Abstract
Poor adherence to standard protocols of blood pressure (BP) measurement in routine clinical practice leads to higher readings than “research-quality” measurements. Whether this phenomenon exists in periodic health examinations was unknown. We aimed to explore the concordance between BP measurements in periodic health examinations and those measured following a standard measurement protocol. We used data from the Kailuan Study, an ongoing longitudinal cohort study in China, of which participants received biennial health examinations in health management centers. In addition, BPs were measured following standard protocols in a workplace-based hypertension management program nested in the Kailuan Study. We compared BP readings of the same person between the two settings using generalized linear mixed-effects models. A total of 3988 men (the mean age was 44.9 years) had at least two BP measurements both in health examinations and management program with a time interval between the two settings that less than 90 days. The mean systolic blood pressures (SBP) and diastolic blood pressures (DBP) in health examinations were 4.2 (95% CI 3.9–4.5) mm Hg and 3.3 (95% CI 3.1–3.5) mm Hg higher than those in the management program, respectively. Bland–Altman analyses showed the wide agreement intervals ranging from − 27.7- to 36.5-mm Hg for SBP and − 18.3- to 24.7-mm Hg for DBP. In conclusion, BP measurements in periodic health examinations were generally higher than BPs measured following a standard protocol. Our findings highlight the importance of standard BP measurement to avoid overestimation of hypertension prevalence and treatment initiation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43657-022-00067-w.
Keywords: Hypertension, Physical examination, Blood pressure determination, Guideline adherence
Introduction
Hypertension is a leading cause of cardiovascular events and all-cause mortality (GBD 2019 Risk factors collaborators 2020; Rapsomaniki et al. 2014). A 20 mm Hg higher systolic blood pressure (SBP) and 10 mm Hg higher diastolic blood pressure (DBP) were each associated with a doubling in the risk of death from vascular disease (Lewington et al. 2002). Periodic health examinations have been an important part of medical practice in disease prevention and early detection since the 1920s (Council on Scientific Affairs 1983; Charap 1981; Edie 1925). Blood pressure (BP), a routine vital check-up item, is widely measured in periodic health examinations for prevention, detection and management of hypertension (Liss et al. 2021). However, it is unclear if BP measures in periodic health examinations are performed according to standard procedure, and the variations of measurement errors are unclear either.
Studies have reported that BP measured in routine clinical practice were typically five to 15 mm Hg higher than the BP measured in trials of antihypertensive medication, partly due to nonadherence to standard protocol for BP measurement in clinical practice (Drawz 2017; Drawz et al. 2020). The landmark Systolic Blood Pressure Intervention Trial (SPRINT) (SPRINT Research Group et al. 2015), which was designed to evaluate the effect of intensive SBP treatment to less than 120 mm Hg on risks of cardiovascular disease (CVD) and all-cause mortality, showed that SBP measurements in routine clinical practice were five to eight mm Hg higher than SBP measured in the trial (Drawz et al. 2020). The difference of BP between trials and clinical practice was part of the reasons for the 2017 American College of Cardiology (ACC) and American Heart Association (AHA) guideline recommendation for SBP target to be less than 130 mm Hg, which was 10 mm Hg higher than the intensive target in the SPRINT (Whelton et al. 2018).
Similar with routine clinical practice (Drawz and Ix 2018), there were obstacles (e.g., space, workload, staffing inertia) to implement standard protocol for BP measurement in periodic health examinations. However, it remains unknown whether BP measurements in health examinations were higher than the true level, which may lead to misdiagnosis and overtreatment of hypertension. In addition, many epidemiological studies measured BPs at baseline in a setting similar to the health examination centers with unknown degrees of adherence to the standard protocol (Lewington et al. 2002; Rapsomaniki et al. 2014). Hence, the accuracy and variability in BP measurements could have a profound impact on the estimations of hypertension prevalence or the association between hypertension and health outcomes.
The Kailuan Study is an ongoing longitudinal cohort study in which participants receive free health examinations every two years since 2006 in hospital-affiliated health management centers (HMCs). Additionally, a workplace-based hypertension management program was initiated among male patients with hypertension since 2009, where BPs were measured by trained community health workers following standard protocol. Therefore, this provides us an unprecedented opportunity to examine the concordance of BP measures in periodic health examinations (health-exam BPs) and the management program (research-quality BPs). We hypothesized that health-exam BPs were generally higher than research-quality BPs. In addition, we tried to explore certain factors (e.g., clinical centers, BP measurement devices, personal characteristics) that might influence the variations between health-exam BPs and research-quality BPs.
Materials and Methods
Study Design and Participants
The Kailuan Study is a large ongoing longitudinal cohort study in Tangshan, China (Wang et al. 2020; Wu et al. 2016), which was based on a functional and comprehensive community owned and managed by the large state-owned coal energy enterprise, Kailuan Group. In the first cycle, between 2006 and 2007 (2006–2007 cycle), a total of 101,510 employees of Kailuan Group aged 18 years or older were enrolled. The participants were followed up biennially, and 25,337, 10,519, 21,651, and 12,396 employees were additionally recruited in the 2008–2009, 2010–2011, 2012–2013, and 2014–2015 cycles, respectively. At each visit, all participants completed questionnaires, laboratory tests, and health examinations.
Since 2009, a workplace-based hypertension management program was initiated to improve the BP control and prevent CVD and other related adverse events, especially sudden CVD events during downhole coal working, and in male patients with hypertension. The detailed study design has been described previously (Zhou et al. 2022). Briefly, the following employees were invited to participate in the management program: men (aged 18–60 years old) with self-reported history of hypertension or current use of antihypertensive medications, or men with BP measurements greater than 140/90 mm Hg in at least two separate visits. Finally, a total of 8984 men with hypertension joined the program between 1 January 2009 and 31 December 2015. The hypertension management program was conducted in 18 worksites, and employees in those worksites were assigned to 10 HMCs for the biennial health examinations. The health professionals and researchers from the Department of Cardiology, Kailuan General Hospital and the community health workers at each worksite jointly initiated and monitored the progress of the program. In the program, participants received health education lectures or health promotion activities at least twice a year, semimonthly visits and BP measures, free antihypertensive medications, and individualized health consultation. Four groups of common antihypertensive medications (nitrendipine & captopril, nitrendipine & antisterone, hydrochlorothiazide & captopril, hydrochlorothiazide & antisterone) were provided free to participants. Other antihypertensive medications were covered by the health insurance of the company and medication costs could be partly reimbursed. The target of BP was less than 140/90 mm Hg (Liu 2011).
BP Measurements
In the management program, BPs were measured in the worksite community health centers by community health workers twice a month using standard measurement protocol according to recommendations from the AHA (Whelton et al. 2018) and the Chinese Hypertension League (CHL) (Liu 2011). However, since the management was voluntary, not all patients had their BPs measured twice a month. All community health workers were medical school graduates and received a four-day interactive training session, on-site field testing, and certifications before the management program, and they were re-trained every two years. After five minutes of rest, at least two BP measurements were taken in the right upper arm in a seated position at one to two minutes intervals. If the difference between the two measurements was greater than five mm Hg (either SBP or DBP), a third measurement would be taken. The average values were used as the final measurements.
In the biennial health examinations, BPs were measured by physicians or nurses who should have followed the same BP measurement protocol. However, due to the high workload, it was unclear whether they were strictly in compliance with the protocol, e.g., five minutes of quiet rest before BP measurements, no talking during the recording, ≥ two BP readings and one to two minutes of measurement interval.
In the management program, about half worksites used calibrated tabletop mercury sphygmomanometers throughout the management period, while the rest half worksites used mercury sphygmomanometers first and then changed to electronic devices (HEM-8102A; Omron Co., Ltd., Dalian, China) around 2014. Mercury sphygmomanometers were used in the biennial health examinations before the 2014–2015 cycle, and automatic electronic devices (Omron HEM-8102A) were used in the 2014–2015 cycle and thereafter. The Omron automatic electronic devices had been certified by the Association for the Advancement of Medical Instrumentation (AAMI) (Takahashi et al. 2015).
To compare health-exam BPs in HMCs and research-quality BPs in hypertension management program, we chose BP readings at each HMC visit, and BP readings in the management program which had the smallest time interval compared to each health-exam BPs no matter which one occurred first.
Assessment of Other Variables
At each biennial visit to the HMCs, participants completed questionnaires, had basic anthropometric measurements and donated fasting blood samples. Data on birth date, sex, educational level, physical activity, smoking status, drinking status, and medical history (diabetes, CVD [coronary artery disease and stroke], and active treatment such as antidiabetic medications, etc.) were collected through self-reported questionnaires. In addition to BP, height and weight were also measured. Body mass index (BMI) was calculated as weight (kg)/height2 (m2). Fasting blood glucose (FBG) was measured using the hexokinase/ glucose-six-phosphate dehydrogenase method (Hitachi 747; Hitachi Co., Ltd., Tokyo, Japan) with an upper limit of detection of 30.07 mmol/L (Jin et al. 2017). Diabetes was defined as either FBG ≥ 7.0 mmol/L, self-report of a physician diagnosis, or self-report use of antidiabetic medication. Urine protein was measured by a semi-quantitative dipstick test (N-600, Dirui Industrial Co., Ltd., Changchun, China), and positive proteinuria was defined as urine protein concentrations ≥ 30 mg/dL. Creatinine was measured by auto-analyzer (Hitachi 747; Hitachi Co., Ltd., Tokyo, Japan), and estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine formula (Levey et al. 2009). Impaired renal function was defined as either positive proteinuria or eGFR < 60 mL/(min·1.73 m2) (Levey et al. 2009). Histories on CVD and cancer diagnoses were obtained via linkage with the Municipal Social Insurance Institution and Hospital Discharge Register and self-reported medical history in questionnaires.
Statistical Analysis
We restricted our main analyses to participants who had at least two health-exam BPs and research-quality BPs with time interval between two settings less than 90 days. The baseline characteristics of those included and excluded from the analyses were described using mean and standard deviation (SD) for continuous variables and using number and percentage (%) for categorical variables. Student t test was used for comparison of continuous variables and Pearson χ2 test was used for comparison of categorical variables.
We used generalized linear mixed-effects models to examine the difference between health-exam BPs and research-quality BPs with random effects for participants and HMC sites. Interaction term between setting of BP measures (health-exam BPs or research-quality BP) and time since management was included in all models. Stratified analyses were conducted by age, BMI, educational level, physical activity, smoking status, drinking status, diabetes, CVD, cancer, impaired renal function, time interval of health-exam and research-quality BPs, order of health-exam and research-quality BPs, antihypertensive drugs, type of sphygmomanometer, and HMC sites. To further identify factors associated with difference between health-exam BPs and research-quality BPs, a generalized estimating equation model, accounting for clustering of multiple BP measurements of the same person, was conducted on those stratified factors showing significant interaction with setting of BP measures (p for interaction < 0.05). In the generalized estimating equation model, BP difference (a binary variable of BP difference > mean difference or not) was used as an independent variable, and those stratified factors showing significant interaction with setting of BP measures were treated as dependent variables and included in the model simultaneously. Finally, the Bland–Altman methods were used to estimate the agreement between health-exam BPs in HMCs and research-quality BPs in management program (Bland and Altman 1999).
We also conducted several sensitivity analyses: (1) changing inclusion criteria of time interval of health-exam and research-quality BPs to less than one, seven, 30, and 180 days, instead of 90 days in the main analysis; (2) including those with only one health-exam BP and research-quality BP record under the time interval inclusion criterion; (3) comparing health-exam BPs with the mean value of those research-quality BPs that had a time interval compared to the health-exam BPs less than 90 days, rather than the one had the smallest time interval in the main analysis. (4) Excluding those zero end-digit BP records, which is a common phenomenon when using mercury device even under a standardized measurement protocol (Broad et al. 2007; Kim et al. 2007).
All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC), and a two-sided p < 0.05 was considered as statistically significant.
Results
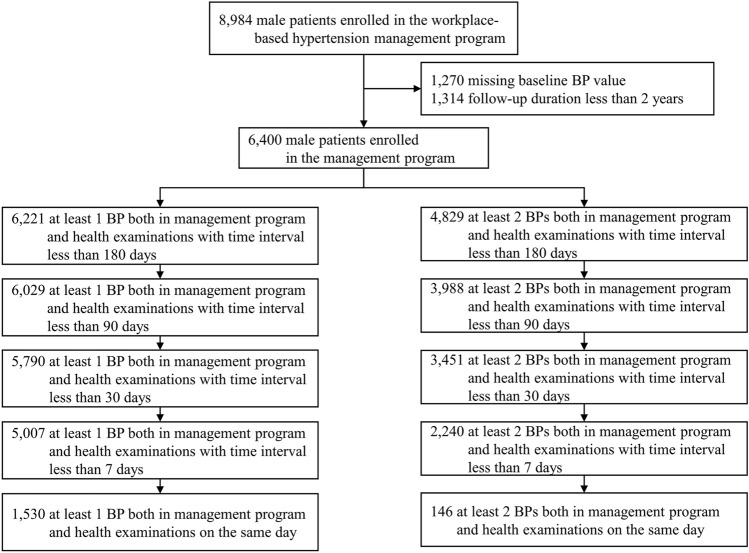
Among the 8984 male participants, those with missing blood pressure at baseline (n = 1270) and with follow-up duration less than two years (n = 1314) were excluded in our analysis. Of the remaining 6400 participants, 3988 were included in the main analyses that had at least two BPs in different settings with a time interval of less than 90 days (Fig. 1). The mean values of baseline age, SBP, and DBP were 44.9 years (SD = 5.9), 142.9 mm Hg (SD = 15.6), and 96.0 mm Hg (SD = 10.1), respectively (Table 1). Compared with those that were excluded from the main analyses, participants included in the analyses had slightly higher SBP and DBP; comparisons of other baseline characters are given in Supplementary Table S1.
Fig. 1.
Flow chart of matching research-quality blood pressure (BP) records in the workplace-based hypertension management program and BPs of the same person in the biennial health examinations
Table 1.
Baseline characteristics of the participants included in the main analyses
| Characteristics | N = 3988 |
|---|---|
| Age, mean (SD), years | 44.9 (5.9) |
| Male, n (%) | 3988 (100) |
| BMI, mean (SD), kg/m2 | 26.2 (3.4) |
| SBP, mean (SD), mm Hg | 142.9 (15.6) |
| DBP, mean (SD), mm Hg | 96.0 (10.1) |
| Education, n (%) | |
| ≤ Middle school | 3305 (82.9) |
| High school | 570 (14.3) |
| ≥ College | 113 (2.8) |
| Physically active, n (%)a | 346 (8.7) |
| Current smoker, n (%) | 2,372 (59.5) |
| Current drinker, n (%) | 2,533 (63.5) |
| CVD, n (%) | 64 (1.6) |
| DM, n (%) | 470 (11.8) |
| Cancer, n (%) | 7 (0.2) |
| Impaired renal function, n (%)b | 558 (14.0) |
BMI body mass index, CVD cardiovascular disease, DBP diastolic blood pressure, DM diabetes, SBP systolic blood pressure
aBeing physically active was defined as moderate or vigorous physical activity for ≥ 80 min per week
bImpaired renal function was defined as the presence of albuminuria or estimated glomerular filtration rate < 60 (ml/min/1.73 m2)
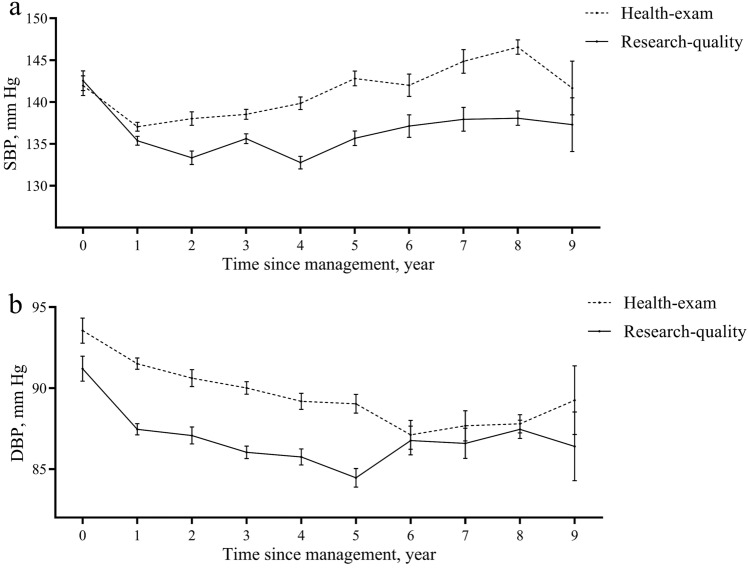
During a median of 5.2 years of follow-up, each participant had two to four health-exam BPs and research-quality BPs to compare. The mean health-exam SBP (139.9 mm Hg) was 4.2 (95% CI 3.9–4.5) mm Hg higher compared with the mean research-quality SBP in hypertension management program (135.7-mm Hg) (Fig. 2; Table 2). The mean difference of DBP was 3.3 (95% CI 3.1–3.5) mm Hg, with a mean health-exam DBP of 90.0 mm Hg and a mean research-quality DBP of 86.8 mm Hg (Fig. 2; Table 2). Significant interactions were found across BMI categories, physical activity levels, history of diabetes, time interval of health-exam and research-quality BPs, type of sphygmomanometer and HMC sites for both SBP and DBP readings, with additional significant interaction across age groups for SBP only and across drink status and antihypertensive drugs for DBP only (all p for interaction < 0.05, Table 2). Larger differences of both SBP and DBP between the two settings were found among individuals with physical inactivity, and among measurements with time interval between the two settings less than 10 days. Compared with using electronic devices in both settings, using mercury sphygmomanometers in both setting also had larger differences of both SBP and DBP. Additionally, larger differences of SBP were found for subgroups of age of 50 to 60, BMI in the normal weight range (18.5–23.9 kg/m2), and patients with diabetes; larger differences of DBP were found for subgroups of BMI in obesity range (≥ 28 kg/m2), current drinker, those without diabetes, and those with drug use of hydrochlorothiazide & antisterone. Further, in the generalized estimating equations model included all abovementioned stratified factors, the odds ratios (ORs) for higher SBP difference were still significant in subgroups of age, BMI, physical activity, time interval of the two BP measurements, and types of sphygmomanometer (Supplementary Figure S1). Significant ORs for higher DBP difference were found in subgroups of types of sphygmomanometer (Supplementary Figure S1).
Fig. 2.
Difference between health-exam blood pressure (BP) and research-quality BP. a systolic blood pressure (SBP); b diastolic blood pressure (DBP). Generalized linear mixed effect model was used to examine the difference between health-exam BP and research-quality BP of the same person with random effects for participants and HMC sites. Interaction term between source (health-exam or research-quality) and time since management was included in models
Table 2.
Differences between health-exam BPs and research-quality BPs of subgroups
| Characteristics | Mean SBP, mm Hg | Mean difference (95% CI) | p for interaction | Mean DBP, mm Hg | Mean difference (95% CI) | p for interaction | ||
|---|---|---|---|---|---|---|---|---|
| Health-exam | Research-quality | Health-exam | Research-quality | |||||
| Overall | 139.9 | 135.7 | 4.2 (3.9, 4.5) | 90.0 | 86.8 | 3.3 (3.1, 3.5) | ||
| Age, years | < 0.001 | 0.64 | ||||||
| 18–39 | 136.9 | 134.9 | 2.0 (0.9, 3.2) | 89.8 | 86.4 | 3.5 (2.7, 4.2) | ||
| 40–49 | 139.2 | 135.2 | 4.0 (3.5, 4.5) | 90.4 | 87.0 | 3.4 (3.0, 3.7) | ||
| 50–60 | 140.9 | 136.2 | 4.8 (4.3, 5.2) | 89.8 | 86.6 | 3.2 (2.8, 3.5) | ||
| BMI, kg/m2 | 0.03 | 0.001 | ||||||
| 18.5–23.9 | 138.9 | 134.1 | 4.8 (4.2, 5.5) | 88.9 | 86.1 | 2.8 (2.4, 3.2) | ||
| 24–27.9 | 139.9 | 135.6 | 4.3 (3.8, 4.8) | 90.0 | 86.8 | 3.2 (2.9, 3.5) | ||
| ≥ 28 | 140.8 | 137.3 | 3.5 (2.9, 4.2) | 91.4 | 87.5 | 3.9 (3.5, 4.4) | ||
| Educational level | 0.16 | 0.10 | ||||||
| ≤ Middle school | 139.8 | 135.6 | 4.3 (4.0, 4.7) | 89.9 | 86.7 | 3.2 (3.0, 3.4) | ||
| > Middle school | 140.1 | 136.5 | 3.7 (2.9, 4.5) | 90.9 | 87.2 | 3.7 (3.2, 4.2) | ||
| Physically activea | 0.005 | 0.002 | ||||||
| Yes | 140.1 | 137.0 | 3.1 (2.3, 4.0) | 90.1 | 87.8 | 2.3 (1.7, 2.9) | ||
| No | 139.6 | 135.1 | 4.5 (4.1, 4.9) | 90.0 | 86.6 | 3.3 (3.1, 3.6) | ||
| Current smoker | 0.96 | 0.06 | ||||||
| Yes | 140.4 | 136.3 | 4.2 (3.7, 4.7) | 90.2 | 87.3 | 3.0 (2.7, 3.3) | ||
| No | 139.2 | 135.0 | 4.2 (3.7, 4.7) | 89.8 | 86.4 | 3.4 (3.1, 3.7) | ||
| Current drinker | 0.29 | < 0.001 | ||||||
| Yes | 140.9 | 136.8 | 4.0 (3.6, 4.5) | 91.0 | 87.3 | 3.7 (3.4, 4.1) | ||
| No | 138.9 | 134.5 | 4.4 (3.9, 4.9) | 89.1 | 86.2 | 2.8 (2.5, 3.1) | ||
| CVD | 0.40 | 0.83 | ||||||
| Yes | 140.7 | 137.3 | 3.5 (1.6, 5.3) | 90.7 | 87.5 | 3.2 (2.0, 4.4) | ||
| No | 139.9 | 135.6 | 4.2 (3.9, 4.6) | 90.0 | 86.7 | 3.3 (3.1, 3.5) | ||
| DM | 0.01 | 0.04 | ||||||
| Yes | 141.6 | 136.5 | 5.0 (4.3, 5.8) | 89.9 | 87.1 | 2.8 (2.4, 3.3) | ||
| No | 139.4 | 135.4 | 4.0 (3.6, 4.4) | 90.1 | 86.7 | 3.4 (3.2, 3.7) | ||
| Cancer | 0.40 | 0.71 | ||||||
| Yes | 138.9 | 136.8 | 2.1 (− 3.2, 7.3) | 90.2 | 86.3 | 3.9 (0.4, 7.4) | ||
| No | 139.9 | 135.7 | 4.2 (3.9, 4.6) | 90.1 | 86.8 | 3.3 (3.0, 3.5) | ||
| Impaired renal functionb | 0.42 | 0.12 | ||||||
| Yes | 140.8 | 136.1 | 4.7 (3.5, 5.9) | 90.9 | 87.0 | 3.9 (3.1, 4.7) | ||
| No | 139.8 | 135.7 | 4.2 (3.8, 4.5) | 90.0 | 86.8 | 3.2 (3.0, 3.5) | ||
| Time interval of health-exam and research-quality BPs | < 0.001 | 0.01 | ||||||
| < 10 days | 139.7 | 135.1 | 4.6 (4.2, 5.0) | 90.0 | 86.5 | 3.5 (3.2, 3.7) | ||
| 10 to < 20 days | 140.1 | 137.1 | 3.0 (1.6, 4.4) | 89.6 | 87.1 | 2.5 (1.6, 3.5) | ||
| ≥ 20 days | 140.8 | 138.4 | 2.3 (1.4, 3.2) | 90.7 | 88.1 | 2.6 (2.0, 3.2) | ||
| Order of health-exam and research-quality BPs | 0.46 | 0.67 | ||||||
| Health-exam before research-quality BPs | 139.5 | 135.4 | 4.1 (3.6, 4.6) | 90.1 | 86.7 | 3.3 (3.0, 3.7) | ||
| Health-exam after research-quality BPs | 140.1 | 135.8 | 4.3 (3.9, 4.8) | 90.0 | 86.8 | 3.2 (2.9, 3.5) | ||
| Use of antihypertensive drugs | 0.07 | < 0.001 | ||||||
| None | 140.4 | 135.9 | 4.5 (3.9, 5.0) | 90.3 | 87.1 | 3.2 (2.9, 3.6) | ||
| Nitrendipine & captoril | 139.3 | 135.3 | 4.0 (3.4, 4.7) | 89.9 | 86.3 | 3.6 (3.2, 4.0) | ||
| Nitrendipine & antisterone | 138.5 | 136.1 | 2.4 (0.8, 4.0) | 90.4 | 86.4 | 4.1 (3.0, 5.1) | ||
| Hydrochlorothiazide & captoril | 139.1 | 135.7 | 3.3 (2.0, 4.7) | 90.4 | 86.3 | 4.0 (3.1, 4.9) | ||
| Hydrochlorothiazide & antisterone | 138.5 | 134.9 | 3.6 (2.1, 5.1) | 90.0 | 85.7 | 4.3 (3.3, 5.2) | ||
| Self-administration | 140.6 | 135.8 | 4.7 (4.0, 5.5) | 89.6 | 87.3 | 2.3 (1.8, 2.8) | ||
| Type of sphygmomanometer | < 0.001 | < 0.001 | ||||||
| Mercury vs mercury | 138.3 | 134.1 | 4.2 (3.6, 4.8) | 90.7 | 86.9 | 3.8 (3.4, 4.2) | ||
| Electronic vs electronic | 142.8 | 140.6 | 2.1 (1.0, 3.2) | 90.7 | 87.1 | 3.6 (2.8, 4.3) | ||
| Mercury vs electronic | 138.1 | 138.9 | − 0.9 (− 2.1, 0.4) | 92.0 | 87.7 | 4.3 (3.5, 5.1) | ||
| Electronic vs mercury | 141.0 | 134.5 | 6.5 (5.7, 7.4) | 87.8 | 85.6 | 2.2 (1.7, 2.8) | ||
| HMC sites | < 0.001 | < 0.001 | ||||||
| One | 140.4 | 139.7 | 0.7 (− 0.1, 1.5) | 91.5 | 87.1 | 4.3 (3.8, 4.9) | ||
| Two | 139.9 | 135.8 | 4.1 (3.1, 5.2) | 91.3 | 88.2 | 3.1 (2.4, 3.8) | ||
| Three | 143.3 | 134.3 | 9.1 (5.6, 12.5) | 95.8 | 84.8 | 11.0 (8.7, 13.2) | ||
| Four | 137.9 | 135.4 | 2.5 (1.8, 3.2) | 87.3 | 86.6 | 0.7 (0.2, 1.1) | ||
| Five | 145.2 | 136.5 | 8.7 (7.2, 10.2) | 93.9 | 87.4 | 6.5 (5.5, 7.5) | ||
| Six | 147.2 | 142.6 | 4.6 (0.2, 8.9) | 91.7 | 89.8 | 1.9 (-1.0, 4.8) | ||
| Seven | 144.4 | 138.0 | 6.3 (5.2, 7.4) | 92.3 | 90.1 | 2.0 (1.5, 3.0) | ||
| Eight | 148.2 | 140.8 | 7.4 (3.1, 11.7) | 97.1 | 91.0 | 6.1 (3.3, 9.0) | ||
| Nine | 135.8 | 127.5 | 8.3 (7.5, 9.0) | 87.3 | 81.9 | 5.4 (4.8, 5.9) | ||
| Ten | 143.8 | 143.3 | 0.6 (− 1.0, 2.1) | 94.7 | 92.1 | 2.7 (1.7, 3.7) | ||
BMI body mass index, BP blood pressure, CVD cardiovascular disease, DBP diastolic blood pressure, DM diabetes, HMCs health management centers, SBP systolic blood pressure
aBeing physically active was defined as moderate or vigorous physical activity for ≥ 80 min per week
bImpaired renal function was defined as the presence of albuminuria or estimated glomerular filtration rate < 60 (ml/min/1.73 m2)
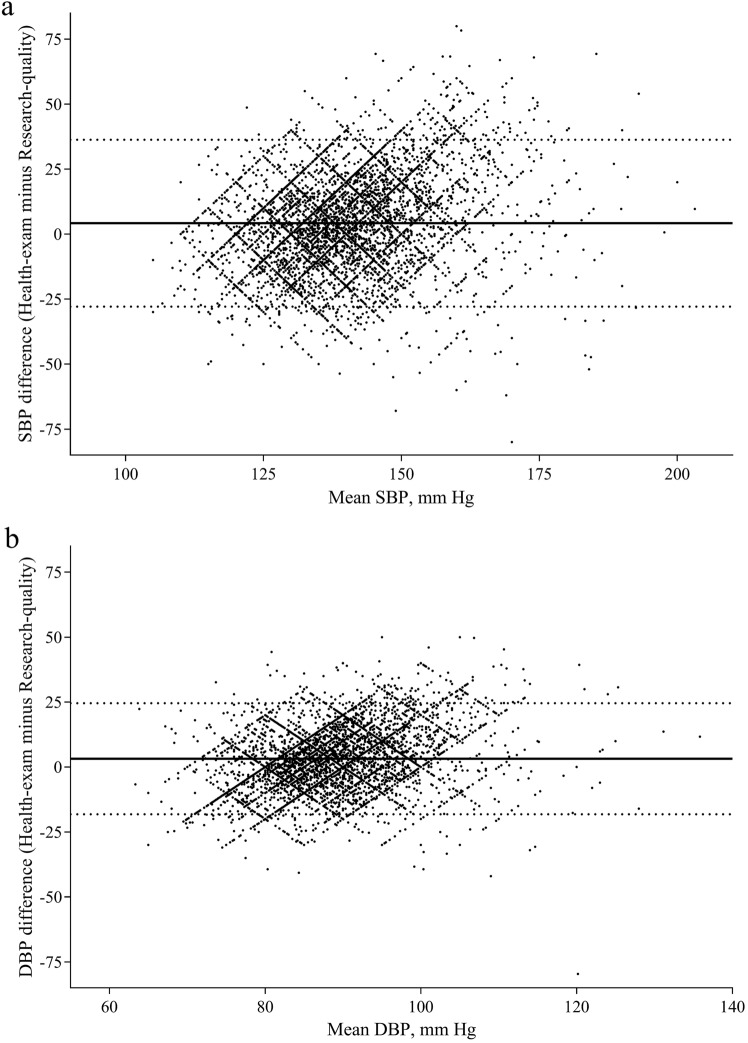
The Bland–Altman plot showed a low agreement between health-exam BPs and research-quality BPs, with wide agreement intervals ranging from − 27.7 to 36.5 mm Hg for SBP readings and − 18.3 to 24.7 mm Hg for DBP readings (Fig. 3). Despite the generally significant higher levels of mean health-exam BPs than research-quality BPs across subgroups, 36.5% (3528 out of 9666) health-exam SBP measurements and 34.7% (3355 out of 9666) health-exam DBP measurements were lower than the corresponding research-quality BP readings. Due to zero end-digit preference in BP measurements using mercury sphygmomanometer, there is a lattice-like distribution in the Bland–Altman plot (Fig. 3).
Fig. 3.
Bland–Altman plot comparing health-exam blood pressure (BP) with research-quality BP. a systolic blood pressure (SBP); b diastolic blood pressure (DBP). Dashed lines indicate 95% limits of agreement; solid lines indicate mean SBP or DBP difference
Similar results were obtained in the various sensitivity analyses, such as changing the inclusion criteria of time interval between health-exam and research-quality BPs of 90 days into one day, seven days, 30 days, and 180 days, excluding those zero end-digit BP records, or using the mean value of those research-quality BPs that had a time interval compared to the health-exam BPs less than 90 days (Table 3).
Table 3.
Sensitivity analysis of difference between health-exam blood pressure (BP) and research-quality BP
| Mean SBP, mm Hg | Mean difference (95% CI) | Mean DBP, mm Hg | Mean difference (95% CI) | |||
|---|---|---|---|---|---|---|
| Health-exam | Research-quality | Health-exam | Research-quality | |||
| Excluding zero end digit BP records | 140.9 | 137.0 | 3.9 (3.4, 4.3) | 90.0 | 87.1 | 2.8 (2.5, 3.1) |
| At least one matched BPs with time interval less than 180 days | 140.7 | 135.4 | 5.3 (5.0, 5.5) | 89.9 | 86.5 | 3.4 (3.2, 3.6) |
| At least two matched BPs with time interval less than 180 days | 140.7 | 135.4 | 5.4 (5.1, 5.7) | 90.0 | 86.5 | 3.5 (3.3, 3.7) |
| At least one matched BPs with time interval less than 90 days | 139.8 | 135.4 | 4.4 (4.1, 4.7) | 89.7 | 86.6 | 3.0 (2.8, 3.2) |
| At least one matched BPs with time interval less than 30 days | 139.9 | 135.1 | 4.8 (4.5, 5.1) | 89.6 | 86.5 | 3.1 (2.9, 3.3) |
| At least two matched BPs with time interval less than 30 days | 139.9 | 135.3 | 4.7 (4.3, 5.0) | 89.9 | 86.5 | 3.4 (3.2, 3.7) |
| At least one matched BPs with time interval less than seven days | 139.9 | 135.0 | 4.9 (4.6, 5.3) | 89.6 | 86.5 | 3.2 (2.9, 3.4) |
| At least two matched BPs with time interval less than seven days | 139.7 | 134.6 | 5.1 (4.6, 5.5) | 89.7 | 86.1 | 3.6 (3.3, 3.9) |
| At least one matched BPs on the same day | 139.0 | 134.2 | 4.8 (4.0, 5.5) | 89.0 | 86.0 | 2.9 (2.4, 3.4) |
| At least two matched BPs on the same day | 139.5 | 134.0 | 5.5 (3.7, 7.2) | 88.9 | 85.8 | 3.1 (1.9, 4.3) |
| Comparing health-exam BPs with mean value of research-quality BPs with time interval less than 90 days | 139.9 | 135.6 | 4.3 (4.0, 4.6) | 90.1 | 86.7 | 3.3 (3.1, 3.5) |
BP blood pressure, DBP diastolic blood pressure, HMCs health management centers, SBP systolic blood pressure
Discussion
In this study comparing BP measurements in periodic health examinations in HMCs with the BPs measured using standard protocol in a hypertension management program, we found that SBP and DBP measured in health examinations were on average, 4.2 mm Hg and 3.2 mm Hg higher than those measured using standard protocol, respectively. Notably, higher mean differences of SBP were observed among individuals who were older, with lower BMI and physically inactive. The mean difference of both SBP and DBP also displayed a substantial variability across different HMC sites and types of sphygmomanometer. Furthermore, there was a low agreement between health-exam BP measurements and research-quality BP measurements in management program.
To our best knowledge, this is the first study to compare the BP measurements in periodic health examinations with research-quality measurements. Prior studies have demonstrated that BPs measured in routine clinical practice were typically five to 15 mm Hg higher than the corresponding BP measured using standard protocol (Bhatt et al. 2016; Drawz 2017; Drawz et al. 2020; Graves et al. 2003; Head et al. 2010; Minor et al. 2012; Ray et al. 2012). There are some similar obstacles between BP measurements in clinical practices and health examinations (Drawz and Ix 2018), e.g., space, workload, staffing inertia. Therefore, our results are in line with previous studies, and showed higher BP values in health examinations than values measured using standard protocol. We also found the variability across different sites and low agreement at individual level, which is also consistent with previous evidence regarding concordance between BP measurements in routine clinical practice and intervention trial (Drawz et al. 2020).
The inconsistent use of standard BP measurement protocol in health examinations and widely acknowledged “white coat effect” are likely the underlying explanations of the differences between health-exam BPs and research-quality BPs (Drawz 2017). Due to the implacable time requirement of standard BP measurement protocol (Whelton et al. 2018), multiple health check-up items and large number of examinees during the examination visits, it is common that BPs are measured in a less strict matter in periodic health examinations. For example, patients may not have three- to five-min rest before BP measurements, or they may even talk during the measurements. While in the management program, guidelines could be better followed. The researchers in the management program are trained to monitor BPs under the guidelines strictly and to provide educational counseling to the patients based on the BP values. In addition, workload for health workers is generally low in management program than that in health examination. Besides, patients were familiar with the observers by frequent contacts and were accustomed to the continual BP measurement in the management program, thus the “white coat effect” in the management program could be less evident.
In addition, we further explored the potential factors that affected the difference between health-exam BPs and research-quality BPs. The mean differences of BP varied by HMC sites, which might due to the variable compliance to protocol in different sites. Greater difference was found when using mercury sphygmomanometers compared with electronic devices with the same settings, which indicated that manual device used in health examination might bring larger incompliance to guideline than automated device. Several individual characteristics, e.g., age, BMI, and physical activity, were also found to contribute to the difference between health-exam BPs and research-quality BPs. Besides, although we demonstrated that the mean level of health-exam BPs was higher than research-quality BPs, a considerable proportion of health-exam BPs (36.4% of SBP and 34.8% of DBP) were still lower than research-quality BP. Within-person variability and variability in BP measurement practices are the potential reasons. Taken together, it is thus inappropriate to apply one common correction factor (e.g., minus five mm Hg) to directly translate the BPs measured at health examinations into BP readings of standard quality at the individual level. At the population level, a correction factor might be used to help estimate the prevalence of hypertension, but the results should still be interpreted with caution given that the differences between health-exam BPs and research-quality BPs varied widely across several factors (sites, devices, personal characteristics, etc.). Therefore, the quality of BP measurement in health examinations and clinical practices should be substantially improved to guide the appropriate management of hypertension. In addition, out-of-office BP monitoring (i.e., home BP measurement or 24-h ambulatory BP monitoring) could be considered in the management of hypertension, which has also been recommended in recent guidelines (Muntner et al. 2019; Siu 2015; Whelton et al. 2018).
Our study also has an implication in understanding the results from other epidemiological studies (Lewington et al. 2002), where a single BP measurement was performed with unknown degrees of adherence to standard measurement protocol. In these studies, it is highly likely to give an overestimate of hypertension prevalence and an imprecise estimate of the association between hypertension and health outcomes. Therefore, a high degree of adherence to standard measurement protocol is also needed in epidemiological studies.
The strengths of the current study included its large sample size, multiple centers, and repeated measures of BP. Moreover, this is the first study to estimate the difference between health-exam BPs and research-quality BPs. Our study also has several limitations. First, we only included males in our research. In prior study (Drawz et al. 2020), the difference between routine clinical BP and well-measured BP was greater in women than men. Second, we did not measure the adherence rate of BP measurements using standard protocol in the management program although the field researchers were instructed to follow the guidelines. Third, the time interval of health-exam BPs in HMCs and research-quality BPs in management program varied in our main analysis (up to 90 days), and there was significant interaction with the time interval. However, sensitivity analyses using different time intervals revealed similar results, indicating the robustness of our findings. Finally, the generalizability of our findings to other populations remains unknown, and more studies on this topic should be conducted.
Conclusion
In conclusion, BP measurements in periodic health examinations were generally higher than BP measurements using standard protocol with a high degree of heterogeneity. Our study highlights the importance of proper BP measurements in periodic health examinations to avoid over-estimation of hypertension prevalence and over-treatment for those who are misclassified as hypertension.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the survey teams of the Kailuan study group for their contribution and the study participants who contributed their information.
Author Contributions
J-XC and Y-FZ contributed equally. Conceptualization: J-XC, Y-FZ, SW and AP; methodology: J-XC and Y-FZ; formal analysis: J-XC; investigation: SC and GW; writing—original draft preparation: J-XC; writing—review and editing: all authors; resources: SW; supervision: SW and AP.
Funding
AP was supported by National Natural Science Foundation of China (81930124 and 82021005) and Shanghai Municipal Science and Technology Major Project (Grant No. 2017SHZDZX01).
Data Availability
The datasets generated during the current study are not publicly available due to data confidentiality but are available from the corresponding author on reasonable request.
Declarations
Conflicts of Interest
The authors declare no competing interests.
Ethical Approval
The study protocol was approved by the Ethics Committee of Kailuan General Hospital.
Consent to Participate
All participants signed written informed consent forms.
Consent for Publication
Not applicable.
Footnotes
Jun-Xiang Chen and Yan-Feng Zhou contributed equally to this work.
Contributor Information
Shouling Wu, Email: drwusl@163.com.
An Pan, Email: panan@hust.edu.cn.
References
- Bhatt H, Siddiqui M, Judd E, Oparil S, Calhoun D. Prevalence of pseudoresistant hypertension due to inaccurate blood pressure measurement. J Am Soc Hypertens. 2016;10(6):493–499. doi: 10.1016/j.jash.2016.03.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- Broad J, Wells S, Marshall R, Jackson R. Zero end-digit preference in recorded blood pressure and its impact on classification of patients for pharmacologic management in primary care - PREDICT-CVD-6. Br J Gen Pract. 2007;57(544):897–903. doi: 10.3399/096016407782317964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charap MH. The periodic health examination: genesis of a myth. Ann Intern Med. 1981;95(6):733–735. doi: 10.7326/0003-4819-95-6-733. [DOI] [PubMed] [Google Scholar]
- Council on Scientific Affairs Medical evaluations of healthy persons. J Am Med Assoc. 1983;249(12):1626–1633. doi: 10.1001/jama.1983.03330360066040. [DOI] [PubMed] [Google Scholar]
- Drawz P. Clinical implications of different blood pressure measurement techniques. Curr Hypertens Rep. 2017;19(7):54. doi: 10.1007/s11906-017-0751-0. [DOI] [PubMed] [Google Scholar]
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol. 2018;29(2):383–388. doi: 10.1681/ASN.2017070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drawz PE, Agarwal A, Dwyer JP, et al. Concordance between blood pressure in the systolic blood pressure intervention trial and in routine clinical practice. JAMA Intern Med. 2020;180(12):1655–1663. doi: 10.1001/jamainternmed.2020.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edie EB. Health examinations past and present and their promotion in pennsylvania. Am J Public Health. 1925;15(7):602–606. doi: 10.2105/ajph.15.7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Risk factors collaborators Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves JW, Nash C, Burger K, Bailey K, Sheps SG. Clinical decision-making in hypertension using an automated (BpTRU™) measurement device. J Hum Hypertens. 2003;17(12):823–827. doi: 10.1038/sj.jhh.1001626. [DOI] [PubMed] [Google Scholar]
- Head GA, Mihailidou AS, Duggan KA, et al. Definition of ambulatory blood pressure targets for diagnosis and treatment of hypertension in relation to clinic blood pressure: prospective cohort study. BMJ. 2010;340:c1104. doi: 10.1136/bmj.c1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Chen S, Vaidya A, et al. Longitudinal change in fasting blood glucose and myocardial infarction risk in a population without diabetes. Diabetes Care. 2017;40(11):1565–1572. doi: 10.2337/dc17-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Samuels TA, Yeh HC, et al. End-digit preference and the quality of blood pressure monitoring in diabetic adults. Diabetes Care. 2007;30(8):1959–1963. doi: 10.2337/dc07-0020. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- Liss DT, Uchida T, Wilkes CL, Radakrishnan A, Linder JA. General health checks in adult primary care: a review. J Am Med Assoc. 2021;325(22):2294–2306. doi: 10.1001/jama.2021.6524. [DOI] [PubMed] [Google Scholar]
- Liu LS, Writing Group of 2010 Chinese guidelines for the management of hypertension [2010 Chinese guidelines for the management of hypertension] Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39(7):579–615. doi: 10.3760/cma.j.issn.0253-3758.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Minor DS, Butler KR, Jr, Artman KL, et al. Evaluation of blood pressure measurement and agreement in an academic health sciences center. J Clin Hypertens. 2012;14(4):222–227. doi: 10.1111/j.1751-7176.2012.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the american heart association. Hypertension. 2019;73(5):e35–e66. doi: 10.1161/HYP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383(9932):1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray GM, Nawarskas JJ, Anderson JR. Blood pressure monitoring technique impacts hypertension treatment. J Gen Intern Med. 2012;27(6):623–629. doi: 10.1007/s11606-011-1937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu AL, U.S. Preventive Services Task Force Screening for high blood pressure in adults: U.S. Preventive services task force recommendation statement. Ann Int Med. 2015;163(10):778–786. doi: 10.7326/M15-2223. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yoshika M, Yokoi T. Validation of two automatic devices for the self-measurement of blood pressure according to the ANSI/AAMI/ISO81060-2:2009 guidelines: the Omron BP765 (HEM-7311-ZSA) and the Omron BP760N (HEM-7320-Z) Vasc Health Risk Manag. 2015;11:49–53. doi: 10.2147/VHRM.S72438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yuan Y, Zheng M, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. 2020;75(23):2921–2930. doi: 10.1016/j.jacc.2020.04.038. [DOI] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- SPRINT Research Group. Wright JT, Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Jin C, Vaidya A, et al. Longitudinal patterns of blood pressure, incident cardiovascular events, and all-cause mortality in normotensive diabetic people. Hypertension. 2016;68(1):71–77. doi: 10.1161/HYPERTENSIONAHA.116.07381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YF, Chen S, Wang G, et al. Effectiveness of a workplace-based, multicomponent hypertension management program in real-world practice: a propensity-matched analysis. Hypertension. 2022;79(1):230–240. doi: 10.1161/HYPERTENSIONAHA.121.18305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are not publicly available due to data confidentiality but are available from the corresponding author on reasonable request.