Abstract
Chalcones are natural substances found in the metabolism of several botanical families. Their structure consists of 1,3-diphenyl-2-propen-1-one and they are characterized by having in their chains an α, β-unsaturated carbonyl system, two phenol rings and a three-carbon chain that unites them. In plants, Chalcones are mainly involved in the biosynthesis of flavonoids and isoflavonoids through the phenylalanine derivation. This group of substances has been shown to be a viable alternative for the investigation of its antibacterial potential, considering the numerous biological activities reported and the increase of the microbial resistance that concern global health agencies. Staphylococcus aureus is a bacterium that has stood out for its ability to adapt and develop resistance to a wide variety of drugs. This literature review aimed to highlight recent advances in the use of Chalcones and derivatives as antibacterial agents against S. aureus, focusing on research articles available on the Science Direct, Pub Med and Scopus data platforms in the period 2015–2021. It was constructed informative tables that provided an overview of which types of Chalcones are being studied more (Natural or Synthetic); its chemical name and main Synthesis Methodology. From the analysis of the data, it was observed that the compounds based on Chalcones have great potential in medicinal chemistry as antibacterial agents and that the molecular skeletons of these compounds as well as their derivatives can be easily obtained through substitutions in the A and B rings of Chalcones, in order to obtain the desired bioactivity. It was verified that Chalcones and derivatives are promising agents for combating the multidrug resistance of S. aureus to drugs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03398-7.
Keywords: Bioactivity, Multi-resistance, Efflux pump, Structure–activity relationship
Introduction
The emergence and availability of antibiotics have revolutionized the treatment of diseases in the 20th century, but this achievement is threatened due to the development of resistance of microorganisms, which reduces the effectiveness of several classes of antibiotics for clinical use (Dan and Dai 2019). There are several factors by which microorganisms acquire or develop resistance to a drug, the most predominant among them is through a process known as natural selection, in which the most sensitive microorganisms to the drug are eliminated and those that manage to survive the action of the antimicrobial develop resistance (Loureiro et al. 2016). Bacterial resistance has become a subject of global concern, and for this reason, more and more researchers around the world are engaged in the search for natural or synthetic products with antibacterial potential (Oliveira et al. 2020; Rezende-Junior et al. 2020).
It is estimated that approximately 700,000 deaths are caused by infections of drug-resistant bacteria per year and this number could reach 10 million deaths per year by 2050 if urgent measures are not taken, according to the report of Neill (2016). In this perspective, the microorganism that has most concerned global health bodies is the gram-positive bacterium Staphylococcus aureus. This pathogenic bacterium belongs to the group of Coccus and is responsible for several diseases that affect humans. It is currently one of the pathogens of greatest clinical interest due to its high ability to colonize and acquire resistance to multiple drugs (Multiple Drug Resistance—MDR) (Grace and Fetsch 2017). Sophisticated resistance mechanisms were developed and acquired by this species after years of exposure to antibacterials (Guo et al. 2020). Enzyme synthesis; Reduction in the absorption of endogenous molecules; Molecular modification of the target; Acquisition of genetic material through mobile genetic elements, such as plasmids; and efflux pumps are examples of resistance mechanisms present in S. aureus (Abushaheen et al. 2020).
Natural compounds are considered a good alternative for the development of new antibiotics because they are products of natural selection, shaped by evolution to interact with cellular targets with high efficiency and selectivity, in addition to presenting important properties that allows interaction with different bacterial cellular targets, preventing cell survival after contact with the antimicrobial (Wright 2017; Rossiter et al. 2017).
Human civilization has long used natural products as the main source of products with therapeutic potential. Currently, more than half of clinical drugs come from natural products and their synthetic derivatives, according to the United States Food and Drug Administration (FDA), which analyzed in detail the introduction of new drugs in the clinic until 2014 (Dan and Dai 2019; Newman and Cragg 2016).
In this sense, Chalcones have gained prominence in medicinal chemistry due to their important pharmacological properties. Chalcones are natural substances present in the secondary metabolism of several botanical species, including the so-called medicinal plants. Medicinal plants are considered by the WHO as any plant that presents in its metabolism compounds with some therapeutic potential (Who 2021). In this sense, several botanical species that have chalcones in their composition and present therapeutic effects of clinical interest are reported in the literature (Ferreira et al. 2018). The Ficus microcarpa species presents a high content of chalcones in its roots and has already had its medicinal and antibacterial properties investigated and proven (Díaz-Tielas 2016; Kalaskar and Surana 2012). They can also be found in the leaves and fruits of species of the genus Artocarpus that is widely used for the treatment or prevention of inflammation, malaria, and vitiligo, mainly in tropical Asian regions (Pereira and Kaplan 2013).
Chemically, Chalcones are known as α, β-unsaturated ketones with a structure consisting in 1,3-diphenyl-2-propen-1-one and are the main precursors of Flavonoids and Isoflavonoids (Fig. 1) (Ferreira et al. 2018). This group of substances has drawn attention for presenting several biological activities of clinical interest, such as antitumor, anti-inflammatory, anti-oxidant and antimicrobial activities, which demonstrates the importance of these compounds (Rashid et al. 2019; Matos et al. 2015).
Fig. 1.
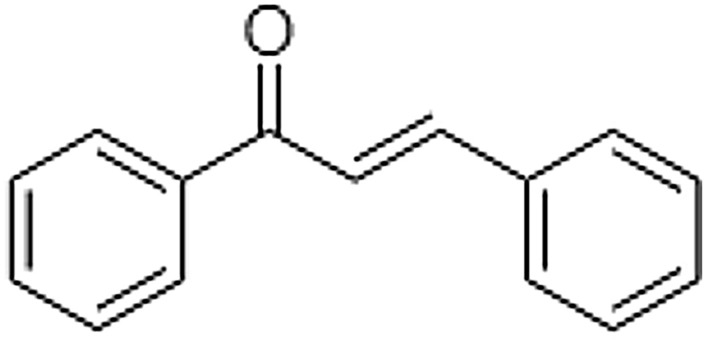
Fundamental structure of chalcones.
Source: Author (2022)
It is important to emphasize that Chalcones are considered important scaffolds for the synthesis of new drugs through the manipulation of their aromatic rings, which can potentiate already proven biological activities or lead to the discovery of new bioactivities, including antibacterial activity (Bitencourt et al. 2019). These compounds are currently being explored with published studies about synthesis, molecular targets and biological activities (Dan and Dai 2019). Within this context, the present review aims to highlight recent advances in the use of natural and synthetic Chalcones as well as their derivatives used as antibacterial agents against S. aureus, focusing on research articles published in the period of 2015–2021.
Methodology
This literature review was carried out by consulting scientific articles available in different databases. All stages of collection, screening and analysis of the articles selected for this review meet a guiding question that follows systematic methods. The research started in October 2021 and ended in January 2022. To achieve the research proposal, the following methodological procedures were applied.
Database
The data platforms used for the collection of material that led to the realization of this review were: (1) Science Direct (https://www.sciencedirect.com); (2) Pub Med (https://pubmed.ncbi.nlm.nih.gov/); (3) Scopus (https://www-periodicos-capes-gov-br.ezl.periodicos.capes.gov.br). The research was carried out with the application of specific descriptors and filters.
Descriptors and filters used
Wide-descriptors were selected in order to compile the largest number of studies and avoid missing out on relevant research to be included. Thus, the descriptors: “Chalconas AND Staphylococcus aureus” were inserted in a combined way in the databases cited above. To better meet the originality of studies on antibacterial activity of Chalcones on S. aureus, were used filters such as: Type of article, Year of publication and Language.
Eligibility criteria
In terms of data mining and the established eligibility criteria, the following inclusion criteria were defined: Original research articles focusing on the investigation of the antibacterial potential of Chalcones of natural and synthetic origin and their derivatives on S. aureus bacteria; in vitro studies; English, Portuguese and Spanish Language; Studies published in the period 2015–2021. The exclusion criteria were: Course Completion Works; Dissertations; Theses; Annals of events; Case reports; in silico and in vivo studies; Book; Bibliographic reviews; Duplicates; Incomplete works, with unavailable access or that do not fit the proposed theme.
Analytical approach
The articles found went through three stages of screening in order to select those that fit the proposal of this review. The first step was defined as general prospecting, where all articles found on each platform were analyzed based on the descriptors and filters used. The three screening processes were based on reading all filtered articles as well as eliminating duplicates found on the same platform and among the three databases.
After analyzing the articles, the analytical approach used consisted of producing informative tables containing the selected articles with the following information: Chemical name of the Chalcone used; Chalcone type (natural or synthetic); Synthesis method; Bacterial Strain, MIC/Inhibition Zone, Activity and Representative Reference. Tabulations were organized in Microsoft Word software. Finally, a descriptive analysis of the bibliographic sample was carried out, accompanied by a discussion on the aspects addressed by the included studies.
Results and discussion
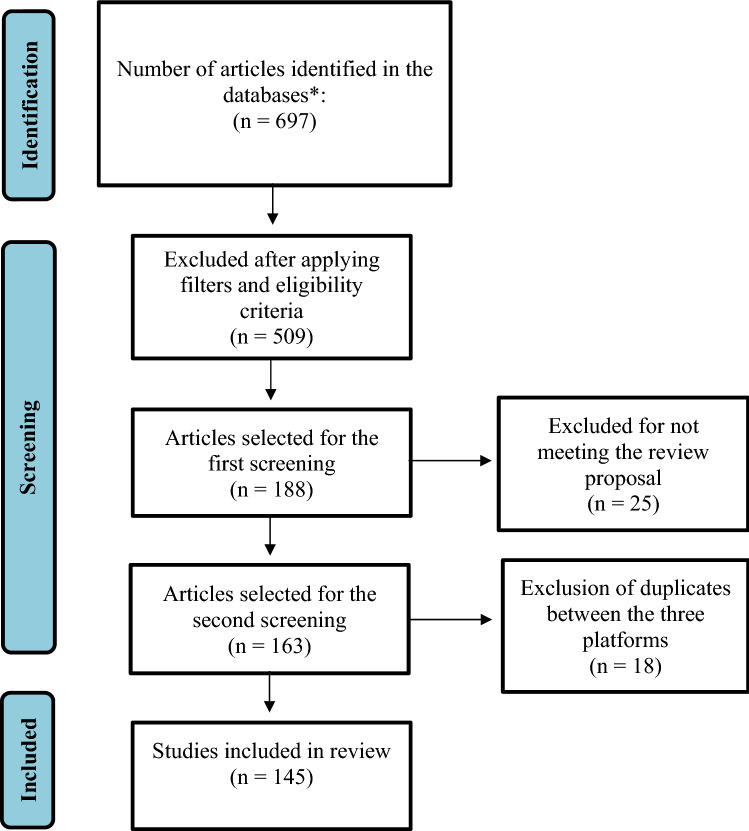
The content of the articles researched allowed us to evaluate recent developments in scientific research involving the use of natural and synthetic Chalcones and their derivatives in the fight against infections caused by S. aureus. The data obtained from the three search platforms resulted in 697 articles using the descriptors and filters as a basis for the research (general prospection). With the application of pre-established eligibility criteria, this step being considered as the first screening, this number was reduced to 188 viable articles for analysis.
The second screening was carried out by reading the articles selected in the first screening and including those that met the proposal of this review, excluding duplicate articles within the same platform. The third and last step was the analysis and exclusion of duplicates between platforms. Table 1 shows in detail the number of articles obtained at each stage of collection.
Table 1.
Number of articles prospected on each platform visited and the screening processes
| Database | General prospection | First screening | Second screening | Exclusion of duplicates |
|---|---|---|---|---|
| Science direct | 320 | 33 | 30 | 18 common articles among the three platforms |
| Pub Med | 100 | 58 | 48 | |
| Scopus | 277 | 97 | 85 | |
| Sum | 697 | 188 | 163 | 145 |
The PRISMA flowchart was assembled to demonstrate the process of metabolizing the results that includes the processes of inclusion and exclusion of articles for each step of the search until reaching the final number of studies considered for this study (Fig. 2).
Fig. 2.
- PRISMA flowchart of the analytical steps for building the review.
Source: Author (2022)
In Tables 2 and 3 below, containing all the articles analyzed and selected to compose the present review, it is possible to observe that 84.83% of the collected studies report the work with synthetic Chalcones to evaluate the antibacterial activity against S. aureus while the natural Chalcones represent 15.17% of the works. This demonstrates the interest of research in the investigation of new compounds from natural substances in order to potentiate known bioactivities or discover new activities, whether in reversing bacterial resistance or reinforcing the antibiotic effect of existing drugs.
Table 2.
Synthesis matrix of articles collected in a literature review that report the antibacterial potential of natural Chalcones against S. aureus
| Chalcone | Type | S. aureus strain(s) | MIC/Inhibition Zone | Activity | Quotes |
|---|---|---|---|---|---|
|
4′-Hydroxy-3–4-dimethoxy-chalcone (1); 3′-Hydroxy-3-acetate, 4-methoxy-chalcone (3); 3′,4′-dihydroxy, 3,4,4′-trimethoxy-chalcone (4); 3,4-dimethoxy-chalcone (5) |
Nature Isolated from Arrabidaea brachypoda |
ATCC 25,923; IS-58; RN-4220; SA1199-B; K2068; K4414; K4100 | 1024; ≥ 1024 μg/mL | Inactive for antibacterial activity | Rezende-JúNIOR et al. (2020) |
| 3′,4′-dihydroxy, 3,4,4′-trimethoxy-chalcone (4) |
Nature Isolated from Arrabidaea brachypoda |
SA1199-B |
16 µg / mL 4X |
NorA efflux pump inhibitor | Rezende-JúNIOR et al. (2020) |
| Lophirones B, C | Nature, isolated from Lophira alata | ATCC 29,213 | 150 – 200 µg/ml | Antibacterial | Ajiboye and Haliru (2016) |
| 4,4′,6′ trihydroxy 3 methoxy 3′ pentene chalcone | Nature, isolated from Elatostema parasiticum | ATCC 6538 | 7.8 μg/ml | Antibacterial | Mariani et al. (2016) |
| Grandiflorone | Nature, isolated from Leptospermum scoparium | - | 16–32 μg/ml | Antibacterial | Killeen et al. (2016) |
| 2ʹ,4ʹ-dihydroxychalcone e 2ʹ,4ʹ-dihydroxy-3ʹ-methoxychalcone |
Nature Isolated from Zuccagnia punctata Cav |
ATCC 29,213, MRSA, MRSCN | 250 µg/mL−1 | Antibacterial | Moreno et al. (2015) |
| Cinnamaldehydo and its Chalcones derivatives |
Nature Isolated from Cinnamomum verum |
MRSA | 3.21 ± 40.0 mm | Antibacterial | Khayyat and Saddiq (2015) |
|
2′,4′-dihydroxychalcone 7-hydroxyflavanone |
Nature Isolated from Flourensia oolepis |
ATCC 6538 |
31 μg/mL 62 μg/mL |
Antibacterial | Joray et al. (2015) |
| Xanthoangelol | Nature, isolated from Angelica keiskei | ATCC 25,923, ATCC 700,699 (MRSA) |
6,25 µM 3,12 µM |
Antibacterial | Meier et al. (2019) |
|
Isobavachalcone; corylifol B |
Nature, isolated from Psoralea corylifolia L | MRSA OM481 OM584 | 8—16 µg/mL | Antibacterial | Cui et al. (2015) |
| 20 -hydroxy-4,40,60 -trimethoxychalcone, 20 -hydroxy-4,40,60 -tetramethoxychalcone, and 3,20 -dihydroxy-4,40,60 -trimethoxychalcone | Nature, isolated from hispidum | ATCC 25,923 | 62,5 mg/mL—125 µg/ml | Antibacterial and anti-biofilm | Costa et al. (2013) |
| Prenylated Chalcones (xanthohumol and Desmethylxanthohumol) | Nature, isolated from Humulus lupulus L | 8146, 8147, CIP 224, T1.1, T25.10, T25.3, T25.9, T26A4, T28.1, T36B1, T47A12, T6.7 | 9,8 – 19,5 µg/ml | Antibacterial, synergism with gentamicin, amoxicillin and vancomycin and anti-biofilm | Bocquet et al. (2019) |
| Lophirones B, C | Nature, isolated from Bamburu village | ATCC 29,213 | Redução da MIC | Antibacterial | Ajiboye and Haliru (2016) |
| Calythropsin | Nature, isolated from Calicotome villosa | - |
18 ± 1.4 mm 6 ± 0.7 mm |
Antibacterial | Alhage et al. (2018) |
| Diuvaretin, Uvaretin and Iso-Uvaretin | Nature, isolated from Uvaria chamae | ATCC 25,923, | 0,0046 mg/mL | Antibacterial | Koudokpon et al. (2018) |
| Basalcone J |
Nature, isolated from Populus Balsamifera |
ATCC 25,923 |
0.61 μM 6 μM |
Antibacterial | Simard et al. (2015) |
| 20 -hydroxy-4,40 -dimethoxy-chalcone and isoliquiritigenin | Nature, isolated from Coreopsis tinctoria Nutt | - | Redução de MIC | Antibacterial | Begmatov et al. (2020) |
| 5′-O-Methyl-3-hydroxyflemingin A and 5′-O-Methylflemingin C | Nature, isolated from Desmodium congestum |
ATCC 43,300, NRSA-NRS 17, NRSA-NRS 1 |
32 μg/mL | Antibacterial | Rees et al. (2015) |
| Isosalipurposide | Nature, isolated from Corylopsis coreana |
693E, MRSA 693E |
1.23 ± 0.06 cm | Antibacterial | Seo et al. (2016) |
| Homoembelin | Nature | ATCC 1026, SA3, SA4, SA11, SA12, MRSA 3, MRSA 4, MRSA 6, MRSA 8 | ≥ 256 µg/mL | Inactive for antibacterial activity | Omosa et al. (2016) |
| Xanthoangelol |
Nature Derived from Amorpha fructicosa |
ATCC 700,699 (MRSA) |
3.125 µM 12,5 µM |
Antibacterial Bactericidal |
Meier et al. (2019) |
|
2', 4'-dihydroxychalcone (DHC) |
Nature Isolated from Zuccagnia punctata Cav |
(F2, F5, F7, F8, F22, F23) and F31 |
(25 µg/mL) *100 µg/mL |
Antibacterial | Nuño et al. (2018) |
|
2', 4'-dihydroxy-3'-methoxycalcone (DMHC) |
Nature Isolated from Zuccagnia punctata Cav |
(F2, F5, F7, F8, F22, F23) and F31 |
(25 µg/mL) *100 µg/mL |
Antibacterial | Nuño et al. (2018) |
|
2', 4'-dihydroxychalcone (DHC) |
Nature Isolated from Zuccagnia punctata Cav |
F7 ATCC 25,923 |
12,5 µg/mL | Anti-biofilm | Nuño et al. (2018)) |
| Isoliquiritigenin |
Nature Isolated from Platymiscium gracile Benth |
ATCC 29,223 | 62,5 µg/mL | Low antibacterial activity | Cuellar et al. (2020) |
a) The spaces filled with (-) mean that the information was absent from the article or was not clear
Table 3.
Synthesis matrix of articles collected in a literature review that report the antibacterial potential of synthetic Chalcones and derivatives against S. aureus
| Chalcone | Type | Synthesis technique | S. aureus strain (s) | MIC/Inhibition Zone | Activity | Quote |
|---|---|---|---|---|---|---|
| (E)-1-(2-hydroxyphenyl)-3-(2,4-dimethoxy-3-methylphenyl)prop-2-en-1-one | Synthetic | – | 1199B |
↓ 50% of the MIC of the antibiotic (128 μg/mL to 64 μg/mL); |
Antibacterial; Synergism with the antibiotic Norfloxacin Inactive as a NorA inhibitor |
Rocha et al. (2021) |
| (E)-1-(2-hydroxyphenyl)-3-(2,4-dimethoxy-3-methylphenyl)prop-2-en-1-one | Synthetic | – | K2068 | ↓ 60.3% of the MIC of BrEt (32 μg/mL to 12.6992 μg/mL) | Inactive for direct antibacterial activity. Synergism with BrEt and potential MepA efflux pump inhibitor | Rocha et al. (2021) |
| (Z)-4-((bis(2-hydroxyethyl)amino)methyl)-7-((1-(4-methoxyphenyl)-3-oxo-3-(thiazol-2-yl) prop-1-en-2-yl) oxy)-2H-chromen-2-one (5i) | Synthetic | – | MRSA | 0.004 mM |
Antibacterial 6X better than the reference antibiotic Norfloxacin |
Hu et al. (2021) |
| 4-bromo-3'-aminochalcone (5f) | Synthetic | Claisen–Schmidt |
MSSA MRSA |
1.9 μg mL; 7.8 µg mL |
Concentration-dependent antibacterial and antibiofilm | Garcia et al. (2021) |
| Chalcones derived from 2-hydroxyacetophenone (A1-A6) | Synthetic | Claisen–Schmidt | RN4220; K4414; 1199B; K2068 | 1024 μg/mL | Clinically irrelevant antibacterial activity; Potential IBEs | Xavier et al. (2021) |
|
Chalcone derivative DBDCPP-3 |
Synthetic | Claisen–Schmidt | – | Over than 15 mm | Bactericide | Shinde et al. (2021) |
|
(E)-3-(furan-2-yl)-1-(2-hydroxy-3,4,6-trimethoxyphenyl)prop 2-en-1-one (Hitfurfural); (E)-1-(2-hydroxy-3,4-dimethoxyphenyl)-3-(thiophen-2-yl)prop-2-en-1-one (Hithiophene) |
Synthetic | Claisen–Schmidt |
1199B K2068 |
1024 μg/mL |
Clinically irrelevant antibacterial activity; Potential NorA and MepA IBEs |
da Silva et al. (2021) |
| (2E)-3-(4-Methoxyphenyl)-1-{4-[(3-phenyl-1,2,4-oxadiazol-5- yl)methoxy]phenyl}prop-2-en-1-one (6a) | Synthetic | – | – |
3.12 μM 2 × more efficient than the standard drug Ciprofloxacin |
Antibacterial | Ibrahim et al. (2021) |
|
(E)-3-(3-bromo-4-methoxyphenyl)-1-(thiazol-2-yl)prop-2-en-1-one (Zn 4 complex) |
Synthetic | Claisen–Schmidt | MTCC 916 | 18 mm | Antibacterial | Johnson and Yardily (2021) |
| (E)-3-(4-(dimethylamino)phenyl)-1-(2-hydroxyphenyl)prop-2-en1-one (3) | Synthetic | Claisen–Schmidt | 1199B | 1000 μg/mL | Clinically irrelevant antibacterial activity; NorA inhibitory potential | Leal et al. (2021) |
| Polymer chalcone (P3) | Synthetic | Claisen–Schmidt | – | 20 mm | Antibacterial | Solmaz et al. (2021) |
| Hybrid Chalcona (1) | Synthetic | Claisen–Schmidt | NCIM 2122 | 10.05 μM | Low antibacterial activity compared to standard drug Ciprofloxacin | Konidala et al. (2021) |
| Chalcone with imide-Zo portion [1,2-a]pyridine (3b, 3d, 3g, 3l and 3m) | Synthetic | Claisen–Schmidt | – | 32 μg/mL and 64 μg/mL | Antibacterial | Soltani et al. (2021) |
| E4,4'-Bromo-4-methylchalcone | Synthetic | Claisen–Schmidt | MRSA | 16 μg/mL | Antibacterial | Aksöz et al. (2021) |
|
Quinoline chalcone: 1-(4-(benzyl sulfonyl) phenyl)- 2,3dibromo-3-(chloroquinoline-3-yl) propane-1- one (7) |
Synthetic | Claisen–Schmidt | – |
At a concentration of 10 mg/disk: 15 mm |
Low antibacterial activity compared to standard drug | Saleh et al. (2020) |
|
Fluorinated chalcone: (E)-3-(1″H-indol-3″-yl)-1-[40 -(trifluoromethyl)phenyl]prop-2-en-1-one |
Synthetic | Claisen–Schmidt | NCIM-2079 | 51 µM | Low antibacterial activity compared to standard drug | Lagu et al. (2020) |
| (E)-1-(4-aminophenyl)-3-(furan-2-yl)-prop-2-en-1-one (AFPO) | Synthetic | Claisen–Schmidt | 10 | ≥ 1024 μg/mL | Irrelevant | Ferraz et al. (2020) |
| (E)-1-(4-aminophenyl)-3-(furan-2-yl)-prop-2-en-1-one (AFPO) | Synthetic | Claisen–Schmidt | 10 |
AFPO + Gentamicin: ↓ 3X AFPO + Penicillin = ↓ 3X |
Synergism (potentiation of antibiotic activity) | Ferraz et al. (2020) |
| Naphthyl Chalcones (3a -3p) | Synthetic | – | MTCC 96 | 24–162 μg/mL | Low antibacterial activity compared to standard drug | Pola et al. (2020) |
| Heteroaromatic Chalcones Derivatives (1a-and 3a-c) | Synthetic | Claisen–Schmidt | N5923 | 13 and 19 mm | Antibacterial | Farooq and Ngaini (2020) |
| Chalcone derivative (1d) | Synthetic | Claisen–Schmidt | – | 11 mm | Antibacterial | Farooq and Ngaini (2020) |
|
Chalcones source of the natural product 2-hydroxy-3,4,6-trimethoxyacetophenone (1–4) |
Synthetic | Claisen–Schmidt |
10 (Multi-resistant); ATCC 25923; |
≥ 1024 μg/mL; 645 μg/mL |
Irrelevant; Low antibacterial activity and synergism with Ciprofloxacin and Gentamicin |
Freitas et al. (2020) |
|
4'-piperazinyl Chalcone—pleuromutilin (14i; 14k) |
Synthetic | Alcoholic condensation |
MRSA (ATCC 33591); MRSA (ATCC 43300); ATCC 25923 |
0.5–1 μg/mL | Antibacterial | Xie et al. (2020) |
| Sulfonamides Chalcones and derivatives: (IV—XXXII) | Synthetic | – | ATCC 25923 | (> 22)—10 (0.156) mm | Inactive for antibacterial activity | Bonakdar et al. (2020) |
| Ferrocenyl Chalcones (Decyl) | Synthetic | Acquired |
NCIMB 8244 MRSA (RCH) |
0.031 mg mL 0.063 mg mL |
Antibacterial | Henry et al. (2020) |
|
Bichalcones and Bispirazoline derivatives (2ª–2d) (3b–3d) |
Synthetic | Acquired | – |
16–32 μg/mL 8 – 32 μg/mL |
Inactive for antibacterial activity | Nisa and Yusuf (2020) |
|
Pyrimidine derived from Chalcone: 9-(Benzo[d][1,3]dioxol-5-yl)- 7-(thiophen-2-yl)pyrido[30,20:4,5] thieno[3,2-d]pyrimidin-4(3H)-one (24a) |
Synthetic | Acquired | ATCC 6538 | 7.81 μg/mL | Antibacterial | Sanad et al. (2020) |
| Triazine Chalcone: 3k | Synthetic | Claisen–Schmidt | NCIM 2178 | 85 mm | Antibacterial | Shinde et al. (2019) |
|
Aminochalcone derivatives: 1–3; 7–8; 11–13; 16 |
Synthetic | Claisen–Schmidt | DSM799 |
0.25 [mg/mL]; 0.125 [mg/mL]; 0.25 [mg/mL]; 0.5 [mg/mL] |
Antibacterial | Kozłowska et al. (2019) |
|
Chalcones and derivatives (6r, 12a); [6s; 12c] 7j–7m |
Synthetic | Claisen–Schmidt |
MSSA (ATCC 29213); MRSA (USA 300) |
(1.56 − 6.25 μg/mL); [3.125 − 6.25 μg/mL]; 1.56 -12.5 μg/mL |
Antibacterial | Zhang et al. (2018b) |
|
Chalcones bonded to Biscoumarine copolyester 4a; 4b; 4c |
Synthetic | Polycondensation | – |
312.5 μg/mL; 625 μg/mL; 156.2 μg/mL |
Antibacterial | Kandaswamy (2019) |
|
1-(2-bromophenyl) ethanone derivatives I1-I5 |
Synthetic | – | NCIM 2079 | They ranged from 31.25 to 500 μg/mL | Significant Antibacterial | Prasad et al. (2018) |
|
Chalcones derivatives 11; 18 |
Synthetic | Claisen–Schmidt |
NTCC8325-4 MRSA 97-7 |
50 and 70 µg/mL; 50 and 90 µg/mL |
Low antibacterial activity | Vásquez-Martínez et al. (2019) |
|
Chalcones derivatives 18; 11 |
Synthetic | Claisen–Schmidt | MRSA 97-7 |
20 μg/mL; 50 μg/mL |
Synergism with the antibiotic methicillin | Vásquez-Martínez et al. (2019) |
|
Chalcone-pyridine hybrids 5j; 5i; 5b; 5f |
Synthetic | – | MTCC 121 |
3.12 μg/mL; 6.25 μg/mL |
Antibacterial | Gondru et al. (2018) |
|
Pyrimidine-2(1H)-ol/-thiol derivatives derived from Chalcones 20–24; 35–39 |
Synthetic | – | ATCC 25923 | 2.1–138.8 µg/mL | Antibacterial | Fandakli et al. (2018) |
|
Chalcones converted to pyrazoles 4h, 4j, 4l, 4m e 4n |
Synthetic | Claisen–Schmidt | – | They ranged from 3.75 to 1.25 µg/mL | Antibacterial | Chowdary et al. (2019) |
|
Benzoxazepines derived from pyrazol-Chalcones 3c; 3h, 5c, 5g; 5b; 5h |
Synthetic | – | – |
30 mm; 28 mm; 27 mm; 32 mm |
Antibacterial | Ashok et al. (2018) |
|
Indolyl Chalcone Derivatives 4b; 5b; 6b, 7; 11 |
Synthetic | – | – |
27 mm; 22 mm; 24 mm, 28 mm |
Antibacterial | Sayed et al. (2018) |
|
Heterocyclic Chalcones 4b* 4c, 4g, 4k |
Synthetic | Claisen–Schmidt | MTCC 096 |
12.5 µg/mL* 6.25 µg/mL |
Antibacterial | Kaushik et al. (2018) |
|
Pyrazole Chalcones 6b; 6c; 7b e 8b |
Synthetic | Claisen–Schmidt | MTCC9886 | 13 ± 0.44 mm; 17 ± 1.10 mm; 15 ± 0.53 mm; 11 ± 2.01 mm | Moderate antibacterial | Kumari et al. (2018) |
|
Triazole-based Chalcones and its derivatives 3f, 5a, 6a, 7b e 8a |
Synthetic | – | – |
44.59 mM; 50.15 mM; 66.61 mM; 76.63 mM; 53.86 mM |
Antibacterial | Santosh et al. (2018) |
|
Anthracene-based Chalcone derivatives ANNP, ANFL; ANID and ANPT |
Synthetic | Claisen–Schmidt | – |
6.25 µg mL; 3.12 µg mL |
Moderate antibacterial | Prakash et al. (2018) |
|
Chalcones derived from acetylpyridines 4; 5; 11; 12 |
Synthetic | – | – | – | Antibacterial | Santra et al. (2018) |
|
β-Carboline Chalcones and their bromide salts 12b, 12c, 12e, 12f, 12g; 13a; 13h |
Synthetic | – | MTCC 96 | Ranged from 440 to > 900 µM | Inactive for antibacterial activity | Reddy et al. (2018) |
| Chalcone-thiazole hybrids (27) | Synthetic | Claisen–Schmidt | ATCC 33592—Methicillin and Gentamicin Resistant | 1.4 µM | Antibacterial | Sashidhara et al. (2015) |
|
1,3- diphenyl-2-propen-1-ones (III-C) |
Synthetic | – | JMC 2151 | 32 ± 1.5 mm | Antibacterial | Alam et al. (2015) |
| Chalcones derivatives: 3ª–3j | Synthetic | – | ATCC 29213; (MRSA) |
16–256 μg/mL 32–64 μg/mL |
Low antibacterial activity | Evranos-Aksöz et al. (2015) |
| 4.5-dihydropyrazol | Synthetic | – | ATCC 29213 | 10–17 mm | Antibacterial | Budak et al. (2017) |
| 2-(4-Methyl-2-oxo-2H-chromen-7-yloxy)-N-(4-((E)-3- (3,4,5-trimethoxyphenyl)acryloyl)phenyl)acetamide | Synthetic | Claisen–Schmidt | ATTC 6538 | 35.8 µg/mL | Antibacterial | El-Sherief et al. (2017) |
|
(4- (4- aryl)-1H- pyrrol-3-yl)(pyren-1-yl)meth- Anone + (3-chloro) |
Synthetic | - | - | 4.1 μg/mL | Antibacterial | Divakar and Shanmugam (2017) |
| monocarbonyl curcuminoids | Synthetic | - | ATCC 25923 | 250 mg/mL | Antibacterial | Ud Din et al. (2017) |
|
2-(2-Hydroxyphenyl)-5-methyl-3-(4-(thiophen-2-yl)-6-(4- methyl-phenyl)-pyrimidin-2-yl) thiazolidin-4-one; 3-Chloro-4-(4-hydroxy-3-methoxyphenyl)-1-(4-(thiophen-2- yl)-6-(4-methyl-phenyl)-pyrimidin-2-yl)azetidin-2-one |
Synthetic | Claisen–Schmidt | – | 100 μg mL | Antibacterial | Patel et al. (2017) |
|
(2E,20 E)-3,30 -(3,30 -(butane-1,4-diylbis(oxy))bis(3,1- phenylene))bis(1-(thiophen-2-yl)prop-2-en-1-one) (2E,20 E)-3,30 -(3,30 -(pentane-1,5-diylbis(oxy))bis(3,1- phenylene))bis(1-(thiophen-2-yl)prop-2-en-1-one) |
Synthetic | Claisen–Schmidt | MTCC 96 | 8 μg/mL | Antibacterial | Yusuf et al. (2017) |
| Chalcones derived from Pyrimidines | Synthetic | – | – | 9–14 mm | Antibacterial | Kumar et al. (2017b) |
| Phenylpropenone pyrrolyl Chalcone | Synthetic | – | – | 6–11 mm | Antibacterial | Kumar et al. (2017a) |
| Chalcones with a triazine nucleus (P1–P3, P5, P6, S6) | Synthetic | – | MTCC 96 | 7–13 mm | Antibacterial | Mahmoodi et al. (2017) |
| Derivatives of (3ar,4S,7R,7as)-2-(4-((E)-3-(3- aryl)acryloyl) phenyl)-3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole1,3(2H)-dione (7e, 7l, 7m and 7n) | Synthetic | Claisen–Schmidt | ATCC 29213 | 14 mm | Antibacterial | Kocyigit et al. (2017) |
| (2E)-3-(2,6- dichlorophenyl)-1-(4-methoxyphenyl) prop-2-en-1-one | Synthetic | – | – | 50 mg/mL | Antibacterial | Sadgir et al. (2020) |
| Chalcones derived from Pyrazoline | Synthetic | – | – | 12.5–25 µg/ml | Antibacterial | Desai and Sastry (2017) |
| 6,6-dimethyl3-aryl-3′,4′,6,7-tetrahydro-1′H,3H-spiro[benzofuran-2,2′-naphthalene]-1′,4(5H)- dione (5a) | Synthetic | Claisen–Schmidt | ATCC 29213 | 16 mm | Antibacterial | Ergüntürk et al. (2017) |
| 2-Pyrazoline (5f) | Synthetic | Claisen–Schmidt | MTCC 737 | 10 mg/mL | Antibacterial | Lokeshwari et al. (2017) |
|
2-bromo-4-[3-(2-hydroxyphenyl)-4,5-dihydro-1h-pyrazol-5-yl]-6-methoxyphenol 1-[5-(3-bromo-4-hydroxy-5-methoxyphenyl)-3-(2-hydrophenyl]-4,5-dihydro-1H-pyrazol-1-yl)ethanone |
Synthetic | – | FNCC 0047 | 7.25 mm | Antibacterial | Setyawati et al. (2017) |
|
Cis-3-4-dihydrohamacanthin B; Bromodeoxytopsentin |
Synthetic | – | RN4220; MRSA 252; ATCC 29213 | 12.5 µg/mL; 6.25 µg/mL | Antibacterial | Labrière et al. (2017) |
|
(2E,2′E)-3,3′-(octane-1,8-diylbis(oxy))bis(3,1- phenylene))bis(1-phenylprop-2-ene-1-one) 1,12-bis(3-(1,3-diphenyl-4,5-dihydro-1H-pyrazol5-yl)phenoxy)dodecane |
Synthetic | Claisen–Schmidt | MTCC 96 | 8 μg/mL | Antibacterial | Yusuf et al. (2017) |
|
Jaceosidin; 5,7,4′-triacetoxy jaceosidin |
Synthetic | – |
ATCC 43,300, VRS 10, NRS 17 NRS 1 |
32–128 µg/mL | Antibacterial | Kumar et al. (2016) |
| 3′-formyl-2′,4′,6′-trihydroxydihydrochalcone | Synthetic | – |
ATCC 29,213 MRSA 33,591 |
17.4 μM 36.6 μM |
Antibacterial | Zaki et al. (2016) |
| Quinoline scaffold (3b, 3d, 3g, 3h–j) | Synthetic | Claisen–Schmidt | - | 200 µg/mL | Antibacterial | Dave and Rahatgaonkar (2016) |
| Ferrocenyl chalcones with O-alkylated vanillins | Synthetic | Claisen–Schmidt | ATCC 25923 | 0.625–5 µg/mL | Antibacterial | Muškinja et al. (2016) |
| Complex Chalcones (3e, 3l) | Synthetic | – | ATCC 43300 | 21.8% Redução de crescimento | Antibacterial | Patil et al. (2016) |
|
(E)-3-{5-[(2-Benzoylbenzofuran-5-yl)methyl]-2- hydroxyphenyl}-1-(3,4-dichlorophenyl)prop-2-en-1- one; {5-[3-(8-Fluoro-4-phenyl-2,3-dihydro-1H-benzo- [b][1,4]diazepin-2-yl)-4-hydroxybenzyl]benzofuran2-yl}(phenyl)methanone |
Synthetic | Claisen–Schmidt | – |
55 µg/mL 34 µg/mL |
Antibacterial | SHankar et al. (2016) |
|
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-chlorophenyl)methanone (4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-bromophenyl)methanone (4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-bromophenyl)methanone |
Synthetic | – | ATCC 6538 | 200–400 µg/mL | No significant antibacterial activity | Patel et al. (2016) |
|
Carbazole-based Chalcones (3a, 3h, 3i, 4a, 4h, 4i, 5a, 5h, 5i) |
Synthetic | Claisen–Schmidt | ATCC 6538 | 20 µg/mL | Antibacterial | Ashok et al. (2016) |
| Glabridin-chalcone hybrid molecules (6h, 7e, 8f) | Synthetic | Claisen–Schmidt |
MTCC-96 MRSA-ST 2071 |
12,5 µg/mL | Antibacterial | Kapkoti et al. (2016) |
| Chalcones derived from 1,3-diaryl pyrazole (6g, 6l and 7l) | Synthetic | – | 4220 | 1–64 μg/mL | Antibacterial | Killeen et al. (2016) |
| Chalcones Derivatives (4ª, 4f) | Synthetic | Claisen–Schmidt | 4220, 209 and 503 | 2 μg/mL | Antibacterial | Wei et al. (2016) |
|
1-[7-(diethylamino)-2H-chromen-2on]-3-(p-chlorophenyl)-2-propen-1-one one; 1-[7-(diethylamino)-2H-chromen-2on]-3-(p-fluorophenyl)-2-propen-1-one |
Synthetic | Claisen–Schmidt | – | 100 µg/mL | Antibacterial | Himangini and Pathak (2016) |
| 4,5-Dihydro-3-(1-benzyl-1H-indol-3-yl)-5-(naphthalene-2-yl) isoxazole | Synthetic | Claisen–Schmidt | RCMB 010028 | 23.4 mm | Antibacterial | Abo-Salem et al. (2016) |
| 1,3-thiazine compounds with a Schiff base portion (5a-5e) | Synthetic | Claisen–Schmidt | – | 09–15 mm | Antibacterial | Babu et al. (2015) |
| 2,8- bis(2,4-Dichlorophenyl)-4H,6H-pyrano[3,2-g]chromene-4,6-dione | Synthetic | Claisen–Schmidt | ATCC 29737 | 12.5 µg/mL | Antibacterial | Husain et al. (2015) |
| 2-Chloro-N-{4-[3-(substitutedphenyl)-acryloyl]-phenyl}-acetamide | Synthetic | Claisen–Schmidt | – | 9 mm | Antibacterial | Jayaramu and Maralihallia (2015) |
| 3-(4′-N,N-dimethyl amino phenyl)-5-(2″, 2″-dimethyl, 7″-hydroxy chroman) isoxazole/3-(3′,4′-chloro-phenyl)-5-(2″, 2″-dimethyl, 7″-hydroxy chroman) isoxazole/3-(4′-methoxy-phenyl)-5-(2″, 2″-dimethyl, 7″-hydroxy chroman) isoxazole/3-(4′-cyano phenyl)-5-(2″, 2″-dimethyl, 7″-hydroxy chroman) isoxazole | Synthetic | Claisen–Schmidt | MTCC 96 | 5–20 µg/mL | Antibacterial | Raju et al. (2015) |
| trans-3-(1H-indol-3-yl)-1-(40-benzyloxyphenyl)-2-propen-1-one (2), 1-(400-biphenyl)-3-(30 40-dihydroxyphenyl)-2-propen-1-one (11), 1-(400-hydroxy-300-methylphenyl)-3-(40 -hydroxyphenyl)-2-propen-1-one (14), 3-(40 -chlorophenyl)-1-(400-hydroxyphenyl)-2-propen-1-one (17), LTG-oxime | Synthetic | Claisen–Schmidt |
ATCC 96 MRSA |
12.5–50 mg mL | Antibacterial | Gaur et al. (2015) |
| 6H-benzo[c]chromen-6-ones and Diazepines (10a,b) | Synthetic | Claisen–Schmidt | ATCC 9144 | 0.625 μg/mL | Antibacterial | Mazimba (2015) |
| Chalcone |
Synthetic acquired |
– |
USA 300; USA 300 ΔSRTA |
53.15 μM 76 μM |
Antibacterial and anti-biofilm | Zhang et al. (2017) |
| Apchal and Achlopheny | Synthetic | Claisen–Schmidt | ATCC 25923, 10, 1199B, K2068 | 70% synergism of gentamicin when associated with Apchal and loss of synergism when associated with Aclo-phenyl | Antibacterial. Synergism and antagonism were observed | Siqueira et al. (2020) |
| Apchal and Achlophenyl | Synthetic | Claisen–Schmidt | SA-1199B; K2068 | Synergism with Norfloxacin and ciprofloxacin; synergism with ciprofloxacin and ethidium bromide | Efflux pump inhibitor | Siqueira et al. (2020) |
| 1,3- Bis-(2-hydroxy-phenyl)-propenone, 3-(3-hydroxy-phenyl)-1-(2-hydroxy-phenyl)-propenone e 3-(4-hydroxy-phenyl)-1-(2-hydroxy-phenyl)-propenone | Synthetic | – | MRSA ATCC 43,300, MRSA CC5, CC8, CC45, CC80, CC152 | 37.5 ± 13.2 μg/mL; 97.5 ± 27.5 μg/mL; 110.8 ± 21.1 μg/mL | Antibacterial | Božić et al. (2015) |
| Chalcones and derivatives (6s e12a) | Synthetic | Claisen–Schmidt | Newman (MSSA); MN8 (MRSA); NRS70 (MRSA) |
3.12 μg/mL; 6.25 μg/mL: 0.8 μg/mL |
Antibacterial, anti-biofilm | Zhang et al. (2018a, b) |
| (E)-N-(3-Aminopropyl)-2-(5-((3-methylbut-2-en-1-yl)oxy)-2-(3-(4-((3-methylbut-2-e n-1-yl)oxy)phenyl)acryloyl)phenoxy)acetamide | Synthetic | – | ATCC29213, MRSA N315, MRSA NCTC10442 | 1.56–3.13 μg/mL | Antibacterial | Lin et al. (2020) |
| Thiazolyl-pyrazoline derivatives (7 a) | Synthetic | Claisen–Schmidt | MSSA (ATCC 25,923), MRSA (ATCC 43,300), VISA |
62.5 µg/mL; 125 µg/mL; 31.5 µg/mL; |
Antibacterial | Cuartas et al. (2020) |
| Derivatives of benzo[4,5]isothiazolo[2,3-a]pyrazine-6,6-dioxide | Synthetic | – | ATCC 6538 | > 1600 µg/mL | Inactive for antibacterial activity | Bassin et al. (2017) |
| Derivatives of 2′,6′-dihydroxy-4′-methoxy-3′,5′-dimethyl chalcone (10) | Synthetic | – | ATCC 43,300, Clinical isolate, VRS 10, NRS 17, NRS 1 |
> 128 µg/mL 64 µg/mL 32 µg/mL |
Antibacterial | Kumar et al. (2016) |
| Derivatives of (E)-3-(4-bromophenyl)-1-(p-tolyl) prop-2-en-1-one (3a, 3e, 3j) | Synthetic | – | ATCC 29,737 |
25.4 mm 27.2 mm 26.4 mm |
Antibacterial | Bhirud et al. (2020) |
| 7,4′-dihydroxy-8,3′-diprenylflavone and erysubin F | Synthetic | Claisen–Schmidt |
MRSA, ATCC 43,300 |
15.4 μM 20.5 μM |
Antibacterial | Kwesiga et al. (2020) |
| (2Z)-3-(3-nitrophenyl)-1-{4-[(E)-(piperidin-1-yl)diazenyl]phenyl}prop-2-en-1-one | Synthetic | Claisen–Schmidt | ATCC 12,598 | 12.5 g/mL | Antibacterial | Sivasankerreddy et al. (2018) |
| Xanthohumol, naringenin, and chalconaringenin | Synthetic | – | PCM 2054 |
3.57 mm 3.77 mm 1.92 mm |
Antibacterial | Stompor and Zarowska (2016) |
|
2′-Hydroxychalcone; 2 0-Hydroxy-2-methoxychalcone; 2 0-Hydroxy-4-methoxychalcone; 2 0-Hydroxy-40 -methoxychalcone-3-yl)(3-bromophenyl)methanone |
Synthetic | Claisen–Schmidt | D1 |
0.26 ± 0.09, 0 ± 0.03, 0.88 ± 0.10 0 ± 0.04 |
Antibacterial | Gładkowski et al. (2019) |
|
4-Hydroxyphenyl 3-Hydroxyphenyl |
Synthetic | Claisen–Schmidt | – |
5 µg/mL 10 µg/mL |
Antibacterial | Desai et al. (2017) |
| Aryl substituted dihydrotriazine derivatives (17h) | Synthetic | – |
4220 QRSA CCARM 3505 |
0.5 µg/mL | Antibacterial | Zhang et al. (2018a) |
| Derivatives of 1,2,3-triazoles (25) | Synthetic | Claisen–Schmidt | – | MIC decrease in 55% | Antibacterial | Syed-Aly et al. (2015) |
|
Derivatives of (2-(Pyridinyl)methylene)-1-tetralone Chalcones (5h) |
Synthetic | – | MRSA | Decrease of 75% | Antibacterial | Gibson et al. (2017) |
|
(E)-N-(4-(3-(4-Chlorophenyl)acryloyl)phenyl)-3-(piperidin-1-yl)propanamide, (E)-N-(4-(3-(4-Methoxyphenyl)acryloyl)phenyl)-3-(piperidin-1-yl)propanamide, (E)-3-(Piperidin-1-yl)-N-(4-(3-(3,4,5-trimethoxyphenyl)acryloyl)phenyl)propanamide |
Synthetic | – | IFO 3060 |
2.0 mg/mL 2.4–8.6 mg/mL |
Antibacterial and anti-biofilm | El-Messery et al. (2018) |
|
(2E)-3-(4-{[1-(2-chloro-4-fluorophenyl)-1H-1,2,3-triazol-4-yl]methoxy}-3- methoxyphenyl)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, (2E)-3-(4-{[1-(2,4-difluorophenyl)-1H-1,2,3-triazol-4-yl]methoxy}-3-methoxyphenyl)-1- (2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, (2E)-3-(4-{[1-(2-chlorophenyl)-1H-1,2,3-triazol-4-yl]methoxy}-3-methoxyphenyl)-1-(2- hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, (2E)-3-[3,4-bis({[1-(2,4-difluorophenyl)-1H-1,2,3-triazol-4-yl]methoxy})phenyl]-1-(2- hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one, 2-[3,4-bis({[1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl]methoxy})phenyl]-5,7-dimethoxy4H-chromen-4-one |
Synthetic | – | ATCC 25323 |
6.25 µg/mL 12.5 µg/mL |
Antibacterial | Kant et al. (2016) |
| 4′-hydroxy-4-methyl chalcone | Synthetic | – | ATCC 29213 | 32 μg/mL | Antibacterial | Evranos-Aksöz et al. (2015) |
| Asymmetric heterocyclic chalcone derivatives and heterocyclic compounds (4, 6, 7, 9) | Synthetic | – | ATCC 25923 | 65–95% of zone of inhibition | Antibacterial | El-Hashash et al. (2015) |
| Derivatives of 3-hydroxy-6-(hydroxymethyl)-2-(2-phenyl-4H-chromen-4-yl)-4H-pyran-4-ones (3a, 3e, 3f e 3 l) | Synthetic | – |
MLS-16 MTCC 2940 |
7.8–15.6 µg/mL | Antibacterial | Bingi et al. (2015) |
| Chalcona derivative: (4[(1E)-3-(pentyloxy)buta-1,3- dien-1-yl]benzene-1,2-diol | Synthetic | Claisen–Schmidt |
ATCC 2592 (MRSA) ATCC 33591 |
0.12 μM | Antibacterial and antibiofilm | Emeri et al. (2019) |
| N-(5a-chloro, 8a-triflouromethyl)-benzyl-N, 1a-dihydro-2H-O, N-isoliquiritigeninoxazine (IMRG4) |
Synthetic Derived from the natural Chalcone Isoliquiritigenin (ISL) |
– | SA-29213; SA-1199;SA-1199B*; SA-K1758; MRSA-P4423; MRSA-P4627; MRSA-P4620; MRSA-B10760; MRSA-ST315; VISA-ST207; VISA-ST1745 | 50 (mg/L); 25(mg/L); 50(mg/L); 25 (mg/L); 50(mg/L); 25(mg/L); 25 (mg/L); 50(mg/L); 25(mg/L); 50 (mg/L); 50(mg/L) |
Antibacterial *NorA efflux pump inhibitor |
Gupta et al. (2019) |
|
(2E)-1-(3′, -methoxy-4′,-hydroxyphenyl)-3-(3-nitrophenyl) prop-2-en1-one (AVMNB) |
Synthetic | Claisen–Schmidt |
ATCC 25923; 10 |
≤ 512 mg/mL | Antibacterial | Garcia et al. (2020) |
|
(2E)-1-(3′, -methoxy-4′,-hydroxyphenyl)-3-(3-nitrophenyl) prop-2-en1-one (AVMNB) |
Synthetic | Claisen–Schmidt |
ATCC 25923; 10 |
MIC↑ | Antagonistic with Cephalexin and Gentamicin antibiotics | Garcia et al. (2020) |
|
2E-1-(2ʹ-hidroxi-3ʹ,4ʹ,6ʹ-trimetoxifenil)-3-(fenil)-prop-2-en-1-ona (HYTPHENYL) |
Synthetic Derived from the natural compound: 2-hydroxy-3,4,6-trimethoxyacetophenone |
Claisen–Schmidt | 358 |
1024 µg/L MIC↓ |
Inactive for antibacterial activity Antibiotic modulator: Synergism with the antibiotic Amikacin |
Teixeira et al. (2019) |
|
(E)-3-(2-methylpyrimidin-5-yl)-1-ferroceynlprop-2-en-1-one (MPFP) |
Synthetic | Claisen–Schmidt | – |
Concentration of 25 μg: 19 mm Concentration of 50 μg: 24 mm Concentration of 75 μg: 31 mm |
Antibacterial | Twinkle et al. (2020) |
|
Cationic derivatives of Chalcones: 5a; 5s; 5b; 5c; 5d, 5g, 5l, 5p, 5q; 5r; 5z; 5ab |
Synthetic | Claisen–Schmidt |
M1-M9 MRSA |
Ranged from 0.25 –32 µg/mL | Antibacterial and synergism with Norfloxacin and Colistin | (CHU et al., 2018) |
| Cationic molecule 5g | Synthetic | Claisen–Schmidt | ATCC29213 | 128 µg/mL | Anti-biofilm | (CHU et al., 2018) |
|
Chalcones derivatives: (E)-2-(4-acetamidophenoxy)-N-(3-(3-(4-methoxyphenyl) acryloyl)phenyl)acetamide (5e); (E)-2-(4-acetamidophenoxy)-N-(3-(3-(4-chlorophenyl)acryloyl) phenyl)acetamide (5b) |
Synthetic | – | – |
Concentration of 100 μg/mL: 9; 10 mm Concentration of 200 μg/mL: 15 mm; 13 mm |
Antibacterial | Babu and Selvaraju (2020) |
|
Chalcones-Sulfonamides 5/6, 8a-f and 9a-f (N = 12 derivatives) |
Synthetic | Claisen–Schmidt | Resistant clinical isolates | > 57 mcM | Inactive for antibacterial activity | Castaño et al. (2019) |
| Thiazole-based Chalcone (Derivative): Compound 18 | Synthetic | – | IFO 3060 | 1 μg/mL | Antibacterial | Alrohily et al. (2019) |
|
Ferrocenyl Chalcone Derivatives: Zn (II) (H1; H5) |
Synthetic | – | ATCC 9144 |
1.349 × 10–8 M/mL 1.268 × 10–7 M/mL |
Significant antibacterial | Liu et al. (2019) |
|
Derivative: (E)-1-(4-brom ophenyl)-3-(4-iodophenyl)prop-2-en-1-one |
Synthetic | Synthesized and characterized using XRD, FT-IR1 H and 13 C NMR | ATCC 12600 | 250 μg/mL | Antibacterial | Zainuri et al. (2017) |
|
Ferrocenyl Chalcone Derivatives: 3a2,3a7 e 3b6 |
Synthetic | – | MRSA, ATCC 43300 |
Concentração de 3 g/L: 11–22 mm |
Antibacterial | Liu et al. (2018) |
|
Chalcones derivatives: 4a; 4e; 4h |
Synthetic | Claisen–Schmidt | ATCC-9144 |
08 µg/mL 08 µg/mL 04 µg/mL |
Antibacterial | Gopi et al. (2016) |
| (E)-1-(4-Bromophenyl)-3-(napthalen-2-yl)prop-2-en-1-one | Synthetic | Claisen–Schmidt | ATCC 12600 | 500 µg/mL | Antibacterial | Thanigaimani et al. (2015) |
| Derivatives if indolyl-4H-chromene-phenylprop-2-en-1-one (5g; 5h) | Synthetic | Claisen–Schmidt | ATCC 700699 | 9.3 µg/mL | Antibacterial | Subbareddy et al. (2018) |
| Chalcone derivative: 1-hydroxynaphth-2-yl pyrazoline (6b) | Synthetic | Claisen–Schmidt | – | 3.9 µg/mL | Antibacterial | El-Desoky et al, (2018) |
| Chalcone derivative: Pyrazoles (5j; 5h) | Synthetic | Claisen–Schmidt | MTCC 3160 | 6.5 µg/mL | Antibacterial | Mishra et al. (2017) |
|
Derivative of Chalcone (4-bromophenyl)-1-(4-chlorophenyl)prop-2-en-1-one (I): 4-[(E){[4-(4-bromophenyl)-6-(4-chlorophenyl)pyrimidin-2-yl]imino}methyl]benzene-1,3-diol (IJ) |
Synthetic | Claisen–Schmidt | NCIM 2079 | 500 µg/mL | Antibacterial | Prasad et al. (2019) |
| [(Chloroquinolin-4-yl)amino]chalcones 8a–f | Synthetic | Claisen–Schmidt |
ATCC 25923 (MSSA) ATCC 43300 (MRSA) |
> 1000 µg/mL | Inactive for antibacterial activity | Ramírez-Prada et al. (2017) |
| Bichalcones 8 (a–h) | Synthetic | Claisen–Schmidt | – | 16–32 µg/mL | Low antibacterial activity | Yusuf and Solanki (2017) |
a) The spaces filled with (–) mean that the information was absent from the article or was not clear
The present review was based on studies on the bacterium S. aureus, justified by the importance of this microorganism as it is currently considered one of the main bacteria of clinical and epidemiological importance. This importance is due to its high ability to persist as a commensal microorganism in addition to its frequent multi-resistance to antimicrobials and its various virulence factors, expressing a countless variety of proteins, toxins and polysaccharides at the extracellular level (Barroso et al. 2014). It is assumed that 80% of the world population is intermittently colonized by this bacterium, acting as the main successful pathogen at hospital and community level, being mainly involved in pyogenic and toxic infections (Silva 2016).
The sequencing of genomes from different strains of S. aureus led to the discovery of a high number of mobile genetic elements, which explains the dissemination of increasingly resistant strains, since horizontal gene transfer is the main cause of multidrug resistance in bacteria (Assef and Neto 2020). This line of reasoning can be justified by the first case of S. aureus resistance to Methicillin that was identified in the United Kingdom 1 year after the introduction of this antibiotic in the clinic, resulting in one of the best known strains today, the MRSA (Methicillin-Resistant Staphylococcus aureus) (Kalenić 2012; Mendes 2010).
The tables also show different types of S. aureus strains, especially MRSA and MSSA (Methicillin-Sensitive Staphylococcus aureus), which are considered the second leading cause of longer hospitalizations, increasing human mortality and morbidity rates, resulting in a economic and political weight worldwide (Mendes 2010; Purrello et al. 2014).
The main methods of synthesis of Chalcones and its derivatives were also observed. Recently, derivation of products from Chalcones has been the subject of investigations due to its relatively simple structure and the variety of biological activities that they present (Ferreira et al. 2018). The set of activities expressed is in part attributed to the immense possibilities of substitutions in the aromatic rings of Chalcones, since the Claisen–Schmidt methodology, which is one of the main methodologies used for the synthesis of these compounds, promotes the obtainment of a large amount of compounds, due to the existence of numerous commercial benzaldehydes and acetophenones that can be combined, providing the structural variety intended (Ducki et al. 1998; Narender and Papi Reddy 2007).
Antibacterial activity of natural Chalcones
Generally, Chalcones can be categorized into single and hybrid Chalcones with the central skeleton of 1,3-diaryl-2-propen-1-one. In plants, Chalcones (cis and trans) are intermediates in the biosynthesis of flavonoids, which increased interest in this substance, and in 1910 occurred its first isolation in the laboratory (Shimokoriyama 1962; Ferreira et al. 2018). Chalcones can be found in dicotyledonous plants, some monocotyledons, pteridophytes and gymnosperms, but they are synthesized as main components in the Leguminosae, Asteracea and Moracea families, which are currently still used in folk medicine in the form of teas (Banoth and Thatikonda 2020).
The biological potential of Chalcones has been investigated since the 1940s, but it was only in the 1970s that researchers became more interested in exploring natural Chalcones when they showed anti-parasitic activities (Nowakowska 2007). With the discovery of antiparasitic activity, the search for more biological activities of Chalcones intensified, since then researchers around the world isolate and test natural Chalcones for various purposes, especially antibacterial activities, which is an emerging issue in a globalized world with so many human deaths by increasingly resistant bacteria.
In this context, Moreno et al. (2015), solated the Chalcones 2ʹ,4ʹ-dihydroxychalcone and 2ʹ,4ʹ-dihydroxy-3ʹ-methoxychalcone from the Zuccagnia punctata plant against a standard strain of S. aureus and a Methicillin-resistant strain (MRSA), which exhibited antibacterial potential with MIC of 250 µg mL. In the following year, Costa et al. (2016), tested the antibacterial potential of the natural Chalcone 20-hydroxy-4,40,60-trimethoxychalcone isolated from the botanical species Piper hyspidium, which obtained an MIC of 125 µg/mL showing potential antibacterial activity. In the same way Mariani et al. (2016) tested the antibacterial potential of natural Chalcone 4,4′,6′ trihydroxy 3 methoxy 3′ pentene chalcone isolated from Elatostema parasiticum which exhibited an MIC of 7.8 μg/mL which was considered to be good antimicrobial activity against a S. aureus clinic strain.
In 2018, Chalcone 2′,4′-dihydroxychalcone was isolated from aerial parts of Zuccagnia punctata which showed noticeable antibacterial and anti-biofilm activity against a series of S. aureus strains, reaching MIC of 25 µg/mL and 12.5 µg/mL, respectively (Nuño et al. 2018). In the following year, Meier et al. (2019) isolated Chalcone Xantoangelol from the fruits of Amorpha fruticosa which demonstrated a potent bactericidal effect against MRSA strain, reaching MIC of 12.5 µM.
In Table 2 below, studies in which natural Chalcones do not show clinically relevant antibacterial activity can be observed, but they were able to inhibit known resistance mechanisms, such as efflux pumps. Results like these are demonstrated in the study by Rezende-Júnior et al. (2020). In the aforementioned study, the natural Chalcone 3′,4′-dihydroxy, 3,4,4′-trimethoxy-chalcone (4) was isolated from the botanical species Arrabidaea brachypoda and tested its antibacterial effect against a series of clinical strains of S. aureus, including a strain carrying the NorA efflux pump, SA-1199B. In the direct antibacterial activity clinical trial, results were determined as clinically irrelevant with an MIC of ≥ 1024 μg/mL. However, in the Ethidium Bromide (BrEt) trial, natural Chalcone reduced the MIC to 16 µg/mL, acting as a potential efflux pump inhibitor.
Antibacterial activity of synthetic Chalcones and derivatives
Due to advances in synthetic organic chemistry, it is possible to obtain bioactive compounds with a diversity of substituents in an increasingly versatile way, such as several Chalcones that are synthesized from the manipulation of the aromatic rings of natural Chalcones (Fonseca 2012). The Claisen–Schmidt condensation methodology between aryl ketones and benzaldehyde derivatives is revealed as the most used methodological strategy for the construction of various chalconic nuclei (Winter 2016).
A series of Chalcones and derivatives were synthesized by the Claisen–Schmidt condensation in the study by Zhang et al. (2018a, b). The antibacterial evaluation revealed that an A ring substituted with R1 hydroxy groups in the 6 series of Chalcones produced active compounds with considerable antibacterial activity, such as the compound 6s ((E)-3-(4-(Diethylamino)phenyl)-1-(2,4-dihydroxyphenyl)prop-2-en-1-one) which exhibited an MIC of 3.12 μg/mL and 6.25 μg/mL against MSSA and MRSA strains, respectively. The α,β-unsaturated ligand between rings A and B was shown to be important for antibacterial activity.
Cuartas et al. (2020) also tested the potential of Chalcones against MSSA and MRSA strains. Chalcones derived from N-substituted pyrazolines proved to be excellent candidates for the development of new antimicrobials. The compound 5-{2-[Bis(2-chloroethyl)amino]-4-chlorothiazol-5-yl}-3-(4-chlorophenyl)-4,5-dihydro-1H-pyrazole-1-carbaldehyde (7a) exhibited a MIC of 61.25 µg/mL for MSSA and 125 µg/mL for MRSA, exhibiting a lower MIC value (31.5 µg/mL) for the VISA strain (Vancomycin—intermediate Staphylococcus aureus).
Zhang et al. (2017), obtained good antibacterial and anti-biofilm activity of a commercially purchased chalcone against two strains of S. aureus carrying an important virulence-related enzyme, Sortase A (SrtA). The use of chalcone against USA 300 and USA 300 ΔSRTA strains expressed an MIC of 53.15 μM and 76 μM, respectively, which can be considered a good anti-virulence strategy in the fight against infections by S. aureus.
In the frame built to show all the studies included in this review, it is possible to observe that there are also many works using hybrid Chalcones. Molecular hybridization is an efficient strategy, widely used for drug design. Hybrid molecules or compounds can provide more biological targets, which facilitates the achievement of the desired bioactivity (Dan and Dai 2019). Therefore, in the study by Kapkoti et al. (2016) ome hybrid molecules of Glabridin-chalcone (6h, 7e, 8f) were synthesized and tested their antibacterial potential against MTCC-96 and MRSA-ST 2071 strains, obtaining a MIC of 12.5 µg/mL, among all compounds tested, compound 8H exhibited marked synergism and reduction of up to 16 times in MICs with Norfloxacin (FICI range from 0.312 to 0.375).
When it comes to synergism, it was observed that several articles report antibiotic potentiation by synthetic Chalcones, such as the work carried out by Siqueira et al. (2020). The Chalcones (2E)-1-(4′-aminophenyl)-3-(phenyl)-prop-2-en-1-one (APCHAL) and (2E)-1-(4′-aminophenyl)-3-(4-Chlorophenyl)-prop-2-en-1-one (ACLO-PHENYL) were synthesized by Claisen–Schmidt condensation and tested against a number of standard and multidrug-resistant strains. It was observed that Chalcone Apchal reduced the MIC by up to 70% of the antibiotic Gentamicin (synergism) while expressing antagonism with the antibiotic Penicillin. Regarding Chalcone Aclo-phenyl, due to the addition of a chlorine group in the substance, a loss of synergism with Gentamicin was observed.
The Chalcones mentioned above were also tested as potential efflux pump inhibitors on SA-1199B strain carrying the NorA efflux pump and K-2068 strain carrying the MepA efflux pump. In the evaluation of inhibition of this resistance mechanism, both Chalcones exhibited a synergistic effect with the antibiotics Norfloxacin and Ciprofloxacin, although Aclo-phenyl is less pronounced. On the other hand, Aphcal showed synergism with both Ciprofloxacin and BrEt, showing good results with the inhibition of the MepA efflux pump. The results also demonstrate that both compounds bind approximately to the same region of the 1199B binding site and that this region overlaps with the preferred binding region of Norfloxacin. Based on these results, the authors state that Chalcone Aphcal can significantly contribute to the prophylaxis or therapy of diseases caused by multidrug-resistant S. aureus.
All the studies, presented and their relevance to the scientific community in terms of public health, reveal the importance of investigating natural compounds and the synthesis of new substances to solve the problem of bacterial resistance worldwide, as well as proving that the bacterium S. aureus is currently one of the bacteria with the greatest investigative focus, given all the complications generated in public health and in the global economy (Table 3).
Chalcones synthesis
Several methodologies are reported in the literature for the preparation of Chalcones, but the best known and most used is the synthesis through the Claisen–Shmidt condensation, in which the reaction of a chosen derivative of acetophenone with suitable aromatic aldehydes occurs, using ethanol or methanol as solvent and sodium hydroxide or potassium hydroxide as catalyst or different reaction conditions, such as aldol condensation in solid phase, by microwave and without the use of solvent. It is a very simple and convenient methodology although, in some cases, it results in a lower income (Mahapatra et al. 2015; Souza 2014).
The general steps for the synthesis of Chalcones using Claisen Schmdth condensation start with aldol condensation followed by basic dehydration (Lindoso and Lindoso 2009). The initial step of reaction is the deprotonation of the ketone group, where the basic catalyst removes the acid alpha hydrogen of the molecule to produce a carbanion which can be stabilized by resonance. From this, a nucleophilic attack occurs against the carbonyl carbon of the aldehyde, structuring a tetrahedral intermediate (alkoxide ion). This intermediate is protonated by a hydrogen in water generating the condensation product and regenerating the basic catalyst. The condensation product occurs by dehydration and for this to happen it is necessary to remove a hydrogen from the alpha position to result in the enolate ion, which by equilibrium eliminates the OH– group, thus forming the Chalcone (Chiaradia 2010).
Other less common methodologies in the synthesis of Chalcones are described in the literature such as Heck, Sonogashira, and SuzukiMiyaura coupling, Friedel–Crafts reaction. However, there is an advantage in using the aldol condensation methodology (Claisen–schmidt) which is its accessibility when compared to the others, as it does not require high temperatures or expensive catalysts. Different methodologies, such as coupling reactions, can become inconvenient by causing environmental impacts caused by metals that are used in the process (Mahapatra et al. 2015; Souza 2014).
Bacterial resistance and efflux pump
Resistance mechanisms and their inhibition by Chalcones is widely cited by many studies in this review. This is due to the emergence of several complex mechanisms and rapid dissemination of multidrug-resistant microorganisms that have been worrying global health agencies, as these characteristics impose a barrier to existing treatments, which many of these treatments have suffered decreases in terms of use and effectiveness (Anvisa 2018). One of the effective resistance mechanisms employed by S. aureus is the active pumping of antimicrobials through membrane transporters, known as efflux pumps (Jang 2016; Rao et al. 2018). Efflux pumps are proteins capable of extruding several types of chemically different substrates, mainly antibiotics (Costa et al. 2013; Hassanzadeh et al. 2020), contributing significantly to a high level of resistance.
With the knowledge about the functionality of efflux pumps and with the goal of reversing bacterial resistance through the inhibition of this mechanism, new Chalcones are being synthesized for the investigation of their inhibitory potential, which can act as efflux pump inhibitors (EPIs) (Labrière et al. 2017; Siqueira et al. 2020). The administration of antibiotics and EPIs simultaneously can reduce the amount needed of a drug to achieve the same effect (Prasch and Bucar 2015; Seukep et al. 2020). Many studies reported in the synthesis tables show the activity of synergisms or antibiotic potentiators of several Chalcones, such as the works carried out by Vásquez-Martínez et al. (2019) and Ferraz et al. (2020).
In the findings of this review, the following studies that worked with pumps were reported: (1) NorA: belonging to the largest and oldest pump family, the Major Facilitator Superfamily (MFS) and is present in the SA-199B strain.; (2) MepA: belonging to the Multidrug and Compound Extrusion Family (Multidrug and Toxic Compound Extrusion—MATE), this pump is expressed in K-2068 strains, both are reported in the works of Rezende-Júnior et al. (2020), Siqueira et al. (2020), Rocha et al. (2021), Xavier et al. (2021) and Da Silva et al. (2021); (3) MsrA: present in the RN-4220 S. aureus strain and belongs to the ABC protein group, this pump was used in the studies by Labrière et al. (2017), Xavier et al. (2021) and Rezende-Júnior et al. (2020); This last author also worked with the efflux pumps (4) QacA/B present in K4414 e (5) QacC overexpressed in K4100 strain, belonging to the family of transporters MFS and SMR, respectively.
The works mentioned above, for the most part, presented relevant results for the research, either by the inhibition of this mechanism by Chalcones or by the reduction of the MIC of the antibiotics used through synergisms, thus potentiating the antibiotic activity.
Structure–activity relationship
In medicinal chemistry, the term structure–activity relationship can be understood as the effect that the chemical structure of a compound has on its biological activity. The main objective of this analysis is to investigate how variation in chemical structure can affect the biological potential of a substance or the ligand/receptor affinity (Marino 2014). According to Guido et al. (2010), the structure–activity relationships can be defined from changes in the prototype molecule and the evaluation of its subsequent biological activities.
In the study by Kozłowska et al. (2019) the effect of structure on the biological activity of synthetic Chalcones is clearly shown. The author of the work synthesized 18 aminochalcones and it was possible to observe that the presence of an amino group in the meta position with the addition of the aromatic ring in compound 14, increased the hydrophobicity of the molecule, facilitating penetration of Chalcone into the cells of microorganisms. Babu and Selavaraju (2020) demonstrated that compounds tested for antibacterial activity that contained the methoxy portion exhibited excellent activity.
In another study also reported in the tables, it demonstrates the synthesis of heterocyclic Chalcones where the reactivity of the produced Chalcones (Chalcones 3) allowed the construction of several heterocyclic systems such as pyrazoline, isoxazoline, benzoflavone and benzocoumarin (El-Desoky et al. 2018). It is possible to observe in the cited study that the modification in the structure of the derivatives of Chalcones 3b to the corresponding flavonone 9b destroyed the antibacterial activity; however, the more the modification to flavone improved in the compound 11b the more the activity was restored beyond the reference antibiotic.
A comparative study was carried out between Chalcones and pyrazole derivatives about the antibacterial activity against S. aureus. There was a great difference in the antibacterial potential between these two compounds due to the presence of a hydroxy group in the fourth position of the A ring. The 2-pyrazoline functions did not increase the antimicrobial activity, while the Chalcones showed better activity (Evranos-Aksöz at al. 2015a, b). In the study by Teixeira et al. (2019) the presence of the hydroxy group in the compound 2E-1-(2ʹ-hydroxy-3ʹ,4ʹ,6ʹ-trimethoxyphenyl)-3-(phenyl)-prop-2-en-1-one (HYTPHENYL) did not determine any antibacterial activity against S. aureus; however, a synergism was noticed when this Chalcone was associated with the antibiotic Amikacin. This change in antimicrobial activity may be related to both the position of the hydroxy group and the presence of the methoxy group in Chalcone.
The Chalcona derivative (E)-1-(4-bromophenyl)-3-(4-iodophenyl)prop-2-en-1-one reported in the work by Zainuri et al. (2017) demonstrated good antibacterial activity against a standard strain of S. aureus with an MIC of 250 µg/mL. However, when the Iodine group was replaced by naphthalene in the study by Thanigaimani et al. (2015), forming the compound (E)-1-(4-Bromophenyl)-3-(napthalen-2-yl)prop-2-en-1-one the antimicrobial activity decreased considerably reaching a MIC of 500 µg/mL, demonstrating the importance of the effects caused by the change in the conformation of a chemical structure or the substitution of functional groups by others.
In the study by Ramírez-Prada et al. (2017) the antibacterial potential of Chalcones and some derivatives is described. Although the Chalcone [(7-Chloroquinolin-4-yl)amino]chalcones (8a–f) described in the table did not show relevant antibacterial activity, compound 6: 3-((7-Chloroquinolin-4-yl)amino)benzaldehyde which contains the parental benzaldehyde in its structure was the most active of the compounds tested, showing inhibitory activity against S. aureus. The results obtained with aldehyde 6 show that the 7-chloro-4-aminoquinoline nucleus confers antibacterial activity, but this activity is abolished when the nucleus is functionalized by other chemical groups.
The structure–activity relationship was summarized based on the antibacterial activity data presented in the summary tables. It was observed during the analysis of the articles that the α, β-unsaturated ketone fraction in Chalcones is essential for the maintenance of antibacterial activity and that the change in the A and B rings can drastically change the biological activity of a compound. It has also been observed that hybrid molecules can significantly improve the desired antimicrobial activity.
Conclusion
This literature review presented the recent advances in the research of natural and synthetic Chalcones with antibacterial potential, organized through informative tables on the articles that constituted the research sample. The main methodology used for the synthesis of Chalcones was described, as well as the main resistance mechanism reported in the articles, how it contributes to the multidrug resistance of S. aureus and the efforts that are being made to inhibit this mechanism. Furthermore, the structure–activity relationship was briefly discussed, to which it can be evidenced that several bioactive portions can be incorporated in the A and B rings and in the α,β-unsaturated ketone fragments. It was observed that the compounds based on Chalcones showed great antibacterial potential and demonstrated the ease of obtaining different skeletons of this group through the Claisen–Schmidt condensation methodology. Future perspectives in the study of Chalcones may be based on the investigation and synthesis of new antibacterial drugs based on Chalcones or substances composed of hybrid molecules, enrich studies about resistance reversal through inhibition of the mechanism cited in the present review and investigate the toxicity of Chalcones as antibacterial agentes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to the State university of Ceará—UECE; Regional University of Cariri—URCA Formatting of funding sources. Fundacąõ Cearense de Apoio ao Desenvolvimento Cientı´fico e Tecnolo´ gico—FUNCAP; Coordenacąõ de Aperfeic¸oamento de Pessoal de Nı´vel Superior—CAPES; Conselho Nacional de Desenvolvimento Cientı´fico e Tecnoloógico—CNPq.
Data availability
All data will be available after a reasonable request to the corresponding author.
Declarations
Conflict of interest
The authors declare that they have no known conflict of interest to disclose.
Ethical statements
This article is according with to the international, national and institutional rules considering biodiversity rights.
References
- Abo-Salem HM, Abdel-aziem A, Islam IE, Yossef MM, El-Sawi ER. Synthesis, antimicrobial activity and molecular docking study of some new N-benzyl and N-benzoyl-3-indolyl heterocycles. Inte J Pharm Pharm Sci. 2016;8(9):224–234. doi: 10.22159/ijpps.2016v8i9.13184. [DOI] [Google Scholar]
- Abushaheen MA, MuzaheedFatani AJ, Alosaimi M, Mansy W, George M, Acharya S, Rathod S, Divakar DD, Jhugroo C, Vellappally S, Khan AA, Shaik J, Jhugroo P. Antimicrobial resistance, mechanisms and its clinical significance. Dis Mon. 2020;66(6):100971. doi: 10.1016/j.disamonth.2020.100971. [DOI] [PubMed] [Google Scholar]
- Ajiboye TO, Haliru FZ. Redox and respiratory chain related alterations in the lophirones B and C-mediated bacterial lethality. Microb Pathog. 2016;100:95–111. doi: 10.1016/j.micpath.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Aksöz BE, Onurdağ FK, Aksöz E, Özgacar SÖ. Biological activity screening of some hydrazone and chalcone derivatives. Turk Hijyen Ve Deneysel Biyoloji Dergisi. 2021;78(2):159–166. doi: 10.5505/TurkHijyen.2020.02439. [DOI] [Google Scholar]
- Alam MS, Rahman SMM, Lee DU. Synthesis, biological evaluation, quantitative-SAR and docking studies of novel chalcone derivatives as antibacterial and antioxidant agents. Chem Pap. 2015;69(8):1118–1129. doi: 10.1515/chempap-2015-0113. [DOI] [Google Scholar]
- Alhage J, Elbitar H, Taha S, Guegan J, Dassouki Z, Vives T, Benvegnu T. Isolation of bioactive compounds from Calicotome villosa stems. Molecules. 2018 doi: 10.3390/molecules23040851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrohily WD, Mahmoud EH, El-Messery SM, Alqurshi A, El-Subbagh H, El-Sayed EH. Antibacterial, antibiofilm and molecular modeling study of some antitumor thiazole based chalcones as a new class of DHFR inhibitors. Microb Pathog. 2019;136(August):103674. doi: 10.1016/j.micpath.2019.103674. [DOI] [PubMed] [Google Scholar]
- Alves Borges Leal AL, Silva PT, Rocha MN, Marinho EM, Marinho ES, Marinho MM, Bandeira PN, Nogueira CES, Barreto HM, Teixeira AMR, Santos HS. Potentiating activity of Norfloxacin by synthetic chalcones against NorA overproducing Staphylococcus aureus. Microb Pathog. 2021;155(February):104894. doi: 10.1016/j.micpath.2021.104894. [DOI] [PubMed] [Google Scholar]
- Anvisa (2018) Antibióticos: uso indiscriminado deve ser controlado. Disponível em: https://tinyurl.com/y5c25lk7. Acesso em: 12 de de Dez de 2021.
- AshoK D, Ravi S, Ganesh A, Vijaya Lakshmi B, Adam S, Murthy SDS. Microwave-assisted synthesis and biological evaluation of carbazole-based chalcones, aurones and flavones. Med Chem Res. 2016;25(5):909–922. doi: 10.1007/s00044-016-1537-7. [DOI] [Google Scholar]
- Ashok D, Radhika G, Rao BA, Sarasija V, Jayashree A, Sadanandam P. Synthesis of benzoxazepine derivatives from pyrazole-chalcone via a simple and convenient protocol using basic alumina as solid support. J Chilean Chem Soc. 2018;63(2):3983–3987. doi: 10.4067/s0717-97072018000203983. [DOI] [Google Scholar]
- Assef APDC, Neto OCC (2020) Bases moleculares da resistência bacteriana. In: Brasil, Agência Nacional de Vigilância Sanitária. Microbiologia Clínica Para O Controle De Infecção Relacionada à Assistência á Saúde, 1° edn. Brasília, Anvisa, pp. 17–28
- Babu AK, Selvaraju K. Synthesis, biological evaluation and docking studies of novel chalcone derivatives as antimicrobial agents. Mater Today Proc. 2020;48(xxxx):382–386. [Google Scholar]
- Babu K, Selvi D, Pitchai D. Synthesis and microbial studies of novel 1, 3-thiazine compounds bearing schiff base moiety. Der Pharma Chemica. 2015;7(10):89–92. [Google Scholar]
- Banoth RK, Thatikonda A. A review on natural chalcones: an update. Int J Pharm Sci Res. 2020;11:546–555. [Google Scholar]
- Barroso H, Meliço-Silvestre A, Taveira N (2014) Microbiologia médica: fundamentos de microbiologia, conceito básicos da resposta imunológica, princípios do diagnóstico, microbiológico médico e bacteriologia. (LIDEL - Edições Técnicas Lda, Ed.). Lisboa
- Bassin P, Botha MJ, Garikipati R, Goyal M, Martin L, Shah S. Synthesis and antibacterial activity of benzo[4,5]isothiazolo[2,3-α]pyrazine-6,6-dioxide derivatives. Molecules. 2017 doi: 10.3390/molecules22111889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begmatov N, Begmatov N, Li N, Bobakulov K, Numonov K, Aisa HA. The chemical components of Coreopsis tinctoria Nutt. and their antioxidant, antidiabetic and antibacterial activities. Nat Product Res. 2020;34(12):1772–1776. doi: 10.1080/14786419.2018.1525377. [DOI] [PubMed] [Google Scholar]
- Bhirud JD, Patil RD, Narkhede HP. Sulfamic acid catalyzed synthesis of new 3,5-[(sub)phenyl]-1H-pyrazole bearing N1-isonicotinoyl: and their pharmacological activity evaluation. Bioorganic Med Chem Lett. 2020;30(23):127558. doi: 10.1016/j.bmcl.2020.127558. [DOI] [PubMed] [Google Scholar]
- Bingi C, Emmadi R, Chennapuram M, Poornachandra Y, Ganesh Kumar C, Nanubolu JB, Atmakur K. One-pot catalyst free synthesis of novel kojic acid tagged 2-aryl/alkyl substituted-4H-chromenes and evaluation of their antimicrobial and anti-biofilm activities. Bioorganic Med Chem Lett. 2015;25(9):1915–1919. doi: 10.1016/j.bmcl.2015.03.034. [DOI] [PubMed] [Google Scholar]
- Bitencourt HR, Marinho AMR, Filho APSS, Pinheiro JC, Tavares MGC, Almeida O, Farias RAF (2019) Síntese de Chalconas. Processos Químicos e Biotecnológicos—V. 6. 10.36229/978-65-5866-009-5
- Bocquet L, Sahpaz S, Bonneau N, Beaufay C, Mahieux S, Samaillie J, Roumy V, Jacquin J, Bordage S, Hennebelle T, Chai F, Quetin-Leclercq J, Neut C, Rivière C. Phenolic compounds from humulus lupulus as natural antimicrobial products: New weapons in the fight against methicillin resistant staphylococcus aureus, leishmania mexicana and trypanosoma brucei strains. Molecules. 2019;24(6):1–25. doi: 10.3390/molecules24061024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdar APS, Sadegui A, Aghaei H, Maal KB. Convenient synthesis of novel chalcone and pyrazoline sulfonamide derivatives as potential antibacterial agents. Russ J Bioorg Chem. 2020;46(3):371–381. doi: 10.1134/S1068162020030048. [DOI] [Google Scholar]
- Božić DD, Božić DD, Milenković MT, Ivković BM, Larsen AR, Cirković IB. Inhibitory effect of newly-synthesized chalcones on hemolytic activity of methicillin-resistant Staphylococcus aureus. Polish J Microbiol. 2015;64(4):379–382. doi: 10.5604/17331331.1185237. [DOI] [PubMed] [Google Scholar]
- Budak Y, Koçyiğit UM, Gürdere MB. Synthesis and investigation of antibacterial activities and carbonic anhydrase and acetyl cholinesterase inhibition profiles of novel 4,5-dihydropyrazol and pyrazolyl-thiazole derivatives containing methanoisoindol-1,3-dion unit. Synth Commun. 2017;47(24):2313–2323. doi: 10.1080/00397911.2017.1373406. [DOI] [Google Scholar]
- Castaño LF, Cuartas V, Bernal A, Insuasty A, Guzman J, Vidal O, Rubio V, Puerto G, Lukáč P, Vimberg V, Balíková-Novtoná G, Vannucci L, Janata J, Quiroga J, Abonia R, Nogueras M, Cobo J, Insuasty B. New chalcone-sulfonamide hybrids exhibiting anticancer and antituberculosis activity. Eur J Med Chem. 2019;176:50–60. doi: 10.1016/j.ejmech.2019.05.013. [DOI] [PubMed] [Google Scholar]
- Chiaradia LD (2010) Síntese, caracterização e Estudo da Relação Estrutura-Atividade (REA) de chalconas e de compostos heterocíclicos biologicamente ativos em doenças negligenciadas, hiperglicemia e leucemia. p. 524
- Chitreddy V, Subbareddy CV, Sundarrajan S, Mohanapriya A, Subashini R, Shanmugam S. Synthesis, antioxidant, antibacterial, solvatochromism and molecular docking studies of indolyl-4H-chromene-phenylprop-2-en-1-one derivatives. J Mol Liq. 2018;251:296–307. doi: 10.1016/j.molliq.2017.12.082. [DOI] [Google Scholar]
- Chowdary Nagendra B, Umashankara M, Dinesh B, Girish K, Baba AR. Development of 5-(Aryl)-3-phenyl-1H-pyrazole derivatives as potent antimicrobial compounds. Asian J Chem. 2019;31(1):45–50. doi: 10.14233/ajchem.2019.21455. [DOI] [Google Scholar]
- Chu WC, Bay PY, Yang Z, Cui D, Hua YG, Yang Y, Yang Q, Zhang E, Qin S. Synthesis and antibacterial evaluation of novel cationic chalcone derivatives possessing broad spectrum antibacterial activity. Eur J Med Chem. 2018;143:905–921. doi: 10.1016/j.ejmech.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Costa SS, Viveiros M, Amaral L, Couto I. Multidrug efflux pumps in Staphylococcus aureus: an update. Open Microbiol J. 2013;7(1):59–71. doi: 10.2174/1874285801307010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa GM, Endo EH, Cortez DAG, Nakamura TU, Nakamura CV, Dias Filho BP. Antimicrobial effects of Piper hispidum extract, fractions and chalcones against Candida albicans and Staphylococcus aureus. J Mycol Med. 2016;26(3):217–226. doi: 10.1016/j.mycmed.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Cuartas V, Robledo SM, Vélez ID, Crespo MDP, Sortino M, Zacchino S, Nogueras M, Cobo J, Upegui Y, Pineda T, Yepes L, Insuast B. New thiazolyl-pyrazoline derivatives bearing nitrogen mustard as potential antimicrobial and antiprotozoal agents. Archiv der Pharmazie. 2020;353(5):e1900351. doi: 10.1002/ardp.201900351. [DOI] [PubMed] [Google Scholar]
- Cuellar JE, Martínez J, Rojano B, Gil JH, Durango D. Chemical composition and antioxidant and antibacterial activity of Platymiscium gracile Benth.: a species threatened by extinction. J King Saud Univ Sci. 2020;32(1):702–708. doi: 10.1016/j.jksus.2018.11.006. [DOI] [Google Scholar]
- Cui Y, Taniguchi S, Kuroda T, Hatano T. Constituents of psoralea corylifolia fruits and their effects on methicillin-resistant Staphylococcus aureus. Molecules. 2015;20(7):12500–12511. doi: 10.3390/molecules200712500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva PT, Xavier JC, Freitas TS, Oliveira MM, Coutinho HDM, Leal ALAB, Barreto HM, Bandeira PN, Nogueira CES, Sena DM. Synthesis, spectroscopic characterization and antibacterial evaluation by chalcones derived of acetophenone isolated from Croton anisodontus Müll. Arg. J Mol Struct. 2021;1226:129403. doi: 10.1016/j.molstruc.2020.129403. [DOI] [Google Scholar]
- Dan W, Dai J. Recent developments of chalcones as potential antibacterial agents in medicinal chemistry. Eur J Med Chem. 2019 doi: 10.1016/j.ejmech.2019.111980. [DOI] [PubMed] [Google Scholar]
- Dave SS, Rahatgaonkar AM. Syntheses and anti-microbial evaluation of new quinoline scaffold derived pyrimidine derivatives. Arab J Chem. 2016;9:S451–S456. doi: 10.1016/j.arabjc.2011.06.009. [DOI] [Google Scholar]
- Desai S, Sastry VG. Synthesis, antimicrobial and antitubercular activity of some pyrazoline derivatives from chalcones of indane-1,3-dione. Int J Pharm Res. 2017;9(1):81–85. [Google Scholar]
- Desai V, Desai S, Gaonkar SN, Palyekar U, Joshi SD, Dixit SK. Novel quinoxalinyl chalcone hybrid scaffolds as enoyl ACP reductase inhibitors: synthesis, molecular docking and biological evaluation. Bioorg Med Chem Lett. 2017;27(10):2174–2180. doi: 10.1016/j.bmcl.2017.03.059. [DOI] [PubMed] [Google Scholar]
- Díaz-Tielas C, Graña E, Reigosa MJ, Sánchez-Moreiras AM. Biological activities and novel applications of chalcones. Planta Daninha Viçosa-MG. 2016;34(3):607–616. doi: 10.1590/s0100-83582016340300022. [DOI] [Google Scholar]
- Divakar MA, Shanmugam S. Live cell imaging of bacterial cells: pyrenoylpyrrole-based fluorescence labeling. Chem Biol Drug Des. 2017;90(4):554–560. doi: 10.1111/cbdd.12978. [DOI] [PubMed] [Google Scholar]
- Ducki S, Forreste S, Hadfield JA, Kendall A, Lawrence NJ, McGown AT, Rennison D. Potent antimitotic and cell growth inhibitory properties of substituted chalcones. Bioorg Med Chem Lett. 1998;8:1051–1056. doi: 10.1016/S0960-894X(98)00162-0. [DOI] [PubMed] [Google Scholar]
- El-desoky ESI, Keshk EM, El-Sawi AA, Abozeide MA, Abouzeide LA, Abdel-Rahman H. Synthesis, biological evaluation and in silico molecular docking of novel 1-hydroxy-naphthyl substituted heterocycles. Saudi Pharm J. 2018;26(6):852–859. doi: 10.1016/j.jsps.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-hashash M, Rizk S, Atta-Allah SR. Synthesis and regioselective reaction of some unsymmetrical heterocyclic chalcone derivatives and spiro heterocyclic compounds as antibacterial agents. Molecules. 2015;20(12):22069–22083. doi: 10.3390/molecules201219827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Messery SM, Habib EE, Al-Rashood STA, Hassan GS. Synthesis, antimicrobial, anti-biofilm evaluation, and molecular modelling study of new chalcone linked amines derivatives. J Enzyme Inhib Med Chem. 2018;33(1):818–832. doi: 10.1080/14756366.2018.1461855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherief HA, Abuo-Rahma GEA, Shoman ME, Beshr EA, Abdel-Baky RM. Design and synthesis of new coumarin–chalcone/NO hybrids of potential biological activity. Med Chem Res. 2017;26(12):3077–3090. doi: 10.1007/s00044-017-2004-9. [DOI] [Google Scholar]
- Emeri FTAS, Fernanda T, Rosalen PL, Paganini ER, Rocha Garcia MA, Nazare AC, Lazarini JG, Alencar SM, Regasini LO, OrlanSdiardi JC. Antimicrobial activity of nitrochalcone and pentyl caffeate against hospital pathogens results in decreased microbial adhesion and biofilm formation. Biofouling. 2019;35(2):129–142. doi: 10.1080/08927014.2019.1574763. [DOI] [PubMed] [Google Scholar]
- Ergüntürk D, Gürdere ME, Budak Y, Ceylan M. Synthesis, characterization, and investigations of antimicrobial activity of 6,6-dimethyl-3-aryl-3′,4′,6,7-tetrahydro-1′H,3H-spiro[benzofuran-2,2′-naphthalene]-1′,4(5H)-dione. Synth Commun. 2017;47(16):1501–1506. doi: 10.1080/00397911.2017.1334918. [DOI] [Google Scholar]
- Evranos-Aksöz B, Onurda FK, Özgacar SÖ. Antibacterial, antifungal and antimycobacterial activities of some pyrazoline, hydrazone and chalcone derivatives. Zeitschrift Fur Naturforschung Sect C J Biosci. 2015;70(7–8):183–189. doi: 10.1515/znc-2014-4195. [DOI] [PubMed] [Google Scholar]
- Evranos-Aksöz B, Onurdağ FK, Özgacar SO. Antibacterial, antifungal and antimycobacterial activities of some pyrazoline, hydrazone and chalcone derivatives. Zeitschrift Fur Naturforschung Sect C J Biosci. 2015;70(7–8):183–189. doi: 10.1515/znc-2014-4195. [DOI] [PubMed] [Google Scholar]
- Fandakli S, Kahriman N, Yücel TB, Karaoğlu SA, Yayli N. Biological evaluation and synthesis of new pyrimidine-2(1H)-ol/-thiol derivatives derived from chalcones using the solid phase microwave method. Turkish J Chem. 2018;42(2):520–535. doi: 10.3906/kim-1711-9. [DOI] [Google Scholar]
- Farooq S, Ngaini Z. Synthesis, molecular docking and antimicrobial activity of α, β-unsaturated ketone exchange moiety for chalcone and pyrazoline derivatives. Chem Select. 2020;5(32):9974–9979. [Google Scholar]
- Ferraz CAN, Tintino SR, Teixeira AMR, Bandeira PN, Santos HS, Cruz BG, Nogueira CES, Moura TF, Pereira RLS, Sena Junior DM, Freitas TS, Rocha JE, Coutinho HD. Potentiation of antibiotic activity by chalcone (E)-1-(4′-aminophenyl)-3-(furan-2-yl)-prop-2-en-1-one against gram-positive and gram-negative MDR strains. Microb Pathogenesis. 2020;148(June):104453. doi: 10.1016/j.micpath.2020.104453. [DOI] [PubMed] [Google Scholar]
- Ferreira MKA, Fontenelle ROS, Magalhães FEA, Bandeira PNS, Menezes JESA, dos Santos H. Potencial Farmacológico de Chalconas: Uma Breve Revisão. Revista Virtual De Quimica. 2018;10(5):1455–1473. [Google Scholar]
- Fonseca PS (2012) Síntese e Caracterização de Chalconas e Dichalconas contendo unidades 1,2,3-triazólicas. p. 214
- Freitas TS, Xavier JC, Pereira RLS, Rocha JE, Muniz DF, Silva PT, Hora JP, Santos HS, Bandeira PN, Nogueira CES, Teixeira AMR, Coutinho HDM. Direct antibacterial and antibiotic resistance modulatory activity of chalcones synthesized from the natural product 2-hydroxy-3,4,6-trimethoxyacetophenone. FEMS Microbiol Lett. 2020;367(15):1–8. doi: 10.1093/femsle/fnaa124. [DOI] [PubMed] [Google Scholar]
- Garcia TR, de Freitas TS, dos Santos HS, Bandeira PN, Julião MSS, Rocha JE, Nogueira CES, Pereira RLS, Barreto ACH, Freire PTC, Coutinho HDM, Teixeira AMR. Structural, vibrational and electrochemical analysis and antibiotic activity study of chalcone (2E)-1-(3ʹ,-methoxy-4ʹ,-hydroxyphenyl)-3-(3-nitrophenyl)prop-2-en-1-one. J Mol Struct. 2020;1216:128358. doi: 10.1016/j.molstruc.2020.128358. [DOI] [Google Scholar]
- Garcia MAR, Theodoro RS, Sardi JCO, Santos MB, Ayusso GM, Pavan FR, Costa AR, Cruz LMR, Rosalen PL, Regasini LO. Design, synthesis and antibacterial activity of chalcones against MSSA and MRSA planktonic cells and biofilms. Química Bioorgânica. 2021;116:105279. doi: 10.1016/j.bioorg.2021.105279. [DOI] [PubMed] [Google Scholar]
- Gaur R, Gupta VK, Pal A, Darokar MP, Bhakuni RS, Kumarc B. In vitro and in vivo synergistic interaction of substituted chalcone derivatives with norfloxacin against methicillin resistant Staphylococcus aureus. RSC Adv. 2015;5(8):5830–5845. doi: 10.1039/C4RA10842F. [DOI] [Google Scholar]
- Gibson MZ, Nguyen MA, Zingales SK. Design, synthesis, and evaluation of (2-(pyridinyl)methylene)-1-tetralone chalcones for anticancer and antimicrobial activity. Med Chem. 2017 doi: 10.2174/1573406413666171020121244. [DOI] [PubMed] [Google Scholar]
- Gładkowski W (2019) Substituted γ-Oxa-ε-lactones. Derived. pp. 1–15
- Gondru R, Saini R, Vaarla K, Singh S, Sirassu N, Bavantula R, Saxen AK. Synthesis and characterization of chalcone-pyridinium hybrids as potential anti-cancer and anti-microbial agents. Chem Select. 2018;3(5):1424–1431. doi: 10.1002/slct.201702971. [DOI] [Google Scholar]
- Gopi C, Sastry VG, Dhanaraju MD. Synthesis and spectroscopic characterisation of novel bioactive molecule of 3-(2-substituted)-1H-indol-3-yl)-1-(thiophen-2yl)prop-2-en-1-one chalcone derivatives as effective anti-oxidant and anti-microbial agents. Beni-Suef Univ J Basic Applied Sci. 2016;5(3):236–243. [Google Scholar]
- Grace D, Fetsch A. Staphylococcus aureus—a foodborne pathogen: Epidemiology, detection, characterization, prevention, and control: an overview. In: Fetsch A, editor. Staphylococcus aureus. Amsterdam: Elsevier; 2017. pp. 3–8. [Google Scholar]
- Guido RV, Andricopulo AD, Oliva G. Planejamento de fármacos, biotecnologia e química medicinal: aplicações em doenças infecciosas. Estudos Avançados. 2010;24(70):81–98. doi: 10.1590/S0103-40142010000300006. [DOI] [Google Scholar]
- Guo Y, et al. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front Cell Infect Microbiol. 2020;10(March):1–11. doi: 10.3389/fcimb.2020.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta VK, Gaurb R, Sharma A, Akthera J, Sainia M, Bhakunib RS, Pathania R. A novel bi-functional chalcone inhibits multi-drug resistant Staphylococcus aureus and potentiates the activity of fluoroquinolones. Bioorg Chem. 2019;83(August 2018):214–225. doi: 10.1016/j.bioorg.2018.10.024. [DOI] [PubMed] [Google Scholar]
- Henry EJ, Bird SJ, Gowland P, Collins M, Cassella JP. Ferrocenyl chalcone derivatives as possible antimicrobial agents. J Antibiotics. 2020;73(5):299–308. doi: 10.1038/s41429-020-0280-y. [DOI] [PubMed] [Google Scholar]
- Himangini D, Pathak DP. Synthesis, characterization and in vitro antibacterial activity of new chalcones linked via coumarin ring. Der Pharma Chemica. 2016;8(15):112–115. [Google Scholar]
- Hu Y, et al. Novel chalcone-conjugated, multi-flexible end-group coumarin thiazole hybrids as potential antibacterial repressors against methicillin-resistant Staphylococcus aureus. Eur J Med Chem. 2021;222:113628. doi: 10.1016/j.ejmech.2021.113628. [DOI] [PubMed] [Google Scholar]
- Husain A, et al. Studies on 1,1′-(4,6-dihydroxy-1,3-phenylene)diethanone based bischalcones and flavones. World J Pharm Sci. 2015;7(8):117–121. [Google Scholar]
- Ibrahim TS, Almalki AJ, Moustafa AH, Allam RM, Abuo-Rahma GEA, El-Subbagh HI, Mohamed MFA. Novel 1,2,4-oxadiazole-chalcone/oxime hybrids as potential antibacterial DNA gyrase inhibitors: design, synthesis, ADMET prediction and molecular docking study. Bioorg Chem. 2021;111(April):104885. doi: 10.1016/j.bioorg.2021.104885. [DOI] [PubMed] [Google Scholar]
- Jang S. Multidrug efflux pumps in Staphylococcus aureus and their clinical implications. J Microbiol. 2016;54(1):1–8. doi: 10.1007/s12275-016-5159-z. [DOI] [PubMed] [Google Scholar]
- Jayaramu PK, Maralihallia RR. Synthesis and in vitro biological activities of chalcones and their heterocyclic derivatives. Der Pharma Chemica. 2015;7(8):30–35. [Google Scholar]
- Joanna Kozłowska J, Potaniec B, Baczyńska D, Żarowska B, Anioł M. Synthesis and biological evaluation of novel aminochalcones as potential anticancer and antimicrobial agents. Molecules. 2019;24(22):1–18. doi: 10.3390/molecules24224129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Yardily A. Spectral, modeling and biological studies on a novel (E)-3-(3-bromo-4-methoxyphenyl)-1-(thiazol-2-yl)prop-2-en-1-one and some bivalent metal(II) complexes. J Mol Struct. 2021;1244:130991. doi: 10.1016/j.molstruc.2021.130991. [DOI] [Google Scholar]
- Joray MB, Trucco LD, González ML, Napal GND, Palacios SM, Bocco JL, Carpinella M. Antibacterial and cytotoxic activity of compounds isolated from Flourensia oolepis. Evid-Based Complement Altern Med. 2015;2015:1–11. doi: 10.1155/2015/912484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaskar MG, Surana SJ. Pharmacognostic and phytochemical studies on Ficus Microcarpa L. fil. Anc Sci Life. 2012;32(2):107–111. doi: 10.4103/0257.7941.118550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalenić S. The importance of methicillin-resistant Staphylococcus aureus in human medicine. Med Sci. 2012;37(November):61–72. [Google Scholar]
- Kandaswamy N. Synthesis, characterisation and antimicrobial evaluation of chalcone coupled biscoumarin copolyesters. Macromol Res. 2019 doi: 10.1007/s13233-019-7082-8. [DOI] [Google Scholar]
- Kant R, Kumar D, Agarwal D, Gupta RD, Tilak R, Awasthi SK, Agarwal A. Synthesis of newer 1,2,3-triazole linked chalcone and flavone hybrid compounds and evaluation of their antimicrobial and cytotoxic activities. Amsterdam: Elsevier Ltd; 2016. [DOI] [PubMed] [Google Scholar]
- Kapkoti DS, Gupta VK, Darokar MP, Bhakuni RS. Glabridin-chalcone hybrid molecules: drug resistance reversal agent against clinical isolates of methicillin-resistant Staphylococcus aureus. MedChemComm. 2016;7(4):693–705. doi: 10.1039/C5MD00527B. [DOI] [Google Scholar]
- Kaushik R, Chand M, Jain SC. Synthesis and antibacterial evaluation of nitrogen containing novel heterocyclic chalcones. Synthetic Commun. 2018;48(11):1308–1315. doi: 10.1080/00397911.2018.1440602. [DOI] [Google Scholar]
- Khayyat SA, Saddiq AA. Photochemical and antimicrobial studies of cinnamaldehyde and its bioactive derivatives. Asian J Chem. 2015;27(8):3023–3027. doi: 10.14233/ajchem.2015.18813. [DOI] [Google Scholar]
- Killeen DP, Larsen L, Dayan FE, Gordon KC, Perry NB, Klink JWV. Nortriketones: antimicrobial trimethylated acylphloroglucinols from Mānuka (Leptospermum scoparium) J Nat Prod. 2016;79(3):564–569. doi: 10.1021/acs.jnatprod.5b00968. [DOI] [PubMed] [Google Scholar]
- Kocyigit UM, Budak Y, Gürdere MB, Tekin S, Köprülü TK, Ertürk F, Özcan K, Gülçin I, Ceylan M. Synthesis, characterization, anticancer, antimicrobial and carbonic anhydrase inhibition profiles of novel (3aR,4S,7R,7aS)-2-(4-((E)-3-(3-aryl)acryloyl) phenyl)-3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3(2H)-dione derivatives. Bioorg Chem. 2017;70:118–125. doi: 10.1016/j.bioorg.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Konidala SK, Dandunga RCSR, Kola PK. Design, multistep synthesis and in-vitro antimicrobial and antioxidant screening of coumarin clubbed chalcone hybrids through molecular hybridization approach. Arab J Chem. 2021;14(6):103154. doi: 10.1016/j.arabjc.2021.103154. [DOI] [Google Scholar]
- Koudokpon H, Armstrong N, Dougnon TV, Fah L, Hounsa E, Bankolé HS, Loko F, Chabrière E, Rolai JM. Antibacterial activity of chalcone and dihydrochalcone compounds from Uvaria chamae roots against multidrug-resistant bacteria. BioMed Res Int. 2018 doi: 10.1155/2018/1453173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Lu Y, Elliott AG, Kavanagh AM, Cooper MA, Davis RA, Rohan A. Semi-synthesis and NMR spectral assignments of flavonoid and chalcone derivatives. Magnetic Resonance Chem. 2016;54(11):880–886. doi: 10.1002/mrc.4482. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kumar A, Pinto JS, Bhashini AG. Synthesis and biological evaluation of pyrimidine derivatives via pyrrolyl chalcones. Res J Pharm Technol. 2017;10(5):1392–1394. doi: 10.5958/0974-360X.2017.00248.7. [DOI] [Google Scholar]
- Kumar P, Kumar A, Deeksha S, Nireeksha G, D’Souza S. Synthesis and antimicrobial evaluation of different substituted phenylpropenone pyrrolyl chalcones. Res J Pharm Technol. 2017;10(5):1426–1428. doi: 10.5958/0974-360X.2017.00252.9. [DOI] [Google Scholar]
- Kumari S, Paliwal SK, Chauhan R. An improved protocol for the synthesis of chalcones containing pyrazole with potential antimicrobial and antioxidant activity. Curr Bioact Compd. 2018;14(1):39–47. doi: 10.2174/1573407212666161101152735. [DOI] [Google Scholar]
- Kwesiga G, Kelling A, Kersting S, Sperlich E, Nickisch-Rosenegk MV, Schmidt B. Total syntheses of prenylated isoflavones from Erythrina sacleuxii and their antibacterial activity: 5-Deoxy-3′-prenylbiochanin A and Erysubin F. J Nat Prod. 2020;83(11):3445–3453. doi: 10.1021/acs.jnatprod.0c00932. [DOI] [PubMed] [Google Scholar]
- Labrière C, Gong H, Finlay BB, Reiner NE, Young RN. Further investigation of inhibitors of MRSA pyruvate kinase: towards the conception of novel antimicrobial agents. Eur J Med Chem. 2017;125:1–13. doi: 10.1016/j.ejmech.2016.09.018. [DOI] [PubMed] [Google Scholar]
- Lagu SB, Yejella RP, Bhandare RR, Shaik AB. Design, synthesis, and antibacterial and antifungal activities of novel trifluoromethyl and trifluoromethoxy substituted chalcone derivatives. Pharmaceuticals. 2020;13(11):1–16. doi: 10.3390/ph13110375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Chen Y, Li H, Liu J, Liu S. Shouping. Design, synthesis, and evaluation of amphiphilic sofalcone derivatives as potent Gram-positive antibacterial agents. Eur J Med Chem. 2020;202:112596. doi: 10.1016/j.ejmech.2020.112596. [DOI] [PubMed] [Google Scholar]
- Lindoso JAL, Lindoso AABP. Neglected tropical diseases in Brazil. Rev Inst Med Trop Sao Paulo. 2009;51:247–253. doi: 10.1590/S0036-46652009000500003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiao S, Yin D, Hong X, Xiaoming Y, Qianyu Q, Yang Z. Ferrocenyl chalcone-based Schiff bases and their metal complexes: highly efficient, solvent-free synthesis, characterization, biological research. J Organometallic Chem. 2018;856:27–33. doi: 10.1016/j.jorganchem.2017.12.022. [DOI] [Google Scholar]
- Liu Y, Yang L, Yin D, Dang Y, Yang L, Zou Q, Li J, Sun J. Solvent-free synthesis, characterization, biological activity of schiff bases and their metal (II) complexes derived from ferrocenyl chalcone. J Organomet Chem. 2019;899:120903. doi: 10.1016/j.jorganchem.2019.120903. [DOI] [Google Scholar]
- Lokeshwari D, Mahadeva S, Kumar AD, Srinivasan B, Shivalingegowda N, Neratur KL, Kumar KA. Synthesis of novel 2-pyrazoline analogues with potent anti-inflammatory effect mediated by inhibition of phospholipase A2: Crystallographic, in silico docking and QSAR analysis. Bioorg Med Chem Lett. 2017;27(16):3806–3811. doi: 10.1016/j.bmcl.2017.06.063. [DOI] [PubMed] [Google Scholar]
- Loureiro RJ, Roque F, Rodrigues AT, Herdeiro MT, Ramalheira E. O uso de antibióticos e as resistências bacterianas: breves notas sobre a sua evolução. Revista Portuguesa De Saúde Pública. 2016;34(1):77–84. doi: 10.1016/j.rpsp.2015.11.003. [DOI] [Google Scholar]
- Mahapatra DK, Bharti SK, Asati V. Chalcone scaffolds as antiinfective agents: structural and molecular target perspectives. Eur J Med Chem. 2015;101:496–524. doi: 10.1016/j.ejmech.2015.06.052. [DOI] [PubMed] [Google Scholar]
- Mahmoodi NO, Zeydi MM, Mamaghani M, Monyazeri N. Synthesis and antibacterial evaluation of several novel tripod pyrazoline with triazine core (TPTC) compounds. Res Chem Intermed. 2017;43(4):2641–2651. doi: 10.1007/s11164-016-2786-2. [DOI] [Google Scholar]
- Mariani R, Suganda AG, Sukandar EY. Drug-drug interactions between griseofulvinand a new prenylated chalcone from elatostema parasiticum and its antibacterial activity nortriptylineat binding sites of bovine serum albumin. Pharmacologyonline. 2016;1:2016. [Google Scholar]
- Marino PA (2014) Estudo de chalconas como antibacterianos potenciais: síntese, avaliação da ação antibacteriana e das propriedades físico-químicas, pp. 1–98
- Matos S, Vazquez-Rodriguez E, Uriarte L. Potential pharmacological uses of chalcones: a patent review (from June 2011–2014) Expert Opin Therap Patents. 2015;25:351–366. doi: 10.1517/13543776.2014.995627. [DOI] [PubMed] [Google Scholar]
- Mazimba O. Antimicrobial activities of heterocycles derived from thienylchalcones. J King Saud Univ Sci. 2015;27(1):42–48. doi: 10.1016/j.jksus.2014.06.003. [DOI] [Google Scholar]
- Meier D, Hernández MV, Van Geelen L, Muharini R, Proksch P, Bandow JE, Kalscheuer R. The plant-derived chalcone Xanthoangelol targets the membrane of Gram-positive bacteria. Bioorg Med Chem. 2019;27(23):115151. doi: 10.1016/j.bmc.2019.115151. [DOI] [PubMed] [Google Scholar]
- Mendes J. Resistência Antibiótica no Staphylococcus aureus; da Investigação Básica à Prática Clínica. Revista Portuguesa De Medicina Intensiva. 2010;17(1):11–15. [Google Scholar]
- Mishra VK, Mishra M, Kashaw V, Kashaw SK. Synthesis of 1,3,5-trisubstituted pyrazolines as potential antimalarial and antimicrobial agents. Bioorg Med Chem. 2017;25(6):1949–1962. doi: 10.1016/j.bmc.2017.02.025. [DOI] [PubMed] [Google Scholar]
- Moreno A, Cuello S, Nuño G, Sayago J. Anti-inflammatory, antioxidant and antimicrobial activity characterization and toxicity studies of flowers of “Jarilla”, a medicinal shrub from Argentina. Nat Prod Commun. 2015;10(6):4–7. doi: 10.1177/1934578X1501000648. [DOI] [PubMed] [Google Scholar]
- Muškinja JM, Burmudžija A, Ratković Z, Ranković B, Kosanić M, Bogdanović G, Novaković S. Ferrocenyl chalcones with O-alkylated vanillins: synthesis, spectral characterization, microbiological evaluation, and single-crystal X-ray analysis. Med Chem Res. 2016;25(9):1744–1753. doi: 10.1007/s00044-016-1609-8. [DOI] [Google Scholar]
- Nagwanshi R, Bakhru M, Jain S. Photodimerization of heteroaryl chalcones: comparative antimicrobial activities of chalcones and their photoproducts. Med Chem Res. 2012;21(8):1587–1596. doi: 10.1007/s00044-011-9667-4. [DOI] [Google Scholar]
- Narender T, Reddy KP. A simple and highly efficient method for the synthesis of chalcones by using borontrifluoride-etherate. Tetrahedron Lett. 2007;48(18):3177–3180. doi: 10.1016/j.tetlet.2007.03.054. [DOI] [Google Scholar]
- Neill JO (2016) Antimicrobial resistance: tackling a crisis for the health and wealth of nations The Review on Antimicrobial Resistance Chaired
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Nisa S, Yusuf M. Synthetic and antimicrobial studies of N-substituted-pyrazoline-based new bisheterocycles. J Heterocycl Chem. 2020;57(4):2024–2036. doi: 10.1002/jhet.3910. [DOI] [Google Scholar]
- Nowakowska Z. A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem. 2007;42:125. doi: 10.1016/j.ejmech.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Nowakowska LJO (2016) Antimicrobial Resistance: tackling a crisis for the health and wealth of nations The Review on Antimicrobial Resistance Chaired
- Nuño G, Alberto MR, Arena ME, Zampini IC, Isla MI. Effect of Zuccagnia punctata Cav (Fabaceae) extract on pro-inflammatory enzymes and on planktonic cells and biofilm from Staphylococcus aureus, Toxicity studies. Saudi J Biol Sci. 2018;25(8):1713–1719. doi: 10.1016/j.sjbs.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M, Pereira K, Zamberlam C. Resistência Bacteriana Pelo Uso Indiscriminado De Antibióticos: Uma Questão De Saúde Pública. Ciências e Educação- REASE: Revista Ibero- Americana de Humanidades; 2020. pp. 183–201. [Google Scholar]
- Omosa LK, Midiwo JO, Mbaveng AT, Tankeo SB, Seukep JA, Voukeng IK, Dzotam JK, Isemeki J, Derese S, Omolle RA, Efferth T, Kuete V. Antibacterial activities and structure–activity relationships of a panel of 48 compounds from Kenyan plants against multidrug resistant phenotypes. Springerplus. 2016;5(1):1–15. doi: 10.1186/s40064-016-2599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel C, Bassin JP, Scott M, Flye J, Hunter AP, Martin L, Goyal M. Synthesis and antimicrobial activity of 1,2-benzothiazine derivatives. Molecules. 2016;21(7):1–16. doi: 10.3390/molecules21070861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Kumari P, Patel NB. Synthesis and biological evaluation of coumarin based isoxazoles, pyrimidinthiones and pyrimidin-2-ones. Arab J Chem. 2017;10:S3990–S4001. doi: 10.1016/j.arabjc.2014.06.010. [DOI] [Google Scholar]
- Patil VP, Barragán E, Patil S, Patil S, Bugarin A. Direct synthesis and antimicrobial evaluation of structurally complex chalcones. ChemistrySelect. 2016;1(13):3647–3650. doi: 10.1002/slct.201600703. [DOI] [Google Scholar]
- Pereira VJ, Kaplan MAC. The high bioactivity of Artocarpus: an exotic genus. Floresta Ambient. 2013 doi: 10.4322/floram.2012.075. [DOI] [Google Scholar]
- Pola S, Banoth KK, Sankaranarayanan M, Ummani R, Garlapati A. Design, synthesis, in silico studies, and evaluation of novel chalcones and their pyrazoline derivatives for antibacterial and antitubercular activities. Med Chem Res. 2020;29:1819–1835. doi: 10.1007/s00044-020-02602-8. [DOI] [Google Scholar]
- Prakash G, Boopathy M, Selvam R, Kumar SJ, Subramanian K. The effect of anthracene-based chalcone derivatives in the resazurin dye reduction assay mechanisms for the investigation of Gram-positive and Gram-negative bacterial and fungal infection. New J Chemi. 2018;42(2):1037–1045. doi: 10.1039/c7nj04125j. [DOI] [Google Scholar]
- Prasad S, Francis SM, Krishnan S, Bharathin P. Synthesis, spectroscopic studies, antibacterial activity, and colorimetric evaluation of the time-killing assay for newly synthesized chalcones using resazurin. Pharm Chem J. 2018;52(6):518–525. doi: 10.1007/s11094-018-1852-z. [DOI] [Google Scholar]
- Prasad S, Radhakrishna V, Ravi TK. Synthesis, spectroscopic and antibacterial studies of some schiff bases of 4-(4-bromophenyl)-6-(4-chlorophenyl)-2-aminopyrimidine. Arab J Chem. 2019;12(8):3943–3947. doi: 10.1016/j.arabjc.2016.03.003. [DOI] [Google Scholar]
- Prasch S, Bucar F. Plant derived inhibitors of bacterial efflux pumps: an update. Phytochem Rev. 2015;14(6):961–974. doi: 10.1007/s11101-015-9436-y. [DOI] [Google Scholar]
- Purrello SM, Daum RS, Edwards GFS, Lina G, Lindsay J, Peters G, Stefani S. Meticillin-resistant Staphylococcus aureus (MRSA) update: new insights into bacterial adaptation and therapeutic targets. J Global Antimicrobial Resistance. 2014;2(2):61–69. doi: 10.1016/j.jgar.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Raju GN, Suresh PV, Nadendla RR, Anusha K. Synthesis, characterization and antimicrobial evaluation of isoxazole derivatives. Der Pharma Chemica. 2015;7(6):346–352. [Google Scholar]
- Ramírez-Prada J, Robledo SM, Vélez ID, Crespo MP, Quiroga J, Abonia R, Montoya A, Svetaz L, Zacchino S, Insuasty B. Synthesis of novel quinoline–based 4,5–dihydro–1H–pyrazoles as potential anticancer, antifungal, antibacterial and antiprotozoal agents. Eur J Med Chem. 2017;131:237–254. doi: 10.1016/j.ejmech.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Rao M, Padyana S, Dipin KM, Kumar S, Nayak BB, Varela MF. Antimicrobial compounds of plant origin as efflux pump inhibitors: new avenues for controlling multidrug resistant pathogens. J Antimicrob Agents. 2018;04(01):1–6. doi: 10.4172/2472-1212.1000159. [DOI] [Google Scholar]
- Rashid HU, Xu Y, Ahmad N, Muhammad Y, Wang LS. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear fator kb activities. Bioorg Chem. 2019;87:335–365. doi: 10.1016/j.bioorg.2019.03.033. [DOI] [PubMed] [Google Scholar]
- Reddy POV, Hridhaya M, Nikhilb K, Khan S, Jha PN, Shah K, Kumar D. Synthesis and investigations into the anticancer and antibacterial activity studies of β-carboline chalcones and their bromide salts. Bioorg Med Chem Lett. 2018;28(8):1278–1282. doi: 10.1016/j.bmcl.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees KA, Bermudez C, Edwards DJ, Elliott AG, Ripen JE, Seta C, Huang JX, Cooper MA, Fraser JA, Yeo TC, Butler MS. Flemingin-type prenylated chalcones from the sarawak rainforest plant Desmodium congestum. J Nat Prod. 2015;78(8):2141–2144. doi: 10.1021/acs.jnatprod.5b00410. [DOI] [PubMed] [Google Scholar]
- Rezende-Júnior LM, Andrade LMS, Leal ALAB, Mesquita ABS, Santos ALPA, Neto JSL, Siqueira-Júnior JP, Nogueira CES, Kaatz GW, Coutinho HDM, Martins N, Rocha CQ, Barreto HM. Chalcones isolated from Arrabidaea brachypoda flowers as inhibitors of nora and mepa multidrug efflux pumps of Staphylococcus aureus. Antibiotics. 2020;9(6):1–12. doi: 10.3390/antibiotics9060351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha JE, Freitas TS, Xavier JC, Pereira RLS, Pereira Junior FN, Nogueira CES, Marinho MM, Bandeira PN, Oliveira MR, Marinho ES, Teixeira AMR, Santos HS, Coutinho HDM. Antibacterial and antibiotic modifying activity, ADMET study and molecular docking of synthetic chalcone (E)-1-(2-hydroxyphenyl)-3-(2,4-dimethoxy-3-methylphenyl)prop-2-en-1-one in strains of Staphylococcus aureus carrying NorA and MepA efflux pumps. Biomed Pharmacotherapy. 2021;140(May):111768. doi: 10.1016/j.biopha.2021.111768. [DOI] [PubMed] [Google Scholar]
- Rossiter SE, Fletcher MH, Wuest WM. Natural products as platforms to overcome antibiotic resistance. Chem Rev. 2017;117:12415–12474. doi: 10.1021/acs.chemrev.7b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadgir NV, Dhonnar SL, Jagdale BS, Sawant AB. Synthesis, spectroscopic characterization, XRD crystal structure, DFT and antimicrobial study of (2E)-3-(2,6-dichlorophenyl)-1-(4-methoxyphenyl)-prop-2-en-1-one. SN Applied Sci. 2020;2(8):1–12. doi: 10.1007/s42452-020-2923-9. [DOI] [Google Scholar]
- Saleh MY, Ayoub AI, Hammady AO. Synthesis biological studies of some new heterocyclic compound derived from 2-chloro-3-formyl quinoline and 4-(benzyl sulfonyl) acetophenone. Egypt J Chem. 2020;63(12):4769–4776. [Google Scholar]
- Sanad SMH, Ahmed AAM, Mekky AEM. Efficient synthesis and molecular docking of novel antibacterial pyrimidines and their related fused heterocyclic derivatives. J Heterocycl Chem. 2020;57(2):590–605. doi: 10.1002/jhet.3789. [DOI] [Google Scholar]
- Santosh R, Selvam MK, Kanekar SU, Nagaraja GK. Synthesis, characterization, antibacterial and antioxidant studies of some heterocyclic compounds from triazole-linked chalcone derivatives. Chem Select. 2018;3(23):6338–6343. doi: 10.1002/slct.201800905. [DOI] [Google Scholar]
- Santra S, Jat B, Santra PK. Synthesis and antimicrobial activities of chalcones and indole drived from acetyl pyridines. Asian J Chem. 2018;30(4):883–888. doi: 10.14233/ajchem.2018.21124. [DOI] [Google Scholar]
- Sashidhara KV, Rao KB, Kushwaha P, Modukuri RK, Singh P, Soni I, Shukla PK, Chopra S, Pasupuleti M. Novel chalcone-thiazole hybrids as potent inhibitors of drug resistant Staphylococcus aureus. ACS Med Chem Lett. 2015;6(7):809–813. doi: 10.1021/acsmedchemlett.5b00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed M, El-Dean AMK, Ahmed M, Hassanein R. Synthesis, characterization, and screening for anti-inflammatory and antimicrobial activity of novel indolyl chalcone derivatives. J Heterocyclic Chem. 2018;55(5):1166–1175. doi: 10.1002/jhet.3149. [DOI] [Google Scholar]
- Seo J, Kim J, Shim J, Yoon G, Bang M, Bae C, Lee K, Park D, Cho S. HPLC analysis, optimization of extraction conditions and biological evaluation of corylopsis coreana uyeki flos. Molecules. 2016;21(1):1–13. doi: 10.3390/molecules21010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setyawati A, Wahyuningsih T, Purwono B. Syntheses of novel pyrazolines as antibacterial agents from natural product vanillin. Asian J Chem. 2017;29(2):454–456. doi: 10.14233/ajchem.2017.20247. [DOI] [Google Scholar]
- Seukep AJ, Kuete V, Nahar L, Sarker SD, Guo M. Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification. Amsterdam: Elsevier Ltd; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar B, Jalapathi P, Ramesh M, Kumar AK, Ragavender M, Bharath G. Synthesis, antimicrobial evaluation, and docking studies of some novel benzofuran based analogues of chalcone and 1,4-benzodiazepine. Russ J Gen Chem. 2016;86(7):1711–1721. doi: 10.1134/S107036321607029X. [DOI] [Google Scholar]
- Shimokoriyama M. Em Flavanones chalcones and aurones. New York: MacMillan Company; 1962. [Google Scholar]
- Shinde RS, Dake SA, Pawar RP. Design, synthesis and antimicrobial activity of some triazine chalcone derivatives. Anti-Infect Agents. 2019;18(4):332–338. doi: 10.2174/2211352517666190710115111. [DOI] [Google Scholar]
- Shinde RA, Adole VA, Jagdale BS, Desale BS. Synthesis, antibacterial and computational studies of Halo Chalcone hybrids from 1-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)ethan-1-one. J Indian Chem Soc. 2021;98(4):100051. doi: 10.1016/j.jics.2021.100051. [DOI] [Google Scholar]
- Silva DACM (2016) Instituto Superior De Ciências Da Saúde Egas Moniz Mestrado Integrado em Ciências Farmacêuticas. Epidemiologia molecular de estirpes bacterianas multirresistentes: Acinetobacter baumannii, Pseudomonas aeruginosa e Staphylococcus aureus, pp. 1–98
- Simard F, Gauthier C, Chiasson E, Lavoie S, Mshvildadze V, Legault J, Pichette A. Antibacterial Balsacones J-M, hydroxycinnamoylated dihydrochalcones from Populus balsamifera Buds. J Nat Prod. 2015;78(5):1147–1153. doi: 10.1021/acs.jnatprod.5b00155. [DOI] [PubMed] [Google Scholar]
- Siqueira MMR, Freire PTC, Cruz BG, Freitas TS, Bandeira PN, Santos HS, Nogueira CES, Teixeira AMR, Pereira RLS, Xavier JC, Campina FF, Barbosa CRS, Neto JBA, Silva MMC, Siqueira-Júnior JP, Coutinho HDM. Aminophenyl chalcones potentiating antibiotic activity and inhibiting bacterial efflux pump. Eur J Pharm Sci. 2021 doi: 10.1016/j.ejps.2020.105695. [DOI] [PubMed] [Google Scholar]
- Sivasankerreddy L, Nagamani B, Rajkumar T, Babu MS, Subbaiah NY, Harika MS, Nageswarao R. Novel diazenyl containing phenyl styryl ketone derivatives as antimicrobial agents. Anti-Infect Agents. 2018;17(1):28–38. doi: 10.2174/2211352516666180927111546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmaz A, İlter Z, Kaya İ. Synthesis, characterization and thermal properties of chalcone methacrylamide polymers containing methoxy group in side chain. J Polym Res. 2021 doi: 10.1007/s10965-021-02592-0. [DOI] [Google Scholar]
- Soltani SS, Farni SMF, Fouromadi A. Síntese e atividade antibacteriana de novas chalconas com uma porção imida-zo[1,2-a]piridina. Biologia Química Atual. 2021;15(2):163–170. doi: 10.2174/2212796815666210223110208. [DOI] [Google Scholar]
- Souza LFS (2014) Síntese de carboxi-Chalconas com ação inibitória de proteínas tirosina fosfatase de Mycobacterium tuberculosis, p. 136
- Stompor M, Zarowska B. Antimicrobial activity of xanthohumol and its selected structural analogues. Molecules. 2016 doi: 10.3390/molecules21050608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Aly MRE, Saad HA, Mohamed MAM. Click reaction based synthesis, antimicrobial, and cytotoxic activities of new 1,2,3-triazoles. Bioorg Med Chem Lett. 2015;25(14):2824–2830. doi: 10.1016/j.bmcl.2015.04.096. [DOI] [PubMed] [Google Scholar]
- Teixeira AMR, Santos HS, Julião M, Bandeira PN. Structural, spectroscopic and microbiological characterization of the chalcone 2E-1-(2ʹ-hydroxy-3ʹ,4ʹ,6ʹ-trimethoxyphenyl)-3-(phenyl)-prop-2-en-1-one derived from the natural product 2-hydroxy-3,4,6-trimethoxyacetophenone. J Mol Struct. 2019;1179:739–748. doi: 10.1016/j.molstruc.2018.11.075. [DOI] [Google Scholar]
- Thanigaimani K, Arshad S, Khalib NC, Razak IA, Arunagiri C, Subashini C, Sulaiman SF, Hashim NS, Ooi KL. A new chalcone structure of (E)-1-(4-Bromophenyl)-3-(napthalen-2-yl)prop-2-en-1-one: Synthesis, structural characterizations, quantum chemical investigations and biological evaluations. Spectrochim Acta Part A Mol and Biomol Spectrosc. 2015;149:90–102. doi: 10.1016/j.saa.2015.04.028. [DOI] [PubMed] [Google Scholar]
- Twinkle AR, Leenaraj DR, Ratkovic Z, Arunsasi BS, Bright KC, Reshma R. Ferrocenyl chalcone derivative (E)-3-(2-methylpyrimidin-5-yl)-1-ferroceynlprop-2-en-1-one: synthesis, structural analysis, docking study and their antibacterial evaluation. J Mol Struct. 2020;1210:128049. doi: 10.1016/j.molstruc.2020.128049. [DOI] [Google Scholar]
- Ud Din Z, Serrano FG, Ademi K, Sousa CP, Deflon VM, Maia PIS, Rodrigues Filho E. Crystal structures, in-silico study and anti-microbial potential of synthetic monocarbonyl curcuminoids. J Mol Struct. 2017;1144:529–534. doi: 10.1016/j.molstruc.2017.05.061. [DOI] [Google Scholar]
- Vásquez-Martínez YA, Osorio ME, San Martín DA, Carvajal MA, Vergara AP, Sanchez E, Raimondi M, Zacchino SA, Mascayano C, Torrent C, Cabezas F, Mejias S, Montoyaf M, Martín MCA, et al. Antimicrobial, anti-inflammatory and antioxidant activities of polyoxygenated chalcones. J Braz Chem Soc. 2019;30(2):286–304. doi: 10.21577/0103-5053.20180177. [DOI] [Google Scholar]
- Wei Z, Chi K, Yu Z, Liu H, Sun L, Zheng C, Piao H. Synthesis and biological evaluation of chalcone derivatives containing aminoguanidine or acylhydrazone moieties. Bioorg Med Chem Lett. 2016;26(24):5920–5925. doi: 10.1016/j.bmcl.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Who. World Health Organization (2021) Traditional, Complementary and Integrative Medicine. http://www.who.int/medicines/areas/traditional/definitions/en/. Accessed 13 Sep 2022
- Winter C (2016) Catalisadores heterogêneos para produção de chalconas: reação de condensação de Claisen-Schmidt, p. 162
- Wright GD. Opportunities for natural products in 21st century antibiotic discovery. Nat Prod Rep. 2017;34:694–701. doi: 10.1039/C7NP00019G. [DOI] [PubMed] [Google Scholar]
- Xavier JC, de Almeida-Neto FWQ, Rocha JE, Freitas TS, Freitas PR, de Araújo ACJ, da Silva PT, Nogueira CES, Bandeira PN, Marinho MM, Marinho ES, Kumar N, Barreto ACH, Coutinho HDM, dos Julião MSS, Santos HS, Teixeira AMR. Spectroscopic analysis by NMR, FT-Raman, ATR-FTIR, and UV-Vis, evaluation of antimicrobial activity, and in silico studies of chalcones derived from 2-hydroxyacetophenone. J Mol Struct. 2021;1241:130647. doi: 10.1016/j.molstruc.2021.130647. [DOI] [Google Scholar]
- Xie C, Zhang S, Zhang W, Wu C, Yong C, Sun Y, Zeng Y, Zhang Q, Huang Z, Chen T, Zhang Y. Synthesis, biological activities, and docking study of novel chalcone-pleuromutilin derivatives. Chem Biol Drug Des. 2020;96(2):836–849. doi: 10.1111/cbdd.13692. [DOI] [PubMed] [Google Scholar]
- Yusuf M, Solanki I. Synthesis and antimicrobial studies of furyl based new bispyrazolines linked via aliphatic chains. J Saudi Chem Soc. 2017;21(3):251–261. doi: 10.1016/j.jscs.2015.02.002. [DOI] [Google Scholar]
- Yusuf M, Kaur A, Abid M. Synthesis, antimicrobial studies, and docking simulations of new bis(4,5-dihydropyrazole) derivatives. J Heterocycl Chem. 2017;54(4):2536–2547. doi: 10.1002/jhet.2835. [DOI] [Google Scholar]
- Zainuri DA, Arshad S, Khalib NC, Razaki IA, Pillai RR, Sulaiman SF, Hashim NS, Ooi KL, Armaković S, Armaković SJ, Panicker CY, Alsenoy CV. Synthesis, XRD crystal structure, spectroscopic characterization (FT-IR, 1H and 13C NMR), DFT studies, chemical reactivity and bond dissociation energy studies using molecular dynamics simulations and evaluation of antimicrobial and antioxidant activities of a novel chalcone derivative, (E)-1-(4-bromophenyl)-3-(4-iodophenyl)prop-2-en-1-one. J Mol Struct. 2017;1128:520–533. doi: 10.1016/j.molstruc.2016.09.022. [DOI] [Google Scholar]
- Zaki MA, Nanayakkara NPD, Hetta MH, Jacob MR, Khan SI, Mohammed R, Ibrahim MA, Coleman C, Fronczek FR, Ferreira D, Muhammad I. Bioactive formylated flavonoids from Eugenia rigida: isolation, synthesis, and X-ray crystallography. J Nat Prod. 2016;79(9):2341–2349. doi: 10.1021/acs.jnatprod.6b00474. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xie Y, Wang Y, Lin Z, Wang L, Li G. Toxicities of monoterpenes against housefly, Musca domestica L. (Diptera: Muscidae) Environ Sci Pollut Res. 2017;24(31):24708–24713. doi: 10.1007/s11356-017-0219-4. [DOI] [PubMed] [Google Scholar]
- Zhang M, Prior AM, Maddox MM, Shen W, Hevener KE, Bruhn DF, Lee RB, Singh AP, Reinicke J, Simmons CJ, Hurdle JG, Lee RE, Sun D. Pharmacophore modeling, synthesis, and antibacterial evaluation of chalcones and derivatives. ACS Omega. 2018;3(12):18343–18360. doi: 10.1021/acsomega.8b03174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Yu Z, Jin X, Li M, Sun LP, Zheng C, Piao H. Synthesis and evaluation of the antibacterial activities of aryl substituted dihydrotriazine derivatives. Bioorg Med Chem Lett. 2018;28(9):1657–1662. doi: 10.1016/j.bmcl.2018.03.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be available after a reasonable request to the corresponding author.