Abstract
Background
This study sought to analyse the trend in smoking-attributable mortality (SAM) in Spain among the population aged ≥35 years across the period 1990–2018.
Methods
SAM was estimated by applying a prevalence-independent method, which uses lung cancer (LC) mortality as a proxy of tobacco consumption. We sourced observed mortality from the National Institute of Statistics (Spain), LC mortality rates in smokers and never smokers from the Cancer Prevention Study I–II, and relative risks from 5 US cohorts. Estimates of annual SAM by cause of death, sex and age are shown, along with crude and annual standardised SAM rates. The trend in standardised all-cause and LC rates was analysed using a joinpoint regression model.
Results
Tobacco caused 1 717 150 deaths in Spain in the period 1990–2018. Among men, cancers replaced cardiovascular diseases–diabetes mellitus (CVD–DM) as the leading group of tobacco-related cause of death in 1994. Among women, CVD–DM remained the leading cause of death throughout the period. Trend analysis of standardised SAM rates due to all causes and LC showed a decrease in men and an increase in women.
Conclusions
The tobacco epidemic in Spain across the period 1990–2018 has had an important impact on mortality and has evolved differently in both genders. SAM is expected to increase dramatically in women in the coming years. SAM data highlight the importance of including a gender perspective in SAM analyses, in designing more effective and comprehensive public health interventions and in developing gender-specific tobacco control policies to curb tobacco consumption.
Introduction
The effects of tobacco consumption on people’s health have long been, and indeed continue to be, a highly relevant research topic. In the 50 years between the publication of the Surgeon General’s first report in 19641 and the latest report, causal relationships between tobacco consumption and more than 20 diseases have been established.2 Among these diseases, three major groups can be discerned, namely, cancers, cardiovascular diseases (CVD) and respiratory diseases. According to the National Statistics Institute (NSI—Spain), in 2020, these three disease groups accounted for 22.8%, 24.3% and 8.6% of total deaths in Spain, respectively.3
In Spain, before 2005, different legislative measures in tobacco control were implemented. Nevertheless, the greatest advance came in 2006 with the implementation of the Law 28/2005 on health measures against smoking and regulating the sale, supply, consumption and advertising of tobacco products.4 This regulation was incomplete, and tobacco consumption was allowed in the hospitality sector under certain assumptions.4,5 In 2010, a comprehensive regulation, the Law 42/2010, amended the Law 28/2005 extending the smoking ban to hospitality venues with no exception.6
Various indicators can be used to ascertain the impact of tobacco consumption on people’s health: the most commonly used is the prevalence of smoking. National Health Surveys, which provide information on the prevalence of tobacco consumption nationwide, have been conducted in Spain since 1987. Notwithstanding this, no year-on-year data are available and the periodicity of such surveys has been irregular. Prevalence data is derivable for 12 years, sourced from both the National Health Survey and the European Health Survey in Spain.7,8 Nonetheless, data for 18 of the 28 years included in the period 1990–2018 is missing, making it impossible to form an accurate picture of the trend in the tobacco epidemic in Spain. Another alternative indicator is the smoking-attributable mortality (SAM). Lopez et al.,9 proposed a model based on the monitoring of prevalence and SAM to analyse the trend in the tobacco epidemic in a given population. The method generally used to estimate SAM is based on the prevalence of tobacco consumption and is known as ‘prevalence-dependent method’. Yet, the lack of data on the prevalence of annual tobacco consumption means that an alternative, prevalence-independent method, must be used.10
The aim of this study was to analyse the trend in SAM in Spain among the population aged ≥35 years across the period 1990–2018, by applying a prevalence-independent method.
Methods
Method of estimation
To estimate SAM, we used a prevalence-independent method of tobacco consumption, developed by Peto et al., which uses lung cancer (LC) mortality as a proxy of tobacco consumption.10
LC SAM was estimated on the basis of observed mortality (OM) due to LC and the difference between the LC mortality rate overall and in never smokers among the study population, year on year, across the period 1990–2018.11
To estimate SAM to the remaining causes associated with tobacco consumption, the smoking impact ratio (SIR) was calculated as follows:
where CLC and NLC are overall and never smokers LC mortality rates in the study population, respectively. S*LC and N*LC are the LC mortality rates among smokers and never smokers in a reference population, respectively.
The population attributable fraction (PAF) for each associated disease is calculated as follows:
where RR refers to the risk posed to smokers of dying of tobacco-related diseases with respect to never smokers.
Lastly, SAM for each cause is obtained as the product of OM multiplied by the PAF.
Data sources
OM was obtained for each year of the period 1990–2018 by sex, age and cause of death from the NSI.3 Cause of death information was extracted between 1990 and 1998 following the coding of the 9th revision of the International Classification of Diseases (ICD) and since 1999 following the coding of the 10th revision of ICD. The codes were harmonized according to the recommendations of Anderson et al.12 The causes of death are divided into three major groups: cancers, cardiovascular diseases-diabetes mellitus (CVD-DM) and respiratory diseases. Detailed information on the causes included in each major group accompanied by their ICD-9 and ICD-10 codes can be found in Supplementary table S1.
The LC mortality rates in the Spanish population were estimated for each year, taking the population resident on 1 July of each year as reference.13
The LC mortality rates in the reference population for smokers and never smokers and the LC mortality rates for never smokers in the study population were drawn from the Cancer Prevention Study Phases I and II.14
Relative risks (RRs) by cause, sex and age group (35–54, 55–64, 65–74, ≥75 years), were obtained from a joint analysis of five cohort studies conducted in the USA.2
Analysis
The following analyses were performed: annual SAM for the period 1990–2018, by sex and cause of death; and annual crude SAM rates, by sex, for 4 causes of death [LC, cancers (including LC), CVD–DM, and respiratory diseases].
Annual SAM rates were standardised by age using the direct method, and calculated for each sex and age group (35–64, ≥65 years). The Eurostat Task Force European standard population was used for rate adjustment.15 We analysed the trend in annual standardised SAM rates using joinpoint regression models, assuming variance to be constant, and setting a maximum of 3 cutpoints (joinpoints) and a significance level of 5%. The annual percentage change (APC) with its 95% confidence interval (CI) was calculated.
Analysis was performed with the statistical package Stata v16.1 and the Joinpoint regression software v4.8.16
Results
In Spain, active smoking caused 1 717 150 deaths between 1990 and 2018 in population aged ≥35 years, accounting for 12.7% of total deaths in this period (22.3% in men and 2.3% in women). Of the total SAM, 1 563 706 deaths occurred in men and 404 174 in population aged ˂65 years, which accounted for 24.8% of total mortality in the same age group during the study period; 31.7% in men and 8.6% in women. Of the total SAM, 42.6% was due to cancers (60.4% due to LC), 35.8% due to CVD–DM (35.1% and 0.7%, respectively), and 21.6% due to respiratory diseases (88.4% due to chronic obstructive pulmonary disease). Among men, the principal group of causes of death comprised cancers (43.6%), followed by CVD–DM (35.1%) and respiratory diseases (21.4%). Among women, CVD–DM (43.2%) was the group of causes with the greatest impact on SAM, followed by cancers (32.5%) and respiratory diseases (24.3%) (tables 1 and 2).
Table 1.
Smoking-attributable mortality (SAM) and its percentage of total SAM in men ≥35 years, by cause of death: Spain, 1990–2018
| Men |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancers |
Cardiovascular diseases–diabetes mellitus |
Respiratory diseases |
Total | ||||||||||
| Lung cancer |
All remaining cancers
a
|
Cardiovascular diseases
b
|
Diabetes mellitus |
COPD |
Other respiratory diseases
c
|
||||||||
| SAM | % | SAM | % | SAM | % | SAM | % | SAM | % | SAM | % | SAM | |
| 1990 | 11 094 | 22.5 | 7388 | 15.0 | 20 429 | 41.5 | 478 | 1.0 | 8715 | 17.7 | 1113 | 2.3 | 49 218 |
| 1991 | 11 529 | 22.7 | 7651 | 15.1 | 21 066 | 41.5 | 460 | 0.9 | 9104 | 17.9 | 979 | 1.9 | 50 789 |
| 1992 | 12 033 | 23.5 | 8000 | 15.6 | 20 837 | 40.7 | 438 | 0.9 | 8849 | 17.3 | 983 | 1.9 | 51 139 |
| 1993 | 12 477 | 23.4 | 8481 | 15.9 | 21 278 | 39.9 | 459 | 0.9 | 9563 | 17.9 | 1040 | 2.0 | 53 299 |
| 1994 | 12 855 | 24.2 | 8684 | 16.3 | 20 815 | 39.1 | 456 | 0.9 | 9410 | 17.7 | 986 | 1.9 | 53 206 |
| 1995 | 13 204 | 24.0 | 8822 | 16.1 | 21 005 | 38.2 | 475 | 0.9 | 10 405 | 18.9 | 1030 | 1.9 | 54 941 |
| 1996 | 12 948 | 23.6 | 8598 | 15.7 | 20 925 | 38.2 | 439 | 0.8 | 10 867 | 19.8 | 1020 | 1.9 | 54 797 |
| 1997 | 13 127 | 24.0 | 8886 | 16.3 | 20 500 | 37.5 | 413 | 0.8 | 10 729 | 19.6 | 1027 | 1.9 | 54 683 |
| 1998 | 13 608 | 24.0 | 9013 | 15.9 | 20 992 | 37.0 | 447 | 0.8 | 11 637 | 20.5 | 1073 | 1.9 | 56 770 |
| 1999 | 14 048 | 23.9 | 9309 | 15.8 | 21 227 | 36.1 | 443 | 0.8 | 12 147 | 20.7 | 1628 | 2.8 | 58 802 |
| 2000 | 13 865 | 25.0 | 9205 | 16.6 | 19 851 | 35.7 | 425 | 0.8 | 10 804 | 19.5 | 1381 | 2.5 | 55 532 |
| 2001 | 14 602 | 26.0 | 9759 | 17.4 | 20 023 | 35.7 | 415 | 0.7 | 9995 | 17.8 | 1345 | 2.4 | 56 139 |
| 2002 | 14 301 | 25.5 | 9395 | 16.8 | 19 529 | 34.8 | 411 | 0.7 | 11 035 | 19.7 | 1401 | 2.5 | 56 072 |
| 2003 | 14 801 | 25.7 | 9669 | 16.8 | 20 097 | 34.8 | 424 | 0.7 | 11 245 | 19.5 | 1435 | 2.5 | 57 670 |
| 2004 | 14 871 | 26.8 | 9754 | 17.6 | 18 971 | 34.2 | 413 | 0.7 | 10 241 | 18.4 | 1262 | 2.3 | 55 511 |
| 2005 | 14 832 | 26.0 | 9558 | 16.7 | 19 123 | 33.5 | 430 | 0.8 | 11 586 | 20.3 | 1589 | 2.8 | 57 117 |
| 2006 | 15 032 | 27.9 | 9587 | 17.8 | 18 149 | 33.6 | 391 | 0.7 | 9507 | 17.6 | 1300 | 2.4 | 53 967 |
| 2007 | 15 278 | 27.5 | 9714 | 17.5 | 18 353 | 33.0 | 396 | 0.7 | 10 411 | 18.7 | 1427 | 2.6 | 55 578 |
| 2008 | 15 199 | 28.2 | 9619 | 17.8 | 17 527 | 32.5 | 377 | 0.7 | 9779 | 18.1 | 1476 | 2.7 | 53 977 |
| 2009 | 15 291 | 28.3 | 9694 | 17.9 | 16 910 | 31.3 | 349 | 0.6 | 10 413 | 19.3 | 1387 | 2.6 | 54 044 |
| 2010 | 15 257 | 28.7 | 10 010 | 18.8 | 16 300 | 30.7 | 327 | 0.6 | 10 153 | 19.1 | 1134 | 2.1 | 53 181 |
| 2011 | 15 410 | 28.9 | 10 085 | 18.9 | 15 980 | 29.9 | 327 | 0.6 | 10 295 | 19.3 | 1278 | 2.4 | 53 375 |
| 2012 | 15 542 | 28.5 | 10 283 | 18.8 | 16 168 | 29.6 | 318 | 0.6 | 10 936 | 20 | 1333 | 2.4 | 54 580 |
| 2013 | 15 415 | 29.4 | 10 118 | 19.3 | 15 497 | 29.6 | 285 | 0.5 | 9888 | 18.9 | 1223 | 2.3 | 52 425 |
| 2014 | 15 032 | 29.4 | 9672 | 18.9 | 14 976 | 29.3 | 301 | 0.6 | 9919 | 19.4 | 1286 | 2.5 | 51 186 |
| 2015 | 15 050 | 28.6 | 9676 | 18.4 | 15 450 | 29.3 | 302 | 0.6 | 10 768 | 20.4 | 1436 | 2.7 | 52 681 |
| 2016 | 15 375 | 29.7 | 9826 | 19.0 | 15 333 | 29.6 | 298 | 0.6 | 9585 | 18.5 | 1415 | 2.7 | 51 831 |
| 2017 | 14 999 | 29.3 | 9557 | 18.7 | 15 172 | 29.6 | 295 | 0.6 | 9712 | 19 | 1482 | 2.9 | 51 217 |
| 2018 | 14 888 | 29.8 | 9328 | 18.7 | 14 775 | 29.6 | 274 | 0.5 | 9113 | 18.2 | 1599 | 3.2 | 49 978 |
| Total | 411 963 | 26.3 | 269 343 | 17.2 | 537 260 | 34.4 | 11 267 | 0.7 | 296 809 | 19 | 37 065 | 2.4 | 1 563 706 |
COPD: Chronic obstructive pulmonary disease.
All remaining cancers: include lip, oral cavity and pharynx, oesophagus, stomach, colon and rectum, liver and intrahepatic bile ducts, pancreas, larynx, cervix uteri, urinary bladder, kidney-renal pelvis and acute myeloid leukaemia.
Cardiovascular diseases: include ischaemic heart disease, rheumatic heart diseases, cardiopulmonary diseases, other types of heart disease, cerebrovascular disease, atherosclerosis, aortic aneurysm and other arterial diseases.
Other respiratory diseases: include influenza–pneumonia–tuberculosis.
Table 2.
Smoking-attributable mortality (SAM) and its percentage of total SAM in women ≥35 years, by cause of death: Spain, 1990–2018
| Women |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancers |
Cardiovascular diseases–diabetes mellitus |
Respiratory diseases |
Total | ||||||||||
| Lung cancer |
All remaining cancersa |
Cardiovascular diseasesb |
Diabetes mellitus |
COPD |
Other respiratory diseasesc |
||||||||
| SAM | % | SAM | % | SAM | % | SAM | % | SAM | % | SAM | % | SAM | |
| 1990 | 68 | 5.0 | 77 | 5.7 | 695 | 51.6 | 11 | 0.8 | 470 | 34.9 | 26 | 1.9 | 1347 |
| 1991 | 103 | 9.1 | 94 | 8.3 | 528 | 46.5 | 18 | 1.6 | 371 | 32.7 | 21 | 1.9 | 1135 |
| 1992 | 81 | 10.5 | 75 | 9.7 | 337 | 43.6 | 13 | 1.6 | 254 | 32.9 | 14 | 1.8 | 773 |
| 1993 | 111 | 5.4 | 128 | 6.2 | 1051 | 51.3 | 15 | 0.7 | 703 | 34.3 | 41 | 2.0 | 2050 |
| 1994 | 123 | 15.7 | 111 | 14.2 | 308 | 39.4 | 19 | 2.5 | 207 | 26.4 | 15 | 1.9 | 783 |
| 1995 | 176 | 9.2 | 154 | 8.0 | 891 | 46.4 | 22 | 1.1 | 640 | 33.3 | 39 | 2.0 | 1922 |
| 1996 | 137 | 10.9 | 114 | 9.1 | 550 | 43.9 | 16 | 1.2 | 408 | 32.5 | 30 | 2.4 | 1254 |
| 1997 | 223 | 11.6 | 172 | 8.9 | 847 | 44.1 | 23 | 1.2 | 610 | 31.7 | 47 | 2.4 | 1922 |
| 1998 | 290 | 9.8 | 226 | 7.6 | 1379 | 46.5 | 29 | 1.0 | 966 | 32.6 | 75 | 2.5 | 2965 |
| 1999 | 311 | 12.0 | 222 | 8.6 | 1082 | 41.9 | 29 | 1.1 | 842 | 32.6 | 95 | 3.7 | 2582 |
| 2000 | 379 | 15.4 | 238 | 9.7 | 1059 | 42.9 | 30 | 1.2 | 666 | 27.0 | 94 | 3.8 | 2466 |
| 2001 | 408 | 17.9 | 251 | 11.0 | 982 | 43.2 | 33 | 1.5 | 525 | 23.1 | 75 | 3.3 | 2274 |
| 2002 | 538 | 15.9 | 346 | 10.2 | 1468 | 43.3 | 42 | 1.2 | 872 | 25.7 | 121 | 3.6 | 3387 |
| 2003 | 651 | 15.0 | 428 | 9.9 | 1942 | 44.8 | 48 | 1.1 | 1113 | 25.7 | 154 | 3.6 | 4337 |
| 2004 | 797 | 18.1 | 503 | 11.4 | 1931 | 43.8 | 52 | 1.2 | 985 | 22.3 | 147 | 3.3 | 4413 |
| 2005 | 792 | 16.3 | 511 | 10.5 | 2107 | 43.3 | 55 | 1.1 | 1208 | 24.8 | 193 | 4.0 | 4865 |
| 2006 | 924 | 20.0 | 562 | 12.1 | 2005 | 43.3 | 56 | 1.2 | 911 | 19.7 | 169 | 3.7 | 4626 |
| 2007 | 1049 | 18.7 | 661 | 11.8 | 2447 | 43.5 | 65 | 1.2 | 1187 | 21.1 | 211 | 3.8 | 5620 |
| 2008 | 1259 | 18.6 | 828 | 12.2 | 2991 | 44.2 | 73 | 1.1 | 1343 | 19.8 | 275 | 4.1 | 6770 |
| 2009 | 1301 | 20.7 | 824 | 13.1 | 2577 | 40.9 | 73 | 1.2 | 1255 | 19.9 | 263 | 4.2 | 6292 |
| 2010 | 1584 | 22.0 | 1037 | 14.4 | 2905 | 40.3 | 80 | 1.1 | 1370 | 19.0 | 237 | 3.3 | 7213 |
| 2011 | 1684 | 19.9 | 1189 | 14.1 | 3525 | 41.7 | 82 | 1.0 | 1646 | 19.5 | 333 | 3.9 | 8459 |
| 2012 | 1891 | 20.8 | 1269 | 14.0 | 3729 | 41.0 | 80 | 0.9 | 1780 | 19.6 | 335 | 3.7 | 9083 |
| 2013 | 2157 | 21.4 | 1503 | 14.9 | 4204 | 41.7 | 92 | 0.9 | 1767 | 17.5 | 370 | 3.7 | 10 092 |
| 2014 | 2083 | 21.9 | 1409 | 14.8 | 3825 | 40.1 | 93 | 1.0 | 1750 | 18.4 | 368 | 3.9 | 9529 |
| 2015 | 2372 | 21.9 | 1596 | 14.8 | 4395 | 40.6 | 101 | 0.9 | 1911 | 17.7 | 437 | 4.0 | 10 812 |
| 2016 | 2541 | 22.9 | 1718 | 15.5 | 4485 | 40.4 | 100 | 0.9 | 1793 | 16.1 | 468 | 4.2 | 11 106 |
| 2017 | 2811 | 22.1 | 1924 | 15.2 | 5259 | 41.4 | 114 | 0.9 | 2027 | 16.0 | 558 | 4.4 | 12 693 |
| 2018 | 2893 | 22.8 | 1936 | 15.3 | 5206 | 41.1 | 115 | 0.9 | 1885 | 14.9 | 639 | 5.0 | 12 673 |
| Total | 29 734 | 19.4 | 20 105 | 13.1 | 64 709 | 42.2 | 1576 | 1.0 | 31 467 | 20.5 | 5853 | 3.8 | 15 3444 |
COPD: chronic obstructive pulmonary disease.
All remaining cancers: include lip, oral cavity and pharynx, oesophagus, stomach, colon and rectum, liver and intrahepatic bile ducts, pancreas, larynx, cervix uteri, urinary bladder, kidney-renal pelvis and acute myeloid leukaemia.
Cardiovascular diseases: include ischaemic heart disease, rheumatic heart diseases, cardiopulmonary diseases, other types of heart disease, cerebrovascular disease, atherosclerosis, aortic aneurysm and other arterial diseases.
Other respiratory diseases: include influenza–pneumonia–tuberculosis.
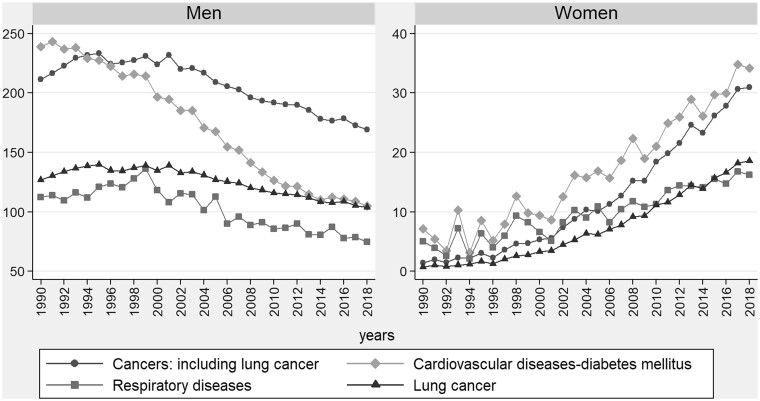
Among men, a downward trend in crude SAM rates was observed in all causes. The crude SAM rate for CVD–DM fell from 239.1 to 105.1 cases per 100 000 population between 1990 and 2018 and was replaced by cancers in 1994. In respiratory diseases, there was a fall in crude SAM rates from 1999 onwards. The crude SAM rates for LC drew level with those for CVD–DM in 2013. Among women, there was a rising trend in crude SAM rates for all causes. In the initial years of the study period, the crude SAM rates for CVD–DM showed wide variability but from 2001 these displayed a rising trend (figure 1). In cancers, an increase was likewise observed in crude SAM rates, going from 1.5 to 31.0 cases per 100 000 population between 1990 and 2018. When the trend was analysed in individualised causes rather than in large groups, LC was observed to be the cause of death with the highest crude SAM rate in men across the period and that which registered the greatest increase in the crude SAM rate in women from 1990 to 2018 (Supplementary tables S2 and S3).
Figure 1.
Crude rates of smoking-attributable mortality in Spain per 100 000 population ≥35 years across the period 1990–2018, by sex and cause of death.
Cancers: include trachea-bronchus-lung, lip, oral cavity, pharynx, oesophagus, stomach, colon and rectum, liver and intrahepatic bile ducts, pancreas, larynx, cervix uteri, urinary bladder, kidney-renal pelvis and acute myeloid leukaemia. Cardiovascular diseases–diabetes mellitus: include ischaemic heart disease, rheumatic heart diseases, cardiopulmonary diseases, other types of heart disease, cerebrovascular disease, atherosclerosis, aortic aneurysm, other arterial diseases and diabetes mellitus. Respiratory diseases: include influenza–pneumonia–tuberculosis and chronic obstructive pulmonary disease
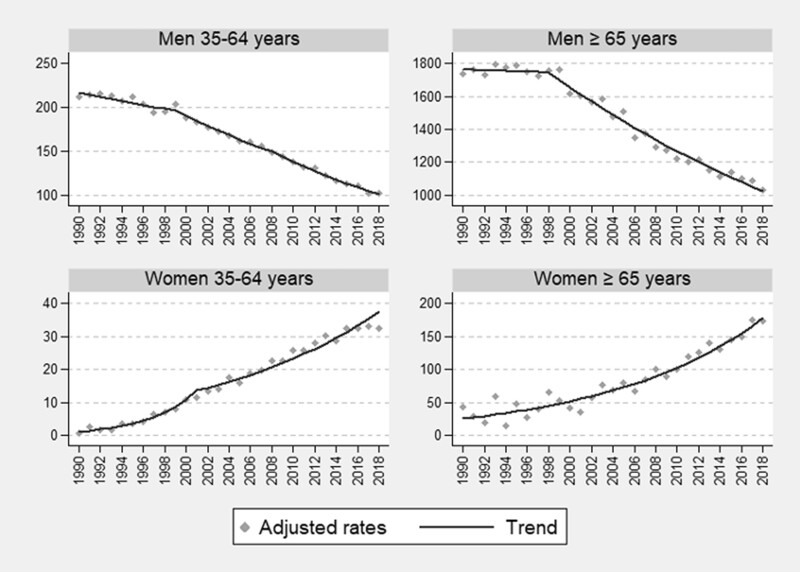
The analysis of the annual standardised SAM rates for all causes revealed opposite scenarios according to sex. Since 1990, the standardised SAM rate independently of age decreased in men and increased in women. The joinpoint model identified three downward periods in men aged 35–64 years. The first change point occurs in 1999, with an APC lower than the second and third periods [APC 1990–99 = −1.1 (95% CI −1.6 to −0.7); APC 1999–2008 = −3.0 (−3.5 to −2.5); APC 2008–18 = −3.8 (−4.2 to −3.5)]. In men aged ≥65 years, two periods with a downward trend were identified, with the APC for the second period being higher than that for the first [APC 1990–98 = −0.1 (−0.9 to 0.7); APC 1998–2018 = −2.6 (−2.8 to −2.4)]. Among women aged 35–64 years, there were two upward periods: the first until 2001 (change point), with a percentage increase in SAM rates which quadrupled that of the second period [APC 1990–2001 = 25.0 (20.3–29.9) and APC 2001–18 = 6.1 (4.1–8.3)]. Among women aged ≥65 years, a single period with a rising trend was identified without any change point [APC 1990–2018 = 7.1 (5.7–8.6)]. The APC differ between sexes, being higher in women and especially in younger women in the first period set by the joinpoint (figure 2 and Supplementary table S4).
Figure 2.
Trends in smoking-attributable all-cause mortality rates adjusted with the joinpoint regression model, by sex (men and women) and age group (35–64 and 65 years and over) across the period 1990–2018 in Spain: rates per 100 000 population
Related to the annual standardised SAM rates for LC, a slightly rising trend was observed in the initial years of the study period among men aged 35–64 and ≥65 years, with this being more marked in the 65-year-and-over group. Among women, a rising trend was observed across the period in both age groups; 35–64 and ≥65 years. In younger women (35–64 years old), joinpoint detected two periods; the first (1990–2002) with an APC that was four times higher than that for the second period. In women aged ≥65 years, no change points were detected (Supplementary figure S1 and Supplementary table S4).
Discussion
In Spain, tobacco consumption caused 1 717 150 deaths in population aged ≥35 years across the period 1990–2018. Although SAM was higher in men throughout the study period, the greater change in SAM figures was observed in women, with an increase of over 10 000 deaths between the first and last years of the series. The principal group of causes of death consisted of cancers in men from 1994 onwards and CVD–DM in women across the study period. LC remained the leading cause of death in men throughout the whole series and in women from 2009 onwards. The standardised SAM rates displayed a downward trend in men and an upward trend in women.
The results obtained in this study reflect the trend in the tobacco epidemic in Spain across the stages proposed by Lopez et al.9 Spanish men started smoking during the Spanish Civil War (1936–39) and its prevalence started to decrease since the 1970s17 and continue to decrease until today.7,8 In women, it is observed that smoking prevalence started to increase in the 1960s17 and decrease in 2006, although this decline has been very slight until the current prevalence data.5,7,8 This places men and women at different stages in the evolution of the tobacco epidemic with men currently at phase IV and women at late phase III.9
In our study, a peak in SAM figures was observed in men in 1999. However, among women, there was an increase in SAM figures across all the series studied. These results are similar to those found by a systematic review of SAM undertaken in Spain, which reported a decrease in SAM figures among men in 2001, and an increase among women ever since the first estimate in 1978.18
According to the Global Burden of Disease study (GBDS),19 the percentage change between the number of deaths attributed to tobacco consumption from 1990 and 2019 in Spain was 1.86%, while in our study it was 23.9%. This difference can be explained by the methodology used in each study. The GBDS uses a methodology based on the current prevalence of tobacco consumption and our study based on the LC mortality rates. The percentage change between smoking prevalence in Spain used by the GBDS between 1990 and 2019 is −23.6% in women and −39.6% in men.19 However, the percentage change between 1990 and 2018 LC mortality rates was 151.7% in women and −12.1% in men.3 The higher percentage change for women and lower for men between LC mortality rates may explain why we observe such differences. Moreover, the GBDS estimated that the percentage of SAM over the total OM in men in 2019 was between 25% and 29% and in women between 5% and 10%.19 In our study, these percentages were 23.3% and 6.0% (data not shown) in men and women in 2018, respectively. In terms of other European countries, France and Italy are in the same range of percentages of SAM in women as Spain, while other European countries such as Portugal have lower percentages and the UK, Denmark and The Netherlands have higher percentages.19,20 In men, it seems that Spain is one of the countries with the highest percentage of SAM, above countries such as France, Italy, the Netherlands, Denmark or the UK,19,20 where the tobacco epidemic started years earlier than in Spain.21
In Spain, estimates of SAM are available for certain years. In all cases, the methodology applied was prevalence-dependent.18 Using a prevalence-independent method, this study has allowed us to observe the pattern described by the trend in SAM over 28 years. It should, however, be borne in mind that previous studies which compared SAM estimates by applying both methods found that the prevalence-independent method yielded higher SAM estimates than the prevalence-dependent method.22–27
In Spain, in 2016 and 2017 SAM was estimated with the prevalence-dependent method, using the same source of RRs and causes of tobacco-related mortality.28,29 Comparing the results of both studies against those obtained in our study, overall the prevalence-independent method has yielded estimates of SAM that were 12% higher in 2016 and 19% higher in 2017. Among men, the variations are smaller, never exceeding 13% in any of the years. The greatest differences are seen in women, particularly in 2017, when the prevalence-independent method estimated 53% more deaths due to tobacco consumption (independent: 12 693 deaths vs. dependent: 8306 deaths). Broken down by cause of death, CVD–DM display the greatest variability between methods, with differences of 24% and 27% in men and 50% and 105% in women in 2016 and 2017, respectively.28,29 The fact that the prevalence-independent method tends to estimate a higher SAM for CVD–DM could be due to the use of LC mortality as a proxy of tobacco consumption. Thus, the epidemiology of tobacco is being assessed as it was decades earlier, whereas the prevalence-dependent method uses prevalences contemporary with OM. Hence, the precedence in time of the exposure variable, tobacco, is avoided. This in turn means that, if prevalence decreases, SAM will also decrease, without taking into account the nature of previous exposures. The fact that the prevalence-independent method relies on the LC mortality rate may mean that, for diseases such as CVD, the time lag of the epidemic might be longer than necessary. As a result of this, the SAM estimates would vary by so much.
In studies that analysed the difference between the two SAM estimation methods, one report small differences22 while others greater than those found it in Spain.23,24,26 In Spain, as in Korea, Italy, Canada and the USA,22,24–26 the most important changes in SAM took place in women, with the prevalence-independent method being the one that estimated higher figures. However, in a study conducted in Lithuania, the greatest variation was observed in men.23 By the cause of death, CVD in both men and women, and respiratory diseases in women displayed the greatest differences between methods. Similar results were also observed in studies undertaken in Lithuania, the USA and Vietnam.23,24,27
According to data sourced from the NSI mortality register,3 over the last 30 years (1990–2018) in Spain, the percentage difference between OM due to cancers and CVD with respect to total annual OM, dropped from 14.8% in 1990 (cancers: 24.0% vs. CVD: 38.8%) to 1.9% in 2018 (cancers: 26.4% vs. CVD: 28.3%). The breakdown by sex shows that among men cancers ranked as the leading cause of death since 2000, surpassing CVD, and that the differences between the two causes were small. Among women, the changes in the percentage of OM due to cancers and CVD were more evident, going from a difference between the two causes of 25.7% in 1990 (cancers: 19.8% vs. CVD: 45.5%) to 9.6% in 2018 (cancers: 21.8% vs. CVD: 30.7%).3 These data are reflected in the trend in figures obtained by our study for SAM due to cancers and CVD. These changes in both OM and SAM are mainly due to the decline in CVD mortality. This decrease can be explained by some reasons; one of the most important is the improvement in secondary prevention of CVD with earlier diagnosis and improved treatment, and to a lesser extent, due to better control of cardiovascular risk factors. Moreover, this decrease can also be explained by differences in the induction time of CVD vs. cancers, in that the time required for tobacco consumption to induce a CVD is less than that required to induce a cancer.30,31
In our study, LC was the cause with the greatest SAM burden in men throughout the period and in women since 2009. These results coincide with what was observed in estimates made in Spain using the prevalence-dependent method.18 These changes in SAM are closely related to the trend in prevalences of tobacco consumption among men and women in previous years. According to NSI data, LC is the leading cause of cancer-related death in men, while in women it is the second leading cause after breast cancer. Among women, it is noteworthy that the percentage difference between OM due to breast cancer and LC with respect to total annual cancer-related OM steadily diminished from 1990 to 2018, going from 13.1 percentage points in 1990 (lung: 4.3% vs. breast: 17.4%) to 3.6 in 2018 (lung: 11.1% vs. breast: 14.7%).3 This increase in LC-related deaths can be accounted for the increase in tobacco consumption among Spanish women.
The SAM estimates obtained in this study are subject to some limitations which have to be identified.32 In Spain, LC mortality rates according to tobacco consumption are not available. Therefore, we are forced to use data from other countries such as the USA and assume that their LC mortality rates for never smokers are the same as in Spain. Another limitation relates to the use of RRs drawn from US cohorts, since the trend in the tobacco epidemic in the USA is different regarding Spain. Due to the lack of risks derived from the follow-up of cohorts in southern Europe, the RRs are derived from 5 contemporary US cohorts since they are the best evidence available to date.2,33 Although the RRs were not adjusted for potential confounding factors, the variation in estimates on applying adjusted RRs is small.34,35 Using LC as a tobacco marker implies assuming that the time of induction of cancers, CVD–DM and respiratory diseases is the same.
This study also exhibits strengths, such as the estimation of SAM across a span of almost 30 years, which enables us to see how the tobacco epidemic has evolved in Spain. Furthermore, although Peto et al.’s method has limitations, it also has strengths. One of the most important is that it respects the criterion of causality of temporal precedence which must exist between exposure to a given risk factor and the effect. This is so because it uses LC as a ‘marker’ of the tobacco epidemic, which reflects tobacco consumption in the population in previous decades. Furthermore, the second leading risk factor for LC, defined by the World Health Organization as being exposure to radon, has a largely irrelevant causal effect at a national level,36 thereby making the use of LC as a marker of the tobacco epidemic that much more reliable. A further strength is that, in the absence of prevalences, it allows for the estimation of SAM.
Tobacco consumption caused more than 1.7 million deaths in Spain across the period 1990–2018. The tobacco epidemic in Spain during this period evolved differently in both sexes: whereas in men there was a fall in SAM figures for all tobacco-related causes, in women the opposite scenario was to be seen. The breakdown by major groups of causes shows that, among men, cancers replaced CVD–DM as the leading group of cause of death due to tobacco consumption in 1994. Among women, while CVD–DM comprised the principal group of cause of death across the entire series, the differences with respect to SAM for cancers became smaller with the passage of years. The trend in standardised SAM rates also displays opposite scenarios in the two sexes, both in the joint analysis of all causes and in LC. Thus, while the standardised rates were observed to fall among men, they were seen to rise among women. Therefore, a substantial increase in women’s SAM figures is expected in the coming years. All data related to SAM highlight the importance of including a gender perspective in the analyses of SAM, in the design of more effective and comprehensive public health interventions and in the development of tobacco control policies to curb tobacco consumption. These policies should consider a gender-specific approach to achieve a higher effectiveness.
Supplementary data
Supplementary data are available at EURPUB online.
Supplementary Material
Acknowledgements
This article forms part of the research conducting to the PhD degree of Julia Rey-Brandariz, who has received a FPU fellowship (reference number FPU20/00926), from the Ministry of Universities of Spain.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request
Conflict of interest
None declared
Funding
This work was supported by the Carlos III Institute of Health [grant number: PI19/00288].
Key points.
Little research is available on the impact of tobacco mortality for a long time span.
We have used a methodology independent of the prevalence of tobacco use.
Through 28 years, tobacco use caused 1 717 150 deaths in Spain.
Attributable mortality differed by sex. Decreases in men and increases in women.
Contributor Information
Julia Rey-Brandariz, Department of Preventive Medicine and Public Health, Universidade de Santiago de Compostela, Santiago de Compostela, Spain.
Mónica Pérez-Ríos, Department of Preventive Medicine and Public Health, Universidade de Santiago de Compostela, Santiago de Compostela, Spain; Consortium for Biomedical Research in Epidemiology and Public Health (CIBER en Epidemiología y Salud Pública/CIBERESP), Madrid, Spain.
María Isolina Santiago-Pérez, Epidemiology Department, Directorate-General of Public Health, Galician Regional Health Authority, Santiago de Compostela, Spain.
Iñaki Galán, National Centre for Epidemiology, Carlos III Institute of Health, Madrid, Spain.
Anna Schiaffino, Directorate-General of Health Planning, Health Department, Catalonian Regional Authority, Barcelona, Spain; Catalonian Institute of Oncology, Barcelona, Spain.
Leonor Varela-Lema, Department of Preventive Medicine and Public Health, Universidade de Santiago de Compostela, Santiago de Compostela, Spain.
Agustín Montes, Department of Preventive Medicine and Public Health, Universidade de Santiago de Compostela, Santiago de Compostela, Spain; Consortium for Biomedical Research in Epidemiology and Public Health (CIBER en Epidemiología y Salud Pública/CIBERESP), Madrid, Spain.
María Esther López-Vizcaíno, Galician Statistics Institute, Santiago de Compostela, Spain.
Alexandra Giraldo-Osorio, Department of Preventive Medicine and Public Health, Universidade de Santiago de Compostela, Santiago de Compostela, Spain; Department of Public Health, University of Caldas, Manizales, Colombia; Carolina Foundation, Madrid, Spain.
Cristina Candal-Pedreira, Department of Preventive Medicine and Public Health, Universidade de Santiago de Compostela, Santiago de Compostela, Spain.
Alberto Ruano-Ravina, Department of Preventive Medicine and Public Health, Universidade de Santiago de Compostela, Santiago de Compostela, Spain; Consortium for Biomedical Research in Epidemiology and Public Health (CIBER en Epidemiología y Salud Pública/CIBERESP), Madrid, Spain.
References
- 1. National Center for Chronic Disease Prevention and Health Promotion. Smoking and Healht: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC: Centers for Disease Control and Prevention, 1964. [Google Scholar]
- 2. U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, 2014. [Google Scholar]
- 3. Instituto Nacional de Estadística. Estadística de defunciones según la causa de muerte, 2021. Available at: https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176780&menu=ultiDatos&idp=1254735573175 (May 2021, date last accessed).
- 4.Boletín Oficial del Estado. Ley 28/2005, de 26 de diciembre, de medidas sanitarias frente al tabaquismo y reguladora de la venta, el suministro, el consumo y la publicidad de los productos del tabaco. Ministerio de la presidencia relaciones con las cortes y memoria democrática, 2005: 42241–50.
- 5. Grupo de Trabajo sobre Tabaquismo de la Sociedad Española de Epidemiología. Evaluación de las políticas de control de tabaquismo en España (Leyes 28/2005 y 42/2010). Madrid: Revisión de la evidencia, 2017.
- 6.Boletín Oficial del Estado. Ley 42/2010, de 30 de diciembre, por la que se modifica la Ley 28/2005, de 26 de diciembre, de medidas sanitarias frente al tabaquismo y reguladora de la venta, el suministro, el consumo y la publicidad de los productos del tabaco. Ministerio de la presidencia relaciones con las cortes y memoria democrática, 2010.
- 7. Ministerio de Sanidad. Encuesta Europea de Salud en España. Available at: https://www.sanidad.gob.es/estadEstudios/estadisticas/EncuestaEuropea/home.htm (February 2021, date last accessed).
- 8. Ministerio de Sanidad. Encuesta Nacional de Salud de España. Available at: https://www.sanidad.gob.es/estadEstudios/estadisticas/encuestaNacional/home.htm (February 2021, date last accessed).
- 9. Lopez AD, Collishaw NE, Piha T.. A descriptive model of the cigarette epidemic in developed countries. Tob Control 1994;3:242–7. [Google Scholar]
- 10. Peto R, Boreham J, Lopez AD, et al. Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet 1992;339:1268–78. [DOI] [PubMed] [Google Scholar]
- 11. Ezzati M, Lopez AD, Rodgers A, Murray CJL.. Comparative Quantification of Health Risks : global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva, Switzerland: World Health Organization, 2004. [Google Scholar]
- 12. Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM.. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep 2001;49:1–32. [PubMed] [Google Scholar]
- 13. Instituto Nacional de Estadística. Población residente por fecha, sexo y edad, 2018. Available at: https://www.ine.es/jaxiT3/Tabla.htm?t=31304&L=0 (June 2021, date last accessed).
- 14. National Cancer Institute. Changes in Cigarette Related Disease Risks and Their Implications for Prevention and Control. Tobacco Control Monograph No 8. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, 1997.
- 15. European Commission. Revision of the European Standard Population Report of Eurostat’s Task Force. Brussels: European Comission, 2013. [Google Scholar]
- 16. National Cancer Institute. Joinpoint Regression Program, version 4.8. Available at: https://surveillance.cancer.gov/joinpoint/ (March 2021, date last accessed).
- 17. Fernandez E, Schiaffino A, Borràs JM, et al. Prevalence of cigarette smoking by birth cohort among males and females in Spain, 1910-1990. Eur J Cancer Prev 2003;12:57–62. Feb [DOI] [PubMed] [Google Scholar]
- 18. Rey-Brandariz J, Pérez-Ríos M, Santiago-Pérez MI, et al. Smoking-attributable mortality in Spain: a systematic review. Adicciones 2021. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 19. GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 2021;397:2337–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stoeldraijer L, Bonneux L, van Duin C, et al. The future of smoking-attributable mortality: the case of England & Wales, Denmark and the Netherlands. Addiction 2015;110:336–45. [DOI] [PubMed] [Google Scholar]
- 21. European Commission. Special Eurobarometer 506. Attitudes of Europeans towards Tobacco and Electronic Cigarettes. Directorate-General for Communication, 2021.
- 22. Kong KA, Jung-Choi KH, Lim D, et al. Comparison of prevalence- and smoking impact ratio-based methods of estimating smoking-attributable fractions of deaths. J Epidemiol 2016;26:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liutkute V, Veryga A, Štelemekas M, Midttun NG.. Burden of smoking in Lithuania: attributable mortality and years of potential life lost. Eur J Public Health 2017;27:736–41. [DOI] [PubMed] [Google Scholar]
- 24. Oza S, Thun MJ, Henley SJ, et al. How many deaths are attributable to smoking in the United States? Comparison of methods for estimating smoking-attributable mortality when smoking prevalence changes. Prev Med 2011;52:428–33. ). [DOI] [PubMed] [Google Scholar]
- 25. Gorini G, Chellini E, Querci A, Costantini AS.. Impact of smoking in Italy in 1998: deaths and years of potential life lost. Epidemiol Prev 2003;27:285–90. [PubMed] [Google Scholar]
- 26. Tanuseputro P, Manuel DG, Schultz SE, et al. Improving population attributable fraction methods: examining smoking-attributable mortality for 87 geographic regions in Canada. Am J Epidemiol 2005;161:787–98. [DOI] [PubMed] [Google Scholar]
- 27. Norman RE, Vos T, Barendregt JJ, et al. Mortality attributable to smoking in Vietnamese men in 2008. Prev Med. 2013;57:232–7. [DOI] [PubMed] [Google Scholar]
- 28. Pérez-Ríos M, Schiaffino A, Montes A, et al. Smoking-attributable mortality in Spain in 2016. Arch Bronconeumol 2020;56:559–63. [DOI] [PubMed] [Google Scholar]
- 29. Rey J, Pérez-Ríos M, Santiago-Pérez MI, et al. Smoking-attributable mortality in the autonomous communities of Spain, 2017. Rev Esp Cardiol (Engl Ed) 2022;75:150–8. Feb [DOI] [PubMed] [Google Scholar]
- 30. Martín-Sánchez JC, Bilal U, Clèries R, et al. Modelling lung cancer mortality rates from smoking prevalence: fill in the gap. Cancer Epidemiol 2017;49:19–23. [DOI] [PubMed] [Google Scholar]
- 31. Roy A, Rawal I, Jabbour S, Prabhakaran D.. Tobacco and cardiovascular disease: a summary of evidence. In: Prabhakaran D, Anand S, Gaziano T, Mbanya J, Wu Y, Nugent R, eds. Disease Control Priorities, Third Edition (Volume 5): Cardiovascular, Respiratory, and Related Disorders. Washington (DC): The World Bank, 2017: 57–77. [PubMed] [Google Scholar]
- 32. Pérez-Ríos M, Rey-Brandariz J, Galán I, et al. Methodological guidelines for the estimation of attributable mortality using a prevalence-based method: the STREAMS-P tool. J Clin Epidemiol 2022;147:101–10. [DOI] [PubMed] [Google Scholar]
- 33. Thun MJ, Carter BD, Feskanich D, et al. 50-Year trends in smoking-related mortality in the United States. N Engl J Med 2013;368:351–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma J, Siegel RL, Jacobs EJ, Jemal A.. Smoking-attributable mortality by state in 2014, U.S. Am J Prev Med 2018;54:661–70. [DOI] [PubMed] [Google Scholar]
- 35. Thun MJ, Apicella LF, Henley SJ.. Smoking vs other risk factors as the cause of smoking-attributable deaths. Confounding in the courtroom. J Am Med Assoc 2000;284:706–12. [DOI] [PubMed] [Google Scholar]
- 36. Ruano-Ravina A, Varela Lema L, García Talavera M, et al. Lung cancer mortality attributable to residential radon exposure in Spain and its regions. Environ Res 2021;199:111372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request