Abstract
Entry of opsonized pathogens into phagocytes may benefit or, paradoxically, harm the host. Opsonization may trigger antimicrobial mechanisms such as reactive oxygen or nitric oxide (NO) production but may also provide a safe haven for intracellular replication. Brucellae are natural intramacrophage pathogens of rodents, ruminants, dogs, marine mammals, and humans. We evaluated the role of opsonins in Brucella-macrophage interactions by challenging cultured murine peritoneal macrophages with Brucella melitensis 16M treated with complement- and/or antibody-rich serum. Mouse serum rich in antibody against Brucella lipopolysaccharide (LPS) (aLPS) and human complement-rich serum (HCS) each enhanced the macrophage uptake of brucellae. Combinations of suboptimal levels of aLPS (0.01%) and HCS (2%) synergistically enhanced uptake. The intracellular fate of ingested bacteria was evaluated with an optimal concentration of gentamicin (2 μg/ml) to control extracellular growth but not kill intracellular bacteria. Bacteria opsonized with aLPS and/or HCS grew equally well inside macrophages in the absence of gamma interferon (IFN-γ). Macrophage activation with IFN-γ inhibited replication of both opsonized and nonopsonized brucellae but was less effective in inhibiting replication of nonopsonized bacteria. IFN-γ treatment of macrophages with opsonized or nonopsonized bacteria enhanced NO production, which was blocked by NG-monomethyl l-arginine (MMLA), an NO synthesis inhibitor. MMLA also partially blocked IFN-γ-mediated bacterial growth inhibition. These studies suggest that primary murine macrophages have limited ability to control infection with B. melitensis, even when activated by IFN-γ in the presence of highly opsonic concentrations of antibody and complement. Additional cellular immune responses, e.g., those mediated by cytotoxic T cells, may play more important roles in the control of murine brucellosis.
Brucella spp., short, nonmotile, nonsporulating, nonencapsulated, gram-negative aerobic rods, are important facultative intracellular pathogens of humans and livestock. Brucella melitensis usually infects sheep, goats, and camels and is the most pathogenic species for humans (1). Like other facultative intracellular pathogenic bacteria (e.g., Francisella tularensis, Listeria monocytogenes, Mycobacterium spp., and Legionella pneumophila), clearance of Brucella infection relies on both cell-mediated immunity (1, 3, 7, 12, 20, 21, 27, 30, 38) and humoral responses (10, 22, 29, 35). The interplay of these two arms of the immune response, however, is not well understood.
Successful infection of the host by Brucella reflects the ability of the bacterium to establish itself in an intracellular environment favorable for its replication. The presence of complement or antibody in the extracellular fluid favors killing of some Brucella strains (35, 38). Opsonization by these humoral factors also enhances uptake by phagocytic cells that shelter the bacteria. The intracellular fate of brucellae may depend on the bacterial species or the kind of phagocyte ingesting them. For example, opsonization with complement in vitro leads to uptake and killing of Brucella abortus by human neutrophils whereas the more virulent B. melitensis survives under these conditions (38). Similarly, B. abortus opsonized with antibody containing fresh or heated bovine serum induces the production of oxidative products in bovine neutrophils (6). Although neutrophils represent an important first line of defense against brucellae, the longer-lived mononuclear phagocyte is the more important effector cell for defense against established infection (1). Bovine blood monocyte-derived macrophages (MDM) ingest more B. abortus bacteria when opsonized with complement-rich bovine serum than with serum rich in both antibody and complement (4), suggesting that complement plays a more important role than specific antibody in uptake and killing of B. abortus by these bovine blood MDM.
In macrophage-pathogen interactions, complement receptors (CR) and complement (2, 3, 27) mediate uptake of intracellular pathogens like L. pneumophila (2, 3, 27), Mycobacterium tuberculosis (32, 33), and Leishmania donovani (3). Ligation to CR does not normally trigger the oxidative burst in phagocytes (33, 36, 37). Consequently, intracellular pathogens gain entry to the cell without the generation of reactive oxygen species, enhancing their survival within host cells (27, 32). A number of investigators (10, 11, 14, 16, 18) have opsonized Brucella with antiserum to ensure the infection of macrophages during in vitro studies. Gross and associates (14) recently showed that both nonopsonized and antibody-opsonized Brucella suis strains induce mRNA for inducible nitric oxide (NO) synthase (iNOS) in the mouse macrophage-like J774A.1 cell line. However, only antibody-opsonized B. suis triggers NO production. Gamma interferon (IFN-γ)-induced, NO-mediated bacteriostasis occurs preferentially if brucellae are opsonized with antibody (14). The contribution of opsonins to the fate of ingested B. melitensis, however, has not been systematically examined. In addition, the interplay of opsonins and macrophage-activating factors in the clearance of brucellae from primary macrophages, rather than cell lines, is unknown.
Endogenous IFN-γ (39) and interleukin-12 (40) can mediate protective immunity to B. abortus infections in CBA/J mice. Treatment of these mice with anti-interleukin-12 increases splenic bacterial burden and reduces NO production by macrophages (40). The IFN-γ-mediated decrease in bacterial burden in antibody-opsonized B. suis-infected J774A.1 cells is at least partly due to NO (14). In other studies (17), however, B. abortus is only minimally affected by NO-mediated antimicrobial activity in proteose-peptone-elicited mouse peritoneal macrophages. This controversy is reminiscent of the controversies enshrouding NO dependence in the control of secondary L. monocytogenes infection in CBA/J mice (31) and NO involvement in macrophage candidacidal potency (34).
In this report, we demonstrate effective ingestion of virulent B. melitensis 16M by resident mouse peritoneal macrophages in the presence of immune serum or human complement-rich serum (HCS). Combinations of slightly effective concentrations of immune serum and HCS are synergistic in this in vitro system. Once internalized, the brucellae grow well in resting (nonactivated) macrophages whether or not the bacteria were previously exposed to opsonins. Activation of macrophages with IFN-γ leads to NO production and inhibition of bacterial growth. IFN-γ-mediated antibacterial activity is partially inhibited by addition of NG-monomethyl l-arginine (MMLA).
MATERIALS AND METHODS
Media.
DME-FBS medium contained 10% fetal bovine serum (FBS) (Hyclone) and 2 mM l-glutamine in Dulbecco's modified Eagle's medium (DME) (BioWhittaker, Walkersville, Md.). DME-FBS-MCSF medium consisted of DME-FBS with 40 ng of recombinant human macrophage colony-stimulating factor per ml, kindly provided by Jay Stoudemire, Genetics Institute, Cambridge, Mass. l-Arginine was present in DME at a concentration of 0.4 mM, except where otherwise indicated.
HCS.
Fresh normal HCS was obtained from a seronegative (by tube agglutination test) healthy adult volunteer. Venous blood was drawn into a Vacutainer and allowed to clot at room temperature for about 30 min. It was then centrifuged for 20 min at 4°C and at 1,880 × g. Serum was aliquoted and stored at −70°C until use (usually within 3 months). Aliquots were thawed and used once.
Antiserum.
Anti-lipopolysaccharide (LPS) serum (aLPS) was obtained by immunizing BALB/c mice with a mixture of B. abortus and B. melitensis LPS (10 μg of each LPS/dose) subcutaneously. Two doses of vaccine were administered 4 weeks apart, and the mice were bled 2 weeks after the second dose. Sera from 5 mice were pooled. Antibody titers to B. melitensis LPS, determined by enzyme-linked immunosorbent assay, were 1:6,000.
Bacteria.
For macrophage challenge, 16M was grown in Brucella broth for 24 h in shaker flasks. Bacteria were pelleted by centrifugation, washed once in 0.9% NaCl, and resuspended to approximately 2 × 108 bacteria/ml in 0.9% NaCl. The actual number of viable organisms was determined in retrospect through dilution and plating for CFU.
Isolation, purification, and bacterial infection of mouse peritoneal macrophages.
Peritoneal cells were isolated from 8- to 16-week-old female BALB/c mice (Jackson Laboratories, Bar Harbor, Maine) by lavage with 10 ml of ice-cold Ca2+,Mg2+-free Hanks balanced salt solution (BioWhittaker). The peritoneal cells were pelleted, resuspended in DME-FBS-MCSF, and plated in 96-well polystyrene microtiter plates (Costar, Cambridge, Mass.) at 0.125 × 106 macrophages per well. The cultures were incubated overnight at 37°C and 5% CO2. The supernatant was aspirated and the adherent macrophage monolayer was washed three times with 37°C DME-FBS and finally covered with 45 μl of DME-FBS-MCSF, with or without HCS or aLPS. Five microliters of bacterial suspension giving a multiplicity of infection (MOI) of 8 was added and cultures were returned to the incubator for 1 h. The concentrations of serum used in these experiments did not cause bacterial agglutination by light microscopy or tube testing. After 1 h at 37°C, the supernatant fluid containing extracellular bacteria was removed and the monolayer was washed three times with 100-μl aliquots of DME-FBS. Monolayers were either lysed to obtain 1-h bacterial counts as described below or replenished with 200 μl of DME-FBS-MCSF routinely containing gentamicin at a concentration of 2 μg/ml. In some instances, this latter medium was supplemented with 10 U of recombinant murine IFN-γ per ml, with or without 1 mM MMLA, a competitive inhibitor of all three forms of l-arginine-dependent NO synthase. Cultures were incubated for 48 h before lysis for determination of bacterial counts. Cultures were inspected by light microscopy before lysis. All monolayers remained intact and cells appeared healthy throughout the 48-h culture period. In preliminary experiments, recombinant human macrophage colony-stimulating factor was required for preservation of the monolayers (data not shown).
Determination of bacterial burden (intracellular CFU) in macrophages.
At appropriate times of incubation in fresh medium after the initial 1-h infection period, bacterial burden was determined as follows: Triton X-100 was added directly to the 200-μl culture (final concentration, 0.1%) to lyse the macrophages. The number of CFU in lysates was determined by serial dilutions and plating on Brucella agar as previously described (8), without separation of supernatants from adherent cells. We had previously determined that, within the short period (10 min) of lysis under these conditions, the 2 μg of gentamicin/ml present in the culture medium did not affect the viability of brucellae liberated to the medium (data not shown).
NO production.
The NO content of culture supernatants was estimated by analysis of nitrite (product of rapid oxidation of NO in aqueous solution) with the Griess reagent (13).
Data analysis.
Macrophages were cultured in quadruplicate or quintuplicate under each experimental condition. CFU and NO analyses were determined independently for each well, so that each raw data point represents CFU or NO content from one well. Data are expressed as means ± standard errors of the mean (SEM) for each treatment group. The significance of differences between treatment groups was determined using the two-tailed Student's t test. In some experiments, percent anti-Brucella activity induced by IFN-γ was calculated as [CFU (minus IFN-γ) − CFU (plus IFN-γ)]/[CFU (minus IFN-γ)] × 100.
RESULTS
Effect of opsonization on the uptake of B. melitensis 16M by mouse peritoneal macrophages.
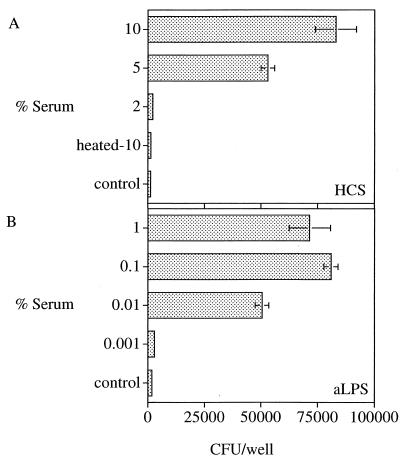
Macrophages took up only a small fraction of unopsonized brucellae. During preliminary investigations in four different experiments, only 1,253 ± 438 (mean ± SEM) CFU were recovered from adherent cells after exposure of macrophages to bacteria at an MOI of 8 (i.e., approximately 106 CFU) in the absence of HCS or aLPS. In contrast, opsonization with HCS profoundly enhanced uptake in a concentration-dependent manner (Fig. 1A); 2% or more HCS led to uptake above the nonopsonized control level (Fig. 1A). Heating HCS to 56°C for 30 min completely abolished enhancement (Fig. 1A). Opsonization of bacteria with aLPS serum from also enhanced uptake in a dose-related manner. Uptake was maximal at ≥0.1% aLPS (Fig. 1B).
FIG. 1.
Effects of opsonization with Brucella aLPS or HCS on uptake of brucellae. Macrophage monolayers were incubated with 8 bacteria per macrophage with or without the indicated opsonins. Controls received DME-FBS-MCSF medium only. After 1 h, monolayers were lysed and the number of cell-associated CFU was determined by serial dilution and agar plating. Data are the mean ± SEM CFU in each well from at least two replicate determinations and represent one of three similar experiments.
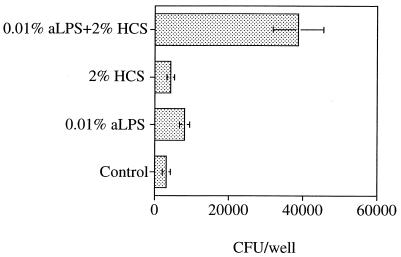
Synergy of complement and antibody.
Combining suboptimal concentrations of HCS (2%) with aLPS (0.01%) had a synergistic effect on bacterial uptake (Fig. 2). There was a 3.2-fold increase in the number of CFU recovered when brucellae were treated with the 2% HCS–plus–0.01% aLPS combination, as compared to the sum of the CFU when bacteria were treated with 2% HCS or 0.01% aLPS alone. At optimal concentrations of either antibody or HCS for infection of macrophages, no synergy between HCS and antibody occurred (data not shown). These observations were consistent with a cooperative antibody and complement-mediated uptake of brucellae at suboptimal levels of either reagent. They further indicated that similar maximal levels of bacterial uptake could be achieved by treatment of bacteria with antibody, complement, or both.
FIG. 2.
Synergistic effect of opsonization with aLPS and HCS on uptake of 16M. Macrophage monolayers were incubated with 8 bacteria per macrophage with or without the indicated opsonins. Controls received DME-FBS-MCSF medium only. After 1 h, monolayers were lysed and the cell-associated CFU were determined by serial dilution and agar plating. Data are the mean ± SEM CFU in each well from five replicate wells and represent one of four similar experiments.
Effects of gentamicin on recovery of bacteria from macrophages during prolonged culture.
Since 16M grows in tissue culture medium at least as well as it does inside macrophages, analysis of the fate of intracellular brucellae for more than the short periods of time required for uptake requires the addition of antibiotics to the medium. Aminoglycosides have traditionally been used for this purpose, because they penetrate poorly into cells. If present in medium at high concentrations, however, gentamicin enters macrophages and kills intracellular bacteria (9). We therefore determined concentrations of gentamicin that would inhibit extracellular, but not intracellular, growth under our culture conditions. Macrophages were cultured with 16M for 1 h and extracellular bacteria were washed off as described above. Monolayers were then replenished with 200 μl of DME-FBS-MCSF containing gentamicin at a concentration of 0, 2, 10, or 100 μg/ml. After 30-min or 24-h incubation, the culture medium was removed and the monolayers were washed three times before lysing and plating to determine the number of CFU. Data in Table 1 show that gentamicin at a concentration of 2 μg/ml was safe for intracellular B. melitensis 16M during long-term incubation. Gentamicin at 2 μg/ml did not significantly affect the number of CFU/well among cultures incubated for 30 min or 24 h (P = 0.17 and P = 0.55, respectively, with respect to cultures incubated without antibiotics). In contrast, culture with 10 or 100 μg of gentamicin per ml led to a significant (P < 0.05 to P < 0.008) dose-related reduction in the number of intracellular CFU at both 30 min and 24 h (Table 1). This bactericidal effect was further evidenced over the next day of culture. At 0 and 2 μg of gentamicin per ml, the number of intracellular bacteria increased by 19 and 27%, respectively, from 30 min to 24 h, while at a concentration of 10 or 100 μg of gentamicin per ml, the number of intracellular CFU declined by 45 and 77%, respectively (Table 1).
TABLE 1.
Inhibition of B. melitensis growth by high-dose gentamicin during long-term incubationa
| Concn of gentamicin (μg/ml) | No. of intracellular B. melitensis (CFU/well)b | P valuec |
|---|---|---|
| 30-min incubation | ||
| 0 (control) | 3,383 ± 138 | |
| 2 | 2,817 ± 405 | 0.170 |
| 10 | 2,633 ± 184 | 0.017 |
| 100 | 2,383 ± 257 | 0.011 |
| 24-h incubation | ||
| 0 (control) | 4,025 ± 194 | |
| 2 | 3,650 ± 125 | 0.550 |
| 10 | 1,450 ± 214 | 0.045 |
| 100 | 550 ± 115 | 0.008 |
Peritoneal macrophages were infected with 16M without opsonins and extracellular bacteria were washed off. Monolayers were replenished with 200 μl of DME-FBS-MCSF containing gentamicin at a concentration of 0, 2, 10, or 100 μg/ml. After 30-min or 24-h incubation, the culture medium was removed and adherent cells were gently washed three times. Bacterial burden was determined by lysis of monolayers, followed by serial dilution and agar plating (i.e., the number of intracellular brucellae).
Values represent mean ± SEM CFU in each well from five replicate wells and represent one of two similar experiments. Data are the mean ± SEM CFU in each well from four determinations from one of two similar experiments.
Student's t test, compared to control wells.
To verify that these low levels of gentamicin, which did not inhibit intracellular replication, were sufficient to inhibit extracellular replication, we examined the number of CFU/ml in supernatants at 48 h in cultures that received 0 to 4 μg of gentamicin per ml after the 1-h infection period (Table 2). In cultures without antibiotics, >540,000 CFU/well were found in supernatants. This number was reduced at least 30-fold by culture with 1 μg of gentamicin per ml and at least 500-fold by culture with 2 μg of gentamicin per ml. In the presence of 1 to 4 μg of gentamicin per ml, the percentage of total CFU/well attributable to bacteria present in the supernatant was less than 5% for 1 μg of antibiotic per ml and less than 1% for a concentration of 2 or 4 μg/ml. There was no difference in total CFU/well among cultures incubated with 1 to 4 μg of gentamicin per ml. Treatment with IFN-γ caused a similar inhibition of replication in cultures treated with all three antibiotic concentrations. These studies indicated that gentamicin concentrations between 1 and 4 μg/ml were sufficient to control extracellular bacterial replication but not so high as to kill intracellular organisms, even in the presence of IFN-γ. For this reason, we performed the rest of our long-term studies with a concentration of 2 μg of gentamicin per ml to inhibit extracellular bacterial replication.
TABLE 2.
Effects of low-dose gentamicin on intracellular and extracellular bacterial growth in macrophages incubated with or without IFN-γa
| Concn of gentamicin (μg/ml) | No. of CFU/wellb
|
|
|---|---|---|
| Without IFN-γ | With IFN-γ | |
| Supernatants | ||
| 0 | >540,000 | >540,000 |
| 1 | 15,048 ± 2,784 | 14,040 ± 1,887 |
| 2 | 1,125 ± 498 | 716 ± 195 |
| 4 | 150 ± 42 | 533 ± 90 |
| Total (supernatants + adherent macrophages) | ||
| 0 | >540,000 | >540,000 |
| 1 | 343,500 ± 26,900 | 184,900 ± 10,400 |
| 2 | 387,700 ± 19,700 | 142,500 ± 3,200 |
| 4 | 306,100 ± 14,600 | 179,900 ± 6,900 |
Peritoneal macrophages were infected with 16M without opsonins and extracellular bacteria were washed off. Monolayers were replenished with DME-FBS-MCSF containing indicated concentrations of gentamicin with or without IFN-γ. After 48 h of incubation, B. melitensis CFU in culture supernatants (extracellular 16M) were determined by serial dilution and plating of supernatants on Brucella agar. The number of intracellular bacterial CFU was determined by lysis of monolayers followed by serial dilution and agar plating.
Values are the mean ± SEM CFU/well from five replicate wells from one of two similar experiments.
Effects of IFN-γ on growth of Brucella in macrophages.
Antibody and complement (35, 38) kill some strains of Brucella. For intracellular bacteria, enhanced ingestion by macrophages may thus provide a safe haven by protecting the bacteria from complement-mediated extracellular killing. On the other hand, antibody and/or complement may synergize with intracellular microbicidal mechanisms to enhance bacterial destruction. To examine these possibilities, we monitored the growth of nonopsonized and opsonized brucellae in resting and IFN-γ-activated macrophages for 48 h. In four preliminary experiments with 1 μg of gentamicin per ml and duplicate wells, cultures incubated with IFN-γ never contained fewer brucellae at 22 to 24 h than at 1 h. Moreover, although IFN-γ-treated cultures tended to have fewer brucellae than cultures incubated with medium alone, this difference was minimal (data not shown). For this reason, the present studies were performed under conditions that focused on IFN-γ-mediated inhibition of growth at 48 h. Both opsonized and nonopsonized bacteria increased in number over this period. In the results shown in Table 3, 16M opsonized with aLPS, HCS, or a combination of HCS and aLPS grew about 30-fold more rapidly than nonopsonized 16M in macrophages that had not been treated with IFN-γ. In three additional experiments, growth of nonopsonized bacteria in the absence of IFN-γ ranged from 55- to 149-fold. Bacteria opsonized with HCS with or without aLPS alone grew 27- to 139-fold, while those opsonized with aLPS alone grew 108- to 434-fold. Addition of IFN-γ to macrophage cultures immediately after infection inhibited bacterial growth but did not reduce the number of CFU below the levels at 1 h. Inhibition occurred regardless of whether brucellae had previously been opsonized. In the experiment shown in Table 3, culture with IFN-γ led to 53% anti-Brucella activity for nonopsonized bacteria and 68 to 89% activity for opsonized bacteria. In three additional experiments, IFN-γ-mediated anti-Brucella activity ranged from 49 to 75% for nonopsonized organisms and 55 to 87% for opsonized organisms. In each of these experiments, IFN-γ-mediated anti-Brucella activity was always greater for each category of opsonized compared to nonopsonized organisms. The choice of opsonin (either aLPS or HCS alone or aLPS plus HCS) did not consistently affect the intensity of this activity (data not shown). By linear regression analysis, fold increase in bacteria in non-IFN-γ-treated cultures did not correlate (P > 0.1) with percent IFN-γ-mediated anti-Brucella activity over all four experiments (r = 0.404; t = 1.654; n = 16).
TABLE 3.
Effects of opsonization on 1-h uptake and 48-h growth of 16M in nonactivated and IFN-γ-activated mouse peritoneal macrophagesa
| Opsonin | No. of brucellae ingested by macrophages after 1-h exposure (103 CFU/well)b | Intracellular brucellae after 48-h incubation (103 CFU/well)bc
|
IFN-γ-mediated macrophage anti-Brucella activity (%)d | |
|---|---|---|---|---|
| Without IFN-γ | With IFN-γ | |||
| None | 3.56±0.45 | 11.17±3.10 (3.13) | 5.21±2.62 (1.46) | 53 |
| 2% HCS | 7.68±0.73 | 872.50±26.81 (113.60) | 275.00±66.41 (35.81) | 68 |
| 0.01% aLPS | 10.90±1.56 | 1,170.00±182.35 (107.34) | 130.00±18.71 (11.93) | 89 |
| 0.01% aLPS + 2% HCS | 22.5±5.44 | 2,512.00±253.96 (111.64) | 312.00±53.63 (13.86) | 88 |
Macrophage monolayers were incubated with 8 bacteria per macrophage with or without 0.01% aLPS and HCS or with a combination of 0.01% aLPS and 2% HCS. Controls received DME-FBS-MCSF medium but without opsonin. The number of intracellular bacteria was determined by lysis of monolayers after 1 and 48 h, followed by serial dilution and plating on agar.
Mean ± SEM CFU per well from five replicate wells in one of four similar experiments.
Numbers in parentheses represent the ratio of CFU at 48 h to CFU at 1 h.
[CFU (minus IFN-γ) − CFU (plus IFN-γ)]/[CFU (minus IFN-γ)] × 100.
The involvement of nitric oxide.
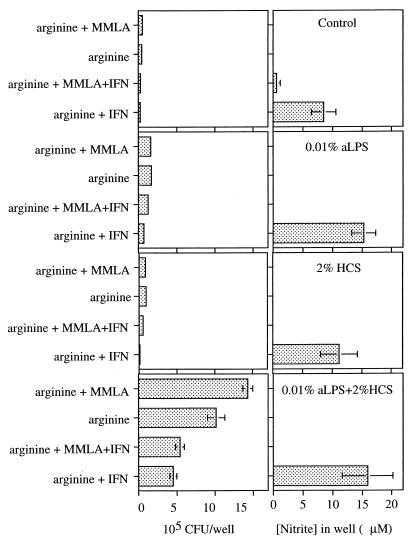
Both NO (14, 17, 40) and reactive oxygen intermediates (17) mediate macrophage anti-Brucella effects. To examine the role of NO in the IFN-γ-mediated anti-Brucella activity described above, we infected macrophages with opsonized brucellae, treated them with IFN-γ in the presence or absence of MMLA in the presence of 0.8 mM l-arginine (twice the concentration in our standard medium), and determined nitrite levels and the number of bacterial CFU (Fig. 3). Macrophages cultured without brucellae or cultured with brucellae but without IFN-γ made ≤0.3 nmol of nitrite/200 μl well (i.e., ≤1.5 μM). Addition of IFN-γ to macrophages cultured without brucellae did not enhance NO production, but NO production by macrophages cultured with bacteria and IFN-γ was profoundly enhanced (>10-fold). Addition of MMLA, which completely abrogated NO production, had inconsistent effects on IFN-γ-mediated anti-Brucella activity (Fig. 3). There was no difference in anti-Brucella activity in MMLA-treated cultures compared to untreated cultures in which brucellae had been opsonized with aLPS plus HCS, but partial MMLA-induced inhibition occurred in cultures in which brucellae had been opsonized with aLPS or HCS alone. Similar partial inhibition occurred in two other experiments (data not shown). These data suggest that anti-Brucella activity is partly NO-dependent but that NO is of minor importance under the present culture conditions.
FIG. 3.
Effects of IFN-γ on 48-h NO production and replication of opsonized or nonopsonized 16M. Macrophage monolayers were incubated for 1 h with bacteria at an MOI of 8 with or without opsonins as indicated. After washing to remove noningested organisms, monolayers received either 2 μg of gentamicin/ml and IFN-γ or control medium with 2 μg of gentamicin/ml but without IFN-γ. Macrophages were lysed at 48 h and cell-associated CFU were determined by serial dilution and agar plating. NO was measured in the supernatants as nitrite. Data are the mean ± SEM CFU in each well from five replicate wells and represent one of four similar experiments.
DISCUSSION
These studies show that antibody and complement independently and synergistically enhance the uptake of B. melitensis in nonactivated murine macrophages. Complement-mediated enhanced uptake is consistent with the findings of Young and associates (38). They reported that virulent and attenuated strains of B. abortus 296 and B. melitensis EP were rapidly ingested by human polymorphonuclear leukocytes after opsonization with normal complement-containing serum, whereas their nonopsonized counterparts were not ingested.
Gross and associates (14) have extended these studies to B. suis. They showed that opsonization of B. suis with specific antibody greatly increases the internalization of the bacteria by the J774A.1 macrophage-like cell line. In other studies on the uptake of nontypeable Haemophilus influenzae by human polymorphonuclear leukocytes (24), only additive opsonic effects with immunoglobulin-rich normal human serum in combination with complement-rich guinea pig serum were observed. The lack of synergy between complement and antibody in that study probably reflects the use of rather high levels of immune serum (1% guinea pig serum and 10% immune serum). Jones and Winter (18) demonstrated enhanced phagocytosis by treatment of smooth B. abortus 2308 with antibody. In our experimental system, rough strains are readily ingested by macrophages (M. O. Eze, unpublished observations). Strain 19, a semirough strain, is also more easily ingested than 2308 (18) and its uptake is independent of antibody.
The receptors used for the uptake of nonopsonized brucellae are unknown. Synergy between the receptor for the Fc domain of immunoglobulin G (FcR) and the receptor for iC3b, a cleavage product of C3 (C3bR), for the uptake of opsonized sheep erythrocytes is well described (25). Synergy in the uptake of intracellular pathogens may involve a combination of additional phagocyte receptors which, on binding ligand, almost always trigger internalization (such as FcR and the mannose receptors) and those that sometimes fail to trigger ingestion (e.g., CR) (19, 25, 33). Thus, virulent strains of M. tuberculosis are phagocytosed by the cooperation of CR and mannose receptors on human MDM surfaces, whereas attenuated strains are internalized by the function only of CR (33). Interestingly, the O-polysaccharides of smooth brucellae have an abundance of mannosyl residues (1, 5) but resist phagocytosis in the absence of opsonins.
Treatment of B. abortus with complement-rich serum prior to exposure to human neutrophils leads to extracellular and intracellular killing of bacteria, but similar treatment of B. melitensis does not lead to killing (38). Cheers and Ho (7) reported an increased uptake of antibody-opsonized L. monocytogenes in mice. Opsonization with antibody did not alter the growth of this organism once it was inside the liver and spleen cells. On the other hand, clearance of Salmonella enterica serovar Typhimurium and B. abortus early in infection increased with opsonization with specific antibody. It was therefore concluded that a nonspecific cellular mechanism was responsible for early enhanced resistance to each infection (7). Our data show that resident mouse peritoneal macrophages, like human neutrophils, fail to kill B. melitensis. As shown by others for B. abortus (16) and B. suis (14), IFN-γ-activated macrophages exerted greater inhibition on the growth of opsonized brucellae than nonopsonized bacteria. It is possible that the relative ineffectiveness of IFN-γ-treated macrophages against nonopsonized bacteria was due to the reduced uptake of nonopsonized organisms and the consequent failure of the fewer ingested bacteria to trigger the release of adequate antimicrobial effector molecules. It is also possible that engagement of macrophage FcR or CR by opsonized brucellae triggers antimicrobial effector responses.
From their work with scavengers and inhibitors of reactive oxygen species, Jiang and associates concluded (17) that superoxide (O2−) and hydrogen peroxide (H2O2) might contribute to control of B. abortus in elicited mouse peritoneal macrophages, whereas NO was of less importance in the process. For several intracellular pathogens, a lack of correlation in the effects of varying NO levels (with NO synthase inhibitors or artificial NO donors) on microbial burden has led to different conclusions regarding the role of NO as an antipathogen effector. For example, murine macrophage NO may act alone or cooperate with other macrophage microbicidal mechanisms to kill Candida (34). For L. monocytogenes, murine macrophage NO may play a direct listericidal role in primary infections but may be of less importance in secondary infections (31). In contrast to the studies of Jiang et al. (17), Gross and associates (14) have shown that NO is involved in the elimination of B. suis from murine cells, provided that both Brucella antibodies and IFN-γ are present. Nonopsonized bacteria did not trigger the production of iNOS or NO, although they did enhance iNOS mRNA levels. Our data most closely resemble those of Jiang et al. (17), in that peritoneal macrophages treated with IFN-γ shortly after infection modestly inhibited the growth of Brucella and the intracellular growth of bacteria in both IFN-γ-treated and control cells was enhanced by the addition of MMLA. The failure of treatment with MMLA to completely reverse the anti-Brucella effect of IFN-γ in both of our studies indicates that factors other than NO must play a predominant role in intramacrophage killing. It is more difficult to compare our data with those of Gross et al. (14), since they used only J774A.1 cells and B. suis, while we used resident peritoneal cells with B. melitensis. It is possible that B. suis is more susceptible to NO-mediated killing than B. melitensis or B. abortus. Alternatively, B. suis may induce higher levels of NO in IFN-γ-treated cells than the other Brucella species. In our system, the levels of NO induced were approximately 1/2 of those induced by B. suis in the studies of Gross et al. (14). The failure of any of these systems to induce the clearance of bacteria from macrophages may be related to failure to achieve the consistent levels of NO actually required to kill Brucella. Strategies that induce more NO production, or favor cooperation between reactive oxygen and reactive nitrogen to produce more potent antimicrobial moieties such as peroxynitrite (15), may remedy this deficiency.
Our culture systems also differ from those of others (10, 11, 14, 16–18) in the concentration of antibiotics used to control the extracellular growth of Brucella. Gentamicin concentrations of ≥50 μg/ml were used in these previous studies to induce rapid killing of extracellular brucellae at the end of the initial infection period. These high concentrations were justified because rapidity of killing by aminoglycosides is related to dose. In standard mean inhibitory concentration or mean bactericidal concentration assays, brucellae are sensitive to 1 to 4 μg of gentamicin per ml (23). These data are consistent with our observations that ≥1 μg of gentamicin/ml was sufficient to prevent growth of 16M over 48 h. In our system, repeated washing of monolayers after the initial 1-h period of infection reduces the number of recoverable extracellular bacteria to ≤100 CFU/well (data not shown). It is possible that the use of high concentrations of antibiotics in previous studies contributed to the decline in CFU in the first 24 h after infection of macrophages with opsonized or nonopsonized bacteria observed by those authors. It is possible that recently ingested bacteria present in membrane-bound compartments are susceptible to gentamicin that penetrates macrophages when it is present in culture fluids at high concentrations (9). After some endosomal maturation has occurred, bacteria may be protected from intramacrophage gentamicin, so growth resumes, even if high aminoglycoside levels are maintained in the culture medium. Trafficking of Brucella-containing phagosomes consistent with this hypothesis has recently been demonstrated in HeLa cells (28). Whether inclusion of high doses of antibiotics in the studies cited influences the anti-Brucella activity attributed to IFN-γ is unknown but must be considered in the interpretation of apparently conflicting results.
These studies are important in light of the consistent observation that the presence of antibody to Brucella, whether derived by active or passive immunization, leads to profound anti-Brucella effects (1, 7, 10, 22, 29, 35) in vivo. Our data suggest that, even when brucellae are opsonized with antibody and complement, the IFN-γ-mediated activation of resting macrophages inhibits the intracellular growth of B. melitensis but does not result in the clearance of organisms from their intracellular niche. These studies support the conclusion that additional cellular immune responses, e.g., those mediated by cytotoxic T cells (26), may also play important roles or that additional signals are provided in vivo to further enhance macrophage brucellacidal activity. We are currently examining mechanisms of antibody effects in vivo with a murine model of intranasal infection with B. melitensis.
ACKNOWLEDGMENTS
M.O.E. was supported by a senior resident research associateship from the U.S. National Research Council.
We are grateful to Joseph Thompson for technical assistance.
REFERENCES
- 1.Baldwin C L, Winter A J. Macrophages and Brucella. Immunol Ser. 1994;60:363–380. [PubMed] [Google Scholar]
- 2.Baumler A J, Heffron F. Microbial resistance of macrophage effector functions: strategies for evading microbicidal mechanisms and scavenging nutrients within mononuclear phagocytes. In: Roth J A, Bolin C A, Brogden K A, Minion F C, Wannemuehler M J, editors. Virulence mechanisms of bacterial pathogens. 2nd ed. Washington, D.C.: ASM Press; 1995. pp. 115–131. [Google Scholar]
- 3.Blackwell J M, Ezekowitz R A, Roberts M B, Channon J Y, Sim R B, Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985;162:324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bounous D I, Enright F M, Gossett K A, Berry C M. Phagocytosis, killing, and oxidant production by bovine monocyte-derived macrophages upon exposure to Brucella abortus strain 2308. Vet Immunol Immunopathol. 1993;37:243–256. doi: 10.1016/0165-2427(93)90197-c. [DOI] [PubMed] [Google Scholar]
- 5.Bundle D R, Cherwonogrodzky J W, Caroff M, Perry M B. The lipopolysaccharides of Brucella abortus and B. melitensis. Ann Inst Pasteur Microbiol. 1987;138:92–98. doi: 10.1016/0769-2609(87)90083-4. [DOI] [PubMed] [Google Scholar]
- 6.Canning P C, Deyoe B L, Roth J A. Opsonin-dependent stimulation of bovine neutrophil oxidative metabolism by Brucella abortus. Am J Vet Res. 1988;49:160–163. [PubMed] [Google Scholar]
- 7.Cheers C, Ho M. Resistance and susceptibility of mice to bacterial infection. IV. Functional specificity in natural resistance to facultative intracellular bacteria. J Reticuloendothel Soc. 1983;34:299–309. [PubMed] [Google Scholar]
- 8.Drazek E S, Houng H S, Crawford R M, Hadfield T L, Hoover D L, Warren R L. Deletion of purE attenuates Brucella melitensis 16M for growth in human monocyte-derived macrophages. Infect Immun. 1995;63:3297–3301. doi: 10.1128/iai.63.9.3297-3301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drevets D A, Canono B P, Leenen P J, Campbell P A. Gentamicin kills intracellular Listeria monocytogenes. Infect Immun. 1994;62:2222–2228. doi: 10.1128/iai.62.6.2222-2228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elzer P H, Jacobson R H, Jones S M, Nielsen K H, Douglas J T, Winter A J. Antibody-mediated protection against Brucella abortus in BALB/c mice at successive periods after infection: variation between virulent strain 2308 and attenuated vaccine strain 19. Immunology. 1994;82:651–658. [PMC free article] [PubMed] [Google Scholar]
- 11.Elzer P H, Phillips R W, Robertson G T, Roop R M., II The HtrA stress response protease contributes to resistance of Brucella abortus to killing by murine phagocytes. Infect Immun. 1996;64:4838–4841. doi: 10.1128/iai.64.11.4838-4841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortier A H, Nacy C A, Polsinelli T, Bhatnagar N. Francisella tularensis, a model pathogen to study the interactin of facultative intracellular bacteria with phagocytic host cells. In: Roth J A, Bolin C A, Brogden K A, Minion F C, Wannemuehler M J, editors. Virulence mechanisms of bacterial pathogens. 2nd ed. Washington, D.C.: ASM Press; 1995. pp. 115–131. [Google Scholar]
- 13.Fortier A H, Polsinelli T, Green S J, Nacy C A. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect Immun. 1992;60:817–825. doi: 10.1128/iai.60.3.817-825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross A, Spiesser S, Terraza A, Rouot B, Caron E, Dornand J. Expression and bactericidal activity of nitric oxide synthase in Brucella suis-infected murine macrophages. Infect Immun. 1998;66:1309–1316. doi: 10.1128/iai.66.4.1309-1316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ischiropoulos H, Zhu L, Beckman J S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Baldwin C L. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993;61:124–134. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X, Leonard B, Benson R, Baldwin C L. Macrophage control of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell Immunol. 1993;151:309–319. doi: 10.1006/cimm.1993.1241. [DOI] [PubMed] [Google Scholar]
- 18.Jones S M, Winter A J. Survival of virulent and attenuated strains of Brucella abortus in normal and gamma interferon-activated murine peritoneal macrophages. Infect Immun. 1992;60:3011–3014. doi: 10.1128/iai.60.7.3011-3014.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levitz S M, Tabuni A. Binding of Cryptococcus neoformans by human cultured macrophages. Requirements for multiple complement receptors and actin. J Clin Investig. 1991;87:528–535. doi: 10.1172/JCI115027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackaness G B. The immunological basis of acquired cellular resistance. J Exp Med. 1964;120:105–120. [PubMed] [Google Scholar]
- 21.Mackaness G B. Resistance to intracellular infection. J Infect Dis. 1971;123:439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- 22.Montaraz J A, Winter A J. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun. 1986;53:245–251. doi: 10.1128/iai.53.2.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortensen J E, Moore D G, Clarridge J E, Young E J. Antimicrobial susceptibility of clinical isolates of Brucella. Diagn Microbiol Infect Dis. 1986;5:163–169. doi: 10.1016/0732-8893(86)90118-5. [DOI] [PubMed] [Google Scholar]
- 24.Musher D M, Hague-Park M, Baughn R E, Wallace R J, Jr, Cowley B. Opsonizing and bactericidal effects of normal human serum on nontypable Haemophilus influenzae. Infect Immun. 1983;39:297–304. doi: 10.1128/iai.39.1.297-304.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman S L, Johnston R B., Jr Role of binding through C3b and IgG in polymorphonuclear neutrophil function: studies with trypsin-generated C3b. J Immunol. 1979;123:1839–1846. [PubMed] [Google Scholar]
- 26.Oliveira S C, Splitter G A. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur J Immunol. 1995;25:2551–2557. doi: 10.1002/eji.1830250922. [DOI] [PubMed] [Google Scholar]
- 27.Payne N R, Horwitz M A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987;166:1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pizarro-Cerda J, Meresse S, Parton R G, van der Goot G, Sola-Landa A, Lopez-Goni I, Moreno E, Gorvel J P. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plommet M, Plommet A M. Immune serum-mediated effects on brucellosis evolution in mice. Infect Immun. 1983;41:97–105. doi: 10.1128/iai.41.1.97-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price R E, Templeton J W, Smith R D, Adams L G. Ability of mononuclear phagocytes from cattle naturally resistant or susceptible to brucellosis to control in vitro intracellular survival of Brucella abortus. Infect Immun. 1990;58:879–886. doi: 10.1128/iai.58.4.879-886.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samsom J N, Langermans J A, Groeneveld P H, van Furth R. Acquired resistance against a secondary infection with Listeria monocytogenes in mice is not dependent on reactive nitrogen intermediates. Infect Immun. 1996;64:1197–1202. doi: 10.1128/iai.64.4.1197-1202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlesinger L S. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 33.Schlesinger L S, Bellinger-Kawahara C G, Payne N R, Horwitz M A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 34.Vazquez-Torres A, Jones-Carson J, Balish E. Nitric oxide production does not directly increase macrophage candidacidal activity. Infect Immun. 1995;63:1142–1144. doi: 10.1128/iai.63.3.1142-1144.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winter A J, Duncan J R, Santisteban C G, Douglas J T, Adams L G. Capacity of passively administered antibody to prevent establishment of Brucella abortus infection in mice. Infect Immun. 1989;57:3438–3444. doi: 10.1128/iai.57.11.3438-3444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright S D, Silverstein S C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983;158:2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto K, Johnston R B., Jr Dissociation of phagocytosis from stimulation of the oxidative metabolic burst in macrophages. J Exp Med. 1984;159:405–416. doi: 10.1084/jem.159.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young E J, Borchert M, Kretzer F L, Musher D M. Phagocytosis and killing of Brucella by human polymorphonuclear leukocytes. J Infect Dis. 1985;151:682–690. doi: 10.1093/infdis/151.4.682. [DOI] [PubMed] [Google Scholar]
- 39.Zhan Y, Cheers C. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect Immun. 1993;61:4899–4901. doi: 10.1128/iai.61.11.4899-4901.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhan Y, Cheers C. Endogenous interleukin-12 is involved in resistance to Brucella abortus infection. Infect Immun. 1995;63:1387–1390. doi: 10.1128/iai.63.4.1387-1390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]