Abstract
As prostate cancer (PCa) is one of the most commonly diagnosed cancer worldwide, identifying potential prognostic biomarkers is crucial. In this study, the survival information, gene expression, and protein expression data of 344 PCa cases were collected from the Cancer Proteome Atlas (TCPA) and the Cancer Genome Atlas (TCGA) to investigate the potential prognostic biomarkers. The integrated prognosis-related proteins (IPRPs) model was constructed based on the risk score of each patients using machine-learning algorithm. IPRPs model suggested that Elevated RAD50 expression (p = 0.016) and down-regulated SMAD4 expression (p = 0.017) were significantly correlated with unfavorable outcomes for PCa patients. Immunohistochemical (IHC) staining and western blot (WB) analysis revealed significant differential expression of SMAD4 and RAD50 protein between tumor and normal tissues in validation cohort. According to the overall IHC score, patients with low SMAD4 (p < 0.0001) expression and high RAD50 expression (p = 0.0001) were significantly correlated with poor outcomes. Besides, expression of SMAD4 showed significantly negative correlation with most immune checkpoint molecules, and the low SMAD4 expression group exhibited significantly high levels of LAG3 (p < 0.05), TGFβ (p < 0.001), and PD-L1 (p < 0.05) compared with the high SMAD4 expression group in the validation cohort. Patients with low SMAD4 expression had significantly higher infiltration of memory B cells (p = 0.002), CD8 + T cells (p < 0.001), regulatory T cells (p = 0.006), M2-type macrophages (p < 0.001), and significantly lower infiltration of naïve B cells (p = 0.002), plasma cells (p < 0.001), resting memory CD4 + T cells (p < 0.001) and eosinophils (p = 0.045). Candidate proteins were mainly involved in antigen processing and presentation, stem cell differentiation, and type I interferon pathways.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43657-022-00070-1.
Keywords: Prostate cancer, Proteomics, Prognosis, SMAD family member 4, Double-strand break repair protein RAD50
Introduction
As the most commonly diagnosed cancer and the fifth leading cause of cancer death with 7.1% mortality, prostate cancer (PCa) has become one of the greatest health issues facing the male population globally (Bray et al. 2018). According to the cancer statistics in America, estimated new cases of PCa may grow to 191,930 in 2020, accounting for more than one in five newly diagnosed cases (Siegel et al. 2020). In recent decades, the high incidence of PCa may be attributed to the popularization of prostate-specific antigen (PSA) early screening, which made a surge in the detection of PCa, while asymptomatic patients received early diagnosis and treatment (Potosky et al. 1995). However, the prognosis of PCa patients has been improved in many countries (Center et al. 2012), which is linked to earlier diagnosis due to PSA screening, the advance in precise treatment, and the application of new drugs (Brawley 2012; Rodrigues et al. 2014). Hence, to further understand the molecular mechanism of proliferation and invasion of PCa, and implement an accurate individualized therapeutic regimen, it is extremely urgent to investigate the new molecules involved in the carcinogenesis and potential prognostic biomarkers of PCa.
Recently, proteomics techniques, as a powerful tool for exploring cancer-related biomarkers, are widely utilized to investigate significant proteins associated with tumorigenesis (Rahim et al. 2015). Furthermore, proteomic analysis can get easier access to the prognostic value of potential biomarkers and reveal functional protein pathways in the cancers (Aslam et al. 2017). Nevertheless, in recent years, some studies have used proteomic analysis to identify potential biomarkers of PCa. Hence, using the unique features of proteomics to investigate potential biomarkers of PCa may provide new prospects for diagnosis and therapy. In this study, we constituted the large-scale PCa proteomic analysis and initially screened certain proteins with prognostic value, including RAD50 and SMAD4.
RAD50 combined with MRE11 and NBS1 as an essential regulative complex (MRN) to respond the cellular damage via repairing DNA double-strand breaks (DSBs) (Bosch et al. 2003; Myler et al. 2017). More recent studies revealed that the mitotic progression of RAD50 deficient fibroblasts was markedly prolonged, indicating that MRN was also involved in the mitotic process (Völkening et al. 2020). Interestingly, it provided increased possibilities for tumorigenesis due to the high frequency of DSBs repair (Jackson and Bartek 2009; O'Connor 2015). Hence, recent research indicated that RAD50 played an essential role as a valuable oncogene in colorectal cancer (Situ et al. 2019) and significantly correlated with overall survival (OS) for patients with PCa (Xu et al. 2020), while as an important and valuable prognosis, RAD50 needs to be further elucidated and verified in PCa patients.
Small Mother Against Decapentaplegic (SMAD) proteins play indispensable roles in TGF-β signaling pathway. A set of intracellular SMAD proteins transfers the TGF-β signaling to phosphorylate the receptor-activated SMADs (R-SMADs) and SMAD3 at the C-terminally located serine residues (Kroon et al. 2017). Ultimately, the activated R-SMADs function with common-mediator SMAD (Co-SMAD) as a heterocomplex to participate in several transcriptional regulations of target genes (Walldén et al. 2017). The impacts of SMAD3 and SMAD4 mutations on tumorigenesis have been clarified in colorectal cancers (CRCs), of which SMAD3 and SMAD4 mutations were detected in approximately 4.3% and 8.6% of CRCs patients, respectively (Fleming et al. 2013; Miyaki et al. 1999). Furthermore, knockout of SMAD4 promoted the expression of miR-301a, which enhanced cell proliferation in vitro and cancer growth in PCa patients (Li et al. 2018). However, the molecular mechanism, diagnostic and prognostic implication of SMAD proteins in the tumorigenesis of PCa need to be further investigated.
The low specificity of PSA screening leads to over-diagnosis and over-treatment of patients. Patients with an indolent, slow-growing PCa are frequently over-treated, while for those with aggressive and life-threatening disease effective therapies are still missing (Martin et al. 2018). Thus, it is necessary to define biomarkers allowing to guide the treatment for those patients with the indolent disease (stay on active surveillance or proceed to treatment), and develop more effective therapies for advanced diseases or biomarkers predicting treatment response.
To investigate the prospects of prognostic proteins significance and establish integrated prognosis-related proteins (IPRPs) model, we used proteomic survival analysis to determine significant proteins and assess the survival risk score of PCa patients from The Cancer Proteome Atlas (TCPA) and The Cancer Genome Atlas (TCGA) cohort. Next, we analyzed the correlation between SMAD4 and immune response. we also investigated the correlations between SMAD4 and downstream genes involved in the TGFβ/SMAD4 signaling pathway, the expression, prognosis, and the potential functions of these genes. In addition, we established the protein interaction network through gene expression profile analysis, which might provide underlying mechanisms and new therapeutic targets for PCa.
Materials and Methods
Raw Biological Microarray Data
Level 4 reverse-phase protein arrays (RPPA) data of prostate cancer were downloaded from TCPA, including 214 proteins. Gene expression data and clinical information, including age at diagnosis, pathologic Tumor Node Metastasis (pTNM) staging, pathological staging, Gleason score, overall survival time, and last known disease status of PCa patients were obtained from the TCGA database. Data for single-cell analysis were obtained from Gene Expression Omnibus (GEO) database (dataset GSE67980). Data analysis began with preprocessing and normalization of raw biological data. After removing bias and matching the sample ID using R software, a total of 344 PCa cases with complete information of protein expression, gene expression, and survival data were enrolled in this study.
Screening of Prognostic Proteins
To evaluate the prognostic value of each protein, survival analyses were performed using the Kaplan–Meier method and univariate Cox regression method by log-rank test with 95% confidence intervals (95% CI). The primary endpoint was overall survival (OS), which was assessed from the date of receiving pathologic diagnosis to the date of death or the last follow-up. Based on the results of univariate Cox regression analysis, a total of 17 proteins significantly correlated with the prognosis of PCa were initially screened as candidate proteins, which were subsequently divided into low- and high-risk protein groups based on the hazard ratio (HR) = 1 as the cut off value. The volcano map was plotted by R software using the ggplot2 package for visualizing the low- and high-risk candidate proteins (Wickham 2016). Next, to further elevate the prognostic accuracy, proteins with p value < 0.05 in both Kaplan–Meier analysis and univariate Cox logistic regression analysis were determined as prognostic proteins, including RAD50, SMAD3, and SMAD4. After multivariate Cox regression analysis, only RAD50 and SMAD4 were identified as proteins with the most prognostic value. Survival curves of PCa patients were plotted based on the expression of each prognostic protein respectively using the survival package of R software (Therneau and Grambsch 2000). The receiver operating characteristic (ROC) curve was constructed and the area under the curve (AUC) was calculated to evaluate the specificity and sensitivity of the prognostic value of proteins.
Construction of IPRPs Model
To construct the IPRPs model, the risk score for each patient was calculated based on the expression of prognostic proteins and the survival coefficient of PCa cases. Integrated risk score = RAD50 expression × 1.18 + SMAD4 expression × (− 6.59). All patients were divided into low- and high-risk groups based on the median risk score as the cutoff value. To evaluate the prognostic value of risk score, survival curves and scatter diagrams were drawn based on risk stratification using the R packages survival and survminer. Heatmap was utilized to visualize the expression of each significant protein in two risk groups.
Univariate and multivariate Cox regression analysis was performed to identify the independent prognostic factors and construct the IPRPs model on this basis. For both statistical analyses, p value < 0.05 was considered significant.
Evaluation of Immunostaining
A total of 92 PCa patients who underwent radical prostatectomy from July 2018 to March 2020 were enrolled from the Fudan University Shanghai Cancer Center (FUSCC, Shanghai, China). Clinical information was obtained from pathology records and electronic medical records. Tumor sections and normal prostate tissues were collected during surgery and then processed and stored at the FUSCC tissue bank. Immunostaining of RAD50, SMAD4, LAG3, TGFβ, CD8 and PD-L1 was implemented using an anti-RAD50 antibody (1:500, ab208019, Abcam), anti-SMAD4 antibody (1:500, ab40759, Abcam), anti-LAG3 antibody (1:500, ab209236, Abcam), anti-TGF beta 1 antibody (1:500, ab215715, Abcam), anti-CD8 alpha antibody (1:500, ab217344, Abcam) and anti-PD-L1 antibody (1:500, ab205921, Abcam), respectively. The immunohistochemistry (IHC) staining of each protein was independently evaluated and scored by two experienced pathologists. Disagreements in scoring were reconciled to reach a consensus. The staining degree score was graded from 0 to 4, based on the coverage percentage of tumor cells (0%, 1–25%, 26–50%, 51–75%, 76–100%). Staining intensity degrees was ranging from 0 to 3, representing samples with no staining, weak, median and strong, respectively. The overall IHC score (from 0 to 12) was calculated according to the multiply of staining degree score and staining intensity.
Based on the median overall IHC score, expressions of RAD50 and SMAD4 were divided into low- and high-expression groups. To verify the prognostic accuracy of prognostic proteins, survival analysis was performed based on RAD50 and SMAD4 expression in the FUSCC cohort.
Western Blot Analysis
Four sample pairs were randomly selected for western blot analysis. Cells extracted from human PCa tissue and adjacent normal tissues were lysed in ice-cold radioimmunoprecipitation (RIPA) buffer (Beyotime Biotechnology Shanghai, China). The cell lysates (10 μg) were separated by electrophoresis on 6% or 10% SDS-Page gel and then transferred onto methanol-activated polyvinylidene fluoride (PVDF) membrane. The blotted membranes were blocked for 1 h at room temperature with 5% bovine serum albumin (5% BSA). After that, the blots were incubated with primary antibodies, anti-SMAD4 antibody (1:1000, ab40759, Abcam), anti-RAD50 antibody (1:1000, ab208019, Abcam), and anti-beta-Actin antibody (1:3,000, ab179467, Abcam) at 4 °C overnight. After washing with Tris HCL buffer + Tween 20 (TBST), membranes were incubated with secondary antibody Goat Anti-Rabbit IgG conjugated with horseradish peroxidase (HRP) (1:3,000, ab205718, Abcam) at room temperature for 1 h. The bands were visualized using ECL-plus™ western blotting chemiluminescence kits (BD Biosciences, New Jersey, USA).
Estimation of Immune Cell Type Fractions
CIBERSORT (https://cibersort.stanford.edu/) is an analytical tool for estimating the cell composition of tissues from their gene expression profiles (Newman et al. 2015). In CIBERSORT, the fraction of 22 human immune cell types including naïve and memory B cells, seven T cell types, NK cells, plasma cells, and myeloid subsets in PCa tissues was estimated using the leukocyte gene signature matrix, termed LM22. PCa patients were divided into high- and low-SMAD4 expression groups, and the correlations between the fraction of each immune cell type and SMAD4 expression level were investigated and visualized using the vioplot package of R software. Pearson’s co-expression analysis was performed to identify the proteins associated with the prognostic model and 0.4 was the cutoff value of the correlation coefficient. Spearman correlation analysis was utilized to investigate the correlated proteins with SMAD4. TGF-β signaling pathways were visualized with BioRender.com.
Gene Set Enrichment Analysis and Functional Enrichment Analysis
GSEA software (version 3.0) was utilized to identify significantly related genes of low- and high-risk cases with 1000 permutations. Adjusted p values (Adj. p) < 0.01 and false discovery rate (FDR) < 0.25 were applied to define the differential expression genes (DEGs) with Benjamini and Hochberg method (Subramanian et al. 2005) using the Limma package (Ritchie et al. 2015). Heatmap was plotted to visualize the difference in gene expression between the high- and low-group. Protein–protein interaction (PPI) network was constructed to reveal the correlations of DEGs using the Search Tool for the Retrieval of Interacting Genes (STRING; http://string-db.org) (version 10.0) (Franceschini et al. 2013) online database. Most significant hub genes were selected using the Molecular Complex Detection (MCODE) plug-in of Cytoscape software (Bandettini et al. 2012). Functional annotations including Kyoto Encyclopedia of Genes And Genomes (KEGG), molecular functions (MF), biological processes (BP), and cellular components (CC) were investigated to identify potential function enrichments of hub genes using the Cluster Profiler package of R software (Yu et al. 2012). Cancer Single-Cell State Atlas (Cancer SCA; http://biocc.hrbmu.edu.cn/CancerSEA) was utilized to investigate the potential function of the molecules involved in the TGFβ/SMAD4 signaling pathway.
Results
Identification of Candidate Protein Biomarkers
A total of 17 proteins were initially screened as candidate prognostic proteins using Kaplan–Meier analysis and univariate Cox regression analysis. As shown in the volcano plot (Fig. 1a), candidate prognostic proteins were separated as high- and low-risk. Next, three proteins, including RAD50, SMAD3, and SMAD4, were identified as prognostic proteins using both Kaplan–Meier analysis and univariate Cox regression analysis (Table 1). After multivariate Cox regression analysis, only RAD50 and SMAD4 were still correlated with PCa prognosis.
Fig. 1.
Screening and identification of significant prognostic protein biomarkers in Prostate cancer. (a) Volcano plot indicated the significant proteins with high- and low-risk. Red and green dots represented significant prognostic proteins with high- and low-risk, respectively. (b, c) Survival curves demonstrated that high expression of RAD50 and down-regulation of SMAD4 significantly correlated with poor OS of PCa patients (p = 0.016, p = 0.017). (d) The blue line represents risk score with AUC of 0.842, indicating the consistent predictive ability
Table 1.
Proteins related to prognosis after K–M analysis and univariate Cox regression analysis (both p value < 0.05)
| Proteins | p value (KM) | p value (unicox) | HR |
|---|---|---|---|
| RAD50 | 0.016 | 0.009 | 4.573 (1.466 to 14.263) |
| SMAD3 | 0.035 | 0.035 | 91.240 (1.399 to > 100) |
| SMAD4 | 0.017 | 0.016 | 0.001 (< 0.001 to 0.016) |
KM Kaplan–Meier analysis, Unicox Univariant Cox regression analysis, HR hazard ratio
Survival Assessment of Significant Proteins
As shown in Fig. 1b, c, survival curves demonstrated that high expression of RAD50 (p = 0.016) and down-regulation of SMAD4 (p = 0.017) were significantly correlated with the poor OS of PCa patients. The risk score for each patient was calculated based on the expression of prognostic proteins and the survival coefficient of PCa cases. ROC curve revealed the prognostic value of risk score with AUC of 0.842, indicating a high predictive ability of the risk model (p < 0.001) (Fig. 1d). Heatmap revealed that RAD50 expression in the high-risk group and SMAD4 expression in the low-risk group was higher than that in each contrast group (Fig. 2a). In addition, the survival time of PCa patients in the high-risk group was significantly shorter than that in the low-risk group (p = 0.015) (Fig. 2b), and the increased risk score also corresponded to a shorter survival time (Fig. 2c, d). However, in both univariate and multivariate Cox regression analysis, only the risk score was significantly correlated with OS (p = 0.003, p = 0.005), and other traditional risk factors such as age, pT stage, pN stage, and Gleason score revealed no correlation with OS, indicating that risk score can be the independent prognostic marker (Fig. 2e, f).
Fig. 2.
Differential expression of integrated prognosis-related proteins (IPRPs) and construction of the prediction model. (a) Heatmap revealed the expression of RAD50 and SMAD4 in high- and low-risk group. (b) The survival time of PCa patients in high-risk group was significantly shorter than that in low-risk group (p = 0.015). (c, d) Increased risk score corresponded to shorter survival of PCa patients. (e, f) Forest plot indicated that risk score was significantly correlated with survival time in both univariant (p = 0.003) and multivariant (p = 0.005) Cox regression analysis. (g) Proteins associated with RAD50 and SMAD4
Expression of Prognostic Proteins in Validation Cohorts
IHC staining indicated low expression of SMAD4 protein in PCa tissues in the Human Protein Atlas (HPA) cohort (Fig. 3a–c). Consistent with this, in the FUSCC cohort, IHC staining revealed high expression of SMAD4 in normal tissue (Fig. 3d) and extremely low expression in tumor tissues (Fig. 3e, f). Western blot analysis also revealed that SMAD4 was low expressed in PCa tissues compared with adjacent normal tissues (Fig. 3g). According to the overall IHC score, patients with low SMAD4 expression were significantly correlated with poor survival times (p < 0.001) (Fig. 3h). Similarly, IHC staining revealed high expression of RAD50 protein in PCa tissue and low expression of RAD50 in normal tissue both in the HPA cohort (Fig. 4a–c) and FUSCC cohort (Fig. 4d–f). Western blot analysis also indicated that RAD50 was highly expressed in tumor tissues and low expressed in adjacent normal tissues (Fig. 4g). Besides, patients with high expression of RAD50 significantly correlated with poor outcomes in the FUSCC cohort (p < 0.001) (Fig. 4h).
Fig. 3.
Validation of the prognostic significance of SMAD4 protein. (a–c) Expression of SMAD4 in PCa tissue in the HPA cohort. Upper scale bar: 100 μm; Bottom scale bar: 50 μm. IHC staining revealed the high expression of SMAD4 in normal tissue (d) and low expression in PCa tissues (e, f) in FUSCC cohort. Scale bar: 50 μm. (g) Western blot analysis indicated that SMAD4 lowly expressed in tumor tissue and highly expressed in normal tissue. (h) Low expression of SMAD4 was significantly correlated with poor outcomes in FUSCC cohort (p < 0.001)
Fig. 4.
Validation of the prognostic significance of RAD50 protein. (a–c) Expression of RAD50 in PCa tissue in the HPA cohort. Upper scale bar: 100 μm; Bottom scale bar: 50 μm. IHC staining revealed the low expression of RAD50 in normal tissue (d) and high expression in PCa tissue (e, f) in FUSCC cohort. Scale bar: 50 μm. Western blot analysis indicated that RAD50 highly expressed in tumor tissue and lowly expressed in normal tissue. (h) High expression of RAD50 was significantly correlated with poor outcomes in FUSCC cohort (p < 0.001)
Investigating of Potentially Correlated Proteins
Various types of proteins associated with RAD50 and SMAD4 were displayed in Fig. 2g. A total of 13 proteins significantly correlated with the expression of RAD50 (correlation coefficients range from − 0.302 to 0.395, p < 0.001), and six with the expression of SMAD4 (correlation coefficients range from 0.306 to 0.394, p < 0.001), and the details are displayed in Supplementary Fig. 1.
Correlation and Immune Infiltration Analysis of SMAD4
As shown in Fig. 5a, the protein–protein interaction network revealed that SMAD4 interacted with 20 related proteins through different types of pathways. Spearman correlation analysis of 498 PCa cases from TCGA cohort indicated significant negative correlation between SMAD4 and LAG3 (r = − 0.232, p < 0.0001), PDCD1 (r = − 0.219, p < 0.0001), TGFB1 (r = − 0.397, p < 0.0001), TNFRSF4 (r = − 0.419, p < 0.0001), TNFRSF18 (r = − 0.488, p < 0.0001), and IL6R (r = 0.269, p < 0.0001) (Fig. 5b). Barplot visualized the infiltration rate of 22 immune cell types in 498 PCa patients (Fig. 5c), and the correlation of each immune cell type infiltration was explained in Fig. 5d. Among them, memory-activated CD4 + T cells and activated dendritic cells (r = 0.49), regulatory T cells and memory B cells (r = 0.43) revealed significantly positive infiltrate correlations. More importantly, as shown in Fig. 5e, PCa patients with low SMAD4 expression level had significantly higher infiltration of memory B cells (p = 0.002), CD8 + T cells (p < 0.001), regulatory T cells (p = 0.006) and M2-type macrophages (p < 0.001), but significantly correlated with lower infiltration of naïve B cells (p = 0.002), plasma cells (p < 0.001), resting memory CD4 + T cells (p < 0.001) and eosinophils (p = 0.045).
Fig. 5.
Correlations between SMAD4 expression and related proteins and immune infiltrations analysis. (a) SMAD4 interact with 20 related proteins through different types of pathways. (b) SMAD4 significantly negative correlated with LAG3, PDCD1, TGFB1, TNFRSF4, TNFRSF18, IL6R (r = − 0.232, − 0.219, − 0.397, − 0.419, − 0.488, 0.269, respectively; p < 0.001). (c) Vioplot demonstrated the correlations between fraction of each immune cell types and SMAD4 expression level. Red indicates high expression group, green indicates low expression group. (d) The correlation coefficient indicated the expression of two kinds of related immune cells in the sample. The depth of color represented the strength of positive and negative correlation. (e) Barplot visualized the infiltration rate of 22 immune cell types. Each column represented a sample, with different colors indicating the infiltration of different immune cells
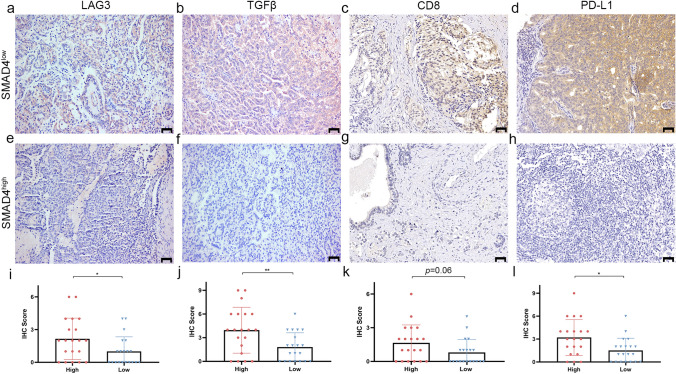
To verify the correlation between SMAD4 and immune response, we compared the expression level of LAG3, TGFβ, CD8, and PD-L1 between low and high expression of SMAD4 in 20 PCa tumor tissues from the FUSCC cohort. As shown in Fig. 6, the low SMAD4 expression group exhibited significantly high levels of LAG3 (p < 0.05), TGFβ (p < 0.001), and PD-L1 (p < 0.05) compared with the high SMAD4 expression group. Although the difference in the CD8 expression was not significant (p = 0.06), there was a trend toward a high expression of CD8 in the low SMAD4 expression group.
Fig. 6.
External validation of immune response-related moleculars in FUSCC cohort. Representive IHC staining images of immune response-related moleculars in SMAD4 low expression group (a–d) and SMAD4 high expression group (e–h). Scale bar: 50 μm. Low SMAD4 expression group exhibited significantly high level of LAG3 (i) (p < 0.05), TGFβ (j) (p < 0.001) and PD-L1 (l) (p < 0.05) compared with the high SMAD4 expression group. There was a trend towards a high expression of CD8 (k) in low SMAD4 expression group
Significantly Involved Pathways and Differential Expressed Genes (DEGs)
As shown in Fig. 7a, the heat map revealed the most significant 100 genes positively and negatively associated with risk score. GSEA results revealed that significant pathways are mainly involved in antigen processing and presentation, regulation of stem cell differentiation, and response to type I interferon (Fig. 7b–d). Protein–protein interaction network of DEGs was constructed and the hub genes in the network were identified, including XIRP1, MYF6, CSRP3, MYOM3, TRON, MYL1, UNC45B, CHRND, TNNI2, TNNC1, and CHRNA1 (Fig. 8a, b). As shown in Fig. 8c, d, functional enrichment analysis demonstrated that hub genes were mostly enriched in cilium movement, axoneme, structural constituent of muscle, and steroid hormone biosynthesis signaling pathway. Details are summarized in Table 2.
Fig. 7.
Potential significantly changed pathways associated with SMAD4 expression level. (a) Heat map displayed the top 100 significant genes positively and negatively correlated with risk score. Significant pathways mainly involved in (b) response to antigen, (c) processing and presentation and (d) regulation of stem cell differentiation
Fig. 8.
Functional enrichment analysis of the DEGs. (a, b) The protein-protein interaction network of DEGs was constructed and the interaction network of hub genes was visualized. (c, d) Functional enrichment analyses of hub genes were visualized in bar plot. Significant genes were significantly enriched in cilium movement, axoneme, structural constituent of muscle and steroid hormone biosynthesis signaling pathway
Table 2.
GO and KEGG pathways enrichment analysis of hub genes
| Term | Description | Count in gene set | p value |
|---|---|---|---|
| GO:0003341 | Cilium movement | 5 | 8.35E−06 |
| GO:0003009 | Skeletal muscle contraction | 4 | 2.77E−05 |
| GO:0036158 | Outer dynein arm assembly | 3 | 4.23E−05 |
| GO:0035082 | Axoneme assembly | 3 | 0.002 |
| GO:0097014 | Ciliary plasm | 6 | 2.36E−05 |
| GO:0005858 | Axonemal dynein complex | 3 | 6.17E−05 |
| GO:0008307 | Structural constituent of muscle | 3 | 0.001 |
| GO:0004866 | Endopeptidase inhibitor activity | 5 | 0.001 |
| GO:0030414 | Peptidase inhibitor activity | 5 | 0.001 |
| hsa00140 | Steroid hormone biosynthesis | 3 | 0.001 |
| hsa00830 | Retinol metabolism | 3 | 0.001 |
| hsa00982 | Drug metabolism—cytochrome P450 | 3 | 0.001 |
GO gene ontology, KEGG Kyoto Encyclopedia of Genes and Genomes
The real biological role of SMAD4 in tumorigenesis is well recognized. SMAD4 is mainly involved in the TGFβ and BMP signaling pathways as a central mediator. The canonical TGF-β/SMAD4 signaling pathway plays a tumor suppressive role at the early stages, mainly by inducing apoptosis and cell cycle arrest. SMAD4 plays a transfer role in TGFβ/SMAD4 signaling pathway, where it binds to SMAD2/3 and accumulated in the nucleus (Massagué et al. 2000). The nucleus localized SMAD2/3/4 complex promotes the expression of target genes, such as pro-apoptotic genes DAPK1, BCL2L11, and KLF10, to induce apoptosis (Tachibana et al. 1997). Besides, it has been reported that some CDK inhibitors, like p15 (CDKN2B), p21 (CDKN1A), and p27 (CDKN1B), were involved in the cell cycle arrest. TGF-β cannot induce the expression of CDK inhibitors in PCa cells, which lack of SMAD4, with the result that cell growth is out of control and tumorigenesis is promoted (Derynck et al. 2001) (Supplementary Fig. 2). Correlation analysis also indicated that the expression of SMAD4 was positively correlated with the expression of CDKN1A, CDKN1B, CDKN2B, DAPK1, BCL2L11, and KLF10 (Supplementary Fig. 3). Besides, the expression of CDKN1A (p = 0.0086), CDKN1B (p = 0.0056), CDKN2B (p = 0.0015), and KLF10 (p = 0.0013) was significantly lower in tumor tissues than that in normal renal tissues, but DAPK1 revealed high expression in tumor tissue (p < 0.0001), and the expression of BCL2L11 (p = 0.9493) showed no significant difference between tumor and normal tissues (Supplementary Fig. 3). Next, to explore the prognostic implication of the molecules involved in the TGFβ/SMAD4 signaling pathway, we performed the survival analysis among patients from the TCGA cohort. The results indicated that low expression of CDKN1A (p = 0.0029) and high expression of CDKN2B (p = 0.0029) and DAPK1 (p = 0.02) were significantly correlated with the poor progression-free interval, and low expression of CDKN1A (p = 0.02) and high expression of DAPK1 (p = 0.0023) were significantly correlated with poor OS (Supplementary Fig. 3), suggesting that CDKN1A and DAPK1 may be the potential prognostic biomarkers and may be used as a therapeutic target of PCa. Subsequently, we analyzed the potential function of the molecules involved in the TGFβ/SMAD4 signaling pathway based on the single-cell dataset. We found that CDKN2B, DAPK1, and BCL2L11 were extremely low expressed in most of the PCa cells, and CDKN1A, CDKN1B, and KLF10 were differentially expressed in different PCa cells (Supplementary Fig. 4). Besides, we found that high expression of CDKN1A (r = 0.35, p < 0.01) and KLF 10 (r = 0.25, p < 0.05) were significantly correlated with invasion-related function state, and high expression of CDKN1B (r = 0.27, p < 0.01) and DAPK1 (r = 0.29, p < 0.05) were significantly correlated with hypoxia-related function state and apoptosis-related function state, respectively (Supplementary Fig. 4). The expression of CDKN2B and BCL2L11 did not detect in any significant function state, possibly due to the extremely low expression of these genes in most of the PCa cells (Supplementary Fig. 4).
Discussion
In our study, proteomic analysis and survival benefits were evaluated to investigate the potential protein biomarkers with prognostic value in PCa patients. RAD50 and SMAD4 were identified as the most significant proteins with prognostic value using proteomic analysis, and as expected, both of SMAD4 and RAD50 were successfully verified in validation cohorts. In the present study, high expression of RAD50 and low expression of SMAD4 were significantly correlated with poor prognosis in PCa patients, suggesting that detection of the expression of these proteins may help to assess the prognosis of patients with PCa.
In several human malignancies, as an essential regulative complex, MRN plays an important role in DNA damage repairing and mitotic process. Hence, genome stability during cell division and proliferation depends to a certain extent on the stable expression of RAD50 in the MRN complex (Bosch et al. 2003). The frequent molecular mutation of the MRN complex provides increased predisposition for malignancies, and RAD50 also responds to tumors with microsatellite instability (Dzikiewicz-Krawczyk 2008; Gao et al. 2008). A previous study indicated that RAD50 mutation did not increase the risk of breast cancer, but the patients with RAD50 mutation had a poor survival outcome, indicating that RAD50 has potential significance in tumor treatment (Fan et al. 2018). Moreover, several studies also demonstrated that elevated expression of MRN complex is also associated with tumor recurrence and chemoresistance (Ho et al. 2018; Kuo et al. 2012; Söderlund et al. 2007). However, the deleterious mutation of RAD50 plays an essential role in tumorigenesis, but the potential prognostic value of RAD50 protein expression in PCa has rarely been reported. In the present study, proteomic analysis in large-scale datasets with survival information supported our hypothesis. The high expression of RAD50 in PCa significantly correlated with unfavorable OS, which is consistent with our previous study (Xu et al. 2020).
TGF- β/SMAD4 signaling pathway transduce the extracellular signal directly to the nucleus. SMAD4 is involved in the TGF- β signaling pathway and is also responsible for several transcriptional regulations of target genes, including differentiation, proliferation, and cancer progression. TGF- β protein inhibits tumor growth through apoptosis and cell cycle arrest in the early stage of tumorigenesis. However, due to the reason that tumor cells become not being sensitive to it, secreted TGF-β protein enhances the immunosuppressive effect of tumor cells and promotes tumor growth (Shi and Massagué 2003). Several studies have indicated the SMAD4 mutation is positively related to approximately a half of pancreatic cancer whether a homozygous deletion or intragenic inactivating mutations (Hahn et al. 1995, 1996). Moreover, the same mutation has also been detected in other tumors, including colorectal cancer, cholangiocarcinoma, gastric cancer as well as prostate cancer (Zhao et al. 2018). However, a previous study indicated that SMAD4 mutations were rarely detected in PCa, but, as a novel marker of disease, SMAD4 methylation of promotor was generally found, which may down-regulate the expression of SMAD4 (Aitchison et al. 2008). In our study, the proteomic analysis demonstrated that low expression of SMAD4 protein in PCa was significantly correlated with unfavorable outcomes, and IHC staining also indicated significant differential expression of SMAD4 protein in PCa and normal tissues. This finding provides prognostic biomarkers and points out an underlying tumorigenesis mechanism for PCa.
The strength of our study is that we attempt to investigate the potential prognostic protein biomarkers using quantitative proteomic analysis for PCa patients. We also investigated the significant signaling pathway, differential gene expressions, PPI network, and immune response of PCa. To verify our results, we detected the protein expression and survival outcomes in PCa patients from the FUSCC, which was consistent with the results of proteomic analysis based on the public dataset. However, the limitations of this study are as follows. Due to the natural limitation of retrospective analysis, our results need to be further verified in multicenter or prospective studies. Moreover, our studies indicated the potential prognostic value of candidate proteins, but the underlying mechanism of these biomarkers did not clarify. However, whether the therapeutic effect can be achieved through our suggestion is still urgently needed to be verified both in vitro and in vivo.
Conclusion
This study constituted a large-scale PCa proteomic analysis and identified protein landscape with prognostic value of PCa from reverse-phase protein arrays. Our findings distinguished RAD50 and SMAD4 as important protein biomarkers with prognostic value and immune response, and outperforming prediction model IPRPs were established with high predictive value, leading to better clinical strategies for PCa patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig 1. Proteins associated with significantly prognostic proteins. (a) 13 proteins significantly correlated with the expression of RAD50, of which PEA15_pS116 revealed the highest positive correlation with 0.395 correlation coefficient. (b) 6 proteins significantly correlated with the expression of SMAD4, and SNAL was correlated with SMAD4 with the highest correlation coefficient 0.394 (TIF 5156 KB)
Supplementary Fig 2. TGFβ/SMAD4 signaling pathway and its roles. In the cytoplasm,SMAD4 plays a transfer role in TGFβ/SMAD4 signaling pathway, where it binds to SMAD2/3 and translocated to the nucleus. The nucleus localized SMAD2/3/4 complex promote the expression of target genes, such as pro-apoptotic genesDAPK, BCL2L11 and KLF10, to induce apoptosis. Besides, SMAD2/3/4 complex combine with FOXO and promote the transcription of CKD inhibitors, such as p15, p21, p27, to induce the cell cycle arrest (JPG 78 KB)
Supplementary Fig 3. Correlations of SMAD4 and TGFβ/SMAD4 signaling pathway involved genes. (a) Correlation of CDKN1A and SMAD4; Expression of CDKN1A between tumor tissues and normal prostate tissues; Progression-free interval and overall survival between low- and high-expression of CDKN1A. (b) Correlation of CDKN1B and SMAD4; Expression of CDKN1B between tumor tissues and normal prostate tissues; Progression-free interval and overall survival between low- and high-expression of CDKN1B. (c) Correlation of CDKN2B and SMAD4; Expression of CDKN2B between tumor tissues and normal prostate tissues; Progression-free interval and overall survival between low- and high-expression of CDKN2B. (d) Correlation of DAPK1 and SMAD4; Expression of DAPK1 between tumor tissues and normal prostate tissues; Progression-free interval and overall survival between low- and high-expression of DAPK1. (e) Correlation of BCL2L11 and SMAD4; Expression of BCL2L11 between tumor tissues and normal prostate tissues; Progression-free interval and overall survival between low- and high-expression of BCL2L11. (f) Correlation of KLF10 and SMAD4; Expression of KLF10 between tumor tissues and normal prostate tissues; Progression-free interval and overall survival between low- and high-expression of KLF10 (TIF 14067 KB)
Supplementary Fig 4. Expressions and functions of TGFβ/SMAD4 signaling pathway involved genes in single cells. Expression of (a) CKDN1A, (b) CDKN1B, (c) CDKN2B, (d) DAPK1, (e) BCL2L11 and (f) KLF10 in PCa single cells, and correlation with 14 function state (TIF 20625 KB)
Acknowledgements
We thank to researchers for providing TCGA, TCPA, and GEO datasets online, and for patients enrolled from the FUSCC cohort.
Abbreviations
- PCa
Prostate cancer
- TCPA
The Cancer Proteome Atlas
- TCGA
The Cancer Genome Atlas
- IPRPs
Integrated prognosis-related proteins
- DEGS
Differential expression genes
- RAD50
Double-strand break repair protein RAD50
- SMAD4
SMAD family member 4
- PSA
Prostate-specific antigen
- MRN
MRE11A-RAD50-NBS1 complex
- DSBs
Double-strand breaks
- TGF- β
Transforming growth factor-β
- CRCs
Colorectal cancers
- GEO
Gene Expression Omnibus
- ROC
Receiver operating characteristic
- AUC
Area under curve
- FUSCC
Fudan University Shanghai Cancer Center
- IHC
Immunohistochemistry
- GSEA
Gene set enrichment analysis
- FDR
False discovery rate
- KEGG
Kyoto Encyclopedia of Genes And Genomes
- MF
Molecular functions
- BP
Biological processes
- CC
Cellular components
- OS
Overall survival
- HPA
Human Protein Atlas
Authors’ Contributions
Conceptualization: AA, S-XZ, XT, W-HX, H-LZ. Data curation and formal analysis: AA, S-XZ, XT, W-HX, YW, MP and W-YW. Funding acquisition: Y-YQ, H-LZ and D-WY. Investigation and methodology: AA, S-XZ, XT and W-HX. Resources and software: AA, XT, W-HX, YW, MP and W-YW. Supervision: G-HS, Y-YQ, H-LZ and D-WY. Validation and visualization: AA, XT, W-HX and YW. Original draft: AA, S-XZ, XT and W-HX. Editing: G-HS, Y-YQ, H-LZ and D-WY.
Funding
This work is supported by National Key Research and Development Project (No. 2019YFC1316000) and National Natural Science Foundation of China (No. 81772706 and No. 81802525).
Availability of Data and Materials
The datasets analyzed in this study were obtained from the corresponding author upon reasonable request or from open-access online databases.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Study procedures were approved by Fudan University Shanghai Cancer Center (Shanghai, China) included in this research (ID: 050432-4-1805C).
Consent to participate
Written informed consents were acquired from open-access online databases.
Consent for publication
The written informed consent was obtained from the online public database and corresponding authors.
Footnotes
Aihetaimujiang Anwaier, Shu-Xuan Zhu, Xi Tian, and Wen-Hao Xu contributed equally.
Contributor Information
Yuan-Yuan Qu, Email: quyy1987@163.com.
Hai-Liang Zhang, Email: zhanghl918@alu.fudan.edu.cn.
Ding-Wei Ye, Email: dwyelie@163.com.
References
- Aitchison AA, Veerakumarasivam A, Vias M, Kumar R, Hamdy FC, Neal DE, Mills IG. Promoter methylation correlates with reduced Smad4 expression in advanced prostate cancer. Prostate. 2008;68(6):661–674. doi: 10.1002/pros.20730. [DOI] [PubMed] [Google Scholar]
- Aslam B, Basit M, Nisar MA, Khurshid M, Rasool MH. Proteomics: technologies and their applications. J Chromatogr Sci. 2017;55(2):182–196. doi: 10.1093/chromsci/bmw167. [DOI] [PubMed] [Google Scholar]
- Bandettini WP, Kellman P, Mancini C, Booker OJ, Vasu S, Leung SW, Wilson JR, Shanbhag SM, Chen MY, Arai AE. MultiContrast Delayed Enhancement (MCODE) improves detection of subendocardial myocardial infarction by late gadolinium enhancement cardiovascular magnetic resonance: a clinical validation study. J Cardiovasc Magn Reson. 2012;14(1):83. doi: 10.1186/1532-429x-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012(45):152–156. doi: 10.1093/jncimonographs/lgs035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61(6):1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- de Kroon LM, Narcisi R, van den Akker GG, Vitters EL, Blaney Davidson EN, van Osch GJ, van der Kraan PM. SMAD3 and SMAD4 have a more dominant role than SMAD2 in TGFβ-induced chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Sci Rep. 2017;7:43164. doi: 10.1038/srep43164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- Dzikiewicz-Krawczyk A. The importance of making ends meet: mutations in genes and altered expression of proteins of the MRN complex and cancer. Mutat Res. 2008;659(3):262–273. doi: 10.1016/j.mrrev.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Fan C, Zhang J, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Xie Y. RAD50 germline mutations are associated with poor survival in BRCA1/2-negative breast cancer patients. Int J Cancer. 2018;143(8):1935–1942. doi: 10.1002/ijc.31579. [DOI] [PubMed] [Google Scholar]
- Fleming NI, Jorissen RN, Mouradov D, Christie M, Sakthianandeswaren A, Palmieri M, Day F, Li S, Tsui C, Lipton L, Desai J, Jones IT, McLaughlin S, Ward RL, Hawkins NJ, Ruszkiewicz AR, Moore J, Zhu HJ, Mariadason JM, Burgess AW, Busam D, Zhao Q, Strausberg RL, Gibbs P, Sieber OM. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Cancer Res. 2013;73(2):725–735. doi: 10.1158/0008-5472.Can-12-2706. [DOI] [PubMed] [Google Scholar]
- Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, Jensen LJ. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Zhang H, Arbman G, Sun XF. RAD50/MRE11/NBS1 proteins in relation to tumour development and prognosis in patients with microsatellite stable colorectal cancer. Histol Histopathol. 2008;23(12):1495–1502. doi: 10.14670/hh-23.1495. [DOI] [PubMed] [Google Scholar]
- Hahn SA, Seymour AB, Hoque AT, Schutte M, da Costa LT, Redston MS, Caldas C, Weinstein CL, Fischer A, Yeo CJ. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res. 1995;55(20):4670–4675. [PubMed] [Google Scholar]
- Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271(5247):350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- Ho V, Chung L, Singh A, Lea V, Abubakar A, Lim SH, Ng W, Lee M, de Souza P, Shin JS, Lee CS. Overexpression of the MRE11-RAD50-NBS1 (MRN) complex in rectal cancer correlates with poor response to neoadjuvant radiotherapy and prognosis. BMC Cancer. 2018;18(1):869. doi: 10.1186/s12885-018-4776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo KT, Chou TY, Hsu HS, Chen WL, Wang LS (2012) Prognostic significance of NBS1 and Snail expression in esophageal squamous cell carcinoma. Ann Surg Oncol 19. 10.1245/s10434-011-2043-2 [DOI] [PubMed]
- Li X, Li J, Cai Y, Peng S, Wang J, Xiao Z, Wang Y, Tao Y, Li J, Leng Q, Wu D, Yang S, Ji Z, Han Y, Li L, Gao X, Zeng C, Wen X. Hyperglycaemia-induced miR-301a promotes cell proliferation by repressing p21 and Smad4 in prostate cancer. Cancer Lett. 2018;418:211–220. doi: 10.1016/j.canlet.2018.01.031. [DOI] [PubMed] [Google Scholar]
- Martin RM, Donovan JL, Turner EL, Metcalfe C, Young GJ, Walsh EI, Lane JA, Noble S, Oliver SE, Evans S, Sterne JAC, Holding P, Ben-Shlomo Y, Brindle P, Williams NJ, Hill EM, Ng SY, Toole J, Tazewell MK, Hughes LJ, Davies CF, Thorn JC, Down E, Davey Smith G, Neal DE, Hamdy FC. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319(9):883–895. doi: 10.1001/jama.2018.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103(2):295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- Miyaki M, Iijima T, Konishi M, Sakai K, Ishii A, Yasuno M, Hishima T, Koike M, Shitara N, Iwama T, Utsunomiya J, Kuroki T, Mori T. Higher frequency of Smad4 gene mutation in human colorectal cancer with distant metastasis. Oncogene. 1999;18(20):3098–3103. doi: 10.1038/sj.onc.1202642. [DOI] [PubMed] [Google Scholar]
- Myler LR, Gallardo IF, Soniat MM, Deshpande RA, Gonzalez XB, Kim Y, Paull TT, Finkelstein IJ. Single-molecule imaging reveals how Mre11-Rad50-Nbs1 initiates DNA break repair. Mol Cell. 2017;67(5):891–898.e894. doi: 10.1016/j.molcel.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60(4):547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Potosky AL, Miller BA, Albertsen PC, Kramer BS. The role of increasing detection in the rising incidence of prostate cancer. JAMA. 1995;273(7):548–552. doi: 10.1001/jama.273.7.548. [DOI] [PubMed] [Google Scholar]
- Rahim MA, Rahim ZH, Ahmad WA, Hashim OH. Can saliva proteins be used to predict the onset of acute myocardial infarction among high-risk patients? Int J Med Sci. 2015;12(4):329–335. doi: 10.7150/ijms.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. J Nucleic Acids Res. 2015;43(7):7. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues DN, Butler LM, Estelles DL, de Bono JS. Molecular pathology and prostate cancer therapeutics: from biology to bedside. J Pathol. 2014;232(2):178–184. doi: 10.1002/path.4272. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- Situ Y, Chung L, Lee CS, Ho V. MRN (MRE11-RAD50-NBS1) complex in human cancer and prognostic implications in colorectal cancer. Int J Mol Sci. 2019 doi: 10.3390/ijms20040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund K, Stål O, Skoog L, Rutqvist LE, Nordenskjöld B, Askmalm MS. Intact Mre11/Rad50/Nbs1 complex predicts good response to radiotherapy in early breast cancer. Int J Radiat Oncol Biol Phys. 2007;68(1):50–58. doi: 10.1016/j.ijrobp.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana I, Imoto M, Adjei PN, Gores GJ, Subramaniam M, Spelsberg TC, Urrutia R. Overexpression of the TGFbeta-regulated zinc finger encoding gene, TIEG, induces apoptosis in pancreatic epithelial cells. J Clin Investig. 1997;99(10):2365–2374. doi: 10.1172/jci119418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau TM, Grambsch PM. Modeling survival data: extending the cox model. New York: Springer; 2000. [Google Scholar]
- van den Bosch M, Bree RT, Lowndes NF. The MRN complex: coordinating and mediating the response to broken chromosomes. EMBO Rep. 2003;4(9):844–849. doi: 10.1038/sj.embor.embor925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völkening L, Vatselia A, Asgedom G, Bastians H, Lavin M, Schindler D, Schambach A, Bousset K, Dörk T. RAD50 regulates mitotic progression independent of DNA repair functions. FASEB J. 2020;34(2):2812–2820. doi: 10.1096/fj.201902318R. [DOI] [PubMed] [Google Scholar]
- Walldén K, Nyman T, Hällberg BM. SnoN stabilizes the SMAD3/SMAD4 protein complex. Sci Rep. 2017;7:46370. doi: 10.1038/srep46370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2016. [Google Scholar]
- Xu WH, Wang J, Sheng HY, Qu YY, Wang HK, Zhu Y, Shi GH, Zhang HL, Ye DW. Prognostic implication and functional annotations of Rad50 expression in patients with prostate cancer. J Cell Biochem. 2020;121:3124–3134. doi: 10.1002/jcb.29580. [DOI] [PubMed] [Google Scholar]
- Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Mishra L, Deng CX. The role of TGF-β/SMAD4 signaling in cancer. Int J Biol Sci. 2018;14(2):111–123. doi: 10.7150/ijbs.23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig 1. Proteins associated with significantly prognostic proteins. (a) 13 proteins significantly correlated with the expression of RAD50, of which PEA15_pS116 revealed the highest positive correlation with 0.395 correlation coefficient. (b) 6 proteins significantly correlated with the expression of SMAD4, and SNAL was correlated with SMAD4 with the highest correlation coefficient 0.394 (TIF 5156 KB)
Supplementary Fig 2. TGFβ/SMAD4 signaling pathway and its roles. In the cytoplasm,SMAD4 plays a transfer role in TGFβ/SMAD4 signaling pathway, where it binds to SMAD2/3 and translocated to the nucleus. The nucleus localized SMAD2/3/4 complex promote the expression of target genes, such as pro-apoptotic genesDAPK, BCL2L11 and KLF10, to induce apoptosis. Besides, SMAD2/3/4 complex combine with FOXO and promote the transcription of CKD inhibitors, such as p15, p21, p27, to induce the cell cycle arrest (JPG 78 KB)
Supplementary Fig 3. Correlations of SMAD4 and TGFβ/SMAD4 signaling pathway involved genes. (a) Correlation of CDKN1A and SMAD4; Expression of CDKN1A between tumor tissues and normal prostate tissues; Progression-free interval and overall survival between low- and high-expression of CDKN1A. (b) Correlation of CDKN1B and SMAD4; Expression of CDKN1B between tumor tissues and normal prostate tissues; Progression-free interval and overall survival between low- and high-expression of CDKN1B. (c) Correlation of CDKN2B and SMAD4; Expression of CDKN2B between tumor tissues and normal prostate tissues; Progression-free interval and overall survival between low- and high-expression of CDKN2B. (d) Correlation of DAPK1 and SMAD4; Expression of DAPK1 between tumor tissues and normal prostate tissues; Progression-free interval and overall survival between low- and high-expression of DAPK1. (e) Correlation of BCL2L11 and SMAD4; Expression of BCL2L11 between tumor tissues and normal prostate tissues; Progression-free interval and overall survival between low- and high-expression of BCL2L11. (f) Correlation of KLF10 and SMAD4; Expression of KLF10 between tumor tissues and normal prostate tissues; Progression-free interval and overall survival between low- and high-expression of KLF10 (TIF 14067 KB)
Supplementary Fig 4. Expressions and functions of TGFβ/SMAD4 signaling pathway involved genes in single cells. Expression of (a) CKDN1A, (b) CDKN1B, (c) CDKN2B, (d) DAPK1, (e) BCL2L11 and (f) KLF10 in PCa single cells, and correlation with 14 function state (TIF 20625 KB)
Data Availability Statement
The datasets analyzed in this study were obtained from the corresponding author upon reasonable request or from open-access online databases.