Abstract
Skin is a complex ecosystem colonized by millions of microorganisms, including bacteria, fungi, and viruses. Skin microbiota is believed to exert critical functions in maintaining host skin health. Profiling the structure of skin microbial community is the first step to overview the ecosystem. However, the community composition is highly individualized and extremely complex. To explore the fundamental factors driving the complexity of the ecosystem, namely the selection pressures, we review the present studies on skin microbiome from the perspectives of ecology. This review summarizes the following: (1) the composition of substances/nutrients in the cutaneous ecological environment that are derived from the host and the environment, highlighting their proposed function on skin microbiota; (2) the features of dominant skin commensals to occupy ecological niches, through self-adaptation and microbe–microbe interactions; (3) how skin microbes, by their structures or bioactive molecules, reshape host skin phenotypes, including skin immunity, maintenance of skin physiology such as pH and hydration, ultraviolet (UV) protection, odor production, and wound healing. This review aims to re-examine the host–microbe interactions from the ecological perspectives and hopefully to give new inspiration to this field.
Keywords: Skin microbiome, Metabolome, Phenome, Microbe–microbe interactions, Ecological niches
Introduction
The skin is considered a barrier organ against the entry of foreign physical, chemical, and biological insults, thereby maintaining the internal homeostasis of the human body. In the past decades, Human Microbiome Project (HMP) has expanded our perception of the skin as not only a piece of placid “soil” but a vast “ecosystem” that harbors a myriad of microbial inhabitants (Human Microbiome Project Consortium 2012). It has been believed that the colonization of diverse microbes resulted from millions of years of mutual adaptation and functional integration (Lousada et al. 2021), and thus the human body forms a complex, synergistic entity, termed a holobiont or meta-organism (Bosch and McFall-Ngai 2011; Rosenberg et al. 2007). The environmental and nutrient conditions define the unique microhabitats for skin microbes (Flowers and Grice 2020), and in turn, these microbes can influence their survival environment (host skin) by stabilizing, mutually beneficial host–microbe interactions (Postler and Ghosh 2017). In various disease conditions, the host–microbe interactions became imbalanced, termed “dysbiosis”, presenting various shifts in microbiome from “healthy” to “diseased” states (Thomas and Jobin 2020).
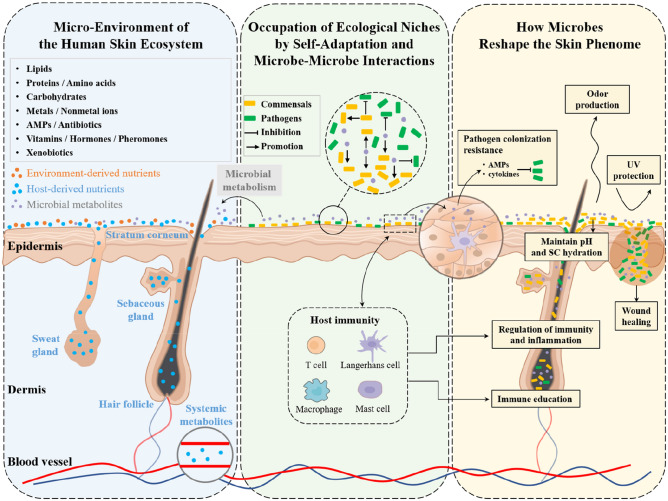
Profiling the structure of skin microbial community is the first step to overview the ecosystem and to address host–microbe interactions. However, this system was proven to be highly individualized and extremely complex. Many factors were identified influencing the composition of the system, including race, gender, age, lifestyle (e.g., occupation, hygiene, skin product and medication usage, and diet) and environment (e.g., climate, geographical location, pollution, UV, and other radiation) (Wei et al. 2022; Grice and Segre 2011; Harris-Tryon and Grice 2022). Nevertheless, from the perspectives of classical ecology, most of these factors may only indirectly influence, but not drive the establishment and maintenance of the system. The primary selection pressures that form the driving forces for the ecosystem, include resource availability (presence of nutrients), environmental conditions (temperature, geographical access) and biological factors (predators and pathogens) (Williams 1996). In this review, we will sum-up related studies centered on these essential selection pressures, including the presence of different types of nutrients and favored micro-environment for dominant skin commensals, the occupation of the ecological niches through self-adaptation or microbe–microbe interactions, and eventually we will discuss how skin microbes, by their structures or bioactive molecules, reshape host skin phenotypes (Fig. 1).
Fig. 1.
Skin microbiome, metabolome and skin phenome, from the perspective of skin as an ecosystem. From left to right: (Blue box) Diverse substances, derived from the host (stratum corneum, skin appendages, and plasma), environment (xenobiotics) and microbial metabolism, cover the skin surface, forming the micro-environment for skin microbiota; (Green box) occupation of ecological niches by self-adaptation and microbe–microbe interactions, promoting commensals or inhibiting pathogens; (Yellow box) the skin microbes, by their own structures or bioactive molecules, reshape the host skin phenotypes
Micro-environment of the Human Skin Ecosystem
The host skin offers nutrients and shelters for microbial survival, competition, and cooperation (Roth and James 1988). Nutrient substances may directly affect microbial colonization, growth and metabolism either through nourishing (Brüggemann et al. 2004) or persecuting (Ferrer et al. 2017); on the other hand, these substances may also finetune the local microenvironment, such as pH or moisture state, and thus exert indirect impact on microbial survival. The microbial energy substances are mainly from the host skin and the outside environment. The host skin-derived nutrients consist of lipids embedded in the “brick and mortar” structure (Chen 2018), piles of dead enucleated corneocytes in the stratum corneum (SC) (Abhishek and Palamadai Krishnan 2016), and the secretions from skin appendages [hair follicles (HFs) and glands]. The environment-derived nutrients include personal skincare products, medication, and other environmental xenobiotics. Here, we summarized the metabolites detected on the skin by various metabolome studies (Table1).
Table 1.
Human skin metabolites: their primary source and functions
| Substances | Functions |
|---|---|
| Metal and non-metal ions from SC and sweat | |
| Sodium, chloride and potassium, calcium, copper, magnesium, zinc, iron, chromium, nickel, lead, manganese, arsenic, mercury, cobalt, molybdenum, strontium, titanium, aluminum, cadmium, lead, nitrogen, iodine, bicarbonate, and phosphorus (Consolazio et al. 1962, 1966; Sears et al. 2012; Minshall et al. 2014; Cohn and Emmett 1978) |
Formation of the high-salt environment (Chen et al. 2018) pH of sweat (Sato 1977; Sato and Sato 1990) Regulation of electrolyte homeostasis (Müller et al. 2019) Microbial growth factors (Constante et al. 2017) NMF: potassium, sodium, magnesium, and calcium (Jokura et al. 1995) |
| Amino acid and its derivatives from SC and sweat glands | |
| l-histidine, threonine, glycine, l-arginine, l-methionine, l-lysine, l-isoleucine, l-leucine, l-valine, l-phenylalanine, tryptophan, l-alanine, l-tyrosine, l-serine, N-acetyl-dl-serine, urocanic acid, uric acid, l-prolinamide, pyroglutamic acid, l-proline, l-carnitine, creatine, l-asparagine, l-glutamine, citrulline, l-glutamate, l-aspartic acid, l-pipecolic acid, ornithine, l-prolinamide, betaine, and taurine (Harshman et al. 2018; Craig et al. 2010) |
NMF: l‐serine, Glycine, l‐alanine, histidine, ornithine, citrulline, arginine, and urocanic acid (Caspers et al. 2001; Burke et al. 1966) Skin barrier integrity and appearance (Solano 2020) Acid–base balance and water retention in SC: urocanic acid, serine, and taurine (Solano 2020; Kim et al. 2012, 2021b) Promote wound healing and restore impaired skin: serine, and arginine (Solano 2020; Badiu et al. 2010) UV protection: urocanic acid, phenylalanine, tyrosine, tryptophan, and taurine (Barresi et al. 2011; Wondrak et al. 2006; Kim et al. 2021b) Antioxidant: methionine, tryptophan (Solano 2020; Sardana and Garg 2010) Defense against pathogens: urocanic acid (Solano 2020) Inflammatory and allergic responses: taurine (Solano 2020; Kim et al. 2021b) Collagen synthesis: isoleucine, leucine, and valine (Yamane et al. 2018) Prevention of acne and cold sore: lysine (Solano 2020) |
| Peptides, proteins and their derivatives | |
| Proteins from SC, viable epidermis and sweat gland | |
| Urea (Caspers et al. 2001); loricrin (Nithya et al. 2015); keratins (Jokura et al. 1995); filaggrin (Arezki et al. 2017); prolactin-inducible protein, clusterin, apolipoprotein D, PIP (Csősz et al. 2015; Myal et al. 1991); serum albumin, cytokeratin I, Zn-α2-glycoprotein, cystatin A; lipophilin B, CatD (Baechle et al. 2006); protease: several members of the major skin desquamatory family of KLKs (such as KLK1, KLK6-11, KLK13) and cathepsins B, D, Z, F, S, L2, β-chain, MMP8 (Baechle et al. 2006; Yu et al. 2017; Baker 2019) |
NMF: filaggrin, urea (Caspers et al. 2001; Arezki et al. 2017) Protect skin from various stresses: keratins, filaggrin, urea, loricrin, apolipoprotein D, and serum albumin (Solano 2020; Nithya et al. 2015; Fluhr et al. 2008; Bajo-Grañeras et al. 2011; Tözsér and Berta 1998) Skin maintenance and protection via desquamation of horny layer, hydrolysis of debris in the ductal lumen, allergen inhibition: proteolytic enzymes (Yokozeki et al. 1991) Tissue regeneration: apolipoprotein D (Bajo-Grañeras et al. 2011) Transport, binding, antioxidant and catalytic activity role: serum albumin, protease (Yu et al. 2017; Gum et al. 2004) Immunological functions: Prolactin-inducible protein bind to IgG, IgG-Fc, CD4-T cell receptor (Autiero et al. 1991; Lee et al. 2002) and also to different species of bacteria such as streptococci (Nistor et al. 2009; Hassan et al. 2009) Chaperone, modulator of MMP9 activity: clusterin (Schenkels et al. 1997; Jeong et al. 2012) |
| Neuropeptides from sweat gland | |
| SP, CGRP (N'Diaye et al. 2017) |
Sense microbes and critical for skin homeostasis (N'Diaye et al. 2017) Modulator of skin microbiome virulence (N'Diaye et al. 2017) Anti-inflammation (Choi et al. 2018): low concentrations of SP |
| Antimicrobial peptides (AMPs) from sweat, sebocytes and keratinocytes (KCs) | |
| RNAse7, S100 proteins (S100A7, S100A8, S100A9, S100A12 and S100A15), hBD-1-3, cathelicidins (Büchau and Gallo 2007); active form of cathelicidin (NL-8, LR-10, KR-10, IK-14, LL-17, LL-23, KR-20, KS-27, KS-30, and LL-37) (Yamasaki et al. 2006; Murakami et al. 2002); DCD (Lousada et al. 2021; Reithmayer et al. 2009); DCD-1L and DCD-1L derived peptides (Schittek et al. 2001); cathelicidin hCAP-18 (Sørensen et al. 2001; Baechle et al. 2006); histone H4 (Lee et al. 2009); LF (Park et al. 2011); sIgA (Imayama et al. 1994); Lcn2 (Takahashi and Yamasaki 2020) |
Participation in epithelial innate defense and defense against pathogens (Serag et al. 2021; Gläser et al. 2005; Nizet et al. 2001; Park et al. 2011) Enhance the antimicrobial action of FFAs in human sebum: histone H4 (Lee et al. 2009) |
| Cytokines/chemokines/antibodies from KCs and sweat | |
| IL-1α, 1β, 6, 8, 25, 31, 36, TNF-α, IFN-β and CXCL10, IgG, IgA (Takahashi and Yamasaki 2020; Dai et al. 2013; Baker 2019) |
Prime and amplify epidermal innate immune signals with the dermal adaptive immune system (Takahashi and Yamasaki 2020; Li et al. 2018b; Xu et al. 2018) |
| Sugar from sweat, cosmetics and extracellular matrix | |
| Lactate (Caspers et al. 2001); glucose, fructose, mannose, and galactose (Roux et al. 2022); β-glucans (Du et al. 2014); hyaluronic acid (Lew and Liong 2013) |
NMF: lactate (Caspers et al. 2001) The elevated glucose level promotes itching and delay the recovery of skin barrier (Ono et al. 2018) Anti-wrinkle, wound healing, antioxidant activity, anti-UV effect, and moisturizing effect: β-Glucans (Du et al. 2014) Epidermal barrier regulation: hyaluronic acid (Lew and Liong 2013) Enhance self-defense of the skin for infection: low molecular weight hyaluronic acid (Gariboldi et al. 2008) |
| Lipid and its metabolites | |
| Sweat-derived lipids | |
| Over 150 lipid mediators, including prostanoids, alcohols, diols, epoxides, ketones, nitrolipids, N-acylethanolamides, monoacylglycerols, and ceramides (Agrawal et al. 2018); lauric acid (C12:0), myristic acid (C14:0), palmitic acid (C16:0), oleic acid (C18:1), and stearic acid (C18:0) (Nunome et al. 2010); lactic acid; pyrrolidone-5-carboxylic acid (Caspers et al. 2001); 5-aminopentanoic acid, and l-pipecolic acid (Harshman et al. 2018) |
Extracellular stimuli response: lipid mediators (Murakami 2011) Antimicrobial, anti-inflammatory effect: lauric acid, oleic acid, and lactic acid (Drake et al. 2008; Fischer et al. 2012; Clayton et al. 2019; Lew and Liong 2013) NMF: lactic acid, pyrrolidone-5-carboxylic acid (McGrath 2008; Caspers et al. 2001) |
| Epidermal (SC) Lipids | |
| Ceramides (Unique to epidermis) (Pappas 2009); FAs: saturated FFAs, monounsaturated FAs, polyunsaturated FAs (PUFAs), and hydroxyl FFAs (Ansari et al. 1970); cholesterol (Cui et al. 2016) | Barrier against the chemical, physical, and microorganism insults (Feingold 2009) |
| Sebaceous lipids from sebum (sebaceous glands) | |
| TG and FAs (Greene et al. 1970); diglycerides, wax esters (Pappas 2009); squalene (Thiboutot 2004; Nicolaides 1974; Thody and Shuster 1989); cholesterol, cholesterol esters (Greene et al. 1970); sapienic acid (C16:1Δ6) (Pappas 2009; Nicolaides 1974); sebaleic acid (18:2Δ5, 8) (Picardo et al. 2009); oleic acid (18:1Δ9) (Lovászi et al. 2017) |
Maintain skin surface moisture permeability: wax ester, FFAs, and squalene (Cui et al. 2016; Pappas 2009) Antimicrobial, antioxidant, anti-inflammatory effect: squalene, wax esters, FFAs, cholesterol ester, sapienic acid, and oleic acid (Nakatsuji et al. 2010; Pappas 2009; Kim and Karadeniz 2012; Cui et al. 2016) Mediate immune responses: FFAs (Cui et al. 2016) UV protection: squalene (Ohsawa et al. 1984) |
| Plasma lipids | |
| Cholesterol, plant sterols, β-sitosterol, campesterol, and stigmasterol (Bhattacharyya et al. 1972); lathosterol and lanosterol (Bhattacharyya et al. 1972); itaconic acid, crotonic acid and heptadecanoic acid, xanthine, d-ribose 5-phosphate, and uric acid (Chen et al. 2021) |
Participation in lipid metabolism: itaconic acid, crotonic acid, and heptadecanoic acid (Chen et al. 2021) Positive correlated with specific skin bacteria: itaconic acid, crotonic acid, and heptadecanoic acid (Chen et al. 2021) Participation in nucleotide metabolism: xanthine, d-ribose 5-phosphate, and uric acid (Chen et al. 2021) |
| Lipids in cosmetic products/personal care products | |
| o-formylbenzoic acid, oleic acid, palmitic acid, and monoacylated glycerols monoolein and monopalmitin (Bouslimani et al. 2015); mineral oils and waxes (Petry et al. 2017) |
Shaping the chemical environment for specific skin microbial communities (Bouslimani et al. 2019) Provide nutrients and promote the growth of lipophilic bacteria (Bouslimani et al. 2015; Unno et al. 2017; Holland et al. 2010) |
| Vitamins mainly from sweat | |
| Niacin (Sargent et al. 1944); vitamin D (Cornbleet et al. 1936; Lugg and Ellis 1954; Dam 1978; van der Beek 1991); l-ascorbic acid (Vitamin C) (Harshman et al. 2018); vitamin E (Cornbleet et al. 1936; Lugg and Ellis 1954; Dam 1978; van der Beek 1991); niacinamide (Gehring 2004) |
Maintenance of epidermal barrier and moisture: niacinamide (Gehring 2004) Anti-inflammatory, anti-aging effect: niacinamide, vitamin C, and vitamin E (Cornbleet et al. 1936; Lugg and Ellis 1954; Dam 1978; van der Beek 1991; Gehring 2004) UV protection: active vitamin D3, and vitamin C (Pullar et al. 2017; Bocheva et al. 2021) |
| Pheromones from sweat glands and sebaceous glands | |
| Releaser/primer/signaler/modulator pheromones (Preti et al. 2003); adrenal glucocorticoids (Nichols and Miller 1948) |
Body odor (Baker 2019) Generate immediate, primarily behavioral responses: releaser pheromones (Preti et al. 2003) Generate slower physiological/endocrine/neuroendocrine responses: primer pheromones (Preti et al. 2003) Mood and multisensory inputs regulation: modulator pheromones (Jacob and McClintock 2000) |
| Other xenobiotics from the environment, i.e. pollutants or personal care products | |
|
PAHs (Leung et al. 2020) POPs (organochlorinated pesticides, polychlorinated biphenyls, perfluorinated compounds) and other toxicants (BPA, heavy metals, phthalate, and polybrominated diphenyl ethers) (Baker 2019) Drugs (griseofulvin, ketoconazole, beta-lactam antibiotics, ceftazidime, ceftriaxone and isotretinoin) (Hoiby et al. 2000; Sato et al. 1989a, 1989b; Tilles 2014) Cosmetics (preservatives, moisturizers, foundation, foot powder, deodorant, topical prebiotics, and topical postbiotics) (Salminen et al. 2021; Pinto et al. 2021; Murphy et al. 2021) Others (e.g., ethanol, pyrrolidine, piperidine, trolamine, and diolamine) (Harshman et al. 2018) |
Influence the function and structure of skin microbiome: PAHs (Leung et al. 2020) Promote premature skin aging, pigmentary disorder, acne, and skin cancer: PAHs (Leung et al. 2020) Cause vitamin D deficiency: POPs (Bocheva et al. 2021) Antibiotics increased antibiotic resistance: drugs Modulation of dihydrotestosterone formation: isotretinoin (Tilles 2014) Cosmetics Influence the function and structure of skin microbiome: foundation and foot powder (Bouslimani et al. 2015, 2019; Elpa et al. 2021; Staudinger et al. 2011; Boxberger et al. 2021) Favor the growth of potential pathogens, such as S. aureus: emulsifiers (Krogsgård Nielsen et al. 2016) Provide nutrients and promote the growth of lipophilic bacteria such as Staphylococcus and Propionibacterium: moisturizers (Bouslimani et al. 2015; Unno et al. 2017; Holland et al. 2010) Preservatives exert antimicrobial effect in vitro (Pinto et al. 2021; Wang et al. 2019a; Murphy et al. 2021), such as inhibit the growth and biofilm formation of S. aureus or pathogenic C. acnes in vitro (Gannesen et al. 2019), but no influence on the skin microbiome in vivo (Murphy et al. 2021) |
NMF natural moisturizing factor, PIP prolactin inducible protein, CatD cathepsin D, KLKs kallikrein-related peptidases, MMP matrix metalloproteinase, SP substance P, CGRP calcitonin gene-related peptide, DCD dermcidin, hBD human β-defensins, LF Lactoferrin, sIgA Secretory form of immunoglobulin A, S100A7 psoriasin, S100A8 calgranulin A, S100A9 calgranulin B, S100A12 calgranulin C, Lcn2 lipocalin-2, IL Interleukin, TNF-α tumor necrosis factor-α, IFN-β interferon-beta, TG triglyceride, FAs fatty acids, FFAs free fatty acids, PAHs polycyclic aromatic hydrocarbons, POPs persistent organic pollutants, BPA bisphenol-A
It is known that individuals, even the same individual at different life stages, vary markedly in regards to the delicate structure or secretion function of the skin and appendages, which produce metabolites consistently and thus play an essential role in shaping diverse microenvironments with distinct pH, salt, moisture, sebum content, and extent of anaerobiosis (Grice and Segre 2011; Capone et al. 2011; Grice et al. 2009). Factors that influence systemic metabolisms, such as diet and gut microbiota, and hormone levels, can also significantly impact the skin’s local microhabitats (Prescott et al. 2017). Furthermore, one’s exposome, such as environmental pollution, UV levels, occupation environment, drug or skincare habits, is highly individualized (Khmaladze et al. 2020). These together form highly complex physical and chemical landscapes on the skin surface, likely to be the real biological explanation that underlies the substantial inter-individual variability in the skin microbiota. Indeed, our previous study showed two robust “cutotypes” of microbial networks on Chinese facial skin, C-cutotype and M-cutotype, possessed distinct patterns of skin properties (Li et al. 2021). The dominant two species, C. acnes and Moraxella osloensis, exhibited vastly varied nutrient-demand: whereas C. acnes was high nutrient demanding, M. osloensis was a non-fastidious bacterium that was able to grow in a mineral medium supplemented with a single organic carbon source (Juni 1974; Juni and Bøvre 2015). This species was shown to be incapable of utilizing any carbohydrates or possessing any saccharolytic activity, but strictly depend on other carbon sources such as acetic or lactic acid (Baumann et al. 1968; Juni 1974; Juni and Bøvre 2015; Moss et al. 1988).
Occupation of Ecological Niches by Self-adaptation and Microbe–Microbe Interactions
The skin surface formed diverse microhabitats, and many studies favored to divide them into four types (sebaceous, moist, dry, and foot) according to the physical properties of anatomical locations (Oh et al. 2014). Although such water/oil-based classification was not delicate enough, some prominent features for the growth and colonization of the microbiota were well identified. Other metabolites and physical properties were also identified in modulating microbial communities. Furthermore, microbe–microbe interactions are essential for shaping the skin ecosystem. In general, microbes deploy strategies to adapt to the living environment and compete for ecological niches via the following: (1) Self-adaptation to the specific environment conditions: skin microbiota changes their characteristic like metabolism pathways to adapt to the skin microenvironment. For example, Staphylococcus synthesized tensioactive agent to withstand the low pH and high salt content of sweat (Hentati et al. 2021; Scharschmidt and Fischbach 2013); (2) Competition for ecological niches through microbe–microbe interactions, for example, coagulase-negative Staphylococcus (CoNS) species can either directly kill or limit the virulence of Staphylococcus aureus through the secretion of different regulators (Flowers and Grice 2020). Here we will sum-up the findings of this part (Table 2).
Table 2.
Features of dominant skin commensals for the occupation of ecological niches
| Favorable microenvironment | Biology basis for self-adaptation | Occupation of ecological niches by microbe–microbe interactions |
|---|---|---|
|
Cutibacterium (gram-positive anaerobic bacilli) C. acnes, C. granulosum, and C. avidum | ||
|
HFs with low oxygen content (Scharschmidt and Fischbach 2013) Sebum-rich areas, i.e. the face, scalp, chest, and back (Scharschmidt and Fischbach 2013; Brown and Shalita 1998) Moist areas: C. avidum (McGinley et al. 1978) |
C. acnes Utilize nutrients from SC, sebum, and sweat (Scharschmidt and Fischbach 2013) by secreting lipase (Brown and Shalita 1998; Brüggemann et al. 2004) and proteases (Holland et al. 1979) Catabolize sebum to FFAs for better skin attachment (Brüggemann et al. 2004; Brown and Shalita 1998; Miskin et al. 1997; Gribbon et al. 1993) Secrete porphyrins to oxidize squalene and lower oxygen tension in HFs (Tilles 2014; Holland et al. 1998) |
C. acnes Secrete propionicin to defend against Gram-positive and Gram-negative anaerobes (Christensen and Bruggemann 2014) Secrete RoxP to facilitate the growth of aerobic bacteria (Allhorn et al. 2016) Produce FFAs to acidify the skin to inhibit colonization by other pathogenic microbes (S. aureus and Streptococcus pyogenes) (Youn et al. 2013) Produce coproporphyrin III to induce S. aureus aggregation and biofilm formation (Wollenberg et al. 2014) Produce CAMP factor to intensify the virulence of S. aureus (Lo et al. 2011) Produce a thiopeptide antibiotic, cutimycin, to limit S. aureus colonization (Claesen et al. 2020) |
|
Staphylococci (gram-positive cocci aerobes or facultative anaerobes) CoNS: S. epidermidis, S. capitis, S. caprae, S. hominis, S. lugdunensis, and S. haemolyticus | ||
|
Highly adaptable: occlude areas (axilla), exposed dry sites (volar forearm) and also low oxygen area of the HFs (Scharschmidt and Fischbach 2013) S. epidermidis favors areas of high eccrine glands density, high moisture, temperature and pH (Scharschmidt and Fischbach 2013) Nasal: S. lugdunensis (Zipperer et al. 2016; Nakatsuji et al. 2017) |
Staphylococci are able to utilize diverse nutrients from SC, sebum and sweat (Scharschmidt and Fischbach 2013) Staphylococcus can synthesize tensioactive agents to withstand the low pH and high salt content of sweat (Hentati et al. 2021; Scharschmidt and Fischbach 2013) S. epidermidis High-salt tolerance (Scharschmidt and Fischbach 2013) Possess various adhesins for colonization (Ginsburg 2002; Scharschmidt and Fischbach 2013; Flowers and Grice 2020) Produce enzymes for esterifying FAs that protect from abundant bactericidal lipids (Chamberlain and Brueggemann 1997) |
S. epidermidis, S. hominis and S. capitis secrete lantibiotics, class II bacteriocins, PSMs or AMPs to inhibit MRSA, Streptococcus pyogenes, S. aureus and C. acnes, and synergize with the human AMP LL-37 to enhance skin defense (Nakatsuji et al. 2017; Bastos et al. 2009; Cogen et al. 2010; O'Neill et al. 2020; Janek et al. 2016) S. epidermidis secrete 6-HAP or SCFAs to inhibit GAS, MRSA and S. aureus growth (Nakatsuji et al. 2018; Wang et al. 2014; Keshari et al. 2019; Kao et al. 2017) S. epidermidis produce and release Esp to inhibit biofilm formation and disrupt the biofilm of S. aureus (Iwase et al. 2010) S. lugdunensis secrete lugdunin to inhibit S. aureus (Zipperer et al. 2016) S. capitis antagonize S. aureus through interference with the agr quorum sensing pathways, which are required for S. aureus virulence (Paharik et al. 2017; Williams et al. 2019) |
| S. aureus (coagulase-positive) | ||
| Moist skin sites (nasal, axillary, inguinal and rectal areas) (Kluytmans et al. 1997; Yang et al. 2010) |
Form biofilm (van Loosdrecht et al. 1990) Multi-drug resistance (Wang et al. 2019b) |
Opportunistic pathogen Acquire ACME horizontally from S. epidermidis to optimize growth conditions for nutrients and survival (Diep et al. 2006; Scharschmidt and Fischbach 2013) |
|
Corynebacteria (gram-positive aerobes or facultative anaerobes belonging to the Phylum Actinobacteria) C. accolens, C. jeikeium, C. urealyticum, C. amycolatum, C. minutissimum, C. striatum, and C. pseudodiphtheriticum | ||
|
Moist and sebaceous skin sites (Scharschmidt and Fischbach 2013) Occluded areas (Flowers and Grice 2020) Nasal cavity: C. pseudodiphtheriticum, C. accolens (Hardy et al. 2019) |
Acquire nutrients from SC, sebum and sweat, depending on lipase (Scharschmidt and Fischbach 2013; Houpt 2005; Flowers and Grice 2020) Halotolerant (high-salt) (Scharschmidt and Fischbach 2013) Generate mycolic acid layer to resist multiple stresses, such as detergents, antimicrobials, and lysozyme, allowing colonization across various conditions (Burkovski 2018; Tauch and Burkovski 2015) C. striatum: multi-drug resistance (Wang et al. 2019b) |
C. accolens produce FFAs to inhibit S. pneumoniae (Bomar et al. 2016) C. striatum modulate the Agr quorum-sensing system and expression of Agr-inducible virulence genes to limit S. aureus (Ramsey et al. 2016) C. pseudodiphtheriticum mediate bactericidal activity against S. aureus (Hardy et al. 2019) |
|
Fungi Malassezia: M. dermatis, M. furfur, M. globosa, M. restricta, and M. sympodialis | ||
|
Relatively stable at different sites (Bouslimani et al. 2019; Findley et al. 2013) Malassezia favored lipid-rich areas, such as the face, scalp, back and outer ears (Kaneko et al. 2010) M. sympodialis (nares, antecubital crease, volar forearm, and hypothenar palm); M. globose (back, occiput, and inguinal crease); M. restricta (external auditory canal, retroauricular crease, and glabella) (Findley et al. 2013); M. obtuse (groin, nasal vestibule) (Grice and Dawson 2017) |
Malassezia enrich glycosyl hydrolases and genes involved in carbohydrate metabolism, concordant with adaptation to a carbohydrate-deficient and lipid-rich environment (Wu et al. 2015) Malassezia acquired a catalase horizontally to protect Malassezia cells from their own secreted hydrogen peroxide generating proteins (Wu et al. 2015) Malassezia aquired flavohemoglobins horizontally from the bacterial genus Corynebacterium, increasing NO resistance (Ianiri et al. 2020; Wisecaver et al. 2016) |
M. globosa secrete protease (MgSAP1) to degrade virulence protein of S. aureus and inhibit its biofilm formation (Li et al. 2018a; Ianiri et al. 2018) Malassezia produce VOCs to inhibit S. aureus, Bacillus subtilis and Escherichia coli (Al-Fatimi et al. 2016) M. sympodialis, M. globosa, and M. slooffiae can form biofilms to be potential pathogens in community (Angiolella et al. 2020) |
|
Others Fungi: Aspergillus, Cryptococcus, Rhodotorula, Epicoccum, and others (Findley et al. 2013) Probiotics: Enterococcus faecalis SL-5, Lactobacillus, Bifidobacteria, and Nitrosomonas eutropha (Kang et al. 2009; Lew et al. 2013; Lee et al. 2018; Notay et al. 2020) | ||
Roxp Radical oxygenase of Propionibacterium acnes, CAMP Christie, Atkins, Munch Peterson, PSMs Phenol-soluble modulins, 6-HAP 6-N-hydroxyaminopurine, SCFAs Short-chain fatty acids, GAS group A Streptococcus, MRSA Methicillin-resistant Staphylococcus aureus, Esp Serine protease, ACME Arginine catabolic mobile element, Agr Accessory gene regulator, MgSAP1 Malassezia globosa Secreted Aspartyl Protease 1, VOCs volatile organic compounds
Compared to the skin surface, HFs provide a more moisture and acidic environment with ultraviolet light protection, facilitating the colonization of multiple bacteria, fungi, and viruses. The most abundant bacteria in the HFs were P. acnes spp. (Lousada et al. 2021). M. restricta and M. globosa are the dominant fungi (Lousada et al. 2021). Meanwhile, the HF virome comprises dependoviruses, Propionibacterium phage P100D and 101A, papillomaviruses and adeno-associated viruses (Hall et al. 2018). In addition, the mite (Demodex folliculorum) groups are often found in the distal infundibulum, usually with their dorsal body oriented against the hair shaft (Elston and Elston 2014).
From Microbes to Host Skin: How Microbes Reshape the Skin Phenome
Skin microbiota leverage “nutrients” from the host skin and environment and produce a series of bioactive molecules with vital functions (Chen et al. 2018). For example, skin microbiota can convert host proteins into amino acids by their protease (Holland et al. 1979; Byrd et al. 2018), ferment carbohydrates into lactic acids (Ong et al. 2020) or decompose sebum lipids such as triglycerides into free fatty acids (FFAs) (Traisaeng et al. 2019; Belkaid and Segre 2014). In addition, skin microbiota produces AMPs, phenol-soluble modulins (PSMs), and antibiotics (Belkaid and Segre 2014; Gallo and Hooper 2012). These metabolism products may further act on the host or other microbes, exert biological effects and reshape the skin phenome.
The most well-studied functions of skin commensals include the following: (1) pathogen colonization resistance by ecological niche blocking for the invasion of opportunistic or pathogenic microbiota, (2) immune education during early phases, and (3) regulation of immunity and inflammation. Given many comprehensive reviews already on these functions, we will take a particular focus on other functions that were usually missed, including the maintenance of skin physiology, such as pH and SC hydration, UV protection, odor production, and wound healing, which were also important functions in skin homeostasis.
Regulation of Immunity and Inflammation
The microbiota is a rich source of short-chain fatty acids (SCFAs) (Traisaeng et al. 2019). For example, C. acnes fermented carbohydrates into propionic acid (Traisaeng et al. 2019); S. epidermidis was able to ferment glycerol to butyric acid and acetic acid in vitro (Traisaeng et al. 2019; Keshari et al. 2019). SCFAs can regulate several immune cell functions, including the production of cytokines (TNF-α, IL-2, IL-6, and IL-10) (Traisaeng et al. 2019), activate resident skin regulatory T (Treg) cells, mitigate inflammatory skin reactions and thus contribute to the preservation of skin homeostasis in mice and human (Schwarz et al. 2017). Butyric acid significantly attenuated lipopolysaccharide (LPS)-induced nuclear factor-κB (NF-κB) activation and nitric oxide production in murine macrophage cell line (Chakravortty et al. 2000), reduced interferon-gamma (IFNγ)-induced proinflammatory IL-6 and TNF-α production in a macrophage cell line (Park et al. 2007) and mediated short-chain fatty acid receptor 2 (FFAR2) to modulate the production of proinflammatory cytokines induced by ultraviolet B (UVB) in mice (Keshari et al. 2019). Furthermore, the ability of immune cells to migrate to the foci of infection can be regulated by SCFAs (Vinolo et al. 2011). Given the potential anti-inflammatory of SCFAs, they are applied on psoriatic skin in vitro. This study found that decreased expression of G-protein-coupled receptors (GPR) GPR43 and GPR109a in psoriatic skin can be restored and expression of inflammatory factors can be inhibited by topical application of sodium butyrate (Krejner et al. 2018). However, SCFAs are not always anti-inflammatory. C. acnes-derived SCFAs inhibit histone deacetylase (HDAC) activity in skin keratinocytes (KCs) and stimulate inflammation through Toll-like receptor (TLR) signaling (Sanford et al. 2016). SCFAs from C. acnes conferred a robust proinflammatory effect in human sebocytes (Sanford et al. 2019). Expression of a major component of the Corynebacterium accolens cell wall, mycolic acid, promotes inflammation in an IL-23-dependent manner under a high-fat diet condition in mice (Ridaura et al. 2018).
The essential amino acid tryptophan (Trp) can be metabolized by human skin microbiota into 5-hydroxytryptophan (5-HTP), indole-3-aldehyde (IAId) and other metabolites (Yu et al. 2019). IAId was able to suppress thymic stromal lymphopoietin (TSLP) and thereby inhibited calcipotriol (MC903)-induced AD-like dermatitis in mice (Yu et al. 2019). IAId can also activate aryl hydrocarbon receptor (AhR), producing indoleamine 2,3-dioxygenase (IDO) and IL-10 in Langerhans cells (LCs), and thus negatively regulate skin inflammation (Liu et al. 2020).
S. epidermidis and other Gram-positive bacteria release adhesion molecules upon bacteriolysis, such as lipoteichoic acid (LTA) (Ginsburg 2002). LTA from Staphylococcal species suppressed inflammation during tissue injury through a Toll-like receptor 2 (TLR2)-dependent mechanism to prevent excessive damage (Lai et al. 2009). Staphylococcal LTA may also have applications in the treatment of inflammatory disease. For example, in an acne model of C. acnes-induced skin inflammation, staphylococcal LTA application abrogated inflammatory effects via induction of a microRNA, miR-143, destabilizes the TLR2 mRNA and decreases protein production (Xia et al. 2016).
In addition, many commensal species contain virulence strains. One major virulence factor of the microorganism is a secretory lipase that acts on triglycerides to release FFAs (Holland et al. 2010). C. acnes exist both in health and patients, but C. acnes from acne patients harbored unique genomic elements encoding virulence factors, including camp5, gehA, sialidases, neuraminidases, endoglicoceraminidases, lipases, proteases and hemolysins that were rarely present in C. acnes genomes from healthy controls (Brüggemann 2005; Burkhart et al. 1999). Several commensals are opportunistic pathogens that encode virulence factors such as toxins, exoenzymes, and adhesins (Brown et al. 2012). Skin microbiota may directly or indirectly mediate inflammatory responses by releasing various virulence factors under unhealthy conditions. Malassezia spp. can be the causative agents in disease. Many Malassezia spp. secrete extracellular vesicles that signal KCs to secrete proinflammatory cytokines (Vallhov et al. 2020; Watanabe et al. 2001; Zhang et al. 2019). Malassezia spp. metabolize sebum to different fatty acids such as phosphatidylcholine (PC) and phosphatidylserine (PS), which then act as irritants, causing flaking and irritation under dandruff, a frequent scalp issue and seborrheic dermatitis conditions (Celis Ramírez et al. 2020; DeAngelis et al. 2005; Han et al. 2019; Johansson et al. 2018).
Pathogen Colonization Resistance
Commensals compete for niches through microbe–microbe interactions, as mentioned above (Table 2). Direct induction of AMPs or cytokine expression in KCs is one of the main strategies used by skin commensals, such as Propionibacterium and S. epidermidis, in defending against pathogen invasion and shaping the skin microbiota community (Midorikawa et al. 2003; Wanke et al. 2011). In addition, commensals function as endogenous cofactors of the skin immune system to promote skin local immune response. Skin harbor considerable commensal-specific T-cell, e.g., Staphylococcus epidermidis-specific IL-17A+ CD8+ T cells (Naik et al. 2015). The activation of these cells can promote AMP production by keratinocytes, thereby promoting heterologous protection against pathogens infections (Braff et al. 2005). Staphylococcus epidermidis can also induce KC to express IL-1α, thus promoting skin αβ T cells to produce IL-17A and IFNγ in mice (Naik et al. 2012). IL-17A induces chemokines that recruit neutrophils and AMP production, thus protecting the host from pathogen infection. In adults, cutaneous mucosal-associated invariant T cells (MAIT cells) are a dominant population of IL-17A-producing lymphocytes (Constantinides et al. 2019). MAIT cells are absent in germ-free (GF) mice, and their development are controlled by microbial metabolites such as vitamin B2 (Treiner et al. 2003; Koay et al. 2016; Legoux et al. 2019). MAIT cells can respond to skin commensals or commensal-derived metabolites in an IL-1-, IL-18-, and antigen-dependent manner (Constantinides et al. 2019), thus enhancing inhibition of pathogen invasion.
Immune Education
The commensals play an essential role in regulating the development, proliferation, maturation and activation of immune cells of innate immunity. A previous study found that GF mice contain mast cells (MCs) that are largely undifferentiated and express abnormally low amounts of stem cell factor (SCF). Commensal bacteria induce KC-produced SCF, promote skin MCs mature. The migration of MCs in the skin is fully dependent on high levels of SCF, as produced by KCs (Wang et al. 2017b). In addition, γδT cells, which play an essential role in recognizing lipids, one of the microbial metabolites (Belkaid and Tamoutounour 2016), significantly reduced IL-17 secretion capacity in GF mice (Naik et al. 2012). Varying from the immune responses to invasive pathogens, adaptive immune responses respond to commensals under noninflammatory conditions, which help build immune homeostasis (Naik et al. 2015).
The skin contains one of the highest frequencies of FOXP3+ Treg cells within the body in mice (Suffia et al. 2006). In the skin of both mice and humans, Tregs reside in the dermis, and a large fraction of these cells can be found in close proximity to HFs, which serve as a natural habitat for skin-resident microorganisms (Ali et al. 2017; Sanchez Rodriguez et al. 2014). Tregs are essential in establishing and regulating immune tolerance to commensal microbes during a defined period of neonatal life in mice (Scharschmidt et al. 2015). S. epidermidis colonization on the skin surface two weeks after birth induces Treg cells’ tolerance to S. epidermidis in adult mice (Scharschmidt et al. 2015). Furthermore, it promotes the accumulation and migration of Treg cells into the skin (Scharschmidt et al. 2017). Further study found that Treg cell migration in Neonatal Skin is influenced by hair follicle development and microbes colonized in the hair follicle. In turn, colonization of microbes in HFs during the early stage is resisted and regulated by Treg cells (Scharschmidt et al. 2017). These results suggest a dynamic balance between microbe and host immune system.
Maintain pH and SC Hydration
Skin microbiota metabolizes dead corneocytes, sweat and sebum components, and other wastes (Pistone et al. 2021) and converts them into amino acids, such as glutamate and aspartate, proteins and various FFAs (Pistone et al. 2021; Timm et al. 2020). They also secrete lactic acid (Ong et al. 2020), a series of SCFAs (Christensen and Brüggemann 2014) and other organic acids (Garrote et al. 2000; Wang et al. 2017a; Bengoa et al. 2019). These acidic metabolites can regulate skin surface pH and SC hydration level (Watabe et al. 2013; McGrath 2008; Caspers et al. 2001; Cui et al. 2016; Pappas 2009).
The skin surface pH is slightly acidic, ranging from 4.5 to 5.5 in human (Braun-Falco and Korting 1986). The pH of the SC is crucial for many vital epidermal functions, including permeability barrier homeostasis, desquamation of corneocytes, initiation of inflammation, processing of secreted lamellar body (LB) polar lipids and antimicrobial defense (Lee and Lee 2014). In addition, variation in pH also affects the SC thickness and pigmentation (Sandby-Møller et al. 2003). These results indicate that many skin traits may intertwine, such as pH, trans-epidermal water loss (TEWL), skin thickness, SC hydration and pigmentation, and thereby may be modulated by skin microbiota and their metabolites.
Our previous study also revealed that cutotypes of microbial networks on Chinese facial skin possess distinct skin traits: C-cutotype skin is more hydrated and more oily, and the levels of skin surface sebum and its microbial metabolite porphyrin are increased; In contrast, M-cutotype skin is dryer and often occurs in the elder (Li et al. 2021). A study on the skin microbiome of Koreans found that Lawsonella had a negative correlation with skin moisture and brown spots; Staphylococcus and Corynebacterium both had negative correlations with the number of UV spots and positive correlations with TEWL; Staphylococcus aureus had a negative correlation with skin moisture parameters (Kim et al. 2021a). Moreover, two studies found a linkage between the skin microbiome and skin metabolites (Howard et al. 2022; Roux et al. 2022). A recent study demonstrated that S. epidermidis can significantly increase skin ceramide levels and thereby prevent water loss of damaged skin dependent on its sphingomyelinase in mice (Zheng et al. 2022).
Skin aging is a dynamic process with a series of changes in the skin phenome (Farage et al. 2008; Pochi et al. 1979; Cotterill et al. 1972; Howard et al. 2022) and skin metabolism, e.g., altered levels of natural moisturizing factors (NMFs), AMPs, vitamins and coenzyme Q10, and many other metabolites (Howard et al. 2022; MacLaughlin and Holick 1985; Kuehne et al. 2017). These changes may underlie the alterations in the microbiome. For example, age-related decrease in sebocyte area is positively correlated with Cutibacterium and negatively correlated with Streptococcus, Acinetobacter, Corynebacterium and Methylobacterium‒Methylorubrum abundance (Howard et al. 2022). Furthermore, anti-aging skincare products were reported able to persist on the skin for weeks and provide long-term contributions to the chemical environment (Bouslimani et al. 2019), thus shaping the specific skin microbial communities (Bouslimani et al. 2015). For example, lipid components of moisturizers could provide nutrients and promote the growth of lipophilic bacteria such as Staphylococcus and Propionibacterium (Bouslimani et al. 2015; Unno et al. 2017; Holland et al. 2010). More details regarding cosmetics can be found in Table 1.
UV Protection
Some skin commensals can protect skin from UV damage by secreting different metabolites (Souak et al. 2021). For example, S. epidermidis can produce 6-HAP to suppress UV-induced tumor in mice (Nakatsuji et al. 2018). Skin microflora produces cis-urocanic acid from l-histidine, affects UV-induced immune suppression and suppresses melanoma growth (Hug et al. 1999; Laihia et al. 2010). Some Streptomyces-derived compounds, such as amides exhibited UV-absorbing, antioxidant, and anti-inflammatory properties (Sánchez-Suárez et al. 2020). Propionic acid produced by Cutibacterium acnes fermentation ameliorates UVB-induced melanin synthesis (Kao et al. 2021). Cyanobacteria develop a diversity of defense mechanisms, including the biosynthesis of UV-absorbing/screening compounds, such as mycosporine-like amino acids (MAAs), and enzymes, including superoxide dismutases (SOD), which counteract oxidative stress (Souak et al. 2021).
Ultraviolet radiation (UV-R) is well known to inhibit the cellular growth of Malassezia furfur (Wikler et al. 1990). On the other hand, Malassezia furfur can produce pityriacitrin, a UV-filtering compound believed to be protective (Machowinski et al. 2006). It is hypothesized that this fungus developed the UV-filter compound to reduce UV damage and compete for survival over other commensals (Machowinski et al. 2006). However, they did not find any adverse effect of pityriacitrin on commensals such as S. aureus, S. epidermidis, or Candida albicans (Machowinski et al. 2006).
Odor Production
The metabolic activities of some skin microbes produce special odors. For example, human body odor is believed to result from bacterial growth and decomposition of secretions from specialized glands in the axillary region (Lam et al. 2018; Decréau et al. 2003; Natsch et al. 2003). Microbes are present in specific scent glands or tissue in mammals and modulate specific odors (Ezenwa et al. 2012). Skin microbes metabolize host sweat and produce volatile metabolites, enhancing the attractiveness of human sweat for the malaria mosquito (Brouwer 1960; Takken and Kline 1989). A recent study specified acetophenone, a volatile from the skin microbiota, promoted mosquito attractiveness in flavivirus-infected hosts (Zhang et al. 2022).
Skin commensal Moraxella osloensis (Li et al. 2021), a species highly tolerant to desiccation and UV irradiation, existed in various living environments, particularly in the laundry. This species has the potential to generate 4-methyl-3-hexenoic acid (4M3H), which is often described as a “wet-and-dirty-dustcloth-like malodor” or an “acidic or sweaty odor” (Kubota et al. 2012). In addition to bacteria, fungi are important sources of many volatile organic compounds (VOCs), including alcohols, aldehydes, esters, FAs, and terpenes (Belinato et al. 2019). In malignant fungating wounds (MFWs), metabolites such as dimethyl trisulfide (DMTS), four fatty acid volatiles (acetic acid, isobutyric acid, butyric acid, and isovaleric acid) and putrescine are linked with components of malignant fungating wound odor (Vardhan et al. 2019).
Wound Healing
Wound healing is a complex but highly regulated process critical for skin barrier function (Han and Ceilley 2017). The presence and abundance of microbes in skin wounds depend on wound type (chronic/acute wound) (Johnson et al. 2018) and shifts over time (Loesche et al. 2017). Studies demonstrated that skin microbiota was also involved in wound healing in multifaceted ways. S. epidermidis promotes rapid KC progression via upregulation of TLR and downstream modulation of TNF-α in skin CD8+ T cells (Linehan et al. 2018; Naik et al. 2015). A study with a wound-induced hair follicle neogenesis (WIHN) mouse model revealed that skin microbiota promoted skin regeneration via IL-1β and KC-dependent IL-1R-MyD88 signaling (Wang et al. 2021). Metabolites from microbiota promote wound healing, e.g., lipoteichoic acid from S. epidermidis can decrease inflammation via TLR2 signaling (Lai et al. 2009). On the other hand, some potential pathogens do not promote cutaneous wound healing. For example, S. aureus (Kirker et al. 2009; den Reijer et al. 2016), Acinetobacter. baumanni and A. junii (de Breij et al. 2012) form biofilms on the SC and have a detrimental effect on human dermal fibroblast migration and ultimately result in cellular apoptosis (Kirker et al. 2012). Microbial stability was believed to be essential for skin health; however, temporal stability in the chronic wound is associated with poor healing as instability in the microbiome reflects effective control of wound bacteria, which prevents any community structure from stabilizing (Loesche et al. 2017).
Conclusion
The present review centers on the current knowledge on skin microbiome from a perspective of skin as an ecosystem and tries to explore the fundamental driving force for the establishment and the balance of the highly personalized microbial feature. We believe that microenvironments that define the physical (e.g., pH, oxygen) and chemical (carbon sources and metabolites) conditions drive the microbiome composition. In turn, these microbes may reshape this environment via microbe–microbe or microbe–host interactions. Skin surface metabolome may be a critical approach to address causative correlations between the skin microbiome and skin phenome; therefore, future skin microbiome research should leverage those multi-omics to reveal these strong correlations and then validate them with the principle of Koch’s postulates. Furthermore, considering the higher complexity of the system due to the host genome and exposome, the longitudinal time-series study should be taken more into consideration for the control of these variables and for addressing the direction of those networks. Based on solid causative correlations, we can develop accurate interventions targeting specific skin microbe(s) and eventually reshape the skin conditions.
Of note, recent studies revealed that microbiota at strain level varies in the local microenvironment (Conwill et al. 2022), suggesting studies on higher resolution should be emphasized, which means deeper sequencing until strain level and more refined sampling sites up to single pore level. However, the greatest challenge for these designs is biomass, including metabolites and metagenomic biomass. This strongly relies on the technology development and iterative update of detection instruments to improve the sensitivity.
The significance of the human skin microbiome is increasingly appreciated. The approach from metagenomic sequencing (profiling) was gradually shifted to isolation/culturomics and function validation (mechanisms). However, some significant issues still exist, such as the lack of ideal ex-vivo skin models (e.g., reconstructed human epidermis (RHEs) and skin explants) that can reliably simulate the complexity of the host–microbe interactions (Harris-Tryon and Grice 2022; Larson et al. 2021). Some recent studies performed the function experiments with three-dimensional (3D) human skin equivalent. For example, a study using 3D skin tissue cultures revealed that a model microbiome or a mixed community of skin microbiome representatives led to pronounced changes in epidermal thickness, epidermal cell proliferation, and filaggrin production (Loomis et al. 2021). Another study investigated the interaction between the skin microbiota and environmental pollutant benzo[a]pyrene (B[a]P), with a microbially competent 3D skin model and demonstrated that commensal metabolism of xenobiotics can influence host toxicity (Lemoine et al. 2021). However, the limitations of these ex-vivo skin models are apparent, i.e., the lack of the histological/physiological/immunological complexity of RHEs, the paucity of inter-donor variability of skin explants, as well as short lifespan and the relatively high costs (Larson et al. 2021). Nevertheless, this is a matter of time to address these issues and push forward the skin microbiota targeted new intervention based on solid experimental evidence.
Acknowledgements
This work was supported by the Shanghai Municipal Science and Technology Major Project (2017SHZDZX01), the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-066), the 111 Project (B13016), and a startup grant from the Greater Bay Area Institute of Precision Medicine (Guangzhou), Fudan University to JX.
Authors’ Contributions
Original draft preparation: HC, JX and QZ; Review and editing: HC, JX, JW, QZ, CD; Scientific supervision: JX, JW, JK. All authors have read and agreed to this version of the manuscript.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Ethical Approval
Not applicable.
Contributor Information
Jiucun Wang, Email: jcwang@fudan.edu.cn.
Jingjing Xia, Email: xiajingjing@fudan.edu.cn.
References
- Abhishek S, Palamadai Krishnan S. Epidermal differentiation complex: a review on its epigenetic regulation and potential drug targets. Cell J. 2016;18:1–6. doi: 10.22074/cellj.2016.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal K, Waller JD, Pedersen TL, et al. Effects of stimulation technique, anatomical region, and time on human sweat lipid mediator profiles. Prostaglandins Other Lipid Mediat. 2018;134:84–92. doi: 10.1016/j.prostaglandins.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Fatimi M, Wurster M, Lindequist U. Chemical composition, antimicrobial and antioxidant activities of the volatile oil of Ganoderma pfeifferi Bres. Medicines. 2016 doi: 10.3390/medicines3020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Zirak B, Rodriguez RS, et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell. 2017;169:1119–29.e11. doi: 10.1016/j.cell.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allhorn M, Arve S, Brüggemann H, et al. A novel enzyme with antioxidant capacity produced by the ubiquitous skin colonizer Propionibacterium acnes. Sci Rep. 2016;6:36412. doi: 10.1038/srep36412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiolella L, Rojas F, Mussin J, et al. Biofilm formation, adherence, and hydrophobicity of M. sympodialis, M. globosa, and M. slooffiae from clinical isolates and normal skin virulence factors of M. sympodialis, M. globosa and M. slooffiae. Med Mycol. 2020;58:1162–1168. doi: 10.1093/mmy/myaa017. [DOI] [PubMed] [Google Scholar]
- Ansari MN, Nicolaides N, Fu HC. Fatty acid composition of the living layer and stratum corneum lipids of human sole skin epidermis. Lipids. 1970;5:838–845. doi: 10.1007/bf02531977. [DOI] [PubMed] [Google Scholar]
- Arezki NR, Williams AC, Cobb AJ, et al. Design, synthesis and characterization of linear unnatural amino acids for skin moisturization. Int J Cosmet Sci. 2017;39:72–82. doi: 10.1111/ics.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autiero M, Abrescia P, Guardiola J. Interaction of seminal plasma proteins with cell surface antigens: presence of a CD4-binding glycoprotein in human seminal plasma. Exp Cell Res. 1991;197:268–271. doi: 10.1016/0014-4827(91)90432-t. [DOI] [PubMed] [Google Scholar]
- Badiu DL, Luque R, Dumitrescu E, et al. Amino acids from Mytilus galloprovincialis (L.) and Rapana venosa molluscs accelerate skin wounds healing via enhancement of dermal and epidermal neoformation. Protein J. 2010;29:81–92. doi: 10.1007/s10930-009-9225-9. [DOI] [PubMed] [Google Scholar]
- Baechle D, Flad T, Cansier A, et al. Cathepsin D is present in human eccrine sweat and involved in the postsecretory processing of the antimicrobial peptide DCD-1L. J Biol Chem. 2006;281:5406–5415. doi: 10.1074/jbc.M504670200. [DOI] [PubMed] [Google Scholar]
- Bajo-Grañeras R, Sanchez D, Gutierrez G, et al. Apolipoprotein D alters the early transcriptional response to oxidative stress in the adult cerebellum. J Neurochem. 2011;117:949–960. doi: 10.1111/j.1471-4159.2011.07266.x. [DOI] [PubMed] [Google Scholar]
- Baker LB. Physiology of sweat gland function: the roles of sweating and sweat composition in human health. Temperature. 2019;6:211–259. doi: 10.1080/23328940.2019.1632145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi C, Stremnitzer C, Mlitz V, et al. Increased sensitivity of histidinemic mice to UVB radiation suggests a crucial role of endogenous urocanic acid in photoprotection. J Investig Dermatol. 2011;131:188–194. doi: 10.1038/jid.2010.231. [DOI] [PubMed] [Google Scholar]
- Bastos MC, Ceotto H, Coelho ML, et al. Staphylococcal antimicrobial peptides: relevant properties and potential biotechnological applications. Curr Pharm Biotechnol. 2009;10:38–61. doi: 10.2174/138920109787048580. [DOI] [PubMed] [Google Scholar]
- Baumann P, Doudoroff M, Stanier RY. Study of the Moraxella group. I. Genus Moraxella and the Neisseria catarrhalis group. J Bacteriol. 1968;95:58–73. doi: 10.1128/jb.95.1.58-73.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinato JR, Silva E, De Souza DS, et al. Rapid discrimination of fungal strains isolated from human skin based on microbial volatile organic profiles. J Chromatogr B Anal Technol Biomed Life Sci. 2019;1110–1111:9–14. doi: 10.1016/j.jchromb.2019.02.007. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346:954–959. doi: 10.1126/science.1260144. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Tamoutounour S. The influence of skin microorganisms on cutaneous immunity. Nat Rev Immunol. 2016;16:353–366. doi: 10.1038/nri.2016.48. [DOI] [PubMed] [Google Scholar]
- Bengoa AA, Iraporda C, Garrote GL, et al. Kefir micro-organisms: their role in grain assembly and health properties of fermented milk. J Appl Microbiol. 2019;126:686–700. doi: 10.1111/jam.14107. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya AK, Connor WE, Spector AA. Excretion of sterols from the skin of normal and hypercholesterolemic humans. implications for sterol balance studies. J Clin Investig. 1972;51:2060–2070. doi: 10.1172/jci107012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocheva G, Slominski RM, Slominski AT. The impact of vitamin D on skin aging. Int J Mol Sci. 2021 doi: 10.3390/ijms22169097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomar L, Brugger SD, Yost BH, et al. Corynebacterium accolens releases antipneumococcal free fatty acids from human nostril and skin surface triacylglycerols. Mbio. 2016;7:e01725-15. doi: 10.1128/mBio.01725-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch TC, Mcfall-Ngai MJ. Metaorganisms as the new frontier. Zoology. 2011;114:185–190. doi: 10.1016/j.zool.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouslimani A, Porto C, Rath CM, et al. Molecular cartography of the human skin surface in 3D. Proc Natl Acad Sci USA. 2015;112:E2120–E2129. doi: 10.1073/pnas.1424409112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouslimani A, Da Silva R, Kosciolek T, et al. The impact of skin care products on skin chemistry and microbiome dynamics. BMC Biol. 2019;17:47. doi: 10.1186/s12915-019-0660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxberger M, Cenizo V, Cassir N, et al. Challenges in exploring and manipulating the human skin microbiome. Microbiome. 2021;9:125. doi: 10.1186/s40168-021-01062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff MH, Zaiou M, Fierer J, et al. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73:6771–6781. doi: 10.1128/iai.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun-Falco O, Korting HC. Normal pH value of human skin. Hautarzt. 1986;37:126–129. [PubMed] [Google Scholar]
- Brouwer R. The attraction of carbon dioxide excreted by the skin of the arm for malaria mosquitoes. Trop Geogr Med. 1960;12:62–66. [PubMed] [Google Scholar]
- Brown SK, Shalita AR. Acne vulgaris. Lancet. 1998;351:1871–1876. doi: 10.1016/s0140-6736(98)01046-0. [DOI] [PubMed] [Google Scholar]
- Brown SP, Cornforth DM, Mideo N. Evolution of virulence in opportunistic pathogens: generalism, plasticity, and control. Trends Microbiol. 2012;20:336–342. doi: 10.1016/j.tim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann H. Insights in the pathogenic potential of Propionibacterium acnes from its complete genome. Semin Cutan Med Surg. 2005;24:67–72. doi: 10.1016/j.sder.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Brüggemann H, Henne A, Hoster F, et al. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science. 2004;305:671–673. doi: 10.1126/science.1100330. [DOI] [PubMed] [Google Scholar]
- Büchau AS, Gallo RL. Innate immunity and antimicrobial defense systems in psoriasis. Clin Dermatol. 2007;25:616–624. doi: 10.1016/j.clindermatol.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RC, Lee TH, Buettner-Janusch V. Free amino acids and water soluble peptides in stratum corneum and skin surface film in human beings. Yale J Biol Med. 1966;38:355–373. [PMC free article] [PubMed] [Google Scholar]
- Burkhart CG, Burkhart CN, Lehmann PF. Acne: a review of immunologic and microbiologic factors. Postgrad Med J. 1999;75:328–331. doi: 10.1136/pgmj.75.884.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkovski A. The role of corynomycolic acids in Corynebacterium-host interaction. Antonie Van Leeuwenhoek. 2018;111:717–725. doi: 10.1007/s10482-018-1036-6. [DOI] [PubMed] [Google Scholar]
- Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16:143–155. doi: 10.1038/nrmicro.2017.157. [DOI] [PubMed] [Google Scholar]
- Capone KA, Dowd SE, Stamatas GN, et al. Diversity of the human skin microbiome early in life. J Investig Dermatol. 2011;131:2026–2032. doi: 10.1038/jid.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers PJ, Lucassen GW, Carter EA, et al. In vivo confocal Raman microspectroscopy of the skin: noninvasive determination of molecular concentration profiles. J Investig Dermatol. 2001;116:434–442. doi: 10.1046/j.1523-1747.2001.01258.x. [DOI] [PubMed] [Google Scholar]
- Celis Ramírez AM, Amézquita A, Cardona Jaramillo JEC, et al. Analysis of malassezia lipidome disclosed differences among the species and reveals presence of unusual yeast lipids. Front Cell Infect Microbiol. 2020;10:338. doi: 10.3389/fcimb.2020.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravortty D, Koide N, Kato Y, et al. The inhibitory action of butyrate on lipopolysaccharide-induced nitric oxide production in RAW 264.7 murine macrophage cells. J Endotoxin Res. 2000;6:243–247. doi: 10.1177/09680519000060030501. [DOI] [PubMed] [Google Scholar]
- Chamberlain NR, Brueggemann SA. Characterisation and expression of fatty acid modifying enzyme produced by Staphylococcus epidermidis. J Med Microbiol. 1997;46:693–697. doi: 10.1099/00222615-46-8-693. [DOI] [PubMed] [Google Scholar]
- Chen X. Current and future technological advances in transdermal gene delivery. Adv Drug Deliv Rev. 2018;127:85–105. doi: 10.1016/j.addr.2017.12.014. [DOI] [PubMed] [Google Scholar]
- Chen YE, Fischbach MA, Belkaid Y. Skin microbiota–host interactions. Nature. 2018;553:427–436. doi: 10.1038/nature25177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, He J, Li J, et al. Microbiome and metabolome analyses reveal novel interplay between the skin microbiota and plasma metabolites in psoriasis. Front Microbiol. 2021;12:643449. doi: 10.3389/fmicb.2021.643449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Kim DJ, Nam S, et al. Substance P restores normal skin architecture and reduces epidermal infiltration of sensory nerve fiber in TNCB-induced atopic dermatitis-like lesions in NC/Nga mice. J Dermatol Sci. 2018;89:248–257. doi: 10.1016/j.jdermsci.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Christensen GJM, Brüggemann H. Bacterial skin commensals and their role as host guardians. Benef Microbes. 2014;5:201–215. doi: 10.3920/bm2012.0062. [DOI] [PubMed] [Google Scholar]
- Claesen J, Spagnolo JB, Ramos SF, et al. A Cutibacterium acnes antibiotic modulates human skin microbiota composition in hair follicles. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.aay5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton RW, Göbel K, Niessen CM, et al. Homeostasis of the sebaceous gland and mechanisms of acne pathogenesis. Br J Dermatol. 2019;181:677–690. doi: 10.1111/bjd.17981. [DOI] [PubMed] [Google Scholar]
- Cogen AL, Yamasaki K, Sanchez KM, et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Investig Dermatol. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn JR, Emmett EA. The excretion of trace metals in human sweat. Ann Clin Lab Sci. 1978;8:270–275. [PubMed] [Google Scholar]
- Consolazio CF, Matoush LO, Nelson RA et al (1962) The dermal excretion of minerals and its possible relation to mineral balance and requirements (Sodium, potassium, iron, magnesium and phosphorus). Rep US Army Med Res Nutr Lab Denver [PubMed]
- Consolazio CF, Matoush LO, Nelson RA, et al. Comparisons of nitrogen, calcium and iodine excretion in arm and total body sweat. Am J Clin Nutr. 1966;18:443–448. doi: 10.1093/ajcn/18.6.443. [DOI] [PubMed] [Google Scholar]
- Constante M, Fragoso G, Lupien-Meilleur J, et al. Iron supplements modulate colon microbiota composition and potentiate the protective effects of probiotics in dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2017;23:753–766. doi: 10.1097/mib.0000000000001089. [DOI] [PubMed] [Google Scholar]
- Constantinides MG, Link VM, Tamoutounour S, et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science. 2019 doi: 10.1126/science.aax6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conwill A, Kuan AC, Damerla R, et al. Anatomy promotes neutral coexistence of strains in the human skin microbiome. Cell Host Microbe. 2022;30:171–82.e7. doi: 10.1016/j.chom.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornbleet T, Klein R, Pace E. Vitamin C content of sweat. Arch Derm Syphilol. 1936;34:253–254. doi: 10.1001/archderm.1936.01470140061009. [DOI] [Google Scholar]
- Cotterill JA, Cunliffe WJ, Williamson B, et al. Age and sex variation in skin surface lipid composition and sebum excretion rate. Br J Dermatol. 1972;87:333–340. doi: 10.1111/j.1365-2133.1972.tb07419.x. [DOI] [PubMed] [Google Scholar]
- Craig SS, Craig SA, Ganio MS, et al. The betaine content of sweat from adolescent females. J Int Soc Sports Nutr. 2010;7:3. doi: 10.1186/1550-2783-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csősz É, Emri G, Kalló G, et al. Highly abundant defense proteins in human sweat as revealed by targeted proteomics and label-free quantification mass spectrometry. J Eur Acad Dermatol Venereol. 2015;29:2024–2031. doi: 10.1111/jdv.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Jia Y, Cheng ZW, et al. Advancements in the maintenance of skin barrier/skin lipid composition and the involvement of metabolic enzymes. J Cosmet Dermatol. 2016;15:549–558. doi: 10.1111/jocd.12245. [DOI] [PubMed] [Google Scholar]
- Dai X, Okazaki H, Hanakawa Y, et al. Eccrine sweat contains IL-1alpha, IL-1beta and IL-31 and activates epidermal keratinocytes as a danger signal. PLoS ONE. 2013;8:e67666. doi: 10.1371/journal.pone.0067666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam B. Vitamins and sport. Br J Sports Med. 1978;12:74–79. doi: 10.1136/bjsm.12.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Breij A, Haisma EM, Rietveld M, et al. Three-dimensional human skin equivalent as a tool to study Acinetobacter baumannii colonization. Antimicrob Agents Chemother. 2012;56:2459–2464. doi: 10.1128/aac.05975-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deangelis YM, Gemmer CM, Kaczvinsky JR, et al. Three etiologic facets of dandruff and seborrheic dermatitis: Malassezia fungi, sebaceous lipids, and individual sensitivity. J Investig Dermatol Symp Proc. 2005;10:295–297. doi: 10.1111/j.1087-0024.2005.10119.x. [DOI] [PubMed] [Google Scholar]
- Decréau RA, Marson CM, Smith KE, et al. Production of malodorous steroids from androsta-5,16-dienes and androsta-4,16-dienes by Corynebacteria and other human axillary bacteria. J Steroid Biochem Mol Biol. 2003;87:327–336. doi: 10.1016/j.jsbmb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Den Reijer PM, Haisma EM, Lemmens-Den Toom NA, et al. Detection of alpha-toxin and other virulence factors in biofilms of Staphylococcus aureus on polystyrene and a human epidermal model. PLoS ONE. 2016;11:e0145722. doi: 10.1371/journal.pone.0145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/s0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- Drake DR, Brogden KA, Dawson DV, et al. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- Du B, Bian Z, Xu B. Skin health promotion effects of natural beta-glucan derived from cereals and microorganisms: a review. Phytother Res. 2014;28:159–166. doi: 10.1002/ptr.4963. [DOI] [PubMed] [Google Scholar]
- Elpa DP, Chiu HY, Wu SP, et al. Skin metabolomics. Trends Endocrinol Metab. 2021;32:66–75. doi: 10.1016/j.tem.2020.11.009. [DOI] [PubMed] [Google Scholar]
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739–743. doi: 10.1016/j.clindermatol.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Ezenwa VO, Gerardo NM, Inouye DW, et al. Microbiology. Anim Behav Microbiome Sci. 2012;338:198–199. doi: 10.1126/science.1227412. [DOI] [PubMed] [Google Scholar]
- Farage MA, Miller KW, Elsner P, et al. Functional and physiological characteristics of the aging skin. Aging Clin Exp Res. 2008;20:195–200. doi: 10.1007/bf03324769. [DOI] [PubMed] [Google Scholar]
- Feingold KR. The outer frontier: the importance of lipid metabolism in the skin. J Lipid Res. 2009;50(Suppl):S417–S422. doi: 10.1194/jlr.R800039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer M, Méndez-García C, Rojo D, et al. Antibiotic use and microbiome function. Biochem Pharmacol. 2017;134:114–126. doi: 10.1016/j.bcp.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Findley K, Oh J, Yang J, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013;498:367–370. doi: 10.1038/nature12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer CL, Drake DR, Dawson DV, et al. Antibacterial activity of sphingoid bases and fatty acids against gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2012;56:1157–1161. doi: 10.1128/aac.05151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers L, Grice EA. The skin microbiota: balancing risk and reward. Cell Host Microbe. 2020;28:190–200. doi: 10.1016/j.chom.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr JW, Darlenski R, Surber C. Glycerol and the skin: holistic approach to its origin and functions. Br J Dermatol. 2008;159:23–34. doi: 10.1111/j.1365-2133.2008.08643.x. [DOI] [PubMed] [Google Scholar]
- Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannesen AV, Borrel V, Lefeuvre L, et al. Effect of two cosmetic compounds on the growth, biofilm formation activity, and surface properties of acneic strains of Cutibacterium acnes and Staphylococcus aureus. Microbiologyopen. 2019;8:e00659. doi: 10.1002/mbo3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariboldi S, Palazzo M, Zanobbio L, et al. Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of beta-defensin 2 via TLR2 and TLR4. J Immunol. 2008;181:2103–2110. doi: 10.4049/jimmunol.181.3.2103. [DOI] [PubMed] [Google Scholar]
- Garrote GL, Abraham AG, De Antoni GL. Inhibitory power of kefir: the role of organic acids. J Food Prot. 2000;63:364–369. doi: 10.4315/0362-028x-63.3.364. [DOI] [PubMed] [Google Scholar]
- Gehring W. Nicotinic acid/niacinamide and the skin. J Cosmet Dermatol. 2004;3:88–93. doi: 10.1111/j.1473-2130.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis. 2002;2:171–179. doi: 10.1016/s1473-3099(02)00226-8. [DOI] [PubMed] [Google Scholar]
- Gläser R, Harder J, Lange H, et al. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- Greene RS, Downing DT, Pochi PE, et al. Anatomical variation in the amount and composition of human skin surface lipid. J Investig Dermatol. 1970;54:240–247. doi: 10.1111/1523-1747.ep12280318. [DOI] [PubMed] [Google Scholar]
- Gribbon EM, Cunliffe WJ, Holland KT. Interaction of Propionibacterium acnes with skin lipids in vitro. J Gen Microbiol. 1993;139:1745–1751. doi: 10.1099/00221287-139-8-1745. [DOI] [PubMed] [Google Scholar]
- Grice EA, Dawson TLJ. Host–microbe interactions: Malassezia and human skin. Curr Opin Microbiol. 2017;40:81–87. doi: 10.1016/j.mib.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum ET, Swanson RA, Alano C, et al. Human serum albumin and its N-terminal tetrapeptide (DAHK) block oxidant-induced neuronal death. Stroke. 2004;35:590–595. doi: 10.1161/01.Str.0000110790.05859.Da. [DOI] [PubMed] [Google Scholar]
- Hall JB, Cong Z, Imamura-Kawasawa Y, et al. Isolation and identification of the follicular microbiome: implications for acne research. J Investig Dermatol. 2018;138:2033–2040. doi: 10.1016/j.jid.2018.02.038. [DOI] [PubMed] [Google Scholar]
- Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34:599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Zhang YJ, Wang HX, et al. Malassezia furfur promoting growth of Staphylococcus epidermidis by increasing pH when cultured in a lipid-free environment. Chin Med J. 2019;132:873–876. doi: 10.1097/cm9.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy BL, Dickey SW, Plaut RD, et al. Corynebacterium pseudodiphtheriticum exploits Staphylococcus aureus virulence components in a novel polymicrobial defense strategy. Mbio. 2019 doi: 10.1128/mBio.02491-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Tryon TA, Grice EA. Microbiota and maintenance of skin barrier function. Science. 2022;376:940–945. doi: 10.1126/science.abo0693. [DOI] [PubMed] [Google Scholar]
- Harshman SW, Pitsch RL, Smith ZK, et al. The proteomic and metabolomic characterization of exercise-induced sweat for human performance monitoring: a pilot investigation. PLoS ONE. 2018;13:e0203133. doi: 10.1371/journal.pone.0203133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MI, Waheed A, Yadav S, et al. Prolactin inducible protein in cancer, fertility and immunoregulation: structure, function and its clinical implications. Cell Mol Life Sci. 2009;66:447–459. doi: 10.1007/s00018-008-8463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentati D, Cheffi M, Hadrich F, et al. Investigation of halotolerant marine Staphylococcus sp. CO100, as a promising hydrocarbon-degrading and biosurfactant-producing bacterium, under saline conditions. J Environ Manag. 2021;277:111480. doi: 10.1016/j.jenvman.2020.111480. [DOI] [PubMed] [Google Scholar]
- Hoiby N, Pers C, Johansen HK, et al. Excretion of beta-lactam antibiotics in sweat—a neglected mechanism for development of antibiotic resistance? Antimicrob Agents Chemother. 2000;44:2855–2857. doi: 10.1128/aac.44.10.2855-2857.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland KT, Greenman J, Cunliffe WJ. Growth of cutaneous propionibacteria on synthetic medium; growth yields and exoenzyme production. J Appl Bacteriol. 1979;47:383–394. doi: 10.1111/j.1365-2672.1979.tb01198.x. [DOI] [PubMed] [Google Scholar]
- Holland KT, Aldana O, Bojar RA, et al. Propionibacterium acnes and acne. Dermatology. 1998;196:67–68. doi: 10.1159/000017870. [DOI] [PubMed] [Google Scholar]
- Holland C, Mak TN, Zimny-Arndt U, et al. Proteomic identification of secreted proteins of Propionibacterium acnes. BMC Microbiol. 2010;10:230. doi: 10.1186/1471-2180-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houpt ER. Microbial inhabitants of humans: their ecology and role in health and disease by Michael Wilson Cambridge, U.K.: Cambridge University Press, 2005. 476 pp., illustrated. $65.00 (cloth) Clin Infect Dis. 2005;41:768–868. doi: 10.1086/432586. [DOI] [Google Scholar]
- Howard B, Bascom CC, Hu P, et al. Aging-associated changes in the adult human skin microbiome and the host factors that affect skin microbiome composition. J Investig Dermatol. 2022;142:1934–46.e21. doi: 10.1016/j.jid.2021.11.029. [DOI] [PubMed] [Google Scholar]
- Hug DH, Dunkerson DD, Hunter JK. The degradation of l-histidine and trans- and cis-urocanic acid by bacteria from skin and the role of bacterial cis-urocanic acid isomerase. J Photochem Photobiol B. 1999;50:66–73. doi: 10.1016/s1011-1344(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianiri G, Heitman J, Scheynius A. The skin commensal yeast Malassezia globosa thwarts bacterial biofilms to benefit the host. J Investig Dermatol. 2018;138:1026–1029. doi: 10.1016/j.jid.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianiri G, Coelho MA, Ruchti F, et al. HGT in the human and skin commensal Malassezia: a bacterially derived flavohemoglobin is required for NO resistance and host interaction. Proc Natl Acad Sci USA. 2020;117:15884–15894. doi: 10.1073/pnas.2003473117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayama S, Shimozono Y, Hoashi M, et al. Reduced secretion of IgA to skin surface of patients with atopic dermatitis. J Allergy Clin Immunol. 1994;94:195–200. doi: 10.1016/0091-6749(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Iwase T, Uehara Y, Shinji H, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465:346–349. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- Jacob S, Mcclintock MK. Psychological state and mood effects of steroidal chemosignals in women and men. Horm Behav. 2000;37:57–78. doi: 10.1006/hbeh.1999.1559. [DOI] [PubMed] [Google Scholar]
- Janek D, Zipperer A, Kulik A, et al. High frequency and diversity of antimicrobial activities produced by nasal staphylococcus strains against bacterial competitors. PLoS Pathog. 2016;12:e1005812. doi: 10.1371/journal.ppat.1005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Ledee DR, Gordon GM, et al. Interaction of clusterin and matrix metalloproteinase-9 and its implication for epithelial homeostasis and inflammation. Am J Pathol. 2012;180:2028–2039. doi: 10.1016/j.ajpath.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson HJ, Vallhov H, Holm T, et al. Extracellular nanovesicles released from the commensal yeast Malassezia sympodialis are enriched in allergens and interact with cells in human skin. Sci Rep. 2018;8:9182. doi: 10.1038/s41598-018-27451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TR, Gómez BI, Mcintyre MK, et al. The cutaneous microbiome and wounds: new molecular targets to promote wound healing. Int J Mol Sci. 2018 doi: 10.3390/ijms19092699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokura Y, Ishikawa S, Tokuda H, et al. Molecular analysis of elastic properties of the stratum corneum by solid-state 13C-nuclear magnetic resonance spectroscopy. J Investig Dermatol. 1995;104:806–812. doi: 10.1111/1523-1747.ep12607005. [DOI] [PubMed] [Google Scholar]
- Juni E. Simple genetic transformation assay for rapid diagnosis of Moraxella osloensis. Appl Microbiol. 1974;27:16–24. doi: 10.1128/am.27.1.16-24.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E, Bøvre K (2015) Bergey's manual of systematics of archaea and bacteria
- Kaneko T, Shiota R, Shibuya S, et al. Human external ear canal as the specific reservoir of Malassezia slooffiae. Med Mycol. 2010;48:824–827. doi: 10.3109/13693780903514880. [DOI] [PubMed] [Google Scholar]
- Kang BS, Seo JG, Lee GS, et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J Microbiol. 2009;47:101–109. doi: 10.1007/s12275-008-0179-y. [DOI] [PubMed] [Google Scholar]
- Kao MS, Huang S, Chang WL, et al. Microbiome precision editing: using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus. Biotechnol J. 2017 doi: 10.1002/biot.201600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao HJ, Wang YH, Keshari S, et al. Propionic acid produced by Cutibacterium acnes fermentation ameliorates ultraviolet B-induced melanin synthesis. Sci Rep. 2021;11:11980. doi: 10.1038/s41598-021-91386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshari S, Balasubramaniam A, Myagmardoloonjin B, et al. Butyric acid from probiotic Staphylococcus epidermidis in the skin microbiome down-regulates the ultraviolet-induced pro-inflammatory IL-6 cytokine via short-chain fatty acid receptor. Int J Mol Sci. 2019 doi: 10.3390/ijms20184477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khmaladze I, Leonardi M, Fabre S, et al. The skin interactome: a holistic, "genome-microbiome-exposome" approach to understand and modulate skin health and aging. Clin Cosmet Investig Dermatol. 2020;13:1021–1040. doi: 10.2147/ccid.S239367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Karadeniz F. Biological importance and applications of squalene and squalane. Adv Food Nutr Res. 2012;65:223–233. doi: 10.1016/b978-0-12-416003-3.00014-7. [DOI] [PubMed] [Google Scholar]
- Kim H, Lim YJ, Park JH, et al. Dietary silk protein, sericin, improves epidermal hydration with increased levels of filaggrins and free amino acids in NC/Nga mice. Br J Nutr. 2012;108:1726–1735. doi: 10.1017/s0007114511007306. [DOI] [PubMed] [Google Scholar]
- Kim JH, Son SM, Park H, et al. Taxonomic profiling of skin microbiome and correlation with clinical skin parameters in healthy Koreans. Sci Rep. 2021;11:16269. doi: 10.1038/s41598-021-95734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Yum HW, Kim SH, et al. Topically applied taurine chloramine protects against UVB-induced oxidative stress and inflammation in mouse skin. Antioxidants. 2021 doi: 10.3390/antiox10060867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirker KR, Secor PR, James GA, et al. Loss of viability and induction of apoptosis in human keratinocytes exposed to Staphylococcus aureus biofilms in vitro. Wound Repair Regen. 2009;17:690–699. doi: 10.1111/j.1524-475X.2009.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirker KR, James GA, Fleckman P, et al. Differential effects of planktonic and biofilm MRSA on human fibroblasts. Wound Repair Regen. 2012;20:253–261. doi: 10.1111/j.1524-475X.2012.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluytmans J, Van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay HF, Gherardin NA, Enders A, et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol. 2016;17:1300–1311. doi: 10.1038/ni.3565. [DOI] [PubMed] [Google Scholar]
- Krejner A, Bruhs A, Mrowietz U, et al. Decreased expression of G-protein-coupled receptors GPR43 and GPR109a in psoriatic skin can be restored by topical application of sodium butyrate. Arch Dermatol Res. 2018;310:751–758. doi: 10.1007/s00403-018-1865-1. [DOI] [PubMed] [Google Scholar]
- Krogsgård Nielsen C, Kjems J, Mygind T, et al. Effects of Tween 80 on growth and biofilm formation in laboratory media. Front Microbiol. 2016 doi: 10.3389/fmicb.2016.01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Mitani A, Niwano Y, et al. Moraxella species are primarily responsible for generating malodor in laundry. Appl Environ Microbiol. 2012;78:3317–3324. doi: 10.1128/aem.07816-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehne A, Hildebrand J, Soehle J, et al. An integrative metabolomics and transcriptomics study to identify metabolic alterations in aged skin of humans in vivo. BMC Genomics. 2017;18:169. doi: 10.1186/s12864-017-3547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Di Nardo A, Nakatsuji T, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laihia JK, Kallio JP, Taimen P, et al. Protodynamic intracellular acidification by cis-urocanic acid promotes apoptosis of melanoma cells in vitro and in vivo. J Investig Dermatol. 2010;130:2431–2439. doi: 10.1038/jid.2010.151. [DOI] [PubMed] [Google Scholar]
- Lam TH, Verzotto D, Brahma P, et al. Understanding the microbial basis of body odor in pre-pubescent children and teenagers. Microbiome. 2018;6:213. doi: 10.1186/s40168-018-0588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson PJ, Chong D, Fleming E, et al. Challenges in developing a human model system for skin microbiome research. J Investig Dermatol. 2021;141:228–231.e4. doi: 10.1016/j.jid.2020.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Lee SH. Epidermal permeability barrier defects and barrier repair therapy in atopic dermatitis. Allergy Asthma Immunol Res. 2014;6:276–287. doi: 10.4168/aair.2014.6.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Bowden GH, Myal Y. Identification of mouse submaxillary gland protein in mouse saliva and its binding to mouse oral bacteria. Arch Oral Biol. 2002;47:327–332. doi: 10.1016/s0003-9969(01)00113-3. [DOI] [PubMed] [Google Scholar]
- Lee DY, Huang CM, Nakatsuji T, et al. Histone H4 is a major component of the antimicrobial action of human sebocytes. J Investig Dermatol. 2009;129:2489–2496. doi: 10.1038/jid.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NY, Ibrahim O, Khetarpal S, et al. Dermal microflora restoration with ammonia-oxidizing bacteria Nitrosomonas eutropha in the treatment of keratosis pilaris: a randomized clinical trial. J Drugs Dermatol. 2018;17:285–288. doi: 10.1001/jamadermatol.2014.2211. [DOI] [PubMed] [Google Scholar]
- Legoux F, Bellet D, Daviaud C, et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science. 2019;366:494–499. doi: 10.1126/science.aaw2719. [DOI] [PubMed] [Google Scholar]
- Lemoine L, Bayrambey D, Roloff A, et al. Commensal-related changes in the epidermal barrier function lead to alterations in the benzo[a]pyrene metabolite profile and its distribution in 3D skin. Mbio. 2021;12:e0122321. doi: 10.1128/mBio.01223-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MHY, Tong X, Bastien P, et al. Changes of the human skin microbiota upon chronic exposure to polycyclic aromatic hydrocarbon pollutants. Microbiome. 2020;8:100. doi: 10.1186/s40168-020-00874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew LC, Liong MT. Bioactives from probiotics for dermal health: functions and benefits. J Appl Microbiol. 2013;114:1241–1253. doi: 10.1111/jam.12137. [DOI] [PubMed] [Google Scholar]
- Lew L-C, Gan C-Y, Liong M-T. Dermal bioactives from lactobacilli and bifidobacteria. Ann Microbiol. 2013;63:1047–1055. doi: 10.1007/s13213-012-0561-1. [DOI] [Google Scholar]
- Li H, Goh BN, Teh WK, et al. Skin commensal Malassezia globosa secreted protease attenuates Staphylococcus aureus biofilm formation. J Investig Dermatol. 2018;138:1137–1145. doi: 10.1016/j.jid.2017.11.034. [DOI] [PubMed] [Google Scholar]
- Li H, Yao Q, Mariscal AG, et al. Epigenetic control of IL-23 expression in keratinocytes is important for chronic skin inflammation. Nat Commun. 2018;9:1420. doi: 10.1038/s41467-018-03704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Xia J, Jiang L, et al. Characterization of the human skin resistome and identification of two microbiota cutotypes. Microbiome. 2021;9:47. doi: 10.1186/s40168-020-00995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan JL, Harrison OJ, Han SJ, et al. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell. 2018;172:784–796.e18. doi: 10.1016/j.cell.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang X, Zhang J, et al. Activation of aryl hydrocarbon receptor in Langerhans cells by a microbial metabolite of tryptophan negatively regulates skin inflammation. J Dermatol Sci. 2020;100:192–200. doi: 10.1016/j.jdermsci.2020.10.004. [DOI] [PubMed] [Google Scholar]
- Lo CW, Lai YK, Liu YT, et al. Staphylococcus aureus hijacks a skin commensal to intensify its virulence: immunization targeting β-hemolysin and CAMP factor. J Investig Dermatol. 2011;131:401–409. doi: 10.1038/jid.2010.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche M, Gardner SE, Kalan L, et al. Temporal stability in chronic wound microbiota is associated with poor healing. J Investig Dermatol. 2017;137:237–244. doi: 10.1016/j.jid.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis KH, Wu SK, Ernlund A, et al. A mixed community of skin microbiome representatives influences cutaneous processes more than individual members. Microbiome. 2021;9:22. doi: 10.1186/s40168-020-00963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lousada MB, Lachnit T, Edelkamp J, et al. Exploring the human hair follicle microbiome. Br J Dermatol. 2021;184:802–815. doi: 10.1111/bjd.19461. [DOI] [PubMed] [Google Scholar]
- Lovászi M, Szegedi A, Zouboulis CC, et al. Sebaceous-immunobiology is orchestrated by sebum lipids. Dermatoendocrinol. 2017;9:e1375636. doi: 10.1080/19381980.2017.1375636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugg JW, Ellis FP. Some water-soluble vitamins in the sweat of tropically acclimatized European men. Br J Nutr. 1954;8:71–77. doi: 10.1079/bjn19540011. [DOI] [PubMed] [Google Scholar]
- Machowinski A, Krämer HJ, Hort W, et al. Pityriacitrin—a potent UV filter produced by Malassezia furfur and its effect on human skin microflora. Mycoses. 2006;49:388–392. doi: 10.1111/j.1439-0507.2006.01265.x. [DOI] [PubMed] [Google Scholar]
- Maclaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Investig. 1985;76:1536–1538. doi: 10.1172/jci112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcginley KJ, Webster GF, Leyden JJ. Regional variations of cutaneous propionibacteria. Appl Environ Microbiol. 1978;35:62–66. doi: 10.1128/aem.35.1.62-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgrath JA. Filaggrin and the great epidermal barrier grief. Australas J Dermatol. 2008;49:67–73. doi: 10.1111/j.1440-0960.2008.00443.x. [DOI] [PubMed] [Google Scholar]
- Midorikawa K, Ouhara K, Komatsuzawa H, et al. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun. 2003;71:3730–3739. doi: 10.1128/iai.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshall C, Nadal J, Exley C. Aluminium in human sweat. J Trace Elem Med Biol. 2014;28:87–88. doi: 10.1016/j.jtemb.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Miskin JE, Farrell AM, Cunliffe WJ, et al. Propionibacterium acnes, a resident of lipid-rich human skin, produces a 33 kDa extracellular lipase encoded by gehA. Microbiology. 1997;143(Pt 5):1745–1755. doi: 10.1099/00221287-143-5-1745. [DOI] [PubMed] [Google Scholar]
- Moss CW, Wallace PL, Hollis DG, et al. Cultural and chemical characterization of CDC groups EO-2, M-5, and M-6, Moraxella (Moraxella) species, Oligella urethralis, Acinetobacter species, and Psychrobacter immobilis. J Clin Microbiol. 1988;26:484–492. doi: 10.1128/jcm.26.3.484-492.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller DN, Wilck N, Haase S, et al. Sodium in the microenvironment regulates immune responses and tissue homeostasis. Nat Rev Immunol. 2019;19:243–254. doi: 10.1038/s41577-018-0113-4. [DOI] [PubMed] [Google Scholar]
- Murakami M. Lipid mediators in life science. Exp Anim. 2011;60:7–20. doi: 10.1538/expanim.60.7. [DOI] [PubMed] [Google Scholar]
- Murakami M, Ohtake T, Dorschner RA, et al. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J Investig Dermatol. 2002;119:1090–1095. doi: 10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- Murphy B, Hoptroff M, Arnold D, et al. In-vivo impact of common cosmetic preservative systems in full formulation on the skin microbiome. PLoS ONE. 2021;16:e0254172. doi: 10.1371/journal.pone.0254172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myal Y, Robinson DB, Iwasiow B, et al. The prolactin-inducible protein (PIP/GCDFP-15) gene: cloning, structure and regulation. Mol Cell Endocrinol. 1991;80:165–175. doi: 10.1016/0303-7207(91)90153-j. [DOI] [PubMed] [Google Scholar]
- N’diaye A, Gannesen A, Borrel V, et al. Substance P and calcitonin gene-related peptide: key regulators of cutaneous microbiota homeostasis. Front Endocrinol. 2017;8:15. doi: 10.3389/fendo.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Linehan JL, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520:104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Kao MC, Zhang L, et al. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression. J Investig Dermatol. 2010;130:985–994. doi: 10.1038/jid.2009.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Narala S, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Butcher AM, et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci Adv. 2018;4:eaao4502. doi: 10.1126/sciadv.aao4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsch A, Gfeller H, Gygax P, et al. A specific bacterial aminoacylase cleaves odorant precursors secreted in the human axilla. J Biol Chem. 2003;278:5718–5727. doi: 10.1074/jbc.M210142200. [DOI] [PubMed] [Google Scholar]
- Nichols J, Miller AT., Jr Excretion of adrenal corticoids in the sweat. Proc Soc Exp Biol Med. 1948;69:448. doi: 10.3181/00379727-69-16751. [DOI] [PubMed] [Google Scholar]
- Nicolaides N. Skin lipids: their biochemical uniqueness. Science. 1974;186:19–26. doi: 10.1126/science.186.4158.19. [DOI] [PubMed] [Google Scholar]
- Nistor A, Bowden G, Blanchard A, et al. Influence of mouse prolactin-inducible protein in saliva on the aggregation of oral bacteria. Oral Microbiol Immunol. 2009;24:510–513. doi: 10.1111/j.1399-302X.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- Nithya S, Radhika T, Jeddy N. Loricrin—an overview. J Oral Maxillofac Pathol. 2015;19:64–68. doi: 10.4103/0973-029x.157204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Notay M, Saric-Bosanac S, Vaughn AR, et al. The use of topical Nitrosomonas eutropha for cosmetic improvement of facial wrinkles. J Cosmet Dermatol. 2020;19:689–693. doi: 10.1111/jocd.13060. [DOI] [PubMed] [Google Scholar]
- Nunome Y, Tsuda T, Kitagawa K. Determination of fatty acids in human sweat during fasting using GC/MS. Anal Sci. 2010;26:917–919. doi: 10.2116/analsci.26.917. [DOI] [PubMed] [Google Scholar]
- O’neill AM, Nakatsuji T, Hayachi A, et al. Identification of a human skin commensal bacterium that selectively kills cutibacterium acnes. J Investig Dermatol. 2020;140:1619–28.e2. doi: 10.1016/j.jid.2019.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Byrd AL, Deming C, et al. Biogeography and individuality shape function in the human skin metagenome. Nature. 2014;514:59–64. doi: 10.1038/nature13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa K, Watanabe T, Matsukawa R, et al. The possible role of squalene and its peroxide of the sebum in the occurrence of sunburn and protection from the damage caused by U.V. irradiation. J Toxicol Sci. 1984;9:151–159. doi: 10.2131/jts.9.151. [DOI] [PubMed] [Google Scholar]
- Ong JS, Taylor TD, Yong CC, et al. Lactobacillus plantarum USM8613 aids in wound healing and suppresses Staphylococcus aureus infection at wound sites. Probiotics Antimicrob Proteins. 2020;12:125–137. doi: 10.1007/s12602-018-9505-9. [DOI] [PubMed] [Google Scholar]
- Ono E, Murota H, Mori Y, et al. Sweat glucose and GLUT2 expression in atopic dermatitis: Implication for clinical manifestation and treatment. PLoS ONE. 2018;13:e0195960. doi: 10.1371/journal.pone.0195960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paharik AE, Parlet CP, Chung N, et al. Coagulase-negative staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe. 2017;22:746–56.e5. doi: 10.1016/j.chom.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas A. Epidermal surface lipids. Dermatoendocrinol. 2009;1:72–76. doi: 10.4161/derm.1.2.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Lee EJ, Lee JC, et al. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int Immunopharmacol. 2007;7:70–77. doi: 10.1016/j.intimp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Park JH, Park GT, Cho IH, et al. An antimicrobial protein, lactoferrin exists in the sweat: proteomic analysis of sweat. Exp Dermatol. 2011;20:369–371. doi: 10.1111/j.1600-0625.2010.01218.x. [DOI] [PubMed] [Google Scholar]
- Petry T, Bury D, Fautz R, et al. Review of data on the dermal penetration of mineral oils and waxes used in cosmetic applications. Toxicol Lett. 2017;280:70–78. doi: 10.1016/j.toxlet.2017.07.899. [DOI] [PubMed] [Google Scholar]
- Picardo M, Ottaviani M, Camera E, et al. Sebaceous gland lipids. Dermatoendocrinology. 2009;1:68–71. doi: 10.4161/derm.1.2.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Ciardiello T, Franzoni M, et al. Effect of commonly used cosmetic preservatives on skin resident microflora dynamics. Sci Rep. 2021;11:8695. doi: 10.1038/s41598-021-88072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistone D, Meroni G, Panelli S, et al. A journey on the skin microbiome: pitfalls and opportunities. Int J Mol Sci. 2021 doi: 10.3390/ijms22189846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochi PE, Strauss JS, Downing DT. Age-related changes in sebaceous gland activity. J Investig Dermatol. 1979;73:108–111. doi: 10.1111/1523-1747.ep12532792. [DOI] [PubMed] [Google Scholar]
- Postler TS, Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 2017;26:110–130. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SL, Larcombe DL, Logan AC, et al. The skin microbiome: impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ J. 2017;10:29. doi: 10.1186/s40413-017-0160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti G, Wysocki CJ, Barnhart KT, et al. Male axillary extracts contain pheromones that affect pulsatile secretion of luteinizing hormone and mood in women recipients. Biol Reprod. 2003;68:2107–2113. doi: 10.1095/biolreprod.102.008268. [DOI] [PubMed] [Google Scholar]
- Pullar JM, Carr AC, Vissers MCM. The roles of vitamin C in skin health. Nutrients. 2017 doi: 10.3390/nu9080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey MM, Freire MO, Gabrilska RA, et al. Staphylococcus aureus shifts toward commensalism in response to Corynebacterium species. Front Microbiol. 2016;7:1230. doi: 10.3389/fmicb.2016.01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmayer K, Meyer KC, Kleditzsch P, et al. Human hair follicle epithelium has an antimicrobial defence system that includes the inducible antimicrobial peptide psoriasin (S100A7) and RNase 7. Br J Dermatol. 2009;161:78–89. doi: 10.1111/j.1365-2133.2009.09154.x. [DOI] [PubMed] [Google Scholar]
- Ridaura VK, Bouladoux N, Claesen J, et al. Contextual control of skin immunity and inflammation by Corynebacterium. J Exp Med. 2018;215:785–799. doi: 10.1084/jem.20171079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E, Koren O, Reshef L, et al. The role of microorganisms in coral health, disease and evolution. Nat Rev Microbiol. 2007;5:355–362. doi: 10.1038/nrmicro1635. [DOI] [PubMed] [Google Scholar]
- Roth RR, James WD. Microbial ecology of the skin. Annu Rev Microbiol. 1988;42:441–464. doi: 10.1146/annurev.mi.42.100188.002301. [DOI] [PubMed] [Google Scholar]
- Roux PF, Oddos T, Stamatas G. Deciphering the role of skin surface microbiome in skin health: an integrative multiomics approach reveals three distinct metabolite-microbe clusters. J Investig Dermatol. 2022;142:469–479.e5. doi: 10.1016/j.jid.2021.07.159. [DOI] [PubMed] [Google Scholar]
- Salminen S, Collado MC, Endo A, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18:649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Rodriguez R, Pauli ML, Neuhaus IM, et al. Memory regulatory T cells reside in human skin. J Clin Investig. 2014;124:1027–1036. doi: 10.1172/jci72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Suárez J, Coy-Barrera E, Villamil L, et al. Streptomyces-derived metabolites with potential photoprotective properties—a systematic literature review and meta-analysis on the reported chemodiversity. Molecules. 2020 doi: 10.3390/molecules25143221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandby-Møller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm Venereol. 2003;83:410–413. doi: 10.1080/00015550310015419. [DOI] [PubMed] [Google Scholar]
- Sanford JA, Zhang LJ, Williams MR, et al. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci Immunol. 2016 doi: 10.1126/sciimmunol.aah4609. [DOI] [PubMed] [Google Scholar]
- Sanford JA, O’neill AM, Zouboulis CC, et al. Short-chain fatty acids from cutibacterium acnes activate both a canonical and epigenetic inflammatory response in human sebocytes. J Immunol. 2019;202:1767–1776. doi: 10.4049/jimmunol.1800893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardana K, Garg VK. An observational study of methionine-bound zinc with antioxidants for mild to moderate acne vulgaris. Dermatol Ther. 2010;23:411–418. doi: 10.1111/j.1529-8019.2010.01342.x. [DOI] [PubMed] [Google Scholar]
- Sargent F, Robinson P, Johnson R. Water-soluble vitamins in sweat. J Biol Chem. 1944;153:285–294. doi: 10.1016/S0021-9258(18)51235-7. [DOI] [Google Scholar]
- Sato K. The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev Physiol Biochem Pharmacol. 1977;79:51–131. doi: 10.1007/BFb0037089. [DOI] [PubMed] [Google Scholar]
- Sato K, Sato F. Na+, K+, H+, Cl−, and Ca2+ concentrations in cystic fibrosis eccrine sweat in vivo and in vitro. J Lab Clin Med. 1990;115:504–511. [PubMed] [Google Scholar]
- Sato K, Kang WH, Saga K, et al. Biology of sweat glands and their disorders. I. Normal sweat gland function. J Am Acad Dermatol. 1989;20:537–563. doi: 10.1016/s0190-9622(89)70063-3. [DOI] [PubMed] [Google Scholar]
- Sato K, Kang WH, Saga K, et al. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol. 1989;20:713–726. doi: 10.1016/s0190-9622(89)70081-5. [DOI] [PubMed] [Google Scholar]
- Scharschmidt TC, Fischbach MA. What lives on our skin: ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discov Today Dis Mech. 2013 doi: 10.1016/j.ddmec.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt TC, Vasquez KS, Truong HA, et al. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity. 2015;43:1011–1021. doi: 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt TC, Vasquez KS, Pauli ML, et al. Commensal microbes and hair follicle morphogenesis coordinately drive Treg migration into neonatal skin. Cell Host Microbe. 2017;21:467–477.e5. doi: 10.1016/j.chom.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkels LC, Walgreen-Weterings E, Oomen LC, et al. In vivo binding of the salivary glycoprotein EP-GP (identical to GCDFP-15) to oral and non-oral bacteria detection and identification of EP-GP binding species. Biol Chem. 1997;378:83–88. doi: 10.1515/bchm.1997.378.2.83. [DOI] [PubMed] [Google Scholar]
- Schittek B, Hipfel R, Sauer B, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–1137. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Bruhs A, Schwarz T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J Investig Dermatol. 2017;137:855–864. doi: 10.1016/j.jid.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Sears ME, Kerr KJ, Bray RI. Arsenic, cadmium, lead, and mercury in sweat: a systematic review. J Environ Public Health. 2012;2012:184745. doi: 10.1155/2012/184745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serag A, Shakkour Z, Halboup AM, et al. Sweat metabolome and proteome: recent trends in analytical advances and potential biological functions. J Proteomics. 2021;246:104310. doi: 10.1016/j.jprot.2021.104310. [DOI] [PubMed] [Google Scholar]
- Solano F. Metabolism and functions of amino acids in the skin. Adv Exp Med Biol. 2020;1265:187–199. doi: 10.1007/978-3-030-45328-2_11. [DOI] [PubMed] [Google Scholar]
- Sørensen OE, Follin P, Johnsen AH, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- Souak D, Barreau M, Courtois A, et al. Challenging cosmetic innovation: the skin microbiota and probiotics protect the skin from UV-induced damage. Microorganisms. 2021 doi: 10.3390/microorganisms9050936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger T, Pipal A, Redl B. Molecular analysis of the prevalent microbiota of human male and female forehead skin compared to forearm skin and the influence of make-up. J Appl Microbiol. 2011;110:1381–1389. doi: 10.1111/j.1365-2672.2011.04991.x. [DOI] [PubMed] [Google Scholar]
- Suffia IJ, Reckling SK, Piccirillo CA, et al. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med. 2006;203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Yamasaki K. Psoriasis and antimicrobial peptides. Int J Mol Sci. 2020 doi: 10.3390/ijms21186791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W, Kline DL. Carbon dioxide and 1-octen-3-ol as mosquito attractants. J Am Mosq Control Assoc. 1989;5:311–316. [PubMed] [Google Scholar]
- Tauch A, Burkovski A. Molecular armory or niche factors: virulence determinants of Corynebacterium species. FEMS Microbiol Lett. 2015;362:fnv185. doi: 10.1093/femsle/fnv185. [DOI] [PubMed] [Google Scholar]
- Thiboutot D. Regulation of human sebaceous glands. J Investig Dermatol. 2004;123:1–12. doi: 10.1111/j.1523-1747.2004.t01-2-.x. [DOI] [PubMed] [Google Scholar]
- Thody AJ, Shuster S. Control and function of sebaceous glands. Physiol Rev. 1989;69:383–416. doi: 10.1152/physrev.1989.69.2.383. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Jobin C. Microbiota in pancreatic health and disease: the next frontier in microbiome research. Nat Rev Gastroenterol Hepatol. 2020;17:53–64. doi: 10.1038/s41575-019-0242-7. [DOI] [PubMed] [Google Scholar]
- Tilles G. Acne pathogenesis: history of concepts. Dermatology. 2014;229:1–46. doi: 10.1159/000364860. [DOI] [PubMed] [Google Scholar]
- Timm CM, Loomis K, Stone W, et al. Isolation and characterization of diverse microbial representatives from the human skin microbiome. Microbiome. 2020;8:58. doi: 10.1186/s40168-020-00831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tözsér J, Berta A. Lactate dehydrogenase activity in pathological human tears obtained with glass capillaries correlates with the albumin content. Int Ophthalmol. 1998;22:289–292. doi: 10.1023/a:1006378613666. [DOI] [PubMed] [Google Scholar]
- Traisaeng S, Herr DR, Kao HJ, et al. A derivative of butyric acid, the fermentation metabolite of Staphylococcus epidermidis, inhibits the growth of a Staphylococcus aureus strain isolated from atopic dermatitis patients. Toxins. 2019 doi: 10.3390/toxins11060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiner E, Duban L, Bahram S, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- Unno M, Cho O, Sugita T. Inhibition of Propionibacterium acnes lipase activity by the antifungal agent ketoconazole. Microbiol Immunol. 2017;61:42–44. doi: 10.1111/1348-0421.12464. [DOI] [PubMed] [Google Scholar]
- Vallhov H, Johansson C, Veerman RE, et al. Extracellular vesicles released from the skin commensal yeast Malassezia sympodialis activate human primary keratinocytes. Front Cell Infect Microbiol. 2020;10:6. doi: 10.3389/fcimb.2020.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Beek EJ. Vitamin supplementation and physical exercise performance. J Sports Sci. 1991;9:77–89. doi: 10.1080/02640419108729868. [DOI] [PubMed] [Google Scholar]
- Van Loosdrecht MC, Lyklema J, Norde W, et al. Influence of interfaces on microbial activity. Microbiol Rev. 1990;54:75–87. doi: 10.1128/mr.54.1.75-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhan M, Flaminio Z, Sapru S, et al. The microbiome, malignant fungating wounds, and palliative care. Front Cell Infect Microbiol. 2019;9:373. doi: 10.3389/fcimb.2019.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinolo MA, Rodrigues HG, Nachbar RT, et al. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kuo S, Shu M, et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol. 2014;98:411–424. doi: 10.1007/s00253-013-5394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu Y, Wang Y, et al. Antioxidant properties of probiotic bacteria. Nutrients. 2017 doi: 10.3390/nu9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Mascarenhas N, Eckmann L, et al. Skin microbiome promotes mast cell maturation by triggering stem cell factor production in keratinocytes. J Allergy Clin Immunol. 2017;139:1205–1216.e6. doi: 10.1016/j.jaci.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Cui S, Zhou L, et al. Effect of cosmetic chemical preservatives on resident flora isolated from healthy facial skin. J Cosmet Dermatol. 2019;18:652–658. doi: 10.1111/jocd.12822. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhou H, Chen D, et al. Whole-genome sequencing reveals a prolonged and persistent intrahospital transmission of Corynebacterium striatum, an emerging multidrug-resistant pathogen. J Clin Microbiol. 2019 doi: 10.1128/jcm.00683-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Sweren E, Liu H, et al. Bacteria induce skin regeneration via IL-1β signaling. Cell Host Microbe. 2021;29:777–791.e6. doi: 10.1016/j.chom.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke I, Steffen H, Christ C, et al. Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J Investig Dermatol. 2011;131:382–390. doi: 10.1038/jid.2010.328. [DOI] [PubMed] [Google Scholar]
- Watabe A, Sugawara T, Kikuchi K, et al. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J Dermatol Sci. 2013;72:177–182. doi: 10.1016/j.jdermsci.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kano R, Sato H, et al. The effects of Malassezia yeasts on cytokine production by human keratinocytes. J Investig Dermatol. 2001;116:769–773. doi: 10.1046/j.1523-1747.2001.01321.x. [DOI] [PubMed] [Google Scholar]
- Wei Q, Li Z, Gu Z, et al. Shotgun metagenomic sequencing reveals skin microbial variability from different facial sites. Front Microbiol. 2022 doi: 10.3389/fmicb.2022.933189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler JR, Janssen N, Bruynzeel DP, et al. The effect of UV-light on pityrosporum yeasts: ultrastructural changes and inhibition of growth. Acta Derm Venereol. 1990;70:69–71. [PubMed] [Google Scholar]
- Williams GC. Adaptation and natural selection. Princeton: Princeton University Press; 1996. [Google Scholar]
- Williams MR, Costa SK, Zaramela LS, et al. Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci Transl Med. 2019 doi: 10.1126/scitranslmed.aat8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisecaver JH, Alexander WG, King SB, et al. Dynamic evolution of nitric oxide detoxifying flavohemoglobins, a family of single-protein metabolic modules in bacteria and eukaryotes. Mol Biol Evol. 2016;33:1979–1987. doi: 10.1093/molbev/msw073. [DOI] [PubMed] [Google Scholar]
- Wollenberg MS, Claesen J, Escapa IF, et al. Propionibacterium-produced coproporphyrin III induces Staphylococcus aureus aggregation and biofilm formation. Mbio. 2014;5:e01286-14. doi: 10.1128/mBio.01286-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondrak GT, Jacobson MK, Jacobson EL. Endogenous UVA-photosensitizers: mediators of skin photodamage and novel targets for skin photoprotection. Photochem Photobiol Sci. 2006;5:215–237. doi: 10.1039/b504573h. [DOI] [PubMed] [Google Scholar]
- Wu G, Zhao H, Li C, et al. Genus-wide comparative genomics of malassezia delineates its phylogeny, physiology, and niche adaptation on human skin. PLoS Genet. 2015;11:e1005614. doi: 10.1371/journal.pgen.1005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Li Z, Liu K, et al. Staphylococcal LTA-induced miR-143 inhibits propionibacterium acnes-mediated inflammatory response in skin. J Investig Dermatol. 2016;136:621–630. doi: 10.1016/j.jid.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Xu M, Lu H, Lee YH, et al. An interleukin-25-mediated autoregulatory circuit in keratinocytes plays a pivotal role in psoriatic skin inflammation. Immunity. 2018;48:787–98.e4. doi: 10.1016/j.immuni.2018.03.019. [DOI] [PubMed] [Google Scholar]
- Yamane T, Morioka Y, Kitaura Y, et al. Branched-chain amino acids regulate type I tropocollagen and type III tropocollagen syntheses via modulation of mTOR in the skin. Biosci Biotechnol Biochem. 2018;82:611–615. doi: 10.1080/09168451.2017.1386084. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Schauber J, Coda A, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- Yang ES, Tan J, Eells S, et al. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin Microbiol Infect. 2010;16:425–431. doi: 10.1111/j.1469-0691.2009.02836.x. [DOI] [PubMed] [Google Scholar]
- Yokozeki H, Hibino T, Takemura T, et al. Cysteine proteinase inhibitor in eccrine sweat is derived from sweat gland. Am J Physiol. 1991;260:R314–R320. doi: 10.1152/ajpregu.1991.260.2.R314. [DOI] [PubMed] [Google Scholar]
- Youn SH, Choi CW, Choi JW, et al. The skin surface pH and its different influence on the development of acne lesion according to gender and age. Skin Res Technol. 2013;19:131–136. doi: 10.1111/srt.12023. [DOI] [PubMed] [Google Scholar]
- Yu Y, Prassas I, Muytjens CM, et al. Proteomic and peptidomic analysis of human sweat with emphasis on proteolysis. J Proteomics. 2017;155:40–48. doi: 10.1016/j.jprot.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Yu J, Luo Y, Zhu Z, et al. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J Allergy Clin Immunol. 2019;143:2108–19.e12. doi: 10.1016/j.jaci.2018.11.036. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Han Y, Sun YZ, et al. Extracellular vesicles derived from Malassezia furfur stimulate IL-6 production in keratinocytes as demonstrated in in vitro and in vivo models. J Dermatol Sci. 2019;93:168–175. doi: 10.1016/j.jdermsci.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhu Y, Liu Z, et al. A volatile from the skin microbiota of flavivirus-infected hosts promotes mosquito attractiveness. Cell. 2022;185:2510–2522.e16. doi: 10.1016/j.cell.2022.05.016. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Hunt RL, Villaruz AE, et al. Commensal Staphylococcus epidermidis contributes to skin barrier homeostasis by generating protective ceramides. Cell Host Microbe. 2022;30:301–13.e9. doi: 10.1016/j.chom.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipperer A, Konnerth MC, Laux C, et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature. 2016;535:511–516. doi: 10.1038/nature18634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.