Abstract
Post-stroke depression (PSD) is the most common mood disorder caused by stroke. Stroke might bring about increased intestinal permeability accompanied by gut microbiota changes. According to the “gut-brain” axis hypothesis, increased intestinal permeability may contribute to PSD. Therefore, we investigated the association between increased intestinal permeability and the occurrence of PSD. Intestinal fatty acid binding protein (iFABP) is responsible for intestinal fatty acid absorption and transport and is often considered a biomarker of gut hyperpermeability, also known as leaky gut. We enrolled 48 healthy controls (HCs), 48 stroke patients without depression, and 48 PSD patients in this study. Plasma iFABP was measured in the three groups. CRP, LBP, and sCD14 were quantified for bacterial infection assessment. In addition, clinical laboratory indicators of lipid metabolism were assessed. The PSD patients exhibited higher iFABP levels compared with HCs and non-depressed stroke patients. Using OPLS discriminant analysis, four proteins (ApoA1, HDL-C, iFABP, and Lp(a)) were identified as potential biomarkers for distinguishing PSD patients from non-depression stroke patients. Our study discovered that elevated plasma iFABP levels in PSD patients correlated with the degree of depression, along with disturbed lipid metabolism. These findings also suggested the need to consider the role of a leaky gut in depression after stroke.
Keywords: Diagnostic biomarker, Leaky gut, Post-stroke depression, Lipid metabolism
Diagnostic biomarker; Leaky gut; Post-stroke depression; Lipid metabolism.
1. Introduction
The neuropsychiatric disorders associated with cerebrovascular disease are of considerable clinical importance. Post-stroke depression (PSD) is the most common mood disorder caused by stroke. Meta-analyses estimated that the prevalence of depression in patients who experienced a stroke was as high as 33% (Mitchell, 2017), resulting in increased disability, morbidity, and mortality in stroke survivors (Bartoli, 2018). Current evidence has identified several similarities in molecular mechanisms between PSD and major depression (Robinson and Jorge, 2016). Nonetheless, additional reliable and specific pathophysiological alterations need to be identified in studies of PSD diagnosis and treatment.
The pathophysiology of PSD is closely linked to stroke. Research has demonstrated that stroke may cause damage to the brain (Pan, 2021) and influence the gastrointestinal (GI) tract, including causing increased intestinal permeability (IP). Increased IP frequently results from intestinal barrier dysfunction. Ye et al. (Ye, 2021) reported that acute stroke led to leucocyte accumulation in the intestinal mucosa, which impaired the intestinal barrier and increased IP. Ischemic stroke also can induce intestinal apoptosis (Ye, 2021) and activate the inflammasome in the intestinal tissues (Kerr, 2022), which might exacerbate gut integrity impairment. Due to stroke-induced IP, the gut microbiota composition has been shown to be altered, and intestinal microbial translocation was observed in other organs (Stanley, 2016; Wei, 2021), which could initiate a systemic immune response following a stroke (Huang and Xia, 2021).
Intestinal fatty acid binding protein (iFABP), also known as FABP2, is specifically expressed throughout the intestine. IFABP is responsible for intestinal fatty acid absorption and metabolism and might influence circulating triglyceride levels (Furuhashi and Hotamisligil, 2008; Gajda and Storch, 2015). On the other hand, iFABP has been associated with intestinal dysfunction and has been reported to be a biomarker for the presence of a leaky gut in intestinal diseases (Wiercinska-Drapalo, 2008; Funaoka, 2010; Sarikaya, 2015) and extra-intestinal disorders. Serum iFABP was significantly elevated in subjects with biliary tract infections and accompanied by elevated high-sensitivity C-reactive protein (CRP) (Weng, 2021). Increased expression of iFABP was observed in patients with Graves' disease compared to healthy controls (Zheng, 2021). Interestingly, recent studies reported that subjects with major depressive disorder (MDD) displayed higher iFABP levels (Stevens, 2018; Ohlsson, 2019).
Therefore, in this study, we measured iFABP levels to determine the presence of a leaky gut in subjects with stroke and PSD. We also assessed CRP, lipopolysaccharide binding protein (LBP) and soluble cluster of differentiation 14 (sCD14) levels as indicators for intestinal bacterial imbalance and translocation. Clinical laboratory assessments that evaluated lipid metabolism also were compared to explore lipid metabolism changes related to the presence of a leaky gut in PSD.
2. Materials and methods
2.1. Study subjects and clinical data collection
All procedures were approved by the Ethics Committee of Yongchuan Hospital of Chongqing Medical University. The participants in this study were enrolled in the Yongchuan Hospital of Chongqing Medical University from May 2020 to December 2021. This study enrolled 144 subjects, including 48 stroke survivors without depression characteristics, 48 antidepressant drug-naïve PSD subjects, and 48 healthy controls (HCs). This study was conducted according to the Declaration of Helsinki for the protection of human participants in medical research. All participants provided written informed consent.
The inclusion criteria for the stroke participants were as follows: (1) diagnosed as either ischemic or hemorrhagic stroke patients who were 18 years or older; (2) a stroke was diagnosed using cranial computed tomography (CT) or magnetic resonance imaging (MRI) within 24h of admission; (3) the patients exhibited clear consciousness and were able to cooperate with the relevant examinations. Patients with the following clinical characteristics were excluded: (1) transient ischemic attack; (2) had neurological diseases other than stroke; (3) exhibited renal, hepatic, or heart failure.
The 24-item Hamilton Depression Rating Scale (HAMD) score was used to assess the depressive symptoms of stroke survivors after stroke onset. Depression severity was categorized as follows: a score of 7 or less indicated the absence of depression, a score between 8 and 19 indicated mild depression, and a score of 20 or higher indicated severe depression. The inclusion criteria for PSD patients were as follows: (1) met the inclusion and exclusion criteria for stroke; (2) had a 24-item HRSD score of 8 or higher; (3) did not have a previous history of psychiatric disorders before the stroke; (4) did not have another mental illness or a cognitive disorder; (5) had not received any antidepressant medication treatment before blood sample collection. The patients’ demographic characteristics were collected within seven days of admission and included age, gender, height, weight, stroke type, smoking and drinking history, as well as the presence of diabetes mellitus, hypertension, or coronary heart disease (CHD).
2.2. Blood sample collection and measurement
Blood samples were collected and processed according to Nikolac's recommendation (Nikolac, 2013). In brief, blood was collected in the morning for all participants using vacutainer tubes containing the chelating agent ethylenediaminetetraacetic acid (EDTA) according to. Plasma was obtained by centrifuging the blood at 3,000rpm for 10min, and iFABP was measured in the three groups. Because a leaky gut is likely to cause bacterial translocation or inflammation, LPS-binding protein (LBP), soluble cluster of differentiation 14 (sCD14), and high-sensitivity C-reactive protein (CRP) also were assessed. The plasma levels of iFABP, LBP, and sCD14 were measured using a solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) kit (MeiMian, Jiangsu, China) according to the manufacturer's specifications. Because iFABP is also related to fatty acid absorption that might influence lipid metabolism, we tested the levels of lipoprotein(a) (Lp(a)), apolipoprotein B (ApoB), apolipoprotein E (ApoE), apolipoprotein A1 (ApoA1), free fatty acid (FFA), total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL) and glucose, which are commonly used laboratory lipid indicators.
2.3. Statistical analysis
A one-way ANOVA and chi-square test were used to determine significant differences in the demographic data. The stroke and PSD patients were randomly assigned to a training set or testing set at a ratio of 2:1. First, an orthogonal projections to latent structures (OPLS) model was constructed using the training set, and a 399-item permutation test was used to validate the OPLS model. Second, the testing set and HCs were used to assess the model's accuracy in predicting independent and blind samples. Third, key molecules responsible for discrimination between stroke and PSD patients were selected based on a combination of a correlation coefficient of |r|>0.349 from the OPLS model and P < 0.05 from the one-way ANOVA. Fourth, the Pearson correlation method was used to identify molecules that were significantly correlated with anxiety or depressive symptoms. Finally, binary logistic regression analysis was used to construct a discriminative model using the identified molecules, and receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance of the model.
3. Results
3.1. Baseline characteristics
This study included 48 non-depressed patients after stroke (stroke group), 48 PSD patients (PSD group), and 48 age- and sex-matched healthy controls (HCs group). The general clinical characteristics of all subjects are shown in Table 1. No significant differences in sex, age, and body mass index (BMI) values were observed among the three groups (P > 0.05). However, a significant difference was observed in the HAMD scores among the groups. The PSD patients displayed mild or moderate depressive symptoms with an average 24-HAMD score of 16.7, while the average 24-HAMD scores were 1.91 and 4.52 in the HCs and stroke patients, respectively. There were no significant differences in the medical therapy (statins, antiplatelet aggregation drugs, anticoagulants) between stroke and PSD groups. Stroke and PSD subjects had no differences in preexisting comorbidities related to depression, including diabetes mellitus (Bădescu, 2016), coronary heart disease (Carney, 2017), hypertension (Li, 2015) and previous stroke. Moreover, the incidences of severe neurological deficits (NIHSS and modified Rankin scores) were higher in the PSD group than in the stroke group. It meant that the PSD group had worse stroke-related neurological status, which implied that stroke symptomatology might be an important indicator in the development of leaky gut in PSD.
Table 1.
Demographic data of the included subjects.a
| HCs | Stroke | PSD | p value (HCs vs. Stroke) | p value (HCs vs. PSD) | p value (PSD vs. Stroke) | |
|---|---|---|---|---|---|---|
| Number | 48 | 48 | 48 | - | - | - |
| Age | 63.4 ± 16.3 | 66.0 ± 9.3 | 63.0 ± 10.0 | 0.70 | 0.99 | 0.35 |
| F/M | 26/22 | 35/13 | 31/17 | 0.09 | 0.41 | 0.51 |
| BMI(kg/m2) | 25.58 ± 2.72 | 26.26 ± 2.46 | 26.65 ± 2.61 | 0.61 | 0.14 | 0.99 |
| 24-HAMD | 1.92 ± 1.54 | 4.52 ± 1.68 | 16.71 ± 6.56 | <0.00001 | <0.00001 | <0.00001 |
| 14-HAMA | 1.23 ± 1.29 | 3.06 ± 1.24 | 12.54 ± 4.41 | <0.00001 | <0.00001 | <0.00001 |
| NIHSS | - | 9 (4,15) | 13 (5.5,19) | 0.026 | ||
| GCS | - | 15 (12,15) | 13 (9,15) | 0.10 | ||
| mRS | - | 4 (3,4) | 4 (3,5) | 0.007 | ||
| Hypertension (%) | - | 34 (70.8) | 31 (64.6) | 0.616 | ||
| Diabetes mellitus (%) | - | 7 (14.5) | 10 (20.8) | 0.344 | ||
| History of stroke (%) | - | 1 (2.1) | 2 (4.1) | 1.000 | ||
| Coronary heart disease (%) | - | 3 (6.25) | 5 (10.4) | 0.208 | ||
| Statins (%) | - | 26 (54.2) | 27 (56.3) | 0.99 | ||
| Antiplatelet aggregation drugs (%) | - | 26 (54.2) | 23 (47.9) | 0.13 | ||
| Anticoagulants (%) | - | 10 (20.6) | 12 (25) | 0.07 | ||
| Antidepressants (%) | 0 (0.0) | 0 (0.0) | - |
one-way ANOVA or chi-square test was used to obtain p-values.
3.2. The OPLS model
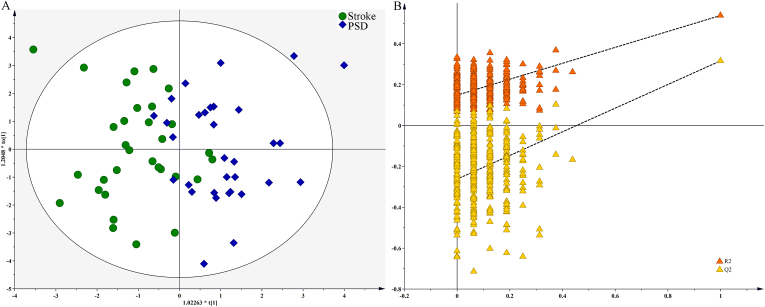
There were 32 stroke and 32 PSD patients included in the training set, which was used to construct the OPLS model. As shown in Figure 1A, the OPLS model demonstrated that the PSD patients were clearly distinguished from the stroke patients. 90.6% of the stroke patients and 84.4% of the PSD patients were correctly recognized in the OPLS model. Furthermore, we used a 399-item permutation test to validate the OPLS model. The results demonstrated that the OPLS model was valid and not over-fitting, as all of the corresponding permutated R2 and Q2 values from the permutation test were significantly lower than the original R2 and Q2 values (Figure 1B).
Figure 1.
OPLS model built using Stroke and PSD patients in training set: A) Stroke (green dot) and PSD (blue diamond) patients were obviously separated with little overlap; B) this model was valid, as the original R2 and Q2 values (top right) were higher than their values from permutation test (bottom left).
3.3. OPLS model validation
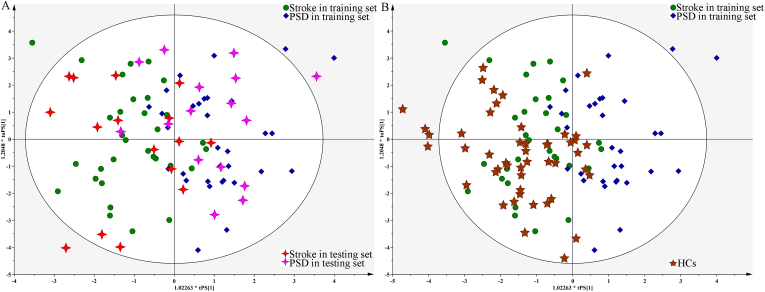
The testing set included 16 stroke and 16 PSD patients that were used to validate the discriminative power of the OPLS model independently. The T-predicted scatter plot from the OPLS model demonstrated that 87.5% of the stroke patients and 81.3% of the PSD patients were correctly predicted by the OPLS model, yielding a predictive accuracy of 84.4% (Figure 2A). In addition, the 48 HCs were used as a blind set to validate whether the OPLS model was specific to patients with stroke versus HCs, and only six HCs were incorrectly identified as PSD patients (Figure 2B). These results indicated that this OPLS model was promising as an objective diagnostic method for PSD.
Figure 2.
T-predicted scatter plot from OPLS model: A) both Stroke (red cross) and PSD (purple cross) patients in testing set were correctly predicted; B) this OPLS model could also effectively separate HCs (brown five-pointed star) from PSD patients.
3.4. Potential biomarkers for PSD
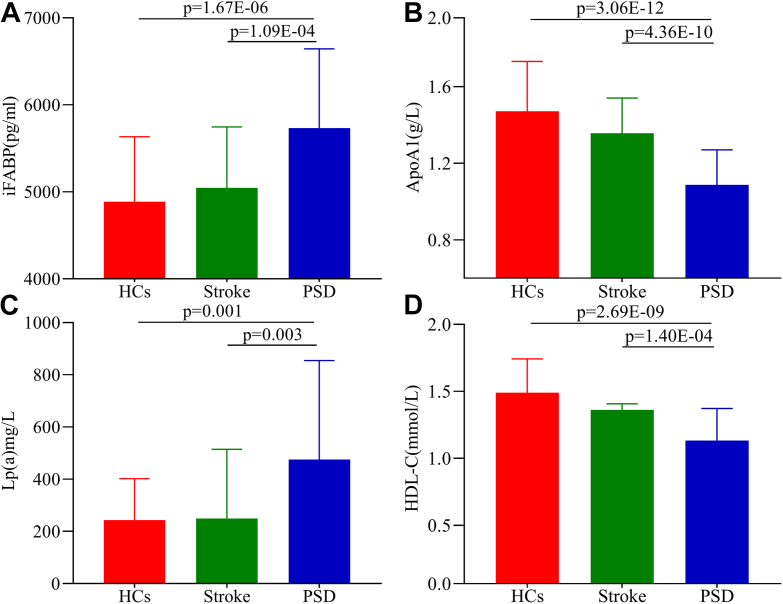
The results of the one-way ANOVA indicated that seven molecules were significantly changed (P < 0.05) between stroke and PSD patients: iFABP, Lp(a), ApoB, TC, ApoA1, HDL-C, and LDL-C. By analyzing the OPLS loading coefficient plots, five molecules with an |r|>0.349 were identified: ApoA1, FFA, HDL-C, iFABP, and Lp(a). Thus, four key molecules responsible for discrimination between stroke and PSD patients were identified and were potential biomarkers for PSD: ApoA1, HDL-C, iFABP, and Lp(a) (Figure 3A-D). When compared to stroke patients and HCs, PSD patients were characterized by lower levels of ApoA1 and HDL-C and higher levels of iFABP and Lp(a). We also found that the ApoA1 (P = 0.042) and HDL-C (P = 0.019) levels were significantly lower in stroke patients than HCs. The detailed information on the assessed molecules is presented in Table 2.
Figure 3.
Potential biomarkers responsible for discrimination between Stroke and PSD patients. Plasma expression levels of A) iFABP and C) Lp (a) in PSD patients were significantly higher than those in HCs and stroke subjects without depression; B) ApopA1 and D) HDL-C displayed a significantly decreased plasma level in PSD patients compared to in HCs and stroke subjects without depression.
Table 2.
Detailed information of the detected molecules.a
| Indicators | HCs | Stroke | PSD | p value (HCs vs. Stroke) | p value (HCs vs. PSD) | p value (PSD vs. Stroke) | Rb |
|---|---|---|---|---|---|---|---|
| iFABP (pg/ml) | 4887.13 | 5045.08 | 5733.85 | 0.98 | 1.67E-06 | 1.09E-04 | 0.74 |
| sCD14 (ng/ml) | 464.68 | 413.56 | 422.08 | 0.13 | 0.28 | 0.99 | 0.06 |
| LBP (μmol/L) | 290.16 | 254.88 | 268.59 | 0.26 | 0.87 | 0.99 | 0.15 |
| CRP (mg/L) | 3.66 | 7.57 | 15.12 | 0.33 | 0.06 | 0.41 | 0.19 |
| Lp(a) (mg/L) | 244.08 | 249.71 | 475.17 | 0.99 | 0.001 | 0.003 | 0.78 |
| ApoB (g/L) | 0.95 | 0.94 | 0.82 | 0.98 | 0.028 | 0.038 | 0.06 |
| FFA (μmol/L) | 444.88 | 604.65 | 522.35 | 0.002 | 0.15 | 0.29 | -0.44 |
| TC (mmol/L) | 5.15 | 4.72 | 3.75 | 0.069 | 7.79E-12 | 3.94E-05 | -0.2 |
| Apo A1 (g/L) | 1.47 | 1.36 | 1.09 | 0.042 | 3.06E-12 | 4.36E-10 | -0.96 |
| HDL-C (mmol/L) | 1.49 | 1.36 | 1.13 | 0.019 | 2.69E-09 | 1.40E-04 | -0.43 |
| LDL-C (mmol/L) | 2.99 | 2.6 | 2.07 | 0.069 | 8.16E-07 | 0.007 | -0.03 |
| TG (mmol/L) | 1.45 | 1.3 | 1.41 | 0.802 | 0.99 | 0.99 | 0.25 |
| HDL/LDL | 0.52 | 0.57 | 0.6 | 0.686 | 0.182 | 0.99 | -0.31 |
| ApoE (mg/L) | 31.69 | 38 | 36.46 | 0.068 | 0.251 | 0.99 | 0.15 |
| Glucose (mmol/L) | 6.19 | 6.43 | 5.85 | 0.927 | 0.623 | 0.384 | -0.22 |
| Creatinine (μmol/L) | 69.44 | 70.67 | 70.13 | 0.984 | 0.99 | 0.99 | -0.28 |
iFABP, intestinal fatty acid binding protein; sCD14, soluble cluster of differentiation 14; LBP, lipopolysaccharide binding protein; CRP, high-sensitivity C-reactive protein; Lp(a), lipoprotein (a); ApoB, apolipoprotein B; FFA, free fatty acid; TC, total cholesterol; Apo A1, apolipoprotein A1; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TG, triglyceride; ApoE, apolipoprotein E.
one-way ANOVA or chi-square test was used to obtain p-values.
correlation coefficient from OPLS loading coefficient plots resulted.
3.5. Correlation analysis
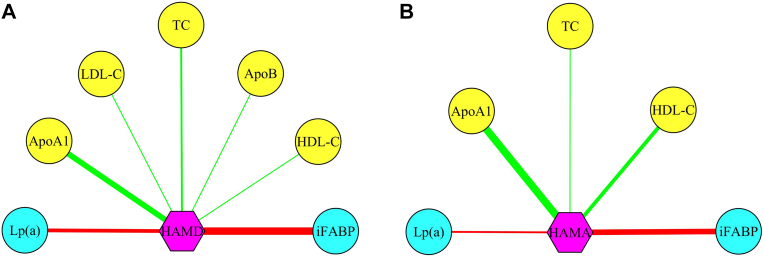
We used the Pearson correlation method to assess significant correlations between the identified molecules and the HAMD and HAMA scores. Two correlation networks were observed. First, seven molecules (ApoA1, HDL-C, TC, Lp(a), iFABP, LDL-C, and ApoB) were significantly correlated with the HAMD score (Figure 4A), and five molecules (ApoA1, HDL-C, TC, iFABP, and Lp(a)) were significantly correlated with the HAMA score (Figure 4B). All four identified potential biomarkers for PSD were significantly correlated with the HAMD and HAMA scores.
Figure 4.
Molecules significantly correlated with anxiety or depressive symptoms. A) ApoA1, HDL-C, TC, Lp(a), iFABP, LDL-C, and ApoB were significantly correlated with the HAMD score; and B) ApoA1, HDL-C, TC, iFABP, and Lp(a) were significantly correlated with the HAMA score. Red and green lines represented positive and negative correlations, respectively. Yellow and cyan circles represented lower and higher levels, respectively, in PSD patients compared to Stroke patients.
3.6. Diagnostic performance assessment
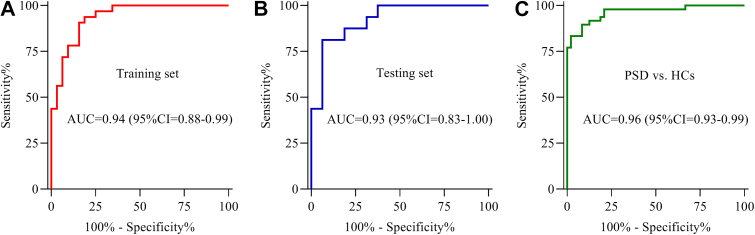
A discriminative model was identified using binary logistic regression analysis: P(Y = 1) = 1/(1 + EXP (-0.001675∗iFABP -0.003169∗Lp(a) +10.070161∗ApoA1+1.219721∗HDL-C -3.600708)). Using this model, we calculated the probability of illness in each patient. Then, the probability of illness in each patient was used to build the ROC curve. The results indicated that this discriminatory model effectively distinguished PSD patients from stroke patients with an area under the ROC curve (AUC) of 0.94 in the training set (Figure 5A). This model also effectively identified PSD patients in the testing set (AUC = 0.93) (Figure 5B) and displayed excellent discriminative power in separating PSD patients from HCs (AUC = 0.96) (Figure 5C). These results suggested that this model might be an excellent method to classify PSD and stroke patients.
Figure 5.
Diagnostic performances of potential biomarkers in diagnosing PSD from Stroke. The panel consisting of iFABP, Lp(a), ApoA1 and HDL-C had excellent diagnostic performances in both A) training and B) testing sets, and C) it was also very effective in separating PSD patients from HCs.
4. Discussion
In clinical assessments, PSD is considered a specific type of depression that occurs after a stroke. One consequence of stroke are changes in the gastrointestinal structure and function, including a leaky gut (Arya and Hu, 2018). IFABP is a protein primarily expressed in the small intestine and helps to maintain intestinal integrity (Furuhashi and Hotamisligil, 2008). When the intestinal barrier is impaired, iFABP is released into the circulation. Camara Lemarroy et al. reported that patients with acute ischemic stroke had significantly higher serum iFABP concentrations compared to controls (Camara-Lemarroy, 2021). At the beginning of this study, we measured the level of iFABP, and it was significantly elevated in all stroke patients (with and without PSD) compared to controls (data not shown). Interestingly, we also found that the PSD patients had significantly higher plasma iFABP levels compared to HCs and stroke patients without depression. Thus, our study indicated that a leaky gut was potentially involved in PSD, and the level of iFABP in plasma was positively correlated with the HAMD scores.
In our study, the “gut-brain” axis is the most likely mechanism to explain how increased iFABP and the related IP are linked to depression after stroke. First, altered gut microbiota in PSD (Ling, 2020; Kang, 2021) together with metabolite alterations could get involved under increased IP condition. Mice that are null for iFABP have increased intestinal permeability, and iFABP ablation results in changes in gut motility and morphology (Lackey, 2021). Moreover, intestinal motility and structure alterations could influence the composition and function of gut microbiota. The microbiota abnormalities could influence emotional behavior by regulating the host metabolism. Several studies have reported that different forms of metabolism, such as amino acid, lipid, and energy metabolism, were significantly disturbed in mice that exhibited microbiota-mediated depressive-like behaviors (Zheng, 2016; Tian, 2021; Liu, 2021). Metabolic disorders associated with plasma and urine of PSD patients have been reported (Hu, 2019; Xie, 2020; Chen, 2020), and fecal metabolism was disturbed in PSD rat models as well (Jiang, 2021) For example, reduced fecal concentrations of short chain fatty acids (SCFAs) have been observed in stroke and PSD animal models (Jiang, 2021). Studies have reported that bacteria-derived SCFAs, such as acetate, butyrate, and propionate may help alleviate depressive symptoms in PSD (Dalile, 2019).
Second, increased immune system activation has been associated with depression (Bai, 2022). The homeostasis of the intestinal barrier immune defenses is critical in preventing the host from undergoing mood and cognitive changes due to bacterial influences (Leclercq, 2012; Maes, 2012). In ischemic stroke rat models, increased T lymphocytes were observed in Peyer's patches, and the IgA and IgM-mediated intestinal immunity was activated (Liu, 2017). Inflammatory cytokines are released into the circulation in large amounts after stroke resulting in “off-site” effects causing increased IP (Ferrara, 2020). As a result, increased inflammatory cytokines such as IL-1 and TNF-ɑ and lipopolysaccharide (LPS) could evoke inflammatory processes in the body, including neuroinflammation in PSD (Mass, 2008; Wijeratne, 2021). LBP is a response to elevated LPS and interacts with CD14 at the cell surface. In our study, we analyzed the plasma levels of LBP and sCD14, and they did not differ significantly among the groups. sCD14 is considered a non-specific monocyte activation marker and does not necessarily indicate increased gut permeability, which could explain why this marker did not show similar changes.
Recent metabolomics studies have reported alterations in fatty acids in PSD patients (Hu, 2019; Xie, 2020; Chen, 2020). We used traditional laboratory indicators to assess the relationship between lipid metabolism and PSD. Lp(a), ApoB, TC, Apo A1, HDL-C, and LDL-C levels were significantly changed in PSD patients. On the one hand, iFABP has long been thought to be involved in the uptake and transport of dietary fatty acids (FAs) in the small intestine (Gajda and Storch, 2015). IFABP can bind more FAs after apical absorption and transport them for TG synthesis and chylomicron formation. Vassileva et al. (Vassileva, 2000) observed that plasma triacylglycerol concentrations in male iFabp−/− mice were 1.3–1.5 fold higher than controls. However, the lipoprotein particles and glucose in the plasma of iFabp−/−mice were not altered. Notably, the iFabp−/− mice had down-regulated the FA levels in the blood, which may explain why the FFA was significantly higher in stroke patients without depression than PSD patients. On the other hand, a leaky gut could affect lipid absorption and delivery across the intestinal barrier. Mice with an impaired intestinal barrier exhibited altered levels of total cholesterol, HDL, and LDL (Wang, 2019). Thus, increased IP is one possible cause of the lipid metabolic disorder observed in PSD. However, additional evidence is needed for validation.
Finally, our findings indicated the presence of leaky gut in PSD patients. In the clinic, several methods might be available for the evaluation of leaky gut. For example, the measurement of urinary lactulose mannitol excretion ratio (LMR) after oral 13C-mannitol or other saccharides (Khoshbin, 2021; Nicholas, 2022) would be helpful to detect for possible leaky gut. Kinds of evidence have provided some gut-directed therapies to improve intestinal barrier impairment. Enteral glutamine could normalize the urinary LMR, although the biological significance of these modifications has not been elucidated (Xi, 2004; Zhou, 2019). Glutamine metabolism is disturbed in subjects with post-stroke cognitive impairment (Liu, 2015); meanwhile, glutamine could protect the brain from ischemic injury (Luo, 2019). Besides, the Western diet with high-fat may increase gut permeability (Leech, 2019). As a result, a high-fiber diet or oral glutamine as a supplement is recommended for stroke patients to avoid gut barrier dysfunction (Agapova, 2018; Krawczyk, 2018). More clinical trials are needed to validate the effects of these methods in PSD in the future.
Several limitations are associated with this study. First, we did not evaluate the plasma iFABP response to antidepressant medications taken by PSD patients. The antidepressant medications could directly affect gut microbiota and the metabolomics profiles. However, whether these medications could affect the intestinal barrier and improve the gut leaky is not clear. Hence, longitudinal studies are needed to provide additional validation of this possibility. We only analyzed plasma iFABP levels as a biological indicator of an increased leaky gut in PSD patients. Additional testing methods using orally administered probe molecules such as sugars (Khoshbin, 2021) could demonstrate the presence of an increased leaky gut in PSD patients. Finally, although we identified several lipid indicators in PSD patients that could be measured clinically, the effects of iFABP on lipid metabolism require additional analysis to find more specific iFABP-related metabolites associated with PSD.
5. Conclusion
In summary, our results suggested that ApoA1, HDL-C, iFABP, and Lp(a) might be predictive blood biomarkers for PSD. We demonstrated that intestinal permeability is increased after stroke and is specifically associated with PSD. Thus, increased intestinal permeability might participate in PSD and affect the lipid metabolism in PSD patients.
Declarations
Author contributions
Chanjuan Zhou: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Libo Zhao: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Jianjun Chen: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jiaju Zhong: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Maolin Cao: Performed the experiments; Analyzed and interpreted the data.
Dan Chen; Liang Fang; Juan Liao; Xiaoli Zhang; Jiaxun Guo; Zhenyu Wang: Performed the experiments.
Funding statement
Dr Chanjuan Zhou was supported by National Natural Science Foundation of China [81701361], Science Foundation funded project of Yongchuan Hospital of Chongqing medical University [YJRC202102].
Libo Zhao was supported by Science Foundation funded project, Yongchuan Hospital of Chongqing Medical University [YJSJ202135].
Jiaju Zhong was supported by Yongchuan Science and Technology Program [2020nb0227].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank all the individuals who participated in the study. We also thank the Edit Springs for providing language help.
Contributor Information
Libo Zhao, Email: zhaolibo_1971@aliyun.com.
Chanjuan Zhou, Email: chanjuan.zhou1987@gmail.com.
References
- Agapova, et al. Additional common bean in the diet of Malawian children does not affect linear growth, but reduces intestinal permeability. J. Nutr. 2018;148(2):267–274. doi: 10.1093/jn/nxx013. [DOI] [PubMed] [Google Scholar]
- Arya, Hu Brain–gut axis after stroke. Brain Circ. 2018;4(4):165. doi: 10.4103/bc.bc_32_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bădescu, et al. The association between diabetes mellitus and depression. J. Med. Life. 2016;9(2):120–125. [PMC free article] [PubMed] [Google Scholar]
- Bai, et al. Gut microbiota-related inflammation factors as a potential biomarker for diagnosing major depressive disorder. Front Cell Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.831186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli, et al. Early post-stroke depression and mortality: meta-analysis and meta-regression. Front. Psychiatr. 2018;530 doi: 10.3389/fpsyt.2018.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara-Lemarroy, et al. D-Lactate and intestinal fatty acid-binding protein are elevated in serum in patients with acute ischemic stroke. Acta Neurol. Belg. 2021;121(1):87–93. doi: 10.1007/s13760-018-0940-x. [DOI] [PubMed] [Google Scholar]
- Carney, Freedland Depression and coronary heart disease. Nat. Rev. Cardiol. 2017;14(3):145–155. doi: 10.1038/nrcardio.2016.181. [DOI] [PubMed] [Google Scholar]
- Chen, et al. Urinary metabolite signatures for predicting elderly stroke survivors with depression. Neuropsychiatric Dis. Treat. 2021;17:925. doi: 10.2147/NDT.S299835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalile, et al. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- Ferrara, et al. Hypothesis and theory: a pathophysiological concept of stroke-induced acute phase response and increased intestinal permeability leading to secondary brain damage. Front. Neurosci. 2020;14:272. doi: 10.3389/fnins.2020.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funaoka ., Kanda ., Fujii Intestinal fatty acid-binding protein (I-FABP) as a new biomarker for intestinal diseases. Rinsho Byori. 2010;58(2):162–168. [PubMed] [Google Scholar]
- Furuhashi, Hotamisligil Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008;7(6):489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajda, Storch Enterocyte fatty acid-binding proteins (FABPs): different functions of liver and intestinal FABPs in the intestine. Prostaglandins Leukot. Essent. Fatty Acids. 2015;93:9–16. doi: 10.1016/j.plefa.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, et al. Objective diagnosis of post-stroke depression using NMR-based plasma metabonomics. Neuropsychiatric Dis. Treat. 2019;15:867. doi: 10.2147/NDT.S192307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Xia Influence of the gut microbiome on inflammatory and immune response after stroke. Neurol. Sci. 2021:1–15. doi: 10.1007/s10072-021-05603-6. [DOI] [PubMed] [Google Scholar]
- Jiang, et al. Alteration of gut microbiome and correlated lipid metabolism in post-stroke depression. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.663967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, et al. Correlation between intestinal flora and serum inflammatory factors in post-stroke depression in ischemic stroke. J. Coll. Phys. Surg. Pak. 2021;31(10):1224–1227. doi: 10.29271/jcpsp.2021.10.1224. [DOI] [PubMed] [Google Scholar]
- Kerr, et al. Inflammasome-regulated pyroptotic cell death in disruption of the gut-brain Axis After stroke. Transl. Stroke Res. 2022:1–15. doi: 10.1007/s12975-022-01005-8. [DOI] [PubMed] [Google Scholar]
- Khoshbin, et al. Development and validation of test for “leaky gut” small intestinal and colonic permeability using sugars in healthy adults. Gastroenterology. 2021;161(2):463–475. doi: 10.1053/j.gastro.2021.04.020. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk, et al. Vol. 10. 2018. Gut permeability might be improved by dietary fiber in individuals with nonalcoholic fatty liver disease (NAFLD) undergoing weight reduction; p. 1793. (11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey, et al. Mechanisms underlying reduced weight gain in intestinal fatty acid-binding protein (IFABP) null mice. Am. J Physiol. Gastrointest. Liver Physiol. 2020;318(3):G518–G530. doi: 10.1152/ajpgi.00120.2019. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq, et al. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav. Immun. 2012;26(6):911–918. doi: 10.1016/j.bbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Leech, et al. Risk factors associated with intestinal permeability in an adult population: a systematic review. Int. J. Clin. Pract. 2019;73(10) doi: 10.1111/ijcp.13385. [DOI] [PubMed] [Google Scholar]
- Li, et al. Prevalence of depression in patients with hypertension: a systematic review and meta-analysis. Medicine. 2015;94(31):e1317. doi: 10.1097/MD.0000000000001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, et al. Structural change of gut microbiota in patients with post-stroke comorbid cognitive impairment and depression and its correlation with clinical features. J. Alzheimers Dis. 2020;77(4):1595–1608. doi: 10.3233/JAD-200315. [DOI] [PubMed] [Google Scholar]
- Liu, et al. Potential of serum metabolites for diagnosing post-stroke cognitive impairment. Mol. Biosyst. 2015;11(12):3287–3296. doi: 10.1039/c5mb00470e. [DOI] [PubMed] [Google Scholar]
- Liu, et al. Ischemic stroke damages the intestinal mucosa and induces alteration of the intestinal lymphocytes and CCL19 mRNA in rats. Neurosci. Lett. 2017;658:165–170. doi: 10.1016/j.neulet.2017.08.061. [DOI] [PubMed] [Google Scholar]
- Liu, et al. Comprehensive analysis of the lysine acetylome and succinylome in the hippocampus of gut microbiota-dysbiosis mice. J. Adv. Res. 2021;30:27–38. doi: 10.1016/j.jare.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, et al. L-glutamine protects mouse brain from ischemic injury via up-regulating heat shock protein 70. CNS Neurosci. Ther. 2019;25(9):1030–1041. doi: 10.1111/cns.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes, et al. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. Int. J. Stroke. 2012;141(1):55–62. doi: 10.1016/j.jad.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Mass, Kubera ., Leunis The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuroendocrinol. Lett. 2008;29(1):117–124. [PubMed] [Google Scholar]
- Mitchell, et al. Prevalence and predictors of post-stroke mood disorders: a meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen. Hosp. Psychiatr. 2017;47:48–60. doi: 10.1016/j.genhosppsych.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Nicholas, et al. Detection of 13C-mannitol and other saccharides using tandem mass spectrometry for evaluation of intestinal permeability or leaky gut. Methods Mol. Biol. 2022;2546:285–294. doi: 10.1007/978-1-0716-2565-1_26. [DOI] [PubMed] [Google Scholar]
- Nikolac, et al. Croatian Society of Medical Biochemistry and Laboratory Medicine: national recommendations for venous blood sampling. Biochem. Med. 2013;23(3):242–254. doi: 10.11613/BM.2013.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson, et al. Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr. Scand. 2019;139(2):185–193. doi: 10.1111/acps.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, et al. Longxuetongluo Capsule protects against cerebral ischemia/reperfusion injury through endoplasmic reticulum stress and MAPK-mediated mechanisms. J. Adv. Res. 2021;33:215–225. doi: 10.1016/j.jare.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R.G., Jorge R.E. Post-stroke depression: a review. Am. J. Psychiatr. 2016;173(3):221–231. doi: 10.1176/appi.ajp.2015.15030363. [DOI] [PubMed] [Google Scholar]
- Sarikaya, et al. Intestinal fatty acid binding protein (I-FABP) as a promising test for Crohn’s disease: a preliminary study. Clin. Lab. 2015;61(1-2):87–91. doi: 10.7754/clin.lab.2014.140518. [DOI] [PubMed] [Google Scholar]
- Stanley, et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 2016;22(11):1277–1284. doi: 10.1038/nm.4194. [DOI] [PubMed] [Google Scholar]
- Stevens, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018;67(8):1555–1557. doi: 10.1136/gutjnl-2017-314759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, et al. Multi-omics data reveals the disturbance of glycerophospholipid metabolism caused by disordered gut microbiota in depressed mice. J. Adv. Res. 2021 doi: 10.1016/j.jare.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva, et al. The intestinal fatty acid binding protein is not essential for dietary fat absorption in mice. Faseb. J. 2000;14(13):2040–2046. doi: 10.1096/fj.99-0959com. [DOI] [PubMed] [Google Scholar]
- Wang, et al. Lactobacillus brevis DM9218 ameliorates fructose-induced hyperuricemia through inosine degradation and manipulation of intestinal dysbiosis. Nutrition. 2019;62:63–73. doi: 10.1016/j.nut.2018.11.018. 2019. [DOI] [PubMed] [Google Scholar]
- Wei, et al. Intestinal barrier dysfunction participates in the pathophysiology of ischemic stroke. CNS Neurol. Disord.: Drug Targets. 2021;20(5):401–416. doi: 10.2174/1871527320666210322115808. [DOI] [PubMed] [Google Scholar]
- Weng, et al. Intestinal fatty acid-binding protein is a biomarker for diagnosis of biliary tract infection. JGH Open. 2021;5(10):1160–1165. doi: 10.1002/jgh3.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiercinska-Drapalo, et al. Intestinal fatty acid binding protein (I-FABP) as a possible biomarker of ileitis in patients with ulcerative colitis. Regul. Pept. 2008;147(1-3):25–28. doi: 10.1016/j.regpep.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Wijeratne ., Sales C. Understanding why post-stroke depression may be the norm rather than the exception: the anatomical and neuroinflammatory correlates of post-stroke depression. J. Clin. Med. 2021;10(8):1674. doi: 10.3390/jcm10081674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi, et al. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns. 2004;30(2):135–139. doi: 10.1016/j.burns.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Xie, et al. Identification of potential metabolite markers for middle-aged patients with post-stroke depression using urine metabolomics. Neuropsychiatric Dis. Treat. 2020;16 doi: 10.2147/NDT.S271990. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, et al. Exploratory investigation of intestinal structure and function after stroke in mice. Mediat. Inflamm. 2021 doi: 10.1155/2021/1315797. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatr. 2016;21(6):786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- Zheng, et al. Elevated levels of circulating biomarkers related to leaky gut syndrome and bacterial translocation are associated with Graves’ disease. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.796212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, et al. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut. 2019;68(6):996–1002. doi: 10.1136/gutjnl-2017-315136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.