Abstract
Cholera toxin (CT) and the heat-labile enterotoxin of Escherichia coli (LT-I) are members of the serogroup I heat-labile enterotoxins (HLT) and can serve as systemic and mucosal adjuvants. However, information is lacking with respect to the structurally related but antigenically distinct serogroup II HLT, LT-IIa and LT-IIb, which have different binding specificities for ganglioside receptors. The purpose of this study was to assess the effectiveness of LT-IIa and LT-IIb as mucosal adjuvants in comparison to the prototypical type I HLT, CT. BALB/c mice were immunized by the intranasal (i.n.) route with the surface protein adhesin AgI/II of Streptococcus mutans alone or supplemented with an adjuvant amount of CT, LT-IIa, or LT-IIb. Antigen-specific antibody responses in saliva, vaginal wash, and plasma were assayed by enzyme-linked immunosorbent assay. Mice given AgI/II with LT-IIa or LT-IIb by the i.n. route had significantly higher mucosal and systemic antibody responses than mice immunized with AgI/II alone. Anti-AgI/II immunoglobulin A (IgA) antibody activity in saliva and vaginal secretions of mice given AgI/II with LT-IIa or LT-IIb was statistically similar in magnitude to that seen in mice given AgI/II and CT. LT-IIb significantly enhanced the number of AgI/II-specific antibody-secreting cells in the draining superficial cervical lymph nodes compared to LT-IIa and CT. LT-IIb and CT induced significantly higher plasma anti-AgI/II IgG titers compared to LT-IIa. When LT-IIb was used as adjuvant, the proportion of plasma IgG2a relative to IgG1 anti-AgI/II antibody was elevated in contrast to the predominance of IgG1 antibodies promoted by AgI/II alone or when CT or LT-IIa was used. In vitro stimulation of AgI/II-specific cells from the superficial lymph nodes and spleen revealed that LT-IIa and LT-IIb induced secretion of interleukin-4 and significantly higher levels of gamma interferon compared to CT. These results demonstrate that the type II HLT LT-IIa and LT-IIb exhibit potent and distinct adjuvant properties for stimulating immune responses to a noncoupled protein immunogen after mucosal immunization.
The heat-labile enterotoxins (HLT) of Vibrio cholerae and Escherichia coli constitute a family of bacterial toxins that are related in structure and function (10, 11, 16, 35). Both are oligomeric protein toxins composed of one A polypeptide and five B polypeptides in which the quaternary structure is maintained by noncovalent bonds between the A polypeptide and a pentameric ring of B subunits (7, 13, 32). The biological effects of the enterotoxins are determined by the binding specificity of the fully assembled B subunits and the enzymatic activity of the A subunit. The pentameric ring formed by the B subunits mediates binding to the sugar residues of gangliosides present on the surface of various eukaryotic cells (3, 18).
Two serogroups of HLT have been distinguished on the basis of distinct immunoreactivity (15, 28). Serogroup I consists of cholera toxin (CT) and the E. coli HLT LT-I, which includes two antigenic variants isolated from humans and pigs, designated LTh-I and LTp-I, respectively (19, 28). Serogroup II enterotoxins include E. coli type II HLT initially designated LT-like toxins and later called LT-II enterotoxins (9). Based on immunoreactivity and amino acid sequence homology, two antigenic variants of LT-II, designated LT-IIa and LT-IIb, have been isolated (9–11, 17). Although serogroup I and serogroup II enterotoxins induce similar morphological effects on Y1 adrenal cells and activate adenylate cyclase in cell cultures, both LT-IIa and LT-IIb appear to be more potent than either CT or LT-I in Y1 adrenal cell assays; however, neither LT-IIa nor LT-IIb induces the typical fluid accumulation in ligated ileal loops observed with CT and LT-I (16). In human T84 intestinal cells, only CT elicited a cyclic AMP-dependent chloride response that is responsible for the massive effusion of water into the lumen of the gut (39).
Comparison of the predicted amino acid sequences of type I and type II enterotoxins reveals a large degree of variability. While the B polypeptides of CT and LT-I exhibit over 80% homology to each other, both CT and LT-I have less than 14% amino acid sequence homology to the B subunits of either LT-IIa or LT-IIb (15, 28–30). The extensive diversity in amino acid sequences between type I and type II HLT not only results in antigenically distinct groups but also imparts different ganglioside binding specificity to the respective B subunits. Specifically, the high-affinity receptor for CT and LT-I has been shown to be the monosialoganglioside GM1, while the B subunit of LT-IIa binds with high affinity to GD1b and less strongly to GM1, GT1b, GQ1b, GD2, GD1a, and GM2 (6). Unlike CT and LT-IIa, LT-IIb lacks affinity for GM1 but has been shown to bind with high affinity to the disialoganglioside GD1a (6).
Gangliosides are sialic acid-containing ceramide oligosaccharides in which the polar head groups consist of carbohydrate moieties with a lipophilic ceramide tail anchored in the lipid bilayer of membranes (23). Gangliosides are primarily components of cell surface membranes, and they vary widely at the cell, tissue, and organ levels as well as between species (23). There is considerable evidence that different gangliosides play important roles in signal transduction pathways involving cellular immunomodulation, proliferation, differentiation, transformation, and suppression (12, 25, 26, 38, 39). The immunological outcome of interactions between type I HLT B subunits and GM1 is well documented. The immunogenicity of type I enterotoxin B subunits depends critically on binding to their high-affinity receptor, GM1, and many of the adjuvant qualities associated with the type I enterotoxins and their B subunits depend on binding GM1 (25, 26, 38). These data suggest that interaction with the cell surface receptor GM1 triggers many of the key immunostimulatory events associated with the type I enterotoxins. However, the effect of different binding specificity on the immunological properties of type I and type II enterotoxins has not been addressed. In this study, we investigated the possibility that receptor-mediated binding differences between type I and type II enterotoxins have a differential effect on the quality or magnitude of the resulting immune response. To determine the immunological effects that LT-IIa and LT-IIb have on mucosal adjuvanticity, we investigated the effects of LT-IIa and LT-IIb in comparison with CT on potentiating antigen-specific immune responses.
MATERIALS AND METHODS
Immunogens.
LT-IIa and LT-IIb were derived from E. coli XL-1 Blue (Stratagene) transformed with plasmids pTDC200 and pTDC101, respectively (2). Growth of recombinant E. coli cells was conducted at 37°C with vigorous shaking (225 rpm) in Luria broth supplemented with ampicillin (150 μg/ml) in the presence of tetracycline (10 μg/ml) or kanamycin (50 μg/ml) for pTDC200 or pTDC101, respectively. Target gene expression was induced at mid-log phase by the addition of isopropyl-β-d-thiogalactoside to 1 mM. Growth was terminated 4 h after induction, and cells were harvested by centrifugation. The bacterial pellet was resuspended to 1/10 the original culture volume in ice-cold 100 mM Tris-HCl (pH 8.0) containing 20% sucrose, 5 mM EDTA, polymyxin B (1 mg/ml), and lysozyme (0.5 mg/ml) to release the periplasm contents. After 30 min of incubation at 4°C, the supernatant was harvested by centrifugation and subjected to 40 or 60% ammonium sulfate saturation for LT-IIa or LT-IIb, respectively. The resulting precipitate was collected by centrifugation and dissolved in 10 mM Tris-HCl (pH 8.0) containing 0.3 M NaCl. The dissolved precipitate was then passed through a 0.45-μm-pore-size syringe filter and subjected to gel filtration using a Sephacryl-100 column (Pharmacia) and anion exchange using a Mono Q column (Pharmacia). Recombinant proteins were analyzed for endotoxin content by means of a quantitative chromagenic Limulus amebocyte lysate assay kit (BioWhittaker, Inc., Walkersville, Md.) using an E. coli K235 lipopolysaccharide (LPS) standard. AgI/II used in this study was purified from the culture supernatants of Streptococcus mutans as previously described (31). CT was purchased from List Biological Laboratories.
Animals and immunizations.
Female BALB/c mice, 8 to 12 weeks of age, were immunized by the intranasal (i.n.) route. Groups of 8 to 10 mice were immunized three times at 10-day intervals with AgI/II (10 μg) alone or coadministered with an adjuvant amount (1 μg) of LT-IIa, LT-IIb, or CT. The vaccines were administered in a standardized volume of 12 μl, applied slowly to both external nares. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Collection of secretions and plasma.
Samples of plasma, saliva, and vaginal washes were collected from individual mice 1 day before the primary immunization (day 0), 8 days after each immunization (days 8, 18, and 28), and 22 days (day 42) and 40 days (day 60) after the third immunization. Saliva was collected with a micropipetter after stimulation of salivary flow by injecting each mouse intraperitoneally with 5 μg of carbachol (Sigma Chemical Company, St. Louis, Mo.). Plasma samples were obtained following centrifugation of blood collected from the tail vein by using a calibrated heparinized capillary tube. Vaginal washes were collected by flushing the vaginal vault five times with 75 μl of sterile phosphate-buffered saline (PBS). Mucosal secretions and plasma samples were stored at −70 and −20°C, respectively, until assayed for antibody activity.
Antibody analysis.
Levels of isotype-specific antibodies in saliva, plasma, and vaginal washes were assayed by enzyme-linked immunosorbent assay (ELISA). Polystyrene microtiter plates (96 well; Nunc, Roskilde, Denmark) were coated overnight at 4°C with AgI/II (5 μg/ml), LT-IIa (3 μg/ml), LT-IIb (3 μg/ml), or CT (3 μg/ml) (Sigma). To determine total immunoglobulin (Ig) isotype concentrations, plates were coated with goat anti-mouse Ig isotype-specific antibodies (Southern Biotechnology, Birmingham, Ala.). Serial twofold dilutions of plasma or secretion samples were added in duplicate, and plates were incubated overnight at 4°C. Plates were then washed with PBS containing 0.1% Tween (PBS-Tw) and incubated at room temperature with the appropriate peroxidase-conjugated goat anti-mouse Ig isotype-specific reagent (Southern Biotechnology). Plates were washed and developed with o-phenylenediamine and hydrogen peroxide. Color reaction was stopped after 15 min, and optical density was measured at 490 nm. Unknown concentrations of antibodies and total Ig levels were calculated by interpolation on calibration curves generated by using a mouse Ig reference serum (ICN Biomedicals, Aurora, Ohio). Mucosal IgA responses are reported as the level of specific antibody IgA/total IgA to compensate for variations arising from salivary flow rate and dilutions of secretions.
Isolation of lymphoid cells.
The cervical lymph nodes (CLN) were identified as the central posterior lymph nodes which lie close to the internal jugular lymph nodes but are dorsal to each brachial plexus and the superficial lymph nodes which lie at the anterior poles of the submandibular salivary glands (37). The nasal lymphoid tissue (NALT) was excised as previously described (40). Lymph nodes, NALT, and spleen were teased apart with syringe needles and dispersed through a 70-μm wire-mesh screen to obtain single-cell suspensions. The cell suspensions were filtered through nylon mesh to remove tissue debris and subjected to centrifugation through Ficoll-Hypaque 1083 (Sigma) to remove erythrocytes and dead cells. All preparations were washed twice and suspended in RPMI 1640 with 10% fetal calf serum (FCS). Total cell yield and viability were enumerated in a hemacytometer using trypan blue (Sigma) staining.
ELISPOT assay.
Enumeration of antigen-specific antibody-secreting cells (ASC) was performed by enzyme-linked immunospot (ELISPOT) analysis using lymphoid cells from NALT, superficial CLN, and spleen isolated from immunized mice 10 days after the third immunization (day 30). Membrane-based 96-well microtiter plates (Millititer HA; Millipore Corp., Bedford, Mass.) were coated with 10 μg of AgI/II per ml or RPMI 1640 with 10% FCS (control), diluted in PBS (pH 8.0), and incubated overnight at room temperature. To detect anti-HLT ASC, plates were coated with 5 μg of CT, LT-IIa, or LT-IIb per ml in PBS overnight at room temperature. Plates were then blocked with RPMI 1640 with 10% FCS for 2 h at 37°C. Lymphoid cell suspensions (105 cells) were incubated in triplicate in the coated plates for 4 h at 37°C in a humidified 5% CO2 incubator. Plates were washed and developed with isotype-specific peroxidase-conjugated anti-mouse Ig antibodies followed by 3-amino-9-ethylcarbazole–hydrogen peroxide substrate. The number of spots was determined with the aid of a stereo dissecting microscope.
Proliferation assay.
Lymphoid cells (2 × 105 cells/well) from the central posterior lymph nodes and spleen were incubated in triplicate for 4 days in 96-well U-bottom tissue culture plates in RPMI 1640 medium (supplemented with 10% FCS, 100 mM sodium pyruvate, 200 mM glutamine, nonessential amino acids, 12 mM HEPES, penicillin, and streptomycin) with different concentrations of AgI/II or in the absence of stimulus. Approximately 18 h before harvesting, the cells were pulsed with 0.5 μCi of [3H]thymidine, and the amount of [3H]thymidine uptake was determined by liquid scintillation counting. The stimulation index was calculated as the ratio of the mean counts per minute in AgI/II-stimulated cultures to the mean counts per minute in nonstimulated control cultures.
Cytokine assays.
Spleen and superficial lymph node cells were plated at 3 × 105 and 2 × 105 cells/well, respectively, and cultured for 3 days in the presence of concanavalin A (2.5 μg/ml) or different concentrations of AgI/II or in the absence of stimulus. Supernatants were collected after centrifugation and stored at −70°C until assayed for the presence of cytokines. The levels of interleukin-4 (IL-4), IL-10, and gamma interferon (IFN-γ) in culture supernatants were determined by a cytokine-specific ELISA (Pharmingen). Briefly, flat-bottomed 96-well microtiter plates (Nunc) were coated with monoclonal anti-IL-4, anti-IL-10, or anti-IFN-γ (Pharmingen) at 2 μg/ml in PBS and incubated overnight at 4°C. Plates were washed with PBS-Tw and blocked to limit nonspecific binding with 10% FCS in PBS for 1 h at 37°C. After washing the plates, supernatants were serially diluted in 1% bovine serum albumin in PBS and added to the wells. A standard curve was generated by using recombinant IL-4 (500 pg/ml), IL-10 (2,000 pg/ml), or IFN-γ (2,000 pg/ml) (Pharmingen). All serial dilutions were incubated at 4°C overnight followed by washing with PBS-Tw. Secondary antibodies consisted of peroxidase-labeled anti-IL-4, biotinylated anti-IL-10, or biotinylated anti-IFN-γ (Pharmingen). In assays using biotinylated antibodies, a 1/1,000 dilution of horseradish peroxidase-conjugated streptavidin in 1% bovine serum albumin in PBS-Tw was added to the appropriate wells, and plates were incubated at room temperature for 2 h. The reaction was developed for 20 min with o-phenylenediamine–H2O2 substrate and stopped with 1 M H2SO4. The color reaction was measured at 490 nm.
Statistical analysis.
Analysis of variance with the Tukey-Kramer multiple test was used for multiple comparisons, and unpaired t tests were performed to analyze differences between two groups. Differences were considered significant at the P < 0.05 level.
RESULTS
Purity of LT-IIa and LT-IIb.
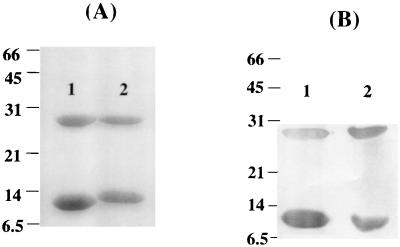
LT-IIa and LT-IIb were purified from periplasmic extracts isolated from pTDC200 and pTDC200, respectively. After conventional chromatography, the homogeneity of each holotoxin preparation was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). SDS dissociated both holotoxins into the A subunit (∼28 kDa) and B-subunit monomers (∼12.5 kDa) (Fig. 1A). No other contaminating bands were observed on the Coomassie blue-stained gel after SDS-PAGE (Fig. 1A). Western blot analysis of the bands with antibodies to LT-IIa or LT-IIb confirmed their identity (Fig. 1B). The endotoxin content for either recombinant protein was less than 1.5 μg/ml, which corresponded to less than 1.5 ng of LPS per μg of purified LT-IIa or LT-IIb. This level of LPS contamination in conjunction with AgI/II has been shown to have no discernible adjuvant effects (42). LT-IIa and LT-IIb were shown to bind to their high-affinity receptors by a ganglioside-dependent ELISA (data not shown) (36).
FIG. 1.
(A) SDS-PAGE of purified LT-IIa (lane 1) and LT-IIb (lane 2) dissociated into the A subunit (∼28 kDa) and B-subunit monomers (∼12.5 kDa). (B) Western blot of recombinant LT-IIa (lane 1) and LT-IIb (lane 2) probed with polyclonal antibodies to LT-IIa and LT-IIb, respectively. Numbers at the left indicate molecular masses in kilodaltons.
Enhancement of mucosal immune responses by CT, LT-IIa, and LT-IIb.
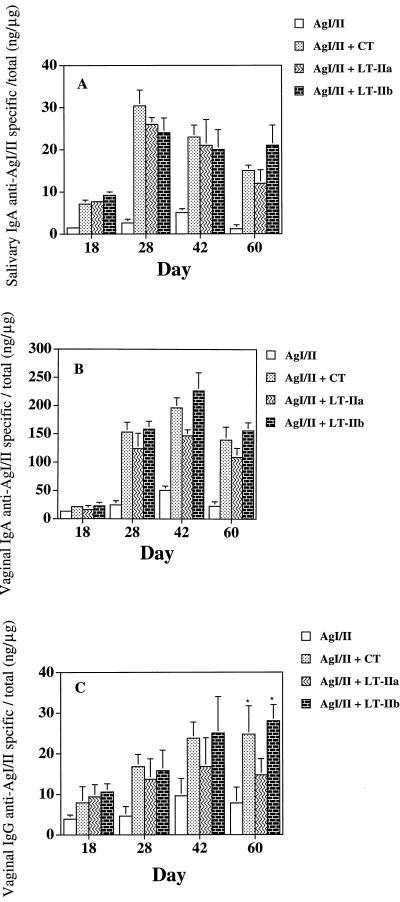
Salivary IgA responses to AgI/II were detected after the second immunization (day 18) and were significantly higher in mice given AgI/II and CT, LT-IIa, or LT-IIb (P < 0.01) compared to responses in mice given AgI/II alone (Fig. 2A). Peak salivary IgA responses to AgI/II occurred on day 28 and persisted through day 60 (Fig. 2A). AgI/II-specific salivary IgA was more than 10-fold higher when CT, LT-IIa or LT-IIb was coadministered with AgI/II.
FIG. 2.
Salivary IgA (A) and vaginal IgA (B) and IgG (C) antibody responses to AgI/II after i.n. immunization of mice with AgI/II alone or with CT, LT-IIa, or LT-IIb as adjuvant. Results are the arithmetic means ± standard deviations for seven mice. Asterisks in panel C indicate statistically significant differences at P < 0.05 compared to LT-IIa.
Vaginal IgA responses to AgI/II were detected after the second immunization, with peak AgI/II-specific IgA antibody responses occurring on day 42 (Fig. 2B). Vaginal IgA anti-AgI/II responses were significantly enhanced (P < 0.001) when LT-IIa, LT-IIb, or CT was used as adjuvant. On days 28, 42, and 60, an approximately 20-fold increase in vaginal IgA anti-AgI/II was observed in mice given LT-IIa, LT-IIb, or CT compared to AgI/II alone. These results demonstrate that LT-IIa, LT-IIb, and CT promote similar levels of salivary and vaginal IgA anti-AgI/II responses.
Vaginal anti-AgI/II IgG responses were detected after the second immunization and were significantly enhanced in mice given AgI/II with either LT-IIa, LT-IIb, or CT (Fig. 2C). Peak vaginal IgG responses to AgI/II occurred on day 42 and persisted through day 60. LT-IIb significantly (P < 0.05) enhanced vaginal anti-AgI/II IgG responses on days 28 and 60 compared to LT-IIa. Moreover, mice given CT or LT-IIb as adjuvant appeared to promote more sustained levels of vaginal anti-AgI/II IgG compared to LT-IIa.
Enhancement of plasma IgG and IgA responses.
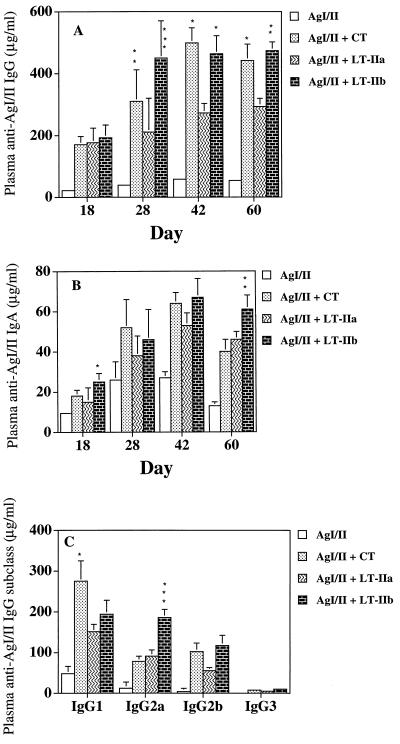
Immunoenhancing activity of LT-IIa and LT-IIb was also seen in the plasma antibody responses to AgI/II (Fig. 3). Maximum plasma IgG (Fig. 3A) and IgA (Fig. 3B) anti-AgI/II levels occurred on day 42. On days 28, 42, and 60, a greater than 10-fold increase was observed in plasma IgG anti-AgI/II antibody activity when CT, LT-IIa, or LT-IIb was used as adjuvant (Fig. 3A). Compared to mice given LT-IIa as adjuvant, AgI/II-specific plasma IgG responses were significantly enhanced in mice given LT-IIb or CT on days 28, 42, and 60 (Fig. 3A).
FIG. 3.
Plasma IgG (A), IgA (B), and IgG subclass (C) antibody responses to AgI/II from mice immunized i.n. with AgI/II alone or with CT, LT-IIa, or LT-IIb. Data represent the arithmetic means ± standard deviations for seven mice. In panel A, ∗, ∗∗, and ∗∗∗ indicate statistically significant differences at P < 0.05, P < 0.01, and P < 0.001, respectively, compared to LT-IIa. In panel B, ∗ and ∗∗ indicate significant differences at P < 0.05 and P < 0.01, respectively, compared to CT and LT-IIa. In panel C, ∗∗∗ indicates significant difference (P < 0.001) compared to LT-IIa and CT and ∗ indicates significant difference (P < 0.05) compared to LT-IIa and LT-IIb.
Plasma IgA anti-AgI/II antibody activity was also significantly increased (P < 0.01) on days 28, 42, and 60 in mice given LT-IIa or LT-IIb as adjuvant compared to AgI/II alone (Fig. 3B). An approximately 5- to 10-fold increase was seen in plasma IgA anti-AgI/II responses when AgI/II was supplemented with LT-IIa, LT-IIb, or CT. Mice given AgI/II with LT-IIb had more sustained and significantly elevated (P < 0.01) levels of plasma AgI/II-specific IgA on day 60 compared to CT or LT-IIa.
Plasma IgG subclass responses.
When AgI/II was given alone by the i.n. route, low levels of plasma antigen-specific IgG1 and much lower levels of antigen-specific IgG2a, IgG2b, and IgG3 were observed (Fig. 3C). When AgI/II was supplemented with CT or LT-IIa, the major plasma IgG subclass response to AgI/II was IgG1 followed by considerably lower levels of IgG2a and IgG2b. CT induced significantly higher levels of anti-AgI/II IgG1 compared to LT-IIa or LT-IIb (Fig. 3C). Although LT-IIa and CT augmented the IgG subclass responses compared to AgI/II alone, the IgG subclass ratios were not significantly altered. In contrast, LT-IIb significantly enhanced (P < 0.001) anti-AgI/II IgG2a in the plasma compared to either CT or LT-IIa (Fig. 3C) and induced similar levels of IgG2a and IgG1 antibodies.
Antiholotoxin responses.
LT-IIa and LT-IIb induced significantly lower (P < 0.001) salivary IgA and vaginal IgA and IgG compared to CT (Table 1). Plasma IgA and IgG anti-CT responses were also statistically (P < 0.001) higher than either anti-LT-IIa or anti-LT-IIb responses.
TABLE 1.
Anti-HLT antibody levels in plasma and secretions of mice immunized by the i.n. route with AgI/II and adjuvant
| Antibody specificitya | Mean ± SD (n = 7)
|
||||
|---|---|---|---|---|---|
| Antibody activity in secretions (% of total IgA)
|
Plasma antibody activity (μg/ml)
|
||||
| Vaginal IgA | Vaginal IgG | Salivary IgA | IgA | IgG | |
| CT | 26.5 ± 6.6b | 3.8 ± 0.7b | 8.2 ± 1.1b | 21.6 ± 3.8b | 262 ± 36b |
| LT-IIa | 8.5 ± 4.3 | 1.1 ± 0.4 | 3.2 ± 0.8 | 7.2 ± 1.4 | 125 ± 21 |
| LT-IIb | 16.3 ± 1.8 | 1.9 ± 0.5 | 2.1 ± 0.6 | 10.2 ± 3.3 | 162 ± 18 |
Samples were collected 40 days after the third immunization. Preimmune samples contained no detectable antibody activity against LT-IIa, LT-IIb, or CT.
Statistically significant differences from LT-IIa and LT-IIb at P < 0.001.
Cellular responses to AgI/II.
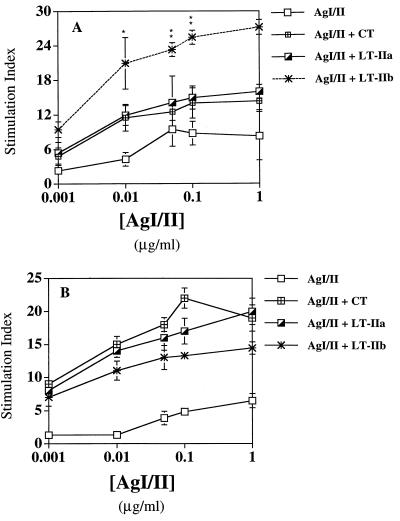
To demonstrate the presence of AgI/II-sensitized cells in the draining lymph nodes (central posterior CLN) and systemic lymphoid tissue (spleen) of mice immunized i.n. with AgI/II supplemented with CT, LT-IIa, or LT-IIb, proliferative responses of lymphoid cell suspensions cultured with various concentrations of AgI/II for 4 days were determined (Fig. 4). Central posterior CLN cells from mice given AgI/II with LT-IIb showed much stronger proliferative responses (P < 0.01) to AgI/II than those from mice given AgI/II alone or supplemented with CT or LT-IIa (Fig. 4A). Splenocytes cultured with various concentrations of AgI/II showed strong stimulation indices in mice supplemented with CT, LT-IIa, or LT-IIb as adjuvant (Fig. 4B). However, no statistical differences were noted between the adjuvant groups. These results demonstrate that the immunostimulatory effects induced by CT, LT-IIa, and LT-IIb on systemic cellular responses are similar, while LT-IIb induces a much stronger antigen-specific response in the local draining lymph nodes of the NALT.
FIG. 4.
Proliferative responses of cells from the central posterior lymph nodes (A) and spleen (B) cultured in vitro with AgI/II for 4 days. Central posterior lymph nodes and spleen were excised 40 days after the last immunization. Results are shown as the stimulation index determined by [3H]thymidine incorporation. The data presented are the mean stimulation index ± standard deviation of quadruplicate cultures. In A, ∗ and ∗∗ indicate statistical differences at P < 0.05 and P < 0.01, respectively, compared to LT-IIa and CT. Background [3H]thymidine incorporation in unstimulated cultures was as follows: for central posterior lymph nodes, AgI/II, 698 ± 136; AgI/II + CT, 3,121 ± 1,402; AgI/II + LT-IIa, 4,166 ± 1,678; and AgI/II + LT-IIb, 3,222 ± 216; for spleen, AgI/II, 1,687 ± 436; AgI/II + CT, 2,849 ± 786; AgI/II + LT-IIa, 2,249 ± 317; and AgI/II + LT-IIb, 2,703 ± 511.
Cytokine production.
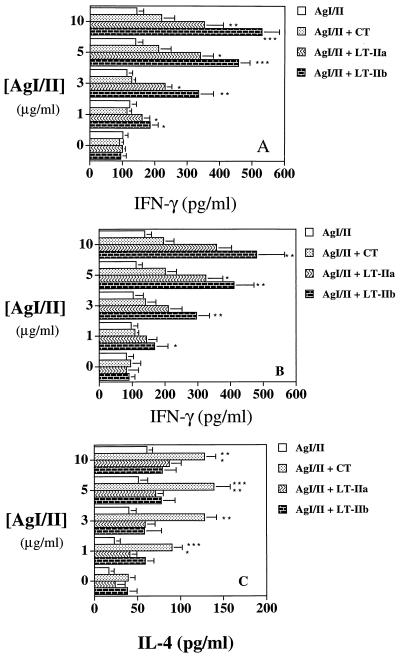
Since the immunoregulatory cytokines IFN-γ and IL-4 are important mediators of IgG2a and IgG1 responses, respectively, culture supernatants from AgI/II-stimulated superficial CLN and splenic cells were analyzed for the presence of IL-4 and IFN-γ (Fig. 5). Cultures of superficial CLN cells stimulated with AgI/II from mice given AgI/II plus LT-IIa or LT-IIb produced significantly larger amounts (P < 0.05 to P < 0.001) of IFN-γ compared to cultures from mice given AgI/II alone or with CT as adjuvant (Fig. 5A). Analysis of AgI/II-stimulated splenocyte cultures revealed a similar pattern (Fig. 5B) in which IFN-γ titers were statistically elevated (P < 0.05 to P < 0.01) when LT-IIa or LT-IIb was used as adjuvant. While LT-IIa and LT-IIb as adjuvants induced similar titers of IL-4, CT induced significantly higher titers of IL-4 in AgI/II-stimulated splenic cultures (Fig. 5C). IL-4 was not detected in supernatants from AgI/II-stimulated cells isolated from the superficial lymph nodes (data not shown). These results demonstrate that CT, LT-IIa, and LT-IIb differ in the ability to stimulate AgI/II-specific cytokine production.
FIG. 5.
Production of IFN-γ and IL-4 by AgI/II-specific cells from superficial lymph nodes (A) and spleens (B and C) of BALB/c mice immunized i.n. with AgI/II with or without CT, LT-IIa, or LT-IIb. Cells were stimulated in vitro with AgI/II at the concentrations shown for 4 days. Data shown are the arithmetic mean values ± standard deviations (n = 4) as determined by cytokine-specific ELISA. In panels A and B, ∗, ∗∗, and ∗∗∗ indicate significant differences at P < 0.05, P < 0.01, and P < 0.001, respectively, compared to CT. In panel C, ∗, ∗∗, and ∗∗∗ indicate significant differences at P < 0.05, P < 0.01, and P < 0.001, respectively, compared to LT-IIa and LT-IIb.
ASC responses.
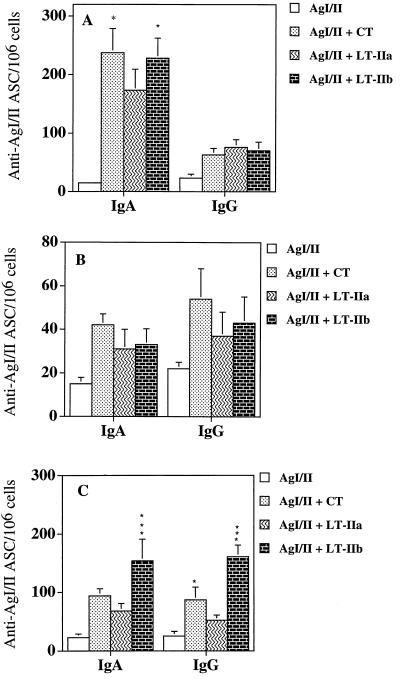
To determine the effects of CT, LT-IIa, and LT-IIb on the number of AgI/II-specific ASC, mice were sacrificed 10 days after the last immunization. Superficial CLN, NALT, and spleens were excised, and the lymphoid cells were examined by ELISPOT assay. Cells from the NALT of mice immunized with AgI/II supplemented with CT or LT-IIb contained significantly (P < 0.05) higher numbers of anti-AgI/II IgA ASC compared to mice given AgI/II in the presence of LT-IIa (Fig. 6A). Mean levels of anti-AgI/II ASC from the spleen were also higher in mice given AgI/II with CT, LT-IIa, or LT-IIb (Fig. 6B). One of the most striking differences between CT, LT-IIa, and LT-IIb on AgI/II-specific ASC was observed in the draining lymph nodes of the nasal mucosa: LT-IIb significantly enhanced (P < 0.01 to P < 0.001) the number of IgA and IgG AgI/II-specific ASC in the superficial CLN compared to CT or LT-IIa (Fig. 6C).
FIG. 6.
Comparison of anti-AgI/II ASC from the NALT (A), spleens (B), and superficial lymph nodes (C) of mice immunized i.n. with AgI/II with or without CT, LT-IIa, or LT-IIb as adjuvant. Data shown are the mean numbers of anti-AgI/II ASC per 106 cells ± standard deviations (n = 3). In panel A, ∗ indicates statistical significance at P < 0.05 compared to LT-IIa; In panel C, ∗∗∗ indicates significant difference at P < 0.001 compared to CT and LT-IIa and ∗ indicates statistical significance at P < 0.05 compared to LT-IIa.
DISCUSSION
In this study, both LT-IIa and LT-IIb possessed strong adjuvant properties for stimulating mucosal IgA as well as systemic IgG immune responses to an unrelated antigen after i.n. immunization. The mucosal inductive site, NALT, as well as the draining CLN represent the regional sites of antigenic stimulation after i.n. immunization (20, 40). 125I-labeled IgG aggregates injected directly into the NALT were recoverable from the central posterior CLN within 30 min (1). Furthermore, it has been shown that antigen that enters via the nasal mucosa drains initially to the superficial CLN (20, 37). Thus, upon i.n. administration, antigen enters via the M-like cells overlying the dome epithelium of the NALT; alternatively, uptake of antigen may occur through the nasal membranes. We found that superficial CLN isolated from mice immunized with LT-IIb as adjuvant showed a significant enhancement in the number of AgI/II-specific IgA and IgG ASC compared to LT-IIa or CT. Potent cellular responses were also observed in the central posterior CLN when AgI/II was coadministered with LT-IIb. Previous studies from this laboratory showed that the central posterior CLN represent a site where memory T cells persist after i.n. immunization (41). These present findings suggest that LT-IIb may enhance antigen uptake in the NALT and nasal mucosa either by enhancing receptor-mediated transport or by affecting the permeability of the nasal mucosa. This latter possibility is supported by previous studies demonstrating that CT can enhance transepithelial flux in the nasal mucosa and also increase the amount of Ag that crosses the mucosal surface and enters the systemic circulation (8, 21).
The anti-AgI/II IgG subclass antibody response patterns potentiated by CT, LT-IIa, and LT-IIb can be attributed to the production of Th1- and Th2-type cytokines provided from mucosal and systemic immune compartments. It is known that cytokines play a major role in selecting the isotype of antibody produced during the immune response. Although no single cytokine alone appears to be required for the generation of IgG subclass responses in vivo, IL-4 and IFN-γ have been shown to enhance production of IgG1 and IgG2a, respectively (27, 33). Previous findings that AgI/II induces a predominantly Th2-mediated immune response are consistent with our observations concerning the anti-AgI/II IgG1 antibody response and cytokine production (H.-Y. Wu, unpublished data). Moreover, while LT-IIa and CT enhanced the anti-AgI/II IgG antibody responses, neither of these adjuvants altered the IgG1/IgG2a ratio compared to AgI/II alone. However, the enhanced IFN-γ production in both systemic and mucosal compartments observed with LT-IIb was associated with elevated anti-AgI/II IgG2a levels so that equivalent levels of IgG1 and IgG2a antibodies were produced, but it did not appear to decrease anti-AgI/II IgG1 responses. Our observations are in agreement with the report that low doses of IFN-γ increase IgG2a production in vivo, while considerably higher levels of IFN-γ decrease IgG1 production (5). Taken together, these findings indicate that LT-IIb effectively stimulates both Th1- and Th2-mediated IgG subclass responses. The ability of the type II enterotoxins to alter both the cytokine and IgG subclass profile suggests that the inherent immunological properties of AgI/II can be significantly changed by the coadministered adjuvant.
While CT and LT-I share the same high-affinity receptor (GM1), the receptor specificity of LT-I has been shown to be more tolerant (6). Experiments with the LT-I B (G33D) mutant that has lost affinity for GM1 demonstrated that both the immunogenicity and adjuvanticity were strongly GM1-dependent (25, 26). Thus, the adjuvanticity of CT and LT-I appeared to depend critically on the GM1 receptor, while the effect of binding to other gangliosides seemed insignificant. Our results indicate that the ability of the type II enterotoxins to target gangliosides other than GM1 results in enhanced systemic and mucosal immune responses and may contribute to the observed differences in adjuvanticity.
The ability of a mucosal adjuvant to potentiate an antigen-specific immune response to a coadministered antigen while not being extremely immunogenic in itself is desirable. The inherent immunogenicity of an adjuvant may result in diminished adjuvant activity upon subsequent use. Thus, high levels of preexisting antibodies to the adjuvant may bind and inactivate it. The present study demonstrated that CT was significantly more immunogenic by the i.n. route in BALB/c mice (H-2d) than LT-IIa or LT-IIb. Previous studies have demonstrated that unlike its adjuvanticity, the immunogenicity of CT depends on the H-2 haplotype (4, 14, 24). Therefore, the observed differences in the anti-HLT antibody responses may be due to the particular genetic background of the mouse strain (BALB/c). However, other studies have shown that the potent immunogenicity of LT-I B subunit depended on interactions with GM1 (26). Mice given wild-type LT-I B subunit produced high levels of anti-B-subunit antibodies compared to the GM1 nonbinding mutant, which induced significantly lower or even nondetectable antibody responses (26). The lack of GM1 binding rather than binding to non-GM1 receptors may have been responsible for the diminished immunogenicity. Thus, the immunogenic properties of the type II HLTs may be due to their affinity for non-GM1 receptors.
Most studies using CT as an adjuvant have demonstrated that it generates predominantly Th2-associated cytokines with subsequent enhancement of IgG1 (22, 34). Our data concerning both the IgG subclass and cytokine profiles induced by CT are in agreement with these studies. However, in contrast to CT, our study demonstrated that LT-IIa and LT-IIb induce a balanced Th1 and Th2 cytokine profile with subsequent enhancement of IgG1, IgG2a, IgG2b, and mucosal IgA responses. Thus, the type II HLTs appear to stimulate both Th1- and Th2-mediated immune responses to a protein immunogen after i.n. immunization.
ACKNOWLEDGMENTS
We thank George Hajishengallis for his critical assessment and helpful comments on the manuscript.
This work was supported in part by grants DE 06746, DE 08182, DE 09081, and T32-AI07051.
REFERENCES
- 1.Biewenga J, Hameleers D M H, Koornstra P J, Kuper C F. Immuno- and enzyme-histochemical and functional studies on nasal associated lymphoid tissue (NALT) in the rat. In: Tsuchiya M, Nagura H, Hibi T, Moro I, editors. Frontiers of mucosal immunology. Vol. 2. Amsterdam, The Netherlands: Excerpta Medica; 1991. pp. 603–604. [Google Scholar]
- 2.Connell T D, Holmes R K. Molecular genetic analysis of ganglioside GD1b-binding activity of Escherichia coli type IIa heat-labile enterotoxin by use of random and site-directed mutagenesis. Infect Immun. 1992;60:63–70. doi: 10.1128/iai.60.1.63-70.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eidels L, Proia R L, Hart D A. Membrane receptors for bacterial toxins. Microbiol Rev. 1983;47:596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elson C O, Ealding W. Genetic control of the murine immune response to cholera toxin. J Immunol. 1985;135:930–932. [PubMed] [Google Scholar]
- 5.Finkelman F D, Holmes J, Katona I M, Urban J F, Jr, Beckmann M P. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 6.Fukuta S, Magnani J L, Twiddy E M, Holmes R K, Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988;56:1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill D M, Clements J D, Robertson D C, Finkelstein R A. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981;33:677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gizurarson S, Tamura S, Aizawa C, Kurata T. Stimulation of the transepithelial flux of influenza HA by cholera toxin B subunit. Vaccine. 1992;10:101–106. doi: 10.1016/0264-410x(92)90025-f. [DOI] [PubMed] [Google Scholar]
- 9.Green B A, Neill R J, Ruyechan W T, Holmes R K. Evidence that a new enterotoxin of Escherichia coli which activates adenylate cyclase in eucaryotic target cells is not plasmid mediated. Infect Immun. 1983;41:383–390. doi: 10.1128/iai.41.1.383-390.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guth B E, Pickett C L, Twiddy E M, Holmes R K, Gomes T A, Lima A A, Guerrant R L, Franco B D, Trabulsi L R. Production of type II heat-labile enterotoxin by Escherichia coli isolated from food and human feces. Infect Immun. 1986;54:587–589. doi: 10.1128/iai.54.2.587-589.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guth B E, Twiddy E M, Trabulsi L R, Holmes R K. Variation in chemical properties and antigenic determinants among type II heat-labile enterotoxins of Escherichia coli. Infect Immun. 1986;54:529–536. doi: 10.1128/iai.54.2.529-536.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannun Y A, Linardic C M. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta. 1993;1154:223–236. doi: 10.1016/0304-4157(93)90001-5. [DOI] [PubMed] [Google Scholar]
- 13.Hardy S J, Holmgren J, Johansson S, Sanchez J, Hirst T R. Coordinated assembly of multisubunit proteins: oligomerization of bacterial enterotoxins in vivo and in vitro. Proc Natl Acad Sci USA. 1988;85:7109–7113. doi: 10.1073/pnas.85.19.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirabayashi Y, Tamura S I, Suzuki Y, Nagamine T, Aizawa C, Shimada K, Kurata T. H-2-unrestricted adjuvant effect of cholera toxin B subunit on murine antibody responses to influenza virus haemagglutinin. Immunology. 1991;72:329–335. [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes R K, Jobling M G, Connell T D. Cholera toxin and related enterotoxins of gram negative bacteria. In: Moss J, Iglewski B, Vaughn M, Tu A T, editors. Handbook of natural toxins. New York, N.Y: Marcel Dekker, Inc.; 1995. pp. 225–255. [Google Scholar]
- 16.Holmes R K, Twiddy E M, Pickett C L. Purification and characterization of type II heat-labile enterotoxin of Escherichia coli. Infect Immun. 1986;53:464–473. doi: 10.1128/iai.53.3.464-473.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes R K, Twiddy E M, Pickett C L, Marcus H, Jobling M G, Pettijean F M J. The Escherichia coli/Vibrio cholerae family of enterotoxins. In: Pohland A E, Dowell V R, Richard J L, editors. Microbial toxins in foods and feeds: cellular and molecular modes of action. New York, N.Y: Plenum Press; 1990. pp. 91–102. [Google Scholar]
- 18.Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981;292:413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- 19.Honda T, Tsuji T, Takeda Y, Miwatani T. Immunological nonidentity of heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1981;34:337–340. doi: 10.1128/iai.34.2.337-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuper C F, Koornstra P J, Hameleers D M H, Biewenga J, Spit B J, Duijvestijn A M, van Breda Vriesman P J C, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 21.Lycke N, Karlsson U, Sjolander A, Magnusson K E. The adjuvant action of cholera toxin is associated with an increased intestinal permeability for luminal antigens. Scand J Immunol. 1991;33:691–698. doi: 10.1111/j.1365-3083.1991.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 22.Marinaro M, Staats H F, Hiroi T, Jackson R J, Coste M, Boyaka P, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee J R. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 23.Nagai Y, Iwamori M. Ganglioside distribution at different levels of organization and its biological implications. Adv Exp Med Biol. 1984;174:135–136. doi: 10.1007/978-1-4684-1200-0_12. [DOI] [PubMed] [Google Scholar]
- 24.Nashar T O, Hirst T R. Immunoregulatory role of H-2 and intra-H-2 alleles on antibody responses to recombinant preparations of B-subunits of Escherichia coli heat-labile enterotoxin (rEtxB) and cholera toxin (rCtxB) Vaccine. 1995;13:803–810. doi: 10.1016/0264-410x(94)00077-z. [DOI] [PubMed] [Google Scholar]
- 25.Nashar T O, Hirst T R, Williams N A. Modulation of B-cell activation by the B subunit of Escherichia coli enterotoxin: receptor interaction up-regulates MHC class II, B7, CD40, CD25 and ICAM-1. Immunology. 1997;91:572–578. doi: 10.1046/j.1365-2567.1997.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nashar T O, Webb H M, Eaglestone S, Williams N A, Hirst T R. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci USA. 1996;93:226–230. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul W E. Interleukin 4/B cell stimulatory factor 1: one lymphokine, many functions. FASEB J. 1987;1:456–461. doi: 10.1096/fasebj.1.6.3315808. [DOI] [PubMed] [Google Scholar]
- 28.Pickett C L, Twiddy E M, Belisle B W, Holmes R K. Cloning of genes that encode a new heat-labile enterotoxin of Escherichia coli. J Bacteriol. 1986;165:348–352. doi: 10.1128/jb.165.2.348-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickett C L, Twiddy E M, Coker C, Holmes R K. Cloning, nucleotide sequence, and hybridization studies of the type IIb heat-labile enterotoxin gene of Escherichia coli. J Bacteriol. 1989;171:4945–4952. doi: 10.1128/jb.171.9.4945-4952.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickett C L, Weinstein D L, Holmes R K. Genetics of type IIa heat-labile enterotoxin of Escherichia coli: operon fusions, nucleotide sequence, and hybridization studies. J Bacteriol. 1987;169:5180–5187. doi: 10.1128/jb.169.11.5180-5187.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell M W, Bergmeier L A, Zanders E D, Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980;28:486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sixma T K, Pronk S E, Kalk K H, Wartna E S, van Zanten B A, Witholt B, Hol W G. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991;351:371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- 33.Snapper C M, Peschel C, Paul W E. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J Immunol. 1988;140:2121–2127. [PubMed] [Google Scholar]
- 34.Snider D P. The mucosal adjuvant activities of ADP-ribosylating bacterial enterotoxins. Crit Rev Immunol. 1995;15:317–348. doi: 10.1615/critrevimmunol.v15.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 35.Spangler B D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svennerholm A M, Holmgren J. Identification of Escherichia coli heat-labile enterotoxin by means of a ganglioside immunosorbent assay (GM1-ELISA) procedure. Curr Microbiol. 1978;1:19–23. [Google Scholar]
- 37.Tilney N. Patterns of lymphatic drainage in the adult laboratory rat. J Anat. 1971;109:369–383. [PMC free article] [PubMed] [Google Scholar]
- 38.Truitt R L, Hanke C, Radke J, Mueller R, Barbieri J. Glycosphingolipids as novel targets for T-cell suppression by the B subunit of recombinant heat-labile enterotoxin. Infect Immun. 1998;66:1299–1308. doi: 10.1128/iai.66.4.1299-1308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf A A, Jobling M G, Wimer-Mackin S, Ferguson-Maltzman M, Madara J L, Holmes R K, Lencer W I. Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia. J Cell Biol. 1998;141:917–927. doi: 10.1083/jcb.141.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H-Y, Nguyen H H, Russell M W. Nasal lymphoid tissue (NALT) as a mucosal inductive site. Scand J Immunol. 1997;46:506–513. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- 41.Wu H-Y, Nikolova E B, Beagley K W, Eldridge J H, Russell M W. Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunization. Infect Immun. 1997;65:227–235. doi: 10.1128/iai.65.1.227-235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu H-Y, Russell M W. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit. Vaccine. 1998;16:286–292. doi: 10.1016/s0264-410x(97)00168-0. [DOI] [PubMed] [Google Scholar]