Abstract
Purpose of Review
Night eating syndrome (NES) is an eating disorder that has historically been under-studied. The current review aims to summarize the most up-to-date research on NES to support better awareness.
Recent Findings
Since NES was recently included as a formal diagnosis, research on the prevalence of NES is ever evolving. Current studies underscore the high comorbidity between NES and other eating disorders, with additional complexities for patient with comorbid eating disorders. Recent findings also support the association between NES and sleep correlates, a relationship that has remained during the COVID-19 pandemic. Emerging research confirms correlates of distress in NES across cultures. There remain mixed findings between NES and BMI. There is also debate around whether age is a risk factor. Bariatric surgery research has focused on the re-emergence of NES post-operatively.
Summary
Our understanding of the correlates of NES is increasing. However, research on the treatment for NES remains particularly under-studied and requires further attention.
Keywords: Night eating syndrome, Nocturnal eating, Delayed food consumption, Evening hyperphagia insomnia, Eating disorder
Introduction
The current article aims to synthesize the growing body of research and clinical knowledge focused on night eating syndrome (NES), with particular emphasis placed on the most recent literature. Although the syndrome was first described in 1955, NES has remained an under-studied disorder [1]. Our current understanding of its complexities is ever evolving. with many correlates of NES not fully understood. It is our hope that an updated review will help providers to identify and treat this less recognized and understood disorder.
Overview
NES is generally classified within the eating disorder domain. It was recently included in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) for the first time under “other specified feeding or eating disorders [2].” NES is conceptualized as a circadian delay in food intake, evidenced by evening hyperphagia (at least 25% of daily food intake ingested after the evening meal) and/or nocturnal awakenings with food ingestion at least two times per week. According to the DSM-V criteria, patients with NES must also experience at least three of five associated features: (a) a lack of desire to eat in the morning and/or breakfast skipped four or more mornings per week; (b) a strong urge to eat between dinner and bedtime and/or during the night; (c) sleep-onset and/or sleep maintenance insomnia at least four nights per week; (d) a belief that one must eat in order to sleep; and (e) depressed or worsening mood in the evening. This constellation of symptoms must be accompanied by marked distress or impairment over a period of 3 months or more [2, 3].
For a NES diagnosis, it is important that a patient has a level of awareness with recall of nocturnal ingestions the following day. Although most patients with NES are easily able to recollect eating episodes, a distinct subset endorse decreased consciousness and seemingly automatic behavior [3]. However, even with this diminished awareness, when prompted, patients with NES should be able to remember some detail, with insight often increasing with treatment [3–5]. According to Lundgren and Pona (2017), “How do you know you have consumed food at night?” can be a key question in assessing awareness [6]. Ultimately, a minimal level of awareness is necessary for a NES diagnosis. For patients without awareness, sleep-related eating disorder (SRED) may be considered (SRED will be reviewed in an upcoming section).
Due to varying criterion utilized across studies, the prevalence of NES is difficult to ascertain. Generally, the estimates of NES within the general population ranges from 1 to 2%. However, the incidence of the disorder has evidenced significant variation within clinical samples. For example, de Zwaan et al. [7] found that across studies, the prevalence of NES in pre-operative bariatric patients ranged from 6 to 64% [7]. In attempt to elucidate the prevalence of NES using only the DSM-5 diagnostic criteria, Kaur et al. [8••] reviewed the most up-to-date empirical literature, concluding that the overall prevalence of NES ranged from 2.8 to 8.2% across eating disorder, obesity, and bariatric surgery populations. Further, Kaur et al. noted that when the cut-off score on the Night Eating Questionnaire (NEQ) (an assessment tool detailed in the assessment section) was decreased from 30 to 25, the prevalence of NES within these clinical populations increased (6.9 to 15.2%) [8••]. These findings underscore the increased frequency of NES across clinical populations, suggesting some populations may be more vulnerable to developing NES.
There is consensus throughout the literature that many sociodemographic factors do not differ significantly between patients with NES and controls, including gender [9, 10•], education level [11, 12], cigarette consumption, and income [13]. The association between age and NES is less clear. While several empirical investigations suggest that age is not a risk factor [9, 10•], recent research indicates that there are higher rates of NES in older individuals [12, 14]. These results are striking given evidence that NES typically first appears in young adulthood [15]. It is possible that Latzer et al. [12] findings capture amplified distress as NES symptoms sustain throughout life. In this way, age may increase the likelihood of disturbance or impairment associated with NES.
Assessment Tools
Several self-report tools have been developed and validated to assess for NES. The NEQ is the most frequently used scale clinically and within research. It is a 14-item questionnaire that uses a 5-point Likert scale. Items assess for symptoms consistent with a diagnosis of NES, such as morning hunger and control over nighttime eating. A total score of 25 or higher is suggestive of NES. There is a positive predictive value (PPV) of 40.7%; meaning, there is a 40.7% chance that a patient who has a positive score has NES. A score of 30 or more is an even stronger indicator of NES, with a PPV of 72.7%. The NEQ is thus able to capture many patients with NES, particularly as scores increase. The questionnaire has also demonstrated good psychometric properties, including internal consistency (Cronbach’s alpha = 0.70), fixed factor structure, and convergent validity [16]. It is limited by a lack of items measuring perceived distress and functional impairment. Two items were added to assess these factors but are not included in the final score [17••]. Overall, the NEQ is a good option to utilize when assessing patient’s risk and to track treatment progress. The NEQ and its scoring are available in the public domain.
The Night Eating Symptom Scale (NESS) was developed based on the NEQ.
On the NESS, each item requires a specific number to assess the frequency of awakening and nocturnal ingestions. The questions are focused on symptoms from the previous week to allow for tracking changes. This scale can be particularly useful in monitoring symptoms across time [15]. The NESS is not available in public domain. In addition, the Night Eating Diagnostic Questionnaire (NEDQ) is another paper-and-pencil option. The NEDQ asks “yes” and “no” questions designed to measure the presence of symptoms. This scale aims to classify patients into categories (non-NES verses NES). When NES is present, the questionnaire further delineates “mild,” “moderate,” and full” NES classifications [15, 17••]. The NEDQ is particularly valuable in regard to quantifying the severity of a patient’s symptoms. The scale is also available in the public domain.
In addition to paper-and-pencil questionnaires, the Night Eating Syndrome History and Inventory (NESHI) is another tool used to evaluate NES symptoms. It was developed as a semi-structured interview to assess a patient’s typical 24-h food intake. The interview includes items from the NEQ while also gathering information about the frequency of nocturnal ingestions, sleep routine, mood, stress, weight and diet history, medical history, and past treatment [15, 17••]. Although one notable downside of this tool is its length, it can provide rich clinical information. It is also important to note the NESHI is unpublished and not available in the public domain.
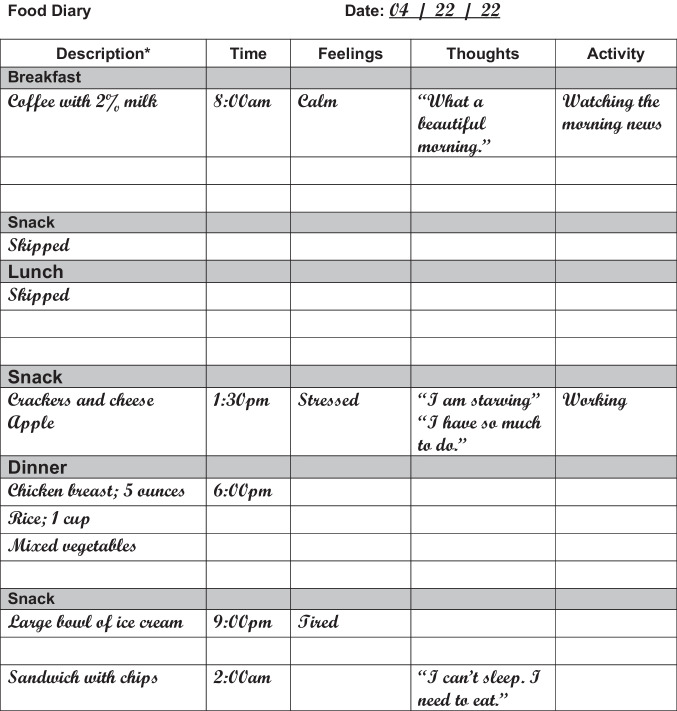
In addition to formal assessment tools, having a patient keep a food record can aid in a provider’s understanding of the clinical picture, elucidating triggers and behavioral patterns. Figure 1 provides an example of a typical food log for a patient with NES.
Fig. 1.
An example of a typical food log for a patient with NES
NES and Other Eating Disorders
When assessing for NES, it is important to consider other eating- and sleep-related disorders that can present with over-lapping symptoms. A large body of literature has specifically explored the relationship between NES and binge eating disorder (BED). While some findings have highlighted marked differences [15, 18], other research has underscored a strong association between the two disorders. In fact, between 18 and 50% of patients with BED have been found to meet criteria for NES [18–20]. Evidence also suggests that there is considerable overlap in genetic factors impacting these two disorders. For instance, one study found that the genetic association between NES and BED was 0.60 in a sample of 11,604 adult twins [21]. These findings suggest that genetic markers may increase a patients’ vulnerability to both disorders. Clinically, this underscores the importance of assessing for BED if a patient meets criteria for NES and vice versa.
Given the rates of comorbidity between NES and BED, recent research has examined correlates of a NES-BED diagnosis. Emerging data indicates that patients with comorbid NES-BED experience significantly higher rates of binge eating days, as well as increased energy and fat consumption when compared to patients with BED-only [12]. In addition, patients with NES-BED may experience significantly more distress than patients with NES only. When comparing these two subpopulations, patients with comorbidity have been found to have greater state anxiety, hunger, shape/weight concerns, disinhibition, and appearance dissatisfaction [18, 20, 22]. Building upon this research, a 2020 study found that female patients with NES-BED endorsed significantly higher rates of childhood physical abuse when compared to patients with BED and BN only [14]. Taken together, these findings highlight the complicated relationship between NES and BED. It appears likely that patients who meet criteria for both disorders represent a higher risk subpopulation, which may necessitate additional intervention.
Given these complexities, it is important that clinicians accurately capture a patient’s disordered eating symptoms. When assessing for NES verses BED, there are key areas to help distinguish between the disorders. More specifically, the size of the nocturnal ingestion can be a central feature separating NES and BED. While nocturnal ingestions are frequently equivalent to the size of a snack or small meal, binge eating episodes require an objectively large amount of food. Studies also highlight how the disorders can be differentiated by the level of weight/shape concern and motivation to eat, with night eating often precipitated by a desire to fall asleep verses a craving or emotional distress [23, 24]. In addition, loss of control can be an important symptom to access. While LOC is needed for a BED diagnosis, only some patients with NES experience LOC. Thus, LOC is not needed for an NES diagnosis. Table 1 further illuminates these clinical differences. Ultimately, carefully assessing the amount of food intake and factors around eating can help elucidate a diagnosis.
Table 1.
Comparison of night eating syndrome and binge eating disorder
| Criterion | Night eating syndrome | Binge eating disorder |
|---|---|---|
| Amount of food consumed | Often the size of a small meal or snack | For a core criterion needs to be objectively large |
| Degree of control | Can range from complete control to minimal (with or without loss of control) | Minimal to none; loss of control is needed |
| Associated mood | Evening dysphoria | Increased depressive symptoms but not associated with time of day |
| Associated sleep concerns | Sleep-onset or maintenance insomnia common | Not associated with diagnosis |
| Motivation/precipitants | Belief that one needs to eat to fall to sleep or back to sleep | Variable; can include urges and emotional eating components |
| Level of weight/shape concern | Typically lower level of concern; may be associated if patient experiences weight gain | Often connected with weight and body image concerns |
Although BES has been the most studied eating disorder in relation to NES, researchers have increasingly explored other eating disorders [25, 26•]. Tu et al. exemplifies this research. Tu and colleagues studied characteristics of NES in patients seeking eating disorder treatment. Similar to previous findings, the researchers found a high prevalence of NES in patients with BN and BED (34.9 and 51.7%, respectively). When comparing NES-only patients to BN-only patients, comparable levels of disordered eating, sleep disturbance, depression, and functional impairment were found. The investigators postulated that the strong urge to eat at night may be akin to the experience of loss of control with BN. Outside of greater eating and weight concerns, the NES-BN group evidenced no significant differences across eating pathology, psychopathology, and functional impairment domains when assessed alongside the BN-only group. These findings underscore similar psychopathology and correlates of distress for patients with NES, BN, and dual diagnoses, contrasting previous research suggesting higher levels of eating disturbance in patients with BN. Given that Tu and colleagues’ study examined patients seeking treatment, it is possible that the NES group was in more distress than the average NES patient. Ultimately, as our understanding continues to grow, it is critical that we elucidate how comorbid eating disorder diagnoses impact symptomatology, severity, and clinical outcomes.
NES and Sleep Correlates
Akin to its relationship with eating disorders, NES shares important over-lapping features with several sleep disorders. In particular, sleep-related eating disorder (SRED) is a parasomnia that serves as an important differential. The most critical diagnostic criteria separating NES from SRED are the nature of the nocturnal ingestions. Specifically, while NES is characterized by awareness, SRED is composed of involuntary eating episodes during sleep with impaired recall. Bruzas and Allison (2022) further note that is not uncommon for patients with SRED to consume unusual food or inedible objects [27]. Thus, there are key features that help distinguish between NES and SRED (see Table 2 for further information).
Table 2.
Comparison of night eating syndrome and sleep-related eating disorder
| Criterion | Night eating syndrome | Sleep-related eating disorder |
|---|---|---|
| Volition | Can be planned episodes but requires a minimal level of intent | Involuntary without volition |
| Awareness | Some level of awareness around episode | Complete lack of awareness |
| Content | Enjoyable or otherwise edible foods | Can include odd combinations or inedible substances |
| Motivation | Triggered by hunger, a compulsion to eat or belief that eating is necessary to sleep | Not associated with hunger or thirst |
| Categorization | Eating disorder; delay in circadian food intake | Parasomnia |
In addition to SRED, an increasing body of literature has examined the relationship between NES and sleep problems. In fact, within their review of the literature, Kaur et al. [8••] contend that being a nocturnal eating disorder, NES is inherently associated with worse sleep-related outcomes [8••]. Research demonstrates that patients with NES experience higher levels of insomnia [9] and poorer sleep quality [10•, 28]. Expanding on this research, when comparing patients with NES with evening hyperphagia to patients with nocturnal ingestions, Loddo et al. found differences in sleep features across NES subgroups. The researchers observed higher levels of total duration of eating episodes, eating latency following wakening, and sleep latency following eating episodes in the evening hyperphagia group [29]. These findings contrast previous research suggesting that sleep duration, rapid eye movement, and sleep efficiency are not affected in NES [28]. It may be that sleep disturbance is heightened in patients with evening hyperphagia. Additional research is needed to continue to fully understand sleep concerns across NES subgroups.
Several recent investigations have explored the association between NES and sleep during the COVID-19 pandemic. More generally, there is research demonstrating increased sleep disturbance during the COVID-19 pandemic, with incidence of insomnia noted to have increased 23% [30]. As a result, there has been concern about the potential for greater vulnerability for NES. Much of the recent research investigating sleep and NES during the pandemic has specifically examined sleep quality [31–33]. Akdevelioglu et al. exemplifies this research. The investigators found that the positive association between poor sleep quality and higher NES scores remained during the COVID-19 pandemic [31]. In addition, Kwan et al. [32] results suggest that poor sleep quality and higher BMI were risk factors for NES during the COVID-19 pandemic [32]. Given this growing body of literature, treatment targeting sleep symptoms may help to reduce risk for NES during times of chronic stress, such as the pandemic. It is important to note that the generalizability of these conclusions is limited by research conducted with university student samples.
NES and Psychological Distress
A large body of research has established a relationship between NES and psychiatric correlates, including increased rates of anxiety, depression, and substance abuse [34]. Research suggests stress and psychiatric symptoms often precede the onset of NES and are associated with symptom maintenance [35]. One recent study examining NES in veterans also demonstrated a significant relationship between NES and posttraumatic stress disorder [9]. Additional research has underscored the increased risk of depression for patients with subclinical and clinically significant NES [9, 12, 26•, 34, 36, 37]. Given this body of research, it is not surprising that many patients with NES present with heightened distress and comorbid psychiatric concerns.
In an attempt to further elucidate the relationship between NES and depression, Riccobono et al. investigated the relationship between NES, depression, and chronotypes within a non-clinical sample [38]. Chronotype is a term used to capture behavioral and biological rhythms in relation to the timing of activity and sleep. While the morning type experiences heightened alertness and energy in the morning, the evening type evidences peak performance and attentiveness later in the day. Riccobono et al. observed a significant relationship between the evening dimension, Beck Depression Inventory (BDI) scores, and NES. These findings suggest that patients with NES may not only experience a circadian delay in food intake, but a delay in overall functioning. Further, it appears that the evening chronotype is a risk factor for depression and NES. The association between these variables may in part help to explain the effectiveness of phototherapy or bright light therapy (BLT) for NES (see “Treatment” section).
To date, most of the studies examining psychological correlates of NES have been conducted in Western societies. Given the impact of sociocultural factors in eating disorders, several researchers have hypothesized that NES may present differently across cultures. For this reason, limited research has begun to explore the relationship between psychiatric correlates and NES within eastern populations [39, 40]. For example, using the Chinese-NEQ, He et al. [40] found that 2.8% of participants met criteria for NES. NES was significantly correlated with psychological distress, including depression, anxiety, and overall stress. He et al.’s findings support emerging research suggesting that the association between NES and psychiatric concerns remains across cultures. More empirical attention is needed to clarify if other clinical associations are evidenced across cultures.
Body Mass Index and NES
The relationship between NES and body mass index (BMI) has been the subject of long-standing interest. Although NES is associated with higher BMI in some studies [14, 32, 41], other research has found no significant relationship [9,10•,36, 42-44]. In a 2012 review, researchers postulated that these conflicting findings might be the result of NES being conceptualized differently across studies [45]. Following an updated review of the empirical literature, Bruzas and Allison concluded that the research remains split [46••]. Given this discrepancy over time, it is likely that moderating variables affect the relationship between NES and BMI. Researchers have suggested the role of several factors, including activity level, dietary restriction, psychopathology, insomnia severity, and genes [45]. There is currently some evidence to suggest that emotional eating and age may also help to explain the variability. Specifically, while a significant relationship was found between NES and weight in individuals with high emotional eating and those between ages 31 to 60, the association did not remain in individuals evidencing low emotional eating or younger/older individuals [47, 48]. Likely, there are other unmeasured or unknown variables that impact this relationship.
Bariatric Patients and NES
Up to 65% of patients undergoing bariatric surgery demonstrate symptoms of NES. Bariatric surgery has shown potential in reducing NES [17••]. However, treatment effects have not been found to have longitudinal value. Despite initial reduction in NES severity after metabolic and bariatric surgery, Nasirzadeh et al. [49] found that symptoms increased significantly 1 to 3 years post-surgery [49]. These results suggest that initial remission of eating pathology is often temporary and does not guarantee long-term resolution. A similar pattern has been found with depression. Studies suggest that depression often improves after surgery but recurs in later years [50]. Ultimately, NES symptoms may re-emerge as the physiological effects of the surgery become less acute. Life stressors may also contribute to the return of symptoms.
Given the temporary remission of NES, recent literature has sought to understand the trajectory and impact of NES symptoms post-operatively. Pinto et al. [51] exemplify this research. The investigators sought to assess the effects of metabolic and bariatric surgery on night eating and depressive symptoms. Data suggested that reductions in depression and night eating were seen predominantly in patients with pre-operative depression [51]. These findings support hypotheses around the important role of low mood in NES. The results are striking given the improvements in depression often observed initially after surgery.
Many bariatric programs require patients who meet criteria for NES to obtain treatment prior to surgery, with the rationale that untreated disordered eating could negatively impact outcomes. However, there is limited evidence that pre-surgical symptoms of NES affect weight loss following surgery [24, 49]. In fact, research suggests that although binge eating at year one is associated with lower total weight loss at 2 years post-surgery, night eating is not predictive of weight outcomes [49]. Clinically, even if NES is not related to poorer weight loss, one must question the clinical and psychological impact of the re-emergence of NES symptoms after surgery.
Supporting this concern, additional research has examined the impact of disordered eating post-operatively. Specifically, Ivezaj et al. [10•] followed 131 patients who sought treatment for eating and weight concerns 6 months following the sleeve gastrectomy. Fifteen percent of the sample met criteria for NES, while another 21.4% were night eating regularly, not meeting full criteria. The researchers concluded that the co-occurrence of loss of control (LOC) eating and night eating following surgery may represent a more severe subgroup with elevated psychopathology, poorer sleep, and lower percent weight loss [10•]. These findings build upon previous research suggesting that LOC in bariatric surgery patients is associated with increased psychological burden, including greater depressive symptoms and poorer mental health-related quality of life [52]. Researchers have postulated that LOC eating could be a potential phenotype marker for NES in bariatric patients. Taken together, these findings may have practical implications when evaluating and following patients for bariatric surgery.
Treatment
The treatment of NES includes pharmacological and behavioral interventions. Treatments targeting the regulation of circadian rhythms, mood, stress, and faulty cognitions have been considered. Pinto et al. [53] conducted a critical review of treatment for NES and concluded that serotonergic agents and psychological interventions, particularly cognitive behavioral therapy (CBT), have shown effectiveness [52]. However, Muscatello et al. contends that there is still a paucity of research in this area, with treatment for NES remaining an emerging field [17••].
Pharmacological Treatment
Studies examining pharmacological treatments largely consider drugs affecting the serotonergic system. Serotonin has a role in eating, sleep, and mood. It is therefore thought to be connected to NES. Decreased serotonin levels have been hypothesized to lead to the alteration of circadian rhythms and increase risk of evening hyperphagia [53]. Night eaters have also been found to have higher levels of serotonin transporter in the temporal lobe, which contributes to changes in circadian rhythms and appetite [54]. Antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), have been the focus of much research for NES treatment [55]. Muscatello’s literature review reports that sertraline has been found to reduce nocturnal ingestions and evening hyperphagia, improving mood, quality of life, and weight [17••]. Paroxetine and fluvoxamine have further demonstrated positive reductions in night eating episodes. Escitalopram has also been studied, but results have been contradictory, with some studies showing no effect [56] and others evidencing significant improvement of NES symptoms [57].
In addition to SSRIs, researchers have examined the off-label use of several medications. For example, topiramate, an anticonvulsant and nerve pain medication, has been studied for NES. There have been sparse reports of its benefit with short-term use, although results have underscored how side effects outweigh benefits from a short-term perspective. In 2015, Macdonald et al. conducted a case study with long-term use of low dose topiramate, with sustained results of remittance of NES symptoms and weight loss over a period of 5 years with no adverse side effects [58]. Melatonin agonists have also been studied to a lesser degree. Matsui et al. [59] conducted the most robust study to date with 49 patients included in the analysis. The investigators found that 42.9% of their participants evidenced a positive response. The researchers also found that the use of ramelteon allowed for significant reductions in benzodiazepine and Z-drugs use [59]. Taken together, these studies suggest increasing support for medications outside of SSRIs.
Non-Pharmacological Treatment
CBT is a well-validated treatment for depression. It has also received increasing empirical support in the treatment of a variety of behavioral health problems, including insomnia, eating disorders, and weight loss. CBT for NES integrates behavioral interventions with cognitive techniques aimed at increasing awareness during eating episodes and addressing faulty cognitions. The treatment also employs thought records, Socratic questioning, stimulus control, and relapse prevention techniques [60]. CBT has evidenced effectiveness in reducing NES symptoms, including evening caloric intake, awakenings, and nocturnal ingestions. A pilot study of a CBT protocol with tailored psychoeducation, nutrition, and sleep hygiene rules was found to be comparable to a randomized controlled trail of sertraline [17••]. Unfortunately, limited data is available on the stability of these improvement over time. Additional studies examining the use of CBT for NES with long-term follow-up are greatly needed.
Research has also explored the use of relaxation techniques. Progressive muscle relaxation (PMR) has been of particular interest. This technique teaches participants to slowly tense and relax muscle groups. Although no known recent studies in the past 5 years have examined this topic, Vander Wal et al. demonstrated that PMR practice results in a reduction of depression and perceived stress. Techniques such as PMR likely help to mitigate the known relationships between stress, insomnia, and NES symptoms [61].
In addition, phototherapy or BLT has been explored as a treatment for NES. Phototherapy involves exposure to certain wavelengths of light by using a light box. Phototherapy has been applied for the treatment of mood disorders and sleep disorders. It’s effects on melatonin and post-synaptic serotonin prompted studies for NES. Though randomized controlled trails are needed, preliminary evidence supports the use of phototherapy for the treatment for NES [59].
Future Directions
Despite NES being described in the 1950s, NES research is still emerging with several areas with conflicting or limited results. Our understanding of NES and the generalizability of previous findings would benefit from continuing to conduct studies across cultures. Additional research elucidating the relationship between NES and BMI is also needed. Further, it would be helpful to clarify how to assess and treat higher risk subsets of patients with NES, such as those with NES-BED and post-operative bariatric surgery patients. Perhaps most importantly, the literature analyzing treatments for NES has been particularly limited and is in dire need of more research. To more effectively treat patients, it is imperative that we continue to explore the long-term outcomes of CBT and other treatments.
Conclusion
It is our hope that the current review sparks clinical curiosity and needed additional research. The most recent literature underscores the complex relationships between NES and other eating and sleep disorders. Additional research is needed to fully appreciate psychiatric, sleep, and physical correlates across NES subgroups and different cultures more broadly. It is likely that subsets of the NES population, such as those with NES-BED, experience greater distress and impairment. It is important that we continue to understand differences within the NES population. Ultimately, the lack of vigorous empirical research regarding the treatment of NES remains perhaps one of the most critical holes in the research, thus limiting our clinical understanding.
Declarations
Conflict of Interest
The authors declare no competing interestss.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Psychological Issues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Megan E. Lavery, Email: Megan.lavery@christianacare.org
Deirdra Frum-Vassallo, Email: Deirdra.Frum-Vassallo@va.gov.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Stunkard AJ, Grace WJ, Wolff HG. The night-eating syndrome: a pattern of food intake among certain obese patients. Am J Med. 1955;19:78–86. doi: 10.1016/0002-9343(55)90276. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 3.Allison KC, Lundgren JD, O'Reardon JP, Geliebter A, Gluck ME, Vinai P, et al. Proposed diagnostic criteria for night eating syndrome. Int J Eat Disord. 2010;43(3):241–247. doi: 10.1002/eat.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison, KC, Stunkard AJ. Self-help for night eating syndrome. In: Latner J, Wilson, GT, editors. Self help for obesity and binge eating. New York: The Guilford Press; 2007.
- 5.Devlin MJ, Allison KC, Goldfein JA, Spanos A. Management of EDNOS (eating disorder not otherwise specified). In: Yager J, Powers P, editors. Clinical management of eating disorders. Washington, DC: American Psychiatric Publishing; 2007.
- 6.Lundgren JD, Pona A. Assessment of night eating. In: Wade T, editors. Encyclopedia of Feeding and Eating Disorders. Singapore: Springer; 2017. 10.1007/978-981-287-104-6_129.
- 7.de Zwaan M, Burgard MA, Schenck CH, Mitchell JE. Night time eating: a review of the literature. Eur Eat Disord Rev. 2003;11(1):7–24. doi: 10.1002/ERV.501. [DOI] [Google Scholar]
- 8.Kaur J, Dang AB, Gan J, An Z, Krug I. Night eating syndrome in patients with obesity and binge eating disorder: a systematic review. Front Psychol. 2022;12:766827. doi: 10.3389/fpsyg.2021.766827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorflinger LM, Ruser CB, Masheb RM. Night eating among veterans with obesity. Appetite. 2017;117:330–334. doi: 10.1016/j.appet.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Ivezaj V, Lawson JL, Lydecker JA, Duffy AJ, Grilo CM. Examination of night eating and loss-of-control eating following bariatric surgery. Eat Weight Disord. 2022;27(1):207–213. doi: 10.1007/s40519-021-01156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zengin-Eroglu M, Sertçelik S, Tamam L. Eating disorders in bariatric surgery candidates admitted to Haydarpa¸sa Numune Training and Research Hospital. Anadolu Psikiyatri Dergisi. 2018;19:355–361. doi: 10.5455/apd.287483. [DOI] [Google Scholar]
- 12.Latzer Y, Yutal AE, Givon M, Kabakov O, Alon S, Zuckerman-Levin N, et al. Dietary patterns of patients with binge eating disorders with and without night eating. Eat Weight Disord. 2020;25:321–328. doi: 10.1007/s40519-018-0590-2. [DOI] [PubMed] [Google Scholar]
- 13.Kara Y, Tuzun S, Oner C, Simsek EE. Night eating syndrome according to obesity groups and the related factors. J Coll Phys Surg Pak 2020:30:833–38. 10.29271/jcpsp.2020.08.833. [DOI] [PubMed]
- 14.Latzer Y, Rozenstain-Hason M, Kabakov O, Givon M, Mizrachi S, Alon S, et al. Childhood maltreatment in patients with binge eating disorder with and without night eating syndrome vs. control. Psych Res. 2020;293:113451. 10.1016/j.psychres.2020113451. [DOI] [PubMed]
- 15.Lundgren JD, Allison KC, Stunkard AJ, editors. Night eating syndrome: research, assessment, and treatment. New York: Guilford Press; 2012. [Google Scholar]
- 16.Allison KC, Lundgren JD, O’Reardon JP. The Night Eating Questionnaire (NEQ): psychometric properties of a measure of severity of the night eating syndrome. Eat Behaviors. 2008;9(1):62–72. doi: 10.1016/j.eatbeh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 17.•• Muscatello MRA, Torre G, Celebre L, Dell'Osso B, Mento C, Zoccali RA, Bruno A. ‘In the night kitchen’: a scoping review on the night eating syndrome. Aust N Z J Psychiatry. 2022;56(2):120–36. 10.1177/00048674211025714. In-depth updated review of NES across several important areas of concern. [DOI] [PubMed]
- 18.Napolitano MA, Head S, Babyak MA, Blumenthal JA. Binge eating disorder and night eating syndrome: psychological and behavioral characteristics. Int J Eat Disord. 2001;30(2):193–203. doi: 10.1002/eat.1072. [DOI] [PubMed] [Google Scholar]
- 19.Allison KC, Crow SJ, Reeves RR, West DS, Foreyt JP, Dilillo VG, et al. Binge eating disorder and night eating syndrome in adults with type 2 diabetes. Obes. 2007;15(5):1287–1293. doi: 10.1038/oby.2007.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colles S, Dixon J, O’brien P. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes. 2007;31(11):1722–30. 10.1038/sj.ijo.0803664. [DOI] [PubMed]
- 21.Root TL, Thornton LM, Lindroos AK, Stunkard AJ, Lichtenstein P, Pedersen NL, et al. Shared and unique genetic and environmental influences on binge eating and night eating: a Swedish twin study. Eat Behav. 2010;11(2):92–98. doi: 10.1016/j.eatbeh.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grilo CM, Masheb RM. Night-time eating in men and women with binge eating disorder. Behav Res Ther. 2004;42(4):397–407. doi: 10.1016/S0005-7967(03)00148-7. [DOI] [PubMed] [Google Scholar]
- 23.Allison KC, Grilo CM, Masheb RM, Stunkard AJ. Binge eating disorder and night eating syndrome: a comparative study of disordered eating. J Consult Clin Psychol. 2005;73(6):1107–1115. doi: 10.1037/0022-006X.73.6.1107. [DOI] [PubMed] [Google Scholar]
- 24.de Zwaan M, Marschollek M, Allison KC. The night eating syndrome (NES) in bariatric surgery patients. Eur Eat Disord Rev. 2015;23(6):426–434. doi: 10.1002/erv.2405. [DOI] [PubMed] [Google Scholar]
- 25.Farhangi MA. Night eating syndrome and its relationship with emotional eating, sleep quality and nutritional status among adolescents’ boys. Community Ment Health J. 2019;55(8):1411–1418. doi: 10.1007/s10597-019-00395-8. [DOI] [PubMed] [Google Scholar]
- 26.Tu CY, Meg Tseng MC, Chang CH. Night eating syndrome in patients with eating disorders: is night eating syndrome distinct from bulimia nervosa? J Formos Med Assoc. 2019;118(6):1038–1046. doi: 10.1016/j.jfma.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Bruzas MB, Allison K.C. Eating disorders: disorders of under- and overnutrition. In: Wilson T, Temple NJ, Bray GA, editors. Nutrition guide for physicians and related healthcare professions. New York: Springer; 2022.
- 28.Cleator J, Abbott J, Judd P, Wilding JP, Sutton CJ. Correlations between night eating, sleep quality, and excessive daytime sleepiness in a severely obese UK population. Sleep Med. 2013;14:1151–1156. doi: 10.1016/j.sleep.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Loddo G, Zanardi M, Caletti MT, Mignani F, Petroni ML, Chiaro G, et al. Searching food during the night: the role of video-polysomnography in the characterization of the night eating syndrome. Sleep Med. 2019;64:85–91. doi: 10.1016/j.sleep.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Zhou SJ, Wang LL, Yang R, Yang XJ, Zhang LG, Guo ZC, et al. Sleep problems among Chinese adolescents and young adults during the coronavirus-2019 pandemic. Sleep Med. 2020;74:39–47. doi: 10.1016/j.sleep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akdevelioglu Y, Sahin TO, Yesildemir O. Sleep quality and its relationship with night eating syndrome, the risk of diabetes, and nutritional status among university students. Prog Nutr. 2020;22(1):304–315. [Google Scholar]
- 32.Kwan YQ, Lee SS, Cheng SH. Night eating syndrome and its association with sleep quality and body mass index among university students during the Covid-19. Malays J J Soc Sci Hum. 2021;6(8):371–83. 10.47405/mjssh.v6i8.944.
- 33.Yildiz MB, Sena S, Temı̇rçı̇n, Ş, Dener BG, Rumeysa RK, Sariyer ET, et al. Night eating syndrome and sleep quality among Turkish University students in COVID-19 pandemic. J Neurobehav Sci. 2021;8:135–41. 10.4103/jnbs.jnbs_27_21.
- 34.Lundgren JD, Allison KC, O’Reardon JP, Stunkard AJ. A descriptive study of non-obese persons with night eating syndrome and a weight-matched comparison group. Eat Behav. 2008;9(3):343–351. doi: 10.1016/j.eatbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchesini G, Calugi R, Marzocchi, Grave RD. Night eating syndrome in obesity. In Preedy VR, Patel VB, Le LA, editors. Handbook of Nutrition, Diet and Sleep. Wageningen: Wageningen Academic Publishers; 2013.
- 36.Cleator J, Judd P, James M, Abbott J, Sutton CJ, Wilding JPH. Characteristics and perspectives of night-eating behaviour in a severely obese population. Clin Obes. 2014;4:30–38. doi: 10.1111/cob.12037. [DOI] [PubMed] [Google Scholar]
- 37.de Zwaan M, Roerig DB, Crosby RD, Karaz S, Mitchell JE. Nighttime eating: a descriptive study. Int J Eat Disord. 2006;39(3):224–232. doi: 10.1002/eat.20246. [DOI] [PubMed] [Google Scholar]
- 38.Riccobono G, Pompili A, Iannitelli A, Pacitti F. The relationship between night eating syndrome, depression and chronotype in a non-clinical adolescent population. Riv Psichiatr. 2019;54(3):115–119. doi: 10.1708/3181.31600. [DOI] [PubMed] [Google Scholar]
- 39.Batra S, Ochani RK, Memon ZA, Shaikh A, Qureshi NE, Bhimani S, et al. Relationship between night eating syndrome and self-esteem: a cross-sectional population-based study in Karachi, Pakistan. Cureus. 2019;11(8):e5540. doi: 10.7759/cureus.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He J, Huang F, Yan J, Wu W, Cai Z, Fan X. Prevalence, demographic correlates, and association with psychological distress of night eating syndrome among Chinese college students. Psychol Health Med. 2018;23(5):578–584. doi: 10.1080/13548506.2017.1400669. [DOI] [PubMed] [Google Scholar]
- 41.Vinai P, Ferri R, Anelli M, Ferini-Strambi L, Zucconi M, Oldani A, et al. New data on psychological traits and sleep profiles of patients affected by nocturnal eating. Sleep Med. 2015;16:746–753. doi: 10.1016/j.sleep.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 42.Dalle Grave R, Calugi S, Marchesini G, Beck-Peccoz P, Bosello O, Compare A, et al. Personality features of obese women in relation to binge eating and night eating. Psychiatry Res. 2013;207:86–91. doi: 10.1016/j.psychres.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Yahia N, Brown C, Potter S, Szymanski H, Smith K, Pringle L, et al. Night eating syndrome and its association with weight status, physical activity, eating habits, smoking status, and sleep patterns among college students. Eat Weight Disord. 2017;22:421–433. doi: 10.1007/s40519-017-0403-z. [DOI] [PubMed] [Google Scholar]
- 44.Zengin-Eroglu M, Sertçelik S, Tamam L. Eating disorders in bariatric surgery candidates admitted to Haydarpaşa Numune Training and Research Hospital. Anadolu Psikiyatri Dergisi. 2018;19:355–361. doi: 10.5455/apd.287483. [DOI] [Google Scholar]
- 45.Gallant AR, Lundgren J, Drapeau V. The night-eating syndrome and obesity. Obes Rev. 2012;13(6):528–536. doi: 10.1111/j.1467-789X.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 46.Bruzas MB, Allison KC. A review of the relationship between night eating syndrome and body mass index. Curr Obes Rep. 2019;8:145–155. doi: 10.1007/s13679-019-00331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meule A, Allison KC, Platte PP. Emotional eating moderates the relationship of night eating with binge eating and body mass. Eur Eat Disord Rev. 2014:22:147–51. 10.1002/erv.227. [DOI] [PubMed]
- 48.Meule A, Allison KC, Brähler E, de Zwaan M. The association between night eating and body mass depends on age. Eat Behav. 2014;15(4):683–685. doi: 10.1016/j.eatbeh.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Nasirzadeh Y, Kantarovich K, Wnuk S, Allan O, Cassin SE, Hawa R, et al. Binge eating, loss of control over eating, emotional eating, and night eating after bariatric surgery: results from the Toronto Bari-PSYCH cohort study. Obes Surg. 2018;28:2032–2039. doi: 10.1007/s11695-018-3137-8. [DOI] [PubMed] [Google Scholar]
- 50.Smith KE, Mason TB, Cao L, Crosby RD, Steffen KJ, Garcia L, et al. Trajectories of depressive symptoms and relationships with weight loss in the seven years after bariatric surgery. Obes Research & Clinical Practice. 2020;14(5):456–461. doi: 10.1016/j.orcp.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinto FT, Carvalhedo de Bruin PF, Sales de Bruin VM, Ney Lemos F, Azevedo Lopes FH, Marcos Lopes P. Effects of bariatric surgery on night eating and depressive symptoms: a prospective study. Surg Obes Relat Dis. 2017;13(6):1057–62. 10.1016/j.soard.2016.12.010. [DOI] [PubMed]
- 52.Royal S, Wnuk S, Warwick K, Hawa R, Sockalingam S. Night eating and loss of control over eating in bariatric surgery candidates. J Clin Psychol Med Settings. 2015;22(1):14–19. doi: 10.1007/s10880-014-9411-6. [DOI] [PubMed] [Google Scholar]
- 53.Pinto TF, Silva FG, Bruin VM, Bruin PF. Night eating syndrome: how to treat it? Rev Assoc Med Bras. 2016;62(7):701–707. doi: 10.1590/1806-9282.62.07.701. [DOI] [PubMed] [Google Scholar]
- 54.Stunkard AJ, Allison KC, Lundgren JD, O'Reardon JP. A biobehavioural model of the night eating syndrome. Obes Rev. 2009;2:69–77. doi: 10.1111/j.1467-789X.2009.00668.x. [DOI] [PubMed] [Google Scholar]
- 55.Kucukgoncu S, Midura M, Tek C. Optimal management of night eating syndrome: challenges and solutions. Neuropsychiatr Dis Treat. 2015;19(11):751–760. doi: 10.2147/NDT.S70312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vander Wal JS, Gang CH, Griffing GT, Gadde KM. Escitalopram for treatment of night eating syndrome: a 12-week, randomized, placebo-controlled trial. J Clin Psychopharmacol. 2012;32(3):341–345. doi: 10.1097/JCP.0b013e318254239b. [DOI] [PubMed] [Google Scholar]
- 57.Allison KC, Studt SK, Berkowitz RI, Hesson LA, Moore RH, Dubroff JG, et al. An open-label efficacy trial of escitalopram for night eating syndrome. Eat Beh. 2013;14(2):199–203. doi: 10.1016/j.eatbeh.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Macdonald G, McMahon C, Tisch S, Campbell L. Topiramate therapy: night eating cure with five year sustained weight loss in an obese patient with type 2 diabetes. J Endocrinol Diab. Obes. 2015;31(1):1067.
- 59.Matsui K, Kuriyama K, Kobayashi M, Inada K, Nishimura K, Inoue Y. The efficacy of add-on ramelteon and subsequent dose reduction in benzodiazepine derivatives/Z-drugs for the treatment of sleep-related eating disorder and night eating syndrome: A retrospective analysis of consecutive patients. J Clin Sleep Med. 2021;17(7):1475–1483. doi: 10.5664/jcsm.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berner LA, Allison KC. Behavioral management of night eating disorders. Psychol Res Behav Manag. 2013;6:1–8. doi: 10.2147/PRBM.S31929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vander Wal JS, Maraldo TM, Vercellone AC, Gagne DA. Education progressive muscle relaxation therapy, and exercise for the treatment of night eating syndrome. A pilot study Appetite. 2015;89(1):136–144. doi: 10.1016/j.appet.2015.01.024. [DOI] [PubMed] [Google Scholar]