Abstract
STUDY QUESTION
Is the chance of childbirth, and risk of infertility, pregnancy loss and need for assisted reproduction different for women with asthma compared to women without asthma?
SUMMARY ANSWER
Women with asthma had comparable chances of giving birth compared to the reference population, however, their risk of both infertility and pregnancy loss, as well their need for medically assisted reproduction, was higher.
WHAT IS KNOWN ALREADY
Reproductive dysfunction has been reported among women with asthma, including longer time to pregnancy, increased risk of pregnancy loss and a higher need of medically assisted reproduction, but their risk of clinical infertility is unknown.
STUDY DESIGN, SIZE, DURATION
This longitudinal register-based cohort study included all women with a healthcare visit for delivery, infertility, pregnancy loss or induced abortion in the southernmost county in Sweden, over the last 20 years.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Using the Skåne Healthcare Register, we identified all women aged 15–45 between 1998 and 2019, who received a diagnosis of asthma before their first reproductive outcome (n = 6445). Chance of childbirth and risk of infertility, pregnancy loss and assisted reproduction were compared to a healthcare seeking population of women without any asthma (n = 200 248), using modified Poisson regressions.
MAIN RESULTS AND THE ROLE OF CHANCE
The chance of childbirth was not different between women with asthma versus those without, adjusted risk ratio (aRR) = 1.02, 95% CI: 1.01–1.03. The risk of seeking care for infertility was increased, aRR = 1.29, 95% CI: 1.21–1.39, and women with asthma more often needed assisted reproduction aRR = 1.34 95% CI: 1.18–1.52. The risk of suffering a pregnancy loss was higher, aRR = 1.21, 95% CI: 1.15–1.28, and induced abortions were more common, aRR = 1.15, 95% CI: 1.11–1.20, among women with asthma.
LIMITATIONS, REASONS FOR CAUTION
The study was an observational study based on healthcare visits and lacked detailed anthropometric data, thus residual confounding cannot be excluded. Only women with a healthcare visit for a reproductive outcome were included, which cannot be translated into pregnancy intention. A misclassification, presumed to be non-differential, may arise from an incorrect or missing diagnosis of asthma or female infertility, biasing the results towards the null.
WIDER IMPLICATIONS OF THE FINDINGS
This study points towards reproductive dysfunction associated with asthma, specifically in regards to the ability to maintain a pregnancy and the risk of needing medically assisted reproduction following clinical infertility, but reassuringly the chance of subsequently giving birth was not lower for these women.
STUDY FUNDING/COMPETING INTEREST(S)
This article is part of the ReproUnion collaborative study, co-financed by EU Interreg ÖKS, Capital Region of Denmark, Region Skåne and Ferring Pharmaceuticals. The authors have no competing interests to disclose.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: infertility, medically assisted reproduction, pregnancy loss, induced abortion, asthma
Introduction
Asthma is one of the most common chronic conditions among women of reproductive age, with a prevalence generally reported at 8–10% (Lötvall et al., 2009; Loftus and Wise, 2015). Chronic inflammation constitutes a fundamental aspect of asthma, and findings suggest that the inflammation could have systemic effects beyond the airways, and thereby potentially affect the reproductive organs (Denburg et al., 2000; Juul Gade et al., 2014). Prior research indicates that asthma could have implications for reproductive function (Juul Gade et al., 2014; Bláfoss et al., 2019; Wasilewska and Małgorzewicz, 2019). Asthma has been found to be more prevalent among women with endometriosis (Sinaii, 2002; Kvaskoff et al., 2015; Peng et al., 2017) and polycystic ovary syndrome (PCOS) (Zierau et al., 2019) and has been associated with both irregular menstruations (Svanes et al., 2005; Wasilewska and Małgorzewicz, 2019) and a higher risk of miscarriages (Blais et al., 2013; Turkeltaub et al., 2019). Other studies have found asthma to be associated with a longer time to pregnancy (Gade et al., 2014), fewer successful pregnancies among fertility patients (Gade et al., 2016) and a higher risk of needing ARTs to achieve a pregnancy (Vejen Hansen et al., 2019). The use of anti-asthmatic drugs has also been linked to subfertility and reduced fecundability (Källén and Otterblad Olausson, 2007; Grzeskowiak et al., 2018). However, most earlier research has been based on small populations (Gade et al., 2016), has relied on a self-reported asthma diagnosis (Gade et al., 2014; Grzeskowiak et al., 2018; Turkeltaub et al., 2019) or were based on women giving birth only (Källén and Otterblad Olausson, 2007; Grzeskowiak et al., 2018; Vejen Hansen et al., 2019). Moreover, the findings are inconsistent, as reviewed by Bláfoss et al. (2019). For example, any severely impaired fertility on a population-level was disputed by one large study reporting equal total fertility rates for women with asthma compared to the general population (Tata et al., 2007). However, a total fertility rate is not readily translated into individual reproductive function, and whether women with asthma have an increased risk of suffering from clinical infertility is not clear.
Given the limited and contradictory evidence summarized above, we aim to describe the chance of childbirth and risk of seeking healthcare for infertility, medically assisted reproduction and pregnancy loss among women who have received a diagnosis of asthma, using population-based healthcare visit data on the total population in the southernmost region in Sweden.
Materials and methods
Data sources
The data for this study were retrieved from the regional Swedish Skåne Healthcare Register. This is an administrative register holding individual-level data on medical diagnoses and procedures from all healthcare consultations in the region of Skåne from 1998 and onwards (Löfvendahl et al., 2020). All healthcare consultations, both in the public and private sector, at all care levels (primary care, specialized in- and out-patient care), to all types of healthcare professionals (physicians, nurses, physiotherapists, midwives, etc) are included in the register. As the entries in the register constitute the basis for economic reimbursement for the healthcare provider, the vast majority of provided care is assumed to be present in the register (Löfvendahl et al., 2020). No anthropometric or life-style related data is collected within the register.
Diagnoses are registered according to the Swedish version of the International Classification of Diseases 10th revision (ICD-10) and a version of ICD-10 adapted for primary care (KSH97-P).
Ethical approval was granted by the Regional Ethics Board at Lund University (DNR 2019-4632).
Study population
Study cohort
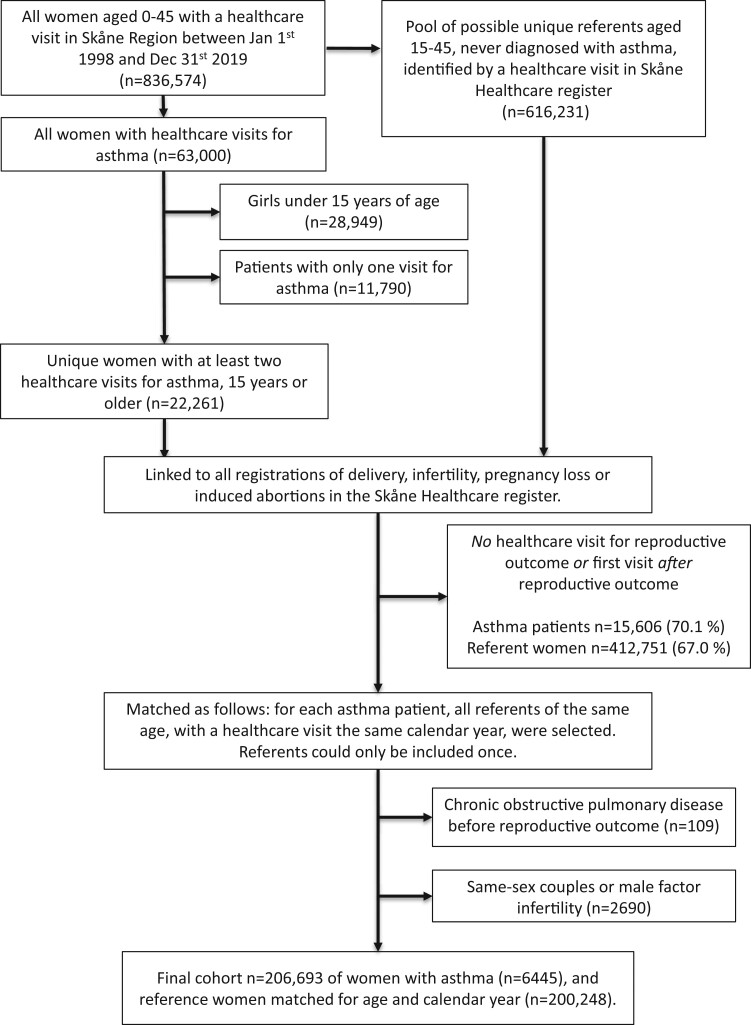
Women were eligible based on their healthcare visits in the Skåne Healthcare register between 1 January 1998 and 31 December 2019, if they were between 15 and 45 years of age at the visit. We identified patients with asthma using the following ICD-10-SE codes: J45–J46 (including all subcategories) and J45-P (code specific to primary care before the successive implementation of ICD-10-SE). To minimize the risk of women being erroneously diagnosed with asthma, only women with at least two healthcare visits with a physician-recorded asthma diagnosis were included, and by this definition, n = 22 261 unique women with asthma were identified.
We wanted to study only women who were reproductively active, for whom reproductive function could be assumed to be relevant. For the sake of causal inference and the ability to adjust for important confounders as described below, we considered the importance of a prospective design where asthma was known to precede the outcomes paramount. We thus applied the following exclusion criteria: (i) no healthcare visit for either delivery, pregnancy loss (miscarriage, ectopic pregnancy or molar pregnancy), induced abortion or infertility or (ii) first asthma diagnosis registered after childbirth, or in the case of no childbirth, after the last infertility visit, pregnancy loss or abortion (excluding n = 15 606 asthma patients). We also excluded women seeking infertility care for social reasons (i.e. same-sex couples) and women with a registered male factor infertility (n = 2690) and patients with a diagnosis of chronic obstructive pulmonary disease (n = 109). The final cohort included a total of n = 6445 women with asthma, as depicted in Fig. 1.
Figure 1.
Flowchart depicting the selection of women with asthma and referents.
Reference population
All women aged 15–45 years with at least one healthcare visit during the study period were eligible to be included in the reference population, provided they did not have any asthma registered at any time. As for women with asthma, reference women were only eligible if they had a healthcare visit before any of the reproductive outcomes, and the same exclusion criteria were applied. The matching was done as follows: for each calendar year, patients with asthma were matched to all women seeking healthcare that same year if they were the same age. Referent women could only be included once even if the hypothetically could be matched to several patients with asthma. The final referent group included n = 200 248 women (Fig. 1).
Definitions of reproductive outcomes
The reproductive outcomes were defined as follows: (i) childbirth, defined as a registered delivery during the study period (ICD-10-SE: O80-O84), (ii) infertility, defined as a healthcare visit for either female infertility (N97), premature ovarian failure (E28.3) or ovarian dysfunction (E28.9), or any visits for assisted reproduction: fertility examination (Z31.4), ovulation stimulation (Z31.8B), intrauterine insemination with partner’s sperm (Z31.1B), IVF (Z31.2A), ICSI (Z31.2B) or embryo transfer (Z31.2C). The definitions were based on the clinical and administrative routines for diagnostic registrations in the region and corroborated with clinicians working in the area, to increase the likelihood of capturing all women with presumed fertility problems, rather than keeping with a strict definition of infertility. This is also the rationale behind including ICSI among the outcomes. ICSI is often used in couples with male factor infertility, but not exclusively. It is also used, in the region and elsewhere, in couples with a combination of male and female causes of infertility, as well as high age of the couple or unsuccessful previous treatments (Nyboe Andersen et al., 2008; Babayev et al., 2014). Thus, excluding patients treated with this procedure would risk excluding those with arguably the most ‘severe’ infertility. Women diagnosed with infertility due to male factors (N97.4) were not included, so couples with clear male factor infertility were not part of the ICSI group included in the study.
Childbirth and risk for infertility among women with asthma were investigated in the following combinations: (i) childbirth, regardless fertility problems, (ii) risk of seeking healthcare for infertility or medically assisted reproduction, and lastly, (iii) chance of childbirth following infertility, where women with asthma and infertility were compared to reference women also experiencing, or treated for, infertility.
Additionally, as separate outcomes (iv) pregnancy loss, defined asmiscarriage (O02–O03), ectopic pregnancy (O00) or molar pregnacy (O01) as well as (v) induced abortion (O04–O07) were investigated. Lastly, (vi) ever pregnant was defined as having registrations of either a delivery, pregnancy loss or induced abortion, according to the codes above.
Covariates
The register does not contain any information on how long the women had been trying to conceive. Although the study population was matched according to year and age at diagnosis, we also approximated a reproductive ‘start-age’ for each outcome as follows: (i) subtracting one and a half years from the age of childbirth, based on an average length of pregnancy and cumulative conception rates of 75–90% after 6–12 months of trying (Taylor, 2003), (ii) subtracting 1 year from first infertility visit, based on advice to patients to seek fertility care after 1 year of unsuccessful pregnancy attempts (if both infertility visit and childbirth, infertility visit takes precedence for the start-age calculation), (iii) subtracting 6 months from the age at pregnancy loss and (iv) subtracting 3 months for induced abortion (as the majority of abortions in Sweden takes place before nine weeks of gestation) (National Board of Health and Welfare, 2020).
We also included endometriosis (N80) and PCOS (E28.3) as potential confounders, provided there were indications that the reason for the healthcare visits were related to fertility issues (i.e. a registration of any infertility diagnosis for the same individual or registrations of endometriosis at a fertility clinic). Lastly, clinical obesity (E.66) registered at any healthcare visit was included, regardless of whether it was registered as a main or auxiliary diagnosis.
Statistical methods
Fertility patterns for the cohort were described according to asthma status by frequencies and percent for categorical variables. We calculated risk ratios (RRs) with 95% CIs between women with asthma and calendar year- and age-matched referents, for the outcomes described above, using modified Poisson regression models. All analyses, both crude and fully adjusted, included an adjustment for calendar year to account for potential cohort effects. The adjusted models further included obesity, endometriosis, PCOS and approximated age of reproductive start as confounders.
As asthma is a disease where symptoms might go into remission, we also wanted to investigate reproductive function in women with a presumed active asthma, defined as women with a healthcare visit with asthma registered as the main diagnosis 3 years or less prior to approximated reproductive start. By this definition, we performed a sub-group analyses for n = 3560 women with active asthma.
The analyses were performed using the statistical analysis software SAS version 9.4 (Copyright c 2013 by SAS Institute Inc., Cary, NC, USA).
Results
The frequency of different reproductive outcomes among women with asthma and in the referent population are shown in Table I. Women with asthma were somewhat younger at childbirth and more often had an obesity diagnosis (P-value for both <0.001).
Table I.
Fertility patterns among women with a registered reproductive outcome between the age of 15–45 years, according to asthma status.
| Total (n = 206 693) | Reference women without asthma (n = 200 248) | Women with asthma (n = 6445) |
|---|---|---|
| Age at index healthcare visit | ||
| <25 | 102 648 (51.3%) | 3976 (61.7%) |
| 25–29 | 45 492 (22.7%) | 1274 (19.8%) |
| 30–34 | 33 770 (16.9%) | 783 (12.1%) |
| 35–39 | 14 882 (7.4%) | 353 (5.5%) |
| 40–45 | 3456 (1.7%) | 59 (0.9%) |
| Approximated age of reproductive start | ||
| <25 | 38 885 (19.4%) | 1456 (22.6%) |
| 25–29 | 60 771 (30.3%) | 2179 (33.8%) |
| 30–34 | 60 187 (30.1%) | 1725 (26.8%) |
| 35–39 | 30 090 (15.0%) | 845 (13.1%) |
| 40–45 | 10 315 (5.2%) | 240 (3.7%) |
| Childbirth | 164 979 (82.4%) | 5329 (82.7%) |
| Age of delivery | ||
| No delivery | 35 269 (17.6%) | 1116 (17.3%) |
| <25 | 84 949 (42.4%) | 3280 (50.9%) |
| 25–29 | 40 183 (20.1%) | 1113 (17.3%) |
| 30–34 | 27 833 (13.9%) | 650 (10.1%) |
| 35–39 | 10 369 (5.2%) | 257 (4.0%) |
| 40–45 | 1645 (0.8%) | 29 (0.4%) |
| Year of delivery | ||
| 1998–2004 | 57 415 (28.7%) | 749 (11.6%) |
| 2005–2009 | 35 652 (17.8%) | 1210 (18.8%) |
| 2010–2014 | 36 886 (18.4%) | 1710 (26.5%) |
| 2015–2019 | 35 026 (17.5%) | 1660 (25.8%) |
| Endometriosis | 393 (0.2%) | 20 (0.3%) |
| PCOS | 1035 (0.5%) | 50 (0.8%) |
| Infertility (diagnosis or treatment)a | 16 220 (8.1%) | 712 (11.0%) |
| With subsequent childbirth | 7599 (3.8%) | 356 (5.5%) |
| Not leading to childbirth | 6672 (3.3%) | 278 (4.3%) |
| Infertility diagnosisb | 14 792 (7.4%) | 642 (10.0%) |
| Unspecified | 8413 (4.2%) | 349 (5.4%) |
| Unexplained after examination | 2155 (1.1%) | 118 (1.8%) |
| Myoma | 99 (0.0%) | 5 (0.1%) |
| Ovarian failure | 211 (0.1%) | 7 (0.1%) |
| Anovulation | 2176 (1.1%) | 88 (1.4%) |
| Tubal factor infertility | 844 (0.4%) | 39 (0.6%) |
| Medically assisted reproductionc | ||
| Ovulation stimulation | 419 (0.2%) | 9 (0.1%) |
| Intrauterine insemination | 596 (0.3%) | 39 (0.6%) |
| Embryo transfer | 43 (0.0%) | 2 (0.0%) |
| In vitro fertilization | 1826 (0.9%) | 87 (1.3%) |
| Intracytoplasmic sperm injection | 1973 (1.0%) | 95 (1.5%) |
| Other treatment | 152 (0.1%) | 7 (0.1%) |
| Pregnancy lossd | 25 119 (12.5%) | 1059 (16.4%) |
| Induced abortion | 35 815 (17.9%) | 1471 (22.8%) |
| Obesity | 13 951 (7.0%) | 1024 (15.9%) |
Women having either infertility diagnoses or undergoing medically assisted reproduction.
Strict clinical infertility diagnosis, excluding women with polycystic ovary syndrome (PCOS) and endometriosis.
Last treatment/procedure registered.
Miscarriage, ectopic pregnancy or molar pregnancy.
Unadjusted and adjusted RRs (aRRs) for the different reproductive outcomes are shown in Table II. The chance of giving birth was not different between women with asthma versus those without: aRR = 1.02 (95% CI: 1.01–1.03). The risk of having to seek healthcare for infertility was increased for women with asthma, aRR = 1.29 (95% CI: 1.21–1.39), but compared to women without asthma also seeking healthcare for infertility, the chance of subsequently giving birth after infertility was not different: aRR for childbirth after infertility = 1.05 (95% CI: 0.99–1.11).
Table II.
Crude and adjusted risk ratios (RRs) and CIs of reproductive outcomes among women with any reproductive healthcare visit.
| No asthma |
Asthma |
Active asthma
a
|
|||||
|---|---|---|---|---|---|---|---|
| No asthma (n = 200 248) | Asthma (n = 6445) | Crude RR (95% CI) | Adjusted RRb (95% CI) | Active asthma (n = 3560) | Crude RR (95% CI) | Adjusted RRb (95% CI) | |
| Childbirth | 164 979 (82.4%) | 5329 (82.7%) | 1.03 (1.02–1.04) | 1.02 (1.01–1.03) | 2892 (81.2%) | 1.00 (0.99–1.02) | 1.01 (0.99–1.02) |
| Infertility (diagnosis or treatment)c | 16 220 (8.1%) | 712 (11.0%) | 1.26 (1.18–1.34) | 1.29 (1.21–1.39) | 372 (10.4%) | 1.17 (1.07–1.28) | 1.24 (1.13–1.37) |
| Childbirth after infertilityd | 7599 (3.8%) | 356 (5.5%) | 1.08 (1.02–1.14) | 1.05 (0.99–1.11) | 178 (5.0%) | 1.05 (0.97–1.14) | 1.03 (0.95–1.12) |
| Pregnancy losse | 25 119 (12.5%) | 1059 (16.4%) | 1.17 (1.11–1.22) | 1.21 (1.15–1.28) | 601 (16.9%) | 1.22 (1.15–1.31) | 1.25 (1.17–1.33) |
| Induced abortion | 35 815 (17.9%) | 1471 (22.8%) | 1.14 (1.11–1.18) | 1.15 (1.11–1.20) | 847 (23.8%) | 1.23 (1.17–1.30) | 1.20 (1.14–1.26) |
Defined as healthcare visit with asthma as main diagnosis <3 years before reproductive start-age.
Adjusted for obesity, endometriosis, polycystic ovary syndrome, approximated age of reproductive start and calendar year.
Includes women with healthcare visits for infertility (endometriosis and polycystic ovary syndrome excluded) or medically assisted reproduction without valid diagnosis.
Compared only to women with a healthcare visit for infertility or medically assisted reproduction, total n = 16 932. Total n = 16 592, when only active asthma included.
That is miscarriage, ectopic pregnancy or molar pregnancy.
The risk of suffering pregnancy loss was increased for women with asthma, aRR = 1.21 (95% CI: 1.15–1.28) and induced abortions were more common, aRR = 1.15 (95% CI: 1.11–1.20).
Restricting the analyses to including only women with a presumed active asthma, i.e. a visit for asthma <3 years before assumed reproductive start, did not change the estimates (Table II).
Investigating the risk of endometriosis, PCOS and specific female infertility diagnoses for women with asthma revealed an increased risk of endometriosis, anovulation, tubal factor infertility and unexplained or unspecified infertility (Table III).
Table III.
Crude and adjusted risk ratios (RRs) and CIs for infertility diagnoses as well as endometriosis and PCOS, according to asthma status.
| No asthma (n = 200 248) | Asthma (n = 6445) | Crude RR (95% CI) | Adjusted RRb (95% CI) | |
|---|---|---|---|---|
| Endometriosis | 393 (0.2%) | 20 (0.3%) | 1.48 (0.94–2.34) | 1.56 (0.99–2.46) |
| PCOS | 1035 (0.5%) | 50 (0.8%) | 1.17 (0.92–1.48) | 1.02 (0.80–1.30) |
| Infertility diagnosisa | 14 792 (7.4%) | 642 (10.0%) | 1.39 (1.29–1.49) | 1.29 (1.18–1.42) |
| Unspecified | 8413 (4.2%) | 349 (5.4%) | 1.33 (1.20–1.48) | 1.33 (1.20–1.48) |
| Unexplained after examination | 2155 (1.1%) | 118 (1.8%) | 1.21 (1.04–1.41) | 1.26 (1.08–1.47) |
| Myoma | 99 (0.0%) | 5 (0.1%) | 1.68 (0.81–3.48) | 1.74 (0.83–3.64) |
| Ovarian failure | 211 (0.1%) | 7 (0.1%) | 0.97 (0.55–1.71) | 0.98 (0.56–1.72) |
| Anovulation | 2176 (1.1%) | 88 (1.4%) | 1.37 (1.15–1.64) | 1.33 (1.11–1.58) |
| Tubal factor infertility | 844 (0.4%) | 39 (0.6%) | 1.42 (1.03–1.96) | 1.49 (1.08–2.05) |
Strict clinical infertility diagnosis, excluding endometriosis and polycystic ovary syndrome (PCOS).
Adjusted for obesity, approximated age of reproductive start and calendar year.
The risk of needing medically assisted reproduction was higher for women with asthma: aRR for any assisted reproduction = 1.34 (95% CI: 1.18–1.52). The risks for specific fertility treatment methods are shown in Table IV.
Table IV.
Crude and adjusted risk ratios (RRs) and CIs for medically assisted reproduction, according to asthma status.
| No asthma (n = 200 248) | Asthma (n = 6445) | Crude RR (95% CI) | Adjusted RRb (95% CI) | |
|---|---|---|---|---|
| Medically assisted reproduction (any) | 5009 (2.5%) | 239 (3.7%) | 1.27 (1.12–1.44) | 1.34 (1.18–1.52) |
| Ovulation stimulation | 419 (0.2%) | 9 (0.1%) | 1.14 (0.70–1.87) | 1.10 (0.67–1.79) |
| Intrauterine insemination | 596 (0.3%) | 39 (0.6%) | 1.92 (1.39–2.66) | 1.98 (1.43–2.74) |
| In vitro fertilization | 1826 (0.9%) | 87 (1.3%) | 1.22 (1.03–1.46) | 1.29 (1.04–1.60) |
| Intracytoplasmic sperm injection | 1973 (1.0%) | 95 (1.5%) | 1.23 (1.00–1.51) | 1.32 (1.07–1.62) |
| Other treatment | 152 (0.1%) | 7 (0.1%) | 1.28 (0.64–2.53) | 1.29 (0.67–2.91) |
Adjusted for calendar year.
Adjusted for obesity, endometriosis, polycystic ovary syndrome, approximated age of reproductive start and calendar year.
Discussion
Principal findings
In this large register-based study based on women with at least one healthcare visit for a reproductive outcome, women diagnosed with asthma had a higher risk of both infertility and pregnancy loss, as well as needing medically assisted reproduction. However, their chance of giving birth was not lower. We further found a higher prevalence of induced abortions in the group of women with asthma compared to the reference population.
Interpretation and comparison to previous studies
The present findings support earlier studies on the association between asthma and the risk of reproductive dysfunction (Bláfoss et al., 2019; Wasilewska and Małgorzewicz, 2019), with some important additions. The risk of clinical infertility among women with asthma has previously been unknown. In their register-study on female twins, Juul Gade et al. reported a longer time to pregnancy for women with asthma, persistent also after adjusting for important confounders such as age, smoking and BMI (Gade et al., 2014). The study was limited however, by relying on patient recall of ever having had asthma and there was no data on other fertility-related diseases. Furthermore, the generalizability of the study is unclear, as it was based on twins and suffered a large amount of missing data from younger individuals (Gade et al., 2014). The same authors also found that fertility treatments in women with asthma more often were unsuccessful and not followed by a live birth (Gade et al., 2016). Although the latter study had the important strengths of confirming the asthma diagnosis with biological testing, and prospectively following time to pregnancy, it included only 245 patients (Gade et al., 2016). Time to pregnancy could not be investigated in the current register-data, but we had access to registered diagnoses of female infertility from the clinical setting. An infertility diagnosis is by its definition (more than 12 months of unprotected intercourse without conceiving) derived from time to pregnancy and might arguably be more relevant from a patient’s perspective.
Other studies have linked the use of anti-asthmatic drugs to subfertility and reduced fecundability (Källén and Otterblad Olausson, 2007; Grzeskowiak et al., 2018), however, these previous studies were completely based on self-report, which might be prone to bias. Our study has confirmed the increased risk for assisted reproduction among women with asthma, as recently found by Vejen Hansen et al. (2019). It is to be noted, however, that the above study only included women with live births (Vejen Hansen et al., 2019). Apart from drawing generalizability into question, conditioning on a future birth might bias the results.
In our study, women with asthma also had a higher risk of pregnancy loss, which is consistent with earlier reports (Blais et al., 2013; Gade et al., 2014; Turkeltaub et al., 2019). The higher rate of induced abortions, however, was not expected, and requires further investigation. Women with asthma tended to be somewhat younger at the estimated reproductive start, which might contribute to more frequent induced abortions, although adjusting for age did not explain away the findings. There is a theoretical possibility that the abortion rate reflects a higher frequency of foetal abnormalities among patients with asthma. Although not consistent, there is some evidence of an increased risk of congenital malformations in offspring to mothers with asthma, including severe types such as gastroschisis and omphalocele (Lin et al., 2012; Murphy et al., 2013; Garne et al., 2016). It was not possible to determine the reason for the abortion, so this question cannot be investigated further in these data.
However, other studies have shown unaffected total birth rates for women with asthma compared to the expected rates (Tata et al., 2007; Turkeltaub et al., 2019), as well as no difference in the mean number of offspring per woman, or proportion of childless women (Gade et al., 2014). The study by Tata et al. (2007) investigating total fertility rates found no evidence of a decreased fertility for women with asthma. In line with this, the chance of subsequently giving birth was not lower for women with asthma in our study, while the risks of infertility, assisted reproduction and pregnancy loss for the same women were increased nonetheless, highlighting that a total fertility rate does not easily translate into the individual reproductive journey.
Potential biological explanations
In terms of biological underpinnings for the results above, Juul Gade et al. propose that asthma might affect reproductive function through systemic inflammatory pathways, as an imbalance of systemic cytokines, e.g. increased levels of interleukin-6, tumor necrosis factor-α (TNF-α), and natural killer cells, seems common to both asthma and reproductive failure (Berry et al., 2007; Juul Gade et al., 2014; Vannuccini et al., 2016; Alijotas-Reig et al., 2017; Lambrecht et al., 2019). TNF-α, regulating diverse cell functions such as proliferation and apoptosis, is central to the inflammatory mechanisms related to implantation, placentation and pregnancy outcomes. Increasing levels have been linked to both recurrent pregnancy loss and implantation failure, and reports have been published where live birth rates have been increased when women with recurrent pregnancy loss were treated with TNF-α blockers (Alijotas-Reig et al., 2017). It might be seen as indirect support of this explanation that well-treated asthmatics (with the inflammatory response subdued) have not been shown to have the same reduction in fecundability or prolongment in time to pregnancy as women with asthma without treatment or those receiving short-term β-antagonists only (Gade et al., 2014; Grzeskowiak et al., 2018).
Strengths and limitations
The present study is one of the largest studies on the subject and includes healthcare data from all care levels, which allows for high coverage and inclusion of data not present in other national health registers. Healthcare in Sweden is predominantly publicly funded and is, apart from a small administrative fee, free for all citizens. This applies also to a certain number of fertility treatments. Since asthma shows a socio-economic gradient, with a higher asthma prevalence in less affluent groups (Uphoff et al., 2015), investigating this question in a context without universal healthcare would entail a high risk of bias. Even in countries with universal healthcare, the use of fertility treatments has been shown to vary according to maternal educational level (Vassard et al., 2018). However, since fertility treatments generally are more common among more highly educated women, the association between asthma and use of fertility treatments in the present study is, by this argument, likely to be underestimated.
Asthma status in our study was based on a physician-recorded clinical diagnosis, as opposed to many of the previous studies based on self-reported asthma (Källén and Otterblad Olausson, 2007; Gade et al., 2014; Grzeskowiak et al., 2018; Turkeltaub et al., 2019). We could include patients diagnosed and treated in primary care, which is not possible in any of the national patient registers. Healthcare in Sweden is organizationally based on primary care, which has taken over an increasing responsibility for the care of diseases such as asthma, referring only certain cases such as e.g. severe or treatment-refractory asthma, to specialized secondary care (Region Skåne Asthma Guidelines, 2020). Although the validity of the asthma diagnosis specifically has not been examined in the Skåne Healthcare register, we tried to increase the accuracy of asthma ascertainment by only including patients with repeated visits with asthma as the main diagnosis.
However, we recognize that there is a risk of underdiagnosing of medical conditions depending on patients’ healthcare seeking behavior, since the register is based on healthcare visits. This might also have led to underdiagnosing of active asthma, since reports have shown a low frequency of healthcare visits (compared to the recommended level) for patients with severe or uncontrolled asthma (Janson et al., 2018; Larsson et al., 2018).
The rate of infertility and use of assisted reproduction could similarly have been underestimated, but we have little reason to believe that the inclination to seek help for fertility issues would be different for women with asthma compared to those without, and no fertility-related examinations are included in standard clinical follow-up of asthma (Region Skåne Asthma Guidelines, 2020). All in all, such misclassification of exposure/outcome is hard to completely avoid when using data of this kind, but based on the reasoning above, we think that this will mainly lead to an issue with ‘noise’ and potential underestimation of results, rather than a serious bias of the results.
For causal inference, we found it paramount to establish that asthma onset preceded the reproductive outcomes. This temporality condition was lacking in some of the earlier studies, e.g. the study by Tata et al. (2007) investigating fertility rates for women with asthma. However, this meant that the study was performed in a selected group of women who had a healthcare visit for at least one reproductive outcome, which must be kept in mind when interpreting the results. These outcomes were chosen with the aim to comprehensively include ‘reproductively active’ women, however, this cannot be translated into pregnancy intention. Some women could have been erroneously excluded (women with a child wish but not achieving a pregnancy nor seeking infertility care), and some women could have been erroneously included (those pregnant without wanting a pregnancy). We had no means of estimating how large these numbers were in the present population, but we had no reason to believe that women with asthma would systematically differ in terms of child wish or inclination to seek infertility services.
Large numbers of both women with asthma (70%) and referent women (67%) were excluded because they had no healthcare visit for any reproductive outcome. These numbers mainly reflect right truncation of the data as well as the comparably short duration (around twenty years) of the Skåne Healthcare Register: asthma is generally diagnosed in childhood, meaning that patients included during later years in the register would not have had the time to start their reproductive journey during follow-up. The same applies for the referents as they were matched according to calendar year and age.
Further, the register includes medical diagnoses and procedures but collects no individual anthropometric or lifestyle-related data. We thus lacked information on e.g. smoking, BMI and socio-economic status, which is a notable limitation. In an attempt to estimate the magnitude of potential unmeasured confounding, e-values were computed (VanderWeele and Ding, 2017; Mathur et al., 2018). With the observed aRRs in this study typically around aRR = 1.30 (up to aRR = 1.98), any unmeasured confounder would have to be associated with both the outcome and the exposure by a risk ratio of 2-fold each (up to 3.4-fold), beyond the measured confounders (age, calendar time, endometriosis, PCOS and clinical obesity), to completely explain away the results. Although such confounders could be conceivable, the direction of the presumed confounding effect must be taken into account. For example, smoking among women of reproductive age (16–44 years) in Sweden ranged from 13–14% in 2006 to 7–11% in 2015 (Swedish Public Health Authority, 2021). The corresponding numbers for asthma patients during the same years were lower: 11% and 6%, respectively (Stegberg et al., 2018). Since smoking negatively affects reproduction, including this covariate in the analyses would thus likely have strengthened the results rather than attenuated them.
BMI has been reported to confer a higher risk of developing asthma by about 50% (Kuruvilla et al., 2019), and increasing BMI is known to detrimentally affect almost all aspects of reproduction (Pasquali, 2003). It was a weakness that we did not have individual measures of BMI, which could perhaps explain the findings to some extent, but the associations were robust towards adjustment for clinical obesity. Also, most fertility clinics in Sweden have BMI restrictions for offering fertility treatment (typically at BMI 30 or 35, depending on region and clinic), leading some patients to seek fertility care elsewhere, e.g. in Denmark, where different regulations apply. These treatments would not show up in our data and considering that obesity was more prevalent among asthma patients, the associations regarding assisted reproduction in this study could hence be underestimated.
Conclusions and potential clinical implications
In summary, we found that asthma has bearings on reproductive function in women, with an increased risk of pregnancy loss, clinical infertility and need to undergo assisted reproduction. Although it is reassuring that the subsequent chance of giving birth was not decreased for women with asthma, the subjective experience (both physically and psychologically) of having to go through pregnancy loss or assisted reproduction should not be overlooked (Cousineau and Domar, 2007; Quenby et al., 2021). In light of this, a 30% risk increase (as found on average in this study) is not necessarily negligible for the woman/couple, in particular since previous research indicate that some of the reproductive difficulties might be mitigated through adequate asthma control (Gade et al., 2014; Alijotas-Reig et al., 2017; Grzeskowiak et al., 2018). This is important as there is a general hesitancy among many women towards medication use during pregnancy, with low adherence also to necessary pharmacological treatment (Ceulemans et al., 2019). From a public health perspective, considering how common asthma is among women in reproductive years, and how high the absolute the risks are for fertility issues (10–15%) (Boivin et al., 2007; Thoma et al., 2013) and pregnancy loss (around 15%, conservatively estimated) (Quenby et al., 2021), the number of potentially affected women is not small.
However, considering the risk of unmeasured confounding in this study, and the selected group of women under study, further investigations are needed to evaluate the robustness of these results.
Contributor Information
Anna Jöud, Division of Occupational and Environmental Medicine, Department of Laboratory Medicine, Faculty of Medicine, Lund University, Medicon Village, Lund, Sweden.
Emma Nilsson-Condori, Reproductive Medicine Center, Skåne University Hospital, Malmö, Sweden.
Lone Schmidt, Department of Public Health, University of Copenhagen, Copenhagen, Denmark.
Søren Ziebe, The Fertility Clinic, Copenhagen University Hospital, Copenhagen Ø, Denmark.
Ditte Vassard, Department of Public Health, University of Copenhagen, Copenhagen, Denmark.
Kristina Mattsson, Division of Occupational and Environmental Medicine, Department of Laboratory Medicine, Faculty of Medicine, Lund University, Medicon Village, Lund, Sweden; Department of Obstetrics and Gynecology, Skåne University Hospital, Lund, Sweden.
Data Availability
The datasets generated or analyzed during the current study are not available publicly, because they are subject to national data protection laws and restrictions imposed by the ethics committee to ensure the privacy of study participants.
Authors’ roles
K.M., E.N.-C. and A.J. conceptualized the study. K.M., A.J. and E.N.-C. contributed to the study design. A.J., S.Z. and L.S. obtained funding. A.J. acquired the data. K.M. drafted the manuscript and did the statistical analyses. All authors interpreted the results. All authors critically revised the manuscript for important intellectual content. K.M. and A.J. had full access to the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analyses.
Funding
The study is part of the ReproUnion collaborative study, co-financed by the European Union, Interreg VÖKS. The funding source had no role in the study design, the collection, analysis and interpretation of data or the writing of the paper and decision to submit it for publication.
Conflict of interest
The authors have no competing interests to declare.
References
- Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, Llurba E, Gris JM.. Tumor necrosis factor-alpha and pregnancy: focus on biologics. an updated and comprehensive review. Clin Rev Allergy Immunol 2017;53:40–53. [DOI] [PubMed] [Google Scholar]
- Babayev SN, Park CW, Bukulmez O.. Intracytoplasmic sperm injection indications: how rigorous? Semin Reprod Med 2014;32:283–290. [DOI] [PubMed] [Google Scholar]
- Berry M, Brightling C, Pavord I, Wardlaw A.. TNF-alpha in asthma. Curr Opin Pharmacol 2007;7:279–282. [DOI] [PubMed] [Google Scholar]
- Bláfoss J, Hansen AV, Malchau Lauesgaard SS, Ali Z, Ulrik CS.. Female asthma and atopy—impact on fertility: a systematic review. J Asthma Allergy 2019;12:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais L, Kettani F-Z, Forget A.. Relationship between maternal asthma, its severity and control and abortion. Hum Reprod 2013;28:908–915. [DOI] [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG.. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 2007;22:1506–1512. [DOI] [PubMed] [Google Scholar]
- Ceulemans M, Lupattelli A, Nordeng H, Odalovic M, Twigg M, Foulon V.. Women’s beliefs about medicines and adherence to pharmacotherapy in pregnancy: opportunities for community pharmacists. Curr Pharm Des 2019;25:469–482. [DOI] [PubMed] [Google Scholar]
- Cousineau TM, Domar AD.. Psychological impact of infertility. Best Pract Res Clin Obstet Gynaecol 2007;21:293–308. [DOI] [PubMed] [Google Scholar]
- Denburg JA, Sehmi R, Saito H, Pil-Seob J, Inman MD, O'Byrne PM.. Systemic aspects of allergic disease: bone marrow responses. J Allergy Clin Immunol 2000;106:S242–S246. [DOI] [PubMed] [Google Scholar]
- Gade EJ, Thomsen SF, Lindenberg S, Backer V.. Fertility outcomes in asthma: a clinical study of 245 women with unexplained infertility. Eur Respir J 2016;47:1144–1151. [DOI] [PubMed] [Google Scholar]
- Gade EJ, Thomsen SF, Lindenberg S, Kyvik KO, Lieberoth S, Backer V.. Asthma affects time to pregnancy and fertility: a register-based twin study. Eur Respir J 2014;43:1077–1085. [DOI] [PubMed] [Google Scholar]
- Garne E, Vinkel Hansen A, Morris J, Jordan S, Klungsøyr K, Engeland A, Tucker D, Thayer DS, Davies GI, Nybo Andersen A-M. et al. Risk of congenital anomalies after exposure to asthma medication in the first trimester of pregnancy—a cohort linkage study. BJOG 2016;123:1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeskowiak LE, Smithers LG, Grieger JA, Bianco-Miotto T, Leemaqz SY, Clifton VL, Poston L, McCowan LM, Kenny LC, Myers J. et al. Asthma treatment impacts time to pregnancy: evidence from the international SCOPE study. Eur Respir J 2018;51:1702035. [DOI] [PubMed] [Google Scholar]
- Janson C, Lisspers K, Ställberg B, Johansson G, Thuresson M, Telg G, Larsson K.. Prevalence, characteristics and management of frequently exacerbating asthma patients: an observational study in Sweden (PACEHR). Eur Respir J 2018;52:1701927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul Gade E, Thomsen SF, Lindenberg S, Backer V.. Female asthma has a negative effect on fertility: what is the connection? ISRN Allergy. 2014;2014:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källén B, Otterblad Olausson P.. Use of anti-asthmatic drugs during pregnancy. 1. Maternal characteristics, pregnancy and delivery complications. Eur J Clin Pharmacol 2007;63:363–373. [DOI] [PubMed] [Google Scholar]
- Kuruvilla ME, Vanijcharoenkarn K, Shih JA, Lee FE-H.. Epidemiology and risk factors for asthma. Respir Med 2019;149:16–22. [DOI] [PubMed] [Google Scholar]
- Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L, Missmer SA.. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update 2015;21:500–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H, Fahy JV.. The cytokines of asthma. Immunity 2019;50:975–991. [DOI] [PubMed] [Google Scholar]
- Larsson K, Ställberg B, Lisspers K, Telg G, Johansson G, Thuresson M, Janson C.. Prevalence and management of severe asthma in primary care: an observational cohort study in Sweden (PACEHR). Respir Res 2018;19:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Munsie JPW, Herdt-Losavio ML, Druschel CM, Campbell K, Browne ML, Romitti PA, Olney RS, Bell EM; National Birth Defects Prevention Study. Maternal asthma medication use and the risk of selected birth defects. Pediatrics 2012;129:e317–e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus PA, Wise SK.. Epidemiology and economic burden of asthma. Int Forum Allergy Rhinol 2015;5:S7–S10. [DOI] [PubMed] [Google Scholar]
- Löfvendahl S, Schelin MEC, Jöud A.. The value of the Skåne Health-care Register: prospectively collected individual-level data for population-based studies. Scand J Public Health 2020;48:56–63. [DOI] [PubMed] [Google Scholar]
- Lötvall J, Ekerljung L, Rönmark EP, Wennergren G, Lindén A, Rönmark E, Torén K, Lundbäck B.. West Sweden Asthma Study: prevalence trends over the last 18 years argues no recent increase in asthma. Respir Res 2009;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur MB, Ding P, Riddell CA, VanderWeele TJ.. Web site and R package for computing E-values. Epidemiology 2018;29:e45–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy VE, Wang G, Namazy JA, Powell H, Gibson PG, Chambers C, Schatz M.. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG 2013;120:812–822. [DOI] [PubMed] [Google Scholar]
- National Board of Health and Welfare. Statistik om aborter—Statistics on Abortions (In Swedish).2020. https://www.socialstyrelsen.se/statistik-och-data/statistik/statistikamnen/aborter/ (09 February 2021, date last accessed).
- Nyboe Andersen A, Carlsen E, Loft A.. Trends in the use of intracytoplasmatic sperm injection marked variability between countries. Hum Reprod Update 2008;14:593–604. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A.. Obesity and reproductive disorders in women. Hum Reprod Update 2003;9:359–372. [DOI] [PubMed] [Google Scholar]
- Peng Y-H, Su S-Y, Liao W-C, Huang C-W, Hsu CY, Chen H-J, Wu T-N, Ho W-C, Wu C-C.. Asthma is associated with endometriosis: a retrospective population-based cohort study. Respir Med 2017;132:112–116. [DOI] [PubMed] [Google Scholar]
- Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, Brosens JJ, Brewin J, Ramhorst R, Lucas ES. et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 2021;397:1658–1667. [DOI] [PubMed] [Google Scholar]
- Region Skåne Asthma Guidelines. Astma—AKO Skåne-riktlinje för primärvården (Asthma—Regional Guidelines for Primary Care). In Swedish. 2020. https://vardgivare.skane.se/vardriktlinjer/lungsjukdomar/ako/astma/ (03 August 2021, date last accessed).
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P.. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod 2002;17:2715–2724. [DOI] [PubMed] [Google Scholar]
- Stegberg M, Hasselgren M, Montgomery S, Lisspers K, Ställberg B, Janson C, Sundh J.. Changes in smoking prevalence and cessation support, and factors associated with successful smoking cessation in Swedish patients with asthma and COPD. Eur Clin Respir J 2018;5:1421389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanes C, Real FG, Gislason T, Jansson C, Jögi R, Norrman E, Nyström L, Torén K, Omenaas E.. Association of asthma and hay fever with irregular menstruation. Thorax 2005;60:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedish Public Health Authority. Daglig tobaksrökning—Daily Tobacco Smoking (In Swedish). 2021. https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/tolkad-rapportering/folkhalsans-utveckling/resultat/levnadsvanor/tobaksrokning-daglig/ (10 March 2022, date last accessed).
- Tata LJ, Hubbard RB, McKeever TM, Smith CJP, Doyle P, Smeeth L, West J, Lewis SA.. Fertility rates in women with asthma, eczema, and hay fever: a general population-based cohort study. Am J Epidemiol 2007;165:1023–1030. [DOI] [PubMed] [Google Scholar]
- Taylor A. ABC of subfertility—extent of the problem. BMJ 2003;327:434–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM.. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324–1331.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PC, Lockey RF, Holmes K, Friedmann E.. Asthma and/or hay fever as predictors of fertility/impaired fecundity in U.S. women: National Survey of Family Growth. Sci Rep 2019;9:1–16. doi: 10.1038/s41598-019-55259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphoff E, Cabieses B, Pinart M, Valdés M, Antó JM, Wright J.. A systematic review of socioeconomic position in relation to asthma and allergic diseases. Eur Respir J 2015;46:364–374. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ, Ding P.. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, Petraglia F.. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update 2016;22:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassard D, Schmidt L, Pinborg A, Petersen GL, Forman JL, Hageman I, Glazer CH, Kamper-Jørgensen M.. Mortality in women treated with assisted reproductive technology-addressing the healthy patient effect. Am J Epidemiol 2018;187:1889–1895. [DOI] [PubMed] [Google Scholar]
- Vejen Hansen A, Ali Z, Malchau SS, Blafoss J, Pinborg A, Ulrik CS.. Fertility treatment among women with asthma: a case–control study of 3689 women with live births. Eur Respir J 2019;53:1800597. [DOI] [PubMed] [Google Scholar]
- Wasilewska E, Małgorzewicz S.. Impact of allergic diseases on fertility. Postepy Dermatol Alergol 2019;36:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierau L, Meteran H, Backer V, Lindenberg S, Skytthe A, Thomsen SF.. The risk of asthma is increased among women with polycystic ovary syndrome: a twin study. ERJ Open Res 2019;5:00018–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study are not available publicly, because they are subject to national data protection laws and restrictions imposed by the ethics committee to ensure the privacy of study participants.