovial inflammation in RA (Li and Wan, 2013; Minhas et al., 2020).
Serum level of NO was effectively decreased in CIA mice with quercetin treatment (Choi et al., 2009). Additionally, apigenin, luteolin, quercetin and kaempferol had an inhibitory effect on NO production stimulated by LPS in RAW 264.7 macrophage cells (Lee and Kim, 2010). In vitro studies showed that magnoflorine, isoliquiritigenin, quercetin, kaempferol, luteolin and apigenin suppressed the level of iNOS (Sun et al., 2020; Lin et al., 2021) and the mechanism may be associated with PI3K/Akt/NF-κB signaling axis and the Keap1-Nrf2/HO-1 signaling pathway (Shen et al., 2022). Furthermore, iNOS was inhibited markedly by quercetin in CIA mice (Yang et al., 2018b) and suppressed by hyperoside in fibroblast-like synoviocytes (FLS) (Fu. et al., 2020).
Immunoregulatory activity
RA is a chronic autoimmune disease characterized as infiltration of the synovial membrane in multiple joints with immune cell such as T cells, B cells, macrophages and DCs (Aletaha and Smolen, 2018). Compounds from EH have immunoregulatory effects via regulating T cells, macrophages, neutrophils, DCs and B cells.
Under antigenic stimulation and cytokine signaling, naive CD4+ T cells activate and differentiate into various Th cell subsets, including Th1, Th2, Th17, follicular Th, and Treg cells. Th1/Th2 and Th17/Treg cells become disproportional in the pathogenesis of RA (Noack and Miossec, 2014; Wang Y et al., 2019). Th1 cells secrete pro-inflammatory factors including interferon-γ (IFN-γ) and TNF-α and Th2 cells secrete anti-inflammatory factors, such as IL-4, IL-10 and IL-13 (Srivastava et al., 2018). Th17 cells accelerate secretion of IL-17 that stimulates production of proinflammatory cytokines including TNF-α, IL-1β, IL-6, matrix metalloproteinases (MMPs) and chemokines (Jin et al., 2018). Treg cells exert anti-inflammatory effects via secreting IL-10 and transforming growth factor-β (TGF-β) (Jiang et al., 2021). Sun et al. found that luteolin and apigenin treatment significantly reduced levels of IFN-γ and IL-2 in concanavalin A -induced splenic T-lymphocyte (Sun et al., 2020). Recent studies have shown that apigenin and chlorogenic acid regulated Th1/Th2 cells balance by inhibiting cytokines of Th1 cells and elevating Th2 cytokines (You. et al., 2009; Chauhan et al., 2012; Zhang. et al., 2015). Quercetin and kaempferol modulated the balance between Th17 and Treg immune response by downregulating Th17 cells and upregulating Treg cells (Lin et al., 2015; Yang et al., 2018b; Lee et al., 2018). Icariin treatment reduced number of Th17 cells in mouse spleens and synovial and suppressed Th17 cell differentiation in vitro experiments via downregulating STAT3 activation (Chi et al., 2014).
Macrophages are commonly divided into two distinct phenotypes, called M1-like macrophages and M2-like macrophages. M1 macrophages are considered as a pro-inflammatory phenotype via expressing pro-inflammatory cytokines and chemokines, while M2 macrophages release anti-inflammatory cytokines including IL-10 and TGF-β (Ross et al., 2021). The number of active M1 macrophages was increased in RA patients while the number of M2 type cells was decreased or inactive (Kennedy et al., 2011). Therefore, blocking M1 macrophage-derived cytokines and modulating the balance between M1 macrophages and M2 macrophages may contribute to the improvement of RA. Icariin and β-sitosterol suppressed M1 macrophage activation and increased M2 macrophage activation (Liu et al., 2019) and the mechanism may be related to inhibit mTOR/S6K1 and NF-κB signaling (Li et al., 2011). In vivo and in vitro studies showed that quercetin inhibited macrophage-derived NO, TNF-α, IL-1β and MCP-1 (Mamani-Matsuda et al., 2006).
In the absence of inflammation, healthy neutrophils circulate in the blood within several hours and undergo apoptosis (McCracken and Allen, 2014). In RA, however, neutrophils inappropriately activated by autoantibodies and inflammatory mediators are characterized by a delayed apoptotic process and migration into the joint (Cecchi et al., 2018). In addition, neutrophils produce reactive oxygen species (ROS) and release diverse cytokines and chemokines, contributing to inflammation and tissue damage (Fresneda Alarcon et al., 2021). The RA synovial microenvironment results in the formation of neutrophil extracellular traps (NETs) that are a source of citrullinated autoantigens and activate FLS, accelerating disease progression and joint damage in RA (Carmona-Rivera et al., 2017). Thus, NETs formation and neutrophil-produced cytokines, chemokines are considered as novel treatment targets (O'Neil and Kaplan, 2019). Quercetin suppressed neutrophil infiltration and NETs formation and increased the apoptosis of activated neutrophils in AIA mice (Yuan et al., 2020). Emodin reduced neutrophil infiltration in AIA mice and increased apoptosis and inhibited autophagy and NETs in vitro (Zhu et al., 2019).
DCs are antigen-presenting cells that link innate and adaptive immune responses. In RA, high concentrations of DCs are recruited in joint synovial fluid and tissues and DCs within the RA synovium are generally mature (Wehr et al., 2019). A recent study showed that synovial microenvironment in RA was responsible for DCs maturation and metabolic reprogramming via up-regulating STAT3 activation (Canavan et al., 2020). In addition, intracellular Zn2+ homeostasis and low oxygen state also impacted the maturation of DCs (Qiao et al., 2022). Inhibition of DCs maturation is an important treatment of DC-targeting in RA. In vivo and in vitro studies showed that apigenin efficiently inhibited DCs maturation and reduced cytokine secretion (Li et al., 2016).
The functions of B cells are closely associated with the pathogenesis of RA, such as antigen presentation, cytokine secretion and autoantibody production (Wu et al., 2021). B cells present autoantigens to T cells and secrete various cytokines including TNF-α, IFN-γ, IL-6, IL-1β, IL-17 and IL-10 (Yanaba et al., 2008). B-cell activating factor (BAFF), a member of the TNF superfamily, promoted the differentiation, proliferation, and activation of B cells via NF-κB signaling pathway (Zhang et al., 2021). Compared with healthy individuals, patients with RA had higher levels of BAFF in the peripheral blood and synovial fluid (Moura et al., 2011). Chlorogenic acid inhibited BAFF Expression in CIA mice and MH7A cells through the NF-κB pathway (Fu et al., 2019).
Osteoprotective activities
Bone and cartilage destruction can be evaluated by morphology, histology, X-ray, and computed tomography scan. Astragalin significantly reduced joint space widening and synovial vascularity by ultrasonography and color doppler and markedly diminished bone destruction of knee and ankle joints by the 3D reconstruction of a micro-CT analysis (Jia et al., 2019b). Icariin inhibited trabecular bone loss and increased bone mineral density in AIA rabbits by micro-CT analysis (Wei et al., 2016). Quercetin treatment attenuated level of 8F-FDG in the ankle and knee joints of CIA mice by 18F-FDG micro-PET imaging, suggesting it could reduce inflammation in joints (Shen et al., 2021). Furthermore, bone erosion and degradation were not serious and the narrowing of joint space was slight with quercetin treatment compared with the arthritis group in the X-ray examination (Haleagrahara et al., 2017). The compounds from EH play osteoprotective roles by decreasing formation and differentiation of osteoclasts, regulating of RANKL/osteoprotegerin (OPG) ratio and downregulating MMPs.
Effects on formation and differentiation of osteoclasts
Formation and differentiation of osteoclasts are the essential elements of bone degradation. Osteoclast differentiation is regulated by the molecular triad RANKL, receptor activator of nuclear factor-κB (RANK) and OPG (Aureal et al., 2020). RANKL-RANK signaling activates osteoclast differentiation and suppresses osteoclast apoptosis (Kitaura et al., 2020). OPG, a RANKL decoy receptor, can prevent RANKL-RANK binding (Boyle et al., 2003). The ratio of RANKL to OPG can be regarded as a marker of progression of osteoclast destruction (van Tuyl et al., 2010).
Ikarisoside A and isoliquiritigenin suppressed osteoclastogenesis in RANKL-stimulated RAW 264.7 cells and bone marrow-derived macrophages via MAPK and NF-κB pathways (Choi et al., 2010; Zhu et al., 2012). Furthermore, in vivo and in vitro studies showed that isoliquiritigenin had an anti-osteoclastogenic activity by suppressing NF-κB-dependent autophagy (Liu et al., 2016).
Emodin inhibited the osteoclast differentiation in bone marrow macrophages (Hwang et al., 2013). Quercetin attenuated IL-17-induced RANKL expression in RAFLS (Kim et al., 2019). Several in vivo models suggested that icariin decreased osteoclasts formation, mechanism of which might be relate to the regulation of RANKL/OPG ratio (Liu. et al., 2013; Wei et al., 2016). Apigenin and luteolin treatment regulated the RANKL/OPG ratio in CIA mice by inhibiting RANKL expression and elevating OPG expression (Liu and Li, 2018; Li et al., 2019).
Effects on cartilage protection
MMPs, belonging to the proteolytic enzymes, are intimately involved in degradation of extracellular matrix in cartilage (Itoh, 2017). Tissue inhibitor of metalloproteinases (TIMPs), a natural inhibitor, specifically inhibit MMPs (Alamgeer et al., 2020). In RA, cytokines promote chondrocytes to secret more cytokines and MMPs that degrade the cartilage and suppress generation of TIMPs (Fang et al., 2020). Furthermore, synovial tissue of RA patients produces diverse MMPs, such as MMP-1, -2, -3, -8, -9, -10, -12, -13 (Itoh, 2017). Previous studies have reported that the serum concentrations of MMP-3 can be considered as predictive marker of inflammation and joint destruction (Yamanaka et al., 2000; Shinozaki et al., 2007).
In vivo and in vitro studies showed that astragalin and quercetin inhibited the expression of MMPs via NF-κB pathway (Jia. et al., 2019b; Wang Z et al., 2019; Wang and Zhao, 2019) and Akt/mTOR pathways (Wang and Zhao, 2019). In vitro studies reported that MMPs were reduced by icariin (Chi et al., 2014), hyperoside (Fu. et al., 2020), kaempferitrin (Jia et al., 2019a), ikarisoside A, kaempferol, apigenin and luteolin treatment (Zhou et al., 2021) via NF-κB and MAPK signaling pathway (Choi et al., 2010; Choi and Lee, 2010; Yoon et al., 2013; Jin et al., 2016) and PI3K/Akt pathways (Hou et al., 2009). MMPs were suppressed with emodin treatment in CIA mice through NF-κB pathway (Hwang et al., 2013).
Effects on FLS proliferation, migration and apoptosis
In RA, FLS results in hyperplasia of the synovial lining, pannus formation, joint destruction through producing cytokines, chemokines, and matrix-degrading molecules and migrating and invading joint cartilage (Bustamante et al., 2017). RAFLS are resistant to apoptosis resulting from up-regulation of anti-apoptotic mediators including Bcl-2, Mcl-2, and FLICE-inhibitory protein (FLIP) and down-regulation of pro-apoptotic proteins including tumor necrosis factor-related apoptosis-inducing ligand and p53 up-regulated modulator of apoptosis (Zhang Q. et al., 2019). Furthermore, FLS produce several enzymes connected with invasive activities of FLS, such as collagenases, aggrecanases, cathepsins, and RANKL (Tu et al., 2018). Expansion of FLS increases oxygen consumption in synovium and forms a hypoxic environment, contributing to synovium angiogenic processes and pannus formation. FLS also stimulate overproduction of MMPs including MMP1, MMP3 and MMP13, leading to degradation of the collagen-rich structures of extracellular matrix (Nygaard and Firestein, 2020). Consequently, FLS can be regarded as hopeful therapeutic target for the treatment of RA (Aletaha and Smolen, 2018).
Icariin, kaempferol, kaempferitrin, chlorogenic acid and apigenin enhanced apoptosis and restrained proliferation of RAFLS via regulating miR-223-3p/NLRP3 signaling pathway (Wu et al., 2020), cell cycle and mitochondrial pathway (Pu et al., 2021), MAPK pathway (Yoon et al., 2013), NF-κB pathways (Yoon et al., 2013; Lou et al., 2015; Wang and Zhao, 2019), JAK/STAT pathways (Lou et al., 2015) and PI3K/Akt/mTOR signaling pathway (Sun et al., 2012; Wang and Zhao, 2019). Quercetin elevated apoptosis and decreased the migration and invasion of FLS through mitochondrial pathway and p53 phosphorylation (Xiao et al., 2013), PI3K/Akt pathway (Pan et al., 2016) and miR-146a/GATA6 axis (Zhao et al., 2020). Kaempferol, hyperoside and luteolin had suppressive effects on either migration or proliferation of FLS by TNF signaling pathway (Ling et al., 2021), fibroblast growth factor receptor 3–ribosomal S6 kinase 2 signaling pathway (Lee et al., 2018), MAPK pathway (Hou et al., 2009; Fu. et al., 2020), NF-κB signaling pathway (Lou et al., 2015; Jin et al., 2016), PI3K/Akt pathways (Hou et al., 2009) and JAK/STAT signaling pathways (Lou et al., 2015). Magnoflorine inhibited migration, invasion, proliferation and induced apoptosis and cell cycle arrest of RAFLS via inhibiting the PI3K/Akt/NF-κB axis signaling pathway and activating the Keap1-Nrf2/HO-1 signaling pathway (Shen et al., 2022).
Antiangiogenic activities
Angiogenesis, the formation of new capillaries, is related to leukocyte ingress into the synovium, synovial hyperplasia and pannus formation (Wang Z et al., 2021). Angiogenesis can be induced by angiogenic mediators including various growth factors, cytokines, chemokines, cell adhesion molecules, etc (Bodolay et al., 2002). Vascular endothelial growth factor (VEGF), one of the most vital growth factors, has a mitogenic and an anti-apoptotic effect on endothelial cells and increases the vascular permeability and cell migration (Melincovici et al., 2018). VEGF is activated by hypoxia and hypoxia-inducible factors 1 (HIF-1) and HIF-2 and pro-inflammatory cytokines including TNF-α and IL-1 (Szekanecz and Koch, 2009).
Histological evaluation demonstrated that quercetin reduced pannus formation in CIA mice (Kawaguchi et al., 2019), the mechanism of which might be associated with inhibition of VEGFA, HIF-1α and capillaries density in synovial tissue of CIA mice (Chu. et al., 2021). The expression of VEGF, VEGFR1 and VEGFR2 in synovial tissues of CIA mice and vascular cell adhesion molecule (VCAM) in human umbilical vein endothelial cells (HUVECs) were significantly inhibited by apigenin (Li et al., 2019; Zhou et al., 2021). A recent study showed that luteolin suppressed the expression of VEGF and HIF-1α in CIA mice and HUVECs (Liu T et al., 2021; Zhou et al., 2021). In vivo and in vitro studies showed that β-sitosterol significantly inhibited the expression and phosphorylation of VEGFR2 (Qian et al., 2021).
Antioxidant activities
Effects on ROS production and mitochondria dysfunction
Generally, it has been reported that oxidative stress is an associated factors in the pathogenesis of RA (Phull et al., 2018). Oxidative stress arises when enhancement of ROS exceeds the normal physiological values (Smallwood et al., 2018). The excessive production of ROS contributes to inflammation, matrix degradation and chondrocytes apoptosis via MAPKs and NF-κB signaling pathway (Phull et al., 2018). Mitochondria are generally regarded as the source of ROS in animal cells and mitochondrial dysfunction is responsible for imbalance of antioxidant systems (Munro and Treberg, 2017).
In vivo studies showed that ROS production was inhibited with quercetin and kaempferol treatment (Santos et al., 2014; Saccol et al., 2020). Apigenin induced intracellular ROS production in MH7A cells, which was associated with activation of ERK1/2 and apoptosis (Shin et al., 2009). Quercetin improved impaired mitochondrial biogenesis and mitochondrial function in CIA mice via regulating the SIRT1/PGC-1α/NRF1/TFAM pathway (Shen et al., 2021). In vitro studies showed that icariin and quercetin treatment induced apoptosis through mitochondrial pathway (Xiao et al., 2013; Pu et al., 2021).
Effects of lipid peroxidation and myeloperoxidase activity
Previous studies have reported that RA patients have increased lipid peroxidation in the synovial fluid and blood serum (Mateen et al., 2016a). Malondialdehyde and thiobarbituric acid-reactive substance (TBARS) are widely used to measure lipid peroxidation (Ghani et al., 2017; Tsikas, 2017). 15-lipoxygenase, a lipid-peroxidizing enzyme, was largely expressed by macrophages, neutrophils and mast cells in RA synovium (Gheorghe et al., 2009).
Luteolin suppressed 15-lipoxygenase in RAW 264.7 macrophage cells (Lee and Kim, 2010). The augmentation in TBARS levels and 15-lipoxygenase were reversed with quercetin treatment (Lee and Kim, 2010; Saccol et al., 2020). Furthermore, other study reported that 12/15-lipoxygenase in lung and liver were inhibited with quercetin treatment in AIA mice (Gardi et al., 2015). Quercetin and kaempferol treatment reduced the myeloperoxidase activity in neutrophils (Santos et al., 2014).
Clinical trials
Although various compounds extracted from EH exert anti-RA effect, few compounds have been used in clinical. In a randomized controlled trial, compared to azathioprine plus placebo or lower doses of quercetin (500, 1000 mg/day), azathioprine plus quercetin (1500 mg/day) in patients with RA obviously downregulated IL-6, intercellular adhesion molecule-1, complement proteins and upregulated IL-10 (Al-Rekabi et al., 2015). Quercetin supplement (500 mg/day) for 8 weeks exerted the beneficial effects on pain, stiffness, disease activity, inflammatory factors and well-being in women with RA in a double-blind, randomized controlled trial (Javadi et al., 2017). However, another study reported that quercetin (500 mg/day) for women with RA after 8 weeks had no significant differences in total antioxidant capacity, oxidized low density lipoprotein, malondialdehyde and high sensitivity C-reactive protein compared to placebo groups (Javadi et al., 2014). The clinical trials of quercetin for the treatment of RA were shown in Table 4.
TABLE 4.
An overview on clinical trials evaluating effects of compounds involved in EH on RA.
| NO. | Flavonoids | Dose | Duration | Size | Results | References |
|---|---|---|---|---|---|---|
| 1 | quercetin | 500 mg/day | 8 weeks | 50 | ↓hs-TNFα, EMS, pain, DAS-28, HAQ | Javadi et al. (2017) |
| 2 | quercetin | 500 mg/day | 8 weeks | 51 | no significant differences in TAC, ox-LDL, MDA, hs-CRP | Javadi et al. (2014) |
| 3 | quercetin (plus azathioprine) | 1,500 mg/day | 8 weeks | 190 | ↓ICAM-1, IL-6, complement proteins, ↑IL-10 | Al-Rekabi et al. (2015) |
hs-TNFα, high sensitivity tumor necrosis factor α, EMS, early morning stiffness; DAS-28, Disease Activity Score–28; HAQ, health assessment questionnaire; ICAM-1, intercellular adhesion molecule I; TAC, total antioxidant capacity; ox-LDL, oxidized low density lipoprotein; MDA, malondialdehyde; ↑ = up-regulation; ↓ = down-regulation.
Alternative strategies for the treatment of RA
Combination with approved drugs
According to European League Against Rheumatism (EULAR) recommendation, DMARDs should be started as soon as the diagnosis of RA (Smolen et al., 2020). Methotrexate (MTX), an efficacious DMARDs, is the first line for the treatment of RA. However, some adverse effects of MTX have limited their extensive clinical application, such as liver dysfunction, renal failure and nausea (Katturajan et al., 2021). In order to overcome the disadvantages and further improve the effective in treatment, it is necessary to explore combination therapy of compounds from EH and DMARDs for the treatment of RA.
Compared to the quercetin (30 mg/kg orally) or MTX (0.75 mg intraperitoneally twice a week) groups, the combination therapy significantly inhibited paw thickness and expression of proinflammatory cytokines including IL-1β, TNF-α, IL-6, and IL-17 in CIA mice (Haleagrahara et al., 2018). Moreover, quercetin reversed the transaminases levels of MTX groups including alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (Costa et al., 2021). However, the other report showed that the concurrent therapy of quercetin (30 mg/kg orally) and MTX (0.5 mg/kg intraperitoneally) did not provide greater protection than a single agent (Haleagrahara et al., 2017).
Bioavailability improvement
EH has been commonly used in various traditional Chinese decoctions for the treatment of RA. Compared to other delivery routes, oral administration has clear advantages including lower pain and less risk of cross-infection (Das and Chaudhury, 2011). Consequently, oral administration was frequently chosen as the primary clinical administration approach. However, poor solubility, low permeability and inferior stability by oral administration result in restriction of their effectiveness (Zhao et al., 2019). For example, bioavailability of quercetin is pharmaceutically characterized as poor solubility, low bioavailability, poor permeability and instability (Cai et al., 2013). Furthermore, low concentration of those components by oral administration exerts curative effects in the joint cavities with systemic circulation. Given the above characteristics and disadvantages, new technologies have drawn increased attention to improve the bioavailability and effectiveness of the components. The new technologies of bioavailability improvement of EH were shown in Figure 4.
FIGURE 4.
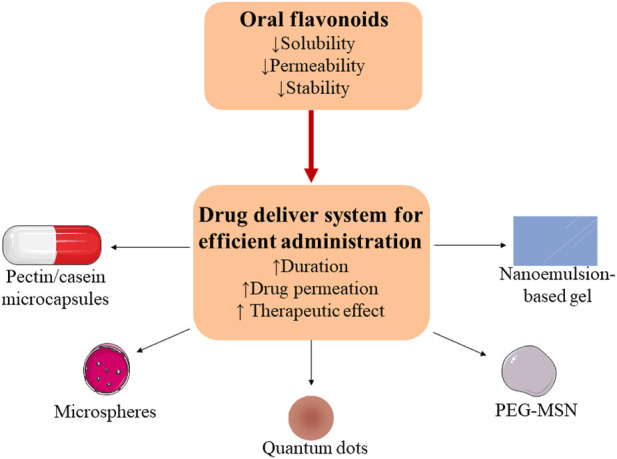
Limitations of flavonoids through oral administration in RA therapy and their delivery systems for a promising option.
Nanoencapsulation technology including liposomes, nanoemulsions, and nanocapsules appear to be a promising option to increase bioavailability of the compounds (Han et al., 2020). Treatment of 10 mg/kg quercetin carried by pectin/casein microcapsules reduced oxidative stress and had no hepatotoxicity and mitochondriotoxicity by oral administration in AIA rats (Souza et al., 2021). In vitro and in vivo studies reported that quercetin loaded in polycaprolactone microspheres increased the duration in joint cavity for more than 30 days (Natarajan et al., 2011). Quercetin loaded by nanoemulsion-based gel had no toxic effect on synoviocytes and improved drug permeation and attenuated paw edema over 24 h in AIA rats (Gokhale et al., 2019). Complexes of quantum dots and quercetin significantly inhibited inflammation and oxidative stress and improved cartilage regeneration in AIA rats (Jeyadevi et al., 2013). Compared with luteolin treatment, combination of polyethylene glycol-mesoporous silica nano-carriers and luteolin was more effective and had longer existence time in AIA rats (Pang et al., 2021).
Safety assessment of EH
EH is an ancient traditional Chinese herbal for 2000 years and has been frequently used to treat various disease. However, it was reported that EH could cause drug-induced liver injury. Wang et al. found that water extract and alcohol extract of Epimedium brevicornu Maxim (20, 40, 80 g/kg) for 8 weeks reduced body weight of mice. Furthermore, the organ coefficient, blood indicators, serum biochemical indicators had a certain degree of change (P<0.05), indicating that EH at 140 times the maximum human dose have certain toxic effects on mice for 8 weeks (Wang. et al., 2018). In vivo study showed that Epimedium koreanum Nakai had liver toxicity which was enhanced by increased dosage and prolonged time (Zhang. et al., 2018). Therefore, although EH is relatively safe for clinical use, it is still necessary to be cautious and do not take large doses or take it for a long time.
Previous research has reported that quercetin at dietary intake levels did not exert harmful effects on human health (Harwood et al., 2007). Recent study also showed that no zebrafish died or had abnormal morphology under 200 μM icariin (Zhong et al., 2019).
In vivo studies reported that hyperoside (65 and 500 mg/kg) had chronic hepatotoxicity and nephrotoxicity in beagle dogs, but the destruction was reversed and returned to normal after withdrawal (Ai. et al., 2015). High concentrations (36 μg/ml) of ikarisoside A induced liver injury in HL-7702 and HepG2 Cells via enhancing oxidative stress and inducing apoptosis (Zhang L. et al., 2019).
Conclusion and future perspectives
RA is a chronic autoimmune disease and causes of RA are unclear. In TCM theory, the patients with RA are affected by “Wind”, “Cold”, “Damp” and “Kidney deficiency”. EH can strengthen bones and muscle, dispel wind chill and tonify the kidney, so it has been widely used in prescriptions of TCM for the treatment of RA. Remarkable curative effects in clinic draw increased attention and pharmacological research to investigate material foundation and pharmacological mechanism.
In this review, 293 components were searched from EH in different database and 15 compounds were filtered for the treatment of RA. Then, therapeutic effect, pharmacological mechanism, bioavailability and toxicity of the components were overall summarized and analyzed. This paper summarized the mechanism of the compounds in the treatment of RA through studies in vivo and in vitro. Studies showed that the components from EH have extensive pharmacological activities including anti-inflammatory, immunoregulatory, antioxidant, antiangiogenic, anti-FLS and osteoprotective effects.
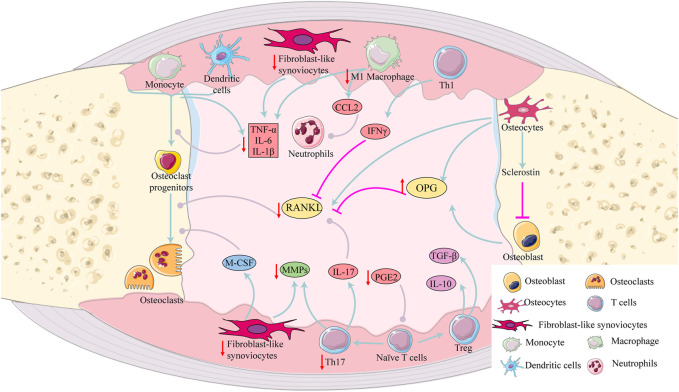
By summarizing, it is no wonder that icariin is prime component and one of chemical markers for quality control of EH, which have bright particularly attention to the health-promoting effects. Icariin has anti-RA effects via anti-inflammatory, immunoregulatory, osteoprotective and antioxidant activities. A schematic view of anti-RA effects of icariin was shown in Figure 5. However, most of data were acquired from laboratory tests in several animal and cellular models. Clinical trials are required to carried out to explore the possible therapeutic effects of icariin for the management of RA.
FIGURE 5.
A schematic view of anti-RA effects of icariin (↑ = up-regulation; ↓ = down-regulation; red arrow = icariin) (images cited from Servier Medical Art images at www.servier.com).
A large number of components from EH were searched and the results indicate that EH may have the multicomponent and multifunctional traits in the treatment of RA. The mechanism of compounds from EH for the treatment of RA was shown in Table 5. However, there are few published studies exploring the anti-RA effects of other compounds isolated from EH. In this review, anti-RA effects of the compounds in other species were inferred to EH. Therefore, it is necessary to investigate the anti-RA effects of other compounds from EH in the future.
TABLE 5.
The mechanism of compounds from EH for the treatment of RA.
| Compounds | Anti-inflammatory activities | Immunoregulatory activities | Osteoprotective activities | FLS | Antiangiogenic activities | Antioxidant activities | |
|---|---|---|---|---|---|---|---|
| Apigenin | ↓TNF-α, IL-12p70, IL-10, CXCR4, COX2, NO, iNOS | ↓Th1 cells, DCs maturation | ↓RANKL, RANK, MMP3 | ↓proliferation, PI3K/Akt | ↓VEGF, VEGFR1, VEGFR2, VCAM | ↑ROS | |
| ↑IL-1β | ↑Th2 cells | ↑OPG | ↑apoptosis, miR-223-3p | ||||
| Astragalin | ↓TNF-α, IL-1β, IL-6, IL-8 | ↓MMP-1, MMP-3, MMP-13, MAPKs, c-Jun/AP-1 | |||||
| Chlorogenic acid | ↓Th1 cells, BAFF, NF-κB | ↓proliferation, NF-κB, JAK/STAT | |||||
| ↑Th2 cells | ↑apoptosis | ||||||
| Emodin | ↓TNF-α, IL-6, PGE2 | ↓NETs | ↓MMP-1, MMP-3, Osteoclastogenesis, NF-κB | ||||
| COX-2 | ↑neutrophil apoptosis | ||||||
| Hyperoside | ↓TNF-α, IL-6, iNOS | ↓MMP-3, MMP-9 | ↓proliferation, migration, NF-κB | ||||
| Icariin | ↓IL-1β, IL-6 and TNF-α, PGE2 | ↓Th17 cells, M1 macrophage | ↓RANKL, MMP-9 | ↓migration, proliferation | ↓mitochondrial transmembrane potential | ||
| ↑M2 macrophage | ↑OPG | ↑apoptosis | ↑ROS | ||||
| Ikarisoside A | ↓MMP9, NF-κB, JNK, Akt, c-Fos, NFATc1 | ||||||
| Isoliquiritigenin | ↓IL-1β, iNOS, COX2 | ↓osteoclastogenesis, MAPK, NF-κB | |||||
| Kaempferitrin | ↓IL-1β, IL-6, TNF-α | ↓MMP1, MMP3 | ↓proliferation, NF-κB, Akt/mTOR | ||||
| ↑apoptosis | |||||||
| Kaempferol | ↓IL-17, IL-21, TNF-α, IL-6, IL-1β, NO, iNOS, COX-2, PGE2 | ↓Th17 cells | ↓MMP1, MMP3 | ↓migration, proliferation, MAPK, NF-κB | ↓ROS myeloperoxidase | ||
| ↑Treg cells | ↑apoptosis | ||||||
| Luteolin | ↓TNF-α, IL-1β, NO, iNOS, COX-2 | ↓MMP1, MMP3, RANKL, MAPKs, AP-1, NF-κB, PI3K-Akt | ↓proliferation, NF-κB, JAK/STAT, PI3K-Akt | ↓VEGF, HIF-1α | ↓Malondialdehyde, 15-lipoxygenase | ||
| ↑OPG | ↑apoptosis | ||||||
| Magnoflorine | ↓IL-6, IL-8, iNOS | ↓migration, invasion, proliferation, Keap1, PI3K/Akt/NF-κB | |||||
| ↑TNF-α, IL-1β | ↑apoptosis, cell cycle arrest, Nrf2, HO-1 | ||||||
| Quercetin | ↓IL-1β, IL-6, TNF-α, IL-8, IL-13, IL-17, MCP-1, PGE2, COX2, NO, iNOS | ↓Th17 cells, neutrophil activity | ↓MMP1, MMP3, MMP-13 RANKL | ↓migration, NF-κB | ↓VEGFA, HIF-1α | ↓ROS, TBARS, 12/15-lipoxygenase, myeloperoxidase | |
| ↑Treg cells | ↑apoptosis | ||||||
| Tricin | ↓TNF-α, IL-6, IL-1β | ||||||
| β-Sitosterol | ↓M1 macrophage | ↓VEGFR2, p-VEGFR2 | |||||
| ↑M2 macrophage | |||||||
Author contributions
L-BZ and YY contributed equally to this work and they collated documents and wrote the manuscript; JH, P-PW, and J-PW polished the language; XC, T-YL, Y-XG, JL, and Z-RY helped to organize the literatures; Q-WT and YX contributed significantly to design and revision of the manuscript.
Funding
The work was supported by National Key R&D Program of China (NO. 2018YFC1705502), National High Level Hospital Clinical Research Funding (NO.2022-NHLHCRF-LX-02-02), Beijing Chinese Medicine Science and Technology Development Fund Project (Youth Planning Project QN-2020-32), Elite Medical Professionals project of China-Japan Friendship Hospital (No. ZRJY2021-TD06).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Ahmed A. H., Abd Elkarim A. S. (2021). Bioactive compounds with significant anti- rheumatoid arthritis effect isolated for the first time from leaves of bougainvillea spectabilis. Curr. Pharm. Biotechnol. 22 (15), 2048–2053. 10.2174/1389201021666201229111825 [DOI] [PubMed] [Google Scholar]
- Ai G., Wang D., Huang Z., Zhang H. (2015). Long-term toxicity of hyperoside in Beagle dogs. Chin. J. New Drugs 24 (14), 1641–1647. [Google Scholar]
- Al-Rekabi M. D., Ali S. H., Al-Basaisi H., Hashim F., Hussein A. H., Abbas H. K. (2015). Immunomodulatory effects of quercetin in patient with active rheumatoid arthritis. Br. J. Med. Health Res. 2 (6), 23–34. 10.3390/ijms19051453 [DOI] [Google Scholar]
- Alamgeer S. T., Hasan U. H., Uttra A. M., Qasim S., Ikram J., Saleem M., et al. (2020). Phytochemicals targeting matrix metalloproteinases regulating tissue degradation in inflammation and rheumatoid arthritis. Phytomedicine 66, 153134. 10.1016/j.phymed.2019.153134 [DOI] [PubMed] [Google Scholar]
- Aletaha D., Smolen J. S. (2018). Diagnosis and management of rheumatoid arthritis: A review. JAMA 320 (13), 1360–1372. 10.1001/jama.2018.13103 [DOI] [PubMed] [Google Scholar]
- Armas-Gonzalez E., Dominguez-Luis M. J., Diaz-Martin A., Arce-Franco M., Castro-Hernandez J., Danelon G., et al. (2018). Role of CXCL13 and CCL20 in the recruitment of B cells to inflammatory foci in chronic arthritis. Arthritis Res. Ther. 20 (1), 114. 10.1186/s13075-018-1611-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aureal M., Machuca-Gayet I., Coury F. (2020). Rheumatoid arthritis in the view of osteoimmunology. Biomolecules 11 (1), 48. 10.3390/biom11010048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodolay E., Koch A. E., Kim J., Szegedi G., Szekanecz Z. (2002). Angiogenesis and chemokines in rheumatoid arthritis and other systemic inflammatory rheumatic diseases. J. Cell. Mol. Med. 6 (3), 357–376. 10.1111/j.1582-4934.2002.tb00514.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Simonet W. S., Lacey D. L. (2003). Osteoclast differentiation and activation. Nature 423 (6937), 337–342. 10.1038/nature01658 [DOI] [PubMed] [Google Scholar]
- Brennan F. M., McInnes I. B. (2008). Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Invest. 118 (11), 3537–3545. 10.1172/JCI36389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Pope J. E. (2017). Novel treatment strategies in rheumatoid arthritis. Lancet 389 (10086), 2338–2348. 10.1016/s0140-6736(17)31491-5 [DOI] [PubMed] [Google Scholar]
- Bustamante M. F., Garcia-Carbonell R., Whisenant K. D., Guma M. (2017). Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 19 (1), 110. 10.1186/s13075-017-1303-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Fang Z., Dou J., Yu A., Zhai G. (2013). Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 20 (20), 2572–2582. 10.2174/09298673113209990120 [DOI] [PubMed] [Google Scholar]
- Canavan M., Marzaioli V., McGarry T., Bhargava V., Nagpal S., Veale D. J., et al. (2020). Rheumatoid arthritis synovial microenvironment induces metabolic and functional adaptations in dendritic cells. Clin. Exp. Immunol. 202 (2), 226–238. 10.1111/cei.13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Rivera C., Carlucci P. M., Moore E., Lingampalli N., Uchtenhagen H., James E., et al. (2017). Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2 (10), eaag3358. 10.1126/sciimmunol.aag3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi I., Arias de la Rosa I., Menegatti E., Roccatello D., Collantes-Estevez E., Lopez-Pedrera C., et al. (2018). Neutrophils: Novel key players in Rheumatoid Arthritis. Current and future therapeutic targets. Autoimmun. Rev. 17 (11), 1138–1149. 10.1016/j.autrev.2018.06.006 [DOI] [PubMed] [Google Scholar]
- Chakraborty D., Gupta K., Biswas S. (2021). A mechanistic insight of phytoestrogens used for Rheumatoid arthritis: An evidence-based review. Biomed. Pharmacother. 133, 111039. 10.1016/j.biopha.2020.111039 [DOI] [PubMed] [Google Scholar]
- Chauhan P. S., Satti N. K., Sharma P., Sharma V. K., Suri K. A., Bani S. (2012). Differential effects of chlorogenic acid on various immunological parameters relevant to rheumatoid arthritis. Phytother. Res. 26 (8), 1156–1165. 10.1002/ptr.3684 [DOI] [PubMed] [Google Scholar]
- Chen L., Yan X., Shi G., Zhao C. (2021). Clinical observation on Wangbi Tablet treating on early rheumatoid arthritis with syndrome of liver and kidney deficiency and syndrome of wind-dampness blocking collaterals. China J. Traditional Chin. Med. Pharm. 36 (04), 2400–2403. [Google Scholar]
- Chen M., Wu J., Luo Q., Mo S., Lyu Y., Wei Y., et al. (2016). The anticancer properties of Herba epimedii and its main bioactive componentsicariin and icariside II. Nutrients 8 (9), 563. 10.3390/nu8090563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Huo X., Yang L., Zhang Y., Zhou K. (2022). Determination of 11 effective components in Epimedium from different primordium by UPLC. J. Tianjin Univ. Traditional Chin. Med. 41 (02), 237–242. [Google Scholar]
- Cheng Y., Wang N., Wang X., Zhang D., Huang W., Yao X. (2006). Chemical constituents of Epimedium koraiensis. J. China Pharm. Univ. 23 (10), 644–647+657. [Google Scholar]
- Chi A., Shen Z., Zhu W., Sun Y., Kang Y., Guo F. (2017). Characterization of a protein-bound polysaccharide from Herba Epimedii and its metabolic mechanism in chronic fatigue syndrome. J. Ethnopharmacol. 203, 241–251. 10.1016/j.jep.2017.03.041 [DOI] [PubMed] [Google Scholar]
- Chi L., Gao W., Shu X., Lu X. (2014). A natural flavonoid glucoside, icariin, regulates Th17 and alleviates rheumatoid arthritis in a murine model. Mediat. Inflamm. 2014, 1–10. 10.1155/2014/392062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J. H., Jung J. Y., Lee B. J., Lee K., Park J. W., Bu Y. (2017). Epimedii Herba: A promising herbal medicine for neuroplasticity. Phytother. Res. 31 (6), 838–848. 10.1002/ptr.5807 [DOI] [PubMed] [Google Scholar]
- Choi E. J., Bae S. C., Yu R., Youn J., Sung M. K. (2009). Dietary vitamin E and quercetin modulate inflammatory responses of collagen-induced arthritis in mice. J. Med. Food 12 (4), 770–775. 10.1089/jmf.2008.1246 [DOI] [PubMed] [Google Scholar]
- Choi E. M., Lee Y. S. (2010). Luteolin suppresses IL-1β-induced cytokines and MMPs production via p38 MAPK, JNK, NF-kappaB and AP-1 activation in human synovial sarcoma cell line, SW982. Food Chem. Toxicol. 48 (10), 2607–2611. 10.1016/j.fct.2010.06.029 [DOI] [PubMed] [Google Scholar]
- Choi H. J., Park Y. R., Nepal M., Choi B. Y., Cho N. P., Choi S. H., et al. (2010). Inhibition of osteoclastogenic differentiation by Ikarisoside A in RAW 264.7 cells via JNK and NF-κB signaling pathways. Eur. J. Pharmacol. 636 (1-3), 28–35. 10.1016/j.ejphar.2010.03.023 [DOI] [PubMed] [Google Scholar]
- Chu X., Chai J., Guo S., Wang Y., Chen C. (2021). Effect of quercetin on synovial angiogenesis in rats with collagen-induced arthritis. J. Shanxi Med. Univ. 52 (03), 301–309. 10.13753/j.issn.1007-6611.2021.03.010 [DOI] [Google Scholar]
- Costa A. C. F., de Sousa L. M., Dos Santos Alves J. M., Goes P., Pereira K. M. A., Alves A., et al. (2021). Anti-inflammatory and hepatoprotective effects of quercetin in an experimental model of rheumatoid arthritis. Inflammation 44 (5), 2033–2043. 10.1007/s10753-021-01479-y [DOI] [PubMed] [Google Scholar]
- Cui X., Deng L., Huang S. (2010). Studies on chemical constituents in chloroform extraction of Epimedium sagittatum. Chin. J. Exp. Traditional Med. Formulae 16 (13), 101–103. 10.13422/j.cnki.syfjx.2010.13.032 [DOI] [Google Scholar]
- Cush J. J. (2021). Rheumatoid arthritis: Early diagnosis and treatment. Med. Clin. North Am. 105 (2), 355–365. 10.1016/j.mcna.2020.10.006 [DOI] [PubMed] [Google Scholar]
- Das S., Chaudhury A. (2011). Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 12 (1), 62–76. 10.1208/s12249-010-9563-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Zou X., Cai T., Ruan F. (2019). Effect of xianling spleen combined with tripterygium wilfordii in the treatment of rheumatoid arthritis. Zhejiang J. Traditional Chin. Med. 54 (12), 884–886. 10.13633/j.cnki.zjtcm.2019.12.014 [DOI] [Google Scholar]
- Fang Q., Zhou C., Nandakumar K. S. (2020). Molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediat. Inflamm. 2020, 1–20. 10.1155/2020/3830212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Maini R. N. (1996). Rheumatoid arthritis. Cell 85 (3), 307–310. 10.1016/s0092-8674(00)81109-5 [DOI] [PubMed] [Google Scholar]
- Fresneda Alarcon M., McLaren Z., Wright H. L. (2021). Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: Same foe different M.O. Front. Immunol. 12, 649693. 10.3389/fimmu.2021.649693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q., Jin X., Gao Y., Wang J., Yan P. (2020). Effect of hyperoside on synoviocytes of rheumatoid arthritis and its mechanism. J. Jinzhou Med. Univ. 41 (03), 7–12. 10.13847/j.cnki.lnmu.2020.03.002 [DOI] [Google Scholar]
- Fu X., Lyu X., Liu H., Zhong D., Xu Z., He F., et al. (2019). Chlorogenic acid inhibits BAFF expression in collagen-induced arthritis and human synoviocyte MH7A cells by modulating the activation of the NF-κB signaling pathway. J. Immunol. Res. 2019, 1–10. 10.1155/2019/8042097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardi C., Bauerova K., Stringa B., Kuncirova V., Slovak L., Ponist S., et al. (2015). Quercetin reduced inflammation and increased antioxidant defense in rat adjuvant arthritis. Arch. Biochem. Biophys. 583, 150–157. 10.1016/j.abb.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Ghani M. A., Barril C., Bedgood D. R., Jr., Prenzler P. D. (2017). Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. x. 230, 195–207. 10.1016/j.foodchem.2017.02.127 [DOI] [PubMed] [Google Scholar]
- Gheorghe K. R., Korotkova M., Catrina A. I., Backman L., af Klint E., Claesson H. E., et al. (2009). Expression of 5-lipoxygenase and 15-lipoxygenase in rheumatoid arthritis synovium and effects of intraarticular glucocorticoids. Arthritis Res. Ther. 11 (3), R83. 10.1186/ar2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale J. P., Mahajan H. S., Surana S. J. (2019). Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomed. Pharmacother. 112, 108622. 10.1016/j.biopha.2019.108622 [DOI] [PubMed] [Google Scholar]
- Guazelli C. F. S., Staurengo-Ferrari L., Zarpelon A. C., Pinho-Ribeiro F. A., Ruiz-Miyazawa K. W., Vicentini F., et al. (2018). Quercetin attenuates zymosan-induced arthritis in mice. Biomed. Pharmacother. 102, 175–184. 10.1016/j.biopha.2018.03.057 [DOI] [PubMed] [Google Scholar]
- Haleagrahara N., Hodgson K., Miranda-Hernandez S., Hughes S., Kulur A. B., Ketheesan N. (2018). Flavonoid quercetin-methotrexate combination inhibits inflammatory mediators and matrix metalloproteinase expression, providing protection to joints in collagen-induced arthritis. Inflammopharmacology 26 (5), 1219–1232. 10.1007/s10787-018-0464-2 [DOI] [PubMed] [Google Scholar]
- Haleagrahara N., Miranda-Hernandez S., Alim M. A., Hayes L., Bird G., Ketheesan N. (2017). Therapeutic effect of quercetin in collagen-induced arthritis. Biomed. Pharmacother. 90, 38–46. 10.1016/j.biopha.2017.03.026 [DOI] [PubMed] [Google Scholar]
- Han D., Chen Q., Chen H. (2020). Food-derived nanoscopic drug delivery systems for treatment of rheumatoid arthritis. Molecules 25 (15), 3506. 10.3390/molecules25153506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. (2022). Comparative study on the content of main flavonoids in Epimedium. Guangming J. Chin. Med. 37 (06), 991–993. [Google Scholar]
- Haque M. A., Jantan I., Harikrishnan H., Abdul Wahab S. M. (2018). Magnoflorine enhances LPS-activated pro-inflammatory responses via MyD88-dependent pathways in U937 macrophages. Planta Med. 84 (17), 1255–1264. 10.1055/a-0637-9936 [DOI] [PubMed] [Google Scholar]
- Harwood M., Danielewska-Nikiel B., Borzelleca J. F., Flamm G. W., Williams G. M., Lines T. C. (2007). A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 45 (11), 2179–2205. 10.1016/j.fct.2007.05.015 [DOI] [PubMed] [Google Scholar]
- Hou Y., Wu J., Huang Q., Guo L. (2009). Luteolin inhibits proliferation and affects the function of stimulated rat synovial fibroblasts. Cell Biol. Int. 33 (2), 135–147. 10.1016/j.cellbi.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Hu Y., Li H., Ji G., Shen X., Wei X., Fu L., et al. (2021). Chemical constituents with HDAC inhibitory effects from Epimedium sagittatum. Nat. Prod. Res. Dev. 33 (10), 1681–1690. 10.16333/j.1001-6880.2021.10.007 [DOI] [Google Scholar]
- Hwang J. K., Noh E. M., Moon S. J., Kim J. M., Kwon K. B., Park B. H., et al. (2013). Emodin suppresses inflammatory responses and joint destruction in collagen-induced arthritic mice. Rheumatol. Oxf. 52 (9), 1583–1591. 10.1093/rheumatology/ket178 [DOI] [PubMed] [Google Scholar]
- Itoh Y. (2017). Metalloproteinases in rheumatoid arthritis: Potential therapeutic targets to improve current therapies. Prog. Mol. Biol. Transl. Sci. 148, 327–338. 10.1016/bs.pmbts.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Javadi F., Ahmadzadeh A., Eghtesadi S., Aryaeian N., Zabihiyeganeh M., Rahimi Foroushani A., et al. (2017). The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double-blind, randomized controlled trial. J. Am. Coll. Nutr. 36 (1), 9–15. 10.1080/07315724.2016.1140093 [DOI] [PubMed] [Google Scholar]
- Javadi F., Eghtesadi S., Ahmadzadeh A., Aryaeian N., Zabihiyeganeh M., Foroushani A. R., et al. (2014). The effect of quercetin on plasma oxidative status, C-reactive protein and blood pressure in women with rheumatoid arthritis. Int. J. Prev. Med. 5 (3), 293–301. [PMC free article] [PubMed] [Google Scholar]
- Jeyadevi R., Sivasudha T., Rameshkumar A., Ananth D. A., Aseervatham G. S., Kumaresan K., et al. (2013). Enhancement of anti arthritic effect of quercetin using thioglycolic acid-capped cadmium telluride quantum dots as nanocarrier in adjuvant induced arthritic Wistar rats. Colloids Surfaces B Biointerfaces 112, 255–263. 10.1016/j.colsurfb.2013.07.065 [DOI] [PubMed] [Google Scholar]
- Ji M., Wang C., Yang T., Meng X., Wang X., Li M. (2021). Integrated phytochemical analysis based on UPLC-MS/MS and network pharmacology approaches to explore the effect of odontites vulgaris moench on rheumatoid arthritis. Front. Pharmacol. 12, 707687. 10.3389/fphar.2021.707687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q., Wang T., Wang X., Xu H., Liu Y., Wang Y., et al. (2019a). Astragalin suppresses inflammatory responses and bone destruction in mice with collagen-induced arthritis and in human fibroblast-like synoviocytes. Front. Pharmacol. 10, 94. 10.3389/fphar.2019.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q., Wang Y., Liang Q., Shi Q. (2019b). Effect of quercetin on the expression of inflammatory factors and matrix metalloproteinases in fibroblast-like synoviocytes of human rheumatoid arthritis. Chin. J. Osteoporos. 25 (06), 738–741. [Google Scholar]
- Jiang Q., Yang G., Liu Q., Wang S., Cui D. (2021). Function and role of regulatory T cells in rheumatoid arthritis. Front. Immunol. 12, 626193. 10.3389/fimmu.2021.626193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C. H., So Y., Nam B., Han S. N., Kim J. B. (2017). Isoegomaketone alleviates the development of collagen antibody-induced arthritis in male balb/c mice. Molecules 22 (7), 1209. 10.3390/molecules22071209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q., Lee C., Lee J. W., Yeon E. T., Lee D., Han S. B., et al. (2014). 2-Phenoxychromones and prenylflavonoids from Epimedium koreanum and their inhibitory effects on LPS-induced nitric oxide and interleukin-1β production. J. Nat. Prod. (Gorakhpur). 77 (7), 1724–1728. 10.1021/np400831p [DOI] [PubMed] [Google Scholar]
- Jin S., Chen H., Li Y., Zhong H., Sun W., Wang J., et al. (2018). Maresin 1 improves the Treg/Th17 imbalance in rheumatoid arthritis through miR-21. Ann. Rheum. Dis. 77 (11), 1644–1652. 10.1136/annrheumdis-2018-213511 [DOI] [PubMed] [Google Scholar]
- Jin X., Gao W., Feng X., Sui H., Fu Q. (2021). Therapeutic effect of hyperoside on mice with collagen-induced arthritis. J. Pract. Med. 37 (17), 2199–2203. [DOI] [Google Scholar]
- Jin X. N., Yan E. Z., Wang H. M., Sui H. J., Liu Z., Gao W., et al. (2016). Hyperoside exerts anti-inflammatory and anti-arthritic effects in LPS-stimulated human fibroblast-like synoviocytes in vitro and in mice with collagen-induced arthritis. Acta Pharmacol. Sin. 37 (5), 674–686. 10.1038/aps.2016.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katturajan R., S V., Rasool M., Evan Prince S. (2021). Molecular toxicity of methotrexate in rheumatoid arthritis treatment: A novel perspective and therapeutic implications. Toxicology 461, 152909. 10.1016/j.tox.2021.152909 [DOI] [PubMed] [Google Scholar]
- Kawaguchi K., Kaneko M., Miyake R., Takimoto H., Kumazawa Y. (2019). Potent inhibitory effects of quercetin on inflammatory responses of collagen-induced arthritis in mice. Endocr. Metab. Immune Disord. Drug Targets 19 (3), 308–315. 10.2174/1871530319666190206225034 [DOI] [PubMed] [Google Scholar]
- Kennedy A., Fearon U., Veale D. J., Godson C. (2011). Macrophages in synovial inflammation. Front. Immunol. 2, 52. 10.3389/fimmu.2011.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. R., Kim B. M., Won J. Y., Lee K. A., Ko H. M., Kang Y. S., et al. (2019). Quercetin, a plant polyphenol, has potential for the prevention of bone destruction in rheumatoid arthritis. J. Med. Food 22 (2), 152–161. 10.1089/jmf.2018.4259 [DOI] [PubMed] [Google Scholar]
- Kim J.-Y., Shim S. H. (2019). Epimedium koreanum extract and its flavonoids reduced atherosclerotic risk via suppressing modification of human HDL. Nutrients 11 (5), 1110. 10.3390/nu11051110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Y., Park S. J., Yun K. J., Cho Y. W., Park H. J., Lee K. T. (2008). Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-κB in RAW 264.7 macrophages. Eur. J. Pharmacol. 584 (1), 175–184. 10.1016/j.ejphar.2008.01.032 [DOI] [PubMed] [Google Scholar]
- Kitaura H., Marahleh A., Ohori F., Noguchi T., Shen W. R., Qi J., et al. (2020). Osteocyte-related cytokines regulate osteoclast formation and bone resorption. Int. J. Mol. Sci. 21 (14), 5169. 10.3390/ijms21145169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. J., Moon S. J., Jeong J. H., Lee S., Lee M. H., Yoo S. M., et al. (2018). Kaempferol targeting on the fibroblast growth factor receptor 3-ribosomal S6 kinase 2 signaling axis prevents the development of rheumatoid arthritis. Cell Death Dis. 9 (3), 401. 10.1038/s41419-018-0433-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Kim B., Jin W. J., Kim H. H., Ha H., Lee Z. H. (2017). Pathogenic roles of CXCL10 signaling through CXCR3 and TLR4 in macrophages and T cells: Relevance for arthritis. Arthritis Res. Ther. 19 (1), 163. 10.1186/s13075-017-1353-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Kim G. H. (2010). Evaluation of antioxidant and inhibitory activities for different subclasses flavonoids on enzymes for rheumatoid arthritis. J. Food Sci. 75 (7), H212–H217. 10.1111/j.1750-3841.2010.01755.x [DOI] [PubMed] [Google Scholar]
- Lei J., Zhang Z., He M., Li H. (2020). Effect of icariin on NLRP3 inflammatory corpuscle signaling pathway in mice with rheumatoid arthritis. Mod. J. Integr. Traditional Chin. West. Med. 29 (29), 3206–3211. [Google Scholar]
- Li F., Du B. W., Lu D. F., Wu W. X., Wongkrajang K., Wang L., et al. (2017). Flavonoid glycosides isolated from Epimedium brevicornum and their estrogen biosynthesis-promoting effects. Sci. Rep. 7 (1), 7760. 10.1038/s41598-017-08203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Chen H. Y., Liu W. X., Jia X. X., Zhang J. G., Ma C. L., et al. (2017). Prostaglandin E2 restrains human Treg cell differentiation via E prostanoid receptor 2-protein kinase A signaling. Immunol. Lett. 191, 63–72. 10.1016/j.imlet.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Li H., Wan A. (2013). Apoptosis of rheumatoid arthritis fibroblast-like synoviocytes: Possible roles of nitric oxide and the thioredoxin 1. Mediat. Inflamm. 2013, 1–8. 10.1155/2013/953462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Peng L., Miao J., Qiu Y., Zhou Y., Gao X., et al. (2011). Icariin induces the expression of toll-like receptor 9 in ana-1 murine macrophages. Phytother. Res. 25 (11), 1732–1735. 10.1002/ptr.3514 [DOI] [PubMed] [Google Scholar]
- Li W., Pan J.-Q., Lü M.-J., Zhang R.-Y., Xiao P.-G. (1995a). A 9, 10-dihydrophenanthrene derivate from Epimedium koreanum. Phytochemistry 39 (1), 231–233. 10.1016/0031-9422(94)00926-k [DOI] [Google Scholar]
- Li W., Xiao P., Zhang R. (1994). Chemical constituents of Epimedium koreanum Nakai. Nat. Prod. Res. 03, 4–8. 10.16333/j.1001-6880.1994.03.002 [DOI] [PubMed] [Google Scholar]
- Li W., Zhang R., Xiao P. (1995b). Study on chemical constituents of Epimedium koraiensis. Chin. Tradit. Herb. Drugs 09, 453–455+503. [Google Scholar]
- Li X., Han Y., Zhou Q., Jie H., He Y., Han J., et al. (2016). Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis. J. Cell. Mol. Med. 20 (1), 170–180. 10.1111/jcmm.12717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Yang B., Bai J. Y., Xia S., Mao M., Li X., et al. (2019). The roles of synovial hyperplasia, angiogenesis and osteoclastogenesis in the protective effect of apigenin on collagen-induced arthritis. Int. Immunopharmacol. 73, 362–369. 10.1016/j.intimp.2019.05.024 [DOI] [PubMed] [Google Scholar]
- Liang Q., Duan Y., Qin C. (2019). Effect of tonifying liver and kidney decoction combined with rutine therapy on serum inflammatory factors and quality of Life for RA patients. J. Sichuan Traditional Chin. Med. 37 (07), 143–146. [Google Scholar]
- Lin F., Luo X., Tsun A., Li Z., Li D., Li B. (2015). Kaempferol enhances the suppressive function of Treg cells by inhibiting FOXP3 phosphorylation. Int. Immunopharmacol. 28 (2), 859–865. 10.1016/j.intimp.2015.03.044 [DOI] [PubMed] [Google Scholar]
- Lin L., Gu X., Chen L., Zhang T., Wang C., Wang Z., et al. (2021). Study on the alleviation of Fengshi Gutong capsule on rheumatoid arthritis through integrating network pharmacology and experimental exploration. J. Ethnopharmacol. 280, 114471. 10.1016/j.jep.2021.114471 [DOI] [PubMed] [Google Scholar]
- Lin Y. J., Anzaghe M., Schulke S. (2020). Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells 9 (4), 880. 10.3390/cells9040880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Xu H., Ren N., Cheng C., Zeng P., Lu D., et al. (2021). Prediction and verification of the major ingredients and molecular targets of Tripterygii Radix against rheumatoid arthritis. Front. Pharmacol. 12, 639382. 10.3389/fphar.2021.639382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li F. (2018). Kidney-tonifying arthralgia-eliminating decoction combined with western medicine in treating 60 Cases of rheumatoid arthritis, West. J. Traditional Chin. Med. 31 (06), 93–95. [Google Scholar]
- Liu R., Hao D., Xu W., Li J., Li X., Shen D., et al. (2019). β-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm. Biol. 57 (1), 161–168. 10.1080/13880209.2019.1577461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhu L., Zhang J., Yu J., Cheng X., Peng B. (2016). Anti-osteoclastogenic activity of isoliquiritigenin via inhibition of NF-κB-dependent autophagic pathway. Biochem. Pharmacol. 106, 82–93. 10.1016/j.bcp.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Liu T., Zhao M., Zhang Y., Qiu Z., Zhang Y., Zhao C., et al. (2021). Pharmacokinetic-pharmacodynamic modeling analysis and anti-inflammatory effect of Wangbi capsule in the treatment of adjuvant-induced arthritis. Biomed. Chromatogr. 35 (7), e5101. 10.1002/bmc.5101 [DOI] [PubMed] [Google Scholar]
- Liu Y., Feng W., He D., Wang Q. (2013). Effect of icariin on bone destruction and serum RANKL/OPG levels in type II collagen-induced arthritis rats. Chin. J. Integr. Traditional West. Med. 33 (09), 1221–1225. [PubMed] [Google Scholar]
- Liu Y., Lu B., Wu Y., Qi Q., Liu M., Yang C., et al. (2021). Inhibitory effect of luteolin on activation of NLRP3 inflammatory corpuscle and protection of joint bone in rheumatoid arthritis rats. China J. Traditional Chin. Med. Pharm. 36 (01), 513–516. [Google Scholar]
- Lou L., Liu Y., Zhou J., Wei Y., Deng J., Dong B., et al. (2015). Chlorogenic acid and luteolin synergistically inhibit the proliferation of interleukin-1β-induced fibroblast-like synoviocytes through regulating the activation of NF-κB and JAK/STAT-signaling pathways. Immunopharmacol. Immunotoxicol. 37 (6), 499–507. 10.3109/08923973.2015.1095763 [DOI] [PubMed] [Google Scholar]
- Luo H., He D., Feng W., Zhu W., Yu J., Qiu M. (2019). 40 cases of rheumatoid arthritis with kidney deficiency and cold-dampness syndrome treatment with Yishen Qubi Tongluo decoction. J. JIANGXI Univ. TCM 31 (05), 35–38. [Google Scholar]
- Ma H., He X., Yang Y., Li M., Hao D., Jia Z. (2011). The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 134 (3), 519–541. 10.1016/j.jep.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Ma W., Yao G., Jia Q., Ouyang H., Chang Y., he J. (2019). Qualitative analysis on chemical constituents from Epimedium brevicornu by UPLC-Q-TOF-MS/MS. J. Chin. Med. Mater. 42 (07), 1554–1559. 10.13863/j.issn1001-4454.2019.07.020 [DOI] [Google Scholar]
- Mamani-Matsuda M., Kauss T., Al-Kharrat A., Rambert J., Fawaz F., Thiolat D., et al. (2006). Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decreased macrophage inflammatory mediators. Biochem. Pharmacol. 72 (10), 1304–1310. 10.1016/j.bcp.2006.08.001 [DOI] [PubMed] [Google Scholar]
- Marahleh A., Kitaura H., Ohori F., Kishikawa A., Ogawa S., Shen W. R., et al. (2019). TNF-Alpha directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front. Immunol. 10, 2925. 10.3389/fimmu.2019.02925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateen S., Moin S., Khan A. Q., Zafar A., Fatima N. (2016a). Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS One 11 (4), e0152925. 10.1371/journal.pone.0152925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateen S., Zafar A., Moin S., Khan A. Q., Zubair S. (2016b). Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 455, 161–171. 10.1016/j.cca.2016.02.010 [DOI] [PubMed] [Google Scholar]
- McCracken J. M., Allen L. A. (2014). Regulation of human neutrophil apoptosis and lifespan in health and disease. J. Cell Death 7, JCD.S11038–23. 10.4137/JCD.S11038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes I. B., Buckley C. D., Isaacs J. D. (2016). Cytokines in rheumatoid arthritis - shaping the immunological landscape. Nat. Rev. Rheumatol. 12 (1), 63–68. 10.1038/nrrheum.2015.171 [DOI] [PubMed] [Google Scholar]
- Meeuwisse C. M., van der Linden M. P., Rullmann T. A., Allaart C. F., Nelissen R., Huizinga T. W., et al. (2011). Identification of CXCL13 as a marker for rheumatoid arthritis outcome using an in silico model of the rheumatic joint. Arthritis Rheum. 63 (5), 1265–1273. 10.1002/art.30273 [DOI] [PubMed] [Google Scholar]
- Melincovici C. S., Boşca A. B., Şuşman S., Mărginean M., Mihu C., Istrate M., et al. (2018). Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 59 (2), 455–467. [PubMed] [Google Scholar]
- Minhas R., Bansal Y., Bansal G. (2020). Inducible nitric oxide synthase inhibitors: A comprehensive update. Med. Res. Rev. 40 (3), 823–855. 10.1002/med.21636 [DOI] [PubMed] [Google Scholar]
- Miyabe Y., Miyabe C., Iwai Y., Luster A. D. (2020). Targeting the chemokine system in rheumatoid arthritis and vasculitis. JMA J. 3 (3), 182–192. 10.31662/jmaj.2020-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabe Y., Miyabe C., Murooka T. T., Kim E. Y., Newton G. A., Kim N. D., et al. (2017). Complement C5a receptor is the key initiator of neutrophil adhesion igniting immune complex-induced arthritis. Sci. Immunol. 2 (7), eaaj2195. 10.1126/sciimmunol.aaj2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura R. A., Cascao R., Perpetuo I., Canhao H., Vieira-Sousa E., Mourao A. F., et al. (2011). Cytokine pattern in very early rheumatoid arthritis favours B-cell activation and survival. Rheumatol. Oxf. 50 (2), 278–282. 10.1093/rheumatology/keq338 [DOI] [PubMed] [Google Scholar]
- Munro D., Treberg J. R. (2017). A radical shift in perspective: Mitochondria as regulators of reactive oxygen species. J. Exp. Biol. 220, 1170–1180. 10.1242/jeb.132142 [DOI] [PubMed] [Google Scholar]
- Natarajan V., Krithica N., Madhan B., Sehgal P. K. (2011). Formulation and evaluation of quercetin polycaprolactone microspheres for the treatment of rheumatoid arthritis. J. Pharm. Sci. 100 (1), 195–205. 10.1002/jps.22266 [DOI] [PubMed] [Google Scholar]
- Niu R. (1989). Action of the drug Herba Epimedii on testosterone of the mouse plasma and its accessory sexual organ before and after processing. Zhongguo Zhong Yao Za Zhi 14 (9), 530574. [PubMed] [Google Scholar]
- Noack M., Miossec P. (2014). Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 13 (6), 668–677. 10.1016/j.autrev.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Nygaard G., Firestein G. S. (2020). Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 16 (6), 316–333. 10.1038/s41584-020-0413-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil L. J., Kaplan M. J. (2019). Neutrophils in rheumatoid arthritis: Breaking immune tolerance and fueling disease. Trends Mol. Med. 25 (3), 215–227. 10.1016/j.molmed.2018.12.008 [DOI] [PubMed] [Google Scholar]
- Okano M., Sugata Y., Fujiwara T., Matsumoto R., Nishibori M., Shimizu K., et al. (2006). E prostanoid 2 (EP2)/EP4-mediated suppression of antigen-specific human T-cell responses by prostaglandin E2. Immunology 118 (3), 343–352. 10.1111/j.1365-2567.2006.02376.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Li N., Liu Y., Xu Q., Liu Q., You Y., et al. (2018). Kaempferol inhibits the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes by blocking activation of the MAPK pathway. Int. Immunopharmacol. 55, 174–182. 10.1016/j.intimp.2017.12.011 [DOI] [PubMed] [Google Scholar]
- Pan F., Zhu L., Lv H., Pei C. (2016). Quercetin promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by upregulating lncRNA MALAT1. Int. J. Mol. Med. 38 (5), 1507–1514. 10.3892/ijmm.2016.2755 [DOI] [PubMed] [Google Scholar]
- Pandya J. M., Lundell A. C., Andersson K., Nordstrom I., Theander E., Rudin A. (2017). Blood chemokine profile in untreated early rheumatoid arthritis: CXCL10 as a disease activity marker. Arthritis Res. Ther. 19 (1), 20. 10.1186/s13075-017-1224-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J., Yang F., Zhang Z., Yang W., Li Y., Xu H. (2021). The role of luteolin nanocomposites in rheumatoid arthritis treatment. Mater. Express 11 (3), 303–309. [Google Scholar]
- Pang X., Yin S. S., Yu H. Y., Zhang Y., Wang T., Hu L. M., et al. (2018). Prenylated flavonoids and dihydrophenanthrenes from the leaves of Epimedium brevicornu and their cytotoxicity against HepG2 cells. Nat. Prod. Res. 32 (19), 2253–2259. 10.1080/14786419.2017.1405410 [DOI] [PubMed] [Google Scholar]
- Park J. Y., Pillinger M. H., Abramson S. B. (2006). Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 119 (3), 229–240. 10.1016/j.clim.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Phull A. R., Nasir B., Haq I. U., Kim S. J. (2018). Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem. Biol. Interact. 281, 121–136. 10.1016/j.cbi.2017.12.024 [DOI] [PubMed] [Google Scholar]
- Pu L., Meng Q., Li S., Liu B., Li F. (2021). Icariin arrests cell cycle progression and induces cell apoptosis through the mitochondrial pathway in human fibroblast-like synoviocytes. Eur. J. Pharmacol. 912, 174585. 10.1016/j.ejphar.2021.174585 [DOI] [PubMed] [Google Scholar]
- Qian K., Zheng X. X., Wang C., Huang W. G., Liu X. B., Xu S. D., et al. (2021). β-Sitosterol inhibits rheumatoid synovial angiogenesis through suppressing VEGF signaling pathway. Front. Pharmacol. 12, 816477. 10.3389/fphar.2021.816477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H., Mei J., Yuan K., Zhang K., Zhou F., Tang T., et al. (2022). Immune-regulating strategy against rheumatoid arthritis by inducing tolerogenic dendritic cells with modified zinc peroxide nanoparticles. J. Nanobiotechnology 20 (1), 323. 10.1186/s12951-022-01536-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F. C., Jiang X. J., Wen S. Z., Wang L. X., Li X. M., Wang F. (2018). Prenylated 2-phenoxychromones and flavonoids from Epimedium brevicornum and revised structures of epimedonins A and B. J. Nat. Prod. (Gorakhpur). 81 (1), 16–21. 10.1021/acs.jnatprod.7b00514 [DOI] [PubMed] [Google Scholar]
- Ross E. A., Devitt A., Johnson J. R. (2021). Macrophages: The good, the bad, and the gluttony. Front. Immunol. 12, 708186. 10.3389/fimmu.2021.708186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccol R., da Silveira K. L., Manzoni A. G., Abdalla F. H., de Oliveira J. S., Dornelles G. L., et al. (2020). Antioxidant, hepatoprotective, genoprotective, and cytoprotective effects of quercetin in a murine model of arthritis. J. Cell. Biochem. 121 (4), 2792–2801. 10.1002/jcb.29502 [DOI] [PubMed] [Google Scholar]
- Santos E. O., Kabeya L. M., Figueiredo-Rinhel A. S., Marchi L. F., Andrade M. F., Piatesi F., et al. (2014). Flavonols modulate the effector functions of healthy individuals' immune complex-stimulated neutrophils: A therapeutic perspective for rheumatoid arthritis. Int. Immunopharmacol. 21 (1), 102–111. 10.1016/j.intimp.2014.04.014 [DOI] [PubMed] [Google Scholar]
- Sheibanie A. F., Khayrullina T., Safadi F. F., Ganea D. (2007). Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum. 56 (8), 2608–2619. 10.1002/art.22794 [DOI] [PubMed] [Google Scholar]
- Shen P., Lin W., Ba X., Huang Y., Chen Z., Han L., et al. (2021). Quercetin-mediated SIRT1 activation attenuates collagen-induced mice arthritis. J. Ethnopharmacol. 279, 114213. 10.1016/j.jep.2021.114213 [DOI] [PubMed] [Google Scholar]
- Shen Y., Fan X., Qu Y., Tang M., Huang Y., Peng Y., et al. (2022). Magnoflorine attenuates inflammatory responses in RA by regulating the PI3K/Akt/NF-κB and Keap1-Nrf2/HO-1 signalling pathways in vivo and in vitro . Phytomedicine 104, 154339. 10.1016/j.phymed.2022.154339 [DOI] [PubMed] [Google Scholar]
- Shin G. C., Kim C., Lee J. M., Cho W. S., Lee S. G., Jeong M., et al. (2009). Apigenin-induced apoptosis is mediated by reactive oxygen species and activation of ERK1/2 in rheumatoid fibroblast-like synoviocytes. Chem. Biol. Interact. 182 (1), 29–36. 10.1016/j.cbi.2009.07.016 [DOI] [PubMed] [Google Scholar]
- Shinozaki M., Inoue E., Nakajima A., Hara M., Tomatsu T., Kamatani N., et al. (2007). Elevation of serum matrix metalloproteinase-3 as a predictive marker for the long-term disability of rheumatoid arthritis patients in a prospective observational cohort IORRA. Mod. Rheumatol. 17 (5), 403–408. 10.1007/s10165-007-0608-5 [DOI] [PubMed] [Google Scholar]
- Shrivastava A. K., Pandey A. (2013). Inflammation and rheumatoid arthritis. J. Physiol. Biochem. 69 (2), 335–347. 10.1007/s13105-012-0216-5 [DOI] [PubMed] [Google Scholar]
- Shu B., Cai F. (2021). Clinical effect of Bushen Tongluo Recipe on rheumatoid arthritis of liver and kidney yin deficiency type and its influence on related cytokines. Beijing J. Traditional Chin. Med. 40 (02), 140–143. 10.16025/j.1674-1307.2021.02.009 [DOI] [Google Scholar]
- Smallwood M. J., Nissim A., Knight A. R., Whiteman M., Haigh R., Winyard P. G. (2018). Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 125, 3–14. 10.1016/j.freeradbiomed.2018.05.086 [DOI] [PubMed] [Google Scholar]
- Smolen J. S., Landewe R. B. M., Bijlsma J. W. J., Burmester G. R., Dougados M., Kerschbaumer A., et al. (2020). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 79 (6), 685–699. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- Souza K. S., Moreira L. S., Silva B. T., Oliveira B. P. M., Carvalho A. S., Silva P. S., et al. (2021). Low dose of quercetin-loaded pectin/casein microparticles reduces the oxidative stress in arthritic rats. Life Sci. 284, 119910. 10.1016/j.lfs.2021.119910 [DOI] [PubMed] [Google Scholar]
- Srivastava R. K., Dar H. Y., Mishra P. K. (2018). Immunoporosis: Immunology of osteoporosis-role of T cells. Front. Immunol. 9, 657. 10.3389/fimmu.2018.00657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X. D., Li W., Ma J. Y., Kim Y. H. (2018). Chemical constituents from Epimedium koreanum Nakai and their chemotaxonomic significance. Nat. Prod. Res. 32 (19), 2347–2351. 10.1080/14786419.2017.1405412 [DOI] [PubMed] [Google Scholar]
- Sun G., Chen X. (2021). Efficacy and safety of Kunxian capsule and Leigongteng Duogan tablets in the treatment of rheumatoid arthritis. China Prac. Med. 16 (33), 138–140. 10.14163/j.cnki.11-5547/r.2021.33.050 [DOI] [Google Scholar]
- Sun M., Zhang D., Ouyang H., Chang Y., He J. (2018). Simultaneous determination of 11 compounds in Epimedium of different places of origin by UPLC. Liaoning J. Traditional Chin. Med. 45 (10), 2146–2149. 10.13192/j.issn.1000-1719.2018.10.042 [DOI] [Google Scholar]
- Sun Q. W., Jiang S. M., Yang K., Zheng J. M., Zhang L., Xu W. D. (2012). Apigenin enhances the cytotoxic effects of tumor necrosis factor-related apoptosis-inducing ligand in human rheumatoid arthritis fibroblast-like synoviocytes. Mol. Biol. Rep. 39 (5), 5529–5535. 10.1007/s11033-011-1356-3 [DOI] [PubMed] [Google Scholar]
- Sun Y. W., Bao Y., Yu H., Chen Q. J., Lu F., Zhai S., et al. (2020). Anti-rheumatoid arthritis effects of flavonoids from Daphne genkwa. Int. Immunopharmacol. 83, 106384. 10.1016/j.intimp.2020.106384 [DOI] [PubMed] [Google Scholar]
- Sung M. S., Lee E. G., Jeon H. S., Chae H. J., Park S. J., Lee Y. C., et al. (2012). Quercetin inhibits IL-1β-induced proliferation and production of MMPs, COX-2, and PGE2 by rheumatoid synovial fibroblast. Inflammation 35 (4), 1585–1594. 10.1007/s10753-012-9473-2 [DOI] [PubMed] [Google Scholar]
- Sze S. C., Tong Y., Ng T. B., Cheng C. L., Cheung H. P. (2010). Herba epimedii: Anti-oxidative properties and its medical implications. Molecules 15 (11), 7861–7870. 10.3390/molecules15117861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekanecz Z., Koch A. E. (2009). Angiogenesis and its targeting in rheumatoid arthritis. Vasc. Pharmacol. 51 (1), 1–7. 10.1016/j.vph.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsikas D. (2017). Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 524, 13–30. 10.1016/j.ab.2016.10.021 [DOI] [PubMed] [Google Scholar]
- Tu J., Hong W., Zhang P., Wang X., Korner H., Wei W. (2018). Ontology and function of fibroblast-like and macrophage-like synoviocytes: How do they talk to each other and can they Be targeted for rheumatoid arthritis therapy? Front. Immunol. 9, 1467. 10.3389/fimmu.2018.01467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Woude D., van der Helm-van Mil A. H. M. (2018). Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best. Pract. Res. Clin. Rheumatol. 32 (2), 174–187. 10.1016/j.berh.2018.10.005 [DOI] [PubMed] [Google Scholar]
- van Tuyl L. H., Voskuyl A. E., Boers M., Geusens P., Landewe R. B., Dijkmans B. A., et al. (2010). Baseline RANKL:OPG ratio and markers of bone and cartilage degradation predict annual radiological progression over 11 years in rheumatoid arthritis. Ann. Rheum. Dis. 69 (9), 1623–1628. 10.1136/ard.2009.121764 [DOI] [PubMed] [Google Scholar]
- Wang G. J., Tsai T. H., Lin L. C. (2007). Prenylflavonol, acylated flavonol glycosides and related compounds from Epimedium sagittatum. Phytochemistry 68 (19), 2455–2464. 10.1016/j.phytochem.2007.05.035 [DOI] [PubMed] [Google Scholar]
- Wang G., Wang T., Dang P. (2021). Clinical observation on 30 cases of rheumatoid arthritis treated with bushen quhan Zhiwang decoction combined with methotrexate. J. Basic Chin. Med. 27 (08), 1298–1300. 10.19945/j.cnki.issn.1006-3250.2021.08.027 [DOI] [Google Scholar]
- Wang J., Zhao Q. (2019). Kaempferitrin inhibits proliferation, induces apoptosis, and ameliorates inflammation in human rheumatoid arthritis fibroblast-like synoviocytes. Phytotherapy Res. 33 (6), 1726–1735. 10.1002/ptr.6364 [DOI] [PubMed] [Google Scholar]
- Wang L., Li Y., Guo Y., Ma R., Fu M., Niu J., et al. (2016). Herba epimedii: An ancient Chinese herbal medicine in the prevention and treatment of osteoporosis. Curr. Pharm. Des. 22 (3), 328–349. 10.2174/1381612822666151112145907 [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhang P., Yuan X., Bi Y., Zhou K., Zhang Y. (2018). Long- term toxicity of different extracts of Epimedium Brevicornu Maxim in mice. Chin. J. Pharmacovigil. 15 (02), 65–69. [Google Scholar]
- Wang Y., Chen S., Mei J. (2019). Quercetin attenuates chondrocyte matrix degradation and apoptosis in rheumatoid arthritis rats by inhibiting NF-κB. Immunol. J. 35 (06), 485–491. 10.13431/j.cnki.immunol.j.20190075 [DOI] [Google Scholar]
- Wang Y., Wu H., Deng R. (2021). Angiogenesis as a potential treatment strategy for rheumatoid arthritis. Eur. J. Pharmacol. 910, 174500. 10.1016/j.ejphar.2021.174500 [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhuo F., Chu P., Yang X., Zhao G. (2019). Germacrone alleviates collagen-induced arthritis via regulating Th1/Th2 balance and NF-κB activation. Biochem. Biophys. Res. Commun. 518 (3), 560–564. 10.1016/j.bbrc.2019.08.084 [DOI] [PubMed] [Google Scholar]
- Wehr P., Purvis H., Law S. C., Thomas R. (2019). Dendritic cells, T cells and their interaction in rheumatoid arthritis. Clin. Exp. Immunol. 196 (1), 12–27. 10.1111/cei.13256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. C., Ping D. Q., You F. T., Qiang C. Y., Tao C. (2016). Icariin prevents cartilage and bone degradation in experimental models of arthritis. Mediat. Inflamm. 2016, 1–10. 10.1155/2016/9529630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Gao J., Kang J., Wang X., Niu Q., Liu J., et al. (2021). B cells in rheumatoid ArthritisPathogenic mechanisms and treatment prospects. Front. Immunol. 12, 750753. 10.3389/fimmu.2021.750753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. M., Luo J., Shi X. D., Zhang S. X., Zhu X. B., Guo J. (2020). Icariin alleviates rheumatoid arthritis via regulating miR-223-3p/NLRP3 signalling axis. Autoimmunity 53 (8), 450–458. 10.1080/08916934.2020.1836488 [DOI] [PubMed] [Google Scholar]
- Xiang L., Tong Y., Tian X., Fang R., Wang F. (2020). The effects of icariin on apoptosis of fibroblast-like synoviocytes and expression of sytokines in rheumatoid arthritis. Curr. Immunol. 40 (05), 360–365+423. [Google Scholar]
- Xiao P., Hao Y., Zhu X., Wu X. (2013). p53 contributes to quercetin-induced apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation 36 (2), 272–278. 10.1007/s10753-012-9543-5 [DOI] [PubMed] [Google Scholar]
- Yamanaka H., Matsuda Y., Tanaka M., Sendo W., Nakajima H., Taniguchi A., et al. (2000). Serum matrix metalloproteinase 3 as a predictor of the degree of joint destruction during the six months after measurement, in patients with early rheumatoid arthritis. Arthritis Rheum. 43 (4), 852–858. [DOI] [PubMed] [Google Scholar]
- Yanaba K., Bouaziz J. D., Haas K. M., Poe J. C., Fujimoto M., Tedder T. F. (2008). A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28 (5), 639–650. 10.1016/j.immuni.2008.03.017 [DOI] [PubMed] [Google Scholar]
- Yang X. H., Li L., Xue Y. B., Zhou X. X., Tang J. H. (2020). Flavonoids from Epimedium pubescens: Extraction and mechanism, antioxidant capacity and effects on CAT and GSH-px of Drosophila melanogaster . PeerJ 8, e8361. 10.7717/peerj.8361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wu Y., Li W., Liu X., Zheng J., Zhang W., et al. (2018a). Determination of geographical origin and icariin content of Herba Epimedii using near infrared spectroscopy and chemometrics. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 191, 233–240. 10.1016/j.saa.2017.10.019 [DOI] [PubMed] [Google Scholar]
- Yang Y., Zhang X., Xu M., Wu X., Zhao F., Zhao C. (2018b). Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int. Immunopharmacol. 54, 153–162. 10.1016/j.intimp.2017.11.013 [DOI] [PubMed] [Google Scholar]
- Yoon H. Y., Lee E. G., Lee H., Cho I. J., Choi Y. J., Sung M. S., et al. (2013). Kaempferol inhibits IL-1β-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. Int. J. Mol. Med. 32 (4), 971–977. 10.3892/ijmm.2013.1468 [DOI] [PubMed] [Google Scholar]
- You W., Liang Q., Zeng G., Wu H., Chen J., Xiong X., et al. (2009). Effects of apigenin on the proliferation and cytokine gene expression of murine T lymphocytes. Chin. Archives Traditional Chin. Med. 27 (06), 1194–1197. 10.13193/j.archtcm.2009.06.75.youwh.045 [DOI] [Google Scholar]
- Yuan K., Zhu Q., Lu Q., Jiang H., Zhu M., Li X., et al. (2020). Quercetin alleviates rheumatoid arthritis by inhibiting neutrophil inflammatory activities. J. Nutr. Biochem. 84, 108454. 10.1016/j.jnutbio.2020.108454 [DOI] [PubMed] [Google Scholar]
- Yuan M., He Y., Dong W., Yang G., Cao Y., Wang J. (2019). Study on treatment of synovial lesions in patients with active rheumatoid arthritis by bushen Jiedu Tongluo decoction combined with methotrexate. Zhejiang J. Traditional Chin. Med. 54 (08), 600–601. 10.13633/j.cnki.zjtcm.2019.08.035 [DOI] [Google Scholar]
- Zhang D., Zhang J., Fong C., Yao X., Yang M. (2012). Herba epimedii flavonoids suppress osteoclastic differentiation and bone resorption by inducing G2/M arrest and apoptosis. Biochimie 94 (12), 2514–2522. 10.1016/j.biochi.2012.06.033 [DOI] [PubMed] [Google Scholar]
- Zhang G., Zhang C., Guo Z. (2020a). Clinical observation on 50 cases of rheumatoid arthritis of phlegm-stasis-obstruction type treated by wanbi xinglei yin combined with leflunomide. Rheumatism Arthritis 9 (06), 24–26. [Google Scholar]
- Zhang H., Wu X., Wang J., Wang M., Wang X., Shen T., et al. (2020). Flavonoids from the leaves of Epimedium Koreanum Nakai and their potential cytotoxic activities. Nat. Prod. Res. 34 (9), 1256–1263. 10.1080/14786419.2018.1560283 [DOI] [PubMed] [Google Scholar]
- Zhang H., Yan L., Zhang Q., Wang Z. (2013). Flavonoids from leaves of Epimedium pubescens. China J. Chin. Materia Medica 38 (12), 1942–1946. [PubMed] [Google Scholar]
- Zhang J. F., Li G., Chan C. Y., Meng C. L., Lin M. C., Chen Y. C., et al. (2010). Flavonoids of Herba Epimedii regulate osteogenesis of human mesenchymal stem cells through BMP and Wnt/β-catenin signaling pathway. Mol. Cell. Endocrinol. 314 (1), 70–74. 10.1016/j.mce.2009.08.012 [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu W., Zhu X., Zhou M., Zhu X. (2015). The effect of acupuncture combined with apigenin treatment on Th1/Th2 cell subsets in mice with collagen-induced arthritis. Chin. J. Gerontology 35 (05), 1353–1355. [Google Scholar]
- Zhang L. L., Xiao H., Zhang F., Wu Y. J., Shu J. L., Li Y., et al. (2021). BAFF, involved in B cell activation through the NF-κB pathway, is related to disease activity and bone destruction in rheumatoid arthritis. Acta Pharmacol. Sin. 42 (10), 1665–1675. 10.1038/s41401-020-00582-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang T., Zhao B. S., Zhang J. X., Yang S., Fan C. L., et al. (2019). Effect of 2''-O-rhamnosyl icariside II, baohuoside I and baohuoside II in Herba epimedii on cytotoxicity indices in HL-7702 and HepG2 cells. Molecules 24 (7), 1263. 10.3390/molecules24071263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhang J., Fan Q., Su Z., Chen C., Peng L., et al. (2018). Hepatoxicity of epimedii folium in rat model based on uniform design and regression analysis. Chin. J. Exp. Traditional Med. Formulae 24 (06), 189–197. 10.13422/j.cnki.syfjx.20180617 [DOI] [Google Scholar]
- Zhang Q., Liu J., Zhang M., Wei S., Li R., Gao Y., et al. (2019). Apoptosis induction of fibroblast-like synoviocytes is an important molecular-mechanism for herbal medicine along with its active components in treating rheumatoid arthritis. Biomolecules 9 (12), 795. 10.3390/biom9120795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Zhu T., Shi J. (2020b). Clinical study on Fugui Gutong capsules for rheumatoid arthritis. J. NEW Chin. Med. 52 (12), 60–63. 10.13457/j.cnki.jncm.2020.12.018 [DOI] [Google Scholar]
- Zhao J., Chen B., Peng X., Wang C., Wang K., Han F., et al. (2020). Quercetin suppresses migration and invasion by targeting miR-146a/GATA6 axis in fibroblast-like synoviocytes of rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 42 (3), 221–227. 10.1080/08923973.2020.1742732 [DOI] [PubMed] [Google Scholar]
- Zhao J., Yang J., Xie Y. (2019). Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. X. 570, 118642. 10.1016/j.ijpharm.2019.118642 [DOI] [PubMed] [Google Scholar]
- Zheng x., Kong L. (2002). Studies on chemical constituents of Epimedium koreanum. Chin. Traditional Herb. Drugs 11, 7–10. [Google Scholar]
- Zheng X., Ye R., Wang H., Zhang Y., Yan Y., Xu Z. (2017). Curative effect of xianzhi fengsui tang for rheumatoid arthritis and its influence on the expression levels of osteoprotegerin in peripheral blood. J. NEW Chin. Med. 49 (12), 70–72. 10.13457/j.cnki.jncm.2017.12.024 [DOI] [Google Scholar]
- Zhong R., Chen Y., Ling J., Xia Z., Zhan Y., Sun E., et al. (2019). The toxicity and metabolism properties of Herba epimedii flavonoids on laval and adult zebrafish. Evidence-Based Complementary Altern. Med. 2019, 3745051–3745059. 10.1155/2019/3745051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. C., Zheng G., Jia L. Y., He X., Zhang C. F., Wang C. Z., et al. (2021). Comprehensive evaluation on anti-inflammatory and anti-angiogenic activities in vitro of fourteen flavonoids from Daphne Genkwa based on the combination of efficacy coefficient method and principal component analysis. J. Ethnopharmacol. 268, 113683. 10.1016/j.jep.2020.113683 [DOI] [PubMed] [Google Scholar]
- Zhu L., Wei H., Wu Y., Yang S., Xiao L., Zhang J., et al. (2012). Licorice isoliquiritigenin suppresses RANKL-induced osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo . Int. J. Biochem. Cell Biol. 44 (7), 1139–1152. 10.1016/j.biocel.2012.04.003 [DOI] [PubMed] [Google Scholar]
- Zhu M., Yuan K., Lu Q., Zhu Q., Zhang S., Li X., et al. (2019). Emodin ameliorates rheumatoid arthritis by promoting neutrophil apoptosis and inhibiting neutrophil extracellular trap formation. Mol. Immunol. 112, 188–197. 10.1016/j.molimm.2019.05.010 [DOI] [PubMed] [Google Scholar]
- Zhu X., Zeng K., Qiu Y., Yan F., Lin C. (2013). Therapeutic effect of emodin on collagen-induced arthritis in mice. Inflammation 36 (6), 1253–1259. 10.1007/s10753-013-9663-6 [DOI] [PubMed] [Google Scholar]