Abstract
A 74-year-old male with a prior left total knee arthroplasty presented with deformity, loosening, pain, and stiffness associated with multiple raised, erythematous, cutaneous nodules about the anterior knee. Workup was concerning for infection, but the skin nodules were atypical. The patient was sent for biopsy which revealed cutaneous diffuse large B-cell lymphoma. The revision surgery was delayed, and the patient underwent chemotherapy/radiation with complete resolution of his lymphoma. He then underwent a successful aseptic revision total knee arthroplasty. Proper identification and treatment of rare cutaneous skin lesions about a prior surgical site can limit morbidity and result in more desirable outcomes.
Keywords: Arthroplasty, Case report, Cutaneous skin lesions knee, Lymphoma
Introduction
Identification and treatment of postoperative complications after total knee arthroplasty (TKA) are undeniably important. While postsurgical skin changes can indicate infection, skin necrosis, or benign etiologies, it is important to be aware and hence identify unusual manifestations. Improper management without a definitive diagnosis can lead to increased morbidity and poor outcomes [1].
Upon review of the literature, there are only 11 case reports of postoperative metallic-implant-associated lymphomas [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. Furthermore, only 2 such reports are concerning a TKA. In this case report, we aim to describe a clinical situation where if one exercises an abundance of caution and obtains an accurate diagnosis, patient morbidity can be avoided with an excellent patient outcome.
Case history
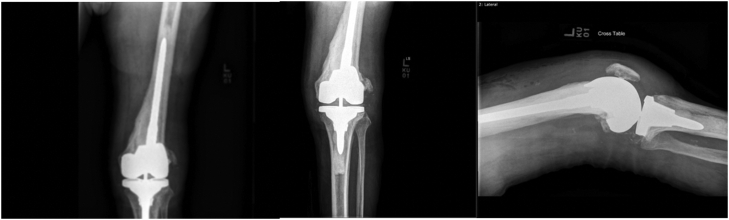
A 74-year-old male presented to our clinic after he had a revision left TKA performed by another surgeon 2 years prior. The patient presented with a valgus deformity, pain, range of motion 15-80 degrees, and anteriorly based erythematous skin nodules (Fig. 1). No deep palpable mass was identified. Radiographs demonstrated a revision TKA (Triathlon TS; Stryker Orthopedics, Mahwah, NJ) implant with lucency around the femoral component and valgus malalignment (Fig. 2). The patients’ implant before his subsequent revision was a Stryker Triathlon primary knee. We obtained standard laboratory workup for infection including aspiration, complete blood count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and bone scan. The knee aspiration was dry, CRP was >10 mg/dl (reported 0-10, >10), ESR 2.0 mm/h, and white blood cell count 6300/mL. Bone scan demonstrated “diffuse uptake about the femoral component concerning for infection.”
Figure 1.
Anterior knee cutaneous nodules upon presentation to clinic.
Figure 2.
Posterior-anterior and lateral radiographs of the left knee with valgus malalignment and lucency about the femoral component (implant: Stryker Triathlon TS).
At this point, our suspicion for infection was high, but the anteriorly based skin nodules were atypical (Fig. 1). We then sent the patient to a dermatology team who biopsied the nodules which revealed a diffuse large B-cell lymphoma with cells being CD20-positive and CD3-, CD4-, and CD30-negative (Figure 3, Figure 4). Computed tomography (CT) of the left lower extremity (LLE) revealed a 14.1 × 8.1-cm soft-tissue mass involving the anterior and posterior distal thigh musculature. A positron emission tomography scan revealed extensive multifocal intraosseous and anterior muscle compartment soft-tissue masses with infiltration into the distal femoral metaphysis (stage III). A bone marrow biopsy at the iliac crest was negative for disease.
Figure 3.
Skin biopsy with sheets of lymphoid-type cells and diffuse nonepidermotropic lymphoid infiltrate (100x).
Figure 4.
High power yielding hyperchromatic and enlarged lymphocytes.
The patient was recommended to undergo chemotherapy and treated with Rituxan-CVP (Genentech, San Francisco, CA) for 5 months. He was not a candidate for Adriamycin (Thymoorgan Pharmazie, Goslar, Germany) due to cardiac comorbidities. At the completion of his chemotherapy, a new CT was obtained, which showed the mass shrunk to 1.5 × 1 cm about the anterior distal thigh musculature.
The patient then received radiation therapy (45 Gy in 25 fractions to residual; 36 Gy to prechemo gross tumor volume) over the course of 1 month. Three months after the completion of the radiation therapy, a repeat CT scan revealed that “the previously described lesion in the distal thigh is not delineated in the current study.” At this point, the patient was deemed clear of disease and a candidate for revision surgery.
Repeat examination of the patients' left knee demonstrated complete resolution of the cutaneous nodules and skin changes (Fig. 5). Repeat ESR, CRP, and complete blood count were normal. Repeat bone scan demonstrated no soft-tissue uptake, but persistent bony uptake in the femur. Repeat knee aspiration was dry. He continued to be symptomatic due to his valgus deformity, pain, and limited range of motion. The patient then underwent left-knee revision TKA to a long-stemmed, cemented femoral component (Stryker Triathlon TS); the tibial side was not revised (Fig. 6). The pathology of bone and soft tissue sent from the surgery revealed no visible tumor/lymphoma. Cultures and frozen sections for infection were negative. The total time from initial presentation to the ultimate revision surgery was 14 months.
Figure 5.
Left knee after the completion of chemotherapy and radiation. Complete resolution of skin nodules and erythema.
Figure 6.
Status after the revision surgery performed on the long-stemmed cemented femur.
At the 12-month follow-up from arthroplasty, there was no recurrence of lymphoma, and the patient returned to all activities of daily living (ADLs). He was working out 6 days a week and pleased with the result. The final range of motion was 3-110 degrees.
The patient was informed the data and images concerning the case would be submitted for publication and provided written consent.
Discussion
Primary non-Hodgkin lymphoma in association with an orthopedic surgery is very rare, with only 12 other reported cases in the literature [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. Of these, only 3 occurred after a TKA [1,2,13]. The median latency to presentation was 8 years (range 7 months to 17 years). Only 4 of the 11 cases presented as soft-tissue manifestations, while the remaining 7 presented as bony radiologic findings [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. Many of the cases provided a diagnostic challenge, with symptoms and signs mimicking other conditions such as loosening or infection.
Whether orthopedic implants have carcinogenic potential can often be a concern of orthopedic surgeons. Particle debris from wear has been shown to promote DNA instability through DNA and chromosomal injuries [[14], [15], [16]]. This damage has theoretical risk to induce malignancy of the surrounding tissues. Multiple early studies established a potential link to orthopedic implants and carcinogenic potential [[17], [18], [19]]. In a study of the rates of malignancy following total hip arthroplasties, Gillespie et al. observed a small increase in the overall prevalence of tumors of lymphatic and hematopoietic origin [20]. Despite the aforementioned studies, no epidemiological study has demonstrated a significant increase in the prevalence of malignancy, including lymphoma in patients who have been treated with orthopedic implants [21,22]. We do not believe the lymphoma in this case was caused by the orthopedic implants utilized in the primary or revision arthroplasties. It is more likely that the lymphoma was present at the time of initial revision or developed spontaneously thereafter.
Long discussions about potential complications after a TKA following radiation therapy should also be conducted with patients in this setting. The effects of radiation on bone have been studied extensively and found to cause osteonecrosis [[23], [24], [25]]. This could lead to increased rates of loosening; however, the addition of cement with stems and augments could help provide more reliable long-term results [26]. In this case, we utilized a long cemented stem with a cone in order to mitigate possible osteonecrosis and, hence, loosening. Furthermore, radiation in the preoperative setting can lead to increased wound-healing problems [27,28]. While radiation was necessary to treat the lymphoma, understanding the potential for these complications is important, and one should be prepared for rotation or free flaps as indicated to mitigate wound and soft-tissue healing problems. Lastly, radiation-associated knee stiffness should be discussed with patients. Stiff patients receive significantly higher mean doses to the suprapatellar bursa/anterior suprapatellar fat pad and quadriceps tendon than nonstiff patients. This suggests that radiation-induced shortening of the quadriceps tendon and damage to the synovial membrane lining the anterior suprapatellar fat pad may be contributing factors [29].
Abnormal presentations of postoperative wound compilations in the setting of a prior TKA, therefore, should be approached with great caution. In our case, initial presentation was certainly concerning for infection. It would be easy to recommend a revision surgery with rotational or free flap in the acute period given the findings. In the only other similar case report published to date by Eskander et al., aggressive surgical management was undertaken [1]. The high suspicion of infection led to the need for both medial and lateral gastrocnemius flaps with knee fusion after it was discovered the patient had a diffuse cutaneous B-cell lymphoma [1].
Current controversies and future considerations
Whether orthopedic implants have carcinogenic potential can often be a concern of orthopedic surgeons. There are no data at this time to support this concern. In this case, it is most likely that the disease was present at the time of the initial operations, and we do not believe the implants caused the tumor. Future considerations, however, could be aimed at studying this potential phenomenon.
Summary
Our case had an initial presentation almost identical to that described by Eskander et al. However, by using an abundance of caution, obtaining an accurate diagnosis, and undergoing proper treatment, the patient was able to have a significantly improved outcome with revision TKA and had no need for flap coverage or fusion. To our knowledge, this is the first case report to demonstrate the diagnosis, treatment, and resolution of diffuse B-cell lymphoma in association with TKA prior to undergoing a revision surgery. The case report demonstrates that a proper identification and treatment can lead to good patient outcomes. The limitation of the study is that we did not perform his prior surgeries and, therefore, do not know if this pathology was present at the time of his initial revision. It is our hope that the information presented will provide surgeons with a framework on how to approach this difficult problem.
Key Points.
-
•
Abnormal cutaneous lesions about a total knee arthroplasty should be approached with caution.
-
•
Good outcomes can be achieved with treatment and resolution of cutaneous B-cell lymphoma about a total knee arthroplasty prior to surgical intervention.
-
•
Lymphoma associated with metallic implants is rare, and current data do not support a cause-and-effect relationship.
Conflicts of interest
Dr. Palumbo receives royalties from DJO and Conformis; is a paid consultant for DJO and Conformis; and holds stock or stock options from Actuos, LLC. Dr. Bernasek receives royalties from DePuy Syntheses, a Johnson & Johnson Company; is a speaker/paid presenter for DePuy Syntheses, a Johnson & Johnson Company; is a paid consultant for DePuy Syntheses, a Johnson & Johnson Company, and Corin Orthopaedics; receives research support from DePuy Syntheses, a Johnson & Johnson Company, and Corin Orthopaedics; and is on the Board of Councilors of American Academy of Orthopaedic Surgeons and Florida Orthopaedic Society. Dr. Lyons receives royalties from Zimmer; is a paid consultant for Zimmer; holds stock or stock options from Amedica and Zimmer; is the editor of the Journal of Arthroplasty; and is on the board of Florida Orthopaedic Society. Dr. Wetzel declares no potential conflicts of interest.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2022.10.010.
Informed patient consent
The authors confirm that informed consent has been obtained from the involved patient or, if appropriate, from the parent, guardian, power of attorney of the involved patient and that they have given approval for this information to be published in this article.
Acknowledgments
The authors would like to acknowledge the Debartolo Center for Adult Reconstruction Research for funding support for this research.
Appendix A. Supplementary data
References
- 1.Eskander M.S., McPhee E., Eskander J.P., Nascimento R., McCormick J.J., Hao S., et al. A left knee wound complication by non-Hodgkins lymphoma in bilateral total knee arthroplasties. Arch Orthop Trauma Surg. 2008;128:1387–1390. doi: 10.1007/s00402-008-0568-z. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhry M.S., Mather H., Marks A., Naresh K. Diffuse large B cell lymphoma complicating total knee arthroplasty: case report and literature review of the association of diffuse large B cell lymphoma with joint replacement. Acta Haematol. 2011;126:141–146. doi: 10.1159/000328202. [DOI] [PubMed] [Google Scholar]
- 3.Cheuk W., Chan A.C., Chan J.K., Lau G.T., Chan V.N., Yiu H.H. Metallic implant-associated lymphoma: a distinct subgroup of large Bcell lymphoma related to pyothorax-associated lymphoma? Am J Surg Pathol. 2005;29:832–836. doi: 10.1097/01.pas.0000157747.10967.f4. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh T.C., Kao C.H., Yen K.Y., Sun S.S. Osteomyelitis-mimicking primary bone lymphoma at hip prosthetic site. Clin Nucl Med. 2007;32:543–544. doi: 10.1097/RLU.0b013e318065a9cf. [DOI] [PubMed] [Google Scholar]
- 5.Ganapathi M., Lake D.N., Griffiths A.P. Periprosthetic high-grade B-cell lymphoma complicating an infected revision total hip arthroplasty. J Arthroplasty. 2001;16:229–232. doi: 10.1054/arth.2001.9827. [DOI] [PubMed] [Google Scholar]
- 6.O’Shea K., Kearns S.R., Blaney A., Murray P., Smyth H.A., McElwain J.P. Periprosthetic malignancy as a mode of failure in total hip arthroplasty. J Arthroplasty. 2006;21:926–930. doi: 10.1016/j.arth.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Radhi J.M., Ibrahiem K., al-Tweigeri T.J. Soft tissue malignant lymphoma at sites of previous surgery. J Clin Pathol. 1998;51:629–632. doi: 10.1136/jcp.51.8.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu A H. Malignant lymphoma at the site of a total hip replacement. Orthopedics. 1999;22:82–84. doi: 10.3928/0147-7447-19990101-11. [DOI] [PubMed] [Google Scholar]
- 9.Syed A.A., Agarwal M., Fenelon G., Toner M. Osseous malignant non-Hodgkin’s B-cell lymphoma associated with total hip replacement. Leuk Lymphoma. 2002;43:2213–2216. doi: 10.1080/1042819021000016140. [DOI] [PubMed] [Google Scholar]
- 10.Dodion P., Putz P., Amiri-Lamraski M.H., Efira A., de Martelaere E., Heimann R. Immunoblastic lymphoma at the site of an infected vitallium bone plate. Histopathology. 1982;6:807–813. doi: 10.1111/j.1365-2559.1982.tb02776.x. [DOI] [PubMed] [Google Scholar]
- 11.McDonald I. Malignant lymphoma associated with internal fixation of a fractured tibia. Cancer. 1981;48:1009–1011. doi: 10.1002/1097-0142(19810815)48:4<1009::aid-cncr2820480426>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Sagaert X., Van Cutsem E., De Hertogh G., Geboes K., Tousseyn T. Gastric MALT lymphoma: a model of chronic inflammation-induced tumour development. Nat Rev Gastroenterol Hepatol. 2010;7:336–346. doi: 10.1038/nrgastro.2010.58. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim I., Haughom B.D., Fillingham Y.A., Brown N., Gitelis S. Primary lymphoma of bone complicating total knee arthroplasty: an unexpected mode of prosthesis failure: a case report. JBJS Case Connect. 2015;5:e34. doi: 10.2106/JBJS.CC.N.00167. [DOI] [PubMed] [Google Scholar]
- 14.Case C.P., Langkamer V.G., Howell R.T., Webb J., Standen G., Palmer M., et al. Preliminary observations on possible premalignant changes in bone marrow adjacent to worn total hip arthroplasty implants. Clin Orthop Relat Res. 1996;329(Suppl):S269–S279. doi: 10.1097/00003086-199608001-00024. [DOI] [PubMed] [Google Scholar]
- 15.Daley B., Doherty A.T., Fairman B., Case C.P. Wear debris from hip or knee re- placements causes chromosomal damage in human cells in tissue culture. J Bone Joint Surg Br. 2004;86:598–606. [PubMed] [Google Scholar]
- 16.Davies A.P., Sood A., Lewis A.C., Newson R., Learmonth I.D., Case C.P. Metal-specific differences in levels of DNA damage caused by synovial fluid recovered at revision arthroplasty. J Bone Joint Surg Br. 2005;87:1439–1444. doi: 10.1302/0301-620X.87B10.16541. [DOI] [PubMed] [Google Scholar]
- 17.Oppenheimer B.S., Oppenheimer E.T., Danishefsky I., Stout A.P. Carcinogenic ef- fect of metals in rodents. Cancer Res. 1956;16:439–441. [PubMed] [Google Scholar]
- 18.Heath J.C., Freeman M.A.R., Swanson S.A.V. Carcinogenic properties of wear par- ticles from prostheses made in cobalt-chromium alloy. Lancet. 1971;1:564–566. doi: 10.1016/s0140-6736(71)91162-7. [DOI] [PubMed] [Google Scholar]
- 19.Memoli V.A., Urban R.M., Alroy J., Galante J.O. Malignant neoplasms associated with orthopedic implant materials in rats. J Orthop Res. 1986;4:346–355. doi: 10.1002/jor.1100040311. [DOI] [PubMed] [Google Scholar]
- 20.Gillespie W.J., Frampton C.M., Henderson R.J., Ryan P.M. The incidence of cancer following total hip replacement. J Bone Joint Surg Br. 1988;70:539–542. doi: 10.1302/0301-620X.70B4.3403594. [DOI] [PubMed] [Google Scholar]
- 21.Ma kela K.T., Visuri T., Pulkkinen P., Eskelinen A., Remes V., Virolainen P., et al. Risk of cancer with metal-on-metal hip replacements: population based study. BMJ. 2012;345:e4646. doi: 10.1136/bmj.e4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Visuri T., Pulkkinen P., Paavolainen P., Pukkala E. Cancer risk is not increased after conventional hip arthroplasty. Acta Orthop. 2010;81:77–81. doi: 10.3109/17453671003667150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bragg D.G., Shidnia H., Chu F.C., Higinbotham N.L. The clinical and radiographic aspects of radiation osteitis. Radiology. 1970;97:103–111. doi: 10.1148/97.1.103. [DOI] [PubMed] [Google Scholar]
- 24.Howland W.J., Loeffler R.K., Starchman D.E., Johnson R.G. Postirradiation atrophic changes of bone and related complications. Radiology. 1975;117(3 pt 1):677–685. doi: 10.1148/117.3.677. [DOI] [PubMed] [Google Scholar]
- 25.Hong S.I., Hong S.K., Wallace J.M., Kohn D.H. Ultrastructural observation of electron irradiation damage of lamellar bone. J Mater Sci Mater Med. 2009;20:959–965. doi: 10.1007/s10856-008-3651-7. [DOI] [PubMed] [Google Scholar]
- 26.Myers T.G., Cui Q., Kuskowski M., Mihalko W.M., Saleh K.J. Outcomes of total and unicompartmental knee arthroplasty for secondary and spontaneous osteonecrosis of the knee. J Bone Joint Surg Am. 2006;88(Suppl 3):76–82. doi: 10.2106/JBJS.F.00568. [DOI] [PubMed] [Google Scholar]
- 27.Cheng E.Y., Dusenbery K.E., Winters M.R., Thompson R.C. Soft tissue sarcomas: preoperative versus postoperative radiotherapy. J Surg Oncol. 1996;61:90–99. doi: 10.1002/(SICI)1096-9098(199602)61:2<90::AID-JSO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 28.Bujko K., Suit H.D., Springfield D.S., Convery K. Wound healing after preoperative radiation for sarcoma of soft tissues. Surg Gynecol Obstet. 1993;176:124–134. [PubMed] [Google Scholar]
- 29.Pak D.J., Griffith K., Bierman J.S. Dose predictors for knee stiffness following postoperative radiathion therapy of soft-tissue sarcomas of the lower extremity. Int J Radiat Oncol. 2011;81:S681. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.