Abstract
Background
The role of consolidation therapy with autologous stem cell transplantation (ASCT) in patients with peripheral T-cell lymphoma (PTCL) in first complete remission (CR1) or partial remission (PR1) remains controversial. The existing data from China are limited. Therefore, we aimed to investigate the effect of ASCT on the survival of Chinese patients with PTCL showing response to induction chemotherapy at our hospital.
Methods
We retrospectively reviewed the data of patients with PTCL (excluding Natural killer/T cell lymphoma) in CR1 or PR1 treated at Peking University Hospital &Institute from 1996 to 2020. Propensity score matching (PSM) was used to balance clinical characteristics between the ASCT and non-ASCT groups. The primary endpoints were event-free survival (EFS) and overall survival (OS).
Results
Of the 414 selected patients, 73 received ASCT consolidation and 341 did not. Over a median follow-up of 5.7 years, survival was significantly better in the ASCT group than in the non-ASCT group (median EFS, 8.1 years vs. 2.8 years, P = 0.002; median OS, 14.9 years vs. 10.2 years, P = 0.007). The 5-year EFS and OS rates were 68.4% and 77.0% in ASCT group, and 43.2% and 57.6% in non-ASCT group, respectively. The survival benefit was confirmed in the propensity score matched cohort (46 patients who received ASCT and 84 patients who did not receive ASCT): P = 0.007 for median EFS and P = 0.022 for the median OS. Cox regression analysis showed that ASCT was independently associated with better survival: hazard ratio (HR) for EFS, 0.46 (95% CI: 0.28-0.76); HR for OS, 0.50 (95% CI: 0.31-0.84). Subgroup analysis showed that ASCT was more likely to benefit higher-risk patients and those with advanced disease. Among the subtypes of PTCL, the benefit was significant in angioimmunoblastic T-cell lymphoma (HR = 0.26 [95% CI: 0.10-0.66] for EFS and 0.29 [95% CI: 0.12-0.74] for OS), but not in the other subtypes.
Conclusion
ASCT may improve the long-term survival of patients with PTCL in first CR or PR, especially for patients with angioimmunoblastic T-cell lymphoma. The specific groups most likely to benefit from upfront ASCT need to be clearly identified.
Keywords: peripheral T-cell lymphoma, survival, autologous hematopoietic stem cell transplantation (autoHCT), outcomes - health care, propensity score matching (PSM)
Introduction
Peripheral T/NK-cell lymphoma (PTCL) is a group of rare and highly heterogeneous malignant lymphoproliferative diseases originating from mature T- and NK-cells. PTCL is classified into subtypes such as angioimmunoblastic T-cell lymphoma (AITL), ALK-positive anaplastic large-cell lymphoma (ALK+ALCL), ALK-negative anaplastic large-cell lymphoma (ALK-ALCL), peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), and so on (1, 2). Most subtypes have a dismal prognosis (3, 4). Due to the rarity and heterogeneity of PTCL, there is still no consensus on the best treatment approach. The role of autologous stem cell transplant (ASCT) consolidation continues to be debated (5–10).
Current guidelines recommended ASCT for most PTCL patients achieving remission after induction chemotherapy (11–13). The National Comprehensive Cancer Network and the American Society for Transplantation and Cellular Therapy recommend ASCT for patients with first-time complete remission (CR1) (11, 12). Meanwhile, the European Society of Medical Oncology guidelines suggests that patients with first-time partial remission (PR1) could also be considered for ASCT (13). However, these recommendations were based on low-quality evidence from retrospective studies (14–17) and a few single-arm prospective studies (5, 18–20). There remains no consensus on the utility of consolidative ASCT.
One real-world study of lymphoma in a Swedish population showed that PTCL patients (excluding those with ALK+ALCL) receiving ASCT had better progression-free survival (HR: 0.56 [0.39-0.80]) and overall survival (OS; HR: 0.58 [0.40-0.84]), but the control group may enroll patients who did not respond to the initial chemotherapy (17). Comprehensive Oncology Measures for Peripheral T-Cell Lymphoma Treatment (COMPLETE) study showed a statistically nonsignificant tendency for better survival following ASCT in PTCL with CR1: the median OS in patients who underwent ASCT was not reached, versus 57.6 months in patients who did not receive ASCT (P = 0.06) (21). The large Lymphoma Study Association (LYSA) Registry-based multicenter retrospective study investigated the effect of ASCT in 134 patients with CR1 or PR1. Neither propensity score-matched analysis (P = 0.90 for PFS, P = 0.66 for OS, respectively) nor Cox multivariate analysis (HR = 1.02 [95% CI: 0.69-1.50] for PFS; HR = 1.08 [95% CI:0.68-1.69] for OS) showed a survival advantage with upfront ASCT consolidation (22).
To the best of our knowledge, no well-designed randomized controlled trial has been conducted so far to evaluate the impact of ASCT in the clinical setting. The data based on Chinese patient population is limited. Therefore, in the present study, we aimed to investigate the effect of ASCT on the survival of patients with PTCL showing response to induction therapy.
Methods
Patients
The study was granted by the ethics committee of Peking University Cancer Hospital & Institute. Informed consent was exempted because the data were analyzed anonymously. A total of 8,826 patients with lymphoma were admitted to Peking University Cancer Hospital & Institute from January 1996 to November 2020. Pathological diagnosis was based on the corresponding criteria (1, 23–25). Patients with mature T-cell lymphoma who achieved CR1/PR1 were included in this study. The main exclusion criteria included patients with natural killer/T cell lymphoma (NK/TCL), receiving allogeneic transplantation, and missing clinical data. Assessment of response to initial treatment was evaluated using the Lugano criteria (26), and 18F-FDG positron emission tomography/computed tomography (PET/CT) was introduced since 2009. A total of 414 patients met the eligibility criteria. Supplementary Figure 1 shows the patient selection process. The selected patients were classified into risk groups using the International Prognostic Index (IPI) (27).
Definitions
Event-free survival (EFS) was defined as the time from the initial diagnosis to the date of occurrence of disease recurrence/progression confirmed by clinical manifestations, imaging or pathological reports, initiation of second-line salvage therapy, or death (whichever occurred first). OS was defined as the time from the initial diagnosis to death due to any cause. None of the above events that occurred at the last follow-up of the patient were censored. Follow-up data were collected via telephone interviews and a review of inpatient and outpatient medical records.
Statistical analysis
Statistical analysis was done using R 4.2.0 (https://www.r-project.org). Categorical variables were summarized as percentages and tested by the chi-square test or Fisher exact test. Propensity score matching (PSM) was performed to compare EFS and OS between ASCT and non-ASCT groups after balancing clinical parameters, which were selected according to previous literature and clinical experience; the parameters were histological subtype, age, sex, lactate dehydrogenase level (LDH), Ann Abor stage, Eastern Cooperative Oncology Group (ECOG) performance status, B symptoms, and response to the initial treatment. The caliper value was set to 0.25, and the matching ratio was 1: 2 (ASCT: non-ASCT). PSM was conducted using the “MatchIt” package in R. Survival analysis was performed before and after matching, using Kaplan–Meier curves and tested by the log-rank test. Univariate and multivariate Cox regression analyses were conducted to determine the independent effect of ASCT on survival after adjusting for other variables (sex, age, Ann Abor stage, IPI risk group, and histological subtype [ALK+ALCL vs. non-ALK+ALCL]). All significant factors from the univariate analysis were included in the multivariate analysis. Survival rates were estimated by the Cox proportional hazards model, Hazard ratios, with 95% confidence intervals, were calculated to quantify risk. Two-sided P < 0.05 was considered statistically significant.
Results
Patient characteristics
Of the 414 selected patients, 73 received ASCT (ASCT group) and 341 did not receive ASCT (non-ASCT group). As shown in Table 1 , the histologic subtypes were ALK+ALCL (n = 85, 20.5%) AITL (n = 116, 28.0%), ALK-ALCL (n = 42, 10.1%), PTCL-NOS (n = 76,18.4%), and others (n = 95, 22.9%). The first-line regimens were mainly CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) and CHOPE (CHOP plus etoposide). The details are available in Supplemental Table 1 and Table 2 . In the ASCT group, the bloodstream infection, febrile neutropenia, and 100-day treatment related mortality rates were 4.4%, 71.1% and 0, respectively.
Table 1.
Baseline characteristics of patients in the ASCT group and the non-ASCT group.
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Non-ASCT | ASCT | P | Non-ASCT | ASCT | P | |
| n = 341 | n = 73 | n = 84 | n = 46 | |||
| Subtypes (%) | 0.540 | 0.860 | ||||
| ALK+ALCL | 67 (19.6) | 18 (24.7) | 19 (22.6) | 12 (26.1) | ||
| ALK-ALCL | 35 (10.3) | 7 (9.6) | 8 (9.5) | 4 (8.7) | ||
| AITL | 92 (27.0) | 24 (32.9) | 32 (38.1) | 17 (37.0) | ||
| PTCL-NOS | 66 (19.4) | 10 (13.7) | 4 (4.8) | 4 (8.7) | ||
| Others* | 81 (23.8) | 14 (19.2) | 21 (25.0) | 9 (19.6) | ||
| Age, years, median (IQR) | 53.0 (35.0, 64.0) | 42.00 (26.0, 51.0) | < 0.001 | 44.0 (25.0, 59.0) | 43.00 (29.0, 54.0) | 0.536 |
| Female (%) | 110 (32.3) | 21 (28.8) | 0.657 | 25 (29.8) | 11 (23.9) | 0.612 |
| LDH ≥ULN (%) | 102 (34.5) | 33 (61.1) | < 0.001 | 42 (50.0) | 26 (56.5) | 0.597 |
| III–IV stage (%) | 226 (66.3) | 61 (83.6) | 0.006 | 70 (83.3) | 39 (84.8) | > 0.99 |
| ECOG ≥2 (%) | 23 (7.2) | 5 (8.2) | 0.993 | 7 (8.3) | 4 (8.7) | > 0.99 |
| B symptoms (%) | 153 (45.1) | 42 (57.5) | 0.073 | 48 (57.1) | 28 (60.9) | 0.821 |
| PR to first treatment (%) | 121 (35.5) | 23 (31.5) | 0.609 | 25 (29.8) | 14 (30.4) | > 0.99 |
AITL, angioimmunoblastic T-cell lymphoma; ALK-ALCL, anaplastic large-cell lymphoma, anaplastic lymphoma kinase negative; ALK+ALCL, anaplastic large-cell lymphoma anaplastic lymphoma kinase positive; PTCL-NOS, PTCL not otherwise specified; LDH, lactate dehydrogenase; ASCT, autologous stem cell transplantation; PR, partial remission; ULN, upper limit of normal; ECOG, Eastern Cooperative Oncology Group performance status.
* Others indicates patients with primary cutaneous anaplastic large cell lymphoma, mycosis fungoides/sézary syndrome, hepatosplenic T-cell lymphoma, enteropathy associated T cell lymphoma, subcutaneous panniculitis-like T-cell lymphoma, monomorphic epitheliotropic intestinal T-cell lymphomas, lymphomatoid papulosis, primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma and other rare subtypes. A portion of patients were classified in this group due to the limitation of the diagnostic measurements in the early era (e.g ALCL with uncertain ALK status).
Survival analysis in unmatched data
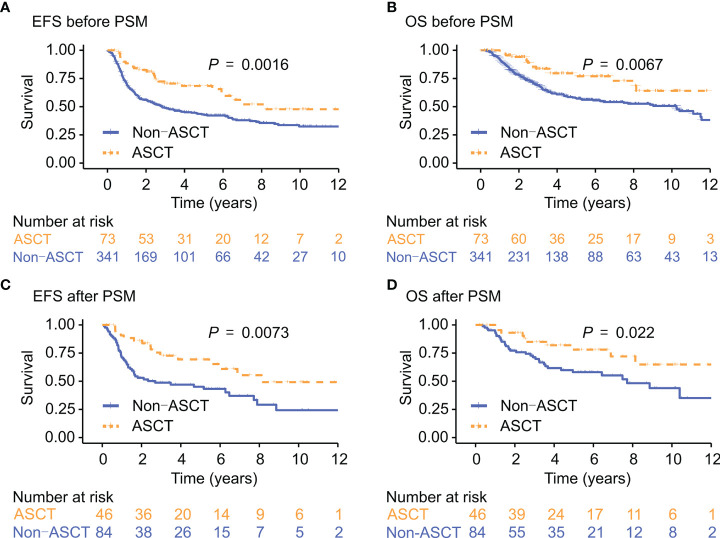
The median follow-up was for 5.7 years. Median EFS and OS were significantly better in the ASCT group than in the non-ASCT group (EFS: 8.1 years vs. 2.8 years, P = 0.002; OS: 14.9 years vs. 10.2 years, P = 0.007). The 5-year EFS and OS rates were 68.4% (95% CI: 57.7%-81.1%) and 77.0% (95% CI: 66.5%-89.1%), respectively, in the ASCT group vs. 43.2% (95% CI: 37.8%-49.3%) and 57.6% (95% CI: 52.0%-63.8%), respectively, in the non-ASCT group (P < 0.001 for all; Figure 1 ).
Figure 1.
EFS and OS in the ASCT group and the non-ASCT group before and after PSM. EFS, event-free survival; OS, overall survival; ASCT, autologous stem cell transplantation; PSM, propensity score matching. Kaplan-Meir analysis of EFS (A, C) and OS (B, D), before and after PSM.
As expected, patients with ALK+ALCL had better survival outcomes ( Supplementary Figures 2A , 2B ; Supplementary Table 3 ). We also compared the survival outcome between patients with CR and PR in Supplementary Figures 3A, B . Patients with CR had better 5-year EFS (74.2% vs. 53.6%) and OS (85.0% vs. 69.0%) than those with PR (57.1% vs. 23.8%; 59.5% vs. 37.2%) in the ASCT and non-ASCT group, respectively ( Supplementary Figures 3C–F ).
Survival analysis in matched data
In the propensity score matched (PSM) cohort, there was no statistically significant differences in clinical characteristics between patients in the ASCT group (n = 46) and the non-ASCT group (n = 84; Table 1 ). In this cohort, median EFS and OS were also better in the ASCT group than in the non-ASCT group (EFS: 8.1 years vs. 2.7 years, P = 0.007; OS: “not reached” vs. 7.7 years, P = 0.022). In addition, the survival difference remained statistically significant when excluding patients with ALK+ALCL after performing PSM method ( Supplementary Figures 2C , 2D ; Supplementary Table 4 ).
Cox regression analysis
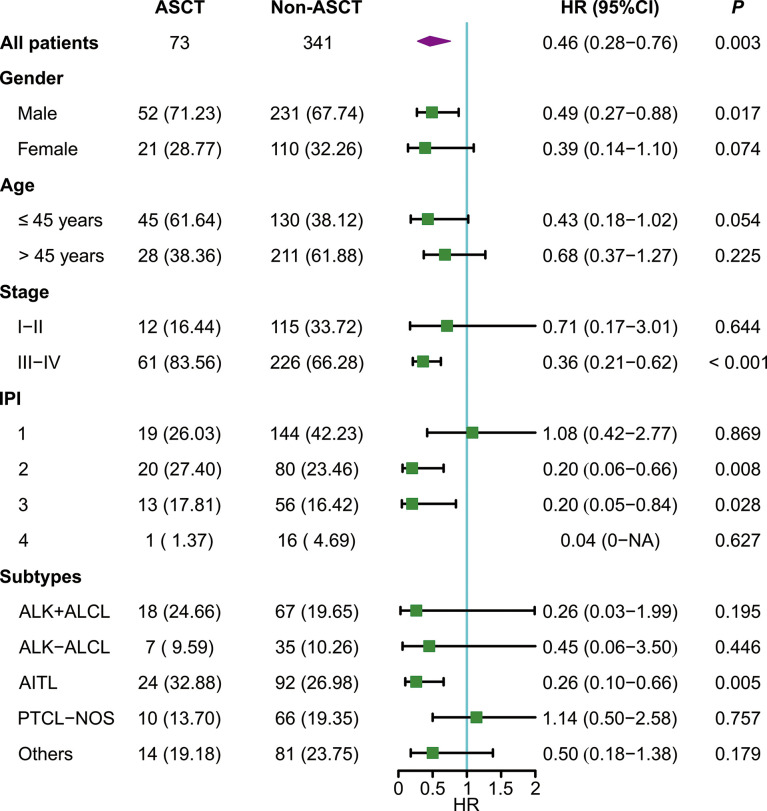
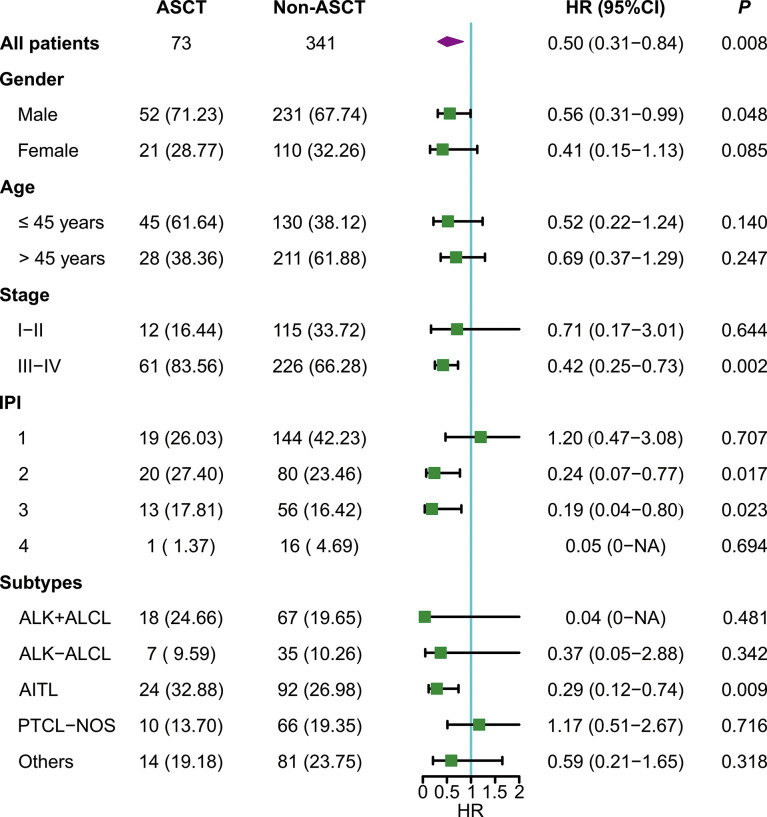
For the whole cohort, ASCT was associated with better EFS (HR: 0.46 [95% CI: 0.28 - 0.76]) and OS (HR: 0.50 [95% CI: 0.31-0.84]) ( Figures 2 , 3 ). Survival of patients with AITL was significantly better in the ASCT group than in the non-ASCT group (HR for EFS: 0.26 [95% CI: 0.10-0.66]; HR for OS: 0.29 [95% CI: 0.12-0.74]). However, there were no significant differences in EFS or OS between the ASCT and non-ASCT groups for ALK+ALCL, ALK-ALCL, AITL, PTCL-NOS, and other subtypes. Further subgroup analysis showed that ASCT was associated with better EFS and OS in advanced-stage disease and in low-intermediate and intermediate-high risk subgroups ( Figures 2 , 3 ). In multivariate Cox regression analysis, ASCT was confirmed as the independent risk factors for OS in PTCL patients with CR1 or PR1 ( Table 2 ).
Figure 2.
Subgroup analysis of event-free survival. ASCT, autologous stem cell transplantation; IPI, International Prognostic Index; AITL, angioimmunoblastic T-cell lymphoma; ALK+ALCL, anaplastic large-cell lymphoma, anaplastic lymphoma kinase positive; ALK-ALCL, anaplastic large-cell lymphoma, anaplastic lymphoma kinase negative; PTCL-NOS, PTCL not otherwise specified; HR, hazard ratio.
Figure 3.
Subgroup analysis of overall survival.
Table 2.
Univariate and multivariate Cox regression analysis for OS.
| Univariate Cox model | Multivariate Cox model | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.034 (1.024-1.045) | < 0.001 | 1.024 (1.011 -1.037) | 0.001 |
| Female | 0.988 (0.705-1.384) | 0.944 | – | – |
| III–IV stage | 2.140 (1.429-3.204) | < 0.001 | 1.899 (1.194 -3.02) | 0.014 |
| ASCT | 0.505 (0.305-0.836) | 0.008 | 0.478 (0.242 -0.945) | 0.034 |
| PR to the 1st treatment | 2.824 (2.060-3.873) | < 0.001 | 2.687 (1.859 -3.884) | < 0.001 |
| ALK+ALCL | 0.101 (0.041-0.245) | < 0.001 | 0.478 (0.242 -0.945) | 0.003 |
| ECOG ≥2 (%) | 1.507 (0.851-2.668) | 0.159 | – | – |
| LDH ≥ULN (%) | 1.531 (1.070-2.191) | 0.020 | 1.138 (0.774 -1.673) | 0.584 |
| B symptoms (%) | 1.112 (0.299-4.128) | 0.875 | – | – |
ALK+ALCL, anaplastic large-cell lymphoma; anaplastic lymphoma kinase positive; ECOG, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; ULN, upper limit of normal; HR, hazard ratio; ASCT, autologous stem cell transplantation; PR, partial response.
Discussion
PTCL has a poorer prognosis than aggressive B-cell lymphomas, with less durable remissions after anthracycline-containing multiagent chemotherapy (4, 28, 29). Treatment is particular challenging due to the relative rarity and biological heterogeneity of PTCL. The value of ASCT in PTCL remains controversial (9, 10, 22, 30), lacking a large sample retrospective study from China. The present study provides evidence in support of the use of ASCT in Chinese chemotherapy responders.
Previous studies suggest that consolidation with ASCT may not offer survival advantages for patients with PTCL except in specific clinical scenarios (10, 21, 22, 31). The COMPLETE study was the first large prospective cohort study to directly compare survival outcomes of patients with nodal PTCL in CR1 treated with or without consolidative ASCT. Eighty-three patients did not undergo ASCT, while 36 underwent consolidative ASCT. After a median follow-up of 2.8 years, no statistically significant difference was found in median OS between the ASCT group and the non-ASCT group (median OS: “not reached” vs. 57.6 months, respectively, P = 0.06). ASCT had no impact on the prognosis of PTCL-NOS but significantly improved OS and progression-free survival for patients with AITL; this finding is consistent with our results. Thus, it appears that patients with AITL are likely to benefit most from ASCT consolidation (16).
Our results were contrary to the findings of the large retrospective multicenter study based on the LYSA registry although both studies had similar designs. The LYSA study evaluated 269 patients with PTCL achieving CR1/PR1. The PTCL subtypes in the LYSA study included PTCL-NOS (29%), AITL (46%), and ALK-ALCL (25%). Among them, 134 patients were allocated to ASCT and 135 did not. Both Cox regression analysis and PSM analysis showed no survival advantage with ASCT consolidation (22). Our study enrolled more histologic subtypes (including ALK+ALCL, Enteropathy-associated T-cell lymphoma (EATL), and others); the differences between the study populations in the two studies may be one of the reasons for the contrary results. In addition, the performance of the intention-to-treat approach in this study may have led to more conservative estimates (32).
Although several earlier studies have reported that PTCL patients who receive upfront ASCT consolidation have a relatively good prognosis (5, 14, 15, 17, 33–35), none of the studies attempted to determine the sub-cohort of patients most likely to benefit. One pooled analysis showed that PTCL-NOS and ALK-ALCL patients without DUSP22 rearrangement could benefit from ASCT (36), indicating ALK-ALCL patients with DUSP22 rearrangement may be a potential transplant-exempt population. Thus, current evidence suggests clear benefits with ASCT consolidation in patients with AITL and uncertain benefits in patients with PTCL-NOS (probably due to the high heterogeneity in this subtype). The benefit of ASCT in different pathological subtypes needs further study.
Our results also demonstrated that upfront consolidative ASCT can improve prognosis in low-intermediate and intermediate-high risk subgroups of PTCL. Because only one patient in the high-risk subgroup underwent ASCT, we did not analyze the high-risk group. The evidence for the long-term efficacy of upfront ASCT in patients with early-stage and low-risk IPI is insufficient (21).
Because some previous studies have suggested that the frontline chemotherapy regimen may be associated with survival outcomes (37), we compared the survival difference between patients receiving CHOP vs. CHOPE as an initial regimen after matching baseline characteristics by PSM (data not shown). No survival difference was observed in our cohort.
In recent years, the discovery and clinical application of molecular biomarkers and signatures have provided unprecedented insights into PTCL (38–41). Novel antibody-drug conjugates and epigenetic modifying agents have shown promising clinical efficacy (42, 43). Of note, The ECHELON-2 study demonstrated a significant improvement in prognosis with frontline brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, and prednisone (A+CHP) compared with CHOP in CD30-positive PTCLs (43). Furthermore, Savage et al. recently conducted an exploratory subgroups analysis of ECHELON-2 trial supporting the role of consolidation consolidative stem cell transplant (autologous or allogeneic) in CD30-positive PTCL who achieve a CR following treatment with A+CHP. Patients who underwent stem cell transplants achieved a 64% reduction in the risk of a PFS event (HR: 0.36 [95% CI, 0.17-0.77]). The results indicate that even in the era of BV, consolidative stem cell transplant is still required (44).
Our results should be carefully interpreted because of some limitations in this study. First, this was a retrospective single-center study. The findings from the subgroup analysis may not be reliable because of the limited number of patients. Second, there is bound to be a discrepancy in response evaluation by PET/CT vs. CT (26). The CR rate may be underestimated for those patients who received response evaluation only by CT in the cohort. Finally, the systems of criteria for diagnosis have undergone changes in recent 20 years (1, 23–25). We enrolled patients according to the diagnostic criteria of that time, but there must be variability around the exact criteria used for diagnosis over long time span. Therefore, pathological review and molecular typing to define the subtypes of PTCL are required in future studies.
Conclusion
ASCT consolidation may prolong survival in Chinese patients with PTCL showing complete or partial response to initial chemotherapy; benefit is most likely in patients with AITL. Patients with higher-risk IPI score and advanced-stage disease may also benefit significantly from ASCT. Our findings need to be confirmed in large controlled prospective studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of Peking University Cancer Hospital. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
HG and WL designed this research. HG collected the data, performed the analysis and prepared the original draft. XJ was involved in data cleaning and analysis. MW and ND provided helpful suggestions for this study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 82070205, 81870154]; the Clinical Research Fund For Distinguished Young Scholars of Beijing Cancer Hospital [grant number QNJJ202106]; and the Capital’s Funds for Health Improvement and Research [grant numbers 2022-1-2152, 2020-2Z-2157].
Acknowledgments
We thank all the colleagues at Peking University Cancer Hospital & Institute for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
| AITL | angioimmunoblastic T-cell lymphoma |
| ALK+ALCL | anaplastic lymphoma kinase positive anaplastic large-cell lymphoma |
| ALK-ALCL | anaplastic lymphoma kinase negative anaplastic large-cell lymphoma |
| ASCT | autologous stem cell transplant |
| CHOP | cyclophosphamide, doxorubicin, vincristine and prednisolone |
| CHOPE | cyclophosphamide, doxorubicin, vincristine, prednisolone, etoposide |
| CI | confidence interval |
| CR1 | first-time complete remission |
| ECOG | Eastern Cooperative Oncology Group |
| EFS | Event-free survival |
| HR | hazard ratio |
| IPI | International Prognostic Index |
| LYSA | Lymphoma Study Association |
| OS | overall survival |
| PET/CT | positron emission tomography/computed tomography |
| PR1 | first-time partial remission |
| PSM | Propensity score matching; |
| PTCL | Peripheral T/NK-cell lymphoma |
| PTCL-NOS | peripheral T-cell lymphoma not otherwise specified |
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1039888/full#supplementary-material
References
- 1. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood. (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu W, Liu J, Song Y, Wang X, Mi L, Cai C, et al. Burden of lymphoma in China, 1990-2019: an analysis of the global burden of diseases, injuries, and risk factors study 2019. Aging (Albany NY). (2022) 14(7):3175–90. doi: 10.18632/aging.204006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao H, Ji X, Liu X, Mi L, Liu W, Wang X, et al. Conditional survival and hazards of death for peripheral T-cell lymphomas. Aging (Albany NY). (2021) 13(7):10225–39. doi: 10.18632/aging.202782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu W, Ji X, Song Y, Wang X, Zheng W, Lin N, et al. Improving survival of 3760 patients with lymphoma: Experience of an academic center over two decades. Cancer Med. (2020) 9(11):3765–3774. doi: 10.1002/cam4.3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. d'Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol (2012) 30(25):3093–9. doi: 10.1200/JCO.2011.40.2719 [DOI] [PubMed] [Google Scholar]
- 6. Blystad AK, Enblad G, Kvaløy S, Berglund A, Delabie J, Holte H, et al. High-dose therapy with autologous stem cell transplantation in patients with peripheral T cell lymphomas. Bone Marrow Transplant. (2001) 27(7):711–6. doi: 10.1038/sj.bmt.1702867 [DOI] [PubMed] [Google Scholar]
- 7. Jantunen E, Wiklund T, Juvonen E, Putkonen M, Lehtinen T, Kuittinen O, et al. Autologous stem cell transplantation in adult patients with peripheral T-cell lymphoma: a nation-wide survey. Bone Marrow Transplant. (2004) 33(4):405–10. doi: 10.1038/sj.bmt.1704367 [DOI] [PubMed] [Google Scholar]
- 8. Feyler S, Prince HM, Pearce R, Towlson K, Nivison-Smith I, Schey S, et al. The role of high-dose therapy and stem cell rescue in the management of T-cell malignant lymphomas: a BSBMT and ABMTRR study. Bone Marrow Transplant. (2007) 40(5):443–50. doi: 10.1038/sj.bmt.1705752 [DOI] [PubMed] [Google Scholar]
- 9. Rodríguez J, Conde E, Gutiérrez A, Arranz R, León A, Marín J, et al. The results of consolidation with autologous stem-cell transplantation in patients with peripheral T-cell lymphoma (PTCL) in first complete remission: the Spanish lymphoma and autologous transplantation group experience. Ann Oncol (2007) 18(4):652–7. doi: 10.1093/annonc/mdl466 [DOI] [PubMed] [Google Scholar]
- 10. Yam C, Landsburg DJ, Nead KT, Lin X, Mato AR, Svoboda J, et al. Autologous stem cell transplantation in first complete remission may not extend progression-free survival in patients with peripheral T cell lymphomas. Am J Hematol (2016) 91(7):672–6. doi: 10.1002/ajh.24372 [DOI] [PubMed] [Google Scholar]
- 11. NCCN Guidelines, T-Cell Lymphomas, Version 2.2022. (2022). Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=: Accessed Oct 10, 2022 [Google Scholar]
- 12. Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: Guidelines from the American society for transplantation and cellular therapy. Biol Blood Marrow Transplant. (2020) 26(7):1247–56. doi: 10.1016/j.bbmt.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 13. d'Amore F, Gaulard P, Trümper L, Corradini P, Kim WS, Specht L, et al. Peripheral T-cell lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2015) 26 Suppl 5:v108–115. doi: 10.1093/annonc/mdv201 [DOI] [PubMed] [Google Scholar]
- 14. Schetelig J, Fetscher S, Reichle A, Berdel WL, Beguin Y, Brunet S, et al. Long-term disease-free survival in patients with angioimmunoblastic T-cell lymphoma after high-dose chemotherapy and autologous stem cell transplantation. Haematologica. (2003) 88(11):1272–8. [PubMed] [Google Scholar]
- 15. Kyriakou C, Canals C, Goldstone A, Caballero D, Metzner B, Kobbe G, et al. High-dose therapy and autologous stem-cell transplantation in angioimmunoblastic lymphoma: complete remission at transplantation is the major determinant of outcome-lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol (2008) 26(2):218–24. doi: 10.1200/JCO.2008.12.6219 [DOI] [PubMed] [Google Scholar]
- 16. Rodríguez J, Conde E, Gutiérrez A, Arranz R, Gandarillas M, Leon A, et al. Prolonged survival of patients with angioimmunoblastic T-cell lymphoma after high-dose chemotherapy and autologous stem cell transplantation: the GELTAMO experience. Eur J Haematol (2007) 78(4):290–6. doi: 10.1111/j.1600-0609.2007.00815.x [DOI] [PubMed] [Google Scholar]
- 17. Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish lymphoma registry. Blood. (2014) 124(10):1570–7. doi: 10.1182/blood-2014-04-573089 [DOI] [PubMed] [Google Scholar]
- 18. Reimer P, Rüdiger T, Geissinger E, Weissinger E, Nerl F, Schmitz N, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol (2009) 27(1):106–13. doi: 10.1200/JCO.2008.17.4870 [DOI] [PubMed] [Google Scholar]
- 19. Corradini P, Tarella C, Zallio F, Dodero P, Zanni M, Valagussa P, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. (2006) 20(9):1533–8. doi: 10.1038/sj.leu.2404306 [DOI] [PubMed] [Google Scholar]
- 20. Mercadal S, Briones J, Xicoy B, Pedro C, Escoda L, Estany C, et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol (2008) 19(5):958–63. doi: 10.1093/annonc/mdn022 [DOI] [PubMed] [Google Scholar]
- 21. Park SI, Horwitz SM, Foss FM, Brown LC, Carson KR, Rosen ST, et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: Report from COMPLETE, a prospective, multicenter cohort study. Cancer. (2019) 125(9):1507–17. doi: 10.1002/cncr.31861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fossard G, Broussais F, Coelho I, Bailly S, Nicolas-Virelizier E, Toussaint E, et al. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: an analysis of patients from LYSA centers. Ann Oncol (2018) 29(3):715–23. doi: 10.1093/annonc/mdx787 [DOI] [PubMed] [Google Scholar]
- 23. Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the international lymphoma study group. Blood. (1994) 84(5):1361–92. doi: 10.1182/blood.V84.5.1361.1361 [DOI] [PubMed] [Google Scholar]
- 24. Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. World health organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the clinical advisory committee meeting-airlie house, Virginia, November 1997. J Clin Oncol (1999) 17(12):3835–49. doi: 10.1200/JCO.1999.17.12.3835 [DOI] [PubMed] [Google Scholar]
- 25. Campo E, Swerdlow SH, Harris N, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood (2011) 117(19):5019–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol (2014) 32(27):3059–68. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. International non-hodgkin's lymphoma prognostic factors project. a predictive model for aggressive non-hodgkin's lymphoma. N Engl J Med (1993) 329(14):987–94. doi: 10.1056/NEJM199309303291402 [DOI] [PubMed] [Google Scholar]
- 28. Liu W, Liu J, Song Y, Wang X, Zhou M, Wang L, et al. Mortality of lymphoma and myeloma in China, 2004-2017: an observational study. J Hematol Oncol (2019) 12(1):22. doi: 10.1186/s13045-019-0706-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abramson JS, Feldman T, Kroll-Desrosiers AR, Muffly LS, Winer E, Flowers CR, et al. Peripheral T-cell lymphomas in a large US multicenter cohort: prognostication in the modern era including impact of frontline therapy. Ann Oncol (2014) 25(11):2211–7. doi: 10.1093/annonc/mdu443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith SM, Burns LJ, van Besien K, Lerademacher J, He W, Fenske TS, et al. Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J Clin Oncol (2013) 31(25):3100–9. doi: 10.1200/JCO.2012.46.0188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kitahara H, Maruyama D, Maeshima AM, Makita S, Miyamoto KI, Fukuhara S, et al. Prognosis of patients with peripheral T cell lymphoma who achieve complete response after CHOP/CHOP-like chemotherapy without autologous stem cell transplantation as an initial treatment. Ann Hematol (2017) 96(3):411–20. doi: 10.1007/s00277-016-2891-8 [DOI] [PubMed] [Google Scholar]
- 32. Bai AD, Komorowski AS, Lo CKL, Tandon P, Li XX, Mokashi V, et al. Intention-to-treat analysis may be more conservative than per protocol analysis in antibiotic non-inferiority trials: a systematic review. BMC Med Res Methodol (2021) 21(1):75. doi: 10.1186/s12874-021-01260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmitz N, Truemper L, Bouabdallah K, Ziepert M, Leclerc M, Cartron G, et al. A randomized phase 3 trial of autologous vs allogeneic transplantation as part of first-line therapy in poor-risk peripheral T-NHL. Blood. (2021) 137(19):2646–56. doi: 10.1182/blood.2020008825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bishton MJ, Haynes AP. Combination chemotherapy followed by autologous stem cell transplant for enteropathy-associated T cell lymphoma. Br J Haematol (2007) 136(1):111–3. doi: 10.1111/j.1365-2141.2006.06371.x [DOI] [PubMed] [Google Scholar]
- 35. Sieniawski M, Angamuthu N, Boyd K, Chasty R, Davies J, Forsyth P, et al. Evaluation of enteropathy-associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood. (2010) 115(18):3664–70. doi: 10.1182/blood-2009-07-231324 [DOI] [PubMed] [Google Scholar]
- 36. Pedersen MB, Hamilton-Dutoit SJ, Bendix K, Ketterling RP, Bedroske PP, Luoma IM, et al. DUSP22 and TP63 rearrangements predict outcome of ALK-negative anaplastic large cell lymphoma: a Danish cohort study. Blood. (2017) 130(4):554–7. doi: 10.1182/blood-2016-12-755496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim J, Cho J, Byeon S, Kim WS, Kim SJ. Comparison of first-line treatments of peripheral T-cell lymphoma according to regimen: A systematic review and meta-analysis. Hematol Oncol (2021) 39(5):664–73. doi: 10.1002/hon.2924 [DOI] [PubMed] [Google Scholar]
- 38. Wang T, Feldman AL, Wada DA, Lu Y, Polk A, Briski R, et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood. (2014) 123(19):3007–15. doi: 10.1182/blood-2013-12-544809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ye Y, Ding N, Mi L, Shi Y, LIu W, Song Y, et al. Correlation of mutational landscape and survival outcome of peripheral T-cell lymphomas. Exp Hematol Oncol (2021) 10(1):9. doi: 10.1186/s40164-021-00200-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iqbal J, Wright G, Wang C, Rosenwald A, Gascoyne RD, Weisenburger DD, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. (2014) 123(19):2915–23. doi: 10.1182/blood-2013-11-536359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heavican TB, Bouska A, Yu J, Lone W, Amador C, Gong Q, et al. Genetic drivers of oncogenic pathways in molecular subgroups of peripheral T-cell lymphoma. Blood. (2019) 133(15):1664–76. doi: 10.1182/blood-2018-09-872549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Horwitz S, O'Connor OA, Pro B, Trümper L, Iyer S, Advani R, et al. The ECHELON-2 trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol (2022) 33(3):288–98. doi: 10.1016/j.annonc.2021.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Horwitz S, O'Connor OA, Pro B, Illidge T, Fanale M, Advani R, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. (2019) 393(10168):229–40. doi: 10.1016/S0140-6736(18)32984-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Savage KJ, Horwitz SM, Advani R, Christensen JH, Domingo-Domenech E, Rossi G, et al. Role of stem cell transplant in CD30-positive PTCL following frontline brentuximab Vedotin+CHP or CHOP in ECHELON-2. Blood Adv (2022) 6(19):5550–5. doi: 10.1182/bloodadvances.2020003971 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.