Abstract
Introduction
Gastric cancer (GC) is one of the most common malignant tumors worldwide. Single-cell sequencing technology can achieve an accurate and unbiased assessment of cell heterogeneity. Therefore, it is necessary to explore the molecular characteristics of GC-related malignant cells at a single-cell resolution.
Methods
Single-cell RNA sequencing GC profiles were collected from the Gene Expression Omnibus database. Moreover, feature gene sets of metabolic pathways and hypoxia signals were collected from the Molecular Signatures Database. The marker genes of specific cell types were collected from the published literature and CellMarker database. The R package InferCNV was used to calculate the copy-number variations of cells and to identify real cancer cells. The weighted relative pathway activity algorithm was used to evaluate the differences in metabolic activity between cell types.
Results
Our study found that cancer epithelial cells exhibited individual differences in molecular features and showed metabolic heterogeneity. Oxidative phosphorylation and glycolytic pathway activity were the major contributors to the metabolic heterogeneity of cancer epithelial cells. Furthermore, we used the hypoxia signaling pathway to indirectly evaluate the oxygen content of cells and found that hypoxia contributed to the heterogeneity of cancer epithelial cells. Finally, functional identification of genes co-expressed with HIF1A showed that the reprogramming of the oxidative stress response contributed to the tumor malignant progression.
Conclusions
This study described hypoxia-induced metabolic reprogramming of GC at a single-cell level, partially addressing the lack of insight into the heterogeneity of cancer cell metabolism when using traditional sequencing technology.
Keywords: Single-cell RNA-Seq, Gastric cancer, Epithelial cell, Metabolic reprogramming, Hypoxia
Single-cell RNA-Seq; Gastric cancer; Epithelial cell; Metabolic reprogramming; Hypoxia.
1. Introduction
As one of the most prevalent cancers, gastric cancer (GC) is the fifth most common malignancy worldwide, with over 1 million new cases diagnosed annually [1]. As most patients are diagnosed at an advanced stage, GC has a very high mortality rate, ranking fourth in mortality. According to the latest statistics, 769,000 people died of GC in 2020 worldwide. The incidence and mortality rate showed downward trends from previous years; however, the positivity rates and prognosis are not yet satisfactory [2, 3]. Existing studies have indicated that GC frequently shows a high degree of heterogeneity in histology, transcriptomics, and genomics [4]. This heterogeneity causes differences in the tumor growth rate, invasion ability, and drug sensitivity, as well as in patient prognosis.
Given that cellular heterogeneity is a common feature of biological systems, including tumors, conventional RNA sequencing (RNA-seq) analysis, which is limited to the assessment of cells in bulk tissues, cannot satisfy all the requirements of research. Hence, analyses of cellular heterogeneity at a single-cell level are particularly important [5]. Single-cell sequencing techniques can help overcome the limitations of traditional techniques and allow an unbiased assessment of cellular heterogeneity, the identification of new cellular states and populations, and the elucidation of dynamic cellular transitions during development and differentiation at an unprecedented resolution and accuracy [6]. Therefore, it is necessary to study the molecular characteristics of malignant cells associated with GC at a single-cell resolution to identify new therapeutic targets for GC.
Although single-cell RNA-seq (scRNA-seq) has been used to clarify the intratumoral heterogeneity of GC [7, 8], this study aimed to explain this heterogeneity from a different perspective. It is well known that metabolic reprogramming can help tumors, including GC, with energy supply, redox maintenance, and cellular communication [9, 10]. In addition, owing to an inadequate supply of blood vessels and blood to tumor tissues, cancerous cells are often starved of oxygen, nutrients, and energy, which promotes their proliferation and malignant transformation, allowing tumor cells to overcome or escape antitumor immune surveillance and bypass cellular senescence and apoptosis [9, 11, 12, 13]. Meanwhile, scRNA-seq could provide insights into metabolic reprogramming of GC at a single-cell level.
In this study, we analyzed the data obtained by scRNA-seq of GC tissues to reveal individual differences among tumor cells and identify the metabolic characteristics of malignant epithelial cells. We also revealed specific coupling relationships between hypoxia, glycolysis, and oxidative phosphorylation (OXPHOS) at a single-cell resolution.
2. Materials and methods
2.1. Data collection
The scRNA-seq profiles of GC cells (accession number GSE112302) were collected from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/gds) [14, 15]. The data represented 10 samples (6 tumor and 4 normal samples). The bulk RNA-seq data and clinical information of patients with stomach adenocarcinoma (STAD) from The Cancer Genome Atlas (TCGA; https://portal.gdc.cancer.gov/) [16] were downloaded from the UCSC Xena database (https://xenabrowser.net/datapages/). The gene expression profiles were derived from 407 samples, including 375 tumor samples and 32 paracancer samples. The feature gene sets used in this work, including metabolic pathways and hypoxia signaling, were collected from the Molecular Signatures Database (http://www.broadinstitute.org/msigdb) [17].
2.2. Data preprocessing and cell clustering
The scRNA-seq profiles were preprocessed, including quality control and normalization. The gene expression profiles and annotation information of single cells were compiled using the R package Seurat [18] for quality control and cell clustering. Cells that expressed fewer than 200 genes were excluded. Genes with detectable expression in more than three cells were retained. Data normalization of the single-cell profiles and the screening of variant genes after quality control were performed using SCTransform in the Seurat package. To avoid the interference of possible batch effects on downstream analysis, Harmony [19] was used to correct the principal component (PC) analysis (PCA) data. Furthermore, the top 3,000 variant genes were analyzed using the standard deviation in PCA. We selected the top 20 PCs that could explain most of the data variability with respect to a cell cluster.
2.3. Cell-type annotation
For each cell cluster, the cell type was defined according to its marker gene. The marker genes of specific cell types in stomach and tumor tissues were collected from the published literature [15] and CellMarker (http://biocc.hrbmu.edu.cn/CellMarker/) database [20].
2.4. Estimation of copy-number variations (CNVs) in cancer cells
The R package InferCNV tool was used to calculate the CNVs of cells derived from tumor tissues and identify true cancer cells [21]. We used the default parameters, and two clusters that mainly contained normal tissue cells were used as the control group.
2.5. Calculation of metabolic pathway activity
The weighted relative pathway activity algorithm [22, 23] was used to evaluate differences in metabolic activities among cell types. First, the relative expression level of a metabolic gene in each cell cluster was defined as the ratio of the average expression value in cells of a specific cluster to the average expression value in all cells. Next, the weighted average of the relative gene expression of a pathway in a specific cell type was considered as an activity score of the pathway. Moreover, the weighting factor of each gene was defined as the reciprocal of the number of pathways that included the gene. To avoid the impact of sequencing quality on pathway activity scores, genes with low expression levels or high deletion rates in pathways were excluded. Additionally, the cell labels were randomly swapped 2,000 times to construct a null distribution model of activity scores. Using this model, we tested the statistical significance of the pathway activity score in each cell type. Thus, a p-value for each pathway score was obtained to assess whether the activity of the pathway was significantly higher or lower than the average.
2.6. Functional enrichment analysis
Gene set enrichment analysis (GSEA) [24] in the R package was used to perform functional analyses of specifically expressed genes in cell clusters. First, the PCs of the expression profile for each cell type were calculated; the importance of each metabolism-related gene was defined as the sum of the absolute values of the gene load in the top PC, which explained at least 80% of the overall variation. Further, genes were sorted in descending order according to their importance.
2.7. Statistical analysis
All statistical analyses and graph generation were carried out in R (version 4.0.2). P < 0.05 was deemed significant
3. Results
3.1. Individual differences among patients at a single-cell resolution
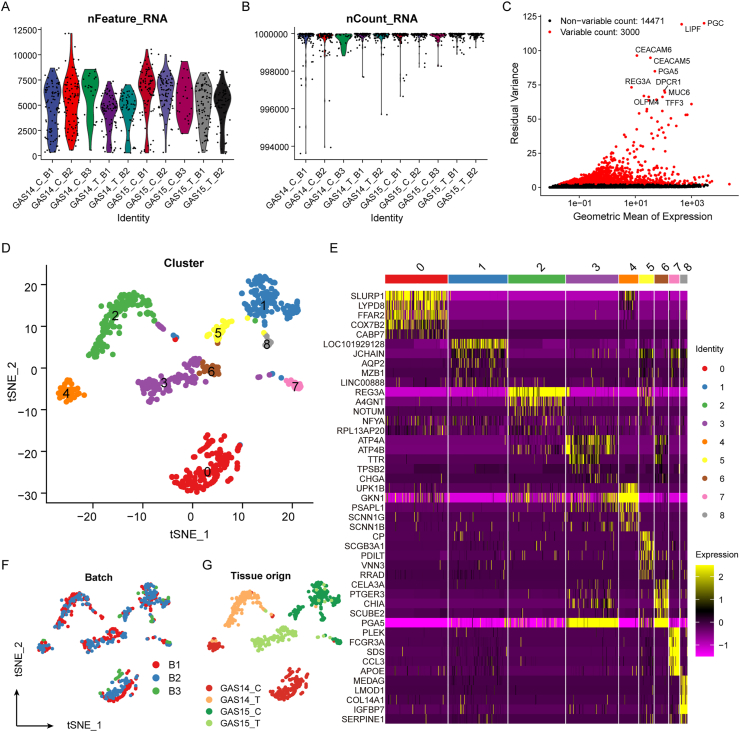
In this study, 707 cells from six GC samples and four normal samples were obtained from the GEO database (Figure 1). We filtered out 16 low-quality cells using quality control and retained 691 cells for further analysis (Figure 2A and B). Genes with undesirable features were filtered out, and 17,471 genes were retained for analysis, of which 14,471 had low and 3,000 had high cell-to-cell variation (Figure 2C). Further, PCA was used for preliminary dimensionality reduction of the scRNA-seq profiles. We selected the top 20 PCs that could explain most of the variation information for further cluster analysis. Using the t-distributed stochastic neighbor embedding (t-SNE) algorithm, these cells were grouped into nine clusters (Figure 2D). Next, differential expression analysis was used to identify specifically expressed genes between clusters. We identified a total of 6,657 genes that were highly expressed between clusters, and the expression levels of the top five upregulated genes in each cluster are displayed in a heatmap (Figure 2E). We found that the expression of PGA5 was significantly higher in clusters 3 and 6 than in the other clusters (Figure 2E); PGA5 encodes pepsin precursor and may be involved in the oncogenic signaling pathway of gastric adenocarcinoma [25]. The clustering results of the cells were combined with the experimental and clinical information of the patients. We found that there was no significant segregation of cells from different batches (Figure 2F), indicating the reliability of the clustering results. Tumor cells of different tissue origins were clearly separated (Figure 2G), indicating the heterogeneity of tumors and individual differences in patients.
Figure 1.
Schematic diagram of the scRNA-seq data analysis pipeline.
Figure 2.
Individual differences among patients at a single-cell resolution (A) Quality control results of single-cell data. The violin chart shows the number of genes detected per cell. The abscissa represents the sample, and the ordinate represents the number of genes (B) Same as in (A) but for the count detected per cell (C) Volcano map of the variation in the expression of 17,471 genes, of which 14,471 showed a low intercellular variation and 3,000 showed a high variation (D) Clustering results of single cells using the t-SNE algorithm. Each cell is marked with a cluster label (E) Heatmap of the top five genes specifically expressed in each cluster (F, G) Same as in (D) but for cells marked with annotation information, including the batch of the experiment and the tissue origin.
3.2. Cell-type identification and CNV patterns of malignant cells
To explore the molecular mechanisms of different cell types in tumors, it is necessary to identify each cell type in cell clusters. According to the classical markers described in previous studies, genes specifically expressed in each cluster were used to annotate cell types. We identified roughly three cell types, namely, epithelial cells, fibroblasts, and macrophages (Figure 3A). The majority of the cells in clusters 0–6 expressed epithelial cell marker genes (EPCAM, KRT18, and KRT8; Figure 3B). Most of the cells in cluster 8 highly expressed ACTA2 and COL1A2 (Figure 3C), which are fibroblast marker genes. Most of the cells in cluster 7 highly expressed CD14 (Figure 3D), which is a marker gene for macrophages. According to the cell-type annotation results, epithelial cells were the main cell type identified. Therefore, further research mainly focused on the reprogramming of the physiological mechanism of epithelial cells in the tumor. The expression levels of immune cell marker genes (CD3D for T cells and CD79A for B cells) in these cells also supported the results (Figure 3E and F). EPCAM+ epithelial cells were grouped into seven clusters, among which the cells in clusters 0, 1, 4, and 5 originated from tumor tissues, whereas those in clusters 2, 3, and 6 were derived from normal tissues. Although the batch effect was eliminated, cancer cells still showed a high heterogeneity, probably caused by CNVs [26]; this assumption was confirmed by the application of InferCNV (Figure 3G). Compared with epithelial cells in normal tissues, cancer epithelial cells showed a higher copy-number amplitude and more abundant CNVs, which usually had chromosomal localization (Figure 3G). Taken together, these results suggested the reliability of the cell-type annotation and also that the CNVs probably acted as the key factor in the heterogeneity of cancer epithelial cells.
Figure 3.
Cell-type identification and CNV patterns of malignant cells (A) Each cell is marked with a cell-type label (B–D) Expression levels of marker genes of epithelial cells, fibroblasts, and macrophages in each cell cluster (E) t-SNE map of expression levels of T-cell marker genes in each cell (F) Same as in (E) but for B-cell marker genes (G) CNV of each cell, as evaluated by InferCNV. Three epithelial clusters derived from normal tissues were used as controls.
3.3. Detecting metabolic reprogramming of tumor cells at a single-cell resolution
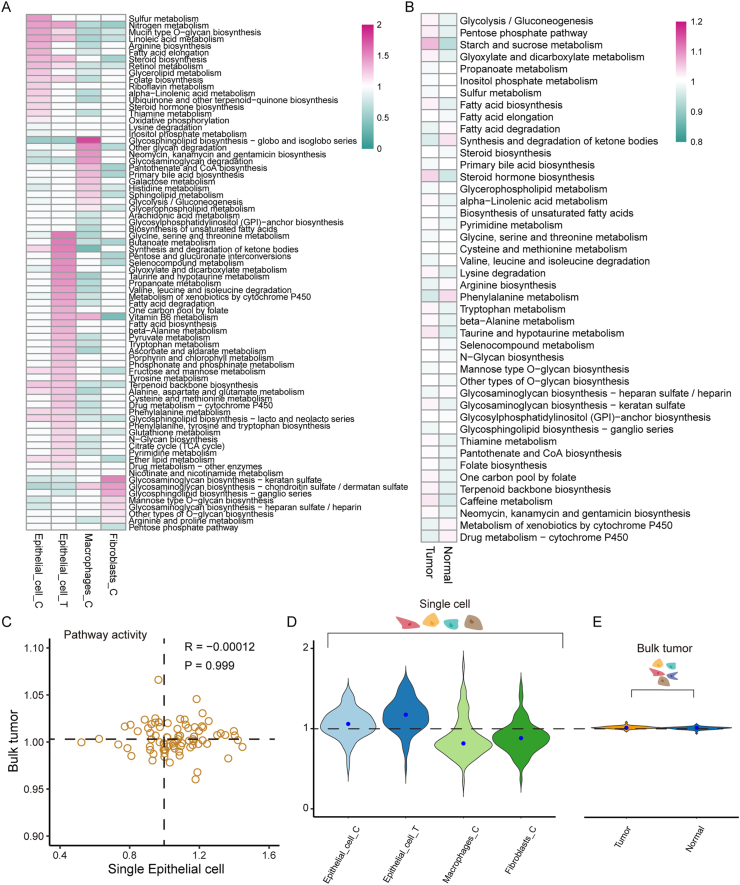
In cancer epithelial cells, the metabolic processes change to meet cell proliferation and metastasis requirements [27]. To explore the metabolic reprogramming mechanism of malignant epithelial cells, the weighted relative pathway activity algorithm was used to analyze the metabolic pathway activity in each cell type. Among the 85 metabolic pathways analyzed, 69 pathways that contained at least five genes had significantly higher activity scores (a pathway activity score >1 and a permutation test p-value <0.05) in at least one cell type than those in the other cell types (Figure 4A). Interestingly, we found that epithelial cells in adjacent tissues had the largest number of upregulated metabolic pathways. A total of 41 upregulated pathways were detected in normal epithelial cells versus 23 in cancer epithelial cells, 13 in tumor-associated macrophages (TAMs), and 7 in cancer-associated fibroblasts (CAFs).
Figure 4.
Detection of metabolic reprogramming of tumor cells at a single-cell resolution (A) Metabolic pathway activities in each cell type for scRNA-seq profiles. Statistically nonsignificant values (random permutation test p > 0.05) are shown as blanks (B) Metabolic pathway activities in STAD tumor samples and matched normal samples from TCGA (C) Scatter plot of pathway activities in bulk STAD tumors from TCGA and in single epithelial cells (D) Distributions of pathway activities in different cell types from the scRNA-seq dataset. Epithelial_cell_C represents cancer epithelial cells; epithelial_cell_T represents normal epithelial cells; fibroblast_C represents CAFs, and macrophage_C represents TAMs (E) Distributions of pathway activities in STAD tumor samples and matched normal samples from TCGA.
Compared with those in normal tissues, the OXPHOS and glycolytic pathway activities were significantly increased in cancer epithelial cells, which could enhance their proliferation. To verify the reliability of the pathway activity scoring algorithm and the metabolic characteristics at a single-cell resolution, this algorithm was further used for the bulk RNA-seq profiles of STAD. In total, 42 metabolic pathways with significantly increased activity scores in tumor or normal tissue were identified (Figure 4B). We further found that tumor samples had 28 upregulated metabolic pathways, while normal samples had 16 upregulated metabolic pathways. Compared with that at a single-cell resolution, the glycolytic pathway, but not OXPHOS, was activated in bulk tumor tissue. Moreover, the correlation between metabolic pathway activities in bulk tumor samples and cancer epithelial cells was not significant (Pearson's r = −1.2e−4, p-value = 0.999; Figure 4C). Comparison of the distribution of metabolic pathway activity scores for the two sequencing technologies showed a greater variation in the distribution of pathway activities in single cells than in bulk tumors (Figure 4D and E). Overall, these results suggest that several metabolic characteristics of the tumor could be revealed only at a single-cell resolution.
3.4. Hypoxia contributes to the heterogeneity of cancer epithelial cells
To identify major contributors to the metabolic heterogeneity of cancer epithelial cells, PCA and GSEA were used to identify metabolic pathways that were significantly enriched in high-weight genes (Figure 5A and B). We found that the OXPHOS and glycolysis/gluconeogenesis pathways had the highest enrichment scores in cancer epithelial cells (Figure 5B), suggesting that the energy supply mechanisms based on OXPHOS and glycolysis were the main contributors to the metabolic heterogeneity of tumors. In this study, we used the activity of the hypoxia signaling pathway to indirectly evaluate the oxygen content of cells. The average expression levels of genes involved in OXPHOS and glycolysis were used to represent energy metabolism. Surprisingly, we found that the activity of OXPHOS significantly correlated with that of glycolysis (Pearson's r = 0.62) but not with hypoxia (Pearson's r = 0.03; Figure 5C); the correlation between glycolysis and hypoxia was relatively higher (Pearson's r = 0.13), consistent with previous studies suggesting that the glycolytic mechanism is activated under hypoxic conditions [28, 29]. Notably, the coupling relationships between OXPHOS, glycolysis, and hypoxia that were detected at a single-cell resolution were not found when using the bulk RNA-seq data (Figure 5D), indicating that the coupling relationship between energy supply and hypoxia could be detected only at a single-cell level. We found that the hypoxia-inducible factor (HIF1A), a responder to reduced oxygen availability in the cellular environment [30], was downregulated in cancer epithelial cells compared to that in normal epithelial cells (Figure 5E). Interestingly, HIF1A was upregulated in TAMs and CAFs compared to that in normal epithelial cells. In the bulk RNA-seq profiles of STAD, the expression level of HIF1A was significantly upregulated in tumor samples (Figure 5F). Together, these results indicate that cancer epithelial cells have a strong oxygen-scavenging capacity, while the hypoxia burden is borne by other functional cells in the tumor microenvironment.
Figure 5.
Contribution of hypoxia to the heterogeneity of cancer epithelial cells (A) PCA of metabolic gene expression levels in different cell types. Top PCs accounting for 80% of the variance are highlighted in red (B) Dot plot of metabolic pathways enriched in genes explaining the heterogeneity between cells (C) Scatter plot of pathway activities involved in glycolysis, OXPHOS, and a response to hypoxia, based on single epithelial cell data. The color indicates the local density of the dots (D) Same as in (C) but for bulk STAD data from TCGA (E) Box plot of the expression levels of HIF1A among different cell types at a single-cell resolution (F) Box plot of the expression levels of HIF1A in STAD tumor samples and the corresponding normal samples.
3.5. Analysis of the reprogramming of the hypoxic stress response
Next, we considered identifying the unique hypoxia regulation mechanism in cancer epithelial cells compared with that in normal epithelial cells. First, the Pearson correlation algorithm was used to identify genes co-expressed with HIF1A in cancer and normal epithelial cells. The genes with a p-value <0.01 and a correlation coefficient R > 0.3 were significantly positively correlated with HIF1A. A total of 588 genes in cancer epithelial cells and 250 genes in normal epithelial cells were found to be significantly related to HIF1A (Figure 6A). Only nine genes were co-expressed with HIF1A in cancer and normal epithelial cells, suggesting a dramatic change in the hypoxic stress response during malignant transformation of cells. Further, the genes that were co-expressed with HIF1A were separately analyzed for functional enrichment using Metascape [31]. We found that the genes co-expressed with HIF1A in cancer epithelial cells had significantly enriched regulatory function in the cytoskeleton, and four functional modules were identified using the MCODE [32] tool (Figure 6B and C). Moreover, the genes co-expressed with HIF1A in normal tissues were mainly responsible for cell metabolism and proliferation activities, and three functional modules were identified (Figure 6D and E). These results indicated that cancer epithelial cells might increase the rate of oxygen consumption to support their metastasis. Similar to the expression of HIF1A, that of NR3C1 was significantly upregulated in TAMs and CAFs and downregulated in cancer epithelial cells compared with that in normal epithelial cells (Figure 6F). In addition, the highly expressed NR3C1 was closely related to a poor patient prognosis (Figure 6G). Taken together, these results suggest that the reprogramming of the oxidative stress response contributes to the malignant progression of tumors.
Figure 6.
Analysis of the reprogramming of the hypoxic stress response (A) Venn diagram of the intersection of genes co-expressed with HIF1A in tumor and normal tissue cells (B) Bar plot of the top 20 functional enrichment results for genes co-expressed with HIF1A in tumor tissue cells (C) Functional modules in the PPI network of genes co-expressed with HIF1A in tumor tissue cells (D) Same as in (B) but for genes co-expressed with HIF1A in normal tissue cells (E) Same as in (C) but for genes co-expressed with HIF1A in normal tissue cells (F) Box plot of the expression levels of NR3C1 in different cell types at a single-cell resolution (G) Kaplan–Meier curves of the survival of patients from TCGA in NR3C1 high- and low-expression categories. A log-rank test was used to calculate statistical significance.
4. Discussion
In this study, we used a computational pipeline to reveal the biological information contained in scRNA-seq profiles of GC cells. Cluster analysis of 707 cells from six GC and four normal samples was used to determine the metabolic characteristics of malignant epithelial cells. In addition, to explore the mechanisms that underlie the metabolic reprogramming of malignant epithelial cells, we adopted the weighted relative pathway activity algorithm to measure the metabolic pathway activity of each cell type. The results showed that the OXPHOS and glycolytic pathway activities both correlated with hypoxia at a single-cell level.
Under normoxic conditions, ATP production during OXPHOS represents the most efficient way to obtain energy. However, under hypoxic conditions, glycolysis is the only process that provides cells with energy [33]. In the hypoxic microenvironment, cancer growth is maintained by metabolic and bioenergetic reprogramming, which is characterized by an adaptive switch from OXPHOS to glycolysis; this phenomenon is denoted as the Warburg effect [34]. Unexpectedly, the sequencing results at a single-cell resolution showed a strong positive correlation between OXPHOS activity and glycolysis; however, this result was not reproduced when using the bulk RNA-seq data. This finding appears to differ from the Warburg effect, as hypoxic conditions triggered the signal transduction pathway for glycolysis and inhibited pathways associated with OXPHOS and other mitochondrial activities for the maintenance of the redox steady state [35]. However, some studies have indicated that certain tumors exhibit two-compartment metabolism, i.e., a reverse Warburg effect that supports the OXPHOS activity in tumor cells. This refers to a metabolic switch to aerobic glycolysis in CAFs that are induced by cancer cells to surround tumors. Secreted energy metabolites, including lactic acid, ketone bodies, or pyruvic acid, can be used by epithelial cancer cells as sources of energy in the mitochondrial tricarboxylic acid cycle, thereby promoting ATP generation via elevated OXPHOS activity, which results in a higher proliferative capacity of tumors and their higher resistance to apoptosis [36, 37]. Therefore, during the progression of GC, two non-mutually exclusive metabolic pathways (OXPHOS and glycolysis) are used by cancer cells to produce energy, depending on different sources of oxygen supply in the tumor microenvironment.
Previous studies have demonstrated that hypoxic conditions can protect tumors against intrinsic antitumor immune responses by promoting an immunosuppressive microenvironment, which can lead to a poor prognosis in patients with GC [38]. In a hypoxic microenvironment, HIF-1 is the main factor mediating cellular oxygen sensing and adaptive responses to hypoxia, as well as a key transcription factor that promotes cancer cell survival and tumor progression in GC [39, 40, 41, 42]. Normally, the HIF1A expression level is significantly upregulated in GC samples, as found by bulk RNA-seq [39, 40]. However, in this study, compared with its expression level in normal epithelial cells, HIF1A was significantly downregulated in cancer epithelial cells and significantly upregulated in TAMs and CAFs at a single-cell resolution. In addition, in cancer and normal epithelial cells, only nine genes were co-expressed with HIF1A, indicating great changes in the responses to hypoxic stress during malignant cell transformation. However, this phenomenon could not be observed using bulk RNA-seq because signals from genes with variable levels of expression would be averaged across cells.
Moreover, we found that the expression of the NR3C1 gene closely followed that of the HIF1A gene, and the high expression of NR3C1 was strongly associated with a poor patient prognosis. The glucocorticoid receptor encoded by the NR3C1 gene can bind to glucocorticoid response elements in the promoters of glucocorticoid-responsive genes to activate their transcription [43]. Some studies have also shown that NR3C1 plays an important role in tumor metabolism and proliferation by interacting with specific response elements in a target gene [44]. Since the primary role of metabolic regulation by glucocorticoids is to respond to stress, the expression of NR3C1 closely follows that of HIF1A, which induces the hypoxic stress response. Nonetheless, the mechanisms underlying the interactions between hypoxia and the glucocorticoid receptor gene (and other related genes) are yet to be elucidated. Therefore, our findings can provide possible directions for further studies on these mechanisms.
As this is a preliminary exploratory study, there are several limitations to our analyses. First, limited numbers of samples and cells were analyzed, and we could not perform separate analyses on the subtypes of GC, which might have affected the accuracy and stability of the results. Consequently, these data must be regarded as hypothesis-generating or discovery data, and studies considering a larger cohort of patients with GC will need to be performed to understand the generalizability of the results of our analysis. Second, tissue separation may have led to the loss of spatial information, thus preventing the elucidation of cell functionality and pathological changes, for example, more accurate evaluation of regional metabolic differences in GC subtypes. To overcome this limitation, spatial RNA-seq can be used in the future, which combines the characteristics of bulk RNA-seq analysis and in situ hybridization, resulting in complete transcriptomic data with spatial information. Finally, scRNA-seq only recovers a few thousand unique transcripts from a single cell, which is much less than the number of transcripts in the entire transcriptome profile. Despite these limitations, using scRNA-seq to describe the metabolic reprogramming of GC is a promising direction for future research.
5. Conclusions
In summary, this study described the hypoxia-induced metabolic reprogramming of GC at a single-cell resolution. Although we analyzed GC only from the perspectives of metabolic pathways and hypoxic stress responses, this study compensates for the shortcomings of conventional RNA-seq analysis, partially overcomes the lack of insight into the heterogeneity of cancer cell metabolism upon use of the conventional approach, and provides a theoretical basis for the study of tumor proliferation, metastasis, and prognosis in patients with GC. Our data may also help identify potential therapeutic targets for the treatment of GC.
Declarations
Author contribution statement
Wenjia Kou; Nianjian Zhao: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Lingyu Zhao; Mengchun Zhang: Performed the experiments; Analyzed and interpreted the data.
Zhihao Yin; Lisha Zhang; Jiaxing Song; Yueying Wang: Performed the experiments.
Cong Qiao; Hongxuan Li: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Dr. Cong Qiao was supported by Innovation Foundation of Higher Education of Heilongjiang Province [900204].
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
www.editage.cnWe would like to thank the Innovation Foundation of Higher Education of Heilongjiang Province (Grant No.990204).
Contributor Information
Cong Qiao, Email: qiaocong@hrbmu.edu.cn.
Hongxuan Li, Email: lihongxuan@hrbmu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C., Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Kumar V., Ramnarayanan K., Sundar R., Padmanbahan N., Srivastava S., Koiwa M., Yasuda T., et al. Single-cell atlas of lineage states, tumor microenvironment and subtype-specific expression programs in gastric cancer. Cancer Discov. 2022;12(3):670–691. doi: 10.1158/2159-8290.CD-21-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heid C.A., Stevens J., Livak K.J., Williams P.M. Real time quantitative PCR. Genome Res. 1996;6(10):986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 6.Potter S.S. Single-cell RNA sequencing for the study of development, physiology and disease. Nat. Rev. Nephrol. 2018;14(8):479–492. doi: 10.1038/s41581-018-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sathe A., Grimes S.M., Lau B.T., Chen J., Suarez C., Huang R.J., Poultsides G., Ji H.P. Single-cell genomic characterization reveals the cellular reprogramming of the gastric tumor microenvironment. Clin. Cancer Res. 2020;26(11):2640–2653. doi: 10.1158/1078-0432.CCR-19-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M., Hu S., Min M., Ni Y., Lu Z., Sun X., Wu J., Liu B., Ying X., Liu Y. Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing. Gut. 2021;70(3):464–475. doi: 10.1136/gutjnl-2019-320368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBerardinis R.J., Chandel N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016;2(5) doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hur H., Paik M.J., Xuan Y., Nguyen D.T., Ham I.H., Yun J., Cho Y.K., Lee G., Han S.U. Quantitative measurement of organic acids in tissues from gastric cancer patients indicates increased glucose metabolism in gastric cancer. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0098581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith K.A., Waypa G.B., Schumacker P.T. Redox signaling during hypoxia in mammalian cells. Redox Biol. 2017;13:228–234. doi: 10.1016/j.redox.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ralph S.J., Rodriguez-Enriquez S., Neuzil J., Saavedra E., Moreno-Sanchez R. The causes of cancer revisited: “mitochondrial malignancy” and ROS-induced oncogenic transformation - why mitochondria are targets for cancer therapy. Mol. Aspect. Med. 2010;31(2):145–170. doi: 10.1016/j.mam.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Vaupel P., Multhoff G. Hypoxia-/HIF-1alpha-Driven factors of the tumor microenvironment impeding antitumor immune responses and promoting malignant progression. Adv. Exp. Med. Biol. 2018;1072:171–175. doi: 10.1007/978-3-319-91287-5_27. [DOI] [PubMed] [Google Scholar]
- 14.Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., Marshall K.A., et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41(Database issue):D991–995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang R., Rong Y., Ge Y., Song W., Ren J., Fu T. Cell differentiation trajectory predicts patient potential immunotherapy response and prognosis in gastric cancer. Aging (Albany NY) 2021;13(4):5928–5945. doi: 10.18632/aging.202515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelin A., Gil-de-Gomez L., Dahiya S., Jiao J., Guo L., Levine M.H., Wang Z., et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metabol. 2017;25(6):1282–1293. doi: 10.1016/j.cmet.2016.12.018. e1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberzon A., Subramanian A., Pinchback R., Thorvaldsdottir H., Tamayo P., Mesirov J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satija R., Farrell J.A., Gennert D., Schier A.F., Regev A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015;33(5):495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korsunsky I., Millard N., Fan J., Slowikowski K., Zhang F., Wei K., Baglaenko Y., Brenner M., Loh P.R., Raychaudhuri S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods. 2019;16(12):1289–1296. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., Lan Y., Xu J., Quan F., Zhao E., Deng C., Luo T., et al. CellMarker: a manually curated resource of cell markers in human and mouse. Nucleic Acids Res. 2019;47(D1):D721–D728. doi: 10.1093/nar/gky900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong Z., Yang Q., Li X. Effect of intra- and inter-tumoral heterogeneity on molecular characteristics of primary IDH-wild type glioblastoma revealed by single-cell analysis. CNS Neurosci. Ther. 2020;26(9):981–989. doi: 10.1111/cns.13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Z., Dai Z., Locasale J.W. Metabolic landscape of the tumor microenvironment at single cell resolution. Nat. Commun. 2019;10(1):3763. doi: 10.1038/s41467-019-11738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Y., Shang S., Guo S., Li X., Zhou H., Liu H., Sun Y., et al. Lnc2Cancer 3.0: an updated resource for experimentally supported lncRNA/circRNA cancer associations and web tools based on RNA-seq and scRNA-seq data. Nucleic Acids Res. 2021;49(D1):D1251–D1258. doi: 10.1093/nar/gkaa1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen S., Li H., Liu J., Sun L., Yuan Y. The panoramic picture of pepsinogen gene family with pan-cancer. Cancer Med. 2020;9(23):9064–9080. doi: 10.1002/cam4.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turajlic S., Sottoriva A., Graham T., Swanton C. Resolving genetic heterogeneity in cancer. Nat. Rev. Genet. 2019;20(7):404–416. doi: 10.1038/s41576-019-0114-6. [DOI] [PubMed] [Google Scholar]
- 27.Rohlenova K., Veys K., Miranda-Santos I., De Bock K., Carmeliet P. Endothelial cell metabolism in health and disease. Trends Cell Biol. 2018;28(3):224–236. doi: 10.1016/j.tcb.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 29.Lunt S.Y., Vander Heiden M.G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 30.Cimmino F., Avitabile M., Lasorsa V.A., Montella A., Pezone L., Cantalupo S., Visconte F., Corrias M.V., Iolascon A., Capasso M. HIF-1 transcription activity: HIF1A driven response in normoxia and in hypoxia. BMC Med. Genet. 2019;20(1):37. doi: 10.1186/s12881-019-0767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A.H., Tanaseichuk O., Benner C., Chanda S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019;10(1):1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng J.L., Xu Y.H., Wang G. Identification of potential crucial genes and key pathways in breast cancer using bioinformatic analysis. Front. Genet. 2019;10:695. doi: 10.3389/fgene.2019.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choudhry H., Harris A.L. Advances in hypoxia-inducible factor biology. Cell Metabol. 2018;27(2):281–298. doi: 10.1016/j.cmet.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Moldogazieva N.T., Mokhosoev I.M., Terentiev A.A. Metabolic heterogeneity of cancer cells: an interplay between HIF-1, GLUTs, and AMPK. Cancers. 2020;12(4) doi: 10.3390/cancers12040862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakazawa M.S., Keith B., Simon M.C. Oxygen availability and metabolic adaptations. Nat. Rev. Cancer. 2016;16(10):663–673. doi: 10.1038/nrc.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavlides S., Whitaker-Menezes D., Castello-Cros R., Flomenberg N., Witkiewicz A.K., Frank P.G., Casimiro M.C., et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8(23):3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 37.Bonuccelli G., Tsirigos A., Whitaker-Menezes D., Pavlides S., Pestell R.G., Chiavarina B., Frank P.G., et al. Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9(17):3506–3514. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pei J.P., Zhang C.D., Yusupu M., Zhang C., Dai D.Q. Screening and validation of the hypoxia-related signature of evaluating tumor immune microenvironment and predicting prognosis in gastric cancer. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.705511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hao L.S., Liu Q., Tian C., Zhang D.X., Wang B., Zhou D.X., Li Z.P., Yuan Z.X. Correlation and expression analysis of hypoxia-inducible factor 1alpha, glucose transporter 1 and lactate dehydrogenase 5 in human gastric cancer. Oncol. Lett. 2019;18(2):1431–1441. doi: 10.3892/ol.2019.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piao H.Y., Liu Y., Kang Y., Wang Y., Meng X.Y., Yang D., Zhang J. Hypoxia associated lncRNA HYPAL promotes proliferation of gastric cancer as ceRNA by sponging miR-431-5p to upregulate CDK14. Gastric Cancer. 2022;25(1):44–63. doi: 10.1007/s10120-021-01213-5. [DOI] [PubMed] [Google Scholar]
- 41.Guzy R.D., Hoyos B., Robin E., Chen H., Liu L., Mansfield K.D., Simon M.C., Hammerling U., Schumacker P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metabol. 2005;1(6):401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J., Xu J., Dong Y., Huang B. Down-regulation of HIF-1alpha inhibits the proliferation, migration, and invasion of gastric cancer by inhibiting PI3K/AKT pathway and VEGF expression. Biosci. Rep. 2018;38(6) doi: 10.1042/BSR20180741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu N.Z., Cidlowski J.A. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol. Cell. 2005;18(3):331–342. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 44.Kim I.K., Kim B.S., Koh C.H., Seok J.W., Park J.S., Shin K.S., Bae E.A., et al. Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells. Nat. Med. 2015;21(9):1010–1017. doi: 10.1038/nm.3922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article.